Abstract
Background
Patient comprehension is fundamental to valid informed consent. Current practices often result in inadequate patient comprehension.
Purpose
An updated review to evaluate the characteristics and outcomes of interventions to improve patient comprehension in clinical informed consent.
Data sources
Systematic searches of MEDLINE and EMBASE (2008-2018).
Study Selection
We included randomized and non-randomized controlled trials evaluating interventions to improve patient comprehension in clinical informed consent.
Data Extraction
Reviewers independently abstracted data using a standardized form, comparing all results and resolving disagreements by consensus.
Data Synthesis
Fifty-two studies of 60 interventions met inclusion criteria. Compared to standard informed consent, a statistically significant improvement in patient comprehension was seen with 43% (6/14) of written interventions, 56% (15/27) of audiovisual interventions, 67% (2/3) of multicomponent interventions, 85% (11/13) of interactive digital interventions, and 100% (3/3) of verbal discussion with test/feedback or teach-back interventions. Eighty-five percent of studies (44/52) evaluated patients’ understanding of risks, 69% (41/52) general knowledge about the procedure, 35% (18/52) understanding of benefits, and 31% (16/52) understanding of alternatives. Participants’ education level was reported heterogeneously, and only 8% (4/52) of studies examined effects according to health literacy. The majority of studies (79%, 41/52) did not specify participants’ race/ethnicity.
Limitations
Variation in interventions and outcome measures precluded conduct of a meta-analysis or calculation of mean effect size. Control group processes were variable and inconsistently characterized. Nearly half of studies (44%, 23/52) had high risk of bias for the patient comprehension outcome.
Conclusions
Interventions to improve patient comprehension in informed consent are heterogeneous. Interactive interventions, particularly with test/feedback or teach-back components, appear superior. Future research should emphasize all key elements of informed consent and explore effects among vulnerable populations.
INTRODUCTION
Clinical informed consent is ethically and legally required prior to invasive medical and surgical procedures. Informed consent occurs when patient-clinician communication results in a patient’s authorization to undergo a specific medical intervention.1 This authorization is only valid if the patient has the capacity to consent, has discussed and understood all relevant information, consents voluntarily, and communicates their decision.2 Relevant information encompasses four key elements of informed consent: risks, benefits, alternatives, and general knowledge about the procedure.3, 4
Standard informed consent generally consists of a patient-clinician discussion followed by the signing of an informed consent document, though specific methods vary. Such practices commonly result in inadequate patient comprehension.3, 5-8 Lack of adequate understanding is particularly common for vulnerable populations such as those who face language barriers or those with limited education or health literacy.8-13 Current practices also generally fail to define adequate patient comprehension and to ensure its assessment as part of the informed consent process.
Internationally, standards of informed consent practice continue to evolve. Explicit protection from non-consensual medical treatments and procedures have long been upheld by international law, though actual practices of informed consent vary by country and may be influenced by cultural context.14-20 Overall, there has been a trend toward more patient-centered informed consent standards in recent years.21-23 There are not, however, international ethical guidelines for clinical informed consent similar to those that have been developed to regulate human subject research.24,25
We previously conducted a systematic review evaluating studies published between 1949 and 2008 of interventions to improve patient comprehension in informed consent for medical and surgical procedures.26 Most included studies (26/44) were fair or poor quality. We found that most interventions resulted in improved patient comprehension compared to standard informed consent, without demonstrable superiority of any particular intervention type. We also found that studies overwhelmingly focused on patient understanding of risks over other key elements of informed consent, suggesting the prioritization of malpractice risk reduction over enhancement of patient autonomy.
This systematic review is an update of our prior report. Since 2008, many relevant studies have been published, including trials evaluating novel interactive digital interventions to improve patient comprehension in informed consent. Additionally, standards for evaluating bias in systematic reviews have evolved to better safeguard against the perpetuation of misleading or biased data within primary studies.27-29 In this review, we sought to evaluate the characteristics (i.e. procedure type, intervention characteristics, comprehension measures, study country, and study population), risk of bias, and outcomes of the recent evidence base for improving patient comprehension in clinical informed consent. As a secondary aim, we assessed the degree to which relevant studies addressed vulnerable populations.
METHODS
We conducted a systematic review of interventions to improve patient comprehension in clinical informed consent. We registered our protocol (PROSPERO ID: CRD42019118264) prior to screening search results and adhered to the study plan therein. This study was exempt from IRB approval.
Study Eligibility
Inclusion criteria included English-language randomized controlled trials (RCTs) and non-randomized controlled trials (NRCTs) that compared the level of comprehension among patients who underwent standard informed consent with those who underwent an enhanced informed consent process for a necessary or recommended clinical procedure or surgery. We considered standard informed consent to include institution-specific standard consent processes and standardized versions of these processes as designed for individual studies (e.g., the use of a checklist to ensure discussion of key points). We selected only studies that utilized a quantitative, objective measure of patient comprehension. We excluded studies described as pilot, feasibility, and exploratory trials, as well as studies evaluating informed consent for research, screening tests, biobanking, genetic testing, advance directives, psychotherapy, and prescription drugs. We also excluded trials in which informed consent was obtained from surrogates, and studies evaluating decision aids designed to help patients choose between various medical management options (i.e. multiple reasonable procedures or medical versus surgical management options for a given medical condition). Interventions that utilized decision aids to enhance patient education in informed consent for a necessary or medically recommended procedure were included.
Study Identification
We conducted a systematic search of MEDLINE (PubMed interface from September 2008 to November 2018) and EMBASE (Elsevier interface from September 2008 to November 2018). A senior reference librarian (M.K.F.) designed the search strategy (Appendix 1). We included search terms aimed to identify interventions designed for vulnerable patient populations such as “English proficiency,” “limited literacy,” and “health literacy.” We examined the reference lists of selected studies identified in the search to enhance the likelihood that all relevant articles were captured.
Study Selection
One reviewer (J.G) screened the initial set of articles by title and abstract. We retrieved all articles identified as potentially relevant in full text. Two reviewers (J.G. and S.N.) performed a full-text review of these articles, comparing inclusion and exclusion results. Discrepancies were resolved by consensus with the senior author (Y.S.).
Data Extraction and Synthesis
Three members of the study team (J.G, S.N, Y.S.) developed a standardized data abstraction form (Appendix 2). Two reviewers (J.G. and S.N.) then independently abstracted data from each study, resolving discrepancies with the senior author (Y.S.).
Study design
All studies were described as RCTs. However, we determined that some employed non-random allocation methods, and reclassified them as NRCTs.
Types of interventions
We grouped interventions into five categories. (1) Written interventions included written materials providing information in addition to standard informed consent as well as simplified versions of standard consent documents. Written interventions could contain limited graphics as long as visual components were not the primary information delivery method. (2) Audiovisual interventions included posters, non-interactive digital images, videos, 3-dimensional models, and audio and video-recordings. Audiovisual interventions could contain limited text as long as written components were not the primary information delivery method. (3) Interactive digital interventions included computer, electronic tablet, and phone applications with interactive features. (4) Verbal discussion with test/feedback or teach-back interventions included interventions in which participants completed an informed consent discussion with a clinician, were tested for comprehension, and then given repeated or further verbal information based on their understanding. (5) Multicomponent interventions included interventions with components spanning two or more of the previous categories for a single study group.
Across all categories, we also grouped interventions according to whether they were interactive or noninteractive. Interactive interventions included those specifically designed to promote active patient involvement and bi-directional communication, such as patients’ physical interaction with 3-dimensional anatomical model during the consent discussion and digital interventions that allowed patients to navigate through educational modules. We further classified interactive interventions by whether they included test/feedback or teach-back components (i.e. explicit communication prompts to assess comprehension of elements of informed consent domains). Noninteractive interventions could include discussion with the consenting clinician but no other interactive features requiring active patient participation.
Study populations
We abstracted the inclusion and exclusion criteria of primary studies as well as study participants’ age, race/ethnicity, proficiency in the study language, educational attainment, and health literacy and numeracy. We additionally abstracted data on the reading level of intervention materials and all sub-analyses conducted on the basis of sociodemographic factors
Outcome measures
Our primary outcome was the difference in patient comprehension scores between study groups. We recorded elements of patient comprehension assessed (risks, benefits, alternatives, and general knowledge about the procedure) and timing of the patient comprehension measure (categorized as immediate (within 1 hour of consent consultation), early (<24 hours but >1 hour after consent consultation) or delayed (>24 hours after consent consultation) to broadly reflect working memory, short-term memory, and long-term memory).30 We also recorded study setting, amount of provider time required to obtain informed consent, patient satisfaction with the informed consent process, and patient anxiety. We only considered an intervention to have resulted in improved patient comprehension if the results were statistically significant.
Risk of bias
We assessed bias using the Cochrane risk-of-bias 2 (RoB 2.0) tool31-33 for the patient comprehension outcome in each trial, including those we reclassified as NRCTs due to use of non-random allocation methods. This tool can be utilized to assign ‘low risk,’ ‘some concerns,’ or ‘high risk’ of bias per study outcome. Overall risk-of-bias judgments are based on assessments in 5 domains. Two reviewers (J.G and S.N.) independently assessed all studies in this manner and resolved any discrepancies.
RESULTS
Literature Search
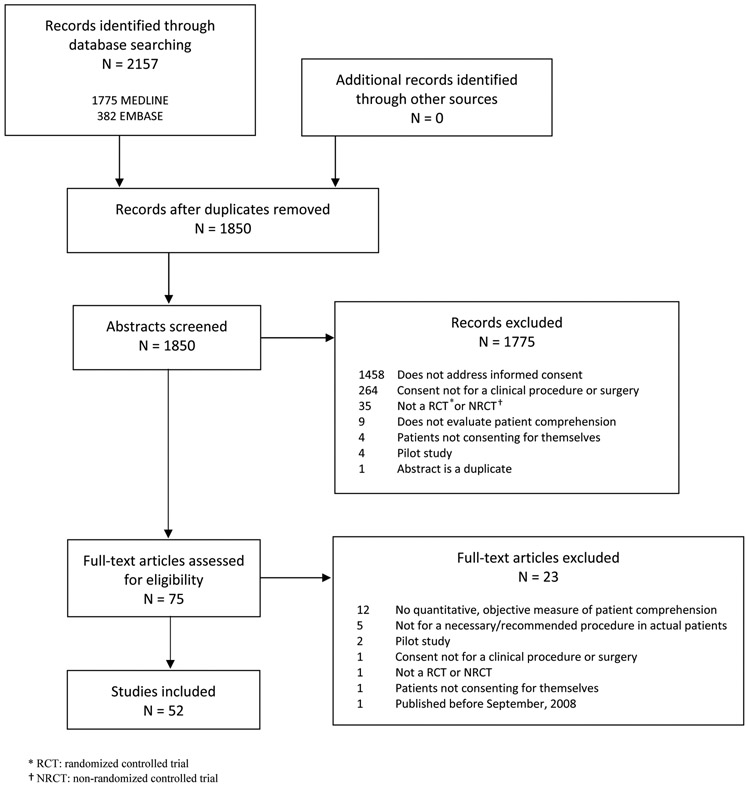
The literature search yielded 2157 citations; 1850 remained after removing duplicates. Initial screening yielded 75 full-text articles, of which 52 studies evaluating 60 interventions were ultimately included (Figure 1).
Figure 1.
Results of literature searches to identify studies of interventions to improve patient comprehension in clinical informed consent
Study and Participant Characteristics
Studies were conducted in 18 countries (Tables 1-6). Most were RCTs; three were NRCTs.34-36 The majority (41/52) addressed informed consent for surgical procedures, 9 for medical procedures, and 2 for a mixture of medical and surgical procedures. Seven studies specified that interventions took place in the outpatient setting,37-43 six in the inpatient setting,44-49 and one in the emergency department.50 The remaining 38 studies did not specify setting.
Table 1.
Results from trials of written interventions to improve patient comprehension in informed consent
| Source | Study country |
N | Mean patient age (and range if specified) |
Patients’ education |
Procedure | Intervention | Elements of patient comprehension assessed |
Comprehension assessment tool |
Timing of patient comprehension assessment relative to informed consent consultation* |
Risk of bias for patient comprehension outcome† |
Results | Group favored‡ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Straessle et al. (2011) | Switzerland | 185 | 54 y | 26% primary education, 37% apprenticeship, 24% secondary education, 12% university | Elective orthopedic surgery | Information form describing types of anesthesia, pre-operative instructions, recovery, and the role of the anesthetist (testing in 20 patients for comprehensibility, legibility, and completeness; reading level not specified) given to patients before the pre-anesthetic consultation versus standard care (control) | General knowledge about procedure | 3 items on a 17-item multiple choice questionnaire adapted from a validated measure (other questions pertained to patient satisfaction) | Immediately | Some concerns for bias | Percentage of correct answers 75% intervention vs. 62% control, P <0.01 | Intervention |
| Borello et al. (2016) | Italy | 70 | Mean age not specified, range 24 - 80 y | 50% did not complete high school, 26% completed high school, 23% college | Laparoscopic cholecystectomy | Standard verbal explanation using a simplified informed consent document with bulleted text and limited anatomical images (reading level not specified) in place of a standard informed consent document versus standard verbal explanation using a “standard text document” (control) | Risks, general knowledge about procedure | 9-item multiple choice questionnaire | Not specified | Some concerns for bias | 33.3% intervention vs 24.3% control answered all questions correctly, “no statistically significant difference" (no P value given) | Neither |
| Wong et al. (2016) | Canada | 60 | 55.8 y | 20% did not complete high school, 27 % completed high school, 49% with some college or trade school, 4% with some graduate school | Carpal tunnel release surgery | Single-sided, single page pamphlet detailing risks at a 7th grade reading level provided following standardized informed consent consultation (detailed, standardized discussion of potential complications) versus standard informed consent consultation alone (control) | Risks | Blinded research team member used standardized script to ask patients to recall risks via telephone | Delayed | Some concerns for bias | Number of risks recalled 1.33 (SD 1.21) intervention vs. 1.45 (SD 1.22) control, P = 0.73 | Neither |
| Aremu et al. (2011) | Nigeria | 50 | 43 y (range 16 - 76) | 52% high school or less, 48% some degree of postsecondary training | Mastoidectomy, tympanoplasty, nasal polypectomy, rhinotomy, maxillectomy, and laryngoscopy | Information handout addressing risks of the specific procedure (reading level not specified) in addition to verbal consent using a standard checklist of risks versus verbal consent using a standard checklist of risks only (control) | Risks | Telephone interview assessing recall of risks discussed | Delayed | Some concerns for bias | Unclear: overall risks correctly recalled reported as both 67% and 62% for intervention vs. 51% control "The difference was significant” (no P value provided) | Intervention |
| Finch et al. (2009) | England | 100 | 73.5 y | Not specified | Transurethral resection of the prostate | Informed consent using British Association of Urological Surgeons (BAUS) procedure-specific consent form including check boxes for frequently occurring risks (reading level not specified) versus informed consent using conventional Department of Health Type 1 consent form (control) | Risks, benefits, general knowledge about procedure | "Short, standardized questionnaire” | Early | High risk of bias | No overall score reported; no significant difference in “median estimation of risk” for 4 independently assessed risks, intervention group accurately predicted risk of redo at 10 years 50% of the time vs. 10% for control, P = 0.007 | Neither |
| Hong et al. (2009) | Canada | 100 | 42 y (range 20 - 68) | 60% no university, 40% university or higher | Rhinoplasty | Written pamphlet outlining the risks of the procedure (reading level not specified) in addition to oral dialogue with surgeons using a checklist of complications versus oral dialogue alone (control) | Risks | Recall of risks via telephone call with guided questions, answers recorded "on a standard set form" | Delayed | High risk of bias | Average risk recall 2.3 out of 5 intervention vs. 1.3 out of 5 control, P < 0.008 | Intervention |
| Khan et al. (2013) | England | 114 | Not specified | Not specified | Hand surgery | A4-sized document (8.27 × 11.69 inches) explaining risks (reading level not specified) in addition to standard verbal information versus standard verbal information only (control) | Risks | "A4 size questionnaire" | Delayed | High risk of bias | Mean percentage of risks recalled 85% intervention vs. 79% control, P = 0.1 | Neither |
| Alsaffar et al. (2016) | Canada | 49 | 49 y (range 27 - 77) | Not specified | Total thyroidectomy | Pamphlet provided at the beginning of the informed consent interview (reading level not specified) and discussed point-by-point in addition to verbal consent with a senior staff surgeon following a standardized script versus verbal consent with a senior staff surgeon following a standardized alone (control) | Risks | 12-item written multiple choice test | Delayed | High risk of bias | Overall correct score 80% intervention vs. 83% control group (no P value provided) | Neither |
| Smith a et al. (2012) | England | 119 | 62.1 y (range 18 - 99) | Not specified | Surgeries for traumatic upper or lower limb fracture requiring surgical fixation | 2-page written information leaflet with details about diagnosis, surgery, risks, post-op care, and rehabilitation (same as information discussed verbally, reading level not specified) given in addition to standard, structured verbal information versus standard, structured verbal information alone (control) | Risks | "Standardized, structured self-administered questionnaire " | Delayed | High risk of bias | Mean score 64% intervention vs. 41% control, P = 0.0014 | Intervention |
| Gangol et al. (2010) | Nepal | 116 | 44.1 y | 7.0% primary (1 to 3 classes), 32.5% secondary (4 to 10 classes) 11.4%, higher (>10 classes) | Elective cholecystectomy | Information leaflet written in Nepali with information about indications, anesthesia, difference between laparoscopy versus open procedure, post-op pain control and diet, and complications (reading level not specified) given in addition to standard verbal counseling versus standard verbal counseling only (control) | Unclear/ not specified | 10 standard questions asked in interviews; each answer scored as 2 (understood well), 1 (satisfactory understanding), or 0 (poor understanding) | Not specified | High risk of bias | Mean score 14.98 out of 20 intervention vs. 8.05 out of 20 control group and level of understanding "good" (score 51-75%) or "satisfactory" (score >75%) in 76.3% intervention vs. 18.2% control, P = 0.000 | Intervention |
Immediately: within 1 hour of informed consent consultation; early: >1 hour but <24 hours of informed consent consultation, delayed: ≥ 24 hours after informed consent consultation
Assessed using the Cochrane Risk of Bias 2.0 tool, see Table 7 for details
If a study reported an improvement in patient comprehension on a single item or multiple items of the comprehension assessment but if overall score did not improve, we considered neither or no group to be favored
Table 6.
Results of trials with multiple study groups receiving different intervention types to improve patient comprehension in informed consent
| Source | Study country |
N | Mean patient age (and range if specified) |
Patients’ education |
Procedure | Intervention | Elements of patient comprehension assessed |
Comprehension assessment tool |
Timing of patient comprehension assessment relative to informed consent consultation* |
Risk of bias for patient comprehension outcome† |
Results | Group favored‡ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Johnson et al. (2011) | USA | 151 | 67 y | Mean education in years: 13 | Total knee arthroplasty | Group 1: control (standardized informed consent using iMedConsent web-based tool plus customized written handout) Group 2: same as control plus a standard video from the American Academy of Orthopedic Surgeons (audiovisual intervention) Group 3: same as group 2 plus a “formal education session” (further details not provided) with a nurse coordinator/ educator additionally reviewing risks, benefits, and expectations of total knee arthroplasty (multicomponent intervention) |
Risks, benefits, alternatives, general knowledge about procedure | 14-item multiple choice questionnaire | Immediately and delayed | High risk of bias | Control: immediate score 10.1 out of 14 (SD 2.4), day of surgery score 10.2 out of 14 (SD 2.3), 6-week post op score 10.5 out of 14 (SD 1.7) Group 2: immediate score 10.8 out of 14 (SD 1.6), day of surgery score 10.3 out of 14 (SD 1.8), 6-week post-op score 10.2 out of 14 (SD 2.7) Group 3: immediate score 11.1 out of 14 (SD 1.5), day of surgery score 11.0 out of 14 (SD 1.2), 6-week post-op score 10.4 out of 14 (SD 2.2) P values 0.11 (immediate), 0.08 (day of surgery), 0.80 (6-weeks post-op) |
None |
| Shukla et al. (2012) | USA | 100 | 74 y | Not specified | Cataract surgery | Group 1: control (conventional resident-administered verbal consent) Group 2: conventional verbal consent plus 2nd grade reading level written information sheet (written intervention) Group 3: conventional verbal consent plus 8th grade reading level written information sheet (written intervention) Group 4: conventional verbal consent plus a 13-minute video from the American Academy of Ophthalmology (audiovisual intervention) |
Risks, benefits, alternatives, general knowledge about procedure | 12-item multiple choice questionnaire | Not specified | High risk of bias | Group 2: mean score 10.8 out of 12 (SD 1.29) vs. 7.68 out of 12 (SD 2.80) control, P = 0.000 Group 3: 9.08 out of 12 (SD 1.60) vs. 7.68 out of 12 (SD 2.80) control, P > 0.05 Group 4 mean score 10.56 out of 12 (SD 1.44) vs. 7.68 out of 12 (SD 2.80) control, P = 0.000 No statistically significant difference between Group 2 and Group 4, P > 0.05 |
Group 4 and group 2 > control |
| Bennett et al. (2009) | USA | 99 | Not specified | Not specified | Imaging-guided spinal epidural steroid, nerve root, and facet joint injections | Group 1: control (standard informed consent which includes “a conversational description of the 12 key points of consent and home-care with the patients given the opportunity to read and sign the printed procedure informed consent document”) Group 2: “teach-the-teacher group” i.e. standard informed consent then participants had to repeat back the 12 key points without error to the physician before informed consent was complete (verbal discussion with teach-back intervention) Group 3: standard informed consent plus participants viewed a set of diagrams illustrating the 12 key points before signing the informed consent form (audiovisual intervention) |
Risks, benefits, general knowledge about procedure | 6-item multiple choice questionnaire with some "circle all that apply" questions (read aloud to any patient with difficulty reading) | Not specified | High risk of bias | Control: 5.5 out of 10 Group 2: 7.4 out of 10 Group 3: 7.3 out of 10 P ≤ 0.05 for each intervention group compared to control |
Group 2 and group 3 > control |
| Cornoiu et al. (2011) | Australia | 61 | 44.2 y (range 20 - 74) | Average grade level: 10.4 | Knee arthroscopy | Group 1: control (standardized verbal consent with a trained resident using a checklist and script based on author consensus of average risk for complications based on literature review plus information about desired information from a focus group of patients who had previously undergone knee arthroscopy) Group 2: 1-page written information at an 8th grade level, without pictures, developed using the same score information as the verbal script, in place of standardized verbal consent (written intervention) Group 3: multimedia education module (pilot tested and revised) covering the same core information as the verbal and the written groups with voice, text, photographs, and 3D computer animations in place of standardized verbal consent, patients could control progression through the module (interactive digital intervention) |
Risks | 10-item questionnaire | Immediately and delayed | High risk of bias | Control: correct answers on immediate testing 88% (SD 14%) Group 2: correct answers on immediate testing 76% (SD 28%) Group 3: correct answers on immediate testing 98% (SD 5%) and answered a higher proportion of questions correctly “at all stages” P < 0.05 |
Group 3 > group 2 and control |
| Goldberger et al. (2011) | USA | 63 | 61 y | 62.3% college or above | Diagnostic cardiac electrophysiology | Group 1: control (standard text read verbatim by physician then questions answered) Group 2: participants given a booklet (no other details provided) then had the opportunity to ask the physician questions (written intervention) Group 3: video narrator read same text as that contained in the booklet then participants had the opportunity to ask the physician questions (audiovisual intervention) |
Risks, general knowledge about procedure | 4 multiple choice questions and patients asked to recall potential complications | Immediately | High risk of bias | Control: mean score: 6 (SD 2) Group 2: mean score 4 (SD 2) Group 3: mean score 5 (SD 2) P = 0.11 |
None |
Immediately: within 1 hour of informed consent consultation; delayed: more than 24 hours after informed consent consultation
Assessed using the Cochrane Risk of Bias 2.0 tool, see Table 7 for details
If a study reported an improvement in patient comprehension on a single item or multiple items of the comprehension assessment but if overall score did not improve, we considered neither or no group to be favored
All studies included control groups that received standard informed consent, though few provided full details of the standard informed consent process (Tables 1-6). When described, standard informed consent generally consisted of a patient-clinician discussion followed by the signing of an institution-specific informed consent form, and sometimes included the provision of standardized written educational materials.
Type and quantity of participant sociodemographic information varied widely. Mean patient age ranged from 22.4 to 74 years old (Tables 1-6). The majority of studies did not specify participants’ health literacy level (88%, 46/52) or race/ethnicity (79%, 41/52) and four studies explicitly excluded participants with low literacy levels.38,51-53 Twenty-four studies (46%) included proficiency in the study language in their eligibility criteria, and only 3 specified inclusion of participants requiring interpreters.37, 54, 55 Education was reported in a variety of ways, including percent of participants having completed certain school levels, or mean education in years (Tables 1-6). Of 29 studies that reported education in a way that could be categorized as “high school or less,” 22 included ≥30% of participants with this level of education.
Variations in Patient Comprehension Measures
Patient comprehension measures ranged from true/false, multiple choice, and open-ended questionnaires to semi-structured interviews (Tables 1-6). The majority (81%, 42/52) were neither validated nor adapted from a validated measure.
Key elements of informed consent assessed by patient comprehension measures varied by study (Tables 1-6). Evaluation of patient understanding of risks was most common (85% of studies, 44/52) followed by general knowledge about the procedure (69%, 41/52), benefits (35%, 18/52) and alternatives (31%, 16/52). Eleven studies (21%) assessed all four key elements of informed consent.36, 51, 56-64 Five studies (10%) did not specify which elements of patient comprehension were assessed.39, 42, 45, 52, 65
Twenty-two studies (41%) evaluated immediate patient comprehension, 5 (10%) evaluated early patient comprehension, 23 (44%) evaluated delayed patient comprehension, and 11 (21%) did not specify timing (Tables 1-6).
Effect of Interventions on Patient Comprehension
Written interventions
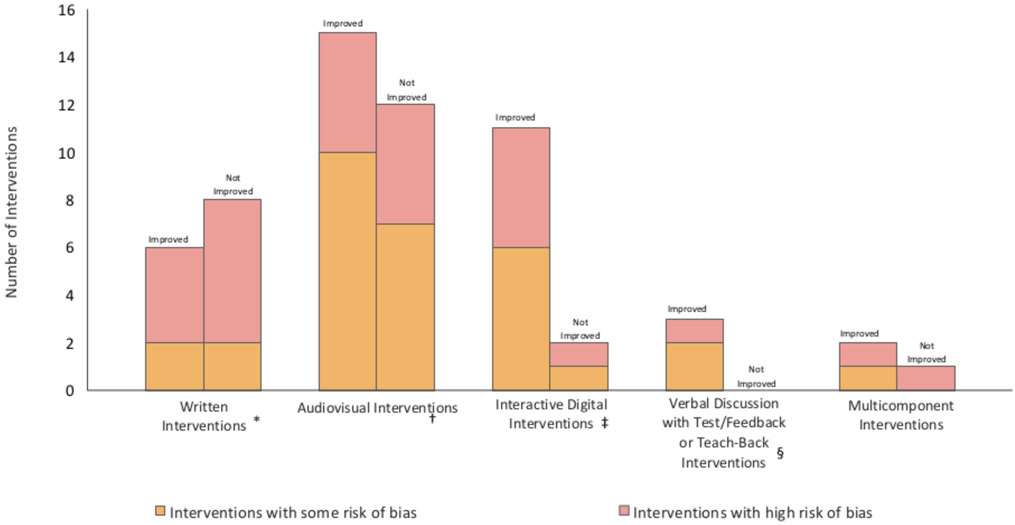
Thirteen studies evaluated 14 written interventions (Tables 1 and 6).38, 44, 45, 49, 54, 60, 66-72 Written interventions ranged from simplified, single-page handouts to multiple-page pamphlets. Six written interventions (43%) resulted in improved patient comprehension compared to standard informed consent (Figure 2)45, 49, 60, 66, 69, 71 and 8 did not38, 44, 54, 60, 67, 68, 70, 72 Fifty percent (2/4) of written intervention trials with some bias risk resulted in improved patient comprehension66, 71 compared with 40% (4/10) of those with high bias risk.45, 49, 69 Ten interventions provided written information in addition to standard informed consent45, 49, 54, 60, 66, 69-72 of which six (60%) improved patient comprehension; four provided written information in place of standard informed consent,38, 44, 67, 68 none of which resulted in improved patient comprehension. Four written interventions indicated reading level, which ranged from 2nd to 8th grade. 38, 60, 72 Only the intervention written at a 2nd grade reading level resulted in improved patient comprehension compared to the control group.60 No written interventions contained interactive, test/feedback, or teach-back components.
Figure 2:
Number of Interventions that Improved or Did Not Improve Patient Comprehension in Informed Consent by Intervention Type
Audiovisual interventions
Twenty-five studies evaluated 27 audiovisual interventions (Tables 2 and 6).34, 36, 37, 39, 40, 42, 43, 46, 48, 50, 60, 63, 64, 65, 68, 73-82 Audiovisual interventions included videos of varying length, visual aids (non-interactive Powerpoint or multimedia presentations, posters, diagrams, or anatomical models), and audio and video-recordings. Fifteen audiovisual interventions (56%) resulted in improved patient comprehension compared to standard informed consent (Figure 2)34, 39, 40, 42, 48, 50, 60, 65, 75, 78-81 and the remaining did not. Fifty-three percent (9/17) of audiovisual intervention trials with some bias risk resulted in improved patient comprehension39, 39, 42, 48, 50, 79, 75, 80 compared with 60% (6/10) of those with high bias risk.34, 40, 60, 65, 78, 81 Seventeen studies utilized audiovisual interventions in addition to standard informed consent,34, 36, 37, 39, 40, 48, 50, 60, 63, 64, 74, 75, 78-82 of which 11 (65%) resulted in improved patient comprehension compared to control. Four studies assessed audiovisual interventions in place of standard informed consent,46, 65, 68, 76 of which 1 (25%) resulted in improved patient comprehension compared to control. One audiovisual intervention was interactive: participants held and touched specified aspects of an anatomical model during the consent discussion.39 No audiovisual interventions contained test/feedback or teach-back components.
Table 2.
Results from trials of audiovisual interventions to improve patient comprehension in informed consent
| Source | Study country |
N | Mean patient age (and range if specified) |
Patients’ education |
Procedure | Intervention | Elements of patient comprehension assessed |
Comprehension assessment tool |
Timing of patient comprehension assessment relative to informed consent consultation* |
Risk of bias for patient comprehension outcome† |
Results | Group favored‡ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Egekeze et al. (2016) | USA | 67 | 54.2 y | All patients had a maximum completed level of academic achievement of a high school diploma | Knee corticosteroid injection | Group 1 : control (10-minute informed consent discussion scripted at an eighth-grade reading level on the basis of information from the knee arthritis section of the patient education website OrthoInfo) Group 2: received the same verbal consent as the control group while watching a 10-minute animated knee anatomy video (played on silent; research staff member pointed at images in the video during the discussion) Group 3: received the same verbal consent as the control group while holding an anatomic knee model; each participant touched specified aspects of the model that corresponded with the discussion |
Unclear/ not specified | 14-item multiple choice questions in interview format with 20 minute time limit | Immediately | Some concerns for bias | Control: mean score 71% (10.0 out of 14) Group 2: mean score 74% (10.3 of 14) Group 3: mean score 84% (11.7 out of 14) P = 0.019 |
Group 3 > group 2 > control |
| Lin et al. (2018) | Taiwan | 142 | Not specified | 15% less than high school, 37% high school, 48% college | Trauma-related debridement surgery | Educational video (~15 minutes duration) developed by a panel of experts describing the surgery, anesthesia, benefits, risks, alternatives, and post-op recovery using advanced 2D graphics, audio narrative, written subtitles and captions in addition to standard consent (verbal information plus written consent form with information about the surgery similar to that on the video plus educational session with health care provider) versus standard consent alone (control) | Risks, general knowledge about procedure | 10-item multiple choice questionnaire | Immediately | Some concerns for bias | Mean knowledge score 72.57% intervention vs. 61.67% control, P < 0.001 Mean improvement in knowledge from baseline 18.71 (SD 16.44) intervention vs. 10.83 (SD 11.23) control, P = 0.001 |
Intervention |
| Huber et al. (2012) | Germany | 220 | 63.4 y | 8.9%, none or basic (missing school-leaving qualification or uncompleted professional training), 52.7%, higher (having passed senior technical college or university), 39.4%, medium (all remaining patients) | Radical prostatectomy | Computer-based multimedia tool (developed by an “interdisciplinary group” and tested with patients and “lay people” before completing the final version) with an interface allowing the consenting physician to navigate between and highlight portions of graphics, illustrations, videos, and pictures in place of standard physician consent using a written consent form containing the same information (control) | Risks | “Remembered quantity” of risks | Early | Some concerns for bias | 2.3 (SD 1.2) intervention vs. 2.4 (SD 1.4) control | Neither |
| Baenninger et al. (2018) | Switzerland | 113 | 35.3 y (range 19.7 - 57.1) | Not specified | Refractive excimer laser treatment for refractive ophthalmologic error | 8-minute video of surgeon giving standard information and treating a patient plus further animations of the procedure in addition to conventional face-to-face consultation with a surgeon versus conventional face-to-face consultation with a surgeon alone (control) | Risks, general knowledge about procedure | 25-item paper-based true/false questionnaire | Not specified | Some concerns for bias | 22 out of 25 mean score for both intervention and control, P = 0.975 | Neither |
| Vo et al. (2018) | USA | 63 | Not specified | Not specified | Cataract Surgery | American Academy of Ophthalmology cataract surgery education video (4 minutes, 9 seconds long) prior to traditional face-to-face counseling with a surgeon versus traditional face-to-face counseling with a surgeon alone (control) | Risks, benefits, alternatives, general knowledge about procedure | Self-administered questionnaire with yes/no and 5 point scale items | Immediately | Some concerns for bias | 79.4% (SD 2.82%) intervention) vs. 79.3% (SD 3.39%) control, P = 0.99 | Neither |
| Pallett a et al. (2018) | USA | 120 | 42.8 y | 0.8% less than 24.6% high school, 74.6% higher education | Hysterectomy | 10-minute video developed by the study investigators with audio script, illustrations, and animations addressing 11 key aspects of the informed consent discussion plus standard physician counseling versus standard physician counseling covering the same 11 key aspects alone (control) | Risks, general knowledge about procedure | Questionnaire with true/false and multiple choice items; 4 versions with each subject given each version in a different order at 4 time points | Immediately and delayed | Some concerns for bias | Immediate: 15.1% improvement in score (SD 2.04%) intervention vs. 5.2% (SD 2.1%) control, P = 0.009 Delayed, day of surgery: 8.3% improvement in score (SD 2.3%) intervention vs. 1.2% (SD 2.01%) control, P = 0.02 Delayed, 6 weeks post consent: scores back to baseline for both groups |
Intervention |
| Lattuca et al. (2018) | France | 843 | 67.3 y | 36% primary school, 48% secondary school, 16% university or higher | Coronary angiography | 5-minute video with 3D animations developed for the study with content derived from the national information and consent form and approved by the scientific committee of the French Society of Cardiology displayed to each patient on an individual 10″ tablet in addition to standard consent (oral information and national standard written form) versus standard consent alone (control) | Risks, general knowledge about procedure | 16-item questionnaire | Immediately | Some concerns for bias | Overall score 11.8 intervention out of 16 (SD 2.8) vs. 9.5 control out of 16 (SD 3.1), P < 0.001 | Intervention |
| Zhang et al. (2017) | China | 80 | Not specified | 66% primary education, 23% secondary education or higher | Cataract surgery | 6-miute educational video based on a video from the American Academy of Ophthalmology that included visual teaching aids, animation, music, and a script written in Mandarin and Cantonese by an “expert in ophthalmological patient-information needs” in addition to traditional verbal consent and consent documents versus traditional verbal consent and consent documents alone (control) | Risks, benefits, alternatives, general knowledge about procedure | 10-item yes/no questionnaire | Immediately | Some concerns for bias | Accuracy rate 80.2% intervention vs. 77.5% control, P = 0.386 | Neither |
| Armstrong et al. (2010) | USA | 84 | 59.0 y | 1.2% 1st through 8th, 17.9% 9th through 12th, 23.8% 1-3 years college, 57.1% 4 or more years college | Skin shave and punch biopsy | Dermatologists “obtained informed consent for skin biopsies using an educational video” with actual footage of shave and punch biopsy procedures displayed on a portable device (unclear whether in place of or in addition to standard consent) versus traditional face-to-face consent with a dermatologist alone (control) | Risks, general knowledge about procedure | 6-item multiple choice questionnaire | Immediately | Some concerns for bias | Knowledge score 1.55 out of 6 (SD 1.71) intervention vs. 1.12 out of 6 (SD 1.71) control, P = 0.259 | Neither |
| Bowers et al. (2015) | Canada | 93 | 60.9 y (range 19 - 89) | Not specified | Endovascular aneurysm repair, peripheral angioplasty, Hickman catheter, and peripherally inserted central catheter insertion | 2 minute computer-generated video presented on an electronic tablet by a medical student with simplistic anatomical visuals and information about the procedure after traditional verbal consent versus traditional verbal consent alone (control) | Risks, general knowledge about procedure | 5-item written true/false test | Not specified | Some concerns for bias | "Intervention group had higher total comprehension scores” (numbers not reported) | Intervention |
| Clark et al. (2011) | USA | 50 | 38.5 y | 36% did not complete high school, 32% completed high school, 24% with some college or trade school, 8% with some graduate school | Elective cholecystectomy | PowerPoint presentation with illustrations of cholelithiasis and laparoscopy cholecystectomy shown after standard informed consent (surgical benefits, risks, and complications explained “in the usual fashion” by general surgery residents using a checklist who had been trained for the study and monitored in a previous 10-patient pilot study) versus standard informed consent alone (control) | Risks, benefits, general knowledge about procedure | 10-item true/false questionnaire | Not specified | Some concerns for bias | Correct response rate 66% intervention vs. 68% control, no P value provided | Neither |
| Mishra et al. (2010) | Scotland | 84 | 66.6 y (range 62 - 71) | Not specified | Coronary artery bypass graft surgery | Group 1: control (standard informed consent consultation) Group 2: informed consent consultation audio-recorded then participants given a tape containing general information about CABG Group 3: informed consent consultation audio-recorded then participants given a tape recording of their own consultation interview Tapes were sent by mail with a letter encouraging patients to listen to them as many times as they wished |
Unclear/ not specified | Validated 16-item questionnaire administered in person with each answer scored on a scale of 0 (inadequate) to 3 (very good) | Not specified | Some concerns for bias | Control: mean score 13.79 out of 48 (SD 5.354) Group 2: mean score 19.64 out of 48 (SD 3.451) Group 3: mean score 31.97 out of 48 (SD 5.922) P < 0.001 |
Group 3 > group 2 > control |
| Ham et al. (2016) | South Korea | 40 | 66.9 y | 37.5% did not complete high school, 32.5% completed high school, 30% university of more | High-performance system photoselective vaporization of the prostate | Multimedia presentation containing the same content as conventional consent forms but utilizing pictures, illustrations, animations, and videos without voiceover explained by a physician in place of conventional consent (verbal explanation plus written document) versus conventional consent alone (control) | Risks, alternatives, general knowledge about procedure | 15-item questionnaire | Immediately | Some concerns for bias | Mean score 10.6 out of 15 (SD 2.8) intervention vs. 9.9 out of 15 (SD 2.3) control, P = 0.332 | Neither |
| Tipotsch-Maca et al. (2016) | Austria | 123 | 71 y (range 56 - 90) | 15% passed graduation examination | Cataract surgery | Computer-animated video in addition to standard consent (standardized face-to-face discussion following a checklist plus information brochure) versus standard consent alone (control) | Risks, alternatives, general knowledge about procedure | 10-item multiple choice questionnaire | Immediately | Some concerns for bias | Mean number of correct answers 8.2 out of 10 (SD 0.5) intervention vs. 7.2 our of 10 (SD 0.7) control | Intervention |
| Wysocki et al. (2012) | Poland | 58 | 57.5 y | Not specified | Mastectomy | 12-minute video with additional information adapted from the National Cancer Institute (www.cancer.gov) presented by a breast cancer survivor in addition to routine informed consent (non-standardized conversation with the attending surgeon, practical information from nurses, basic informed consent form) versus routine informed consent alone (control) | Alternatives, general knowledge about procedure | "Self-administered questionnaire” | Early and delayed | Some concerns for bias | No overall score reported; greater percentage of intervention group correctly answered a question regarding treatment options within 24 hours and 7 days post-op (P = 0.010 and 0.036, respectively) with no difference at 30 days post-op, and no difference between groups on two other questionnaire items | Neither |
| Choi et al. (2015) | South Korea | 51 | 22.4 y (range 18 - 27) | 19.6% up to secondary education, 80.4% postsecondary education | Impacted mandibular third molar removal | Narrated slideshow with simple illustrations created in PowerPoint by “personnel at the Korean Academy of Dental Science” including audio and visual cues plus standard informed consent document versus standard informed consent (verbal explanation plus Korean Dental Association Informed Consent document) | Risks | Open-ended questionnaire form assessing recall of risks | Delayed | High risk of bias | No overall score reported; intervention group showed improvement over control in recall of only 2 out of 8 individual risks, P < 0.05, no significant difference in recall of all other risks, P > 0.1 | Neither |
| Ellett et al. (2014) | Australia | 41 | 36.1 y (range 19 - 51) | 3% primary, 20% secondary including year 10, 34% secondary years 11 and 12, 26% graduate degree, 8% postgraduate degree | Operative laparoscopy for investigation and treatment of pelvic pain | Routine, standardized surgical consent followed by a 15-minute educational multimedia module containing voice, script developed by the study authors, and 3D computer animations versus routine surgical consent alone (control) | Risks, benefits, general knowledge about procedure | 14-item true/ false/ unsure questionnaire | Immediately and delayed | High risk of bias | Immediate: mean score 11.3 out of 14 (SD 0.49) intervention vs. 7.9 out of 14 (SD 0.50) on immediate testing, P < 0.001 Delayed: no difference |
Intervention |
| Karan a et al. (2011) | India | 60 | 63.1 y | Mean formal education in years: 3.0 | Cataract surgery | 24″ × 36″ poster displaying nine images in addition to scripted verbal informed consent read by a native Tamil speaker versus scripted verbal informed consent read by a native Tamil speaker alone (control) | Risks, benefits, general knowledge about procedure | Oral 11-item true/false/don’t know quiz | Immediately and early | High risk of bias | Immediate: mean score 8.17 out of 11 intervention vs. 8.13 out of 11 control, P = 0.9361 Within 24 hours: mean score 8.71 out of 11 intervention vs. 7.39 out of 11 control, P = 0.0049, and mean improvement in score 3.6 intervention vs. 1.3 control, P = 0.002 |
Intervention |
| Winter et al. (2016) | Australia | 88 | 54 y | 27% did not complete high school, 73% secondary education or above | Cystoscopy and ureteric stent insertion | 7:07-minute educational video with cartoon animation presented on an electronic tablet versus standard verbal consent with “urology registrar” | Unclear/ not specified | True/false and multiple choice questionnaire | Not specified | High risk of bias | 23.26 out of 32 intervention vs. 20.13 out of 32 control, P < 0.001 17.8% increase in score when standard verbal consent group was crossed over to the intervention, P < 0.001 No significant difference in score when intervention group was crossed over to the control, P = 0.621 |
Intervention |
| Sharma et al. (2018) | USA | 101 | Not specified | Not specified | Functional endoscopic sinus surgery | One pre-operative and one immediate post-operative visit were video-recorded; intervention group was given access to their recordings via secured internet server to watch at their discretion, control group was not given access to their recordings | Risks, general knowledge about procedure | Paper-based questionnaire with multiple choice and yes/no items | Delayed | High risk of bias | Accurate recall of risks 66% intervention vs. 63% control Correct answers to questions regarding extent of surgery 4.46 out of 5 intervention vs. 4.27 out of 5 control “Not statistically significant” (no P value given) |
Neither |
| Wilhelm et al. (2009) | Germany | 212 | 53.2 y | 59.9% did not complete high school, 40.1% high school degree or higher | Laparoscopic cholecystectomy | 26-minute DVD designed by the Institute of Media Informatics of the University of Munich and the Department of Surgery of the Technische Universtät of Munich with text, 3D computer animations, video sequences, audio commentary, and an “education dialogue” between a surgeon and actor posing as a patient in addition to standard informed consent with a surgeon versus standard informed consent with a surgeon alone (control) | Risks, general knowledge about procedure | 25-item multiple choice questionnaire | Delayed | High risk of bias | Mean score 19.88 out of 25 intervention vs 17.58 out of 25 control | Intervention |
Immediately: within 1 hour of informed consent consultation; early: >1 hour but <24 hours of informed consent consultation, delayed: ≥ 24 hours after informed consent consultation
Assessed using the Cochrane Risk of Bias 2.0 tool, see Table 7 for details
If a study reported an improvement in patient comprehension on a single item or multiple items of the comprehension assessment but if overall score did not improve, we considered neither or no group to be favored
Interactive digital interventions
Thirteen studies evaluated 13 interactive digital interventions (Tables 3 and 6), including computer, electronic tablet, and mobile phone applications that had features requiring active patient participation. The majority of interactive digital interventions (85%, 11/13) resulted in improved patient comprehension compared to standard informed consent (Figure 2).38, 41, 51, 52, 55, 58, 61, 62, 83-85 Six used computer-based programs, 4 used tablet applications, 2 used web modules, and one used a mobile phone application. Eighty-six percent (6/7) of interactive digital intervention trials with some bias risk resulted in improved patient comprehension51, 52, 55, 61, 62, 85 compared with 83% (5/6) of those with high bias risk.38, 41, 58, 83, 84 Nine were provided in addition to standard informed consent,41, 51-53, 55, 83-85 of which seven (78%) resulted in improved patient comprehension; two were provided in place of standard informed consent, both of which resulted in improved patient comprehension,61, 62 and one study did not specify.58 All of the interactive digital interventions contained interactive features and 5 included a test/feedback component.52, 58, 61, 62, 84
Table 3.
Results from trials of interactive digital interventions to improve patient comprehension in informed consent
| Source | Study country |
N | Mean patient age (and range if specified) |
Patients’ education |
Procedure | Intervention | Elements of patient comprehension assessed |
Comprehension assessment tool |
Timing of patient comprehension assessment relative to informed consent consultation* |
Risk of bias for patient comprehension outcome† |
Results | Group favored‡ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bethune et al. (2018) | Canada | 38 | 62.2 y | Not specified | Lumbar spine or cervical spine decompression for degenerative disease, craniotomy for brain tumor, trigeminal neuralgia treatment | E-book interactive multimedia application lasting 7-10 minutes (specific interactive features not specified, pilot tested among “student volunteers with no medical training”) with written explanations, pictures, and short videos relevant to the specific procedure provided on an electronic tablet prior to a standard informed consent discussion with a surgeon versus standard consent discussion with a surgeon alone (control) | Risks, alternatives, general knowledge about procedure | 14-item questionnaire adapted from a validated measure with each item rated on a scale of 0-3 (max score 42) | Immediately | Some concerns for bias | 23.2 out of 42 (SD 4.9) intervention vs. 20.2 out of 42 (SD 4.0) control, P = 0.02 | Intervention |
| Gordon et al. (2017) | USA | 288 | 51 y (range 22 – 77) | 72.2% less than college, 27.8% BA or higher | Kidney transplant with increased risk donors | “Inform Me” electronic tablet application provided (average duration 47 minutes) in addition to routine transplant education versus routine transplant education alone (control); application utilized “low literacy and low numeracy messages” and adaptive learning to personalize educational content in 5 interactive chapters with textual explanations, graphics, videos, photographs and test questions between chapters (based on accuracy of answers the application presented additional information specific to incorrect answers, correct answers were required to progress to the next chapter) | Unclear/ not specified | 31-item multiple choice test completed electronically by the intervention group and on paper by the control group | Immediately and delayed | Some concerns for bias | Immediate: mean score 20.69 out of 31 intervention vs. 13.94 out of 31 control, P <0.001 Delayed: mean score 17.94 out of 31 intervention vs. 14.7 out of 31 control, P <0.001 |
Intervention |
| Kinman et al. (2017) | USA | 60 | 55.6 y | 61.4% completed high school, 19.3% some college or trade school, 17.5% some graduate school | Pelvic organ prolapse surgery | Interactive electronic tablet application (“developed and internally validated” at the study institution after being administered to “a separate group of 32 patients from the same clinical setting” as study participants) with illustrations of a female pelvis with normal anatomy, examples of pelvic organ prolapse, and possible surgical treatments for prolapse plus standard consent (verbal consent using a “standardized protocol script” plus time to ask questions plus signing of consent document) versus standard consent alone (control) | Risks, alternatives, general knowledge about procedure | 20-item validated multiple choice questionnaire | Immediately and delayed | Some concerns for bias | Immediate: “no significant difference in mean improvement,” P = 0.22 Delayed: significant decrease in score in intervention group only, P < 0.01 |
Immediate: neither, delayed: control |
| Wollinger et al. (2012) | Austria | 90 | 73.7 y (range 48 – 94) | Not specified | Cataract surgery | “CatInfo” computer-based program with touch-screen monitor and headphones using a stepwise approach through modules via a “traffic light” system (patients instructed to select green if they understood everything within the module, yellow for further questions to discuss with physician, and red to repeat the module) in addition to standard face-to-face consent versus standard face-to-face consent plus a short sham computer presentation (control) | Risks, general knowledge about procedure | Validated 23-item multiple choice questionnaire | Not specified | Some concerns for bias | Questions correctly answered 15 out of 19 intervention vs. 12 out of 19 control, P < 0.01 | Intervention |
| Fraval et al. (2015) | Australia | 211 | 54.0 y | Not specified | Total knee arthroplasty, total hip arthroplasty, knee arthroscopy, shoulder arthroscopy and anterior cruciate ligament reconstruction | Online education resource developed by the Western Health Orthopaedic department (www.orthoanswer.org, no commercial funding, website contributed to by “orthopaedic residents and registrars, physiotherapists, occupational therapists and medical students” and “reviewed by consultant orthopaedic surgeons”) for patients with a 5th grade reading level or in addition to standard discussion with the treating surgeon versus standard discussion with the treating surgeon alone (control) | Risks, benefits, alternatives, general knowledge about procedure | Operation-specific questionnaire adapted from a validated survey | Immediately | Some concerns for bias | Average correct answers 69.25% intervention vs. 47.38% control, P < 0.01 | Intervention |
| Tait et al. (2009) | USA | 135 | 60.5 y | 26.7% ≤ high school, 23.0% some college or trade school, 43.0% ≥ bachelor’s degree | Diagnostic cardiac catheterization | 10-12 minute interactive computer program with text, narration, 2D and 3D graphics, and ability to type in questions at any point to be relayed to the cardiologist, and a short optional quiz to ascertain understanding of the key elements of the procedure (content based on existing consent documents, relevant literature, and input from cardiologists and computer graphic designers reviewed by cardiologists, “informed consent experts,” nurses, students and patients prior to use) versus standard verbal and written informed consent with a cardiology fellow or physician’s assistant (control) | Risks, benefits, alternatives, general knowledge about procedure | Semi-structured interview with 6 responses written down verbatim and scored from 0 (no understanding) to 2 (complete understanding), based on the validated Deaconess Informed Consent Comprehension Test | Delayed | Some concerns for bias | Understanding score 9.3 out of 12 (SD 2.2) intervention vs. 8.1 out of 12 (SD 2.3) control immediately post procedure, P < 0.05 Understanding score 8.6 out of 12 (SD 2.7) intervention vs. 7.9 out of 12 (SD 2.2) control 2 weeks post procedure, “not statistically significant” (no P value given) |
Intervention |
| Tait et al. (2014) | USA | 151 | 61.9 y | 27.8% ≤ high school graduate, 28.4% some college or trade school, 41.7% ≥ Bachelor’s degree | Diagnostic cardiac catheterization | 10-12 minute electronic tablet interactive multimedia program with in-line exercises and corrected feedback, 2D and 3D computer models, dynamic visualization of anatomical and physiologic functions, informational text inserts, voice over, and ability to click icons for additional information (content based on “information from medical textbooks, media, and expert opinion) versus standard verbal and written informed consent information (control) | Risks, benefits, alternatives, general knowledge about procedure | Semi-structured interview with 6 responses written down verbatim and scored from 0 (no understanding) to 2 (complete understanding), based on the validated Deaconess Informed Consent Comprehension Test | Delayed | Some concerns of bias | Understanding score 8.3 out of 12 (SD 2.4) intervention vs. 7.4 out of 12 (SD 2.5) control immediately post procedure, P < 0.05 Understanding score 7.6 out of 12 (SD 2.2) intervention vs. 6.6 out of 12 (SD 2.5) control 2 weeks post procedure, P < 0.05 |
Intervention |
| Heller et al. (2008) | USA | 133 | 47.0 y | 46.6% some college or less, 50.4% college or more | Breast reconstruction | Menu-driven interactive software program with animated graphics, patient testimonials, before-and-after photographs, and video explanations from plastic surgeons and clinical specialists (content selected in light of focus groups and faculty discussion at the University of Texas M.R. Anderson Cancer Center) in addition to routine education versus routine education alone (control) | General knowledge about procedure | 12-item multiple choice questionnaire | Delayed | High risk of bias | Mean increase from baseline 14% intervention vs. 8% control, P = 0.02 | Intervention |
| Gyomber et al. (2010) | Australia | 40 | 61 y | 27.5% did not complete high school, 42.5% completed high school, 30% tertiary education or technical school | Radical prostatectomy | Interactive multimedia PowerPoint presentation containing animated information and multiple-choice questions (developed with input from urologists, nurses, and patients and tested on 5 volunteers) probing understanding of key points at a 7th grade reading level; progression through the module required a correct response to each question, incorrect responses prompted a review of the information before repeating the question versus standard consent consisting of a verbal discussion with a doctor and nurse at a pre-admission clinic using a checklist of issues to cover and an informational booklet provided 3 weeks prior to the consent discussion and again at the time of the consent discussion (control) Groups were crossed over immediately after consent and initial evaluation of comprehension |
Risks, benefits, alternatives, general knowledge about procedure | 26-item multiple choice and true/false questionnaire | Immediately | High risk of bias | Average score 78% intervention vs. 57% control, P < 0.001 On crossover, control improved on average by 11% while intervention group was unchanged, P < 0.05 |
Intervention |
| Kim et al. (2018) | South Korea | 60 | Not specified | 5% less than middle school, 35% completed high school, 60% college/university | Nasal bone fracture reduction surgery | Mobile phone application with information about the surgery, post op management, and alerts such as “nasal packing will be removed today” in addition to traditional informed consent (“verbal descriptions and paper permission”) versus traditional informed consent alone (control) | Risks | Number of recalled surgical risks out of 6 | Delayed | High risk of bias | 1.72 out of 6 (SD 0.52) intervention vs. 1.49 out of 6 (SD 0.57) control, P = 0.047 | Intervention |
| Siu et al. (2015) | Canada | 50 | 48.3 y (range 21 – 85) | 20% did not complete high school, 46% some college or trade school, 34% Bachelor’s or higher | Endoscopic sinus surgery | 6-minute interactive computer multimedia module at an 8th grade reading level based on information from current standardized informed consent documents with voice-over, images, figures, animations, and knowledge checkpoints with quiz-type questions, in addition to routine verbal informed consent versus routine verbal informed consent alone (control) | Risks | Immediate: “participants were asked to complete a written questionnaire listing as many of the risks discussed at the consultation visit that they could recall.” Delayed: “patients were asked to recall as many of the risks of the procedure as possible” via telephone |
Immediately and delayed | High risk of bias | Immediate: risks recalled 4.88 intervention vs. 3.5 control, P = 0.0036 Delayed: risks recalled 3.2 intervention vs. 2.9 control, P = 0.222 |
Intervention |
| Brandel et al. (2017) | USA | 65 | 49.37 y | 6.2% completed high school, 72.3 with some college or trade school, 21.5% with some graduate school | Breast reconstruction, breast reduction, and abdominoplasty | Standard patient education plus procedure-specific interactive Web-based educational module (details about type of interactive features not specified) versus standard patient education plus generic safety Web-based module (control) | Risks, benefits, alternatives, general knowledge about procedure | Surgically focused, modified version of the Shared Decision-making 25 index tool | Not specified | High risk of bias | “No differences between experimental groups” (no P value given) | Neither |
Immediately: within 1 hour of informed consent consultation; early: >1 hour but <24 hours of informed consent consultation, delayed: ≥ 24 hours after informed consent consultation
Assessed using the Cochrane Risk of Bias 2.0 tool, see Table 7 for details
If a study reported an improvement in patient comprehension on a single item or multiple items of the comprehension assessment but if overall score did not improve, we considered neither or no group to be favored
Verbal discussion with test/feedback or teach-back interventions
Three studies evaluated 3 verbal discussion with test/feedback or teach-back interventions (Tables 4 and 6).34, 57, 59, In one intervention, patients received routine preoperative education followed by a knowledge test. They then received a corrected copy of their test followed by a telephone call with a nurse who reviewed the test and provided additional information tailored to their level of understanding.59 In the other two interventions, patients underwent standard informed consent and were then prompted to teach back key information to the consenting physician.34, 57 All of the verbal discussion with test/feedback or teach-back interventions (3/3, 100%) resulted in improved patient comprehension compared to standard informed consent. Two of these trials had some bias risk57,59 and one had high bias risk.34
Table 4.
Results from trials of verbal discussion with test/feedback or teach-back interventions to improve patient comprehension in informed consent
| Source | Study country |
N | Mean patient age (and range if specified) |
Patients’ education |
Procedure | Intervention | Elements of patient comprehension assessed |
Comprehension assessment tool |
Timing of patient comprehension assessment relative to informed consent consultation* |
Risk of bias for patient comprehension outcome† |
Results | Group favored‡ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kesänen et al. (2016) | Finland | 100 | 62.5 y | 76% did not complete high school, 24% completed high school | Spinal stenosis surgery | Routine, preoperative education ( face-to-face discussion with a surgeon and nurse plus written material) followed by a “Knowledge Test Feedback Intervention” (patients took a test, received results with corrections, then completed an "empowering telephone discourse based on the patients' existing knowledge" with a nurse) versus routine preoperative education along (control) | Risks, benefits, alternatives, general knowledge about procedure | 27-item true/ false/ do not know test | Delayed | Some concerns for bias | At admission: 21.6 out of 27 (SD 3.4) intervention vs. 15.4 out of 27 (SD 4.1), P < 0.0001 At discharge: 21.4 out of 27 (SD 3.4) intervention vs. 15.2 out of 27 (SD 4.2) control, P < 0.0001 At 6 months: 20.3 out of 27 (SD 4.5) intervention vs. 14.6 out of 27 (SD 3.9) control, P < 0.0001 |
Intervention |
| Fink et al. (2010) | USA | 575 | 61.9 y | 12.1% did not complete high school, 34.2% completed high school, 35.8% some college or trade school, 17.8% college graduate | Carotid endarterectomy, laparoscopic cholecystectomy, radical prostatectomy, and total hip arthroplasty | Standard informed consent using iMedConsent web-based tool but when the participants were ready to sign the consent a repeat back dialog was initiated that prompted the provider to test the participant on key information (the provider could then provide additional information and education depending on the participants’ responses) versus standard informed consent using iMedConsent along (control) | Risks, benefits, alternatives, general knowledge about procedure | 23 to 26-item questionnaire depending on the procedure | Immediately | Some concerns for bias | Total mean comprehension scores for all operations 71.4% intervention vs. 68.2% control, P = 0.03 | Intervention |
Immediately: within 1 hour of informed consent consultation; delayed: more than 24 hours after informed consent consultation
Assessed using the Cochrane Risk of Bias 2.0 tool, see Table 7 for details
If a study reported an improvement in patient comprehension on a single item or multiple items of the comprehension assessment but if overall score did not improve, we considered neither or no group to be favored
Multicomponent interventions
Three studies evaluated 3 multicomponent interventions (Tables 5 and 6).35, 36, 47 One utilized a paper-based decision aid written at an 8th grade reading level plus access to a website with supplemental videos,47 one provided a written pamphlet and showed patients an anatomical model during the consent discussion (no patient interaction with the model was described)35, and one provided an educational video plus an additional education session with a nurse coordinator/educator (further details were not provided).36 The first two (67%) resulted in improved patient comprehension. Of these, one had some risk of bias47 and one had high risk of bias.78 The third did not result in improved patient comprehension. This intervention had high risk of bias.36 All multicomponent interventions were provided in addition to rather than in place of standard informed consent; none contained interactive, test/feedback, or teach-back components.
Table 5.
Results from trials of multicomponent interventions to improve patient comprehension in informed consent
| Source | Study country |
N | Mean patient age (and range if specified) |
Patients’ education |
Procedure | Intervention* | Elements of patient comprehension assessed |
Comprehension assessment tool |
Timing of patient comprehension assessment relative to informed consent consultation* |
Risk of bias for patient comprehension outcome† |
Results | Group favored‡ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kostick et al. (2018) | USA | 98 | 59.77 y (range 20 - 84) | 60% ≤ high school degree/ GED, 40% ≥ some college | Left ventricular assist device implantation | Paper-based decision aid written at an 8th-grade reading level (took on average 59 minutes to review) with information about living with an LVAD, resources for patients and caregivers, narratives provided by patients and caregivers, risk and benefit information, and links to a website with supplemental informational videos developed with “extensive input of intended end users” (patients, caregivers, candidates and decliners of LVAD treatment) and clinicians involved in shared decision-making via in-depth interviews then drafted and tested following International Patient Decision Aid Standards quality indicators versus “standard education” (control) | Risks, benefits, general knowledge about procedure | 20-item multiple choice LVAD knowledge scale developed and validated by the research team | Immediately and delayed | Some concerns for bias | 68% intervention vs. 59% control at one week follow up, P = 0.02 No significant difference at 1 month |
Intervention |
| Karan et al. (2014) | India | 97 | Not specified | Not specified | Cataract surgery | 3-fold pamphlet designed by Unite for Sight, an ophthalmologist in Chennai, and a team of “visual communication specialists at a US-based university” plus patients were shown a 3D model of the eye in addition to scripted verbal informed consent read by a native Tamil speaker versus scripted verbal informed consent read by a native Tamil speaker only (control) | Risks, benefits, general knowledge about procedure | Validated 11-item true/false/don’t know quiz | Immediately and early | High risk of bias | Immediate: average difference in score from baseline 5.17 intervention vs. 1.52 control Within 24 hours: average difference in score from baseline 5.43 intervention vs. 1.06 control “P value on the order of 10−6” |
Intervention |
Immediately: within 1 hour of informed consent consultation; early: >1 hour but <24 hours of informed consent consultation, delayed: ≥ 24 hours after informed consent consultation
Assessed using the Cochrane Risk of Bias 2.0 tool, see Table 7 for details
If a study reported an improvement in patient comprehension on a single item or multiple items of the comprehension assessment but if overall score did not improve, we considered neither or no group to be favored
Interactive versus Noninteractive Interventions
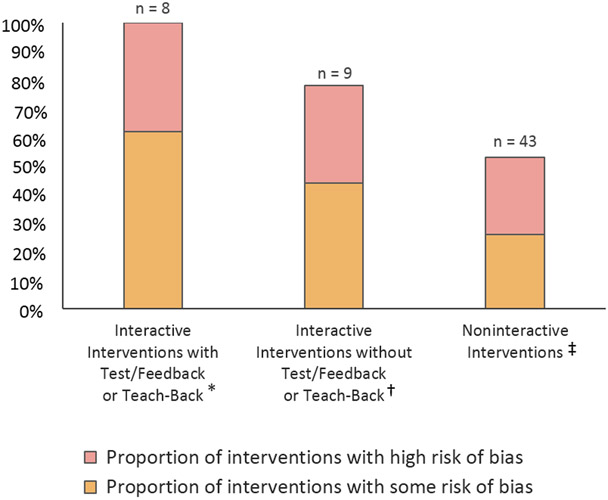
Across the above categories, a total of 43 interventions (72%) were noninteractive and 17 (28%) were interactive. Eight of the interactive interventions included test/feedback or teach-back components including one audiovisual intervention,39 5 digital interventions 52, 58, 61, 62, 84 and 3 verbal discussion with test/feedback or teach-back interventions. 34, 57, 59 Fifty-three percent (23/43) of noninteractive interventions, 78% (7/9) of interactive interventions without test/feedback or teach-back components, and 100% (8/8) of interactive interventions with test/feedback or teach-back components resulted in improved patient comprehension compared to standard informed consent (Figure 3).
Figure 3.
Percent of Interventions that Improved Patient Comprehension in Informed Consent by Degree of Interactivity
Timing of Comprehension Measures
In general, improvements in patient comprehension were more pronounced on immediate testing, though two studies found improvements on early testing35, 78 and fourteen studies found improvements on delayed testing.40, 41, 47, 49, 52, 59, 66, 69, 79, 81, 61, 62, 83, 84
Effect of Interventions on Other Outcomes
Twenty-seven studies evaluated patient satisfaction with the informed consent process. Eight (30%) demonstrated higher satisfaction among the intervention group compared to standard informed consent46, 48, 50, 51, 64, 71, 75, 77 and 19 (70%) found no difference in satisfaction between groups.
Fourteen studies evaluated patient anxiety. Twelve (86%) found no difference in anxiety between intervention and control groups,34, 38, 40, 48, 51, 57, 65, 68, 74, 77, 80, 82 and 2 (14%) found less anxiety among intervention group patients.42, 76
Ten studies evaluated amount of provider time required to obtain informed consent. Two found no difference in the intervention group compared to standard informed consent.54, 77 Five interventions reduced consultation time with the consenting provider by an average of 4–6.7 minutes.63, 64, 68, 74, 79 One intervention with reduced consultation time resulted in improved patient comprehension.79 Three verbal discussion with test-feedback or teach-back interventions required longer consultation times, by an average of 2.3, 2.6, and 21 minutes, and each resulted in improved patient comprehension compared to standard informed consent. 34, 57, 59
Informed Consent and Vulnerable Populations
Some studies conducted sub-analyses on the basis of sociodemographic data, most commonly examining education (19 studies), age (16 studies), and gender (12 studies). Lower education (10/19 studies), older age (6/16 studies), and limited health literacy (3/4 studies) were associated with lower patient comprehension scores; however, these studies did not clearly specify whether interventions were differentially effective based on these factors. Only four studies examined effects according to health literacy52, 57, 61, 62 of which one reported a statistically significant improvement in comprehension scores among participants with limited health literacy receiving the intervention.52 Reading level of intervention materials was reported in seven studies, and ranged from 2nd to 8th grade. 30, 47, 51, 58, 60, 72, 84 One study assessed the efficacy of an informed consent intervention for patients with language barriers, finding that non-native speakers of the study language showed decreased comprehension compared to native speakers in both the control and intervention groups.37
Risk of Bias
No study had a low overall bias risk for the patient comprehension outcome; 56% (29/52) had some concerns and 44% (23/52) had high overall risk (Appendix 3). Factors that most commonly increased risk of bias were non-blinding of study participants, those delivering the intervention, or outcome assessors, lack of allocation concealment, data availability for <90% of participants randomized, and lack of a pre-specified trial registration or protocol.
DISCUSSION
In this systematic review, we identified 52 studies evaluating 60 interventions to improve patient comprehension in informed consent for medical and surgical procedures published between 2008 and 2018. Studies included written, audiovisual, interactive digital, verbal discussion with test/feedback or teach-back, and multicomponent interventions. Overall, about two thirds of interventions improved patient comprehension. A higher proportion of verbal discussion with test/feedback or teach-back and interactive digital interventions improved patient comprehension than did multicomponent, audiovisual, or written interventions. Effective interventions could be delivered without placing undue time burdens on providers or negatively impacting patient satisfaction or anxiety, though less than a third of studies reported the impact of interventions on time required of providers or on patient anxiety. Our results show that a variety of interventions may be helpful in improving patient comprehension in clinical informed consent.
Our findings suggest, however, that interactive informed consent interventions, i.e. those that intentionally promote active patient involvement and bi-directional communication, may be superior to noninteractive interventions. Among interactive interventions, those that utilize test/feedback and teach-back techniques appear particularly effective. This finding is in accordance with previous literature,12, 86 and multiple organizations already include teach-back recommendations in their quality guidelines for clinical informed consent.21, 87, 88 Non-interactive interventions, such as those in which patients independently read additional information or reviewed visual aids, were less likely to improve patient comprehension. Non-interactive interventions provided in place of rather than in addition to standard informed consent appeared to be the least affective. Interactive digital interventions may promote improved patient comprehension regardless of whether they are provided in place of or in addition to standard informed consent. These interventions could potentially be developed to meet quality standards, though additional studies assessing dissemination and implementation are needed. Additionally, interactive digital interventions can help ensure efficiency of clinician-patient informed consent discussions by allowing patients to independently navigate through digital information, gaining understanding and recording questions prior to clinician involvement.
As in our original review, the most commonly assessed element of patient comprehension was understanding of procedural risks, with fewer studies evaluating patients’ understanding of benefits, alternatives, and general knowledge about the procedure. Only about a fifth of studies evaluated understanding of all four elements. This suggests a continued emphasis on malpractice risk reduction over patient autonomy and the use of informed consent in a physician-centered rather than a patient-centered manner. Future studies examining efficacy of informed consent interventions should equally emphasize and assess understanding of all four key elements of informed consent.
Sociodemographic information provided by studies addressing language proficiency, education, and health literacy was sparse and highly variable. The majority of studies did not explicitly address effectiveness of interventions for patients representing vulnerable populations, for whom current informed consent practices are particularly inadequate. This represents an important gap in current research.
The majority of tools used to assess patient comprehension were neither validated nor adapted from validated measures. This reflects a lack of standards defining adequate patient comprehension in informed consent and of practices ensuring that adequate patient comprehension is achieved.
We recommend further research addressing patient comprehension in informed consent for vulnerable populations. Specific quality standards for informed consent should be developed, including 1) defining adequate patient comprehension encompassing all four key elements of informed consent, 2) developing validated measures to assess attainment of adequate patient comprehension within routine informed consent, 3) consistently using easy-to-understand, linguistically and culturally appropriate materials to specifically ensure adequate comprehension for patients facing language barriers and for patients with limited education and health literacy, and 4) including interactive components within the informed consent process, particularly test/feedback and teach-back techniques.
Our study has limitations. First, the accuracy of our findings is limited by the quality of included studies; none achieved a low overall risk-of-bias assessment for the patient comprehension outcome. This, however, is likely the result of increasingly rigorous standards for risk-of-bias evaluations in systematic reviews, which have evolved to better safeguard against the perpetuation of bias in primary studies. Additionally, results from studies within our review with lower risk for bias showed a similar pattern of benefit compared to results from all included studies. Second, wide variation in patient comprehension assessment tools and reporting precluded conduct of a meta-analysis or calculation of mean effect size. We were therefore unable to compare the magnitude of effects by intervention type or other factors. Developing and validating standardized patient comprehension measures is an important area of future research to enable comparisons of this type. Third, standard informed consent for control groups was inconsistent across studies and often not clearly described, making it difficult to determine the exact processes to which interventions were compared. Fourth, although some included studies conducted sub-analyses on the basis of sociodemographic factors, there was not sufficient data to draw meaningful conclusions about whether interventions provided equal benefit to vulnerable populations. Fifth, our review may be biased by exclusion of non-English language studies, though study countries were relatively diverse. Finally, our review may be subject to publication bias, though about a third of evaluated interventions had negative findings.
In summary, we found evidence of the efficacy of a variety of interventions to improve patient comprehension in informed consent for medical and surgical procedures. Interactive features appear especially helpful in improving patient understanding, particularly those incorporating test/feedback and teach-back components. Novel interactive digital interventions are particularly promising. Further research should better address vulnerable populations to ensure that interventions equally benefit patients facing language barriers and those with limited education and health literacy.
Supplementary Material
Acknowledgments
Johanna Glaser is supported by the National Center for Complementary and Integrative Health (T32AT003997). Dr. Sarah Nouri is supported by the National Research Service Award (NRSA) (T32HP19025). Dr. Alicia Fernandez is supported by the NIH (K24DK102057). Dr. Rebecca Sudore is supported by the NIH National Institute on Aging (K24AG054415). Michele Klein-Fedyshin is supported by the National Institute on Alcohol Abuse and Alcoholism (R21AA025484). Funding agreements ensure the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
References
- 1.American Medical Association. Informed Consent, http://www.ama-assn.org/delivering-care/ethics/informed-consent. (Accessed Sep 8, 2019).
- 2.Department of Health. Reference guide to consent for examination or treatment, second edition. London: Department of Health (UK) July 2009. [Google Scholar]
- 3.<b/>The Joint Commission, Division of Health Care Improvement Informed consent: more than getting a signature. Quick Safety, Issue 21 February 2016. [Google Scholar]
- 4.Jonsen AR, Siegler M, Winslade WJ, eds. Preferences of Patients. 8th ed. New York, NY: McGraw-Hill Education; 2015. [Google Scholar]
- 5.Brezis M, Israel S, Weinstein-Birenshtock A, et al. Quality of informed consent for invasive procedures. Int J Qual Health Care 2008. DOI: 10.1093/intqhc/mzn025. [DOI] [PubMed] [Google Scholar]
- 6.Kinnersley P, Phillips K, Savage K, et al. Interventions to promote informed consent for patients undergoing surgical and other invasive healthcare procedures. Cochrane Database Syst Rev 2013. DOI: 10.1002/14651858.CD009445.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherlock A, Brownie S. Patients' recollection and understanding of informed consent: a literature review. ANZ Journal of Surgery. 2014;84(4):207–210. doi: 10.1111/ans.12555. [DOI] [PubMed] [Google Scholar]
- 8.Matiasek J, Wynia MK. Reconceptualizing the Informed Consent Process at Eight Innovative Hospitals. The Joint Commission Journal on Quality and Patient safety. 2008;34(3):127–137. doi: 10.1016/S1553-7250(08)34015-X. [DOI] [PubMed] [Google Scholar]
- 9.Ankuda CK, Block SD, Cooper Z, et al. Measuring critical deficits in shared decision making before elective surgery. Patient Educ Couns 2014. DOI: 10.1016/j.pec.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Literacy, Institute of Medicine (US) Committee on Health, Nielsen-Bohlman L, Panzer AM, et al. What Is Health Literacy?: National Academies Press (US), 2004. [PubMed] [Google Scholar]
- 11.Bickmore TW, Pfeifer LM and Paasche-Orlow MK. Using computer agents to explain medical documents to patients with low health literacy. Patient Educ Couns 2009. DOI: 10.1016/j.pec.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudore RL, Landefeld CS, Williams BA, et al. Use of a modified informed consent process among vulnerable patients: a descriptive study. J Gen Intern Med 2006. DOI: 10.1111/j.1525-1497.2006.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krankl JT, Shaykevich S, Lipsitz S, et al. Patient predictors of colposcopy comprehension of consent among English- and Spanish-speaking women. Womens Health Issues 2011. DOI: 10.1016/j.whi.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Raposo VL. Lost in ‘Culturation’: medical informed consent in China (from a Western perspective). Med Health Care Philos. 2019;22(1):17–30. DOI: 10.1007/s11019-018-9835-0. [DOI] [PubMed] [Google Scholar]
- 15.Masaki S, Ishimoto H and Asai A. Contemporary issues concerning informed consent in Japan based on a review of court decisions and characteristics of Japanese culture. BMC Med Ethics 2014. DOI 10.1186/1472-6939-15-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawal YZ, Garba ES, Ogirima MO, et al. The doctrine of informed consent in surgical practice. Annals of African Medicine 2011. DOI: 10.4103/1596-3519.76558. [DOI] [PubMed] [Google Scholar]
- 17.Chima SC. Evaluating the quality of informed consent and contemporary clinical practices by doctors in South Africa: an empirical study. BMC Med Ethics 2013. DOI: 10.1186/1472-6939-14-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nandimath OV. Consent and medical treatment: the legal paradigm in India. Indian J Urol 2009. DOI: 10.4103/0970-1591.56202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunin De Skrzynno SC and Di Maggio F. Surgical consent in sub-Saharan Africa: a modern challenge for the humanitarian surgeon. Trop Doct 2018. DOI: 10.1177/0049475518780531. [DOI] [PubMed] [Google Scholar]
- 20.Hammami MM, Al-Jawarneh Y, Hammami MB, et al. Information disclosure in clinical informed consent: “reasonable” patient’s perception of norm in high-context communication culture. BMC Med Ethics 2014. DOI: 10.1186/1472-6939-15-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Quality Forum (NQF). Implementing a National Voluntary Consensus Standard for Informed Consent: A User’s Guide for Healthcare Professionals. Washington, DC: NQF; 2005. [Google Scholar]
- 22.Salzburg Global Seminar. Salzburg statement on shared decision making. BMJ 2011. DOI: 10.1136/bmj.d1745. [DOI] [PubMed] [Google Scholar]
- 23.Spatz ES, Krumholz HM, and Moulton BW. The new era of informed consent: getting to a reasonable-patient standard through shared decision making. JAMA 2016. DOI: 10.1001/jama.2016.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013. DOI: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 25.Council for International Organizations of Medical Sciences. International ethical guidelines for health-related research involving humans. CIOMS; 2016. 978–929036088-9. [Google Scholar]
- 26.Schenker Y, Fernandez A, Sudore R, et al. Interventions to improve patient comprehension in informed consent for medical and surgical procedures: a systematic review. Med Decis Making 2011. DOI: 10.1177/0272989X10364247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner L, Boutron I, Hróbjartsson A, et al. The evolution of assessing bias in Cochrane systematic reviews of interventions: celebrating methodological contributions of the Cochrane Collaboration Systematic reviews 2013. DOI: 10.1186/2046-4053-2-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viswanathan M, Patnode CD, Berkman ND, et al. Assessing the Risk of Bias in Systematic Reviews of Health Care Interventions In: Anonymous Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US), 2008. [PubMed] [Google Scholar]
- 29.Ioannidis JPA. The Mass Production of Redundant, Misleading, and Conflicted Systematic Reviews and Meta-analyses. Milbank Q 2016. DOI: 10.1111/1468-0009.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cowan N What are the differences between long-term, short-term, and working memory?. Progress in brain research 2008. DOI: 10.1016/S0079-6123(07)00020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JP, Savovicć J, Page MJ, et al. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2.0). Cochrane Database of Systematic Reviews 2018. [Google Scholar]
- 32.Higgins JP. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2.0) additional consideration for cross-over trials. Cochrane Database of Systematic Reviews 2016. [Google Scholar]
- 33.Eldridge S, Campbell M, Campbell M, et al. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2.0) additional consideration for cluster-randomized trials. Cochrane Database of Systematic Reviews 2016. [Google Scholar]
- 34.Bennett DL, Dharia CV, Ferguson KJ, et al. Patient-Physician Communication: Informed Consent for Imaging Guided Spine Injections 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karan A, Somasundaram P, Michael H, et al. The effect of multimedia interventions on the informed consent process for cataract surgery in rural South India. Indian J Ophthalmol 2014. DOI: 10.4103/0301-4738.116488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson MR, Singh JA, Stewart T, et al. Patient understanding and satisfaction in informed consent for total knee arthroplasty: a randomized study. Arthritis Care Res (Hoboken) 2011. DOI: 10.1002/acr.20475. [DOI] [PubMed] [Google Scholar]
- 37.Clark S, Mangram A, Ernest D, et al. The Informed Consent: A Study of the Efficacy of Informed Consents and the Associated Role of Language Barriers. Journal of Surgical Education 2011. DOI: 10.1016/j.jsurg.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Cornoiu A, Beischer AD, Donnan L, et al. Multimedia patient education to assist the informed consent process for knee arthroscopy. ANZ Journal of Surgery 2011. DOI: 10.1111/j.1445-2197.2010.05487.x. [DOI] [PubMed] [Google Scholar]
- 39.Egekeze N, Dubin J, Williams K, et al. The Age of OrthoInfo: A Randomized Controlled Trial Evaluating Patient Comprehension of Informed Consent. The Journal of bone and joint surgery. American volume 2016. DOI: 10.2106/JBJS.15.01291. [DOI] [PubMed] [Google Scholar]
- 40.Ellett L, Villegas R, Beischer A, et al. Use of a Multimedia Module to Aid the Informed Consent Process in Patients Undergoing Gynecologic Laparoscopy for Pelvic Pain: Randomized Controlled Trial. Journal of Minimally Invasive Gynecology 2014. DOI: 10.1016/j.jmig.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Heller L, Parker PA, Youssef A, et al. Interactive Digital Education Aid in Breast Reconstruction. Plastic and reconstructive surgery 2008. DOI: 10.1097/PRS.0b013e318180ed06. [DOI] [PubMed] [Google Scholar]
- 42.Mishra PK, Mathias H, Millar K, et al. A Randomized Controlled Trial to Assess the Effect of Audiotaped Consultations on the Quality of Informed Consent in Cardiac Surgery. Archives of Surgery 2010. DOI: 10.1001/archsurg.2010.45. [DOI] [PubMed] [Google Scholar]
- 43.Sharma S, McCrary H, Romero E, et al. A prospective, randomized, single-blinded trial for improving health outcomes in rhinology by the use of personalized video recordings. International forum of allergy & rhinology 2018. DOI: 10.1002/alr.22145. [DOI] [PubMed] [Google Scholar]
- 44.Finch WJG, Rochester MA and Mills RD. A randomised trial of conventional versus BAUS procedure-specific consent forms for transurethral resection of prostate. Annals of The Royal College of Surgeons of England 2009. DOI: 10.1308/003588409X359277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gangol R and Maharjan D. Information leaflet as an adjunct to verbal counseling in obtaining informed consent. JNMA J Nepal Med Assoc 2010. [PubMed] [Google Scholar]
- 46.Ham DY, Choi WS, Song SH, et al. Prospective Randomized Controlled Study on the Efficacy of Multimedia Informed Consent for Patients Scheduled to Undergo Green-Light High-Performance System Photoselective Vaporization of the Prostate. The world journal of men’s health 2016. DOI: 10.5534/wjmh.2016.34.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kostick KM, Bruce CR, Minard CG, et al. A Multisite Randomized Controlled Trial of a Patient-Centered Ventricular Assist Device Decision Aid (VADDA Trial). Journal of cardiac failure 2018. DOI: 10.1016/j.cardfail.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Lattuca B, Barber-Chamoux N, Alos B, et al. Impact of video on the understanding and satisfaction of patients receiving informed consent before elective inpatient coronary angiography: A randomized trial. American Heart Journal 2018. DOI: 10.1016/j.ahj.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Smith HK, Manjaly JG, Yousri T, et al. Informed consent in trauma: Does written information improve patient recall of risks? A prospective randomised study. Injury 2011. DOI: 10.1016/j.injury.2011.06.419. [DOI] [PubMed] [Google Scholar]
- 50.Lin Y, Chen C, Lee W, et al. Educational video-assisted versus conventional informed consent for trauma-related debridement surgery: a parallel group randomized controlled trial. BMC medical ethics 2018. DOI: 10.1186/s12910-018-0264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fraval A, Chandrananth J, Chong YM, et al. Internet based patient education improves informed consent for elective orthopaedic surgery: a randomized controlled trial. BMC musculoskeletal disorders 2015. DOI: 10.1186/s12891-015-0466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gordon EJ, Sohn M, Chang C, et al. Effect of A Mobile Web App on Kidney Transplant Candidates’ Knowledge About Increased Risk Donor Kidneys: A Randomized Controlled Trial. Transplantation 2017; 101: 1167–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kinman C, Meriwether K, Powell C, et al. Use of an iPad™ application in preoperative counseling for pelvic reconstructive surgery: a randomized trial. Int Urogynecol J 2018. DOI: 10.1007/s00192-017-3513-2. [DOI] [PubMed] [Google Scholar]
- 54.Alsaffar H, Wilson L, Kamdar DP, et al. Informed consent: do information pamphlets improve post-operative risk-recall in patients undergoing total thyroidectomy: prospective randomized control study. Journal of otolaryngology - head & neck surgery 2016. DOI: 10.1186/s40463-016-0127-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bethune A, Marisol D, Mikaeel V, et al. e-Consent: approaching surgical consent with mobile technology. Canadian journal of surgery. Journal canadien de chirurgie 2018. DOI: 10.1503/cjs.016017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brandel M, Reid C, Parmeshwar N, et al. Efficacy of a Procedure-Specific Education Module on Informed Consent in Plastic Surgery. Annals of Plastic Surgery 2017. DOI: 10.1097/SAP.0000000000000970. [DOI] [PubMed] [Google Scholar]
- 57.Fink AS, Prochazka AV, Henderson WG, et al. Enhancement of surgical informed consent by addition of repeat back: a multicenter, randomized controlled clinical trial. Annals of surgery 2010. DOI: 10.1097/SLA.0b013e3181e3ec61. [DOI] [PubMed] [Google Scholar]
- 58.Gyomber D, Lawrentschuk N, Wong P, et al. Improving informed consent for patients undergoing radical prostatectomy using multimedia techniques: a prospective randomized crossover study. BJU Int 2010. DOI: 10.1111/j.1464-410X.2010.09309.x. [DOI] [PubMed] [Google Scholar]
- 59.Kesänen J, Leino-Kilpi H, Lund T, et al. The Knowledge Test Feedback Intervention (KTFI) increases knowledge level of spinal stenosis patients before operation − a randomized controlled follow-up trial. Patient Education and Counseling 2016. DOI: 10.1016/j.pec.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 60.Shukla A, Mary K, and Legutko P. Informed consent for cataract surgery: Patient understanding of verbal, written, and videotaped information. Journal of Cataract and Refractive Surgery 2012. DOI: 10.1016/j.jcrs.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 61.Tait AR, Voepel-Lewis T, Chetcuti SJ, et al. Enhancing patient understanding of medical procedures: Evaluation of an interactive multimedia program with in-line exercises. International Journal of Medical Informatics 2014. DOI: 10.1016/j.ijmedinf.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tait AR, Voepel-Lewis T, Moscucci M, et al. Patient Comprehension of an Interactive, Computer-Based Informsation Program for Cardiac Catheterization: A Comparison With Standard Information. Archives of Internal Medicine 2009. DOI: 10.1001/archinternmed.2009.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vo TA, Ngai P and Tao JP. A randomized trial of multimedia-facilitated informed consent for cataract surgery. Clinical ophthalmology (Auckland, N.Z.) 2018. DOI: 10.2147/OPTH.S150670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Ruan X, Tang H, et al. Video-Assisted Informed Consent for Cataract Surgery: A Randomized Controlled Trial. Journal of ophthalmology 2017. DOI: 10.1155/2017/9593631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winter M, Kam J, Nalavenkata S, et al. The use of portable video media vs standard verbal communication in the urological consent process: a multicentre, randomised controlled, crossover trial. BJU Int 2016. DOI: 10.1111/bju.13595. [DOI] [PubMed] [Google Scholar]
- 66.Aremu SK, Alabi BS, and Segun-Busari S. The role of informed consent in risks recall in otorhinolaryngology surgeries: verbal (nonintervention) vs written (intervention) summaries of risks. American Journal of Otolaryngology--Head and Neck Medicine and Surgery 2011. DOI: 10.1016/j.amjoto.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 67.Borello A, Ferrarese A, Passera R, et al. Use of a simplified consent form to facilitate patient understanding of informed consent for laparoscopic cholecystectomy. Open Medicine 2016. DOI: 10.1515/med-2016-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goldberger JJ, Kruse J, Kadish AH, et al. Effect of informed consent format on patient anxiety, knowledge, and satisfaction. American Heart Journal 2011. DOI: 10.1016/j.ahj.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 69.Hong P, Makdessian AS, Ellis DAF, et al. Informed consent in rhinoplasty: prospective randomized study of risk recall in patients who are given written disclosure of risks versus traditional oral discussion groups. J Otolaryngol Head Neck Surg 2009. [PubMed] [Google Scholar]
- 70.Khan Z, Sayers AE, Khattak MU, et al. A Prospective Randomized Control Study on Patient’s Recall of Consent after Hand Surgery: How Much They Want to Know?. Orthop Rev (Pavia) 2013. DOI: 10.4081/or.2013.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Straessle R, Gilliard N, Frascarolo P, et al. Is a pre-anaesthetic information form really useful?. Acta Anaesthesiol Scand 2011. DOI: 10.1111/j.1399-6576.2011.02422.x. [DOI] [PubMed] [Google Scholar]
- 72.Wong A, Martin J, Tang D, et al. The Effect of Written Information on Recall of Surgical Risks of Carpal Tunnel Release Surgery: A Randomized Controlled Study. Plastic and Reconstructive Surgery 2016. DOI: 10.1097/PRS.0000000000002771. [DOI] [PubMed] [Google Scholar]
- 73.Armstrong AW, Alikhan A, Cheng LS, Schupp C, Kurlinkus C, Eisen DB. Portable video media for presenting informed consent and wound care instructions for skin biopsies: a randomized controlled trial. Br J Dermatol. 2010;163(5):1014–1019. doi: 10.1111/j.1365-2133.2010.10067.x. [DOI] [PubMed] [Google Scholar]
- 74.Baenninger PB, Faes L, Kaufmann C, et al. Efficiency of video-presented information about excimer laser treatment on ametropic patients’ knowledge and satisfaction with the informed consent process. Journal of Cataract & Refractive Surgery 2018. DOI: 10.1016/j.jcrs.2018.07.052. [DOI] [PubMed] [Google Scholar]
- 75.Bowers N, Eisenberg E, Montbriand J, et al. Using a multimedia presentation to improve patient understanding and satisfaction with informed consent for minimally invasive vascular procedures. Surgeon, The 2015. DOI: 10.1016/j.surge.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 76.Choi SH, Won JH, Cha JY, et al. Effect of Audiovisual Treatment Information on Relieving Anxiety in Patients Undergoing Impacted Mandibular Third Molar Removal. Journal of Oral and Maxillofacial Surgery 2015. DOI: 10.1016/j.joms.2015.06.175. [DOI] [PubMed] [Google Scholar]
- 77.Huber J, Ihrig A, Yass M, et al. Multimedia Support for Improving Preoperative Patient Education: A Randomized Controlled Trial Using the Example of Radical Prostatectomy. Ann Surg Oncol 2013. DOI: 10.1245/s10434-012-2536-7. [DOI] [PubMed] [Google Scholar]
- 78.Karan AM, Campbell DJ and Mayer HR. The effect of a visual aid on the comprehension of cataract surgery in a rural, indigent South Indian population. Digital journal of ophthalmology : DJO 2011. DOI: 10.5693/djo.01.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pallett AC, Nguyen BT, Klein NM, et al. A randomized controlled trial to determine whether a video presentation improves informed consent for hysterectomy. American Journal of Obstetrics and Gynecology 2018. DOI: 10.1016/j.ajog.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 80.Tipotsch-Maca SM, Varsits RM, Ginzel C, et al. Effect of a multimedia-assisted informed consent procedure on the information gain, satisfaction, and anxiety of cataract surgery patients. Journal of Cataract and Refractive Surgery 2016. DOI: 10.1016/j.jcrs.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 81.Wilhelm D, Gillen S, Wirnhier H, et al. Extended preoperative patient education using a multimedia DVD—impact on patients receiving a laparoscopic cholecystectomy: a randomised controlled trial. Langenbecks Arch Surg 2009. DOI: 10.1007/s00423-008-0460-x. [DOI] [PubMed] [Google Scholar]
- 82.Wysocki WM, Mituś J, Komorowski AL, et al. Impact of preoperative information on anxiety and disease-related knowledge in women undergoing mastectomy for breast cancer: a randomized clinical trial. Acta Chir Belg 2012. [DOI] [PubMed] [Google Scholar]
- 83.Kim CH, Cheon JS, Choi WY, et al. The efficacy of mobile application use on recall of surgical risks in nasal bone fracture reduction surgery. Archives of craniofacial surgery 2018. DOI: 10.7181/acfs.2018.19.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siu JM, Rotenberg BW, Franklin JH, et al. Multimedia in the informed consent process for endoscopic sinus surgery: A randomized control trial. The Laryngoscope 2016. DOI: 10.1002/lary.25793. [DOI] [PubMed] [Google Scholar]
- 85.Wollinger C, Hirnschall N, and Findl O. Computer-based tutorial to enhance the quality and efficiency of the informed-consent process for cataract surgery. Journal of Cataract and Refractive Surgery 2012. DOI: 10.1016/j.jcrs.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 86.Shoemaker SJ, Brach C, Edwards A, et al. Opportunities to Improve Informed Consent with AHRQ Training Modules. Jt Comm J Qual Patient Saf 2018. DOI: 10.1016/j.jcjq.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Agency for Healthcare Research and Quality (AHRQ). The AHRQ Informed Consent and Authorization Toolkit for Minimal Risk Research. Agency for Healthcare Research and Quality, Rockville, MD; 2019. [Google Scholar]
- 88.Fleisher L, Raivitch S, Miller SM, et al. A Practical Guide to Informed Consent. Temple University Health System 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.