Abstract
Objectives
Patients with autoimmune gastritis (AIG) are reported to have an increased risk of developing gastric cancer (GC). In this study, we assess the characteristics and outcomes of GC patients with AIG in a multicenter case-control study.
Methods
Between April 2013 and May 2017, patients with GC, including cancers of the esophagogastric junction (EGJ) Siewert type II and III, were recruited. Patients with histological characteristics of AIG were identified and matched in a 1:2 fashion for age and gender to GC patients with no AIG. Presenting symptoms were documented using a self-administered questionnaire.
Results
Histological assessment of gastric mucosa was available for 572/759 GC patients. Overall, 28 (4.9%) of GC patients had AIG (67 ± 9 years, female-to-male ratio 1.3:1). In patients with AIG, GC was more likely to be localized in the proximal (i.e. EGJ, fundus, corpus) stomach (odds ratio (OR) 2.7, 95% confidence interval (CI) 1.0–7.1). In GC patients with AIG, pernicious anemia was the leading clinical sign (OR 22.0, 95% CI 2.6–187.2), and the most common indication for esophagogastroduodenoscopy (OR 29.0, 95% CI 7.2–116.4). GC patients with AIG were more likely to present without distant metastases (OR 6.2, 95% CI 1.3–28.8) and to be treated with curative intention (OR 3.0, 95% CI 1.0–9.0). The five-year survival rates with 95% CI in GC patients with and with no AIG were 84.7% (83.8–85.6) and 53.5% (50.9–56.1), respectively (OR 0.25, 95% CI 0.08–0.75, p = 0.001).
Conclusions
Pernicious anemia leads to earlier diagnosis of GC in AIG patients and contributes significantly to a better clinical outcome.
Keywords: Gastric cancer, autoimmune gastritis, Helicobacter pylori, survival, symptoms
Introduction
Gastric cancer (GC) is responsible for over 1,000,000 new cases in 2018 and an estimated 783,000 deaths, making it the fifth most frequently diagnosed cancer and the third leading cause of cancer deaths worldwide.1 Helicobacter pylori (H. pylori) gastritis is the main risk factor for GC,2 whereas the risk for GC development in patients with autoimmune gastritis (AIG) has not been precisely defined.3–5
AIG accounts for less than 5% of all cases of chronic gastritis.6 AIG is caused by an autoimmune T-cell-driven process that destroys the oxyntic mucosa of the proximal stomach via autoantibodies against parietal cells (APCA) and intrinsic factor (AIFA).7 Autoreactive T cells directed against the H+/K+-ATPase (proton pump) may also play a role in the development of AIG.8,9 AIG presents with atrophy of the oxyntic gastric mucosa accompanied by hypo- or achlorhydria. Long-term sequelae of AIG include iron-deficiency anemia and decreased production of intrinsic factor, with vitamin B12 malabsorption and pernicious anemia (PA).2,5 Furthermore, the impaired acid production leads to hypergastrinemia and enterochromaffin-like cell hyperplasia, which may progress to a neuroendocrine tumor.10 Involvement of H. pylori infection in the pathogenesis of AIG has been proposed,5 but at present it is not clear whether H. pylori is the cause of AIG or rather an “innocent bystander”.11
PA, which may eventually develop in patients with atrophy of the oxyntic gastric mucosa due to either H. pylori or AIG, is associated with a roughly seven-fold increased GC risk.12 On the other hand, 5% of patients with AIG and no concomitant H. pylori infection may develop GC irrespective of PA status.13 However, the prevalence of AIG in patients with GC has not been investigated so far.
Current epidemiological trends suggest a possible reversal of both declining incidence and male predominance among patients with GC. The decline in H. pylori infections and the increase in the incidence of autoimmune diseases, such as AIG reported in the western world,2 may contribute to explaining the observed trends.14 Thus, the characterization and early identification of patients with increased risk of GC, particularly those resulting from AIG, is of considerable relevance for early diagnosis and reduction of GC mortality.
The aim of our study is to assess the characteristics and outcomes of GC patients with and without AIG in a multicenter case-control study.
Materials and methods
Study population
The staR (Gastric Cancer Research) consortium consists of physicians and scientists from different European countries, who recruit patients with GC, including cancers of the esophagogastric junction (EGJ) Siewert type II and III.15,16
Within the staR project, a cohort of 759 patients treated in different German centers, with current or past diagnosis of GC, was recruited between April 2013 and May 2017. Patients with gastric neoplasia other than adenocarcinoma were excluded. Discharge letters and medical reports of esophagogastroduodenoscopy (EGD) with histology were obtained from the respective treatment centers for each study participant. Serum samples of all patients were collected by their primary care physician or treating centers and stored at –80 °. Patient data were managed in the database REDCap© (version 4.8.13).
Study design
GC patients from the German staR centers with complete histological assessment of non-neoplastic gastric mucosa were selected. Histology records were reviewed by FW and MV to identify typical histological findings of AIG. Controls were GC patients with no AIG, matched for age and sex in a 1:2 fashion. Paraffin-embedded specimens of gastric mucosa from GC patients with and with no AIG were submitted to a reference GI pathologist (MiV) for central assessment.
Gastrointestinal symptoms occurring within 12 months prior to GC diagnosis were documented using a self-administered questionnaire and telephone interview. Survival data of GC patients were obtained from family members or registration offices. The study was approved by the Ethics Committee of the Otto-von-Guericke University Hospital of Magdeburg on 29 January 2013 (approval number 170/12) and was in accordance with the Helsinki Declaration of 1975, as revised in 1983. All patients provided written informed consent.
Histology
Histology is considered the most reliable method for assessing the presence of AIG.17 The Sydney System classification of gastritis defines AIG as inflammation restricted to the oxyntic mucosa associated with diffuse complete glandular atrophy in the corpus, in an H. pylori-negative subject.18 If biopsies of the gastric antrum and body are available, the presence of chronic gastritis, atrophy and intestinal metaplasia (IM) in the corpus, with a relatively normal antral mucosa in the absence of H. pylori infection, should raise suspicion of AIG.2
Histopathological assessment of gastric mucosa (biopsies or stomach after gastrectomy) is scored by default according to the Sydney System classification in Germany and in other countries. Briefly, different morphological variables, including H. pylori density, neutrophil activity, chronic inflammation (density of mononuclear cells), atrophy of the antrum and corpus and IM, are scored based on a visual analog scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe).18
AIG can also appear in H. pylori-positive patients.19 Indeed, previous studies have shown that H. pylori antibodies are associated with parietal cell antigens, such as H+/K+-ATPase.20 As AIG-associated atrophy of the gastric body is markedly different from H. pylori-associated atrophic gastritis, the two diagnoses are not mutually exclusive and may coexist.18
Autoantibodies
Positivity for antibodies against APCA and/or AIFA helps to define AIG, although a subset of AIG patients may have negative APCA and/or AIFA serology.21 Due to destruction of the oxyntic mucosa and target autoantigen (H+/K+-ATPase), the autoantibody levels fall as the disease progresses.22 Furthermore, a seroconversion of APCA and/or AIFA after gastrectomy is plausible.23
Considering the fact that most patients were recruited several years after GC diagnosis, that a proportion of them had received gastrectomy and that seroconversion of the APCA and/or AIFA may have occurred, the diagnosis of AIG was made on a pathological basis.
Self-administered questionnaires and telephone interviews
All study participants were interviewed using a structured questionnaire providing information on demographics and medical conditions/clinical abnormalities. Patients with AIG were later contacted again for a telephone interview and specifically asked about previous H. pylori infection and eradication therapy, as well as gastrointestinal symptoms occurring within 12 months prior to GC diagnosis. These were categorized into (1) alarm symptoms (vomiting, melena, dysphagia, loss of weight); (2) dyspeptic symptoms (nausea, feeling of increased abdominal fullness, upper abdominal pain, lower abdominal pain, lack of appetite, heartburn); (3) asthenia, fatigue, weakness; and (4) back pain.
Helicobacter pylori status
GC patients with at least one positive test among histology (from records), H. pylori serology (from records), cytotoxin-associated gene A protein (CagA) IgG serology (performed on all recruited GC patients) or an eradication therapy documented in the past (records, questionnaire or interview) were considered H. pylori-positive. Patients with negative results in all tests were classified as H. pylori-negative.
CagA determination
CagA was determined in all study participants (GC patients both with AIG and with no AIG) using a CagA IgG kit (GD33, Genesis Diagnostics, London, UK), according to the manufacturers' instructions. Patients who had anti-CagA IgG ≥ 6.25 U/mL were classified as H. pylori-positive. All serological examinations were carried out in a blinded fashion in the same laboratory.
Determination of APCA and AIFA
APCAs against H+/K+-ATPase antigen were detected by immunofluorescence tests using rat liver, kidney and stomach as a substrate (Generic Assays GmbH, Dahlewitz/Berlin, Germany). Bound IgG was detected using anti-human IgG fluorescein isothiocyanate at a screening dilution of 1 in 20. APCAs were reported as negative or positive if there was cytoplasmic staining of parietal cells. Presence of AIFA was assayed using a quantitative enzyme-linked immunosorbent assay (ELISA) method (Alegria System, ORGENTEC Diagnostika GmbH, Mainz, Germany). The cut-off used was 6 U/mL. Test results were interpreted according to the manufacturer's instructions. All serological examinations were carried out in a blinded fashion in the same laboratory.
Evaluation of PA and iron-deficiency anemia
The presence of PA and iron-deficiency anemia was assessed according to WHO criteria,24,25 based on histology records and laboratory findings at initial diagnosis of GC. Anemia was defined by hemoglobin concentration <13 g/dL in men and <12 g/dL in women. PA was defined as macrocytic anemia (mean corpuscular volume >100 fL) with a serum vitamin B12 level <200 pg/ml. Iron-deficiency anemia was defined as microcytic anemia (mean corpuscular volume <80 fL) with a transferrin saturation <15% and a serum ferritin level <15 μg/L. In cases in which the diagnosis of PA was known prior to the GC diagnosis, the data were transferred.
Statistical analysis
Data of GC patients with and with no AIG were compared by the chi-squared test and odds ratios (ORs) with corresponding 95% confidence intervals (CIs). For all comparisons, a statistical p-value < 0.05 (two-sided) was considered significant. These tests were estimated using SPSS® (version 23.0) and online calculators (https://www.socscistatistics.com/tests/ and http://www.hutchon.net/ConfidOR.htm). The overall and five-year survival rates of GC patients with and with no AIG were determined by Kaplan–Meier survival analysis using Microsoft® Excel (version 16.20) and compared by the log-rank test using SPSS® (version 23.0). Overall survival time was the time from the date of GC diagnosis to the date of death or last follow-up (14 November 2016).
Results
Study population characteristics
Figure 1 shows the recruitment of study patients. Complete histological assessment according to the Sydney classification was possible in 26/28 GC patients with AIG (93%). In 2/28 (7%) of GC patients with AIG, the Sydney classification could not be applied unambiguously. In one patient in particular, the diagnoses of AIG and PA were only mentioned in a discharge letter. In this case, no other clinical findings could be retrieved to support the diagnosis. However, the patient had not undergone gastrectomy, and a positive APCA serology confirming the diagnosis was obtained. In the other case, a histology report on the entire stomach was available after gastrectomy. The diagnosis of AIG was described but a detailed grading according to the Sydney classification was not reported in the original histology report.
Figure 1.
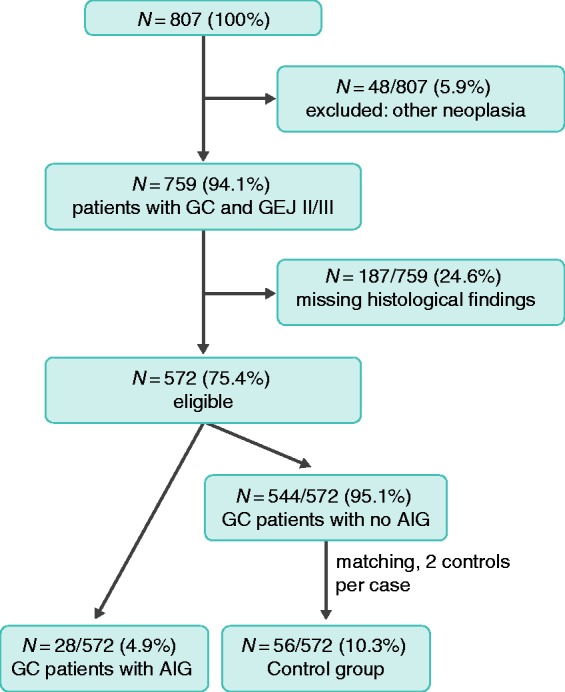
Flow diagram of the enrollment of study patients.
GC: gastric cancer; GEJ: adenocarcinoma of the gastroesophageal junction; AIG: autoimmune gastritis.
For six out of 28 GC patients with AIG, central assessment was impossible as the paraffin sections were already older than 10 years and no longer available. For these six patients, the original histological findings were used for statistical evaluation.
Clinical, serological and histopathological characteristics
The clinical and serological characteristics of GC patients with and without AIG are shown in Table 1. The histopathological parameters are shown in Table 2. Staging of GC patients with and with no AIG is shown in Table 3.
Table 1.
Comparison of clinical and serological characteristics for gastric cancer (GC) patients with and with no autoimmune gastritis (AIG).
| Parameter | AIG | No AIG | p-value | OR (95% CI) |
|---|---|---|---|---|
| N | 28 | 56 | – | – |
| Age at GC diagnosis ± SD (range) | 67 ± 9 (58–76) | 67 ± 9 (58–76) | – | – |
| Sex w:m (%) | 1.3:1 | 1.3:1 | – | – |
| Helicobacter pylori-positive (%) (from histology and/or from patient history) | 10 (35) | 43 (77) | 0.0002 | 6.0 (2.2–16.0) |
| Active H. pylori gastritis (%) | 4 (14) | 18 (32) | 0.079 | 2.8 (0.86–9.4) |
| Successful H. pylori eradication (%) | 6 (21) | 25 (45) | 0.038 | 3.1 (1.1–9.7) |
| CagA-positive (%) | 6 (21) | 9 (17) | 0.546 | 1.42 (0.5–4.5) |
| H. pylori-positive: total (%) (CagA and/or histology and/or from patient history) | 14 (50) | 43 (77) | 0.013 | 3.3 (1.3–8.7) |
| Localization: proximal (%)a | 20 (71) | 27 (48) | 0.043 | 2.7 (1.0–7.1) |
| NET/ECL cell hyperplasia | 2 (7) | 0 (0) | – | 20.8 (1.1–401.2) |
| Pernicious anemia (%) | 8 (29) | 1 (2) | 0.0002 | 22.0 (2.6–187.2) |
| Iron-deficiency anemia (%) | 9 (32) | 10 (18) | 0.140 | 0.5 (0.2–1.3) |
| Laurén classification: intestinal (%) | 15 (54) | 28 (50) | 0.758 | 1.2 (0.5–2.9) |
| Seropositivity (%)b | 8/28 (28) | 0/56 (0) | – | 26.5 (5.7–123.0) |
| APCA (%) | 6 (21) | 0 (0) | – | 24.3 (4.2–140.2) |
| AIFA (%) | 2 (7) | 0 (0) | – | 21.0 (1.1–401.2) |
| Seropositivity (%)c | 8/8 (100) | 0/16 (0) | – | 74.6 (12.8–434.8) |
| APCA (%) | 6 (75) | 0 (0) | – | 46.2 (6.8–314.8) |
| AIFA (%) | 2 (25) | 0 (0) | – | 23.0 (1.1–465.2) |
| Autoimmune diseases: any disease (%) | 7 (25) | 2 (4) | 0.003 | 9.0 (1.7–46.9) |
| Autoimmune thyroid disease (%) | 4 (14) | 0 (0) | – | 22.5 (2.7–186.8) |
| Rheumatoid disease (%) | 3 (11) | 2 (4) | 0.192 | 3.2 (0.5–20.6) |
Localized in esophagogastric junction (EGJ), fundus or corpus.
N = 15/28 patients received gastrectomy with possible seroconversion, N = 5/28 patients with negative serology for anti-parietal cell-antibody (APCA) and anti-intrinsic factor-antibody (AIFA).
Subgroup analysis: N = 8/8 patients with positive serology for APCA and AIFA.
p-values ≤ 0.05 (bold) are statistically significant (X2 test).
CI: confidence interval; OR: odds ratio; SD: standard deviation; ECL: enterochromaffin-like cells; NET: neuroendocrine tumor; CagA: cytotoxin-associated gene A protein.
Table 2.
Comparison of histopathological parameters for gastric cancer patients with and with no autoimmune gastritis (AIG).
| Parameter | AIG | No AIG | p-value | OR (95% CI) |
|---|---|---|---|---|
| N a | 26 | 52 | ||
| Atrophy of corpus grade 1–3 (%) | 26 (100) | 15 (27) | 0.000 | 16.7 (6.6–42.7) |
| No atrophy (%) | 0 (0) | 38 (73) | – | – |
| Grade 1 (%) | 0 (0) | 8 (15) | – | – |
| Grade 2 (%) | 7 (27) | 5 (10) | – | – |
| Grade 3 (%) | 19 (73) | 1 (2) | 0.000 | 138.4 (15.6–1201.0) |
| Atrophy of antrum grade 1–3 (%) | 9 (34) | 23 (44) | 0.416 | 1.5 (0.6–4.0) |
| No atrophy (%) | 17 (66) | 29 (56) | – | – |
| Grade 1 (%) | 7 (27) | 13 (25) | – | – |
| Grade 2 (%) | 2 (7) | 6 (11) | – | – |
| Grade 3 (%) | 0 (0) | 4 (8) | – | – |
| IM of corpus grade 1–3 (%) | 18 (69) | 4 (8) | 0.000 | 27.0 (7.2–100.8) |
| No IM (%) | 8 (31) | 48 (92) | – | – |
| Grade 1 (%) | 4 (15) | 2 (4) | – | – |
| Grade 2 (%) | 7 (27) | 1 (2) | – | – |
| Grade 3 (%) | 7 (27) | 1 (2) | 0.0006 | 18.8 (2.2–163.0) |
| IM of antrum grade 1–3 (%) | 12 (46) | 13 (25) | 0.059 | 2.6 (0.9–6.9) |
| No IM (%) | 14 (54) | 39 (75) | – | – |
| Grade 1 (%) | 6 (24) | 4 (8) | – | – |
| Grade 2 (%) | 5 (19) | 9 (17) | – | – |
| Grade 3 (%) | 1 (3) | 0 (0) | – | – |
26/28 (93%) with appropriate scoring according to the Sydney classification.
CI: confidence interval; OR: odds ratio; IM: intestinal metaplasia.
p-values ≤ 0.05 (bold) are statistically significant (X2 test).
Table 3.
Comparison of tumor data for gastric cancer patients with and without autoimmune gastritis (AIG).
| Parameter | AIG | No AIG | p-value | OR (95% CI) |
|---|---|---|---|---|
| N | 28 | 56 | – | – |
| Early gastric cancer (%) | 18 (64) | 11 (20) | 0.00005 | 7.4 (2.7–20.3) |
| UICC: I–II (%) | 22 (79) | 22 (39) | 0.0007 | 5.7 (2.0–16.2) |
| UICC: I–III (%) | 26 (93) | 38 (68) | 0.011 | 6.2 (1.3–28.8) |
| Grading: G1–G2 (%) | 13 (46) | 21 (38) | 0.432 | 1.4 (0.6–3.6) |
| Treatment with curative intention (%) | 23 (82) | 34 (61) | 0.047 | 3.0 (1.0–9.0) |
UICC: Union for International Cancer Control; CI: confidence interval; OR: odds ratio.
p-values ≤ 0.05 (bold) are statistically significant (X2 test).
Comparison of gastrointestinal symptoms for GC patients with and with no AIG
Table 4 shows the gastrointestinal symptoms occurring within one year prior to GC diagnosis. GC patients with AIG were more often symptom-free (OR 5.6, 95% CI 1.7–19.0), and the most common indication for EGD was PA (36%, OR 29.0, 95% CI 7.2–116.4), which is shown in Table 5. In contrast, upper abdominal pain was the most common indication for EGD, leading to the diagnosis of GC in patients with no AIG (43%, OR 3.5, 95% CI 1.1–10.4). Dyspeptic and alarm symptoms also occurred more frequently in GC patients with no AIG (OR 2.6, 95% CI 1.0–6.7 and OR 2.8, 95% CI 1.1–7.1, respectively).
Table 4.
Comparison of gastrointestinal symptoms (<1 year) for gastric cancer patients with and with no autoimmune gastritis (AIG).
| Parameter | AIG | No AIG | p-value | OR (95% CI) |
|---|---|---|---|---|
| N | 28 | 56 | ||
| No symptoms (%) | 9 (32) | 5 (9) | 0.003 | 5.6 (1.7–19.0) |
| Alarm symptoms (%) | 11 (39) | 36 (64) | 0.029 | 2.8 (1.1–7.1) |
| Vomiting (%) | 6 (21) | 11 (20) | 0.848 | 1.2 (0.4–3.4) |
| Melena (%) | 1 (4) | 7 (13) | 0.189 | 0.3 (0.0–2.2) |
| Dysphagia (%) | 3 (11) | 10 (18) | 0.394 | 0.6 (0.1–2.2) |
| Weight loss (%) | 5 (18) | 26 (46) | 0.007 | 3.4 (1.1–10.3) |
| Dyspeptic symptoms (%) | 13 (46) | 39 (70) | 0.039 | 2.6 (1.0–6.7) |
| Nausea (%) | 5 (18) | 12 (21) | 0.701 | 0.8 (0.3–2.5) |
| Feeling of increased abdominal fullness (%) | 5 (18) | 16 (29) | 0.285 | 0.5 (0.2–1.7) |
| Upper abdominal pain (%) | 6 (21) | 27 (48) | 0.042 | 2.9 (1.0–8.3) |
| Lower abdominal pain (%) | 0 (0) | 4 (7) | – | 0.2 (0.0–1.8) |
| Lack of appetite (%) | 5 (18) | 17 (30) | 0.219 | 0.5 (0.2–1.5) |
| Heartburn (%) | 1 (4) | 7 (13) | 0.189 | 0.3 (0.0–2.2) |
| Back pain (%) | 0 (0) | 6 (11) | – | 0.2 (0.0–1.2) |
| Asthenia, fatigue, weakness (%) | 9 (32) | 18 (32) | 1.000 | 1 (0.4–2.6) |
CI: confidence interval; OR: odds ratio.
p-values ≤ 0.05 (bold) are statistically significant (X2 test).
Table 5.
Indication for esophagogastroduodenoscopy, which led to gastric cancer diagnosis.
| Parameter | AIG | No AIG | p-value | OR (95% CI) |
|---|---|---|---|---|
| N | 28 | 56 | ||
| Pernicious anemia (%) | 10 (36) | 0 (0) | – | 290 (7.2–116.4) |
| Upper abdominal pain (%) | 5 (18) | 24 (43) | 0.023 | 3.5 (1.1–10.4) |
| Asthenia, fatigue, weakness (%) | 5 (18) | 16 (29) | 0.285 | 1.8 (0.6–5.7) |
| Feeling of increased abdominal fullness (%) | 5 (18) | 14 (25) | 0.461 | 1.5 (0.5–4.8) |
| Nausea (%) | 5 (18) | 8 (14) | 0.670 | 1.3 (0.4–4.4) |
| Loss of weight (%) | 4 (14) | 20 (36) | 0.040 | 3.3 (1.0–11.0) |
| Lack of appetite (%) | 4 (14) | 14 (25) | 0.259 | 0.5 (0.1–1.7) |
| Vomiting (%) | 4 (14) | 8 (14) | 1.000 | 1.0 (0.3–3.7) |
| Dysphagia (%) | 3 (11) | 7 (13) | 0.812 | 0.8 (0.2–3.5) |
| Incidental finding (%) | 2 (7) | 6 (11) | 0.599 | 0.6 (0.1–3.4) |
| Melena (%) | 2 (7) | 6 (11) | 0.599 | 0.6 (0.1–3.4) |
| Heartburn (%) | 2 (7) | 4 (7) | 1.000 | 0.8 (0.1–4.4) |
| Backpain (%) | 0 (0) | 5 (9) | – | 0.2 (0.0–1.4) |
| Lower abdominal pain (%) | 0 (0) | 4 (7) | – | 0.2 (0.0–1.8) |
AIG: autoimmune gastritis; CI: confidence interval; OR: odds ratio.
p-values ≤ 0.05 (bold) are statistically significant (X2 test).
Survival analysis
Median follow-up duration for all patients was 30 months and ranged from 0 to 142 months. Five GC patients with AIG (17.9%) and 26 GC patients with no AIG (46.4%) had died at the last follow-up (14 November 2016).
Subgroup analyses were carried out according to patient gender. Median follow-up duration for women was 34 months and ranged from 0 to 122 months. One (6.3%) GC patient with AIG and 10 (31.3%) GC patients with no AIG had died at the last follow-up.
Median follow-up duration for men was 19 months and ranged from 0 to 142 months. At the last follow-up there were four (33.3%) and 16 (66.7%) deaths in GC patients with AIG and with no AIG, respectively.
The five-year survival rate of GC patients with AIG was 84.7% (95% CI 83.8–85.6) and 53.5% (95% CI 50.9–56.1) for GC patients with no AIG (OR 0.25, 95% CI 0.08–0.75, p = 0.001; Figure 2). In women, the five-year survival rate was 92.3% (95% CI 87.6–97.0) in GC patients with AIG and 66.3% (95% CI 63.6–69.0) in those with no AIG (p = 0.025, data not shown). In men, the five-year survival rate was 73.3% (95% CI 71.8–74.8) in GC patients with AIG and 36.7% (95% CI 33.8–39.5) in those with no AIG (p = 0.010, data not shown).
Figure 2.
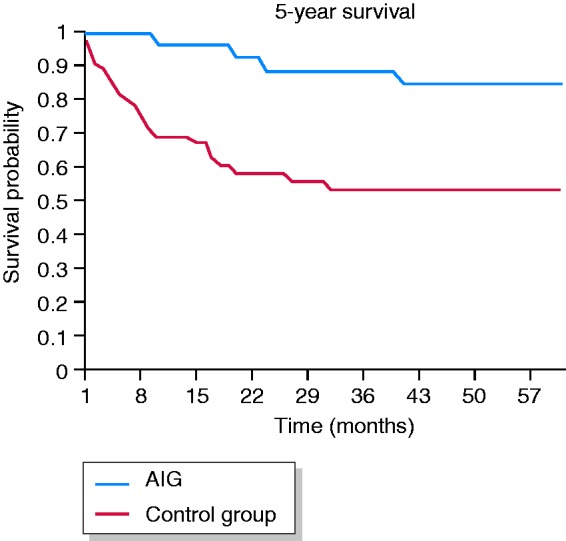
Kaplan–Meier five-year survival curves of gastric cancer patients with and with no autoimmune gastritis (AIG). Five-year survival: AIG: 84.7% (95% CI 83.8–85.6); no AIG: 53.5% (95% CI 50.9–56.1), p = 0.001.
The subgroup analysis confirmed the results obtained in a comparison of clinical, serological and tumor data, histopathological parameters and gastrointestinal symptoms (<1 year) between GC patients with and with no AIG. Patients who received gastrectomy with possible seroconversion (N = 15) and those with negative serology for APCA and AIFA (N = 5, data not shown) were excluded. This also applies to the analyses of the overall and five-year survival probabilities for all patients, as well as for women and men.
Discussion
GC patients with AIG have histopathological, serological and clinical characteristics, as well as outcomes that are distinct from GC associated with H. pylori gastritis. In particular, GC patients with AIG have a better prognosis than GC patients with no AIG. The main reason for this is that the presence of PA, though not a typical sign of GC, prompts EGD and, therefore, leads to an early diagnosis of GC in patients with AIG. Thus, the cause of better outcomes in GC patients with AIG is presumably the typical AIG-phenotype rather than another cancer biology in this particular population. Our findings support the recommendation that PA should prompt EGD even in the absence of gastrointestinal symptoms.13,24
Previously, we have shown that H. pylori-negative AIG differs significantly from H. pylori-induced oxyntic atrophic gastritis in terms of histopathological, serological and clinical characteristics.26 In the present study, we confirm that these differences also apply to GC patients with and with no AIG. GC patients with AIG were more likely to have severe (total) atrophy and IM of the gastric corpus compared to those with no AIG.26 As described by Correa, atrophy followed by IM may further progress to intraepithelial neoplasia and, finally, to invasive GC.27 Following the multi-step process of gastric carcinogenesis, patients with AIG were more likely to develop GC in the proximal stomach (EGJ, fundus or corpus) within a milieu of severe atrophy and IM. Recently, GC development according to Correa's cascade in the absence of H. pylori has been questioned.3 Contrary to this opinion, our data support an increased GC risk in patients with AIG in the absence of H. pylori infection. Notably, anti-H. pylori CagA antibodies, which persist longer in serum, were employed as well.28 Our observation that patients with H. pylori-negative AIG can develop GC further confirms the European MAPS II guideline update 2019, which recommends endoscopic surveillance at three- to five-year intervals for patients with AIG.29
The seroprevalence of APCA was higher in GC patients with H. pylori-negative AIG. The seropositivity for APCA – but also for other autoantibodies (i.e. thyroglobulin antibodies, thyroid peroxidase antibodies, anti-smooth muscle antibodies and antimitochondrial antibodies, data not shown) – further strengthens the hypothesis of a distinct autoimmune etiology that is possibly independent of H. pylori in at least a percentage of GC patients with AIG.
In line with our previous reports on AIG patients without GC, AIG patients with GC were more likely to have another autoimmune disease (OR 9.0; 95% CI 1.7–46.9), most commonly an autoimmune thyroid disease (14%).26,30 Furthermore, we confirm that hematological abnormalities are frequent findings in AIG. Indeed, PA was more likely to occur in GC patients with AIG compared to those with no AIG (29% vs 2%, respectively).5,17,31
In our cohort, the average age at the initial diagnosis of GC was 67 ± 9 years in patients with AIG, which is in line with overall epidemiological data.32 However, in contrast to the overall GC epidemiology with male predominance, GC arising in patients with AIG is more likely to occur in females (female-to-male ratio 1.3:1).
To the best of our knowledge, this is the first study to report the prevalence of histological AIG in GC patients, which was 4.9%. A prospective study taking biopsies from gastric antrum and body mucosa can provide more precise estimates of AIG prevalence in GC patients. The prevalence of AIG in our cohort of patients with GC was similar to the 2–5% prevalence of AIG reported in the general population.2
The prevalence of AIG in patients with GC reported in our study is higher than the PA rate in patients with GC. For example, in a Danish study, PA was diagnosed in 19/877 (2.2%) patients with GC.33 However, PA is a late manifestation of AIG and does not represent a surrogate for the actual prevalence of AIG. In the general population, the prevalence of PA is also lower than the prevalence of AIG (0.15–1% vs 2–5%, respectively).17
In addition to PA, iron-deficiency anemia is also a common hematological finding of AIG, especially in the earlier stages.4 Accordingly, 32% of our GC patients with AIG showed iron-deficiency anemia compared to 18% of GC patients with no AIG. It is difficult in clinical practice to distinguish whether the cause of iron-deficiency anemia in this specific population is cancer-related, AIG-related5 or both.
The high five-year survival rate observed in our cohort of patients with GC even in the absence of AIG can be explained by the predominance of surgical patients with better survival.
One limitation of our study is the fact that the identification of patients with AIG was based on pathology records. As the characterization of the underlying gastritis is not explicitly recommended for the work-up of GC patients, the diagnosis of AIG among GC patients might have been underestimated. Furthermore, the small sample number resulted in ORs with wide CIs and prevented us from performing a multivariate analysis. However, our patients were matched a priori for gender and age, and study results were confirmed in subgroup analyses.
In conclusion, in this study, PA was associated with earlier diagnosis of GC in AIG patients compared to non-AIG patients and contributes significantly to a better clinical outcome. A stronger awareness of a GC risk in patients with AIG is crucial to further improve the outcome of this selected group of patients.
Acknowledgements
The authors thank all patients and their families for participating in this study. The authors also thank Ursula Stolz and Marion Holley (Department of Gastroenterology, Otto-von-Guericke University, Magdeburg) for their experimental work. This work contains substantial parts of the doctoral thesis of FW.
Declaration of conflicting interests
MiV received honoraria by FALK, Malesci, Olympus, Shire. JH is a member of the advisory board of Ipsen, and received travel grants from Ipsen and Shire; he also received honoraria from Merck. PPG is proctor for Intuitive Surgical. OW received honoraria from Bayer, BMS, Celgene, Ipsen, Novartis, Roche and Shire; he is a member of the advisory boards of Amgen, Bayer, BMS, Celgene, Eisai, Falk, Merck, Novartis, Roche, Servier and Shire, and received support for conducting clinical trials from Medac, Novartis and MSD. MM received personal fees and/or grants from Falk foundation, MSD, Lilly, Roche, Pfitzer, Amgen, Bristol-Myers Squibb, Merck Serono, MCI group. FL received personal fees from Amgen, Astellas, Astra Zeneca, Biontech, Eli Lilly, Elsevier, Infomedica, Merck, MSD, Roche, Servier, grants, personal fees and non-financial support from BMS. PM is involved in speakers’ bureau or consulting: Biocodex, Biohit, Danone, Mayoly-Spindler. MaV received honoraria from Nordic Pharma, Merck Serono, Bayer Vital, Lilly and Sirtex, and is a member of the advisory boards of Ipsen, Lilly, Nordic Pharma, BMS, MSD, Eisai and Amgen.
Ethics approval
This article does not contain any studies with animals performed by any of the authors. The study was carried out in accordance with the Declaration of Helsinki. Ethical approval was granted by the Ethics Committee of the Otto-von-Guericke University Hospital of Magdeburg on (approval number 170/12).
Funding
MaV and JS received support for this work from the Deutsche Forschungsgemeinschaft (project number 275016020). The work content is independent of the funding.
Informed consent
Informed consent was obtained from all individual participants included in the study. All participants included in thestudy consented to the publication of the data extracted from the statistical study. No individual patient data are reported.
ORCID iD
Marino Venerito https://orcid.org/0000-0001-8581-0974
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6): 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Coati I, Fassan M, Farinati F, et al. Autoimmune gastritis: pathologist's viewpoint. World J Gastroenterol 2015; 21(42): 12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham DY, Zou WY. Guilt by association: intestinal metaplasia does not progress to gastric cancer. Curr Opin Gastroenterol 2018; 34(6): 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy G, Dawsey SM, Engels EA, et al. Cancer risk after pernicious anemia in the US elderly population. Clin Gastroenterol Hepatol 2015; 13(13): 2282–2289.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hershko C, Ronson A, Souroujon M, et al. Variable hematologic presentation of autoimmune gastritis: age-related progression from iron deficiency to cobalamin depletion. Blood 2006; 107(4): 1673–1679. [DOI] [PubMed] [Google Scholar]
- 6.Lahner E, Esposito G, Galli G, et al. Atrophic gastritis and pre-malignant gastric lesions. Transl Gastrointest Cancer 2015; 4(4): 272–281. [Google Scholar]
- 7.Zhang Y, Weck MN, Schottker B, et al. Gastric parietal cell antibodies, helicobacter pylori infection, and chronic atrophic gastritis: evidence from a large population-based study in Germany. Cancer Epidemiol Biomarkers Prev 2013; 22(5): 821–826. [DOI] [PubMed] [Google Scholar]
- 8.Toh BH, Sentry JW, Alderuccio F. The causative H+/K + ATPase antigen in the pathogenesis of autoimmune gastritis. Immunol Today 2000; 21(7): 348–354. [DOI] [PubMed] [Google Scholar]
- 9.D'elios MM, Bergman MP, Azzurri A, et al. H+,K+-ATPase (proton pump) is the target autoantigen of Th1-type cytotoxic T cells in autoimmune gastritis. Gastroenterology 2001; 120(2): 377–386. [DOI] [PubMed] [Google Scholar]
- 10.Bordi C, D'Adda T, Azzoni C, et al. Hypergastrinemia and gastric enterochromaffin-like cells. Am J Surg Pathol 1995; 19(Suppl 1): S8–S19. [PubMed] [Google Scholar]
- 11.Smyk DS, Koutsoumpas AL, Mytilinaiou MG, et al. Helicobacter pylori and autoimmune disease: cause or bystander. World J Gastroenterol 2014; 20(3): 613–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vannella L, Lahner E, Osborn J, et al. Systematic review: gastric cancer incidence in pernicious anaemia. Aliment Pharmacol Ther 2013; 37(4): 375–382. [DOI] [PubMed] [Google Scholar]
- 13.Mahmud N, Stashek K, Katona BW, et al. The incidence of neoplasia in patients with autoimmune metaplastic atrophic gastritis: a renewed call for surveillance. Ann Gastroenterol 2018; 32(1): 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson WF, Rabkin CS, Turner N, et al. The changing face of noncardia gastric cancer incidence among US non-Hispanic whites. J Natl Cancer Inst 2018; 110(6): 608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 1998; 85(11): 1457–1459. [DOI] [PubMed] [Google Scholar]
- 16.Heinrichs SKM, Hess T, Becker J, et al. Evidence for PTGER4, PSCA, and MBOAT7 as risk genes for gastric cancer on the genome and transcriptome level. Cancer Med 2018; 7(10): 5057–5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massironi S, Zilli A, Elvevi A, et al. The changing face of chronic autoimmune atrophic gastritis: an updated comprehensive perspective. Autoimmun Rev 2019; 18(3): 215–222. [DOI] [PubMed] [Google Scholar]
- 18.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996; 20(10): 1161–1181. [DOI] [PubMed] [Google Scholar]
- 19.Veijola LI, Oksanen AM, Sipponen PI, et al. Association of autoimmune type atrophic corpus gastritis with Helicobacter pylori infection. World J Gastroenterol 2010; 16(1): 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Negrini R, Savio A, Poiesi C, et al. Antigenic mimicry between Helicobacter pylori and gastric mucosa in the pathogenesis of body atrophic gastritis. Gastroenterology 1996; 111(3): 655–665. [DOI] [PubMed] [Google Scholar]
- 21.Rusak E, Chobot A, Krzywicka A, et al. Anti-parietal cell antibodies - diagnostic significance. Adv Med Sci 2016; 61(2): 175–179. [DOI] [PubMed] [Google Scholar]
- 22.Tozzoli R, Kodermaz G, Perosa AR, et al. Autoantibodies to parietal cells as predictors of atrophic body gastritis: a five-year prospective study in patients with autoimmune thyroid diseases. Autoimmun Rev 2010; 10(2): 80–83. [DOI] [PubMed] [Google Scholar]
- 23.Bizzaro N, Antico A, Villalta D. Autoimmunity and gastric cancer. Int J Mol Sci 2018; 19(2): 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lahner E, Annibale B. Pernicious anemia: new insights from a gastroenterological point of view. World J Gastroenterol 2009; 15(41): 5121–5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goddard AF, James MW, McIntyre AS, et al. British Society of Gastroenterology. Guidelines for the management of iron deficiency anaemia. Gut 2011; 60(10): 1309–1316. [DOI] [PubMed] [Google Scholar]
- 26.Venerito M, Varbanova M, Röhl F-W, et al. Oxyntic gastric atrophy in Helicobacter pylori gastritis is distinct from autoimmune gastritis. J Clin Pathol 2016; 69(8): 677–685. [DOI] [PubMed] [Google Scholar]
- 27.Correa P. Chronic gastritis: a clinico-pathological classification. Am J Gastroenterol 1988; 83(5): 504–509. [PubMed] [Google Scholar]
- 28.Ekström AM, Held M, Hansson L-E, et al. Helicobacter pylori in gastric cancer established by CagA immunoblot as a marker of past infection. Gastroenterology 2001; 121(4): 784–791. [DOI] [PubMed] [Google Scholar]
- 29.Pimentel-Nunes P, Libânio D, Marcos-Pinto R, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019; 51(04): 365–388. [DOI] [PubMed] [Google Scholar]
- 30.Venerito M, Radünz M, Reschke K, et al. Autoimmune gastritis in autoimmune thyroid disease. Aliment Pharmacol Ther 2015; 41(7): 686–693 . [DOI] [PubMed] [Google Scholar]
- 31.Miceli E, Lenti MV, Padula D, et al. Common features of patients with autoimmune atrophic gastritis. Clin Gastroenterol Hepatol 2012; 10(7): 812–814. [DOI] [PubMed] [Google Scholar]
- 32.Marqués-Lespier JM, González-Pons M, Cruz-Correa M. Current perspectives on gastric cancer. Gastroenterol Clin North Am 2016; 45(3): 413–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elsborg L, Mosbech J. Pernicious anaemia as a risk factor in gastric cancer. Acta Med Scand 2009; 206(1–6): 315–318. [DOI] [PubMed] [Google Scholar]


