Abstract
The utility of any model system for toxicity screening depends on the level of correlation between test responses and toxic reactions in humans. Assays in C. elegans can be fast and inexpensive, however few studies have been done comparing toxic responses in this easily cultured nematode with data on mammalian toxicity. Here we report that a screening assay for acute toxicity, using adult C. elegans grown in axenic liquid culture, replicated LD50 toxicity ranking in rat for five metals. This assay utilized the COPAS Biosort and propidium iodide (PI) as a fluorescent indicator of morbidity and mortality after 30-hour exposures. We found that chronic toxicity assays of 2-week treatment duration, followed by analysis of PI induced red fluorescence levels, produced less consistent results than the acute assays. However, other chronic toxicity endpoints were compound and concentration specific, including changes in vulval and gonadal morphology, intestinal thickness and integrity, and the presence of retained internal eggs in post-reproductive animals. Some of these endpoints reflect similar findings in mammals, indicating that measurements of morbidity and mortality in conjunction with morphology analyses in C. elegans may have the potential to predict mammalian toxic responses.
Keywords: alternative animal model, C. elegans, toxicity ranking, heavy metals, propidium iodide, liquid culture
1. Introduction
Toxicology studies are a vital step in ensuring the public safety. Safety studies for evaluating adverse effects of substances often utilize large numbers of rodents and are extremely expensive and time consuming. This has resulted in a backlog of over 10,000 compounds that are in need of prioritization for further testing (Dix et al., 2007). Through various laws, agreements, and memoranda of understanding (MOU), several government agencies have collaborated to outline the need for expedited development of fast, reliable alternative methods for screening potential toxins (Congress, 2000; National Research Council, 2000). A recent MOU between the National Institutes of Health, U.S. Environmental Protection Agency, and the U.S. FDA, stressed exploration of high-throughput assays and tests that utilize phylogenetically lower animal species such as fish and worms to reduce the cost and time of toxicity testing, and to prioritize large numbers of chemicals for further, more extensive toxicological evaluation (HHS/NIH et al., 2010).
One well studied model organism, the nematode Caenorhabditis elegans, with its small size, short lifecycle, and transparent body, has the potential to provide valuable toxicity data in a whole organism with the speed and lower expense ratios of in vitro testing (Boyd et al., 2007; Cole et al., 2004; Leung et al., 2008). As with any model system, C. elegans will only prove useful in toxicity screening if results are predictive of toxic responses in other organisms, yet very few studies have been done to evaluate correlations between responses in C. elegans and toxicity ranking in mammals (Boyd et al., 2010a). In one such study, ranking of median lethal concentration (LC50) values in C. elegans for eight metal salts paralleled ranking for rat median lethal dose (LD50) values, and the LC50 values from C. elegans were found to be equally predictive of relative toxicity in rats as LD50 values were from mice to rats (Williams and Dusenbery, 1988). Another study analyzing motility in adult C. elegans found that for fifteen organophosphate compounds, toxicity ranking order was significantly correlated to rat LD50s (Cole et al., 2004). Both of these studies utilized approximately 100 animals or less per condition and did not use methods that would lend themselves to scaling for high-throughput screening.
The COPAS™ Biosort (Union Biometrica) is a microfluidic device that automates the analysis, sorting, and dispensing of hundreds of C. elegans nematodes per minute. As each worm passes the sensor, axial length, extinction (a measure of optical density), and fluorescence excitation in three wavelength ranges can be evaluated (Pulak, 2006). Axial length and extinction increase as C. elegans hatch from eggs and develop through the four larval stages (L1-L4) into an adult (Sprando et al., 2009), and we have shown that COPAS measurements of growth can be used to significantly correlate toxicity ranking in C. elegans to rat LD50 ranking (Ferguson et al., 2010). In previously published reports of COPAS analyses of growth, various sophisticated methods of statistical analysis were utilized to evaluate the differences among treatment group data sets, each comprised of thousands of measurements (Boyd et al., 2010b; Boyd et al., 2009; Ferguson et al., 2010). One of the goals of this study was to evaluate the reliability of simpler methods of analyzing COPAS outputs. Additionally, in an effort to compare C. elegans data to rat LD50 ranking in a similar assay, we sought a means to measure C. elegans death that would lend itself to use in high-throughput techniques. Studies have been done using a fluorescent marker of death in C. elegans (Gill et al., 2003), but we found that the fluorescent dye used in these studies only identifies C. elegans that have died from heat shock. Thus, the objectives for this work were threefold: 1) to develop a COPAS method to quantitatively assess toxicity by measuring death in adult C. elegans using a dye that will fluoresce in worms that died from any cause, 2) to identify a simple, fast, and reliable method to analyze the resultant COPAS data output from thousands of worms per condition, and 3) to assess the usefulness of this method in ranking compounds based on their known toxicity.
2. Materials and Methods
2.1. C. elegans strains and culture
The C. elegans N2 Bristol strain used in these experiments was obtained from the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources. Worm cultures were maintained in vented tissue culture flasks in C. elegans habitation media (CeHM), an axenic liquid culture media containing C. elegans habitation reagent (CeHR) (Rao et al., 2005; Sprando et al., 2009) and nonfat cows’ milk, and maintained in incubators at 22°C on shakers set to 80 rpm. Synchronized cultures were obtained by bleaching gravid adult worms, collecting the eggs, and hatching the eggs overnight in M9 buffer (adapted from (Nass and Hamza, 2007)). At 22°C, cohorts reached mid-fourth larval stage (L4) between 54 and 56 hours after switching the hatchlings from M9 to CeHM. In all assays of synchronized cohorts, 25 μM 5-fluoro-2’-deoxyuridine (FUdR) was added to all tested cultures at 56 hours post hatchling feeding to prevent progeny production, and this time point was counted as day 0 of adulthood.
Dosing was accomplished by preparing metal solutions in distilled water at twice the desired final concentration; (CeHR) was also prepared in concentrated form and then mixed with dosing solution or water as previously described (Sprando et al., 2009). All CeHM-dosing mixtures tested neutral with pHydrion™ paper (Micro Essential Laboratory, Brooklyn, New York). For one-day trials of acute toxicity, 30-hour treatments began at day 4 of adulthood in CeHM or CeHR, as indicated. For two-week trials of chronic toxicity, treatment began at the 56 hour post-hatchling feeding time point, and CeHM containing 25 μM FUdR plus indicated concentrations of metals was changed every 3 to 4 days. All experimental animals were maintained at approximately 5 μL dosing mixture per worm. In 25 cm flasks, this provided a view of 10-30 worms per 4x-objective field. Rough estimates of live vs. dead fractions in treatment flasks were obtained by observing all worms in three fields. Unanaesthetized adult C. elegans that appeared healthy were not observed to be immobile at any time, therefore, worms that were immobile for 30 seconds were assumed dead.
2.2. Dye Testing
SYTOX green nucleic acid stain (Molecular Probes), was used according to the manufacturer’s instructions. For propidium iodide (PI) staining, 750 μL of 1 mg/mL PI was added to C. elegans in 10 mL CeHM without treatment metals added 17 to 24 hours before analysis. All cultures were incubated in CeHM without additives for at least 2 hours prior to analysis to flush excess PI from the intestinal tract of live worms.
2.3. Reagents
Cadmium chloride, copper chloride, mercury chloride, potassium chloride, sodium arsenite, PI, and FUdR were purchased from Sigma-Aldrich (St. Louis, MO). Stock solutions were prepared in filtered distilled water and further diluted for use.
2.4. Microscopy Image Analysis
Images of C. elegans were obtained using a Nikon ECLIPSE 80i microscope, Intensilight C-HGFI illuminator (Nikon Instruments, Melville, NY), and SPOT camera and software (SPOT Imaging Solutions, Sterling Heights, MI). Images were analyzed using SPOT and ImageJ (National Institutes of Health, USA) software. For presentation, phase contrast (oblique illumination) images were balanced for brightness and contrast and, when necessary, rotated for a consistent anterior-posterior, dorsal-ventral presentation format in Photoshop CS5. All fluorescence images within a single figure received identical brightness/contrast manipulations. All morphology analysis was done using images taken with a 10x objective of sodium azide anesthetized C. elegans held between a coverslip and a 2% agarose pad. Live vs. dead microscopy analyses were done without anesthetic. Death was assumed when no movement was detected over a 30 second observation period.
2.5. COPAS
COPAS sample pressure was calibrated using GP Control Particles (Union Biometrica, Holliston, MA) according to the manufacturer’s instructions. Briefly, with sample pressure at 4.95 instrument specific units, 15 to 20 GP Control Particles passed the sensors per second. This sample pressure was then maintained throughout the study. Live, unanaesthetized C. elegans were washed twice and then diluted in M9 buffer to obtain a maximum of 15 readings per second. A reading refers to the data obtained when an object passes the COPAS sensors. A minimum of 3,000 readings were obtained from each treatment sample. Each reading included red fluorescence, extinction (EXT), and the time of flight (TOF) information. Red fluorescence indicates the level of PI uptake. EXT measures the decrease in laser light when a particle or organism passed through the laser beam, giving an indication of optical density. TOF measures the amount of time the instrument microprocessor was detained in the analysis of a signal, giving a relative indication of the length of an object.
3. Results
3.1. Dye testing
Standard methods for determining death in C. elegans involve assessment of individual worms for response to light pressure from a thin platinum wire or eyelash probe, or assessment of small populations for movement using images in conjunction with tracking software. In order to utilize the COPAS to screen for the live/dead fraction in a population, we first needed to identify a universal fluorescent marker of death. SYTOX™ (Molecular Probes) has been successfully used with the COPAS to measure death in heat shocked populations of C. elegans (Gill et al., 2003). However, we found that SYTOX did not increase fluorescence in worms that had died from causes other than heat stress (data not shown), limiting its usefulness. PI is a red fluorescent DNA intercalating dye that is excluded from live cells in culture. We found that incubation with PI caused worms that had died of heat shock (Fig. 1A–B), sodium arsenite (NaAs) toxicity (Fig. 1C–D), and old age (Fig 1E–F) to fluoresce brightly. Additionally, only very low levels of staining were observed in live, healthy worms. Together, these data indicate that PI can be used as a marker of death from a variety of causes.
Figure 1:
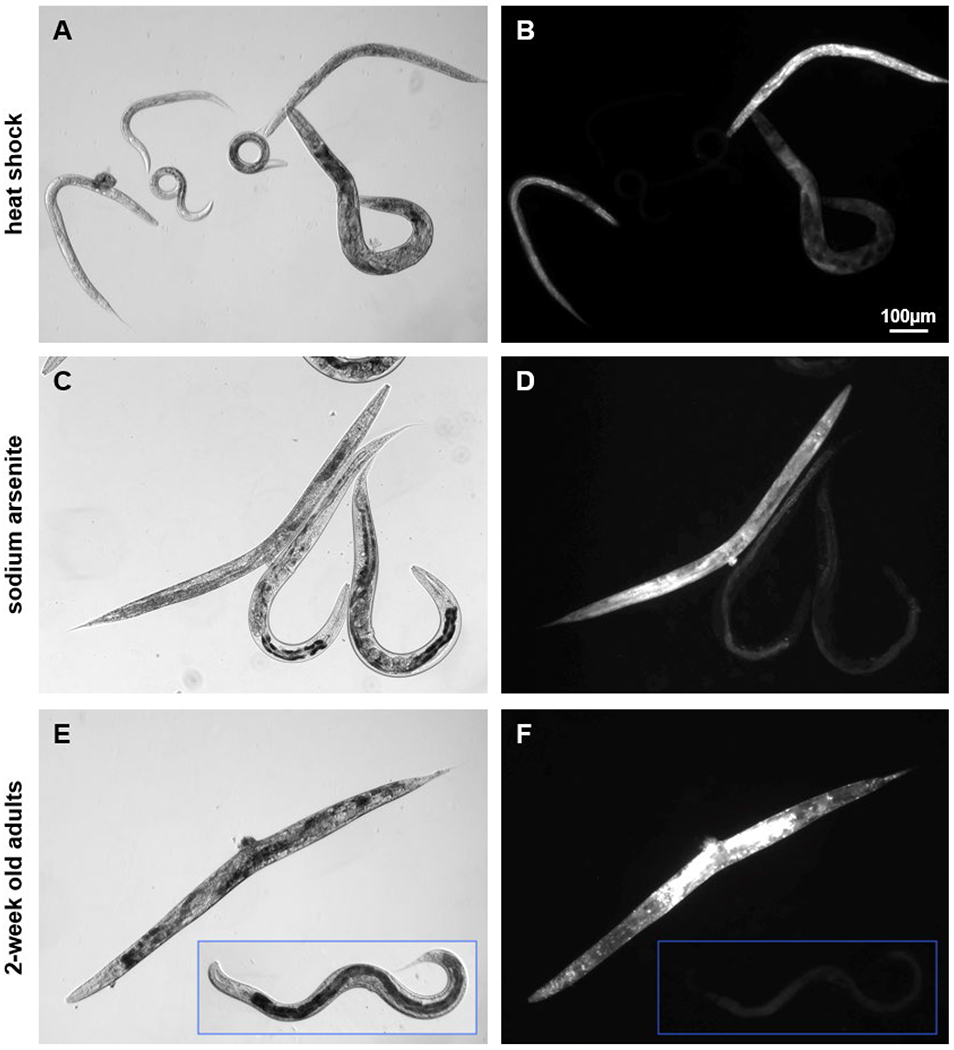
C. elegans that died of heat shock, sodium arsenite exposure, and old age, fluoresced after propidium iodide treatment.
(A) Mixed age C. elegans after a one-hour heat shock at 37°C followed by 18 hours incubation with PI in CeHM, plus an additional 2 hours in CeHM to allow for intestinal flushing. (B) 100ms exposure shows that three mobile worms (A, center) did not fluoresce, while three immobile (dead) worms had varying levels of fluorescence intensity. (C) Age synchronized adult C. elegans treated with 65 ppm NaAs for 30 hours followed by PI treatment as above. (D) 100ms exposure shows bright fluorescence in the single dead worm on the left, but not in the live worms. (E) Two adult day-14 C. elegans maintained post-L4 in CeHM containing FUdR imaged from the same slide. (F) Image and inset are both 1s exposures of the worms pictured in (E).
Levels of PI fluorescence in C. elegans determined to be dead by observed immobility varied greatly (Fig. 1B). In order to determine a PI red fluorescence level cut off for toxicity, we utilized the COPAS sorting feature on two week old adults. We found that when sorting for PI treated C. elegans with red fluorescence values of up to 50 instrument specific units, the COPAS only selected live, healthy worms (Fig. 2A–B). COPAS PI red fluorescence values above 50 (CRF>50) were associated with dead worms, or live worms with structural damage. A small fraction of control animals were observed to have gonadal lesions at 2 weeks of adulthood, but only those live animals that also had vulvar abnormalities were observed to have PI fluorescence in gonadal tissues (Fig. 2C–D). Sorting for COPAS red fluorescence values over 100 selected live animals with a great deal of structural damage or degradation (Fig. 2E–F) and dead animals (Fig. 2G–H). In further analyses, we therefore utilized the fraction of COPAS readings with red fluorescence values greater than 50 as a measure of morbidity and mortality in each sample.
Figure 2:
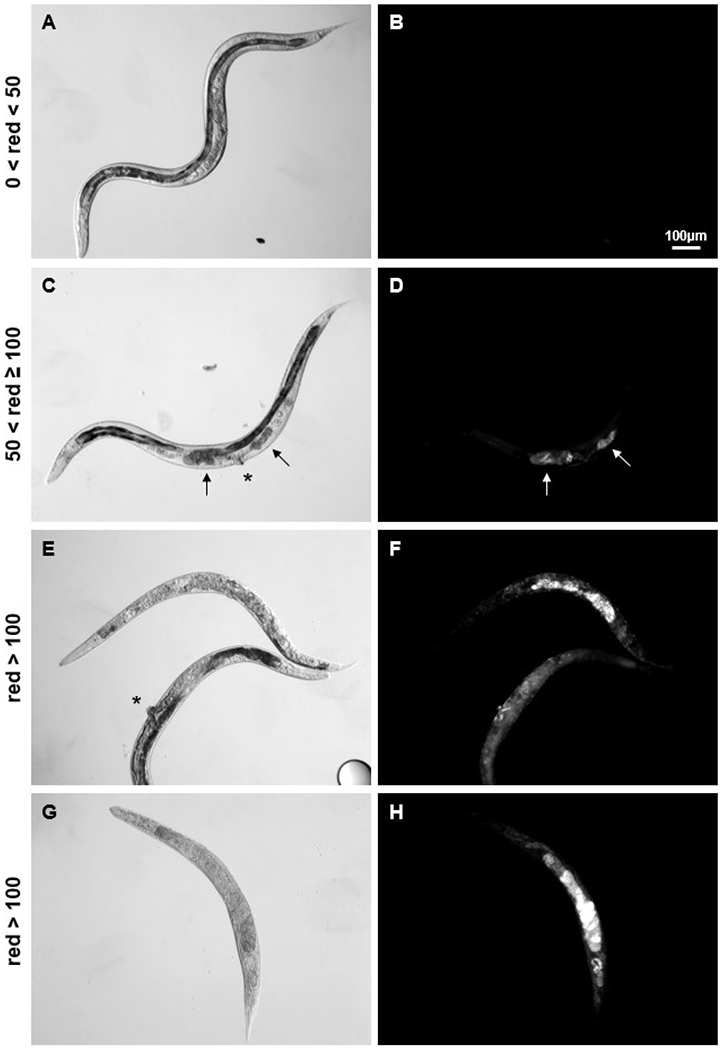
COPAS PI red fluorescence values above 50 (instrument specific units) corresponded to damaged and dead C. elegans.
Using the sorting feature of the COPAS on adult day-14 C. elegans, worms were selected for the red fluorescence values indicated at left. (A-B) COPAS sorting for units with red fluorescence readings of 50 and below selected apparently healthy, mobile worms. The majority had dark anterior intestines which nearly fill the body cavity, and normal age-associated gonadal atrophy. (C-D) PI staining of dead tissue within gonadal lesions (arrows) is apparent in live worms with vulvar abnormalities (asterisk). (E-F) Pictured worms are alive but have severe internal degradation and no longer move normally. (G-H) Dead worms do not necessarily fluoresce more brightly than very sick worms. Fluorescence images are all 100ms exposures with identical brightness and contrast manipulations for presentation.
3.2. Refining COPAS data analysis
The strength of the COPAS is that it can analyze hundreds of C. elegans nematodes per minute. Technical difficulties with the system include: 1. inclusion of background noise as a low diagonal line of readings in recorded data (Fig. 3A, lower right quadrant), 2. eggs and other debris with low EXT and TOF values are not distinguished from worms (Fig. 3A, lower left corner), and 3. overlapping worms can pass the sensors resulting in a single reading with artificially high TOF and EXT values (Fig. 3A, upper right quadrant). Small changes in conditions such as temperature, handling and age of salt solutions, efficiency of aspiration during wash steps, and worm concentration in analyzed samples can have large effects on the number of these artifactual readings. Therefore, defined limits are required to exclude these readings from analyses.
Figure 3:
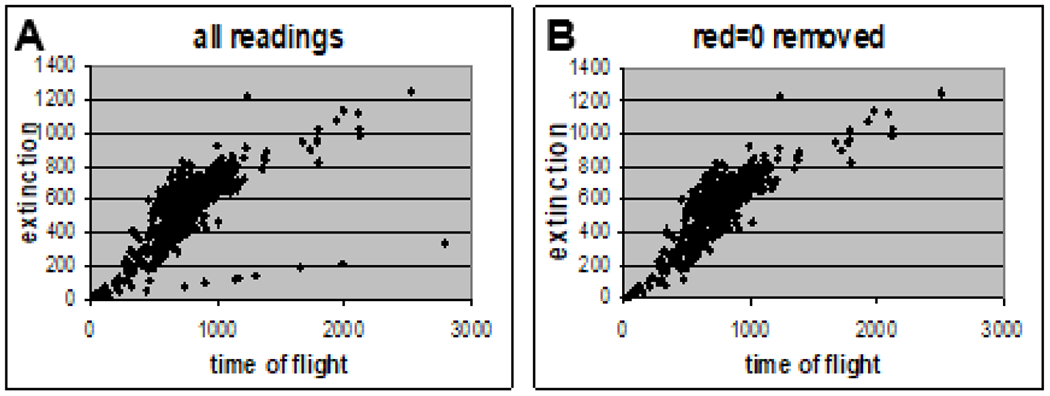
COPAS analysis: distinguishing data from artifacts.
(A) All readings from age synchronized, PI treated adult day-5 C. elegans, plotted for EXT vs. TOF. (B) The same data after readings with red fluorescence values of zero were removed from the data set.
Using COPAS data from PI treated control C. elegans in scatter charts to plot TOF vs. EXT, we noted that all of the data points in the low “line” of background noise had red fluorescence values of zero. We then utilized the COPAS sorting feature on adult day-14 C. elegans treated with PI to select for units with red values of zero to one and found that only media was selected (data not shown). We therefore excluded readings with red values of zero from data analyses (Fig. 3B).
While average TOF values are excellent indications of differences in axial length between groups, individual TOF values do not correlate precisely to length. This may be due to some worms passing the sensors sideways or folded, or to small changes in pressure and flow which can alter the time of flight for two worms of the same length. However, we found that sorting for units with TOF values below 200 did not select whole adult worms. Additionally, sorting for five units with TOF values above 1500 resulted in the selection of ten worms, indicating that each reading with a TOF value above 1500 was for two worms together. Therefore, by utilizing only TOF values between 200 and 1500, COPAS data can be limited to the size of single adult worms.
3.3. Acute sodium arsenite toxicity is detected by PI fluorescence
The CERCLA Priority List of Hazardous Substances, maintained jointly by the Agency for Toxic Substances and Disease Registry and the Environmental Protection Agency, ranks arsenic first among substances that pose significant threats to human health (ATSDR and DHHS, 2007). Sodium arsenite (NaAs) is toxic to C. elegans, with an approximate survival rate of 70% after exposure to 195 ppm for 24 hours on young adult worms (Liao et al., 2010), and as little as 7 ppm altering larval growth rates (Liao et al., 2010; Sprando et al., 2009). Using NaAs as a model toxicant with 195 ppm as an intermediate dose, we tested whether COPAS analysis would correlate red fluorescence values from PI with NaAs concentration. After a 30-hour treatment in CeHM using age synchronized adult day-4 C. elegans, we found that increasing concentrations of NaAs from zero to 585 ppm did not induce changes in observed size or optical density (Fig. 4A&C), which was reflected in similar graphs of COPAS EXT vs. TOF values for control and 585 ppm NaAs treated populations (Fig. 4E–F). When looking at red fluorescence levels however, the differences between the four treatment groups of 0, 65 ppm, 195 ppm and 585 ppm NaAs were marked. For the control group, only two out of every hundred worms had a CRF>50 (Fig. 4B&G). The fraction of the 65 and 195 ppm NaAs treatment groups with CRF>50 increased to 0.07 and 0.22 respectively (Fig. 4I). At 585 ppm NaAs, the fraction of the treated population with CRF>50 increased to 0.96 (Fig. 4D, H–I). These data demonstrate that PI fluorescence can be used to assess acute toxicity and dose response in C. elegans.
Figure 4:
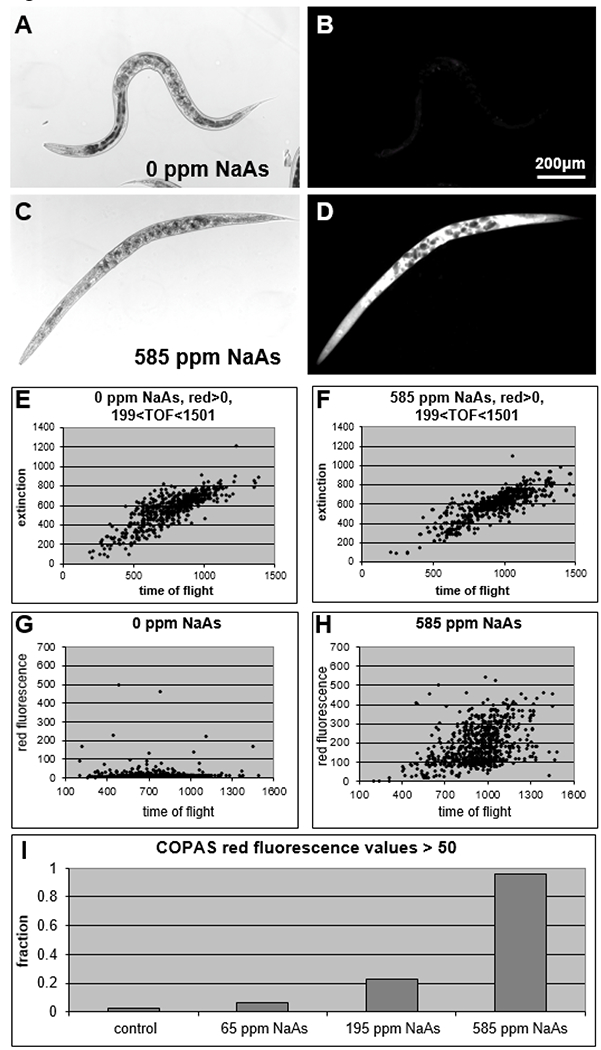
Acute sodium arsenite toxicity was detected by COPAS PI red fluorescence values.
(A) Control adult day-5 C. elegans. (B) 100ms exposure of the same field. (C-D) After 30 hours in 585 ppm NaAs followed by PI staining, most worms were dead and fluoresced brightly. (E) The same data as shown in Fig. 3A, with readings removed that had zero for red fluorescence and/or TOF values of less than 200 or greater than 1500. (F) In the 585 ppm NaAs treated group, readings for EXT and TOF were similar to controls. (G) COPAS values of red fluorescence plotted against TOF show that PI staining was generally low in controls, and (H) increased dramatically after treatment with 585 ppm NaAs. (I) Data plotted as the fraction of each population with CRF>50.
3.4. Acute toxicity ranking in adult C. elegans using PI and COPAS analysis mimics rat LD50 ranking.
To determine if PI fluorescence as measured by the COPAS can be used with adult C. elegans to rank toxicity, we selected five metal salts, cadmium chloride (CdCl2), copper chloride (CuCl2), mercury chloride (HgCl2), potassium chloride (KCl), and sodium arsenite (NaAs), for further testing using a 30-hour treatment on day-4 adults. These compounds have rat LD50s ranging from 1 to 26,000 mg/kg (Table 1). M9, a buffered salt solution commonly used in C. elegans manipulations, when combined with high concentrations of CdCl2 or CuCl2, induced the formation of apparently crystalline precipitates on the order of 10 to 100 gm in diameter. High concentrations of CdCl2 in CeHM induced formation of precipitated matter in clumps of 1 mm or more. Therefore, this 30-hour assay was performed in CeHR. In three separate trials, at all concentrations tested, nearly all of HgCl2 treated C. elegans had CRF>50, making HgCl2 the most toxic compound tested in this assay (Fig. 5A). At 1000 and 500 ppm, NaAs also killed most of the worms, with both concentrations producing CRF>50 in a minimum 0.98 of the treated populations. A dose response was seen at the lower NaAs concentrations, with 250 and 125 ppm giving CRF>50 fractions of 0.89 +/−0.01 (mean +/−SD) and 0.22 +/−0.05 respectively. CdCl2 treatment induced a clear dose response at all tested concentrations, with 1000, 500, 250 and 125 ppm inducing CRF>50 fractions of 0.62 +/−0.08, 0.40 +/−0.04, 0.23 +/−0.06, and 0.15 +/− 0.03 respectively. At 1000 ppm CuCl2, the CRF>50 fraction was 0.36 (+/−16). Lower CuCl2 concentrations had CRF>50 fractions between 0.05 and 0.07 as did all KCl treatment groups and controls. Taken together, these data indicate a ranking in C. elegans from most toxic to least toxic of HgCl2>NaAs>CdCl2>CuCl2>KCl. This ranking mimics the toxicity ranking for these compounds in rat (Table 1).
Table 1:
Toxicity ranking as measured by rat oral LD50s (ScienceLab, 2010)
| Test Compound | Rat Oral LD50 (mg/kg) |
|---|---|
| Mercuric Chloride | 1 |
| Sodium Arsenite | 41 |
| Cadmium Chloride | 88 |
| Copper Chloride | 584 |
| Potassium Chloride | 2,600 |
Figure 5:
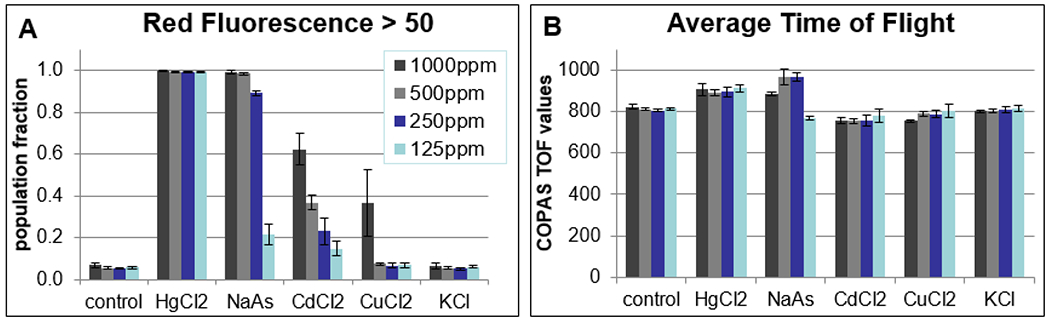
30-hour treatments of adult C. elegans reproduce mammalian toxicity ranking.
(A) The fraction of each population with CRF>50 at decreasing concentrations of 5 different metal salts. (B) In acute toxicity assays, some average TOF values increase for highly toxic compounds, possibly due to rigor mortis. Values and error bars reflect the mean and standard deviation from three trials, with a minimum of 3000 readings for each condition in each trial.
As expected, TOF and EXT values did not change significantly for most treatments in this acute toxicity assay in adult C. elegans, with the exception of slightly higher TOF values for some conditions with the highest CRF>50 values (Fig. 5B). This is likely due to rigor mortis preventing worms from passing the sensors bent or folded as would happen with a proportion of live, healthy animals or those dead long enough to start to degrade.
3.5. Evaluation of chronic toxicity testing with metal chlorides
The symptoms of acute and chronic poisoning with the same compound differ, as do recommended tests and treatments (Lussier et al., 1985; Ratnaike, 2003). Additionally, relative toxicity ranking differs among species used as environmental indicators of contamination (Lussier et al., 1985). Having demonstrated the utility of screening C. elegans using the COPAS with PI to measure acute toxicity, we next wanted to test this model’s effectiveness in measuring chronic toxicity. Depending on environmental conditions such as temperature and nutrient availability, wild type C. elegans have a life span of approximately three to five weeks. We therefore conducted a preliminary two-week chronic toxicity trial using metal chlorides HgCl2, CdCl2, CuCl2 and KCl at four concentrations in 4-fold steps from 7.8 to 500 ppm in CeHM to determine concentrations that would maximally affect adult C. elegans without killing all of them before the end of the test.
One limitation of COPAS analysis of PI fluorescence levels at a single two-week time point was that C. elegans that had been dead for extended periods did not fluoresce as brightly as those that had died recently (data not shown). Additionally, differences in the efficiency of centrifugation between live and dead animals favors recovery of live animals in wash steps that are required for changing media and COPAS analysis. Therefore, samples in which all animals were dead in 11 days or less were not analyzed.
HgCl2 at 500 and 125 ppm killed all adult C. elegans within 2 days. About half of the 500 ppm CdCl2 treated animals were dead by day 8 and all were dead by day 11. At least half of the animals at 125 ppm CdCl2 were dead by day 11 (data not shown). Unfortunately, the sample with 125 ppm CdCl2 could not be analyzed, as CdCl2 at concentrations above 62.5 ppm caused elements in CeHM to agglomerate, preventing the wash steps that are required prior to COPAS analysis. As dietary deprivation in post-larval C. elegans extends rather than shortens lifespan (Lee et al., 2006; Szewczyk et al., 2006), death in samples with agglomerated matter was likely caused by cadmium toxicosis rather than by reduced nutrient availability. CuCl2 at 500 ppm also killed all of the animals by day 11, however at 125 ppm CuCl2 approximately 80 to 90% of animals were observed to still be moving on day 11. By visual inspection, KCl did not have an apparent effect at any tested concentration.
Using the four controls in this experiment to determine a baseline of variability from sample to sample within the same experiment, we found the fractional population with CRF>50 varied from 0.06 to 0.07, average EXT from 809 to 848, and average TOF from 914 to 969 (Table 2). COPAS values for 500 ppm KCl fell within the range or very close to those for controls, indicating that it is not toxic to C. elegans at tested concentrations. As CeHR contains over 5000 ppm potassium in the form of potassium citrate and potassium phosphate, this finding is not surprising.
Table 2:
COPAS measurements on wild type C. elegans after a 2-week treatment with metal chlorides and subsequent PI staining.
| Compound | ppm | na | CRF>50b | TOFc | st. dev | EXTC | st. dev |
|---|---|---|---|---|---|---|---|
| HgCl2 | 0 | 2887 | 0.06 | 969 | 189 | 848 | 148 |
| HgCl2 | 7.8 | 2912 | 0.16 | 981 | 186 | 825 | 147 |
| HgCl2 | 31.25 | 2934 | 0.81 | 815 | 195 | 583 | 134 |
| HgCl2 | 125 | d | |||||
| HgCl2 | 500 | d | |||||
| CdCl2 | 0 | 2960 | 0.06 | 914 | 205 | 809 | 153 |
| CdCl2 | 7.8 | 2932 | 0.13 | 940 | 157 | 683 | 118 |
| CdCl2 | 31.25 | 2992 | 0.66 | 681 | 167 | 449 | 106 |
| CdCl2 | 125 | e | |||||
| CdCl2 | 500 | d | |||||
| CuCl2 | 0 | 2856 | 0.07 | 949 | 194 | 840 | 160 |
| CuCl2 | 7.8 | 2765 | 0.11 | 952 | 229 | 820 | 203 |
| CuCl2 | 31.25 | 2850 | 0.19 | 968 | 180 | 822 | 150 |
| CuCl2 | 125 | 2935 | 0.56 | 683 | 184 | 496 | 134 |
| CuCl2 | 500 | d | |||||
| KCl | 0 | 2920 | 0.07 | 942 | 192 | 829 | 147 |
| KCl | 500 | 2939 | 0.06 | 899 | 196 | 816 | 143 |
n represents the number of readings with red values greater than zero and TOF values between 199 and 1501 for each condition analyzed by COPAS in a single experiment
the fraction of worms in each sample with a COPAS red fluorescence value from PI greater than 50 (instrument specific units)
the average and standard deviation of EXT and TOF values for each sample condition
all animals were dead before the experiment was completed and were not analyzed by COPAS
CdCl2 induced agglomeration of media components at high concentrations. While some animals were still moving after two weeks at 125 ppm CdCl2, they could not be separated from the media by centrifugation and were not analyzed.
At 7.8 ppm, the most striking difference in COPAS values among tested compounds was for CdCl2 with a reduced average EXT, which corresponded to observed intestinal thinning in this treatment group (Fig. 6A–B). Intestinal abnormalities, including disrupted cytosomes and shortened microvilli, have been reported in C. elegans treated chronically with as little as 1 ppm CdCl2 (Popham and Webster, 1979). In several other trials not reported here, we have found that low EXT values correspond with thinning or other abnormalities of the intestine, indicating that for adult C. elegans grown in liquid culture, COPAS EXT values have the potential to be used as an indication of intestinal health.
Figure 6: 2-week treatments with metal chlorides induce morphologic changes in adult C. elegans.
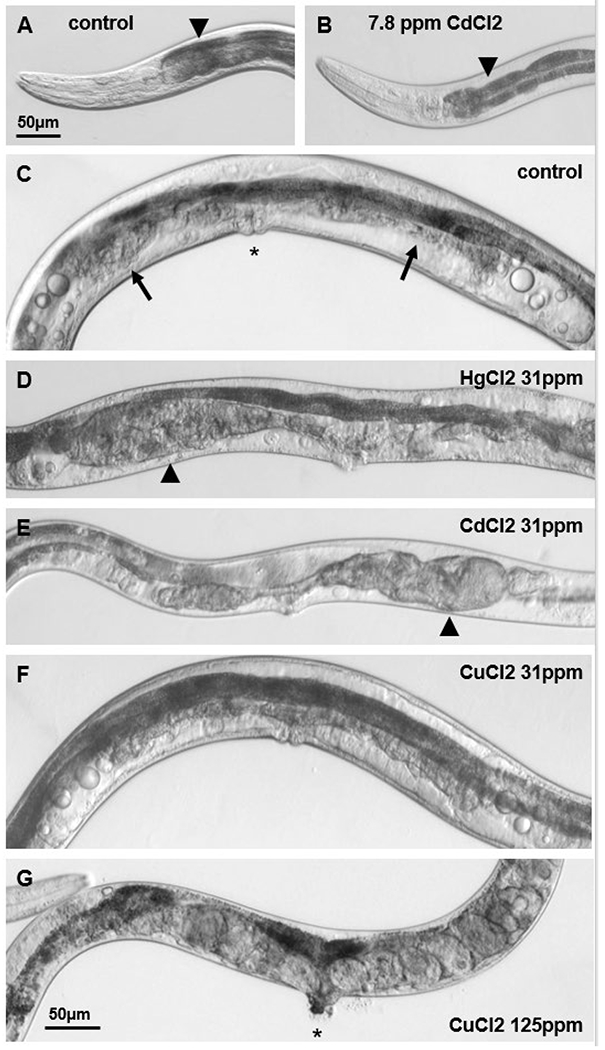
(A) In most adult day-14 C. elegans from control groups, the anterior intestine (arrowhead) is dark, relatively smooth, and nearly fills the body. Scale bar in (A) is for images A & B. (B) Representative image demonstrates that anterior intestines were thinner in the group treated with 7.8 ppm CdCl2 for two weeks. (C) Image of control FUdR-treated C. elegans shows intact vulva (asterisk), normal age-associated atrophy of the uterus (arrows), and oocytic material visible as circles at the sides of the image. (D) Vulvar abnormalities and gonadal lesions (arrowheads) were frequently observed in the 31.25 ppm HgCl2 treatment group. (E) 31.25 ppm CdCl2 also induced vulvar and gonadal changes. Thin, pale intestines correspond to low COPAS EXT values for this group. (F) The majority of C. elegans treated with 31.25 ppm CuCl2 for two weeks appeared similar to controls. (E) Vulvar abnormalities (asterisk) and internal eggs were frequent in the 125 ppm CuCl2 treatment group. Scale bar in (G) is for images C-G. All images presented are of live adult day-14 C. elegans.
At 31.25 ppm, HgCl2 had the greatest effect on fractional CRF>50 values (Table 2) and by microscopy observation, most HgCl2 treated worms were dead at this concentration. All CdCl2 treated worms observed by microscopy appeared small and pale at this concentration (Fig. 6E), and this was reflected in low TOF and EXT values. The fractional CRF>50 for HgCl2, CdCl2, and CuCl2 was 0.81, 0.66, and 0.19 respectively. The fractional CRF>50 for 500 ppm KCl was 0.06, therefore the toxicity ranking in this initial 2-week trial was HgCl2>CdCl2>CuCl2>KCl.
3.6. Morphological observations subsequent to chronic heavy metal exposure
Among the control and 31.25 ppm CuCl2 treatment groups after two weeks of treatment, encapsulated gonadal lesions were observed in 3 of 20 and 2 of 11 respectively, with the remainder showing normal gonadal atrophy (Fig. 6C&F). In contrast, among the 31.25 ppm HgCl2 and CdCl2 treatment groups, 9 of 20 and 17 of 20 were observed to have encapsulated gonadal lesions, respectively (Fig. 6D&E). Thin but intact intestines were observed in 3 of 20 controls, and 4 of 11 from the 31.25 ppm CuCl2 treatment group with the remainder having smoothly outlined anterior intestines which nearly filled the body cavity, while thin or apparently degraded intestines were observed in 11 of 20 and 18 of 20 from the 31.25 ppm HgCl2 and CdCl2 treatment groups, respectively. These intestinal differences were reflected in COPAS EXT values for each group (Table 2). The 2-week treatment with 125 ppm CuCl2 also induced a very low average EXT, and correspondingly, 21 out of 25 analyzed by light microscopy from this group had anterior intestines that appeared granular or degraded (far left, Fig. 6G). 8 of 25 from the 125 ppm CuCl2 treatment group had encapsulated gonadal lesions, and 17 had protruding vulva. Consistent with a previous report of egg-laying defects observed at high CuCl2 concentration (Calafato et al., 2008), many animals treated with 125 ppm CuCl2 contained internal eggs, with 16 of 25 containing an average of 7 visible eggs (Fig. 6G). In comparison, four to six visible eggs were observed in 3 of 20 from the 31.25 ppm CdCl2, and in only 1 of 20 from the 31.25 ppm HgCl2 treatment groups, while no internal eggs were observed in the control or 31.25 ppm CuCl2 groups.
3.7. In C. elegans, evaluations of chronic toxicity using COPAS data from a single concentration at a single two-week time point produce inconsistent results
For further chronic toxicity testing, we selected a single concentration of 31.25 ppm. In four trials, HgCl2 consistently produced the highest CRF>50, but TOF and EXT values were highly variable (Fig. 7). In contrast, while TOF and EXT values for CuCl2 were consistently close to control values, CRF>50 fractions varied from control to CdCl2 levels. COPAS values for CdCl2 fell between HgCl2 and CuCl2 values for CRF>50, TOF and EXT in most, but not all trials. Every effort was made to maintain consistent conditions and handling, however, small changes in temperature can have large effects on C. elegans lifespan. Additionally, C. elegans that have been dead for several days do not settle during centrifugation steps with the same efficiency as live and recently dead animals, therefore media changes and wash steps may be selective. We conclude that COPAS analysis of PI fluorescence in C. elegans at a single time point after a two-week treatment begun at the fourth larval stage is a less reliable method to rank toxicity than 30-hour treatments on adults.
Figure 7:
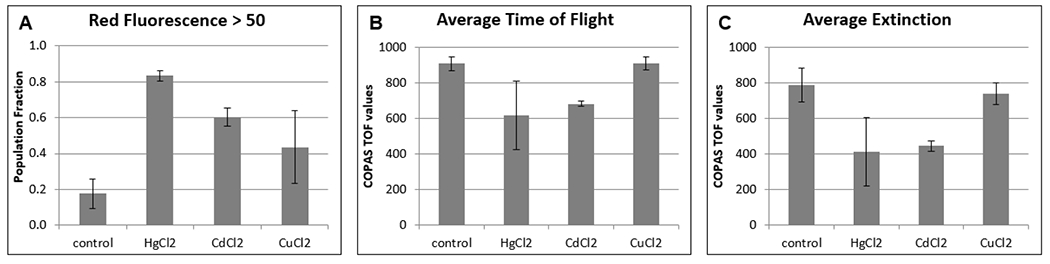
Chronic toxicity trials with three metal chlorides produced inconsistent results from COPAS data obtained at a single time-point after two-week treatments at 31.25 ppm.
(A) The average value of the fraction of the population that is dead or dying, as represented by COPAS red fluorescence values greater than 50. (B) The average TOF for each condition. (C) The average EXT for each condition. Data is from four 2-week trials with heavy metal chlorides at 31.25 ppm, with approximately 3,000 animals analyzed per condition per trial. Error bars represent the standard deviation of the average over the four trials.
4. Discussion
The three Rs strategy of alternatives to animal testing involves reduction in the use of higher animals, refinement of current techniques, and ultimately replacement with alternative methods (Russell and Burch, 1959). To further these goals, a collaborative effort to develop new methods of toxicity analysis was begun in 2008 by the National Institutes of Environmental Health Sciences, the National Human Genome Research Institute, and the U.S. Environmental Protection Agency, and joined by the U.S. Food and Drug Administration in 2010. One of the central tenets of this collaboration, informally called Tox21, is the exploration of novel high throughput screening assays and tests using phylogenetically lower animal species such as C. elegans.
In developing replacements for testing in higher animals, it is essential to define the limits within which alternative animal models have the potential to provide human relevant results. Advantages of utilizing C. elegans as a model organism include low maintenance liquid culture protocols, a rapid generation time of approximately three days, hermaphroditic reproduction which can quickly produce vast numbers of near clonal organisms, and clear external structures which allow microscopic observation of internal organs in live animals. For these reasons, C. elegans offers the convenience and speed of in vitro type laboratory techniques in a whole organism complete with distinct yet communicating neuronal, digestive, and reproductive tissues. Additionally, C. elegans was the first multicellular organism to have its complete genome sequenced, and genetic analysis indicates a high degree of conservation with mammals (Bargmann, 1998; Consortium, 1998). Disadvantages of using C. elegans for toxicity screening include a lack of many structures common to higher organisms, such as respiratory and cardiovascular systems. Adult somatic cells are post-mitotic, therefore C. elegans is not likely to be a good model for the assessment of carcinogens. Additionally, C. elegans has a tough collagenous cuticle which protects it from the environment, limiting its use to the study of potential toxins that can be orally ingested.
Wild type C. elegans hermaphrodites lay approximately 275 eggs by self-fertilization (Byerly et al., 1976). There are several methods for maintaining a C. elegans experimental cohort without progeny, and each has disadvantages. One method is to individually transfer adult worms away from eggs and hatchlings to a fresh environment daily during the reproductive period (Johnson and Wood, 1982). This process is time consuming, and manual manipulations carry the potential for structural damage to the worms. Another method utilizes temperature sensitive mutant strains that are fertile at permissive temperatures and infertile at non-permissive temperatures, but apparently wild type in all other respects (Nelson et al., 1978). Strain stocks are maintained at permissive temperatures while experimental cohorts are transferred to non-permissive temperatures during development so that testing is carried out on sterile adults. However, the low yet significant rate of approximately 0.1 progeny per adult raised at the non-permissive temperature (Mitchell et al., 1979) is unacceptable in liquid culture experiments with thousands of worms per flask. The thymidylate synthetase inhibitor FUdR (Cohen et al., 1958) completely blocks embryogenesis in C. elegans grown in axenic liquid culture, and has been reported to have no significant effects on the rates of growth, aging, or pharyngeal pumping when used at 25 μM on wild type worms (Gandhi et al., 1980). Recently however, the widespread use of FUdR in lifespan analyses of C. elegans has been called into question due to studies which have shown that FUdR at concentrations of 50 μM and above in agar based media can significantly increase the lifespan of specific genetic mutant strains (Aitlhadj and Sturzenbaum, 2010; Van Raamsdonk and Hekimi, 2011), and that FUdR is protective in some models of stress (Suzanne Angeli and colleagues, personal communication). We selected FUdR for synchrony maintenance due to its widespread use and ease of application in large scale experiments but acknowledge that it may have metabolic effects with the potential to interact with specific toxic responses.
We found that PI can be used as an indicator of morbidity and mortality resulting from a variety of causes, in both FUdR synchronized and mixed age populations. Given that the intensity of red fluorescence varied considerably among dead and dying worms, with structurally damaged live worms and those dead for intermediate periods fluorescing more brightly than those recently and long dead, we found that measuring the fraction of C. elegans with red fluorescence from PI of greater than 50 COPAS specific units provided a measure of morbidity and mortality within a treatment population. Our results with increasing concentrations of NaAs indicate that PI used in conjunction with the COPAS can provide a sensitive method to measure acute toxicity in C. elegans. We also demonstrated that population fractions with CRF>50 after a 30-hour treatment using four different concentrations of five metals with a wide range of rat LD50s on adult C. elegans could rank these metals the same order as they are ranked in mammals, with nearly identical results from three separate trials. Further testing is required to determine if finer distinctions in toxicity ranking can be made with this method.
Results from analyses of PI staining levels by COPAS from chronic treatments were less consistent than those from the 30-hour assays. While the average fractional populations with CRF>50 ranked the three metal chlorides we studied by this method in the order HgCl2>CdCl2>CuCl2, there was a good deal of variability from one trial to the next, especially for copper. Part of this variability may be due to the loss of dead animals during centrifugation steps required for the two to three times weekly changes in media. Since small changes in temperature result in significant changes in C. elegans lifespan, it may be that differences in ambient temperature during the short time that worms were removed from the incubator for manipulations and examination resulted in these differences from one experiment to the next. It may be that chronic toxicity assays in C. elegans that measure CRF>50 could provide consistent results if certain experimental parameters were altered from the ones we used here, such as multiple concentrations, multiple time-points for COPAS analysis, and/or a later initiation of treatment, as we used in the acute toxicity assays.
Our morphology analyses from chronic toxicity trials did provide potentially interesting mammalian-correlative data. For the more toxic heavy metal chlorides in our two-week study, HgCl2 and CdCl2 at 31.25 ppm, abnormal intestinal, gonadal, and vulvar morphology were indicators of chronic toxicity, while visible oocytic material was associated with health in adult day-14 control and CuCl2 treated C. elegans. Cadmium toxicity is associated with histopathology of the intestine across phyla, including cellular necrosis and abnormalities of the villi in mice (Valberg et al., 1977), Paneth cell vacuolation and abnormalities of the villi in rats (Phillpotts, 1986), cellular necrosis and vacuolation of mucosa and enterocytes in fish (Kruatrachue et al., 2003), and abnormalities of cytosomes and microvilli in C. elegans (Popham and Webster, 1979). After a two-week treatment begun at the fourth larval stage, we observed intestinal thinning in C. elegans treated with 7.8 and 31.25 ppm CdCl2, and these findings were corroborated by low COPAS EXT values. Copper is known to inhibit reproduction in water fleas (Dave, 1984) and sheep (Murawski et al., 2006), and to inhibit egg laying in C. elegans (Calafato et al., 2008). We observed high levels of retained eggs in 125 ppm CuCl2 chronically treated C. elegans. Taken together, these data indicate that C. elegans may be a useful predictor of some toxic effects in mammals.
Measuring growth rates, frequency of protruding vulva, brood size, and levels of internal hatching are established methods of determining toxicity in C. elegans (Anderson et al., 2001; Boyd et al., 2010a; Calafato et al., 2008; Sprando et al., 2009). To this list, we add analysis of intestinal width and integrity, gonadal lesions, internal eggs, and lack of visible oocytic material in post-reproductive FUdR treated C. elegans as measures of chronic toxicity. Some morphological differences were reflected in COPAS values, as in the observed correlation between intestinal thinning or disruption and low EXT, while others such as gonadal lesions in the absence of vulvar disruption were not, stressing the importance of visual analysis, especially when analyzing potential toxins with unknown effects. Thus, toxicity screens using C. elegans should include morphology analysis in addition to methods such as the one detailed here, metabolic testing, and/or gene expression analyses.
In summary, we have developed a method to quantitatively assess toxicity by measuring morbidity and mortality in C. elegans using axenic liquid culture, a fluorescent dye, and the COPAS. We assessed the usefulness of this method in ranking a small number of compounds of varied but known toxicity. We found that 30-hour assays replicated rat LD50 toxicity ranking for five metals and were highly reproducible. While chronic toxicity assays with this method were less consistent, interesting morphology observations provided potentially mammalian-relevant data. We are presently evaluating the usefulness of this method in assessing the toxicity of a number of different chemical compounds including other heavy metals, mycotoxins and sea food toxins. It is our hope that, using C. elegans as an experimental model, we will be able to develop a rapid and low-cost screen to identify potentially toxic compounds. The identification of these potentially hazardous compounds would facilitate a more rational choice for further in vivo testing which would ultimately have the potential to result in reduced toxicity testing in mammals.
Highlights:
Propidium iodide can be used as a fluorescent marker of morbidity and mortality in toxicity assays using C. elegans
Toxicity screen with C. elegans, PI and COPAS demonstrates the potential of this model to predict mammalian outcomes
C. elegans morphology analyses in conjunction with other types of testing can increase the sensitivity of toxicity screens
Acknowledgements
Funding Source: United States Food and Drug Administration/Center for Food Safety and Applied Nutrition/Office of Applied Research and Safety Assessment/Division of Toxicology. The authors would like to thank Mr. Thomas Black and Mr. Michael Scott for skilled technical assistance and Dr. Jeffrey Yourick for helpful suggestions in manuscript preparation.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References:
- Aitlhadj L, and Sturzenbaum SR. 2010. The use of FUdR can cause prolonged longevity in mutant nematodes. Mech Ageing Dev. 131:364–365. [DOI] [PubMed] [Google Scholar]
- Anderson GL, Boyd WA, and Williams PL. 2001. Assessment of sublethal endpoints for toxicity testing with the nematode Caenorhabditis elegans. Environ Toxicol Chem. 20:833–838. [PubMed] [Google Scholar]
- ATSDR, and U.S. DHHS. 2007. CERCLA Priority List of Hazardous Substances. Agency for Toxic Substances and Disease Registry. [Google Scholar]
- Bargmann CI 1998. Neurobiology of the Caenorhabditis elegans genome. Science. 282:2028–2033. [DOI] [PubMed] [Google Scholar]
- Boyd WA, McBride SJ, and Freedman JH. 2007. Effects of genetic mutations and chemical exposures on Caenorhabditis elegans feeding: evaluation of a novel, high-throughput screening assay. PLoS One. 2:e1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd WA, McBride SJ, Rice JR, Snyder DW, and Freedman JH. 2010a. A high-throughput method for assessing chemical toxicity using a Caenorhabditis elegans reproduction assay. Toxicol Appl Pharmacol. 245:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd WA, Smith MV, Kissling GE, and Freedman JH. 2010b. Medium- and high-throughput screening of neurotoxicants using C. elegans. Neurotoxicol Teratol. 32:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd WA, Smith MV, Kissling GE, Rice JR, Snyder DW, Portier CJ, and Freedman JH. 2009. Application of a mathematical model to describe the effects of chlorpyrifos on Caenorhabditis elegans development. PLoS One. 4:e7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L, Cassada RC, and Russell RL. 1976. The life cycle of the nematode Caenorhabditis elegans. I. Wild-type growth and reproduction. Dev Biol. 51:23–33. [DOI] [PubMed] [Google Scholar]
- Calafato S, Swain S, Hughes S, Kille P, and Sturzenbaum SR. 2008. Knock down of Caenorhabditis elegans cutc-1 exacerbates the sensitivity toward high levels of copper. Toxicol Sci. 106:384–391. [DOI] [PubMed] [Google Scholar]
- Cohen SS, Flaks JG, Barner HD, Loeb MR, and Lichtenstein J. 1958. The Mode of Action of 5-Fluorouracil and Its Derivatives. Proc Natl Acad Sci U S A. 44:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RD, Anderson GL, and Williams PL. 2004. The nematode Caenorhabditis elegans as a model of organophosphate-induced mammalian neurotoxicity. Toxicol Appl Pharmacol. 194:248–256. [DOI] [PubMed] [Google Scholar]
- Congress. 2000. ICCVAM (Interagency Coordinating Committee on the Validation of Alternative Methods) Authorization Act of 2000 (Public Law 106-545). [Google Scholar]
- Consortium C.e. S. 1998. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 282:2012–2018. [DOI] [PubMed] [Google Scholar]
- Dave G 1984. Effects of copper on growth, reproduction, survival and haemoglobin in Daphnia magna. Comp Biochem Physiol C. 78:439–443. [DOI] [PubMed] [Google Scholar]
- Dix DJ, Houck KA, Martin MT, Richard AM, Setzer RW, and Kavlock RJ. 2007. The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicol Sci. 95:5–12. [DOI] [PubMed] [Google Scholar]
- Ferguson M, Boyer M, and Sprando R. 2010. A method for ranking compounds based on their relative toxicity using neural networking, C. elegans, axenic liquid culture, and the COPAS parameters TOF and EXT. Open Access Bioinformatics. 2010:139–144. [Google Scholar]
- Gandhi S, Santelli J, Mitchell DH, Stiles JW, and Sanadi DR. 1980. A simple method for maintaining large, aging populations of Caenorhabditis elegans. Mech Ageing Dev. 12:137–150. [DOI] [PubMed] [Google Scholar]
- Gill MS, Olsen A, Sampayo JN, and Lithgow GJ. 2003. An automated high-throughput assay for survival of the nematode Caenorhabditis elegans. Free Radic Biol Med. 35:558–565. [DOI] [PubMed] [Google Scholar]
- HHS/NIH, HIEHS/NTP, NHGRI/NCGC, EPA/ORD, and FDA. 2010. MEMORANDUM OF UNDERSTANDING on High Throughput Screening, Toxicity Pathway Profiling, and Biological Interpretation of Findings. [Google Scholar]
- Johnson TE, and Wood WB. 1982. Genetic analysis of life-span in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 79:6603–6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruatrachue M, Rangsayatorn N, Pokethitiyook P, Upatham ES, and Singhakaew S. 2003. Histopathological changes in the gastrointestinal tract of fish, Puntius gonionotus, fed on dietary cadmium. Bull Environ Contam Toxicol. 71:561–569. [DOI] [PubMed] [Google Scholar]
- Lee GD, Wilson MA, Zhu M, Wolkow CA, de Cabo R, Ingram DK, and Zou S. 2006. Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging Cell. 5:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung MCK, Williams PL, Benedetto A, Au C, Helmcke KJ, Aschner M, and Meyer JN. 2008. Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol Sci. 106:5–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao VH, Liu JT, Li WH, Yu CW, and Hsieh YC. 2010. Caenorhabditis elegans bicarbonate transporter ABTS-1 is involved in arsenite toxicity and cholinergic signaling. Chem Res Toxicol. 23:926–932. [DOI] [PubMed] [Google Scholar]
- Lussier SM, Gentile JH, and Walker J. 1985. Acute and chronic effects of heavy metals and cyanide on Mysidopsis Bahia (Crustacea:Mysidacea). Aquat Toxicol. 7:25–35. [Google Scholar]
- Mitchell DH, Stiles JW, Santelli J, and Sanadi DR. 1979. Synchronous growth and aging of Caenorhabditis elegans in the presence of fluorodeoxyuridine. J Gerontol. 34:28–36. [DOI] [PubMed] [Google Scholar]
- Murawski M, Bydlon G, Sawicka-Kapusta K, Wierzchos E, Zakrzewska M, Wlodarczyk S, Molik E, and Zieba D. 2006. The effect of long term exposure to copper on physiological condition and reproduction of sheep. Reprod Biol. 6 Suppl 1:201–206. [PubMed] [Google Scholar]
- Nass R, and Hamza I. 2007. The nematode C. elegans as an animal model to explore toxicology in vivo: solid and axenic growth culture conditions and compound exposure parameters. Curr Protoc Toxicol. Chapter 1:Unit1 9. [DOI] [PubMed] [Google Scholar]
- National Research Council, N. 2000. Scientific Frontiers in Developmental Toxicology and Risk Assessment. National Academy Press, Washington, DC. [PubMed] [Google Scholar]
- Nelson GA, Lew KK, and Ward S. 1978. Intersex, a temperature-sensitive mutant of the nematode Caenorhabditis elegans. Dev Biol. 66:386–409. [DOI] [PubMed] [Google Scholar]
- Phillpotts CJ 1986. Histopathological changes in the epithelial cells of rat duodenum following chronic dietary exposure to cadmium, with particular reference to Paneth cells. Br J Exp Pathol. 67:505–516. [PMC free article] [PubMed] [Google Scholar]
- Popham JD, and Webster JM. 1979. Cadmium toxicity in the free-living nematode, Caenorhabditis elegans. Environ Res. 20:183–191. [DOI] [PubMed] [Google Scholar]
- Pulak R 2006. Techniques for analysis, sorting, and dispensing of C. elegans on the COPAS flow-sorting system. Methods Mol Biol. 351:275–286. [DOI] [PubMed] [Google Scholar]
- Rao AU, Carta LK, Lesuisse E, and Hamza I. 2005. Lack of heme synthesis in a free-living eukaryote. Proc Natl Acad Sci U S A. 102:4270–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnaike RN 2003. Acute and chronic arsenic toxicity. Postgrad Med J. 79:391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W, and Burch R. 1959. The principles of humane experimental technique. Methuen, London. [Google Scholar]
- ScienceLab. 2010. Material Safety Data Sheets. Sciencelab.com, Inc. [Google Scholar]
- Sprando RL, Olejnik N, Cinar HN, and Ferguson M. 2009. A method to rank order water soluble compounds according to their toxicity using Caenorhabditis elegans, a Complex Object Parametric Analyzer and Sorter, and axenic liquid media. Food Chem Toxicol. 47:722–728. [DOI] [PubMed] [Google Scholar]
- Szewczyk NJ, Udranszky IA, Kozak E, Sunga J, Kim SK, Jacobson LA, and Conley CA. 2006. Delayed development and lifespan extension as features of metabolic lifestyle alteration in C. elegans under dietary restriction. J Exp Biol. 209:4129–4139. [DOI] [PubMed] [Google Scholar]
- Valberg LS, Haist J, Cherian MG, Delaquerriere-Richardson L, and Goyer RA. 1977. Cadmium-induced enteropathy: comparative toxicity of cadmium chloride and cadmium-thionein. J Toxicol Environ Health. 2:963–975. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk JM, and Hekimi S. 2011. FUdR causes a twofold increase in the lifespan of the mitochondrial mutant gas-1. Mech Ageing Dev. 132:519–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PL, and Dusenbery DB. 1988. Using the nematode Caenorhabditis elegans to predict mammalian acute lethality to metallic salts. Toxicol Ind Health. 4:469–478. [DOI] [PubMed] [Google Scholar]


