Abstract
Oral squamous cell carcinoma (OSCC) is the leading cause of mortality for oral cancer. Numerous risk factors mainly related to unhealthy habits and responsible for chronic inflammation and infections have been recognized as predisposing factors for oral carcinogenesis. Recently, even microbiota alterations have been associated with the development of human cancers. In particular, some specific bacterial strains have been recognized and strongly associated with oral cancer development (Capnocytophaga gingivalis, Fusobacterium spp., Streptococcus spp., Peptostreptococcus spp., Porphyromonas gingivalis and Prevotella spp.). Several hypotheses have been proposed to explain how the oral microbiota could be involved in cancer pathogenesis by mainly paying attention to chronic inflammation, microbial synthesis of cancerogenic substances, and alteration of epithelial barrier integrity. Based on knowledge of the carcinogenic effects of dysbiosis, it was recently suggested that probiotics may have anti-tumoral activity. Nevertheless, few data exist with regard to probiotic effects on oral cancer. On this basis, the association between the development of oral cancer and oral dysbiosis is discussed focusing attention on the potential benefits of probiotics administration in cancer prevention.
Keywords: microbiota, microbiome, oral cancer, carcinogenesis, chronic inflammation, dysbiosis, oral squamous cell carcinoma, probiotics
1. Introduction
Oral squamous cell carcinoma (OSCC) is the leading cause of death among all oral cancers. This tumor originates from the oral mucosa and accounted for over 350,000 new diagnoses and more than 175,000 recorded deaths worldwide in 2018 (1). It was widely demonstrated that the development of tumors, including that of OSCC, is sustained by several risk factors and predisposing conditions such as fibers, chemicals, pesticides and heavy metals able to induce pro-oncogenic genetic and epigenetic alterations (2–6). Other factors, including oral injuries, inflammatory diseases, infections, and bacterial dysbiosis are now recognized as risk factors for cancer development (7–10).
Regarding OSCC, it was demonstrated that its development is strictly influenced by host-related and lifestyle factors mainly represented by smoking, alcohol abuse, tobacco and tobacco-derivate chewing and oral virus infections (HPV) (11,12). In addition, it was demonstrated that the combination of smoke, alcohol drinking and poor oral hygiene increase the risk of oral cancer onset due to chronic inflammation and infection which constitute the principal factors involved in cancer pathogenesis, influencing the resident microbiota that are involved in the homeostasis of the oral environment (13–17). Importantly, a precise distinction must be made between microbiota and microbiome: the former is comprised of all the bacteria species hosted within the oral cavity, while with the latter term is used to define the collective genomes of microorganisms inhabiting the oral mucosa (18–20).
Oral microbiota changes are able to modulate the connection between oral bacteria and humans causing diseases (21,22). Oral microbiota seems to influence OSCC through the carcinogenetic modulation of cell metabolism (such as modifying the concentration of nutrients and vitamins), thereby promoting the production of different cytokines known to be involved in several pathological conditions (23–27).
Over 600 bacterial species constitute the oral microbiota. However, the majority of these species are uncultivated (19). The availability of new sequencing technology allowed the identification of bacterial communities that harbor the oral cavity and that are involved in human health (28) (Table I).
Table I.
Most common microbial species represented in a normal bacterial flora of oral cavity.a
| Bacterial species | Characteristics | Localization | Distribution |
|---|---|---|---|
| Streptococcus mitis | Gram-positive coccus, | Buccal surface | High |
| facultative anaerobe. | Vestibule | High | |
| It has been associated with: | Tongue | High | |
| i) Bacterial endocarditis, especially in | Palate | High | |
| patients with prosthetic valves; | Tonsils | High | |
| ii) Infection in immunocompromised | Tooth surfaces | High | |
| patients, particularly immediately after tissue transplants and in neutropenic cancer patients. | Subgingival surface | High | |
| Streptococcus | Gram-positive coccus, | Buccal surface | Medium |
| sanguis | facultative anaerobe. | Tongue lateral | Medium |
| It has been associated with: | Palate | Medium | |
| i) Bacterial endocarditis, especially in | Tooth surfaces | High | |
| patients with prosthetic valves; ii) Infection in immunocompromised patients. | Subgingival surface | Medium | |
| Streptococcus | Gram-positive coccus, | Buccal surface | Medium |
| gordonii | facultative anaerobe. | Vestibule | Medium |
| It has been associated with: | Palate | Medium | |
| i) Bacterial endocarditis, especially in | Tooth surfaces | High | |
| patients with prosthetic valves; ii) Infection in immunocompromised patients. | Subgingival surface | Medium | |
| Gemella sanguinis | Gram-positive coccus, | Buccal surface | High |
| facultative anaerobe. | Vestibule | High | |
| It has been associated with: | Tongue lateral | High | |
| i) Bacterial endocarditis. | Palate | High | |
| Gemella | Gram-positive coccus, | Tonsils | High |
| haemolysans | facultative anaerobe. | Tooth surfaces | High |
| It has been associated with: i) Bacterial endocarditis. | Subgingival surface | Medium | |
| Granulicatella | Gram-positive coccus, | Buccal surface | Medium |
| elegans | facultative anaerobe. | Vestibule | High |
| Tongue lateral | Medium | ||
| It has been associated with: | Hard palate | High | |
| i) Infective endocarditis. | Soft palate | Medium | |
| Tonsils | Medium | ||
| Subgingival surface | Medium | ||
| Granulicatella adiacens | Gram-positive coccus, facultative anaerobe. | Buccal surface Vestibule | High Medium |
| Tongue | High | ||
| It has been associated with: | Hard palate | Medium | |
| i) Infective endocarditis. | Soft palate | High | |
| Tooth surfaces | High | ||
| Subgingival surface | Medium | ||
| Neisseria spp. | Gram-negative diplococci, aerobic. | Buccal surface | Medium |
| Tongue | Medium | ||
| Most gonococcal infections are | Palate | High | |
| asymptomatic and self-resolving | Tonsils | Medium | |
| except for N. meningitidis and | Tooth surfaces | High | |
| N. gonorrhoeae. | |||
| Streptococcus mitis | Gram-negative rod-shaped bacteria, | Buccal surface | Medium |
| anaerobic. | Tongue | Medium | |
| It is a fundamental human pathogen | Soft palate | Medium | |
| in various anaerobic infections | Tonsils | Medium | |
| (i.e. transmissible subcutaneous | Tooth surfaces | Medium | |
| infections). | Subgingival surface | Medium |
Microbial species as indicated (205).
Researchers have investigated the possible association between microbes and the alteration of physiological conditions. In this context, it was demonstrated that gut microbiota predisposes individuals for the development of different diseases including celiac disease, neurological disorders and blood pressure alterations (29–31). All these pathological conditions are involved in the development of severe health conditions mainly represented by neurovascular disorders, chronic degenerative diseases, and cancer (32–46). Among all cancers, oral cancer is particularly related with oral and gut microbiota composition as widely demonstrated. Among the different bacteria strongly associated with OSCC, Fusobacterium nucleatum, Porphyromonas gingivalis, and Prevotella intermedia are considered the most represented bacteria types (47–51). Moreover, other bacterial genera, such as Actinomyces, Clostridium, Enterobacteriaceae, Fusobacterium, Haemophilus, Porphyromonas, Prevotella, Streptococcus spp. and Veillonella are associated with pre-cancerous lesions and oral cancer (52). According to other studies, a high bacterial load of Prevotella melaninogenica, Streptococcus mitis and Capnocytophaga gingivalis are identifiable in saliva samples of OSCC patients (Table II) (50,51).
Table II.
Predominant microbial communities associated with OSCC.
| Bacterial species | Localization (206) | Refs. | Type of sample |
|---|---|---|---|
| Actinomyces spp | Tooth surface | (52) | Saliva samples |
| Bacteroides spp | Gingival crevice | (110,111,187) | Saliva samples |
| Bifidobacterium spp | Tooth surface | (112,113) | Plaque biofilm samples |
| Capnocytophaga spp | Gingival crevice Tongue | (49) | Samples of gingival SCC and normal gingiva |
| (50) | Oral rinse | ||
| (51) | Saliva samples | ||
| (53) | Oral swabs | ||
| (117) | Oral rinse | ||
| (181–184) | Samples of oral tumour and precancerous leukoplakia Samples of tongue and floor SCC and normal tissues | ||
| Catonella spp | Gingival crevice | (175) | Oral swabs |
| Clostridium spp | Gingival crevice | (52) | Saliva samples |
| Dialister spp | Gingival crevice | (175) | Oral swabs |
| Enterobacteriaceae spp | Gingival crevice | (52) | Saliva samples |
| Enterococcus spp | Tongue, tooth surface | (53) | Saliva samples |
| Filifactor spp | Gingival crevice | (175) | Oral swabs |
| Firmicutes spp | Gingival crevice | (187) | Saliva samples |
| Fusobacteria spp | Gingival crevice | (51) | Saliva samples |
| (117) | Oral swabs | ||
| (175) | OSCC biopsies and deep- | ||
| (176) | epithelium swabs | ||
| (180) | Oral rinse | ||
| Haemophilus spp | Oropharynx | (52) | Saliva samples |
| Tonsil | (181) | Oral rinse | |
| Lactobacillus spp | Tooth surface | (112,113) | Plaque biofilm samples |
| Lactococcus spp | Tooth surface | (112,113) | Plaque biofilm samples |
| Leuconostoc spp | Tooth surface | (112,113) | Plaque biofilm samples |
| Oribacterium spp | Gingival crevice Tooth surface | (181) | Oral rinse |
| Paludibacter spp | Gingival crevice Tooth surface | (181) | Oral rinse |
| Parvimonas spp | Gingival crevice | (53) | Saliva samples |
| Tooth surface | (175) | Oral swabs | |
| Pediococcus spp | Tooth surface | (112,113) | Plaque biofilm samples |
| Peptococcus spp | Gingival crevice | (175) | Oral swabs |
| Peptostreptococcus spp | Tooth surface | (51) | Saliva samples |
| Gingival crevice | (53) | Samples of dental abscess, | |
| (90) | endodontic or pericoronal | ||
| (114) | infection, periodontal pocket | ||
| (175) | Oral swabs | ||
| (183) | Samples of tongue and floor | ||
| (184) | SCC and normal tissues | ||
| Porphyromonas gingivalis | Tongue | (47–49) | Samples of gingival SCC |
| Gingival crevice | (51,53) | and normal gingiva | |
| (90) | Saliva samples | ||
| (183) | Samples of tongue and floor | ||
| (184) | SCC and normal tissues | ||
| Prevotella intermedia | Gingival crevice | (50,51) | Oral rinse |
| (90) | Saliva samples | ||
| Rothia spp | Gingival crevice | (122) | Oral rinse |
| Tooth surface | |||
| Slackia spp | Gingival crevice | (53) | Saliva samples |
| Streptococcus spp | Gingival crevice | (50) | Oral rinse |
| Oropharynx | (51) | Saliva samples | |
| Tooth surface | (52) | Samples of oral tumour and | |
| Tonsil | (90) | precancerous leukoplakia | |
| Gingival crevice | (122) | ||
| Oropharynx | (124) | ||
| Tooth Surface | (125) | ||
| Tonsil | (182) |
Although the association between some species and oral cancer was already established, the complexity of the relationship occurring between cancer and oral microbiota remains unexplained and cannot be limited to the evaluation of a single pathogen (53). Moreover, there are no concordant analytical protocols for the analysis of oral microbiota and microbiome. Therefore, it is difficult to establish oral cavity-associated microbial patterns in cancer patients and healthy subjects. Consequently, there is a lack of novel microbial biomarkers for the early identification of oral carcinoma (54,55).
Previous findings demonstrated that a strong association between oral microbiota and oral cancer exists. Starting from this supposition, a number of studies focused on the prevention of neoplastic transformation and retardation of cancer progression by modulating the carcinogenic or protective microbiome. In this context, probiotics administration was recently considered a good cancer preventive strategy due to the immunological effects (8,9,25). The beneficial effects of lactic ferments and probiotics were identified in the 19th century by Dr Ilya Metchnikoff. Nevertheless, only in recent years have these products been widely used for the treatment of several diseases (56,57). Numerous studies have shown the potential positive effects of probiotics on cancer through several mechanisms that include immune modulation, the prevention of pathogen infections, inflammatory modulation, reduced cancer formation and metastatic process (9,25). To the best of our knowledge, few data have been generated about the effects of probiotics in oral cancer development. Of note, the results of a previous study demonstrated that the administration of Lactobacillus rhamnosus GG (LGG) was able to increase the effects of geniposide, an anticancer molecule tested on human oral squamous carcinoma cells (HSC-3), demonstrating the beneficial role of LGG as potential adjuvant of geniposide treatment (58).
The aim of this review was to describe the scientific evidence collected during the years pertaining to oral microbiota and neoplastic transformation with special attention for OSCC. Finally, a brief overview on the anti-tumoral effect of probiotics and their applications in oral cancer was reported.
2. Impact of oral health dysregulation on oral cancer development
Observational studies have shown a link among oral cancer and infrequent tooth brushing, infrequent dental visits and loss of or missing teeth (59–62). These findings, however, pertain only to non-smokers and non-drinkers (13–14). Another study revealed that periodontal illnesses are correlated with an increased risk for oral tumors (63). Furthermore, research performed on 51 tongue cancer patients and 54 normal controls cases revealed that chronic periodontal inflammation is a cancer risk factor (64). In addition, periodontitis patients showed an increased risk for OSCC compared to healthy controls (65). Another observational study conducted on a wide cohort of individuals in the USA investigated the use of dental care and oral cancer risk. The analysis of covariates and dental care appointments demonstrated that individuals with a dental appointment during the past 12 months had a lower (62%) oral cancer risk compared with subjects that had not used dental care procedures in the past year (66).
According to these results, the research group of Börnigen et al (67) analyzed the role of oral microbiome and its composition by analyzing the biological samples of 121 oral cancer patients and 242 healthy controls matched for age and sex. The multivariate analyses highlighted significant variations of the oral microbiome in subjects with poor dental hygiene, in smokers, and oral cancer patients. In particular, although the microbiome alterations in cancer patients were significant, the alterations were more evident after tooth loss. Therefore, findings of that study showed that both oral microbiome alterations and tooth loss constitute important risk factors for oral cancer development due to the molecular and microenvironmental changes occurring in the oral cavity after these events (67).
3. Possible mechanisms of carcinogenesis induced by dysbiosis
The association between gut microbiota and gastric cancer is well known (68). However, the association between oral cancer and oral dysbiosis is not fully understood (69). Different mechanisms of action to elucidate the oral microbiota influence on cancer pathogenesis, including bacterial stimulation of chronic inflammation have been reported. This process causes the production of inflammatory mediators that can cause or facilitate mutagenesis, uncontrolled cell proliferation, angiogenesis and cell degeneration responsible for neurodegenerative disorders and cancer (70–72). In addition, bacteria are able to modulate cell proliferation through activation of the nuclear factor κB (NF-κB) and the inhibition of cell apoptosis promoting or inhibiting the development of several cancer types (73–75).
Moreover, Pang et al specified that the integration of virus oncogenes into host genomes or the alteration of epithelial barrier integrity could promote genome instability and favor irreversible cellular damage (76). In this context, it is noteworthy that the complex interaction among microbiota, epithelial barriers, and inflammation could assume a key role in the carcinogenic process (77–80).
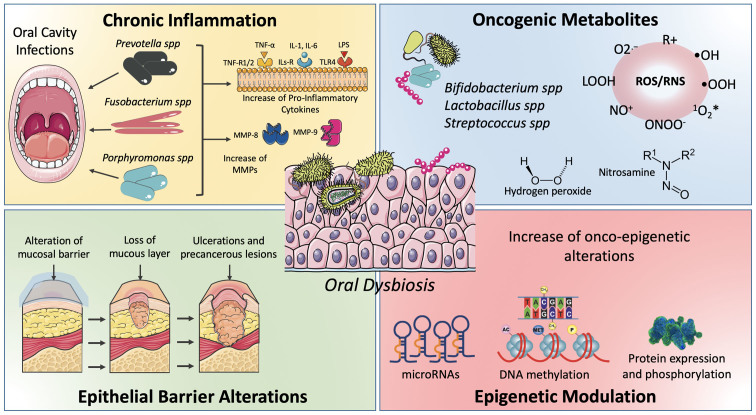
Finally, it was recently demonstrated that microbiota and oral mucosa dysbiosis lead to the accumulation of different epigenetic alterations predisposing for neoplastic transformation (Fig. 1) (81).
Figure 1.
Oral microbiota dysbiosis is associated with oral cancer development through different mechanisms. Oral infections and dysbiosis are responsible for the instauration of a pro-inflammatory microenvironment of which inflammatory cytokines and matrix metalloproteinases favor the development and progression of tumors. Furthermore, the bacteria host in the oral cavity produces oxygen and nitrogen reactive species, as well as oncogenic metabolites (e.g., nitrosamines) to induce genetic damage to cells composing the oral mucosa. Another mechanism of neoplastic transformation mediated by oral dysbiosis is the alteration of the epithelial barriers predisposing the individuals for the development of chronic pre-cancerous lesions. Finally, oral dysbiosis is responsible for several epigenetic alterations predisposing the development of tumors (e.g., alteration of onco-miR or DNA methylation phenomena).
Chronic inflammation. According to data reported in the literature, approximately 25% of human cancer shares chronic inflammation as a risk factor, indicating that inflammation is one of the most important hallmarks of cancer (82). Several processes including cell proliferation, angiogenesis, mutagenesis and oncogene activation may be caused or facilitated by chronic inflammatory mediators that alter the normal homeostasis of cells and tissues (82).
Some anaerobic oral bacteria including Fusobacterium, Porphyromonas and Prevotella species are associated with periodontal diseases and lead to chronic inflammation. The inflammatory mediators secreted by bacterial cells are able to interact with the cells of different tissues inducing diffused inflammatory processes. Periodontal bacteria influence the paracrine production of different pro-inflammatory mediators, such as interleukins (IL-1, IL-6, IL-17, IL-23), tumor-necrosis factor-α (TNF-α), and proteinases able to deteriorate the extracellular matrix (MMP-8, −9 and −13) (83,84). The increased production of these inflammatory proteins is responsible for several types of cancer and the overexpression of these proteins (especially IL-6 and MMP-9) predict for a worse prognosis and an aggressive tumor phenotype (85–87).
The upregulation of cytokines and other inflammatory factors lead to the alteration of different molecular pathways, including metabolic pathways, responsible for the modulation of cell metabolism and proliferation. For example, RAGE protein expression changes significantly after periodontal diseases mediated by oral microbiota alterations, leading to carcinogenesis (88). Moreover, gram-negative bacteria release a pro-inflammatory lipopolysaccharide endotoxin (LPS) able to stimulate the production of IL1-β, IL-6 ant TNF-α by binding the TLR receptor of leucocytes (89,90). In particular, these inflammatory cytokines lead to the overexpression of other pro-inflammatory proteins stimulating the release of phospholipase A2, prostaglandins (PG) and acute phase proteins (91,92). Other studies demonstrated that high IL-1 levels favor a pro-angiogenetic microenvironment, supporting tumor spread (93–95). Simultaneously, IL-1 induces MMP-9, which has been associated with more aggressive phenotypes of carcinoma, higher invasiveness, and low patient survival (96,97).
Other studies demonstrated that tumor spread is also sustained by the overexpression of IL-6, which in turn leads to the upregulation of matrix-metalloproteinases, adhesion molecules and endothelial leukocyte adhesion molecules (98,99). All these data showed that interleukins, and in particular IL-6, are strictly involved in neoplastic transformation (100). Besides interleukins, altered levels of TNF-α, mediated by the alteration of Wnt and NF-κB pathways, were associated with the development of tumors (101,102). These data further corroborate the importance of NF-κB in cancer. Indeed, NF-κB acts as an immunostimulant factor against neoplastic cells; however, its protein expression is increased in several cancers acting as an oncogene (103,104).
The abovementioned evidence suggested that dysbiosis-associated cancer may rely on the abnormal activation of NF-κB (68). Thus, a fundamental role is played also by the immune system, which in the presence of pathogens stimulates the production of NF-κB (105).
Oncogenic substances production. Numerous substances produced by bacteria have been suggested to possess a carcinogenic action. Bacterial metabolism leads to the production of sulfur compounds, acids and free radicals, mainly nitric and oxygen reactive species, able to induce pro-tumoral genetic damage. Furthermore, several bacteria have an alcoholic metabolism responsible for the production of acetaldehyde, which sustains neoplastic transformation (90).
Regarding reactive oxygen species (ROS) and reactive nitrogen species (RNS), it is well established that alteration of NADPH oxidase and nitric oxide synthase (NOS) activity leads to the accumulation of these harmful substances which promote chronic inflammation and, as described in the above chapter, cancer development (106,107). Bacteria also play fundamental roles in these processes. Some peroxygenase oral microorganisms are involved in this process producing hydrogen peroxide (H2O2) and include Bifidobacterium adolescentis, Lactobacillus acidophilus, L. fermentum, L. jensenii, L. minutus (90,108), Streptococcus gordonii, S. mitis, S. oligofermentans, S. oralis, and S. sanguinis (109).
Other oncogenic substances produced by oral bacteria are represented by sulfides and nitrosamines. Bacteria including Bacteroides and Firmicutes species are capable to ferment the host excessive protein into sulfides and nitrosamines. These harmful substances are able to induce DNA damage in the oncogene or onco-suppressor genes (110,111).
In addition, oral microorganisms, including Bifidobacterium, Lactobacillus, Lactococcus, Leuconostoc, Pediococcus, Peptostreptococcus stomatis and Streptococcus produce several types of acids (lactic, acetic, butyric, isobutyric, isovaleric, and isocaproic acids), which reduce the environmental pH (112–114). These acids contribute to the establishment of an ideal tissue microenvironment favorable for cancer cell proliferation and metastatic spread (115,116).
Other studies highlighted the importance of superoxide dismutase (SOD) activity and its expression through the analysis of microbiomes detected in cancer samples and normal mucosa of oral cancer patients (117). The SOD activity is fundamental for inhibiting the detrimental effects of O2.−. In particular, it was shown that in tumor samples the presence of Fe2+ reacts with H2O2 leading to the production of harmful reactive species that promote neoplastic transformation by inducing DNA mutations affecting key genes involved in the regulation of cell cycle (118).
Yost et al reported that in cancer patients, both in tumor or normal sites, microbiome tryptophanase activities are a possible carcinogenetic mechanism. In particular, the higher metabolism of L-tryptophan to secondary metabolites (indole, pyruvate and ammonium) seems to be related to cancer development (117). In this context, the role of aryl hydrocarbon receptor (AHR) is fundamental (119). Similarly, other enzymes, such as glutamate dehydrogenase (GDH), are overexpressed in oral cancer patients and their imbalance may contribute to the alteration of the cellular redox state (120,121).
Finally, as stated above, several oral microorganisms (S. gordonii, S. mitis, S. oralis, S. salivarius, S. sanguinis (122), and Candida yeasts (123) are involved in alcohol metabolizing to acetaldehyde, which has a carcinogenic potential (68,123). All these bacteria constitute a risk for OSCC development due to their acetaldehyde production (124,125).
Integrity alteration of epithelial barrier. In a recent review, Pang et al described the role of dysbiosis in the alteration of epithelial barriers in homeostasis and immune activation, as well as the relationship with carcinogenesis (76). Changes taking place in anatomic structure or in microbial composition and mucus production may lead to an epithelial barrier dysfunction and microenvironment alterations (126). The consequent imbalance between epithelia/microbiota are key factors both in infections and other microbial diseases, including tumor (127,128).
Moreover, as aforementioned, pro-inflammatory conditions sustained by microbial alterations contribute to epithelial barrier alteration. According to Virchow (1881) (129), the inflammatory events are linked to microbiota and cancer. In addition, inflammation may modify the bacteria population, favor microbial translocation and induce the growth of specific bacteria (126,129,130).
Specific microbial metabolites (i.e., ROS and hydroxyl radical) and toxins [such as cytolethal distending toxin (CDT)] generate genomic damages inducing the neoplastic transformation of epithelial cells. Moreover, bacteria activate several signal transduction pathways through virulence genes, e.g., AvrA virulence factor. Another factor stimulated by bacteriocins and bacterial proteins is the transforming growth factor β (TGF-β), which induces abnormal cell proliferation (76,131).
Furthermore, TGF-β plays a role as an immunomodulating factor inhibiting dendritic cells (DCs); T-receptor cells thus act as tumor-promoter factors (132). Notably, the mechanism of TGF-β signaling in promoting tumorigenesis is also associated with the dysregulated inflammation microenvironment actuated by microbiota (76).
Microbiota-induced epigenetic modulation
It has been widely demonstrated that environmental factors, including diet, lifestyle habits, and natural compounds, are responsible for both genetic and epigenetic alterations predisposing the development of several diseases, and have beneficial effects in preventing the development of chronic-degenerative disorders (133–140). In particular, dietary intake and food consumption modulate several cellular and molecular processes acting in a multifactorial manner (141–143). Indeed, the consumption of specific food and nutrients may modulate inflammatory and cell cycle regulatory pathways to maintain the optimal cell homeostasis, preventing the development of diseases (144,145). On the contrary, imbalances of nutrient consumption and/or absorption lead to epigenetic changes associated with certain pathologies, including cancer. The way by which food and nutrient intake is able to influence the onset of certain pathologies remains to be determined. However, findings have shown how foods can change the individual's redox state, the oral and gut microbiota, the DNA methylation status and the alteration of microRNA (miRNAs) expression levels (146–149).
In particular, the two latter epigenetic events are now been recognized as key mechanisms of neoplastic transformation and a plethora of diseases (12,87,150). In this context, recent studies have identified different miRNAs, a class of non-coding RNA of 20–22 nucleotides, associated with the development and progression of different tumors (151–157). In addition, numerous studies have demonstrated the presence of a dual relationship between microbiota and host microRNAs (miRNAs) and vice versa (158–160). Liu and co-workers showed that fecal miRNAs produced by epithelial cells and Hopx-positive cells were able to penetrate bacteria (F. nucleatum and E coli) modulating the gene expressions of bacteria and altering the microbiota composition and bacterial cell growth (161). These preliminary observations, obtained in Dicer1-deficient mice, allowed the researchers to conclude that fecal miRNAs exert an important role in the regulation of gut microbiota and microbiome suggesting their possible use as novel therapeutic strategies. Fecal miRNAs are not derived only from intestinal cells. Different studies demonstrated that fecal miRNAs can be derived from foods and can be absorbed by intestinal epithelia modulating the expression levels of host genes (162,163). These miRNAs are mainly planted exosome-derived miRNAs; however, several studies showed that milk-derived miRNAs play key roles (164–166).
All these food-derived miRNAs can presumably interact with oral and gut microbiota (167,168). On this basis, exogenous miRNAs may act as bacterial small RNA to interfere with bacterial gene expression modulating the entire microbiome (169,170). However, further studies are needed to deepen the knowledge on interactions between host-miRNAs and oral/gut microbiota. On the other hand, even the microbiota resident in the oral and intestinal mucosa may modulate the expression of specific miRNAs, thus highlighting a dual relationship between miRNAs and microbiota and their ability to influence each other (171).
One of the mechanisms by which microbiota alters the expression levels of host-epithelial miRNAs is the production of different metabolites leading to significant changes in host-cell metabolism resulting in the alteration of the gene and miRNA expressions (172–174). Pang et al investigated the association among dysbiosis, dysfunction of epithelial barrier and alteration of immune system to evaluate how dysbiosis stimulates carcinogenesis (76).
4. Oral bacteria with potential carcinogenetic activity
According to Zhao et al (175), oral cancer samples exhibited more bacterial species compared to healthy individuals. Specifically, Catonella, Dialister, Filifactor, Fusobacterium, Parvimonas, Peptococcus and Peptostreptococcus are the most overexpressed bacteria in OSCC samples with previous periodontitis. Notably, the evaluation of Fusobacterium bacteria was proposed as a diagnostic criterium for OSCC (175). In particular, Fusobacterium nucleatum is responsible, not only for opportunistic infections, but it was recently associated with several kind of cancers (175–179).
In addition, Yost et al (117) performed a preliminary meta-transcriptomic study in order to analyze the oral microbiome in oral squamous cell carcinoma patients and establish the connection with the molecular features of this tumor. Authors of that study found increased expression levels of Fusobacteria transcripts in both tumor and peritumoral tissue samples when compared to healthy individuals. Interestingly, they also showed that Fusobacteria virulence factors may be involved in the pathogenesis of oral cancer (117). Outcomes of that study are in agreement with findings of Nagy et al (50) who observed some oral bacteria correlated with keratinizing squamous cell carcinomas, including Fusobacterium sp. Moreover, Yang et al (180) correlated the microbiome variations to cancer progression. The Fusobacteria abundance was significantly higher in oral cancer patients showing progression and these data were more evident in stage 4 patients (7.92%) compared to healthy controls (2.98%) (180).
Lim et al (181) analyzed oral rinse to evaluate the microbiome variations and their correlation oral and head and neck cancers. Those authors identified a panel of bacteria (Capnocytophaga, Corynebacterium, Haemophilus, Oribacterium, Paludibacter, Porphyromonas, and Rothia) to discern oral cavity cancer patients (OCC), oropharyngeal cancer patients (OPC) and healthy subjects (181). On the basis of results obtained, the authors proposed the detection of these bacteria to predict the risk of OCC and OPC, reaching 100 and 90% sensitivity and specificity, respectively (182). The outcomes of that study are in agreement with previous studies (49,51,53,182–184) that indicated Capnocytophaga gingivalis, Peptostreptococcus sp., Porphyromonas gingivalis, Prevotella sp., and Streptococcus sp. as the oral microorganism mostly associated with OSCCs.
Yost and collaborators demonstrated that among various examined species, Capnocytophaga gingivalis was the more represented in non-tumoral sites. These contrasted results could be due to the different samples examined, as well as to the number of cases enrolled (117). Other bacteria associated with head, neck, and esophageal cancers were streptococci, of which Streptococcus anginosus represents the most important bacterium (51,185,186).
Recently, Yang et al (187) investigated the functional role of microbiota changes related to the genetic alterations observed in OSCC patients. The analysis of saliva samples demonstrated an imbalance in the oral cavity taxa showing a relative abundance of Bacteroidetes and Firmicutes in three different groups of oral cancer clustered according to the mutational status. Moreover, significant variations of microorganism diversity were highlighted through analysis of the three groups of patients. Based on these results, the authors proposed a possible association between mutation in OSCC and alteration of microbiota and microbiome (187). Differences in bacterial composition were also detected between precancerous lesions and cancer samples (53). The results of that study showed that salivary microbiota patterns were importantly modulated in the three analyzed groups. In particular, the genera Bacillus, Enterococcus, Parvimonas, Peptostreptococcus, and Slackia showed a predictive value for discrimination between precancerous and neoplastic lesions (53).
5. Anti-tumoral effects of probiotics
In recent years, it was widely demonstrated that the consumption of healthy foods enriched with probiotic acid lactic bacteria has positive effects regarding tumor prevention. Probiotics are able to reduce the mutagenic effects of harmful substances while modulating the expression of proteins involved in cell proliferation, apoptosis, inflammation, or immune system activation (188). Several in vitro experiments performed on cancer cells showed that probiotics possess anti-proliferative and pro-apoptotic effects in these tumor models (189). Lee and co-workers demonstrated that the cytoplasmic elements of Bifidobacterium longum, L. acidophilus and L. casei exert anti-neoplastic effects in different tumor in vitro models (190). Besides these probiotics, Bacillus polyfermenticus (191), Lactobacillus acidophilus 606 (192), LGG/Bb12 (193), LGG/Bifidobacterium animalis subsp. lactis (194), and Lactobacillus rhamnosus GG (9) possessed anti-neoplastic effects in colorectal cancer cell lines.
Few data are available on the effects of probiotics on oral cancer. In a recent study, HSC-3 OSCC cell lines were used to determine the effects of Lactobacillus rhamnosus GG (LGG) in increasing the antiblastic effects of geniposide, a derivate of Gardenia jasminoides which in preclinical studies showed important anticancer effects (58,195). The results obtained by the authors showed that the combined treatment with geniposide and LGG increased the apoptotic rate of HSC-3 cells. In particular, in a synergistic manner, LGG intensified the antineoplastic action of geniposide, supporting the tentative use of this combined therapy also in clinical practice. In another study, Asoudeh-Fard et al (196) demonstrated that Lactobacillus plantarum was able to inhibit and activate the MAPKs and PTEN pathways, respectively, playing a potential role in the regulation of cancer. Indeed, it is well known that PTEN and MAPKs are associated with the inhibition and the initiation of cancer development, respectively (196). Consequently, a possible use of L. plantarum for probiotics cancer therapy was proposed.
In addition, Aghazadeh et al (197) showed that Acetobacter syzygii strain secretions possess anticancer activity promoting the apoptosis induction in oral cancer cells. Interestingly, Acetobacter syzygii products were not involved in the alteration of homeostasis of the epithelial cell line (197). These findings seem to show the crucial role represented by the microorganism in controlling cancer development in oral tissues and encourage further investigations on the effect of probiotics on OSCC development.
6. Conclusions
This review widely discussed how the dysregulation of oral microbiota and oral mucosa homeostasis may represent modifiable risk factors associated with the development of OSCC. In addition, it was shown that certain bacterial strains may play a protective role against oral neoplastic transformation suggesting the possible use of probiotics administration as novel preventive and therapeutic strategies. In this scenario, several species have been strongly correlated with oral carcinoma, such as Capnocytophaga gingivalis, Fusobacterium sp., Streptococcus sp., Peptostreptococcus sp., Porphyromonas gingivalis and Prevotella sp., due to the fact that these bacteria may promote inflammation, cell proliferation and the production of some oncogenic substances (90). Recent findings have shown that the evaluation of oral microbiota and microbiome may provide important information on oral cancer oncogenesis, outcome prediction and therapeutic response (including immunotherapy) (198,199). In addition, thanks to the new high-throughput molecular technologies it was possible to define the precise composition and gene expression (microbiome) of oral bacteria in OSCC patients and normal controls identifying specific strains associated with an increased risk of OSCC development (1,200). Therefore, the analysis of circulating biomarkers (miRNAs, circulating DNA, specific proteins) represents a good approach for the assessment of oral cancer risk (201–203).
In this respect, the use of probiotics that in appropriate amounts give a health benefit to the host, including anti-tumoral effects, could be useful to promote cancer therapies representing a new step of the evolution of anticancer pharmacological treatments (190,204). Although in vitro and in vivo experiments demonstrated the beneficial effects probiotics (7–9), few data are available about the efficacy of probiotics in oral cancer. It is reasonable to hypothesize that the beneficial effects exert by probiotics in intestinal and colorectal cancer is similar to those acted in the oral mucosa. Starting from this assumption, future studies are required to explore the involvement of oral microbiota and its relationship with oral cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
GRMLR, DN and MS conceived the study. GG, EP provided the information about microbiota and oral cancer. GRMLR, GG and EP organized the Tables. GG and DN were involved in the preparation of the figure. GRMLR, EP, ER and MS were involved in the preparation of the original draft of the manuscript, while DN, ER and MS reviewed and edited the article. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Fenga C, Gangemi S, Di Salvatore V, Falzone L, Libra M. Immunological effects of occupational exposure to lead (Review) Mol Med Rep. 2017;15:3355–3360. doi: 10.3892/mmr.2017.6381. (Review) [DOI] [PubMed] [Google Scholar]
- 3.Rapisarda V, Ledda C, Matera S, Fago L, Arrabito G, Falzone L, Marconi A, Libra M, Loreto C. Absence of t(14;18) chromosome translocation in agricultural workers after short-term exposure to pesticides. Mol Med Rep. 2017;15:3379–3382. doi: 10.3892/mmr.2017.6385. [DOI] [PubMed] [Google Scholar]
- 4.Rapisarda V, Salemi R, Marconi A, Loreto C, Graziano AC, Cardile V, Basile MS, Candido S, Falzone L, Spandidos DA, et al. Fluoro-edenite induces fibulin-3 overexpression in non-malignant human mesothelial cells. Oncol Lett. 2016;12:3363–3367. doi: 10.3892/ol.2016.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falzone L, Marconi A, Loreto C, Franco S, Spandidos DA, Libra M. Occupational exposure to carcinogens: Benzene, pesticides and fibers (Review) Mol Med Rep. 2016;14:4467–4474. doi: 10.3892/mmr.2016.5791. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malfa GA, Tomasello B, Sinatra F, Villaggio G, Amenta F, Avola R, Renis M. ‘Reactive’ response evaluation of primary human astrocytes after methylmercury exposure. J Neurosci Res. 2014;92:95–103. doi: 10.1002/jnr.23290. [DOI] [PubMed] [Google Scholar]
- 7.Vivarelli S, Salemi R, Candido S, Falzone L, Santagati M, Stefani S, Torino F, Banna GL, Tonini G, Libra M. Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers (Basel) 2019;11:E38. doi: 10.3390/cancers11010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banna GL, Torino F, Marletta F, Santagati M, Salemi R, Cannarozzo E, Falzone L, Ferraù F, Libra M. Lactobacillus rhamnosus GG: An overview to explore the rationale of its use in cancer. Front Pharmacol. 2017;8:603. doi: 10.3389/fphar.2017.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vivarelli S, Falzone L, Basile MS, Nicolosi D, Genovese C, Libra M, Salmeri M. Benefits of using probiotics as adjuvants in anticancer therapy. World Ac Sci J. 2019;1:125–135. (Review) [Google Scholar]
- 10.Garozzo A, Falzone L, Rapisarda V, Marconi A, Cinà D, Fenga C, Spandidos DA, Libra M. The risk of HCV infection among health-care workers and its association with extrahepatic manifestations (Review) Mol Med Rep. 2017;15:3336–3339. doi: 10.3892/mmr.2017.6378. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng JH, Iyer NG, Tan MH, Edgren G. Changing epidemiology of oral squamous cell carcinoma of the tongue: A global study. Head Neck. 2017;39:297–304. doi: 10.1002/hed.24589. [DOI] [PubMed] [Google Scholar]
- 12.Falzone L, Lupo G, La Rosa GRM, Crimi S, Anfuso CD, Salemi R, Rapisarda E, Libra M, Candido S. Identification of novel micrornas and their diagnostic and prognostic significance in oral cancer. Cancers (Basel) 2019;11:E610. doi: 10.3390/cancers11050610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham S, Dayal H, Rohrer T, Swanson M, Sultz H, Shedd D, Fischman S. Dentition, diet, tobacco, and alcohol in the epidemiology of oral cancer. J Natl Cancer Inst. 1977;59:1611–1618. doi: 10.1093/jnci/59.6.1611. [DOI] [PubMed] [Google Scholar]
- 14.Marshall JR, Graham S, Haughey BP, Shedd D, O'Shea R, Brasure J, Wilkinson GS, West D. Smoking, alcohol, dentition and diet in the epidemiology of oral cancer. Eur J Cancer B Oral Oncol. 1992;28B:9–15. doi: 10.1016/0964-1955(92)90005-L. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: Short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 16.Read SA, Douglas MW. Virus induced inflammation and cancer development. Cancer Lett. 2014;345:174–181. doi: 10.1016/j.canlet.2013.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196–202. doi: 10.1016/j.canlet.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJJ., Jr Communication among oral bacteria. Microbiol Mol Biol Rev. 2002;66:486–505. doi: 10.1128/MMBR.66.3.486-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Maweri SA, Warnakulasuriya S, Samran A. Khat (Catha edulis) and its oral health effects: An updated review. https://doi.org/10.1111/jicd.12288. J Investig Clin Dent. 2018;9 doi: 10.1111/jicd.12288. [DOI] [PubMed] [Google Scholar]
- 22.Leonardi R, Loreto C, Barbato E, Polimeni A, Caltabiano R, Lo Muzio L. A histochemical survey of the human temporomandibular joint disc of patients with internal derangement without reduction. J Craniofac Surg. 2007;18:1429–1433. doi: 10.1097/scs.0b013e31814fb72a. [DOI] [PubMed] [Google Scholar]
- 23.Petralia MC, Mazzon E, Fagone P, Falzone L, Bramanti P, Nicoletti F, Basile MS. Retrospective follow-up analysis of the transcriptomic patterns of cytokines, cytokine receptors and chemokines at preconception and during pregnancy, in women with post-partum depression. Exp Ther Med. 2019;18:2055–2062. doi: 10.3892/etm.2019.7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vesty A, Gear K, Biswas K, Radcliff FJ, Taylor MW, Douglas RG. Microbial and inflammatory-based salivary biomarkers of head and neck squamous cell carcinoma. Clin Exp Dent Res. 2018;4:255–262. doi: 10.1002/cre2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meurman JH. Oral microbiota and cancer. J Oral Microbiol. 2010;2:2. doi: 10.3402/jom.v2i0.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pennisi M, Malaguarnera G, Bartolo GD, Lanza G, Bella R, Chisari EM, Cauli O, Vicari E, Malaguarnera M. Decrease in Serum Vitamin D Level of Older Patients with Fatigue. Nutrients. 2019;11:E2531. doi: 10.3390/nu11102531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pennisi M, Di Bartolo G, Malaguarnera G, Bella R, Lanza G, Malaguarnera M, Vitamin D. Vitamin D serum levels in patients with statin-induced musculoskeletal pain. Dis Markers. 2019;2019:3549402. doi: 10.1155/2019/3549402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Human Microbiome Project Consortium: A framework for human microbiome research. Nature. 2012;486:215–221. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marasco G, Di Biase AR, Schiumerini R, Eusebi LH, Iughetti L, Ravaioli F, Scaioli E, Colecchia A, Festi D. Gut microbiota and celiac disease. Dig Dis Sci. 2016;61:1461–1472. doi: 10.1007/s10620-015-4020-2. [DOI] [PubMed] [Google Scholar]
- 30.Quigley EMM. Microbiota-brain-gut axis and neurodegenerative diseases. Curr Neurol Neurosci Rep. 2017;17:94. doi: 10.1007/s11910-017-0802-6. [DOI] [PubMed] [Google Scholar]
- 31.Yang T, Zubcevic J. Gut-brain axis in regulation of blood pressure. Front Physiol. 2017;8:845. doi: 10.3389/fphys.2017.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinciguerra L, Lanza G, Puglisi V, Pennisi M, Cantone M, Bramanti A, Pennisi G, Bella R. Transcranial Doppler ultrasound in vascular cognitive impairment-no dementia. PLoS One. 2019;14:e0216162. doi: 10.1371/journal.pone.0216162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puglisi V, Bramanti A, Lanza G, Cantone M, Vinciguerra L, Pennisi M, Bonanno L, Pennisi G, Bella R. Impaired cerebral haemodynamics in vascular depression: insights from transcranial doppler ultrasonography. Front Psychiatry. 2018;9:316. doi: 10.3389/fpsyt.2018.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lanza G, Cantone M, Musso S, Borgione E, Scuderi C, Ferri R. Early-onset subcortical ischemic vascular dementia in an adult with mtDNA mutation 3316G>A. J Neurol. 2018;265:968–969. doi: 10.1007/s00415-018-8795-x. [DOI] [PubMed] [Google Scholar]
- 35.Bordet R, Ihl R, Korczyn AD, Lanza G, Jansa J, Hoerr R, Guekht A. Towards the concept of disease-modifier in post-stroke or vascular cognitive impairment: A consensus report. BMC Med. 2017;15:107. doi: 10.1186/s12916-017-0869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lanza G, Bramanti P, Cantone M, Pennisi M, Pennisi G, Bella R. Vascular cognitive impairment through the looking glass of transcranial magnetic stimulation. Behav Neurol. 2017;2017:1421326. doi: 10.1155/2017/1421326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bella R, Cantone M, Lanza G, Ferri R, Vinciguerra L, Puglisi V, Pennisi M, Ricceri R, Di Lazzaro V, Pennisi G. Cholinergic circuitry functioning in patients with vascular cognitive impairment - no dementia. Brain Stimul. 2016;9:225–233. doi: 10.1016/j.brs.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 38.Pennisi M, Lanza G, Cantone M, Ricceri R, Spampinato C, Pennisi G, Di Lazzaro V, Bella R. Correlation between motor cortex excitability changes and cognitive impairment in vascular depression: Pathophysiological insights from a longitudinal TMS study. Neural Plast. 2016;2016:8154969. doi: 10.1155/2016/8154969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pennisi G, Bella R, Lanza G. Motor cortex plasticity in subcortical ischemic vascular dementia: What can TMS say? Clin Neurophysiol. 2015;126:851–852. doi: 10.1016/j.clinph.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Lanza G, Papotto M, Pennisi G, Bella R, Ferri R. Epileptic seizure as a precipitating factor of vascular progressive supranuclear palsy: A case report. J Stroke Cerebrovasc Dis. 2014;23:e379–e381. doi: 10.1016/j.jstrokecerebrovasdis.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 41.Concerto C, Lanza G, Cantone M, Pennisi M, Giordano D, Spampinato C, Ricceri R, Pennisi G, Aguglia E, Bella R. Different patterns of cortical excitability in major depression and vascular depression: A transcranial magnetic stimulation study. BMC Psychiatry. 2013;13:300. doi: 10.1186/1471-244X-13-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bella R, Ferri R, Lanza G, Cantone M, Pennisi M, Puglisi V, Vinciguerra L, Spampinato C, Mazza T, Malaguarnera G, et al. TMS follow-up study in patients with vascular cognitive impairment-no dementia. Neurosci Lett. 2013;534:155–159. doi: 10.1016/j.neulet.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 43.Bella R, Ferri R, Cantone M, Pennisi M, Lanza G, Malaguarnera G, Spampinato C, Giordano D, Raggi A, Pennisi G. Motor cortex excitability in vascular depression. Int J Psychophysiol. 2011;82:248–253. doi: 10.1016/j.ijpsycho.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Bella R, Ferri R, Pennisi M, Cantone M, Lanza G, Malaguarnera G, Spampinato C, Giordano D, Alagona G, Pennisi G. Enhanced motor cortex facilitation in patients with vascular cognitive impairment-no dementia. Neurosci Lett. 2011;503:171–175. doi: 10.1016/j.neulet.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 45.Pennisi G, Ferri R, Cantone M, Lanza G, Pennisi M, Vinciguerra L, Malaguarnera G, Bella R. A review of transcranial magnetic stimulation in vascular dementia. Dement Geriatr Cogn Disord. 2011;31:71–80. doi: 10.1159/000322798. [DOI] [PubMed] [Google Scholar]
- 46.Bella R, Pennisi G, Cantone M, Palermo F, Pennisi M, Lanza G, Zappia M, Paolucci S. Clinical presentation and outcome of geriatric depression in subcortical ischemic vascular disease. Gerontology. 2010;56:298–302. doi: 10.1159/000272003. [DOI] [PubMed] [Google Scholar]
- 47.Atanasova KR, Yilmaz O. Looking in the Porphyromonas gingivalis cabinet of curiosities: The microbium, the host and cancer association. Mol Oral Microbiol. 2014;29:55–66. doi: 10.1111/omi.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yilmaz O. The chronicles of Porphyromonas gingivalis: The microbium, the human oral epithelium and their interplay. Microbiology. 2008;154:2897–2903. doi: 10.1099/mic.0.2008/021220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katz J, Onate MD, Pauley KM, Bhattacharyya I, Cha S. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int J Oral Sci. 2011;3:209–215. doi: 10.4248/IJOS11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagy KN, Sonkodi I, Szöke I, Nagy E, Newman HN. The microflora associated with human oral carcinomas. Oral Oncol. 1998;34:304–308. doi: 10.1016/S1368-8375(98)80012-2. [DOI] [PubMed] [Google Scholar]
- 51.Mager DL, Haffajee AD, Devlin PM, Norris CM, Posner MR, Goodson JM. The salivary microbiota as a diagnostic indicator of oral cancer: A descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J Transl Med. 2005;3:27. doi: 10.1186/1479-5876-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu X, Zhang Q, Hua H, Chen F. Changes in the salivary microbiota of oral leukoplakia and oral cancer. Oral Oncol. 2016;56:e6–e8. doi: 10.1016/j.oraloncology.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 53.Lee WH, Chen HM, Yang SF, Liang C, Peng CY, Lin FM, Tsai LL, Wu BC, Hsin CH, Chuang CY, et al. Bacterial alterations in salivary microbiota and their association in oral cancer. Sci Rep. 2017;7:16540. doi: 10.1038/s41598-017-16418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, Winget M, Yasui Y. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–1061. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 55.Lucs A, Saltman B, Chung CH, Steinberg BM, Schwartz DL. Opportunities and challenges facing biomarker development for personalized head and neck cancer treatment. Head Neck. 2013;35:294–306. doi: 10.1002/hed.21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meurman JH, Stamatova I. Probiotics: Contributions to oral health. Oral Dis. 2007;13:443–451. doi: 10.1111/j.1601-0825.2007.01386.x. [DOI] [PubMed] [Google Scholar]
- 57.Stamatova I, Meurman JH. Probiotics: Health benefits in the mouth. Am J Dent. 2009;22:329–338. [PubMed] [Google Scholar]
- 58.Cheng Z, Xu H, Wang X, Liu Z. Lactobacillus raises in vitro anticancer effect of geniposide in HSC-3 human oral squamous cell carcinoma cells. Exp Ther Med. 2017;14:4586–4594. doi: 10.3892/etm.2017.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang JS, Lo HI, Wong TY, Huang CC, Lee WT, Tsai ST, Chen KC, Yen CJ, Wu YH, Hsueh WT, et al. Investigating the association between oral hygiene and head and neck cancer. Oral Oncol. 2013;49:1010–1017. doi: 10.1016/j.oraloncology.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Rosenquist K, Wennerberg J, Schildt EB, Bladström A, Göran Hansson B, Andersson G. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population-based case-control study in southern Sweden. Acta Otolaryngol. 2005;125:1327–1336. doi: 10.1080/00016480510012273. [DOI] [PubMed] [Google Scholar]
- 61.Divaris K, Olshan AF, Smith J, Bell ME, Weissler MC, Funkhouser WK, Bradshaw PT. Oral health and risk for head and neck squamous cell carcinoma: The Carolina Head and Neck Cancer Study. Cancer Causes Control. 2010;21:567–575. doi: 10.1007/s10552-009-9486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garrote LF, Herrero R, Reyes RM, Vaccarella S, Anta JL, Ferbeye L, Muñoz N, Franceschi S. Risk factors for cancer of the oral cavity and oro-pharynx in Cuba. Br J Cancer. 2001;85:46–54. doi: 10.1054/bjoc.2000.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michaud DS, Liu Y, Meyer M, Giovannucci E, Joshipura K. Periodontal disease, tooth loss, and cancer risk in male health professionals: A prospective cohort study. Lancet Oncol. 2008;9:550–558. doi: 10.1016/S1470-2045(08)70106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tezal M, Sullivan MA, Reid ME, Marshall JR, Hyland A, Loree T, Lillis C, Hauck L, Wactawski-Wende J, Scannapieco FA. Chronic periodontitis and the risk of tongue cancer. Arch Otolaryngol Head Neck Surg. 2007;133:450–454. doi: 10.1001/archotol.133.5.450. [DOI] [PubMed] [Google Scholar]
- 65.Tezal M, Sullivan MA, Hyland A, Marshall JR, Stoler D, Reid ME, Loree TR, Rigual NR, Merzianu M, Hauck L, et al. Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18:2406–2412. doi: 10.1158/1055-9965.EPI-09-0334. [DOI] [PubMed] [Google Scholar]
- 66.Holmes L, Jr, desVignes-Kendrick M, Slomka J, Mahabir S, Beeravolu S, Emani SR. Is dental care utilization associated with oral cavity cancer in a large sample of community-based United States residents? Community Dent Oral Epidemiol. 2009;37:134–142. doi: 10.1111/j.1600-0528.2008.00450.x. [DOI] [PubMed] [Google Scholar]
- 67.Börnigen D, Ren B, Pickard R, Li J, Ozer E, Hartmann EM, Xiao W, Tickle T, Rider J, Gevers D, et al. Alterations in oral bacterial communities are associated with risk factors for oral and oropharyngeal cancer. Sci Rep. 2017;7:17686. doi: 10.1038/s41598-017-17795-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vogelmann R, Amieva MR. The role of bacterial pathogens in cancer. Curr Opin Microbiol. 2007;10:76–81. doi: 10.1016/j.mib.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 69.Lim Y, Totsika M, Morrison M, Punyadeera C. Oral microbiome: A new biomarker reservoir for oral and oropharyngeal cancers. Theranostics. 2017;7:4313–4321. doi: 10.7150/thno.21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cali F, Cantone M, Cosentino FII, Lanza G, Ruggeri G, Chiavetta V, Salluzzo R, Ragalmuto A, Vinci M, Ferri R. Interpreting genetic variants: hints from a family cluster of Parkinson's disease. J Parkinsons Dis. 2019;9:203–206. doi: 10.3233/JPD-171292. [DOI] [PubMed] [Google Scholar]
- 71.Pennisi M, Lanza G, Cantone M, Schepis C, Ferri R, Barone R, Bella R. Unusual neurological presentation of nevoid basal cell carcinoma syndrome (Gorlin-Goltz Syndrome) J Clin Neurol. 2017;13:439–441. doi: 10.3988/jcn.2017.13.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Wang X, Li H, Ni C, Du Z, Yan F. Human oral microbiota and its modulation for oral health. Biomed Pharmacother. 2018;99:883–893. doi: 10.1016/j.biopha.2018.01.146. [DOI] [PubMed] [Google Scholar]
- 73.Guarneri C, Bevelacqua V, Polesel J, Falzone L, Cannavò PS, Spandidos DA, Malaponte G, Libra M. NF-κB inhibition is associated with OPN/MMP 9 downregulation in cutaneous melanoma. Oncol Rep. 2017;37:737–746. doi: 10.3892/or.2017.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leonardi GC, Falzone L, Salemi R, Zanghì A, Spandidos DA, Mccubrey JA, Candido S, Libra M. Cutaneous melanoma: From pathogenesis to therapy (Review) Int J Oncol. 2018;52:1071–1080. doi: 10.3892/ijo.2018.4287. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-κB in development and progression of human cancer. Virchows Arch. 2005;446:475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 76.Pang X, Tang YJ, Ren XH, Chen QM, Tang YL, Liang XH. Microbiota, epithelium, inflammation, and TGF-β signaling: An intricate interaction in oncogenesis. Front Microbiol. 2018;9:1353. doi: 10.3389/fmicb.2018.01353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Couturier-Maillard A, Secher T, Rehman A, Normand S, De Arcangelis A, Haesler R, Huot L, Grandjean T, Bressenot A, Delanoye-Crespin A, et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest. 2013;123:700–711. doi: 10.1172/JCI62236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu B, Elinav E, Huber S, Strowig T, Hao L, Hafemann A, Jin C, Wunderlich C, Wunderlich T, Eisenbarth SC, et al. Microbiota-induced activation of epithelial IL-6 signaling links inflammasome-driven inflammation with transmissible cancer. Proc Natl Acad Sci USA. 2013;110:9862–9867. doi: 10.1073/pnas.1307575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Francescone R, Hou V, Grivennikov SI. Microbiome, inflammation, and cancer. Cancer J. 2014;20:181–189. doi: 10.1097/PPO.0000000000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ojesina AI, Lichtenstein L, Freeman SS, Pedamallu CS, Imaz-Rosshandler I, Pugh TJ, Cherniack AD, Ambrogio L, Cibulskis K, Bertelsen B, et al. Landscape of genomic alterations in cervical carcinomas. Nature. 2014;506:371–375. doi: 10.1038/nature12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Allen J, Sears CL. Impact of the gut microbiome on the genome and epigenome of colon epithelial cells: Contributions to colorectal cancer development. Genome Med. 2019;11:11. doi: 10.1186/s13073-019-0621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2012;2:98. doi: 10.3389/fimmu.2011.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Szkaradkiewicz AK. Karpiński TM: Microbiology of chronic periodontitis. J Biol Earth Sci. 2013;3:M14–M20. [Google Scholar]
- 84.Leonardi R, Talic NF, Loreto C. MMP-13 (collagenase 3) immunolocalisation during initial orthodontic tooth movement in rats. Acta Histochem. 2007;109:215–220. doi: 10.1016/j.acthis.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 85.Salemi R, Falzone L, Madonna G, Polesel J, Cinà D, Mallardo D, Ascierto PA, Libra M, Candido S. MMP-9 as a candidate marker of response to BRAF inhibitors in melanoma patients with BRAFV600E mutation detected in circulating-free DNA. Front Pharmacol. 2018;9:856. doi: 10.3389/fphar.2018.00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Silva EM, Mariano VS, Pastrez PRA, Pinto MC, Castro AG, Syrjanen KJ, Longatto-Filho A. High systemic IL-6 is associated with worse prognosis in patients with non-small cell lung cancer. PLoS One. 2017;12:e0181125. doi: 10.1371/journal.pone.0181125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Falzone L, Salemi R, Travali S, Scalisi A, McCubrey JA, Candido S, Libra M. MMP-9 overexpression is associated with intragenic hypermethylation of MMP9 gene in melanoma. Aging (Albany NY) 2016;8:933–944. doi: 10.18632/aging.100951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Katz J, Wallet S, Cha S. Periodontal disease and the oral-systemic connection: ‘is it all the RAGE?’. Quintessence Int. 2010;41:229–237. [PubMed] [Google Scholar]
- 89.Zhang G, Ghosh S. Molecular mechanisms of NF-kappaB activation induced by bacterial lipopolysaccharide through Toll-like receptors. J Endotoxin Res. 2000;6:453–457. doi: 10.1179/096805100101532414. [DOI] [PubMed] [Google Scholar]
- 90.Karpiński TM. Role of oral microbiota in cancer development. Microorganisms. 2019;7:E20. doi: 10.3390/microorganisms7010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hou LT, Liu CM, Liu BY, Lin SJ, Liao CS, Rossomando EF. Interleukin-1beta, clinical parameters and matched cellular-histopathologic changes of biopsied gingival tissue from periodontitis patients. J Periodontal Res. 2003;38:247–254. doi: 10.1034/j.1600-0765.2003.02601.x. [DOI] [PubMed] [Google Scholar]
- 92.Konopka Ł, Brzezinska Błaszczyk E. Cytokines in gingival crevicular fluidas potential diagnostic and prognostic markers of periodontitis. Dent Med Probl. 2010;47:206–213. [Google Scholar]
- 93.Carmi Y, Dotan S, Rider P, Kaplanov I, White MR, Baron R, Abutbul S, Huszar M, Dinarello CA, Apte RN, et al. The role of IL-1β in the early tumor cell-induced angiogenic response. J Immunol. 2013;190:3500–3509. doi: 10.4049/jimmunol.1202769. [DOI] [PubMed] [Google Scholar]
- 94.Jin L, Yuan RQ, Fuchs A, Yao Y, Joseph A, Schwall R, Schnitt SJ, Guida A, Hastings HM, Andres J, et al. Expression of interleukin-1beta in human breast carcinoma. Cancer. 1997;80:421–434. doi: 10.1002/(SICI)1097-0142(19970801)80:3<421::AID-CNCR10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 95.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pannone G, Santoro A, Feola A, Bufo P, Papagerakis P, Lo Muzio L, Staibano S, Ionna F, Longo F, Franco R, et al. The role of E-cadherin down-regulation in oral cancer: CDH1 gene expression and epigenetic blockage. Curr Cancer Drug Targets. 2014;14:115–127. doi: 10.2174/1568009613666131126115012. [DOI] [PubMed] [Google Scholar]
- 97.Wong SHM, Fang CM, Chuah LH, Leong CO, Ngai SC. E-cadherin: Its dysregulation in carcinogenesis and clinical implications. Crit Rev Oncol Hematol. 2018;121:11–22. doi: 10.1016/j.critrevonc.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 98.Kossakowska AE, Edwards DR, Prusinkiewicz C, Zhang MC, Guo D, Urbanski SJ, Grogan T, Marquez LA, Janowska-Wieczorek A. Interleukin-6 regulation of matrix metalloproteinase (MMP-2 and MMP-9) and tissue inhibitor of metalloproteinase (TIMP-1) expression in malignant non-Hodgkin's lymphomas. Blood. 1999;94:2080–2089. doi: 10.1182/blood.V94.6.2080. [DOI] [PubMed] [Google Scholar]
- 99.Natali P, Nicotra MR, Cavaliere R, Bigotti A, Romano G, Temponi M, Ferrone S. Differential expression of intercellular adhesion molecule 1 in primary and metastatic melanoma lesions. Cancer Res. 1990;50:1271–1278. [PubMed] [Google Scholar]
- 100.Haura EB, Turkson J, Jove R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol. 2005;2:315–324. doi: 10.1038/ncponc0195. [DOI] [PubMed] [Google Scholar]
- 101.Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor-alpha as a tumour promoter. Eur J Cancer. 2006;42:745–750. doi: 10.1016/j.ejca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 102.Rivas MA, Carnevale RP, Proietti CJ, Rosemblit C, Beguelin W, Salatino M, Charreau EH, Frahm I, Sapia S, Brouckaert P, et al. TNF alpha acting on TNFR1 promotes breast cancer growth via p42/P44 MAPK, JNK, Akt and NF-kappa B-dependent pathways. Exp Cell Res. 2008;314:509–529. doi: 10.1016/j.yexcr.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 103.Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Agassandian M, Shurin GV. Bacterial Infections and Cancar Development. In: Shurin MR, Thanavala Y, Ismail N, editors Infection and Cancaer: Bi-Directorial Interactions; 1 ed. Springer International Publishing; 2015;2015:408. [Google Scholar]
- 106.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 107.Piao JY, Lee HG, Kim SJ, Kim DH, Han HJ, Ngo HK, Park SA, Woo JH, Lee JS, Na HK, et al. Helicobacter pylori activates IL-6-STAT3 signaling in human gastric cancer cells: Potential roles for reactive oxygen species. Helicobacter. 2016;21:405–416. doi: 10.1111/hel.12298. [DOI] [PubMed] [Google Scholar]
- 108.Brauncajs M, Sakowska D. Krzemiński Z: Production of hydrogen peroxide by lactobacilli colonising the human oral cavity. Med Dosw Mikrobiol. 2001;53:331–336. [Google Scholar]
- 109.Abranches J, Zeng L, Kajfasz JK, Palmer SR, Chakraborty B, Wen ZT, Richards VP, Brady LJ, Lemos JA. Biology of oral streptococci. Microbiol Spectr. 2018;6:6. doi: 10.1128/microbiolspec.GPP3-0042-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Carbonero F, Benefiel AC, Alizadeh-Ghamsari AH, Gaskins HR. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front Physiol. 2012;3:448. doi: 10.3389/fphys.2012.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bhatt AP, Redinbo MR, Bultman SJ. The role of the microbiome in cancer development and therapy. CA Cancer J Clin. 2017;67:326–344. doi: 10.3322/caac.21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Karpiński TM, Szkaradkiewicz AK. Karpiński TM, Szkaradkiewicz AK: Characteristic of bacteriocines and their application. Pol J Microbiol. 2013;62:223–235. doi: 10.33073/pjm-2013-030. [DOI] [PubMed] [Google Scholar]
- 113.Senneby A, Davies JR, Svensäter G, Neilands J. Acid tolerance properties of dental biofilms in vivo. BMC Microbiol. 2017;17:165. doi: 10.1186/s12866-017-1074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Downes J, Wade WG. Peptostreptococcus stomatis sp. nov., isolated from the human oral cavity. Int J Syst Evol Microbiol. 2006;56:751–754. doi: 10.1099/ijs.0.64041-0. [DOI] [PubMed] [Google Scholar]
- 115.Lunt SJ, Chaudary N, Hill RP. The tumor microenvironment and metastatic disease. Clin Exp Metastasis. 2009;26:19–34. doi: 10.1007/s10585-008-9182-2. [DOI] [PubMed] [Google Scholar]
- 116.Mazzio EA, Smith B, Soliman KF. Evaluation of endogenous acidic metabolic products associated with carbohydrate metabolism in tumor cells. Cell Biol Toxicol. 2010;26:177–188. doi: 10.1007/s10565-009-9138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yost S, Stashenko P, Choi Y, Kukuruzinska M, Genco CA, Salama A, Weinberg EO, Kramer CD, Frias-Lopez J. Increased virulence of the oral microbiome in oral squamous cell carcinoma revealed by metatranscriptome analyses. Int J Oral Sci. 2018;10:32. doi: 10.1038/s41368-018-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Franco R, Schoneveld O, Georgakilas AG, Panayiotidis MI. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008;266:6–11. doi: 10.1016/j.canlet.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 119.Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat Rev Cancer. 2014;14:801–814. doi: 10.1038/nrc3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jin L, Li D, Alesi GN, Fan J, Kang HB, Lu Z, Boggon TJ, Jin P, Yi H, Wright ER, et al. Glutamate dehydrogenase 1 signals through antioxidant glutathione peroxidase 1 to regulate redox homeostasis and tumor growth. Cancer Cell. 2015;27:257–270. doi: 10.1016/j.ccell.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu G, Zhu J, Yu M, Cai C, Zhou Y, Yu M, Fu Z, Gong Y, Yang B, Li Y, et al. Glutamate dehydrogenase is a novel prognostic marker and predicts metastases in colorectal cancer patients. J Transl Med. 2015;13:144. doi: 10.1186/s12967-015-0500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pavlova SI, Jin L, Gasparovich SR, Tao L. Multiple alcohol dehydrogenases but no functional acetaldehyde dehydrogenase causing excessive acetaldehyde production from ethanol by oral streptococci. Microbiology. 2013;159((159Pt 7)):1437–1446. doi: 10.1099/mic.0.066258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marttila E, Bowyer P, Sanglard D, Uittamo J, Kaihovaara P, Salaspuro M, Richardson M, Rautemaa R. Fermentative 2-carbon metabolism produces carcinogenic levels of acetaldehyde in Candida albicans. Mol Oral Microbiol. 2013;28:281–291. doi: 10.1111/omi.12024. [DOI] [PubMed] [Google Scholar]
- 124.Salaspuro M. Microbial metabolism of ethanol and acetaldehyde and clinical consequences. Addict Biol. 1997;2:35–46. doi: 10.1080/13556219772840. [DOI] [PubMed] [Google Scholar]
- 125.Meurman JH, Uittamo J. Oral micro-organisms in the etiology of cancer. Acta Odontol Scand. 2008;66:321–326. doi: 10.1080/00016350802446527. [DOI] [PubMed] [Google Scholar]
- 126.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sellers RS, Morton D. The colon: From banal to brilliant. Toxicol Pathol. 2014;42:67–81. doi: 10.1177/0192623313505930. [DOI] [PubMed] [Google Scholar]
- 128.Taur Y, Pamer EG. Microbiome mediation of infections in the cancer setting. Genome Med. 2016;8:40. doi: 10.1186/s13073-016-0306-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 130.Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.White RA, Malkoski SP, Wang XJ. TGFβ signaling in head and neck squamous cell carcinoma. Oncogene. 2010;29:5437–5446. doi: 10.1038/onc.2010.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang L. TGFbeta and cancer metastasis: An inflammation link. Cancer Metastasis Rev. 2010;29:263–271. doi: 10.1007/s10555-010-9226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lanza G, Centonze SS, Destro G, Vella V, Bellomo M, Pennisi M, Bella R, Ciavardelli D. Shiatsu as an adjuvant therapy for depression in patients with Alzheimer's disease: A pilot study. Complement Ther Med. 2018;38:74–78. doi: 10.1016/j.ctim.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 134.Lanza G, Bella R, Cantone M, Pennisi G, Ferri R, Pennisi M. Cognitive impairment and celiac disease: Is transcranial magnetic stimulation a trait d'union between gut and brain? Int J Mol Sci. 2018;19:E2243. doi: 10.3390/ijms19082243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pennisi M, Bramanti A, Cantone M, Pennisi G, Bella R, Lanza G. Neurophysiology of the ‘Celiac Brain’: Disentangling Gut-Brain Connections. Front Neurosci. 2017;11:498. doi: 10.3389/fnins.2017.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bella R, Lanza G, Cantone M, Giuffrida S, Puglisi V, Vinciguerra L, Pennisi M, Ricceri R, D'Agate CC, Malaguarnera G, et al. Effect of a gluten-free diet on cortical excitability in adults with celiac disease. PLoS One. 2015;10:e0129218. doi: 10.1371/journal.pone.0129218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pennisi G, Lanza G, Giuffrida S, Vinciguerra L, Puglisi V, Cantone M, Pennisi M, D'Agate CC, Naso P, Aprile G, et al. Excitability of the motor cortex in de novo patients with celiac disease. PLoS One. 2014;9:e102790. doi: 10.1371/journal.pone.0102790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pennisi M, Lanza G, Cantone M, Ricceri R, Ferri R, D'Agate CC, Pennisi G, Di Lazzaro V, Bella R. Cortical involvement in celiac disease before and after long-term gluten-free diet: A transcranial magnetic stimulation study. PLoS One. 2017;12:e0177560. doi: 10.1371/journal.pone.0177560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tiffon C. The impact of nutrition and environmental epigenetics on human health and disease. Int J Mol Sci. 2018;19:E3425. doi: 10.3390/ijms19113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Acquaviva R, Sorrenti V, Santangelo R, Cardile V, Tomasello B, Malfa G, Vanella L, Amodeo A, Genovese C, Mastrojeni S, et al. Effects of an extract of Celtis aetnensis (Tornab.) Strobl twigs on human colon cancer cell cultures. Oncol Rep. 2016;36:2298–2304. doi: 10.3892/or.2016.5035. [DOI] [PubMed] [Google Scholar]
- 141.Malfa GA, Tomasello B, Acquaviva R, Genovese C, La Mantia A, Cammarata FP, Ragusa M, Renis M, Di Giacomo C. Betula etnensis Raf. (Betulaceae) extract induced HO-1 expression and ferroptosis cell death in human colon cancer cells. Int J Mol Sci. 2019;20:E2723. doi: 10.3390/ijms20112723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Soldati L, Di Renzo L, Jirillo E, Ascierto PA, Marincola FM, De Lorenzo A. The influence of diet on anti-cancer immune responsiveness. J Transl Med. 2018;16:75. doi: 10.1186/s12967-018-1448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Farhud D, Zarif Yeganeh M, Zarif Yeganeh M. Nutrigenomics and nutrigenetics. Iran J Public Health. 2010;39:1–14. [PMC free article] [PubMed] [Google Scholar]
- 144.Martucci M, Ostan R, Biondi F, Bellavista E, Fabbri C, Bertarelli C, Salvioli S, Capri M, Franceschi C, Santoro A. Mediterranean diet and inflammaging within the hormesis paradigm. Nutr Rev. 2017;75:442–455. doi: 10.1093/nutrit/nux013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, Teeling JL, Blaak EE, Fenech M, Vauzour D, et al. Low-grade inflammation, diet composition and health: Current research evidence and its translation. Br J Nutr. 2015;114:999–1012. doi: 10.1017/S0007114515002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Murtaza N, Burke LM, Vlahovich N, Charlesson B, O'Neill HM, Ross ML, Campbell KL, Krause L, Morrison M. Analysis of the effects of dietary pattern on the oral microbiome of elite endurance athletes. Nutrients. 2019;11:E614. doi: 10.3390/nu11030614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kadayifci FZ, Zheng S, Pan YX. Molecular mechanisms underlying the link between diet and DNA methylation. Int J Mol Sci. 2018;19:E4055. doi: 10.3390/ijms19124055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Guillemin GJ, Essa MM, Song BJ, Manivasagam T. Dietary supplements/antioxidants: impact on redox status in brain diseases. Oxid Med Cell Longev. 2017;2017:5048432. doi: 10.1155/2017/5048432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Quintanilha BJ, Reis BZ, Duarte GBS, Cozzolino SMF, Rogero MM. Nutrimiromics: Role of microRNAs and nutrition in modulating inflammation and chronic diseases. Nutrients. 2017;9:E1168. doi: 10.3390/nu9111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Battaglia R, Palini S, Vento ME, La Ferlita A, Lo Faro MJ, Caroppo E, Borzì P, Falzone L, Barbagallo D, Ragusa M, et al. Identification of extracellular vesicles and characterization of miRNA expression profiles in human blastocoel fluid. Sci Rep. 2019;9:84. doi: 10.1038/s41598-018-36452-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Candido S, Lupo G, Pennisi M, Basile MS, Anfuso CD, Petralia MC, Gattuso G, Vivarelli S, Spandidos DA, Libra M, et al. The analysis of miRNA expression profiling datasets reveals inverse microRNA patterns in glioblastoma and Alzheimer's disease. Oncol Rep. 2019;42:911–922. doi: 10.3892/or.2019.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Falzone L, Romano GL, Salemi R, Bucolo C, Tomasello B, Lupo G, Anfuso CD, Spandidos DA, Libra M, Candido S. Prognostic significance of deregulated microRNAs in uveal melanomas. Mol Med Rep. 2019;19:2599–2610. doi: 10.3892/mmr.2019.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Falzone L, Scola L, Zanghì A, Biondi A, Di Cataldo A, Libra M, Candido S. Integrated analysis of colorectal cancer microRNA datasets: Identification of microRNAs associated with tumor development. Aging (Albany NY) 2018;10:1000–1014. doi: 10.18632/aging.101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Polo A, Crispo A, Cerino P, Falzone L, Candido S, Giudice A, De Petro G, Ciliberto G, Montella M, Budillon A, et al. Environment and bladder cancer: Molecular analysis by interaction networks. Oncotarget. 2017;8:65240–65252. doi: 10.18632/oncotarget.18222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.McCubrey JA, Fitzgerald TL, Yang LV, Lertpiriyapong K, Steelman LS, Abrams SL, Montalto G, Cervello M, Neri LM, Cocco L, et al. Roles of GSK-3 and microRNAs on epithelial mesenchymal transition and cancer stem cells. Oncotarget. 2017;8:14221–14250. doi: 10.18632/oncotarget.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Falzone L, Candido S, Salemi R, Basile MS, Scalisi A, McCubrey JA, Torino F, Signorelli SS, Montella M, Libra M. Computational identification of microRNAs associated to both epithelial to mesenchymal transition and NGAL/MMP-9 pathways in bladder cancer. Oncotarget. 2016;7:72758–72766. doi: 10.18632/oncotarget.11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hafsi S, Candido S, Maestro R, Falzone L, Soua Z, Bonavida B, Spandidos DA, Libra M. Correlation between the overexpression of Yin Yang 1 and the expression levels of miRNAs in Burkitt's lymphoma: A computational study. Oncol Lett. 2016;11:1021–1025. doi: 10.3892/ol.2015.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dalmasso G, Nguyen HTT, Yan Y, Laroui H, Charania MA, Ayyadurai S, Sitaraman SV, Merlin D. Microbiota modulate host gene expression via microRNAs. PLoS One. 2011;6:e19293. doi: 10.1371/journal.pone.0019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Peck BCE, Mah AT, Pitman WA, Ding S, Lund PK, Sethupathy P. Functional transcriptomics in diverse intestinal epithelial cell types reveals robust microrna sensitivity in intestinal stem cells to microbial status. J Biol Chem. 2017;292:2586–2600. doi: 10.1074/jbc.M116.770099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yuan C, Subramanian S. MicroRNA mediated tumor-microbiota metabolic interactions in colorectal cancer. DNA Cell Biol. 2019;38:281–285. doi: 10.1089/dna.2018.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu S, da Cunha AP, Rezende RM, Cialic R, Wei Z, Bry L, Comstock LE, Gandhi R, Weiner HL. The host shapes the gut microbiota via fecal microrna. Cell Host Microbe. 2016;19:32–43. doi: 10.1016/j.chom.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang L, Chen T, Yin Y, Zhang CY, Zhang YL. Dietary microRNA-A Novel Functional Component of Food. Adv Nutr. 2019;10:711–721. doi: 10.1093/advances/nmy127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Teodori L, Petrignani I, Giuliani A, Prattichizzo F, Gurău F, Matacchione G, Olivieri F, Coppari S, Albertini MC. Inflamm-aging microRNAs may integrate signals from food and gut microbiota by modulating common signalling pathways. Mech Ageing Dev. 2019;182:111127. doi: 10.1016/j.mad.2019.111127. [DOI] [PubMed] [Google Scholar]
- 164.Lang C, Karunairetnam S, Lo KR, Kralicek AV, Crowhurst RN, Gleave AP, MacDiarmid RM, Ingram JR. Common Variants of the Plant microRNA-168a Exhibit Differing Silencing Efficacy for Human Low-Density Lipoprotein Receptor Adaptor Protein 1 (LDLRAP1) MicroRNA. 2019;8:166–170. doi: 10.2174/2211536608666181203103233. [DOI] [PubMed] [Google Scholar]
- 165.Golan-Gerstl R, Elbaum Shiff Y, Moshayoff V, Schecter D, Leshkowitz D, Reif S. Characterization and biological function of milk-derived miRNAs. Mol Nutr Food Res. 2017;61:1700009. doi: 10.1002/mnfr.201700009. [DOI] [PubMed] [Google Scholar]
- 166.Wagner AE, Piegholdt S, Ferraro M, Pallauf K, Rimbach G. Food derived microRNAs. Food Funct. 2015;6:714–718. doi: 10.1039/C4FO01119H. [DOI] [PubMed] [Google Scholar]
- 167.Teng Y, Ren Y, Sayed M, Hu X, Lei C, Kumar A, Hutchins E, Mu J, Deng Z, Luo C, et al. Plant-derived exosomal micrornas shape the gut microbiota. Cell Host Microbe. 2018;24:637–652.e8. doi: 10.1016/j.chom.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Adami GR, Tangney CC, Tang JL, Zhou Y, Ghaffari S, Naqib A, Sinha S, Green SJ, Schwartz JL. Effects of green tea on miRNA and microbiome of oral epithelium. Sci Rep. 2018;8:5873. doi: 10.1038/s41598-018-22994-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Felden B, Gilot D. Modulation of bacterial srnas activity by epigenetic modifications: Inputs from the eukaryotic miRNAs. Genes (Basel) 2018;10:E22. doi: 10.3390/genes10010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bloch S, Węgrzyn A, Węgrzyn G, Nejman-Faleńczyk B. Small and smaller-sRNAs and microRNAs in the regulation of toxin gene expression in prokaryotic cells: A mini-review. Toxins (Basel) 2017;9:E181. doi: 10.3390/toxins9060181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yuan C, Steer CJ, Subramanian S. Host-microRNA-microbiota interactions in colorectal cancer. Genes (Basel) 2019;10:E270. doi: 10.3390/genes10040270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Miro-Blanch J, Yanes O. Epigenetic regulation at the interplay between gut microbiota and host metabolism. Front Genet. 2019;10:638. doi: 10.3389/fgene.2019.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Patrignani P, Tacconelli S, Bruno A. Gut microbiota, host gene expression, and aging. J Clin Gastroenterol. 2014;48(Suppl 1):S28–S31. doi: 10.1097/MCG.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 174.Masotti A. Interplays between gut microbiota and gene expression regulation by miRNAs. Front Cell Infect Microbiol. 2012;2:137. doi: 10.3389/fcimb.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhao H, Chu M, Huang Z, Yang X, Ran S, Hu B, Zhang C, Liang J. Variations in oral microbiota associated with oral cancer. Sci Rep. 2017;7:11773. doi: 10.1038/s41598-017-11779-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Al-Hebshi NN, Nasher AT, Maryoud MY, Homeida HE, Chen T, Idris AM, Johnson NW. Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci Rep. 2017;7:1834. doi: 10.1038/s41598-017-02079-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Audirac-Chalifour A, Torres-Poveda K, Bahena-Román M, Téllez-Sosa J, Martínez-Barnetche J, Cortina-Ceballos B, López-Estrada G, Delgado-Romero K, Burguete-García AI, Cantú D, et al. Cervical microbiome and cytokine profile at various stages of cervical cancer: A pilot study. PLoS One. 2016;11:e0153274. doi: 10.1371/journal.pone.0153274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hsieh YY, Tung SY, Pan HY, Yen CW, Xu HW, Lin YJ, Deng YF, Hsu WT, Wu CS, Li C. Increased abundance of Clostridium and Fusobacterium in gastric microbiota of patients with gastric cancer in Taiwan. Sci Rep. 2018;8:158. doi: 10.1038/s41598-017-18596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yamamura K, Baba Y, Nakagawa S, Mima K, Miyake K, Nakamura K, Sawayama H, Kinoshita K, Ishimoto T, Iwatsuki M, et al. Human microbiome Fusobacterium nucleatum in esophageal cancer tissue is associated with prognosis. Clin Cancer Res. 2016;22:5574–5581. doi: 10.1158/1078-0432.CCR-16-1786. [DOI] [PubMed] [Google Scholar]
- 180.Yang CY, Yeh YM, Yu HY, Chin CY, Hsu CW, Liu H, Huang PJ, Hu SN, Liao CT, Chang KP, et al. Oral microbiota community dynamics associated with oral squamous cell carcinoma staging. Front Microbiol. 2018;9:862. doi: 10.3389/fmicb.2018.00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lim Y, Fukuma N, Totsika M, Kenny L, Morrison M, Punyadeera C. The performance of an oral microbiome biomarker panel in predicting oral cavity and oropharyngeal cancers. Front Cell Infect Microbiol. 2018;8:267. doi: 10.3389/fcimb.2018.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sasaki M, Yamaura C, Ohara-Nemoto Y, Tajika S, Kodama Y, Ohya T, Harada R, Kimura S. Streptococcus anginosus infection in oral cancer and its infection route. Oral Dis. 2005;11:151–156. doi: 10.1111/j.1601-0825.2005.01051.x. [DOI] [PubMed] [Google Scholar]
- 183.Pushalkar S, Ji X, Li Y, Estilo C, Yegnanarayana R, Singh B, Li X, Saxena D. Comparison of oral microbiota in tumor and non-tumor tissues of patients with oral squamous cell carcinoma. BMC Microbiol. 2012;12:144. doi: 10.1186/1471-2180-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Galvão-Moreira LV, da Cruz MC. Oral microbiome, periodontitis and risk of head and neck cancer. Oral Oncol. 2016;53:17–19. doi: 10.1016/j.oraloncology.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 185.Shiga K, Tateda M, Saijo S, Hori T, Sato I, Tateno H, Matsuura K, Takasaka T, Miyagi T. Presence of Streptococcus infection in extra-oropharyngeal head and neck squamous cell carcinoma and its implication in carcinogenesis. Oncol Rep. 2001;8:245–248. [PubMed] [Google Scholar]
- 186.Narikiyo M, Tanabe C, Yamada Y, Igaki H, Tachimori Y, Kato H, Muto M, Montesano R, Sakamoto H, Nakajima Y, et al. Frequent and preferential infection of Treponema denticola, Streptococcus mitis, and Streptococcus anginosus in esophageal cancers. Cancer Sci. 2004;95:569–574. doi: 10.1111/j.1349-7006.2004.tb02488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yang SF, Huang HD, Fan WL, Jong YJ, Chen MK, Huang CN, Chuang CY, Kuo YL, Chung WH, Su SC. Compositional and functional variations of oral microbiota associated with the mutational changes in oral cancer. Oral Oncol. 2018;77:1–8. doi: 10.1016/j.oraloncology.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 188.Kumar M, Kumar A, Nagpal R, Mohania D, Behare P, Verma V, Kumar P, Poddar D, Aggarwal PK, Henry CJ, et al. Cancer-preventing attributes of probiotics: An update. Int J Food Sci Nutr. 2010;61:473–496. doi: 10.3109/09637480903455971. [DOI] [PubMed] [Google Scholar]
- 189.Yu AQ, Li L. The potential role of probiotics in cancer prevention and treatment. Nutr Cancer. 2016;68:535–544. doi: 10.1080/01635581.2016.1158300. [DOI] [PubMed] [Google Scholar]
- 190.Lee JW, Shin JG, Kim EH, Kang HE, Yim IB, Kim JY, Joo HG, Woo HJ. Immunomodulatory and antitumor effects in vivo by the cytoplasmic fraction of Lactobacillus casei and Bifidobacterium longum. J Vet Sci. 2004;5:41–48. doi: 10.4142/jvs.2004.5.1.41. [DOI] [PubMed] [Google Scholar]
- 191.Ma EL, Choi YJ, Choi J, Pothoulakis C, Rhee SH, Im E. The anticancer effect of probiotic Bacillus polyfermenticus on human colon cancer cells is mediated through ErbB2 and ErbB3 inhibition. Int J Cancer. 2010;127:780–790. doi: 10.1002/ijc.25011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kim Y, Oh S, Yun HS, Oh S, Kim SH. Cell-bound exopolysaccharide from probiotic bacteria induces autophagic cell death of tumour cells. Lett Appl Microbiol. 2010;51:123–130. doi: 10.1111/j.1472-765X.2010.02859.x. [DOI] [PubMed] [Google Scholar]
- 193.Borowicki A, Michelmann A, Stein K, Scharlau D, Scheu K, Obst U, Glei M. Fermented wheat aleurone enriched with probiotic strains LGG and Bb12 modulates markers of tumor progression in human colon cells. Nutr Cancer. 2011;63:151–160. doi: 10.1080/01635581.2010.516874. [DOI] [PubMed] [Google Scholar]
- 194.Stein K, Borowicki A, Scharlau D, Schettler A, Scheu K, Obst U, Glei M. Effects of synbiotic fermentation products on primary chemoprevention in human colon cells. J Nutr Biochem. 2012;23:777–784. doi: 10.1016/j.jnutbio.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 195.Wan LH, Yao Z, Ni F, Wei M, Zhou Z, Wang HQ, Sun Y, Zhong ZX. Biosynthesis of genipin from gardenoside catalyzed by β-glucosidase in two-phase medium. CIESC J. 2014;65:3583–3591. [Google Scholar]
- 196.Asoudeh-Fard A, Barzegari A, Dehnad A, Bastani S, Golchin A, Omidi Y. Lactobacillus plantarum induces apoptosis in oral cancer KB cells through upregulation of PTEN and downregulation of MAPK signalling pathways. Bioimpacts. 2017;7:193–198. doi: 10.15171/bi.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Aghazadeh Z, Pouralibaba F, Yari Khosroushahi A. The prophylactic effect of Acetobacter syzygii probiotic species against squamous cell carcinoma. J Dent Res Dent Clin Dent Prospects. 2017;11:208–214. doi: 10.15171/joddd.2017.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Orlandi E, Iacovelli NA, Tombolini V, Rancati T, Polimeni A, De Cecco L, Valdagni R, De Felice F. Potential role of microbiome in oncogenesis, outcome prediction and therapeutic targeting for head and neck cancer. Oral Oncol. 2019;99:104453. doi: 10.1016/j.oraloncology.2019.104453. [DOI] [PubMed] [Google Scholar]
- 199.Christofi T, Baritaki S, Falzone L, Libra M, Zaravinos A. Current perspectives in cancer immunotherapy. Cancers (Basel) 2019;11:E1472. doi: 10.3390/cancers11101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Guerrero-Preston R, Godoy-Vitorino F, Jedlicka A, Rodríguez-Hilario A, González H, Bondy J, Lawson F, Folawiyo O, Michailidi C, Dziedzic A, et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget. 2016;7:51320–51334. doi: 10.18632/oncotarget.9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tuaeva NO, Falzone L, Porozov YB, Nosyrev AE, Trukhan VM, Kovatsi L, Spandidos DA, Drakoulis N, Kalogeraki A, Mamoulakis C, et al. Translational application of circulating DNA in oncology: Review of the last decades achievements. Cells. 2019;8:E1251. doi: 10.3390/cells8101251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Silantyev AS, Falzone L, Libra M, Gurina OI, Kardashova KS, Nikolouzakis TK, Nosyrev AE, Sutton CW, Mitsias PD, Tsatsakis A. Current and future trends on diagnosis and prognosis of glioblastoma: From molecular biology to proteomics. Cells. 2019;8:E863. doi: 10.3390/cells8080863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ribeiro IP, de Melo JB, Carreira IM. Head and neck cancer: Searching for genomic and epigenetic biomarkers in body fluids - the state of art. Mol Cytogenet. 2019;12:33. doi: 10.1186/s13039-019-0447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Falzone L, Salomone S, Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol. 2018;9:1300. doi: 10.3389/fphar.2018.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Chattopadhyay I, Verma M, Panda M. Role of oral microbiome signatures in diagnosis and prognosis of oral cancer. Technol Cancer Res Treat. 2019;18:1533033819867354. doi: 10.1177/1533033819867354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.