Abstract
Background
In Crohn's disease, higher adalimumab trough levels and negative anti-adalimumab-antibodies associate with better clinical and endoscopic outcomes. Intestinal ultrasound has become a relevant non-invasive method to monitor treatment. However, data on the association between adalimumab levels and bowel wall thickness measured with ultrasound is limited.
Objective
The purpose of this study was to examine the possible association between the sonographic transmural-thickness and adalimumab trough levels.
Methods
This prospective observational cohort study was conducted at Sheba Medical Center in 2014–2018. Crohn's disease patients on adalimumab maintenance therapy with intestinal ultrasound performed within <30 days of trough level measurement were included. Associations between terminal ileum and colonic thickness, adalimumab levels and therapy retention were assessed.
Results
Fifty events of ultrasound with concomitant adalimumab trough level measurements in 44 Crohn's disease patients were included. Patients with trough level <3 μg/ml had significantly higher bowel wall thickness, both for terminal ileum (p = 0.04) and colon (p = 0.02). Thirty-two patients continued adalimumab therapy over one year. The adalimumab retention rate was higher among those with terminal ileum thickness <4 mm (p = 0.03).
Conclusion
Lower adalimumab trough levels were associated with higher bowel wall thickness indicating poorer therapy outcome. Transmural thickness measurement with ultrasound may be a useful target for guiding biologic therapy in Crohn's disease.
Keywords: Crohn's disease, biological therapy, biologics, anti-tumor necrosis factor, adalimumab, pharmacokinetics, intestinal ultrasonography, transmural thickness
Key summary
Summarize the established knowledge on this subject
In Crohn's disease, patients treated with adalimumab, higher trough levels and no anti-adalimumab-antibodies have been associated with better clinical and endoscopic outcome.
Data on the association between adalimumab trough levels and intestinal-transmural-thickness is currently very limited.
What are the significant and/or new findings of this study?
Patients with adalimumab trough levels <3 μg/ml had significantly thicker bowel wall, both for TI and colon (p = 0.04, 0.02 respectively).
Of the patients, 72% (32/44) continued adalimumab therapy over one year.
On survival analysis, the adalimumab retention rate was higher among patients with terminal ileum thickness<4 mm (p = 0.03).
Introduction
Adalimumab is an anti-tumor necrosis factor (TNF)α monoclonal antibody, administered for moderate to severe Crohn's disease (CD). Higher adalimumab trough levels have been associated with favorable clinical, inflammatory and endoscopic outcomes in CD, while lower levels have been associated with disease exacerbations and loss of response to adalimumab therapy.1–4 Thus, therapeutic drug monitoring of anti-TNF therapy, is applied as part of routine patient management in many centers worldwide. Measuring drug and antibody trough levels upon clinical worsening, in order to adjust dosage and therapy intervals, has been shown to improve patient care and save costs.5,6
Intestinal ultrasonography (IUS) is a non-invasive imaging modality, which has become accessible and clinically relevant in CD. Ultrasonographic assessment of the mucosal, submucosal, and muscular layers, as well as visualization of possible intestinal and extra-intestinal pathologies (such as luminal stenosis, abscesses, or fistulas) can be attained clearly using intestinal IUS.7–10 Thus, IUS is one of the diagnostic modalities recommended by the European Crohn's and Colitis Organization (ECCO) for monitoring treatment in CD.11 Thickening of the bowel wall is the most significant ultrasonographic feature of CD, found to be a valid marker of inflammation in CD.12 Other ultrasonographic signs including pseudo-stratification of the bowel wall (the disappearance of the normal bowel layers), mesenteric and adipose hypertrophy, enlargement of lymph nodes, and enhancement of blood flow in the bowel wall.13
The data on the association of trough levels and ultrasonographic transmural thickness is extremely limited. Therefore, we decided to examine whether an association exists between the sonographic thickness for the terminal ileum (TI) and the colon and adalimumab trough levels, obtained concurrently.
Methods
Patient population
This was a prospective cross-sectional observational cohort study of CD patients receiving scheduled adalimumab therapy at Sheba Medical Center between April 2014–December 2018. Patients were prospectively monitored for adalimumab drug and AAA levels. Thirty of 50 (60%) of the samples analyzed were prospectively collected as part of the POETIC study3 and the rest of the sera were collected later on. IUS was scheduled prospectively during the course of maintenance therapy, in proximity to the retrieval of trough serum samples, as part of the current study. Patients' demographics and clinical characteristics were obtained from their medical charts.
Eligible patients were individuals older than 18 years, treated with scheduled maintenance adalimumab therapy, after completion of the standard induction 160–180 mg course (>4 weeks since commencement of adalimumab therapy).
Patients were excluded if they previously underwent ileo-cecectomy, if they had not suffered from small intestinal CD (i.e. solely colonic CD patients were excluded), or if they did not have adalimumab trough levels obtained within one month of performance of the IUS.
The final analysis was a per-event analysis, so that patients who had undergone IUS on two events, within >6 months apart, with trough level measurements in proximity to the IUS, could be included more than once.
The study was approved by the Sheba Medical Center ethics committee and written, informed consent was obtained from each patient included in the study. Approval was granted for Helsinki protocol SMC-5598-08 on 28 January 2009. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
Intestinal ultrasound
All examinations were performed by a single experienced operator (DC) using a BK 3000 ultrasound machine (BK Ultrasound, Peabody, USA) with low frequency (2.5–6 MHz) curved array transducer enabling all abdominal quadrants to be examined for fill levels, potential pathological distension, motility, and para-intestinal structures such as abscesses, followed by examination using a high resolution linear array transducer (6.0–12 MHz) for detailed examination of the bowel wall structure. All examinations were performed without any preceding preparation, using a consistent technique and protocol, beginning the examination with the proximal to distal colon, followed by complete examination of the small bowel. Assessment was performed for features of inflammation, especially bowel wall thickness of the TI and the colon.13 The ultrosonographer was blinded to trough levels, disease location, and all other patients' baseline characteristics.
TI thickness was measured at least in two different areas in a minimal distance of 1 cm from each other. Colonic bowel thickness was measured at the right colon, transverse coon, left colon and sigmoid colon. At each segment, thickness was measured at least in two different areas at a minimal distance of 1 cm from each other. The thickest segment of the colon was chosen for the purpose of analysis (according to the International Bowel Society Scientific Committee Proceedings, the reported measurements should include the thickest points, representing the worse place of inflammation).
Adalimumab trough level measurement
Serum samples were collected at trough, before adalimumab injections. Adalimumab levels were measured by a previously described drug-tolerant assay at the Sheba Medical Center.14,15
Clinical scores
Adalimumab retention was defined as regular adalimumab maintenance therapy, which was continued for at least one year beyond the time of IUS. Clinical status was assessed as part of the retention analysis, and determined by the Harvey-Bradshaw index (HBI).16–18
Statistical analysis
Continuous variables were expressed as the median and interquartile range (IQR). The Mann-Whitney test was used to compare continuous variables and Fischer's exact test was used for categorical data. Receiver-operating characteristic (ROC) analyses cutoffs were determined by the Youden most accurate point. Kaplan-Meier curves were plotted to assess the temporal rate of events and log rank test was computed for the comparison between survival free durations. All reported p values were two-sided, and a p value less than 0.05 was considered statistically significant. All statistics were performed with MedCalc software (version 12.2.1.0, Mariakerke, Belgium).
Results
Demography and clinical outcomes
In total, 50 IUS with concomitant prospective adalimumab trough level measurements were included in the study (obtained from 44 CD patients). Hence, six patients had two events included, all during maintenance therapy, with at least six months between events. The median time period between the IUS and trough level measurements was 13 days (IQR 6–21 days). Median age at induction was 33.5 years (IQR 25.7–39.2 years), male to female ratio was 0.91. Twelve patients (27.2%) received concomitant immunomodulator therapy. Fourteen patients (32.5%) previously received anti-TNF therapy. Median time from commencement of adalimumab therapy to IUS was 10.8 months (IQR 5.7–17.5 months). The patients' clinical and demographic characteristics are depicted in Table 1.
Table 1.
Patients' demographic and clinical characteristics.
| n a | 50 |
| Number of patients | 44 |
| CD, n (%) | 44 (100) |
| Age at induction, years (median, IQR) | 33.5 (25.7–39.2) |
| Age at diagnosis, years (median, IQR) | 27 (19–36) |
| Disease duration, years (median, IQR) | 2 (1–7.5) |
| Male/female ratio | 0.91 |
| Smoking at induction, n (%) | 12 (27.2) |
| Past smoker, n (%) | 6 (13.6) |
| Previous surgery, n (%) | 10 (22.7) |
| Weight at induction (median, IQR) | 65 (59–82.5) |
| Comorbidities, n (%) | 21 (47.7) |
| Concomitant immunomodulator therapy, n (%) | 12 (27.2) |
| Disease location, n (%) | Ileal - 22 (50) Ileo-colonic - 22 (50) |
| Disease behavior, n (%) | Inflammatory - 22 (50) Stricturing - 14 (31.8) Fistulizing - 8 (18.2) |
| Perianal involvement, n (%) | 12 (27.2) |
| CD upper GI involvement, n (%) | 12 (27.2) |
| Extra-intestinal manifestations, n (%) | 23 (52.2) |
| Previous adalimumab therapy, n (%) | 3 (6) |
| Previous anti-TNF therapy, n (%) | 14 (32.5) |
| Previous immunomodulator therapy, n (%) | 26 (60.4) |
| Once weekly adalimumab therapy | 10 (20) |
CD: Crohn's disease; GI: gastrointestinal; IQR: interquartile range; TNF: tumor necrosis factor.
Number of events of adalimumab measurement within 30 days of intestinal ultrasound.
Association of adalimumab trough serum levels and bowel wall thickness
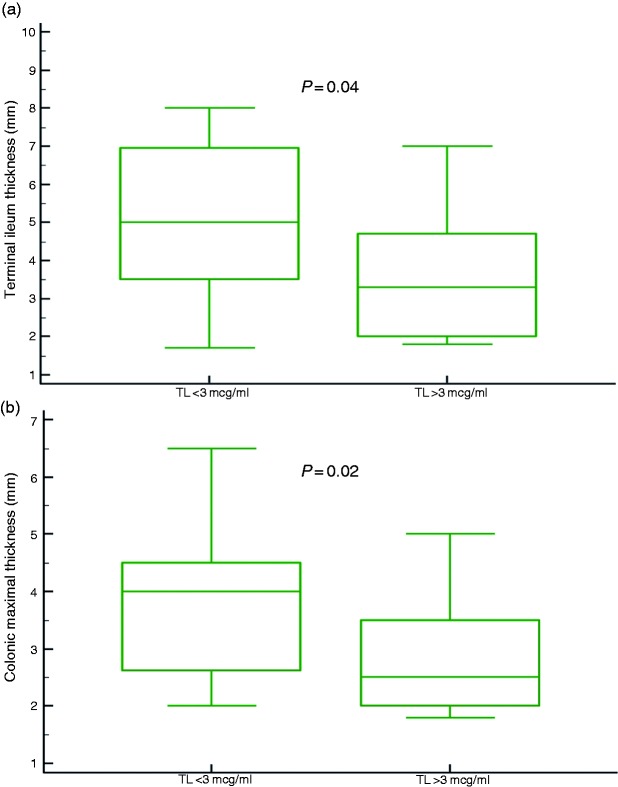
There was a significant negative association between adalimumab trough level and TI wall thickness on concurrent IUS; Patients with adalimumab levels below 3 μg/ml had significantly higher TI wall thickness than patients with adalimumab levels above 3 μg/ml (median TI thickness 5, 3.5 mm, IQR 3.5–7, 2–4.7 mm, respectively, p = 0.04, Figure 1(a)). Thresholds of 1 and 2 μg/ml were explored as well, although no statistical significant association with bowel wall thickness was detected (data not shown). Moreover, a statistically significant difference was noted also between the first (adalimumab level<3.1 μl/ml) and fourth quartile (>6.7 μl/ml) of adalimumab levels (median thickness 5, 2.9 mm, confidence interval (CI) 4.1–7, 2–3.3 mm for first and fourth quartiles respectively, p = 0.002), and between the first and second quartiles (median thickness for second quartile 3.45 mm, CI 3–4.7 mm, p = 0.01, Supplementary Material Figure 1).
Figure 1.
(a) Terminal ileum thickness was significantly higher in patients with adalimumab trough level (TL) below, rather than above 3 μg/ml. (b) Colonic maximal thickness was significantly higher in patients with adalimumab TL below, rather than above 3 μg/ml.
Similar negative association was observed for colonic wall thickness; Patients with adalimumab levels below 3 μg/ml had significantly higher colonic wall thickness than patients with adalimumab levels above 3 μg/ml (median colonic wall thickness 4, 2.5 mm, IQR 2.6–4.5, 2–3.5 mm, respectively, p = 0.02, Figure 1(b)). Similarly, a statistically significant difference was noted also between the first and fourth quartile. Hence, Large Intestinal (LI) was significantly thicker among patients with adalimumab levels in the first, compared to the last quartile (median thickness 4, 2.35 mm, CI 2.9–4.5, 2–2.8 mm for first and fourth quartiles respectively, p = 0.04).
ROC curve analysis was performed to define adalimumab trough level, which is best associated with ultrasonographic transmural healing (i.e. normalization of bowel thickness). For the TI, a cut-off of 3 mm was used. Trough level > 2 μg/ml was best associated with TI wall thickness below 3 mm, but this trend did not reach statistical significance (p = 0.1, area under the curve (AUC) 0.63, sensitivity 33%, specificity 95%). For the colon, a trough level >3.8 μg/ml was best associated with colonic wall thickness below 4 mm, defined as tissue healing threshold (p = 0.0001, AUC 0.812, sensitivity 85%, specificity 75%, Figure 2).
Figure 2.
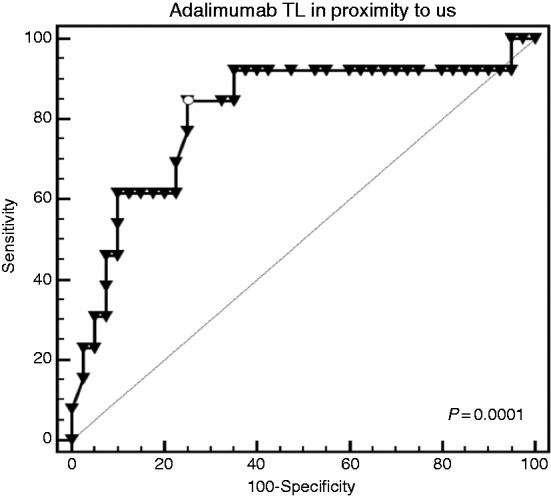
On receiver-operating characteristic (ROC) curve analysis, adalimumab trough level (TL) below 3.8 μg/ml was significantly associated with colonic thickness <4 mm (p = 0.0001, area under the curve (AUC)=0.812, sensitivity 85%, specificity 75%).
A sub-analysis was performed for the association of anti-adalimumab-antibodies (AAAs) with bowel wall thickness. Out of the total cohort (n = 50), positive antibodies were detected only in seven (14%) patients. In those seven patients, median LI and SI thickness were 2.8, 4.5 mm respectively, compared to 2.8, 3.5 mm in antibody-negative cases (n = 50). The difference was statistically significant only for the latter (p = 0.74, 0.01 respectively).
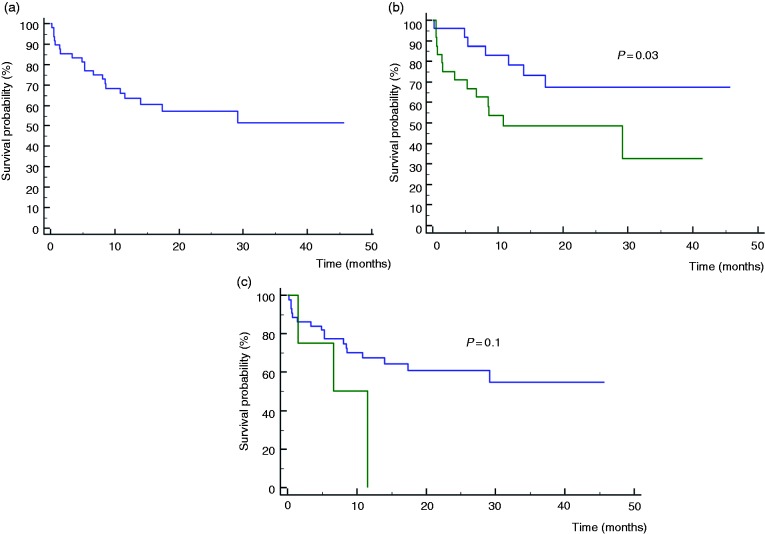
Association of bowel wall thickness with adalimumab therapy retention
In total, 72% (32/44 patients) continued adalimumab therapy for over one year (Figure 3(a)). Median time between performance of IUS and therapy failure/end of follow up was 13.5 months (IQR 7–27 months). An analysis of the association between sonographic TI and colonic wall thickness, respectively, versus subsequent adalimumab therapy retention, showed that TI wall thickness <5 mm and colonic wall thickness <4 mm were both associated with a higher rate of adalimumab therapy retention, although statistical significance was not reached (p = 0.07, 0.08, OR=0.28, 0.26, 95% CI 0.07–1.1, 0.06–1.2, respectively). On survival analysis, the rate of adalimumab retention was significantly higher among patients with TI wall thickness <4 mm, than those with TI wall thickness >4 mm (p = 0.03, Figure 3(b)). As for the colon, thickness <5 mm was associated with a higher rate of therapy retention, but this trend did not reach statistical significance (p = 0.1, Figure 3(c)).
Figure 3.
(a) Adalimumab therapy retention among the total study cohort (n = 44). (b) Adalimumab therapy retention among patients with terminal ileum (TI) wall thickness lower versus higher than 4 mm (p = 0.03). Blue line: patients with TI thickness below 4 mm. Green line: patients with TI thickness above 4 mm. (c) Adalimumab therapy retention among patients with colonic wall thickness below versus above 5 mm (p = 0.1). Blue line: patients with colonic wall thickness below 5 mm. Green line: patients with colonic wall thickness above 5 mm.
Discussion
IUS has been extensively associated with clinical outcome in IBD patients. A recent study has shown that the sensitivity and specificity of IUS were 89%, 94% for disease location; 79%, 94% for stricturing complications; 85%, 94% for detecting abscesses; and 70%, 95% for detecting fistulas, respectively.19 Systematic reviews as well as a recent prospective study, have showed that IUS is as accurate as magnetic resonance enterography (MRE) in assessing disease activity and complications in CD patients.20–24 In fact, IUS-driven management of CD patients, such as continuing, changing, or optimizing therapy, demonstrated a good concordance with clinical decisions based on all findings, including clinical manifestations, biochemical markers, MRE, and colonoscopy.25,26 Specifically, a prospective study has also demonstrated IUS to alter clinical decisions in CD patients.27
An association between higher adalimumab trough levels and better clinical outcomes has been previously demonstrated in several studies.5,28,29 Specifically, in the recently published POETIC study, a cut-off of 6.7 μg/ml for adalimumab trough levels has been associated with clinical remission by the end of induction therapy.3 Measurement of adalimumab trough levels has also been associated with optimal inflammatory and endoscopic outcomes.4,30 Notwithstanding, although IUS has been extensively validated as a monitoring tool for CD patients, and incorporated into the European Crohn's and Colitis Organization (ECCO) guidelines, associations between anti-TNFα trough levels and sonographic findings, not previously been examined.
In our study, patients with adalimumab levels over 3 μg/ml at trough, had significantly lower transmural bowel wall thickness, both for TI and for the colon. ROC curves, which were significant for the colon, demonstrated that trough levels >3 μg/ml were significantly associated with normalization of bowel wall thickness. Furthermore, in this study, out of 50 IUS with concomitant prospective drug and antibody level measurements, positive antibodies were detected only in seven (14%). This proportion was lower than reported in previous studies (20–30%), probably because, contrary to previous publications, only maintenance period sera were included, whereas it is known that most antibodies develop during the induction period.3 To our knowledge, this study is the first to report an association between formation of AAAs and higher TI thickness, although the findings are limited by the relatively small sub-group of antibody-positive patients.
These novel findings strengthen the paradigm that non-invasive periodic monitoring of intestinal wall thickness together with therapeutic drug monitoring are complementary and associate with the patient's clinical status. Therefore, we assume that prospective monitoring of serial IUS and drug level measurements could improve CD patients' outcomes, by early detecting of exacerbations and thereby avoiding hospitalizations and surgeries. Furthermore, when combined with serial IUS monitoring, proactive Therapeutic Drug Monitoring (TDM) could be proven efficient and cost-saving. Additional studies are required in order to explore this further.
In the current study we also examined associations between ultrasonographic transmural bowel wall thicknesses and adalimumab therapy retention. Seventy-seven per cent of the total cohort continued therapy for over one year following the IUS (performed itself during maintenance). Previous studies have defined 60–80% as rate of adalimumab retention for one year at least.31 This goes in line with the fact that secondary loss of response seems to occur among approximately 40% of regular anti-TNF therapy patients.32 Associations between ultrasonographic transmural bowel wall thickness and therapy retention were especially significant for the TI and had only borderline significance for the colon. We assume the findings were less robust for the colon due to the fact that IUS was not performed at induction, but during maintenance therapy, when bowel wall inflammation has potentially already improved, at least partly. In addition, while all patients included had evidence of ileal CD, only 50% had colonic involvement, which might affect the analysis.
Our study has several limitations. First, although all IUS examinations have been performed during a maintenance period, the time between therapy commencement and IUS was variable, which might have affected the results. Secondly, therapy retention was chosen to represent clinical outcome. On the one hand, this is an objective parameter, with less patient-associated variability. On the other hand, therapy retention is also affected by drug adverse events and parameters such as patient preferences and life necessities (co-morbidities, pregnancies, financial issues etc.). Third, although the study included prospective IUS and adalimumab trough levels measurements and clinical follow-up for one year afterwards, all other parameters were extracted from the patients' medical records and this study did not include prospective measurements of calprotectin, imaging, or endoscopies. Finally, the study was of modest size, with 50 events (44 patients) possibly leading to under-selection of some associations.
In conclusion, the current study demonstrated that adalimumab trough level lower than 3 μl/ml was significantly associated with lack of normalization of bowel wall (TI and colonic) thickness. Lower wall thickness, in turn, was associated with a higher rate of adalimumab therapy retention, especially for the TI. Thus, both therapeutic drug monitoring and IUS monitoring in CD patients treated with adalimumab are associated with better patient outcomes and are probably complementary modalities. If corroborated by additional studies, these findings strengthen the notion that transmural healing on IUS might serve as a novel therapeutic target in CD.
Supplemental Material
Supplemental material, UEG878974 Supplemental Material for Lower adalimumab trough levels are associated with higher bowel wall thickness in Crohn's disease by Bella Ungar, Zohar Ben-Shatach, Limor Selinger, Alona Malik, Ahmad Albshesh, Shomron Ben-Horin, Rami Eliakim, Uri Kopylov and Dan Carter in United European Gastroenterology Journal
Acknowledgements
The following author contributions were made: DC conceived the study and drafted the manuscript; BU was involved in study conception, analysis and interpretation of data and manuscript drafting; ZBS, AM, LS and AA participated in acquisition of data; SBH, UK and RE participated in data interpretation and in critical revision of the manuscript for important intellectual property. All authors have approved the final draft submitted.
Declaration of conflicting interests
SBH has received consulting and advisory board fees and/or research support from Abbvie, MSD, Janssen, Takeda, and CellTrion; UK has received speaker fees from Abbvie, Janssen, Medtronic, and Takeda, research support from Takeda, Medtronic and Janssen, and consulting fees from Takeda, Medtronic and Abbvie; DC has received speakers fees from Takeda, Janssen, Abbvie, and Tarp and consultancy fees from Takeda and Taro. RE has received consultant and speaker fees from Janssen, Abbvie, Takeda, and Medtronic; and BU has received consultation fees from Takeda, Neopharm, Janssen and Abbvie. The remaining authors disclose no conflicts of interest.
Ethics approval
The study was approved by the Sheba Medical Center ethics committee. Approval was granted for Helsinki protocol SMC-5598-08 on 28 January 2009.
Funding
This work was supported in part by the 7th Dr Pinchas Borenstein Talpiot Medical Leadership Program, The Chaim Sheba Medical Center (to BU).
Informed consent
Written informed consent was obtained from each patient included in the study.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Karmiris K, Paintaud G, Noman M, et al. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn's disease. Gastroenterology 2009. 137: 1628–1640. [DOI] [PubMed] [Google Scholar]
- 2.Yarur AJ, Jain A, Hauenstein SI, et al. Higher adalimumab levels are associated with histologic and endoscopic remission in patients with crohn's disease and ulcerative colitis. Inflamm Bowel Dis 2016. 22: 409–415. [DOI] [PubMed] [Google Scholar]
- 3.Ungar B, Engel T, Yablecovitch D, et al. Prospective observational evaluation of time-dependency of adalimumab immunogenicity and drug concentrations: The POETIC study. Am J Gastroenterol 2018. 113: 890–898. [DOI] [PubMed] [Google Scholar]
- 4.Mazor Y, Almog R, Kopylov U, et al. Adalimumab drug and antibody levels as predictors of clinical and laboratory response in patients with Crohn's disease. Aliment Pharmacol Ther 2014. 40: 620–628. [DOI] [PubMed] [Google Scholar]
- 5.Yanai H, Lichtenstein L, Assa A, et al. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol 2015. 13: 522–530. [DOI] [PubMed] [Google Scholar]
- 6.Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn's disease who lose response to anti-TNF treatment: A randomised, controlled trial. Gut 2014. 63: 919–927. [DOI] [PubMed] [Google Scholar]
- 7.Fraquelli M, Colli A, Casazza G, et al. Role of US in detection of Crohn disease: Meta-analysis. Radiology 2005. 236: 95–101. [DOI] [PubMed] [Google Scholar]
- 8.Parente F, Maconi G, Bollani S, et al. Bowel ultrasound in assessment of Crohn's disease and detection of related small bowel strictures: A prospective comparative study versus x ray and intraoperative findings. Gut 2002. 50: 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rieder F, Zimmermann EM, Remzi FH, et al. Crohn's disease complicated by strictures: A systematic review. Gut 2013. 62: 1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maconi G, Sampietro GM, Parente F, et al. Contrast radiology, computed tomography and ultrasonography in detecting internal fistulas and intra-abdominal abscesses in Crohn's disease: A prospective comparative study. Am J Gastroenterol 2003. 98: 1545–1555. [DOI] [PubMed] [Google Scholar]
- 11.Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn's disease 2016: Part 1: Diagnosis and medical management. J Crohn's Colitis 2017; 11: 3–25. [DOI] [PubMed] [Google Scholar]
- 12.Wilkens R, Hagemann-Madsen RH, Peters DA, et al. Validity of contrast-enhanced ultrasonography and dynamic contrast-enhanced MR enterography in the assessment of transmural activity and fibrosis in Crohn's disease. J Crohn's Colitis 2018. 12: 48–56. [DOI] [PubMed] [Google Scholar]
- 13.Carter D, Eliakim R. Feasibility of bedside bowel ultrasound performed by a gastroenterologist for detection and follow-up of inflammatory bowel disease. Isr Med Assoc J 2017. 19: 139–142. [PubMed] [Google Scholar]
- 14.Ben-Horin S, Yavzori M, Katz L, et al. The immunogenic part of infliximab is the F(ab′)2, but measuring antibodies to the intact infliximab molecule is more clinically useful. Gut 2011. 60: 41–48. [DOI] [PubMed] [Google Scholar]
- 15.Roblin X, Rinaudo M, Del Tedesco E, et al. Development of an algorithm incorporating pharmacokinetics of adalimumab in inflammatory bowel diseases. Am J Gastroenterol 2014. 109: 1250–1256. [DOI] [PubMed] [Google Scholar]
- 16.Higgins PDR, Schwartz M, Mapili J, et al. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut 2005; 54: 782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvey RF, Bradshaw JM. Simple index of Crohn's disease activity. Lancet 1980. 315: 514. . [DOI] [PubMed] [Google Scholar]
- 18.Vermeire S, Schreiber S, Sandborn WJ, et al. Correlation between the Crohn's disease activity and Harvey-Bradshaw indices in assessing Crohn's disease severity. Clin Gastroenterol Hepatol 2010. 8: 357–363. [DOI] [PubMed] [Google Scholar]
- 19.Allocca M, Fiorino G, Bonifacio C, et al. Comparative accuracy of bowel ultrasound versus magnetic resonance enterography in combination with colonoscopy in assessing Crohn's disease and guiding clinical decision-making. J Crohns Colitis 2018. 12: 1280–1287. [DOI] [PubMed] [Google Scholar]
- 20.Rispo A, Imperatore N, Testa A, et al. Diagnostic accuracy of ultrasonography in the detection of postsurgical recurrence in Crohn's disease: A systematic review with meta-analysis. Inflamm Bowel Dis 2018. 24: 977–988. [DOI] [PubMed] [Google Scholar]
- 21.Kopylov U, Yung DE, Engel T, et al. Diagnostic yield of capsule endoscopy versus magnetic resonance enterography and small bowel contrast ultrasound in the evaluation of small bowel Crohn's disease: Systematic review and meta-analysis. Digestive and Liver Disease 2017; 49: 854–863. [DOI] [PubMed] [Google Scholar]
- 22.Horsthuis K, Bipat S, Bennink RJ, et al. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: Meta-analysis of prospective studies. Radiology 2009. 247: 64–79. [DOI] [PubMed] [Google Scholar]
- 23.Panés J, Bouzas R, Chaparro M, et al. Systematic review: The use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn's disease. Aliment Pharmacol Ther 2011. 34: 125–145. [DOI] [PubMed] [Google Scholar]
- 24.Greenup AJ, Bressler B, Rosenfeld G. Medical imaging in small bowel Crohn's disease - Computer tomography enterography, magnetic resonance enterography, and ultrasound: “Which one is the best for what”. Inflamm Bowel Dis 2016. 22: 1246–1261. [DOI] [PubMed] [Google Scholar]
- 25.Calabrese E, Maaser C, Zorzi F, et al. Bowel ultrasonography in the management of Crohn's disease. A review with recommendations of an international panel of experts. Inflamm Bowel Dis 2016. 22: 1168–1183. [DOI] [PubMed] [Google Scholar]
- 26.Kucharzik T, Wittig BM, Helwig U, et al. Use of intestinal ultrasound to monitor Crohn's disease activity. Clin Gastroenterol Hepatol 2017. 15: 535–542. [DOI] [PubMed] [Google Scholar]
- 27.Novak K, Tanyingoh D, Petersen F, Kucharzik T, Panaccione R, Ghosh S, et al. Clinic-based point of care transabdominal ultrasound for monitoring Crohn's disease: Impact on clinical decision making. J Crohns Colitis 2015. 9: 795–801. [DOI] [PubMed] [Google Scholar]
- 28.Bodini G, Giannini EG, Savarino EV, et al. Adalimumab trough levels and response to biological treatment in patients with inflammatory bowel disease: A useful cutoff in clinical practice. Am J Gastroenterol 2015. 110: 472–473. [DOI] [PubMed] [Google Scholar]
- 29.Zittan E, Kabakchiev B, Milgrom R, et al. Higher adalimumab drug levels are associated with mucosal healing in patients with Crohn's disease. J Crohns Colitis 2016. 10: 510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe K, Matsumoto T, Hisamatsu T, et al. Clinical and pharmacokinetic factors associated with adalimumab-induced mucosal healing in patients with Crohn's disease. Clin Gastroenterol Hepatol 2018. 16: 542–549. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka H, Kamata N, Yamada A, et al. Long-term retention of adalimumab treatment and associated prognostic factors for 1189 patients with Crohn's disease. J Gastroenterol Hepatol 2018. 33: 1031–1038. [DOI] [PubMed] [Google Scholar]
- 32.Ungar B, Chowers Y, Yavzori M, et al. The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut 2014; 63: 1258–1264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, UEG878974 Supplemental Material for Lower adalimumab trough levels are associated with higher bowel wall thickness in Crohn's disease by Bella Ungar, Zohar Ben-Shatach, Limor Selinger, Alona Malik, Ahmad Albshesh, Shomron Ben-Horin, Rami Eliakim, Uri Kopylov and Dan Carter in United European Gastroenterology Journal