Summary
The main objectives in Crohn's disease are to avoid disease complications and preserve the patient's quality of life. Early disease control and close monitoring with specific targets to reach might be the only way to change the disease course. In two decades, we have moved from clinical response to full remission (clinical and endoscopic remission) requiring a tight monitoring of both symptoms and objective signs of inflammation. This review summarizes the concepts of tight control and treat-to-target and their potential for disease modification.
Keywords: Tight control, Crohn's disease, treat-to-target
Clinical case
A 31-years old female with smoking habit presented with chronic diarrhoea and abdominal pain. Diagnosis of moderate ileocaecal Crohn's disease (CD) was made based on the presence of five aphthous erosions at initial colonoscopy. The first line of treatment was oral budesonide. Given the persistence of elevated C-reactive protein (CRP) at 10mg/L and faecal calprotectin (FC) at 350 µg/g at 3 months and despite the absence of symptoms, adalimumab treatment was initiated. Because of the presence of persistent erosions at colonoscopy at 6 months, the treatment was optimized with adalimumab 80 mg every other week. Ten years later, the patient has no disability, normal biomarkers (CRP and FC), no bowel damage at magnetic resonance imaging and did not undergo surgery.
Introduction
CD is a chronic and progressive state of the digestive tract, which can lead to gradual and cumulative bowel damage by altering the parietal architecture resulting in complications such as strictures, fistulae, surgery, intestinal failure and cancer, causing subsequent disability.1 The Lémann Index is a validated score to assess and quantify bowel damage, which can be used to evaluate the impact of therapeutic strategies on CD course.2 Historically, the primary objective of treatment in therapeutic trials and clinical practice in CD was to induce and maintain symptomatic remission. This approach failed to clearly modify the natural course of CD.3 Similar to other inflammatory diseases such as rheumatoid arthritis,4 new theories such as treat-to-target (T2T) and tight control have emerged. T2T involves identification of a pre-specified target to be reached with therapy, followed by adapted modifications of treatment and repeated monitoring until the target is reached, in a tailored way for the patients regarding their individual needs.5 With the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) consensus, treatment goals in CD have moved to ‘deep remission’, which is defined by reaching both symptomatic and endoscopic remission (defined as no ulceration at ileocolonoscopy). There are well known discrepancies between clinical symptoms and endoscopical lesions in CD6 and clinical evaluation is not a reliable criteria to lead modification of treatment to control persistent mucosal inflammation, underling the need for an accurate target in order to assess treatment response. Biomarkers (CRP and FC) were not targets in STRIDE but only adjunctive measures of inflammation for monitoring in CD due to insufficient evidence to recommend treatment optimization using biomarkers alone.5 As it is impossible to repeat colonoscopy, a trial called CALM investigated the effectiveness and safety of two treatment strategies in achieving endoscopical remission in patients with CD by optimizing treatment according to predefined failure criteria: clinical evaluation with adjunctive measures of inflammation (CRP and FC) in the tight control group or clinical evaluation alone in the clinical management group.7 This trial allowed a prospective validation of tight control strategies based on careful and continuous surveillance of the disease activity by validated composite measurements, and early therapeutic optimization or change of treatment if necessary7 (Figure 1). This review will discuss four challenging questions: what are optimal targets in CD? Can endoscopy be replaced in the context of tight monitoring? How can the course of CD be modified? Should poor prognostic factors be abandoned in the era of tight monitoring?
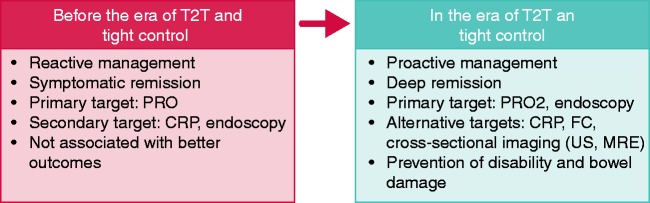
Figure 1.
Changes in Crohn's disease management over the past decade.
Before the era of T2T and tight control, patient management was guided by symptoms and only CRP was routinely measured. Other examinations were performed on demand. Residual inflammation could progressively alter the bowel wall and lead to significant damage, with strictures or fistulas requiring disabling surgery.
T2T = treat-to-target; PRO = patient reported outcomes; CRP = C-reactive protein; FC = faecal calprotectin; US = ultrasonography; MRE = magnetic resonance enterography.
What is the optimal target in CD?
Patient reported outcomes
Patient reported outcome (PRO) is the assessment of the patient perception about their symptoms, functional status and well-being. In CD, symptom-based PRO measures (PRO-2) are composed of the two most prominent symptoms, which are abdominal pain and stool frequency.8 The PRO-2 goal should be resolution of abdominal pain and normalization of bowel habit.5 The CD Activity Index is commonly used in inflammatory bowel disease (IBD) trials to assess clinical activity of the disease.9,10 A patient should undergo clinical evaluation every 3 months during active disease and every 6–12 months for quiescent disease.5 In order to evaluate disability resulting from IBD, the IBD Disability Index (IBD-DI) was developed in 201211,12 and validated in a French population-based study with high internal consistency, inter-observer reliability and construct validity, and moderate intra-observer reliability.13 Disability is a major stake and should be prevented in IBD. Therefore the IBD-DI should be integrated in therapeutic trials and clinical practice as a principal secondary endpoint.13 However because of the poor reliability of clinical evaluation to guide treatment decisions and the lack of change in the disease course with symptoms-based strategies more objective targets are necessary to prevent bowel damage and resultant disability.
Endoscopy
Endoscopy remains the gold standard to assess disease activity in ileocolonic CD. There are two endoscopic scoring systems used in CD: the CD Endoscopic Index of Severity (CDEIS) and the Simple Endoscopic Score for CD (SES-CD). SES-CD was developed to respond to the practical limitations of the original score, which is complex and time-consuming to use. The two scores are highly correlated.14,15 Both scores were found to be responsive to change in a prospective study.16 The definition of endoscopic remission commonly admitted is a CDEIS <3 or a SES-CD ≤2.17 These scores remain little-used in practice,18 so STRIDE adopted a simpler goal (resolution of ulceration).5 Endoscopic evaluation should be made at a minimum of 3 months after initiation of treatment and preferably between 6 and 9 months, because lower rates of mucosal healing have been found with early evaluation.19,20 Achieving deep remission is the goal in CD5 because it is associated with better outcomes so endoscopic remission became a major stake. In the ACCENT I trial testing infliximab for moderate to severe CD, there was a trend towards fewer hospitalizations and surgeries in patients with mucosal healing at weeks 10 and 54.21 Likewise, the absence of mucosal ulceration at ileocolonoscopy within 1 year of diagnosis or initiating therapy has been associated with reduced corticosteroid use and decreased clinical disease activity, fewer abdominal surgeries related to CD,22 and predicts sustained steroid-free remission 3 and 4 years after therapy initiation.23 Endoscopic remission (or mucosal healing) on the first post-treatment endoscopy was associated with a higher rate of sustained clinical remission, maintenance of mucosal healing, and lower risk of CD-related surgery.24 Endoscopy is the main target but PRO should not be neglected because deep remission was associated with lower risk of major adverse events compared with endoscopic remission alone.25
Histology
Data concerning the role of histologic remission on disease outcomes are scare and mainly retrospective. Compared with endoscopic remission, histologic remission was associated with a lower risk of clinical relapse.26 In the recent trial comparing ustekinumab and placebo in CD, histologic response at week 8 was significantly associated with long-term outcomes of clinical response, clinical remission, mucosal healing, and endoscopic remission at week 44.27 Given the scant data concerning the predictive role of histological remission in CD, as well as concerns of sampling issues due to the patchy and transmural nature of the disease and the absence of a validated histologic scoring system, histology is not yet recommended as a target in CD.5
Can we replace endoscopy in the context of tight monitoring?
Radiologic targets
Cross-sectional imaging techniques are not primary targets in CD but are complementary tools to endoscopy, especially if the diseased segment cannot be accessed.5 Radiological assessment is less invasive, can evaluate the small bowel, and provide information about the transmural nature of inflammation. Ultrasonography (US), computed tomography enterography (CTE), and magnetic resonance enterography (MRE) can be used according to the patient situation with an equivalent accuracy.28 CTE has shown high accuracy in the assessment of disease but exposes subjects to ionizing radiation29 and should be abandoned outside the emergency setting. MRE is a non-ionizing technique that has been found to be predictive for disease outcomes in CD. In a prospective study including 214 patients with inactive disease on MRE, rates of therapy optimization, hospitalization and surgery at 1 year were significantly lower.30 The Nancy score is an MRE score that has been shown to accurately detect endoscopic healing in CD, and was found to be responsive to change after treatment.31 It showed good accuracy with 80% specificity and 70% sensitivity for the diagnosis of endoscopic healing.31 Mucosal healing on MR after treatment corresponding to a Nancy score <6 was also associated with lower risk of surgery.31The Nancy score is usable in practice for tight monitoring as it does not require fasting or colonic preparation. The simplified MaRIA score (Magnetic Resonance Index of Activity) is another score that strongly correlates with endoscopical findings32 but requires bowel preparation and its predictive value is pending. US is a widely available, cheap, non-invasive, time-efficient and well-tolerated technique. It was found to be highly correlated with MRE, and US-guided strategies showed good concordance with both cross-sectional imaging and colonoscopy, demonstrating its decision-making relevance for CD patients.33 Several US scores are available for CD but most of them have been developed through suboptimal processes or their predictive value has not been demonstrated.34
CRP
CRP is a broadly used and studied biomarker in CD. When CD patients have raised CRP levels at diagnosis, variation of CRP concentration may help to monitor response to treatment. In CD, elevated CRP is correlated with clinically active disease and endoscopic and histologic inflammations.35 CRP was found to be predictive of relapse in CD patients with elevated levels at diagnosis36 but approximately 20% of CD patients do not have increased CRP during flares37 and an elevated CRP level may be provoked by an extra-intestinal cause.37 A CRP level ≥5 mg/L was found to have a 92% specificity for predicting active endoscopic CD but only 49% sensitivity.38 A decrease of CRP might be a better predictor of long-term outcomes than the baseline level. In a post hoc analysis of the ACCENT I randomized controlled trial (RCT), patients with CRP ≥5 mg/L at week 14 had a probability of sustained response of 37.2% compared with 56.6% in patients with CRP <5 mg/L.39 In another study, CRP normalization <10 mg/L at week 12 was also predictive of endoscopic response at week 52 with a positive predictive value of 79%.40 However, CRP is not indicative of CD as it reflects systemic inflammation and is poorly correlated with endoscopical healing, but remains a complimentary tool to endoscopy and FC.
Faecal calprotectin
In CD an FC level <250 µg/g is predictive of mucosal healing (corresponding to CDEIS < 3) with a sensitivity of 94% and a specificity of 62%.41 Conversely, an FC concentration >250 µg/g has a positive predictive value of 78.4% for the presence of ulcers in CD patients with colonic involvement.41 After surgery, available evidence showed that FC values <100 µg/g strongly suggest no recurrent disease.42 In CD asymptomatic patients, two consecutive elevated FC levels were associated with a higher risk of relapse within 3 months.43 In a cohort of patients with CD treatment with tumor-necrosis factor (TNF) antagonists, a concentration of FC ≤100 µg/g after induction therapy was highly associated with clinical remission at 1 year.44 FC concentration may vary with disease location with FC levels lower in patients with ileal disease compared with those with colonic involvement45; however, any abnormal FC value must guide treatment decision regardless of ileal or colonic involvement, and initial investigations in CD should include FC at the first colonoscopy.46 FC might also guide decisions for de-escalation.
How to modify the course of CD? From early intervention to early disease control
Two RCTs (RAPID and AZTEC) studied early initiation of azathioprine in CD (before 6 months compared with conventional management and before 8 weeks compared with placebo respectively), but neither of these trials showed a clear benefit.47,48 Early introduction of combined immunosuppression (before 12 weeks) was studied in the RCT REACT, including 1819 patients first treated with corticosteroids.49 In this study, combination therapy with adalimumab or infliximab associated with an immunosuppressant (thiopurine or methotrexate) did not result in a better clinical remission (corticosteroid-free remission, defined by the Harvey–Bradshaw Index ≤4) at 1 year compared with conventional management,49 however, many patients in the trial had had CD for several years. Conversely, patients treated with early combination therapy had a significant reduction in serious adverse events such as surgery,49 suggesting that early initiation of potent agents might change the natural history of CD. Other trials showed that patients with recent disease reached remission more frequently than patients with longstanding disease whether treated with adalimumab alone,50 certolizumab51 or vedolizumab.52 As already shown in rheumatology,53 these studies underline the importance of early intervention in CD. More importantly, in the light of the CALM results, early control of disease activity more than early intervention might be the key to changing the natural history of CD. The results were consistent across studies regardless of the mechanism of action of the treatments administered, suggesting that the nature of the first treatment might no be determinant. Ten to twenty percent of patients with mild to moderate CD will have an uncomplicated evolution,54 and tight control might prevent overtreatment in this population. These patients, who are more likely to remain quiescent after treatment for the first flair, might, for example, be treated with one course of budesonide for mild ileitis or one course of prednisolone for mild colitis associated with close monitoring.
Should we abandon poor prognostic factors in the era of tight monitoring?
Current guidelines in CD identified four poor prognosis factors: perianal involvement, ileocolonic and jejunal location, diagnosis of CD before the age of 40 years, and the need to treat the initial flare with steroids.46 Current smoking and penetrating or stricturing disease behaviour are also risk factors for surgery in CD.55 Patients with more than one poor prognosis factor could benefit from early introduction of biologics.46 This is a field of active research because reliable risk factors might individualize patients that need intensive therapy in order to prevent complications and, conversely, avoid overtreatment in patients with good disease prognosis. More prognosis factors (such as serologic or genetic factors) were identified, especially in children56 but were never implemented in clinical practice mainly due to their insufficient predictive value. These prognostic factors appear to be even less significant if patients are closely monitored; exempting patients with multiple risk factors, tight monitoring with a rapid step-up strategy should be recommended with the ultimate aim of preventing disabilities and bowel damage.
Discussion
The primary goal of CD management should be the prevention of long-term disability and bowel damage. In the past decade patients have been undertreated because of strategies targeting only the symptoms. In 2010 the concepts of early CD and the window of opportunity emerged,57,58 leading broadly to the early use of potent agents (mainly anti-TNF therapy) in a top-down strategy. However, this strategy might lead to overtreatment in the 10–20% of patients with a benign natural history,54 and raises both economic and safety concerns. Early disease control based on close monitoring using non-invasive radiologic and/or biological markers might be the answer to altering the natural course of CD and maximizing the risk–benefit ratio of such a strategy. Proactive therapeutic drug monitoring was also recently validated in a prospective study and appears to be associated with better long-term outcomes.59 It might be a useful complementary monitoring tool. We refer the readers to recent review articles on the clinical utility of drug monitoring in IBD.60 Many poor prognostic factors have been described in CD,61–63 which may guide treatment decisions in an attempt to avoid complications or overtreatment; however, this approach appears less relevant if there is tight control of disease activity, except in patients with multiple risk factors who should benefit from early introduction of biologics. Some factors may restrain the applicability and acceptance of T2T in everyday practice. The ongoing REACT2 study (ClinicalTrials.gov NCT01698307)64 comparing clinical remission associated with endoscopical healing versus clinical remission alone using objective outcomes may allow prospective validation of T2T in the future. In the years to come we might be even more ambitious by achieving transmural and histological healing given the increase in our armamentarium. The ongoing CURE trial (Clinical Trial NCT03306446),65 for example, will explore whether drug de-escalation can be considered in CD patients who benefited from early disease control with anti-TNF therapy and tight monitoring.
Declaration of conflicting interests
Thomas Chateau has no conflicts of interest to disclose. Peyrin-Biroulet reports personal fees from AbbVie, Janssen, Genentech, Ferring, Tillots, Pharmacosmos, Celltrion, Takeda, Boerhinger Ingelheim, Pfizer, Index Pharmaceuticals, Sandoz, Celgene, Biogen, Samsung Bioepis, Alma, Sterna, Nestle, Enterome, Allergan, MSD, Roche, Arena, Gilead, Hikma, Amgen; grants from AbbVie, MSD and Takeda; and stock options: CTMA.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Torres J, Mehandru S, Colombel JF, et al. Crohn's disease. Lancet 2017; 389: 1741–1755. [DOI] [PubMed] [Google Scholar]
- 2.Pariente B, Mary J-Y, Danese S, et al. Development of the Lémann Index to assess digestive tract Damage in patients With Crohn's Disease. Gastroenterology 2014; 148: 52–63. . [DOI] [PubMed] [Google Scholar]
- 3.Bouguen G, Peyrin-Biroulet L. Surgery for adult Crohn's disease: what is the actual risk?. Gut 2011; 60: 1178–1181. [DOI] [PubMed] [Google Scholar]
- 4.Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 2010; 69: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peyrin-Biroulet L, Sandborn W, Sand BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol 2015; 110: 1324–1338. [DOI] [PubMed]
- 6.Peyrin-Biroulet L, Reinisch W, Colombel J-F, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn's disease in the SONIC trial. Gut 2014; 63: 88–95. [DOI] [PubMed] [Google Scholar]
- 7.Colombel J-F, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn's disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 2017; 390: 2779–2789. [DOI] [PubMed] [Google Scholar]
- 8.Bojic D, Bodger K, Travis S, et al. Patient reported outcome measures (PROMs) in inflammatory bowel disease: new data. J Crohns Colitis 2017; 1: S576– S585. . [DOI] [PubMed] [Google Scholar]
- 9.Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology 1976; 70: 439–444. [PubMed] [Google Scholar]
- 10.Thia KT, Sandborn WJ, Lewis JD, et al. Defining the optimal response criteria for the Crohn's Disease Activity Index for induction studies in patients with mildly to moderately active Crohn's disease. Am J Gastroenterol 2008; 103: 3123–3131. [DOI] [PubMed] [Google Scholar]
- 11.Peyrin-Biroulet L, Cieza A, Sandborn WJ, et al. Development of the first disability index for inflammatory bowel disease based on the International Classification of Functioning, Disability and Health. Gut 2012; 61: 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gower-Rousseau C, Sarter H, Savoye G, et al. Validation of the Inflammatory Bowel Disease Disability Index in a population-based cohort. Gut 2017; 66: 588–596. [DOI] [PubMed] [Google Scholar]
- 13.Peyrin-Biroulet L, Gower-Rousseau C. The IBD Disability Index should become a major secondary endpoint in clinical practice and in clinical trials. J Crohns Colitis 2016; 10: 1375–1377. [DOI] [PubMed] [Google Scholar]
- 14.Daperno M, D'Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc 2004; 60: 505–512. [DOI] [PubMed] [Google Scholar]
- 15.Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn's disease: a prospective multicentre study. Groupe d'Etudes Therapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut 1989; 30: 983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khanna R, Zou G, Stitt L, et al. Responsiveness of endoscopic indices of disease activity for Crohn's disease. Am J Gastroenterol 2017; 112: 1584–1592. [DOI] [PubMed] [Google Scholar]
- 17.Vuitton L, Marteau P, Sandborn WJ, et al. IOIBD technical review on endoscopic indices for Crohn's disease clinical trials. Gut 2016; 65: 1447–1455. [DOI] [PubMed] [Google Scholar]
- 18.Duchesne C, Faure P, Kohler F, et al. Management of inflammatory bowel disease in France: a nationwide survey among private gastroenterologists. Dig Liver Dis 2014; 46: 675–681. [DOI] [PubMed] [Google Scholar]
- 19.Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn's disease: data from the EXTEND trial. Gastroenterology 2012; 142: 1102–1111. [DOI] [PubMed] [Google Scholar]
- 20.Rutgeerts P, Diamond RH, Bala M, et al. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn's disease. Gastrointest Endosc 2006; 63: 433–442. [DOI] [PubMed] [Google Scholar]
- 21.Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011; 141: 1194–1201. [DOI] [PubMed] [Google Scholar]
- 22.Colombel JF, Rutgeerts PJ, Sandborn WJ, et al. Adalimumab induces deep remission in patients with Crohn's disease. Clin Gastroenterol Hepatol 2014; 12: 414–422. [DOI] [PubMed] [Google Scholar]
- 23.Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn's disease. Gastroenterology 2010; 138: 463–468. [DOI] [PubMed] [Google Scholar]
- 24.Shah SC, Colombel JF, Sands BE, et al. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn's disease. Aliment Pharmacol Ther 2016; 43: 317–333. [DOI] [PubMed] [Google Scholar]
- 25.Ungaro R, Yzet C, Bossuyt P, et al. Endoscopic and Deep Remission At 1 Year Prevents Disease Progression in Early Crohn's Disease: Long-Term Data from Calm. Gastroenterology 2019; 156: 411. [DOI] [PMC free article] [PubMed]
- 26.Christensen B, Erlich J, Gibson PR, et al. 603 – histological healing is associated with decreased clinical relapse in patients with ileal Crohn's disease. Gastroenterology 2018; 154: S-128–S-129. [Google Scholar]
- 27.Li K, Friedman JR, Chan D, et al. Effects of ustekinumab on histologic disease activity in patients with Crohn's disease. Gastroenterology 2019; 57: 1019–1031.e7. [DOI] [PubMed] [Google Scholar]
- 28.Ordas I, Rimola J, Rodriguez S, et al. Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn's disease. Gastroenterology 2014; 146: 374–382. [DOI] [PubMed] [Google Scholar]
- 29.Panes J, Bouzas R, Chaparro M, et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn's disease. Aliment Pharmacol Ther 2011; 34: 125–145. [DOI] [PubMed] [Google Scholar]
- 30.Fernandes SR, Rodrigues RV, Bernardo S, et al. Transmural healing is associated with improved long-term outcomes of patients with Crohn's disease. Inflamm Bowel Dis 2017; 23: 1403–1409. [DOI] [PubMed] [Google Scholar]
- 31.Thierry M-L, Rousseau H, Pouillon L, et al. Accuracy of diffusion-weighted magnetic resonance imaging in detecting mucosal healing and treatment response, and in predicting surgery, in Crohn's disease. J Crohns Colitis 2018; 12: 1180–1190. [DOI] [PubMed] [Google Scholar]
- 32.Ordás I, Rimola J, Alfaro I, et al. Development and validation of a simplified magnetic resonance index of activity for Crohn's disease. Gastroenterology 2019; 157: 432–439. [DOI] [PubMed] [Google Scholar]
- 33.Allocca M, Fiorino G, Bonifacio C, et al. Comparative accuracy of bowel ultrasound versus magnetic resonance enterography in combination with colonoscopy in assessing Crohn's disease and guiding clinical decision-making. J Crohns Colitis 2018; 12: 1280–1287. [DOI] [PubMed] [Google Scholar]
- 34.Bots S, Nylund K, Löwenberg M, et al. Ultrasound for assessing disease activity in IBD patients: a systematic review of activity scores. J Crohns Colitis 2018; 12: 920–929. [DOI] [PubMed] [Google Scholar]
- 35.Solem CA, Loftus EV Jr, Tremaine WJ, et al. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis 2005; 11: 707–712. [DOI] [PubMed] [Google Scholar]
- 36.Liverani E, Scaioli E, Digby RJ, et al. How to predict clinical relapse in inflammatory bowel disease patients. World J Gastroenterol 2016; 22: 1017–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones J, Loftus EV Jr, Panaccione R, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn's disease. Clin Gastroenterol Hepatol 2008; 6: 1218–1224. [DOI] [PubMed] [Google Scholar]
- 38.Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol 2015; 110: 802–819. [DOI] [PubMed] [Google Scholar]
- 39.Reinisch W, Wang Y, Oddens BJ, et al. C-reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn's disease: a post-hoc analysis from ACCENT I. Aliment Pharmacol Ther 2012; 35: 568–576. [DOI] [PubMed] [Google Scholar]
- 40.Kiss LS, Szamosi T, Molnar T, et al. Early clinical remission and normalisation of CRP are the strongest predictors of efficacy, mucosal healing and dose escalation during the first year of adalimumab therapy in Crohn's disease. Aliment Pharmacol Ther 2011; 34: 911–922. [DOI] [PubMed] [Google Scholar]
- 41.D'Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012; 18: 2218–2224. [DOI] [PubMed] [Google Scholar]
- 42.Wright EK, Kamm MA, De Cruz P, et al. Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn's disease after surgery. Gastroenterology 2015; 148: 938–947. [DOI] [PubMed] [Google Scholar]
- 43.De Vos M, Louis EJ, Jahnsen J, et al. Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm Bowel Dis 2013; 19: 2111–2117. [DOI] [PubMed] [Google Scholar]
- 44.Molander P, Af Bjorkesten CG, Mustonen H, et al. Fecal calprotectin concentration predicts outcome in inflammatory bowel disease after induction therapy with TNF alpha blocking agents. Inflamm Bowel Dis 2012; 18: 2011–2017. [DOI] [PubMed] [Google Scholar]
- 45.Gecse KB, Brandse JF, van Wilpe S, et al. Impact of disease location on fecal calprotectin levels in Crohn's disease. Scand J Gastroenterol 2015; 50: 841–847. [DOI] [PubMed] [Google Scholar]
- 46.Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn's disease 2016: part 1: diagnosis and medical management. J Crohn Colitis 2017; 11: 3–25. [DOI] [PubMed] [Google Scholar]
- 47.Cosnes J, Bourrier A, Laharie D, et al. Early administration of azathioprine vs conventional management of Crohn's disease: a randomized controlled trial. Gastroenterology 2013; 145: 758–765. [DOI] [PubMed] [Google Scholar]
- 48.Panés J, López–SanRomán A, Bermejo F, et al. Early azathioprine therapy is no more effective than placebo for newly diagnosed Crohn's disease. Gastroenterology 2013; 145: 766–774. [DOI] [PubMed] [Google Scholar]
- 49.Khanna R, Bressler B, Levesque BG, et al. Early combined immunosuppression for the management of Crohn's disease (REACT): a cluster randomised controlled trial. Lancet 2015; 386: 1825–1834. [DOI] [PubMed] [Google Scholar]
- 50.Schreiber S, Reinisch W, Colombel JF, et al. Early Crohn's disease shows high levels of remission to therapy with adalimumab: sub-analysis of CHARM. J Crohns Colitis 2007; 1: 49 . [Google Scholar]
- 51.Schriber S, Colombel JF, Bloomfield R, et al. Increased response and remission rates in short-duration Crohn's disease with subcutaneous certolizumab pegol: an analysis of PRECiSE 2 randomized maintenance trial data. Am J Gastroenterol 2010; 105: 1574–1582. [DOI] [PubMed] [Google Scholar]
- 52.Faleck DM, Winters A, Chablaney S, et al. Shorter disease duration is associated with higher rates of response to vedolizumab in patients with Crohn's disease but not ulcerative colitis. Clin Gastroenterol Hepatol 2019; S1542–3565: 30013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young A and Huizinga T. Early rheumatoid arthritis. Best Pract Res Clin Rheumatol 2009; 23: 1–2. [DOI] [PubMed]
- 54.Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, et al. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol 2010; 105: 289–297. [DOI] [PubMed] [Google Scholar]
- 55.Bemelman W, Warusavitarne J, Sampietro G, et al. ECCO-ESCP consensus on surgery for Crohn's disease. J Crohn's Colitis 2018; 12: 1–16. [DOI] [PubMed] [Google Scholar]
- 56.Kugathasan S, Denson LA, Walters TD, et al. Prediction of complicated disease course for children newly diagnosed with Crohn's disease: a multicentre inception cohort study. Lancet 2017; 389: 1710–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peyrin-Biroulet L, Loftus EV, Colombel JF, et al. Early Crohn disease: a proposed definition for use in disease-modification trials. Gut 2010; 59: 141–147. [DOI] [PubMed] [Google Scholar]
- 58.Peyrin-Biroulet L, Billioud V, D'Haens G, et al. Development of the Paris definition of early Crohn's disease for disease-modification trials: results of an international expert opinion process. Am J Gastroenterol 2012; 107: 1770–1776. [DOI] [PubMed] [Google Scholar]
- 59.Fernandes SR, Bernardo S, Simoes C, et al. proactive infliximab drug monitoring is superior to conventional management in inflammatory bowel disease. Inflamm Bowel Dis. Epub ahead of print June 2019. DOI: 10.1093/ibd/izz131. [DOI] [PubMed] [Google Scholar]
- 60.Ricciuto A, Dhaliwal J, Walters TD, et al. Clinical outcomes with therapeutic drug monitoring in inflammatory bowel disease: a systematic review with meta-analysis. J Crohns Colitis 2018; 12: 1302–1315. [DOI] [PubMed] [Google Scholar]
- 61.Beaugerie L, Seksik P, Nion-Larmurier I, et al. Predictors of Crohn's disease. Gastroenterology 2006; 130: 650–656. [DOI] [PubMed] [Google Scholar]
- 62.Solberg IC, Cvancarova M, Vatn MH, et al. Risk matrix for prediction of advanced disease in a population-based study of patients with Crohn's disease (the IBSEN Study). Inflamm Bowel Dis 2014; 20: 60–68. [DOI] [PubMed] [Google Scholar]
- 63.Loly C, Belaiche J, Louis E. Predictors of severe Crohn's disease. Scand J Gastroenterol 2008; 43: 948–954. [DOI] [PubMed] [Google Scholar]
- 64.Enhanced algorithm for Crohn's treatment incorporating early combination therapy (REACT2), https://clinicaltrials.gov/ct2/show/NCT01698307 (2019, accessed 24 October 2019).
- 65.Changing the course of Crohn's disease with an early use of adalimumab (CURE), https://clinicaltrials.gov/ct2/show/NCT03306446 (2019, accessed 24 October 2019).