Abstract
Background
Osteoblast differentiation is a critical process to maintain the stability of the bone homeostasis. Zingerone, 4-(4-hydroxy-3-methoxyphenyl)-2-butanone (ZG), isolated from ginger, performs a wide range of biological functions in human diseases. The objective of this paper was to clarify the role of ZG in human bone mesenchymal stem cells (hBMSCs) and associated mechanisms of ZG promoting osteoblast differentiation.
Material/Methods
The cytotoxicity of ZG was detected by MTT assay. The expression levels of miR-200c-3p, smad7, and osteoblast differentiation markers (alkaline phosphatase [ALP], osteocalcin [OC], osterix [OSX] and runt-related transcription factor 2 [RUNX2]) were assessed by quantitative real-time polymerase chain reaction (qRT-PCR). The protein levels of smad7, ALP, OC, OSX, and RUNX2 were quantified by western blot analysis. The target mRNAs were predicted by bioinformatics tools TargetScan. The interaction between miR-200c-3p and smad7 was verified by luciferase reporter assay and RIP assay.
Results
ZG was nontoxic to hBMSCs, and it accelerated osteoblast differentiation by inducing the expression of ALP, OC, OSX, and RUNX2. MiR-200c-3p was upregulated, but smad7 was downregulated in hBMSCs treated with ZG at different concentrations at different periods. Besides, miR-200c-3p positively regulated the expression of ALP, OC, OSX, and RUNX2 in ZG-induced hBMSCs. Moreover, miR-200c-3p targeted smad7 and strengthened the expression of ALP, OC, OSX, and RUNX2 in ZG-induced hBMSCs by downregulating smad7.
Conclusions
ZG contributed to osteoblast differentiation via miR-200c-3p/smad7 regulatory axis by promoting the expression of ALP, OC, OSX, and RUNX2 in hBMSCs.
MeSH Keywords: Mesenchymal Stromal Cells, Osteoblasts, Smad7 Protein
Background
Bone is the organ of the skeletal system and plays irreplaceable functions in shaping the body, mechanically supporting and protecting the body, and promoting movement [1]. In addition, bones contribute to the balance of mineral ions and regulate metabolism [2,3]. The diverse functions of bones depend on the maintenance of bone homeostasis. Human bone marrow mesenchymal stem cells (hBMSCs) are compatible implants in bone tissue project and have a variety of properties that can be differentiated into osteoblasts, nerve cells, chondrocytes, and cardiomyocytes [4,5]. Osteoblast differentiation is a vital process in maintaining bone homeostasis [6]. Hence, exploring the mechanisms involved in osteoblast differentiation of hBMSCs is of incredible value in bone homeostasis.
Zingerone, 4-(4-hydroxy-3-methoxyphenyl)-2-butanone (ZG) is a non-volatile compound produced directly during the drying of ginger. It has been reported that ZG has positive effects on human health including inhibiting tumor growth [7], anti-inflammatory [8], and antioxidant [9] effects against radiation-induced genetic damage and apoptosis [10]. However, the research on ZG on osteogenic differentiation is extremely limited. Srinaath et al. reported that ZG had a stimulatory action on the differentiation of mouse mesenchymal stem cells (mMSCs) towards osteoblasts, suggesting its unique role in bone tissue regeneration [11].
The microRNAs (miRNAs) are single-stranded, noncoding RNAs that are 18~22 nucleotide in length, and which function through competitively combining with the 3′-untranslated region (3′UTR) of target mRNAs [12]. Interestingly, recent research has hinted the capacity of certain miRNAs to conduct differentiation and behavior in the microenvironment of bone tissues. For example, overexpression of miR-96 promoted osteoblast differentiation and bone formation via exciting Wnt signaling pathway in ankylosing spondylitis (AS) mice [13]. Overexpression of miR-214 inhibited the osteoblast differentiation of BMSCs through suppressing β-catenin expression and attenuating Wnt/β-catenin signaling pathway activity [14]. MiR-33a-5p contributed to tumor necrosis factor-α (TNF-α)-inhibited osteogenic differentiation through downregulating the level of special AT-rich sequence-binding protein 2 (SATB2) in hBMSCs [15]. These findings revealed the essential roles of miRNAs in the processes of osteoblast differentiation and bone formation. Previous reports noticed the aberrant downregulation of miR-200c-3p in cemento-ossifying fibroma (COF) [16], and the role of miR-200c in osteogenic differentiation has been mentioned a lot [17,18]. However, the role of miR-200c-3p in hBMSCs and associated mechanism in osteogenic differentiation is still not clear.
Recently, smad7 has been identified to inhibit transforming growth factor β (TGF-β) signals transduction by acting downstream of TGF-βreceptor [19]. The role of smad7 in TGF-β-mediated physiology and pathology is diverse. For instance, smad7 has been shown to function in the processes of tumorigenesis, metastasis and invasion of colorectal cancer [20], human breast cancer [21], melanoma [22] and endometrial carcinomas [23]. Smad7 could also mediate the inflammatory response and autoimmune inflammation of TGF-β in some situations, such as inflammatory bowel disease [24,25]. Additionally, the involvement of smad7 in osteogenic differentiation was partly identified [26,27]. Unfortunately, the role of smad7 in osteogenic differentiation and regulatory mechanism were still limited and needed further investigation.
In our present study, we aimed to verify the impact of ZG on osteoblast differentiation and the expression levels of miR-200c-3p and smad7 in ZG-induced hBMSCs. Moreover, we detected the relationship between miR-200c-3p and smad7, and their functional roles in ZG-induced hBMSCs, aiming to provide an underlying mechanism of ZG in the process of osteoblast differentiation.
Material and Methods
Cell culture and cell treatment
The hBMSCs were isolated as described in a previous study [28] and were maintained in Dulbecco’s Modified Eagle Medium (DMEM; Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Life Technologies) at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
Osteogenesis was induced by ZG (Sigma-Aldrich, St. Louis, MO, USA) according to the previous description [11]. Briefly, hBMSCs were treated with ZG at concentrations of 50 μM, 100 μM, and 200 μM for 48 hours, and the untreated group was considered as control. In addition, hBMSCs were treated with 200 μM ZG at different stages (0, 24, 48, and 72 hours) for time-course analysis. For functional analysis, hBMSCs were treated with 200 μM ZG for further investigation.
Cell transfection
MiR-200c-3p mimics (miR-200c-3p) and miR-200c-3p inhibitor (in-miR-200c-3p) together with their corresponding negative control (miR-NC and in-miR-NC) were purchased from Ribobio (Guangzhou, China). Overexpression vector of smad7 pcDNA3.1-smad7 (smad7) was constructed by Hanbio Biotechnology Co., Ltd. (Shanghai, China). All transfection reactions in hBMSCs were conducted by using Lipofectamine 2000 reagent (Thermo Fisher Scientific, Waltham, MA, USA). Following experiments were performed at 48 hours post-transfection.
3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-H-tetrazolium bromide (MTT) assay
MTT assay was conducted to check the cytotoxicity of ZG as described by previous research [29]. Specifically, hBMSCs treated with ZG were cultured in DMEM in 24-well plates for 48 hours. Then the supernatant was obtained by centrifugation and then 200 μL of 0.05% MTT solution (Beyotime, Shanghai, China) was sucked in every well. After incubating for continuing 1 hour at room temperature, the upper solution was discarded, and dimethyl sulfoxide (DMSO; Sigma-Aldrich) was used to dissolve the formazan crystal in each well. Finally, the optical density (OD) value at 570 nm was recorded by using spectrophotometer (Thermo Fisher Scientific).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from hBMSCs treated with ZG at different concentrations or at different time points using TRIzol reagent (Sigma-Aldrich). For miR-200c-3p quantification, TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA) was used to conduct reverse transcription reaction. For smad7 quantification, cDNA synthesis was done with High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Then the qRT-PCR reaction was performed with the Power SYBR Green PCR Master Mix (Applied Biosystems) on 7900HT thermocycler (Applied Biosystems). The relative expression was calculated by using the 2−ΔΔCt method. U6 small nucleolar RNA and β-actin were used as internal controls. Primers were listed as below:
alkaline phosphatase (ALP),
forward (F): 5′-TTGTGCCAGAGAAAGAGAGAGA-3′ and
reverse (R): 5′-GTTTCAGGGCATTTTTCAAGGT-3′;
osteocalcin (OC), F: 5′-ATGGCTTGAAGACCGCCTAC-3′ and
R: 5′-AGGGCAGAGAGAGAGGACAG-3′;
osterix (OSX), F: 5′-ACTGGCTAGGTGGTGGTCAG-3′ and
R: 5′-GGTAGGGAGCTGGGTTAAGG-3′;
runt-related transcription factor 2 (RUNX2),
F: 5′-AGATGATGACACTGCCACCTCTG-3′
and R: 5′-GGGATGAAATGCTTGGGAACTGC-3′;
β-actin, F: 5′-GCACCACACCTTCTACAATG-3′ and
R: 5′-TGCTTGCTGATCCACATCTG-3′; U6,
F: 5′-CGCTTCGGCAGCACATATAC-3′ and
R: 5′-TTCACGAATTTGCGTGTCAT-3′.
The primers of miR-200c-3p were directly purchased from Ribobio (Guangzhou).
Western blot assay
RIPA lysis buffer (Bio Basic, Markham, Ontario, Canada) was used to obtain total protein from hBMSCs. Next, total protein was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) after detecting the concentration and then transferred on polyvinylidene fluoride (PVDF) membranes (Bio-Rad, Hercules, California, USA). Afterwards, the membranes were blocked at room temperature by tris-buffered saline (TBS; Sigma-Aldrich) containing 5% skim milk for 2 hours and incubated with the primary antibodies against ALP (ab83259, 1: 1000; Abcam, Cambridge, UK), OC (ab93876, 1: 500; Abcam), OSX (ab22552, 1: 1000; Abcam), RUNX2 (ab23981, 1: 1000; Abcam), smad7 (ab90086, 1: 1000; Abcam), or β-actin (ab8226, 1: 1000; Abcam). Next day, the membranes were washed and incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (ab205718, 1: 5000; Abcam) for 2 hours. Finally, the blots were visualized using an enhanced chemiluminescence (ECL) kit (Millipore, Billerica, MA, USA) on the ECL detection system (Millipore).
MiRNA target sites prediction
The target genes and binding sites of miR-200c-3p were identified through the online bioinformatics analysis tool TargetScan (http://www.targetscan.org).
Luciferase reporter assay
Smad7 3′UTR wild type (smad7 3′UTR-WT) sequences containing the binding sites of miR-200c-3p and smad7 3′UTR mutant (smad7 3′UTR-MUT) sequences designed by smad7 3′UTR-WT were amplified and inserted into the pGL3-basic vector. Then smad7 3′-UTR-WT or smad7 3′-UTR-MUT and miR-200c-3p or in-miR-200c-3p were co-transfected into hBMSCs, respectively, miR-NC and in-miR-NC acting as controls. After 48-hour transfection, cell lysates were collected, and luciferase activity was measured by using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) on a FLUOstar Omega (BMG Labtech, Ortenberg, Germany).
RNA immunoprecipitation (RIP) assay
Magna RIPTM RNA-Binding Protein Immunoprecipitation Kit (Millipore) was adopted to carry out RIP assay in agreement with the manufacturer’s instructions. Specifically, hBMSCs were lysed with RIP lysis solution. Then cell lysate was disposed of RIP buffer containing magnetic beads conjugated to human anti-AGO2 antibody or IgG control. The co-precipitated RNAs were purified and quantified by qRT-PCR.
Statistical analysis
Statistical analysis was operated using SPSS 15.0 software (SPSS, Chicago, IL, USA). All data are presented as mean±standard deviation (SD) based on the results of at least 3 independent experiment. Differences were analyzed using Student’s t-test to compare 2 groups or one-way ANOVA for multiple groups. The difference (P<0.05) was considered to be statistically significant.
Results
ZG was non-toxic nature and promoted osteoblast differentiation in hBMSCs
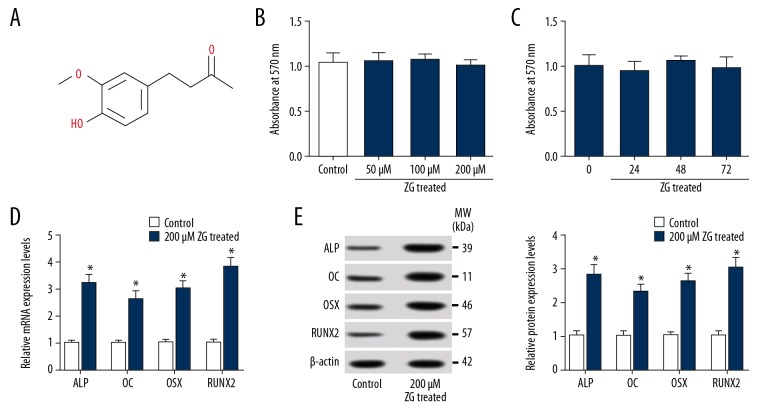
To determine the effects on osteoblast differentiation of ZG, hBMSCs were treated with ZG of 200 μM for 48 hours, and then typical osteoblast differentiation markers, such as ALP, OC, OSX, and RUNX2, were quantified by qRT-PCR and western blot assay. The chemical structural formula of ZG was shown in Figure 1A. Beforehand, the cytotoxicity of ZG to hBMSCs was checked. The results showed that cells treated with ZG at a concentration of 50 μM, 100 μM, or 200 μM had no obvious change in OD value compared with control (Figure 1B). Besides, the cells treated with 200 μM for 24, 48, and 72 hours had no significant change in OD value (Figure 1C). These data suggested that ZG was a non-toxic nature to hBMSCs. Furthermore, the expression of ALP, OC, OSX, and RUNX2 was congruously promoted at both mRNA (Figure 1D) and protein (Figure 1E) levels in hBMSCs treated with ZG. The data suggested that osteoblast differentiation was promoted under ZG treatment conditions.
Figure 1.
ZG was non-toxic nature and promoted osteogenesis in hBMSCs. (A) The chemical structural formula of ZG was shown. (B) Cytotoxicity assessment was performed in hBMSCs treated with ZG at different concentrations (50 μM, 100 μM and 200 μM) by MTT assay. (C) Cytotoxicity detection of hBMSCs treated with 200 μM ZG at different stages (0, 24, 48, and 72 hour) was carried out by MTT assay. (D, E) The mRNA (D) and protein (E) expression levels of osteoblast differentiation markers (ALP, OC, OSX, and RUNX2) were measured by qRT-PCR or western blot in hBMSCs treated with ZG, respectively. Data were presented as mean±standard deviation (SD) based on 3 independent replicates. * P<0.05. ZG – zingerone; hBMSCs – human bone mesenchymal stem cells; MTT assay – 3-(4,5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-H-tetrazolium bromide assay; qRT-PCR – quantitative real-time polymerase chain reaction; ALP – alkaline phosphatase; OC – osteocalcin; OSX – osterix; RUNX2 – runt-related transcription factor 2.
MiR-200c-3p was upregulated, but smad7 was downregulated in ZG-induced hBMSCs at different concentration for different stages
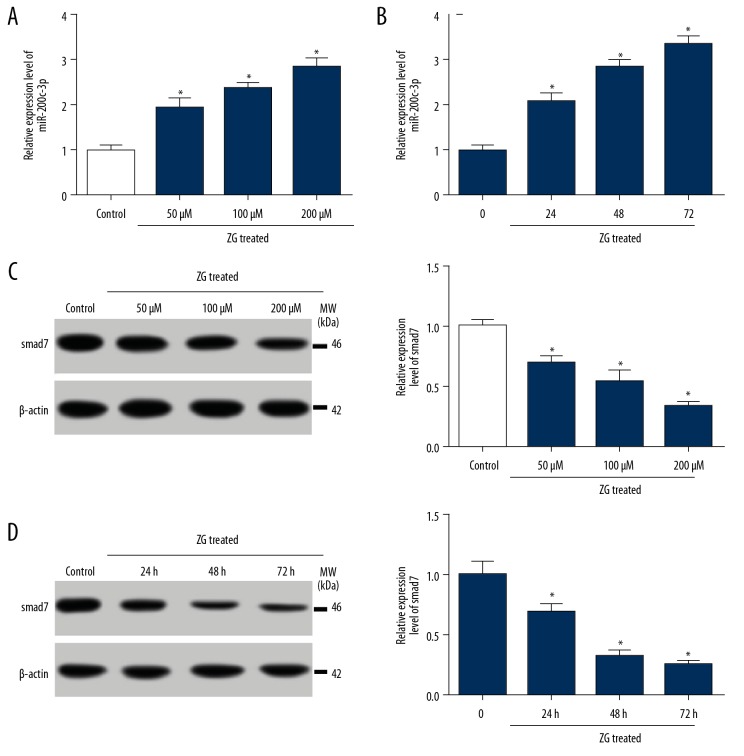
To detect the effect of ZG on the expression of miR-200c-3p and smad7 in hBMSCs, qRT-PCR or western blot was performed. The expression of miR-200c-3p was significantly increased in hBMSCs treated with ZG (50 μM, 100 μM, and 200 μM) following the dose-dependent manner compared with control (Figure 2A). Besides, the expression of miR-200c-3p was increasingly strengthened with the increase of time (24, 48, and 72 hours) after treatment with 200 μM ZG compared with untreated cells (Figure 2B). On the contrary, the protein level of smad7 was gradually reduced with the enrichment of ZG concentration (Figure 2C). Similarly, the level of smad7 was declined inch by inch with the increase of time (Figure 2D). These data claimed that miR-200c-3p and smad7 might partly function ZG-induced hBMSCs.
Figure 2.
MiR-200c-3p was upregulated, but smad7 was downregulated in hBMSCs treated with ZG. (A) The expression of miR-200c-3p in hBMSCs treated with ZG at different concentrations (50 μM, 100 μM, and 200 μM) was assessed by qRT-PCR. (B) The expression of miR-200c-3p in hBMSCs treated with 200 μM ZG at different stages (0, 24, 48, and 72 hours) was assessed by qRT-PCR. (C) The expression of smad7 in hBMSCs treated with ZG at different concentrations (50 μM, 100 μM, and 200 μM) was quantified by western blot. (D) The expression of smad7 in hBMSCs treated with 200 μM ZG at the different stage (0, 24, 48, and 72 hours) was quantified by western blot. Data were presented as mean±standard deviation (SD) based on 3 independent replicates. * P<0.05. ZG – zingerone; hBMSCs – human bone mesenchymal stem cells; NC – negative control; qRT-PCR – quantitative real-time polymerase chain reaction.
MiR-200c-3p contributed to osteoblast differentiation in ZG-induced hBMSCs
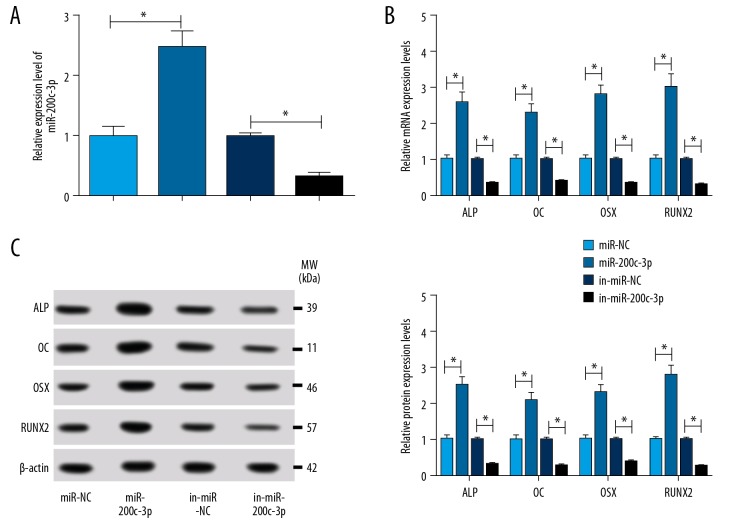
To verify the role of miR-200c-3p on osteoblast differentiation, ZG-induced hBMSCs were transfected with miR-200c-3p or in-miR-200c-3p, miR-NC or in-miR-NC as the control. Firstly, the efficiency of miR-200c-3p overexpression or inhibition was examined and the result showed that the expression of miR-200c-3p was rapidly picked up in ZG-induced cells transfected with miR-200c-3p compared with miR-NC, while the expression of miR-200c-3p was rapidly plummeted in cells treated with in-miR-200c-3p (Figure 3A). Next, the result of qRT-PCR indicated that the accumulation of miR-200c-3p facilitated the expression of ALP, OC, OSX, and RUNX2 compared with miR-NC (Figure 3B). Not surprisingly, the inhibition of miR-200c-3p sequestered the expression of ALP, OC, OSX, and RUNX2 (Figure 3B). Simultaneously, the protein levels of ALP, OC, OSX and RUNX2 assessed by western blot revealed the coincident result with their mRNA levels assessed by qRT-PCR (Figure 3C). This data implied that miR-200c-3p played an active role in the process of osteoblast differentiation.
Figure 3.
MiR-200c-3p contributed to osteoblast differentiation in ZG-induced hBMSCs. ZG-induced hBMSCs were transfected with miR-200c-3p mimics or miR-200c-3p inhibitor, miR-NC or in-miR-NC acting as a control. (A) The efficiency of miR-200c-3p overexpression or inhibition was measured by qRT-PCR. (B, C) The effects of miR-200c-3p overexpression or inhibition on the expression of osteoblast differentiation markers (ALP, OC, OSX, and RUNX2) were monitored by qRT-PCR (B) or western blot (C). Data were presented as mean±standard deviation (SD) based on 3 independent replicates. * P<0.05. ZG – zingerone; hBMSCs – human bone mesenchymal stem cells; NC – negative control; qRT-PCR – quantative real-time polymerase chain reaction; ALP – alkaline phosphatase; OC – osteocalcin; OSX – osterix; RUNX2 – runt-related transcription factor 2.
Smad7 was a target of miR-200c-3p
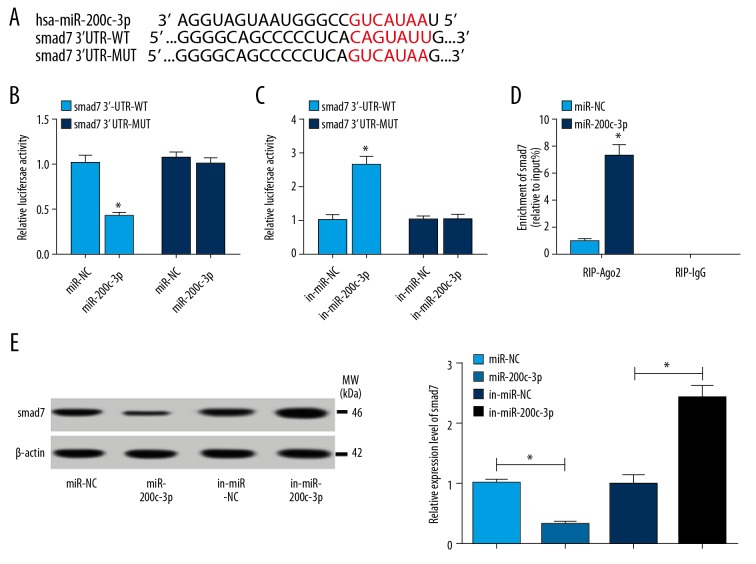
To ascertain the interaction between miR-200c-3p and smad7, online software TargetScan was used to analyze the binding sites between miR-200c-3p and smad7. The binding sites between miR-200c-3p and smad7 3′ UTR were exhibited in Figure 4A, then the wild type sequences of smad7 3′ UTR (smad7 3′UTR-WT) containing the binding sites with miR-200c-3p were mutated to generate smad7 3′UTR mutant sequences (smad7 3′UTR-MUT). Afterwards, smad7 3′UTR-WT and smad7 3′UTR-MUT were amplified and cloned into pGL3-basic vector to construct fusion plasmids, namely smad7 3′-UTR-WT and 3′-UTR-MUT, respectively. Luciferase reporter assay showed that the luciferase activity was remarkably decreased in cells co-transfected with smad7 3′-UTR-WT and miR-200c-3p, while there was no significant change in luciferase activity of cells co-transfected with smad7 3′-UTR-MUT and miR-200c-3p, miR-NC as a control (Figure 4B). Inversely, luciferase activity was obviously enhanced in cells co-transfected with smad7 3′-UTR-WT and in-miR-200c-3p, while luciferase activity had no noticeable change in cells co-transfected with smad7 3′-UTR-MUT and in-miR-200c-3p, in-miR-NC as a control (Figure 4C). Synchronously, RIP assay observed that miR-200c-3p mimics could reinforce the enrichment of smad7 in Ago2 RIP compared with miR-NC, whereas there was no difference of smad7 expression in IgG RIP (Figure 4D). Moreover, western blot analysis proved that the expression of smad7 was significantly diminished in cells transfected with miR-200c-3p, while it was sharply intensified in cells transfected with in-miR-200c-3p, miR-NC or in-miR-NC acting as the control (Figure 4E). This data manifested that miR-200c-3p regulated smad7 expression by directly binding to smad7.
Figure 4.
(A–E) smad7 was a target of miR-200c-3p. (A) The binding sites between miR-200c-3p and smad7 were analyzed by online software TargetScan. (B, C) Luciferase reporter assay was executed to verify the interaction of miR-200c-3p and smad7 in hBMSCs. (B) hBMSCs were transfected with smad7 3′UTR-WT sequences containing binding sites of miR-200c-3p or smad7 3′UTR-MUT sequences and miR-200c-3p mimics, miR-NC as a control. (C) hBMSCs were transfected with smad7 3′UTR-WT sequences containing binding sites of miR-200c-3p or smad7 3′UTR-MUT sequences and miR-200c-3p inhibitor, in-miR-NC as a control. (D) RIP assay was conducted to further confirmed the relationship between miR-200c-3p and smad7. (D) The protein level of smad7 was determined by western blot assay after miR-200c-3p overexpression or inhibition. Data were presented as mean±standard deviation (SD) based on 3 independent replicates. * P<0.05. hBMSCs – human bone mesenchymal stem cells; RIP – RNA immunoprecipitation.
MiR-200c-3p promoted the expression of ALP, OC, OSX, and RUNX2 through downregulating smad7 in ZG-induced hBMSCs
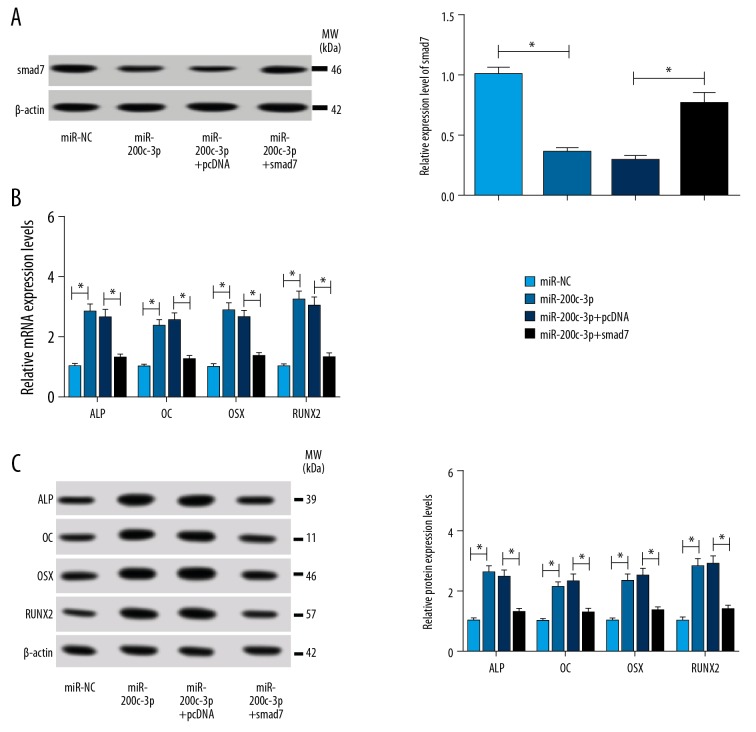
To elucidate the underlying mechanism of miR-200c-3p regulating osteoblast differentiation in ZG-induced hBMSCs, miR-200c-3p, miR-NC, miR-200c-3p +smad7, and miR-200c-3p+pcDNA was transfected into ZG-induced hBMSCs, respectively. First, western blot analysis showed that the transfection of miR-200c-3p+smad7 recovered the expression of smad7 inhibited by miR-200c-3p overexpression (Figure 5A). The analysis of qRT-PCR suggested that overexpression of miR-200c-3p in hBMSCs resulted in the promotion of osteoblast differentiation markers (ALP, OC, OSX, and RUNX2). However, the simultaneous ectopic overexpression of smad7 reversed the accelerative effects of miR-200c-3p on the expression of osteoblast differentiation markers (Figure 5B). The protein levels of these markers presented the uniform tendency with the mRNA expression level (Figure 5C). These results indicated that miR-200c-3p promoted osteoblast differentiation in ZG-induced hBMSCs by inhibiting smad7 expression.
Figure 5.
MiR-200c-3p promoted osteoblast differentiation in ZG-induced hBMSCs by downregulating smad7 expression. hBMSCs treated with 200 μM ZG for 48 hours were transfected with miR-200c-3p mimics, NC, miR-200c-3p mimics+ pcDNA-smad7, or miR-200c-3p mimics+ pcDNA, respectively. (A) The relative protein level of smad7 was measured by western blot. (B) qRT-PCR was performed to detect changes in the mRNA levels of ALP, OC, OSX, and RUNX2. (C) The protein levels of ALP, OC, OSX, and RUNX2 were quantified by western blot analysis. Data were presented as mean±standard deviation (SD) based on 3 independent replicates. * P<0.05. ZG – zingerone; hBMSCs – human bone mesenchymal stem cells; NC – negative control; qRT-PCR – quantitative real-time polymerase chain reaction; ALP – alkaline phosphatase; OC – osteocalcin; OSX – osterix; RUNX2 – runt-related transcription factor 2.
Discussion
Osteoblast differentiation holds its value in the maintenance of bone homeostasis. ZG is a compound isolated from ginger and has positive effects on a variety of biological processes. This study evaluated the toxicity of ZG to hBMSCs and explored the effects of ZG treatment on the expression of osteoblast differentiation markers. The study also identified the significantly upregulated miR-200c-3p and significantly downregulated smad7 in ZG-induced cells and investigated their interrelationship and their roles on the expression of osteoblast differentiation markers in ZG-induced hBMSCs. This report revealed a possible mechanism for ZG to promote osteoblast differentiation in hBMSCs.
With the change of lifestyles and concepts, people are more inclined to use natural substances for disease treatment [30]. ZG is a natural compound separated from the drying process of ginger. As an immunostimulant, ZG has various biological functions, including anti-inflammatory activity, antioxidant activity, antidiarrhoeic, phagocytic activity and anti-pathogenic activity [30–33]. Particularly, ZG is positive and has a stimulating effect on the differentiation of mMSCs into osteoblasts at the cellular and molecular levels [11]. Similarly, our research suggested that ZG contributed to osteoblast differentiation by stimulating the expression osteoblast differentiation markers, including RUNX2, ALP, OSX, and OC. Runx2, expressed in osteoblast lineage cells and chondrocytes, has been reported as an inducer of osteoblast and chondrocyte differentiation [34]. Most of ALP isoenzymes are derived from the bones and liver [35]. ALP is regarded as a key marker for osteoblast activity during the early stage of osteoblast differentiation [36]. OSX, a zinc-finger-containing transcription factor, is necessary for bone formation and osteoblast differentiation [37,38]. OC, also known as bone Gla protein, is the most affluent non-collagen in the bone. It was synthesized and secreted by mature osteoblasts and osteocytes and is currently served as a sign for bone formation [39,40]. In agreement with the previous study, this paper elucidated the function of ZG to promote osteoblast differentiation, and this function was partly accomplished by reinforcing the expression of miR-200c-3p.
MiR-200c-3p is a member of miR-200c family. A recent study reported that overexpression of miR-200c promoted osteogenic differentiation by targeting Myd88 via activating the AKT/β-catenin signaling pathway [4]. Another research reported that miR-200c improved osteogenic differentiation through directly regulating IL-6, IL-8, and CCL-5 expression by binding to their 3′UTRs [41]. Consistent with these papers, our study demonstrated that miR-200c-3p mimics effectively promoted osteoblast differentiation via enhancing the expression of several osteoblast differentiation markers. Additionally, smad7, acting as a target of miR-200c-3p, was observed in this paper. Smad7 has been reported as a corepressor of Runx2 and inhibits osteoblast differentiation through acting as a target of certain miRNAs. According to the reports, miR-590-5p weakened the depleted effect of high glucose (HG) on osteoblast differentiation by inhibiting the abundance of smad7 in MC3T3-E1 osteoblastic cells [42]. Smad7 inhibited proliferation, differentiation and mineralization of mouse osteoblastic cells [43]. MiR-17-5p might modulate osteoblastic cell proliferation partly through suppressing SMAD7 expression [44]. These findings suggest that smad7 might serve as a negative factor in the process of osteoblast differentiation.
Conclusions
Collectively, our study firstly indicated that ZG led to the facilitation of osteoblast differentiation via miR-200c-3p/smad7 regulatory axis, which provided a novel route for the stimulation of bone formation and regeneration.
Footnotes
Source of support: Departmental sources
Conflict of interests
None.
References
- 1.Oryan A, Monazzah S, Bigham-Sadegh A. Bone injury and fracture healing biology. Biomed Environ Sci. 2015;28:57–71. doi: 10.3967/bes2015.006. [DOI] [PubMed] [Google Scholar]
- 2.Marolt D, Knezevic M, Novakovic GV. Bone tissue engineering with human stem cells. Stem Cell Res Ther. 2010;1:10. doi: 10.1186/scrt10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bighamsadegh A, Oryan A. Basic concepts regarding fracture healing and the current options and future directions in managing bone fractures. Int Wound J. 2015;12:238–47. doi: 10.1111/iwj.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia P, Gu R, Zhang W, et al. MicroRNA-200c promotes osteogenic differentiation of human bone mesenchymal stem cells through activating the AKT/β-Catenin signaling pathway via downregulating Myd88. J Cell Physiol. 2019;234(12):22675–86. doi: 10.1002/jcp.28834. [DOI] [PubMed] [Google Scholar]
- 5.Toshihisa K. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem. 2010;99:1233–39. doi: 10.1002/jcb.20958. [DOI] [PubMed] [Google Scholar]
- 6.Xiao WZ, Gu XC, Hu B, et al. Role of microRNA-129-5p in osteoblast differentiation from bone marrow mesenchymal stem cells. Cell Mol Biol (Noisy-le-grand) 2016;62:95–99. [PubMed] [Google Scholar]
- 7.Choi JS, Ryu J, Bae WY, et al. Zingerone suppresses tumor development through decreasing cyclin D1 expression and inducing mitotic arrest. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19092832. pii: E2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lokender K, Sanjay C, Kusum H. Zingerone suppresses liver inflammation induced by antibiotic mediated endotoxemia through down regulating hepatic mRNA expression of inflammatory markers in Pseudomonas aeruginosa peritonitis mouse model. PLoS One. 2014;9:e106536. doi: 10.1371/journal.pone.0106536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sang-Guk S, Young KJ, Hae Young C, Ji-Cheon J. Zingerone as an antioxidant against peroxynitrite. J Agric Food Chem. 2005;53:7617–22. doi: 10.1021/jf051014x. [DOI] [PubMed] [Google Scholar]
- 10.Rao BN, Archana PR, Aithal BK, Rao BS. Protective effect of zingerone, a dietary compound against radiation induced genetic damage and apoptosis in human lymphocytes. Eur J Pharmacol. 2011;657:59–66. doi: 10.1016/j.ejphar.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Srinaath N, Balagangadharan K, Pooja V, et al. Osteogenic potential of zingerone, a phenolic compound in mouse mesenchymal stem cells. Biofactors. 2019;45(4):575–82. doi: 10.1002/biof.1515. [DOI] [PubMed] [Google Scholar]
- 12.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma S, Wang DD, Ma CY, Zhang YD. microRNA-96 promotes osteoblast differentiation and bone formation in ankylosing spondylitis mice through activating the Wnt signaling pathway by binding to SOST. J Cell Biochem. 2019;120:15429–42. doi: 10.1002/jcb.28810. [DOI] [PubMed] [Google Scholar]
- 14.Li JP, Zhuang HT, Xin MY, Zhou YL. MiR-214 inhibits human mesenchymal stem cells differentiating into osteoblasts through targeting β-catenin. Eur Rev Med Pharmacol Sci. 2017;21:4777–83. [PubMed] [Google Scholar]
- 15.Mi W, Shi Q, Chen X, et al. miR-33a-5p modulates TNF-α-inhibited osteogenic differentiation by targeting SATB2 expression in hBMSCs. FEBS Lett. 2016;590:396–407. doi: 10.1002/1873-3468.12064. [DOI] [PubMed] [Google Scholar]
- 16.Pereira T, Brito JAR, Guimaraes ALS, et al. MicroRNA profiling reveals dysregulated microRNAs and their target gene regulatory networks in cemento-ossifying fibroma. J Oral Pathol Med. 2018;47:78–85. doi: 10.1111/jop.12650. [DOI] [PubMed] [Google Scholar]
- 17.Akkouch A, Eliason S, Sweat ME, et al. Enhancement of microRNA-200c on osteogenic differentiation and bone regeneration by targeting Sox2-mediated Wnt signaling and Klf4. Hum Gene Ther. 2019;30(11):1405–18. doi: 10.1089/hum.2019.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia P, Gu R, Zhang W, et al. MicroRNA-200c promotes osteogenic differentiation of human bone mesenchymal stem cells through activating the AKT/beta-Catenin signaling pathway via downregulating Myd88. J Cell Physiol. 2019;234:22675–86. doi: 10.1002/jcp.28834. [DOI] [PubMed] [Google Scholar]
- 19.Stopa M, Anhuf D, Terstegen L, et al. Participation of Smad2, Smad3, and Smad4 in transforming growth factor beta (TGF-beta)-induced activation of Smad7. THE TGF-beta response element of the promoter requires functional Smad binding element and E-box sequences for transcriptional regulation. J Biol Chem. 2000;275:29308–17. doi: 10.1074/jbc.M003282200. [DOI] [PubMed] [Google Scholar]
- 20.Broderick P, Carvajalcarmona L, Pittman AM, et al. A genome-wide association study shows that common alleles of SMAD7 influence colorectal cancer risk. Nat Genet. 2007;39:1315–17. doi: 10.1038/ng.2007.18. [DOI] [PubMed] [Google Scholar]
- 21.Yin JJ, Selander K, Chirgwin JM, et al. TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Javelaud D, Mohammad KS, Mckenna CR, et al. Stable overexpression of Smad7 in human melanoma cells impairs bone metastasis. Cancer Res. 2007;67:2317–24. doi: 10.1158/0008-5472.CAN-06-3950. [DOI] [PubMed] [Google Scholar]
- 23.Dowdy SC, Mariani A, Reinholz MM, et al. Overexpression of the TGF-beta antagonist Smad7 in endometrial cancer. Gynecol Oncol. 2005;96:368–73. doi: 10.1016/j.ygyno.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Monteleone G, Kumberova A, Croft NM, et al. Blocking Smad7 restores TGF-β1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001;108:601–9. doi: 10.1172/JCI12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang W, Huang XR, Li AG, et al. Signaling mechanism of TGF-β1 in prevention of renal inflammation: role of Smad7. J Am Soc Nephrol. 2005;16:1371–83. doi: 10.1681/ASN.2004121070. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Yang F, Wang Z, Fu Q, Liang A. MicroRNA-21 promotes osteogenic differentiation by targeting small mothers against decapentaplegic 7. Mol Med Rep. 2015;12:1561–67. doi: 10.3892/mmr.2015.3497. [DOI] [PubMed] [Google Scholar]
- 27.Jiang LB, Tian L, Zhang CG. Bone marrow stem cells-derived exosomes extracted from osteoporosis patients inhibit osteogenesis via microRNA-21/SMAD7. Eur Rev Med Pharmacol Sci. 2018;22:6221–29. doi: 10.26355/eurrev_201810_16028. [DOI] [PubMed] [Google Scholar]
- 28.Zou L, Zou X, Li H, et al. Molecular mechanism of osteochondroprogenitor fate determination during bone formation. Adv Exp Med Biol. 2006;585:431–41. doi: 10.1007/978-0-387-34133-0_28. [DOI] [PubMed] [Google Scholar]
- 29.Arumugam B, Balagangadharan K, Selvamurugan N. Syringic acid, a phenolic acid, promotes osteoblast differentiation by stimulation of Runx2 expression and targeting of Smad7 by miR-21 in mouse mesenchymal stem cells. J Cell Commun Signal. 2018;12:561–73. doi: 10.1007/s12079-018-0449-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmad B, Rehman MU, Amin I, et al. A review on pharmacological properties of zingerone (4-(4-hydroxy-3-methoxyphenyl)-2-butanone) ScientificWorldJournal. 2015;2015 doi: 10.1155/2015/816364. 816364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar L, Chhibber S, Harjai K. Hepatoprotective effect of zingerone (4-(4-hydroxy-3-methoxyphenyl) butan-2-one) in lipopolysaccharide induced liver injury mouse model through down regulation of inflammatory mediators. Int J Pharm Phyt Res. 2014;6:308–14. [Google Scholar]
- 32.Iyappan R, Nithya N, Remitha R, et al. Zingerone protects against stannous chloride-induced and hydrogen peroxide-induced oxidative DNA damage in vitro. Biol Trace Elem Res. 2013;155:455–59. doi: 10.1007/s12011-013-9801-x. [DOI] [PubMed] [Google Scholar]
- 33.Jaw-Chyun C, Li-Jiau H, Shih-Lu W, et al. Ginger and its bioactive component inhibit enterotoxigenic Escherichia coli heat-labile enterotoxin-induced diarrhea in mice. J Agric Food Chem. 2007;55:8390–97. doi: 10.1021/jf071460f. [DOI] [PubMed] [Google Scholar]
- 34.Komori T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem Cell Biol. 2018;149:313–23. doi: 10.1007/s00418-018-1640-6. [DOI] [PubMed] [Google Scholar]
- 35.Mukaiyama K, Kamimura M, Uchiyama S, et al. Elevation of serum alkaline phosphatase (ALP) level in postmenopausal women is caused by high bone turnover. Aging Clin Exp Res. 2015;27:413–18. doi: 10.1007/s40520-014-0296-x. [DOI] [PubMed] [Google Scholar]
- 36.Lim EK, Keem JO, Yun HS, et al. Smart nanoprobes for the detection of alkaline phosphatase activity during osteoblast differentiation. Chem Commun (Camb) 2015;51:3270–72. doi: 10.1039/c4cc09620g. [DOI] [PubMed] [Google Scholar]
- 37.Nakashima K, Zhou X, Kunkel G, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 38.Han Y, Kim YM, Kim HS, Lee KY. Melatonin promotes osteoblast differentiation by regulating Osterix protein stability and expression. Sci Rep. 2017;7:5716. doi: 10.1038/s41598-017-06304-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karsenty G. Transcriptional regulation of osteoblast differentiation during development. Front Biosci. 1998;3:d834–37. doi: 10.2741/a326. [DOI] [PubMed] [Google Scholar]
- 40.Brennan-Speranza TC, Conigrave AD. Osteocalcin: an osteoblast-derived polypeptide hormone that modulates whole body energy metabolism. Calcif Tissue Int. 2015;96:1–10. doi: 10.1007/s00223-014-9931-y. [DOI] [PubMed] [Google Scholar]
- 41.Hong L, Sharp T, Khorsand B, et al. MicroRNA-200c represses IL-6, IL-8, and CCL-5 expression and enhances osteogenic differentiation. PLoS One. 2016;11:e0160915. doi: 10.1371/journal.pone.0160915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vishal M, Vimalraj S, Ajeetha R, et al. MicroRNA-590-5p stabilizes Runx2 by targeting Smad7 during osteoblast differentiation. J Cell Physiol. 2017;232:371–80. doi: 10.1002/jcp.25434. [DOI] [PubMed] [Google Scholar]
- 43.Masato Y, Yoshifumi I, Takako T, et al. Smad7 inhibits differentiation and mineralization of mouse osteoblastic cells. Endocr J. 2012;59:653–62. doi: 10.1507/endocrj.ej12-0022. [DOI] [PubMed] [Google Scholar]
- 44.Jie J, Xiaobo F, Weihua X, et al. MiR-17-5p modulates osteoblastic differentiation and cell proliferation by targeting SMAD7 in non-traumatic osteonecrosis. Exp Mol Med. 2014;46:e107. doi: 10.1038/emm.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]