Summary
Background
Non‐alcoholic fatty liver disease (NAFLD), prediabetes and type 2 diabetes mellitus are known to be closely linked with obesity as early as during childhood.
Objectives
The study aimed to determine the prevalence of prediabetes and T2DM in children with obesity with or without increased transaminases.
Methods
Data from the observational multicentre (n = 51), cross‐sectional Adipositas Patienten Verlaufsbeobachtung registry were analyzed. Mild increase (mild group) was defined by alanine transaminase (ALT) >24 to ≤50 U/L and moderate to severe increase (advanced group) by ALT > 50 U/L. Prediabetes and T2DM were defined according to recent IDF/ISPAD guidelines.
Results
The prevalence of prediabetes and T2DM was 11.9% (95% CI: 11.0–12.8) and 1.4% (95% CI: 1.1–1.7) among all participants (n = 4932; male = 2481; mean age 12.9 ± 2.7 years; BMI‐SDS 2.1 ± 0.5; Tanner stage 3.2 ± 1.5). The prevalence of impaired glucose metabolism (prediabetes and T2DM) was 13.8% (95% CI: 12.1–15.4) in the mild, 21.9% (95% CI: 18.8–25.1) in the advanced group, 10.7% (95% CI: 9.4–11.9) in the control group. Mild and advanced groups had greater odds ratios for prediabetes [1.42; 95% CI: 1.17–1.72, 2.26‐fold; (1.78–2.86), respectively], the advanced group also for T2DM [2.39 (1.36–4.21)] compared to controls. While an increase in transaminases predominantly affected boys, girls within the advanced group had a higher T2DM prevalence than males (5.4 vs. male 2.1%).
Conclusions
Children with obesity and increased liver transaminases as surrogates of NAFLD should be screened for T2DM.
Keywords: Blood glucose, childhood obesity, diabetes, impaired glucose metabolism, non‐alcoholic fatty liver disease, prediabetes
Abbreviations
- NAFLD
non‐alcoholic fatty liver disease
- T2DM
type 2 diabetes mellitus
- ALT
alanine amino transaminase
- US
United States
- NASH
non‐alcoholic steatohepatitis
- APV
Adipositas Patienten Verlaufsbeobachtung registry
- OGTT
oral glucose tolerance test
- min
minutes
- mild increase group
mild group
- IFG
impaired fasting glucose
- BMI
body mass index
- BMI‐SDS
BMI as standard deviation score
- HOMA‐IR
homeostatic model assessment for insulin resistance
- Matsuda‐ISI
insulin sensitivity index‐Matsuda
- INSAUC
area under the curve of insulin
- INSpeak
insulin peak
- HOMA‐SC
homeostatic model assessment for insulin secretion
- SD
standard deviation
- %
percentage
- χ2‐Test
chi‐square test
- OR
odds ratio
- CI
confidence interval
- NGT
normal glucose tolerance
- ULN
upper limit of the norm
1. INTRODUCTION
Non‐alcoholic fatty liver disease (NAFLD) has become the most common cause of chronic liver disease among children and adults, and is known to be closely linked to obesity, sedentary lifestyle and hypercaloric diets.1, 2 The severity of NAFLD ranges from isolated steatosis to steatohepatitis, fibrosis and cirrhosis.3 NAFLD is associated with several other metabolic comorbidities including dyslipidemia, hypertension and type 2 diabetes mellitus (T2DM).4 Ultimately, patients with NAFLD carry an increased risk for cardiovascular disease (5). In severe cases, hepatocellular carcinoma and liver failure might develop in children with obesity‐related NAFLD, requiring liver transplantation (5, 6).
In addition, a strong association between insulin resistance, hepatic steatosis and impaired glucose metabolism has been shown to be present already in youth (7). Even adolescents with merely moderately elevated alanine transaminase (ALT) values were shown to have deteriorations in lipid‐ and glucose metabolism (8). Providing the only reliable available epidemiological data so far, a recent study demonstrated a prevalence of impaired glucose metabolism among children with histologically diagnosed NAFLD of around 30% (6.5% T2DM) in the United States (US)9 as compared to only 5% of children without NAFLD (less than 1% T2DM) (10, 11). Children with T2DM were shown to have greater risk of having non‐alcoholic steatohepatitis (NASH), and thus greater long‐term‐risk for an adverse hepatic outcome.9
To date, there has been no study with sufficient sample size allowing an estimate of the prevalence of prediabetes and T2DM in children depending on the presence of NAFLD in European German‐speaking countries. There is a substantial difference in the prevalence and incidence of pediatric T2DM between certain ethno‐racial groups in the United States and cohorts from other regions worldwide.12 In addition, a recent meta‐analysis of adult data showed that the association between NAFLD and the risk of incident T2DM is stronger in some ethnic groups and that more severe forms of NAFLD seemed to be linked to an even greater risk of developing T2DM.13 Thus, the aim of this large European multicentre cross‐sectional study was to assess the prevalence of prediabetes and T2DM and in patients with varying degrees of increased transaminases as surrogates of NAFLD.
2. MATERIALS AND METHODS
2.1. The Adipositas Patienten Verlaufsbeobachtung registry
Data of the Adipositas Patienten Verlaufsbeobachtung (APV) registry were collected on the basis of German guidelines, defining how to diagnose and treat children and adolescents with overweight or obesity.14 Standardised longitudinal documentation of patients with obesity was managed by a computer software using the visual FoxPro 9.0 compiler. Participation in this study was only allowed for specialised obesity treatment centres. Collected data were anonymised and sent to the department of epidemiology at the University of Ulm for central analysis. The consortium of the APV registry has access to all data and warrants its integrity. Ethical as well as data management guidelines corresponded to local standards.
2.2. Selection of patients
This study included clinical and laboratory data collected in 51 centres in Germany, Austria and Switzerland from the APV database (http://www.a-p-v.de) (15). Between 2000 and 2018, 109.772 patient visits of patients with overweight or obesity were documented. Totally, 7620 underwent an oral glucose tolerance test (OGTT) and 4932 also had fasting blood samples taken for biochemical analysis. The following inclusion criteria of patients were applied: (i) age 2–20 years, (ii) OGTT with venous blood glucose levels at 0 and 120 min and (iii) assessment of liver enzymes within one month of OGTT. Exclusion criteria were (i) underweight, (ii) syndromal or endocrine obesity and (iii)) metformin treatment. OGTT‐derived measures of insulin sensitivity and secretion were analyzed in a subgroup of 1528 patients with complete glucose and insulin data sets.
2.3. Definition of non‐alcoholic fatty liver disease, prediabetes and type 2 diabetes mellitus
All participants were stratified based on their ALT value, as a surrogate for NAFLD, into (i) control group (ALT ≤ 24 U/L, n = 2506), (ii) mild increase group (mild group, ALT > 24 U/L – ≤ 50 U/L, n = 1760) and (iii) advanced group (ALT > 50 U/L, n = 666).16 Prediabetes (n = 586) was defined by impaired fasting glucose (IFG) with a fasting glucose value between 5.6 mmol/L and ≤ 7 mmol/L and/or a 120 min blood glucose level in OGTT ≥ 7.8 mmol/L and < 11.1 mmol/L (IGT). T2DM (n = 69) was defined by a fasting glucose value ≥7 mmol/L and/or a 120 min OGTT value ≥11.1 mmol/L and exclusion of other diabetes types by local clinical physician.17
2.4. Characterisation of weight status
Height and weight were assessed by standardised and calibrated scales and stadiometers wearing light clothing without shoes by trained staff. Normal weight was defined by a body mass index (BMI) smaller or equal to the 90th percentile, overweight above the 90th percentile and below or equal to the 97th percentile, obesity above the 97th percentile and less or equal to 99.7th percentile and morbid obesity above the 99.7th percentile, respectively, based on percentiles for German children and adolescents (18, 19), and BMI was also expressed as standard deviation score (BMI‐SDS).20
2.5. Characterisation of insulin sensitivity and secretion
Insulin sensitivity was characterised by homeostatic model assessment for insulin resistance (HOMA‐IR) and insulin sensitivity index‐Matsuda (MATSUDA‐ISI) (21, 22). Insulin secretion was described by area under the curve of insulin (INSAUC), insulin peak (INSpeak) and homeostatic model assessment for insulin secretion (HOMA‐SC) (22, 23).
2.6. Statistical methods
All statistical analyses were performed with SAS 9.2 (Statistical Analysis Software, SAS Institute Inc., Cary, NC, USA). A p‐value of <0.01 was considered as statistically significant. Continuous parameters were described by mean and standard deviation (SD) and binary variables by percentages (%). To show differences in mean, significance levels were tested using Wilcoxon tests for continuous variables and chi‐square tests (χ2‐test) for binominal data. Correlations were tested via Spearman´s correlation test. Multiple logistic regression models were used to identify the odds ratio (OR) and 95% Wald confidence interval (CI) of different NAFLD groups. OR was adjusted for sex, age and BMI‐SDS.
3. RESULTS
Characteristics of different transaminase groups in regard to pubertal stage, anthropometry, liver enzymes and measures of glucose metabolism (Table 1).
Table 1.
Characteristics of patients in different transaminase groups (controls, mild increased transaminases group, advanced group)
| Control group | Mild increase group | Advanced group | p‐Value* | p‐Value** | |
|---|---|---|---|---|---|
| Total (N) | 2506 | 1760 | 666 | ||
| Mean age (years) (Std. Dev.). | 12.7 (2.7) | 12.8 (2.7) | 13.6 (2.7) | .37 | <.01 |
| Male sex (%, 95% CI) | 39.5 (37.6–41.5) | 57.5 (55.2–59.8) | 72.1 (68.7–75.5) | <.01 | <.01 |
| BMI (kg/m2) (Std. Dev.) | 30.4 (6) | 32.3 (6.4) | 34.7 (7.4) | <.01 | <.01 |
| BMI‐SDS (Std. Dev.) | 2.0 (0.5) | 2.2 (0.5) | 2.3 (0.5) | <.01 | <.01 |
| Tanner stage (Std. Dev.) | 3.2 (1.6) | 3.1 (1.5) | 3.3 (1.5) | .26 | .66 |
| Normal weight (%, 95% CI) | 5.4 (4.5–6.3) | 2.4 (1.7–3.1) | 1.4 (0.5–2.2) | ||
| Overweight (%, 95% CI) | 30.0 (28.2–31.8) | 19.0 (17.1–20.8) | 13.8 (11.2–16.4) | ||
| Obesity (%, 95% CI) | 53.3 (51.3–55.2) | 59.3 (57.0–61.6) | 55.9 (52.1–59.6) | ||
| Extreme obesity (%, 95% CI) | 11.3 (0.1–12.6) | 19.3 (17.5–21.2) | 29.0 (25.5–32.4) | ||
| Fasting glucose (mmol/L) | 4.71 | 4.70 | 4.86 | .20 | <.01 |
| AST (U/L) (Std. Dev.) | 22.8 (8.7) | 29.5 (7.8) | 54.1 (59.0) | <.01 | <.01 |
| ALT (U/L) (Std. Dev.) | 17.4 (5.1) | 33.4 (7.0) | 88.0 (63.5) | <.01 | <.01 |
| GGT (U/L) (Std. Dev.) | 16.7 (10.5) | 22.9 (12.0) | 40.9 (46.3) | <.01 | <.01 |
| HOMA‐IR (Std. Dev.) | 3.7 (3.7) | 4.5 (3.3) | 5.9 (5.2) | ||
| MATSUDA‐ISI (Std. Dev.) | 2.6 (2.4) | 2.1 (1.7) | 1.7 (1.4) | ||
| INSAUC [(mass/volume) × time] (Std. Dev.) | 20 834.1 (17 613.3) | 21 605 (14 536.7) | 26 076.5 (17 956.2) | ||
| INSpeak (Um/L) (Std. Dev.) | 98.0 (172.9) | 115.2 ( 158.6) | 172.2 (196.1) | ||
| IGI (Std. Dev.) | −1.1 ( 150.1) | 3.2 (3.0) | 5.1 (24.6) | ||
| HOMA‐SC (Std. Dev.) | 0.7 ( 0.5) | 0.9 (0.5) | 1.1 (0.6) |
Abbreviations: BMI, body mass index; AST, aspartate transaminase; ALT, alanine transaminase; GGT, gamma‐glutamyl transpeptidase; BMI‐SDS, standardised BMI; 95% CI, 95% confidence interval; HOMA‐IR, homeostatic model assessment for insulin resistance; MATSUDA‐ISI, indicates values which are comparable to rate of disappearance of plasma glucose measured by insulin clamp with glucose tracer; INSAUC, area under the curve insulin; INSpeak, insulin peak; HOMA‐SC, homeo‐static model assessment for insulin secretion; IGI, insulinogenic index; 0–30 INSAUC/GluAUC., 0–30 min area under the curve insulin over area under the curve glucose; 30–120 INSAUC/GluAUC., 30–120 min area under the curve insulin over area under the curve glucose; Std. Dev, standard deviation.
p‐Value: Differences between control and mild increase group.
p‐Value: Differences between control and advanced group.
The demographic characteristics and concentrations of fasting glucose, fasting insulin and liver enzymes of the different transaminase groups are given in Table 1. Patients in the advanced group were older than those in the other groups. While there were more girls in the control group, more than half of the participants in the mild and almost 3 out of 4 participants of the advanced group were male. BMI and BMI‐SDS values were higher among patients in the mild group and the advanced group as compared to those in the control group. While there was no difference in the prevalence of patients classified with obesity between transaminase groups, less patients with mild and advanced severity were classified with overweight and more of them with extreme obesity as compared to the controls. Tanner stage did not differ between subjects of different transaminase groups. As by definition, liver enzymes were the highest in the advanced group, followed by the mild and the control group, respectively.
Patients in the mild group as well as in the advanced group had higher glucose levels than in the control group during OGTT (Fig. 1). Compared to the control group, this difference was significant in the advanced group at baseline and at all time points of the OGTT. Only in the mild group, the fasting and 60 min glucose value had no significant difference in comparison to the control group. Comparing all three transaminase categories, blood insulin values were higher in both increase groups than in control patients at all time points of OGTT (Fig. 1).
Figure 1.
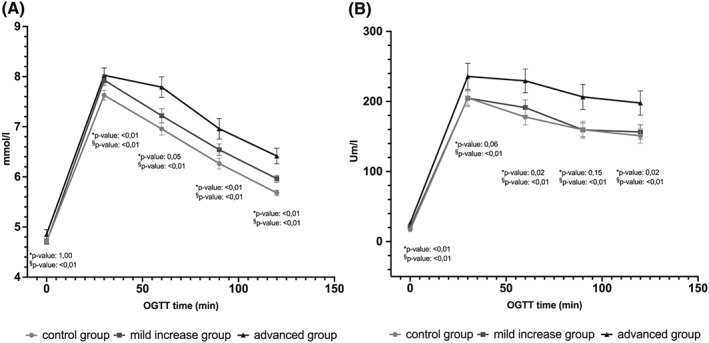
Blood glucose levels (a, mmol/L) and insulin level during oral glucose tolerance test (OGTT) (b, Um/L) in the control group, mild increased transaminase group and advanced group, shown with lower and upper 95% CI for mean
Among patients with normal glucose tolerance (NGT), prediabetes and T2DM, 50% (95% CI: 48.5–51.5), 53.4% (95% CI: 49.4–57.5) and 46.4% (95% CI: 34.3–58.4), respectively, were male. In the NGT, prediabetes and T2DM group, 47.7% (95% CI: 46.2–49.1), 59.9% (95% CI: 55.9–63.9) and 53.6% (95% CI: 41.6–65.7) of the patients were in the mild group and 12.2% (95% CI: 11.2–13.1), 21.5% (95% CI: 18.2–24.6) and 29% (95% CI: 18.0–40.0) in the advanced group, respectively.
3.1. Measures of insulin sensitivity and secretion
As indicated by HOMA‐IR and MATSUDA‐ISI, patients in the mild and advanced group were more insulin resistant than controls. Insulin secretion described by HOMA‐SC was higher in both increase groups as compared to controls. INSAUC, INSpeak, and insulinogenic index (IGI) tended to be higher with rising ALT‐values, but did not reach significance (Table 1). In addition, ALT values were negatively correlated with MATSUDA‐ISI (r = −0.26; p < 0.01) and positively with HOMA‐IR (r = 0.23; p < 0.01), HOMA‐SC (r = 0.24; p < 0.01) and peak insulin (r = 0.21, p < 0.01) in the entire cohort.
3.2. Prevalences of prediabetes and type 2 diabetes mellitus in children within different transaminase groups
The prevalence of prediabetes and T2DM was 11.9% (95% CI: 11.0–12.8) and 1.4% (95% CI: 1.1–1.7) among all participants, respectively (Table 2). The prevalence of prediabetes was significantly higher in the advanced group as compared to the control group. Patients in the advanced group had a significantly higher prevalence of T2DM than controls. Prevalence of prediabetes increased with deteriorating transaminase degree in both girls and boys. However, girls within the advanced group had a higher T2DM prevalence than males within the same group. There was a significant difference in the prevalence of prediabetes between boys and girls of all groups, while there was no difference in the prevalence of T2DM between boys and girls referring to all groups.
Table 2.
Prevalence and 95% confidence interval of prediabetes and type 2 diabetes mellitus in different groups (controls, mild increase group and advanced group)
| Controls | Mild increase group | Advanced group | p‐Value* | p‐Value** | |
|---|---|---|---|---|---|
| Total (N) | 2506 | 1760 | 666 | ||
|
Prediabetes (%, 95% CI) |
9.4 (8.2–10.5) | 12.8 (11.2–14.3) | 18.9 (15.9–21.9) | <.01 | <.01 |
| ♂ (%, 95% CI) | 9.7 (7.8–11.5) | 12.5 (10.4–14.5) | 19 (15.4–22.5) | ||
| ♀ (%, 95% CI) | 9.2 (7.7–10.6) | 13.2 (10.8–15.7) | 18.8 (13.1–24.5) | ||
| T2DM (%, 95% CI) | 1.3 (0.8–1.7) | 1 (0.5–1.4) | 3.0 (1.7–4.3) | .35 | <.01 |
| ♂ (%, 95% CI) | 1.1 (0.5–1.8) | 1.1 (0.4–1.7) | 2.1 (0.8–3.4) | ||
| ♀ (%, 95% CI) | 1.4 (0.8–2.0) | 0.8 (0.2–1.4) | 5.4 (2.1–8.6) |
Abbreviations: OGTT, oral glucose tolerance test; T2DM, type 2 diabetes mellitus.
p‐Value: Differences between the controls and mild increase group.
p‐Value: Differences between the controls and advanced group.
3.3. Odds ratios of prediabetes and type 2 diabetes mellitus in children within different transaminase groups
Children in the mild and the advanced group had significantly greater odds ratios of being classified with prediabetes as compared to controls, whereby adjustment for age, sex and BMI‐SDS had little influence (Table 3). Children in the advanced group had greater odds ratios of being classified with T2DM compared to children within controls. These odds ratios remained similar after adjustment. There was no significant difference in the risk for T2DM between the mild and the control group.
Table 3.
OR and 95% CI of T2DM and prediabetes according to level of ALT
| T2DM (n = 69) | Prediabetes (n = 586) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Crude | Adjusted* | Crude | Adjusted* | |||||||
| OR | 95% CI | OR | 95% CI | p‐Value** | OR | 95% CI | OR | 95% CI | p‐Value** | |
| Advanced group | 2.4 | 1.4–4.2 | 2.2 | 1.2–4 | 0.01 | 2.3 | 1.8–2.9 | 1.9 | 1.5–2.4 | <.01 |
| Mild increase group | 0.8 | 0.4–1.4 | 0.7 | 0.4–1.4 | .02 | 1.4 | 1.2–1.7 | 1.3 | 1.1–1.6 | <.01 |
| Controls | 1 | – | 1 | – | – | 1 | 1 | – | – | |
Note: Logistic regression models were used to estimate the OR and 95% CI, adjusted for age, sex and BMI‐SDS.
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval; BMI‐SDS, BMI as standard deviation score; OGTT, oral glucose tolerance test; T2DM, type 2 diabetes mellitus.
p‐Value: Difference in adjusted odds ratios for T2DM between different groups with elevated transaminases compared to control group.
p‐Value: Difference in adjusted odds ratios for prediabetes between different groups with elevated transaminases compared to control group.
4. DISCUSSION
Our cross‐sectional analysis of data derived from one of the largest pediatric European obesity multicentre studies shows that children with increased liver transaminases as surrogates of NAFLD show higher prevalences for prediabetes and T2DM as compared to those with normal transaminases.
Current estimated worldwide healthcare cost in diabetes treatment and complications of more than USD 720 billion and an approximate 50% increase in the number of people with diabetes by 2045 underscore the serious global challenge of the diabetes pandemic.24 Until less than 30 years ago, T2DM was regarded an adult disease.25 However, since the early 1990s, a trend of increasing cases of T2DM in children has been witnessed, particularly in North America, paralleling the staggering increase in the prevalence of obesity.25 However, while it is projected that by 2050 there may be a fourfold increase in the prevalence of youth‐onset T2DM in the United States, in a recent follow‐up survey of a cross‐sectional study, Neu et al. reported that the prevalence of youth‐onset T2DM in Germany remained stable over the past 10 years, and that the majority of youth‐onset T2DM cases were part of specific ethnic minorities (26, 27). Given the asymptomatic nature at T2DM followed by rapid progression with beta‐cell function deteriorating by 15–20% per year when diagnosed in young people and complications arising within 5 years, identification of individuals at high risk is crucial.28 Youth with NAFLD have recently been shown to have substantially higher risk of T2DM than youth in general, with as many as one in three children with NAFLD having abnormal glucose metabolism in a U.S. cohort (9, 29, 30). In a 5‐year follow‐up of adults with newly diagnosed diabetes, hepatocellular lipid content was higher in patients assigned to a severe insulin‐resistant cluster as compared to patients with mild age‐related diabetes, mild obesity‐related diabetes, severe autoimmune diabetes and severe insulin‐deficient diabetes at baseline.31 Therefore, the results of our study underscore the need for youth with obesity and elevated liver enzymes to undergo comprehensive metabolic assessment including an OGTT.
We based our diagnostic criteria for increased ALT values on those suggested by Schwimmer et al. and considered elevations up to two times the upper limit of the norm (ULN) as surrogate of 'mild NAFLD'. While no increased risk for T2DM could be detected in this group, an increase in the odds ratios for prediabetes by ~30% was evident. Three percent of youth within the advanced group (ALT 2‐ to 4.5‐fold the ULN) were diagnosed with T2DM and 22% with abnormal glucose metabolism in our cohort. These patients had two times the odds ratios for T2DM. The cross‐sectional nature of our study prevents us from drawing any conclusions in regard to direction of causality. Serum liver enzyme concentrations are considered to have a relatively poor sensitivity and specificity in the diagnosis of NAFLD.32 However, our data are in line with recently published systematic reviews and metanalyses on prospective studies in adult populations. Ballestri et al. pooled data of more than 117.000 adults diagnosed with NAFLD by serum liver enzymes which demonstrated an increased incident risk of T2DM with a pooled relative risk of 1.97 for ALT (last vs. first quartile or quintile).33 Similar results were shown by Mantovani et al., who analysed data of almost 300.000 adult individuals of whom 30% had been diagnosed with NAFLD by imaging methods.13 Further, Sung and coworkers demonstrated that patients with NAFLD had twice the risk of developing T2DM compared to patients without NAFLD during five years of follow‐up, while resolution of fatty liver prevented them from developing T2DM.34 In keeping with this, a recent retrospective study demonstrated a strong and independent association between NAFLD improvement and reduced incidence of T2DM in adult patients.35 In addition, an elevated risk of incident T2DM was even shown for patients with normal weight and NAFLD as compared to patients with normal to overweight without NAFLD.36 To date, published adult data regarding the association between histologically confirmed‐NAFLD and the risk of incident T2DM are rare, with no such longitudinal data available in the pediatric age group. In a retrospective analysis of Swedish adults, approximately 80% of individuals with biopsy‐proven NAFLD developed T2DM (58%) or pre‐diabetes (20%) within a 14‐year follow‐up period.37 These data provide evidence of biological plausibility that NAFLD may increase risk of incident T2DM.
Our study confirms previous studies that pediatric NAFLD is associated with decreased insulin sensitivity and a compensatory insulin response.38 In NAFLD, accumulation of intrahepatic fat predominantly results from increased influx of esterified free fatty acids from maladapted white adipose tissue (60%), de‐novo lipogenesis (25%) and diet (15%) and drives hepatic gluconeogenesis and insulin resistance already in childhood.39, 40 In addition, oxidised fatty acids have been suggested to be involved in the pathogenesis of T2DM in adolescents with obesity by mediating hepatic injury and impairing beta‐cell function. Further, hepatic insulin resistance has been shown to trigger the synthesis of several proinflammatory mediators and prodiabetogenic hepatokines (e.g., fetuin‐A, fetuin‐B, fibroblast growth factor‐21, retinol‐binding protein 4, selenoprotein P) that may favour the development of T2DM.41, 42
In line with data from previous studies, our results show significant differences in NAFLD risk between both sexes with a clear male predominance.43, 44 However, among youth with prediabetes and T2DM, there were significantly more girls than boys. In addition to this general female predominance in abnormal glucose metabolism, girls within the advanced group exhibited more than double the prevalence in T2DM than boys (5.4% vs. 2.1%). These data are in line with those published by Newton et al.: among 675 U.S. adolescents with histologically proven NAFLD, 71.1% were male.9 Contrary to this, girls with increased transaminases were significantly more likely to have T2DM than boys (13.7% vs. 3.5%). Although the cause for this gender difference remains elusive, it seems relevant that girls with NAFLD are more likely to have additional associated comorbidities such as arterial hypertension.45
In general, during reproductive age, males were shown to have a higher prevalence and severity of NAFLD compared to females. On the contrary, postmenopausal women are known to carry a greater risk of NAFLD than men, suggesting a protective role of oestrogens. Further, the role of NAFLD as a sexual dimorphic disease is also supported by animal models that demonstrate a disposition of male individuals to more advanced fatty liver disease compared to females that is linked to a state of subclinical inflammation and increased hepatic morbidity.46 A study applying a computational model concluded that due to sex‐specific metabolic demands, female and male livers are metabolically diverse and therefore differently regulated.47 In addition, a recent review stated that most published clinical and epidemiological studies fail to examine sex differences appropriately' and suggested to consider sex differences, sex hormones, age and other reproductive information in clinical investigation and gene association studies of NAFLD 'in order to fill current gaps and implement precision medicine for patients with NAFLD'.46
There are some strengths and limitations that need to be acknowledged. The primary strength of this study is its large sample size of patients. In addition, the availability of fasting and OGTT data improves the validity of the diagnosis of T2DM. Nevertheless, although investigators at the different study sites of the APV study group are requested to adhere to most recent T2DM screening recommendations, which includes islet cell antibody testing, some uncertainty in regard to accurate diagnostic testing remains.48 The same applies to the exclusion of other causes of elevated liver enzymes such as Wilson's disease, celiac disease, autoimmune or other metabolic diseases including alcohol abuse, which may not have been adequately excluded by clinicians in all patients entered into the APV study database. NAFLD was diagnosed based on elevated liver enzyme levels although the gold standard of diagnosis is liver biopsy. Thus, there may be some limitations in detecting NAFLD in children with obesity having completely normal liver enzyme levels in the present study. However, since widely used 'normal' ranges of liver transaminases may underestimate the presence of NAFLD, we chose to use biology‐based thresholds providing higher sensitivity and sufficient specificity.16 Similarly, OGTT data in part reflect diagnostic management strategies of the study centres, which may not always adhere to current diagnostic guidelines as outlined above. There is also no centralised measurement of laboratory parameters, particularly glucose and insulin, and we have to acknowledge differences in assay procedures. Further, information about race and ethnicity were not available. Finally, given the high prevalence of prediabetes in our sample, calculated OR may overestimate the relative risk of prediabetes.
In conclusion, children with increased transaminases as surrogates of NAFLD have a two to threefold higher risk of prediabetes and T2DM as compared to controls. While NAFLD in youth predominantly affects boys, girls with NAFLD are at higher risk for T2DM. This study confirms that youth with NAFLD, in particular when associated with obesity, should undergo a detailed clinical assessment of glucose metabolism, independent of the background epidemiology of T2DM.
CONFLICT OF INTEREST STATEMENT
No conflict of interest was declared.
ACKNOWLEDGEMENTS
For our study, we received grant support from the German 'Competence Network Adipositas' (project funding reference number 01 GI 0839) and from the 'German Center of Diabetes Research' (reference number 82DZD01402). This work was also supported in part by grants of the German Federal Ministry for Education and Research (reference numbers 01GI1120A and 01GI1401). FK was involved in planning of the study design, statistical analysis, generation of figures and tables, data interpretation and writing of the manuscript. DW was involved in planning of the study design, statistical analysis, data interpretation, manuscript writing and editing of the manuscript. RWH and EB were involved in implementing the APV registry, statistical analysis and editing of the manuscript. SGP, KH, AK, TR, MR, GSS, MW, KW and SW were involved in implementing the APV registry and editing of the manuscript. All authors were involved in the paper's process and approved the final version.
Koutny F, Weghuber D, Bollow E, et al. Prevalence of prediabetes and type 2 diabetes in children with obesity and increased transaminases in European German‐speaking countries. Analysis of the APV initiative. Pediatric Obesity. 2020;15:e12601 10.1111/ijpo.12601
REFERENCES
- 1. Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The prevalence of non‐alcoholic fatty liver disease in children and adolescents: a systematic review and meta‐analysis. PLoS One. 2015;10(10):e0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alisi A, Manco M, Vania A, Nobili V. Pediatric nonalcoholic fatty liver disease in 2009. J Pediatr. 2009;155(4):469‐474. [DOI] [PubMed] [Google Scholar]
- 3. Rashid M, Roberts EA. Nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr. 2000;30(1):48‐53. [DOI] [PubMed] [Google Scholar]
- 4. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73‐84. [DOI] [PubMed] [Google Scholar]
- 5. Lindbäck SM, Gabbert C, Johnson BL, et al. Pediatric nonalcoholic fatty liver disease: a comprehensive review. Adv Pediatr. 2010;57(1):85‐140. [DOI] [PubMed] [Google Scholar]
- 6. Nobili V, Alisi A, Grimaldi C, et al. Non‐alcoholic fatty liver disease and hepatocellular carcinoma in a 7‐year‐old obese boy: coincidence or comorbidity? Pediatr Obes. 2014;9(5):e99‐e102. [DOI] [PubMed] [Google Scholar]
- 7. Di Bonito P, Pacifico L, Chiesa C, et al. Impaired fasting glucose and impaired glucose tolerance in children and adolescents with overweight/obesity. J Endocrinol Invest. 2017;40(4):409‐416. [DOI] [PubMed] [Google Scholar]
- 8. Burgert TS, Taksali SE, Dziura J, et al. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab. 2006;91(11):4287‐4294. [DOI] [PubMed] [Google Scholar]
- 9. Newton KP, Hou J, Crimmins NA, et al. Prevalence of prediabetes and type 2 diabetes in children with nonalcoholic fatty liver disease. JAMA Pediatr. 2016;170(10):e161971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liese AD, D'Agostino RB, Hamman RF, et al. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118(4):1510‐1518. [DOI] [PubMed] [Google Scholar]
- 11. Lee AM, Fermin CR, Filipp SL, Gurka MJ, DeBoer MD. Examining trends in prediabetes and its relationship with the metabolic syndrome in US adolescents, 1999‐2014. Acta Diabetol. 2017;54(4):373‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. (NCD‐RisC) NRFC . Worldwide trends in diabetes since 1980: a pooled analysis of 751 population‐based studies with 4.4 million participants. Lancet. 2016;387(10027):1513‐1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta‐analysis. Diabetes Care. 2018;41(2):372‐382. [DOI] [PubMed] [Google Scholar]
- 14. Reinehr T, Holl RW, Wabitsch M. The German Working Group of Obesity in Childhood and Adolescence (AGA): improving the quality of care for overweight and obese children in Germany. Obes Facts. 2008;1(1):26‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flechtner‐Mors M, Neuhauser H, Holl RW, et al. Blood pressure in 57,915 pediatric patients who are overweight or obese based on five reference systems. Am J Cardiol. 2015;115(11):1587‐1594. [DOI] [PubMed] [Google Scholar]
- 16. Schwimmer JB, Dunn W, Norman GJ, et al. SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology. 2010;138(4):1357‐1364. 1364.e1351–1364.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mayer‐Davis EJ, Kahkoska AR, Jefferies C, et al. ISPAD Clinical Practice Consensus Guidelines 2018: definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes. 2018. Oct;19(Suppl 27):7‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hölling H, Schlack R, Kamtsiuris P, Butschalowsky H, Schlaud M, Kurth BM. The KiGGS study. Nationwide representative longitudinal and cross‐sectional study on the health of children and adolescents within the framework of health monitoring at the Robert Koch Institute. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55(6–7):836‐842. [DOI] [PubMed] [Google Scholar]
- 19. Plachta‐Danielzik S et al. Body fat percentiles for German children and adolescents. Obes Facts. 2012;5:77‐90. [DOI] [PubMed] [Google Scholar]
- 20. Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44(1):45‐60. [PubMed] [Google Scholar]
- 21. Henderson M, Rabasa‐Lhoret R, Bastard JP, et al. Measuring insulin sensitivity in youth: how do the different indices compare with the gold‐standard method? Diabetes Metab. 2011;37(1):72‐78. [DOI] [PubMed] [Google Scholar]
- 22. Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91(12):4753‐4761. [DOI] [PubMed] [Google Scholar]
- 23. Angelopoulos NG, Zervas A, Livadas S, et al. Reduced insulin secretion in normoglycaemic patients with beta‐thalassaemia major. Diabet Med. 2006;23(12):1327‐1331. [DOI] [PubMed] [Google Scholar]
- 24. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40‐50. [DOI] [PubMed] [Google Scholar]
- 25. Arslanian S. Type 2 diabetes in children: clinical aspects and risk factors. Horm Res. 2002;57(Suppl 1):19‐28. [DOI] [PubMed] [Google Scholar]
- 26. Neu A, Feldhahn L, Ehehalt S, et al. No change in type 2 diabetes prevalence in children and adolescents over 10 years: update of a population‐based survey in South Germany. Pediatr Diabetes. 2018;19(4):637‐639. [DOI] [PubMed] [Google Scholar]
- 27. Imperatore G, Boyle JP, Thompson TJ, et al. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012;35(12):2515‐2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hannon TS, Arslanian SA. The changing face of diabetes in youth: lessons learned from studies of type 2 diabetes. Ann N Y Acad Sci. 2015;1353:113‐137. [DOI] [PubMed] [Google Scholar]
- 29. Cali AM, De Oliveira AM, Kim H, et al. Glucose dysregulation and hepatic steatosis in obese adolescents: is there a link? Hepatology. 2009;49(6):1896‐1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shah S, Kublaoui BM, Oden JD, White PC. Screening for type 2 diabetes in obese youth. Pediatrics. 2009;124(2):573‐579. [DOI] [PubMed] [Google Scholar]
- 31. Zaharia OP, Strassburger K, Strom A, et al. Risk of diabetes‐associated diseases in subgroups of patients with recent‐onset diabetes: a 5‐year follow‐up study. Lancet Diabetes Endocrinol. 2019;7(9):684‐694. [DOI] [PubMed] [Google Scholar]
- 32. Patton HM, Lavine JE, Van Natta ML, et al. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology. 2008;135(6):1961.e1962‐1971.e1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ballestri S, Nascimbeni F, Romagnoli D, Baldelli E, Targher G, Lonardo A. Type 2 diabetes in non‐alcoholic fatty liver disease and hepatitis C virus infection–liver: the "Musketeer" in the spotlight. Int J Mol Sci. 2016;17(3):355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sung KC, Wild SH, Byrne CD. Resolution of fatty liver and risk of incident diabetes. J Clin Endocrinol Metab. 2013;98(9):3637‐3643. [DOI] [PubMed] [Google Scholar]
- 35. Yamazaki H, Tsuboya T, Tsuji K, Dohke M, Maguchi H. Independent association between improvement of nonalcoholic fatty liver disease and reduced incidence of type 2 diabetes. Diabetes Care. 2015;38(9):1673‐1679. [DOI] [PubMed] [Google Scholar]
- 36. Fukuda T, Hamaguchi M, Kojima T, et al. The impact of non‐alcoholic fatty liver disease on incident type 2 diabetes mellitus in non‐overweight individuals. Liver Int. 2016;36(2):275‐283. [DOI] [PubMed] [Google Scholar]
- 37. Ekstedt M, Franzén LE, Mathiesen UL, et al. Long‐term follow‐up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865‐873. [DOI] [PubMed] [Google Scholar]
- 38. Mameli C, Brunetti D, Colombo V, et al. Combined use of a wristband and a smartphone to reduce body weight in obese children: randomized controlled trial. Pediatr Obes. 2018;13(2):81‐87. [DOI] [PubMed] [Google Scholar]
- 39. Haas JT, Francque S, Staels B. Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Annu Rev Physiol. 2016;78:181‐205. [DOI] [PubMed] [Google Scholar]
- 40. Caprio S, Perry R, Kursawe R. Adolescent obesity and insulin resistance: roles of ectopic fat accumulation and adipose inflammation. Gastroenterology. 2017;152(7):1638‐1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meex RCR, Watt MJ. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol. 2017;13(9):509‐520. [DOI] [PubMed] [Google Scholar]
- 42. Meex RC, Hoy AJ, Morris A, et al. Fetuin B is a secreted hepatocyte factor linking steatosis to impaired glucose metabolism. Cell Metab. 2015;22(6):1078‐1089. [DOI] [PubMed] [Google Scholar]
- 43. Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115(5):561‐565. [DOI] [PubMed] [Google Scholar]
- 44. Denzer C, Thiere D, Muche R, et al. Gender‐specific prevalences of fatty liver in obese children and adolescents: roles of body fat distribution, sex steroids, and insulin resistance. J Clin Endocrinol Metab. 2009;94(10):3872‐3881. [DOI] [PubMed] [Google Scholar]
- 45. Schwimmer JB, Zepeda A, Newton KP, et al. Longitudinal assessment of high blood pressure in children with nonalcoholic fatty liver disease. PLoS One. 2014;9(11):e112569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther. 2017;34(6):1291‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cvitanović Tomaš T, Urlep Ž, Moškon M, Mraz M, Rozman D. Computational Model: Sexual Aspects in Hepatic Metabolism and Abnormalities. Front Physiol. 2018;9:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. American Diabetes Association . Standards of medical care in diabetes – 2014. Diabetes Care. 2014;37(Suppl 1):S14‐S80. [DOI] [PubMed] [Google Scholar]


