Abstract
Background
Colorectal cancer (CRC) and its precursor lesions are detected at an early stage by CRC screening programmes, which reduce CRC-related mortality. An important quality indicator for CRC screening is the occurrence of interval CRC (IC) between screening rounds. Currently there is no guideline regarding acceptable levels of ICs in CRC screening programmes, and ICs reported in prior work vary considerably.
Methods
This study describes the occurrence of screen-detected (SD) CRC and non–screen-detected CRC within the population-based CRC screening programme of Flanders, stratified by multiple variables such as sex, age, tumour location and tumour stage between October 2013 and July 2017. In addition, faecal immunochemical test (FIT) IC proportions over the sum of SD-CRCs and FIT-ICs are calculated, together with FIT sensitivity and programme sensitivity to display the effectiveness of detecting CRC by the screening programme.
Results
Of 1,212,354 FIT participants, 4094 were diagnosed with SD-CRC, whereas 772 participants were diagnosed with CRC between FIT-screening rounds. Significant associations were shown between people not being SD for CRC and women, older individuals, right-sided tumour location and more advanced tumour stage. Furthermore, a clear distinction was shown between the right-sided and the left-sided colorectum concerning all above-mentioned variables and distributions of tumour stages.
Conclusion
The Flemish FIT-interval CRC proportion of 15.9% was within the limits of previously published results. In addition, calculations show that the effectiveness of the screening programme is dependent on tumour location, suggesting that future research should report results stratified by location.
Keywords: Colorectal cancer screening, FIT, interval cancers, non–screen-detected colorectal cancers
Key summary
- What is the established knowledge about this subject?
- • The faecal immunochemical test (FIT) interval cancer (IC) proportion relative to screen-detected (SD) colorectal cancer (CRC) cases varies considerably among countries.
- • Non–SD-CRCs help to contextualise the effectiveness of the population-based screening programme.
- • Although some determinants are associated with the occurrence of ICs, such as increasing FIT results and tumour stage, these associations are often not reproduced because of small sample sizes.
- What are the new findings?
- • Significant associations were shown between the occurrence of non–SD-CRC and female sex, older age, increased tumour stage and right-sided tumour location in the colorectum.
- • The proportion of FIT-interval CRC and the sensitivity of the screening programme are dependent on CRC tumour location and stage.
- • FIT screening is much more effective for detecting left-sided CRC compared with right-sided CRC, but also for detecting tumour-node-metastasis stage 0 compared with invasive CRC.
Introduction
Worldwide, more than 1.8 million estimated new colorectal cancer (CRC) cases and 881,000 deaths occurred in 2018, accounting for about 1 in 10 cancer cases and deaths.1 Between 2012 and 2018, both the incidence and the mortality for CRC increased; as for Europe, the estimated CRC incidence increased from 447,000 to 500,000 and the estimated mortality from 215,000 to 242,000.1 Population-based CRC screening programmes have been implemented in multiple countries where the faecal immunochemical test for haemoglobin (FIT) is used most often, compared with other options.2 These population-based CRC screening programmes enable detection of precancerous lesions and CRC at an earlier stage and have been shown to reduce CRC-related mortality compared with no screening at all.3 In Flanders (one of the three regions in Belgium), population-based CRC screening was initiated in 2013,4 coordinated by the Flemish Centre for Cancer Detection (CCD), which sends out a biennial quantitative FIT to the screening-eligible target population by mail. The quantitative FIT (OC-sensor, Eiken Chemical Co, Tokyo, Japan), with a positivity threshold of 15 or more haemoglobin per gram of faeces (μgHb/g), was chosen as the screening test in Flanders. The quality control both of the FIT and the rest of the screening programme is reported elsewhere.5
An important quality issue of population-based CRC screening programmes is the occurrence of interval cancer (IC), defined as a ‘CRC diagnosed after a screening test or examination in which no cancer is detected and before the date of the next recommended examination’.6 The effectiveness of CRC screening is directly dependent on the occurrence of ICs and is often reported as the IC proportion, calculated by the IC over the sum of both the ICs and screen-detected (SD) cancers.7–9
Two notable, randomised controlled trials regarding CRC screening using the guaiac faecal occult blood test (gFOBT) showed a significant mortality reduction compared with no screening.3,10 However, the IC proportions over all SD-CRCs in the above-mentioned studies were 51.3% and 55.2%, providing evidence that ICs account for about half of CRCs diagnosed in populations screened biennially with the gFOBT. Because the FIT is considered superior over the gFOBT, studies started using the FIT and reporting ICs. The importance of investigating ICs became clear when several studies described varying FIT-IC proportions between 6.9% and 50.8% (24-month invitation interval and FIT-positivity thresholds between 10 and 80 μgHb/g).7,9,11–13 In addition, ICs are associated with more severe outcome and reduced chances of survival,9,14 which is substantiated by the strong relation between tumour stage and survival. In Flanders (2011–2015) the five-year survival for CRC in the general population was 96% for tumour-node-metastasis (TNM) stage I CRC compared with 19% for stage IV CRC.15 Considering the 2018 article by Allemani et al., which shows an average five-year survival rate between 50 and 70% for CRC in the European Union, there is room for improvement by early detection.16
In Belgium a unique linkage of screening data with Belgian Cancer Registry (BCR) data and reimbursement data related to diagnostic and therapeutic procedures from health insurance companies (HICs) is present. This makes it possible to evaluate SD and non-SD cancers in the target population in detail.
The aim of this study is to obtain a better understanding of non–SD-CRC which could reveal new information on potential limitations of the current screening method and how to tackle them in future.
Methods
Study cohort and definitions
The study population cohort consists of data from the Flemish population-based CRC screening programme,4 including FIT participants and FIT non-participants between October 2013 and July 2017 who were subsequently diagnosed with CRC. CRC data provided by the BCR were available until incidence year 2016 at the time of analysis and provided information on incidence date, tumour location and stage. Stage was assessed by combining the clinical and pathological TNM staging.17 For tumour location, the International Classification of Diseases for Oncology, third revision topography codes were used.18 Primary tumours located in the caecum to the splenic flexure were coded as right sided, whereas tumours located from the splenic flexure to the rectum were categorised as left sided. Screening data from the CCD is recurrently linked with the BCR databases by a unique patient identifier to evaluate different quality indicators for screening according to European guidelines.17 Alongside CRC and patient characteristics at the population level, the BCR collects all pathology results of colorectal specimens, regardless of diagnosis, merging them into a cyto-histopathological database (CHP) from 2008 onward. Additionally, CHP data are completed with reimbursement data related to diagnostic and therapeutic procedures from the HICs.
The CHP registry provided additional cancer data until July 2017, limited to tumour behaviour (invasive or in situ) and location together with data on normal or non-(pre)cancerous lesions and adenomas with or without a villous component. Differentiation between advanced and non-advanced adenomas is impossible because size and amount of lesions are not registered.
For the purpose of standardisation and future benchmarking, the IC definition of Sanduleanu et al. is used.6 Because no centralised colonoscopy quality register exists in Belgium, results of colonoscopies are not available, and the reimbursement HIC data for colonoscopies are used to assess the date of subsequent colonoscopies after a positive FIT. A quality assessment of performed colonoscopies in Belgium is reported elsewhere.19 Based on the delay between the dates of subsequent colonoscopies and CRC incidence, SD cancers could be distinguished from post-colonoscopy ICs. Definitions of SD-CRC and non–SD-CRC for this study are listed in Table 1. For future comparison, this study also reports on non-invasive carcinoma (TNM stage 0, carcinoma in situ or CIS) separately from invasive carcinoma (TNM stage ≥1).20 In case of multiple lesions, only the most advanced finding was included. For evenly advanced lesions on both sides of the colorectum, left-sided lesions were withheld.
Table 1.
Definitions of screen-detected (SD) CRC and non–screen-detected CRC.
| Nomenclature | Definition |
|---|---|
| SD-CRC | CRC diagnosed within the Flemish CRC screening programme after a positive FIT (≥15 µg/g), within six months after first subsequent colonoscopy and before next recommended FIT invitation (24 months) |
| Non–SD-CRC | FIT interval cancer (FIT IC): CRC diagnosed after a negative FIT (<15 µgHB/g) and before the next FIT invitation (24 months) |
| Post-colonoscopy interval cancer within a FIT screening programme: CRC diagnosed after a positive FIT (≥15 µgHB/g), more than six months after first subsequent colonoscopy and before recommended follow-up (10 years) | |
| Colonoscopy non-responder CRC: CRC diagnosed in participants who are screened positive by FIT but do not adhere to colonoscopy follow-up, and diagnosis is determined by other means than colonoscopy within two years | |
| FIT non-participant CRC: CRC diagnosed in individuals from the screening target population who did not participate within 10 years after first FIT invitation |
CRC: colorectal cancer; FIT: faecal immunochemical test.
According to the definitions in Table 1, CRC data are necessary until 24 months after the FIT to determine SD-CRC and FIT-IC cases. Complete cancer stage data were available until the year 2016; the results related to the screening years 2015, 2016 and 2017 contain missing values with regard to tumour staging. Regarding the definitions, post-colonoscopy ICs are incomplete as well because a follow-up time of 10 years has not been reached yet.
Data analyses
The proportion of the FIT IC is calculated by the amount of FIT ICs relative to the sum of SD-CRCs and FIT ICs (1). The sensitivity of the FIT is considered as FIT being positive when CRC is present (2), also called the detection method,21 whereas the programme's sensitivity includes the colonoscopy non-responder CRC and post-colonoscopy IC to the calculations (3) as shown in Table 2. The above-mentioned calculations were performed for the stratified variables location of tumour, distinguishing left-sided from right-sided and between CIS (TNM stage 0) and CRC (TNM stage ≥1), distinguishing between severity. Furthermore, time from FIT participation to CRC diagnosis is described, as is the screening round. The FIT non-participant CRC is not considered in the programme's sensitivity calculation nor in the odds ratio (OR) calculations because this group could consist of people with family history or CRC symptoms (which is currently unknown) because they are advised not to participate and would bias the calculations. Point estimates and confidence intervals (CI) of the ORs are calculated using Wald between not being SD and sex, age, tumour location and tumour stage.22 Statistical significance is reached if the p value falls to less than .01. Missing values are assumed to be missing at random because of the data delay, which is substantiated by the tested non-significant relation between missing data, sex, age and FIT results (available data). For the three calculations shown in Table 2, exhaustive data (2013–2015) are used because it is expected that data from 2015–2017 consist of relatively more SD cases compared with ICs because the latter take longer to occur. Data were analysed in R (version 3.4.3) on a Windows server 2016 standard (R Core Team, 2017).23
Table 2.
Different calculation methods for screening accuracy.
| • Proportion of FIT IC | 1: FIT IC/(FIT IC + SD-CRC) |
| • Sensitivity of FIT (detection method) | 2: SD-CRC/(FIT IC + SD-CRC) |
| • Programme's sensitivity | 3: SD CRC/(SD CRC + FIT IC + post-colonoscopy IC + colonoscopy non-responder CRC) |
CRC: colorectal cancer; FIT: faecal immunochemical test; IC: interval cancer; SD: screen-detected.
Privacy and ethics
Approval for the general Flemish CRC screening programme and the data exchange with the BCR, the HIC and the CCD was given 17 September 2013 by the Belgian Privacy Commission with reference SCSZG/13/194, number 13/09124 to organise and evaluate the screening programme. When participating in the screening programme, all participants filled out a written informed consent explaining that personal information can be used for scientific research and evaluation to improve the CRC screening programme. Anonymised data were accessible for the researchers at the secured BCR environment, strictly in line with all applicable privacy restrictions. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Results
Between October 2013 and July 2017, after respecting the exclusion criteria,4 an average of 52% of the invited individuals participated in the Flemish CRC screening programme (Figure 1). There were 4094 CRCs, 2999 CIS and 36,187 adenomas detected by FIT followed-up by colonoscopy, whereas 8873 normal or non-(pre)cancerous findings were detected. Additionally, 6725 people who did not respond to their FIT invitation had a CRC diagnosis afterward (Figure 1). Thus 10% (8873/88,875) of the participants with a positive FIT resulted in a normal or non-(pre)cancerous result. Figure 1 shows screening data combined with colonoscopy outcome data for the years 2013–2017. Next to the absolute values over this time period, the screening data are also shown as a yearly average to contextualise the screening numbers per screening round.
Figure 1.
Flowchart of the Flemish CRC screening programme and FIT non-participant outcomes between October 2013 and July 2017 with screen-detected and non–screen-detected CRCs.
(a) Exclusion from screening applies if people are screened by either a FIT or gFOBT two years prior to invitation, had a colectomy in the past, or a colonoscopy or CRC diagnosis in the last 10 years. (b) No follow-up performed or another follow-up procedure performed such as another FIT, other imaging, surgery, or virtual colonoscopy.
CRC: colorectal cancer; FIT: faecal immunochemical test; gFOBT: guaiac faecal occult blood test; Y-Avg.: yearly average.
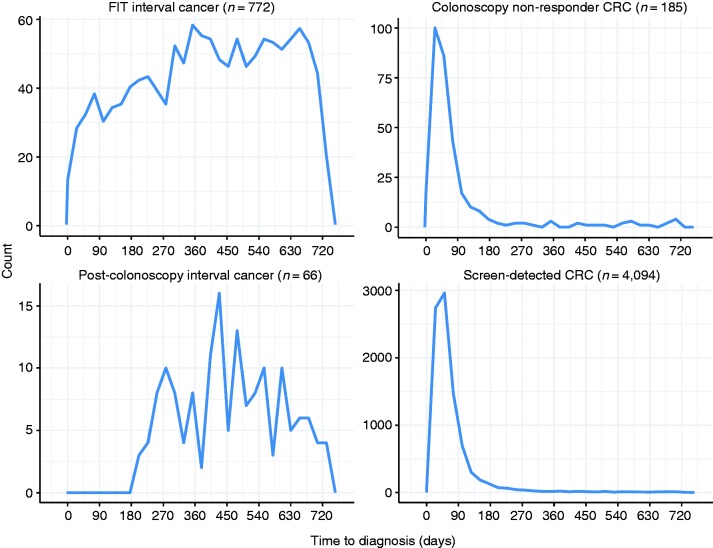
Figure 2 shows the time between FIT participation and CRC diagnosis according to the different subgroups. SD-CRCs and colonoscopy non-responder CRCs were most frequently diagnosed within the first three months after the positive FIT.5 A sensitivity analysis showed that 3% of the colonoscopy non- responder CRCs had had another FIT, 6% underwent a surgical intervention, 11% imaging (radiology or magnetic resonance imaging) and for the remaining 79% there were no data on examinations.
Figure 2.
Time from faecal immunochemical test (FIT) participation to colorectal cancer (CRC) diagnosis.
The Y-axis is free in this figure and needs to be taken into account for interpretation.
Although FIT screening detects many precursor lesions and CRCs, a significant number are missed. In Flanders, between October 2013 and July 2017, 772 CRCs were detected between a negative FIT result and before the next screening round was due. Sixty-six participants with a positive FIT and a negative colonoscopy follow-up were diagnosed with CRC six months after this colonoscopy. Furthermore, 185 participants were diagnosed with CRC after a positive FIT without a colonoscopy follow-up.
Regarding the odds of being SD (Table 3), non–SD-CRCs are significantly more often diagnosed in women compared with men (OR 1.49) and in older age groups (1.38 and 1.71). Furthermore, right-sided tumours were at increased significant odds of not being SD (OR 2.14), and a trend was found between not being SD and tumour stage because advanced tumours were more likely to be missed by screening (OR 1.86 for stage II and OR 5.58 for stage IV).
Table 3.
Target population demographics and tumour characteristics per screen-detected (SD) and non–screen-detected categories.
| SD |
Non-SD |
|||||
|---|---|---|---|---|---|---|
| SD-CRC (n = 4,094) (%) | FIT IC (n = 772) (%) | Post-colonoscopy IC (n = 66) (%) | Colonoscopy non-responder (n = 185) (%) | FIT non-participant (n = 6725) (%) | Not SD Odds ratioa (99% CI) | |
| Sex | ||||||
| Male | 2606 (63.7%) | 417 (54.0%) | 38 (58.1%) | 121 (65.6%) | 3497 (52%) | 1 (Reference) |
| Female | 1488 (36.3%) | 355 (46.0%) | 28 (41.9%) | 64 (34.4%) | 3228 (48%) | 1.49 (1.24, 1.79) |
| Median age at diagnosis, y (IQR) | 66 (62–70) | 68 (63–72) | 68 (64.5–72) | 68 (62–71) | 66 (61–70) | |
| 56–59 | 630 (15.4%) | 85 (11%) | 5 (7.7%) | 27 (14.6%) | 1157 (17.2%) | 1 (Reference) |
| 60–64 | 1044 (25.5%) | 163 (21.1%) | 11 (17.4%) | 36 (19.4%) | 1715 (25.5%) | 1.08 (0.78, 1.50) |
| 65–69 | 1171 (28.6%) | 227 (29.4%) | 24 (36.1%) | 49 (26.3%) | 2017 (30%) | 1.38 (1.01, 1.88) |
| 70–74 | 1249 (30.5%) | 297 (38.5%) | 26 (38.7%) | 73 (39.7%) | 1836 (27.3%) | 1.71 (1.27, 2.30) |
| Tumour location | ||||||
| Left (N, %) | 3190 (77.9%) | 457 (59.2%) | 37 (56.1%) | 143 (77.3%) | 4739 (70.5%) | 1 (Reference) |
| Right (N, %) | 904 (22.1%) | 315 (40.8%) | 29 (43.9%) | 42 (22.7%) | 1986 (29.5%) | 2.14 (1.76, 2.59) |
| Overlapping locationb | 248 | 44 | 5 | 11 | 80 | – |
| Location unknown | 226 | 47 | 2 | 6 | 80 | – |
| Tumour stage | ||||||
| Stage 0c | 2999 | 234 | 46 | 80 | 1896 | |
| Stage I | 2170 (53%) | 240 (31%) | 23 (36%) | 72 (39%) | 1681 (25%) | 1 (Reference) |
| Stage II | 778 (19%) | 154 (20%) | 19 (29%) | 50 (27%) | 1777 (26%) | 1.86 (1.45, 2.38) |
| Stage III | 900 (22%) | 193 (25%) | 17 (26%) | 43 (23%) | 1749 (26%) | 1.82 (1.44, 2.31) |
| Stage IV | 246 (6%) | 185 (24%) | 7 (10%) | 20 (11%) | 1518 (23%) | 5.58 (4.20, 7.42) |
| Stage uncleard | 1013 | 123 | 24 | 21 | 1249 | – |
| Staging not yet knowne | 1640 | 448 | 43 | 61 | 0 | – |
| Screening round | ||||||
| First round | 5519 | 680 | 86 | 201 | – | |
| Second round | 790 | 45 | 2 | 32 | – | |
| Median time from FIT until CRC diagnosis (IQR), d | 50 (34–78) | 406 (237–572) | 460 (356–557) | 49 (27–83.5) | NA | NA |
| FIT (µgHB/g) median (IQR) | 112 (42.4–200) | 10 (10–10) | 62.4 (38.5–198.5) | 122 (43–200) | NA | NA |
| <10 µgHB/g | 1147 | |||||
| 10–14 µgHB/g | 157 | |||||
CI: confidence interval; CRC: colorectal cancer; FIT: faecal immunochemical test; IC: interval cancer; IQR: interquartile range; NA: not available.
FIT non-participant is not considered in calculating the odds ratio, preventing possible bias, as described in the Methods section.
An overlapping location is registered by the pathologist, which makes it impossible to categorise it as left or right sided.
Stage 0 not included in calculations.
Stage is not known, which could be caused by neoadjuvant therapy before staging by the pathologist.
CRC data collected from the cyto-histopathological database, which does not contain information on stage.
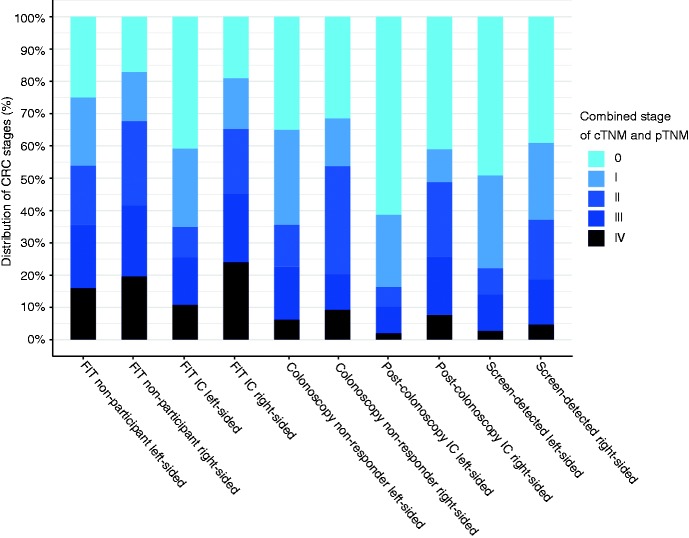
When analysing tumour location according to the SD or non-SD categories, a clear difference in severity of the tumour is observed between the left- and the right-sided colorectum as illustrated in Figure 3. Specifically, the right side compared with the left side is associated with a more severe outcome across every category.
Figure 3.
Distribution of CRC stage according to the different categories and tumour location from 2013 to 2015.
CRC: colorectal cancer; cTNM: clinical tumour-node-metastasis staging; FIT: faecal immunochemical test; IC: interval cancer; pTNM: pathological tumour-node-metastasis staging.
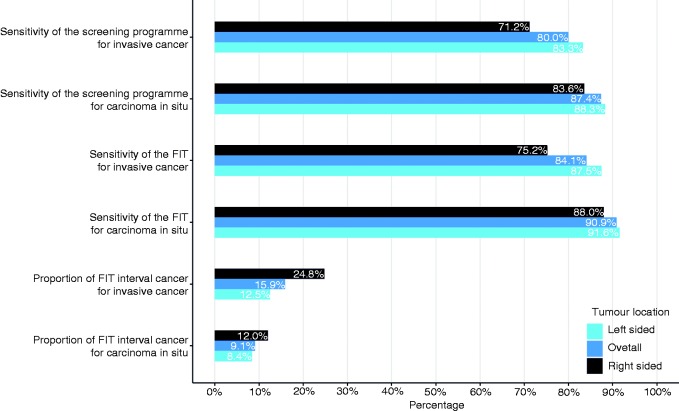
Because tumour location is associated with not being SD, this is taken into account in the calculations of the FIT-ICs proportion, FIT sensitivity and programme sensitivity as shown in Figure 4. Again, there is a consistent difference between the left- and right-sided colorectum with regard to missing CRC across all calculations. The proportion of FIT ICs compared with SD-CRCs is 24.8% of all invasive CRCs in the right-sided colorectum, which is almost double the 12.5% in the left-sided colorectum. This proportion would be 15.9% if location were not considered, which it often is. In addition, if the CIS and the invasive CRCs are compared, the screening programme is more effective in detecting CIS than invasive CRC. The presented results suggest that if the combination of both the right-sided location and tumour stage of 1 or greater are considered, the FIT sensitivity could decrease even more (Figure 4).
Figure 4.
Calculations of colorectal cancer (CRC) screening programme sensitivity, faecal immunochemical test (FIT) sensitivity and FIT interval cancer (IC) proportions, according to carcinoma in situ or CRC and tumour location.
These calculations are based on data from 2013 until 2015 for valid calculation due to complete data on interval cancers.
Discussion
This study describes the occurrence and characteristics of SD-CRCs, FIT ICs, colonoscopy non-responder CRCs, FIT non-participant CRCs and to some extent post-colonoscopy ICs. When comparing the occurrence of CIS and CRC in FIT non-participants with the SD-CRCs in Figure 1 and Table 2, the number of invasive CRC diagnoses are skewed toward the FIT non-participants (n = 4094 vs n = 4728, respectively). Participation in FIT screening in Flanders is 52%, which explains why many CRCs are detected outside the screening programme. Non-participation could be due to symptoms, family history, socioeconomic status25 or a recent CRC diagnosis (possibly fast growing). The CCD information flyer underscores that individuals with symptoms or CRC family history (increased risk for CRC) should seek advice from their general practitioner instead of participating in the FIT screening.
These possible reasons limit some of the calculations in this study because no correction in the FIT non-participant group for family history (generally 10% to 30% in the general population26) or symptoms was possible. This advocates for the registration of family history of CRC and the presence of symptoms to check whether people do not adhere to the FIT for the correct reasons, although complying with FIT is always the preferred course of action for CRC prevention.
Sex was associated with not being SD in this study: Women had an OR of 1.49 (CI 99% 1.24–1.79) compared with men. Compared with other studies, this result is of interest because multiple studies report opposite or insignificant results, which for most prior work is probably explained by small sample sizes.7,9,11,27,28 A Scottish CRC study, however, does agree with our findings regarding the difference in sex when comparing SD participants to interval CRC.8 Although this latter study is based on the gFOBT, the authors strongly suggest that the stool-based approach is less sensitive for CRC in women compared with men, as our results suggest as well. With respect to age, our study found that older age was associated with not being SD. Most studies considering age did not find an associating trend because of reasons discussed previously or the binary use of age.12,27–29 However, another Scottish study agreed with our findings, with a significant trend regarding age.7
Comparing studies in this way should be considered with caution because screening programmes and study protocols do not always adhere to the same inclusion criteria for screening, and differences in target population, FIT-positivity threshold, screening interval or CRC incidence exist. This problem is currently considered by monitoring ICs during screening and colonoscopy surveillance to facilitate benchmarking and comparison across studies and programmes.30 Taking these considerations into account and comparing our FIT-IC proportion of invasive CRC of 15.9% with prior research using FIT (range: 6.9–50.8),7,9,11–13 our results are consistent.
Regarding the location and severity of the detected tumour, the results indicate that a detected CRC on the right-sided colorectum is more advanced compared with the left and this severity increases even more in FIT ICs or in a FIT non-participant as seen in Figure 3. Because the full follow-up length necessary for post-colonoscopy IC is not considered, the results concerning post-colonoscopy IC need to be interpreted with caution. Prior research shows variable results considering the stage distribution of CRC for SD-CRCs, FIT IC and post-colonoscopy IC, which are again caused by previously mentioned limitations.7,9,12,13,28,29 Prior work that did use adequate sample sizes shows comparable results with this study, namely that ICs are found more on the right-sided colorectum compared with the left, and the opposite is true for SD-CRCs.8,11,31 The interaction between location and tumour stage as shown in Figure 3 is not always mentioned in prior works, though its importance is shown through differences in prognosis between right- and left-sided CRCs.29 Furthermore, calculations show that FIT screening is much more effective for detecting left-sided CRC compared with right-sided CRC, but also for detecting CIS compared with invasive CRC.
A limitation of this study is that a data delay is present which currently causes screening round 2 or greater data to not be up to date in the BCR. Normally a trend of increasing FIT IC would be observed in subsequent screening rounds because of a peak in detected CRCs at the start of a screening programme.8,32 Because the BCR has full coverage over the first years of the detected CRCs in the screening programme, all FIT ICs are included in the data for this period and used for calculations.
Conclusions
This study describes the occurrence of SD and non–SD-CRC in participants in the Flemish CRC screening programme. Additionally, this study describes CRC in FIT non-participants who did not respond to a FIT invitation or did not comply with the recommended colonoscopy after a positive FIT. These results are shown stratified by location and severity of tumour. More severe tumours were located in the right-sided colorectum compared with the left side, and more CRC was missed by FIT screening if located on the right side. This became evident because not being SD was clearly associated with sex, age, tumour location and stage. Future research should go in the direction of benchmarking ICs between different regions and countries, a practice not currently taking place. After comparing ICs between countries, consensus can be reached on what is an acceptable IC rate and what information concerning IC should be communicated to professionals, policy makers and participants. At this moment, however, communication concerning IC in a screening programme should be organised per country for professionals and participants.
Acknowledgements
We acknowledge the Flemish Colorectal Cancer Screening Task Force for functioning as a sounding board. Additionally, we thank Mr Marlon van Loo for proofreading our manuscript.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Anonymised data were accessible for the researchers at the secured BCR environment, strictly in line with all applicable privacy restrictions. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Informed consent
When participating in the screening programme, all participants filled out a written informed consent explaining that personal information can be used for scientific research and evaluation to improve the CRC screening programme.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: A global overview of existing programmes. Gut 2015; 64: 1637–1649. [DOI] [PubMed] [Google Scholar]
- 3.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996; 348: 1472–1477. [DOI] [PubMed] [Google Scholar]
- 4.Hoeck S, Pringels S, Kellen E, et al. First results of the Flemish colorectal cancer screening program: Start-up- period late 2013. Acta Gastroenterol Belg 2016; 79: 421–428. [PubMed] [Google Scholar]
- 5.The Centre for Cancer Detection and The Belgian Cancer Registry. Monitoring report of the Flemish Colorectal Cancer Screening Programme, https://dikkedarmkanker.bevolkingsonderzoek.be/sites/default/files/atoms/files/Jaarrapport2017_DEF_0.pdf (2017, accessed 4 January 2019).
- 6.Sanduleanu S, le Clercq CM, Dekker E, et al. Definition and taxonomy of interval colorectal cancers: A proposal for standardising nomenclature. Gut 2015; 64: 1257–1267. [DOI] [PubMed] [Google Scholar]
- 7.Digby J, Fraser CG, Carey FA, et al. Interval cancers using a quantitative faecal immunochemical test (FIT) for haemoglobin when colonoscopy capacity is limited. J Med Screen 2016; 23: 130–134. [DOI] [PubMed] [Google Scholar]
- 8.Steele RJ, McClements P, Watling C, et al. Interval cancers in a FOBT-based colorectal cancer population screening programme: Implications for stage, gender and tumour site. Gut 2012; 61: 576–581. [DOI] [PubMed] [Google Scholar]
- 9.van der Vlugt M, Grobbee EJ, Bossuyt PMM, et al. Interval colorectal cancer incidence among subjects undergoing multiple rounds of fecal immunochemical testing. Gastroenterology 2017; 153: 439–447.e2. [DOI] [PubMed] [Google Scholar]
- 10.Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996; 348: 1467–1471. [DOI] [PubMed] [Google Scholar]
- 11.Australian Institute of Health and Welfare. Analysis of bowel cancer outcomes for the National Bowel Cancer Screening Program: 2018, Canberra: Australian Institute of Health and Welfare, 2018. [Google Scholar]
- 12.Portillo I, Arana-Arri E, Idigoras I, et al. Colorectal and interval cancers of the Colorectal Cancer Screening Program in the Basque Country (Spain). World J Gastroenterol 2017; 23: 2731–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stegeman I, van Doorn SC, Mundt MW, et al. Participation, yield, and interval carcinomas in three rounds of biennial FIT-based colorectal cancer screening. Cancer Epidemiol 2015; 39: 388–393. [DOI] [PubMed] [Google Scholar]
- 14.Asteria CR, Lucchini G, Guarda L, et al. The detection of interval colorectal cancers following screening by fecal immunochemical test may predict worse outcomes and prompt ethical concerns: A 6-year population-based cohort study in a full district. Eur J Cancer Prev 2019; 28: 17–26. [DOI] [PubMed] [Google Scholar]
- 15.Belgian Cancer Registry. Cancer Survival in Belgium, Brussels 2012.https://kankerregister.org/media/docs/publications/CancerSurvivalinBelgium.PDF (accessed 4 January 2019).
- 16.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018; 391: 1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC Cancer Staging Manual and the future of TNM. Ann Surg Oncol 2010; 17: 1471–1474. [DOI] [PubMed] [Google Scholar]
- 18.The International Agency for Research on Cancer. WHO Classification of Tumours of the Digestive System, 4th ed Lyon: IARC, 2010. [Google Scholar]
- 19.Macken E, Van Dongen S, Francque S, et al. Parameters influencing the quality of colonoscopy in Belgium: A critical evaluation. Acta Gastroenterol Belg 2018; 81: 29–38. [PubMed] [Google Scholar]
- 20.Amin M, Edge S, Greene F, et al. (eds) American Joint Committee on Cancer Staging Manual. 8th ed. New York: Springer International Publishing, 2017.
- 21.Fletcher RH, Fletcher SW and Fletcher GS. Detection and incidence methods for calculating sensitivity. In: Rhyner S. (ed.) Clinical epidemiology: The essentials. 5th ed. Baltimore: Lippincott Williams & Wilkins, 2014, pp.163-164.
- 22.Wald A. Tests of statistical hypotheses concerning several parameters when the number of observations is large. Trans Am Math Soc 1943; 54: 426–482. [Google Scholar]
- 23.R Core Team. R: A language and environment for statistical computing, https://www.R-project.org/ (2017, accessed 15 April 2019).
- 24.Belgian Privacy Commission, 17-09-2013, https://www.gegevensbeschermingsautoriteit.be/sites/privacycommission/files/documents/beraadslaging_AG_091_2013_0.pdf ( accessed 4 January 2019).
- 25.Hoeck S, van de Veerdonk W, De Brabander I, et al. Does the Flemish colorectal cancer screening programme reach equity in FIT uptake?. Eur J Public Health. 10.1093/eurpub/ckz043. [DOI] [PubMed] [Google Scholar]
- 26.Lowery JT, Ahnen DJ, Schroy PC 3rd, et al. Understanding the contribution of family history to colorectal cancer risk and its clinical implications: A state-of-the-science review. Cancer 2016; 122: 2633–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu SY, Chuang SL, Chen SL, et al. Faecal haemoglobin concentration influences risk prediction of interval cancers resulting from inadequate colonoscopy quality: Analysis of the Taiwanese Nationwide Colorectal Cancer Screening Program. Gut 2017; 66: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia M, Domènech X, Vidal C, et al. Interval cancers in a population-based screening program for colorectal cancer in Catalonia, Spain. Gastroenterol Res Prac 2015; 2015: 672410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.George AT, Aggarwal S, Dharmavaram S, et al. Faecal occult blood testing screening for colorectal cancer and ‘missed’ interval cancers: Are we ignoring the elephant in the room? Results of a multicentre study. Colorectal Dis 2017; 19: O108–O114. [DOI] [PubMed] [Google Scholar]
- 30.World Endoscopy Organization. The Expert Working Group ‘Right-Sided Lesions and Interval Cancers’ of the Colorectal Cancer (CRC) Screening Committee of the World Endoscopy, http://www.worldendo.org/about-us/committees/colorectal-cancer-screening/ccs-testpage2-level4/right-sided-lesions-and-interval-colorectal-cancers/ (2019, assessed 12 February 2019).
- 31.Blanks R, Burón Pust A, Alison R, et al. Screen-detected and interval colorectal cancers in England: Associations with lifestyle and other factors in women in a large UK prospective cohort. Int J Cancer 2019; 145: 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zorzi M, Fedato C, Grazzini G, et al. High sensitivity of five colorectal screening programmes with faecal immunochemical test in the Veneto Region, Italy. Gut 2011; 60: 944–949. [DOI] [PubMed] [Google Scholar]