Abstract
Background
Antimuscarinics are often used for treatment of overactive bladder (OAB), but exposure to medications such as antimuscarinics that have anticholinergic properties has been linked to adverse cognitive effects. A phase 4 placebo-controlled study (PILLAR; NCT02216214) described the efficacy and safety of mirabegron, a β3-adrenoreceptor agonist, for treatment of wet OAB in patients aged ≥65 years. This pre-planned analysis aimed to measure differences in cognitive function between mirabegron and placebo, using a rapid screening instrument for mild cognitive impairment: the Montreal Cognitive Assessment (MoCA).
Methods
Outpatients aged ≥65 years with wet OAB were randomized 1:1 to mirabegron or placebo, stratified by age (<75/≥75 years). There were no exclusion criteria regarding cognitive status. Patients randomized to mirabegron initially received 25 mg/day with an optional increase to 50 mg/day after week 4/8 based on patient/investigator discretion. The MoCA was administered at baseline and end of treatment (EoT, week 12). The study protocol was Independent Ethics Committee/Institutional Review Board-approved.
Results
Of the 887 randomized patients who received ≥1 dose of study drug, 72.3% were female, 79.5% were white, and 28.1% were aged ≥75 years. All patients had ≥1 comorbidity and 94.3% were receiving ≥1 concomitant medication. One third of patients had a history of psychiatric disorders, the most common being depression (17.2%), insomnia (15.7%), and anxiety (11.4%). Baseline mean (standard error, SE) MoCA total scores were 26.9 (0.1) and 26.8 (0.1) in the mirabegron and placebo groups, respectively. Among patients with MoCA data available at baseline/EoT, 27.1% (115/425) and 25.8% (106/411) of mirabegron and placebo group patients, respectively, had impaired cognitive function at baseline (MoCA total score <26). There was no statistically significant change in adjusted mean (SE) MoCA total score from baseline to EoT in the mirabegron group (−0.2 [0.1]) or the placebo group (−0.1 [0.1]).
Conclusions
Treatment with mirabegron for 12 weeks did not contribute to drug-related cognitive side effects in patients aged ≥65 years, as measured by the MoCA. Furthermore, the pattern of change in cognition over time in an older OAB trial population does not appear to differ from that of subjects receiving placebo.
Trial registration
NCT02216214 (prospectively registered August 13, 2014).
Keywords: Aged, Clinical trial, Phase 4, Cognition, Lower urinary tract symptoms, Urinary bladder, Overactive, Elderly, Geriatric
Background
Overactive bladder (OAB) is a symptom complex of storage lower urinary tract symptoms (LUTS) characterized by the presence of urgency. It affects all ages, but is increasingly prevalent as age increases [1–4]. According to the National Overactive Bladder Evaluation (NOBLE) study, prevalence of OAB in those ≥65 years is around 30%, twice as prevalent as in those ≤45 years [2]. By 2025, it is estimated that there will be 52 million adults in the USA with LUTS [5]. The older patient population with OAB has high levels of concomitant medication use and comorbidity, is more likely to experience falls and fractures, and is at increased risk of impairment of activities of daily living [6–8]. However, frail elderly patients with multiple comorbidities are often excluded from clinical trials [9–11].
Antimuscarinics are used to treat OAB, however, the risk of anticholinergic adverse events (AEs) such as dry mouth and constipation increases with age [12]. Exposure to medications with anticholinergic properties has also been linked to adverse cognitive effects, in particular in patients ≥65 years [13]. Furthermore, older people are particularly sensitive to anticholinergic effects as a result of significant age-related decrease in cholinergic neurons/receptors in the brain, reduction in hepatic and renal clearance of medications, and increase in blood-brain barrier (BBB) permeability [14]. In addition, the older patient population is likely to be receiving polypharmacy including other drugs with anticholinergic activity (e.g. tricyclic antidepressants, bronchodilators, ACE inhibitors, and antipsychotics), potentially resulting in a problematic anticholinergic burden, with negative effects on cognitive performance [15]. As a result, the Beers criteria lists all antimuscarinic drugs used for the treatment of OAB as potentially inappropriate for first-line treatment in those ≥65 years of age with dementia or cognitive impairment [16].
There are several screening instruments to detect dementia and cognitive impairment, including the Mini-Mental State Examination (MMSE) [17], the Geriatric Mental State Examination [18] and the Montreal Cognitive Assessment (MoCA) [19]. The 30-item MoCA, a brief cognitive screening tool for mild cognitive impairment (MCI), is recognized as a sensitive measure of cognitive function that can capture declines in cognition over repeated administrations. The MoCA is scaled from 1 to 30, with higher MoCA scores indicating better cognitive function; a MoCA score <26 indicates impaired cognitive function [19]. The MoCA includes six cognitive domains, including visuospatial abilities; language; combined attention, concentration, and working memory; executive function; short-term memory recall; and orientation to time and place, although subsequent consensus has emerged that the total score is most meaningful. The MoCA was conceptualized as an improvement over the MMSE, which does not allow for discrimination between those with MCI and elderly adults with no cognitive impairment. The original context for the MoCA was as a clinical tool, serving front-line physicians in making an initial assessment of MCI.
Mirabegron is a β3-adrenoreceptor agonist that represents an alternative OAB treatment to antimuscarinics, and potentially has a more favorable benefit-to-risk ratio in older patient populations [20–23]. The PILLAR study compared flexibly-dosed mirabegron versus placebo in community-dwelling elderly patients ≥65 years with OAB and incontinence [24]. Statistically significant mean improvements in bladder diary parameters were observed for mirabegron versus placebo, and safety and tolerability were in line with the mirabegron safety profile. The PILLAR study is the first to use MoCA to detect potential cognitive decline related to OAB treatment and aimed to measure any differences in change from baseline of MoCA scores between mirabegron and placebo.
Methods
Full methods are described in the PILLAR primary paper [24], but, in brief, PILLAR was a phase 4, multicentre, 12-week study in the US and Canada. Independent Ethics Committee/Institutional Review Board-approved written informed consent was obtained from all participants or their legally authorized representatives. This study adheres to CONSORT guidelines. Community-dwelling patients aged ≥65 years with wet OAB (≥1 incontinence episode and ≥3 urgency episodes during the 3-day diary, plus an average of ≥8 micturitions/24 h), were randomized in a 1:1 fashion to receive mirabegron or placebo. There were no specific exclusion criteria regarding cognitive status, although patients needed to be able to complete the micturition diaries and questionnaires. Patient-reported medical/surgical history was used to identify comorbidities. Patients who were randomized to mirabegron initially received 25 mg/day, with an optional increase to 50 mg/day after week 4/8 based on patient/investigator discretion. The study was designed and powered to detect a difference on incontinence and micturition frequency between combined mirabegron and placebo groups, and was not powered to detect a difference in MoCA scores. Data are displayed as placebo versus total mirabegron and according to treatment group. The safety analysis set (SAF) included all randomized patients receiving at least 1 dose of study medication. AEs were recorded throughout the study until week 16 (4 weeks post end of treatment [EoT]). The MoCA was the sole safety endpoint for which inferential comparison was pre-specified in the PILLAR statistical analysis plan. Stratified rank analysis of covariance with change in score from baseline was the response variable, with treatment group and gender as fixed factors. An additional ANCOVA included treatment, gender, and MoCA score at baseline as covariates. The MoCA was conducted at baseline and EoT, week 12, by clinic staff trained in its administration, in either English or Canadian French. Information on education level was not collected directly; however, clinic staff were instructed to add 1 point for any participants with ≤12 years education, as per the original MoCA scoring instructions [19]. Differences in change from baseline of MoCA scores between mirabegron and placebo were analyzed using a stratified ANCOVA model. As a complement to the pre-specified analyses, a post hoc analysis of relative risk was conducted to determine the relative risk of change in MoCA score by at least 4 points, which is deemed the minimum detectable change (MDC) [25]. Other post hoc analyses were change from baseline to EoT by baseline medical history of depression or antidepressant medication use, or by presence of ≥1 strong anticholinergic concomitant medication.
Results
Baseline
Of the 2380 patients screened, 445 were randomized to mirabegron and 443 to placebo; one patient in the placebo group did not receive treatment (Table 1). Of the 887 randomized patients who received ≥1 dose of study drug, overall 72.3% were female, 79.5% were white, and 28.1% were aged ≥75 years. In total, 226 patients received mirabegron 25 mg and only 219/445 patients (49.2%) uptitrated to 50 mg. There were no meaningful differences in demographic characteristics at baseline between the total mirabegron group and the placebo group. All patients had ≥1 comorbidity and 94.3% were receiving ≥1 concomitant medication; those relevant to cognition are shown in Table 2. One third of patients in the SAF had a history of psychiatric disorders, the most common being depression (17.2%), insomnia (15.7%), and anxiety (11.4%). Charlson Comorbidity Index scores (mean ± SD) were low: 2.3 ± 1.2 for both groups (Table 1).
Table 1.
Baseline characteristics (safety analysis set)
| Placebo (n = 442) | Mirabegron Total (n = 445) | |
|---|---|---|
| Female sex, n (%) | 324 (73.3) | 317 (71.2) |
| Age, mean ± SD | 71.9 ± 6.0 | 71.7 ± 5.5 |
| Age ≥75 years, n (%) | 124 (28.1) | 125 (28.1) |
| BMI, kg/m2, mean ± SD | 30.2 ± 6.4 | 29.7 ± 6.3 |
| Category, n (%) | ||
| <25 | 91 (20.6) | 108 (24.3) |
| ≥25–<30 | 150 (33.9) | 157 (35.3) |
| ≥30 | 201 (45.5) | 180 (40.4) |
| Ethnicity, n (%) | ||
| Not Hispanic or Latino | 395 (89.4) | 401 (90.1) |
| Hispanic or Latino | 43 (9.7) | 41 (9.2) |
| Unknown | 4 (0.9) | 3 (0.7) |
| Race, n (%) | ||
| White | 357 (80.8) | 348 (78.2) |
| Asian | 54 (12.2) | 59 (13.3) |
| Black or African American | 25 (5.7) | 33 (7.4) |
| Other | 6 (1.4) | 5 (1.1) |
| Country, n (%) | ||
| United States | 389 (88.0) | 385 (86.5) |
| Canada | 53 (12.0) | 60 (13.5) |
| Charlson Comorbidity Index score, mean ± SD | 2.3 (1.2) | 2.3 (1.2) |
| History of psychiatric disorders | ||
| Depression | 72 (16.3) | 81 (18.2) |
| Insomnia | 82 (18.6) | 57 (12.8) |
| Anxiety | 42 (9.5) | 59 (13.3) |
| Sleep disorder | 5 (1.1) | 6 (1.3) |
| Attention deficit/hyperactivity disorder | 4 (0.9) | 4 (0.9) |
| Libido decreased | 4 (0.9) | 4 (0.9) |
| Bipolar disorder | 3 (0.7) | 4 (0.9) |
| Nicotine dependence | 5 (1.1) | 1 (0.2) |
| Adjustment disorder with depressed mood | 2 (0.5) | 1 (0.2) |
| Initial insomnia | 0 | 2 (0.4) |
| Persistent depressive disorder | 0 | 2 (0.4) |
| Stress | 0 | 2 (0.4) |
| Major depression | 1 (0.2) | 1 (0.2) |
| Adjustment disorder | 0 | 1 (0.2) |
| Alcoholism | 0 | 1 (0.2) |
| Burnout syndrome | 0 | 1 (0.2) |
| Depressed mood | 0 | 1 (0.2) |
| Drug abuse | 0 | 1 (0.2) |
| Drug dependence | 0 | 1 (0.2) |
| Emotional disorder | 0 | 1 (0.2) |
| Mood swings | 0 | 1 (0.2) |
| Nervousness | 0 | 1 (0.2) |
| Post-traumatic stress disorder | 0 | 1 (0.2) |
| Premature ejaculation | 0 | 1 (0.2) |
| Anxiety disorder | 1 (0.2) | 0 |
| Claustrophobia | 1 (0.2) | 0 |
| Obsessive-compulsive disorder | 1 (0.2) | 0 |
| MoCA total scorea, n (%) | ||
| Category, n (%) | ||
| Normal (≥26) | 305 (69.3) | 310 (70.0) |
| Mild (18–25) | 103 (23.4) | 112 (25.3) |
| Moderate (10–17) | 3 (0.7) | 3 (0.7) |
| Severe (<10) | 0 | 0 |
| Missing | 29 (6.6) | 18 (4.1) |
Safety analysis set (SAF): all randomized subjects who received ≥1 dose of study medication
MoCA Montreal Cognitive Assessment, SD standard deviation
aN = 440 for placebo and 443 for mirabegron total
Table 2.
Concomitant non-OAB medications relevant to cognition during double-blind treatment period
| n (%) | Placebo (n = 442) | Mirabegron Total (n = 445) |
|---|---|---|
| CONCOMITANT ANTIDEPRESSANTS | ||
| SSRIs | 54 (12.2) | 53 (11.9) |
| Citalopram | 14 (3.2) | 9 (2.0) |
| Sertraline | 11 (2.5) | 4 (0.9) |
| Escitalopram oxalate | 5 (1.1) | 8 (1.8) |
| Escitalopram | 5 (1.1) | 7 (1.6) |
| Fluoxetine | 8 (1.8) | 4 (0.9) |
| Citalopram hydrobromide | 3 (0.7) | 6 (1.3) |
| Paroxetine | 1 (0.2) | 6 (1.3) |
| Sertraline hydrochloride | 4 (0.9) | 3 (0.7) |
| Fluoxetine hydrochloride | 3 (0.7) | 3 (0.7) |
| Paroxetine hydrochloride | 0 | 3 (0.7) |
| Other antidepressants | 23 (5.2) | 31 (7.0) |
| Trazodone | 13 (2.9) | 17 (3.8) |
| Duloxetine | 3 (0.7) | 4 (0.9) |
| Duloxetine hydrochloride | 4 (0.9) | 2 (0.4) |
| Mirtazapine | 2 (0.5) | 3 (0.7) |
| Trazodone hydrochloride | 0 | 3 (0.7) |
| Venlafaxine hydrochloride | 2 (0.5) | 1 (0.2) |
| Desvenlafaxine succinate | 0 | 1 (0.2) |
| Nefazodone | 0 | 1 (0.2) |
| Oxitriptan | 0 | 1 (0.2) |
| Venlafaxine | 1 (0.2) | 0 |
| Vortioxetine | 1 (0.2) | 0 |
| Non-selective monoamine reuptake inhibitors | 7 (1.6) | 9 (2.0) |
| Amitriptyline | 2 (0.5) | 7 (1.6) |
| Doxepin | 1 (0.2) | 1 (0.2) |
| Nortriptyline | 2 (0.5) | 0 |
| Amitriptyline hydrochloride | 0 | 1 (0.2) |
| Imipramine | 1 (0.2) | 0 |
| Nortriptyline hydrochloride | 1 (0.2) | 0 |
| Diazepines, oxazepines, thiazepines and oxepines | 3 (0.7) | 1 (0.2) |
| Quetiapine fumarate | 3 (0.7) | 1 (0.2) |
| Other antipsychotics | 0 | 2 (0.4) |
| Aripiprazole | 0 | 1 (0.2) |
| Risperidone | 0 | 1 (0.2) |
| CONCOMITANT ANTICHOLINERGICS | ||
| Antihistamines for systemic use | 38 (8.6) | 41 (9.2) |
| Hydroxyzine | 2 (0.5) | 2 (0.4) |
| Hydroxyzine hydrochloride | 1 (0.2) | 1 (0.2) |
| Aminoalkyl ethers | 15 (3.4) | 16 (3.6) |
| Diphenhydramine hydrochloride | 10 (2.3) | 11 (2.5) |
| Diphenhydramine | 4 (0.9) | 4 (0.9) |
| Dimenhydrinate | 1 (0.2) | 1 (0.2) |
| Carbinoxamine maleate | 0 | 1 (0.2) |
| Piperazine derivatives | 3 (0.7) | 2 (0.4) |
| Meclozine | 2 (0.5) | 1 (0.2) |
| Phenothiazine derivatives | 1 (0.2) | 1 (0.2) |
| Promethazine | 1 (0.2) | 0 |
| Promethazine hydrochloride | 0 | 1 (0.2) |
| Substituted alkylamines | 0 | 2 (0.4) |
| Chlorphenamine maleate | 0 | 2 (0.4) |
| Cough and cold preparations | 17 (3.8) | 20 (4.5) |
| Promethazine with codeine | 0 | 1 (0.2) |
| Carbamic acid esters | 5 (1.1) | 1 (0.2) |
| Carisoprodol | 2 (0.5) | 1 (0.2) |
| Synthetic anticholinergics, esters with tertiary amine groups | 0 | 2 (0.4) |
| Dicycloverine hydrochloride | 0 | 2 (0.4) |
OAB overactive bladder, SSRIs selective serotonin reuptake inhibitors
Baseline mean (standard error, SE) MoCA total scores were 26.9 (0.1) and 26.8 (0.1) in the mirabegron and placebo groups, respectively (Table 3). Among patients with MoCA data available at baseline/EoT, 27.1% (115/425) and 25.8% (106/411) of mirabegron and placebo group patients, respectively, had impaired cognitive function (MoCA total score <26) at baseline (Fig. 1).
Table 3.
Change from baseline to EoT in MoCA test total score (SAF)
| Placebo | Mirabegron Total | |
|---|---|---|
| Baseline, mean (SE)a | 26.8 (0.1) | 26.9 (0.1) |
| EoT, mean (SE)b | 27.0 (0.1) | 26.9 (0.1) |
| Adjusted change from baseline, mean (SE) | −0.1 (0.1) | −0.2 (0.1) |
| 95% two-sided CI | (−0.4, 0.2) | (−0.4, 0.1) |
| p-value | 0.471 |
CI confidence interval, EoT end of treatment, MoCA Montreal Cognitive Assessment, SAF safety analysis set, SE standard error
aN = 440 for placebo and 443 for mirabegron total
bN = 413 for placebo and 427 for mirabegron total
Fig. 1.
MoCA score at baseline and week 12/EoT. EoT end of treatment, MoCA Montreal Cognitive Assessment. Impaired cognitive function = MoCA total score <26 [19]
End of treatment
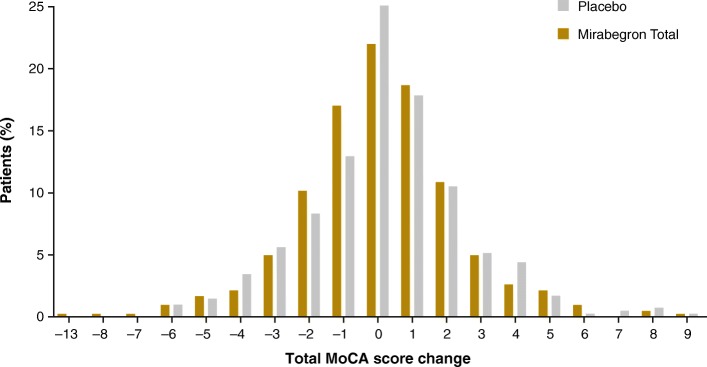
There were no changes in adjusted mean (SE) MoCA total score from baseline to EoT in the mirabegron group (−0.2 [0.1]) or the placebo group (−0.1 [0.1]) (Table 3). Changes in subscale scores are shown in Table 4. The number of patients missing scores at EoT were 29 for placebo and 18 for the mirabegron total group. Of the 411 patients receiving placebo and 425 patients receiving mirabegron, 48 patients (24 in each group) had declines of MoCA score of ≥4 points at week 12. The distribution of score changes are shown in Fig. 2. At week 12/EoT, 24.5% (104/425) and 25.8% (106/411) of mirabegron and placebo patients, respectively, had impaired cognitive function (Fig. 1). Post hoc analyses showed mirabegron and placebo to be similar with respect to the likelihood of experiencing cognitive decline (Fig. 3), using the MDC for a community-living elder population found in the literature [25]. MoCA scores showed no differences in change from baseline to EoT by baseline medical history of depression or antidepressant medication use, or by presence of ≥1 strong anticholinergic concomitant medication as recognized by the Anticholinergic Cognitive Burden scale [26] (Table 5).
Table 4.
Change from baseline to EoT in MoCA subscale scores (SAF)
| Change, mean (SD) | Placebo (n = 411) | Mirabegron Total (n = 425) |
|---|---|---|
| Attention Points | −0.0 (0.9) | −0.1 (0.9) |
| Language Points | 0.0 (0.7) | 0.0 (0.7) |
| Naming Points | −0.0 (0.4) | 0.0 (0.4) |
| Visuospatial/Executive Points | 0.0 (1.0) | −0.1 (1.2) |
| Abstraction Points | 0.0 (0.5) | −0.0 (0.5) |
| Delayed Recall Points | 0.2 (1.4) | 0.2 (1.3) |
| Orientation Points | −0.0 (0.4) | −0.0 (0.4) |
EoT end of treatment, MoCA Montreal Cognitive Assessment, SAF safety analysis set, SD standard deviation
Fig. 2.
Percentage of patients with indicated MoCA score change at week 12/EoT. EoT end of treatment, MoCA Montreal Cognitive Assessment
Fig. 3.
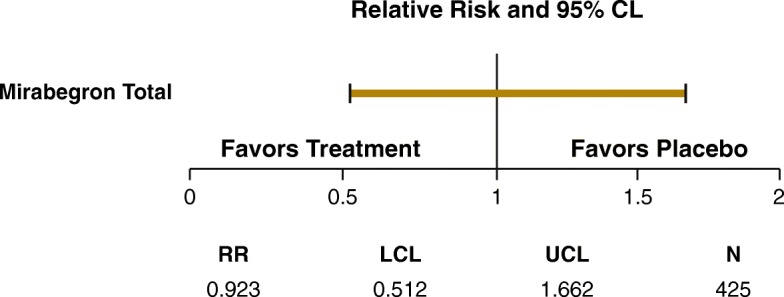
Forest plot for MCD of at least −4 points in total MoCA score. CL confidence limit, EoT end of treatment, LCL lower confidence limit, MCD minimally clinically important difference, MoCA Montreal Cognitive Assessment, N number of observations, RR relative risk ratio, UCL upper confidence limit
Table 5.
MoCA scores for patients with/without use of antidepressant medication, depression diagnosis from medical history, and by use of strong anticholinergic burden concomitant medication
| Placebo (n = 411) | Mirabegron Total (n = 425) | |||||
|---|---|---|---|---|---|---|
| Total score at BL, mean (SD) | Total score at EoT, mean (SD) | Change in Total score, mean | Total score at BL, mean (SD) | Total score at EoT, mean (SD) | Change in Total score, mean | |
| Use of antidepressant medicationa | ||||||
| No (n = 695) | 26.9 (2.9) | 27.1 (2.8) | 0.2 | 26.9 (2.7) | 27.0 (2.7) | 0.0 |
| Yes (n = 141) | 26.6 (2.6) | 26.9 (2.4) | 0.3 | 26.5 (3.0) | 26.6 (2.8) | 0.2 |
| Depression diagnosis from medical historyb | ||||||
| No (n = 683) | 26.8 (2.9) | 27.0 (2.7) | 0.2 | 27.0 (2.5) | 27.0 (2.7) | 0.0 |
| Yes (n = 153) | 26.9 (2.6) | 27.1 (2.6) | 0.1 | 26.2 (3.3) | 26.7 (2.8) | 0.4 |
| Use of anticholinergic burden concomitant medicationc | ||||||
| No (n = 767) | 26.8 (2.8) | 27.1 (2.7) | 0.2 | 26.8 (2.7) | 26.9 (2.7) | 0.0 |
| Yes (n = 69) | 26.6 (3.5) | 26.6 (3.1) | 0 | 27.0 (2.9) | 27.4 (2.4) | 0.4 |
aN = 345 for placebo and 350 for mirabegron total for No, N = 66 for placebo and 75 for mirabegron total for Yes
bN = 341 for placebo and 342 for mirabegron total for No, N = 70 for placebo and 83 for mirabegron total for Yes
cN = 380 for placebo and 387 for mirabegron total for No, N = 31 for placebo and 38 for mirabegron total for Yes
Of note, over 300 study participants, or nearly 41%, experienced an increase from one to nine points in MoCA score (Fig. 2). There were no differences between treatment and placebo groups in MoCA score improvements. The frequency of AEs that are typically seen with antimuscarinics was low for both mirabegron and placebo (Table 6).
Table 6.
Drug-related treatment-emergent adverse events ≥1% in either treatment group (SAF)
| TEAEs, n (%) | Placebo (n = 442) | Mirabegron Total (n = 445) |
|---|---|---|
| Drug-related TEAEsa | 57 (12.9) | 84 (18.9) |
| Dry mouth | 7 (1.6) | 6 (1.3) |
| Nausea | 3 (0.7) | 6 (1.3) |
| Constipation | 4 (0.9) | 3 (0.7) |
| Diarrhea | 1 (0.2) | 5 (1.1) |
| Headache | 7 (1.6) | 18 (4.0) |
| Dizziness | 6 (1.4) | 2 (0.4) |
| Somnolence | 1 (0.2) | 2 (0.4) |
| Escherichia urinary tract infection | 7 (1.6) | 9 (2.0) |
| Fatigue | 10 (2.3) | 7 (1.6) |
aPossible or probable, as assessed by the investigator, or where relationship was missing
SAF safety analysis set, TEAEs treatment-emergent adverse events
Discussion
Treatment with mirabegron for 12 weeks had no adverse impact on cognitive function in patients aged ≥65 years, as measured by the MoCA and compared with those randomized to placebo. Together with the efficacy demonstrated during the PILLAR study, these data suggest that mirabegron does not worsen cognition in older adults treated for OAB who are at risk of or concerned about cognitive impairment. Therefore, mirabegron represents a viable alternative for treatment of older patients with OAB, especially for those on anticholinergic medications for other diseases.
In comparison, a systematic review and several subsequent publications have found a strong link between cognitive impairment and anticholinergic potency of medications [14, 15, 26, 27]. There appears to be a differential effect on cognition between antimuscarinics used in the treatment of OAB, although there is substantial variability in scientific approaches such as duration of observation, outcome measures employed, and populations studied. Several studies have reported that oral oxybutynin has a significant negative effect on cognitive function in healthy adults ≥60 years of age [28–31]. Cognitive impairment has also been reported with tolterodine at therapeutic doses in small numbers of cognitively intact [32–34] and cognitively impaired older people [35]. In separate studies, cumulative exposure to strong anticholinergics increased the odds of transitioning from normal cognition to MCI in adults ≥65 years [36] and was associated with an increased risk for dementia and Alzheimer’s disease [15]. Anticholinergics have also been suggested to reduce the likelihood of reverting to normal cognition among those with a diagnosis of MCI [36]. However, conflicting evidence in older people with dementia suggests that anticholinergics may not affect cognition in these individuals [27, 37, 38].
It is worth noting that oxybutynin and tolterodine made up the majority of OAB medication use in these prior reports. Other studies support the lack of cognitive effects for trospium in patients ≥75 years [39–42]. Solifenacin at a dose of 10 mg also had no detectable effect on cognition in healthy elderly volunteers ≥65 years [29] or in those ≥75 years with MCI [31]. With the exception of a case report of delirium following fesoterodine treatment in an 89-year-old man with renal failure [43], there do not appear to be reports of cognitive impairment with fesoterodine in patients ≥65 years [44, 45], or with darifenacin in patients ≥60 years [30] or ≥65 years [46]. Newer, longer-acting antimuscarinics may therefore not be associated with cognitive effects; however, caution is warranted because of overall anticholinergic load.
As people aged ≥65 years are frequently under-represented in clinical trials, the results in the PILLAR study, which included a substantial proportion ≥75 years, are clinically relevant. Patients in this study were community dwelling and relatively healthy, as shown by low baseline Charlson Comorbidity Index scores. However, the number of concomitant medications and comorbid conditions observed at baseline in this study are similar to those in the general older population [8].
The MoCA presents several advantages compared with the MMSE. For example, it includes more executive function and visuospatial items than the MMSE, thus the MoCA provides a more comprehensive assessment across cognitive domains. In addition, the MoCA 5-word recall task is better at probing for subtle memory changes than the MMSE 3-word task [47]. The improvement in sensitivity in detecting cognitive decline compared with the MMSE has been clearly demonstrated, particularly in stroke studies [48, 49]. Other advantages of the MoCA are that it can be administered in the clinical setting by a trained rater in 10 min (although training occurred for all sites it is worth noting that this study took place before mandatory MoCA training was introduced by the developers), without relying on specific expertise in neurology, psychiatry, or clinical psychology. The MoCA may therefore be useful to practitioners if they are concerned about cognitive decline in their patients due to an increased anticholinergic burden resulting from multiple medications [50].
A number of studies have assessed the ability of the MoCA to detect change in healthy and impaired populations. Feeney et al. provided a distribution-based estimate of change from a sample of community-living people aged ≥55 years in Ireland [25]. This study provided a calculation of the MDC, with the implication that an individual’s score would need to change by ≥4 points on the MoCA to be confident that the change was not due to chance or measurement error. In the current study, post hoc analyses utilizing the MDC showed that mirabegron and placebo were similar with respect to the likelihood of experiencing cognitive decline.
Although initially developed for capture of MCI in patients presenting with cognitive complaints due to Alzheimer’s disease [19], the MoCA has also been used to assess cognitive decline associated with other conditions. These include stroke [51], acquired brain injury [52], Parkinson’s disease [53, 54], chronic obstructive pulmonary disease [55], heart failure [56], complications of diabetes [57], chronic hemodialysis [58], and Huntington’s disease [59]. The Huntington Study Group used the MoCA in a trial of dutetrabenazine for treatment of chorea in patients with Huntington’s disease. They reported that there was no significant difference in the mean change on the total MoCA score between placebo and the treatment of interest across the 12 weeks of the trial [59]. This outcome reflects the use of the MoCA in the current study.
The MoCA was developed specifically for detecting decrements in the performance of basic neurocognitive tasks, although it has also been used to track improvements. The current study supports the use of the MoCA for measuring decline in cognitive function as a side effect of OAB treatment and also demonstrates the similarity in general cognitive function outcomes between mirabegron and placebo. While the MoCA has well-established utility as a screening tool for cognitive impairment, it is not a diagnostic test and provides incomplete measurement of the individual core cognitive domains. It should therefore not be viewed as a substitute for more in-depth neuropsychological assessment when domain-specific information is required [60].
Limitations of the current study include the short study duration and the use of the same version of the MoCA 12 weeks after first administration. This use of the same version is likely reflected in the 41% of patients who improved their MoCA score on subsequent administration (i.e. a training effect). In addition, the study enrolled patients with wet OAB based in the community, so the results are not representative of other elderly patient groups, such as those in nursing homes or hospitalized. Also, Charlson Comorbidity Index scores were very low at baseline, indicating that these community-dwelling patients were generally healthy and unlikely to be frail; thus these findings do not apply to all elderly individuals with OAB.
Conclusions
Cognitive assessment over 12 weeks using the MoCA in the PILLAR study demonstrates that mirabegron treatment does not contribute to drug-related cognitive side-effects. Furthermore, the pattern of change in cognition over the trial duration in an older OAB trial population does not appear to differ from that of subjects receiving placebo.
Acknowledgments
The authors would like to thank the PILLAR study investigators, and all patients and their family/legal representatives who took part in the study. Preliminary results of this research were presented previously at EAU in 2019 (10.1016/S1569-9056(19)31134-0).
Abbreviations
- AEs
Adverse events
- BBB
Blood-brain barrier
- EoT
End of treatment
- LUTS
Lower urinary tract symptoms
- MCI
Mild cognitive impairment
- MDC
Minimum detectable change
- MMSE
Mini-Mental State Examination
- MoCA
Montreal Cognitive Assessment
- NOBLE
National Overactive Bladder Evaluation
- OAB
Overactive bladder
Authors’ contributions
DE performed statistical analysis. TLG, NLC, JM, DS, SH, DE and CRS were involved in study design, analysis and interpretation of data and drafting the manuscript, and read and approved the final manuscript.
Funding
Astellas Pharma Global Development, Inc. initiated and funded the study and contributed to the design, analysis and interpretation of data and provided funding for medical writing. Medical writing support was provided by Sue Cooper, CMPP, of Envision Scientific Solutions.
Availability of data and materials
Access to anonymized individual participant-level data collected during the trial, in addition to supporting clinical documentation, is planned for trials conducted with approved product indications and formulations, as well as compounds terminated during development. Conditions and exceptions are described under the Sponsor Specific Details for Astellas on www.clinicalstudydatarequest.com. Study-related supporting documentation is redacted and provided if available, such as the protocol and amendments, statistical analysis plan and clinical study report. Access to participant-level data is offered to researchers after publication of the primary manuscript (if applicable) and is available as long as Astellas has legal authority to provide the data. Researchers must submit a proposal to conduct a scientifically relevant analysis of the study data. The research proposal is reviewed by an Independent Research Panel. If the proposal is approved, access to the study data is provided in a secure data sharing environment after receipt of a signed Data Sharing Agreement.
Ethics approval and consent to participate
The study protocol was reviewed by an Independent Ethics Committee/Institutional Review Board for the study sites. Approval for the study protocol was obtained from the relevant competent authority prior to study initiation. Institutional Review Board/Independent Ethics Committee-approved written informed consent was obtained from all participants or their legally authorized representative prior to any study-related procedures.
Consent for publication
Not applicable as no individual data are reported, all data has been anonymized and participants are not identifiable.
Competing interests
TLG is a consultant for Astellas, and has received grants from the National Institute on Aging and the National Institutes of Health. NLC is a consultant for Astellas. JM has received grants from Astellas. DS has received grants and personal fees for services as an investigator, consultant, and speaker for Astellas. SH has received grants from Allergan, Astellas, Ipsen, and Ixaltis, and personal fees from Allergan, Astellas, and Pfizer. DE and CRS are employees of Astellas Pharma Global Development, Inc.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Milsom I, Stewart W, Thüroff J. The prevalence of overactive bladder. Am J Manag Care. 2000;6(11 Suppl):S565–S573. [PubMed] [Google Scholar]
- 2.Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- 3.Coyne KS, Sexton CC, Thompson CL, Milsom I, Irwin D, Kopp ZS, et al. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: results from the Epidemiology of LUTS (EpiLUTS) study. BJU Int. 2009;104(3):352–360. doi: 10.1111/j.1464-410X.2009.08427.x. [DOI] [PubMed] [Google Scholar]
- 4.Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50(6):1306–1315. doi: 10.1016/j.eururo.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Litman HJ, McKinlay JB. The future magnitude of urological symptoms in the USA: projections using the Boston Area Community Health survey. BJU Int. 2007;100(4):820–825. doi: 10.1111/j.1464-410X.2007.07018.x. [DOI] [PubMed] [Google Scholar]
- 6.Zarowitz BJ, Allen C, O'Shea T, Tangalos E, Berner T, Ouslander JG. Clinical burden and nonpharmacologic management of nursing facility residents with overactive bladder and/or urinary incontinence. Consult Pharm. 2015;30(9):533–542. doi: 10.4140/TCP.n.2015.533. [DOI] [PubMed] [Google Scholar]
- 7.Soliman Y, Meyer R, Baum N. Falls in the elderly secondary to urinary symptoms. Rev Urol. 2016;18(1):28–32. [PMC free article] [PubMed] [Google Scholar]
- 8.Ganz ML, Liu J, Zou KH, Bhagnani T, Luo X. Real-world characteristics of elderly patients with overactive bladder in the United States. Curr Med Res Opin. 2016;32(12):1997–2005. doi: 10.1080/03007995.2016.1226167. [DOI] [PubMed] [Google Scholar]
- 9.Wolff GF, Kuchel GA, Smith PP. Overactive bladder in the vulnerable elderly. Res Rep Urol. 2014;6:131–138. doi: 10.2147/RRU.S41843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kistler KD, Xu Y, Zou KH, Ntanios F, Chapman DS, Luo X. Systematic literature review of clinical trials evaluating pharmacotherapy for overactive bladder in elderly patients: an assessment of trial quality. Neurourol Urodyn. 2018;37(1):54–66. doi: 10.1002/nau.23309. [DOI] [PubMed] [Google Scholar]
- 11.DuBeau Catherine E., Kuchel George A., Johnson II Theodore, Palmer Mary H., Wagg Adrian. Incontinence in the frail elderly: Report from the 4th international consultation on incontinence. Neurourology and Urodynamics. 2010;29(1):165–178. doi: 10.1002/nau.20842. [DOI] [PubMed] [Google Scholar]
- 12.Fortin M-P, Rouch I, Dauphinot V, Gédéon C, Genthon S, Bonnefoy M, et al. Effects of anticholinergic drugs on verbal episodic memory function in the elderly: a retrospective, cross-sectional study. Drugs Aging. 2011;28(3):195–204. doi: 10.2165/11586580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Ancelin ML, Artero S, Portet F, Dupuy A-M, Touchon J, Ritchie K. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ. 2006;332:455. doi: 10.1136/bmj.38740.439664.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell N, Boustani M, Limbil T, Ott C, Fox C, Maidment I, et al. The cognitive impact of anticholinergics: A clinical review. Clin Interv Aging. 2009;4:225–233. doi: 10.2147/cia.s5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med. 2015;175(3):401–407. doi: 10.1001/jamainternmed.2014.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–46. [DOI] [PubMed]
- 17.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state": A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Copeland JRM, Dewey ME, Henderson AS, Kay DWK, Neal CD, Harrison MAM, et al. The Geriatric Mental State (GMS) used in the community: replication studies of the computerized diagnosis AGECAT. Psychol Med. 1988;18(1):219–223. doi: 10.1017/S003329170000204X. [DOI] [PubMed] [Google Scholar]
- 19.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 20.Drake MJ, MacDiarmid S, Chapple CR, Esen A, Athanasiou S, Cambronero Santos J, et al. Cardiovascular safety in refractory incontinent patients with overactive bladder receiving add-on mirabegron therapy to solifenacin (BESIDE) Int J Clin Pract. 2017;71(5):e12944. doi: 10.1111/ijcp.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshida M, Nozawa Y, Kato D, Tabuchi H, Kuroishi K. Safety and effectiveness of mirabegron in patients with overactive bladder aged ≥75 years: analysis of a Japanese post-marketing study. Low Urin Tract Symptoms. 2019;11(1):30–38. doi: 10.1111/luts.12190. [DOI] [PubMed] [Google Scholar]
- 22.Wagg A, Cardozo L, Nitti VW, Castro-Diaz D, Auerbach S, Blauwet MB, et al. The efficacy and tolerability of the β3-adrenoceptor agonist mirabegron for the treatment of symptoms of overactive bladder in older patients. Age Ageing. 2014;43(5):666–675. doi: 10.1093/ageing/afu017. [DOI] [PubMed] [Google Scholar]
- 23.Wagg A, Nitti VW, Kelleher C, Castro-Diaz D, Siddiqui E, Berner T. Oral pharmacotherapy for overactive bladder in older patients: mirabegron as a potential alternative to antimuscarinics. Curr Med Res Opin. 2016;32(4):621–638. doi: 10.1185/03007995.2016.1149806. [DOI] [PubMed] [Google Scholar]
- 24.Wagg Adrian, Staskin David, Engel Eli, Herschorn Sender, Kristy Rita M., Schermer Carol R. Efficacy, safety, and tolerability of mirabegron in patients aged ≥65 yr with overactive bladder wet: a phase IV, double-blind, randomised, placebo-controlled study (PILLAR) European Urology. 2020;77(2):211–220. doi: 10.1016/j.eururo.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Feeney J, Savva GM, O'Regan C, King-Kallimanis B, Cronin H, Kenny RA. Measurement error, reliability, and minimum detectable change in the Mini-Mental State Examination, Montreal Cognitive Assessment, and Color Trails Test among community living middle-aged and older adults. J Alzheimers Dis. 2016;53(3):1107–1114. doi: 10.3233/JAD-160248. [DOI] [PubMed] [Google Scholar]
- 26.Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311–320. doi: 10.2217/1745509X.4.3.311. [DOI] [Google Scholar]
- 27.Fox C, Richardson K, Maidment ID, Savva GM, Matthews FE, Smithard D, et al. Anticholinergic medication use and cognitive impairment in the older population: the Medical Research Council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477–1483. doi: 10.1111/j.1532-5415.2011.03491.x. [DOI] [PubMed] [Google Scholar]
- 28.Katz IR, Sands LP, Bilker W, DiFilippo S, Boyce A, D'Angelo K. Identification of medications that cause cognitive impairment in older people: the case of oxybutynin chloride. J Am Geriatr Soc. 1998;46(1):8–13. doi: 10.1111/j.1532-5415.1998.tb01006.x. [DOI] [PubMed] [Google Scholar]
- 29.Wesnes KA, Edgar C, Tretter RN, Bolodeoku J. Exploratory pilot study assessing the risk of cognitive impairment or sedation in the elderly following single doses of solifenacin 10 mg. Expert Opin Drug Saf. 2009;8(6):615–626. doi: 10.1517/14740330903260790. [DOI] [PubMed] [Google Scholar]
- 30.Kay G, Crook T, Rekeda L, Lima R, Ebinger U, Arguinzoniz M, et al. Differential effects of the antimuscarinic agents darifenacin and oxybutynin ER on memory in older subjects. Eur Urol. 2006;50(2):317–326. doi: 10.1016/j.eururo.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 31.Wagg A, Dale M, Tretter R, Stow B, Compion G. Randomised, multicentre, placebo-controlled, double-blind crossover study investigating the effect of solifenacin and oxybutynin in elderly people with mild cognitive impairment: the SENIOR study. Eur Urol. 2013;64(1):74–81. doi: 10.1016/j.eururo.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Tsao JW, Heilman KM. Transient memory impairment and hallucinations associated with tolterodine use. N Engl J Med. 2003;349(23):2274–2275. doi: 10.1056/NEJM200312043492325. [DOI] [PubMed] [Google Scholar]
- 33.Salvatore S, Serati M, Cardozo L, Uccella S, Bolis P. Cognitive dysfunction with tolterodine use. Am J Obstet Gynecol. 2007;197(2):e8. doi: 10.1016/j.ajog.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 34.Womack KB, Heilman KM. Tolterodine and memory: dry but forgetful. Arch Neurol. 2003;60(5):771–773. doi: 10.1001/archneur.60.5.771. [DOI] [PubMed] [Google Scholar]
- 35.Jewart RD, Green J, Lu C-J, Cellar J, Tune LE. Cognitive, behavioral, and physiological changes in Alzheimer disease patients as a function of incontinence medications. Am J Geriatr Psychiatry. 2005;13(4):324–328. doi: 10.1097/00019442-200504000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Campbell NL, Lane KA, Gao S, Boustani MA, Unverzagt F. Anticholinergics influence transition from normal cognition to mild cognitive impairment in older adults in primary care. Pharmacotherapy. 2018;38(5):511–519. doi: 10.1002/phar.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lackner TE, Wyman JF, McCarthy TC, Monigold M, Davey C. Randomized, placebo-controlled trial of the cognitive effect, safety, and tolerability of oral extended-release oxybutynin in cognitively impaired nursing home residents with urge urinary incontinence. J Am Geriatr Soc. 2008;56(5):862–870. doi: 10.1111/j.1532-5415.2008.01680.x. [DOI] [PubMed] [Google Scholar]
- 38.Sink KM, Thomas J, 3rd, Xu H, Craig B, Kritchevsky S, Sands LP. Dual use of bladder anticholinergics and cholinesterase inhibitors: long-term functional and cognitive outcomes. J Am Geriatr Soc. 2008;56(5):847–853. doi: 10.1111/j.1532-5415.2008.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chancellor M, Boone T. Anticholinergics for overactive bladder therapy: central nervous system effects. CNS Neurosci Ther. 2012;18(2):167–174. doi: 10.1111/j.1755-5949.2011.00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staskin D, Kay G, Tannenbaum C, Goldman HB, Bhashi K, Ling J, et al. Trospium chloride is undetectable in the older human central nervous system. J Am Geriatr Soc. 2010;58(8):1618–1619. doi: 10.1111/j.1532-5415.2010.02988.x. [DOI] [PubMed] [Google Scholar]
- 41.Geller EJ, Dumond JB, Bowling JM, Khandelwal CM, Wu JM, Busby-Whitehead J, et al. Effect of trospium chloride on cognitive function in women aged 50 and older: a randomized trial. Female Pelvic Med Reconstr Surg. 2017;23(2):118–123. doi: 10.1097/SPV.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 42.Staskin D, Kay G, Tannenbaum C, Goldman HB, Bhashi K, Ling J, et al. Trospium chloride has no effect on memory testing and is assay undetectable in the central nervous system of older patients with overactive bladder. Int J Clin Pract. 2010;64(9):1294–1300. doi: 10.1111/j.1742-1241.2010.02433.x. [DOI] [PubMed] [Google Scholar]
- 43.Charbonneau JM, Bisset R, Nguyen PV-Q. Delirium following fesoterodine treatment for urgency incontinence in an 89-year old man. Can Urol Assoc J. 2016;10(7–8):E261–E2E3. doi: 10.5489/cuaj.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DuBeau CE, Kraus SR, Griebling TL, Newman DK, Wyman JF, Johnson TM, 2nd, et al. Effect of fesoterodine in vulnerable elderly subjects with urgency incontinence: a double-blind, placebo controlled trial. J Urol. 2014;191(2):395–404. doi: 10.1016/j.juro.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 45.Kay GG, Maruff P, Scholfield D, Malhotra B, Whelan L, Darekar A, et al. Evaluation of cognitive function in healthy older subjects treated with fesoterodine. Postgrad Med. 2012;124(3):7–15. doi: 10.3810/pgm.2012.05.2543. [DOI] [PubMed] [Google Scholar]
- 46.Lipton RB, Kolodner K, Wesnes K. Assessment of cognitive function of the elderly population: effects of darifenacin. J Urol. 2005;173(2):493–498. doi: 10.1097/01.ju.0000148963.21096.5d. [DOI] [PubMed] [Google Scholar]
- 47.Lam B, Middleton LE, Masellis M, Stuss DT, Harry RD, Kiss A, et al. Criterion and convergent validity of the Montreal cognitive assessment with screening and standardized neuropsychological testing. J Am Geriatr Soc. 2013;61(12):2181–2185. doi: 10.1111/jgs.12541. [DOI] [PubMed] [Google Scholar]
- 48.Koski L. Validity and applications of the Montreal cognitive assessment for the assessment of vascular cognitive impairment. Cerebrovasc Dis. 2013;36(1):6–18. doi: 10.1159/000352051. [DOI] [PubMed] [Google Scholar]
- 49.Lees R, Selvarajah J, Fenton C, Pendlebury ST, Langhorne P, Stott DJ, et al. Test accuracy of cognitive screening tests for diagnosis of dementia and multidomain cognitive impairment in stroke. Stroke. 2014;45(10):3008–3018. doi: 10.1161/STROKEAHA.114.005842. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida M, Kato D, Nishimura T, Van Schyndle J, Uno S, Kimura T. Anticholinergic burden in the Japanese elderly population: Use of antimuscarinic medications for overactive bladder patients. Int J Urol. 2018;25(10):855–862. doi: 10.1111/iju.13758. [DOI] [PubMed] [Google Scholar]
- 51.Burton L, Tyson SF. Screening for cognitive impairment after stroke: a systematic review of psychometric properties and clinical utility. J Rehabil Med. 2015;47(3):193–203. doi: 10.2340/16501977-1930. [DOI] [PubMed] [Google Scholar]
- 52.Lim PA, McLean AM, Kilpatrick C, DeForge D, Iverson GL, Silverberg ND. Temporal stability and responsiveness of the Montreal Cognitive Assessment following acquired brain injury. Brain Inj. 2016;30(1):29–35. doi: 10.3109/02699052.2015.1079732. [DOI] [PubMed] [Google Scholar]
- 53.Biundo R, Weis L, Bostantjopoulou S, Stefanova E, Falup-Pecurariu C, Kramberger MG, et al. MMSE and MoCA in Parkinson's disease and dementia with Lewy bodies: a multicenter 1-year follow-up study. J Neural Transm (Vienna) 2016;123(4):431–438. doi: 10.1007/s00702-016-1517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fiorenzato E, Weis L, Falup-Pecurariu C, Diaconu S, Siri C, Reali E, et al. Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE) performance in progressive supranuclear palsy and multiple system atrophy. J Neural Transm (Vienna) 2016;123(12):1435–1442. doi: 10.1007/s00702-016-1589-3. [DOI] [PubMed] [Google Scholar]
- 55.Villeneuve S, Pepin V, Rahayel S, Bertrand J-A, de Lorimier M, Rizk A, et al. Mild cognitive impairment in moderate to severe COPD: a preliminary study. Chest. 2012;142(6):1516–1523. doi: 10.1378/chest.11-3035. [DOI] [PubMed] [Google Scholar]
- 56.Cameron J, Worrall-Carter L, Page K, Baker SS, Ski CF. Screening for mild cognitive impairment in patients with heart failure: Montreal Cognitive Assessment versus Mini Mental State Exam. Eur J Cardiovasc Nurs. 2013;12(3):252–260. doi: 10.1177/1474515111435606. [DOI] [PubMed] [Google Scholar]
- 57.Oğurel T, Oğurel R, Özer MA, Türkel Y, Dağ E, Örnek K. Mini-mental state exam versus Montreal Cognitive Assessment in patients with diabetic retinopathy. Niger J Clin Pract. 2015;18(6):786–789. doi: 10.4103/1119-3077.163274. [DOI] [PubMed] [Google Scholar]
- 58.Tiffin-Richards FE, Costa AS, Holschbach B, Frank RD, Vassiliadou A, Krüger T, et al. The Montreal Cognitive Assessment (MoCA) - a sensitive screening instrument for detecting cognitive impairment in chronic hemodialysis patients. PLoS One. 2014;9(10):e106700. doi: 10.1371/journal.pone.0106700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huntington Study Group. Effect of deutetrabenazine on chorea among patients with Huntington disease: a randomized clinical trial. JAMA. 2016;316(1):40–50. [DOI] [PubMed]
- 60.Coen RF, Robertson DA, Kenny RA, King-Kallimanis BL. Strengths and limitations of the MoCA for assessing cognitive functioning: findings from a large representative sample of Irish older adults. J Geriatr Psychiatry Neurol. 2016;29(1):18–24. doi: 10.1177/0891988715598236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Access to anonymized individual participant-level data collected during the trial, in addition to supporting clinical documentation, is planned for trials conducted with approved product indications and formulations, as well as compounds terminated during development. Conditions and exceptions are described under the Sponsor Specific Details for Astellas on www.clinicalstudydatarequest.com. Study-related supporting documentation is redacted and provided if available, such as the protocol and amendments, statistical analysis plan and clinical study report. Access to participant-level data is offered to researchers after publication of the primary manuscript (if applicable) and is available as long as Astellas has legal authority to provide the data. Researchers must submit a proposal to conduct a scientifically relevant analysis of the study data. The research proposal is reviewed by an Independent Research Panel. If the proposal is approved, access to the study data is provided in a secure data sharing environment after receipt of a signed Data Sharing Agreement.