Abstract
Free‐range poultry farms have a high risk of introduction of avian influenza viruses (AIV), and it is presumed that wild (water) birds are the source of introduction. There is very scarce quantitative data on wild fauna visiting free‐range poultry farms. We quantified visits of wild fauna to a free‐range area of a layer farm, situated in an AIV hot‐spot area, assessed by video‐camera monitoring. A total of 5,016 hr (209 days) of video recordings, covering all 12 months of a year, were analysed. A total of 16 families of wild birds and five families of mammals visited the free‐range area of the layer farm. Wild birds, except for the dabbling ducks, visited the free‐range area almost exclusively in the period between sunrise and the moment the chickens entered the free‐range area. Known carriers of AIV visited the outdoor facility regularly: species of gulls almost daily in the period January–August; dabbling ducks only in the night in the period November–May, with a distinct peak in the period December–February. Only a small fraction of visits of wild fauna had overlap with the presence of chickens at the same time in the free‐range area. No direct contact between chickens and wild birds was observed. It is hypothesized that AIV transmission to poultry on free‐range poultry farms will predominantly take place via indirect contact: taking up AIV by chickens via wild‐bird‐faeces‐contaminated water or soil in the free‐range area. The free‐range poultry farmer has several possibilities to potentially lower the attractiveness of the free‐range area for wild (bird) fauna: daily inspection of the free‐range area and removal of carcasses and eggs; prevention of forming of water pools in the free‐range facility. Furthermore, there are ways to scare‐off wild birds, for example use of laser equipment or trained dogs.
Keywords: avian influenza, ducks, free‐range poultry, gulls, water pools, wild fauna
1. INTRODUCTION
Consumer concern for animal welfare and demand for a more (alleged) ecological way of production (Rabobank, 2019) have led to considerable growth in free‐range production systems worldwide: retail market share of free‐range table eggs has increased from 10% in 2000 to 46% in 2018 in Australia (AECL, 2018); in the United Kingdom, production of free‐range table eggs has increased more than twofold from 2006 to 2018 (UK.gov, 2018). In the Netherlands, the number of free‐range laying hens increased with more than 40% between 2005 and 2013 (PVE, 2005; 2013). Poultry farms with free‐range facilities offer birds the choice between indoor and outdoor areas during daytime and with that the opportunity to exhibit natural behaviour like foraging and dust‐bathing. Furthermore, it provides access to natural light, fresh air, variable weather conditions and a potentially higher space allowance resulting in a lower density of birds when the birds visit the free‐range area. In addition, the diversity of plant species present in a free‐range area may elicit pecking, scratching, tearing, biting and harvesting of seeds. Small animals such as insects and worms may stimulate hunting and digging of chickens (Knierim, 2006).
At the same time, free‐range layer farms have—compared to indoor layer farms—a higher risk of introduction of low (LPAIV) and highly pathogenic avian influenza viruses (HPAIV) (Bouwstra et al., 2017; Gonzales, Stegeman, Koch, Wit, & Elbers, 2013; Kirunda et al., 2015; Terregino et al., 2007; Welby, Berg, et al., 2010a). The sheer majority of LPAIV introductions on poultry farms in the Netherlands takes place on free‐range layer farms (Bouwstra et al., 2017; Gonzales et al., 2013). Avian influenza viruses (AIV) are categorized as LPAIV or HPAIV, based on the pathobiological effects of the virus in chickens: in general LPAIV infections may be asymptomatic and produce no or mild disease in chickens (Gonzales & Elbers, 2018), while HPAIV infections produce high morbidity and mortality in poultry (Pantin‐Jackwood & Swayne, 2009). Influenza viruses carry two glycoproteins on their surface: haemagglutinin (HA) and neuraminidase (NA) and on the basis of these glycoproteins are divided into subtypes (Webster, Bean, Gorman, Chambers, & Kawaoka, 1992). Among the known HA subtypes affecting birds (H1–H16), H5 and H7 virus subtypes can be either LPAIV or HPAIV and are notifiable to the World Organization for Animal Health (OIE).
Wild birds are involved in the long‐distance spread of both LPAIV and HPAIV (Gilbert et al., 2006; Verhagen, Herfst, & Fouchier, 2015) and are the main natural reservoir for LPAIV. Extensive wild bird surveillance studies have revealed the highest LPAIV prevalence in birds of the orders Anseriformes (ducks, geese, swans) and Charadriiformes (gulls, terns, waders). High seroprevalence was observed particularly in dabbling ducks: shallow water ducks (such as mallard, teal and pintail) that feed primarily along the surface of the water or by tipping headfirst into the water to graze on aquatic plants, vegetation, larvae and insects (Grillo et al., 2015; Haynes et al., 2009; Lewis et al., 2013; Munster et al., 2007; Olsen et al., 2006; Stallknecht & Shane, 1988). Therefore, several wild bird species of these orders are considered AIV higher risk host species (EFSA et al., 2017; Munster et al., 2007; Veen et al., 2007). There are three lines of evidence suggesting wild birds can be the source of AIV infection in poultry: (a) temporal associations between AIV isolated from wild birds and from outbreaks in poultry flocks (East, Ainsworth, Warner, Dunowska, & Azuolas, 2010; Fouchier et al., 2004; Gilbert et al., 2006; Halvorson, 2009); (b) genetic similarity between AIV strains isolated from wild birds and from poultry (Campitelli et al., 2004; Chen et al., 2016; Marche, Borm, Lambrecht, Houdart, & Berg, 2014; Verhagen et al., 2015) and only recently (c) a combination of (a) and (b) (Beerens et al., 2018; Bouwstra et al., 2015; Lycett, Bodewes, & Pohlmann, 2016). Furthermore, a recent study indicates that other wild bird orders can act as potential local spreaders or bridge species for AIV between aquatic water birds and domestic poultry (Caron, Grosbois, Etter, Gaidet, & Garine‐Wichatitsky, 2014).
Typically, LPAIV replicates in cells lining the respiratory and digestive tracts and virus can be excreted in high concentrations in bird faeces (Pantin‐Jackwood & Swayne, 2009). Influenza viruses can remain infectious in surface water for prolonged periods of time and the relatively high virus prevalence in aquatic wild birds may in part be due to the stability of the virus in surface waters, enabling effective transmission via the faecal‐oral route (Fouchier & Munster, 2009). Wild birds and wild fauna that visit the free‐range facility of a poultry farm can either infect poultry by direct or indirect contact (Alexander, 2007): direct contact defined as physical contact between an infected wild bird/animal and a chicken, and indirect contact defined as a chicken coming into contact with the virus via a medium, for example contamination of the soil surface by wild bird faeces (von Waldburg‐Zeil, Staaveren, & Harlander‐Matauschek, 2019) or contamination of the water by wild bird faeces in pools of water (Markwell & Shortridge, 1982) present in the free‐range facility, or by fomites: fomites contaminated with wild bird faeces that come into contact with chickens or deliver by movement‐contaminated wild bird faeces into the barn to be picked up by chicken (e.g. coveralls, boots and equipment). Biological vectors like insects, mice and rats may become infected and may shed the virus in the neighbourhood of chickens or be consumed by chickens (Velkers, Blokhuis, Veldhuis Kroeze, & Burt, 2017). However, the relative roles of direct contact versus environmental contamination in the transmission of AIV remain poorly understood (Achenbach & Bowen, 2011).
There is scarce data on wild birds visiting poultry farms and in particular free‐range poultry farms. Voslamber (2005, 2006) counted visiting wild birds once for one hour during daytime at 60 free‐range poultry farms in the Netherlands, both in summer (July‐August 2005) and in the fall period (September‐November 2006). In both periods, sometimes a large amount of wild birds could be observed in the free‐range facility or in the surroundings of poultry farms. However, presumed AIV high‐risk wild birds were seen only in very small quantities during daytime in the free‐range facility or near the poultry barn, but they kept themselves at considerable distance (>100 m) from the chickens and no direct contact was observed. Veen et al. (2007) reported on visual bird counts performed twice for about one hour in the period January – May on and around free‐range and non‐free‐range poultry farms in England, Turkey, Germany and Italy to identify AIV higher risk wild bird species. A recent study has indicated that mallards (Anas plathyrhynchos), a keystone water bird with respect to AIV transmission, are visiting foraging sites, sometimes located on or around animal farms, particularly at night and not at daytime (Kleyheeg et al., 2017), thereby they are often missed in visual bird counting performed during daytime. Burns et al. (2012) counted visits of wild birds to 20 poultry farms in British Columbia and 21 poultry farms in Ontario, Canada, for 30 min once, 1 hr after sunrise. These farms had no free‐range facilities.
Several methods have been used to study visits and interactions between wild fauna and livestock. First of all manual count by biologists of wild fauna visiting farms (Veen et al., 2007; Voslamber, 2005, 2006). Furthermore, the use of motion sensor trap cameras (cameras that are remotely activated via an active or passive sensor) has become increasingly popular due to low equipment costs and allowing minimum invasive capture of animal behaviour without human presence (Caravaggi et al., 2017; Kukielkaa et al., 2013; Scott, Phalen, et al., 2018a). Continuous video‐camera recording in an experimental setting in the field to investigate interactions between wild birds and a small group of backyard poultry was used by Welby, Poncin, et al. (2010b) in Belgium. Although labour‐intensive and thus costly, the advantage of continuous video recording includes round‐the‐clock monitoring with a high level of precision capturing animal behaviour.
The objective of our investigation was to quantify visits of wild fauna to a free‐range area of a layer farm, situated in an AIV hot‐spot area, assessed by video‐camera monitoring. This basic information is needed in order to develop and scientifically test intervention strategies to prevent or decrease contact between wild birds and the free‐range area of layer farms.
2. METHODS
2.1. Commercial free‐range layer farm
2.1.1. Selection and AIV history
The poultry farm was selected at random from a group of a total of 20 free‐range layer farms in the Netherlands that experienced three or more introductions of LPAIV since they started production (so‐called avian influenza hot‐spot farms). Our study farm experienced six LPAIV introductions since its start of production in 2008: subtype H6N1 in 2010, H6N2 in 2012, H9N2 in 2013, H6N2 in 2014 and H10N7 in 2017 and 2018. This free‐range farm was serologically monitored for the presence of AIV antibodies every three months as part of the normal procedure within the national serological surveillance programme (Bouwstra et al., 2017).
2.1.2. Location and layout
For an aerial overview of the farm and surroundings (made with Google Earth Pro, 2019), see Figure 1. The poultry barn has on both long sides a fenced free‐range area (A and B in Figure 1), to be accessed by the hens through locks in the side‐walls of the poultry barn. At the start of a production round, with new layer chickens, the free‐range area is covered by grass and small weeds; during the production round, the soil of the free‐range area becomes barren due to chicken activity/foraging. In the free‐range area, there are a few small trees (covering a few per cent of the total free‐range area), protected against chicken attacks and foraging by fencing (Figure 2). The area surrounding the poultry farm including the free‐range facility, consists of pastures for dairy cattle and sheep, without any trees; there are some large (oak) trees surrounding the private house of the poultry farmer (K in Figure 1). One side of the fenced free‐range area (A in Figure 1) is surrounded by a waterway ditch (about 1m wide, filled with water) that separates the free‐range poultry area and a large area of grass pastures for cows and sheep. This waterway ditch is connected to a canal (about 4 m wide), and this canal is again connected to a larger waterway (about 12 m wide); both waterways are situated approximately 500 m north‐east from the poultry farm.
Figure 1.

Aerial map of location of free‐range layer farm (source: Google Earth Pro, 2019). A: free‐range area on north‐side of poultry barn; B: free‐range area on south‐side of the poultry barn; C: ditch with surface water; D: ditch with surface water; E: ditch with surface water; F: canal with surface water; G: wide canal with surface water; H: neighbour free‐range layer farm; I: poultry barn; J: a few small trees (fenced) in free‐range‐area; K: a few large trees around the private house of poultry farmer
Figure 2.

North‐side of the fenced free‐range area, on the right side the poultry barn
2.1.3. Management
The commercial free‐range layer farm has a flock size of approximately 38,000 laying hens, housed in a poultry barn measuring approximately 100 m length × 25 m wide. It is managed by the farmer and his wife, with part‐time help of a technician. As a biosecurity measure—after experiencing the first LPAIV introduction in 2010 when the chickens could wander as far as possible into the surrounding pastures and could drink water from the waterway ditches (C, D, E and F in Figure 1) in which dabbling ducks and swans were observed swimming—the free‐range areas were fenced (2m high) on both sides of the barn, the fence connected to the poultry barn (see Figure 2). The free‐range area was made available to the layers by the farmer mostly from the end of the morning until sunset, and depending on the weather conditions and season. The layers were fed and watered indoors, never in the free‐range area. On the same premises, the poultry farmer is also responsible for a dairy herd (60 dairy cows and 60 young stock) and 50 Texelaar sheep. Both livestock species use pastures situated on the outside of the fenced free‐range layer area. Rodent pest control measures were in operation (rodent bait stations against the outside wall of the stable). Chicken carcass and egg removal from the free‐range area by the farmer was done on an irregular basis (never on a daily basis), sometimes with intervals between removal activities of a couple of weeks.
2.1.4. Wild bird presence in surroundings outside the free‐range area
In the waterways surrounding the outside of the fenced free‐range area of the poultry farm (C, D, E, F and G in Figure 1), congregations of water birds can be found, like mute swans (Cygnus olor), mallards (Anas platyrhynchos) and Eurasian wigeons (Mareca penelope). In the grass pastures surrounding the outside of the fenced free‐range area of the poultry farm, congregations of several migrating wild birds species, depending on the season, can be found: barnacle goose (Branta leucopsis), greyleg goose (Anser anser), greater white‐fronted goose (Anser albifrons); lesser black‐backed gull (Larus fuscus), black‐headed gull (Chroicocephalus ridibundus); mute swan (Cygnus olor). The poultry farm is located close (<2 km) to the coastline in the northern part of the Netherlands in a wild bird friendly area (policy of tolerance towards geese and other wild waterfowl by the provincial government). The poultry farm is under a flyway of migrating wild waterfowl flying along the coastline visiting the Netherlands in the Autumn and Spring.
2.2. Video‐camera equipment
In order to cover accurately the total free‐range area, a total of eight 1.3 Mpx TruVision IP 1/3" CMOS video cameras (Interlogix, United Technologies Corporated) with variable focus objective 2.8–12 mm were installed at a height of 4m above ground‐level alongside the outer wall of the poultry barn: six cameras monitored the free‐range area A (Figure 1) and two cameras covered the free‐range area B (Figure 1). Cameras were connected to a TruVision NVR10 network video recorder with HDMI/VGA video output and a 4TB hard disk for storage. The cameras were equipped with IR LEDs enabling night recording. Recording was done at a speed of 2 frames/s, 24 hr/day, 7 days/week, enabling recording of about 41 observation days per recorder before a recorder had to be refreshed. All 12 months of a year were covered by recordings.
2.3. Converting video recordings into analysable data
For the sheer amount of work involved, it was not possible to convert all possible 365 recording days of a year into analysable data, so we used as much as possible a random sample of the population data (sometimes not all days of an observation month were available, for example because the hard disk of the recorder was full). Unfortunately, there were no data in literature available on estimates of for example mean bird count, mean visit time and accompanying standard errors for visits of wild fauna to a free‐range area of poultry farm on which we could base a sample size calculation for the number of observation days needed. So we based our sample size calculation on estimation of a proportion, for example with respect to estimation of the distribution of activities of the wild fauna visiting the free‐range area. Given a population size of 365 days (one year covering all months and possible seasonality of visits of wild fauna), the largest sample size needed to estimate a proportion of activity (using an a priori estimate of 50% because of lack of prior knowledge) with 95% confidence and a maximum allowable error in the estimate of 5% is 187 observation days (Snedecor & Cochran, 1980), which translates in approximately 15 observation days per observation month of 30 days. We sampled on average 17 days (range: 10–23 days) per observation month, analysing in total 209 days of video recordings. Video recording was replayed on large (32–42”) LCD monitors: all eight camera images on the screen, with the possibility to focus and show only one camera image and even zoom in to get more visible detail. Recordings could be replayed at different speed, and there was a possibility to archive snapshots of specific video recordings. Specified characteristics of a wild fauna visit were entered into a MS Excel database: date of visit; identification of visiting fauna (Family, Order, Species); number of specific fauna visiting; time (hh:mm:ss) of entering/landing in the free‐range area; time (hh:mm:ss) of exiting the free‐range area; activities by fauna exhibited during the visit: foraging; swimming/bathing in water pool; drinking water; sitting in tree or bushes, observing surroundings; eating from chicken carcass; eating from egg; grooming; courting; and playing with each other. These data made a detailed characterization (length of time of a visit, what species involved, what activity involved etc.) of each wild fauna visit possible. In addition, the time (hh:mm:ss) of entrance of layers into the free‐range facility (in general in the morning) was recorded as well as the time (hh:mm:ss) the last layers moved out of the free‐range facility and entered the barn (in general in the evening). Furthermore, if direct physical contact between poultry and wild birds was observed, this was recorded.
A wild fauna visit was defined as one or more of wild birds or a wild animal from the same species landing in/entering the free‐range area (or sitting on the fence), and subsequently staying until leaving the free‐range area. The time period of a wild fauna visit was calculated as the number of wild birds/animals of the same species, multiplied by the total time of this visit. If possibly the same or other wild birds/animals landed in/entered the free‐range area again at a later time during the day, this was counted as a new wild fauna visit and the time period of this wild fauna visit was calculated again. On the same day, more than one wild fauna visit of the same bird/animal species may be observed.
2.4. Observer agreement
Five observers (author and four biology and veterinary science students) converted the video‐data into analysable data. The identification of wildlife species was conducted by the observers, and if needed with support of a wild bird field guide of Europe (Svensson, Mullarney, & Zetterström, 2012) and if needed by a colleague (Kees Veldman, Wageningen Bioveterinary Research), who has had many years of experience with field identification of birds. If exact species determination was not possible from the video recordings, the general species category was indicated with the addition of an unspecified mark, for example gull unspecified or mouse unspecified. Inter‐observer agreement in observations of wild fauna as a measure of data quality was calculated using Cohen's Kappa statistic (Landis & Koch, 1977) by having pairs of observers independently noting visits of wild fauna for one or more of the same observation days. Cohen's Kappa was calculated for a) all wild fauna species observations on a given day and b) for observations on a given day specifically on birds of the orders Anseriformes and Charadriiformes, which are considered AI risk wild birds. Based on an a priori estimated inter‐observer agreement of approximately 0.95 and a maximum acceptable error in the estimated inter‐observer agreement of 0.1 and a 95% confidence level, a sample size of approximately 20 observation days is required (Snedecor & Cochran, 1980).
2.5. Statistical analysis
Data analysis targeted investigation of factors considered relevant for the potential risks for introduction of avian influenza from wild birds to poultry. It was assumed that these factors would be (a) the number of wild birds visiting the free‐range area, (b) how often they visit (frequency) and (c) how long they stay when visiting the free‐range area; this variable combines the number of birds and duration of the visit. The first two (response) variables were assessed by fitting generalized linear models (GLM) with a Poisson or negative binomial (when Poisson models were overdispersed) error distributions and the third (response) variable was assessed by fitting a linear regression model. Explanatory variables included in these models were the wild bird taxonomic Order and the month of the year. The latter was included to investigate the temporal dependency of the response variables. To better understand the models’ outcomes, regarding relationships between order (Anseriformes, Charadriiformes and Passeriformes) and month of the year, we performed a correspondence analysis (CA). CA is a multivariate statistical technique that provides a means of displaying a set of data in a two‐dimensional graphical form, by decomposing associations into orthogonal factors. Additionally, temporal relationships were further assessed by fitting models (for mean number of birds and duration of the visit) to data subsets of each wild bird Order. Hence, only month was used as explanatory variable and January was used as reference month for comparison. Because of the multiple comparisons carried out between months (11 pairwise between month comparisons), a Bonferroni correction was used (p < .05/11) to set the threshold for significance. All models were fitted using the statistical software R (R Development Core Team, 2018).
3. RESULTS
3.1. AIV diagnostic testing
During the study period, the free‐range layer farm was tested every three months for antibodies against AIV in the framework of the routine national AIV surveillance programme; all test results were negative.
3.2. Observer agreement
Mean Cohen's Kappa statistic measuring the inter‐observer agreement for observations on all wild fauna species was 0.95 (SD: 0.026; range: 0.89–1) based on 20 observation days. Disagreement was present in only a few bird observations on a given day where small birds like house sparrow (Passer domesticus) or common starling (Sturnus vulgaris) were missed by one of the observers. For observations on wild birds of the orders Anseriformes and Charadriiformes, mean Cohen's Kappa was 0.97 (SD: 0.054: range 0.80–1) based on 20 observation days with these birds present.
3.3. Wild fauna visits
Our study base consisted of 6,058 wild fauna visits, covering all months of the year. The median number of daily visits in each month varied from seven visits in September and October, when the lowest numbers of visits were observed, to 39 visits in March (Figure 3). There was considerable variation in total number of wild fauna visits per observation day within and between months; for the complete study base, there was a range of 4 to 134 wild fauna visits per observation day.
Figure 3.
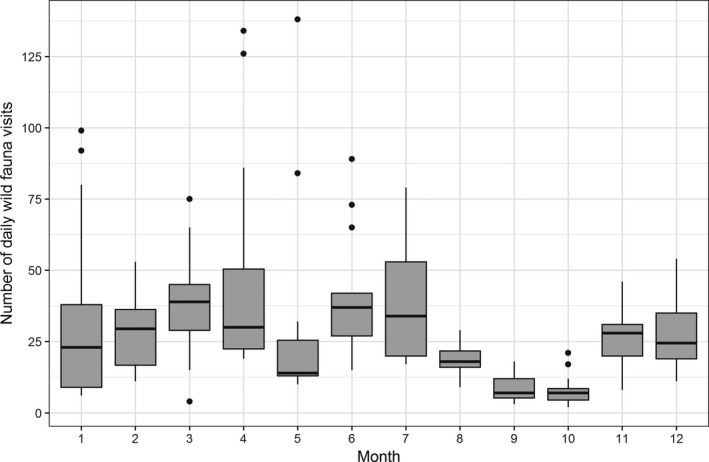
Distribution of the total daily wild fauna visits (unit of measurement: total daily wild fauna visits) to the free‐range area of the layer farm by month (fat dark line in the box: median; lower end of the box: 25% quantile; higher end of the box: 75% quantile; highest bullet or high end of the vertical line coming out of the box: highest value; lowest bullet or low end of vertical line coming out of the box: lowest value). A wild fauna visit was defined as one or more of wild birds from the same species landing, or wild animals (e.g. rat, mouse) entering, and subsequent staying for a continuous time period in the free‐range area (or sitting on the fence) until departing
3.4. Visiting wild fauna species
A total of 16 families of wild birds and five families of mammals visited the free‐range area of the layer farm.
Of the order Anseriformes, only members of the subfamily Anatini or dabbling ducks were seen visiting the free‐range area in the period November–May, and only during the night (from about one hour after sunset to sunrise the next day). Peak frequency of visiting dabbling ducks was in the period December–January, median number of visiting dabbling ducks per day was 4.5 (range: 0–20) and 6.0 (range: 0–34), respectively (Figure 4). The correspondence analysis (Figure 5, right graphs) showed a marked association between the frequency of visits and the month of December (visits were seen almost daily) (Figure 5a) and a strong positive association between the number of birds of the order Anseriformes and the months of December, January and February (Figure 5b). This was further confirmed when the mean number of visiting birds of the order Anseriformes each month was compared pairwise (within these Order). The mean number of birds of the order Anseriformes in January was significantly (p < .0045) higher than all other months except for December and February (Figure 4, Table S1).
Figure 4.
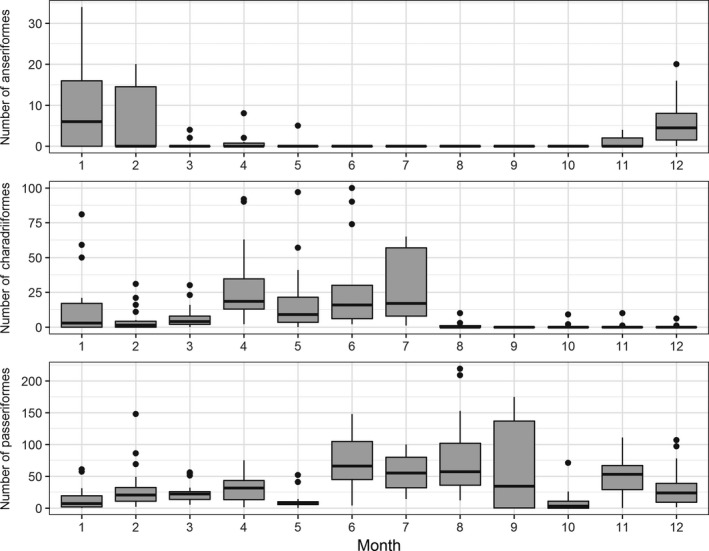
Distribution of total daily bird counts (unit of measurement: total daily bird count) of members of the order Anseriformes, Charadriiformes and Passeriformes visiting the free‐range area of the layer farm by month (fat dark line in the box: median; lower end of the box: 25% quantile; higher end of the box: 75% quantile; highest bullet or high end of the vertical line coming out of the box: highest value; lowest bullet or low end of vertical line coming out of the box: lowest value)
Figure 5.
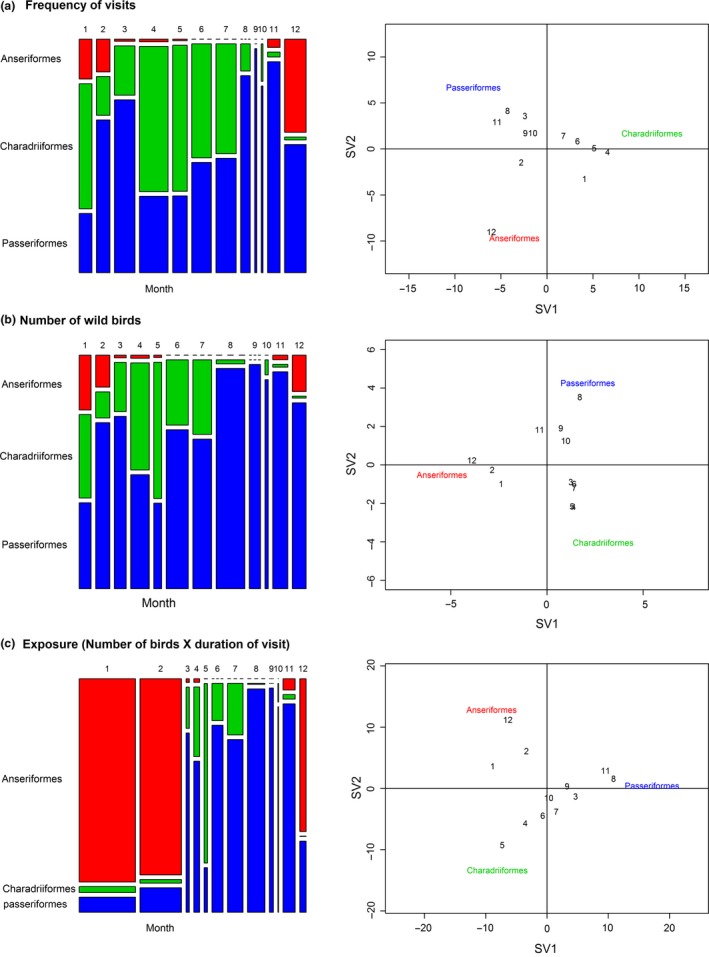
Mosaic plots (graphs on the left side) and correspondence analysis plots (graphs on the right side) displaying: (a) the frequency of visits (mean number of visit per month), (b) number of wild birds visiting the outdoor facility and (c) exposure time, which is the number of birds visiting the outdoor facility times the duration of their visit (bird hours)
On average, the frequency of visits of birds of the orders Charadriiformes and Passeriformes were 1.26 (95% CI: 1.11–1.43) and 1.34 (1.19–1.52) times higher (p < .001) than that of birds of the order Anseriformes, respectively. Similarly, the mean number of birds of the orders Charadriiformes and Passeriformes was on average 5.4 (95% CI: 3.4–8.5) and 32.2 (95% CI: 20.8–50.2) times higher (p < .001) than the mean number of daily visiting birds of the order Anseriformes, respectively. The mosaic plots in Figure 5a,b show the relative comparison among Orders of the frequency of visits and mean number of birds (visiting per day) for each month of the study period.
Although massive amounts of migrating geese species like barnacle goose (Branta leucopsis), greyleg goose (Anser anser) and greater white‐fronted goose (Anser albifrons), and large aggregations of mute swans (Cygnus olor) were observed in the pastures surrounding the free‐range area, they never landed into the fenced free‐range area itself (and thus not counted in the data because they never contacted the free‐range area of the poultry farm). Of the order Charadriiformes, predominantly several species of gulls visited the free‐range area: lesser black‐backed gull (Larus fuscus graellsii), black‐headed gull (Chroicocephalus ridibundus) and unspecified species of gulls. The main visiting period was between January and July, with a peak visiting frequency observed in the months of April and May (Figure 5a). The distribution of the number of visiting birds per day for each month is shown in Figure 4. Differences in the mean number of visiting birds per day were only observed for the period August to December. The mean number of visiting birds these months were significantly lower (p < .0045) than January (Table S2).
Several species of the order Passeriformes like magpie (Pica pica), black crow (Corvus corone), jackdaw (Corvus monedula), house sparrow (Passer domesticus) and blackbird (Turdus merula) visited the free‐range area in almost every month of the year. Correspondence analysis identified the months mostly associated with higher frequency of visits of this order (in relation to the other orders) being March and the period from August to November (Figure 5a). The number of visiting birds per day is summarized in Figure 4. The period between August to November was associated with higher numbers of visiting birds from this order (Figure 5b), with the mean number of visiting birds (of the same order) being significantly higher (p < .0045) in the months June to September and November than January. No significant differences (p > .05) were found for the other months (Figure 4, Table S3).
The buzzard (Buteo buteo), a bird of prey, visited the free‐range area every month of the year. There was a stone marten (Martes foina) couple that occupied a burrow (unknown to the poultry farmer) inside the free‐range area in a fenced area to protect some trees from being destroyed by the chickens, so were permanently present in the free‐range area. The free‐range area was irregularly visited by a fox (Vulpes vulpes). Furthermore, rodents like unspecified species of rats and mice visited the free‐range area and poultry barn during the night, almost on all observation days and during all months of the year.
3.5. Wild fauna exposure time
The largest amount of exposure time of wild fauna to the free‐range area was by far realized by the dabbling ducks and specifically in the period December ‐ February (Table 1 and Figure 5c). This is due to the fact that the dabbling ducks, when visiting the free‐range facility, are present not only in higher numbers (see above) but also in the majority of the time period between sunset and sunrise, which lasts in winter approximately 14–15 hr. Overall, the yearly exposure time for birds of the order Anseriformes was significantly higher (17% higher, p = .01) than that of birds of the order Passeriformes and significantly higher (47% higher, p < .001) than that of birds of the order Charadriiformes. Months associated with higher exposure times from birds of the order Passeriformes were March and the period from July to November, while April and June were mostly associated with birds of the order Charadriiformes (Figure 5c).
Table 1.
Total visit time (in hh:mm:ss) by month of wild fauna visiting the free‐range area of the layer farm (data ordered alphabetically by Order and species name)
| Family | Order | Species (Latin) | Species name | Month | Total visit time of wild fauna (hh:mm:ss) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jan | Febr | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | |||||
| Total observation days | 17 | 20 | 23 | 18 | 15 | 13 | 13 | 22 | 10 | 19 | 17 | 22 | ||||
| Accipitridae | Accipitriformes | Buteo buteo | Buzzard | 0:32:41 | 12:43:02 | 6:12:27 | 0:00:41 | 0:36:24 | 0:01:43 | 1:03:09 | 17:29:58 | 3:36:35 | 15:02:19 | 7:59:15 | 10:55:48 | |
| Anatidae | Anseriformes | Anas platyrhynchos | Mallard | 1,593:13:02 | 1,135:58:56 | 1:41:34 | 2:50:48 | 0:03:42 | 8:54:32 | 656:11:59 | ||||||
| Anatidae | Anseriformes | unspecified | Unspecified wild duck species | 0:08:48 | 10:05:16 | 0:55:48 | ||||||||||
| Laridae | Charadriiformes | Chroicocephalus ridibundus | Black‐headed gull | 1:50:29 | 0:32:12 | 8:25:09 | 28:21:09 | |||||||||
| Charadriidae | Charadriiformes | Pluvialis squatarola | Grey plover | 0:03:18 | 0:44:59 | 7:34:26 | ||||||||||
| Laridae | Charadriiformes | Larus fuscus graellsii | Lesser black‐backed gull | 0:28:11 | 5:37:51 | 8:19:17 | 11:56:30 | 82:35:13 | 2:13:57 | 1:00:36 | 0:05:53 | 0:01:30 | ||||
| Haematopodidae | Charadriiformes | Haematopus ostralegus | Oyster catcher | 0:24:20 | 31:36:08 | 58:54:56 | 13:57:33 | 0:27:53 | ||||||||
| Laridae | Charadriiformes | unspecified | Unspecified gull species | 45:37:42 | 20:09:29 | 18:35:42 | 17:24:55 | 14:06:22 | 22:46:20 | 0:00:11 | 0:00:12 | 0:14:04 | ||||
| Columidae | Columbiformes | Streptopelia decaocto | Eurasian collared‐dove | 0:03:28 | 0:14:54 | |||||||||||
| Columidae | Columbiformes | Columba palumbus | Ringdove | 2:10:08 | 0:12:20 | 1:49:03 | 0:46:17 | 0:01:59 | ||||||||
| Columidae | Columbiformes | unspecified | Unspecified dove species | 0:36:10 | 0:48:51 | 38:59:15 | 36:16:51 | 96:22:02 | 0:19:34 | 0:07:15 | 4:03:20 | |||||
| Falconidae | Falconiformes | Falco tinnunculus | Common kestrel | 0:15:43 | 0:00:51 | |||||||||||
| Rallidae | Gruiformes | Gallimula chloropus | Common Moor‐hen | 40:01:57 | ||||||||||||
| Turdidae | Passeriformes | Turdus merula | Blackbird | 55:58:16 | 7:55:01 | 51:37:52 | 1:51:17 | 0:09:36 | 0:25:58 | 53:08:04 | 7:38:57 | 0:08:23 | 0:14:46 | 125:58:48 | 8:00:52 | |
| Corvidae | Passeriformes | Corvus corone | Black crow | 0:13:33 | 3:49:34 | 1:21:53 | 10:55:14 | 6:49:30 | 11:57:26 | 71:36:31 | 42:39:24 | 0:32:44 | 0:22:35 | 0:28:02 | ||
| Sturnidae | Passeriformes | Sturnus vulgaris | Common starling | 0:31:45 | 17:23:30 | 57:45:56 | 114:34:51 | 105:31:25 | 4:06:17 | 20:03:28 | 0:07:47 | |||||
| Paridae | Passeriformes | Parus major | Great tit | 0:07:47 | ||||||||||||
| Passeridae | Passeriformes | Passer domesticus | House sparrow | 61:10:56 | 98:42:31 | 2:46:01 | 109:54:45 | 2:15:43 | 254:57:38 | 112:24:35 | 348:59:45 | 15:35:39 | 10:31:47 | 61:14:10 | 39:03:30 | |
| Corvidae | Passeriformes | Corvus monedula | Jackdaw | 0:06:11 | 0:02:16 | 1:14:22 | 0:09:28 | 39:56:27 | 7:14:24 | 2:08:30 | 1:13:21 | 6:37:21 | ||||
| Corvidae | Passeriformes | Pica pica | Magpie | 0:38:08 | 31:06:44 | 25:09:08 | 0:06:12 | 9:15:28 | 1:16:17 | 33:43:36 | 19:24:47 | 10:17:24 | 8:13:25 | |||
| Motacillidae | Passeriformes | Motacilla flava | Western yellow wagtail | 0:17:42 | ||||||||||||
| Motacillidae | Passeriformes | Motacilla alba | White wagtail | 0:02:30 | 0:36:56 | 28:52:06 | 132:48:12 | |||||||||
| Ardeidae | Pelecaniformes | Ardea cinerea | Grey heron | 0:08:12 | 1:06:59 | |||||||||||
| Tytonidae | Strigiformes | unspecified | Unspecified owl species | 0:00:05 | 1:00:44 | 3:07:48 | 0:00:06 | |||||||||
| Canidae | Carnivora | Vulpes vulpes | Fox | 0:00:28 | 0:09:20 | 0:00:17 | 0:08:59 | 0:15:17 | 0:00:58 | |||||||
| Felidae | Carnivora | Felis silvestris catus | House cat | 0:02:43 | 0:00:29 | 0:05:29 | ||||||||||
| Mustelidae | Carnivora | Martes foina | Stone marten | 2:59:45 | 1:53:00 | 12:11:24 | 0:56:21 | 0:25:40 | 0:14:16 | 1:22:31 | 5:55:18 | 0:31:43 | 6:11:20 | 7:16:25 | 3:36:24 | |
| Leporidae | Lagomorpha | Lepus europaeus | Hare | 0:12:43 | 6:35:34 | |||||||||||
| Leporidae | Lagomorpha | Oryctolagus cuniculus | Rabbit | 00:00:07 | 2:20:34 | |||||||||||
| Muridae | Rodentia | unspecified | Unspecified mouse species | 8:12:54 | 2:29:44 | 4:44:16 | 1:35:01 | 0:11:13 | 0:01:44 | 0:29:45 | 0:21:40 | 0:44:34 | 0:25:19 | 1:04:21 | 0:25:23 | |
| Muridae | Rodentia | unspecified | Unspecified rat species | 0:16:56 | 0:06:10 | 0:03:44 | 0:08:44 | 0:45:06 | 0:00:30 | 0:04:15 | 0:02:42 | 0:04:30 | 0:00:55 | |||
In Table 1, the exposure time for the different observed wild bird species within each order is shown. Specific members of the orders Passeriformes and Charadriiformes with high exposure times were the house sparrow (largest amount of exposure time in April, June – August), black bird (largest amount of exposure time in January, March, July and November), common starling (largest amount of exposure time in July‐September); and the gull species: largest amount of exposure time in the period January ‐ August.
3.6. Temporal patterns in visits
Wild birds, except for the dabbling ducks and unspecified species of owl, visited the free‐range area almost exclusively in the morning: in the period between sunrise and the moment the chickens entered the free‐range area. In majority, the free‐range area was made available to the chickens in the morning at around 10:00–11:00 a.m. In the autumn and winter period with sometimes freezing temperatures or with heavy rain and wind, the chickens stayed inside the poultry barn. When the chickens massively poured out from the barn into the free‐range area, wild birds disappeared, often hunted away by groups of chickens. Some of the wild bird species disappeared into branches of trees or onto the fence surrounding the free‐range area, but most of them disappeared altogether out of the free‐range area. Stone marten (Martes foina), fox (Vulpes vulpes), house cat (Felis silvestris catus), unspecified species of rats and mice, dabbling ducks and the unspecified owl species visited the free‐range facility only between approximately 1–2 hr after sunset and sunrise the next morning, without chickens being present in the free‐range facility.
3.7. Wild fauna – poultry interaction
Only 8% of the total number of recorded visits of wild fauna had overlap with the presence of chickens at the same time in the free‐range area; the sheer majority of the wild birds were sitting on the fence or in trees or bushes, on a considerable distance from the chickens. In decreasing order, the distribution of visiting wild fauna species, overlapping with chicken presence, was house sparrow (Passer domesticus) (17.4%), black crow (Corvus corone) (14.7%), gull—unspecified species (13.4%), buzzard (Buteo buteo) (13.0%), dove—unspecified species (10.5%), blackbird (Turdus merula) (9.7%), common starling (Sturnus vulgaris) (6.0%), black‐headed gull (Chroicocephalus ridibundus) (4.7%), magpie (Pica pica) (3.3%), lesser black‐backed gull (Larus fuscus graellsii) (3.1%), oyster catcher (Haematopus ostralegus) (2.3%), jackdaw (Corvus monedula) (0.6%), mouse—unspecified species (0.7%), rat—unspecified species and mallard (Anas platyrhynchos) (0.2%). However, never direct contact between live poultry and wild birds was observed during these sparse overlapping periods.
3.8. Wild fauna activity
The most prominent activity of all visiting wild fauna to the free‐range area was foraging (Table 2). Of all wild bird species, only the dabbling ducks exhibited a considerable portion of the time swimming and bathing in water pools. The buzzard (Buteo buteo) was predominantly busy with observation of the surroundings while positioned on the fence and was never seen attacking live chickens. Lesser black‐backed gull (Larus fuscus graellsii), black‐headed gull (Chroicocephalus ridibundus), magpie (Pica pica), black crow (Corvus corone), buzzard (Buteo buteo), stone marten (Martes foina) and fox (Vulpes vulpes) were seen eating from chicken carcasses present in the free‐range area, while magpie (Pica pica) and black crow (Corvus corone) were also observed eating eggs present in the free‐range area. The stone marten was seen dragging carcasses within the free‐range area to its burrow, situated in the free‐range area itself; on occasion, the fox was seen taking carcasses from the free‐range area in the mouth and transporting it to outside the free‐range area.
Table 2.
Distribution of activities by wild fauna visiting the free‐range area of the layer farm (data ordered alphabetically by Order and species name). Simultaneous activities within a wild fauna visit were recorded separately (total activities could therefore equate to more than 100%)
| Family | Order | Species (Latin) | Species name | Swimming/ bathing (%) | Drinking water (%) | Foraging (%) | Sit in tree/ bushes/on fence and observing (%) | Eating from carcass (%) | Eating from egg (%) | Grooming (%) | Courtiing (%) | Playing with Eachother (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accipitridae | Accipitriformes | Buteo buteo | Buzzard | 0.0 | 0.3 | 8.7 | 93.9 | 4.2 | 0.6 | 0.0 | 0.0 | 0.0 |
| Anatidae | Anseriformes | Anas platyrhynchos | Mallard | 46.3 | 34.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Anatidae | Anseriformes | unspecified | Unspecified wild duck species | 22.2 | 22.2 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Laridae | Charadriiformes | Chroicocephalus ridibundus | Black‐headed gull | 0.0 | 5.3 | 97.4 | 3.5 | 1.8 | 0.0 | 0.0 | 0.9 | 0.0 |
| Charadriidae | Charadriiformes | Pluvialis squatarola | Grey plover | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Laridae | Charadriiformes | Larus fuscus graellsii | Lesser black‐backed gull | 0.0 | 2.0 | 97.7 | 2.5 | 17.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| Haematopodidae | Charadriiformes | Haematopus ostralegus | Oyster catcher | 0.0 | 0.5 | 97.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Laridae | Charadriiformes | unspecified | Unspecified gull species | 0.0 | 12.2 | 83.5 | 25.0 | 1.9 | 0.0 | 0.2 | 0.5 | 0.0 |
| Columidae | Columbiformes | Streptopelia decaocto | Eurasian collared‐dove | 0.0 | 0.0 | 75.0 | 75.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Columidae | Columbiformes | Columba palumbus | Ringdove | 0.0 | 9.1 | 45.5 | 54.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Columidae | Columbiformes | unspecified | Unspecified dove species | 0.0 | 0.4 | 65.0 | 38.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.0 |
| Falconidae | Falconiformes | Falco tinnunculus | Common kestrel | 0.0 | 0.0 | 25.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Rallidae | Gruiformes | Gallimula chloropus | Common Moor‐hen | 0.0 | 50.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Turdidae | Passeriformes | Turdus merula | Blackbird | 0.2 | 4.7 | 80.1 | 36.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Corvidae | Passeriformes | Corvus corone | Black crow | 0.0 | 5.0 | 59.6 | 47.6 | 9.1 | 0.8 | 0.0 | 0.0 | 0.0 |
| Sturnidae | Passeriformes | Sturnus vulgaris | Common starling | 0.0 | 2.4 | 50.6 | 65.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Paridae | Passeriformes | Parus major | Great tit | 0.0 | 0.0 | 100.0 | 50.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Passeridae | Passeriformes | Passer domesticus | House sparrow | 0.4 | 7.0 | 64.0 | 64.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Corvidae | Passeriformes | Corvus monedula | Jackdaw | 0.0 | 0.0 | 63.0 | 40.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Corvidae | Passeriformes | Pica pica | Magpie | 0.0 | 19.5 | 100.0 | 17.2 | 30.3 | 0.5 | 0.0 | 0.0 | 0.0 |
| Motacillidae | Passeriformes | Motacilla flava | Western yellow wagtail | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Motacillidae | Passeriformes | Motacilla alba | White wagtail | 0.0 | 0.0 | 97.1 | 5.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Ardeidae | Pelecaniformes | Ardea cinerea | Grey heron | 0.0 | 20.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Tytonidae | Strigiformes | unspecified | Unspecified owl species | 0.0 | 0.0 | 8.8 | 91.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Canidae | Carnivora | Vulpes vulpes | Fox | 0.0 | 0.0 | 94.1 | 0.0 | 5.9 | 0.0 | 0.0 | 0.0 | 0.0 |
| Felidae | Carnivora | Felis silvestris catus | House cat | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Mustelidae | Carnivora | Martes foina | Stone marten | 0.0 | 3.8 | 95.2 | 0.0 | 1.5 | 0.0 | 0.0 | 0.0 | 1.3 |
| Leporidae | Lagomorpha | Lepus europaeus | Hare | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Leporidae | Lagomorpha | Oryctolagus cuniculus | Rabbit | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Muridae | Rodentia | unspecified | Unspecified mouse species | 0.0 | 1.3 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Muridae | Rodentia | unspecified | Unspecified mouse species | 0.0 | 20.7 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
4. DISCUSSION
The measures of inter‐observer agreement in this study are considered nearly perfect (Landis & Koch, 1977) or excellent (Fleiss, 1981), indicating the availability of high‐quality data for analysis.
The objective of this study was to quantify visits of wild fauna to a free‐range area of a layer farm, situated in an avian influenza hot‐spot area. A total of 16 families of wild birds and five families of mammals visited the free‐range area of the layer farm exclusively at times that chickens were not present in the free‐range facility (still locked‐up in the poultry barn): in the period between sunrise and the moment the layers enter the free‐range area in the morning. The free‐range poultry farmer can lower the attractiveness of the free‐range area for wild fauna by daily inspection of the free‐range facility and removal of carcasses and eggs. This will predominantly limit the attractiveness of the free‐range area to scavengers such as gulls, magpies, black crows, buzzards, owls and stone martens. This is of importance because scavengers might introduce AIV to the free‐range area of the poultry farm.
Known carriers of AI viruses visit the outdoor facility regularly. Several species of gulls almost daily during the period January – August in the morning before the chickens go out in the free‐range facility. Dabbling ducks visited the outdoor facilities in the period between sunset and sunrise when the chickens are housed and locked‐up in the barn between November and April, with a distinct peak in both frequency of visits and number of birds during the period December – February. Consequently, the highest exposure times, which may also represent a period of highest risk for introduction of AI, were estimated in these months.
We observed in our study high variation in wild fauna visits between observation days (range: 4–134 wild fauna visits per day). This means that, for a precise and well‐based quantitative judgment on wild bird visits and exposure time, one has to include a high number of observation days in one's study. Unfortunately, this requirement is often neglected for practical and resource reasons.
Scott, Phalen, et al. (2018a) counted on average 2.4 wildlife visits per day per farm using motion‐sensing camera traps (a total of 30 poultry farms in the study, camera traps operated for one week per farm in the period June‐February), this is at least 10 times less than we have observed in our study. The order Passeriformes were the most frequent visitors to poultry farms in the Australian study (Scott, Phalen, et al., 2018a), with 1.6% of total wild bird visits identified by cameras by members of the orders Anseriformes and Charadriiformes. In our study, members of the order Passeriformes were also frequent visitors. But in contrast to the Australian study, dabbling ducks and gull species were very frequent visitors in our study too. These findings might be related to the fact that the farm in our study is located in an AIV hot‐spot area (presence of abundant water bodies), and it is therefore logical that a much higher number of wildlife visits can be anticipated, in particular by AIV high‐risk host birds like dabbling ducks and gulls.
The peak in visits (frequency and numbers) by dabbling ducks to the free‐range area of the layer farm in the period December – February in our study was similarly observed by Welby, Poncin, et al. (2010b) in Belgium in a field experiment with two small flocks of chickens (10 and 20–30 chickens in experimental pens). The peak in visits by dabbling ducks to the free‐range area of the layer farm between December – February correlates well with annual counting results of dabbling ducks by the national wild water bird monitoring (Hornman et al., 2019). The presence of waterways or even water pools in the free‐range area of the farm is an important attractant for these water birds. The water pools in our study farm are formed in the autumn–winter period in the free‐range area due to abundant and prolonged periods of rain, which is normal for that time of the year in the Netherlands. As can be seen in Figure 6, most precipitation in the area was in the period October ‐ February, resulting in large water pools in the free‐range area (Figure 7). These water pools are attractive to wild water birds and chickens alike: for drinking, bathing and swimming, but in those processes also potential AIV contamination of the water takes place due to defecation. AIV excreted by ducks in surface water can survive for months at low temperatures in experiments (Breban, Drake, Stallknecht, & Rohani, 2009; Stallknecht & Brown, 2009; VanDalen, Franklin, Mooers, Sullivan, & Shriner, 2010). Prevention of water pool forming in the free‐range area can be done by drainage and by equalizing the soil area (filling the lower‐lying holes in the free‐range area with soil).
Figure 6.
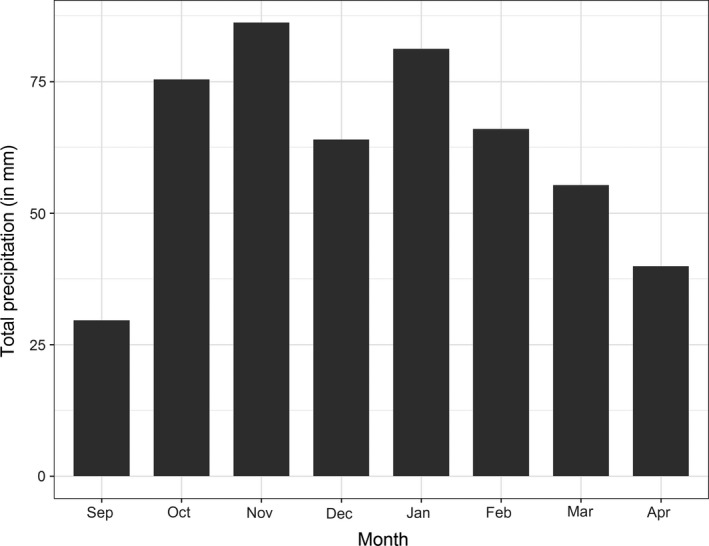
Total precipitation (in mm) by month from an official KNMI weather station located approximately 1 km from the free‐range layer farm in this study (source: Royal Netherlands Meteorological Institute (KNMI); https://www.knmi.nl/nederland-nu/klimatologie-metingen-en-waarnemingen)
Figure 7.

Water pools in the free‐range area of the layer farm (second half of November)
The high number of wild birds involved, high frequency of visits and exposure time by members of the order Passeriformes to the free‐range area of the layer farm could indicate a possible potential role in AIV transmission. However, several studies have detected only very low levels of LPAIV in birds of the order Passeriformes (Gronesova, Kabat, Trnka, & Betakova, 2008; Hansbro et al., 2010; Munster et al., 2007; Peterson, Bush, Spackman, Swayne, & Ip, 2008; Rutz et al., 2007; Slusher et al., 2014).
The stone marten (Martes foina) belongs to the family of Mustelidae like the ferret (Mustela putorius furo). Ferrets are known to be susceptible to AIV infection and are used as an experimental mammalian model to study influenza virus pathogenicity, including HPAIV and evaluate vaccines because disease in ferrets closely resembles that of humans (Kreijtz et al., 2013; Zitzow et al., 2002). During the Asian HPAI H5N1 epidemic, a naturally infected stone marten (Martes foina) with encephalitis signs was found in a rural area with numerous confirmed cases of HPAI H5N1 infection in wild birds in Germany in 2006 (Klopfleisch et al., 2007). Since the stone marten roams and forages the surrounding pastures with the presence of carcasses of dead wild birds, it may act as a reservoir and vector, bringing AIV to the free‐range facility of the poultry farm.
Rodents like mice and rats can be abundant around poultry houses, share their habitat with water birds and regularly enter poultry houses (Velkers et al., 2017). Our study also shows that rats and mice were frequent visitors of the free‐range area of the layer farm. Avian influenza viruses replicate efficiently in wild‐caught house mice under experimental conditions, indicating mice might play a role as a vector for AIV on poultry farms (Shriner et al., 2012). However, several attempts made to isolate virus from small rodents caught in conjunction with AIV outbreaks in commercial poultry were not very successful (Velkers et al., 2017).
No direct contact between live poultry and wild birds in the free‐range area was seen in our study, the same lack of interaction was observed in a recent field study with 20 free‐range poultry farms in Australia (Scott, Phalen, et al., 2018a). It is hypothesized that AIV transmission to poultry on free‐range poultry farms will predominantly take place via indirect contact: taking up AIV by chickens via wild‐bird‐faeces‐contaminated water or soil in the free‐range area. AIV are stable and persist for a long period of time in water, especially at low temperatures (Stallknecht & Brown, 2009), and it is shown that AIV can be isolated from mud and soil (Breban et al., 2009).
One might think of discouraging the start of new free‐range poultry farms in AI high‐risk areas—areas that are close to waterways and nature areas with waterfowl (Bouwstra et al., 2017)—in order to lower the risk of AIV introductions on poultry farms. If a free‐range farm, with a history of repeated introductions of AIV, is already located in a high‐risk area, one can think about possibilities to scare away wild birds after sunrise in the morning before entrance of the chickens into the free‐range area but in particular dabbling ducks between sunset and sunrise, from the free‐range facility using trained dogs or laser equipment. Trained dogs (e.g. border collie) have been used to scare off wild birds (Castelli & Sleggs, 2000; Holevinski, Curtis, & Malecki, 2007). In Australia, some free‐range poultry farms use trained Maremma breed dogs to limit interaction between wild fauna and poultry (AGDAFF, 2009; Gibbs, ; Scott, Singh, et al., 2018b). Laser equipment is used for several years to scare off wild birds, in particular geese (Blackwell, Bernhardt, & Dolbeer, 2002; Gorenzel, Blackwell, Simmons, Salmon, & Dolbeer, 2002; Werner & Clark, 2006). In the Netherlands, there is experience with lasers to scare off wild birds around oil‐rig platforms in the sea, airports, fruit orchids, in the aquaculture sector, and garbage landfills (BCG, 2018). There are recent examples of free‐range poultry farmers that used laser technology to scare off wild birds during the HPAI H5N8 epidemic in Europe in 2017 (Bijleveld, 2017).
Another option to prevent contact between chickens and wild life, and at the same time address consumer demands with respect to welfare and an ecological way of production, is using other housing designs like the ‘Rondeel’ concept (http://www.rondeeleieren.nl/). In this innovative chicken farm design, chickens have the possibility to be exposed to sunlight and fresh air in an outdoor area, but the outdoor area is totally fenced and together with the translucent but solid roofing offers shelter and protection, preventing contact with wildlife and their excrements in the environment. Another aviary housing option used more and more is the winter garden that can be built as a subsidiary to a normal poultry house (https://www.bigdutchman.com/en/egg-production/news/photos/aviary-systems/). In this system, the chickens also have the possibility to be exposed to sunlight and fresh air in a totally fenced outdoor area offering shelter and protection against contact to wildlife. The eggs produced in the ‘Rondeel’ concept do not certify for the label free‐range egg, but are marketed with its own brand ‘Rondeel egg’ at a price, in the Netherlands, equal or slightly higher than certified free‐range eggs. The eggs produced with the winter garden concept are marketed under the requirements of ‘one star better life’ production at a price that is lower than the free‐range eggs and the Rondeel egg.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the authors have complied, if applicable, with EU directive 2010/63/EU. No ethical approval was required as this study only observed wild fauna visits to the free‐range area of a commercial layer farm using fixed video‐camera monitoring; there was no handling of—or any direct human contact with—wild fauna.
Supporting information
ACKNOWLEDGEMENTS
This study was funded by the Netherlands' Ministry of Agriculture, Nature and Food Quality (Veterinary Epidemiology and Risk Analysis project WOT‐01‐001‐004). Lynn de Boer and Nina Bakker (students of Aeres University of Applied Sciences, Dronten), Gerdien van Dijk and Nhlanhla Makatini (students of Aeres University of Applied Sciences, Almere), Joeri Baak (student of Faculty of Veterinary Medicine, Utrecht) and Steven Venema (student of van Hall Larenstein University of Applied Sciences, Velp) are acknowledged for helping out during practical work at the study farm and with converting the video recordings into analysable data. Kees Veldman (Wageningen Bioveterinary Research) is thanked for helping with the identification of wild bird species. The poultry farmer is gratefully acknowledged for cooperation and giving access to his poultry farm.
Elbers ARW, Gonzales JL. Quantification of visits of wild fauna to a commercial free‐range layer farm in the Netherlands located in an avian influenza hot‐spot area assessed by video‐camera monitoring. Transbound Emerg Dis. 2020;67:661–677. 10.1111/tbed.13382
REFERENCES
- Achenbach, J. E. , & Bowen, R. A. (2011). Transmission of avian influenza A viruses among species in an artificial barnyard. PLoS ONE, 6, e17643 10.1371/journal.pone.0017643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, D. J. (2007). An overview of the epidemiology of avian influenza. Vaccine, 25, 5637–5644. 10.1016/j.vaccine.2006.10.051 [DOI] [PubMed] [Google Scholar]
- Australian Egg Corporation Limited (AECL) (2018). AECL annual report 2018. North Sydney, NSW, Australia: Retrieved from https://www.australianeggs.org.au/who-we-are/annual-reports/ [Google Scholar]
- Australian Government ‐ Department of Agriculture, Fisheries and Forestry (AGDAFF) (2009). National farm biosecurity manual, poultry production. Retrieved from http://www.agriculture.gov.au/pests-diseases-weeds/protect-animal-plant/bird-owners/poultry_biosecurity_manual [Google Scholar]
- BCG . (2018). Bird Control Group (BCG), Delft, The Netherlands. Retrieved from http://birdcontrolgroup.com [Google Scholar]
- Beerens, N. , Koch, G. , Heutink, R. , Harders, F. , Vries, D. P. E. , Ho, C. , … Elbers, A. (2018). Novel highly pathogenic avian influenza A(H5N6) virus in the Netherlands, December 2017. Emerging Infectious Diseases, 24, 770–773. 10.3201/eid2404.172124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijleveld, H. (2017). Laser to scare off wild birds from free‐range poultry farms. Pluimveehouderij 47 (23 March 2017), 18 (in Dutch). [Google Scholar]
- Blackwell, B. F. , Bernhardt, G. R. , & Dolbeer, R. A. (2002). Lasers as nonlethal avian repellents. The Journal of Wildlife Management, 66, 250–258. 10.2307/3802891 [DOI] [Google Scholar]
- Bouwstra, R. , Gonzales, J. L. , de Wit, J. J. , Stahl, J. , Fouchier, R. A. M. , & Elbers, A. R. W. (2017). Risk for low pathogenicity avian influenza virus on poultry farms, the Netherlands, 2007–2013. Emerging Infectious Diseases, 23, 1510–1516. 10.3201/eid2309.170276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwstra, R. , Heutink, R. , Bossers, A. , Harders, F. , Koch, G. , & Elbers, A. R. W. (2015). Full‐genome sequence of influenza A(H5N8) virus in poultry linked to sequences of strains from Asia, the Netherlands, 2014. Emerging Infectious Diseases, 21, 872–874. 10.3201/eid2105.141839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breban, R. , Drake, J. M. , Stallknecht, D. E. , & Rohani, P. (2009). The role of environmental transmission in recurrent avian influenza epidemics. PLoS Computational Biology, 5, e1000346 10.1371/journal.pcbi.1000346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns, T. E. , Ribble, C. , Stephen, C. , Kelton, D. , Toews, L. , Osterhold, J. , & Wheeler, H. (2012). Use of observed wild bird activity on poultry farms and a literature review to target species as high priority for avian influenza testing in 2 regions of Canada. Canadian Veterinary Journal, 53, 158–166. [PMC free article] [PubMed] [Google Scholar]
- Campitelli, L. , Mogavero, E. , De Marco, M. A. , Delogu, M. , Puzelli, S. , Frezza, F. , … Donatelli, I. (2004). Interspecies transmission of a H7N3 influenza virus from wild birds to intensively reared domestic poultry in Italy. Virology, 323, 24–36. 10.1016/j.virol.2004.02.015 [DOI] [PubMed] [Google Scholar]
- Caravaggi, A. , Banks, P. B. , Burton, A. C. , Finlay, C. M. V. , Haswell, P. M. , Hayward, M. W. , … Wood, M. D. (2017). A review of camera trapping for conservation behaviour research. Remote Sensing in Ecology and Conservation, 3, 109–122. 10.1002/rse2.48 [DOI] [Google Scholar]
- Caron, A. , Grosbois, V. , Etter, E. , Gaidet, N. , & de Garine‐Wichatitsky, M. (2014). Bridge hosts for avian influenza viruses at the wildlife/domestic interface: An eco‐epidemiological framework implemented in southern Africa. Preventive Veterinary Medicine, 117, 590–600. 10.1016/j.prevetmed.2014.09.014 [DOI] [PubMed] [Google Scholar]
- Castelli, P. M. , & Sleggs, S. E. (2000). Efficacy of border collies to control nuisance Canada geese. Wildlife Society Bulletin, 28, 385–392. [Google Scholar]
- Chen, L.‐J. , Yu, B. , Lin, X.‐D. , Yang, Z.‐Q. , Guo, W.‐P. , Shi, M. , … Zhang, Y.‐Z. (2016). Diversity and evolution of avian influenza viruses in live poultry markets, free‐range poultry and wild wetland birds in China. Journal of General Virology, 97, 844–854. 10.1099/jgv.0.000399 [DOI] [PubMed] [Google Scholar]
- East, I. J. , Ainsworth, C. , Warner, S. , Dunowska, M. , & Azuolas, J. K. (2010). Seroconversion to avian influenza virus in free‐range chickens in the Riverland region of Victoria. Australian Veterinary Journal, 88, 290–293. 10.1111/j.1751-0813.2010.00601.x [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority (EFSA) (2017). Avian influenza overview September – November 2017. EFSA Journal, 15, e05141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiss, J. L. (1981). Statistical methods for rates and proportions, 2nd ed. (pp. 38–46). (New York: John Wiley). [Google Scholar]
- Fouchier, R. A. M. , & Munster, V. J. (2009). Epidemiology of low pathogenic avian influenza viruses in wild birds. Revue Scientifique Et Technique / Office International Des Épizooties, 28, 49–58. 10.20506/rst.28.1.1863 [DOI] [PubMed] [Google Scholar]
- Fouchier, R. A. M. , Schneeberger, P. M. , Rozendaal, F. W. , Broekman, J. M. , Kemink, S. A. G. , Munster, V. , & Osterhaus, A. D. M. E. (2004). Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proceedings of the National Academy of Sciences, 101(5), 1356–1361. 10.1073/pnas.0308352100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs, B. (2017). Maremma breed tough but worthwhile, Canberra chicken farmer Bruce Gibbs says. Retrieved from https://www.smh.com.au/environment/conservation/maremma-breed-tough-but-worthwhile-canberra-chicken-farmer-bruce-gibbs-says-20170210-guahry.html. [Google Scholar]
- Gilbert, M. , Xiao, X. , Domenech, J. , Lubroth, J. , Martin, V. , & Slingenbergh, V. (2006). Anatidae migration in the western Palearctic and spread of highly pathogenic avian influenza H5N1 virus. Emerging Infectious Diseases, 12, 1650–1656. 10.3201/eid1211.060223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales, J. L. , & Elbers, A. R. W. (2018). Effective thresholds for reporting suspicions and improve early detection of avian influenza outbreaks in layer chickens. Scientific Reports, 8, 8533 10.1038/s41598-018-26954-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales, J. L. , Stegeman, J. A. , Koch, G. , de Wit, J. J. , & Elbers, A. R. W. (2013). Rate of introduction of a low pathogenic avian influenza virus infection in different poultry production sectors in the Netherlands. Influenza and Other Respiratory Viruses, 7, 6–10. 10.1111/j.1750-2659.2012.00348.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Google Earth Pro (2019). Google Earth Pro, version 7.3.2.5776. US Department of State Geographer, Google LLC. [Google Scholar]
- Gorenzel, W. P. , Blackwell, B. F. , Simmons, G. D. , Salmon, T. P. , & Dolbeer, R. A. (2002). Evaluation of lasers to disperse American crows, Corvus brachyrhynchos, from urban night roosts. USDA National Wildlife Research Center ‐ Staff Publications, 466. Retrieved from https://digitalcommons.unl.edu/icwdm_usdanwrc/466 [Google Scholar]
- Grillo, V. L. , Arzey, K. E. , Hansbro, P. M. , Hurt, A. C. , Warner, S. , Bergfeld, J. , … Post, L. (2015). Avian influenza in Australia: A summary of 5 years of wild bird surveillance. Australian Veterinary Journal, 93, 387–393. 10.1111/avj.12379 [DOI] [PubMed] [Google Scholar]
- Gronesova, P. , Kabat, P. , Trnka, A. , & Betakova, T. (2008). Using nested RT‐PCR analyses to determine the prevalence of avian influenza viruses in Passarines in western Slovakia, during summer 2007. Scandinavian Journal of Infectious Diseases, 40, 954–957. 10.1080/00365540802400576 [DOI] [PubMed] [Google Scholar]
- Halvorson, D. A. (2009). Prevention and management of avian influenza outbreaks: Experiences from the United States of America. Revue Scientifique Et Technique / Office International Des Épizooties, 28, 359–369. 10.20506/rst.28.1.1866 [DOI] [PubMed] [Google Scholar]
- Hansbro, P. M. , Warner, S. , Tracey, J. P. , Arzey, K. E. , Selleck, P. , O’Riley, K. , … Hurt, A. C. (2010). Surveillance and Analysis of Avian Influenza Viruses, Australia. Emerging Infectious Diseases, 16, 1896–1904. 10.3201/eid1612.100776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes, L. , Arzey, E. , Bell, C. , Buchanan, N. , Burgess, G. , Cronan, V. , … Bunn, C. (2009). Australian surveillance for avian influenza viruses in wild birds between July 2005 and June 2007. Australian Veterinary Journal, 87, 266–272. 10.1111/j.1751-0813.2009.00446.x [DOI] [PubMed] [Google Scholar]
- Holevinski, R. A. , Curtis, P. D. , & Malecki, R. A. (2007). Hazing of Canada geese is unlikely to reduce nuisance populations in urban and suburban communities. Human‐Wildlife Conflicts, 1, 257–264. [Google Scholar]
- Hornman, M. , Hustings, F. , Koffijberg, K. , van Winden, E. , van Els, P. , … Soldaat, L. (2019). Water birds in the Netherlands in 2016/2017. Sovon rapport 2019/01, RWS‐rapport BM 19.01. Sovon Vogelonderzoek Nederland, Nijmegen (in Dutch). [Google Scholar]
- Kirunda, H. , Kabi, F. , Muwereza, N. , Kabuuka, T. , Kerfua, S. D. , Kasaija, P. D. , … Wabwire‐Mangen, F. (2015). Seroprevalence and risk factors for exposure of free‐range poultry to avian influenza viruses in important bird areas in Uganda. Avian Diseases, 59, 64–70. 10.1637/10874-052714-Reg [DOI] [PubMed] [Google Scholar]
- Kleyheeg, E. , van Dijk, J. G. B. , Tsopoglou‐Gkina, D. , Woud, T. Y. , Boonstra, D. K. , Nolet, B. A. , & Soons, M. B. (2017). Movement patterns of a keystone waterbird species are highly predictable from landscape configuration. Movement Ecology, 5, 2 10.1186/s40462-016-0092-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfleisch, R. , Wolf, P. U. , Wolf, C. , Harder, T. , Starick, E. , Niebuhr, M. , … Teifke, J. P. (2007). Encephalitis in a Stone Marten (Martes foina) after Natural Infection with Highly Pathogenic Avian Influenza Virus Subtype H5N1. Journal of Comparative Pathology, 137, 155–159. 10.1016/j.jcpa.2007.06.001 [DOI] [PubMed] [Google Scholar]
- Knierim, U. (2006). Animal welfare aspects of outdoor runs for laying hens: A review. Netherlands Journal of Agricultural Science, 54, 133–145. 10.1016/S1573-5214(06)80017-5 [DOI] [Google Scholar]
- Kreijtz, J. , Kroeze, E. J. B. V. , Stittelaar, K. J. , de Waal, L. , van Amerongen, G. , van Trierum, S. , … Osterhaus, A. (2013). Low pathogenic avian influenza A(H7N9) virus causes high mortality in ferrets upon intratracheal challenge: A model to study intervention strategies. Vaccine, 31, 4995–4999. 10.1016/j.vaccine.2013.06.071 [DOI] [PubMed] [Google Scholar]
- Kukielkaa, E. , Barasona, J. A. , Cowie, C. E. , Drewe, J. A. , Gortazar, C. , Cotarelo, I. , & Vicenteca, J. (2013). Spatial and temporal interactions between livestock and wildlife in South Central Spain assessed by camera traps. Preventive Veterinary Medicine, 112, 213–221. 10.1016/j.prevetmed.2013.08.008 [DOI] [PubMed] [Google Scholar]
- Landis, J. R. , & Koch, G. G. (1977). The measurement of observer agreement for categorical data. Biometrics, 33, 159–174. 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- Lewis, N. S. , Javakhishvili, Z. , Russell, C. A. , Machablishvili, A. , Lexmond, P. , Verhagen, J. H. , … Fouchier, R. A. M. (2013). Avian Influenza virus surveillance in wild birds in Georgia: 2009–2011. PLoS ONE, 8(3), e58534 10.1371/journal.pone.0058534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycett, S. J. , Bodewes, R. , Pohlmann, A. & the Global Consortium for H5N8 and Related Influenza Viruses (2016). Role for migratory wild birds in the global spread of avian influenza H5N8. Science, 354, 213–217. 10.1126/science.aaf8852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marche, S. , Van Borm, S. , Lambrecht, B. , Houdart, P. , & van den Berg, T. (2014). Chasing notifiable avian influenza in domestic poultry: A case report of LPAI H5 viruses in two Belgian holdings. Transboundary and Emerging Diseases, 61, 526–536. 10.1111/tbed.12056 [DOI] [PubMed] [Google Scholar]
- Markwell, D. D. , & Shortridge, K. F. (1982). Possible Waterborne transmission and maintenance of influenza viruses in domestic ducks. Applied and Environmental Microbiology 43, 110‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster, V. J. , Baas, C. , Lexmond, P. , Waldenström, J. , Wallensten, A. , Fransson, T. , & Fouchier, R. A. M. (2007). Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Path 3, e61 10.1371/journal.ppat.0030061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, B. , Munster, V. J. , Wallensten, A. , Waldenström, J. , Osterhaus, A. D. M. E. , & Fouchier, R. A. M. (2006). Global Patterns of Influenza A Virus in Wild Birds. Science, 312, 384–388. 10.1126/science.1122438 [DOI] [PubMed] [Google Scholar]
- Pantin‐Jackwood, M. J. , & Swayne, D. E. (2009). Pathogenesis and pathobiology of avian influenza virus infection in birds. Revue Scientifique Et Technique / Office International Des Épizooties, 28, 113–136. 10.20506/rst.28.1.1869 [DOI] [PubMed] [Google Scholar]
- Peterson, A. T. , Bush, S. E. , Spackman, E. , Swayne, D. E. , & Ip, H. S. (2008). Influenza A virus infections in land birds, People’s Republic of China. Emerging Infectious Diseases, 14, 1644–1646. 10.3201/eid1410.080169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Product Board of Livestock, Meat and Eggs (PVE) . Poultry Flock Information System, 2005 and 2013. Zoetermeer, The Netherlands. [Google Scholar]
- R Development Core Team (2018). A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from https://www.R-project.org [Google Scholar]
- Rabobank (2019). Cijfers en trends in de legpluimveehouderij (in Dutch). https://www.rabobank.nl/bedrijven/cijfers-en-trends/veehouderij/legpluimveehouderij/ [Google Scholar]
- Rutz, C. , Dalessi, S. , Baumer, A. , Kestenholz, M. , Engels, M. , & Hoop, R. (2007). Avian influenza: Wild bird monitoring in Switzerland between 2003–2006 (in German). Schweizer Archiv Fur Tierheilkunde, 149, 501–509. 10.1024/0036-7281.149.11.501. [DOI] [PubMed] [Google Scholar]
- Scott, A. B. , Phalen, D. , Hernandez‐Jover, M. , Singh, M. , Groves, P. , & Toribio, J.‐A.‐L.‐M.‐L. (2018a). Wildlife presence and interactions with chickens on Australian commercial chicken farms assessed by camera traps. Avian Diseases, 62, 65–72. 10.1637/11761-101917-Reg.1 [DOI] [PubMed] [Google Scholar]
- Scott, A. B. , Singh, M. , Groves, P. , Hernandez‐Jover, M. , Barnes, B. , Glass, K. , … Toribio, J.‐A. (2018b). Biosecurity practices on Australian commercial layer and meat chicken farms: Performance and perceptions of farmers. PLoS ONE, 13, e0195582 10.1371/journal.pone.0195582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriner, S. A. , VanDalen, K. K. , Mooers, N. L. , Ellis, J. W. , Sullivan, H. J. , Root, J. J. , … Franklin, A. B. (2012). Low‐pathogenic avian influenza viruses in wild house mice. PLoS ONE, 7, e39206 10.1371/journal.pone.0039206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusher, M. J. , Wilcox, B. R. , Lutrell, M. P. , Poulson, R. L. , Brown, J. D. , Yabsley, M. J. , & Stallknecht, D. E. (2014). Are Passarine birds reservoirs for influenza A viruses ? Journal of Wildlife Diseases, 50, 792–809. 10.7589/2014-02-043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedecor, G. W. , & Cochran, W. C. (1980). Statistical methods, 7th ed Ames, IA: The Iowa State University Press. [Google Scholar]
- Stallknecht, D. E. , & Brown, J. D. (2009). Tenacity of avian influenza viruses. Revue Scientifique Et Technique / Office International Des Épizooties, 28, 59–67. 10.20506/rst.28.1.1880 [DOI] [PubMed] [Google Scholar]
- Stallknecht, D. E. , & Shane, S. M. (1988). Host range of avian influenza virus in free‐living birds. Veterinary Research Communications, 12, 125–141. 10.1007/BF00362792 [DOI] [PubMed] [Google Scholar]
- Svensson, L. , Mullarney, K. , & Zetterström, D. (2012). Vogelgids van Europa, 5th Edition. Dutch translation of “Fågelguiden Europas och Medelhavsområdets fågler i fält”, Vogelbescherming Nederland en ANWB. Tirion Uitgevers, Utrecht, The Netherlands: 447 pp. [Google Scholar]
- Terregino, C. , De Nardi, R. , Guberti, V. , Scremin, M. , Raffini, E. , Moreno Martin, A. , … Capua, I. (2007). Active surveillance for avian influenza viruses in wild birds and backyard flocks in Northern Italy during 2004 to 2006. Avian Pathology, 36, 337–344. 10.1080/03079450701488345 [DOI] [PubMed] [Google Scholar]
- United Kingdom Latest egg Statistics (2018). UK.gov. 2018. Retrieved from https://www.gov.uk/government/statistics/historical-statistic-notices-on-uk-egg-production-and-prices-2018 [Google Scholar]
- VanDalen, K. K. , Franklin, A. B. , Mooers, N. L. , Sullivan, H. J. , & Shriner, S. A. (2010). Shedding light on avian influenza H4N6 infection in mallards: Modes of transmission and implications for surveillance. PLoS ONE, 5, e12851 10.1371/journal.pone.0012851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veen, J. , Brouwer, J. , Atkinson, P. , Bilgin, C. , Blew, J. , Eksioglu, S. , … Delany, S. (2007). Ornithological data relevant to the spread of Avian Influenza in Europe: Further identification and first assessment of higher risk species (p. 60). Wageningen, The Netherlands: Wetlands International. [Google Scholar]
- Velkers, F. C. , Blokhuis, S. J. , Veldhuis Kroeze, E. J. B. , & Burt, S. A. (2017). The role of rodents in avian influenza outbreaks in poultry farms: A review. Veterinary Quarterly, 37, 182–194. 10.1080/01652176.2017.1325537 [DOI] [PubMed] [Google Scholar]
- Verhagen, J. H. , Herfst, S. , & Fouchier, R. A. M. (2015). How a virus travels the world. Science, 347, 616–617. 10.1126/science.1259924 [DOI] [PubMed] [Google Scholar]
- von Waldburg‐Zeil, C. G. , van Staaveren, N. , & Harlander‐Matauschek, A. (2019). Do laying hens eat and forage in excreta from other hens? Animal, 13(2), 367–373. 10.1017/S1751731118001143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voslamber, B. (2005). Wild birds on and around free‐range poultry farms. Sovon Dutch Center for Field Ornithology, Nijmegen, the Netherlands: Report 2005/18 (in Dutch), 16 pp. [Google Scholar]
- Voslamber, B. (2006). Wild birds on and around free‐range poultry farms, July‐August 2006. Sovon Dutch Center for Field Ornithology, Nijmegen, the Netherlands: Report 2006/08 (in Dutch), 15 pp. [Google Scholar]
- Webster, R. G. , Bean, W. J. , Gorman, O. T. , Chambers, T. M. , & Kawaoka, Y. (1992). Evolution and ecology of influenza A viruses. Microbiological Reviews, 56, 152–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welby, S. , Poncin, O. , Claes, G. , van der Stede, Y. , Vangeluwe, D. , Marché, S. , … van den Berg, T. (2010b). Empirical approach for risk based model to enable detection and measures against spread of Low Pathogenic Avian Influenza, preliminary results of wild bird contacts with outdoor poultry pens. Veterinary and Agrochemical Research Centre (CODA‐CERVA). Scientific Report, 2009–2010, 36–38. [Google Scholar]
- Welby, S. , van den Berg, T. , Marché, S. , Houdart, P. , Hooyberghs, J. , & Mintiens, K. (2010a). Redesigning the serological surveillance program for notifiable avian influenza in Belgian professional poultry holdings. Avian Diseases, 54(Suppl. 1), 597–605. 10.1637/8749-033009-Reg.1 [DOI] [PubMed] [Google Scholar]
- Werner, S. J. , & Clark, L. (2006). Effectiveness of a motion‐activated laser hazing system for repelling captive Canada geese. Wildlife Society Bulletin, 34, 2–7. 10.2193/0091-7648(2006)34[2:EOAMLH]2.0.CO;2 [DOI] [Google Scholar]
- Zitzow, L. A. , Rowe, T. , Morken, T. , Shieh, W.‐J. , Zaki, S. , & Katz, J. M. (2002). Pathogenesis of avian influenza A (H5N1) viruses in ferrets. Journal of Virology, 76, 4420–4429. 10.1128/JVI.76.9.4420-4429.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


