Abstract
The transcription factor Bhlhe40 is required for the homeostatic proliferation of peritoneal macrophages and their expansion during type 2 immunity
Circulating monocytes can contribute to the ontogeny of macrophages in tissues such as the gut or the dermis. However, in most tissues, resident macrophage populations are derived from embryonic precursors 1–3. These precursors infiltrate the tissue before birth. A priori, tissue resident macrophages have to cycle to self-renew to maintain their population locally in the adult tissue. In the context of active immune response, tissue resident macrophages cycle to expand in numbers. Importantly, during self-renewal and rapid proliferation, these cells maintain their identity. In this issue of Nature Immunology, Jarjour et al. identify a transcription factor Bhlhe40 that is required for not only the self-renewal of serous cavity macrophages, such as large peritoneal macrophages (LPM), but also for their expansion following Heligmosomoides polygyrus bakeri infection 4. Similarly, IL-4 was found to drive the expansion of LPMs and pleural macrophages in a Bhlhe40-dependent manner. The authors note that the genetic ablation of Bhlhe40 resulted in failure of LPMs to cycle through the G1 phase of the cell cycle and led to reduced numbers of LPM, but didn’t affect other tissue resident macrophage populations such as those in the alveoli, spleen, kidney or liver. This study provides a glimpse of the subset-specific machinery of tissue-resident macrophages that enable their maintenance and expansion in adult tissues.
Differentiated cells are usually not in cell cycle. Tissue resident macrophages are primarily quiescent cells. For example, ~17% of resident pleural macrophages in naïve mice were described to be Ki67 positive, while the rest were quiescent 5. The decision point where these quiescent cells are licensed to enter the cell cycle can be driven by colony stimulating factor-1 receptor (CSF1R) signaling 6, while cytokines, such as IL-4, are the predominant drivers of local macrophage proliferation during type 2 immunity 5. Bhlhe40 appears to regulate cell cycle transit for LPMs. Self-renewal or proliferation requires progression through the various phases of the cell cycle including G1, S, G2 and M. LPM in Bhlhe40−/− mice failed to successfully complete the G1 phase of the cell cycle to move to S phase. Passage through the cell cycle requires overcoming a number of tightly guarded checkpoints, including the G1 checkpoints. Quiescent cells entering the cell cycle, as well as dividing cells, will have to pass a G1 checkpoint, also called Restriction point or R, to proceed through G1. Passage through this G1 checkpoint involves de-repression of E2F which is required for the expression of genes necessary for the S phase 7. E2F is normally sequestered by the retinoblastoma protein Rb. This transition is typically triggered by growth-factor signaling, which regulate Cyclin D1 and p21 transcription and protein stability. The symphony of Cyclin and Cyclin-dependent kinase (Cdk) function reaches a crescendo wherein Rb gets hyperphosphorylated. The Cyclin D1:Cdk4 complex phosphorylates Rb. The Cyclin D1:Cdk4 and the Cyclin D1:Cdk6 complexes also sequester the cell cycle inhibitors p21 and p27 away from the Cyclin E:Cdk2 complex, allowing this later complex to phosphorylate Rb. Cyclin E:Cdk2 also phosphorylates and degrades p27. Hyperphosphorylation of Rb results in its degradation and the release of active E2F. Apart from this R point, some investigators have reported late G1 checkpoints, analogous to the yeast START checkpoint, mediated by amino acid availability and mTOR 8. Once cells pass through these checkpoints, they can complete G1 and enter the S phase of the cell cycle. The exact location of the arrest in the absence of Bhlhe40 relative to the G1 checkpoints remains unclear. Notwithstanding, Bhlhe40 function is indispensable for successful completion of G1. Of note, genetic ablation of a different transcription factor, GATA6, was previously shown result in a significant reduction in the number of LPM 9,10. Yet, the transcriptional changes in Bhlhe40−/− LPM were distinct from those in Gata6−/− LPM, indicating that these transcription factors control different steps in the life of LPMs.
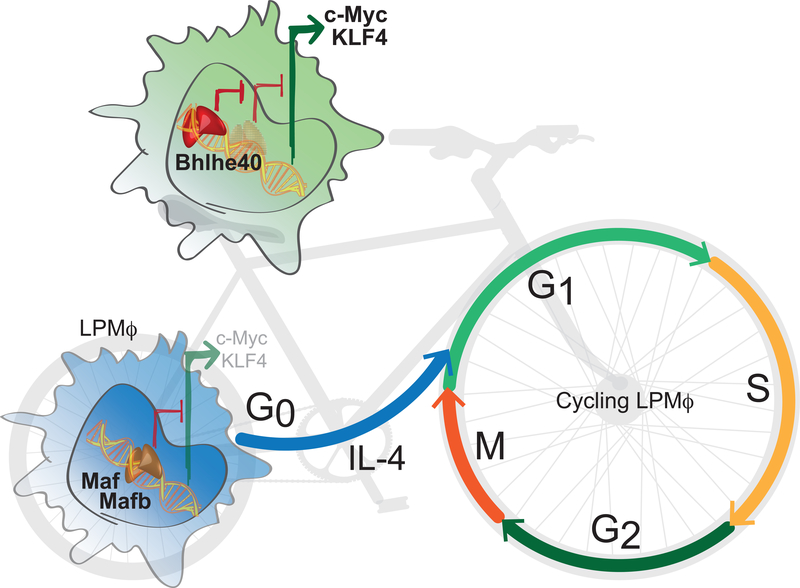
Jarjour et al. find that, mechanistically, Bhlhe40 binds the promoter elements of Maf and Mafb locus and represses their transcription (Fig. 1). This leads to cell cycle progression and upregulation of E2F target genes. The transcription factors Maf and Mafb play a role in pulling differentiated cells out of the cell cycle. Silencing Maf and Mafb allows the extended expansion of macrophages without the loss of their differentiated phenotype and function 11. Importantly, c-myc and KLF4 were reported to be upregulated in Maf −/−Mafb−/− mouse. c-myc and KLF4, along with Oct-3/4 and Sox-2 constitute the Yamanaka factors for induced pluripotency of differentiated cells. Interestingly, re-entry of LPMs into cell cycle occurs without the loss of their differentiation status.
Figure 1. Proliferation of peritoneal macrophages is dependent on Bhlhe40.
In quiescent large peritoneal macrophages (LPMs), the transcription factors Maf and Mafb inhibit the expression of KLF4 and c-Myc, limiting self-renewal. Cytokines, such as IL-4, induce the entry of LPMs into the cell cycle. The progression of LPMs through the cell cycle is dependent on the transcription factor Bhlhe40 and its ability to bind the promoter elements of Maf and Mafb locus and represses their transcription, thus leading to the increased expression of the pro-proliferative transcription factors KLF4 and c-Myc.
The regulation of Bhlhe40 itself is not well understood. Do CSF-1 or IL-4 regulate Bhlhe40 expression or its activity during homeostasis or type II immunity, leading to Maf and Mafb repression and cell cycle entry of LPM? The identification of IL-4-dependent Bhlhe40-binding to a site downstream of the Bhlhe40 locus suggests complex autoregulatory transcriptional regulation 4. Since the function of Bhlhe40 was found to be required for the IL-4-dependent expansion of LPMs as well as pleural macrophages, it would be interesting to explore whether this shared dependency of serous macrophages relates to a common epigenetic landscape or environmental signals in these cavities. It is also important to note that Bhlhe40 was only required for IL-4 induced proliferation, but not IL-4 induced polarization of these macrophages. Bhlhe40 deletion did not affect thioglycolate-induced recruitment of monocyte-derived macrophages in the peritoneum, illustrating its cell-type specific function. Whether other cytokines can stimulate LPM proliferation also through Bhlhe40 remains to be described.
To test the requirement of Bhlhe40 in the proliferation of macrophages during type II immunity, the authors made use of the helminth Heligmosomoides polygyrus bakeri known to establish a chronic infection in mice. Other helminth infections, such as Nippostrongylus brasiliensis, are known to resolve. What happens to Bhlhe40 after resolution of type II immunity? LPMs should exit the cell cycle upon resolution. How proliferation (continuing on to G1) versus cell cycle exit (going to G0) is decided is an area of active investigation. For dividing cells, Cdk2 activity, for example, has been associated with G1 versus G0 decisions 12. The fate of Bhlhe40 as LPMs exit the cell cycle and the signaling that regulates this would be another intriguing area to investigate.
The identification of Bhlhe40 as a tissue-specific regulator of peritoneal macrophages self-renewal and expansion illustrates the significance of local signals in macrophage function. These signals can be both intrinsic and part of the macrophage hardwire, such as Bhlhe40, as well as dynamic and extrinsic such as the secreted surfactant protein A and C1q as recently described by Minutti et al 13. The spatial and temporal coincidence of extrinsic signals 14, together with the intrinsic transcriptional program of the macrophage, directs the complex biology of maintenance through self-renewal and rapid expansion while maintaining polarization during an immune response. This also allows the differential function of discrete macrophage populations coming under the influence of identical local signals.
REFERENCES
- 1.Merad M. et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol 3, 1135–1141, doi: 10.1038/ni852 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schulz C. et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90, doi: 10.1126/science.1219179 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Yona S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91, doi: 10.1016/j.immuni.2012.12.001 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarjour N. et al. Bhlhe40 mediates tissue-specific control of macrophage proliferation in homeostasis and type 2 immunity. Nature Immunology (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenkins SJ et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332, 1284–1288, doi: 10.1126/science.1204351 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hume DA & MacDonald KP Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 119, 1810–1820, doi: 10.1182/blood-2011-09-379214 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Duronio RJ & Xiong Y. Signaling pathways that control cell proliferation. Cold Spring Harb Perspect Biol 5, a008904, doi: 10.1101/cshperspect.a008904 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster DA, Yellen P, Xu L. & Saqcena M. Regulation of G1 Cell Cycle Progression: Distinguishing the Restriction Point from a Nutrient-Sensing Cell Growth Checkpoint(s). Genes Cancer 1, 1124–1131, doi: 10.1177/1947601910392989 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okabe Y. & Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell 157, 832–844, doi: 10.1016/j.cell.2014.04.016 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosas M. et al. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science 344, 645–648, doi: 10.1126/science.1251414 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aziz A, Soucie E, Sarrazin S. & Sieweke MH MafB/c-Maf deficiency enables self-renewal of differentiated functional macrophages. Science 326, 867–871, doi: 10.1126/science.1176056 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Spencer SL et al. The proliferation-quiescence decision is controlled by a bifurcation in CDK2 activity at mitotic exit. Cell 155, 369–383, doi: 10.1016/j.cell.2013.08.062 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minutti CM et al. Local amplifiers of IL-4Ralpha-mediated macrophage activation promote repair in lung and liver. Science 356, 1076–1080, doi: 10.1126/science.aaj2067 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosurgi L. et al. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 356, 1072–1076, doi: 10.1126/science.aai8132 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]