Abstract
Background
Electronic cigarettes (E-cigarettes) generate aerosol containing metal contaminants. Our goals were to quantify aerosol metal concentrations and to compare the effects of power setting and device type (closed-system vs. open-system) on metal release.
Methods
Aerosol samples were collected from two closed-system devices (a cigalike and pod) and two open-system devices (mods). Each open-system device was operated at three different power settings to examine the effect of device power on metal release. Concentrations of 14 metals in e-cigarette aerosol collected via droplet deposition were measured using inductively coupled plasma mass spectroscopy. Aerosol metal concentrations were reported as mass fractions (μg/kg) in the e-liquid.
Results
For open-system device 1 (OD1), median arsenic (As), chromium (Cr), copper (Cu), iron (Fe), manganese (Mn), nickel (Ni), lead (Pb), antimony (Sb), tin (Sn), and zinc (Zn) concentrations increased 14, 54, 17, 30, 41, 96, 14, 81, 631, and 7-fold when the device power was increased from low (20 W) to intermediate (40 W) setting. When the power was further increased from intermediate (40 W) to high (80 W) setting, concentrations of As, Cr, Cu, Mn, Ni, and Sb did not change significantly. For open-system device 2 (OD2), Cr and Mn concentrations increased significantly when device power was increased from low (40 W) to intermediate (120 W) setting, and then decreased significantly when power was further increased from intermediate (120 W) to high (200 W) setting. Among the four devices, aerosol metal concentrations were higher for the open-system than the closed-system devices, except for aluminum (Al) and uranium (U). For Cr, median (interquartile range) concentrations (μg/kg) from the open-system devices were 2.51 (1.55, 4.23) and 15.6 (7.88, 54.5) vs. 0.39 (0.05, 0.72) and 0.41 (0.34, 0.57) for the closed-system devices. For Ni, concentrations (μg/kg) from the open-system devices were 793 (508, 1169) and 2148 (851, 3397) vs. 1.32 (0.39, 3.35) and 11.9 (10.7, 22.7) from the closed-system devices. Inhalation of 0% and 100% of samples from OD1, 7.4% and 88.9% from OD2 by typical e-cigarette users would exceed chronic minimum risk levels (MRL) of Mn and Ni, respectively. No MRL exceedance was predicted for the closed-system devices. A large fraction of users of OD1 (100%) and OD2 (77.8%) would be exposed to Ni levels higher than those from reference tobacco cigarette 3R4F.
Conclusions
Our findings suggest that power setting and device type affect metal release from devices to aerosol which would subsequently be inhaled by users. Metal concentrations from open-system devices first increased with device power, and then leveled off for most metals. Open-system devices generate aerosol with higher metal concentrations than closed-system devices. These findings inform tobacco regulatory science, policy makers and health professionals on potential metal health risks associated with e-cigarette use, design and manufacturing.
Keywords: Toxic metals, E-cigarettes, Aerosol, Open-system, Closed-system
1. Introduction
Electronic cigarettes (e-cigarettes) have become increasingly popular since current e-cigarette users perceive them as a safer alternative to tobacco cigarettes (Glasser et al., 2017; Grana et al., 2014; Shang et al., 2017; Walley et al., 2015). A recent study indicates that blood cadmium levels among smokers who quit tobacco-burning cigarettes and switched completely to e-cigarettes for 6 months were lower than levels of those who continued smoking (Prokopowicz et al. 2018). In a cross-sectional study in Romania, higher serum concentrations of selenium, silver, and vanadium were detected among e-cigarette users compared to cigarette smokers (Badea et al., 2018). In e-cigarette users from Maryland, USA, chromium and nickel concentrations in aerosol samples collected from their personal e-cigarettes were positively associated with metal internal dose as measured in saliva and urine (Aherrera et al., 2017). Thus while potentially reducing cadmium exposure, e-cigarettes could contribute to substantial exposure to other toxic metals.
Metals may leach from several parts of an e-cigarette, including the heating coil, joints and wires (Williams et al. 2015; Williams et al. 2017; Olmedo et al., 2018). Kim et al. (2017) reported that lead and manganese aerosol levels were below the limit of detection (LOD) when a new e-cigarette was used, while lead (0.097±0.003 mg/L) and manganese (0.001±0.000 mg/L) were detected in aerosols after 4 months of testing (20 h total usage), demonstrating that the metals originated from the e-cigarette device and not from the e-liquid. Similarly, Olmedo et al. (2018) reported that metal contamination of the e-liquid from the tank and of the aerosol was markedly higher than in the dispenser e-liquid, demonstrating that contact of unused e-liquid with the device (e.g., heating coil) resulted in e-liquid and aerosol contamination. However, some metals or metalloids such as arsenic can be transferred from the e-liquid into the aerosol (Olmedo et al., 2018). Williams et al. (2015) showed that for some devices tin aerosol levels could be attributed to tin used in joints and tin-coated wires and that tin aerosol levels could be reduced by coating the wires with silver rather than tin. Since device design including coil composition and electrical power is highly variable, metal concentrations in aerosols generated by different devices need to be studied to inform manufacturers, regulators and consumers.
In January 2014, a total of 466 brands of e-cigarettes could be purchased on the Internet (Zhu et al., 2014). E-cigarettes can be broadly divided into two distinct categories: closed-system and open-system devices (Chen et al. 2016). Closed-system devices can resemble tobacco cigarettes in terms of size and shape. They are commonly disposable or can be reloaded with a prefilled cartridge or tank of their own brand with limited choices of flavors and nicotine concentrations, and limited ability to change power (Grana et al. 2014). Closed-system devices are commonly referred to as cigalikes and USB shaped devices (or pods) (e.g. JUUL), which are most commonly used by youth or new e-cigarette users (Bhatnagar et al., 2014; Qasim et al., 2017; Shang et al., 2017).
Open-system devices are larger than tobacco cigarettes and resemble a pen or tank, which allow users to refill an “atomizer” with an increasingly wide variety of e-liquids differing in flavor, nicotine content and manufacturer (Chen et al. 2016). These devices are referred to as tanks, e-vapors, or mods depending on their characteristics, and are most commonly utilized by daily e-cigarette users, typically former smokers (Qasim et al., 2017). The most distinguishing difference between closed-system and open-system devices is that the latter allow users to adjust device power, change (and make) the heating coil, and mix their own e-liquid, resulting in higher puff volume (Talih et al. 2015; Brown et al. 2014). Even though numerous studies have measured metals in aerosols, most of them focused on one type of e-cigarette device. No direct comparison in aerosol metal concentrations between open-system and closed-system devices is available.
Sleiman et al. (2016) studied the effects of voltage on aldehyde emissions from a mod device of EGO brand with tobacco-flavored e-liquid, finding a 3-fold increase in total aldehydes emissions when voltage was increased from 3.3 to 4.8 V. Kosmider et al. (2014) found that increasing voltage of a mod device of eGo-3 brand from 3.2 to 4.8 V resulted in a 4- to more than 200-fold increase in formaldehyde, acetaldehyde, and acetone levels. Using the same mod device, Kosmider et al. (2018) found that the mean nicotine yield generated by one puff increased 1.5–2.8 fold for three different liquids when device power was increased from 4.3 to 9.6 W. However, the impact of electrical power on e-cigarette metal emissions is unknown.
Overall, the effect(s) of device power settings and device type (open- and closed-system) on metal concentration in aerosol is unknown. The main objective of this study was to investigate the effect of three different power outputs of open-system devices on metals in aerosols, and provide a direct comparison of metal concentrations in aerosols released by two closed- and two open-system devices. Moreover, we determined daily inhaled metal doses of typical e-cigarette users for the devices we tested and compared them to exposure limits set by the Agency for Toxic Substances and Disease Registry (ATSDR) of the US Department of Health and Human Services.
2. Materials and methods
2.1. E-cigarette devices and e-liquid characteristics
Four e-cigarette devices and 16 e-liquids differing in flavor and nicotine content were purchased online. Two popular closed-system devices, a BLU (BLU Products, Miami, FL) and a JUUL (JUUL Labs, San Francisco, CA) identified as CD1 and CD2, respectively, were studied (Tayyarah and Long, 2014; Mikheev et al. 2016; Kavuluru et al. 2018; Huang et al. 2018). The open-system devices were Istick 25 (Eleaf Electronics Co., Ltd, Shenzhen, PRC) and SMOK (Smoktech, Shenzhen, PRC), identified as OD1 and OD2, respectively. These open-system device brands were selected because they were very popular in a previous study on e-cigarette contaminant emissions from devices used by recruited daily e-cigarette users (Olmedo et al., 2018). In that study, about 11% and 7% of participants used Istick 25 and SMOK, respectively. Device characteristics are summarized in Table 1. For the closed-system devices, e-liquids prior to contact with the devices were unavailable for metal analysis, because tanks were prefilled by the manufacturers. Thus, we were also unable to determine the pH value of those e-liquids.
Table 1.
Device and e-liquid characteristics.
| Device ID | Commercial Name | Coil | Shape | Power (W) | Resistance (Ohm) | Battery voltage | Flavor | Nicotine content (mg/mL) | Aerosol samples (n) | pH |
|---|---|---|---|---|---|---|---|---|---|---|
| CD1 | BLU | Nichrome | Cigalike | N/A | N/A | 3.7 V | Blueberry | 24 | 7 | ND |
| Tobacco | 24 | 14 | ND | |||||||
| Menthol | 0 | 7 | ND | |||||||
| Menthol | 24 | 9 | ND | |||||||
| CD2 | JUUL | Nichrome | Pod | N/A | N/A | N/A | Fruit medley | 59 | 3 | ND |
| Cool mint | 59 | 3 | ND | |||||||
| Tobacco | 59 | 3 | ND | |||||||
| OD1 | Istick 25 | Kanthal | Tank | 1–85 | 0.2 | 3.7 V | Blueberry | 0 | 3 | 5 |
| Blueberry | 6 | 3 | 6.1 | |||||||
| Blueberry | 24 | 3 | 6.5 | |||||||
| Tobacco | 0 | 3 | 4 | |||||||
| Tobacco | 6 | 3 | 7.9 | |||||||
| Tobacco | 24 | 3 | 8.6 | |||||||
| Menthol | 0 | 3 | 6.2 | |||||||
| Menthol | 6 | 3 | 8.4 | |||||||
| Menthol | 24 | 3 | 8.5 | |||||||
| OD2 | SMOK | Stainless steel | Tank | 6–220 | 0.6 | 2 × 3.7 V | Blueberry | 0 | 3 | 5 |
| Blueberry | 6 | 3 | 6.1 | |||||||
| Blueberry | 24 | 3 | 6.5 | |||||||
| Tobacco | 0 | 3 | 4 | |||||||
| Tobacco | 6 | 3 | 7.9 | |||||||
| Tobacco | 24 | 3 | 8.6 | |||||||
| Menthol | 0 | 3 | 6.2 | |||||||
| Menthol | 6 | 3 | 8.4 | |||||||
| Menthol | 24 | 3 | 8.5 |
N/A=not available (Information not indicated directly on packages, not available on the internet, and not obtained after direct contact with the manufacturer). For open-system devices (OD1, OD2), n denotes the number of aerosol samples collected at intermediate power setting.
Each open device was tested with three different e-liquids/flavors: blueberry, tobacco, and menthol (all from “Import Eliquid” MVS, MyVaporStore Inc, www.myvaporstore.com). These flavors were chosen as 93.4% and 92.1% of all 466 e-cigarette brands offered tobacco and menthol flavors, followed by fruit flavor (84.2%) (Zhu et al. 2014). Each of the three “Import Eliquid” flavors were purchased with three different nicotine contents: 0, 6, and 24 mg/mL as indicated by the manufacturer. Three vials were collected for each e-liquid, thus, a total of 27 samples were obtained for open-system devices. We note that the three “Import Eliquid” flavors came in packaged bottles that only listed the manufacturing country (PRC) and the US distributor (MV Store Inc.) but not the manufacturer’s name.
For closed devices, we selected disposable tanks among those available for each product as follows. For CD1, we obtained e-liquid with blueberry, tobacco and menthol flavors with a nicotine content of 24 mg/mL in manufacturer-specific disposable tanks called cartomizers. In addition, we obtained menthol-flavored e-liquid without nicotine. More than three vials were collected for each e-liquid, resulting in a total of 37 samples. For CD2, we obtained fruit medley, cool mint, and Virginia tobacco flavors, all with a 59 mg/mL nicotine content, in manufacturer-specific disposable tanks called pods. Three vials were collected for each e-liquid, thus, a total of 9 samples were obtained. The reason for the markedly smaller number of samples for CD2 is that this device generates relatively little aerosol, making aerosol collection cumbersome as a much larger number of puffs was required than for the other devices. Detailed information about each e-liquid is provided in Table 1.
To examine the effects of device power, each open-system device was operated at three different power settings. The adjustable power of OD1 and OD2 ranged between 1 and 85 W and between 6 and 220 W, respectively. Power settings for our sample collection were chosen slightly above and below the minimum and maximum settings to ensure stable device performance: 20 and 80 W for OD1 and 40 and 200 W for OD2, as well as an intermediate power setting, i.e., 40 W for OD1 and 120 W for OD2. Testing both devices at 40 W allowed for a direct comparison between the two open-system devices. Coil resistance was 0.2 Ohm for OD1 and 0.6 Ohm for OD2. The closed-system device manufacturers did not disclose device power and resistance, and these parameters could not be changed.
2.2. Aerosol collection
All e-cigarettes were puffed inside a fume hood by connecting the mouthpiece via flexible tubing to a peristaltic pump. Aerosol was directly collected from all devices using the method developed by Olmedo et al. (2016), and for each aerosol sample collected the aerosol collection efficiency was determined from a mass balance. That aerosol collection method has been used in several other studies (Olmedo et al. 2018; Aherrera et al. 2017). On the downstream side of the pump, the aerosol was directed into a series of tubing sections and collected in a 1.5 mL centrifuge tube. The flow rate was 1 L/min.
For the closed-system devices, one 5-cm long pipette tip was connected to three 2-cm long tubing sections (internal diameter of 0.16 cm, outside diameter of 0.32 cm), three 2-cm long cut pipette tips, and one piece of a 5-cm long tubing section (internal diameter of 0.16 cm, outside diameter of 0.32 cm). A total of 50–100 and 290–330 puffs were required to collect approximately 0.3–0.6 mL of aerosol from CD1 and CD2, respectively, with a puff duration of 4 s and an inter-puff time of 11 s (Olmedo et al. 2016). To collect sufficient amounts of aerosol for the analytical laboratory, we had to generate a higher number of puffs for CD2, as this device releases less aerosol than the other devices. These 290 to 330 puffs were not executed in a single session as the battery needed to be charged after 50 puffs.
Aerosol from the open-system devices was collected by slightly modifying Olmedo’s method to increase the aerosol recovery rate. The collection system consisted of one 5-cm long pipette tip and one 12-cm long tubing section (internal diameter of 0.38 cm, outside diameter of 0.5 cm). The puff duration was 4 s, with a 26 s inter-puff time (Olmedo et al., 2018; Talih et al., 2015). A total of 15–120 and 25–120 puffs were needed to collect enough aerosol (approximately 0.3–0.6 mL) for OD1 and OD2 for metal analysis. The large range of puff numbers was due to the different power settings; more puffs were needed to collect enough aerosol at low power settings. E-cigarette batteries were charged for 24 hr before each session and replaced when devices indicated battery depletion.
To increase recovery of aerosol for chemical analysis, some of the liquid, which remained in the tubing after the desired number of puffs was administered for a given e-liquid, was collected by manually flicking the tubing, placing it back into the pump, and pumping an additional five puffs of non-aerosol containing air with the e-cigarette being disconnected. To determine the recovery fraction of aerosolized e-liquid, we employed the mass balance law based on the measured weights of the tank, the collection vial, and the tubing before and after each session. The mean±standard deviation (SD) aerosol recovery was 71.7% ±11.2%, 83.2%±7.38%, 77.6% ±6.97%, and 82.9% ±8.46% for CD1, CD2, OD1, and OD2, respectively. The aerosol recovery was similar for open-system (78–83%) and closed-system (72–83%) devices, supporting that aerosol levels from the two device types can be compared to each other.
In a biomarker study (Aherrera et al. 2017), metal levels in the aerosol collected using this method were highly correlated with metal internal dose. In adjusted models, the third tertiles of aerosol Ni and Cr concentrations were associated with saliva Ni and Cr levels that were 321% and 193% higher than those of the first tertiles, suggesting that our collection method collects the aerosol that is inhaled by the user.
2.3. Metal analyses
Metal concentrations in collected e-cigarette aerosol samples were determined as previously described (Olmedo et al., 2018) at the Columbia University Trace Metal Core Lab using inductively coupled plasma mass spectrometry (ICP-MS, NexION 350S, PerkinElmer) with a dynamic reaction cell. Briefly, a 0.1–0.2 g aliquot of each sample was diluted with 5 mL diluent (2% HNO3, 1% methanol, 0.02% Triton X-100). We selected the following 14 metals for aerosol sample analysis based on previous studies and feasibility at our laboratory: aluminum (Al), arsenic (As), cadmium (Cd), chromium (Cr), copper (Cu), iron (Fe), manganese (Mn), nickel (Ni), lead (Pb), antimony (Sb), tin (Sn), uranium (U), tungsten (W), and zinc (Zn). Multi-element calibration standards were prepared by serial dilution of the stock solution. Internal standards were added to both samples and calibration standards to control for long-term signal drifts; we used gallium (Ga) for low- (Al, Cr, Cu, Fe, Mn, Ni, Zn), rhodium (Rh) for medium- (As, Cd, Sb, Sn), and iridium (Ir) for high-molecular mass metals (Pb, U, W). Quality assurance involved using reference material SRM 1640a (Trace Elements in Natural Water, NIST, USA). Matrix blanks consisted of 70% propylene glycol (high purity, Amresco, USA) and 30% glycerol (Ultra pure, ICN Biochemicals, USA) were analyzed to account for matrix effects. Blank e-liquid samples were passed through the tubing system using the peristaltic pump to account for potential background air contamination. Median concentrations of the blanks were used to correct concentrations in the aerosol samples. Limits of detection (LOD) in μg/kg were 6.7 for Al, 0.15 for As, 0.1 for Cd, 0.1 for Cr, 0.4 for Cu, 5.55 for Fe, 0.05 for Mn, 0.2 for Ni, 0.05 for Pb, 0.05 for Sb, 0.6 for Sn, 0.05 for U, 0.05 for W and 0.7 for Zn. Concentrations lower than the LOD were substituted by LOD/√2 for statistical analysis.
2.4. Comparison to exposure limits
Metal concentrations of agglomerated aerosol were reported as mass fractions of metal in the collected liquid (μg/kg). Agglomerated aerosol concentrations were converted into air aerosol concentrations ca (mg/m3) as described in Olmedo et al. (2018). Air concentrations were compared to chronic minimum risk levels (MRLs) for metal inhalation: 3×10−4 mg/m3 for Mn and 9×10−5 mg/m3 for Ni where for Mn D = 5 days of exposure per week and H = 8 hrs of exposure per day are assumed whereas for Ni D = 5 days and H = 6 hrs (ATSDR 2012; ATSDR 2005; U.S. EPA 2000; U.S. EPA 2016). As exposure windows of e-cigarette use differ from MRL windows, aerosol concentrations ca were converted into concentrations c* comparable to the MRLs. We assumed the daily metal dose (mg) inhaled by a representative e-cigarette user (puff volume PV = 70 mL; Np = 120 puffs per day) to be equal to the dose received by a person inhaling the metal at concentration c* at a representative minute ventilation (MV = 6 L/min) during MRL exposure windows (Qasim et al., 2017; Goniewicz et al., 2014; Kent, 2006). Therefore c* can be estimated from
| Eq.(1) |
We interpreted c* > MRL as a MRL exceedance, and we report for each device and each of the three metals the daily inhaled metal dose (μg/d), which can be calculated from either the left- or right-hand side of Eq. (1).
Even though no MRL is available for Pb, the daily Pb dose was calculated as there is no safe level of Pb exposure. Daily doses of Mn, Ni and Pb were compared to daily metal doses received by an individual smoking an equivalent number of reference tobacco cigarettes 3R4F. To determine this number, it was assumed that 15 puffs from an e-cigarette are equivalent to smoking one cigarette (St Helen et al. 2016). For each metal, the daily dose received by the smoker was obtained by multiplying the published metal concentration (ng/cigarette) (Pappas et al. 2014) by the number of daily cigarettes.
2.5. Statistical analyses
Medians and interquartile ranges (IQR) were used to summarize quantitative variables. We used box plots to visualize metal concentration distributions stratified by device type. For open-system devices, we also show aerosol metal levels stratified by device power. Differences in log-transformed metal concentrations across different device types and device powers were analyzed by one-way ANOVA. We ran some sensitivity analyses to assess the impact of puff number in our analysis by fitting linear regression models using the log-transformed metal concentrations as the outcome and device type or device power as predictor variables, with and without adjusting the models for puff number. Both p-values and beta coefficients (95% confidence intervals) from linear regression were determined. All the analyses were run in R version 3.4.2.
3. Results
3.1. Aerosol metal concentrations by device power
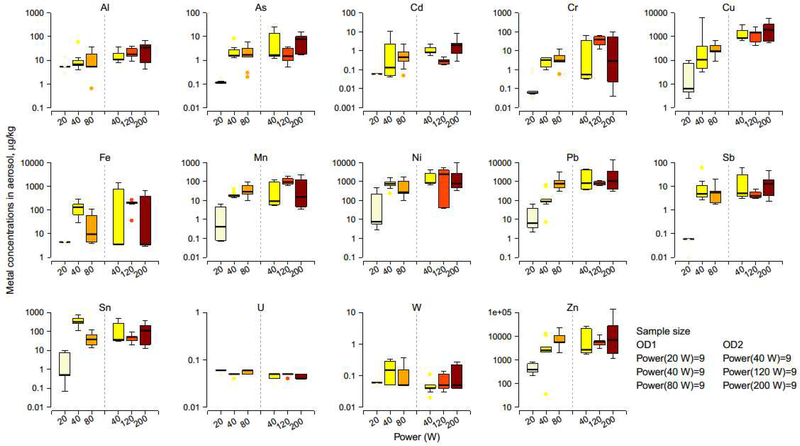
For OD1, significantly higher metal concentrations were found when the power was increased from the low (20 W) to the intermediate (40 W) setting, resulting in 14, 54, 17, 30, 41, 96, 14, 81, 631, and 7-fold increase in median As, Cr, Cu, Fe, Mn, Ni, Pb, Sb, Sn, and Zn concentrations, respectively (Table 2; Figure 1). When the power was increased from the intermediate (40 W) to the high (80 W) setting, the concentrations of As, Cr, Cu, Mn, Ni, and Sb did not change significantly. However, significant decreases were observed for Fe from 131 to 9.5 and for Sn from 322 to 38.0 μg/kg, whereas Pb levels increased from 89.5 to 792 and Zn levels from 2,536 to 5,555 μg/kg.
Table 2.
Median (interquartile range) metal concentrations (μg/kg) in aerosols by device power.
| Median (IQR) in OD1 | Overall p-value | Linear regression p-value | Beta value (95% CI) | |||||||
| 20 W (n=9) | 40 W (n=9) | 80 W (n=9) | 20 vs. 40 W | 20 vs. 80 W | 40 vs. 80 W | 20 vs. 40 W | 20 vs. 80 W | 40 vs. 80 W | ||
| Al | 5.41 (5.23–5.52) | 6.70 (5.96–9.94) | 5.39 (5.15–18.2) | 0.53 | 0.27 | 0.59 | 0.56 | 0.46 (−0.37, 1.28) | 0.22 (−0.61, 1.05) | −0.24 (−1.06, 0.59) |
| As | 0.11 (0.11–0.12) | 1.57 (1.48–2.85) | 1.70 (1.40–3.13) | <0.001 | <0.001 | <0.001 | 0.31 | 2.87 (2.15, 3.59) | 2.51 (1.80, 3.23) | −0.36 (−1.07, 0.35) |
| Cd | 0.06 (0.06–0.06) | 0.13 (0.05–2.29) | 0.46 (0.28–0.85) | 0.02 | 0.03 | 0.007 | 0.48 | 1.50 (0.11, 2.87) | 1.97 (0.59, 3.35) | 0.48 (−0.90, 1.85) |
| Cr | 0.06 (0.06–0.07) | 3.22 (1.42–4.15) | 3.03 (2.51–5.40) | <0.001 | <0.001 | <0.001 | 0.76 | 3.23 (2.25, 4.19) | 3.37 (2.40, 4.34) | 0.15 (−0.82, 1.11) |
| Cu | 6.3 (4.64–73.1) | 107 (46.6–403) | 244 (222–412) | <0.001 | <0.001 | <0.001 | 0.61 | 2.57 (1.22, 3.92) | 2.91 (1.56, 4.27) | 0.34 (−1.01, 1.70) |
| Fe | 4.44 (4.38–4.54) | 131 (64.2–174) | 9.5 (4.43–58.5) | <0.001 | <0.001 | 0.009 | <0.001 | 3.17 (2.30, 4.05) | 1.21 (0.32, 2.08) | −1.97 (−2.84, −1.09) |
| Mn | 0.42 (0.08–4.76) | 17.4 (16.2–19.6) | 29.5 (21.5–63.0) | <0.001 | <0.001 | <0.001 | 0.44 | 3.34 (2.10, 4.57) | 3.80 (2.56, 5.04) | 0.47 (−0.77, 1.70) |
| Ni | 8.00 (5.89–222) | 768 (610–978) | 277 (235–1,031) | <0.001 | <0.001 | <0.001 | 0.33 | 3.29 (1.96, 4.61) | 2.65 (1.32, 3.97) | −0.64 (−1.96, 0.68) |
| Pb | 6.50 (3.80–39.5) | 89.5 (84.6–115) | 792 (471–1,170) | <0.001 | <0.001 | <0.001 | 0.001 | 2.22 (1.07, 3.36) | 4.24 (3.10, 5.39) | 2.02 (0.88, 3.17) |
| Sb | 0.06 (0.06–0.06) | 4.84 (3.63–11.0) | 5.48 (1.98–6.06) | <0.001 | <0.001 | <0.001 | 0.23 | 4.99 (4.19, 5.79) | 4.51 (3.71, 5.31) | −0.48 (−1.28, 0.31) |
| Sn | 0.51 (0.45–7.73) | 322 (265–501 | 38.0 (19.2–64.3) | <0.001 | <0.001 | <0.001 | <0.001 | 5.78 (4.57, 6.98) | 3.58 (2.38, 4.79) | −2.19 (−3.40, −0.99) |
| U | 0.06 (0.06–0.06) | 0.05* (0.05*–0.05*) | 0.06 (0.05*–0.06) | <0.001 | <0.001 | 0.11 | 0.002 | −0.19 (−0.26, −0.11) | −0.06 (−0.14, 0.02) | 0.13 (0.05, 0.20) |
| W | 0.06 (0.06–0.06) | 0.15 (0.05–0.29) | 0.05** (0.05*–0.15) | 0.06 | 0.02 | 0.24 | 0.19 | 0.79 (0.15, 1.43) | 0.37 (−0.27, 1.01) | −0.42 (−1.06, 0.22) |
| Zn | 387 (303–684) | 2,536 (2,183–3,432) | 5,555 (5,183–10,264) | <0.001 | 0.004 | <0.001 | 0.04 | 1.64 (0.56, 2.72) | 2.79 (1.71, 3.87) | 1.15 (0.07, 2.23) |
| Median (IQR) in OD2 | Overall p-value | Linear regression p-value | Beta value (95% CI) | |||||||
| 40 W (n=9) | 120 W (n=9) | 200 W (n=9) | 40 vs. 120 W | 40 vs. 200 W | 120 vs. 200 W | 40 vs. 120 W | 40 vs. 200 W | 120 vs. 200 W | ||
| Al | 10.7 (10.3–19.7) | 17.9 (15.0–30.7) | 34.7 (7.65–44.8) | 0.4 | 0.23 | 0.26 | 0.94 | 0.43 (−0.29, 1.14) | 0.40 (−0.32, 1.12) | −0.03 (−0.74, 0.69) |
| As | 1.67 (1.50–13.8) | 1.51 (0.98–3.25) | 7.76 (1.79–10.3) | 0.03 | 0.08 | 0.36 | 0.01 | −0.82 (−1.75, 0.10) | 0.42 (−0.51, 1.35) | 1.24 (0.31, 2.17) |
| Cd | 0.83 (0.79–1.43) | 0.28 (0.20–0.33) | 1.98 (0.74–2.50) | <0.001 | <0.001 | 0.19 | <0.001 | −1.26 (−1.93, −0.60) | 0.44 (−0.23, 1.11) | 1.70 (1.03, 2.37) |
| Cr | 0.56 (0.36–34.6) | 39.4 (20.8–57.3) | 2.90 (0.23–54.7) | 0.02 | 0.01 | 0.86 | 0.02 | 2.78 (0.60, 4.96) | 0.19 (−1.99, 2.37) | −2.59 (−4.77, −0.41) |
| Cu | 868 (815–1,788) | 1,480 (602–1,591) | 1,936 (648–3,286) | 0.3 | 0.8 | 0.23 | 0.15 | −0.90 (−0.80, 0.62) | 0.42 (−0.29, 1.14) | 0.51 (−0.20, 1.23) |
| Fe | 3.57 (3.52–778) | 200 (188–214) | 3.50 (3.28–371) | 0.09 | 0.06 | 0.91 | 0.05 | 2.00 (−0.12, 4.12) | −0.11 (−2.23, 2.00) | −2.11 (−4.24, 0.005) |
| Mn | 9.42 (6.15–92.8) | 96.1 (75.6–143) | 15.9 (4.7–124) | 0.01 | 0.006 | 0.77 | 0.01 | 1.74 (0.54, 2.94) | 0.17 (−1.02, 1.37) | −1.56 (−2.76, −0.37) |
| Ni | 863 (842–2,601) | 2,491 (41.1–4,068) | 789 (462–2,628) | 0.75 | 0.5 | 0.95 | 0.54 | −0.50 (−1.99, 1.00) | −0.05 (−1.54, 1.44) | 0.45 (−1.05, 1.94) |
| Pb | 848 (402–4,141) | 839 (660–1007) | 1,079 (395–3,550) | 0.62 | 0.58 | 0.67 | 0.33 | −0.27 (−1.26, 0.72) | 0.21 (−0.79, 1.20) | 0.48 (−0.52, 1.47) |
| Sb | 5.15 (3.77–31.1) | 3.9 (3.4–5.7) | 13.2 (4.08–19.4) | 0.14 | 0.12 | 0.79 | 0.07 | −0.70 (−1.58, 0.19) | 0.11 (−0.77, 1.00) | 0.81 (−0.07, 1.70) |
| Sn | 36.2 (33.6–275) | 51.3 (31.4–52.1) | 111 (19.7–203) | 0.39 | 0.25 | 0.95 | 0.23 | −0.57 (−1.56, 0.42) | 0.03 (−0.96, 1.02) | 0.60 (−0.39, 1.59) |
| U | 0.05* (0.04–0.05*) | 0.05* (0.05*–0.05*) | 0.04 (0.04–0.05*) | 0.05 | 0.14 | 0.32 | 0.02 | 0.07 (−0.03, 0.17) | −0.05 (−0.15, 0.05) | −0.12 (−0.22, −0.02) |
| W | 0.04 (0.04–0.05*) | 0.05* (0.04–0.11) | 0.05* (0.04–0.22) | 0.07 | 0.27 | 0.02 | 0.2 | 0.35 (−0.29, 0.99) | 0.75 (0.12, 1.39) | 0.40 (−0.23, 1.04) |
| Zn | 2,664 (1,997–20,838) | 5,784 (4,236–6,337) | 6,952 (1,910–26,969) | 0.64 | 0.81 | 0.37 | 0.51 | 0.14 (−1.03, 1.31) | 0.52 (−0.65, 1.69) | 0.38 (−0.79, 1.58) |
indicate LOD values
Figure 1.
Boxplots of metal concentrations in aerosols by device power. The horizontal lines within boxes indicate medians; boxes, interquartile ranges; whiskers, values within 1.5 times the interquartile range from boxes; solid circles outside the box, outlier data values. Aerosols were generated using three powers in OD1 (20, 40 and 80 W) and OD2 (40, 120 and 200 W) with tobacco, blueberry, and menthol flavored e-liquids.
For OD2, concentrations of Cr and Mn increased significantly when device power was increased from the low (40 W) to the intermediate (120 W) setting, and then decreased significantly when power was further increased from the intermediate (120 W) to the high (200 W) setting.
Sensitivity analyses, in which we adjusted models for the log-transformed metal concentrations for puff number, confirmed the observed trends in metal levels with increasing device power.
3.2. Aerosol metal concentrations by device types
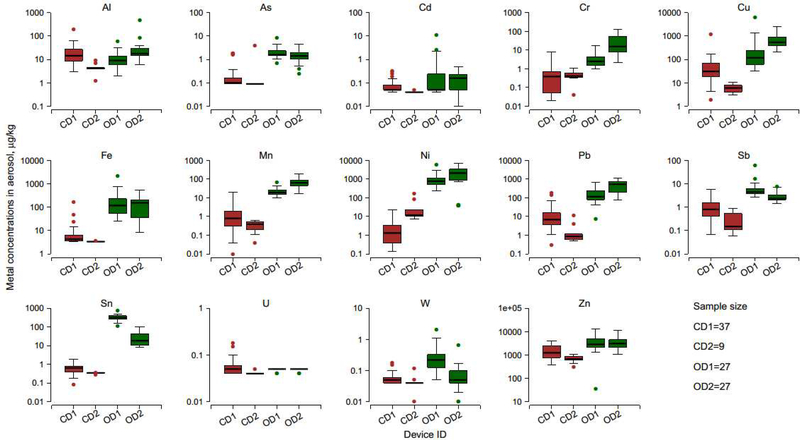
Of the 14 metals analyzed, concentrations were higher for open-system than for closed-system devices, except for Al and U for which levels were similar (Table 3,Figure 2). For Cd and U, 62% and 95% of all samples were <LOD, respectively. For Sn and W, 48% and 26% of closed-system device samples were <LOD, respectively. For Cr, only one sample from CD2 was <LOD. Other metals were detectable in all samples.
Table 3.
Median (interquartile range) metal concentrations (μg/kg) in aerosols by device type.
| Median (IQR) |
Linear regression p-value |
Beta value (95% CI) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD1 (n=37) | CD2 (n=9) | OD1 (n=27) | OD2 (n=27) | Overall p-value | CD vs. OD | CD1 vs. CD2 | OD1 vs. OD2 | CD vs. OD | CD1 vs. CD2 | OD1 vs. OD2 | |
| Al | 14.4 (8.63–27.2) | 4.12 (4.05–4.40) | 9.13 (5.96–13.9) | 17.7 (14.7–29.6) | <0.001 | 0.34 | <0.001 | <0.001 | 0.18 (−0.19, 0.54) | −1.30 (−1.89, −0.70) | 0.82 (0.38,1.26) |
| As | 0.10 (0.09–0.16) | 0.09 (0.09–0.09) | 1.59 (1.47–2.39) | 1.43 (1.04–1.98) | <0.001 | <0.001 | 0.65 | 0.11 | 2.35 (2.03, 2.67) | −0.14 (−0.73, 045) | −0.35 (−0.79, 0.09) |
| Cd | 0.05 (0.05–0.08) | 0.04 (0.04–0.04) | 0.05 (0.05–0.26) | 0.16 (0.05–0.23) | 0.008 | 0.002 | 0.22 | 0.45 | 0.66 (0.25, 1.07) | −0.47 (−1.22, 0.28) | −0.21 (−0.76, 0.34) |
| Cr | 0.39 (0.05–0.72) | 0.41 (0.34–0.57) | 2.51 (1.55–4.23) | 15.6 (7.88–54.5) | <0.001 | <0.001 | 0.42 | <0.001 | 3.25 (2.68, 3.81) | 0.37 (−0.53, 1.27) | 1.94 (1.27, 2.59) |
| Cu | 31.0 (18.2–67.2) | 6.02 (4.05–7.88) | 118 (60.6–247) | 542 (379–913) | <0.001 | <0.001 | <0.001 | <0.001 | 2.53 (2.04, 3.02) | −1.78 (−2.54, −1.01) | 1.39 (0.84, 1.95) |
| Fe | 4.28 (3.50–6.44) | 3.38 (3.36–3.42) | 118 (55.0–229) | 153 (36.6–211) | <0.001 | <0.001 | 0.14 | 0.27 | 3.00 (2.60, 3.40) | −0.55 (−1.28, 0.18) | −0.30 (−0.83, 0.24) |
| Mn | 0.80 (0.30–1.93) | 0.39 (0.20–0.53) | 19.6 (15.3–27.1) | 64.0 (45.5–90.6) | <0.001 | <0.001 | 0.03 | <0.001 | 4.14 (3.69, 4.60) | −0.87 (−1.64, −0.10) | 1.09 (0.53, 1.65) |
| Ni | 1.32 (0.39–3.35) | 11.9 (10.7–22.7) | 793 (508–1,169) | 2,148 (851–3,397) | <0.001 | <0.001 | <0.001 | 0.61 | 6.04 (5.41, 6.67) | 2.73 (1.69, 3.77) | 0.19 (−0.57, 0.96) |
| Pb | 6.88 (3.65–15.6) | 0.88 (0.57–1.15) | 115 (80.4–253) | 541 (193–749) | <0.001 | <0.001 | <0.001 | <0.001 | 3.74 (3.24, 4.25) | −1.89 (−2.69, −1.09) | 1.17 (0.58, 1.76) |
| Sb | 0.81 (0.40–1.52) | 0.15 (0.11–0.53) | 4.56 (3.68–6.23) | 2.33 (2.07–3.40) | <0.001 | <0.001 | <0.001 | 0.003 | 1.90 (1.53, 2.26) | −1.23 (−1.83, −0.62) | −0.68 (−1.12, −0.24) |
| Sn | 0.64 (0.39–0.81) | 0.35 (0.34–0.35) | 322 (269–391) | 18.6 (10.4–42.1) | <0.001 | <0.001 | 0.01 | <0.001 | 5.05 (4.58, 5.52) | −0.54 (−0.98, −0.11) | −2.74 (−3.07, −2.43) |
| U | 0.05* (0.04–0.06) | 0.04 (0.04–0.04) | 0.05* (0.05*–0.05*) | 0.05* (0.05*–0.05*) | 0.002 | 0.16 | <0.001 | 1.00 | −0.07 (−0.16, 0.03) | −0.31 (−0.48, −0.14) | 0.00 (−0.12, 0.12) |
| W | 0.05* (0.04–0.06) | 0.04 (0.04–0.04) | 0.22 (0.13–0.33) | 0.05* (0.04–0.10) | <0.001 | <0.001 | 0.21 | <0.001 | 0.76 (0.41, 1.11) | −0.34 (−0.87, 0.19) | −1.37 (−1.76, −0.98) |
| Zn | 1,247 (769–2,470) | 683 (597–864) | 2,888 (2,130–4,915) | 3,114 (2,075–4,444) | <0.001 | <0.001 | 0.02 | 0.68 | 0.97 (0.65, 1.29) | −0.67 (−1.24, −0.09) | 0.09 (−0.33, 0.51) |
indicate LOD values
Figure 2.
Boxplots of metal concentrations in aerosols by device type. The horizontal lines within boxes indicate medians; boxes, interquartile ranges; whiskers, values within 1.5 times the interquartile range from boxes; solid circles outside the box, outlier data values. For open-system devices (OD1, OD2), only samples collected at the intermediate power setting were analyzed.
For Cr and Ni, known components of coil alloys, higher levels were found for open-system devices. Median concentrations (μg/kg) of Cr were 2.51 for OD1, 15.6 for OD2, 0.39 for CD1, and 0.41 for CD2. For Ni, median concentrations were 793 for OD1, 2,148 for OD2, 1.32 for CD1 and 11.9 for CD2. For Pb, the median concentrations of 115 and 541 μg/kg for OD1 and OD2 were 17 to 615 times higher compared to 6.88 and 0.88 μg/kg for CD1 and CD2. Similarly, Al, As, Cu, Fe, Mn, Sb, Sn, U, W and Zn concentrations were higher for open-system than closed-system devices.
We found statistically significant differences in aerosol concentrations of eight metals between the two open-system devices. Median Al, Cr, Cu, Mn and Pb levels were ~2, 6, 5, 3 and 5 times higher for OD2 than for OD1 (Table 3). In contrast, median Sb, Sn and W levels were ~2, 17 and 4 times higher for OD1 than for OD2, respectively. No significant differences in As, Cd, Fe, Ni, U and Zn levels were found between the open-system devices. Among the closed-system devices, Al, Cu, Fe, Pb, Sb, Sn, U and Zn concentrations were higher for CD1 than CD2; CD2 exhibited ~9 times higher Ni concentrations than CD1.
Aerosol metal concentrations spanned several orders of magnitude within e-cigarette devices. For example, Pb levels (μg/kg) ranged from 7.73 to 703.6 for OD1, and from 79.5 to 1,187 for OD2. Similarly, Fe levels (μg/kg) ranged from 24.7 to 2,236 for OD1, and from 8.49 to 546 for OD2.
The sensitivity analysis adjusting for puff number also found significantly higher metal levels for open-system compared to closed-system devices. However, a difference was found in the comparison between the closed-system devices: differences in metal concentrations between CD1 and CD2 after adjustment were not significant, except for zinc (Table S2).
3.3. Metal exposure limits
For Mn, the daily inhaled dose of typical e-cigarette users would range from 0.02 to 0.13 μg/d for OD1, and from 0.10 to 0.67 μg/d for OD2; no samples from OD1 and 7.4% from OD2 would cause MRL exceedance (Table 4). For Ni, all samples from OD1 and 88.9% from OD2 would cause MRL exceedance, with the daily inhaled dose (μg/d) ranging from 0.59 to 9.10 for OD1, and from 0.10 to 32 for OD2. Aerosols from the closed-system devices would not cause MRL exceedance for Mn and Ni. Compared to daily inhaled dose from reference cigarette 3R4F, 100% of the aerosol samples from OD1 and 77.8% from OD2 would result in Ni exposure higher than from the reference cigarette; Mn doses from all the aerosol samples were lower than those from the reference cigarette. The daily inhaled Pb dose ranged from 8.4×10−5 to 1.0×10−1 for CD1 and from 9.0×10−5 to 2.2×10−3 μg/d for CD2. Higher daily inhaled Pb doses were obtained for open-system devices, ranging from 0.013 to 0.15 for OD1 and from 0.42 to 7.8 μg/d for OD2.
Table 4.
Median (range) of daily inhaled metal dose (μg/d) in aerosols with related exposure limits for Mn, Ni, and Pb.
| Value | Mn | Ni | Pb | |
|---|---|---|---|---|
| CD1 | Median (Range) |
3.2×10−4
(6.2×10−6–8.9×10−3) |
6.1×10−4
(4.1×10−5–7.4×10−3) |
2.9×10−3
(8.4×10−5–1.0×10−1) |
| Percent exceeding limit (%) | 0 | 0 | ||
| CD2 | Median (Range) |
8.2×10−5
(6.9×10−6–1.1×10−4) |
2.2×10−3
(1.5×10−3–3.1×10−2) |
1.6×10−4
(9.0×10−5–2.2×10−3) |
| Percent exceeding limit (%) | 0 | 0 | ||
| OD1 | Median (Range) |
3.8×10−2
(2.0×10−2–1.3×10−1) |
1.4 (5.9×10−1–9.11) |
2.2×10−1
(1.3×10−2–1.5) |
| Percent exceeding limit (%) | 0 | 100 | ||
| OD2 | Median (Range) |
2.8×10−1
(9.5×10−2–6.7×10−1) |
9 (1.0×10−1–32) |
2.4 (4.2×10−1–7.8) |
| Percent exceeding limit (%) | 7.4 | 88.9 | ||
| Limits | 0.62 a | 0.14 b | ||
| Reference cigarette 3R4F | 2.1 | 0.59 |
MRL for Mn (ATSDR 2012). MRLs are daily averages
ATSDR MRL for Ni (ATSDR 2005; U.S. EPA 2000).
4. Discussion
Vaping practices such as selecting device power is a determining factor of metal concentrations in aerosol generated by e-cigarettes. Notably, for many but not all of the metals measured, aerosol concentrations generally increased with power. At higher power, metal levels appeared to level off. Aerosol metal concentrations varied considerably between e-cigarette devices. Levels of most metals were higher for open-system than for closed-system devices.
Device power potentially affects metal emission, because it affects coil temperature. Zhao et al. (2018) found that coil temperature increased from 106.8 to 265.8°C when voltage was increased from 2.2 to 5.7 V (along with an increase in power). It is conceivable that coil degradation and associated metal emissions increase with temperature and therefore device power. A dependence of toxicant emissions on power has also been found for other chemicals: Sleiman et al. (2016) found that the e-liquid consumed per puff increased linearly when voltage was increased from 3.3 to 4.3 V, and then remained almost constant when the voltage was further increased to 4.8 V. They suggested that below 4.3 V most of the power was used to evaporate e-liquid. Voltages above 4.3 V led to only a marginally higher evaporation but caused enhanced decomposition of the e-liquid solvent thereby forming organic toxicants such as aldehydes.
Differences in aerosol metal levels among the four devices are potentially related to coil composition. According to the manufacturers, the coils of the closed-system devices were made of Nichrome, an alloy consisting of 80% Ni and 20% Cr (Palazzolo et al., 2017). Coils of OD1 consisted of Kanthal, which contains mainly Al, Cr, and Fe (Williams et al., 2017). Coils of OD2 consisted of stainless steel, primarily containing Cr, Fe, and Mn (Farsalinos et al., 2015; Williams et al., 2013). Consistently, aerosol Fe levels were higher for open-system than for closed-system devices. Interestingly, Ni levels were higher in open-system than in closed-system devices, even though the coils of the closed-system devices contained 80% Ni. This could be due to Ni releases from other parts of the devices such as joints and wires. Higher available power in open-system devices could also explain the higher Ni levels. Aerosol Cr levels were also higher for the open-system devices, but is not clear whether this can be attributed to coil composition, because Cr content in both Kanthal and stainless steel can vary substantially and be less or greater than the 20% Cr content of Nichrome. More Mn was detected in aerosols from OD2 compared to OD1, likely because Mn is a major ingredient in stainless steel. However, less Al was detected in aerosols from OD1 compared to OD2, even though the coils of OD1 contained substantial amounts of Al, potentially indicating that other parts of the device released Al.
Metals in the aerosols could also originate from the e-liquid. For open-system devices, the median (interquartile range) As, U, and W concentrations in the e-liquid were 0.90 (0.43, 1.35), 0.05 (0.04, 0.05), and 0.15 (0.10, 0.42) μg/kg, while in the aerosol samples they were 1.57 (1.26, 2.30), 0.05 (0.05, 0.05), and 0.11 (0.05, 0.22) μg/kg, respectively. The fact that concentrations in the e-liquid were similar to those in the aerosol samples supports that most As, U, and W was transferred together with the solvent (e.g., propylene glycol or vegetable glycerin) from the e-liquid into the aerosol, rather than been transferred from the coil or other parts of the device. For As, a similar finding was reported in a study of e-liquids and aerosol samples from daily e-cigarette users from Maryland (Olmedo et al. 2018). For Sb, the median (interquartile range) concentration of 3.43 (2.36, 4.94) in aerosol was two-fold higher than that in the e-liquid of 1.26 (0.74, 1.67) μg/kg; however, the additional source of Sb is unknown.
Metal concentrations varied largely within e-cigarette devices, which is consistent with findings from other studies (Hess et al. 2017; Williams et al. 2015). To examine how emissions of metals varied with time passed since aerosol collection was started with a device initially at room temperature, three vials of aerosol were collected consecutively in a single session. We found no clear patterns of aerosol metal concentrations over time, and for most metals the aerosol concentrations were relatively steady (Figure S1). Aging of the devices could contribute to deterioration of the coil and other metallic parts of the device, resulting in gradual release of metals to the aerosol. More research is needed to better understand the high variability in metal releases within devices.
Differences in aerosol metal concentrations were also found between flavors (results not shown). It was not apparent, however, which e-liquid characteristics were associated with increased aerosol metal levels. We found that a flavor group (e.g., “tobacco” which is offered as “Tobacco” flavor for CD1 and as “Virginia tobacco” for CD2) is not a predictor for aerosol metal levels, perhaps because different e-liquid manufacturers use different formulas and different ingredient purity to produce the same (or similar) flavor.
Our aerosol sampling protocol was not always representative of typical e-cigarette use, for which the average number of puffs in a single vaping session is on the order of 20 or less (National Academies of Sciences, Engineering, and Medicine, 2018). For open devices we used up to 120 puffs at the lowest power setting. However, there is no indication that the larger number of puffs would affect metal levels as shown by our sensitivity analyses as well as the fact that metal levels in consecutively collected aerosol samples did not show a clear trend.
The two closed-system devices showed statistically significant differences between metal concentrations before adjustment for number of puffs, and the significance of those differences disappeared with adjustment for the number of puffs. Because the number of puffs was very different for both types of devices due to the need to increase the number of puffs to collect sufficient aerosol for CD2, collinearity between device type (CD1 and CD2) and the number of puffs collected could increase the standard errors and lead to an underestimation of the association.
In recent years, use of CD2 (JUUL brand) has increased rapidly in middle and high schools (Hammond et al., 2018; Huang et al., 2018). In a national online survey conducted in 2017, almost 8% of 1012 young people aged 15–24 reported ever use of JUUL in the past 30 days (Willett et al., 2018). The current use rate of JUUL is 47.1% among 875 ever e-cigarette users from 4 high schools (Krishnan-Sarin et al. 2019). Although JUUL might be safer compared to open-system devices from the metal toxicity perspective, we found higher Ni and Cr levels in JUUL aerosol compared to CD1. These two metals have been associated with respiratory diseases and lung cancer (IARC 2012a, 2012b; Jaishankar et al. 2014). Unintended metal exposure to developing youth could thus result in detrimental health consequences due to JUUL use.
A large portion of aerosol samples from open-system devices exceeded metal exposure limits. Nickel represents a serious problem as 89% to 100% of aerosol samples exceeded the MRL and 78% to 100% of the samples would result in daily Ni doses higher than those from the reference cigarette. Even though no MRL for Pb is available, daily inhaled Pb dose was calculated since low Pb exposure can result in adverse health effects (Jusko et al. 2008; Fadrowski et al. 2010). Aerosols from the closed-system devices would not exceed the MRLs for Mn and Ni, and daily inhaled doses of Mn and Ni from these aerosols would be lower than those from the mainstream smoke from the reference cigarette.
A recent review found that 68 countries around the world have regulations on e-cigarettes, mainly focusing on advertising, minimum age of consumers, and restrictions of places where e-cigarette use is permitted (Kennedy et al., 2017). The Family Smoking Prevention and Tobacco Control Act regulates manufacturing, distribution, and marketing of tobacco products in the US since 2009. Since 2016, e-cigarettes in the USA have been deemed tobacco products and under the regulatory authority of the US Food and Drug Administration (FDA) (FDA 2016). FDA has recently announced stricter regulations on the sales of JUUL and other e-cigarettes to reduce youth access (FDA 2018). However, device design including coil composition and resistance, as well as device power, has so far not being regulated by FDA.
This study has several limitations. First, a relatively small number of devices was investigated. Even though open-system devices were found to generate higher metal concentrations than closed-system devices, due to limited funding, we only tested four devices. Differences in metal concentrations by device type therefore need to be interpreted cautiously, and more research is needed to investigate the effects of device type on metal emissions. Second, the open-system devices we tested permit continuous control of power, but we only examined three power settings. The effects of power on metal emissions should be studied more systematically with special emphasis on a potential threshold at which metal emissions may level off. Third, metal leaching from device components such as the coil, wires, brass clamps, and joints was not evaluated directly, although this information would be crucial for policy makers. Lastly, different metal species can have widely differing human health hazards. Our metals analyses did not provide any information on speciation, e.g., the proportions of Cr(III) and Cr(VI) in our samples.
Notwithstanding these limitations, the findings may help inform legislators, policy makers, and healthcare providers on potential health risks associated with e-cigarette design and manufacturing, providing novel information on which device type and power setting are most likely to increase metal exposure. We also provide a direct comparison of aerosol metal levels between open- and closed-system devices, finding that exposure to aerosolized metals can be largely reduced by using e-cigarettes with lower power settings. Inhalation of a high percentage of aerosol samples from the open-system devices by typical e-cigarette users would cause exceedance of the MRLs for Mn and Ni, and potentially of Cr(VI). The MRLs would not be exceeded for the closed-system devices. Therefore, open-system devices (mods) may place users at increased health risks due to exposure to high levels of toxic metals as compared to closed-system devices (cigalikes and pods).
5. Conclusions
Levels of harmful metals in aerosols were found to be largely dependent on device power and type. Most notably, higher device power was associated with higher aerosol metal levels. Open-system devices (mods) may place users at increased health risks due to exposure to high levels of toxic metals as compared to closed-system devices (cigalikes and pods). Regulations on device composition and operation parameters should be considered.
Supplementary Material
Open-system e-cigarettes can be refilled with e-liquid unlike closed-system devices (85 characters)
Users of open-system e-cigarettes are exposed to higher levels of toxic metals (81 characters)
Open–system devices typically emit more toxic metals when device power is increased (85 characters)
We evaluated metal emissions from the most popular closed-system device, a “pod” (82 characters)
Acknowledgments
Research reported in this publication was supported by NIEHS and FDA Center for Tobacco Products (CTP) (grant 1R21ES029777-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration. Moreover, this study was supported by NIEHS grant P30 ES009089 and by a Johns Hopkins University Technology Transfer Seed Award. DZ was supported by the China Scholarship Council (201706190116). PO was supported by the Alfonso Martín Escudero Foundation (Postdoctoral Fellowship 2014). AA was supported by a grant from the Cigarette Restitution Fund (State of Maryland; grant PHPA-G2034).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aherrera A, Olmedo P, Grau-Perez M, et al. , 2017. The association of e-cigarette use with exposure to nickel and chromium: A preliminary study of non-invasive biomarkers. Environ. Res. 159, 313–320. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). 2012. Toxicological profile for manganese. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=102&tid=23 [accessed 1 November 2017]. [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). 2005. Toxicological profile for nickel. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=245&tid=44 [accessed 1 November 2017]. [Google Scholar]
- Badea M, Luzardo OP, Gonzalez-Antuna A, et al. , 2018. Body burden of toxic metals and rare earth elements in non-smokers, cigarette smokers and electronic cigarette users. Environ Res. 166, 269–275 [DOI] [PubMed] [Google Scholar]
- Bhatnagar A, Whitsel LP, Ribisl KM, et al. , 2014. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 130, 1418–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CJ, Cheng JM, 2014. Electronic cigarettes: product characterisation and design considerations. Tob Control. 23 Suppl 2, ii4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Zhuang YL, Zhu SH, et al. , 2016. E-Cigarette Design Preference and Smoking Cessation: A U.S. Population Study. Am J Prev Med. 51, 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Voudris V, Poulas K, et al. , 2015. Are metals emitted from electronic cigarettes a reason for health concern? A risk-assessment analysis of currently available literature. Int. J. Environ. Res. Public Health 12, 5215–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadrowski JJ, Navas-Acien A, Tellez-Plaza M, et al. , 2010. Blood lead level and kidney function in US adolescents: The Third National Health and Nutrition Examination Survey. Arch Intern Med. 170, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. 2018. Warning Letters and Civil Money Penalties Issued to Retailers for Selling JUUL and Other E-Cigarettes to Minors. https://www.fda.gov/TobaccoProducts/NewsEvents/ucm605278.htm
- Food and Drug Administration. 2016. Deeming tobacco products to be subject to the federal food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products; Final Rule. Fed. Reqist 81(90):28973–29106. [PubMed] [Google Scholar]
- Glasser AM, Collins L, Pearson JL, et al. , 2017. Overview of Electronic Nicotine Delivery Systems: A Systematic Review. Am. J. Prev. Med 52, 33–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Knysak J, Gawron M, et al. , 2014. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 23, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grana R, Benowitz N, Glantz SA et al. , 2014. E-cigarettes: a scientific review. Circulation. 129, 1972–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond D, Wackowski OA, Reid JL, et al. , 2018. Use of Juul E-Cigarettes Among Youth in the United States. Nicotine Tob. Res. 10.1093/ntr/nty237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess CA, Olmedo P, Navas-Acien A, et al. , 2017. E-cigarettes as a source of toxic and potentially carcinogenic metals. Environ Res. 152, 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Duan Z, Kwok J, et al. , 2018. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob. Control doi: 10.1136/tobaccocontrol-2018-054382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer). 2012a. Chromium (VI) compounds. IARC Monographs 100C:147–167. [Google Scholar]
- IARC. 2012b. Nickel and nickel compounds. IARC Monographs 100C:169–218. [Google Scholar]
- Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. 2014. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7(2):60–72, PMID: 26109881, 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaishankar M, Tseten T, Anbalagan N, et al. , 2014. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 7, 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusko TA, Henderson CR, Lanphear BP, et al. , 2008. Blood lead concentrations < 10 microg/dL and child intelligence at 6 years of age. Environ Health Perspect. 116, 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavuluru R, Han S, Hahn EJ 2018. On the popularity of the USB flash drive-shaped electronic cigarette Juul. Tob. Control doi: 10.1136/tobaccocontrol-2018-054259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RD, Awopegba A, De Leon E, et al. , 2017. Global approaches to regulating electronic cigarettes. Tob. Control 26, 440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent M 2006. The Oxford Dictionary of Sports Science & Medicine. 3rd Edition. Oxford University Press. [Google Scholar]
- Kim JJ, Sabatelli N, Tutak W, et al. , 2017. Universal electronic-cigarette test: physiochemical characterization of reference e-liquid. Tob Induc Dis. 15, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmider L, Sobczak A, Fik M, et al. , 2014. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res. 16, 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmider L, Spindle TR, Gawron M, et al. , 2018. Nicotine emissions from electronic cigarettes: Individual and interactive effects of propylene glycol to vegetable glycerin composition and device power output. Food Chem. Toxicol 115, 302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Jackson A, Morean M, et al. , 2019. E-cigarette devices used by high-school youth. Drug Alcohol Depend. 194, 395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheev VB, Brinkman MC, Granville CA, et al. , 2016. Real-Time Measurement of Electronic Cigarette Aerosol Size Distribution and Metals Content Analysis. Nicotine Tob Res. 18, 1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine, 2018. Public Health Consequences of E-Cigarettes. Editors: Eaton DL, Kwan LY, and Stratton K, Washington (DC), National Academies Press (US). [PubMed] [Google Scholar]
- Olmedo P, Goessler W, Tanda S, et al. , 2018. Metal Concentrations in e-Cigarette Liquid and Aerosol Samples: The Contribution of Metallic Coils. Environ. Health Perspect. 126, 10.1289/EHP2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmedo P, Navas-Acien A, Hess C, et al. , 2016. A direct method for e-cigarette aerosol sample collection. Environ. Res. 149, 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas RS, et al. , 2014. Toxic Metal Concentrations in Mainstream Smoke from Cigarettes Available in the USA. J Anal Toxicol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzolo DL, Crow AP, Nelson JM, et al. , 2017. Trace Metals Derived from Electronic Cigarette (ECIG) Generated Aerosol: Potential Problem of ECIG Devices That Contain Nickel. Front Physiol. 7, 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopowicz A, Sobczak A, Szula-Chraplewska M, et al. , 2018. Exposure to Cadmium and Lead in Cigarette Smokers Who Switched to Electronic Cigarettes. Nicotine Tob Res. nty161, 10.1093/ntr/nty161 [DOI] [PubMed] [Google Scholar]
- Qasim H, Karim ZA, Rivera JO, et al. , 2017. Impact of Electronic Cigarettes on the Cardiovascular System. J. Am. Heart Assoc. 6, 006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang C, Huang J, Chaloupka FJ, et al. , 2017. The impact of flavour, device type and warning messages on youth preferences for electronic nicotine delivery systems: evidence from an online discrete choice experiment. Tob. Control 0, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleiman M, Logue JM, Montesinos VN, et al. , 2016. Emissions from Electronic Cigarettes: Key Parameters Affecting the Release of Harmful Chemicals. Environ. Sci. Technol. 50, 9644–9651. [DOI] [PubMed] [Google Scholar]
- St Helen G, Havel C, Dempsey DA, Jacob P III., Benowitz NL, 2016. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction 111(3):535–544, PMID: 26430813, 10.1111/add.13183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statista, 2019. Dollar sales share of electronic cigarettes (e-cigarettes) in U.S. convenience stores (C-stores) in 2017, by brand 2019. Available from: https://www.statista.com/statistics/285116/us-e-cigarettes-companies-trend-in-market-share/ Accessed on 1/14/2019.
- Tayyarah R, Long GA, 2014. Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Regul Toxicol Pharmacol. 70, 704–710. [DOI] [PubMed] [Google Scholar]
- Talih S, Balhas Z, Eissenberg T, et al. , 2015. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine Tob. Res 17, 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2000. Nickel Compounds. https://www.epa.gov/sites/production/files/2016-09/documents/nickle-compounds.pdf [accessed 19 December 2017].
- U.S. EPA (U.S. Environmental Protection Agency). 2016. NAAQS Table. https://www.epa.gov/criteria-air-pollutants/naaqs-table [accessed 5 October 2017].
- Walley SC, et al. , 2015. Electronic Nicotine Delivery Systems. Pediatrics. 136, 1018–1026. [DOI] [PubMed] [Google Scholar]
- Willett JG, Bennett M, Hair EC, et al. , 2018. Recognition, use and perceptions of JUUL among youth and young adults. Tob. Control doi: 10.1136/tobaccocontrol-2018-054273 [DOI] [PubMed] [Google Scholar]
- Williams M, Bozhilov K, Ghai S, et al. , 2017. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS One. 12, e0175430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, To A, Bozhilov K, et al. , 2015. Strategies to Reduce Tin and Other Metals in Electronic Cigarette Aerosol. Plos One. 10, e0138933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Ghai S, Talbot P, et al. , 2013. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One. 8, e57987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Nelson J, Dada O, et al. , 2018. Assessing electronic cigarette emissions: linking physico-chemical properties to product brand, e-liquid flavoring additives, operational voltage and user puffing patterns. Inhal. Toxicol 30, 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu SH, Sun JY, Bonnevie E, et al. , 2014. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob. Control 23 Suppl 3, iii3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.