Dear Editors,
The ability of lasers to selectively destroy the melanin pigment responsible for solar/senile lentigines (SL) (age spots) without damaging the surrounding tissue makes laser therapy a preferred option for treating SL.1 However, laser therapies typically utilize a high energy output to achieve thermal ablation of their selective targets—and therefore can be associated with a period of recovery, healing, and down time, depending on the modality. The Kleresca® biophotonic platform, which generates fluorescent light energy (FLE), is a non‐invasive form of photobiomodulation with multiple applications.2 FLE has clinical efficacy in treating inflammatory skin conditions such as acne vulgaris and rosacea,3, 4, 5, 6 in addition to its healing and normalizing properties, rejuvenating the skin.7 It has previously been combined with other invasive treatment techniques, for example, following picosecond laser treatment of SL, where it enhanced the overall appearance of the skin.8
A common observation with the Kleresca® biophotonic platform is the transient emergence of underlying hyperpigmented spots.3 This case sought to investigate the use of the Kleresca® treatment to intensify and demask underlying SL prior to their targeting with laser therapy.
Here, we present a 67‐year‐old woman with histologically confirmed SL (Figure 1A) treated with the Kleresca® treatment (FB Dermatology, Ireland). Treatment comprised the application of a 2‐mm layer of the Kleresca® pre–post photoconverter gel followed by irradiation with a multi‐LED lamp (447 nm), as per the instructions for use. A double/stacking treatment consisting of one 9‐minute treatment session, a 10‐minute interval, and a subsequent 9‐minute treatment session was completed once a week for 2 weeks. One week after the second Kleresca® treatment session, the patient was treated with a 694 nm Qs‐ruby laser using a 4‐mm spot with an energy output of 5 J/cm2 (Tattoo Star, Asclepion Laser technologies). Standardized pictures were obtained using a VISIA camera system (Candfield), before the Kleresca® treatment (Figure 1A), 1 week after the 2 Kleresca® sessions and immediately prior to the treatment with the QS‐ruby laser (Figure 1B), and 3 weeks after the QS‐ruby laser treatment (Figure 1C). Pictures were analyzed using ImageJ (NIH), the percentage area of pigmentation was measured (Figure 1D), and from this, the percentage change in pigmentation between treatments was calculated (Table 1).
Figure 1.
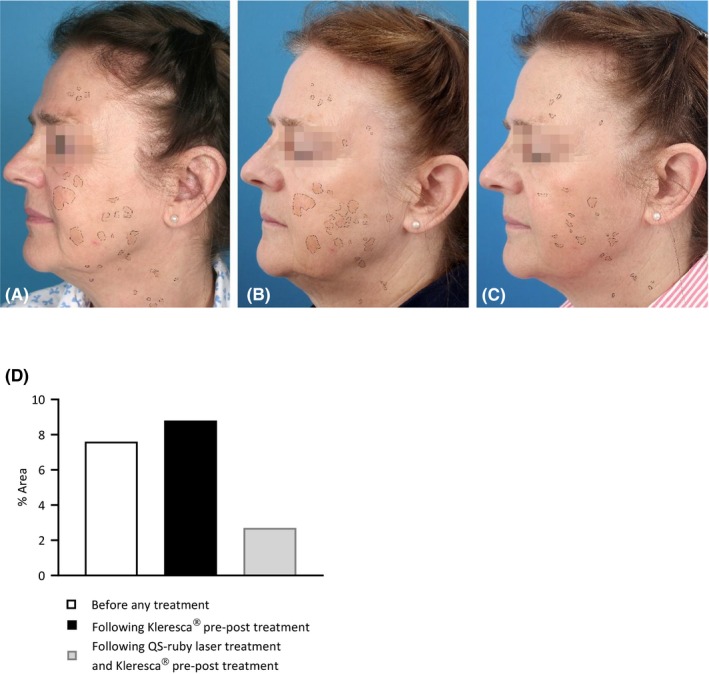
Standardized pictures obtained using a Visia camera system (Candfield); before the Kleresca® pre–post treatment (A), 1 wk after the second Kleresca® session and immediately prior to the treatment with the QS‐ruby laser (B), and 3 wk after the QS‐ruby laser treatment (C) were used to track the changes in pigmentation. (D) Graph shows the % area of pigmentation identified from the patient pictures
Table 1.
Showing the percentage change in pigmentation calculated following the Kleresca® treatment compared with baseline (before any treatment) and following the subsequent laser treatment compared with baseline
| Following Kleresca® pre–post | Following QS‐ruby laser treatment |
|---|---|
| +14% | −232% |
Following the Kleresca® treatment, there was an increase in the % area of pigmentation (Figure 1B; Table 1) compared with the baseline (before any treatment; Figure 1A; Table 1). Following the subsequent QS‐ruby laser treatment, the pigmentation decreased by 232%, (Figure 1C; Table 1).
By increasing the % area of pigmentation, the Kleresca® treatment highlighted the SL spots to be targeted by the QS‐ruby laser. The complete mechanism for the emergence or darkening of underlying pigmented areas following the Kleresca® treatment has not been fully elucidated. However, since the pigment can become visible during a treatment session of only 9 minutes, it is clear that this is an immediate and transient pigmentation response acting on melanin already present in the skin, as opposed to inducing new melanin synthesis. Further, it differs from damage associated UV‐induced hyperpigmentation.
Only one single session of the laser treatment was required to ablate the visible SL lesions. Hence, the treatment essentially prepared the skin by activating the skin cells and intensified the key areas to be targeted with the QS‐ruby laser as well as enhancing its efficacy.
Since laser fluence, power and pulse duration are key determinants of the efficacy of laser treatment for SL,1 the use of Kleresca® before typically invasive laser therapies, may reduce the number of laser sessions required, reduce the fluency, while enhancing their efficacy, leading to an overall reduction in down time and common associated side effects of common laser treatments required.
To conclude, the Kleresca® treatment activated the skin cells to successfully intensify all SL areas to be targeted by the laser treatment. Only one laser session was required to successfully target and ablate the lesions. Kleresca® pre–post treatment is a useful adjunct therapy preparing the skin before and rejuvenating the skin after more invasive therapies, favoring a fast recovery, and enhancing the overall esthetic results.
CONFLICTS OF INTEREST
MCE. Nielsen and DE are employees of FB Dermatology Ltd/Kleresca®.
REFERENCES
- 1. Todd MM, Rallis TM, Gerwels JW, Hata TR. A comparison of 3 lasers and liquid nitrogen in the treatment of solar lentigines: a randomized, controlled, comparative trial. Arch Dermatol. 2000;136(7):841‐846. [DOI] [PubMed] [Google Scholar]
- 2. Jalili A. Chromophore gel‐assisted phototherapy. J für Ästhetische Chir. 2019;12(S1):1‐5. [Google Scholar]
- 3. Antoniou C, Dessinioti C, Sotiriadis D, et al. A multicenter, randomized, split‐face clinical trial evaluating the efficacy and safety of chromophore gel‐assisted blue light phototherapy for the treatment of acne. Int J Dermatol. 2016;55(12):1321‐1328. [DOI] [PubMed] [Google Scholar]
- 4. Nikolis A, Fauverghe S, Scapagnini G, et al. An extension of a multicenter, randomized, split‐face clinical trial evaluating the efficacy and safety of chromophore gel‐assisted blue light phototherapy for the treatment of acne. Int J Dermatol. 2018;57(1):94‐103. [DOI] [PubMed] [Google Scholar]
- 5. Braun SA, Gerber PA. A photoconverter gel‐assisted blue light therapy for the treatment of rosacea. Int J Dermatol. 2017;56(12):1489‐1490. [DOI] [PubMed] [Google Scholar]
- 6. Sannino M, Lodi G, Dethlefsen MW, et al. Fluorescent light energy: treating rosacea subtypes 1, 2, and 3. Clin Case Reports. 2018;6(12):2385‐2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nikolis A, Bernstein S, Kinney B, Scuderi N, Rastogi S, Sampalis JS. A randomized, placebo‐controlled, single‐blinded, split‐faced clinical trial evaluating the efficacy and safety of KLOX‐001 gel formulation with KLOX light‐emitting diode light on facial rejuvenation. Clin Cosmet Investig Dermatol. 2016;9:115‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scarcella G, Dethlefsen M, Nielsen M. Treatment of solar lentigines using a combination of picosecond laser and biophotonic treatment. Clin Case Reports. 2018;6(9):1868‐1870. [DOI] [PMC free article] [PubMed] [Google Scholar]


