Abstract
The optimal development of novel molecularly targeted agents for the treatment of cancer requires a re-evaluation of the current drug development paradigm. selection of patients, optimal biologic dose versus maximum tolerated dose, definition of response and clinical benefit and trial designs that address these considerations are the focus of debate in the field of early cancer therapeutics. we present a review of the opportunities and challenges facing drug development in oncology through the phases of clinical development starting with first-in-human trials.
Introduction
Increased understanding of the biology of cancer and the availability of new technologies, such as micorarrays, proteomics, and novel imaging, has allowed the identification of molecular targets for cancer drug development. The high costs, prolonged timelines and lack of predictive preclinical models for targeted agents continue to pose challenges for the development of new drugs.1 The shift from using cytotoxic chemotherapeutic agents to the new generation of molecularly targeted agents, however, requires re-evaluation of the current drug development paradigm. Once an agent is brought forward for clinical development, questions about how to optimally evaluate the agent remain; for example, which population of patients would benefit from the drug (selection of patients) and whether the agent should be administered alone or evaluated in a rational drug combination for potential synergism.
Basis for maximum tolerated dose
Phase I clinical trials of cytotoxic agents in oncology are traditionally dose-finding studies involving a relatively small number (that is, 20–60) of participants, for which the principal objectives are to define the safety and toxicity profile of an agent, evaluate the pharmacokinetics and determine the recommended phase II dose.2 This is achieved by establishing the maximum tolerated dose for the agent. The basis for recommending the maximum tolerated dose for phase II clinical testing is the assumption that the therapeutic effect and the associated toxic effects are correlated, and that the mechanism of action of both the toxic and therapeutic effects is the same.3 In addition, phase II trials frequently use objective response rate as a surrogate for clinical benefit, providing the basis for proceeding with further development of the agent. Such an assumption might not be appropriate for the new generation of molecularly targeted agents. These agents presumably have a wider therapeutic index, the proposed mechanism of therapeutic versus toxic effects could be different (off-target effects), they might require prolonged doses at relatively low levels to provide clinical benefit (such as progression-free survival [PFS]), and they could be cytostatic rather than cytotoxic (therefore might not cause tumor shrinkage) compared with conventional chemotherapeutic agents. In this case, an optimal biologic dose associated with the desired drug target effect could be a more-relevant end point in early phase trials. Defining optimal biologic dose, however, requires extensive preclinical evaluations to validate the drug target, define ‘optimal target inhibition, and incorporate analytically validated assays into early phase trials of the agent to assess for target modulation.4
Toxic effects associated with targeted agents include rash with anti-EGFR agents, or hypertension associated with anti-VEGF therapies. These adverse effect outcomes could aid in establishing a proof-of-concept drug target effect and help define the optimal target inhibition. The optimal biologic dose, however, needs to be defined by drug target effects in the tumor at a dose and schedule that is associated with tolerable toxicities (such as grades 1 or 2 by the Common Terminology Criteria for Adverse Events [CTCAE] v 3.0).5–7
Time-to-progression versus response rate
Phase II trials are generally small (30–70 participants), single-arm studies for which the primary end point is to determine the efficacy of the compound against a specific histology. Tumor shrinkage provides objective evidence of an antitumor effect, which is used as a surrogate for clinical benefit. objective tumor response can usually be measured easily with imaging techniques such as CT, and standardized criteria have been developed and used in clinical trials such as the WHO response criteria and the Response Evaluation Criteria in solid Tumors (RECIST).8,9 The RECIST define objective tumor response as a 30% reduction in the sum of the longest diameters of all target lesions from baseline. Tumor progression is defined as a 20% increase in size compared with baseline or best response or development of a new lesion. Although objective response rate is considered the standard primary end point for most phase II trials of cytotoxic agents, it might not be adequate for targeted agents when inhibition of tumor growth rather than tumor regression is the primary outcome. PFS (that is, duration of time alive without progression) and time to progression (TTP; that is, duration of time from the start of given therapy until the evidence of tumor progression) could represent useful end points for trials assessing targeted therapies as they are measures of efficacy that do not depend on tumor shrinkage. To be able to accurately assess clinical benefit using PFS or TTP as end points, however, requires randomized, controlled trials that, for example, compare the new agent with an established regimen. In an effort to accurately assess the efficacy of a targeted agent other novel end points have been proposed, such as water fall plots, spaghetti plots of tumor size over time for individual patients, and functional imaging;10 however, these end points have to be validated and their use might depend on the agent under consideration. These end points could be incorporated as additional end points into an early phase trial to evaluate for preliminary evidence of activity. The ability to draw firm conclusions from these additional end points may require the evaluation of a larger number of patients in a given trial, or comparing effects across multiple trials, and needs to be carefully discussed with experienced biostatisticians before making decisions about future agent development.
Clinical trial objectives and end points
Overall survival is the gold standard end point in comparative phase III trials of patients with malignant disease. Assessment of overall survival, however, requires a prolonged observation period, depending on the type and stage of the disease, and can potentially be confounded by treatment with subsequent lines of therapies at the time of progression.11 Thus, TTP or PFS remain useful end points for such types of trials. Selection of patients becomes very important in certain trials of targeted agents; in cases where the agent provides benefit for only a subset of patients, selection is essential as administration of the agent in an unselected population could mask the potential benefit seen in only a subset of patients (Figure 1).
Figure 1 |.
Effect of selection of patients in phase III studies.49 a | Model for a phase III trial in which 100% of patients show a treatment effect. b | Model for a phase III trial in which 25% of patients show a treatment effect. Permission obtained from the American Society of Clinical Oncology © Pegram, M. D. et al. Targeted therapy: wave of the future. J. Clin. Oncol. 23, 1776–1781 (2005).
Choosing an appropriate end point to evaluate in definitive trials is dependent on the disease and the expected drug effect (on the basis of data from previous clinical trials). Treatment with targeted agents can result in three outcomes. First, it could result in tumor shrinkage in the majority of the patients treated, such as imatinib in patients with chronic myelogenous leukemia and gastrointestinal stromal tumors (GISTs).12,13 Second, tumor shrinkage might be observed in only a small subset of patients; for example, EGFR tyrosine kinase inhibitors (TKIs) in patients with non-small-cell lung cancer (NSCLC);14,15 and thirdly tumor stabilization, such as treatment with single agent bevacizumab in several tumor types.16–18 Initial phase I/II trials of single-agent bevacizumab in renal, breast and ovarian cancer showed modest antitumor activity, as shown by RECIST- defined responses (that is, more than 30% shrinkage in sum of the longest dimension of the target lesions) (9–15%); however, it was associated with prolonged TTP.16–18 Such an agent can still be developed in conjunction with chemotherapy with response rate as a primary objective in phase II trials. Imatinib is the first example of the development of a selective TKI with potent anti-cancer activity against selected malignancies. The effects of imatinib in BCR-ABL positive versus negative cells have been demonstrated in preclinical models,19 and subsequently, dramatic response rates in phase III clinical trials established imatinib as an effective molecularly targeted therapy for patients with chronic myelogenous leukemia.20–22 The BCR-ABL fusion protein is formed by the translocation of chromosomes 9 and 22, and has a causal role in the pathogenesis of chronic myelogenous leukemia. Imatinib is also a potent inhibitor of other tyrosine kinases including platelet-derived growth factor receptor (PDGFR) and KIT, and it is highly effective in the treatment of patients with GIST-bearing activating c-KIT mutations. In a clinical trial of 147 patients with unresectable metastatic GIST, the presence of KIT mutations in exon 11 conferred a response rate to imatinib of up to 78%, versus 28% for patients with wild-type c-KIT tumors.20 This raises the question about appropriate selection of patients and enrichment in early phase trials to adequately assess targeted therapies.
Genetic abnormalities and targeted agents
Unfortunately, the majority of human cancers have numerous genetic abnormalities driving tumor growth and survival and there is rarely a single critical target. Thus, the agent of interest might be effective in only a subset of patients in whom the cancer is dependent on the drug target for growth or survival. This is exemplified by the clinical development and eventual role of EGFR TKIs in advanced NSCLC.23
Gefitinib is an orally active selective EGFR TKI. Two large, randomized, double-blind, multicenter, phase II trials14,15 examined the efficacy of gefitinib in patients with advanced NSCLC who were previously treated with one or two chemotherapy regimens. The response rate ranged from 9% to 19% in patients with platinum refractory disease, and median survival was 6–8 months. On the basis of the results of these two phase II trials gefitinib became the first oral agent approved for patients with refractory NSCLC. In a confirmatory phase III trial 1,692 patients with NSCLC who had refractory disease or were intolerant to their latest chemotherapy were randomized to receive gefitinib (250 mg daily) plus best supportive care or placebo plus best supportive care. Gefitinib failed to show a benefit in either the survival or median time-to-treatment failure when compared with placebo.24 A subset analysis revealed that the increased TTP and survival occurred predominantly in patients of Asian origin and in never-smokers compared with non-Asians and former or current smokers. Analysis of the entire unselected population of patients had obscured the benefit of gefitinib.25 Similar clinical benefit for these subsets of patients with NSCLC was also observed in trials of another EGFR TKI, erlotinib.26 It has since been demonstrated that EGFR mutations are present in 12% of Caucasian patients and can predict response to small-molecule EGFR inhibitors. Interestingly, these mutations are more frequent in Asians, never-smokers, women and patients with adenocarcinoma or bronchioloalveolar cancer. Activating EGFR mutations are rare or absent in other tumor types.
Tamura et al.27 prospectively assessed whether specific mutations in the EGFR gene affected the clinical outcomes of patients with NSCLC treated with gefitinib. Patients with recurrent or metastatic NSCLC who harbored EGFR mutations in exons 18, 19, or 21 and had received fewer than two previous chemotherapy regimens were treated with oral gefitinib 250 mg once daily. Of 104 patients recruited, EGFR mutations were detected in 25; 12 patients had deletion mutations in exon 19, and 13 patients had missense mutations (L858R) in exon 21. Overall response rate was 75% (95% CI 57.6–91.0%). Partial responses were seen in 71.4% of patients. Among patients with EGFR mutations, the response rate did not differ significantly between current or former smokers and those who had never smoked (67% versus 79%) or between chemotherapy-naive and post-chemotherapy patients (77% versus 73%). Female patients with a deletion mutation in exon 19 of EGFR tended to have a higher response rate than male patients with the same mutation (89% versus 50%). The median PFS time was 11.5 months and the 1-year survival was 79% (95% CI 63.4–93.8%).27
Another consideration in the clinical use of anticancer therapies is the presence or eventual development of resistance to the targeted agent. Multiple abnormalities (for example, KRAS mutation confers resistance to EGFR-directed therapies in colon cancer)28 and activation of other pathways (for example, insulin-like growth factor receptor 1 mediates resistance to anti-EGFR therapy)29 explain the observed resistance to molecular targeted therapies and provide a rationale for treatment strategies that combine two or more targeted agents30 or the use of less-selective agents that could inhibit multiple signal transduction pathways. Possible factors controlling sensitivity or resistance to molecular targeted therapies include increased transcriptional expression of the target because of gene amplification, the presence of mutations downstream from the intended target (for example, KRAS mutations conferring resistance to anti-EGFR antibody therapy28), and the emergence of additional mutations in the target (for example, secondary mutation in exon 20 [T790M] of EGFR that confers resistance after initial response to EGFR TKIs in NSCLC). It is not only the presence but also the type of mutation that can confer resistance, as shown by responses to sunitinib in imatinib-resistant GIST, which correlate with KIT and PDGFRA mutation status.31 In a report on the clinical benefit observed with sunitinib in patients with imatinib-resistant GIST, PFS and overall survival were significantly longer for patients with either a primary KIT exon 9 mutation or wild-type KIT/PDGFRA compared with patients with a KIT exon 11 mutation.31 Another mechanism of resistance could be caused by overexpression of multidrug transporter membrane proteins.22,32
Use of a randomized discontinuation design
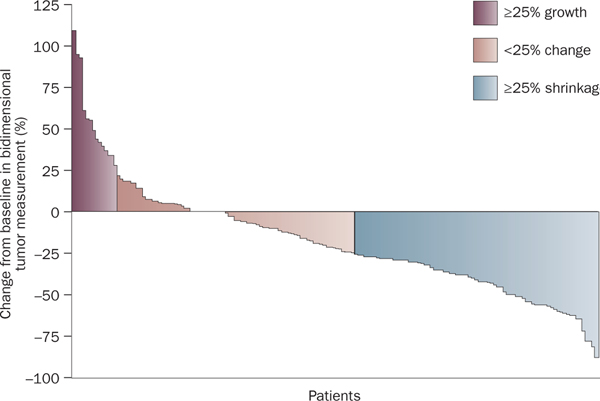
A drug with limited tumor efficacy but increased non-progression rate and TTP is the rationale for a randomized discontinuation (or withdrawal) trial (RDT). The best example of this scenario is the RDT of sorafenib in patients with metastatic renal-cell carcinoma. This study was initially proposed in 1975 and employed an enrichment design to evaluate the activity of a drug while use of placebo was decreased.33 Patients received the study drug for an initial period, then, upon restaging were assigned to one of three different categories, on the basis of measurement of target lesions compared with baseline: tumor shrinkage >25%; tumor growth or shrinkage <25%; and tumor growth >25%. Patients assigned to the tumor growth or shrinkage <25% category were randomly assigned to continue on the study drug or receive placebo and then followed for PFS.34 In this RDT study33 sorafenib was initially administered to all patients in a 12-week, open-label run-in period at a dose of 400 mg twice daily. After the 12-week run-in period and evaluation of disease status, 73 patients (36%) had achieved tumor shrinkage ≥25% compared with baseline, 69 patients (34%) had tumor measurements that remained within 25% of baseline levels, and 51 patients (25%) showed either tumor growth ≥25% or other evidence of progression at or before week 12 (Figure 2). Of the 69 patients identified at 12 weeks with tumor growth or shrinkage of <25% who were eligible for entry into the randomized phase, a total of 65 patients were randomly assigned to receive sorafenib (32 patients) or placebo (33 patients). At 12 weeks post-randomization (24 weeks from study entry), 50% of patients (16 of 32 patients) who received sorafenib were progression free, compared with only 18% of patients (6 of 33) who received placebo (P = 0.0077). Median PFS from the 12-week randomization was also significantly longer in the sorafenib group (24 weeks) compared with the placebo group (6 weeks).35 This study led to a randomized, placebo-controlled phase III trial of patients with metastatic renal-cell carcinoma; 451 patients received 400 mg sorafenib twice daily and 452 patients received placebo. Median PFS was 5.5 months in the sorafenib group and 2.8 months in the placebo group (HR 0.44; 95% CI 0.35 to 0.55, P <0.01). The first interim analysis of overall survival showed that sorafenib reduced the risk of death compared with placebo (HR 0.72; 95% CI, 0.54–0.94, P = 0.02) and prolonged PFS. Partial responses were reported as the best response in 10% of patients who received sorafenib and in 2% of those who received placebo (P <0.001). The positive results of the phase II RDT study by Ratain et al. were confirmed by Escudier et al.36 in a placebo-controlled phase III trial, resulting in FDA approval of sorafenib.
Figure 2 |.
Bidimensional tumor measurements in a randomized discontinuation phase II clinical trial of 193 patients.35 Permission obtained from the American Society of Clinical Oncology © Ratain, M. J. et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 24, 2505–2512 (2006).
An example of an RDT that did not produce confirmatory phase III data is the use of carboxyaminoimidazole (CAI) in renal-cell carcinoma, in which a total of 368 patients were accrued and randomly allocated to receive therapy with 250 mg/day CAI (trial CALGB 69,901). Patients with stable disease at the 16-week evaluation point were randomly assigned in a double-blind manner to continued CAI or placebo. At the randomization point, 51% of patients had progressive disease, 30% withdrew, 1% had partial response, and 17% had stable disease. Those with stable disease were randomly assigned to treatment. The trial was halted after the first 49 patients randomly assigned did not respond to CAI.37 There are multiple limitations to applying the RDT design, especially in an unselected population of patients with slow-growing tumors.38 The ability to enrich the population of patients on the basis of the presence of a specific target might increase usefulness and applicability of RDT trials.39
Single versus combination regimens
Additive or synergistic effects of a drug combination have been observed in trials that include bevacizumab, a monoclonal antibody to VEGF. Initial phase I and II trials of single-agent bevacizumab failed to produce tumor regressions in the majority of patients, but there was evidence of clinical benefit as noted by a prolongation of TTP.16–18 Preclinical data indicate that this agent should be studied in combination with multiple cytotoxics. The results revealed significant synergism that resulted in improved response rate and overall survival in patients with metastatic colorectal cancer and NSCLC. In a randomized phase II trial, patients with metastatic colorectal cancer were randomly allocated to 5-fluorouracil (5-FU 500 mg/m2) or leucovorin (LV 500 mg/m2) alone, 5-FU combined with LV plus low-dose bevacizumab (5 mg/kg every two weeks), or to 5-FU combined with LV plus high-dose bevacizumab (10 mg/kg every two weeks). The addition of low-dose or high-dose bevaci- zumab in combination with Fu and LV resulted in higher response rates (40%, and 24%, respectively) compared with a 17% response rate in the control arm (FU and LV alone). Increased median time-to-disease progression and median survival rates were also reported with the addition of bevacizumab.40 These results led to a randomized phase III study of bevacizumab plus chemotherapy as first-line therapy for patients with metastatic colorectal cancer. Hurwitz et al. compared irinotecan, bolus 5-FU, and LV (IFL) plus bevacizumab (5 mg/kg every 2 weeks) with IFL plus placebo. The addition of bevacizumab resulted in a significant improvement in survival (20.3 months versus 15.6 months, HR for death of 0.66, P <0.001).41
The drug works best in the adjuvant setting
Some targeted agents may show limited activity in the metastatic setting but demonstrate survival advantages when used in patients with early-stage disease. This can be exemplified by trials evaluating immune-based therapies that are thought to be more efficacious in low-volume disease. For instance, interferon α (IFN-α) has been evaluated as an adjuvant therapy for high-risk melanoma. The actual clinical benefit of adjuvant IFN-α and its effect on overall survival is, however, controversial. Two randomized trials were initiated to investigate the effect of IFN-α on overall survival and disease-free survival in patients with high-risk or intermediate-risk melanoma. In both trials the significant effect of IFN-α on overall survival at 5 years was lost with longer-term follow up.42,43 A report on the efficacy and toxicity of ganglioside GM2-KLH21 in the adjuvant treatment of melanoma raised concerns about the detrimental effects of this vaccine in the adjuvant setting.44 Therefore, even though the concept of therapies being more effective in the minimal disease state has been postulated, therapies need to be carefully evaluated in adequately powered trials in the adjuvant setting with a special emphasis on the risks associated with the study therapy.
Future directions
There is an increasing need for novel trial designs that can effectively evaluate the new generation of molecularly targeted therapies. The design of randomized, controlled trials needs to be revised to determine objectives such as optimal biologic dose, which requires incorporation of analytically validated assays performed on uniformly handled tissues into clinical human trials. Preclinical investigations, therefore, need to focus on qualifying the assay methodology, tissue handling, processing and storage that is then incorporated into the trial design so that reliable data can be obtained from trials to form a basis for further decisions regarding the clinical development of the agent.45,46 These first-inhuman trials incorporating such assays can be used to select the most promising agent among analogs developed to target a particular pathway during preclinical development and evaluation, and can provide essential pharmacokinetic and target modulation data that inform further agent development.45 Some of these pilot trials could be conducted earlier than the traditional phase I trials under the ‘exploratory investigational new drug’ guidance proposed by the FDA.45,47
An important consideration while designing early phase trials of molecularly targeted agents is to have meaningful end points such as PFS or TTP as opposed to objective response rate. Depending on the agent under study, appropriate trial designs and end points will need to be chosen to optimize and develop promising agents and limit the further testing of agents that are unlikely to show clinical benefit.
As shown with bevacizumab, designing combination trials in the era of molecularly targeted agents does not require single-agent activity as proof of principle to progress to the next stage of drug development. However, as combinations of molecularly targeted agents affect multiple cellular pathways, it is important for reliable new technologies (that is, microarrays, proteomics) to be validated in preclinical models. This validation will help to define the multiple intracellular pathways affected by combinations of novel molecularly targeted agents.
Conclusions
Incorporation of such assays into early phase trials (phase I) will allow an early assessment of target modulation in humans at different drug dose levels in both tumor biopsies and surrogate tissues. The design of subsequent studies will need to take various factors into account (Box 1). Phase II studies must consider the biological expectations of the molecular target agent (tumor shrinkage versus nonshrinkage), the modulation of the target documented in previous studies (exploratory investigational new drug and phase I studies), appropriate selection of patients (enrichment studies)48 and early determination of activity (fluorodeoxyglucose or [18F]-3’-fluoro-3’-deoxy-L-thymidme-PET, dMRI, dCT, svEGF) for a given target. Phase III studies should be pursued only in the presence of sufficient signs of activity (tumor responses or biological activity), possibly after randomized phase II studies of adequate power in subset groups of patients. these strategies will hopefully reduce the risk of negative large phase III studies, and increase the number of new drug approvals for the treatment of cancer.
Box 1 |. Drug development pathways after phase I studies.
Randomized discontinuation design
The drug has minimal antitumor efficacy but increases non-progression rate and time to progression
Randomized phase ii design
The drug has minimal antitumor efficacy but is additive to or synergistic with chemotherapy, radiotherapy, or other agents
The drug is a tumor stabilizer
Phase iii study
The drug is a tumor stabilizer
Adjuvant studies
The drug works better at earlier stages of the disease
Thus, the various considerations underlying the optimal development plan for a molecularly targeted agent include decisions regarding the appropriate population of patients to study in early versus late phase trials (unselected patients, trials in a given histology, selection of patients on the basis of the presence of a known mutation), single-agent versus combination trials (two targeted therapies or a targeted agent combined with a chemotherapeutic agent) and the appropriate end points for such trials.
Key points.
The design of trials to test molecularly targeted agents need to be revised to achieve objectives such as optimal biologic dose, which might require incorporation of analytically validated assays into initial clinical studies
The design of early phase trials of molecularly targeted agents might need to have end points such as progression-free survival or time to progression as opposed to objective response rate
The design of subsequent phase II studies will need to take into consideration the biological expectations of the molecular target agent (tumor shrinkage versus nonshrinkage)
Phase III studies should be pursued only in the presence of sufficient signs of activity, and ideally only initiated after randomized phase ii studies of adequate power
References
- 1.Kola I & Landis J Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 3, 711–716 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Kummar S et al. Drug development in oncology: classical cytotoxics and molecularly targeted agents. Br. J. Clin. Pharmacol. 62, 15–26 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox E et al. Clinical Trial Design for Target-Based Therapy. Oncologist 7, 401–409 (2002). [DOI] [PubMed] [Google Scholar]
- 4.[No authors listed] Steps to consider in pharmacodynamic assay development http://dtp.nci.nih.gov/docs/phase0/PharmacoDynamicAssayDeveloment.html (accessed 25 september (2008).
- 5.Perez-Soler R et al. Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. J. Clin. Oncol. 22, 3238–3247 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Ciardiello F et al. Antitumor effect and potentiation of cytotoxic drug activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin. Cancer Res. 6, 2053–2063 (2000). [PubMed] [Google Scholar]
- 7.Herbst RS et al. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J. Clin. Oncol. 23, 2544–2555 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Therasse P et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United states, National Cancer Institute of Canada. J. Natl Cancer Inst. 92, 205–216 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Park JO et al. Measuring response in solid tumors: comparison of RECIST and WHO response criteria. Jpn J. Clin. Oncol. 33, 533–537 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Schuetze SM et al. Selection of Response Criteria for Clinical Trials of sarcoma Treatment. Oncologist 13 (Suppl. 2), 32–40 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Millar AW & Lynch LP Rethinking clinical trials for cytostatic drugs. Nat. Rev. Cancer 3, 540–545 (2003). [DOI] [PubMed] [Google Scholar]
- 12.weinstein i. B & Joe AK Mechanisms of disease: oncogene addiction—a rationale for molecular targeting in cancer therapy. Nat. Clin. Pract. Oncol. 3, 448–457 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Sharma SV et al. A common signalling cascade may underlie “addiction” to the src, BCR-ABL, and EGF receptor oncogenes. Cancer Cell 10, 425–435 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kris MG et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: A randomized trial. JAMA 290, 2149–2158 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Fukuoka M et al. Multi-institutional randomized phase ii trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 21, 2237–2246 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Yang JC et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N. Engl. J. Med. 349, 427–434 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rugo HS Bevacizumab in the treatment of breast cancer: rationale and current data. Oncologist 9 (Suppl. 1), 43–49 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Cannistra SA et al. Bevacizumab in patients with advanced platinum-resistant ovarian cancer [Abstract]. ASCO Meeting Abstracts 24, 5006 (2006). [Google Scholar]
- 19.Gambacorti-Passerini C et al. inhibition of the ABL kinase activity blocks the proliferation of BCR/ABL+ leukemic cells and induces apoptosis. Blood Cells Mol. Dis. 23, 380–394 (1997). [DOI] [PubMed] [Google Scholar]
- 20.Blanke CD et al. Evaluation of the safety and efficacy of an oral molecularly-targeted therapy, STI571, in patients (Pts) with unresectable or metastatic gastrointestinal stromal tumors (GISTS) expressing C-KIT (CD117) [Abstract]. Proc. Am. Soc. Clin. Oncol. 20, 1 (2001). [Google Scholar]
- 21.Deininger M et al. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood 105, 2640–2653 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Deininger M. w. & Druker BJ specific targeted therapy of chronic myelogenous leukemia with imatinib. Pharmacol. Rev. 55, 401–423 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Sequist LV et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J. Clin. Oncol. 26, 2442–2449 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Thatcher N et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 366, 1527–1537 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Chang A et al. Gefitinib (IRESSA) in patients of Asian origin with refractory advanced non-small cell lung cancer: subset analysis from the ISEL study. J. Thorac. Oncol. 1, 847–855 (2006). [PubMed] [Google Scholar]
- 26.Clark GM et al. smoking history and epidermal growth factor receptor expression as predictors of survival benefit from erlotinib for patients with non-small-cell lung cancer in the National Cancer Institute of Canada Clinical Trials Group study Br.21. Clin. Lung Cancer 7, 389–394 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Tamura K et al. Multicentre prospective phase II trial of gefitinib for advanced non-small cell lung cancer with epidermal growth factor receptor mutations: results of the west Japan Thoracic Oncology Group trial (wJTOG0403). Br. J. Cancer 98, 907–914 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amado RG et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 1626–1634 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Morgillo F et al. Implication of the insulin-like growth factor-IR pathway in the resistance of non-small cell lung cancer cells to treatment with gefitinib. Clin. Cancer Res. 13, 2795–2803 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Dancey JE & Chen HX strategies for optimizing combinations of molecularly targeted anticancer agents. Nat. Rev. Drug Discov. 5, 649–659 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Heinrich MC et al. Sunitinib (SU) response in imatinib-resistant (IM-R) GIST correlates with KIT and PDGFRA mutation status [Abstract]. ASCO Meeting Abstracts 24, 9502 (2006). [Google Scholar]
- 32.Xu J et al. Human multidrug transporter ABCG2, a target for sensitizing drug resistance in cancer chemotherapy. Curr. Med. Chem. 14, 689–701 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Amery W & Dony J A clinical trial design avoiding undue placebo treatment. J. Clin. Pharmacol. 15, 674–679 (1975). [DOI] [PubMed] [Google Scholar]
- 34.Kopec JA et al. Randomized discontinuation trials: utility and efficiency. J. Clin. Epidemiol. 46, 959–971 (1993). [DOI] [PubMed] [Google Scholar]
- 35.Ratain MJ et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 24, 2505–2512 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Escudier B et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 356, 125–134 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Stadler WM et al. Successful implementation of the randomized discontinuation trial design: an application to the study of the putative antiangiogenic agent carboxyaminoimidazole in renal cell carcinoma—CALGB 69901. J. Clin. Oncol. 23, 3726–3732 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Freidlin B & Simon R Evaluation of randomized discontinuation design. J. Clin. Oncol. 23, 5094–5098 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Freidlin B & Simon R Adaptive signature design: an adaptive clinical trial design for generating and prospectively testing a gene expression signature for sensitive patients. Clin. Cancer Res. 11, 7872–7878 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Kabbinavar F et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (Lv) with FU/Lv alone in patients with metastatic colorectal cancer. J. Clin. Oncol. 21, 60–65 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Hurwitz H et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335–2342 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Kirkwood JM et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial esT 1684. J. Clin. Oncol. 14, 7–17 (1996). [DOI] [PubMed] [Google Scholar]
- 43.Cascinelli N et al. Effect of long-term adjuvant therapy with interferon alpha-2a in patients with regional node metastases from cutaneous melanoma: a randomised trial. Lancet 358, 866–869 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Eggermont AM et al. EORTC 18961: Post-operative adjuvant ganglioside GM2-KLH21 vaccination treatment vs observation in stage II (T3-T4N0M0) melanoma: 2nd interim analysis led to an early disclosure of the results [Abstract]. ASCO Meeting Abstracts 26, 9004 (2008). [Google Scholar]
- 45.Kummar S et al. Compressing drug development timelines in oncology using phase ‘0’ trials. Nat. Rev. Cancer 7, 131–139 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Murgo AJ et al. Designing phase 0 cancer clinical trials. Clin. Cancer Res. 14, 3675–3682 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.FDA Center for Drug evaluation and research (CDER). Guidance for industry, investigators, and reviewers exploratory IND studies [2006] http://www.fda.gov/cder/guidance/7086fnl.htm (accessed 25 september 2008).
- 48.Collins JM imaging and other biomarkers in early clinical studies: one step at a time or re-engineering drug development? J. Clin. Oncol.23, 5417–5419 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Pegram MD et al. Targeted therapy: wave of the future. J. Clin. Oncol. 23, 1776–1781 (2005). [DOI] [PubMed] [Google Scholar]