Abstract
Introduction
Percutaneous peripheral nerve stimulation (PNS) provides an opportunity to relieve chronic low back pain and reduce opioid analgesic consumption as an alternative to radiofrequency ablation and permanently implanted neurostimulation systems. Traditionally, the use of neurostimulation earlier in the treatment continuum has been limited by its associated risk, invasiveness, and cost.
Methods
Percutaneous PNS leads (SPRINT MicroLead) were placed bilaterally to target the medial branches of the dorsal rami nerves under image guidance. The percutaneous leads were connected to miniature wearable stimulators (SPRINT PNS System) for the 1‐month therapy period, after which the leads were removed. Pain and disability were assessed long‐term up to 12 months after lead removal.
Results
Substantial, clinically significant reductions in average pain intensity (≥50% reduction as measured by the Brief Pain Inventory Short Form) were experienced by a majority of subjects (67%) at end of treatment compared to baseline (average 80% reduction among responders; P < 0.05, analysis of variance; n = 9). Twelve months after the end of PNS treatment, a majority of subjects who completed the long‐term follow‐up visits experienced sustained, clinically significant reductions in pain and/or disability (67%, n = 6; average 63% reduction in pain intensity and 32‐point reduction in disability among responders). No serious or unanticipated adverse events were reported.
Conclusions
This study challenges the long‐held notion that a positive trial of PNS should be followed by a permanent implant in responders. Percutaneous PNS may serve as an effective neurostimulation therapy for patients with chronic low back pain and should be considered earlier in the treatment continuum as a motor‐sparing means of avoiding opioids, denervation, and permanently implanted neurostimulation systems.
Keywords: low back pain, percutaneous peripheral nerve stimulation, chronic pain, disability
Introduction
Chronic low back pain (LBP) is a leading cause of disability among adults and is one of the most prevalent musculoskeletal conditions that is challenging to treat.1, 2, 3 Disability as a result of chronic LBP is a common complaint, as pain often decreases quality of life by interfering with function and reducing the patient’s ability to complete normal activities of daily living, such as walking, housework, or personal care.4, 5, 6 Further, because chronic LBP is difficult to diagnose and treat, it represents a significant healthcare burden. In many cases (up to 85%), chronic LBP may be nonspecific or have an unidentified cause.7 After LBP has become chronic (typically defined as pain lasting longer than 12 weeks), pain and disability can intensify as the result of a cycle of central sensitization, whereby changes in central pain processing result in hypersensitivity to normal inputs and persistent pain, even if injuries have healed.8, 9, 10, 11 It is the presence of both peripheral and central pain generators in chronic LBP that has made successful treatment with conventional methods challenging.12
Chronic LBP and the Opioid Crisis
Despite changes in recent years to LBP treatment guidelines, which suggest that opioids should not be offered for the treatment of chronic LBP, opioids continue to be commonly prescribed and a diagnosis of chronic LBP is associated with an increased likelihood of opioid use and abuse.13, 14, 15 Because there is little evidence supporting the efficacy of opioids in treating chronic LBP, improving the patient’s ability to return to work, or reducing the need for other pain therapies,16 it is widely accepted that an effective, non‐opioid treatment is needed to treat chronic back pain and limit opioid use to prevent dependence and addiction.
Treatments for Chronic LBP
Historically, the treatment paradigm for non‐opioid pain management has included a wide range of approaches of increasing invasiveness, from medications (eg, non‐opioid analgesics such as nonsteroidal anti‐inflammatory drugs, muscle relaxants, tricyclic antidepressants, or corticosteroids17, 18), to transcutaneous electrical nerve stimulation (TENS), physical therapy, and interventions such as anesthetic or steroid injections, radiofrequency ablation, permanently implanted neurostimulation or intrathecal drug delivery systems, or surgery.
Although transcutaneous electrical nerve stimulation (TENS; applied via surface electrodes) is used as a minimally invasive treatment option by patients, it may cause discomfort due to activation of cutaneous nerve endings or skin irritation at the stimulation intensities required to activate the deep pain‐relieving nerve fibers, leading to low rates of patient compliance or ineffective treatment at comfortable intensities.19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 Interestingly, although some payer coverage policies require that a patient have a positive response to TENS prior to undergoing peripheral nerve stimulation (PNS), TENS has not been found to be a reliable predictor of PNS efficacy.35 Physical therapy can reduce back pain and disability, and thereby opioid usage, but patients may be unwilling or unable to comply with prescribed regimens.36, 37, 38, 39, 40, 41, 42 Anesthetic and/or corticosteroid injections usually only provide short‐term relief and must be repeated.43, 44, 45, 46, 47, 48 Radiofrequency ablation can provide pain relief in well‐selected patients with facetogenic pain, but is highly dependent on physician expertise, destroys nerve tissue and/or denervates key paraspinal muscles, and is often followed by a return of pain after several months.49
Surgery and many permanently implanted neurostimulation systems can be highly invasive, complex, irreversible, and carry risks of complications. Surgical procedures (eg, spinal fusion, disc replacement) for back pain that attempt to repair physical deformities frequently fail to reduce pain or disability50, 51, 52 and may result in failed back surgery syndrome53 or a need for reoperation.54, 55, 56, 57 Permanently implanted neurostimulation (eg, spinal cord stimulation [SCS]) systems can reduce pain, opioid use, and disability,58, 59, 60, 61, 62, 63, 64, 65, 66, 67 but traditionally multifactorial etiology pain, as is common among those with chronic LBP, has been difficult to successfully treat conventionally.68, 69, 70 Due in part to the invasiveness, risks of the surgery and implantation of leads near the spinal cord, high complication rate,71, 72, 73, 74, 75, 76 and associated expense, SCS is typically relegated to a treatment of last resort (ie, only employed after other therapies have failed) and only used in about 5% of candidates.77
An effective and less invasive system is needed that does not have the costs, risks, complications, and delayed care associated with previous therapies,78, 79 especially if such a system may permit short‐term use to interrupt the cycle of chronic pain and provides long‐term pain relief that prevents the recurrence of pain or the need for surgery or a permanent implant.80, 81 Percutaneous PNS consists of 1 or 2 fine‐wire leads (Figure 1), which are implanted via a percutaneous introducer and connected to a miniature wearable stimulator that is programmed by the clinician and adjusted by the patient. The system enables delivery of electrical stimulation to nerves innervating the region of pain (eg, the low back), while avoiding the challenges associated with permanently implanted neurostimulation systems. Studies suggest that use of neurostimulation earlier in the treatment continuum could improve patient outcomes, for example, by reducing the number of hospitalizations and clinic visits, or reducing opioid usage.82 An effective option is needed earlier in the treatment continuum that can reduce pain, opioid use, and disability, with the benefits of a neurostimulation system, while avoiding the need for a permanently implanted system and the associated risks of complications.78, 79 Percutaneous PNS provides an opportunity as an earlier neurostimulation intervention that may preclude the need for opioids, denervation, surgery, and permanently implanted systems, particularly given evidence of long‐term relief in patients with chronic pain.80, 81, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97 A recently published double‐blinded randomized controlled trial (RCT) assessing the efficacy of percutaneous PNS in the treatment of chronic postamputation pain demonstrated significant and durable pain relief and improvement in quality of life with results sustained through 1 year.81, 97 The present investigation was designed as a prospective case series study to determine the feasibility of generating similar sustainable reductions in pain and disability and improvements in quality of life in patients with LBP using the same device.
Figure 1.
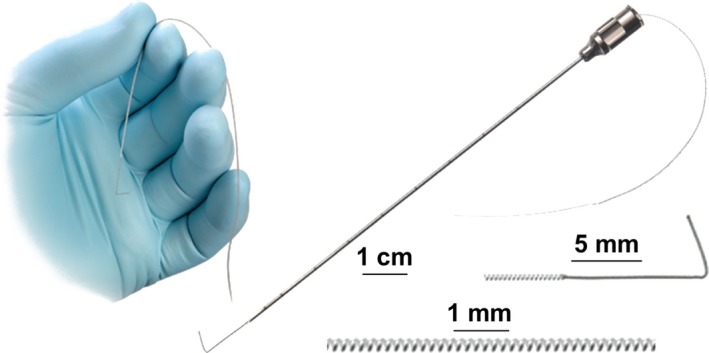
Percutaneous peripheral nerve stimulation (PNS) lead used for treatment of chronic low back pain (LBP). Percutaneous PNS was delivered using a coiled, fine‐wire MicroLead (SPR Therapeutics, Inc.) for 1 month for the treatment of chronic LBP. Figure used with permission from SPR Therapeutics, Inc.
Methods
Individuals with chronic LBP were screened for enrollment in a prospective case series study approved by U.S. Food and Drug Administration (FDA) investigational device exemption and the institutional review board (IRB; Quorum IRB, Seattle, WA, U.S.A.; registered on ClinicalTrials.gov), and written informed consent was obtained from participants. Although the device was investigational at the time of enrollment, the SPRINT PNS System (SPR Therapeutics, Inc., Cleveland, OH, U.S.A.) has since received FDA 510(k) clearance and is indicated for use “up to 60 days in the back and/or extremities for: (1) Symptomatic relief of chronic, intractable pain, post‐surgical and post‐traumatic acute pain; (2) Symptomatic relief of post‐traumatic pain; and (3) Symptomatic relief of post‐operative pain. It is not intended to treat pain in the craniofacial region.”
Participants must have had chronic axial LBP (ie, lumbar pain with a duration ≥ 12 weeks) with at least 4 weeks of stable analgesic medication usage. Subjects completed a 7‐day baseline diary by recording their daily average and worst pain intensities on a numeric rating scale from 0 to 10 (Brief Pain Inventory Short Form, BPI‐3, BPI‐5). To enroll, subjects must have had a baseline average BPI‐5 score (average pain intensity) ≥ 4. Subjects were excluded from participation if they had any of the following: radicular pain; previous lumbar surgery; signs of a serious underlying cause of LBP (eg, cancer, chronic infection, metabolic bone disorder); anesthetic injections within 3 months of baseline; radiofrequency ablation within 6 months of baseline; conditions such as fibromyalgia, multiple sclerosis, or spinal cord injury; pending litigation; signs of infection on or around the low back or other conditions that increase the risk for infection; allergy to medical‐grade adhesives or tapes; an implanted pacemaker/defibrillator or neurostimulator; body mass index (BMI) ≥ 40; or depression (score > 20 on the Beck Depression Inventory [BDI‐II]). Due to the feasibility nature of this prospective case series study, participants were not required to have previously received a specific LBP diagnosis or to have completed prior diagnostic testing or imaging confirming a particular etiology of LBP.
Percutaneous, open‐coil PNS leads (MicroLead; SPR Therapeutics) were implanted bilaterally under sterile conditions with the subject in a prone position to target the medial branches of the dorsal rami nerves. The location for lead placement in each participant was selected following physical examination and manual palpation by the physician to determine the location of axial LBP. Leads were implanted at the segmental level corresponding with the center of each subject’s painful region, confirmed by ultrasound.
To identify the correct location of lead placement, a stimulating probe was first placed into the tissue under ultrasound guidance, using known anatomical landmarks to target the medial branches of the dorsal rami after the nerve exits the intervertebral foramen as it lies along the lamina, medial and inferior to the facet joint (Figure 2). Stimulation of the nerve target (medial branch of the dorsal ramus nerve) was confirmed with selective activation of the lumbar multifidus and comfortable contractions overlapping the region of pain. Upon successful stimulation of the medial branches of the dorsal ramus, the stimulating probe was removed and the percutaneous PNS lead was implanted with the same approach via a preloaded introducer. Multifidus contractions at the final location of PNS lead placement were verified with ultrasound visualization and patient‐reported sensations of stimulation.
Figure 2.
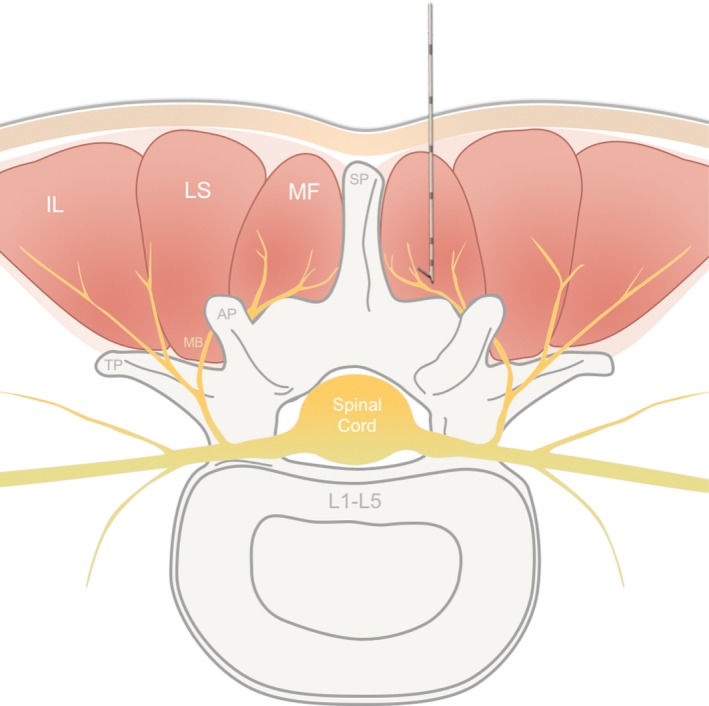
Anatomical target of percutaneous peripheral nerve stimulation lead placement for treatment of chronic low back pain. Percutaneous fine‐wire leads (SPRINT MicroLead, SPR Therapeutics, Inc.) were placed to target the medial branch (MB) of the dorsal ramus, medial and inferior to the facet joint in the center of the region of pain. Selective activation of the multifidus (MF) overlapping the area of pain confirmed appropriate lead placement. A cross‐sectional view of the lumbar paraspinal anatomy is shown, with the lead and introducer placed targeting the MB of the dorsal ramus. AP, articular process; IL, iliocostalis; LS, longissimus; SP, spinous process; TP, transverse process.
The percutaneous leads remained implanted for the duration of the 1‐month therapy and were connected to the miniature wearable stimulators (SPRINT PNS System; SPR Therapeutics). Stimulation was programmed to selectively stimulate the medial branches of the dorsal rami, the nerves innervating the multifidi, to result in comfortable cycling activation in the region of pain (frequency = 12 Hz). The stimulators were programmed such that each subject received a customized range of intensities, which generated strong, but comfortable sensations. Subjects were encouraged to use stimulation for 6 hours per day for each day of the 1‐month treatment period, while continuing their normal daily routines and activities.
During treatment with percutaneous PNS, subjects were not allowed to engage in or receive any other treatments for LBP, apart from their baseline medications. Each week of treatment, subjects recorded daily pain levels and analgesic medication consumption in diaries and returned to the clinic for evaluation and assessments (eg, disability via the Oswestry Disability Index [ODI]; patient global impression of change [PGIC]; adverse events). Leads were removed at the end of the 1‐month therapy (end of treatment). Subjects later returned to the clinic for follow‐up visits and assessments at 3, 6, and 12 months after the end of treatment.
Results
This report reviews the long‐term results of 9 subjects enrolled in this prospective case series study who met the eligibility criteria and received percutaneous PNS for the treatment of chronic LBP. This report is the first to describe the sustained results among responders at 1 year after the end of the PNS therapy.
Baseline Characteristics and PNS Treatment
At enrollment, subjects were on average 53.3 years old with an average BMI of 28.9. The average duration of chronic LBP prior to enrollment was 10 years, despite use of several therapies for LBP, such as opioids, non‐opioids, physical therapy, and injections. After the physical examination and review of LBP‐related history at the baseline visit, the etiology of chronic LBP for 1 participant was determined to be degenerative disc disease, but a majority of the participants (n = 8/9) had nonspecific axial LBP, or pain of an unknown cause. Each subject underwent implantation of fine‐wire percutaneous PNS leads without complications, as outlined in the Methods section. All subjects received bilateral percutaneous PNS with 2 leads, except for 1 subject with unilateral, right‐sided pain who received only 1 PNS lead ipsilateral to the side of pain. Subjects reported that stimulation of the medial branches of the dorsal rami nerves resulted in comfortable sensations in regions overlapping their LBP.
Correct lead positioning was confirmed during weekly visits by inspection of the lead exit site, queries for changes in sensation with stimulation, and evaluation of stimulation thresholds for muscle activation. At the end of the 1‐month therapy, the percutaneous PNS leads were removed without discomfort or complication.
Results at End of PNS Treatment
After 1 month of treatment with percutaneous PNS, substantial, clinically significant reductions in average pain intensity (≥ 50% as measured on the BPI‐5) compared to baseline were experienced by a majority of subjects (67%, average 80% reduction among responders; P < 0.05, analysis of variance; n = 9; 95% confidence interval [CI] [0.36, 0.97]). Clinically significant reductions in disability (≥ 10‐point reduction on the ODI) were also experienced by a majority of subjects (67%, average 22.9‐point reduction among responders; n = 9; 95% CI [0.36, 0.97]).6 These subjects also reported substantial reductions in analgesic usage, as 83% of subjects (n = 5/6 taking medications at baseline; 95% CI [0.53, 1.13]) reported a 50% or greater reduction in total analgesic medication usage (ie, both opioids and non‐opioids). Importantly, all subjects also either successfully avoided opioids during treatment with PNS (n = 8) or ceased using opioids with PNS (n = 1). On average, subjects reported that their quality of life was “much improved” with PNS treatment (PGIC, on a scale of very much worse to very much improved). A subject satisfaction survey assessed at the end of treatment found that a majority (88%) would recommend PNS to a friend with LBP and a majority (88%) also preferred to use PNS over analgesic medications (n = 8; 95% CI [0.65, 1.10]).
Sustained Results at 1 Year Post‐Treatment
Twelve months after the end of PNS treatment, a majority (67%) of subjects completing the long‐term follow‐up visits reported clinically significant reductions in pain intensity and/or disability (n = 6; 95% CI [0.28, 1.04]). Among those completing long‐term follow‐up visits, 50% experienced substantial clinically significant reductions (≥ 50%) in pain intensity, which were sustained at 12 months (n = 6; average 63% reduction in pain intensity among responders; Figure 3). Further, 50% also experienced clinically significant reductions in disability at 12 months after the end of PNS treatment (average 32‐point reduction in ODI score among responders; Figure 4).
Figure 3.
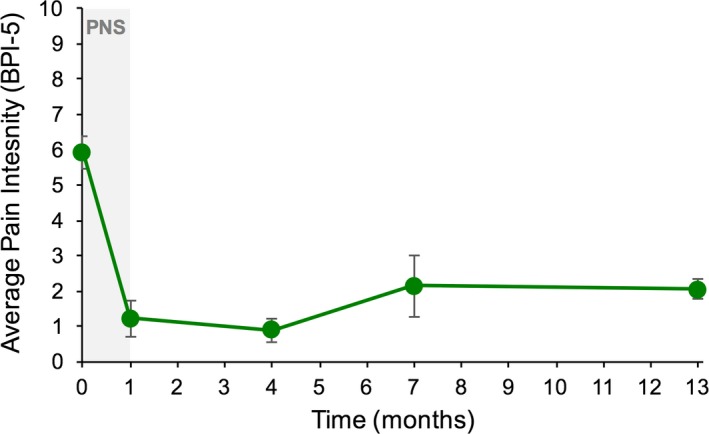
Average pain intensity among responders over time. Subjects experiencing a clinically significant reduction in pain at the end of treatment (≥ 50% reduction) continued to experience sustained results long term, through at least 12 months after the end of peripheral nerve stimulation (PNS) treatment. The average reduction among responders 1 year after end of treatment with PNS was 63%. BPI‐5, Brief Pain Inventory Short Form.
Figure 4.
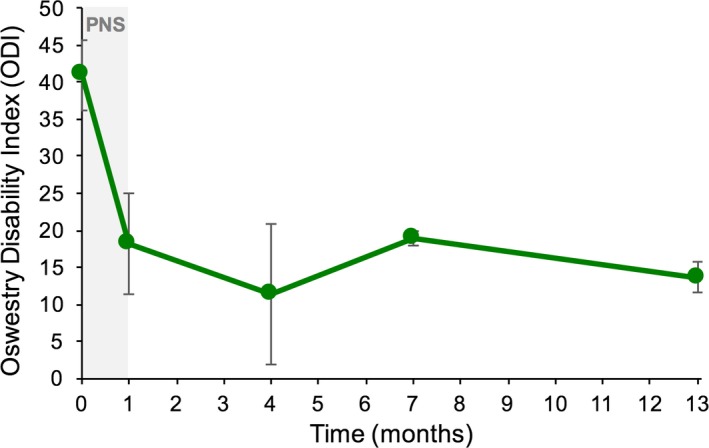
Oswestry Disability Index results among responders over time. Subjects experiencing a clinically significant reduction in disability (≥ 10 points) continued to experience sustained results long term, through at least 12 months after the end of peripheral nerve stimulation (PNS) treatment. The average reduction in disability among responders 1 year after the end of treatment with PNS was 32 points. A 10‐point improvement in the Oswestry Disability Index score is considered clinically significant.
A year after treatment with PNS, a majority of subjects (83%) reported improvement in their quality of life since enrollment due to PNS (PGIC; 95% CI [0.54, 1.13]). Results of a subject satisfaction survey at 12 months after end of treatment revealed that all subjects completing the 12‐month follow‐up visit (100%) were either satisfied or very satisfied with the pain relief they received following stimulation therapy. All subjects (100%) also reported that had this method of percutaneous PNS been previously available, they would prefer to have pursued PNS earlier in the treatment for their LBP.
Safety
No serious or unanticipated adverse events occurred in this study of percutaneous PNS for the treatment of chronic LBP. Reports of skin irritation were the only adverse events related to the device or procedure and occurred in 2 subjects. These subjects reported redness and itching at the location where dressings were located on the skin. These subjects were provided with latex‐free dressings to help reduce the risk for skin irritation.
Discussion
This report is the first to explore the long‐term effects of percutaneous PNS of the medial branches of the dorsal rami for the treatment of chronic LBP 1 year after the end of treatment and lead removal. The medial branches of the dorsal ramus were selected as the target of percutaneous PNS with this approach due to their purported role in chronic nonspecific LBP and innervation of the multifidus overlapping regions of axial LBP.98 While the use of ultrasound for needle guidance for other pain management approaches, such as anesthetic nerve blocks,99 has become common, this study demonstrates the feasibility of successfully targeting the medial branches of the dorsal rami with percutaneous PNS under ultrasound guidance, without requiring fluoroscopy, as is commonly utilized in other neurostimulation applications.
Percutaneous PNS treatment produced sustained, clinically meaningful improvements in chronic LBP and secondary outcomes at 12 months. A majority of subjects experienced substantial (≥50%) reductions in average pain intensity with treatment, with an average reduction of 80% among responders. A majority of subjects also experienced significant reductions in LBP‐related disability (≥10‐point reduction in ODI score), with an average 23‐point reduction among responders with treatment. Importantly, these clinically significant reductions were sustained long‐term at 1 year after PNS lead removal. Of subjects completing long‐term follow‐up visits at 12 months after the end of treatment, a majority (67%) reported sustained substantial reductions in pain intensity and/or disability.
Percutaneous PNS enabled reductions in both opioid and non‐opioid analgesic medication consumption during treatment, which were sustained in the long‐term follow‐up. Given the ongoing opioid crisis, it was important to note that all subjects also successfully either avoided opioid consumption (n = 8) or ceased opioid consumption (n = 1) with PNS treatment, which was sustained long term. Subjects’ improvements in chronic LBP were also corroborated by improvements in quality of life via the PGIC, demonstrating the potential for PNS to significantly improve quality of life and physical functioning, by relieving pain long‐term, out to at least 1 year after treatment.
Proposed Mechanism of Action
The sustained improvements reported here are consistent with results from previously published studies where percutaneous PNS provided sustained clinically significant pain relief in several other pain conditions, such as chronic shoulder pain, neuropathic pain, post‐surgical pain, and back pain.8, 9, 10, 11, 12 One of the key elements of the mechanism of action proposed to be responsible for the sustained analgesic effects of percutaneous PNS is thought to be the modulation of central sensitization, often thought to occur among patients with chronic back pain and other painful conditions with both nociceptive and neuropathic characteristics.8, 9, 100 In addition to stimulation of afferent fibers, which engage the gate mechanism directly to reduce pain signaling, stimulation of efferent nerve fibers activates muscles and thereby generates physiological proprioceptive afferent signals from the muscle spindles and Golgi tendon organs activated in those muscles.101, 102 Together, these afferent signals may help to normalize or partially reverse membrane excitability of neurons and circuits in the pain processing pathways.103 This reduction in pain signals with PNS may also disrupt the cycle of centrally mediated pain, permitting greater levels of activity, which may further reduce pain via activity‐dependent neuroplasticity even long after therapy delivery has ended.104, 105, 106 This mechanism could explain the pain reduction experienced during treatment with percutaneous PNS, and it may also support the durability of the effect on pain and improvements in disability long term.14
Percutaneous PNS may serve as an effective early intervention neurostimulation therapy for the treatment of LBP that preserves motor function and obviates the need for a permanent implant. The fine‐wire percutaneous PNS leads were designed to reduce invasiveness, adverse events, and some of the technical challenges that were previously associated with traditional systems and applications for PNS. Because this PNS system was designed purposely for use as a percutaneous therapy, it has an excellent safety profile and fewer complications than permanently implanted neurostimulation systems.15, 16, 17, 18 Studies suggest that use of neurostimulation earlier in the treatment continuum could also improve patient outcomes, for example, by reducing the number of hospitalizations and clinic visits, or reducing opioid usage.82 This approach of percutaneous PNS provides a unique opportunity to be used as an earlier neurostimulation intervention that may preclude the need for opioids, denervation, and permanently implanted neurostimulation systems, due to the less invasive nature of the intervention in conjunction with evidence of long‐term relief in patients with many types of chronic pain.80, 81, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97 In addition to 2 previously published RCTs using this same device for the treatment of chronic shoulder pain,86, 94 a third double‐blinded RCT was recently published and demonstrated successful relief of chronic neuropathic pain and improvement in quality of life, with results sustained through 1 year.97
Limitations
Although the results presented here are promising and consistent with previous studies of percutaneous PNS for other types of pain,80, 81, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97 this study has limitations, which should be considered in interpretation of the results. In particular, the population size was limited (n = 9) and did not include a control group or explore placebo effect; additional studies could help confirm these results in a larger population of patients, including studies that might compare the effects of percutaneous PNS to other standard interventional approaches used for patients with chronic LBP. Because chronic LBP can include a heterogeneous population (eg, facetogenic, discogenic, arthritic, or myofascial pain) and the selection criteria for inclusion in this study were broad, additional studies and analyses of larger populations, including larger, prospective multicenter case series studies, may determine LBP subtypes or characteristics that are more likely to benefit from percutaneous PNS, as well as if specific types of diagnostic tests or imaging are predictive of success.
The results from the present investigation suggest that percutaneous PNS can produce significant reductions in pain and disability and improvements in quality of life among patients with chronic LBP out to at least 1 year after treatment. The large population of patients with chronic LBP is in need of less invasive, non‐opioid pain management therapies that could be provided earlier in the treatment continuum, before more invasive, destructive, or expensive therapies, such as surgery or permanent neurostimulation system implantation. As such, percutaneous PNS may offer substantial advantages and an opportunity to shift the paradigm by offering effective neurostimulation to these patients earlier in the treatment continuum.
Conclusion
The results reported here showing sustained relief of chronic LBP and disability for at least 12 months highlight the potential for percutaneous PNS to obviate the need for more invasive permanently implanted systems. This is consistent with previously published studies and RCTs of percutaneous PNS in other pain indications (eg, neuropathic pain, chronic shoulder pain), where clinically significant reductions in pain and improvements in pain‐related disability were also sustained long‐term.80, 81, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97 Together, these studies reveal the potential for percutaneous PNS to be used as an alternative to existing treatment modalities for chronic pain, effectively reducing pain and opioid use, while reducing disability and invasiveness. This approach has the potential to significantly influence the care continuum for chronic back pain by providing the benefits of an effective neurostimulation therapy to patients earlier than has been previously possible.
Conflicts of Interest
C.G. and L.K. have consulted for SPR Therapeutics. M.M. and J.B. are employees of SPR Therapeutics.
Acknowledgements
The authors thank the staff at the Center for Clinical Research for the referral, recruitment, and care of all subjects participating in this study. This study was funded by SPR Therapeutics, Inc.
References
- 1. Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hardt J, Jacobsen C, Goldberg J, Nickel R, Buchwald D. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803–812. [DOI] [PubMed] [Google Scholar]
- 3. Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an internet‐based survey. J Pain. 2010;11:1230–1239. [DOI] [PubMed] [Google Scholar]
- 4. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25:2940–2952. [DOI] [PubMed] [Google Scholar]
- 5. Bentsen SB, Hanestad BR, Rustoen T, Wahl AK. Quality of life in chronic low back pain patients treated with instrumented fusion. J Clin Nurs. 2008;17:2061–2069. [DOI] [PubMed] [Google Scholar]
- 6. Anderson VC, Carlson C, Shatin D. Outcomes of spinal cord stimulation: patient validation. Neuromodulation. 2001;4:11–17. [DOI] [PubMed] [Google Scholar]
- 7. Deyo RA, Phillips WR. Low back pain. A primary care challenge. Spine (Phila Pa 1976). 1996;21:2826–2832. [DOI] [PubMed] [Google Scholar]
- 8. Nijs J, Van Houdenhove B, Oostendorp RAB. Recognition of central sensitization in patients with musculoskeletal pain: application of pain neurophysiology in manual therapy practice. Man Ther. 2010;15:135–141. [DOI] [PubMed] [Google Scholar]
- 9. Roussel NA, Nijs J, Meeus M, Mylius V, Fayt C, Oostendorp R. Central sensitization and altered central pain processing in chronic low back pain: fact or myth? Clin J Pain. 2013;29:625–638. [DOI] [PubMed] [Google Scholar]
- 10. Young Casey C, Greenberg MA, Nicassio PM, Harpin RE, Hubbard D. Transition from acute to chronic pain and disability: a model including cognitive, affective, and trauma factors. Pain. 2008;134:69–79. [DOI] [PubMed] [Google Scholar]
- 11. Giesecke T, Gracely RH, Grant MA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613–623. [DOI] [PubMed] [Google Scholar]
- 12. Clauw DJ. Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s). Best Pract Res Clin Rheumatol. 2015;29:6–19. [DOI] [PubMed] [Google Scholar]
- 13. Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ. 2015;350:g6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deyo RA, Smith DHM, Johnson ES, et al. Opioids for back pain patients: primary care prescribing patterns and use of services. J Am Board Fam Med. 2011;24:717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Campos TF. Low back pain and sciatica in over 16s: assessment and management NICE Guideline [NG59]. J Physiother. 2017;63:120. [DOI] [PubMed] [Google Scholar]
- 16. Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ. 2015;350:g6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chou R, Huffman LH. Medications for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:505–514. [DOI] [PubMed] [Google Scholar]
- 18. Chou R. Pharmacological management of low back pain. Drugs. 2010;70:387–402. [DOI] [PubMed] [Google Scholar]
- 19. Ford KS, Shrader MW, Smith J, McLean TJ, Dahm DL. Full‐thickness burn formation after the use of electrical stimulation for rehabilitation of unicompartmental knee arthroplasty. J Arthroplasty. 2005;20:950–953. [DOI] [PubMed] [Google Scholar]
- 20. Nadler SF, Prybicien M, Malanga GA, Sicher D. Complications from therapeutic modalities: results of a national survey of athletic trainers. Arch Phys Med Rehabil. 2003;84:849–853. [DOI] [PubMed] [Google Scholar]
- 21. Deyo RA, Walsh NE, Martin DC, Schoenfeld LS, Ramamurthy S. A controlled trial of transcutaneous electrical nerve stimulation (TENS) and exercise for chronic low back pain. N Engl J Med. 1990;322:1627–1634. [DOI] [PubMed] [Google Scholar]
- 22. Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta‐analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain. 2003;7:181–188. [DOI] [PubMed] [Google Scholar]
- 23. Galloway DJ, Boyle P, Burns HJ, Davidson PM, George WD. A clinical assessment of electroanalgesia following abdominal operations. Surg Gynecol Obstet. 1984;159:453–456. [PubMed] [Google Scholar]
- 24. Warfield CA, Stein JM, Frank HA. The effect of transcutaneous electrical nerve stimulation on pain after thoracotomy. Ann Thorac Surg. 1985;39:462–465. [DOI] [PubMed] [Google Scholar]
- 25. Davies JR. Ineffective transcutaneous nerve stimulation following epidural anaesthesia. Anaesthesia. 1982;37:453–454. [DOI] [PubMed] [Google Scholar]
- 26. Navarathnam RG, Wang IY, Thomas D, Klineberg PL. Evaluation of the transcutaneous electrical nerve stimulator for postoperative analgesia following cardiac surgery. Anaesth Intensive Care. 1984;12:345–350. [DOI] [PubMed] [Google Scholar]
- 27. Cuschieri RJ, Morran CG, McArdle CS. Transcutaneous electrical stimulation for postoperative pain. Ann R Coll Surg Engl. 1985;67:127–129. [PMC free article] [PubMed] [Google Scholar]
- 28. Smedley F, Taube M, Wastell C. Transcutaneous electrical nerve stimulation for pain relief following inguinal hernia repair: a controlled trial. Eur Surg Res. 1988;20:233–237. [DOI] [PubMed] [Google Scholar]
- 29. Conn IG, Marshall AH, Yadav SN, Daly JC, Jaffer M. Transcutaneous electrical nerve stimulation following appendicectomy: the placebo effect. Ann R Coll Surg Engl. 1986;68:191–192. [PMC free article] [PubMed] [Google Scholar]
- 30. McCallum MI, Glynn CJ, Moore RA, Lammer P, Phillips AM. Transcutaneous electrical nerve stimulation in the management of acute postoperative pain. Br J Anaesth. 1988;61:308–312. [DOI] [PubMed] [Google Scholar]
- 31. Sim DT. Effectiveness of transcutaneous electrical nerve stimulation following cholecystectomy. Physiotherapy. 1991;77:715–722. [Google Scholar]
- 32. Forster EL, Kramer JF, Lucy SD, Scudds RA, Novick RJ. Effect of TENS on pain, medications, and pulmonary function following coronary artery bypass graft surgery. Chest. 1994;106:1343–1348. [DOI] [PubMed] [Google Scholar]
- 33. DeSantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Curr Rheumatol Rep. 2008;10:492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Radhakrishnan R, Sluka KA. Deep tissue afferents, but not cutaneous afferents, mediate transcutaneous electrical nerve stimulation‐induced antihyperalgesia. J Pain. 2005;6:673–680. [DOI] [PubMed] [Google Scholar]
- 35. Kirsch W, Lewis J, Simon RJ. Experiences with electrical stimulation devices for the control of chronic. Pain. 1975;9:217–220. [PubMed] [Google Scholar]
- 36. Frih ZBS, Fendri Y, Jellad A, Boudoukhane S, Rejeb N. Efficacy and treatment compliance of a home‐based rehabilitation programme for chronic low back pain: a randomized, controlled study. Ann Phys Rehabil Med. 2009;52:485–496. [DOI] [PubMed] [Google Scholar]
- 37. Liddle SD, Baxter GD, Gracey JH. Exercise and chronic low back pain: what works? Pain. 2004;107:176–190. [DOI] [PubMed] [Google Scholar]
- 38. Friedrich M, Gittler G, Halberstadt Y, Cermak T, Heiller I. Combined exercise and motivation program: effect on the compliance and level of disability of patients with chronic low back pain: a randomized controlled trial. Arch Phys Med Rehabil. 1998;79:475–487. [DOI] [PubMed] [Google Scholar]
- 39. Childs JD, Fritz JM, Wu SS, et al. Implications of early and guideline adherent physical therapy for low back pain on utilization and costs. BMC Health Serv Res. 2015;15:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sluijs EM, Kok GJ, van der Zee J. Correlates of exercise compliance in physical therapy. Phys Ther. 1993;73:771–782; discussion 783–786. [DOI] [PubMed] [Google Scholar]
- 41. Nelson BW, O’Reilly E, Miller M, Hogan M, Wegner JA, Kelly C. The clinical effects of intensive, specific exercise on chronic low back pain: a controlled study of 895 consecutive patients with 1‐year follow up. Orthopedics. 1995;18:971–981. [DOI] [PubMed] [Google Scholar]
- 42. Alexandre NM, Nordin M, Hiebert R, Campello M. Predictors of compliance with short‐term treatment among patients with back pain. Rev Panam Salud Publica. 2002;12:86–94. [DOI] [PubMed] [Google Scholar]
- 43. Leggett LE, Soril LJJ, Lorenzetti DL, et al. Radiofrequency ablation for chronic low back pain: a systematic review of randomized controlled trials. Pain Res Manag. 2014;19:e146–e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Staal JB, de Bie R, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low‐back pain. Cochrane Database Syst Rev. 2008;(3):CD001824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shen FH, Samartzis D, Andersson GBJ. Nonsurgical management of acute and chronic low back pain. J Am Acad Orthop Surgeons. 2006;14:477–487. [DOI] [PubMed] [Google Scholar]
- 46. Conn A, Buenaventura RM, Datta S, Abdi S, Diwan S. Systematic review of caudal epidural injections in the management of chronic low back pain. Pain Physician. 2009;12:109–135. [PubMed] [Google Scholar]
- 47. Koes BW, Scholten RJPM, Mens JMA, Bouter LM. Efficacy of epidural steroid injections for low‐back pain and sciatica: a systematic review of randomized clinical trials. Pain. 1995;63:279–288. [DOI] [PubMed] [Google Scholar]
- 48. Huston CW, Slipman CW, Garvin C. Complications and side effects of cervical and lumbosacral selective nerve root injections. Arch Phys Med Rehabil. 2005;86:277–283. [DOI] [PubMed] [Google Scholar]
- 49. Juch JN, Maas ET, Ostelo RW, et al. Effect of radiofrequency denervation on pain intensity among patients with chronic low back pain: the MINT randomized clinical trials. JAMA. 2017;318:68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chotai S, Parker SL, Sivaganesan A, et al. Effect of complications within 90 days on patient‐reported outcomes 3 months and 12 months following elective surgery for lumbar degenerative disease. Neurosurg Focus. 2015;39:E8. [DOI] [PubMed] [Google Scholar]
- 51. Parker SL, Mendenhall SK, Shau DN, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis: comparative effectiveness and cost‐utility analysis. World Neurosurg. 2014;82:230–238. [DOI] [PubMed] [Google Scholar]
- 52. Abbott AD, Tyni‐Lenné R, Hedlund R. Early rehabilitation targeting cognition, behavior, and motor function after lumbar fusion: a randomized controlled trial. Spine. 2010;35:848–857. [DOI] [PubMed] [Google Scholar]
- 53. Shapiro CM. The failed back surgery syndrome: pitfalls surrounding evaluation and treatment. Phys Med Rehabil Clin North Am. 2014;25:319–340. [DOI] [PubMed] [Google Scholar]
- 54. Fritzell P, Hagg O, Wessberg P, Nordwall A. 2001 Volvo Award Winner in Clinical Studies: lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine (Phila Pa 1976). 2001;26:2521–2532; discussion 2532–2534. [DOI] [PubMed] [Google Scholar]
- 55. Martin BI, Mirza SK, Comstock BA, Gray DT, Kreuter W, Deyo RA. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine (Phila Pa 1976). 2007;32:382–387. [DOI] [PubMed] [Google Scholar]
- 56. Cloyd JM, Acosta FL Jr, Ames CP. Complications and outcomes of lumbar spine surgery in elderly people: a review of the literature. J Am Geriatr Soc. 2008;56:1318–1327. [DOI] [PubMed] [Google Scholar]
- 57. Brox JI, Reikerås O, Nygaard Ø, et al. Lumbar instrumented fusion compared with cognitive intervention and exercises in patients with chronic back pain after previous surgery for disc herniation: a prospective randomized controlled study. Pain. 2006;122:145–155. [DOI] [PubMed] [Google Scholar]
- 58. Sharan AD, Riley J, Falowski S, et al. Association of opioid usage with spinal cord stimulation outcomes. Pain Med. 2017;19:699–707. [DOI] [PubMed] [Google Scholar]
- 59. Rainville J, Hartigan C, Martinez E, Limke J, Jouve C, Finno M. Exercise as a treatment for chronic low back pain. Spine J. 2004;4:106–115. [DOI] [PubMed] [Google Scholar]
- 60. van der Velde G, Mierau D. The effect of exercise on percentile rank aerobic capacity, pain, and self‐rated disability in patients with chronic low‐back pain: a retrospective chart review. Arch Phys Med Rehabil. 2000;81:1457–1463. [DOI] [PubMed] [Google Scholar]
- 61. Rainville J, Ahern DK, Phalen L, Childs LA, Sutherland R. The association of pain with physical activities in chronic low back pain. Spine (Phila Pa 1976). 1992;17:1060–1064. [DOI] [PubMed] [Google Scholar]
- 62. Mayer TG, Gatchel RJ, Mayer H, Kishino ND, Keeley J, Mooney V. A prospective two‐year study of functional restoration in industrial low back injury. An objective assessment procedure. JAMA. 1987;258:1763–1767. [PubMed] [Google Scholar]
- 63. Hazard RG, Fenwick JW, Kalisch SM, et al. Functional restoration with behavioral support. A one‐year prospective study of patients with chronic low‐back pain. Spine (Phila Pa 1976). 1989;14:157–161. [PubMed] [Google Scholar]
- 64. Wittink H, Rogers W, Gascon C, Sukiennik A, Cynn D, Carr DB. Relative contribution of mental health and exercise‐related pain increment to treadmill test intolerance in patients with chronic low back pain. Spine (Phila Pa 1976). 2001;26:2368–2374.11679823 [Google Scholar]
- 65. Edwards BC, Zusman M, Hardcastle P, Twomey L, O’Sullivan P, McLean N. A physical approach to the rehabilitation of patients disabled by chronic low back pain. Med J Aust. 1992;156:167–172. [DOI] [PubMed] [Google Scholar]
- 66. Frost H, Klaber Moffett JA, Moser JS, Fairbank JC. Randomised controlled trial for evaluation of fitness programme for patients with chronic low back pain. BMJ. 1995;310:151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Barolat G, Oakley JC, Law JD, North RB, Ketcik B, Sharan A. Epidural spinal cord stimulation with a multiple electrode paddle lead is effective in treating intractable low back pain. Neuromodulation. 2001;4:59–66. [DOI] [PubMed] [Google Scholar]
- 68. Oakley JC. Spinal cord stimulation in axial low back pain: solving the dilemma. Pain Med. 2006;7:S58–S63. [Google Scholar]
- 69. Deer T, Pope J, Hayek S, et al. Neurostimulation for the treatment of axial back pain: a review of mechanisms. Tech Outcome Future Adv. 2014;17:52–68. [DOI] [PubMed] [Google Scholar]
- 70. Rigoard P, Basu S, Desai M, et al. Multicolumn spinal cord stimulation for predominant back pain in failed back surgery syndrome patients: a multicenter randomized controlled trial. Pain. 2019;160:1410–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20‐year literature review. J Neurosurg. 2004;100:254–267. [DOI] [PubMed] [Google Scholar]
- 72. Heidecke V, Rainov NG, Burkert W. Hardware failures in spinal cord stimulation for failed back surgery syndrome. Neuromodulation. 2000;3:27–30. [DOI] [PubMed] [Google Scholar]
- 73. Turner JA, Hollingworth W, Comstock BA, Deyo RA. Spinal cord stimulation for failed back surgery syndrome: outcomes in a workers’ compensation setting. Pain. 2010;148:14–25. [DOI] [PubMed] [Google Scholar]
- 74. Turner JA, Loeser JD, Deyo RA, Sanders SB. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain. 2004;108:137–147. [DOI] [PubMed] [Google Scholar]
- 75. Mekhail NA, Mathews M, Nageeb F, Guirguis M, Mekhail MN, Cheng J. Retrospective review of 707 cases of spinal cord stimulation: indications and complications. Pain Pract. 2011;11:148–153. [DOI] [PubMed] [Google Scholar]
- 76. Rosenow JM, Stanton‐Hicks M, Rezai AR, Henderson JM. Failure modes of spinal cord stimulation hardware. J Neurosurg. 2006;5:183–190. [DOI] [PubMed] [Google Scholar]
- 77. Vakkala M, Järvimäki V, Kautiainen H, Haanpää M, Alahuhta S. Incidence and predictive factors of spinal cord stimulation treatment after lumbar spine surgery. J Pain Res. 2017;10:2405–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Narouze SN, Zakari A, Vydyanathan A. Ultrasound‐guided placement of a permanent percutaneous femoral nerve stimulator leads for the treatment of intractable femoral neuropathy. Pain Physician. 2009;12:E305–E308. [PubMed] [Google Scholar]
- 79. Slavin KV. Technical aspects of peripheral nerve stimulation: hardware and complications. Prog Neurol Surg. 2011;24:189–202. [DOI] [PubMed] [Google Scholar]
- 80. Rauck RL, Cohen SP, Gilmore CA, et al. Treatment of post‐amputation pain with peripheral nerve stimulation. Neuromodulation. 2014;17:188–197. [DOI] [PubMed] [Google Scholar]
- 81. Gilmore CA, Ilfeld BM, Rosenow JM, et al. Percutaneous 60-day peripheral nerve stimulation implant provides sustained relief of chronic pain following amputation: 12-month follow-up of a randomized, double-blind, placebo-controlled trial. Reg Anesth Pain Med. 10.1136/rapm-2019-100937 [DOI] [PubMed] [Google Scholar]
- 82. Lad SP, Petraglia FW, Kent AR, et al. Longer delay from chronic pain to spinal cord stimulation results in higher healthcare resource utilization. Neuromodulation. 2016;19:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chae J, Ng A, Yu DT, et al. Intramuscular electrical stimulation for shoulder pain in hemiplegia: does time from stroke onset predict treatment success? Neurorehabil Neural Repair. 2007;21:561–567. [DOI] [PubMed] [Google Scholar]
- 84. Chae J, Wilson RD, Bennett ME, Lechman TE, Stager KW. Single‐lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case series. Pain Pract. 2013;13:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chae J, Yu DT, Walker ME. Percutaneous, intramuscular, neuromuscular electrical stimulation for the treatment of shoulder subluxation and pain in chronic hemiplegia. Am J Phys Med Rehabil. 2001;80:296–301. [DOI] [PubMed] [Google Scholar]
- 86. Chae J, Yu DT, Walker ME, et al. Intramuscular electrical stimulation for hemiplegic shoulder pain. Am J Phys Med Rehabil. 2005;84:832–842. [DOI] [PubMed] [Google Scholar]
- 87. Gilmore CA, Kapural L, McGee MJ, Boggs JW. 30-Day Percutaneous PNS of Medial Branch Nerves Provides Sustained Relief of Chronic Back Pain for 1 Year Following Treatment. 2018 Regional Anesthesiology and Acute Pain Medicine Meeting. New York, NY: American Society of Regional Anesthesia and Pain Medicine. [Google Scholar]
- 88. Ilfeld BM, Gabriel RA, Saulino MF, et al. Infection rates of electrical leads used for percutaneous neurostimulation of the peripheral nervous system. Pain Pract. 2017;17:753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ilfeld BM, Gilmore CA, Grant SA, et al. Ultrasound‐guided percutaneous peripheral nerve stimulation for analgesia following total knee arthroplasty: a prospective feasibility study. J Orthop Surg Res. 2017;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ilfeld BM, Grant SA. Ultrasound‐guided percutaneous peripheral nerve stimulation for postoperative analgesia: could neurostimulation replace continuous peripheral nerve blocks? Reg Anesth Pain Med. 2016;41:720–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ilfeld BM, Grant SA, Gilmore CA, et al. Neurostimulation for postsurgical analgesia: a novel system enabling ultrasound‐guided percutaneous peripheral nerve stimulation. Pain Pract. 2017;17:892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Renzenbrink GJ, Ijzerman MJ. Percutaneous neuromuscular electrical stimulation (P‐NMES) for treating shoulder pain in chronic hemiplegia. Effects on shoulder pain and quality of life. Clin Rehabil. 2004;18:359–365. [DOI] [PubMed] [Google Scholar]
- 93. Wilson RD, Bennett ME, Lechman TE, Stager KW, Chae J. Single‐lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case report. Arch Phys Med Rehabil. 2011;92:837–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wilson RD, Gunzler DD, Bennett ME, Chae J. Peripheral nerve stimulation compared with usual care for pain relief of hemiplegic shoulder pain: a randomized controlled trial. Am J Phys Med Rehabil. 2014;93:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yu DT, Chae J, Walker ME, Fang ZP. Percutaneous intramuscular neuromuscular electric stimulation for the treatment of shoulder subluxation and pain in patients with chronic hemiplegia: a pilot study. Arch Phys Med Rehabil. 2001;82:20–25. [DOI] [PubMed] [Google Scholar]
- 96. Yu DT, Chae J, Walker ME, et al. Intramuscular neuromuscular electric stimulation for poststroke shoulder pain: a multicenter randomized clinical trial. Arch Phys Med Rehabil. 2004;85:695–704. [DOI] [PubMed] [Google Scholar]
- 97. Gilmore CA, Ilfeld BM, Rosenow JM, et al. Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic post‐amputation pain: a multi‐center randomized placebo‐controlled trial. Reg Anesth Pain Med 2019;44:637–645. [DOI] [PubMed] [Google Scholar]
- 98. Freeman MD, Woodham MA, Woodham AW. The role of the lumbar multifidus in chronic low back pain: a review. PM&R. 2010;2:142–146. [DOI] [PubMed] [Google Scholar]
- 99. Narouze S, Peng PW. Ultrasound‐guided interventional procedures in pain medicine: a review of anatomy, sonoanatomy, and procedures: part ii: axial structures. Reg Anesth Pain Med. 2010;35:386–396. [DOI] [PubMed] [Google Scholar]
- 100. Nijs J, Apeldoorn A, Hallegraeff H, et al. Low back pain: guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Physician. 2015;18:E333–E346. [PubMed] [Google Scholar]
- 101. Proske U. What is the role of muscle receptors in proprioception? Am Assoc Electrodiagnostic Med. 2005;31:780–787. [DOI] [PubMed] [Google Scholar]
- 102. Windhorst U. Muscle proprioceptive feedback and spinal networks. Brain Res Bull. 2007;73:155–202. [DOI] [PubMed] [Google Scholar]
- 103. Bentley LD, Duarte RV, Furlong PL, Ashford RL, Raphael JH. Brain activity modifications following spinal cord stimulation for chronic neuropathic pain: a systematic review. Eur J Pain. 2016;20:499–511. [DOI] [PubMed] [Google Scholar]
- 104. Birbaumer N, Lutzenberger W, Montoya P, et al. Effects of regional anesthesia on phantom limb pain are mirrored in changes in cortical reorganization. J Neurosci. 1997;17:5503–5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Preißler S, Dietrich C, Meissner W, et al. Brachial plexus block in phantom limb pain: a case report. Pain Med. 2011;12:1649–1654. [DOI] [PubMed] [Google Scholar]
- 106. Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. [DOI] [PubMed] [Google Scholar]


