Abstract
Background:
Asthma-chronic obstructive pulmonary disease (COPD) overlap (ACO) represents the confluence of bronchial airway hyperreactivity and chronic airflow limitation and has been described as leading to worse lung function and quality of life than found with either singular disease process.
Objective:
We aimed to describe the prevalence and risk factors for ACO among adults across 6 low- and middle-income countries (LMICs).
Methods:
We compiled cross-sectional data for 11,923 participants aged 35 to 92 years from 4 population-based studies in 12 settings. We defined COPD as postbronchodilator FEV1/forced vital capacity ratio below the lower limit of normal, asthma as wheeze or medication use in 12 months or self-reported physician diagnosis, and ACO as having both.
Results:
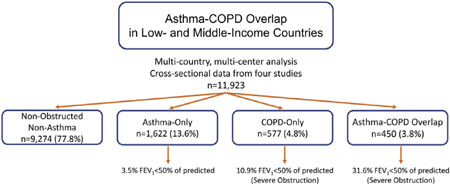
The prevalence of ACO was 3.8% (0% in rural Puno, Peru, to 7.8% in Matlab, Bangladesh). The odds of having ACO were higher with household exposure to biomass fuel smoke (odds ratio [OR], 1.48; 95% CI, 0.98–2.23), smoking tobacco (OR, 1.28 per 10 pack-years; 95% CI, 1.22–1.34), and having primary or less education (OR, 1.35; 95% CI, 1.07–1.70) as compared to nonobstructed nonasthma individuals. ACO was associated with severe obstruction (FEV1 %, <50; 31.6% of ACO vs 10.9% of COPD alone) and severe spirometric deficits compared with participants with asthma (−1.61 z scores FEV1; 95% CI, −1.48 to −1.75) or COPD alone (−0.94 z scores; 95% CI, −0.78 to −1.10).
Conclusions:
ACO may be as prevalent and more severe in LMICs than has been reported in high-income settings. Exposure to biomass fuel smoke may be an overlooked risk factor, and we favor diagnostic criteria for ACO that include environmental exposures common to LMICs.
Keywords: Asthma, chronic obstructive pulmonary disease, COPD, overlap, asthma-COPD overlap, ACO, epidemiology, risk factors, health outcomes, spirometry, population-based
GRAPHICAL ABSTRACT
Asthma-chronic obstructive pulmonary disease (COPD) overlap (ACO) refers to chronic respiratory disease composed of clinical and biological markers of both asthma and COPD, each of which is a leading cause of disability and death worldwide.1–4 Although COPD and asthma have been well defined, the mechanism of injury in ACO has not.5 COPD is characterized by poorly reversible airflow obstruction via an abnormal inflammatory response in the lungs, marked by innate and adaptive immune responses to noxious exposures such as cigarette smoke.6 Asthma is characterized by airway hyperresponsiveness and inflammation that results in reversible airway obstruction.7 Current opinions largely agree that ACO is not a standalone condition but lack consensus on whether the underlying mechanism of injury represents a synergistic result of unique phenotypes of asthma and COPD or a spectrum of eosinophilic and neutrophilic inflammatory illness.8–11 In general, ACO has been defined as increased variability of airflow in association with an incompletely reversible airway obstruction, a symptomatic description that could be representative of many underlying physiologies.12
To date, research has less often focused on individuals whose illness classification lies in between COPD and asthma. Most randomized controlled trials focusing on COPD and asthma have included only those participants who had clearly defined cases of disease, failing to represent the breadth of respiratory disease that exists in populations.13–16 ACO refers to people who fail to fall into these strictly defined sets.
The only known population-based studies of ACO have been performed in high-income settings (Korea, Italy, Norway, the Netherlands) or in metropolitan areas of low- and middle-income countries (LMICs), such as the PLATINO study.17–22 Little is known about ACO in other resource-poor settings. Because ACO may represent the confluence of eosinophilic and neutrophilic inflammation, risk factors unique to LMICs—unplanned urbanization, biomass fuel exposure, and increasing tobacco use—need to be examined to understand the driving forces behind ACO in LMICs.23–26 With these factors in mind, the potential exists for there to be a significant population of undiagnosed ACO in the low- and middle-income world.
A recent workshop led by the American Thoracic Society and the United States National Heart, Lung, and Blood Institute called for further epidemiological investigation of ACO.27 We hypothesize that due to exposures common to LMIC settings, ACO will be comparably prevalent to high-income areas. To that aim, we characterize ACO in a population-based sample of adults from multiple LMICs, which represent a broad range of geographical settings including wide degrees of urbanization and ethnic diversity. In addition, we compare individuals with ACO to those without respiratory disease, those with asthma-only, and those with COPD-only, examining risk factors and lifestyle outcomes between groups.
METHODS
Study setting
We compiled data for this analysis from 4 population-based studies conducted in LMICs under the sponsorship of the United States National Institutes of Health. These included the CRONICAS Cohort Study in Peru,28 the Pulmonary Risk in South America (PRISA) study in Argentina, Chile, and Uruguay,29 a longitudinal study in Bangladesh,30 and the Lung Function in Nakaseke and Kampala (LiNK) study in Uganda.31 In total, the data represent 12 sites of varying geography and socioeconomic status in 6 different LMICs.
Study design
Multistage age- and sex-stratified random sampling was used in the PRISA and CRONICAS studies, whereas the Bangladesh study used simple random sampling. The LiNK study used population proportional to size sampling, as outlined by the World Health Organization.32,33 Recruitment began in 2010 for the CRONICAS Cohort Study, in 2011 for the PRISA study and the Bangladesh study, and in 2015 for the LiNK study. The lower age cutoffs were 35 years for the LiNK and CRONICAS studies and 40 years for the Bangladesh study with no upper limit, whereas the PRISA study included participants aged 45 to 75 years. Each study required residency of the area and the ability to give informed consent. Common exclusion criteria included pregnancy, active pulmonary tuberculosis, recent myocardial infarction, and recent surgery of the chest, lungs, heart, or eyes. Each study limited enrollment to 1 person per household. Field workers completed confidentiality training, and informed consent was collected for all research participants. Each study obtained research approval from their local and international internal review boards.
Field workers performed spirometry following American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines using flow-based, portable Easy-On-PC spirometers (CRONICAS and LiNK studies) and EasyOne spirometers (Bangladesh and PRISA studies), both of which use the same technology developed by ndd Medical Technologies (Zurich, Switzerland).34 Lung function was recorded as forced vital capacity (FVC), FEV1, and FEV1/FVC ratio. These measures were transformed into z scores on the basis of Global Lung Function Initiative mixed-ethnic reference population.35 Postbronchodilator spirometry was conducted 15 minutes after inhaled salbutamol in the Bangladesh, LiNK, and CRONICAS studies, whereas albuterol was used in the PRISA study. CRONICAS and PRISA studies conducted postbronchodilator assessment on all participants, whereas the LiNK study and the Bangladesh study conducted postbronchodilator testing only on those participants who were obstructed following initial testing. Specific methods for collection of other data are outlined in previous publications.28–31
Outcome measures
We defined COPD as a postbronchodilator FEV1/FVC ratio below the lower limit of normal (defined as the lowest 5th percentile) of the Global Lung Function Initiative mixed-ethnic reference population; asthma as fulfilling 1 of 3 criteria: self-report of wheezing in 1 year, self-report of medication use for asthma in 1 year, or self-report of a physician diagnosis of asthma; and ACO as the presence of both conditions.20,21,36–38 On the basis of these criteria, participants were then categorized into 4 groups: participants without asthma or COPD (nonobstructed nonasthma), participants with asthma and no COPD (asthma-only), participants with COPD and no asthma (COPD-only), and participants with both (ACO).
We defined hospitalization as self-report of hospitalization for primarily pulmonary reason and lifestyle impairment as self-report of impairment with daily activities due to breathing, both over the last year. Percent of reversibility was defined as % (post-bronchodilator FEV1 [or FVC] - prebronchodilator FEV1 [or FVC]) /prebronchodilator FEV1 [or FVC]). Bronchodilator reversibility (as a dichotomous outcome) was defined as greater than or equal to 12% improvement and greater than or equal to 200 mL increase in FEV1 or FVC according to ATS/ERS criteria.39 Severe obstruction on spirometry was defined as an FEV1 less than 50% of predicted and a FEV1/FVC ratio below the lower limit of normal based on reference values.40
Risk factors
We defined low education as having completed primary schooling or less. We defined exposure to biomass fuel smoke as belonging to a household that burns natural solid fuels (wood, charcoal, crop waste, or similar) for heating or cooking. Tobacco exposure history was quantified by pack-years of smoking. We defined 1 pack-year as having smoked, on average, 20 cigarettes per day for 1 year. We defined history of tuberculosis as self-report of posttreatment pulmonary tuberculosis. We calculated body mass index (BMI) as weight/height2 (kg/m2) and defined obesity as greater than or equal to 30 kg/m2.
Biostatistical methods
Our primary aim was to estimate the prevalence of ACO and describe its risk factors. To test for differences between sites and respiratory health groups, categorical and continuous data were compared via chi-square and ANOVA tests, respectively. Because of the clustered nature of our data, we used multivariable alternating logistic regression for binary outcomes and mixed effects models with random intercepts for continuous outcomes to account for correlation within sites. Alternating logistic regression is a variant of generalized estimating equations where the association between participants for a particular site is modeled with log odds ratios (ORs) instead of correlations.41 These were used to characterize ACO against asthma-only, COPD-only, nonobstructed nonasthma, and general non-ACO groups. For risk factors, all multivariable models for ACO were adjusted by age, sex, BMI, education, biomass exposure, and pack-years of smoking. For lifestyle outcomes, the selected measure was modeled as the independent variable while respiratory health status was included with the same set of predictors to reduce confounding. Interquartile ORs compare the odds of having the outcome at the 75th percentile of the variable range to the odds at the 25th percentile. We also calculated mean percent of reversibility and the proportion fulfilling ATS/ERS reversibility criteria for each respiratory health group, as well as their corresponding 95% CIs. Sensitivity analyses for spirometric outcomes were conducted among those studies that included postbronchodilator spirometry for all participants (n = 7277). Further sensitivity analyses were conducted according to other studies’ definitions of COPD, asthma, and ACO. Analyses were performed in STATA 13 (StataCorp, College Station, Tex) and R (www.r-project.org).
RESULTS
Participant characteristics
The 4 studies contributed a total of 12,435 participants, 11,923 (95.9%) of whom were included in this analysis. Of those excluded, 13 were missing basic demographic information, an additional 47 fell outside the spirometry reference or individual study inclusion criteria for age, and a final 452 were missing data required to classify respiratory health status. Participants excluded from this analysis were younger, more likely to be female, less likely to be obese, more likely to be exposed to biomass fuel smoke, had fewer pack-years of smoking history, and had lower education than those who remained (see Table E1 in this article’s Online Repository at www.jacionline.org). A summary of participants’ demographic characteristics can be found in Table I.
TABLE I.
Comparison of demographic factors between field sites*
| Demographic characteristic: % (n) or median (IQR) | Lima (8.4%; n = 997) |
Tumbes (7.9%; n = 946) |
Urban Puno (4.2%; n = 503) |
Rural Puno (4.2%; n = 503) |
Marcos Paz (10.4%; n = 1,240) |
Bariloche (9.2%; n = 1,094) |
Temuco (8.9%; n = 5 1,059) |
Canelones (7.8%; n = 5 935) |
Dhaka (14.6%; n = 5 1,746) |
Matlab (15.3%; n = 5 1,829) |
Kampala (4.2%; n = 5 498) |
Nakaseke (4.8%; n = 5 573) |
Overall (n = 5 11,923) |
P value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | 54.7 (45.3–63.7) | 54.7 (44.6–64.3) | 54.8 (44.9–64.4) | 54.8 (45.1–63.9) | 58.4 (51.7–65.4) | 56.6 (51.0–63.5) | 51.6 (58.8–66.1) | 59.1 (52.3–66.4) | 50 (44–58) | 53 (47–63) | 41.1 (36.8–48.1) | 46.8 (40.3–56.2) | 54.2 (47–63.0) | <.001 |
| Female | 50.7% (505) | 49.8% (471) | 50.5% (254) | 52.7% (265) | 40.1% (497) | 39.0% (427) | 45.4% (481) | 40.0% (374) | 54.7% (955) | 53.9% (986) | 50.2% (250) | 52.7% (302) | 48.4% (5,767) | <.001 |
| BMI (kg/m2) | 28.0 (25.3–31.1) | 27.9 (25.1–30.8) | 27.5 (25.1–30.2) | 24.7 (22.5–27.5) | 29.5 (26.1–33.4) | 28.3 (24.9–32.2) | 28.7 (26.0–31.5) | 29.0 (25.5–32.6) | 24.4 (21.3–27.4) | 20.1 (18.1–22.9) | 25.0 (22.0–29.4) | 22.8 (20.6–26.0) | 26.2 (22.4–30.0) | <.001 |
| Obese (BMI ≥30 kg/m2) | 32.4% (323) | 31.5% (298) | 26.4% (133) | 10.7% (54) | 46.5% (576) | 37.8% (414) | 36.5% (386) | 42.4% (396) | 12.5% (218) | 1.6% (29) | 21.5% (107) | 9.8% (56) | 25.1% (2,990) | <.001 |
| Pack-years smoking | 0 (0–0.2) | 0 (0–0.3) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–9.2) | 0 (0–9.5) | 0 (0–0) | 0 (0–0) | 0 (0–0.24) | <.001 |
| Daily smoker | 3.2% (32) | 5.6% (53) | 2.2% (11) | 0.2% (1) | 22.8% (283) | 23.6% (258) | 15.2% (161) | 21.8% (204) | 6.2% (109) | 9.0% (165) | 8.6% (43) | 8.0% (46) | 11.5% (1,366) | <.001 |
| Household using biomass | 2.4% (24) | 16.7% (158) | 2.0% (10) | 95.2% (479) | 0.5% (6) | 23.2% (253) | 22.3% (231) | 5.1% (43) | 3.7% (61) | 98.1% (1,789) | 93.1% (461) | 99.7% (570) | 34.9% (4,085) | <.001 |
| Primary education or less | 54.2% (540) | 64.0% (605) | 34.8% (175) | 66.6% (335) | 65.7% (814) | 50.1% (547) | 29.3% (310) | 50.0% (466) | 56.0% (978) | 81.2% (1,485) | 79.1% (394) | 92.5% (530) | 60.2% (7,179) | <.001 |
IQR, Interquartile range.
P value refers to inequality of metric across all site groups based on ANOVA or χ2 test.
Prevalence of ACO and associated risk factors
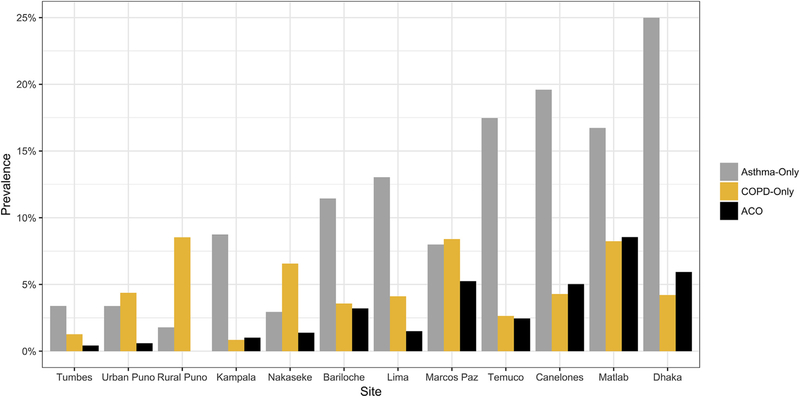
For the full sample, we found a 3.8% (95% CI, 3.4%−4.1%) prevalence of ACO, which varied from 0% in Rural Puno, Peru, to 7.8% in Matlab, Bangladesh (Fig 1; see Table E2 in this article’s Online Repository at www.jacionline.org). Participants with ACO were older, less likely to be female or obese, more likely to smoke, more likely to be exposed to biomass fuel smoke, and had less education than the nonobstructed nonasthma or asthma-only population (Table II). Nearly half (43.8%) of those with COPD and 21.7% of those with asthma had ACO.
FIG 1.
Health status by site. Because ACO is defined in this analysis as a combination of fulfilling the criteria for both asthma and COPD, categories are not discrete; an individual may fall into only 1 category. The prevalence of asthma and COPD as separate conditions (along with ACO) can be found in Table E2.
TABLE II.
Comparison of demographic factors between disease statuses*
| Demographic characteristic: % (n) or median (IQR) | Nonobstructed nonasthma (77.8%; n = 9274) |
Asthma-only (13.6%; n = 1622) |
COPD-only (4.8%; n = 577) |
ACO (3.8%; n = 450) |
P value |
|---|---|---|---|---|---|
| Age (y) | 53.9 (46.7–62.9) | 53.9 (46.8–62) | 59 (51–66.9) | 58 (51.6–66) | <.001 |
| Female | 49.2% (4561) | 49.6% (804) | 37.1% (214) | 41.8% (188) | <.001 |
| BMI (kg/m2) | 26.4 (22.8–30.1) | 26.6 (22.5–30.6) | 23.4 (19.3–27.6) | 23.1 (19.0–28.5) | <.001 |
| Obese (BMI ≥30 kg/m2) | 25.5% (2465) | 28.9% (469) | 13.0% (75) | 18.0% (81) | <.001 |
| Pack-years smoking | 0 (0–0) | 0 (0–2.8) | 0.3 (0–19.7) | 0 (0–20.3) | <.001 |
| Daily smoker | 9.8% (904) | 13.6% (220) | 22.9% (132) | 24.4% (110) | <.001 |
| Household using biomass fuels | 34.5% (3148) | 31.3% (492) | 44.7% (257) | 42.7% (188) | <.001 |
| Primary education or less | 59.5% (5513) | 59.3% (961) | 67.1% (387) | 70.7% (318) | <.001 |
IQR, Interquartile range.
P value refers to inequality of metric across all disease status groups from ANOVA or χ2 test.
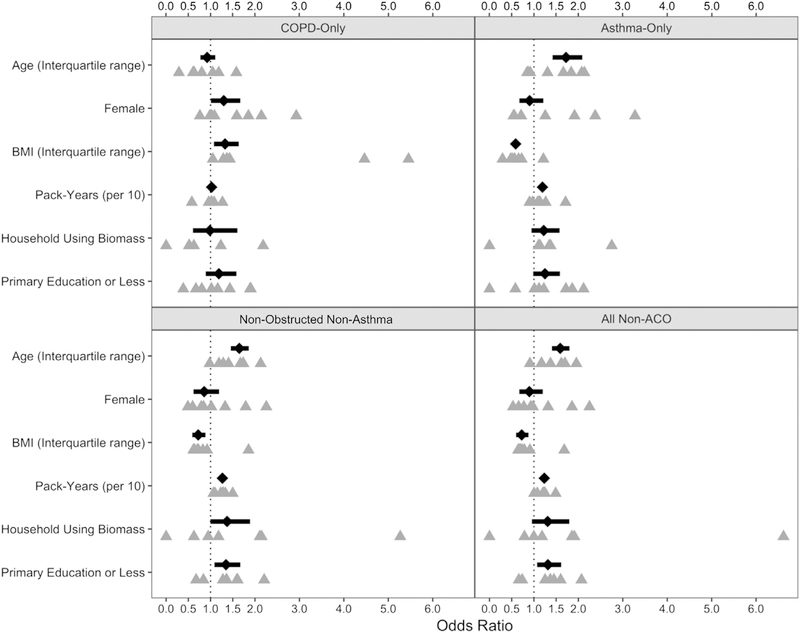
Risk factors for ACO compared with those for the nonobstructed nonasthma, asthma-only, COPD-only, and general non-ACO population are outlined in Fig 2 and Table E3 (in this article’s Online Repository at www.jacionline.org). Age, pack-years smoking, low education, and biomass exposure were all risk factors for ACO compared with the asthma-only, nonobstructed nonasthma, and general population groups, whereas higher BMI was protective. Higher BMI was the only risk factor for ACO when compared with COPD. The prevalence of ACO was higher with older age: 1.1% (95% CI, 0.8%−1.7%) for those younger than 45 years, 3.9% (95% CI, 3.4%−4.4%) for those aged 45 to 59 years, 4.9% (95% CI, 4.2%−5.7%) for those aged 60 to 74 years, and 5.7% (3.9%−8.3%) for those aged 75 years or older (see Fig E1 in this article’s Online Repository at www.jacionline.org).
FIG 2.
Forest plot of risk factors for ACO. Risk factors are presented as ORs for presence of ACO compared with reference populations represented by each panel (ACO vs COPD-Only, ACO vs Asthma-Only, ACO vs Nonobstructed nonasthma, and ACO vs all without ACO). ORs are represented by black diamonds while the 95% CI is represented by horizontal bars. Site-specific estimates are represented by gray triangles directly under the overall estimate. Overall estimates were generated via alternating logistic regressions, which accounted for clustering by site. All models included each risk factor presented here.
Lifestyle impairment and ACO
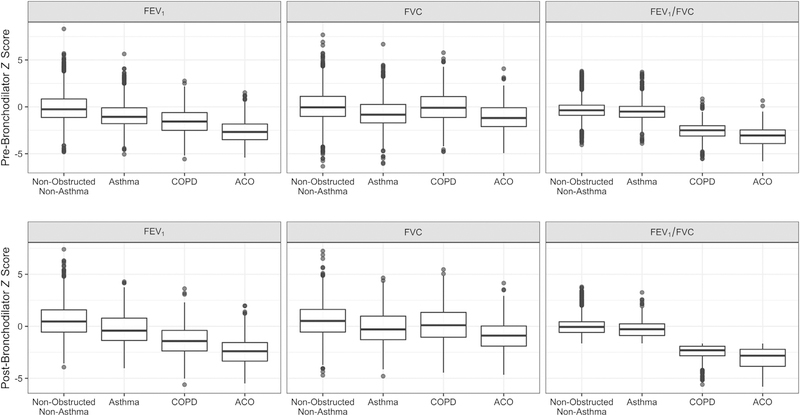
Measures of lifestyle impairment for participants with ACO can also be seen in Table E3. In unadjusted comparisons, 6.6% (95% CI, 4.6%−9.3%) of participants with ACO reported being hospitalized for breathing difficulty in 1 year compared with 1.9% (95% CI, 1.3%−2.7%) of those with asthma-only and 0.5% (95% CI, 0.2%−1.6%) of those with COPD-only. Among those with ACO, 44.7% (95% CI, 40.1%−49.3%) reported impairment of daily activities due to breathing compared with 29.6% (95% CI, 27.4%−31.9%) of those with asthma-only and 8.1% (95% CI, 6.2%−10.7%) of those with COPD-only. The odds ratio for hospitalization was higher for participants with ACO (OR, 32.4; 95% CI, 17.3–60.8) than for those with COPD-only (OR, 1.62; 95% CI, 0.47–5.63) or with asthma-only (OR, 8.30; 95% CI, 4.75–14.5) when compared to nonobstructed nonasthma participants. Similarly, the odds ratio for life impairment was higher for participants with ACO (OR, 21.8; 95% CI, 16.2–29.3) than for those with COPD-only (OR, 1.46; 95% CI, 1.02–2.11) or with asthma-only (OR, 7.29; 95% CI, 6.13–8.67) when compared to nonobstructed nonasthma participants. The ACO group had a prebronchodilator FEV1/FVC ratio that was approximately 2.5 z scores lower than the nonobstructed nonasthma, asthma-only, and general non-ACO groups, and 0.61 z scores lower than the COPD-only group (Fig 3; see Table E3). The difference in FEV1/FVC ratio z score between the ACO group and the nonobstructed nonasthma population was higher than the difference between COPD-only (−2.03; 95% CI, −2.11 to −1.95) and asthma-only (−0.24; 95% CI, −0.29 to −0.19) subsets compared with the same. Sensitivity testing with postbronchodilator z scores (n = 7277) revealed very similar results (see Table E3).
FIG 3.
Box plots of prebronchodilator and postbronchodilator spirometry z scores by disease status. The top and bottom of the box represent the 75th and 25th percentile values of the distribution, respectively, while the center line represents the median. The circles on the top and bottom represent outlying values. Z scores were calculated on the basis of Global Lung Function Initiative mixed-ethnic reference population and are presented here unadjusted. FVC refers to the maximum total volume of air exhaled. FEV1/FVC refers to their ratio. FEV1 and FVC may come from different qualifying spirometry trials.
Severity of obstruction and reversibility in ACO
Among those with ACO, 31.6% (95% CI, 27.4%−36.0%) of participants had severe obstruction (an FEV1 < 50% of predicted) on prebronchodilator spirometry compared with 10.9% (95% CI, 8.6%−13.7%) of those with COPD-only. Comparatively, 3.5% (95% CI, 2.7%−4.5%) of those with asthma-only had an FEV1 <50% of predicted. In adjusted models of prebronchodilator FEV1/FVC ratio among the full sample, both age and disease status remained independently associated factors (see Table E4 in this article’s Online Repository at www.jacionline.org).
When using postbronchodilator spirometry, 23.0% (95% CI, 17.6%−29.4%) of those with ACO had severe obstruction (FEV1 <50% of predicted) after administration of a bronchodilator compared with 5.8% (95% CI, 3.7%−8.9%) of those with COPD-only. A total of 0.4% (95% CI, 0.1%−1.2%) of those with asthma-only had an FEV1 <50% of predicted. The average percent reversibility of FEV1 was 10.8% (95% CI, 8.6%−13.0%) for ACO, 5.6% (95% CI, 4.4%−6.7%) for COPD-only, and 4.3% (95% CI, 3.8%−4.8%) for the asthma-only group. A total of 37.8% (95% CI, 31.2%−44.8%) of participants with ACO met reversibility criteria compared with 22.5% (95% CI, 18.3%-%) of those with COPD-only and 10.8% (95% CI, 8.8%−13.1%) of those with asthma-only.
DISCUSSION
In this multistudy analysis, we estimated the prevalence of ACO in a sample of nearly 12,000 adults in LMICs and compared risk factors and lifestyle impairment outcomes between participants with ACO and those with asthma-only or COPD-only. Although comparisons with previous studies are difficult due to variable case definitions and differing reference populations, our data indicate that there may be a high prevalence of ACO found in LMICs: settings that are known to include environmental risk factors strongly linked to asthma and COPD, such as biomass fuel smoke exposure, rapid urbanization, increasing tobacco smoking, and periurban sprawl.23,25,42
There are limited data on ACO prevalence and risk factors in LMICs. In a 2014 analysis of the PLATINO study cohort, Menezes et al20 report a 1.8% prevalence of ACO over 5 Latin and South American sites; however, only urban centers were sampled and a more restrictive definition of asthma was used. Using the same criteria among studies that conducted universal postbronchodilator spirometry, we found a prevalence of ACO of 0.71% (n = 6916), although it should be noted that the prevalence of asthma would be expected to be higher in urban settings.25 An analysis of the large European Community Respiratory Health Survey in Europe reported ACO in 3.1% of participants.19 It is notable that this population was younger when compared with those most at risk for COPD and ACO (mean age, 34.3 years) but located in high-income settings, which increased the likelihood of ever having had an asthma diagnosis by a physician.19 Our data are consistent with a previous analysis of the general population of Italy, which found a prevalence of ACO with older age.18 In our analysis, 43.8% of participants with COPD had ACO, which is higher than previous estimates of 13% to 20% from high-income populations, barring Norway, which stands out at 56% using self-report criteria.22,43–47
Our participants with ACO were of similar age to those with COPD-only and were around 4 years older than those with asthma-only, results that have varied in previous research.19,20,47,48 In agreement with Menezes et al20 and Chung et al,21 our ACO and COPD populations had a lower BMI than did nonobstructed nonasthma or asthma-only participants.36,48 Women comprised a higher percentage of our ACO population than the COPD-only group, but less than the asthma-only or nonobstructed nonasthma group, in line with other population-based studies.19–21
Many studies have characterized ACO by clinical or quality-of-life outcomes, often describing participants with overlap as more disabled than those with either asthma or COPD alone.18,20,21,36,47,49–51 In our sample, the ACO population was also associated with greater reductions in spirometric outcomes and higher odds of hospitalization or impairment of daily activities compared with the asthma-only and COPD-only populations, seen in some but not all previous studies.19–21,36,47,51,52 Moreover, the presence of both asthma and COPD in a participant (the overlap group) was associated with worse outcomes than would be expected by combining the effects of asthma and COPD alone, an effect not seen in an analysis of high-income countries by de Marco et al.19 For example, the ACO group had worse lung function by prebronchodilator FEV1/FVC than expected when adding the deficits associated with having COPD-only and asthma-only compared with the nonobstructed nonasthma population. This remained the case for postbronchodilator testing. Our data agree with the pattern of higher prevalence of severe obstruction on spirometry in ACO compared with asthma- or COPD-only populations noted by Chung et al; however, the prevalence in our sample of LMIC settings was much higher than in their South Korean sample (31.8% vs 12%).21 It should be noted that these estimates were produced via different reference populations, making for an imperfect comparison.
It is known that cross-sectional lung function is lower in older ages and because of our finding of an association between age and ACO prevalence, we investigated the possibility of confounding. We conducted a sensitivity analysis whereby lung function was modeled on both disease status (categorical) and age (continuous) among other factors noted above. We found that both remained independently associated factors and no confounding was seen. Our finding of a 0.18-unit decline in FEV1/FVC ratio per year falls closely in line with previously published reference values.53
As an observation, we call for the consideration of household air pollution as an environmental exposure in future case definitions of ACO. Some publications, including a recent Spanish consensus document, have included a history of tobacco smoking as a criterion for the diagnosis of ACO.19,54–56 In many settings, household air pollution is a prevalent and important risk factor of chronic respiratory illness, and its population-attributable risk may be higher than that of tobacco smoking.57 Golpe et al58 concluded that exposure to biomass fuel smoke raises systemic inflammation in a manner similar to tobacco smoking. Other studies have shown raised pulmonary inflammation profiles due to biomass fuel smoke exposure.59,60 Our analysis shows both tobacco smoking and biomass fuel smoke exposure to be risk factors for the development of ACO compared with nonobstructed nonasthma populations. We favor inclusive criteria such as those found in the 2015 GINA/GOLD guidelines—which take into account exposures to any respiratory risk factor—as a more globally focused set of guidelines.61
Strengths of this analysis include a large and demographically diverse sample of adults living in LMICs across the southern hemisphere, benefitting from high-quality data collection and spirometry. The diversity of our sample, which covered a wide range of ages (35–92 years) and settings (rural areas and cities of all sizes on 3 continents), leads us to believe that our results are generalizable to other LMIC settings. However, this analysis has some potential shortcomings. First, we presented prebronchodilator spirometry testing for our main analysis of lung function outcomes because postbronchodilator measurements were not available across the entire study sample. This allowed us to analyze a larger sample and potentially increase the heterogeneity among participants. Sensitivity testing with a smaller sample of postbronchodilator tests validated those results, although it should be noted that the studies that provided bronchodilators to every participant were both located in South America and, therefore, results from our sensitivity analyses should be compared with the full cohort with care. Second, because of the challenges in collating data across different studies, we were also limited in our ability to investigate asthma-specific risk factors as well as a wider variety of health outcomes, such as quality-of-life questionnaires. In addition, among studies over the past decade, the prevalence and characteristics of ACO have varied, in large part because of variable case definitions.17 Our definition of ACO was limited by an inability to optimally diagnose asthma as outlined by the Global Initiative for Asthma.62 It is not feasible for studies of this nature to review medical records or even collect postbronchodilator reversibility on every participant. However, our strategy of combining appropriate and available indicators across studies to identify probable asthma cases (such as self-report of wheeze and past physician diagnosis of asthma) has been used in past large-scale studies of ACO.20,21,36 As opposed to those studies, which used a fixed FEV1/FVC ratio cutoff of 0.7 to diagnose COPD, we opted to define COPD as an FEV1/FVC ratio below the lower limit of normal based on the Global Lung Function Initiative mixed-ethnic reference population. Finally, although an established international reference population was used for this analysis, it cannot function as the best reference for our entire, diverse study sample. As with variable case definitions, the use of different reference populations across studies makes direct comparisons between our analysis and existing literature difficult. However, until validated references exist for each population, we decided it was a reasonable option.
In the first large-scale, population-based analysis of ACO in diverse areas of LMICs, we found a high prevalence of severe respiratory disease—marked by increased hospitalization and deficits in lung function—that varied by site and was higher with older age. Importantly, we found an increased proportion of those with asthma or COPD who show features consistent with overlap and a high rate of severe obstruction among those with ACO. Thus, the results of this analysis indicate that ACO may be as prevalent and the associated respiratory deficits more severe in LMICs, which are experiencing the simultaneous burdens of household air pollution exposure and high rates of urbanization and sprawl, than in high-income countries. Research should focus on more accurately defining ACO or the underlying continuum of respiratory illness that it represents. In addition, important exposures common in LMICs, such as biomass fuel use, should be considered for diagnostic criteria.
Supplementary Material
Clinical implications:
Because of risk factors common to low- and middle-income countries, such as rapid urbanization and biomass exposure, asthma-COPD overlap may be as common and more severe than in high-income settings.
Acknowledgments
The Pulmonary Risk in South America Study was sponsored and funded by the National Heart, Lung, and Blood Institute (NHLBI), a division of the National Institutes of Health (NIH) in the United States (contract no. 268200900029C). The CRONICAS study was supported by the NHLBI (contract no. HHSN268200900033C). The Lung Function in Nakaseke and Kampala study was supported in part by the Fogarty International Center (grant no. 5R25TW009340) and by a COPD Discovery Award from Johns Hopkins University. W.C. is supported in part by the NIH (grant no. UM1HL134590). T.S. was supported by a National Research Service Award through the National Institute of Environmental Health Sciences of the NIH (grant no. 1F32ES028577). A.R. was supported by the NIH Office of the Director, Fogarty International Center, and the NHLBI through the International Clinical Research Fellows Program at Vanderbilt University (grant no. R24 TW007988) and the American Relief and Recovery Act.
We express appreciation for the efforts of our field staff and thank all the participants of our studies for their contributions.
Abbreviations used
- ACO
Asthma-COPD overlap
- ATS/ERS
American Thoracic Society/European Respiratory Society
- BMI
Body mass index
- COPD
Chronic obstructive pulmonary disease
- FVC
Forced vital capacity
- LiNK
Lung Function in Nakaseke and Kampala
- LMICs
Low- and middle-income countries
- OR
Odds ratio
- PRISA
Pulmonary Risk in South America
Footnotes
Disclosure of potential conflict of interest: R. A. Wise reports grants and/or personal fees from AstraZeneca/Medimmune, Boehringer Ingelheim, Contrafect, GlaxoSmith-Kline, Pfizer, Pulmonx, Roche, Spiration, Sunovion, Teva, Pearl Therapeutics, Merck, and Bonti outside the submitted work. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Braman SS. The global burden of asthma. Chest 2006;130:4S–12S. [DOI] [PubMed] [Google Scholar]
- 2.Chan-Yeung M, Ait-Khaled N, White N, Ip MS, Tan WC. The burden and impact of COPD in Asia and Africa. Int J Tuberc Lung Dis 2004;8:2–14. [PubMed] [Google Scholar]
- 3.The Global Asthma Report 2014. Auckland, New Zealand: Global Asthma Network; 2014. [Google Scholar]
- 4.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223. [DOI] [PubMed] [Google Scholar]
- 5.Leung JM, Sin DD. Asthma-COPD overlap syndrome: pathogenesis, clinical features, and therapeutic targets. BMJ 2017;358:j3772. [DOI] [PubMed] [Google Scholar]
- 6.MacNee W Pathology, pathogenesis, and pathophysiology. BMJ 2006;332: 1202–4. [Google Scholar]
- 7.Hogg JC. Pathology of asthma. J Allergy Clin Immunol 1993;92:1–5. [DOI] [PubMed] [Google Scholar]
- 8.Zeki AA, Schivo M, Chan A, Albertson TE, Louie S. The asthma-COPD overlap syndrome: a common clinical problem in the elderly. J Allergy (Cairo) 2011; 2011:861926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orie NG, Sluiter HJ, De Vries K, Tammeling GJ, Witkop J. The host factor in bronchitis In: Orie NG, Sluiter HJ, editors. Bronchitis. Assen: Royal van Gorcum; 1961. pp. 43–59. [Google Scholar]
- 10.Putcha N, Wise RA. Asthma-chronic obstructive pulmonary disease overlap syndrome: nothing new under the sun. Immunol Allergy Clin North Am 2016;36: 515–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977;1:1645–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax 2009;64:728–35. [DOI] [PubMed] [Google Scholar]
- 13.Travers J, Marsh S, Caldwell B, Williams M, Aldington S, Weatherall M, et al. External validity of randomized controlled trials in COPD. Respir Med 2007; 101:1313–20. [DOI] [PubMed] [Google Scholar]
- 14.Postma DS, van den Berge M. The different faces of the asthma-COPD overlap syndrome. Eur Respir J 2015;46:587–90. [DOI] [PubMed] [Google Scholar]
- 15.Abramson MJ, Perret JL, Dharmage SC, McDonald VM, McDonald CF. Distinguishing adult-onset asthma from COPD: a review and a new approach. Int J Chron Obstruct Pulmon Dis 2014;9:945–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabbri LM, Romagnoli M, Corbetta L, Casoni G, Busljetic K, Turato G, et al. Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;167:418–24. [DOI] [PubMed] [Google Scholar]
- 17.Bonten TN, Kasteleyn MJ, de Mutsert R, Hiemstra PS, Rosendaal FR, Chavannes NH, et al. Defining asthma-COPD overlap syndrome: a population-based study. Eur Respir J 2017;49:pii:1602008. [DOI] [PubMed] [Google Scholar]
- 18.de Marco R, Pesce G, Marcon A, Accordini S, Antonicelli L, Bugiani M, et al. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): prevalence and risk factors in young, middle-aged and elderly people from the general population. PLoS One 2013;8:e62985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Marco R, Marcon A, Rossi A, Anto JM, Cerveri I, Gislason T, et al. Asthma, COPD and overlap syndrome: a longitudinal study in young European adults. Eur Respir J 2015;46:671–9. [DOI] [PubMed] [Google Scholar]
- 20.Menezes AM, Montes de Oca M, Perez-Padilla R, Nadeau G, Wehrmeister FC, Lopez-Varela MV, et al. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest 2014;145:297–304. [DOI] [PubMed] [Google Scholar]
- 21.Chung JW, Kong KA, Lee JH, Lee SJ, Ryu YJ, Chang JH. Characteristics and self-rated health of overlap syndrome. Int J Chron Obstruct Pulmon Dis 2014; 9:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henriksen AH, Langhammer A, Steinshamn S, Mai XM, Brumpton BM. The prevalence and symptom profile of asthma-COPD overlap: the HUNT study. COPD 2018;15:27–35. [DOI] [PubMed] [Google Scholar]
- 23.Wjst M, Boakye D. Asthma in Africa. PLoS Med 2007;4:e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997;349:1498–504. [DOI] [PubMed] [Google Scholar]
- 25.Robinson CL, Baumann LM, Romero K, Combe JM, Gomez A, Gilman RH, et al. Effect of urbanisation on asthma, allergy and airways inflammation in a developing country setting. Thorax 2011;66:1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baumann LM, Robinson CL, Combe JM, Gomez A, Romero K, Gilman RH, et al. Effects of distance from a heavily transited avenue on asthma and atopy in a periurban shantytown in Lima, Peru. J Allergy Clin Immunol 2011;127: 875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodruff PG, van den Berge M, Boucher RC, Brightling C, Burchard EG, Christenson SA, et al. American Thoracic Society/National Heart, Lung, and Blood Institute Asthma-Chronic Obstructive Pulmonary Disease Overlap Workshop Report. Am J Respir Crit Care Med 2017;196:375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miranda JJ, Bernabe-Ortiz A, Smeeth L, Gilman RH, Checkley W, Group CCS. Addressing geographical variation in the progression of non-communicable diseases in Peru: the CRONICAS cohort study protocol. BMJ Open 2012;2:e000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubinstein AL Irazola VE, Bazzano LA, Sobrino E, Calandrelli M, Lanas F, et al. Detection and follow-up of chronic obstructive pulmonary disease (COPD) and risk factors in the Southern Cone of Latin America: the pulmonary risk in South America (PRISA) study. BMC Pulm Med 2011;11:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alam DS, Chowdhury MA, Siddiquee AT, Ahmed S, Clemens JD. Prevalence and determinants of chronic obstructive pulmonary disease (COPD) in Bangladesh. COPD 2015;12:658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siddharthan T, Grigsby M, Morgan B, Kalyesubula R, Wise RA, Kirenga B, et al. Chronic respiratory diseases and the urban divide: a population-based study examining prevalence and attributable risk factors for chronic respiratory disease in Uganda. Am J Respir Crit Care Med 2017;195:A1390. [Google Scholar]
- 32.Bostoen K, Chalabi Z. Optimization of household survey sampling without sample frames. Int J Epidemiol 2006;35:751–5. [DOI] [PubMed] [Google Scholar]
- 33.Chao LW, Szrek H, Peltzer K, Ramlagan S, Fleming P, Leite R, et al. A comparison of EPI sampling, probability sampling, and compact segment sampling methods for micro and small enterprises. J Dev Econ 2012;98:94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–38. [DOI] [PubMed] [Google Scholar]
- 35.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012;40:1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miravitlles M, Soriano JB, Ancochea J, Munoz L, Duran-Tauleria E, Sanchez G, et al. Characterisation of the overlap COPD-asthma phenotype: focus on physical activity and health status. Respir Med 2013;107:1053–60. [DOI] [PubMed] [Google Scholar]
- 37.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347–65. [DOI] [PubMed] [Google Scholar]
- 38.European Community Respiratory Health Survey. Variations in the prevalence of respiratory symptoms, self-reported asthma attacks and use of asthma medication in the European Community Respiratory Health Survey (ECRHS). Eur Respir J 1996;9:687–95. [DOI] [PubMed] [Google Scholar]
- 39.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948–68. [DOI] [PubMed] [Google Scholar]
- 40.Pocket guide to COPD diagnosis, management, and prevention: a guide for health care professionals. Global Initiative for Chronic Obstructive Lung Disease, Inc; 2017. Available at: http://www.goldcopd.org.
- 41.Carey V, Zeger SL, Diggle P. Modelling multivariate binary data with alternating logistic regressions. Biometrika 1993;80:517–26. [Google Scholar]
- 42.Hu G, Zhou Y, Tian J, Yao W, Li J, Li B, et al. Risk of COPD from exposure to biomass smoke: a metaanalysis. Chest 2010;138:20–31. [DOI] [PubMed] [Google Scholar]
- 43.Tamada T, Sugiura H, Takahashi T, Matsunaga K, Kimura K, Katsumata U, et al. Biomarker-based detection of asthma-COPD overlap syndrome in COPD populations. Int J Chron Obstruct Pulmon Dis 2015;10:2169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung WS, Lin CL, Kao CH. Comparison of acute respiratory events between asthma-COPD overlap syndrome and COPD patients: a population-based cohort study. Medicine (Baltimore) 2015;94:e755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hardin M, Silverman EK, Barr RG, Hansel NN, Schroeder JD, Make BJ, et al. The clinical features of the overlap between COPD and asthma. Respir Res 2011;12:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soriano JB, Davis KJ, Coleman B, Visick G, Mannino D, Pride NB. The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United Kingdom. Chest 2003;124:474–81. [DOI] [PubMed] [Google Scholar]
- 47.Kauppi P, Kupiainen H, Lindqvist A, Tammilehto L, Kilpelainen M, Kinnula VL, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma 2011;48:279–85. [DOI] [PubMed] [Google Scholar]
- 48.Hardin M, Cho M, McDonald ML, Beaty T, Ramsdell J, Bhatt S, et al. The clinical and genetic features of COPD-asthma overlap syndrome. Eur Respir J 2014; 44:341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaya FT, Dongyi D, Akazawa MO, Blanchette CM, Wang J, Mapel DW, et al. Burden of concomitant asthma and COPD in a Medicaid population. Chest 2008;134:14–9. [DOI] [PubMed] [Google Scholar]
- 50.Brzostek D, Kokot M. Asthma-chronic obstructive pulmonary disease overlap syndrome in Poland: findings of an epidemiological study. Postepy Dermatol Alergol 2014;31:372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milanese M, Di Marco F, Corsico AG, Rolla G, Sposato B, Chieco-Bianchi F, et al. Asthma control in elderly asthmatics: an Italian observational study. Respir Med 2014;108:1091–9. [DOI] [PubMed] [Google Scholar]
- 52.Barrecheguren M, Roman-Rodriguez M, Miravitlles M. Is a previous diagnosis of asthma a reliable criterion for asthma-COPD overlap syndrome in a patient with COPD? Int J Chron Obstruct Pulmon Dis 2015;10:1745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159:179–87. [DOI] [PubMed] [Google Scholar]
- 54.Mir Viladrich I, Dauden Tello E, Solano-Lopez G, Lopez Longo FJ, Taxonera Samso C, Sanchez Martinez P, et al. Consensus document on prevention and treatment of tuberculosis in patients for biological treatment [in English, Spanish]. Arch Bronconeumol 2016;52:36–45. [DOI] [PubMed] [Google Scholar]
- 55.Cataldo D, Corhay JL, Derom E, Louis R, Marchand E, Michils A, et al. A Belgian survey on the diagnosis of asthma-COPD overlap syndrome. Int J Chron Obstruct Pulmon Dis 2017;12:601–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slats A, Taube C. Asthma and chronic obstructive pulmonary disease overlap: asthmatic chronic obstructive pulmonary disease or chronic obstructive asthma? Ther Adv Respir Dis 2016;10:57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siddharthan T, Grigsby MR, Goodman D, Chowdhury M, Rubinstein A, Irazola V, et al. Association between household air pollution exposure and chronic obstructive pulmonary disease outcomes in 13 low- and middle-income country settings. Am J Respir Crit Care Med 2018;197:611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Golpe R, Martin-Robles I, Sanjuan-Lopez P, Perez-de-Llano L, Gonzalez-Juanatey C, Lopez-Campos JL, et al. Differences in systemic inflammation between cigarette and biomass smoke-induced COPD. Int J Chron Obstruct Pulmon Dis 2017;12:2639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guarnieri MJ, Diaz JV, Basu C, Diaz A, Pope D, Smith KR, et al. Effects of woodsmoke exposure on airway inflammation in rural Guatemalan women. PLoS One 2014;9:e88455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sussan TE, Ingole V, Kim JH, McCormick S, Negherbon J, Fallica J, et al. Source of biomass cooking fuel determines pulmonary response to household air pollution. Am J Respir Cell Mol Biol 2014;50:538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.GINA/GOLD. Diagnosis of diseases of chronic airflow limitation: asthma, COPD and asthma-COPD overlap syndrome (ACOS). 2015. Available at: http://www.ginasthma.org.
- 62.Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma; 2017. Available at: http://www.ginasthma.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.