Abstract
Introduction
Since it became available in the mid‐2010s, dorsal root ganglion (DRG) stimulation has become part of the armamentarium to treat chronic pain. To date, one randomized controlled trial, and several studies of moderate sample size and various etiologies have been published on this topic. We conducted a pooled analysis to investigate the generalizability of individual studies and to identify differences in outcome between chronic pain etiologic subgroups and/or pain location.
Materials and Methods
One prospective, randomized comparative trial and six prospective, single‐arm, observational studies were identified that met pre‐defined acceptance criteria. Pain scores and patient‐reported outcome (PRO) measures were weighted by study sample sizes and pooled. Safety data are reported in aggregate form.
Results
Our analysis included 217 patients with a permanent implant at 12‐month follow‐up. Analysis of pooled data showed an overall weighted mean pain score of 3.4, with 63% of patients reporting ≥50% pain relief. Effectiveness sub‐analyses in CRPS‐I, causalgia, and back pain resulted in a mean reduction in pain intensity of 4.9, 4.6, and 3.9 points, respectively. Our pooled analysis showed a pain score for primary affected region ranging from 1.7 (groin) to 3.0 (buttocks) and responder rates of 80% for foot and groin, 75% for leg, and 70% for back. A substantial improvement in all PROs was observed at 12 months. The most commonly reported procedural or device complications were pain at the IPG pocket site, lead fracture, lead migration, and infection.
Conclusions
DRG stimulation is an effective and safe therapy for various etiologies of chronic pain.
Keywords: Causalgia, complex regional pain syndrome type I, dorsal root ganglion stimulation, failed back surgery syndrome, pooled analysis
INTRODUCTION
Chronic pain is defined as pain persisting past normal healing time, lasting or recurring for more than six months 1. Chronic pain affects approximately 20–30% of the population in the United States and Europe 2, 3. It is often a debilitating condition that substantially diminishes quality of life. Post‐surgical complications and trauma (25%) and spine problems (20%) are responsible for almost half the incidence of chronic pain 4. Chronic pain has a wide range of etiologies that can be neuropathic, nociceptive, or nociplastic in nature 5, 6. Neuropathic pain is caused by a lesion or disease of the somatosensory system. Nociceptive pain arises from harmful stimuli to non‐neural tissue and is due to the activation of nociceptors 6. Nociplastic pain is a relatively new term; the International Association for the Study of Pain describes it as “altered nociception despite no clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system causing the pain” 6.
Spinal nerves, formed from afferent sensory axons (the dorsal root) and motor efferent axons (the ventral root), emerge from the intervertebral neural foramina between adjacent vertebral segments 7. The dorsal root ganglion (DRG) is located at both sides of the spinal cord on the distal end of the dorsal root in the lateral epidural space. The DRG houses the cell bodies of sensory neurons and is an active participant in the development of certain forms of chronic pain 8, 9, 10, 11, 12, 13. Because of this, the DRG might be an attractive target for electrical stimulation.
Koopmeiners et al were the first to establish that low‐frequency electrical stimulation increases Ca2+ influx into DRG neurons, decreases the frequency of multiple action potentials, and significantly reduces conduction velocity 14. A review by Krames summarized various other mechanisms of action of DRG stimulation that have been hypothesized over the years, such as modification of growth factor release, reversal of cytokine release and genetic changes, downregulation of irregular ion channels, and restoration of normal ion flux 15. The end result of electrical stimulation on the DRG neurons is to stabilize and decrease hyperexcitability 15. In addition, to our knowledge, all in vitro and in vivo animal studies have shown that DRG stimulation has positive effects on pain‐related outcomes without causing any inflammation or DRG tissue damage and may actually be anti‐inflammatory 16, 17.
Conventional spinal cord stimulation (SCS, which applies electrical stimulation to the dorsal column) can result in sub‐optimal effectiveness for treating different chronic pain etiologies 18, 19, 20. In the last decade, the DRG has become a focus for electrical stimulation with the potential advantage of better target control. The cerebrospinal fluid layer surrounding the DRG has much lower volume than the one that surrounds the spinal cord. Therefore, lower stimulation amplitudes are required with DRG stimulation compared with SCS, resulting in less postural variation. A recent study with laser‐evoked potentials has shown that DRG stimulation might even result in restorative processes by normalizing pain signal transfer from the periphery to supra‐spinal levels 21.
We conducted a pooled analysis of published, prospective studies to identify differences in effectiveness of DRG stimulation by pain etiology or location and to investigate the generalizability and reproducibility of individual studies that followed patients for at least 12 months. This pooled analysis evaluates a substantial patient population with complex regional pain syndrome (CRPS). CRPS type I (CRPS‐I) is a syndrome characterized by a continuous (spontaneous and/or evoked) pain that is seemingly disproportionate in time or degree to the usual course of pain after trauma or other lesion. The pain is regional (not in a specific nerve territory or dermatome) and usually has a distal predominance of abnormal sensory, motor, sudomotor, vasomotor edema, and/or trophic findings. A second subtype, causalgia, is a chronic pain condition that can develop after injury or trauma to a peripheral nerve.
This study also set out to give an overview of the DRG stimulation literature, including real‐world observations. Finally, pooling the data in analyses may provide a more realistic presentation of outcomes with DRG stimulation therapy.
MATERIALS AND METHODS
A literature search of PubMed was carried out using the search terms “dorsal root ganglion”[All Fields] AND “chronic pain”[All Fields] AND “neuromodulation”[All Fields] AND “prospective”[All Fields]. Google Scholar was also searched using keywords “Dorsal Root ganglion,” “neuromodulation,” and “chronic pain,” Criteria for inclusion were 1) human studies with five or more subjects, 2) studies in an adult population (age ≥ 18 years), and 3) at least 12 months follow‐up of prospectively collected clinical outcomes. Articles presenting subgroups or sub‐analyses of larger studies were excluded. All studies had to be conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. The study protocols and informed consent documentation had to be reviewed and approved by an ethics committee or institutional review board at each site. Written informed consent had to be obtained from patients before initiation of any protocol‐specified procedures.
Pain scores collected using visual analog scale (VAS) or numeric rating scale were considered equivalent 22. When collected in millimeter, a VAS score was converted to centimeter such that all pain scores ranged between 0 and 10. Pain score summary statistics (mean and standard deviation) were provided by the authors of two prospective, independent, physician‐initiated studies. We calculated a single pooled mean by weighting the average pain score for each study by sample size. The responder rate within this pooled analysis was defined as ≥50% reduction in pain score.
Patient‐reported outcome (PRO) data were extracted from each study and those reported in at least two studies were pooled. Four PROs met these criteria. The EuroQol‐5D (EQ‐5D index) is a measure of health‐related quality of life that measures mobility, self‐care, usual activities, pain, and anxiety, and summarizes into one score. The Profile of Mood States (POMS) assesses an individual's transient psychologic state. Brief Pain Inventory (BPI) uses two scales; 1) pain severity and 2) the degree to which pain interferes with function. The Oswestry Disability Index (ODI) assesses back‐related disability. This instrument groups patients by minimal disability (≤20%), moderate disability (21%–40%), severe disability (41%–60%), crippled (61%–80%), and bed‐bound (>80%). Similar to pain scores, a weighted mean was calculated for each of these instruments. When available, each PRO was compared to the published population normal value and/or minimum clinically important difference (MCID).
Reported device‐ and procedure‐related complications were collated across articles and were reported in aggregated form.
RESULTS
The PubMed Database Search and Google Scholar Keyword Search identified 34 unique records. Of these, 27 were excluded based on the criteria above. Seven prospective studies of independent subject populations were identified; one randomized trial 23, and six prospective, observational studies 24, 25, 26, 27, 28, 29. Thus, seven studies were reviewed for this pooled analysis; five were industry‐initiated (sponsored by Spinal Modulation [now Abbott, Plano, TX]) 23, 24, 25, 26, 27 and two were physician‐initiated studies 28, 29 (Fig. 1). A total of 256 subjects received a permanent implant. Twelve‐month follow‐up data were available for 217 subjects (85%). Standard inclusion and exclusion criteria for neuromodulation were applied across all studies. All studies required patients to have persistent pain for at least six months, pain intensity of at least 6 on 0‐10 scale, failed conservative treatments, and be physically and psychologically suitable for implantation of a neurostimulator. Patients with unstable pain condition, recent corticosteroid or radiofrequency treatment at the intended site of stimulation, presence of an active implantable device, coagulation disorder or use of anticoagulants, and current/planned pregnancy were excluded. An overview of the design, sample size, age, sex, pain etiologies, and outcome measures for each study is provided in Table 1.
Figure 1.
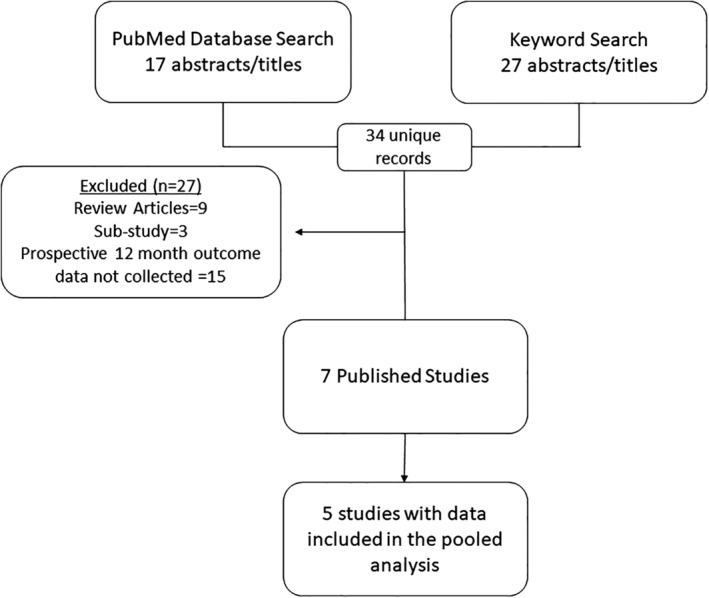
Summary of article selection.
Table 1.
Characteristics of All Prospective Studies.
| Study | Study design | Age | Sex | Indications | Number of implants | Number of subjects at 12 months | Outcomes |
|---|---|---|---|---|---|---|---|
| Liem et al.27 | Prospective, single‐arm, observational | 54.3 ± 13.3 | 27 F/24 M | Study total | 32 | 25 | Pain (VAS), physical functioning (BPI), quality of life (EQ‐5D), mood (POMS), quality and intensity of pain (McGill Pain Questionnaire), paresthesia distribution, patient satisfaction, global impression of change, and safety |
| FBSS | 8 | ||||||
| CRPS‐I | 8 | ||||||
| Causalgia | 6 | ||||||
| Disc‐related pain | 4 | ||||||
| Radicular Pain | 2 | ||||||
| Lumbar Stenosis | 2 | ||||||
| Other | 2 | ||||||
| Deer et al.23 | Prospective, controlled, randomized | 52.4 ± 12.7 | 39 F/37 M | Study total | 61 | 55 |
Primary: treatment success rates for DRG vs SCS Secondary: positional effects on paresthesia intensity Non‐powered: pain (VAS), quality of life (SF‐36), mood (POMS), physical functioning (BPI), patient satisfaction, and safety |
| CRPS‐I | 30 | ||||||
| Causalgia | 25 | ||||||
| Morgalla et al.28 | Prospective, single‐arm, observational | 50.4 ± 13.4 | 13 F/21 M | Causalgia | 30 | 25 | Pain (VAS), disability (PDI), catastrophizing (PCS), physical functioning (BPI), depression (BDI), and safety |
| Morgalla et al.29 | Prospective, single‐arm, observational | 56.8 ± 14.4 | 27 F/35 M | Causalgia | 51 | 40 | Pain (VAS), disability (PDI), catastrophizing (PCS), physical functioning (BPI), depression (BDI), and safety |
| Huygen et al.24 | Prospective, single‐arm, observational | 52.0 ± 11.5 | 42 F/24 M | Study total | 56 | 49 | Pain (VAS), physical functioning (BPI), mood (POMS), quality of life (EQ‐5D), and safety |
| FBSS | 25 | 24 | |||||
| CRPS‐I | 11 | 9 | |||||
| Causalgia | 15 | 10 | |||||
| Post‐amputation | 2 | 2 | |||||
| Radicular pain | 2 | 2 | |||||
| Other | 2 | 2 | |||||
| Kallewaard et al.25 | Prospective, single‐arm, observational | 47.5 ± 13.4 | 13 F/7 M |
Nonoperated Discogenic Low back pain |
15 | 14 | Pain (NRS), quality of life (EQ‐5D), disability (ODI), mood (POMS), patient satisfaction, and safety |
| Kallewaard et al.26 | Prospective, single‐arm, observational | 51.0* ± 12.2 | 7 F/4 M* | Lumbar Discectomy | 11 | 9 | Pain (NRS), physical functioning (BPI), quality of life (EQ‐5D), disability (ODI), mood (POMS), and safety |
| Total in seven studies | 256 | 217 | |||||
At permanent implant.
Pooled Analysis of 12‐Month Follow‐up Subjects
The weighted mean pain score for all patients decreased from 7.8 at baseline to 3.4 at 12 months. Figure 2 presents the mean score for each study at baseline and 12‐month follow‐up. The most common pain etiologies were causalgia (N = 104; 48%), back pain (N = 53; 24%), and CRPS‐I (N = 46; 21%).
Figure 2.
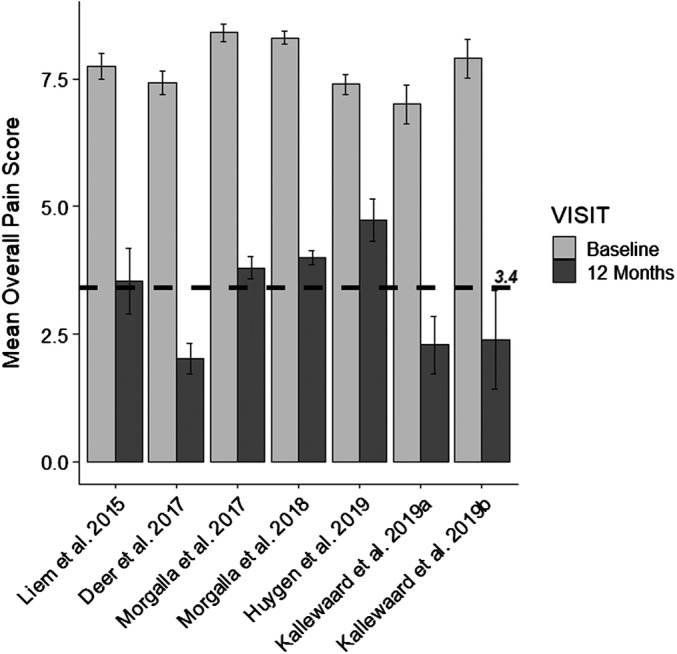
The mean score for each study is shown at baseline and at 12‐month follow‐up with the standard error of the mean. The mean weighted score across all studies at 12 months (3.4) is shown by a dashed line.
Five studies collected data on patients diagnosed with causalgia, either post‐trauma or post‐surgical nerve damage. Multiple nerves are represented in the data set including the femoral, sciatic, ilioinguinal, peroneal, and tibial. The pooled pain score decreased by 58% (4.6 points) from a weighted mean of 8.0 at baseline to 3.4 at 12 months (Table 2).
Table 2.
Pain Score Sub‐Analysis in CRPS‐I, Causalgia, and FBSS Subjects.
| Study | N (at 12 months follow‐up) | Overall pain at baseline | Overall pain at 12 months | Responder rates |
|---|---|---|---|---|
| Causalgia | ||||
| Liem et al.27 | 4 | 7.3 ± 1.0 | 4.0 ± 3.4 | |
| Deer et al.23 | 25 | 7.3 ± 2.0 | 1.9 ± 2.3 | |
| Morgalla et al.28 | 25 | 8.4 ± 1.0 | 3.8 ± 1.1 | |
| Morgalla et al.29 | 40 | 8.3 ± 0.9 | 4.0 ± 0.82 | |
| Huygen et al.24 | 10 | 7.0 ± 1.6 | 4.0 ± 2.5 | |
| 104 | 8.0 ± 1.2* | 3.4 ± 1.5* | 68% | |
| CRPS‐I | ||||
| Liem et al.27 | 7 | 7.7 ± 1.0 | 2.7 ± 3.3 | |
| Deer et al.23 | 30 | 7.6 ± 1.4 | 2.2 ± 2.3 | |
| Huygen et al.24 | 9 | 7.8 ± 1.7 | 5.0 ± 2.8 | |
| 46 | 7.7 ± 1.4* | 2.8 ± 2.6* | 71% | |
| FBSS and CLBP | ||||
| Liem et al.27 | 8 | 8.2 ± 0.97 | 4.8 ± 3.2 | |
| Huygen et al.24 | 22 | 7.3 ± 2.6 | 4.5 ± 2.7 | |
| Kallewaard et al.25 | 14 | 7.0 ± 1.4 | 2.3 ± 2.1 | |
| Kallewaard et al.26 | 9 | 7.9 ± 1.1 | 2.4 ± 2.9 | |
| 53 | 7.5 ± 1.8* | 3.6 ± 2.8* | 55% | |
Weighted mean and standard deviation.
Four studies reported outcomes on DRG stimulation for back pain. The group was heterogeneous and consisted of pain following lumbar discectomy, non‐operated discogenic low back pain, and failed back surgery syndrome (FBSS). A total of 53 subjects had 12‐month follow‐up data available. The weighted average pain score decreased 52% (3.9 points) from a mean of 7.5 at baseline to 3.6 at 12 months (Table 2). Most back pain was reported in combination with foot, groin, buttock, or leg pain (N = 35; 66%). Nine patients (17%) had back pain only and two (4%) reported leg pain only.
Outcomes in patients with CRPS‐I of the lower extremities were collected in three studies. The weighted average pain score of this patient population decreased by 64% (4.9 points) from a mean of 7.7 at baseline to 2.8 at 12 months (Table 2).
The most frequently reported areas of primary pain were leg (N = 64), foot (N = 50), back (N = 37), buttocks (N = 18), and groin (N = 11). Pain scores for these discrete regions at 12 months ranged from 1.7 (groin) to 3.0 (buttocks). Responder rates for primary pain areas were 80% for both foot and groin, 75% for leg, 70% for back, and 59% for buttocks. An overview of percentage pain relief per patient for these primary pain areas is shown in Fig. 3. Morgalla et al reported a 73% responder rate at three years for a subset of patients with groin pain 28. A second study from this group collected data from subjects presenting with knee, hand, foot, back, and leg pain and reported an overall 82.5% response rate 29.
Figure 3.
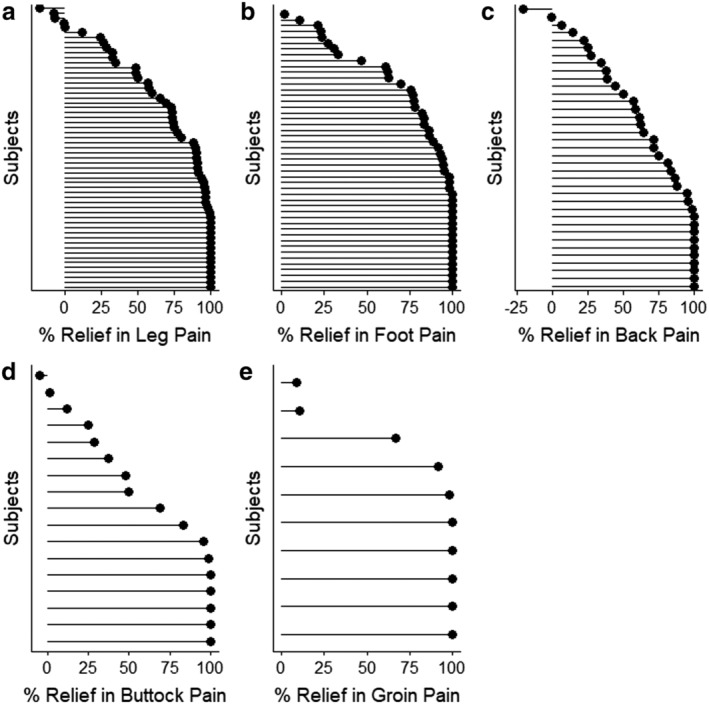
The top five pain locations are shown (A–E) in order of the number of subjects. The line informs the change from baseline and the circle is lined up at the percent relief calculated. Subjects are ordered in increasing order within each panel.
Four PROs describing quality of life (EQ‐5D), mood state (POMS, therapy satisfaction), and physical function (BPI, ODI) were available. Each instrument showed a substantial improvement at the 12‐month follow‐up visit. EQ‐5D index increased from 0.38 to 0.69. Mood disturbance assessed on POMS decreased from 25.6 at baseline to 7.3. As measured on BPI, pain severity decreased by 3.1 points, and pain interference decreased by 2.8. In patients with low back pain, disability reported on ODI reduced from severe disability at baseline (mean score of 43.8) to minimal disability (mean score of 17.5) (Table 3).
Table 3.
Patient‐Reported Outcomes Common Across Studies Summarized by a Weighted Mean.
| Outcome measure | Number of subjects at 12 months | Baseline | 12 months |
|---|---|---|---|
| Quality of Life (EQ‐5D Index) | 90 | 0.38 ± 0.09 | 0.69 ± 0.09 |
| Mood (POMS) | 97 | 25.6 ± 14.4 | 7.3 ± 13.0 |
| Function (BPI: Severity) | 138 | 7.0 ± 1.3 | 3.9 ± 2.0 |
| Function (BPI: Interference) | 138 | 6.1 ± 3.3 | 3.3 ± 2.3 |
| Oswestry Disability Index | 23 | 43.8 ± 11.8 | 17.5 ± 14.4 |
All studies reported procedure and device related complications. The most frequently reported complication was pain at the IPG pocket site (N = 26, 10.2%). A total of 15 lead fractures and 15 lead migrations (both 5.9%) were reported. Thirteen infections (5.1%) occurred across studies; seven of which at the IPG site. Additional reported complications were temporary motor stimulation (N = 12, 4.7%), all reported in one study, and dural puncture (N = 11, 4.3%) (Table 4). Of the 256 permanent implants, a total of 16 (6.3%) were explanted, and were due to infection (8/16), lack of pain relief (5/16), noncompliance (2/16), and by patient request (1/16).
Table 4.
Complications Due to the Device or Procedure in the Pooled Studies.
| Complications related to device or procedure | Number of events |
|---|---|
| Pain at IPG site | 26 |
| Lead Fracture | 15 |
| Lead Migration | 15 |
| Infection | 13 |
| Temporary motor stimulation | 12 |
| Dural puncture | 11 |
| Increased lead impedance | 5 |
| Loss of stimulation | 2 |
| Buzzing sound in one ear | 1 |
| Changes in sensation related to stimulation | 1 |
| Disconnection of the external trial stimulator | 1 |
| Increased pain after the trial implant procedure | 1 |
| Fell due to weakness in one leg | 1 |
| Transient motor deficit | 1 |
DISCUSSION
The aim of this study was to identify differences in effectiveness of DRG stimulation by pain etiology/or location and to investigate the generalizability and reproducibility of individual prospective studies following patients for 12 months. Our pooled analysis showed high responder rates and effectiveness of DRG neurostimulation for various pain etiologies, mainly CRPS‐I, causalgia, and low back pain. These results are consistent with independent, retrospective studies evaluating DRG stimulation in chronic pain conditions such as phantom limb pain, chronic pelvic pain, and groin pain 30, 31, 32.
Patients suffering with CRPS‐I and causalgia showed a 64% and 58% decrease in pain score at 12‐month follow‐up, respectively. Effective treatment options in severe CRPS‐I are limited, and first‐line treatments such as pharmacologic intervention or rehabilitation therapies are often unsuccessful in the long‐term, or the evidence is generally absent or unclear 33, 34. Kemler et al showed that SCS and physical therapy resulted in pain reduction and health‐related quality of life improvement in carefully selected CRPS‐I patients up to two years post‐permanent implant 35. The reduction in pain intensity was less than 50% (2.1 vs. 4.9) compared to what was obtained for CRPS‐I patients in current analysis. The ACCURATE trial showed superiority of DRG over SCS in CRPS‐I and causalgia of the lower extremity at 3‐ and 12‐month follow‐up 23.
Patients with FBSS (N = 39) showed a 3.5‐point decrease in overall pain score at 12 months (7.6–4.1). Comparatively, the recent PROMISE trial compared SCS plus optimal medical management (OMM) treatment to OMM only in a FBSS population 36. In the as‐treated analysis, SCS subjects reported a 2.0‐point reduction in mean back intensity (7.5–5.4) and a 1.6‐point reduction in leg pain intensity (5.2–3.7). The discrepancy in efficacy between our pooled analysis and the PROMISE trial suggests the utility of performing a comparative trial DRG vs. SCS in this patient population.
Two studies in the low back pain group examined more homogenous patient cohorts: pain after lumbar discectomy, and patients with non‐operated discogenic back pain 25, 26. Recurrent back pain can occur in up to 25% of patients post discectomy within two years 37. Both groups in these studies had high responder rates independent of a surgical intervention; 78% and 79%, respectively. Bilateral L2 placement was used exclusively in the discogenic back pain group and in most patients in the lumbar discectomy cohort. Patients were carefully selected to increase the success of accurate lead placement; those with extensive intra‐foraminal fibrosis or epidural adhesions were excluded.
PROs provide information on the impact of treatment on quality of life and physical function from the patient's perspective. One year after implantation, all patients exhibited a substantial improvement in functional outcomes (BPI, ODI), and psychological impact measures as reported on POMS. Patient reported quality of life was within the normal range; the 12 month EQ‐5D index was 0.69 compared with population norm of 0.86 ± 0.23 38. Furthermore, MCID of 0.074 was achieved by 67% of patients 39. Mood disturbance decreased to 7.3, which is below the score of unaffected individuals (12.7) 40. The MCID for BPI subscales pain severity and pain interference were reported as a 2.2‐ and 2.1‐point improvement 41; 58% and 50% of patients met these clinical impact scores, respectively. A clinically meaningful change in ODI (decrease by ≥13 points) was achieved by 61% of back pain patients 42. Park et al calculated a MCID of 9‐points in patients with pain post‐lumbar surgery 43; 83% of this patient population in our analysis met or exceeded MCID. Furthermore, 70% of patients reported an improvement by at least one disability category.
DRG neurostimulation has been shown to effectively treat discrete areas of pain, such as the groin and foot, which are typically more difficult to treat with traditional SCS without causing discomfort in non‐painful regions 27, 32, 44, 45. In these specific areas, our pooled analysis showed an 80% responder rate at one year. These results are comparable to other published literature. Morgalla et al reported >70% responder rate for focal (mainly groin and knee) pain at three years 28, 29. Moreover, a large retrospective study of 271 patients showed similar pain reduction regardless of focal area being treated 46. The results are consistent with those reported herein; the responder rate across the five most common focal areas treated was 74%. Their analysis also highlighted the importance of obtaining as much coverage over the painful area(s) as possible. This can be achieved by placing additional leads to recruit additional DRGs and authors recommended that a minimum of 2 leads be placed in any DRG stimulation trial 46.
Post‐market studies collect real world data, whereas randomized controlled trials are conducted under strict inclusion and exclusion criteria and tightly controlled settings. The only randomized controlled study assessing the efficacy of DRG stimulation to date is the ACCURATE trial 23. This trial showed an overall mean pain score reduction of 69% at 12 months follow‐up; 78% of the subjects responded to the therapy compared to a mean pain score reduction of 56% and a 63% responder rate in our pooled analysis. This slightly lower effectiveness in current analysis is not unexpected, given the addition of post‐market data collected in typical clinical settings and heterogeneous patient populations.
The procedural complications reported across the seven studies in our pooled analysis are in line to those observed for other neurostimulation therapies 47. Pain at IPG pocket site was the most commonly complication. A subset of these were due to infection, of which all but one was resolved with antibiotic treatment. However, it is unlikely that all possible complications have been identified in our pooled sample of 256 patients. A recent safety analysis of post‐market surveillance data collected from an internal complaint reporting and handling database showed a 3.2% event rate for DRG stimulation devices similar to that reported for SCS in the same time frame (3.1%) 48. The most frequently observed events in this surveillance study are also those identified by our analysis.
Study Limitations
Data of most patients in our analysis come from industry‐sponsored studies; there is a need to verify current results in independent studies. Our study was set up to present pooled data up to one‐year post‐permanent implant as this is the most common follow‐up period in published literature. The field is moving toward collecting longer term outcomes as exemplified by recent congress presentations 49, 50, 51, 52. These independent studies support effective pain relief and quality of life improvements up to three years post‐implant. Future studies should continue to collect longer term outcomes to support a sustained therapeutic effect and ascribe to the initiative on methods, measurement, and pain assessment in clinical trials recommendations 53, 54. Furthermore, for a full review of patient experience, additional investigations should be made publicly available such as audits of clinical practice as well as national medical device reporting databases and registries.
Pooling of data has disadvantages as it is difficult to minimize various sources of bias and studies are often heterogeneous. However, eligibility criteria, study design, and follow‐up durations were similar across the studies included herein. Although the mean weighted by sample size does not approach meta‐analysis methodology, this is the first study to present combined DRG effectiveness of a substantial patient population.
CONCLUSIONS
DRG stimulation is an effective therapy for multiple chronic pain disorders. It is a safe and widely used therapy for patients that have failed to receive pain relief and quality of life improvements from other interventions. With the advent of new stimulation modulations in recent years, it is important that treatments are tailored to specific diagnoses and conditions. The DRG stimulation therapy is most successful for treating focal pain in carefully selected patients.
Authorship Statement
Marie Fahey and Robyn Capobianco designed the study and were involved in drafting/revising the manuscript, data collection, and the statistical analysis. Bram Blomme was involved in drafting/revising the manuscript, data collection, and the statistical analysis. Frank Huygen, Jan Willem Kallewaard, Harold Nijhuis, Liong Liem, Jan Vesper, Matthias Morgalla, and Timothy Deer were involved in data collection, and drafting/revising the manuscript. All authors approved the final manuscript.
COMMENTS
This paper is an important for several reasons. Firstly, it shows the pooled results of prospective studies for DRG stimulation and they are robust with a low complication rate. Secondly, as 5 of the 7 studies were industry sponsored it shows what we know, namely that early prospective studies of new technology are likely to have industry sponsorship and a minority of sponsorship independent investigator studies. This, when conjoined with a combined industry/clinician authorship does not demonstrate bias per se but does cause one to seek additional ways to have the therapy assessed as the authors correctly outlined. For myself, I look for stages of proof of a therapy. First comes the pivotal trial, then replication trials. Then, pooled analysis of prospective trials. Then, real world audits of clinical practice. Then, registries of outcomes for efficacy and complications. Only then do I feel I ‘know’ a therapy. Clearly we are progressing along that path with DRG stimulation, but have further to go.
Apropos of that is that rare complications are unlikely to show up in early prospective studies. In Australia, we have had one paraplegia and one tetraplegia after DRG stimulator implant. Rare and devastating complications such as these more commonly appear in registries and explain the almost universal clinician desire for such data collection. DRG stimulation provides a unique way to access and modulate the nervous system. How it will compare with spinal cord stimulation will very much depend on the progress made in both areas over the next ten years. Whilst the advances in SCS are coming thick and fast, I would like to see a similar surge in research and development in the DRG sphere. I believe there can be more benefit to come.
Marc Russo, MBBS
Sydney, Australia
***
DRG Stimulation has been nothing short of a breakthrough for the field. Conditions like amputee pain, pelvic pain, post herniorrhaphy pain, post thoracotomy pain, and post mastectomy pain were largely out reach before DRG stimulation became available, but now we have a powerful tool that has put these conditions well within reach insofar as our ability to offer an effective treatment and give our patients hope. But as the past has taught us, every medical breakthrough comes with questions and hesitation. DRG stimulation is a departure from traditional SCS on many levels, but none is more apparent than the means by which the leads are deployed within the epidural space. Since it became available in the United States in 2016, much has been made of whether or not DRG stimulation is “safe” or “worth the risk.” This publication is crucial for assuaging those fears and putting apprehension to rest with black and white data that not only proves once again how effective this therapy is, but that it is safe.
Corey Hunter, MD
New York City, NY, USA
***
I commend Frank Huygen and colleagues for their excellent work summarizing the available clinical evidence on the effectiveness and safety of dorsal root ganglion stimulation. Their paper will prove very useful to all of who use this therapy with our patients.
Christopher Gilligan, MD
Boston, MA, USA
Comments not included in the Early View version of this paper.
Conflict of Interest: Frank Huygen serves as a paid consultant for Abbott, Medtronic, and Grunenthal. Jan Willem Kallewaard serves as a paid consultant for Abbott, Boston Scientific, and Saluda. Harold Nijhuis serves as a paid consultant for Saluda Medical and Abbott. Liong Liem has no disclosures. Jan Vesper serves as a paid consultant for Abbott, Boston Scientific, Medtronic, and Biotronic. Marie Fahey, Bram Blomme, and Robyn Capobianco are employees of Abbott (formerly St. Jude Medical). Matthias Morgalla serves as a paid consultant for Abbott. Timothy Deer serves as a paid consultant for Abbott, Axonics, Bioness, Flowonix, Mainstay, Nalu, Saluda, SpineThera, Vertiflex, and Vertos.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Sources of Financial Support: Not applicable.
[The copyright line for this article was changed on 18 February 2020 after original online publication.]
REFERENCES
- 1. Becker A, Held H, Redaelli M et al. Low back pain in primary care: Costs of care and prediction of future health care utilization. Spine (Phila Pa 1976) 2010;35:1714–1720. [DOI] [PubMed] [Google Scholar]
- 2. Dahlhamer J, Lucas J, Zelaya C et al. Prevalence of chronic pain and high‐impact chronic pain among adults — United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67:1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Hecke O, Torrance N, Smith BH. Chronic pain epidemiology and its clinical relevance. Br J Anaesth 2013;111:13–18. [DOI] [PubMed] [Google Scholar]
- 4. International Association for the Study of Pain . Unrelieved pain is a major global healthcare problem. Washington, D.C.: International Association for the Study of Pain; 2013.
- 5. Nicholson B. Differential diagnosis: Nociceptive and neuropathic pain. Am J Manag Care 2006;12:S256–S262. [PubMed] [Google Scholar]
- 6. International organisation for the Study of Pain . IASP Terminology 2019. https://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698.
- 7. Sheng SR, Wang XY, Xu HZ, Zhu GQ, Zhou YF. Anatomy of large animal spines and its comparison to the human spine: A systematic review. Eur Spine J 2010;19:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung JM, Chung K. Importance of hyperexcitability of DRG neurons in neuropathic pain. Pain Pract 2002;2:87–97. [DOI] [PubMed] [Google Scholar]
- 9. Sapunar D, Ljubkovic M, Lirk P, McCallum JB, Hogan QH. Distinct membrane effects of spinal nerve ligation on injured and adjacent dorsal root ganglion neurons in rats. Anesthesiology 2005;103:360–376. [DOI] [PubMed] [Google Scholar]
- 10. Cregg R, Momin A, Rugiero F, Wood JN, Zhao J. Pain channelopathies. J Physiol 2010;588:1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gemes G, Koopmeiners A, Rigaud M et al. Failure of action potential propagation in sensory neurons: Mechanisms and loss of afferent filtering in C‐type units after painful nerve injury. J Physiol 2013;591:1111–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res 2009;196:115–128. [DOI] [PubMed] [Google Scholar]
- 13. Nakamura SI, Myers RR. Injury to dorsal root ganglia alters innervation of spinal cord dorsal horn lamina involved in nociception. Spine (Phila Pa 1976) 2000;25:537–542. [DOI] [PubMed] [Google Scholar]
- 14. Koopmeiners AS, Mueller S, Kramer J, Hogan QH. Effect of electrical field stimulation on dorsal root ganglion neuronal function. Neuromodulation 2013;16:304–311. [DOI] [PubMed] [Google Scholar]
- 15. Krames ES. The dorsal root ganglion in chronic pain and as a target for neuromodulation: A review. Neuromodulation 2015;18:24–32. [DOI] [PubMed] [Google Scholar]
- 16. Vuka I, Vucic K, Repic T, Ferhatovic Hamzic L, Sapunar D, Puljak L. Electrical stimulation of dorsal root ganglion in the context of pain: A systematic review of in vitro and in vivo animal model studies. Neuromodulation 2018;21:213–224. [DOI] [PubMed] [Google Scholar]
- 17. Gravius N, Chaudhry SR, Muhammad S et al. Selective L4 dorsal root ganglion stimulation evokes pain relief and changes of inflammatory markers: Part I profiling of saliva and serum molecular patterns. Neuromodulation 2019;22:44–52. [DOI] [PubMed] [Google Scholar]
- 18. Lagauche D, Facione J, Albert T, Fattal C. The chronic neuropathic pain of spinal cord injury: Which efficiency of neuropathic stimulation? Ann Phys Rehabil Med 2009;52:180–187. [DOI] [PubMed] [Google Scholar]
- 19. Turner JA, Hollingworth W, Comstock BA, Deyo RA. Spinal cord stimulation for failed back surgery syndrome: Outcomes in a workers' compensation setting. Pain 2010;148:14–25. [DOI] [PubMed] [Google Scholar]
- 20. Ubbink DT, Vermeulen H. Spinal cord stimulation for non‐reconstructable chronic critical leg ischaemia. Cochrane Database Syst Rev 2013;2:CD004001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morgalla MH, de Barros Filho MF, Chander BS, Soekadar SR, Tatagiba M, Lepski G. Neurophysiological effects of dorsal root ganglion stimulation (DRGS) in pain processing at the cortical level. Neuromodulation 2019;22:36–43. [DOI] [PubMed] [Google Scholar]
- 22. Thong ISK, Jensen MP, Miro J, Tan G. The validity of pain intensity measures: What do the NRS, VAS, VRS, and FPS‐R measure? Scand J Pain 2018;18:99–107. [DOI] [PubMed] [Google Scholar]
- 23. Deer TR, Levy RM, Kramer J et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: A randomized comparative trial. Pain 2017;158:669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huygen F, Liem L, Nijhuis H, Cusack W, Kramer J. Evaluating dorsal root ganglion stimulation in a prospective Dutch cohort. Neuromodulation 2019;22:80–86. [DOI] [PubMed] [Google Scholar]
- 25. Kallewaard JW, Edelbroek C, Terheggen M, Raza A, Geurts JW. A prospective study of dorsal root ganglion stimulation for non‐operated discogenic low back pain. Neuromodulation: 2019. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 26. Kallewaard JW, Nijhuis H, Huygen F et al. Prospective cohort analysis of DRG stimulation for failed Back surgery syndrome pain following lumbar discectomy. Pain Pract 2019;19:204–210. [DOI] [PubMed] [Google Scholar]
- 27. Liem L, Russo M, Huygen FJ et al. One‐year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic pain. Neuromodulation 2015;18:41–48. [DOI] [PubMed] [Google Scholar]
- 28. Morgalla MH, Bolat A, Fortunato M, Lepski G, Chander BS. Dorsal root ganglion stimulation used for the treatment of chronic neuropathic pain in the groin: A single‐center study with long‐term prospective results in 34 cases. Neuromodulation 2017;20:753–760. [DOI] [PubMed] [Google Scholar]
- 29. Morgalla MH, Fortunato M, Lepski G, Chander BS. Dorsal root ganglion stimulation (DRGS) for the treatment of chronic neuropathic pain: A single‐center study with long‐term prospective results in 62 cases. Pain Physician 2018;21:E377–E387. [PubMed] [Google Scholar]
- 30. Eldabe S, Burger K, Moser H et al. Dorsal root ganglion (DRG) stimulation in the treatment of phantom limb pain (PLP). Neuromodulation 2015;18:610–616. [DOI] [PubMed] [Google Scholar]
- 31. Hunter CW, Yang A. Dorsal root ganglion stimulation for chronic pelvic pain: A case series and technical report on a novel Lead configuration. Neuromodulation 2019;22:87–95. [DOI] [PubMed] [Google Scholar]
- 32. Schu S, Gulve A, Eldabe S et al. Spinal cord stimulation of the dorsal root ganglion for groin pain‐a retrospective review. Pain Pract 2015;15:293–299. [DOI] [PubMed] [Google Scholar]
- 33. Harden RN, Oaklander AL, Burton AW et al. Complex regional pain syndrome: Practical diagnostic and treatment guidelines, 4th edition. Pain Med 2013;14:180–229. [DOI] [PubMed] [Google Scholar]
- 34. Smart KM, Wand BM, O'Connell NE. Physiotherapy for pain and disability in adults with complex regional pain syndrome (CRPS) types I and II. Cochrane Database Syst Rev 2016;2:CD010853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kemler MA, De Vet HC, Barendse GA, Van Den Wildenberg FA, Van Kleef M. The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: Two years' follow‐up of the randomized controlled trial. Ann Neurol 2004;55:13–18. [DOI] [PubMed] [Google Scholar]
- 36. Rigoard P, Basu S, Desai M et al. Multicolumn spinal cord stimulation for predominant back pain in failed back surgery syndrome patients: A multicenter randomized controlled trial. Pain 2019;160:1410–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parker SL, Mendenhall SK, Godil SS et al. Incidence of low Back pain after lumbar discectomy for herniated disc and its effect on patient‐reported outcomes. Clin Orthop Relat Res 2015;473:1988–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kind P, Hardman G, Macran S. UK Population norms for EQ‐5D: The University of York. Centre for Health Economics; 1999. https://www.york.ac.uk/che/pdf/DP172.pdf.
- 39. Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ‐5D and SF‐6D. Qual Life Res 2005;14:1523–1532. [DOI] [PubMed] [Google Scholar]
- 40. Nyenhuis DL, Yamamoto C, Luchetta T, Terrien A, Parmentier A. Adult and geriatric normative data and validation of the profile of mood states. J Clin Psychol 1999;55:79–86. [DOI] [PubMed] [Google Scholar]
- 41. Mease PJ, Spaeth M, Clauw DJ et al. Estimation of minimum clinically important difference for pain in fibromyalgia. Arthritis Care Res (Hoboken) 2011;63:821–826. [DOI] [PubMed] [Google Scholar]
- 42. Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY. Minimum clinically important difference in lumbar spine surgery patients: A choice of methods using the Oswestry disability index, medical outcomes study questionnaire short form 36, and pain scales. Spine J 2008;8:968–974. [DOI] [PubMed] [Google Scholar]
- 43. Park KB, Shin JS, Lee J et al. Minimum clinically important difference and substantial clinical benefit in pain, functional, and quality of life scales in failed Back surgery syndrome patients. Spine (Phila Pa 1976) 2017;42:E474–E481. [DOI] [PubMed] [Google Scholar]
- 44. Zuidema X, Breel J, Wille F. Paresthesia mapping: A practical workup for successful implantation of the dorsal root ganglion stimulator in refractory groin pain. Neuromodulation 2014;17:665–669. [DOI] [PubMed] [Google Scholar]
- 45. Liem L, Mekhail N. Management of Postherniorrhaphy chronic neuropathic groin pain: A role for dorsal root ganglion stimulation. Pain Pract 2016;16:915–923. [DOI] [PubMed] [Google Scholar]
- 46. Hunter CW, Sayed D, Lubenow T et al. DRG FOCUS: A multicenter study evaluating dorsal root ganglion stimulation and predictors for trial success. Neuromodulation 2019;22:61–79. [DOI] [PubMed] [Google Scholar]
- 47. Eldabe S, Buchser E, Duarte RV. Complications of spinal cord stimulation and peripheral nerve stimulation techniques: A review of the literature. Pain Med 2016;17:325–336. [DOI] [PubMed] [Google Scholar]
- 48. Deer T, Pope J, Hunter C et al. Safety analysis of dorsal root ganglion stimulation in the Treatment of chronic pain. Neuromodulation 2019; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kretzschmar M, Reining M, Jünemann T, Kielstein S, Felthöfer L. Dorsal Root Ganglion Stimulation (DRGS) in the treatment of neuropathic pain after peripheral nerve injury of upper and lower extremities ‐ three‐years folow‐up in 21 patients. 14th World Congress International Neuromodulation Society, Sydney, Australia, May 25–30, 2019.
- 50. Kretzschmar M. Treatment of chronic post‐surgical knee pain after total knee endoprosthesis with dorsal root ganglion stimulation (DRGS) ‐ retrospective analysis after 36 months. 14th World Congress International Neuromodulation Society, Sydney, Australia, May 25–30, 2019.
- 51. Peña I, Casado G, Jiménez P, Vancamp T, Portillo G. Dorsal Root Ganglion Stimulation (DRG): Long‐Term Experience with 100 Patients. 14th World Congress International Neuromodulation Society, Sydney, Australia, May 25–30, 2019.
- 52. Slotty P, Schu S, Chatzikalfas A, Vesper J. Dorsal Root Ganglion Stimulation under Real Life Conditions ‐ a Single‐center Case Series of 115 Patients. 14th World Congress International Neuromodulation Society, Sydney, Australia, May 25–30, 2019.
- 53. Dworkin RH, Turk DC, Peirce‐Sandner S et al. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain 2010;149:177–193. [DOI] [PubMed] [Google Scholar]
- 54. Gewandter JS, Dworkin RH, Turk DC et al. Research design considerations for chronic pain prevention clinical trials: IMMPACT recommendations. Pain 2015;156:1184–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]


