Abstract
Cell-cell adhesions serve to mechanically couple cells, allowing for long-range transmission of forces across cells in development, disease and homeostasis. Recent work has shown that such contacts also play a role in transducing mechanical cues into a wide variety of cellular behaviors important to tissue function. As such, understanding the mechanical regulation of cells through their adhesion molecules has become a point of intense focus. This review will highlight the existing and emerging technologies and models that allow for exploration of cadherin-based adhesions as sites of mechanotransduction.
Keywords: Cadherins, adherens junctions, mechanotransduction, cell adhesions, traction force microscopy, force transduction
Introduction:
It is well established that mechanical forces generated by contractile activity within the actomyosin cytoskeleton of individual cells are critical not only for driving structural changes inside those cells, but also for propagating forces outside those cells to control tissue-level organization(Wozniak and Chen, 2009, DuFort et al., 2011). Through the coupling of the contractile cystoskeleton to adhesion molecules at the cell surface, cellular forces can transmit long distances across multiple cells via cell-cell adhesion receptors, and to extracellular matrix (ECM) via integrins, to drive large-scale physical movements during morphogenetic events such as folding, twisting, and tissue compaction. In adult life such long-range forces are critical for functionality of muscles, blood vessels, integument, amongst others. With the advent several decades ago of substrates to measure the traction forces cells apply against their integrin-mediated cell-matrix adhesions, early efforts in mechanobiology primarily focused on defining mechanical characteristics of integrin-mediated binding. More recently, several studies have highlighted the importance of mechanical coupling across cell-cell adhesion receptors and the actin cytoskeleton for successful tissue development and morphogenesis (Rauzi et al., 2010, Rauzi et al., 2008, Martin et al., 2010, Sawyer et al., 2009, Maitre et al., 2012).
Of the many cell-cell adhesion molecules coupled to the cytoskeleton, including desmosomes, claudins and occludins, classical cadherins are perhaps the most important for dynamically regulating the transmission of forces between cells. Classical cadherins are Ca2+-dependent transmembrane adhesion proteins that mediate the formation of adherens junctions through homophilic binding of their extracellular domains, and are exemplified by N (neural)-, E (epithelial)- and P (placental)- cadherins. Cadherin-based adherens junctions (AJs) are not only critical for the formation of cell-cell contacts but are also crucial for initiating and dynamically regulating the formation of other cell junctional complexes such as tight and gap junctions, and desmosomes (Gumbiner et al., 1988, Tunggal et al., 2005, Frenzel and Johnson, 1996, Lewis et al., 1994, Capaldo and Macara, 2007). Cadherins have been shown to be linked intracellularly to the actin cystokeleton by the dynamic binding of the intracellular cadherin domain to the actin cytoskeleton via molecular complexes consisting of α-catenins (Aberle et al., 1994), β-catenins (Jou et al., 1995), p120-catenin (Reynolds et al., 1994), and other molecular partners (Fig 1a). While the molecular basis for the connections between the complex and the actin cytoskeleton are still being defined (Dufour et al., 2013, Yamada et al., 2005), these complexes mechanically couple cadherins with the cytoskeleton, and such coupling is necessary for force transmission through these complexes and across numerous cells. Interestingly, cytoskeletal contractility has been shown to differentially regulate adhesion strengthening and disruption, highlighting the importance of this coupling in the dynamic adhesions required for tissue morphogenesis (Conti et al., 2004, Miyake et al., 2006, Shewan et al., 2005, Smutny et al., 2010, Maddugoda et al., 2007, Yamada and Nelson, 2007, Krishnan et al., 2011, Krieg et al., 2008).
Figure 1.
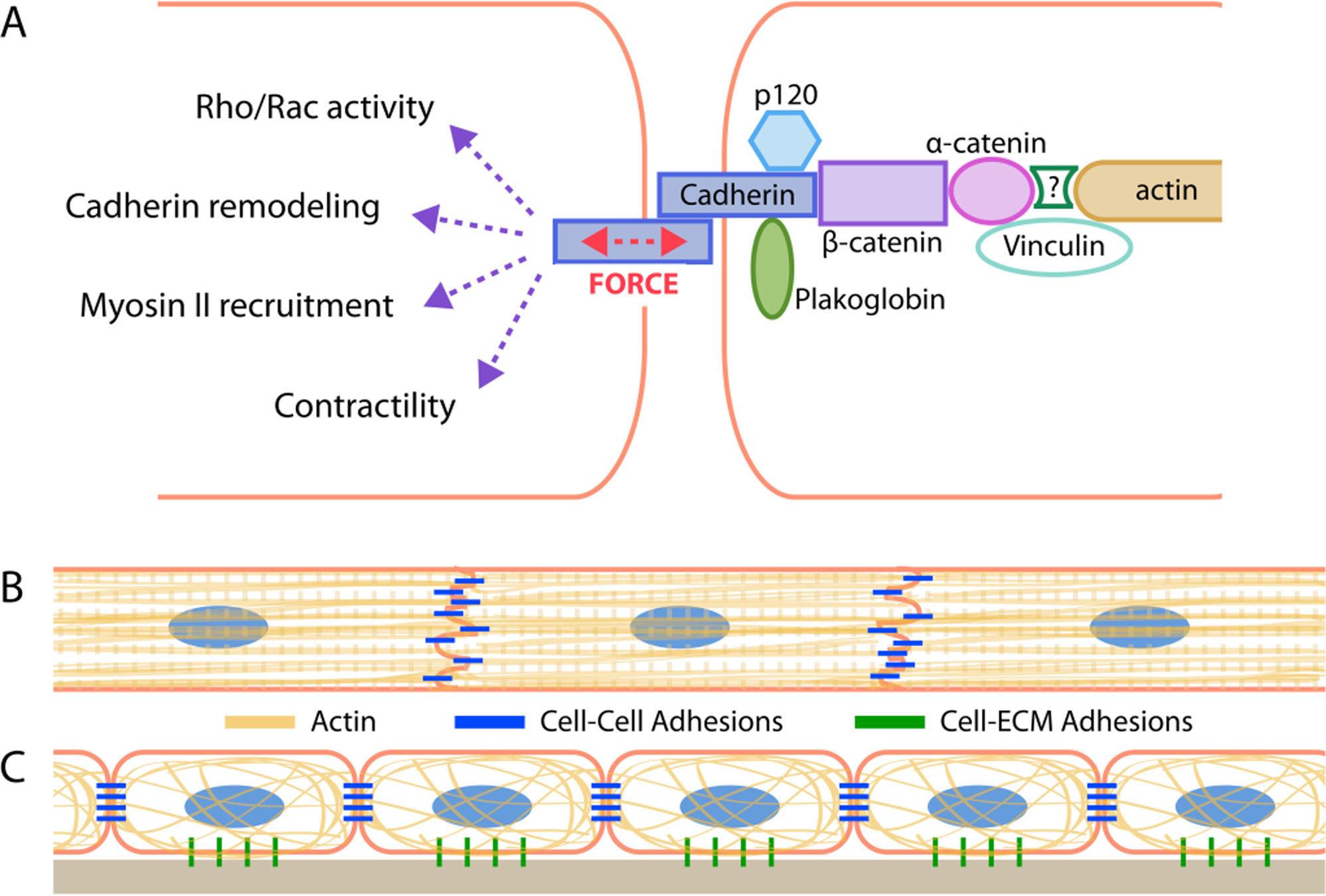
Classical-cadherin-mediated adhesions mechanically connect cells in tissues A) Schematic depicting homophilic cadherin adhesion between two cells and their cytoskeleton. Cadherin-mediated mechanotransduction drives many biological-signaling events. Schematic of cytoskeletal organizaton and its coupling with cadherin-mediated adhesions in B) cardiac muscle and C) epithelial layer of the skin.
The severe morphogenetic and functional defects caused by disruption of cadherin-mediated junctions are highlighted by a brief look at in vivo studies of the skin and heart. In the heart, N-cadherin junctions form a part of the intercalated disc that mechanically links myocytes together end-to-end and serves as an attachment site for myofibrils spanning adjacent cells (Fig 1b) (Goncharova et al., 1992, Geisler et al., 2010). Conditional knockout of the N-cadherin gene in the adult mouse heart leads to disruption of the intercalated discs and distorted myofibrils; reduction in the gap junction protein connexin 43; impaired mechanical function; and arrhythmia-induced sudden death (Kostetskii et al., 2005). Studies have conversely demonstrated the importance of N-cadherin to cytoskeletal maturation of cardiomyocytes, multicellular assembly and tissue structure in the heart (Radice et al., 1997, Piven et al., 2011). Cadherins are critical to the assembly and structure of many tissues, including the skin, in which E-cadherin regulates cell-cell adhesions in the epithelial cell sheets (Fig 1c) (Hirai et al., 1989, Fujita et al., 1992). Functionally blocking cadherins causes severe defects in skin tissue morphogenesis and organization during development (Hirai et al., 1989) and disrupts actin filament cables and coordinated cell migration necessary for wound healing (Danjo and Gipson, 1998). These studies illustrate the importance of cadherin adhesions in regulating mechanical and structural function of the heart and skin.
Although the requirement of cadherins for tissue formation and maintenance has clearly been demonstrated, it has until recently been difficult to characterize how mechanical forces and their propagation through cell-cell junctions contribute to these biological processes. Recent progress in the development of methods to apply and measure forces across cell-cell junctions have highlighted the truly enabling nature of biophysical tools in the investigation of mechanotransduction by cadherin complexes.
Given the close co-dependency between new biophysical tools and our ability to further understand mechanical regulation of cell-cell junctions, this review is structured around the different approaches that have been developed to measure and apply forces to junctions. We will discuss our developing understanding of cadherin-based junctions as both force transmission and transduction structures in the context of the advancing engineering techniques. We begin with methods involving coating cadherins onto surfaces in order to drive single cells to form AJ-like structures across which forces can be measured or applied, then examine methods to measure cell-cell forces on cells cultured on a planar surface, and end with methods with the potential for extending force measurements to 3D in vitro culture models.
Forces between immobilized cadherins and single cells
The large variety and dynamic nature of adhesions paired with their general inaccessibility to mechanical characterization in vivo make it difficult to isolate the effects of forces on specific junctions, and consequently methods to investigate the mechanics of specific cell-cell adhesion receptors have been slow to develop. An important early approach for isolating cadherin binding involved immobilization of dimers of an N-cadherin-Fc chimera (extracellular domain of N-cadherin coupled to IgG Fc fragment) on rigid surfaces (Fig 2a), which at the time allowed the exploration of the effects of cadherin engagement in cells (Lambert et al., 2000). A natural extension of immobilizing cadherins on a deformable substrate provided the first direct evidence of significant forces transmitted across cadherins (Ganz et al., 2006). Previously, researchers had developed culture substrates of microfabricated arrays of flexible, vertical polydimethylsiloxane (PDMS) microneedles on which cells would attach and spread across multiple microneedle tips (Fig 2b) (Tan et al., 2003). Forces across these cell-substrate attachments could then be measured by the cell-mediated deflections in these spring-like needles. Although the technique was originally used to characterize forces at cell-matrix adhesions, culturing cells on such substrates coated with the N-cadherin-Fc chimera allowed one to demonstrate forces exerted at homophilic cadherin adhesions. In this case, C2 myogenic cells were shown to spread and generate forces through cadherin binding comparable in magnitude to cell-ECM adhesions, suggesting that cells are able to transmit forces through these junctions (Fig 2c) (Ganz et al., 2006).
Figure 2.

Cadherin-coated tools to apply and measure force in a single cell in vitro. A) Cadherin-Fc chimera (cadherin extracellular domain (C-ECD) coupled to IgG Fc fragment (purple)) is immobilized on rigid surface. Binding occurs between the C-ECD of the chimera and the C-ECD of the cell. B) A cell seeded on microneedles coated with cadherin-FC chimera applies traction forces through cadherin-mediated adhesions that cause measurable deflections of the microdneedles. C) Single C2 myogenic cells on microneedle substrate spread and exert mapable traction forces through cadherin-mediated binding. Arrows indicate magnitude and direction of traction forces exerted by the cell (Ganz et al., 2006). D) Deformable polyacrylamide gels with immobilized cadherin-Fc chimera on their surface allow cells to bind and exert traction forces through cadherin-mediated adhesions. E) Single myocyte cells plated on cadherin-coated substrates (polyacrylamide gels or glass) of varying rigidity exhibit stiffness-dependent cell spreading. F-actin (red); α-actinin (green). Scale bars = 10 μm (Chopra et al., 2011). F) Magnetic beads with immobilized cadherin-FC chimera bind to cell surface and exert a shear force through cadherin adhesions when rotated. G) (i) The measured displacement of an E-cadherin(E-cad)- or poly-L-lysine(PL)-coated bead on the surface of an F9 cell under a 0.3 Hz oscillatory magnetic field for one minute. (ii) Stiffening of the cell as a result of cadherin-coated bead-applied shear relative to unperturbed cadherin-coated bead-cell adhesion was modulated by treatment with blebbistatin, to inhibit actin contractility, or Latrunculin B (LatB) or Cytochalasin D (CytoD) to disrupt actin polymerization (le Duc et al., 2010).
Two additional studies addressed whether the forces that are transmitted through cadherin adhesions could modulate cellular responses, by examining cells cultured on N-Cadherin-coated polyacrylamide substrates of varying stiffnesses (Fig 2d) (Chopra et al., 2011, Ladoux et al., 2010). By varying substrate rigidity, one could modulate the mechanical resistance to deformation by cellular forces applied through adherens junctions, testing the ability of the cells to sense and respond to these forces. Measuring displacement of fluorescent beads embedded near the surface of the gel before and after lysing of the cell allows for calculation of the traction forces exerted on the substrate surface. In both studies the cells (C2 mouse myogenic cells and primary cardiac myocytes) responded to changes in substrate rigidity by changing their morphology and cytoskeleton assembly. Both cell types on stiff substrates displayed a highly spread morphology with the formation of large cadherin adhesion complexes and robust stress fiber assembly. On soft substrates cells appeared rounded with disorganized and poor actin stress fiber bundling (Fig 2e). Myocytes have been shown to elongate and assemble contractile units (sarcomeres) on ECM-coated flexible substrates that match in vivo cardiac tissue elasticity (Jacot et al., 2008, Chopra et al., 2012). A similar change in shape and enhancement of sarcomere assembly was observed on cadherin-coated substrates that matched cardiac tissue elasticity (Chopra et al., 2011), suggesting that myocytes are able to sense and adapt their cytoskeleton to forces via cell-cell or cell-ECM adhesions. Such responses were lost when cells were treated with blebbistatin, a myosin II inhibitor (Ladoux et al., 2010), further supporting the notion that mechanical stresses were responsible for the observed changes. These studies provided some of the first evidence that cadherins were not only force transmitters, but were also part of an actomyosin-machinery-reliant mechanotransductive complex through which cells respond to mechanical stimuli.
Both of the studies discussed above utilized polyacrylamide gel substrates for which stiffness can be varied by degree of crosslinking. Of note, recent studies have raised important concerns about whether polyacrylamide gels are in fact a good model for varying mechanical interactions with cells. The change in degree of crosslinking used to vary gel stiffness also modulates the nanoscale pore structure of these gels such that less stiff gels also present fewer sites for immobilizing proteins (Trappmann et al., 2012). This decreased density of immobilization sites appears to affect how proteins are coupled to the substrate, and in the case of cell-matrix adhesions, the authors found that this decreased density alone can cause changes in cell spreading and adhesions assembly. In Ladoux et al.’s study, they recapitulated the stiffness effects using PDMS microneedle substrates in place of polyacrylamide gels. Unlike in gels, stiffness of the microneedle substrate is varied by changing pillar height, reducing the flexibility of each individual needle. As such, ligand density has no potential dependence on substrate stiffness.
Rather than relying on cell-generated forces on substrates to study mechanotransduction, others have taken a more direct approach by applying forces at junctions using magnetic beads (Wang et al., 1993). Of note, optical tweezers and atomic force microscopy have been used to measure and apply forces as well but the range of forces is significantly lower (0.1–100pN and 10pN-10nN, respectively), making this approach generally more attractive for single molecule studies (Baumgartner et al., 2000, Neuman and Nagy, 2008). In a formative study Le Duc et al. covalently bound E-cadherin-Fc chimeras to the surface of magnetic beads, and subsequently incubated these beads with a confluent monolayer of embryonic carcinoma F9 cells (le Duc et al., 2010). Applying an oscillatory magnetic field perpendicular to the beads’ magnetic moment rotated the beads on the cell surface to apply a shear force (Fig 2f). By measuring the displacement of the beads in response to this imposed force, they were able to measure the local stiffness of the cell and reported that cell cytoskeleton stiffened in response to a cyclic (0.3 Hz) force within a minute of force application through cadherin-coated beads. This stiffening response was abrogated in cells treated with blebbistatin to block myosin-mediated contractility, Latrunculin B, or Cytochalasin D, to disrupt actin depolymerization and/or polymerization (Fig 2g). Cells also did not stiffen when treated with calcium chelating reagent ethylene glycol tetraacetic acid (EGTA) to disrupt cadherin binding. When cells were treated with hepatocyte growth factor (HGF) to increase cell contractility, immunofluorescence staining revealed vinculin recruitment to actin-anchored cadherin adhesions (while localization of the adhesion complex proteins E-cadherin, α-catenin, β-catenin and p120-catenin did not appear to change with increased contractility). Vinculin recruitment was blocked when cells were treated with blebbistatin, but returned upon rinsing away of the contractility inhibitor. Although cell-cell adhesions and matrix adhesions exhibit a similar stiffening and vinculin-recruitment response to forces, some of the molecular players that mediate these responses are likely different. For example, Le Duc et al. showed cadherin-induced stiffening was only partially inhibited in vinculin knockout cells and did not involve the localization of paxillin at cell-cell contacts, which is important for vinculin recruitment at integrin-mediated focal adhesions (Humphries et al., 2007, Pasapera et al., 2010). The consequences of such differences are not yet clear, but these early studies suggest that mechanotransduction at adherens junctions may have different functions than at cell-ECM adhesions.
Such applied forces at cadherins not only appear to locally stiffen the cell, but also impact broader cell polarization. Weber et al., coated beads with C-cadherin-Fc and let these beads bind to individual Xenopus mesendoderm cells (Weber et al., 2012). Binding itself had no effect on cell phenotype, but upon application of tensile force to the adhesions through magnetic tweezers, cells polarized and migrated directionally, with protrusions extending away from the direction of bead pulling.
In addition to force transmission and transduction findings discussed above, studies of single cells with cadherin-coated force-sensing substrates may actually provide some insight into how cells interact with each other. The developmental process of cell sorting that enables tissue morphogenesis has been historically attributed to differential adhesion affinity, in which cells expressing similar cadherins tend to segregate together (Friedlander et al., 1989). Explanations for why this segregation occurred however, remained unclear for some time as it was shown that heterophilic cadherin extracellular domain binding can occur in vitro (Prakasam et al., 2006) and that binding affinity alone could not always account for the segregation. Using magnetic twisting cytometry to apply forces across cadherins, Tabdili et al. examined binding affinity as well as cell cytoskeletal stiffening responses to homophilic and heterophilic cadherin binding (Tabdili et al., 2012). This study tested cadherin binding in four different cell types against N-, E-, C-cadherin coated beads. Endogenous N-cadherin expressing C2C12 mouse myoblast cells and MDA-MB-435 cells, and E-cadherin expressing MCF7 human breast epithelial cells and Madin-Darby canine kidney (MDCK) cells were tested. In all cell types binding affinity appeared relatively indifferent to the binding event (homophilic or heterophilic), whereas cell stiffening only occurred when cells were bound to beads through homophilic cadherin binding (e.g. E-cadherin expressing cells only stiffened in response to beads coated with E-cadherin-Fc). Thus, it is possible that mechanotransduction initiated by homophillic and not heterophillic cadherin binding drives a necessary reinforcement for cells to maintain and stabilize their adheren junctions, and could explain the sorting behavior of cell types.
Spatial micropatterning of cadherin and integrin surfaces
While cells actively bind to each other, they are also in contact with the extracellular matrix. A number of studies have explored the possible interplay between integrin and cadherin binding, but only recently have such studies focused on how forces impact that interaction. To test whether rigidity regulates cell binding to substrates coated with either cadherins or fibronectin, Tsai et al. patterned islands of fibronectin surrounded by immobilized E-cadherin-Fc on PDMS surfaces of varying mechanical properties (Tsai and Kam, 2009). While MDCK-epithelial cells were able to form both cadherin and integrin-mediated adhesions, on stiff surfaces, integrin engagement blocked cadherin binding of MCF-7 (adenocarcinoma cell line) cells. This study suggests a cross-talk between cadherin and integrin adhesions that appears to be mechanically regulated. In another study of epithelial cells plated on alternating patterned lines of E-cadherin-Fc and collagen type IV, Borghi et al. found that the presence of E-cadherin-Fc decreased, in a concentration dependent manner, lamellipodia formation throughout the cell as well as biased cell migration (Borghi et al., 2010). Cells preferentially applied traction forces on the ECM stripes, and in the presence of E-cadherin the traction stresses on the ECM were anisotropic. Interestingly, concentration and presence of cadherin binding did not affect migration rate of the cells but did influence directional bias, and without ECM presence cell migration was absent. A similar rearrangement of ECM-applied traction force directionality was observed in keratinocyte colonies when cell-cell adhesion formation was initiated in the presence of calcium (Mertz et al., 2013). Such force-dependent crosstalk between cell-cell and cell-ECM adhesion molecules seems to suggest both physical and biochemical regulatory pathways play a role in coordinating these two systems.
Studies of immobilized cadherin-Fc and, in some cases ECM, on surfaces provided insight into the ability of these adhesions to transmit and transduce force under tightly controlled conditions, but they are limited in capturing the active cell-to-cell aspects of cell-cell adhesions, such as allowing cadherins to move laterally on both cell surfaces, or the mechanics of two cells pulling on each other. To model lateral mobility of cadherins using a substrate, Perez et al. tethered E-cadherin-Fc molecules through glycosylphosphatidyl inositol to supported lipid bilayers (Perez et al., 2005). Islands of anchored fibronectin were patterned into the bilayer. While only 30–60% of the E-cadherin-Fc proteins were diffusively mobile, without the fibronectin islands cells could not spread and their ability to cluster the cadherins was diminished. This approach may eventually provide additional insights into the clustering behavior of cadherins, but studies of the forces involved have not yet been reported. As such, much of our most recent understanding of junctional forces is derived from measurements between two attached cells.
Forces between cells in 2D culture
Often building off insights from cadherin-coated substrate studies, groups have recently employed cell-cell doublets to capture the dynamic complexities of cell-cell adhesions. The dual pipette assay was an early method developed to explore the strength of adhesions formed between two cells. In such an assay, two cells at the end of pipette tips are brought into contact and then the separation force, or force required to break the junction, is measured (Fig 3a). Using this assay, Chu et al. measured the force to separate two E-cadherin transfected murine sarcoma S180 cells, which do not express any endogenous cadherins (Chu et al., 2004). After cadherin transfection, cells adhered to each other and E-cadherin and β-catenin localized to adhesion sites. When two E-cadherin expressing S180 cells were brought into contact, the force required to break the cell-cell adhesion increased rapidly with time over approximately 30 minutes and then stabilized (around 200nN max separation force). Similar results were obtained using two N-cadherin expressing S180 cells. However, when one E-cadherin cell was paired with one N-cadherin cell while cells would maintain contact for up to 30 minutes, the force to separate the cells was undetectable, again supporting Tabdili et al.’s conclusion that only homophilic binding appears to lead to adhesion strengthening and stabilization.
Figure 3.
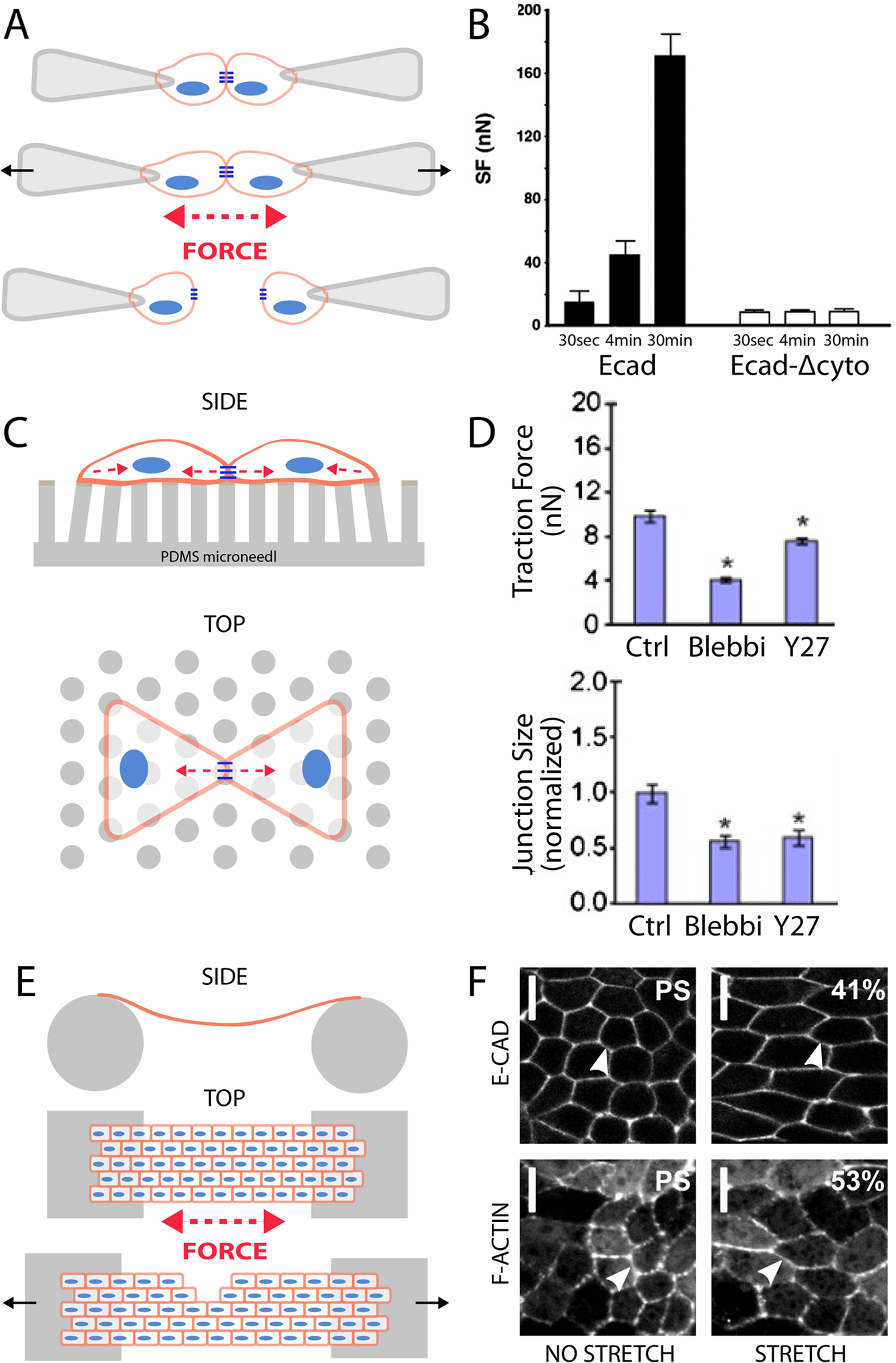
Tools to study mechanotransduction in cell-to-cell adhesions in vitro. A) In the dual pipette assay two cells held by suction at the end of separate pipettes are brought into contact. After adhesions have formed between the cells the force required to separate the cells is measured. B) The force required to break the cell-cell adhesion (SF) between two S180 cells expressing E-cadherin increased over 30 minutes. Adhesions between S180 cells transfected with an E-cadherin lacking the cytoplasmic domain (Ecad-Δcyto) did not demonstrate such time-dependent adhesion strengthening (Chu et al., 2004). C) Cells on microneedles are constrained to patterned ECM to create a repeatable, straight cell-cell adhesion. Cells exert forces through cell-ECM and cell-cell adhesions. D) “Tugging” forces across the cell-cell adhesion as well as adhesion size were reduced when endothelial cells were treated with actin contractility inhibitors, Blebbistatin (Blebbi) or Y27632 (Y27) (Liu et al., 2010). E) A monolayer of cells suspended between two rods is stretched until cell-cell adhesions are disrupted causing a fissure in the monolayer. The force required to cause the monolayer failure is measured. F) Fluorescent images of a monolayer of MDCK II epithelial cells pre-stretch and during stretch. Top row: Cells expressing E-cadherin GFP. Bottom row: Cells expressing Life-act GFP, an F-actin marker (Harris et al., 2012).
To examine the role of the cytoplasmic cadherin complex in this adhesion strengthening Chu et al. transfected cells with either E-cadherin constructs that lacked the cytoplasmic domain, and thus the β-catenin binding domain, (Ecad-Δcyto) or with chimeric E-cadherin-α-catenin (EαMC) also lacking β-catenin binding site. It appears all cells were able to form adhesions, but that adhesion strengthening occurred in the EαMC and E-cadherin expressing cell doublets, while not in the Ecad-Δcyto expressing cells (Fig 3b). This finding suggests that connection to the actin cytoskeleton is required for adhesion strengthening, but not for initial adhesion formation (characterized as having an approximate 20nN separation force and forming within the first 30 seconds). Additionally, it appears that the cadherin domain is required for β-catenin coexpression as no such expression was found in either of the cells expressing E-cadherins with cytoplasmic edits. Treatment of cells with actin-dysregulation drugs Latrunculin B or Jasplakinolide also blocked adhesion strengthening, but not initial adhesion formation, again providing evidence for the link between the cell cytoskeleton and cadherin adhesion dynamics. This study aligns with the important finding that α-catenin undergoes a conformational change allowing for vinculin binding and consequently adherens junction stability and force transmission (Yonemura et al., 2010). In a related dual pipette study, Thomas et al. found that over expression of mutant forms of the α-catenin vinculin-binding domain or depletion of vinculin significantly reduced adhesion strength of S180 cells and that localization of vinculin was dependent on the stretching of, or increasing the force between cells in the assay (Thomas et al., 2013). Such findings provide evidence that the mechanotransductive properties of cadherin adhesions may be modulated by the cytoplasmic complex through which cadherins are linked to the actin cytoskeleton.
As the dual pipette assay uses cell doublets that are suspended between two micropipettes, it is difficult to study the influence of cell shape and cell-ECM generated forces on cadherin-mediated mechanotransduction and the separation force reported by this assay does not indicate what levels of force cells normally exert across these junctions. To overcome these issues, Liu et al. developed a method for calculating the endogenous forces between two cells plated on ECM-coated microneedles (Fig 3c) (Liu et al., 2010). They reasoned that the force across the cell-cell adhesion in a cell doublet is equal to the vector sum of cell-matrix traction forces experienced in either of the two cells (which are equal and opposite), described as the cell-cell “tugging force.” This insight allowed them to examine the relationship between cell-cell forces and cell-ECM forces. They found that this tugging force was approximately perpendicular to the plane of endothelial cell-cell adhesion. Importantly, they reported that as tugging forces increased, the size of the cell-cell junction grew, while inhibiting these forces led to AJ disassembly. Using approaches to modulate myosin-mediated contractile forces or by directly pulling on junctions, the authors showed changes in junction size (Fig 3d). Further, the dynamic response to forces appeared to require Rac-mediated signaling, with Rac being a well known regulator of actin cytoskeleton organization (Nobes and Hall, 1995).
Employing a similar approach of deriving tugging forces from traction force measurements, Maruthamuthu et al. studied randomly coupled epithelial cells on collagen-coated polyacrylamide gels (Maruthamuthu et al., 2011). By separately increasing substrate stiffness and ligand density they found cell-ECM forces increased and the calculated cell-cell forces were a constant fraction of cell-ECM forces. This finding suggested a direct link between integrin binding and cadherin binding, providing further support for the notion that both adhesion molecules are mechanically connected via the same actin cytoskeletal networks. Interestingly, in this study, increased junction size was not observed with increased cell-cell forces. A number of differences between the two studies could explain these contrasting responses, including a difference in cell type (endothelial versus epithelial), cadherin (VE-cadherin versus E-cadherin), the geometry of the junctions (endothelial junctions were small compared to the cell diameter whereas epithelial junctions already reached the same width of the cells), and structure of the underlying actin (stress fibers in endothelial cells versus cortical actin in epithelia). Using the same approach to study cardiac myocytes, McCain et al. also observed that forces between myocytes increased with substrate stiffness, though at the highest forces cell-ECM adhesions began to form under the cell-cell contacts and were hypothesized to de-stabilize the cell-cell adhesions (McCain et al., 2012). Across all of these studies, the endogenous cell-generated magnitude of forces and stresses across AJs are of the same nanonewton order as those experienced at cell-matrix adhesions. Thus, while these forces are significantly lower than the fracture forces reported using dual pipettes, they suggest that AJs nonetheless experience substantial physiological forces in their homeostatic settings.
Studies of single cells on pattered ECM and cadherin substrates provided important first evidence of a force-dependent cross talk between integrin and cadherin-mediated adhesions. However, these studies were unable to explore the dynamic feedback between cell-cell adhesions. Tseng et al. examined mammary epithelial cell doublet adhesions when seeded on ECM-patterned polyacrylamide gels to explore cell-cell adhesions in the context of anisotropic ECM-patterned substrates (Tseng et al., 2012). They found that cell-cell adhesions remain stable and spatially confined to regions that are ECM deprived. Cell-cell junctions positioned close to ECM regions experienced large perpendicular tensional forces that led to destabilization of the cadherin junctions. Interestingly, the relative localization pattern of the two adhesion types was perturbed when cells were exposed to blebbistatin. In support of the single cell findings, this study suggests a role for the mechanical linkage between integrin and cadherin binding that drives cell function, and eventual tissue morphogenesis.
Other studies have attempted to examine cell forces under a more complex environment employing multiple cells. It has been shown that forces across cells within a monolayer differ greatly from those across single pairs of cells (Trepat et al., 2009). In their study, Trepat et al. computed cell-ECM traction forces as well as cell-cell stresses in a migrating sheet of MDCK (strain II) cells on polyacrylamide gels. The authors suggest that collective cell migration is not driven purely by a dragging front of leading cell, nor by individually propelled cells. Instead they find forces through cell-ECM and cell-cell adhesions engage in a tug-of-war type relationship to create collective motion. In a separate study employing a suspended 2D sheet of epithelial cells (Fig 3e), Harris et al. was able to examine the effects of stretch on monolayer integrity, cell-cell adhesions and cytoskeletal tension (Fig 3f) (Harris et al., 2012). Surprisingly the average force needed to separate a pair of cells within monolayers was near ninefold larger than measured in pairs of isolated cells. These studies have just begun to reveal the potential differences in cell-cell interactions as a result of culture methods. Thus, we see the continued extension of our ability to study force in progressively more complex settings is key to improved understanding of force transmission across cell-cell adhesions.
FRET studies for cadherin mechanics
While studies have provided valuable insight into the potential molecular regulation and makeup of the cadherin-actin complex, much is not understood about how this complex is mechanically regulated. While the engineering techniques above have begun to shed light on cadherins for mechanotransduction, they lack the ability to characterize the force that is actually experienced within the cadherin complex versus other cell-cell receptors.
To address this, investigators have begun to develop single molecule force sensors. Insertion of a random coiled-coil spring-like sequence separating a Förster Resonance Energy Transfer (FRET) pair into a molecule can reveal whether and when that molecule is under tension. This FRET method was applied in integrin force studies (Grashoff et al., 2010) and later adapted for cadherin studies. To explore the cadherin complex under tension in epithelial cell doublets in vitro, Borghi et al. employed a FRET sensor inserted into the cytoplasmic domain of E-cadherin between the transmembrane domain and the β-catenin binding domain (Fig 4a) (Borghi et al., 2012). Thus, when the cadherin was under tension the FRET signal would be undetectable. Borghi, et al. found that E-cadherin is under constant tension by the actomyosin cytoskeleton both at cell-cell junctions as well as throughout the free plasma membrane. Treatment of cells with Cytochalasin B or ML-7 (a myosin II inhibitor) resulted in a detectable FRET signal (Fig 4b). They were also able to show α-E-catenin (and the α-catenin binding domain of the cadherin) is required for E-cadherin to carry this tension, supporting the theory that α-catenin provides a mechanical link between the cadherin and the actin cytoskeleton (direct or otherwise e.g. through vinculin). This study provides powerful support for the physical link between the cadherins and the actin cytoskeleton and shows that the cadherin catenin complex is upstream of the mechanotransduction process similar to that observed for integrins.
Figure 4.

Emerging force measurement tools may allow mechanotransduction studies in 3D in vitro cell culture models. A) FRET complexes are inserted between the transmembrane domain and the cytoplasmic β-catenin binding domain of the cadherin molecule. The FRET signal is observable when the molecule is not under tension. Signal is absent when the FRET-containing cadherin is under tension. B) FRET index of MDCK epithelial cells expressing the tension sensitive FRET-cadherin (EcadTSMod) indicates cadherin molecules are under tension both at cell-cell junctions and in the free plasma membrane. This tension is lost when cells are treated with actin polymerization inhibitor, Cytochalasin B (Borghi et al., 2012). C) In vitro 3D cardiac microtissues. Immunofluorescence stainings of cytoskeletal and ECM proteins demonstrate cell alignment and cell-cell and cell-ECM adhesions (i) F-actin (green), Fibronectin (Red), Tenascin-C (Yellow) (Legant et al., 2009). (ii) Troponin-T (red) and nuclei (blue). Scale bar: 10μm (Boudou et al., 2012). (iii) N-cadherin (red), α-actinin (green) and nuclei (blue). Scale bar: 20μm. D) (i) Histochemistry and (ii) immunofluorescence staining of a 3D in vitro skin model adapted to explore tumor cells migration. β-Gal expressing tumor cells line the top of an epithelium of human epidermal keratinocytes, which lies above a collagen matrix that contains dermal fibroblasts. β-catenin (red), β-gal (green) and nuclei (blue). E) (i) Bead displacement trajectories around a volume rendering of a 3T3 fibroblast spreading in a 3D hydrogel are color-coded by magnitude. Scale bar: 50 μm. (ii) Contour plot of the magnitude of traction forces exerted by the cell in one protrusion. Range of approximately 0 kPa (blue) to 2 kPa (red) of force (Legant et al., 2010).
FRET sensors open up a window of opportunity for studying adhesion tension under a variety of settings. Recently, Conway et al. employed a FRET VE-cadherin in 2D endothelial sheets, and found that VE-cadherin was under myosin-dependent tension in static culture(Conway et al., 2013). Surprisingly, when shear stress was applied to these cells, tension across VE-cadherin was reduced as was total cell-cell and cell-matrix adhesion tension. Their measurements of tension on non-engaged VE-cadherin were approximately zero, suggesting that perhaps unbound cadherins are disconnected from the actin cytoskeleton, and that only their engagement leads to this connection. This finding differs partially from the FRET study by Borghi et al., and it raises questions as to the differences between endothelial and epithelial cadherin signaling, 2D sheets of cells versus cell pairs, and study-specific analysis techniques. FRET technology provides us the potential to study mechanotransduction in cadherins in a more complex setting as the technology is widely adoptable and adaptable to different settings (e.g. in vitro single cells, monolayers or 3D models, or in vivo) and molecules (cadherin, β-catenin, α-catenin, etc.). Thus, advances in FRET application may allow us to understand piece-by-piece how mechanical forces are transmitted within the cell and eventually converted to a biochemical signal. Of note, a limitation of current FRET technology is the inability to determine directionality of the force, as the optical signal only provides evidence of strain within an adhesion complex.
Future studies: Advancing current technologies to three dimensional culture systems
We now know that cells plated on planar substrates behave differently than those cultured within 3D hydrogels (Baker and Chen, 2012, Cukierman et al., 2001). Cell shape (Beningo et al., 2004), focal adhesion assembly (Beningo et al., 2004), migration modes (Friedl, 2004), and differentiation (Benya and Shaffer, 1982, Huebsch et al., 2010, Khetan et al., 2013) can all be affected by the dimensionality of their extracellular environment. Not surprisingly, cell-cell interactions are also greatly altered depending on environment. For example, epithelial cells on 2D surfaces form a sheet-like monolayer while those cultured in 3D arrange into polarized spheroids with a central lumen, similar to the acini structures seen in vivo (Emerman and Pitelka, 1977, Weigelt and Bissell, 2008).
To create a more relevant setting in which to study cell-cell interactions, many 3D in vitro model systems have been developed with tunable ECM, high structural control, and accessibility for imaging. Microtissues of aligned cardiac cells suspended in ECM between flexible cantilevers allow whole tissue force measurements while cells engage in cell-cell and cell-ECM adhesions in 3D (Fig 4c)(Boudou et al., 2012, Legant et al., 2009). Three dimensional in vitro angiogenesis models including the bead sprouting assay (Nakatsu et al., 2003), lumen formation assay (Koh et al., 2008) and more complex models such as those recently developed by Zheng et al. and Nguyen et al. are currently limited in their ability to examine mechanical regulation of vessel homeostasis and the angiogenic process (Zheng et al., 2012, Nguyen et al., 2013). Another widely used 3D model, the in vitro skin model, has provided an excellent platform to study homophilic and heterophilic cell interactions within a multi-layer, structured 3D environment that recapitulates the native setting (Fig 3d) (Boelsma et al., 2000, Alt-Holland et al., 2008).
While these systems all provide a setting to explore the importance of cell-cell adhesions in a more-native 3D environment, they lack the ability to measure or apply force at the cellular level. Such systems today are generally confined to immunofluorescence staining or dosing with inhibitors to investigate cell-cell interactions. It is possible that the combination of 3D in vitro models with emerging force sensing technologies could allow for cadherin mechanotransduction studies in 3D, providing novel insight into the relationship between individual cell-cell adhesions and larger scale tissue force transmission. As we discussed above, FRET technology holds great potential for application in a variety of systems and cells, and could potentially provide insight into cell-cell force transmission in previously inaccessible 3D in vitro models. Alternatively, traction force microscopy, which provided great early insight into directionality and magnitude of cell-ECM and cell-cell force transmission in 2D, has recently advanced to single cell studies in 3D (Fig 4f) (Legant et al., 2010, Franck et al., 2011). This approach involves the seeding of individual cells in defined and degradable hydrogels that contain fluorescent beads. Measurement of the beads’ displacement before and after lysing the encapsulated cell allows for the determination of cellular traction forces exerted on the surrounding matrix. While the method has thus far been established for measuring cell-matrix traction forces, deriving cell-cell tugging forces is likely not far off. Force measurements at cell-cell adhesions in 3D could provide new appreciation of the multicellular mechanics behind tissue morphogenesis.
Conclusions
Developmental models provided early insight into the role of force propagation in tissue morphogenesis. More recently, in vitro cell culture paired with force sensing technologies have shown that both cell-ECM and cell-cell adhesions can serve as mechanotransductive complexes. As previous studies have clearly linked mechanical stimulus through ECM adhesions to cell activities including differentiation (Engler et al., 2006), proliferation (Klein et al., 2009, Paszek et al., 2005) and death (Chen et al., 1997), it seems likely such stimulus through cadherins (or other potentially mechanotransductive cell-cell adhesion molecules such as desmosomes or occludins) could drive similar cell responses. Advancements and collaborations in force measurement technology will open doors to the many remaining questions surrounding the cadherin complex. Particularly, of interest is understanding how tension across cadherins is converted into biochemical signals and how such biochemical signaling in turn leads to changes at the adhesion site itself. The cues behind dynamic remodeling and differential strengthening and destabilization of cell-cell adhesions as required by processes such as cell sorting and tissue morphogenesis remain somewhat a mystery. Finally, crosstalk between cadherins and integrins has only begun to be appreciated be it through their attachment to same actin cytoskeleton or force driven biochemical signaling. It is quite possible that as we advance toward force application and measurement in 3D models new insights into cadherin adhesion remodeling and cross-adhesion signaling will arise. We look forward to the continued progress of a field that in only the last few years has covered so much territory in defining cadherins as an important player in mechanotransduction and tissue function.
Acknowledgements:
The authors thank Jeroen Eyckmans, Britta Trappmann, Evangelia Bellas, and William Polacheck for helpful discussions.
Footnotes
Declaration of Interest Statement:
The authors declare no conflict of interest.
REFERENCES:
- ABERLE H, BUTZ S, STAPPERT J, WEISSIG H, KEMLER R & HOSCHUETZKY H 1994. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci, 107 (Pt 12), 3655–63. [DOI] [PubMed] [Google Scholar]
- ALT-HOLLAND A, SHAMIS Y, RILEY KN, DESROCHERS TM, FUSENIG NE, HERMAN IM & GARLICK JA 2008. E-cadherin suppression directs cytoskeletal rearrangement and intraepithelial tumor cell migration in 3D human skin equivalents. Journal of Investigative Dermatology, 128, 2498–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER BM & CHEN CS 2012. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci, 125, 3015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUMGARTNER W, HINTERDORFER P, NESS W, RAAB A, VESTWEBER D, SCHINDLER H & DRENCKHAHN D 2000. Cadherin interaction probed by atomic force microscopy. Proceedings of the National Academy of Sciences of the United States of America, 97, 4005–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENINGO KA, DEMBO M & WANG YI 2004. Responses of fibroblasts to anchorage of dorsal extracellular matrix receptors. Proceedings of the National Academy of Sciences of the United States of America, 101, 18024–18029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENYA PD & SHAFFER JD 1982. Dedifferentiated Chondrocytes Reexpress the Differentiated Collagen Phenotype When Cultured in Agarose Gels. Cell, 30, 215–224. [DOI] [PubMed] [Google Scholar]
- BOELSMA E, GIBBS S, FALLER C & PONEC M 2000. Characterization and comparison of reconstructed skin models: Morphological and immunohistochemical evaluation. Acta Dermato-Venereologica, 80, 82–88. [PubMed] [Google Scholar]
- BORGHI N, LOWNDES M, MARUTHAMUTHU V, GARDEL ML & NELSON WJ 2010. Regulation of cell motile behavior by crosstalk between cadherin- and integrin-mediated adhesions. Proceedings of the National Academy of Sciences of the United States of America, 107, 13324–13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORGHI N, SOROKINA M, SHCHERBAKOVA OG, WEIS WI, PRUITT BL, NELSON WJ & DUNN AR 2012. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch (vol 109, 12568, 2012). Proceedings of the National Academy of Sciences of the United States of America, 109, 19034–19034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUDOU T, LEGANT WR, MU AB, BOROCHIN MA, THAVANDIRAN N, RADISIC M, ZANDSTRA PW, EPSTEIN JA, MARGULIES KB & CHEN CS 2012. A Microfabricated Platform to Measure and Manipulate the Mechanics of Engineered Cardiac Microtissues. Tissue Engineering Part A, 18, 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAPALDO CT & MACARA IG 2007. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell, 18, 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN CS, MRKSICH M, HUANG S, WHITESIDES GM & INGBER DE 1997. Geometric control of cell life and death. Science, 276, 1425–1428. [DOI] [PubMed] [Google Scholar]
- CHOPRA A, LIN V, MCCOLLOUGH A, ATZET S, PRESTWICH GD, WECHSLER AS, MURRAY ME, OAKE SA, KRESH JY & JANMEY PA 2012. Reprogramming cardiomyocyte mechanosensing by crosstalk between integrins and hyaluronic acid receptors. J Biomech, 45, 824–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOPRA A, TABDANOV E, PATEL H, JANMEY PA & KRESH JY 2011. Cardiac myocyte remodeling mediated by N-cadherin-dependent mechanosensing. Am J Physiol Heart Circ Physiol, 300, H1252–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU YS, THOMAS WA, EDER O, PINCET F, PEREZ E, THIERY JP & DUFOUR S 2004. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J Cell Biol, 167, 1183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONTI MA, EVEN-RAM S, LIU C, YAMADA KM & ADELSTEIN RS 2004. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J Biol Chem, 279, 41263–6. [DOI] [PubMed] [Google Scholar]
- CONWAY DE, BRECKENRIDGE MT, HINDE E, GRATTON E, CHEN CS & SCHWARTZ MA 2013. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol, 23, 1024–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUKIERMAN E, PANKOV R, STEVENS DR & YAMADA KM 2001. Taking cell-matrix adhesions to the third dimension. Science, 294, 1708–12. [DOI] [PubMed] [Google Scholar]
- DANJO Y & GIPSON IK 1998. Actin ‘purse string’ filaments are anchored by E-cadherin-mediated adherens junctions at the leading edge of the epithelial wound, providing coordinated cell movement. J Cell Sci, 111 (Pt 22), 3323–32. [DOI] [PubMed] [Google Scholar]
- DUFORT CC, PASZEK MJ & WEAVER VM 2011. Balancing forces: architectural control of mechanotransduction. Nature Reviews Molecular Cell Biology, 12, 308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUFOUR S, MEGE RM & THIERY JP 2013. alpha-catenin, vinculin, and F-actin in strengthening E-cadherin cell-cell adhesions and mechanosensing. Cell Adh Migr, 7, 345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMERMAN JT & PITELKA DR 1977. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro, 13, 316–28. [DOI] [PubMed] [Google Scholar]
- ENGLER AJ, SEN S, SWEENEY HL & DISCHER DE 2006. Matrix elasticity directs stem cell lineage specification. Cell, 126, 677–89. [DOI] [PubMed] [Google Scholar]
- FRANCK C, MASKARINEC SA, TIRRELL DA & RAVICHANDRAN G 2011. Three-dimensional traction force microscopy: a new tool for quantifying cell-matrix interactions. PLoS One, 6, e17833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENZEL EM & JOHNSON RG 1996. Gap junction formation between cultured embryonic lens cells is inhibited by antibody to N-cadherin. Developmental Biology, 179, 1–16. [DOI] [PubMed] [Google Scholar]
- FRIEDL P 2004. Prespecification and plasticity: shifting mechanisms of cell migration. Current Opinion in Cell Biology, 16, 14–23. [DOI] [PubMed] [Google Scholar]
- FRIEDLANDER DR, MEGE RM, CUNNINGHAM BA & EDELMAN GM 1989. Cell Sorting-out Is Modulated by Both the Specificity and Amount of Different Cell-Adhesion Molecules (Cams) Expressed on Cell-Surfaces. Proceedings of the National Academy of Sciences of the United States of America, 86, 7043–7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJITA M, FURUKAWA F, FUJII K, HORIGUCHI Y, TAKEICHI M & IMAMURA S 1992. Expression of cadherin cell adhesion molecules during human skin development: morphogenesis of epidermis, hair follicles and eccrine sweat ducts. Arch Dermatol Res, 284, 159–66. [DOI] [PubMed] [Google Scholar]
- GANZ A, LAMBERT M, SAEZ A, SILBERZAN P, BUGUIN A, MEGE RM & LADOUX B 2006. Traction forces exerted through N-cadherin contacts. Biology of the Cell, 98, 721–730. [DOI] [PubMed] [Google Scholar]
- GEISLER SB, GREEN KJ, ISOM LL, MESHINCHI S, MARTENS JR, DELMAR M & RUSSELL MW 2010. Ordered Assembly of the Adhesive and Electrochemical Connections within Newly Formed Intercalated Disks in Primary Cultures of Adult Rat Cardiomyocytes. Journal of Biomedicine and Biotechnology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONCHAROVA EJ, KAM Z & GEIGER B 1992. The involvement of adherens junction components in myofibrillogenesis in cultured cardiac myocytes. Development, 114, 173–83. [DOI] [PubMed] [Google Scholar]
- GRASHOFF C, HOFFMAN BD, BRENNER MD, ZHOU RB, PARSONS M, YANG MT, MCLEAN MA, SLIGAR SG, CHEN CS, HA T & SCHWARTZ MA 2010. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature, 466, 263–U143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUMBINER B, STEVENSON B & GRIMALDI A 1988. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol, 107, 1575–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS AR, PETER L, BELLIS J, BAUM B, KABLA AJ & CHARRAS GT 2012. Characterizing the mechanics of cultured cell monolayers. Proceedings of the National Academy of Sciences of the United States of America, 109, 16449–16454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRAI Y, NOSE A, KOBAYASHI S & TAKEICHI M 1989. Expression and role of E- and P-cadherin adhesion molecules in embryonic histogenesis. I. Lung epithelial morphogenesis. Development, 105, 263–70. [DOI] [PubMed] [Google Scholar]
- HUEBSCH N, ARANY PR, MAO AS, SHVARTSMAN D, ALI OA, BENCHERIF SA, RIVERA-FELICIANO J & MOONEY DJ 2010. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nature Materials, 9, 518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMPHRIES JD, WANG P, STREULI C, GEIGER B, HUMPHRIES MJ & BALLESTREM C 2007. Vinculin controls focal adhesion formation by direct interactions with talin and actin. Journal of Cell Biology, 179, 1043–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOT JG, MCCULLOCH AD & OMENS JH 2008. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J, 95, 3479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOU TS, STEWART DB, STAPPERT J, NELSON WJ & MARRS JA 1995. Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc Natl Acad Sci U S A, 92, 5067–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHETAN S, GUVENDIREN M, LEGANT WR, COHEN DM, CHEN CS & BURDICK JA 2013. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nature Materials, 12, 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN EA, YIN LQ, KOTHAPALLI D, CASTAGNINO P, BYFIELD FJ, XU TN, LEVENTAL I, HAWTHORNE E, JANMEY PA & ASSOIAN RK 2009. Cell-Cycle Control by Physiological Matrix Elasticity and In Vivo Tissue Stiffening. Current Biology, 19, 1511–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOH W, STRATMAN AN, SACHARIDOU A & DAVIS GE 2008. In vitro three dimensional collagen matrix models of endothelial lumen formation during vasculogenesis and angiogenesis. Angiogenesis: In Vitro Systems, 443, 83–101. [DOI] [PubMed] [Google Scholar]
- KOSTETSKII I, LI J, XIONG Y, ZHOU R, FERRARI VA, PATEL VV, MOLKENTIN JD & RADICE GL 2005. Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure. Circ Res, 96, 346–54. [DOI] [PubMed] [Google Scholar]
- KRIEG M, ARBOLEDA-ESTUDILLO Y, PUECH PH, KAFER J, GRANER F, MULLER DJ & HEISENBERG CP 2008. Tensile forces govern germ-layer organization in zebrafish. Nature Cell Biology, 10, 429–U122. [DOI] [PubMed] [Google Scholar]
- KRISHNAN R, KLUMPERS DD, PARK CY, RAJENDRAN K, TREPAT X, VAN BEZU J, VAN HINSBERGH VWM, CARMAN CV, BRAIN JD, FREDBERG JJ, BUTLER JP & AMERONGEN GPV 2011. Substrate stiffening promotes endothelial monolayer disruption through enhanced physical forces. American Journal of Physiology-Cell Physiology, 300, C146–C154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LADOUX B, ANON E, LAMBERT M, RABODZEY A, HERSEN P, BUGUIN A, SILBERZAN P & MEGE RM 2010. Strength Dependence of Cadherin-Mediated Adhesions. Biophysical Journal, 98, 534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMBERT M, PADILLA F & MEGE RM 2000. Immobilized dimers of N-cadherin-Fc chimera mimic cadherin-mediated cell contact formation: contribution of both outside-in and inside-out signals. Journal of Cell Science, 113, 2207–2219. [DOI] [PubMed] [Google Scholar]
- LE DUC Q, SHI QM, BLONK I, SONNENBERG A, WANG N, LECKBAND D & DE ROOIJ J 2010. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner (vol 189, pg 1107, 2010). Journal of Cell Biology, 191, 891–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEGANT WR, MILLER JS, BLAKELY BL, COHEN DM, GENIN GM & CHEN CS 2010. Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nature Methods, 7, 969–U113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEGANT WR, PATHAK A, YANG MT, DESHPANDE VS, MCMEEKING RM & CHEN CS 2009. Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues. Proceedings of the National Academy of Sciences of the United States of America, 106, 10097–10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS JE, JENSEN PJ & WHEELOCK MJ 1994. Cadherin Function Is Required for Human Keratinocytes to Assemble Desmosomes and Stratify in Response to Calcium. Journal of Investigative Dermatology, 102, 870–877. [DOI] [PubMed] [Google Scholar]
- LIU ZJ, TAN JL, COHEN DM, YANG MT, SNIADECKI NJ, RUIZ SA, NELSON CM & CHEN CS 2010. Mechanical tugging force regulates the size of cell-cell junctions. Proceedings of the National Academy of Sciences of the United States of America, 107, 9944–9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADDUGODA MP, CRAMPTON MS, SHEWAN AM & YAP AS 2007. Myosin VI and vinculin cooperate during the morphogenesis of cadherin cell cell contacts in mammalian epithelial cells. J Cell Biol, 178, 529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAITRE JL, BERTHOUMIEUX H, KRENS SFG, SALBREUX G, JULICHER F, PALUCH E & HEISENBERG CP 2012. Adhesion Functions in Cell Sorting by Mechanically Coupling the Cortices of Adhering Cells. Science, 338, 253–256. [DOI] [PubMed] [Google Scholar]
- MARTIN AC, GELBART M, FERNANDEZ-GONZALEZ R, KASCHUBE M & WIESCHAUS EF 2010. Integration of contractile forces during tissue invagination. Journal of Cell Biology, 188, 735–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARUTHAMUTHU V, SABASS B, SCHWARZ US & GARDEL ML 2011. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proceedings of the National Academy of Sciences of the United States of America, 108, 4708–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCAIN ML, LEE H, ARATYN-SCHAUS Y, KLEBER AG & PARKER KK 2012. Cooperative coupling of cell-matrix and cell-cell adhesions in cardiac muscle. Proceedings of the National Academy of Sciences of the United States of America, 109, 9881–9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERTZ AF, CHE Y, BANERJEE S, GOLDSTEIN JM, ROSOWSKI KA, REVILLA SF, NIESSEN CM, MARCHETTI MC, DUFRESNE ER & HORSLEY V 2013. Cadherin-based intercellular adhesions organize epithelial cell-matrix traction forces. Proc Natl Acad Sci U S A, 110, 842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYAKE Y, INOUE N, NISHIMURA K, KINOSHITA N, HOSOYA H & YONEMURA S 2006. Actomyosin tension is required for correct recruitment of adherens junction components and zonula occludens formation. Experimental Cell Research, 312, 1637–1650. [DOI] [PubMed] [Google Scholar]
- NAKATSU MN, SAINSON RCA, AOTO JN, TAYLOR KL, AITKENHEAD M, PEREZ-DEL-PULGAR S, CARPENTER PM & HUGHES CCW 2003. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1. Microvascular Research, 66, 102–112. [DOI] [PubMed] [Google Scholar]
- NEUMAN KC & NAGY A 2008. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nature Methods, 5, 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NGUYEN DH, STAPLETON SC, YANG MT, CHA SS, CHOI CK, GALIE PA & CHEN CS 2013. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc Natl Acad Sci U S A, 110, 6712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOBES CD & HALL A 1995. Rho, Rac, and Cdc42 Gtpases Regulate the Assembly of Multimolecular Focal Complexes Associated with Actin Stress Fibers, Lamellipodia, and Filopodia. Cell, 81, 53–62. [DOI] [PubMed] [Google Scholar]
- PASAPERA AM, SCHNEIDER IC, RERICHA E, SCHLAEPFER DD & WATERMAN CM 2010. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. Journal of Cell Biology, 188, 877–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASZEK MJ, ZAHIR N, JOHNSON KR, LAKINS JN, ROZENBERG GI, GEFEN A, REINHART-KING CA, MARGULIES SS, DEMBO M, BOETTIGER D, HAMMER DA & WEAVER VM 2005. Tensional homeostasis and the malignant phenotype. Cancer Cell, 8, 241–54. [DOI] [PubMed] [Google Scholar]
- PEREZ TD, NELSON WJ, BOXER SG & KAM L 2005. E-cadherin tethered to micropatterned supported lipid bilayers as a model for cell adhesion. Langmuir, 21, 11963–11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIVEN OO, KOSTETSKII IE, MACEWICZ LL, KOLOMIETS YM, RADICE GL & LUKASH LL 2011. Requirement for N-cadherin-catenin complex in heart development. Experimental Biology and Medicine, 236, 816–822. [DOI] [PubMed] [Google Scholar]
- PRAKASAM AK, MARUTHAMUTHU V & LECKBAND DE 2006. Similarities between heterophilic and homophilic cadherin adhesion. Proceedings of the National Academy of Sciences of the United States of America, 103, 15434–15439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADICE GL, RAYBURN H, MATSUNAMI H, KNUDSEN KA, TAKEICHI M & HYNES RO 1997. Developmental defects in mouse embryos lacking N-cadherin. Developmental Biology, 181, 64–78. [DOI] [PubMed] [Google Scholar]
- RAUZI M, LENNE PF & LECUIT T 2010. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature, 468, 1110–U515. [DOI] [PubMed] [Google Scholar]
- RAUZI M, VERANT P, LECUIT T & LENNE PF 2008. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nature Cell Biology, 10, 1401–U57. [DOI] [PubMed] [Google Scholar]
- REYNOLDS AB, DANIEL J, MCCREA PD, WHEELOCK MJ, WU J & ZHANG Z 1994. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol, 14, 8333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAWYER JK, HARRIS NJ, SLEP KC, GAUL U & PEIFER M 2009. The Drosophila afadin homologue Canoe regulates linkage of the actin cytoskeleton to adherens junctions during apical constriction. Journal of Cell Biology, 186, 57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEWAN AM, MADDUGODA M, KRAEMER A, STEHBENS SJ, VERMA S, KOVACS EM & YAP AS 2005. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Molecular Biology of the Cell, 16, 4531–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMUTNY M, COX HL, LEERBERG JM, KOVACS EM, CONTI MA, FERGUSON C, HAMILTON NA, PARTON RG, ADELSTEIN RS & YAP AS 2010. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nature Cell Biology, 12, 696–U147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABDILI H, LANGER M, SHI QM, POH YC, WANG N & LECKBAND D 2012. Cadherin-dependent mechanotransduction depends on ligand identity but not affinity. Journal of Cell Science, 125, 4362–4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAN JL, TIEN J, PIRONE DM, GRAY DS, BHADRIRAJU K & CHEN CS 2003. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proceedings of the National Academy of Sciences of the United States of America, 100, 1484–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS WA, BOSCHER C, CHU YS, CUVELIER D, MARTINEZ-RICO C, SEDDIKI R, HEYSCH J, LADOUX B, THIERY JP, MEGE RM & DUFOUR S 2013. alpha-Catenin and vinculin cooperate to promote high E-cadherin-based adhesion strength. J Biol Chem, 288, 4957–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAPPMANN B, GAUTROT JE, CONNELLY JT, STRANGE DGT, LI Y, OYEN ML, STUART MAC, BOEHM H, LI BJ, VOGEL V, SPATZ JP, WATT FM & HUCK WTS 2012. Extracellular-matrix tethering regulates stem-cell fate (vol 11, pg 642, 2012). Nature Materials, 11, 742–742. [DOI] [PubMed] [Google Scholar]
- TREPAT X, WASSERMAN MR, ANGELINI TE, MILLET E, WEITZ DA, BUTLER JP & FREDBERG JJ 2009. Physical forces during collective cell migration. Nature Physics, 5, 426–430. [Google Scholar]
- TSAI J & KAM L 2009. Rigidity-Dependent Cross Talk between Integrin and Cadherin Signaling. Biophysical Journal, 96, L39–L41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSENG QZ, DUCHEMIN-PELLETIER E, DESHIERE A, BALLAND M, GUILLOU H, FILHOL O & THERY M 2012. Spatial organization of the extracellular matrix regulates cell-cell junction positioning. Proceedings of the National Academy of Sciences of the United States of America, 109, 1506–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUNGGAL JA, HELFRICH I, SCHMITZ A, SCHWARZ H, GUNZEL D, FROMM M, KEMLER R, KRIEG T & NIESSEN CM 2005. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. Embo Journal, 24, 1146–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG N, BUTLER JP & INGBER DE 1993. Mechanotransduction across the Cell-Surface and through the Cytoskeleton. Science, 260, 1124–1127. [DOI] [PubMed] [Google Scholar]
- WEBER GF, BJERKE MA & DESIMONE DW 2012. A Mechanoresponsive Cadherin-Keratin Complex Directs Polarized Protrusive Behavior and Collective Cell Migration. Developmental Cell, 22, 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIGELT B & BISSELL MJ 2008. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Seminars in Cancer Biology, 18, 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOZNIAK MA & CHEN CS 2009. Mechanotransduction in development: a growing role for contractility. Nature Reviews Molecular Cell Biology, 10, 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMADA S & NELSON WJ 2007. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol, 178, 517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMADA S, POKUTTA S, DREES F, WEIS WI & NELSON WJ 2005. Deconstructing the cadherin-catenin-actin complex. Cell, 123, 889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YONEMURA S, WADA Y, WATANABE T, NAGAFUCHI A & SHIBATA M 2010. alpha-Catenin as a tension transducer that induces adherens junction development. Nature Cell Biology, 12, 533–U35. [DOI] [PubMed] [Google Scholar]
- ZHENG Y, CHEN JM, CRAVEN M, CHOI NW, TOTORICA S, DIAZ-SANTANA A, KERMANI P, HEMPSTEAD B, FISCHBACH-TESCHL C, LOPEZ JA & STROOCK AD 2012. In vitro microvessels for the study of angiogenesis and thrombosis. Proceedings of the National Academy of Sciences of the United States of America, 109, 9342–9347. [DOI] [PMC free article] [PubMed] [Google Scholar]


