Abstract
Investigations into the human 8-oxodGTPase, MutT Homolog 1 (MTH1), have risen sharply since the first-in-class MTH1 inhibitors were reported to be highly tumoricidal. However, MTH1 as a cancer therapeutic target is currently controversial because subsequently developed inhibitors did not exhibit similar cytotoxic effects. Here, we provide the first direct evidence for MTH1-independent 8-oxodGTPase function in human cancer cells and human tumors, using a novel ATP-releasing guanine-oxidized (ARGO) chemical probe. Our studies show that this functionally redundant 8-oxodGTPase activity is not decreased by five different published MTH1-targeting small molecules or by MTH1 depletion. Significantly, while only the two first-in-class inhibitors, TH588 and TH287, reduced cancer cell viability, all five inhibitors evaluated in our studies decreased 8-oxodGTPase activity to a similar extent. Thus, the reported efficacy of the first-in-class MTH1 inhibitors does not arise from their inhibition of MTH1-specific 8-oxodGTPase activity. Comparison of DNA strand breaks, genomic 8-oxoguanine incorporation, or alterations in cellular oxidative state by TH287 versus the noncytotoxic inhibitor, IACS-4759, contradict that the cytotoxicity of the former results solely from increased levels of oxidatively damaged genomic DNA. Thus, our findings indicate that mechanisms unrelated to oxidative stress or DNA damage likely underlie the reported efficacy of the first-in-class inhibitors. Our study suggests that MTH1 functional redundancy, existing to different extents in all cancer lines and human tumors evaluated in our study, is a thus far undefined factor which is likely to be critical in understanding the importance of MTH1 and its clinical targeting in cancer.
Introduction
Many tumors sustain high reactive oxygen species (ROS) levels due to aberrant metabolism and constitutive oncogenic signaling (1-3). ROS are major effectors of DNA damage and concomitant tumor suppression (4). Therefore, aggressive tumors acquire mechanisms that are protective against oxidatively damaged DNA and its consequences (4, 5). Although oxidative damage can occur directly on genomic DNA, several studies have reported that the nucleotide pool, due to its relatively greater accessibility by mitochondrially generated ROS and redox cycling pro-oxidant factors, is more vulnerable to oxidation (6-8). Thus, unless detoxified by nucleotide pool–cleansing enzymes, these oxidatively damaged DNA precursors can be readily incorporated into the genome by DNA polymerases. We previously reported the human nucleotide pool–sanitizing enzyme and functional 8-oxodGTPase, MutT Homolog 1 (MTH1, also known as hMTH1 or NUDT1; ref. 9), is elevated in oncogenic RAS-driven tumor cells, and inhibits oxidative DNA damage and its tumor-suppressive effects (10-14). Published data from our laboratory (11, 12) and others (15), as well as examination of public tumor datasets (16, 17) indicate tumors possess higher MTH1 mRNA and protein levels than adjacent normal tissue, and that tumors with elevated MTH1 mRNA levels have significantly lower overall and disease-free survival (reviewed in refs. 18, 19).
Our published studies were the first to show that shRNA-mediated MTH1 depletion induces genomic DNA breaks and a persistent DNA damage response, elicits cellular senescence, and inhibits xenograft tumor formation by KRAS-driven non-small cell lung cancer (NSCLC) lines (12, 13). Studies with the first-in-class MTH1 inhibitors, TH287 and TH588 (20), suggested MTH1 to be a potent broad-spectrum chemotherapeutic target. Since then, interest in MTH1 as a therapeutic target has skyrocketed, with multiple studies evaluating MTH1 inhibition-induced phenomena in different cancer lines, consequently spurring the development of additional MTH1 inhibitors (21-25). The therapeutic index for MTH1 inhibitors was expected to be excellent as MTH1-null mice are developmentally and phenotypically normal, with minimal increases in mutagenic →T transversions (26), and only show low instances of spontaneous tumors with late age (>18 months; ref. 27). Although our study and others support MTH1 inhibition as an effective tumor-suppressive strategy (12, 20, 28), a number of studies using second-generation inhibitors have drawn the opposite conclusion (reviewed in ref. 18), casting doubt on MTH1 as a bona fide therapeutic target.
We believe that these current contradictions stem from variability in model systems used across the studies, inconsistency in assays used to assess drug treatment outcomes, possible compensatory mechanisms that are functionally redundant with MTH1, and off-target cytotoxic effects of the first-in-class inhibitors. The inability to clarify these issues has been compounded by the lack of a sensitive and specific readout for native cell line/tissue MTH1 8-oxodGTPase enzymatic activity. MTH1 studies to date have utilized the inorganic pyrophosphate release assay to determine the inhibitory potency (IC50) of their lead compounds against recombinant MTH1 enzyme. However, this assay cannot be reliably used to measure endogenous cell line or tissue-specific 8-oxodGTPase activity, as pyrophosphate release is not unique to the 8-oxodGTPase enzymatic pathway.
Accordingly, using a panel of cancer cell lines, we have established endogenous cellular 8-oxodGTPase activity and the MTH1-specific contribution to this activity by using the newly developed ATP-releasing guanine-oxidized (ARGO) probe-based assay. The chimeric ARGO probe generates ATP following its cleavage by 8-oxodGTPase activity, which can then be directly detected as luminescence (29, 30). Combining this assay with MTH1 depletion or with its pharmacologic inhibition allows determination of MTH1-specific contributions to total 8-oxodGTPase activity (29). Herein, we have assessed the effects of five different MTH1 inhibitors on cellular 8-oxodGTPase activity: the first-in-class inhibitors TH287 and TH588, which reportedly possess high tumoricidal activity (20) as well as three independently developed MTH1 inhibitors, IACS-4759 (ref. 21; denoted here as IACS), AstraZeneca compound 21 (ref. 24; denoted here as AZ-21), and BAY-707 (22), which do not appear to affect cell viability. Although MTH1 is considered to be the main human 8-oxodGTPase, our studies here reveal for the first time that its contribution to total endogenous 8-oxodGTPase activity differs among cancer cell lines as well as among human tumor specimens. Indeed, we have found some cancer cell lines possess 8-oxodGTPase activity that is nearly completely independent of MTH1, and is furthermore unaffected by either MTH1 depletion or MTH1 inhibitors. This finding demonstrates redundancy within the 8-oxodGTPase pathway, and the potential to compensate for the loss of MTH1. Importantly, our studies also independently establish that, despite nearly identical degrees of 8-oxodGTPase activity inhibition by all five inhibitors across a panel of cancer cell lines, only TH588 and TH287 induce significant cytotoxicity. Thus our results suggest that these first-in-class inhibitors reduce cell viability through off-target mechanisms. This is a critical finding with strong clinical implications, as the first-in-man clinical trial ( NCT03036228) with Karonudib, an MTH1 inhibitor based on the same drug scaffold as the first-in-class inhibitors, is currently underway in patients with advanced solid tumors.
Materials and Methods
Human tissue-derived experiments
All human subjects research was carried out following written consent obtained from the patients and according to the protocols approved by the University of Miami Institutional Review Board (IRB# 20100200). Retrospective frozen tissue samples consisting of deidentified matched normal and tumor pairs were obtained from untreated patients diagnosed with stage 1, 2, or 3 NSCLC at the Sylvester Comprehensive Cancer Center (Miami, FL).
Cell lines
BEAS2B (CRL-9609), H1563 (CRL-5875), A549 (CCL-185), H23 (CRL-5800), H358 (CRL-5807), PC3 (CRL-1435), U2OS (HTB-96), and HeLa (CCL-2) were obtained from ATCC. Cell lines were authenticated by short tandem repeat profiling and Mycoplasma testing via PCR (Genetica Corp) in August 2018 (A549, H23,H358,H1563) or in October 2019 (U2OS). PC3 and HeLa cells were used within four passages following thaw from original ATCC purchased stocks. All cell culture reagents were purchased from Gibco/Life Technologies Inc., except for the FBS used to supplement the media, which was obtained from Hyclone. Cell lines were cultured in RPMI-1640 base medium (11875) with the exception of U2OS, which were cultured in DMEM (11965), supplemented with 10% FBS and 100 U/mL penicillin/streptomycin (15140122). Cells were cultured at 37°C in a humidified incubator at 21% oxygen or 5% oxygen, 5% CO2 (HeraCell Tri-Gas, Thermo Fisher Scientific, Inc.).
MTH1 inhibitors
TH588 and TH287 were commercially obtained (Sigma, SML1096, SML1069, respectively). IACS-4759 (IACS) was a kind gift from Dr. Alessia Petrocchi (MD Anderson Cancer Center, Houston, TX). The AstraZeneca inhibitor compound 21 (AZ-21) was synthesized in the Kool laboratory as per the original reported structure and synthesis methodology (24). BAY-707 (22) was obtained from the Structural Genomics Consortium (SGC).
Recombinant MTH1 and MTH2 proteins
Recombinant human MTH1 (NUDT1) was purchased from Abcam (ab99390). Recombinant human MTH2 (NUDT15) was obtained commercially from Origene, TP310477. The recombinant proteins were diluted in nuclease-free water (Ambion, AM9937) to achieve the concentrations tested for ARGO activity in the relevant figures.
DNA constructs and viral transduction
The pLKO.shGFP, pLKO.shMTH1, and pLKO.shp53 lentiviral constructs as well as the pBh.MTH1 and pBp.KRASV12 retroviral constructs have been described previously (11, 12). Plasmids were sequenced prior to use (Source Biosciences). Lentiviral and retroviral production was carried out in HEK 293T cells (ATCC), and infection of target cells was performed as described previously (12, 13). Prior to carrying out experiments, transduced cells were selected in media containing either 2.5 μg/mL puromycin (Sigma, P7255) or 100 μg/mL hygromycin (Sigma, H3274), for a minimum duration corresponding to the time taken for untransduced cells to die in antibiotic-supplemented media. Protein knockdown or overexpression in the transduced cells was verified via Western blotting.
Drug treatment and cell viability assays
Between 150 to 750 cells/well (optimized for each specific cell line and duration of drug treatment) were plated in triplicate in 96-well plates. Twenty-four hours after plating, culture media were replaced in the wells and either TH287, TH588, IACS-4759, or AZ-21 added. Vehicle wells were treated with DMSO (Sigma, D2650). At 72 hours postdrug treatment, viability was established using the CellTiter-Glo assay (Promega, G7571) with luminescence values read on a FilterMax F5 Microplate Reader (Molecular Devices, LLC). Data were normalized to luminescence values from vehicle-treated controls and plotted as % relative luminescence units (RLU) against a log-transformed x-axis. The drug IC50 values were generated using the GraphPad Prism software (version 6) using a nonlinear regression model, choosing log (inhibitor) versus response–variable slope as the equation, and robust fit as the curve-fitting method. IC50 values were not determined (N.D.) when the dose response did not achieve 50% loss of viability.
Preparation of ATP-depleted cell and tissue lysates for the ARGO assay
Lysates were prepared as described previously (29) with minor modifications. Approximately, 107 cells were washed with DPBS (Gibco, 14190), and harvested by scraping prior to lysis. Tissues (approximately 20 mg) were lysed using 500 μL hypotonic buffer, by pulsing with a handheld battery-operated homogenizer (VWR Pellet Mixer) over ice. The hypotonic lysis buffer components are as follows: 10 mmol/L HEPES, pH 7.9, 1.5 mmol/L MgCl2, 10 mmol/L KCl, 0.5 mmol/L DTT, 1× Protease Inhibitor Cocktail (Roche, 11697498001), and 1 mmol/L Na3VO4, as described previously (29). Lysates were incubated on ice for 30 minutes with gentle mixing every 10 minutes, followed by centrifugation at 13,000 rpm for 10 minutes, at 4° C. To deplete ATP, supernatants were then transferred to Amicon 3K Centrifugal Filters (EMD Millipore UFC500324) and centrifuged for 10–30 minutes at 13,000 rpm at 4°C, until the lysate had filtered through. The filtrate was discarded, and the remaining concentrate reconstituted to the original sample volume with hypotonic buffer. Centrifugation and reconstitution were repeated twice more for a total of three ATP depletions. After the final centrifugation, the filter was inverted in an unused, chilled tube and centrifuged at 1,000 × g for 2 minutes at 4°C to collect the concentrated, ATP-depleted lysate. Protein concentrations were determined using the Protein Assay Dye Reagent (Bio-Rad, 5000006). Lysates were used at 1 μg/mL and diluted in hypotonic buffer.
Measurement of total and MTH1-specific 8-oxo-d-GTPase activity via the ARGO assay
The ARGO assay was performed as described previously (29). Briefly, the reaction buffer (50 mmol/L NaCl, 10 mmol/L Tris-HCl, pH 7.9, 10 mmol/L MgCl2, 1 mmol/L Na3VO4, 1 mmol/L DTT) containing 40 μmol/L ARGO probe was prepared in nuclease-free water (Ambion, AM9937). Individual reaction tubes were prepared on ice to a final volume of 22 μL, containing 2 μL(2 μg) of cell/tissue lysate or indicated concentrations of recombinant MTH1 or MTH2 diluted in 2 μL and 20 μmol/L MTH1 inhibitor (TH588, TH287, IACS-4759, AZ-21, or BAY-707) or 0.2% DMSO. Reactions were incubated at 30°C for 1 hour, and 5 μL of each reaction was pipetted in triplicate into the wells of a prechilled white flat-bottomed 96-well plate (Greiner). Subsequently, 95 μL of CellTiter-Glo luciferin/luciferase solution (Promega, G7571) was added to each well and luminescence was read on a Spectramax i3x microplate reader (Molecular Devices) at the endpoint (10 minutes after incubation in CellTiter-Glo luciferin/luciferase solution) or at 1-minute intervals for 30 minutes. The raw luminescence values obtained correspond to total 8-oxodGTPase activity. To calculate MTH1-specific 8-oxodGTPase activity, RLU values from MTH1 inhibitor–treated lysate were subtracted from the RLU value of the counterpart DMSO-treated control sample, and the resulting averaged RLU value plotted as a measure of MTH1-specific 8-oxodGTPase activity. Where indicated, data were normalized to luminescence values from DMSO-treated controls and plotted as percent luminescence against a log-transformed x-axis.
Western blotting
Cells in culture were harvested by mechanical scraping on ice and lysed in a sodium fluoride buffer as described previously (31). Protein lysates from human lung specimens were made using RIPA buffer (Thermo Fisher Scientific, 89900), supplemented with a protease inhibitor cocktail (Roche, 11697498001). Protein concentrations were measured using the Protein Assay Dye Reagent (Bio-Rad, 5000006). Approximately 5–25 μg of total protein was run on a 4%–12% Bis-Tris pre-cast NuPage gel (Life Technologies, NP0321 or NP0322) on the Novex gel system, and subsequently transferred onto polyvinylidene difluoride membrane (Immobilon, EMD Millipore, IPVH000010) at 30 V at 4°C. Blots were washed in 0.1% TBST and then probed with antibodies against the following proteins: MTH1 (1:2,000, Novus Biologicals 100–109), actin (1:2,000, Sigma A2066), p53 (1:1,000, Santa Cruz Biotechnology, SC126), cleaved PARP (1:1,000, Cell Signaling Technology, 9541), catalase (1:2,000, Abcam, ab16731), SOD2 (1:1,000, Enzo Life Sciences, ADI-SOD-110), GAPDH (1:20,000, Abcam, ab9485), γ-tubulin (1:10,000, Sigma T6557). Appropriate secondary horseradish peroxidase antibodies (GE Healthcare) were used and blots were developed using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, 34095) on film (Denville Scientific, E3018). Immunoblots in the figures are representative of data from a minimum of two independent runs. Pixel intensity for the bands was measured via the ImageJ Analyze Gels (NIH) module and normalized to the loading signal for each band.
ROS measurement
The ROS assay was carried out via staining with 5- (and-6)-chloromethyl-2′,7′-dichlorofluorescein diacetate (CM-H2DCF-DA; Molecular Probes/Life Technologies, C6827) as described previously (31). Briefly, cells at equivalent confluency under the indicated conditions were collected through trypsinization and incubated with freshly prepared 10 mmol/L CM-H2DCF-DA in Ca2+- and Mg2+-free 1× Hank balanced salt solution (HBSS, Gibco, 14175), for 30 minutes at 37°C. The cells were then washed and resuspended in 1× HBSS before detection of fluorescent signal. The x-axis represents FITC channel (FL1) fluorescence intensity in log-scale and the y-axis represents the number of cells. The flow cytometric profiles were generated and analyzed on a BD Accuri C6 Cytometer and included software (BD Biosciences).
Polarographic evaluation of mitochondrial respiratory chain function
The acute and chronic effects of the MTH1 inhibitors, TH287 and IACS, on mitochondrial respiratory chain function were evaluated at 37°C using a polarographic chamber (Hansatech Instruments Limited), as described previously (32). To test whether the MTH1 inhibitors function through acute inhibition of mitochondrial enzymes, endogenous intact cell respiration was measured using approximately 106 H358 cells suspended in respiration buffer (0.3 mol/L mannitol, 10 mmol/L KCl, 5 mmol/L MgCl2, 0.5 mmol/L EDTA, 0.5 mmol/L EGTA, 1 mg/mL BSA, 10 mmol/L K3PO4, pH 7.4) after sequential injection of DMSO or the indicated MTH1 inhibitors into the polarographic chamber. Potassium cyanide (KCN, 320 μmol/L) was used to assess the specificity of the measurements. The oxygen consumption rate (OCR) for each sample was calculated by dividing the nmol O2 consumed/minute after each injection point, by the total number of live cells in the assay.
To evaluate the chronic effects of MTH1 inhibition on mitochondrial respiration and oxidative phosphorylation, H358 cells were treated with 0.1% DMSO, 10 μmol/L TH287, or 10 μmol/L IACS for 22 hours. Endogenous respiration was measured in intact cells suspended in respiration buffer (supplemented with 2 mmol/L ADP and 7 U/mL hexokinase), after which the cells were permeabilized using 4.92 μg/mL digitonin. Respiration rates were then measured after each sequential injection of glutamate and malate at 10 mmol/L each, 1 μmol/L oligomycin, 0.2 μmol/L carbonyl cyanide m-chlorophenylhydrazone (CCCP), and 320 μmol/L KCN. The OCR was calculated as above, after which the KCN-sensitive OCR was obtained by subtracting from the KCN-insensitive OCR for each run. Two independent samples were run for each DMSO/MTH1 inhibitor condition for all polarographic experiments.
Comet assay and determination of genomic 8-oxodG
U2OS, H358, and A549 cells were plated at a density of 1.25 × 105 cells in 6-well culture dishes, treated with either 0.1% DMSO (vehicle) or the indicated drugs (10 μmol/L) for 24 or 36 hours prior to being harvested via trypsinization, and the pellets snap frozen in liquid nitrogen. The alkaline-modified comet assay, with or without human OGG1 (hOGG1) treatment (New England BioLabs, M0241) in matched counterparts, was performed using two independent samples per condition, as described previously (33-35). Each comet slide gel had approximately 15,000 cells. Hydrogen peroxide (H2O2)-treated cells (50 μmol/L for 30 minutes, 37°C in the dark) or etoposide-treated cells (C3, immortalized human lymphocyte line) provided by Trevigen (4256-010-CC) were used as positive controls to ensure technical accuracy of the assay conditions. A minimum of 100 individual cells in total were scored across the two independent samples per condition and the % tail DNA was calculated via Comet Assay IV software version 4.2 (Perceptive Instruments). The OGG1 treatment excises genomically incorporated 8-oxodG leaving apurinic sites or alkali labile sites (ALS) which, under alkaline comet assay conditions, are converted into single-strand breaks (SB). The P values were established using an unpaired Student t test, with Welch correction applied if variances were found to be unequal.
Statistical analyses
Specifics regarding experimental replicates and statistical tests are listed in the figure legends or methods, as applicable. Unless otherwise stated in the figure legend, all data are presented as mean ± SD. Unless specified otherwise, an unpaired Student t test was performed to assess significance. If variances were found to be unequal, an unpaired Student t test with Welch correction was performed. P values < 0.05 were considered statistically significant. Statistical analyses were performed using GraphPad Prism software (version 6).
Results
The ARGO assay accurately establishes MTH1-specific contributions to cellular 8-oxodGTPase activity via MTH1 depletion or MTH1 inhibitor treatment
All studies to date involving MTH1 inhibitors (reviewed in ref. 18) have utilized the release of inorganic pyrophosphate to measure inhibitor potency against recombinant MTH1 enzyme. This assay, however, cannot be used reliably to evaluate endogenous cell or tissue MTH1 activity given the large number of enzymatic functions that release this moiety. Recently, a more sensitive and direct assay for detecting endogenous cell/tissue 8-oxodGTPase activity has been developed (29). This assay employs luminescence-based detection of ATP in stringently ATP-depleted cell or tumor lysates, released through 8-oxodGTPase activity at ARGO, a chimeric nucleotide probe containing both 8-oxo-dGTP and ATP (Fig. 1A).
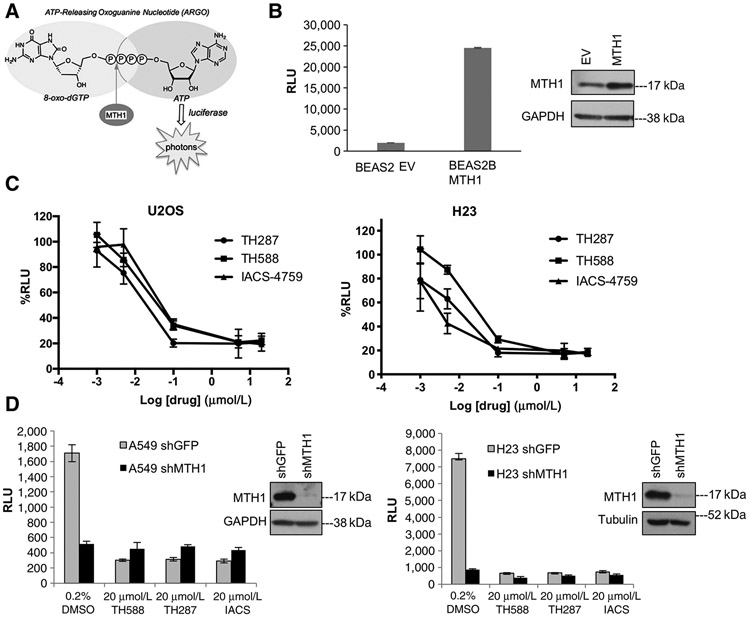
Figure 1.
The ARGO probe–based assay is an accurate measure of MTH1-specific 8-oxodGTPase activity. A, Schematic showing how cleavage of the chimeric ARGO probe (with 8-oxodG and A base moieties) by MTH1 8-oxodGTPase activity generates ATP that can then be detected via a luciferase-based luminescent signal. B, ARGO probe–generated luminescence (in relative luminescence units, RLU) from lysates made from stable MTH1-ovexpressing BEAS2B cells or their empty vector (EV)-transduced counterparts. Each sample was run in triplicate and data are representative of a minimum of n = 2.Arepresentative Western blot run on 10 μg total protein lysates from these cell lines, with the MTH1 signal and GAPDH loading control, is shown to the right of the graph. Error bars, ± SD. C, The percent decreases in ARGO probe-generated luminescence in U2OS and H23 lysates by the three indicated MTH1 inhibitors. The log-transformed drug concentration range is indicated on the x-axis. Each concentration was run in triplicate, and the signal at each concentration is normalized to signal from the counterpart vehicle-treated lysates. Error bars, ± SD. D, Comparison of effects on ARGO probe–generated luminescence produced by shRNA-mediated MTH1 depletion versus the first-in-class MTH1 inhibitors. Data are representative of a minimum of n = 2, with each sample run in triplicate. Western blotting on 10 μg of total protein from A549 lysates and 20 mg total protein from H23 lysates, with the MTH1 signal and GAPDH or tubulin as loading controls, is shown to the right of the graph, to establish levels of MTH1 protein knockdown.
We independently validated the ability of the ARGO assay to accurately measure MTH1-specific 8-oxodGTPase activity through several experiments. First, we verified that an MTH1-overexpressing BEAS2B cell line developed in our laboratory (12) showed substantially higher ARGO activity–based luminescence compared with its empty vector–transduced counterpart (Fig. 1B). We then established the dose dependency of 8-oxodGTPase activity against a range of concentrations of three MTH1 inhibitors, TH588, TH287, or IACS, in U2OS, a cell line previously shown to have very high MTH1-specific activity (29), and which was used extensively in characterizing the cellular effects of the first-in-class inhibitors (20). This was accomplished by incubating the ATP-depleted U2OS cell lysates with the respective inhibitors, which then exhibited a marked MTH1 inhibitor concentration–dependent decrease in 8-oxodGTPase activity across all three inhibitors (Fig. 1C, left). We also established MTH1 inhibitor dose–response curves on total 8-oxodGTPase activity for the NSCLC line H23 (Fig. 1C, right), which we previously found had the most striking tumor-suppressive response to MTH1 depletion in a xenograft tumor model (12). Our results show that TH287 suppressed ARGO/8-oxodGTPase activity more strongly than TH588 at all but the two highest concentrations (5 μmol/L, 20 μmol/L), consistent with its reported lower IC50 over TH588 (20). In both U2OS and H23 cells, both IACS and TH287 showed maximal suppression of 8-oxodGTPase activity at the sub-1 μmol/L range (Fig. 1C). Note that we have utilized 20 μmol/L MTH1 inhibitor to pretreat lysates for subsequent ARGO-based experiments in this study. This dose was selected to ensure maximal inhibition of MTH1-specific activity via all MTH1 inhibitors, to most stringently establish residual (MTH1-independent) 8-oxodGTPase activity.
We next assessed whether addition of the small-molecule MTH1 inhibitors to ATP-depleted lysates was a valid and accurate method to establish MTH1-specific 8-oxodGTPase activity. To do so, we compared the decrease in ARGO cleavage-induced luminescence produced by the inhibitors vis-à-vis MTH1 depletion using a validated and efficacious shRNA (ref.12; Fig. 1D). As anticipated, we found that lysates from MTH1-depleted A549 and H23 cells exhibited dramatically lower luminescent signal in the ARGO assay compared with their control shGFP counterparts (Fig. 1D, DMSO groups). We then compared this result against MTH1 inhibitor–induced decreases in ARGO cleavage–induced luminescence. In both A549 and H23, we found that the MTH1 inhibitors decreased ARGO-based luminescence to an extent that was virtually identical to that observed in counterpart lysates from MTH1 shRNA–transduced cells (Fig. 1D). These results verified that the difference between total ARGO luminescence and the residual (MTH1-independent) signal following TH588, TH287, or IACS treatment accurately reflects MTH1-specific (or druggable) 8-oxodGTPase activity. These results also suggested that the residual luminescence observed in the shMTH1 samples is unlikely to be a consequence of incomplete suppression of MTH1 activity, as in each case the drugs were able to inhibit 8-oxodGTPase activity at least as well as the shRNA. Finally, we showed that addition of equimolar 8-oxodGTP (but not dGTP) relative to ARGO was able to compete away a substantial fraction of ARGO cleavage–induced luminescence (Supplementary Fig. S1A). The fact that the ARGO probe functions as an MTH1 substrate with relatively high efficiency (only ~8-fold lower than native 8-oxo-dGTP; ref. 29) makes it difficult to compete the ARGO signal to baseline. Collectively, our results herein provide strong evidence that the ARGO assay provides an accurate measure of total endogenous 8-oxodGTPase activities, and of MTH1-specific 8-oxodGTPase activity through establishing the fraction of total ARGO-derived luminescence that is sensitive to MTH1 inhibitors or MTH1 shRNA.
Cellular 8-oxodGTPase activity is inhibited equivalently by the different MTH1 inhibitors in multiple cancer cell lines revealing MTH1-independent 8-oxodGTPase activity
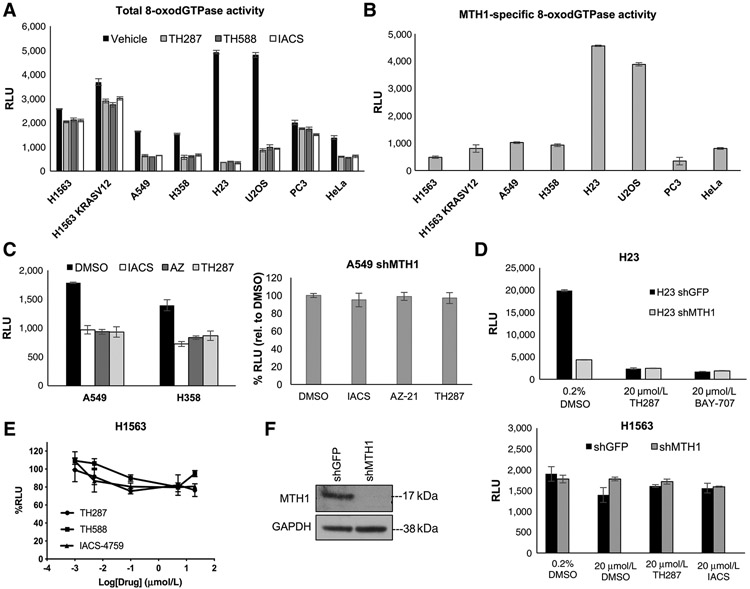
We next sought to assess whether the tumoricidal efficacy of TH588 and TH287 (20) versus IACS (ref. 21; reportedly not cytotoxic to tumor cells) was due to differences in the respective abilities of these small molecules to inhibit cellular 8-oxodGTPase activity. Accordingly, we established total (Fig. 2A) and MTH1-specific (Fig. 2B) 8-oxodGTPase activity in a panel of cancer cell lines, including several NSCLC cell lines in which we have extensively characterized the effects of MTH1 depletion via shRNA (12) as well as in cell lines used in the original study describing the ARGO assay (29). To further assess whether the efficacy of MTH1 small-molecule inhibitors was determined by the extent to which they inhibit 8-oxodGTPase activity, we also utilized two additional noncytotoxic MTH1 inhibitors, AZ-21 (compound 21; ref. 24) as well as BAY-707 (22) in the ARGO assay. Our results verified that AZ-21 and BAY-707 were also able to inhibit 8-oxodGTPase activity to a virtually identical extent as TH287 or IACS (Fig. 2C and D). Furthermore, similar to the other three inhibitors (Fig. 1D), AZ-21 and BAY-707 inhibition of MTH1-specific 8-oxodGTPase activity was comparable with that achieved by MTH1 depletion via shRNA (Fig. 2C and D). On the basis of our results, we noted two things: first, the five inhibitors we utilized all inhibited 8-oxodGTPase activity to a near identical extent, and second, some cancer lines (such as H1563 or PC3) had very little contribution from MTH1 to their endogenous 8-oxodGTPase activity, as measured by the ARGO probe. This finding suggested that some cells may possess an alternate enzyme with 8-oxodGTPase activity that can hydrolyze the ARGO probe, in the absence of functional MTH1.
Figure 2.
Five different MTH1 inhibitors, regardless of reported cytotoxicity, similarly inhibit 8-oxodGTPase activity across a panel of cancer cell lines, revealing a “nondruggable” activity component. A, Levelsof total cellular 8-oxodGTPase activity in the indicated panel of cancer cell lines. Results are derived from two independent experiments (n = 2) with each sample run in triplicate. Error bars, ± SD. B, Levels of MTH1-specific 8-oxodGTPase activity in the panel of cancer cell lines from A. The MTH1-specific values were generated by subtracting the average of triplicate RLU values from each of the three inhibitors shown in A from the RLU value i nthe vehicle counterpart and averaged across the different inhibitors used. Results are derived from two independent experiments (n = 2) with each sample run in triplicate. Error bars, ± SD. C, Left, total cellular 8-oxodGTPase activity under vehicle and different MTH1 inhibitors in A549 and H358 cells. Baseline (DMSO) luminescence values are shown next to the RLU values obtained following lysate treatment with either TH287, IACS-4759, or AZ-21 inhibitors for each cell line. Results are derived from two independent experiments (n = 2) with each sample run in triplicate. Right, percentage change in ARGO assay–generated luminescence (relative to vehicle-treated control) in MTH1-depleted A549 cell lysates, following treatment with the three indicated MTH1 inhibitors. Note that AZ-21 suppresses activity similarly to the other two inhibitors and none of the three treatments produce any significant difference in 8-oxodGTPase activity relative to shMTH1 lysates. Each sample was run in triplicate. Error bars, ± SD. D, Comparison of how total 8-oxodGTPase activity in H23 shGFP versus shMTH1 cells is affected by the reportedly cytotoxic inhibitor TH287 versus the noncytotoxic inhibitor BAY-707. Each sample was run in triplicate. Error bars, ± SD. E, The percentage decreases in ARGO probe–generated luminescence in H1563 lysates by the three indicated MTH1 inhibitors. The log-transformed drug concentration range is indicated on the x-axis. Each concentration was run in triplicate, and the luminescent signal at each concentration is normalized to the signal from its counterpart vehicle-treated sample. Error bars, ± SD. F, Comparison of how total 8-oxodGTPase activity in H1563 cells is affected by shRNA-mediated MTH1 depletion versus the three indicated MTH1 inhibitors, run in triplicate. Error bars, ± SD. Western blotting on 10 μg of total protein lysates confirming MTH1 knockdown in the shMTH1 samples, with GAPDH as the loading control, is shown to the left of the 8-oxodGTPase activity graph.
We next confirmed that the H1563 line was singularly lacking in druggable 8-oxodGTPase activity across a range of concentrations for three MTH1 inhibitors (Fig. 2E). We verified this finding by assessing ARGO cleavage in H1563 cells depleted of MTH1 via shRNA relative to control (shGFP) counterparts (Fig. 2F). Unlike A549 and H23 depleted of MTH1 (Fig. 1D), the majority of 8-oxodGTPase activity remained intact in H1563 shMTH1 lysates, recapitulating the results with the MTH1 inhibitors. We confirmed efficient MTH1 knockdown in counterpart H1563 lysates via immunoblotting (Fig. 2F). We note that, in our prior studies (12), H1563 was also the cell line most refractory to MTH1 depletion–associated DNA breaks and tumor-suppressive effects, compared with the other NSCLC lines used in this study.
Collectively, our findings unequivocally demonstrate the presence of MTH1-independent 8-oxodGTPase activity, as measured by the ARGO probe, which cannot be depleted by shRNA or inhibited by MTH1-targeting small molecules. This redundant activity varies in extent among the multiple cancer lines tested in our study but remains consistent in each cell line across experiments, suggesting this variation is unlikely to be a technical artefact. Significantly, because none of the five inhibitors showed significant differences in their relative ability to suppress 8-oxodGTPase activity in these cell lines, their on-target suppression of 8-oxodGTPase activity is unlikely to be the source of their reportedly discrepant tumoricidal efficacies (18, 20-22, 24).
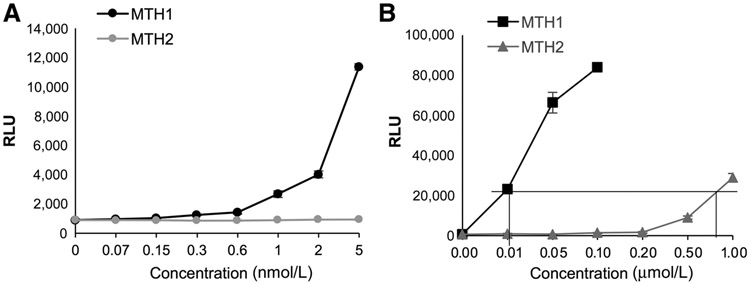
Phylogenetic trees and hierarchical clustering of the extensive Nudix family of hydrolases show that NUDT15 (MTH2) and NUDT18 (MTH3) share the same sequence similarity as NUDT1 (MTH1; ref. 36). Although MTH1 is known to be the most efficient mammalian 8-oxodGTPase, human MTH2 (NUDT15) has also been reported to exhibit weak 8-oxo-dGTPase activity (about 40-fold lower) relative to MTH1, in cell-free systems (36, 37). Analysis of publicly available RNA-seq data from the four main NSCLC lines used in this study, A549, H23, H358, and H1563, suggests that MTH2 is robustly transcriptionally expressed in these cell lines (Supplementary Fig. S1B). In contrast, MTH3 has much lower transcriptional levels than either MTH1 or MTH2 in these cell lines (Supplementary Fig. S1B). Few validated reagents are currently available for genetic modulation or detection of endogenous MTH2. We therefore assessed whether recombinant MTH2 could cleave the ARGO probe. We could not detect any ARGO luminescence from addition of recombinant MTH2 at the nanomolar range, at which comparable concentrations of recombinant MTH1 robustly produced signal (Fig. 3A). However, at about an approximately 80-fold higher concentration, we found recombinant MTH2 protein could hydrolyze ARGO to generate a luminescent signal comparable with recombinant MTH1 (Fig. 3B), consistent with the reported weaker 8-oxodGTPase activity of MTH2. The ARGO signal from this higher MTH2 concentration could be reduced to a similar extent as the corresponding lower MTH1 concentration by addition of exogenous 8-oxodGTP, showing the specificity of the ARGO probe for detecting relative 8-oxodGTPase activity from these proteins at their respective reactive concentrations (Supplementary Fig. S1C). Collectively, these observations raise the possibility that MTH2, which is not druggable by the first-in-class inhibitors (20), may be the source of non-MTH1-dependent ARGO luminescence we observe in cancer cell lines. However, at this time, we cannot definitively rule out the possibility that there are other functional 8-oxodGTPases capable of generating the residual ARGO signal we observe in the cancer lines.
Figure 3.
MTH2 can generate ARGO cleavage–induced luminescence. A, Comparison of ARGO cleavage–generated luminescence in response to increasing concentrations of human recombinant MTH1 and MTH2, up to 5 nmol/L. Each concentration was run in triplicate. Error bars, ± SD. B, Comparison of ARGO cleavage–generated luminescence in response to increasing concentrations of human recombinant MTH1 and MTH2 (up to 1 μM/L). Each concentration was run in triplicate. The boxed lines indicate the concentrations of MTH1 versus MTH2 at which equivalent ARGO cleavage–induced luminescence is observed. Error bars, ± SD.
The MTH1-specific contribution to total 8-oxodGTPase activity differs among human NSCLC specimens and is distinct from the nondruggable component of this activity
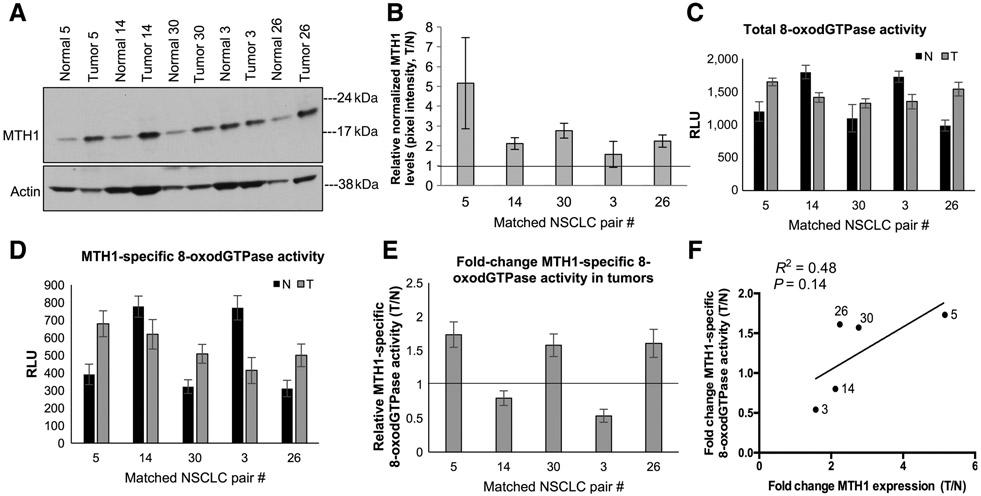
Several studies have proposed using MTH1 expression in tumors as a prognostic or progression biomarker and to rationalize MTH1 inhibition as a therapeutic approach (38-41). To determine whether there is undruggable MTH1 8-oxodGTPase activity in human tumors similar to cancer cell lines, we utilized five matched normal/NSCLC patient specimen pairs that we had previously profiled via qPCR and found to have higher MTH1 mRNA levels in the tumor counterparts (12). Consistent with previous findings by us and others (8, 11, 15, 42), MTH1 protein expression in these samples was also found to be higher in the tumor versus the normal tissue (between ~2 and 4 fold; Fig. 4A and B; Supplementary Fig. S2A and S2B). Using the ARGO assay on these patient samples to establish total 8-oxodGTPase activity (Fig. 4C) with TH287 addition to the lysates, to deduce MTH1-specific 8-oxodGTPase activity (Fig. 4D), we observed variable extents of residual ARGO activity among the tumors that could not be reduced by TH287. We ensured that the ARGO-generated luminescence in these samples was within the linear range of the assay (Supplementary Fig. S2C). In this limited sample size, we did not find elevated MTH1-specific 8-oxodGTPase across all tumor specimens versus their normal counterparts (Fig. 4E). However, we did observe a general correlation between the fold change in protein expression and the fold change in MTH1-specific 8-oxodGTPase activity in the tumor versus normal counterparts (Fig. 4F). We saw a similar trend of correlation between MTH1 expression and MTH1-specific activity in NSCLC cell lines (Supplementary Fig. S2D). These data are consistent with what we reported recently in a separate collaborative publication using the ARGO assay in more extensive patient-derived tumor/normal specimens (30).
Figure 4.
Comparison of MTH1-specific 8-oxodGTPase activity in non–small cell lung cancer (NSCLC) human specimen with their respective MTH1 protein expression levels. A, Immunoblot showing MTH1 protein levels in matched human normal lung/lung cancer specimens derived from untreated stage I/stage II patients with NSCLC. Actin is shown as the loading control. B, Fold changes in tumor (T) versus normal (N) MTH1 protein expression. Pixel density from the immunoblot MTH1 signals from A and Supplementary Fig.2A, normalized to the loading control, were quantified via ImageJ. The horizontal line represents the normal tissue baseline set at 1 for each pair. Error bars, ±SEM. C, Total 8-oxodGTPase activity in each matched pair of NSCLC tumors. Results are derived from two independent experiments (n = 2) with each sample run in triplicate. D, Corresponding MTH1-specific 8-oxodGTPase activity, calculated by subtracting the luminescent ARGO signal from TH287-treated counterpart lysates. Results are derived from two independent experiments (n = 2) with each sample run in triplicate. Error bars, ± SD. E, Relative MTH1-specific 8-oxodGTPase activity changes in the tumor versus adjacent normal tissues is shown as a fold change between tumor (T)/normal (N) values from D. The horizontal line represents the normal tissue baseline set at 1 for each pair. Error bars, ± SD. F, Linear regression analysis comparing fold change (T/N) MTH1 protein expression against fold change (T/N) MTH1-specific 8-oxodGTPase activity in the matched normal versus tumor patient tissue. Robustness of fit and P values, calculated in GraphPad Prism, are shown.
Although we note this is a limited number of human clinical samples, our findings suggest that MTH1 expression alone may not accurately reflect MTH1 activity in human tumors as the former is almost always higher relative to normal counterpart tissue. Whereas MTH1-specific 8-oxodGTPase activity (measured in tumor samples by the ARGO assay in conjunction with the current MTH1 inhibitors) may not always match MTH1 expression, and thus could potentially identify tumors that may not respond well to clinical MTH1 targeting. More importantly, our results underscore that MTH1-independent (undruggable) 8-oxodGTPase activity is also present in human tumors and adjacent normal tissue and is likely to be an important factor underlying response to clinical MTH1 inhibition.
The antitumor efficacy of MTH1-targeting small molecules does not depend on suppression of 8-oxodGTPase activity
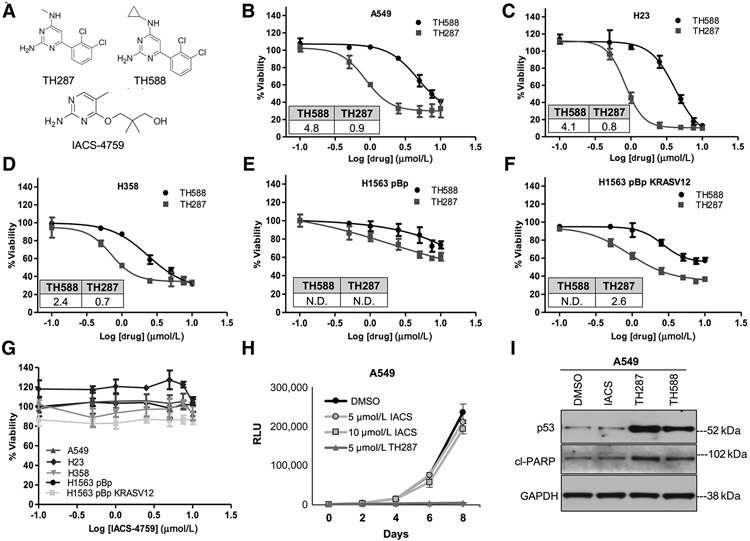
We noted a lack of published studies that have evaluated the cytotoxicity and other molecular effects of multiple first- and second-generation MTH1 inhibitors across the same panel of cell lines. To carry out this comparison, we identically treated NSCLC cell lines with varying KRAS and p53 mutational status (A549, H23, H358, H1563, H1563 KRASV12), in which we had previously established cellular and molecular effects of MTH1 depletion (12), with TH588, TH287 (20), or IACS-4759 (ref. 21; published structures; Fig. 5A). The first-in-class inhibitors were able to induce cytotoxicity in all these lines, with TH287 showing a lower IC50 than TH588 (Fig. 5B-F), as anticipated from the original report describing these inhibitors (20). Curiously, as we had noted in our shMTH1 study (12), the p53 wt and KRAS wt line, H1563, was much more resistant to these inhibitors (Fig. 5E) than its KRASV12-transduced isogenic counterpart (Fig. 5F). However, no defects in cell viability were observed with a 72-hour IACS-4759 treatment in any of the above cell lines (Fig. 5G). Nor were any adverse effects observed with a prolonged 10-day treatment at concentrations up to 20 μmol/L IACS-4759 in the three most TH588- and TH287-responsive cell lines, A549, H23, and H358 (Supplementary Fig. S3A). Similarly, as reported, we could not observe any adverse effects on cell viability through AZ-21 treatment of two TH588- and TH287-responsive cell lines (A549, H358; Supplementary Fig. S3B).
Figure 5.
Relative effects on the viabilities of NSCLC cell lines produced by three different MTH1 inhibitors, TH588, TH287, or IACS-4759. A, Published chemical structures of TH287, TH588, and IACS-4759. BμF, Approximately 200–750 cells (optimized per cell line based on their proliferation rates) were plated per well in a 96-well plate. The next day, the cell lines were treated with vehicle (0.1% DMSO) or the indicated first-in-class MTH1 inhibitor for 72 hours. Viability in response to drug treatment was assessed via luminescence signal from CellTiter-Glo normalized to DMSO control. The x-axis represents the log-transformed drug treatment doses. The percent viability plotted for each data point are an average of two independent experiments, with each data point treated in triplicate. Error bars, ± SD. The IC50 values (in μmol/L) for TH588 and TH287 are shown as insets within each dose–response curve. IC50 values were generated using GraphPad Prism software as described in the Materials and Methods section. IC50 values were not determined (N.D.) for H1563 pBp treated with TH287 and TH588, for H1563 pBp KRASV12 treated with TH588 due to the viability remaining above 50%. G, The denoted cell lines were treated for 72 hours with the indicated doses of IACS-4759 and processed as in B–F. Error bars, ± SD. No IC50s could be determined for any of the cell lines. H, Comparison of cell growth following IACS versus TH287 treatment. A549 cells (approximately 150 cells per well in a 96-well plate) were treated with vehicle or the indicated drug doses in triplicate, and growth curves established via luminescence signal from CellTiter-Glo over the duration of the experiment. Vehicle or drug was replenished every 24 hours. Note that the y-axis plots raw RLU values over the 8-day time course rather than % viability normalized to a control group, to reflect overall changes in cell numbers over the 8-day treatments. Error bars, ± SD. I, Western blot analysis of MTH1 inhibitor–treated A549 cells to assess molecular markers of cell death. Cells were treated with 10 μmol/L of the indicated inhibitors for 72 hours before being harvested and lysed for total protein. Approximately 10 μg of total protein lysates from each sample was probed with p53-specific or cleaved PARP–specific antibodies. GAPDH is shown as the loading control.
A head-to-head comparison of potential chronic effects on overall cell growth from IACS versus TH287 treatment in A549 cells, across 8 days of treatment, further confirmed the striking inability of IACS to affect cell growth relative to the DMSO control (Fig. 5H). In contrast, the 5 μmol/L TH287 treatment started manifesting as reduced cell numbers (relative to IACS- or DMSO-treated counterparts) at approximately day 3 and its cytotoxic effects were highly evident by the end of the treatment duration. Moreover, immunoblotting of the p53-wt A549 cells treated with IACS-4759, TH588, or TH287 showed that, unlike the first-in-class inhibitors, IACS treatment did not elevate either p53 expression or the cell death marker, cleaved (cl) PARP following treatment (Fig. 5I).
Because the original first-in-class inhibitor study utilized U2OS cells (20), which possess very high MTH1-specific 8-oxodGTPase activity (Fig. 2B), and because PC3 have a relatively much lower MTH1-specific 8-oxodGTPase activity (Fig. 2B; ref. 29), we also tested how TH287 versus IACS affected viability in these two lines. We found again that IACS did not affect either line (Supplementary Fig. S3C), whereas TH287 killed both cell lines with equal efficacy (Supplementary Fig. S3D). Collectively, these findings conclusively show that the efficacy of the first-in-class inhibitors does not arise from their on-target inhibition of MTH1-specific 8-oxodGTPase activity.
Our original studies in NSCLC lines showed that the predominant effect of genetic MTH1 depletion, both in vitro and in vivo, is the induction of senescence or proliferative defects rather than cell death (12). This finding was independently supported by another study that evaluated the cellular effects of decreased MTH1 expression through miR-145 transfection in lung cancer lines (43). We, therefore, assessed whether IACS might be inducing cell senescence rather than cell death. Because A549 showed the most dramatic senescence phenotype under MTH1 depletion of the cell lines used in this study (12), we established growth curves in this cell line over 8 days and saw the expected growth arrest in the shMTH1 A549 cells relative to their shGFP controls (Supplementary Fig. S4A). We then compared the morphology and senescence-associated β-galactosidase (SA-β-gal) staining in the shMTH1 A549 cells versus the IACS-treated A549 cells. Whereas the MTH1-depleted A549 cells developed the flattened morphology and enhanced SA-β-gal staining consistent with cellular senescence (Supplementary Fig. S4B, brightfield images, top), the IACS-treated cells showed very few focally stained cells (<5% of total cells; Supplementary Fig. S4B, brightfield images, bottom), which could be a consequence of the high density of these cultures. The lack of pervasive senescence induction in these cells is verified by the lack of p53 increase in the IACS-treated cells (Fig. 5I). In contrast, treatment with TH588 robustly induced both p53 levels (Fig. 5I) and morphology consistent with cell death (Supplementary Fig. S4C, which was also not seen with IACS treatment). These results confirm that, unlike TH588 or TH287, IACS-4759 does not induce any tumor-suppressive outcomes despite its equally effective on-target inhibition of MTH1 8-oxodGTPase activity.
The first-in-class MTH1 inhibitors (but not IACS-4759) Increase cellular ROS but their cytotoxic effects do not arise as a result of elevated oxidative stress
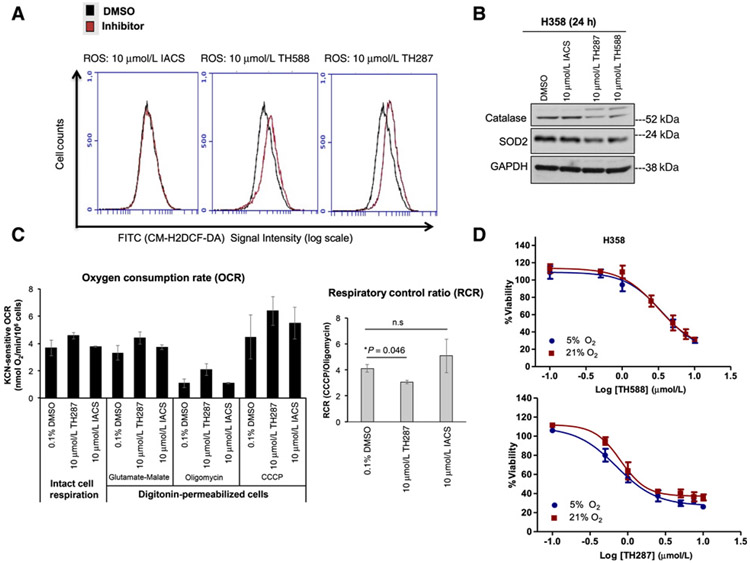
Recent studies (44, 45) have noted that the first-in-class inhibitor TH588 elevates cellular ROS and that its cytotoxic effects stem from this off-target phenomenon. However, no prior study has compared ROS production between the first-in-class MTH1 inhibitors to a noncytotoxic MTH1 inhibitor, to ascertain whether the elevated ROS occur due to inhibition of MTH1 8-oxodGTPase activity. Here we measured total cellular ROS levels using the cell-permeant oxidation-sensitive probe, CM-H2DCF-DA, in H358 cells at 17 hours, following equivalent treatments with TH588, TH287, or IACS. We found that both TH588 and TH287 elevated cellular ROS levels, whereas IACS did not (Fig. 6A), suggesting that the ROS production is indeed an off-target effect specific to the first-in-class inhibitors. While we did not observe any treatment-associated changes in the expression of global redox-regulatory proteins such as Nrf2 or thioredoxin-1, we found a small decrease in mitochondrial superoxide dismutase (SOD2) expression as well as a noticeable reduction in catalase signal following 24 hours of treatment with TH588 and TH287, but not IACS-4759 (Fig. 6B). This decrease in catalase expression was accompanied by a supershifted band in the TH588 and TH287-treated samples, consistent with reported posttranslationally modified versions of catalase that are targeted for degradation via the ubiquitin pathway (46). Regardless, this decrease in catalase, which detoxifies hydrogen peroxide into water, is consistent with the observed elevation in CM-H2DCF-DA signal, which reflects intracellular hydroperoxide levels.
Figure 6.
The first-in-class MTH1 inhibitors produce ROS and decrease OXPHOS efficiency relative to the noncytotoxic MTH1 inhibitor, IACS-4759. A, ROS levels in untreated and MTH1 inhibitor–treated cells. H358 cells were plated at an equivalent density and treated with either DMSO or the indicated inhibitors for 17 hours. All samples were harvested via trypsinization and, following incubation with CM-H2DCF-DA, concurrently assessed for total ROS levels via signal detection in the FITC channel. The flow cytometric profiles shown are representative of two independent runs. B, Immunoblotting for antioxidant proteins in H358 cells treated with DMSO or 10 μmol/L MTH1 inhibitors for 24 hours. Approximately 10 μg of total protein lysates were immunoblotted and probed with the indicated antibodies. GAPDH is shown as a loading control. This blot is representative of two independently run blots. C, The first-in-class MTH1 inhibitor, TH287, shows indications of decreased OXPHOS efficiency. H358 cells were treated for 22 hours with the indicated MTH1 inhibitors (10 μmol/L) or vehicle (0.1% DMSO). To assess mitochondrial respiratory capacity, the treated cells were subjected to polarographic measurements of intact cell respiration. Subsequently, the cells were permeabilized with digitonin, followed by the subsequent injection of the indicated substrates, prior to measurement of respiration. Oxygen consumption rates (OCR) were calculated as specified in the Materials and Methods section (left). The respiratory control ratio (RCR, right) was calculated by dividing the KCN-sensitive CCCP OCR by the KCN-sensitive oligomycin OCR. Error bars, ± SD. D, Cells cultured at 5% oxygen tension are not protected from the cytotoxic effects of TH588 or TH287. H358 cells were cultured at 5% or 21% oxygen for 12 days prior to plating for the indicated drug treatments in triplicate. Cells were kept at their respective oxygen conditions during the 72-hour treatment period. Viability was assessed via the CellTiter-Glo assay and drug responses curves established as in Fig. 5. Error bars, ± SD.
We next assessed whether these elevated ROS could result from a direct or secondary effect of the MTH1 inhibitors on the mitochondrial respiratory chain (MRC) and oxidative phosphorylation (OXPHOS) system. The possibility of an acute effect was discarded by measuring cellular respiration continuously in the absence and then incremental additions of IACS or TH287 (up to 35 μmol/L), which did not show any significant difference relative to the vehicle control (Supplementary Fig. S5A). Thus, TH287 does not directly inhibit mitochondrial respiratory chain enzymes, for instance, like the mitochondrial poison potassium cyanide (KCN; Supplementary Fig. S5A).
However, the chronic 22-hour treatment of H358 cells with 10 μmol/L TH287 resulted in a trend of enhanced endogenous cellular respiration in intact cells, relative to DMSO or IACS-treated cells (Fig. 6C). To further examine this effect, the cells were subsequently permeabilized with digitonin, at concentrations that do not affect the integrity of the mitochondrial membranes, and we measured the ability of the cells to oxidize glutamate plus malate, substrates that feed electrons to the MRC via complex I in the presence of excess ADP. The assay was followed by additions of oligomycin to inhibit the mitochondrial F1Fo-ATPase, the uncoupler CCCP to measure maximal respiratory capacity, and the MRC inhibitor KCN to eliminate non-MRC oxygen consumption. Whereas the coupled and uncoupled rates with glutamate plus malate oxidation were slightly enhanced in TH287-treated cells, oligomycin was clearly less effective in inhibiting oxygen consumption in these conditions, in comparison with DMSO- and IACS-treated cells. Oligomycin inhibits ATP synthase by blocking its proton channel (Fo subunit), which is necessary for OXPHOS of ADP to ATP. In general, the inhibition of ATP synthesis by oligomycin would also stop the MRC. Because the high proton concentration buildup in the intermembrane space is not dissipated, the free energy released by substrate oxidation is not enough to further pump protons against the steep gradient. Decreased oligomycin effects in TH287-treated cells resulted in a significantly decreased respiratory control ratio (RCR, Fig. 6C). This observation indicates increased proton leak and lower respiratory efficiency, which may explain the enhanced ROS production in TH287-treated cells.
Elevated ROS production, in and of itself, is not a source of enhanced cytotoxicity, which occurs through oxidative stress, and may instead be funneled into oxidative signaling rather than damage (3). We and others have shown that the in vitro tumor-suppressive effects of oxidative stress (including through MTH1 depletion) can be mitigated by culturing cells in lower but pre-hypoxic (3%–5%) oxygen tensions (12-14, 31, 47, 48). To determine whether the first-in-class inhibitors exert their cytotoxic effects through oxidative stress, we cultured H358 cells for 12 days under 5% or 21% oxygen tension prior to TH588 or TH287 treatment and subsequent measurements of cell viability. Counterpart cultures from 5% versus 21% O2 were assessed for ROS levels and, as anticipated, the former was found to have both lower baseline levels of CM-H2DCF-DA signal as well as lower signal following H2O2 treatment (Supplementary Fig. S5B). However, we did not find any significant differences in the viability of TH588- or TH287-treated cells under 5% versus 21% O2 (Fig. 6D), suggesting that these inhibitors do not kill cells through oxidative stress-associated mechanisms. A similar finding was recently reported for TH588 treatment of three colorectal cancer cell cultures, showing that modulation of ROS levels in these culture models does not affect TH588-induced viability defects or DNA damage (44). Thus, the ROS produced by TH588 and TH287 are off-target effects potentially arising from indirect impairment of mitochondrial respiration. More importantly, these ROS do not appear to be the direct source of the first-in-class inhibitor cytotoxicity, and may instead be affecting some ROS signaling–associated process that indirectly contributes to their antitumor effects.
The first-in-class cytotoxic MTH1 inhibitor TH287 does not produce genomic 8-oxodG incorporation to a greater extent than the noncytotoxic inhibitor IACS-4759
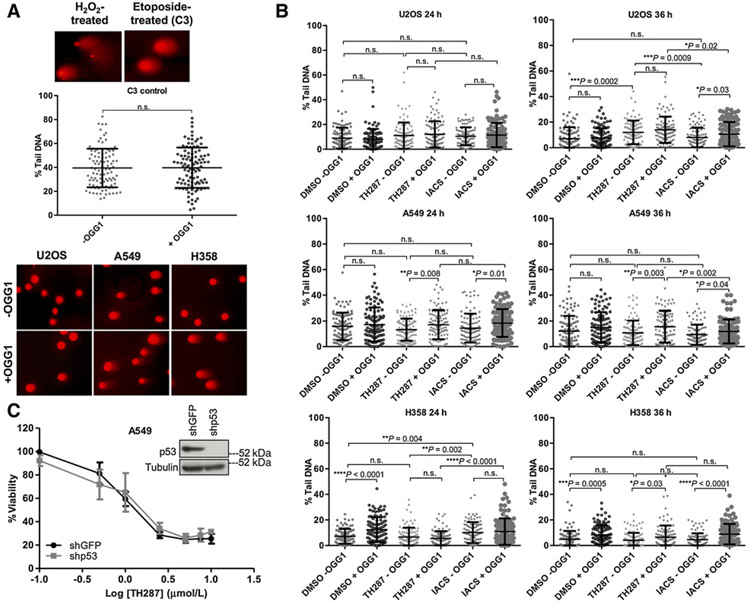
Prior studies have posited that the tumoricidal effects of the first-in-class MTH1 inhibitors result critically from their ability to increase nuclear genomic 8-oxodG (20, 49), in contrast with the noncytotoxic independently developed inhibitors (49). Using the alkaline single-cell gel electrophoresis (“comet”) assay in conjunction with the OGG1-modified version (33-35), we therefore compared whether there were differences in alkaline-labile sites as well as genomic 8-oxodG incorporation (manifested as increased % tail DNA in OGG1-treated over untreated samples) produced by TH287 versus IACS. These experiments were performed in U2OS, A549, and H358 cells under treatment conditions consistent with those reported for similar assays in the original first-in-class MTH1 inhibitor study as well as in a follow-up study (20, 49). As anticipated, even without OGG1 treatment, DNA alkaline-labile sites, single- and double-strand breaks were noticeably increased in H2O2- and etoposide-treated positive controls (Fig. 7A). Furthermore, under baseline (vehicle-treated) conditions, in situ OGG1 treatment of lysed, low melting point agarose-embedded cells resulted in a greater number of cells with elevated % tail DNA from cleavage 3′ to the unrepaired 8-oxodG sites, albeit to variable extents among the cell lines (Fig. 7A and B). The most striking baseline differences in % tail DNA between OGG1-treated and untreated counterparts were seen in vehicle-treated H358 cells (Fig. 7A and B), which likely possess low endogenous OGG1 expression as well as activity due to their p53-null status (50). In contrast, U2OS cells, which exhibit the most robust 8-oxodGTPase activity of the three cell lines (Fig. 2A), showed the least differences between OGG1-treated and untreated counterpart samples, consistent with low genomic incorporation of 8-oxodGTP (Fig. 7B).
Figure 7.
The cytotoxic effects of the first-in-class inhibitors do not arise from an enhanced ability to introduce genomic 8-oxodG incorporation relative to IACS-4759. A, Top, representative images of H2O2− or etoposide-treated positive controls from the comet assay. Middle, scatter plots showing quantification of % tail DNA between untreated (−OGG1) and treated (+OGG1) C3 cells (n= 2). Each point on the graph is the %tail DNA quantified from a single cell, and 100 cells in total were quantified for each condition, over two independent samples (n.s., nonsignificant). Error bars, ± SD. Bottom, representative examples of comets from −OGG1 and +OGG1 U2OS, A549, and H358 cells (DMSO vehicle-treated), showing the spectrum of % tail DNA scored in Fig. 7B. Images are shown at a 10× magnification. B, Quantitation of % tail DNA. One-hundred individual determinations from U2OS, A549, and H358 cells treated for 24 or 36 hours with DMSO, 10 μm/L TH287 or 10 mm/L IACS, either with or without OGG1 treatment (n = 2) are shown. Each point on the graph is the % tail DNA of a single cell, and 100 cells were quantified per each condition. The P values were calculated by an unpaired Student t test with Welch correction applied if variances were found to be unequal, and are indicated above the relevant comparison groups (n.s., nonsignificant). Error bars, ± SD. C, TH287 treatment–induced loss of viability in the p53 wild type A549 cells is not rescued by p53 knockdown. Cells were transduced with shGFP or shp53, selected in puromycin-containing media, and then plated in triplicate for TH287 treatment over 72 hours. Viability was assessed via the CellTiter-Glo assay and drug responses curves established. Error bars, ± SD. The extent of p53 knockdown is shown via immunoblot (inset).
However, despite prior studies (20, 49) showing that U2OS cells exhibit enhanced 8-oxodG incorporation following 24-hour treatment with TH588, TH287, or inhibitors derived from these scaffolds, we did not observe significant differences following TH287 treatment between OGG1-treated and untreated U2OS counterparts at either time point (Fig. 7B). At 36 hours, the TH287-treated U2OS cells exhibited small but statistically significant increases in alkaline-labile sites (−OGG1) as well as correspondingly higher 8-oxodG excision (+OGG1) relative to the IACS-4759-treated U2OS cells. However, in A549 and H358 cells, the effects of TH287 on alkaline labile sites (−OGG1) or genomic 8-oxodG incorporation (+OGG1) were largely comparable or smaller to what was observed in the counterpart IACS-4759-treated cells. Furthermore, given that ROS elevation by TH287 was observed most robustly at under 24 hours following treatment, we did not observe any correspondingly enhanced effects of TH287 treatment at this time point, either relative to IACS-4759 or relative to its own 36-hour time point (Fig. 7B). This observation is consistent with results described in the previous section, suggesting that while the first-in-class inhibitors elevate ROS, the ensuing increase is not sufficient to evoke oxidative stress-associated outcomes such as DNA damage. Indeed, overall, IACS-4759 showed a modest advantage over TH287 in enhancing genomic 8-oxodG incorporation (Fig. 7B), consistent with its cleaner on-target effects on MTH1 8-oxodGTPase activity. Thus, our comet assay data (Fig. 7B) contradict the explanation that the cytotoxicity of TH287 (and likely TH588, the relatively less cytotoxic first-in-class inhibitor; ref. 20) versus IACS-4759 results from its enhanced incorporation of 8-oxodG (49). Consistent with the comet data, shRNA-mediated depletion of p53 in A549 cells did not rescue the cytotoxic effects of treatment with either TH588 or TH287 (Fig. 7C). Collectively, these observations suggest that the tumoricidal efficacy of the first-in-class inhibitors is not due to enhanced genomic incorporation of nuclear 8-oxodG nor indeed due to induction of genotoxic stress.
Discussion
Examination of multiple human cancer datasets (cbioportal.org) reveals that MTH1 is either amplified or overexpressed in tumors, and that these alterations significantly correlate adversely with overall survival as well as progression-free survival (18, 19), supporting a tumor-promoting role for MTH1 function in cancer. Nevertheless, despite promising results with MTH1 depletion and the first-in-class inhibitors in cell lines and xenograft models (11, 12, 14, 20), MTH1 has become a controversial target for cancer therapeutic development due to the apparent lack of tumoricidal effects of multiple second-generation MTH1 inhibitors on cancer cell lines (18, 21, 22, 24, 25). Our objective here was to perform a comprehensive, side-by-side assessment of different MTH1 inhibitors in an attempt to understand this discrepancy. Accordingly, in this study, we have assessed the reportedly efficacious first-in-class MTH1 inhibitors, TH588 and TH287, as well as the noncytotoxic, independently developed inhibitors, IACS-4759, AZ-21, and BAY-707, in a panel of relevant cancer cell lines, including the NSCLC lines in which we had characterized the molecular and cellular effects of MTH1 depletion (12). Because these drugs were all developed to target the MTH1 active site, our study addresses a critical gap in prior studies by directly measuring total endogenous 8-oxodGTPase activity as well as the MTH1-specific (druggable) component of this activity, through application of the novel ARGO probe-based assay (29) in cancer cell lines and human tumor-derived specimen. Moreover, in conjunction, we have compared the first-in-class inhibitors vis-à-vis independently developed MTH1-targeting small molecules against their effects on cell viability, cellular ROS levels, and nuclear genomic 8-oxodG incorporation.
Our findings conclusively show that suppression of MTH1-specific 8-oxodGTPase activity does not underlie the efficacy (or lack thereof) of MTH1-targeting small molecules, as the two first-in-class and three independently-developed MTH1 inhibitors are able to equivalently suppress cellular 8-oxodGTPase activity in the same set of cell lines and to a similar extent as shRNA-mediated MTH1 depletion. Furthermore, our study reveals that cancer cell lines as well as human tumor samples exhibit MTH1-independent (or undruggable) 8-oxodGTPase activity to varying extents, with some cells possessing 8-oxodGTPase activity with very little contribution from MTH1, for example, H1563 lung cancer cells and PC3 prostate cancer cells.
Our results here support the existence of alternative mechanisms that can replace MTH1 8-oxodGTPase function in human tumor cells. There are other Nudix enzymes that exhibit substrate and biological redundancy to MTH1, therefore potentially substituting for MTH1 function. We postulate MTH2 could be a contributor to this redundancy as it is robustly expressed transcriptionally in the NSCLC cell lines used in our study, and due to its ability to cleave the ARGO probe. Further in-depth studies are required to determine whether its weak 8-oxodGTPase activity suffices to compensate for loss of MTH1 or whether other functional 8-oxodGTPases underlie the observed redundancy. Additional candidates for alternative 8-oxodGTPases may derive from other members of the Nudix hydrolase family for which clear substrates have not yet been identified, for instance NUDT17, which is a close familial relative of MTH1 (37). Alternate possibilities include as yet unidentified posttranslationally modified or mutated forms of MTH1, which no longer bind to the first-in-class inhibitors, but are still able to bind and hydrolyze the ARGO probe. We note that urinary levels of 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodGuo), a byproduct of both genomic DNA repair and oxidized deoxynucleotide hydrolysis, are reported to be similar between wild type and MTH1 knockout animals, indicative of potential redundancy in murine MTH1 (51), supporting our findings with human MTH1.
Prior reports have suggested that ROS generation (44,45) or nuclear 8-oxodG incorporation (49) by the first-in-class inhibitors are the source of their cytotoxicity. However, our experiments in U2OS cells (used in the original publication reporting the first-in-class inhibitors; ref. 20) as well as in NSCLC lines, A549 and H358 (characterized in our prior shMTH1 studies; ref. 12), suggest that the efficacy of TH588 and TH287 does not arise from either elevated 8-oxodG incorporation or from oxidative or p53-dependent genotoxic stress (Figs. 6 and 7). Our prior studies used multiple shRNAs against MTH1 (12) (Supplementary Figs. S1, S7, and S9 therein), isogenic wt/KRASV12 lines to mimic contrasting conditions of oncogenic oxidative stress, and extensive rescue experiments (including culturing cells at low oxygen tension or depleting p53) to demonstrate the critical role of endogenous oxidative stress in producing on-target shMTH1-induced tumor-suppressive effects. These included irreparable genotoxic damage and p53-dependent induction of cellular senescence. In this study, we show that despite their cytotoxicity, the first-in-class inhibitors do not evoke congruent tumor-inhibitory mechanisms in culture models and conditions equivalent to those employed in our MTH1 depletion studies.
Our results instead suggest that the first-in-class inhibitors exert their potent cytotoxic effects largely through mechanisms unrelated to MTH1 8-oxodGTPase function, genomic 8-oxodG incorporation or oxidative stress. In this eventuality, the noted discrepancies among the MTH1 inhibitors can be explained by the existence of the MTH1- independent 8-oxodGTPase activity that we have observed via the ARGO assay. This redundant activity is present to some extent in every cancer line we evaluated in this study, and moreover is unaffected by any of the five tested MTH1 inhibitors. Thus, it may compensate wholly or partially for MTH1 enzymatic function and consequently negate the effects from those inhibitors that only suppress MTH1 8-oxodGTPase function, but not the off-target cytotoxic effects from TH588 and TH287.
We note that MTH1 depletion via shRNA also does not inhibit this MTH1-independent activity although, as discussed above, shMTH1 does produce in vitro and in vivo tumor-suppressive effects consistent with on-target loss of MTH1 function (12). Moreover, unlike TH588 and TH287, shMTH1 does not induce cytotoxicity (12), which generally requires higher levels of cellular stress and p53 induction than senescence (reviewed in ref. 52). Furthermore, we reported that MTH1 depletion selects for cell subpopulations with decreased malignancy, marked by low ROS levels as well as decreased RAS oncoprotein and oncogenic signaling, over a longer time frame (one to three weeks in vitro, three to four weeks in vivo) than used in the pharmacologic targeting studies (11, 12). Possibly, longer duration in vivo treatment with the ostensibly noncytotoxic MTH1 inhibitors in spontaneous cancer models (e.g., in RAS or other oxidant signaling-driven transgenic models of autochthonous tumor growth) will uncover their true therapeutic utility.
Curiously, TH588 and TH287 exhibited higher efficacy (lower IC50) in KRASV12-transduced H1563 cells relative to their wt KRAS parental counterparts (Fig. 5E vs. F), similar to what we observed in these lines with MTH1 depletion (12). These first-in-class inhibitors have been reported to affect microtubule dynamics and metabolic pathways (25,45, 49), which are also altered by RAS-driven signaling. Thus, the first-in-class inhibitors (but not the subsequently developed inhibitors) could putatively be exerting their tumoricidal effects by taxing oncogenic stress responses that synergize with 8-oxodGTPase inhibition. For instance, a recent study found that TH588 showed synergistic killing in combination with Polo-like kinase (Plk1) inhibitors through MTH1-independent off-target effects on the mitotic spindle (53).
Regardless, the additional questions raised by our study underlie as-yet undiscovered complexities of MTH1 function and the molecular contexts that modulate its efficacy as a therapeutic target. Without a full understanding of these details through carefully controlled molecular characterization in appropriate experimental systems, it would be premature to dismiss the potential of MTH1 as a therapeutic target in cancer. We recommend that future models of MTH1 inhibition in cancer utilize measurements of MTH1-specific and -independent 8-oxodGTPase activities. We further put forth the possibility that the clinical utility of MTH1 inhibitors may not lie in their use as single and/or acute agents but rather as part of judicious combinatorial regimens that enhance their therapeutic index and durability of effect.
Supplementary Material
Acknowledgments
We thank Nisha Sharma and Laura Misiara for technical assistance. Dr. Sion Williams and the Sylvester Oncogenomics Core Facility are thanked for assistance with arranging STR profiling of cell lines. BAY-707 was supplied by the Structural Genomics Consortium under an Open Science Trust Agreement: http://www.thesgc.org/click-trust. This work was supported by the NIH/NCI grants R01CA175086 (to P. Rai), R01CA217809 (E.T. Kool) and an NIEHS grant R15ES02719 (to M.S. Cooke), as well as by Sylvester Comprehensive Cancer Center research support funding (to P. Rai).
Footnotes
Disclosure of Potential Conflicts of Interest
MSC is subcontracted by the Thomas Helleday Foundation for Medical Research for analyzing samples from the first-in-man trial with Karonudib, an MTH1 inhibitor not utilized in this study. No potential conflicts of interest were disclosed by the other authors.
Supplementary data for this article are available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
References
- 1.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 2013;12:931–47. [DOI] [PubMed] [Google Scholar]
- 2.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 2009;8: 579–91. [DOI] [PubMed] [Google Scholar]
- 3.Rai P Human Mut THomolog 1 (MTH1):a roadblock for the tumor-suppressive effects of oncogenic RAS-induced ROS. Small GTPases 2012;3:13–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res 2010;44: 479–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtin NJ. DNA repair dysregulation from cancer driver to therapeutic target. Nat Rev Cancer 2012;12:801–17. [DOI] [PubMed] [Google Scholar]
- 6.Haghdoost S, Sjolander L, Czene S, Harms-Ringdahl M. The nucleotide pool is a significant target for oxidative stress. Free Radic Biol Med 2006;41:620–6. [DOI] [PubMed] [Google Scholar]
- 7.Kamiya H, Kasai H. Formation of 2-hydroxydeoxyadenosine triphosphate, an oxidatively damaged nucleotide, and its incorporation by DNA polymerases. Steady-state kinetics of the incorporation. J Biol Chem 1995;270:19446–50. [DOI] [PubMed] [Google Scholar]
- 8.Rai P. Oxidation in the nucleotide pool, the DNA damage response and cellular senescence: defective bricks build a defective house. Mutat Res 2010;703:71–81. [DOI] [PubMed] [Google Scholar]
- 9.Sakumi K, Furuichi M, Tsuzuki T, Kakuma T, Kawabata S, Maki H, et al. Cloning and expression of cDNA for a human enzyme that hydrolyzes 8-oxo-dGTP, a mutagenic substrate for DNA synthesis. J Biol Chem 1993;268:23524–30. [PubMed] [Google Scholar]
- 10.Burton DG, Rai P. MTH1 counteracts oncogenic oxidative stress. Oncoscience 2015;2:785–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giribaldi MG, Munoz A, Halvorsen K, Patel A, Rai P. MTH1 expression is required for effective transformation by oncogenic HRAS. Oncotarget 2015;6: 11519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel A, Burton DG, Halvorsen K, Balkan W, Reiner T, Perez-Stable C, et al. MutT Homolog 1 (MTH1) maintains multiple KRAS-driven pro-malignant pathways. Oncogene 2015;34:2586–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rai P, Onder TT, Young JJ, McFaline JL, Pang B, Dedon PC, et al. Continuous elimination of oxidized nucleotides is necessary to prevent rapid onset of cellular senescence. Proc Natl Acad Sci U S A 2009;106:169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rai P, Young JJ, Burton DG, Giribaldi MG, Onder TT, Weinberg RA. Enhanced elimination of oxidized guanine nucleotides inhibits oncogenic RAS-induced DNA damage and premature senescence. Oncogene 2011;30:1489–96. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy CH, Pass HI, Mitchell JB. Expression of human MutT homologue (hMTH1) protein in primary non-small-cell lung carcinomas and histologically normal surrounding tissue. Free Radic Biol Med 2003;34:1447–57. [DOI] [PubMed] [Google Scholar]
- 16.Buchholz M, Braun M, Heidenblut A, Kestler HA, Kloppel G, Schmiegel W, et al. Transcriptome analysis of microdissected pancreatic intraepithelial neoplastic lesions. Oncogene 2005;24:6626–36. [DOI] [PubMed] [Google Scholar]
- 17.Garber ME, Troyanskaya OG, Schluens K, Petersen S, Thaesler Z, Pacyna-Gengelbach M, et al. Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci U S A 2001;98:13784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samaranayake GJ, Huynh M, Rai P. MTH1 as a chemotherapeutic target: the elephant in the room. Cancers 2017;9:pii:E47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rai P, Sobol RW. Mechanisms of MTH1 inhibition-induced DNA strand breaks: the slippery slope from the oxidized nucleotide pool to genotoxic damage. DNA Repair 2019;77:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gad H, Koolmeister T, Jemth AS, Eshtad S, Jacques SA, Strom CE, et al. MTH1 inhibition eradicates cancer by preventing sanitation of the dNTP pool. Nature 2014;508:215–21. [DOI] [PubMed] [Google Scholar]
- 21.Petrocchi A, Leo E, Reyna NJ, Hamilton MM, Shi X, Parker CA, et al. Identification of potent and selective MTH1 inhibitors. Bioorg Med Chem Lett 2016;26:1503–7. [DOI] [PubMed] [Google Scholar]
- 22.Ellermann M, Eheim A, Rahm F, Viklund J, Guenther J, Andersson M, et al. Novel class of potent and cellularly active inhibitors devalidates MTH1 as broad-spectrum cancer target. ACS Chem Biol 2017;12:1986–92. [DOI] [PubMed] [Google Scholar]
- 23.Rahm F, Viklund J, Tresaugues L, Ellermann M, Giese A, Ericsson U, et al. Creation of a novel class of potent and selective MutT homologue 1 (MTH1) inhibitors using fragment-based screening and structure-based drug design. J Med Chem 2018;61:2533–51. [DOI] [PubMed] [Google Scholar]
- 24.Kettle JG, Alwan H, Bista M, Breed J, Davies NL, Eckersley K, et al. Potent and selective inhibitors of MTH1 probe its role in cancer cell survival. J Med Chem 2016;59:2346–61. [DOI] [PubMed] [Google Scholar]
- 25.Kawamura T, Kawatani M, Muroi M, Kondoh Y, Futamura Y, Aono H, et al. Proteomic profiling of small-molecule inhibitors reveals dispensability of MTH1 for cancer cell survival. Sci Rep 2016;6:26521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egashira A, Yamauchi K, Yoshiyama K, Kawate H, Katsuki M, Sekiguchi M, et al. Mutational specificity of mice defective in the MTH1 and/or the MSH2 genes. DNA Repair (Amst) 2002;1:881–93. [DOI] [PubMed] [Google Scholar]
- 27.Tsuzuki T, Egashira A, Igarashi H, Iwakuma T, Nakatsuru Y, Tominaga Y, et al. Spontaneous tumorigenesis in mice defective in the MTH1 gene encoding 8-oxo-dGTPase. Proc Natl Acad Sci U S A 2001;98:11456–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huber KV, Salah E, Radic B, Gridling M, Elkins JM, Stukalov A, et al. Stereo-specific targeting of MTH1 by (S)-crizotinib as an anticancer strategy. Nature 2014;508:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji D, Beharry AA, Ford JM, Kool ET. A chimeric ATP-linked nucleotide enables luminescence signaling of damage surveillance by MTH1, a cancer target. J Am Chem Soc 2016;138:9005–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McPherson LA, Troccoli CI, Ji D, Bowles AE, Gardiner ML, Mohsen MG, et al. Increased MTH1-specific 8-oxodGTPase activity is a hallmark of cancer in colon, lung and pancreatic tissue. DNA Repair 2019;83:102644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samaranayake GJ, Troccoli CI, Huynh M, Lyles RDZ, Kage K, Win A, et al. Thioredoxin-1 protects against androgen receptor-induced redox vulnerability in castration-resistant prostate cancer. Nat Commun 2017;8:1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrientos A, Fontanesi F, Diaz F. Evaluation of the mitochondrial respiratory chain and oxidative phosphorylation system using polarography and spectro-photometric enzyme assays. Curr Protoc Hum Genet 2009;Chapter 19:Unit19.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooke MS, Duarte TL, Cooper D, Chen J, Nandagopal S, Evans MD. Combination of azathioprine and UVA irradiation is a major source of cellular 8-oxo-7,8-dihydro-2′-deoxyguanosine. DNA Repair 2008;7:1982–9. [DOI] [PubMed] [Google Scholar]
- 34.Schoenmakers E, Agostini M, Mitchell C, Schoenmakers N, Papp L, Rajanayagam O, et al. Mutations in the selenocysteine insertion sequence-binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. J Clin Invest 2010;120:4220–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karbaschi M, Cooke MS. Novel method for the high-throughput processing of slides for the comet assay. Sci Rep 2014;4:7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carreras-Puigvert J, Zitnik M, Jemth AS, Carter M, Unterlass JE, Hallstrom B, et al. A comprehensive structural, biochemical and biological profiling of the human NUDIX hydrolase family. Nat Commun 2017;8:1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takagi Y, Setoyama D, Ito R, Kamiya H, Yamagata Y, Sekiguchi M. Human MTH3 (NUDT18) protein hydrolyzes oxidized forms of guanosine and deoxyguanosine diphosphates: comparison with MTH1 and MTH2. J Biol Chem 2012;287:21541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akiyama S, Saeki H, Nakashima Y, Iimori M, Kitao H, Oki E, et al. Prognostic impact of MutT homolog-1 expression on esophageal squamous cell carcinoma. Cancer Med 2017;6:258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Yang CC, Tian XY, Li YX, Cui J, Chen Z, et al. MutT-related proteins are novel progression and prognostic markers for colorectal cancer. Oncotarget 2017;8:105714–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou H, Jian Y, Leng Y, Liu N, Tian Y, Wang G, et al. Human MutT homologue 1 mRNA overexpression correlates to poor response of multiple myeloma. Int J Hematol 2017;105:318–25. [DOI] [PubMed] [Google Scholar]
- 41.Fujishita T, Okamoto T, Akamine T, Takamori S, Takada K, Katsura M, et al. Association of MTH1 expression with the tumor malignant potential and poor prognosis in patients with resected lung cancer. Lung Cancer 2017;109:52–7. [DOI] [PubMed] [Google Scholar]
- 42.Speina E, Arczewska KD, Gackowski D, Zielinska M, Siomek A, Kowalewski J, et al. Contribution of hMTH1 to the maintenance of 8-oxoguanine levels in lung DNA of non-small-cell lung cancer patients. J Natl Cancer Inst 2005;97:384–95. [DOI] [PubMed] [Google Scholar]
- 43.Cho WC, Chow AS, Au JS. MiR-145 inhibits cell proliferation of human lung adenocarcinoma by targeting EGFR and NUDT1. RNA Biol 2011;8:125–31. [DOI] [PubMed] [Google Scholar]
- 44.van der Waals LM, Laoukili J, Jongen JMJ, Raats DA, Borel Rinkes IHM, Kranenburg O. Differential anti-tumour effects of MTH1 inhibitors in patient-derived 3D colorectal cancer cultures. Sci Rep 2019;9:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang JY, Jin L, Yan XG, Sherwin S, Farrelly M, Zhang YY, et al. Reactive oxygen species dictate the apoptotic response of melanoma cells to TH588. J Invest Dermatol 2016;136:2277–86. [DOI] [PubMed] [Google Scholar]
- 46.Cao C, Leng Y, Liu X, Yi Y, Li P, Kufe D. Catalase is regulated by ubiquitination and proteosomal degradation. Role of the c-Abl and Arg tyrosine kinases. Biochemistry 2003;42:10348–53. [DOI] [PubMed] [Google Scholar]
- 47.Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol 2003;5:741–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ho HY, Cheng ML, Cheng PF, Chiu DT. Low oxygen tension alleviates oxidative damage and delays cellular senescence in G6PD-deficient cells. Free Radic Res 2007;41:571–9. [DOI] [PubMed] [Google Scholar]
- 49.Warpman Berglund U, Sanjiv K, Gad H, Kalderen C, Koolmeister T, Pham T, et al. Validation and development of MTH1 inhibitors for treatment of cancer. Ann Oncol 2016;27:2275–83. [DOI] [PubMed] [Google Scholar]
- 50.Chatterjee A, Mambo E, Osada M, Upadhyay S, Sidransky D. The effect of p53-RNAi and p53 knockout on human 8-oxoguanine DNA glycosylase (hOgg1) activity. FASEB J 2006;20:112–4. [DOI] [PubMed] [Google Scholar]
- 51.Evans MD, Mistry V, Singh R, Gackowski D, Rozalski R, Siomek-Gorecka A, et al. Nucleotide excision repair of oxidised genomic DNA is not a source of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine. Free Radic Biol Med 2016;99:385–91. [DOI] [PubMed] [Google Scholar]
- 52.Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep 2014;15:1139–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patterson JC, Joughin BA, Prota AE, Muhlethaler T, Jonas OH, Whitman MA, et al. VISAGE reveals a targetable mitotic spindle vulnerability in cancer cells. Cell Syst 2019;9:74–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.