Abstract
Scope
We prospectively evaluated the association between self-reported dietary intake and urinary metabolomic markers of habitual nut exposure with cognitive decline over a 3-year follow-up in an older Italian population.
Methods and results
We selected 119 older participants, from the InCHIANTI cohort based on self referred nut intake: the non-nut consumer (n=72) and the regular nut consumer (≥2.9 g/d, n=47) groups. Nut exposure was measured at baseline either with the use of a validated food frequency questionnaire or with an HPLC-Q-ToF-MS metabolomic approach. Three years after, 28 from non-consumers group and 10 from consumers group experienced cognitive decline. Dietary nut exposure was characterized by urinary metabolites of polyphenols and fatty acids pathways. Nut consumption estimated either by the dietary marker or by the urinary marker model was in both cases associated with less cognitive decline (OR: 0.78, 95% CI: 0.61,0.99; P = 0.043 and OR: 0.995, 95% CI: 0.991,0.999; P = 0.016, respectively) with AUCs 73.2 (95% CI: 62.9,83.6) and 73.1 (62.5,83.7), respectively.
Conclusions
A high intake of nuts may protect older adults from cognitive decline. The use of a panel of metabolites provides accurate and complementary information of the nut exposure and reinforces the results obtained using dietary information.
Keywords: cognition, diet, InCHIANTI, metabolomics, nuts
Graphical Abstract
Nut consumption estimated either by the dietary marker or by the urinary marker model was in both cases associated with less cognitive decline in older adults
1. Introduction
A decline in cognitive function is a core feature of dementia, which represents a growing health problem worldwide [1]. The World Health Organization has predicted that the total number of people with dementia will rise up to 115.4 million by 2050 [2]. Accordingly, identifying effective strategies for preventing dementia or slowing down its progression is becoming a major public health priority. Accumulating evidence suggests an association between healthy cognitive function and dietary factors, in particular high vegetable and fruit intake, low saturated and trans fat intake, high long-chain omega-3 fatty acid intake, and intake of vitamins E and B12 [3, 4]. Recent studies suggested that dietary intake of nuts is protective against cognitive decline [5]. Nuts contain large amounts of plant protein and unsaturated fatty acids, dietary fibre, vitamins (e.g., folic acid, niacin, tocopherols, vitamin B6), minerals (e.g., calcium, magnesium, potassium, zinc), and bioactive compounds such as phytosterols and phenolic compounds [6]. Its peculiar chemical composition is critical for providing their beneficial health effects. Indeed, epidemiological and clinical studies have associated exposure to nuts with a reduced risk of cardiovascular diseases, type 2 diabetes, cancer, metabolic syndrome and total mortality [7, 8]. Because of these data, nut intake is recommended in several dietary guidelines worldwide [9]. However, evidence that nut consumption is protective against cognitive decline is still scant [10].
In addition, a general problem of the studies that evaluated the protective role of nut dietary consumption on cognitive decline is that nut exposure was only based on the assessment of dietary questionnaires, which lack precision and tend to be biased [11]. In order to better evaluate the association between dietary exposure and health outcomes, an accurate and objective assessment of the exposure is needed. The use of food biomarkers may overcome these limitations. For example, untargeted metabolomic approaches are progressively more used in nutritional studies, alone or in combination to self-reported dietary questionnaires [11].
The aim of this study is to investigate the association between dietary and urinary markers of habitual nut exposure with cognitive decline over a 3-year follow-up in a cohort of Italian participants aged 65 years and older in the InCHIANTI (Invecchiare in Chianti (Aging in Chianti)) study. For this propose, we measured the nut exposure either by dietary questionnaire or by applying an untargeted metabolomic approach. We characterized the food metabolome associated with nut intake, and developed a potential model of urinary biomarkers for assessing habitual nut exposure accurately in the InCHIANTI cohort.
2. Experimental Section
STUDY POPULATION
The InCHIANTI study is a population-based prospective cohort study located in two municipalities adjacent to the city of Florence (Italy), and it is described in detail elsewhere [12]. The study randomly sampled 1260 participants aged ≥ 65 years, of whom 1155 agreed to be enrolled. The participation rate was 91.7%. Of these, 82 participants (7.1%) with dementia at baseline, according to criteria set out in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition), were excluded. A total of 609 dementia-free participants aged ≥ 65 years were selected based on the availability of baseline 24 h urine samples (collected in 1998–2000), as well as baseline and 3-year follow-up cognitive assessment (administered in 1998–2000 and 2001–2003, respectively) (Supporting Information Figure 1). Finally, 119 subjects were selected based on baseline nut intake (see dietary assessment section for details) and randomly selected for metabolomics analysis based on previous experience [13]. The Italian National Institute of Research and Care of Aging Ethical Committee approved the study protocol, and all participants provided informed consent to participate.
DIETARY ASSESSMENT
Nut intake was assessed at baseline with the use of the Italian version of the food frequency questionnaire (FFQ) developed and validated in the European Prospective Study into Cancer and Nutrition (EPIC) [14]. This questionnaire includes one item regarding the consumption of walnuts, almonds, hazelnuts and peanuts with four categories for the frequency of consumption (never, rarely, times per week or per month). In the present study, total nut intake was converted to g/d. Regarding the nut intake of the selected participants, 72 patients never consumed nuts (0 g/d) (non consumers (NC)), and 47 patients were regular consumers (RC), i.e. participants with an intake of ≥2.9 g/d of nuts in the preceding year (nut serving size, 28 g). The groups NC and RC were frequency matched group by sex, age and smoking status (Supporting Information Figure 1). In the present study, sporadic consumers (between >0g/d and < 2.9 g/d of nut intake) were excluded to reduce “biological noise”.
URINARY NUT METABOLOME ASSESSMENT
At baseline, a single 24 h urine sample was collected from each participant. Urine samples were divided into aliquots, coded and stored at −80 ºC until analysis. Sample preparation was based on previously published methodology and was applied for the metabolomic analysis [13, 15]. Briefly, the samples were centrifuged for 5 min at 12000 g. A 50 μL aliquot of the supernatant was mixed with an equal volume of Milli-Q water, and transferred into a 96-well plate for metabolomics analyses.
Metabolomic analyses were conducted using an HPLC-Q-ToF-MS [Agilent 1200 Series Rapid Resolution HPLC system coupled to a hybrid quadrupole TOF (Q-ToF) QSTAR Elite mass spectrometer (Applied Biosystems/MDS Sciex, Framingham, MA, USA)] in negative ion mode due to the chemical characteristics of the metabolites (observed before in our previous articles [13, 15]) and following our previously published protocol [13]. A linear gradient elution was performed with a binary system consisting of [A] Milli-Q water 0.1% HCOOH (v/v) and [B] acetonitrile 0.1% HCOOH (v/v), at a constant flow rate of 600 μL min−1. The gradient elution (v/v) of [B] used was as follows (time, min; B, %): (0, 1), (4, 20), (6, 95), (7.5, 95), (8, 1), (12, 1).
Data were extracted and aligned using MarkerView TM 1.2.1 (Applied Biosystems, MDS Sciex, Toronto, Ontario, Canada). The parameters used for the processing of raw data are listed in (Supporting Information Table 1. Before multivariate statistical analysis, mass feature data sets were log-transformed and Pareto-scaled using SIMCA-P +13.0 software (Umetrics, Umeå, Sweden). The mass features with coefficient of variation (CV) in urine samples greater than CV in pool of urine samples were removed to minimize the analytical variation. The mass features that were missing in at least 25% of samples from both groups were considered to be noise and they were excluded in the future analyses.
The differences in the urine metabolome between the groups of study (NC versus RC) were explored using partial least-squares discriminant analysis with orthogonal signal correction (OSC-PLS-DA) (Supporting Information Figure 3 [16]. The quality of the models was evaluated through the goodness-of-fit parameter (R2X), the proportion of the variance of the response variable that is explained by the model (R2Y), and the predictive ability parameter (Q2). The validation of the models was evaluated with the permutation test (n=200) (20). As a final quality test, the whole data set was randomly split into four equal-size subsamples (25% of the sample each), three of which were used as the training set while the remaining one was used as the validation set. This process was repeated four times, each subsample being used as the validation set at least once, and the correctly classified participants in each validation set (%) were calculated (Supporting Information Tables 2). Those mass features with the highest variable importance projection (VIP) values in the RC group (cut-off ≥ 2) were selected as the most relevant to explain the differences in urinary metabolomics profiles associated with nut intake. After that, a multistep procedure was used to annotate putative urinary markers of nut intake from the selected mass features [15]. Putatively annotated compounds were carried out by matching mass features with mass spectral databases (Human Metabolome Database [17], Metlin [18], MetFrag [19]) with a mass error tolerance of ±10 mDa (assigning a level 2 of the evidence in the identification in accordance with Metabolomics Standards Initiative criteria [20]).
COGNITIVE FUNCTION ASSESSMENT
Cognitive function was measured using the Mini-Mental State Examination (MMSE), which was administered at baseline and at 3-year follow-up. The MMSE is a validated method for assessing global cognitive function, and is widely used in both clinical practice and research [21, 22]. It evaluates five areas of cognitive functioning: orientation, registration, attention and calculation, recall, and language abilities. Overall scores range from 0 to 30, with high scores indicating better cognitive functioning (continuous variable).
To calculate change in cognitive function, MMSE scores at baseline were subtracted from MMSE scores at 3-year follow-up. Thus, the subjects were divided into those with cognitive decline, defined as a decrease of two points or more from baseline assessment to 3-year follow-up [21, 23] and those without it (dichotomous variable).
OTHER BASELINE COVARIATE ASSESSMENT
Trained geriatricians conducted a comprehensive assessment of health, functional status and anthropometric measures using standardized protocols [12]. Dietary intake of total energy (kcal/d) and alcohol (g/d) in the previous year were estimated using the FFQ [14] and an Italian food composition table [24]. Physical activity in the previous year was self-reported and was classified as [25]: 1) sedentary (completely inactive or light-intensity physical activity, e.g. walking), 2) light (light-intensity physical activity for 2 to 4 h/wk), and 3) moderate to intense (light-intensity physical activity of at least > 4 h/wk or moderate-intensity physical activity of at least 1–2 h/wk). Smoking status (current, former or never-smoker), age, sex, BMI and education (years of schooling) were reported or calculated. Functional status was assessed using Katz’s Activities of Daily Living (ADL) [26], and the Lawton and Brody Instrumental Activities of Daily Living (IADL) [27] scales. Depressive symptoms were evaluated using the Center for Epidemiologic Studies Depression Scale (CES-D). A CES-D score of ≥16 was defined as a depressed mood [28]. Diseases were ascertained by combining information from self-reported physician diagnoses, pharmacological treatments, medical history, clinical examinations and blood tests. Diseases considered in this analysis were renal impairment, diabetes, cardiovascular diseases (myocardial infarction, angina pectoris and peripheral arterial disease), hypertension and stroke [29]. Inflammatory markers were measured in serum samples. Interleukin (IL)-6 and IL-1 receptor antagonist (IL-1ra) were measured by high-sensitivity enzyme-linked immunoabsorbent assays (ELISAs) using commercial kits (BIOSOURCE International Inc., Camarillo, CA). The high-sensitivity C-reactive protein (hsCRP) was measured by an ELISA colorimetric competitive immunoassay that used purified protein and polyclonal anti-CRP antibodies. TNF-α was measured using multiplex technology (Human Serum Adipokine Panel B LINCOplex kit; Linco Research, Inc., St. Charles, MO) [12].
STATISTICAL ANALYSIS
Descriptive analyses were performed to summarize information about the baseline characteristics of the study population. Differences between nut exposure groups, as well as differences between groups of cognitive function and differences between included and excluded participants groups, were tested by using a Student’s t-test, Mann-Whitney test or Chi-square test.
Nut exposure was assessed using either dietary or urinary markers. To explore the relationships between dietary and urinary markers of nut intake, Spearman’s rank correlation coefficients were used.
Multimetabolite prediction biomarker model of nut exposure
To design multimetabolite biomarker panels associated with habitual dietary nut exposure, Tobit models [30], a class of censored regression model designed to mitigate the problem of zero-inflated data, were conducted to estimate β coefficients and their SEE with the identified urinary markers. The continuous variable on dietary marker of nut intake was used as dependent variable, where the threshold was a value of 0 g/d. Two full and two reduced multimetabolite biomarker panels related to nut intake were proposed using identified urinary metabolites. The combination of individual biomarkers’ performance was evaluated using ROC curves. The likelihood ratio test was used for comparing the goodness of fit between the models.
Associations between nut exposure and cognitive decline
Associations between nut exposure, dietary and urinary markers (individuals and panels) and cognitive decline were analysed using two different but complementary statistical approaches: 1) associations with a change in cognitive function (continuous variable) over 3 years of follow-up, which were evaluated by estimating the standardized β coefficients and their 95% CI in linear regression models; and 2) associations with a decline in cognitive function (dichotomous variable) over 3 years of follow-up, which were analysed by estimating the OR and its 95% CI in logistic regression models. Covariates in these statistical models were identified a priori as known risk factors or potential confounders. Four separate statistical models were performed: Model 1, adjusted for the baseline cognitive score (in order to correct for the “regression toward the mean”), for sex and age; Model 2, additionally adjusted for BMI, energy intake (except for urinary markers), alcohol consumption, education, physical activity and smoking status; and Model 3, additionally adjusted for depressive symptoms, stroke, cardiovascular diseases, hypertension and diabetes. Interactions with sex, age, BMI, education, smoking status and physical activity were evaluated between dietary nut marker and change in cognitive function by including product terms in the fully adjusted model. Moreover, the global performance of the associations between dietary and urinary markers of nut intake, adjusted for covariates (Model 3), and decline in cognitive function was evaluated using ROC curves and estimating the AUC (95% CI).
All P values presented are two-tailed and were considered to be statistically significant when P<0.05. Data from metabolomic and epidemiologic analyses were analysed using R software version 3.2.4 (http://www.r-project.org) and the SPSS package program version 21.0 (SPSS, Chicago, IL).
3. Results
Descriptive study and dietary markers of nut exposure
The baseline characteristics of the study population according to groups of dietary nut intake are presented in Table 1. There were 72 NC (0 g/d) and 47 RC (≥2.9 g/d). This study included 57 women and 62 men, with a mean age of 73 years. Participants in the RC group had a lower prevalence of disability in ADL and had a smaller decline (change) in cognitive function than those in the NC group. Of the 119 participants, 38 had cognitive decline (Supporting Information Table 3).
Table 1.
Baseline characteristics of the study population according to groups of habitual dietary nut intake1
| Total | Non-consumers | Regular Consumers | P | |
|---|---|---|---|---|
| Participants, n (%) | 119 | 72 (60.5) | 47 (39.5) | – |
| Nut, g/d | 1.9 ± 3.22 | 0.0 ± 0.0 | 4.9 ± 3.3 | <0.001 |
| Energy, kcal/d | 2014 ± 589 | 1804 ± 541 | 2337 ± 511 | <0.001 |
| Alcohol, g/d | 17.3 ± 22.9 | 14.0 ± 18.7 | 22.3 ± 27.7 | 0.054 |
| Demographics | ||||
| Sex, F, n (%) | 57 (47.9) | 36 (50.0) | 21 (44.7) | 0.57 |
| Age, y | 73.0 ± 5.9 | 73.5 ± 6.2 | 72.2 ± 5.2 | 0.23 |
| BMI, kg/m2 | 27.5 ± 3.8 | 27.1 ± 3.7 | 27.9 ± 3.8 | 0.27 |
| Physical Activity, n (%) | 0.53 | |||
| Sedentary | 18 (15.1) | 9 (12.5) | 9 (19.1) | |
| Light | 55 (46.2) | 33 (45.8) | 22 (46.8) | |
| Moderate to high | 46 (38.7) | 30 (41.7) | 16 (34.0) | |
| Smoking status, n (%) | 0.18 | |||
| Never | 51 (42.9) | 26 (36.1) | 25 (53.2) | |
| Current | 35 (29.4) | 24 (33.3) | 11 (23.4) | |
| Former | 33 (27.7) | 22 (30.6) | 11 (23.4) | |
| Education (years of school) | 6.0 ± 3.5 | 5.9 ± 3.7 | 6.1 ± 4.2 | 0.84 |
| Disability in ≥1 ADL, n (%) | 6 (5.0) | 6 (8.3) | 0 (0.0) | 0.042 |
| Disability in ≥1 IADL, n (%) | 14 (11.8) | 8 (11.1) | 6 (12.8) | 0.78 |
| Inflammatory markers | ||||
| IL-6, pg/mL | 2.8 (1.9–3.9)3 | 2.4 (1.8–3.8) | 3.0 (2.2–4.9) | 0.11 |
| TNF-α, pg/mL | 4.4 (2.9–5.6) | 4.2 (2.8–5.4) | 4.8 (3.6–5.9) | 0.30 |
| hsCRP, μg/mL | 2.7 (1.5–4.8) | 2.5 (1.4–5.0) | 3.0 (1.5–4.8) | 0.36 |
| IL-1ra, pg/mL | 121.3 (93.4–187.1) | 120.0 (84.7–193.9) | 125.8 (93.8–178.5) | 0.99 |
| Diseases and conditions | ||||
| Renal impairment, n (%) | 67 (57.8) | 43 (61.4) | 24 (52.2) | 0.32 |
| Diabetes, n (%) | 12 (10.1) | 9 (12.5) | 3 (6.4) | 0.28 |
| Cardiovascular disease, n (%) | 26 (21.8) | 12 (16.7) | 14 (29.8) | 0.09 |
| Hypertension, n (%) | 62 (52.1) | 38 (52.8) | 24 (51.1) | 0.86 |
| Stroke, n (%) | 11 (9.2) | 7 (9.7) | 4 (8.5) | 0.82 |
| Depressive symptoms, CES-D score ≥16, n (%) | 33 (27.7) | 19 (26.4) | 14 (29.8) | 0.69 |
| Cognitive function | ||||
| Subjects with cognitive decline, n (%) | 38 (31.9) | 28 (38.0) | 10 (21.0) | 0.04 |
| Baseline global cognitive function, MMSE score | 25.7 ± 2.5 | 24.6 ± 4.4 | 25.7 ± 2.7 | 0.87 |
| Global cognitive function change | −0.5 ± 3.4 | −1.1 ± 3.7 | 0.4 ± 2.6 | 0.012 |
Descriptive analyses were compared between groups of nut exposure with the use of a Student’s t-test, Mann-Whitney test or Chi-square test as appropriate. No missing participants. ADL, Activities of Daily Living; CES-D, Center for Epidemiologic Studies Depression scale; hsCRP, high-sensitivity C-Reactive Protein; IADL, Instrumental Activities of Daily Living; IL-1ra, Interleukin-1 receptor antagonist; IL-6, Interleukin-6; MMSE, Mini-Mental State Examination; TNF-α, Tumors Necrosis Factor-α.
Mean ± SD (all such values).
Median (IQR) (all such values).
Urinary metabolomic markers assessment
The results of the data acquisition quality and the quality of the models of metabolic profiling differentiation in the study are included in the Supporting Information Figure 2 and 3).
A total of 18 urinary metabolites were identified or tentatively identified as discriminatory urinary metabolites related to dietary nut exposure including: 1) markers of the polyphenol microbial metabolism (urolithin A (glucuronide, sulphate and sluphoglucuronide), urolithin B (glucuronide), hydroxyhippuric acid, hydroxyphenylacetic acid); 2) a marker of the polyphenol intestinal metabolism (resveratrol-sulphate); 3) markers of the fatty acid metabolism (dodecanedioic and dimethylglutaric acids); 4) markers of the tryptophan metabolism (indole-3-acetic acid glucuronide and indoxyl sulphate / indoxylsulphuric acid); 5) a marker of the benzoxazinoid biosynthesis (dihydroxy-benzoxazinone) and 6) four unidentified markers (Table 2). According to the FFQ, there were statistically significant differences in wine consumption (P = 0.011) between the groups of dietary nut intake. Although among nuts, peanuts can also contain small amounts of resveratrol; it was assumed that resveratrol-sulphate is a biomarker of wine more than nut consumption [31]. Therefore, resveratrol-suphate metabolite was excluded from the subsequent analyses
Table 2.
Urinary metabolites tentatively identified as potential biomarkers of nut intake1
| N. Cluster | RT (min) | Detected mass (m/z) | Theoretical mass (m/z) | Error (mDa) | Assignation | Potential biomarker | VIP |
|---|---|---|---|---|---|---|---|
| Markers of the polyphenol microbial metabolism | |||||||
| 1 | 6.45 | 227.0327 | 227.0350 | 2.3 | [M – H]− | Urolithin A | 3.75 |
| 228.0365 | 228.0384 | 1.9 | 13C[M – H]− | 2.56 | |||
| 2 | 5.38 | 403.0626 | 403.0671 | 4.5 | [M – H]− | Urolithin A glucuronide | 4.78 |
| 404.0663 | 404.0705 | 4.1 | 13C[M – H]− | 4.38 | |||
| 227.0329 | 227.0350 | 2.1 | [M – H – glucuronide]− | 3.43 | |||
| 3 | 6.64 | 306.9884 | 306.9918 | 3.4 | [M – H]− | Urolithin A sulphate | 3.96 |
| 4 | 5.23 | 483.0187 | 483.0239 | 5.2 | [M – H]− | Urolithin A sulphoglucuronide | 3.01 |
| 5 | 6.80 | 211.0387 | 211.0401 | 1.4 | [M – H]− | Urolithin B | 2.00 |
| 6 | 6.30 | 387.0650 | 387.0721 | 7.1 | [M – H]− | Urolithin B glucuronide | 2.44 |
| - | 388.0708 | 388.0755 | 4.7 | 13C[M – H]− | 2.23 | ||
| - | - | 211.0386 | 211.0401 | 1,5 | [M – H – glucuronide]− | - | - |
| 7 | 5.00 | 151.0579 | 151.0594 | 1.5 | 13C[M – H – COO]− | Hydroxyhippuric acid | 2.66 |
| - | - | 150.0529 | 150.0560 | 3,1 | [M – H – COO]- | - | - |
| - | 4.98 | 194.0367 | 194.0459 | 9,2 | [M – H]- | - | - |
| 8 | 4.50 | 151.0383 | 151.0401 | 1.8 | [M – H]− | 2-Hydroxyphenylacetic acid | 3.59 |
| Markers of the polyphenol intestinal metabolism | |||||||
| 9 | 6.95 | 307.0265 | 307.0282 | 1.7 | [M – H]− | Resveratrol-sulphate | 2.99 |
| - | - | 308.0285 | 308.0316 | 3.1 | 13C[M – H]- | - | - |
| 227.0702 | 227.0714 | 1.2 | [M – H – sulphate]− | 2.72 | |||
| - | - | 228.0732 | 228.0748 | 1.6 | 13C[M – H – sulphate]− | - | - |
| Markers of the fatty acid metabolism | |||||||
| 10 | 6.77 | 229.1429 | 229.1445 | 1.6 | [M – H]− | Dodecanedioic acid | 2.15 |
| 230.1468 | 230.1479 | 1.1 | 13C[M – H]− | 2.06 | |||
| 11 | 4.07 | 160.0700 | 160.0697 | 0.3 | 13C[M – H]− | Dimethylglutaric acid | 2.70 |
| - | - | 159.0664 | 159.0663 | 0.1 | [M – H]- | - | - |
| 115.0769 | 115.0764 | 0.5 | [M – H – COO]− | 2.09 | |||
| - | - | 97.0657 | 97.0659 | 0.2 | [M – H – COO – H2O]- | - | - |
| Markers of the tryptophan metabolism | |||||||
| 12 | 5.28 | 175.0265 | 175.0594 | 0.3 | 13C[M – H – glucuronide]− | Indole-3-acetic acid glucuronide | 2.08 |
| - | - | 174.0569 | 174.0560 | 0.9 | [M – H – glucuronide]- | - | - |
| - | - | 351.0911 | 351.0915 | 0.4 | 13C[M – H]- | - | - |
| - | - | 350.0878 | 350.0881 | 0.3 | [M – H]- | - | - |
| 13 | 4.55 | 212.0018 | 212.0023 | 0.5 | [M – H]− | Indoxyl sulphate / Indoxylsulphuric acid | 2.58 |
| 213.0048 | 213.0057 | 0.9 | 13C[M – H]− | 2.05 | |||
| 132.0448 | 132.0455 | 0.7 | [M – H – sulphate]− | 2.13 | |||
| - | - | 425.0117 | 425.0119 | 0.2 | [2M– H]− | - | - |
| Markers of the benzoxazinoid biosynthesis | |||||||
| 14 | 4.93 | 180.0373 | 180.0302 | 7.1 | [M – H]− | Dihydroxy-benzoxazinone | 3.14 |
| Unidentified markers | |||||||
| 15 | 6.70 | 309.0409 | [M – H]− | Unknown A (sulphate derivative) | 3.19 | ||
| 310.0437 | 13C[M – H]− | 2.27 | |||||
| 311.0381 | 213C[M – H]− | 2.48 | |||||
| 16 | 6.15 | 359.1329 | [M – H]− | Unknown B (glucuronide derivative) | 2.04 | ||
| - | - | 183.1024 | - | - | [M – H – glucuronide]- | - | - |
| 17 | 6.54 | 397.1102 | [M – H]− | Unknown C | 2.66 | ||
| - | - | 398.1141 | 398,1136 | 0,5 | 13C[M – H]- | - | - |
| 18 | 6.22 | 373.1150 | [M – H]− | Unknown D | 2.25 | ||
| 374.1126 | 13C[M – H]− | 2.34 | |||||
One-class OSC-PLS-DA model (NC versus RC) was used. NC, non-nut consumers; RT, retention time; VIP, variable importance projection; RC, regular nut consumers. Most important MS/MS fragments that were used in the identification fo the metabolites with VIP <2 are also included in the table.
In order to improve the discrimination between the two groups (NC and RC), two full multimetabolite biomarker panels of nut intake were evaluated using Tobit models to estimate β coefficients and their SEE: Model A) all 13 known identified urinary metabolites; and Model B) a selection of 7 identified urinary metabolite biomarkers (urolithin A and its three conjugates, urolithin B and its glucuronide, and dodecanedioic acid), which were previously associated with nut exposure as well (11, 12, 34). Two reduced biomarker panels were also evaluated: Model C) a reduced panel with the 4 metabolite urinary biomarkers from Model A with the highest β coefficient (urolithin A glucuronide, hydroxyhippuric acid, hydroxyphenylacetic acid and dimethylglutaric acid); and Model D) a reduced panel with 2 metabolite biomarkers from Model B (urolithin A and its glucuronide) (Table 3). The reduced models were not statistically different from the full models in a likelihood ratio test (Models A and C: P =0.30; Models B and D: P = 0.35). Furthermore, the panels for Models A, B, C and D displayed 80.9, 74.5, 83.0 and 70.2 % sensitivity and 87.5, 69.4, 84.7 and 70.8 % specificity, with an AUC of 90.7, 76.7, 93.3 and 78.2 % (all P<0.001), respectively (Supporting Information Figure 4).
Table 3.
Multimetabolite prediction biomarker models associated with habitual nut intakea
| Model | Dietary nut exposure urinary biomarker | β ± SEE | P | Model | Dietary nut exposure biomarker | β ± SEE | P |
|---|---|---|---|---|---|---|---|
| A | Urolithin A | −0.033 ± 1.196 | 0.98 | C | Urolithin A glucuronide | 2.905 ± 0.676 | <0.001 |
| Urolithin A glucuronide | 2.193 ± 1.032 | 0.034 | Hydroxyhippuric acid | 3.436 ± 0.894 | <0.001 | ||
| Urolithin A sulphate | 0.088 ± 1.332 | 0.95 | Hydroxyphenylacetic acid | 3.263 ± 0.763 | <0.001 | ||
| Urolithin A sulphoglucuronide | 0.011 ± 1.242 | 0.99 | Dimethylglutaric acid | 3.452 ± 1.124 | <0.001 | ||
| Urolithin B | 0.240 ± 1.392 | 0.86 | |||||
| Urolithin B glucuronide | 0.670 ± 1.212 | 0.58 | |||||
| Hydroxyhippuric acid | 3.453± 0.971 | <0.001 | |||||
| Hydroxyphenylacetic acid | 2.887 ± 0.795 | <0.001 | |||||
| Dodecanedioic acid | 1.102 ± 0.850 | 0.19 | |||||
| Dimethylglutaric acid | 2.339 ± 1.220 | 0.055 | |||||
| Indole-3-acetic-acid-O-glucuronide | 1.834± 0.965 | 0.057 | |||||
| Indoxyl sulphate/Indoxylsulphuric acid | 0.688 ± 0.564 | 0.22 | |||||
| Dihydroxy-benzoxazinone | −0.186 ± 0.881 | 0.83 | |||||
| B | Urolithin A | 1.380 ± 1.319 | 0.29 | D | Urolithin A | 1.899 ± 0.914 | 0.038 |
| Urolithin A glucuronide | 3.023 ± 1.191 | 0.011 | Urolithin A glucuronide | 2.578 ± 0.921 | 0.005 | ||
| Urolithin A sulphate | 1.811 ± 1.381 | 0.19 | |||||
| Urolithin A sulphoglucuronide | −2.163 ± 1.423 | 0.13 | |||||
| Urolithin B | −0.917 ± 1.570 | 0.56 | |||||
| Urolithin B glucuronide | 0.791 ± 1.368 | 0.56 | |||||
| Dodecanedioic acid | 1.245 ± 0.895 | 0.16 |
Tobit models were used.
Association between habitual nut exposure and cognitive function
The associations between dietary and urinary (individual and combined) markers of nut intake and the change or the decline in the MMSE score at 3-year follow-up are reported in Table 4. In the fully adjusted linear regression models (Model 3), participants who reported a higher amount of nut intake in the FFQ (1 SD of difference) had better cognitive function (β: 0.25; 95% CI: 0.04, 0.46; P = 0.018). No statistically significant interactions were found between dietary nut intake and sex (P = 0.70), age (P = 0.66), BMI (P = 0.67), education (P = 0.47), smoking status (P = 0.40) and physical activity (P = 0.74) in relation to change in cognitive function (data not tabulated). Moreover, a statistically significant inverse association between nut intake and cognitive decline risk (OR: 0.78; 95% CI: 0.61, 0.99; P = 0.043) was observed.
Table 4.
Associations between nut exposure and cognitive declinea
| Nut exposure | Change in cognitive function | Decline in cognitive function | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |||||||
| β (95% CI) | P-value | β (95% CI) | P-value | β (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Dietary marker | ||||||||||||
| Nut intake | 0.19 (0.00, 0.38) | 0.046 | 0.25 (0.05, 0.46) | 0.014 | 0.25 (0.04, 0.46) | 0.018 | 0.87 (0.73, 1.04) | 0.12 | 0.79 (0.63, 1.00) | 0.05 | 0.78 (0.61, 0.99) | 0.043 |
| Urinary markers | ||||||||||||
| Urolithin A | 0.86 (0.10, 1.61) | 0.026 | 1.00 (0.24, 1.76) | 0.010 | 0.85 (0.04, 1.66) | 0.040 | 0.41 (0.19, 0.90) | 0.027 | 0.37 (0.16, 0.82) | 0.015 | 0.37 (0.15, 0.88) | 0.024 |
| Urolithin A glucuronide | 0.64 (−0.04, 1.33) | 0.06 | 0.90 (0.20, 1.60) | 0.013 | 0.86 (0.15, 1.56) | 0.018 | 0.74 (0.45, 1.20) | 0.22 | 0.68 (0.40, 1.16) | 0.16 | 0.69 (0.40, 1.19) | 0.18 |
| Urolithin A sulphate | 0.96 (0.17, 1.75) | 0.017 | 1.21 (0.40, 2.01) | 0.004 | 1.14 (0.32, 1.95) | 0.007 | 0.52 (0.27, 1.01) | 0.05 | 0.41 (0.19, 0.86) | 0.019 | 0.43 (0.20, 0.91) | 0.028 |
| Urolithin A sulphoglucuronide | 0.64 (−0.12, 1.41) | 0.10 | 0.97 (0.18, 1.76) | 0.017 | 0.86 (0.05, 1.68) | 0.039 | 0.69 (0.38, 1.25) | 0.23 | 0.54 (0.27, 1.08) | 0.08 | 0.57 (0.28, 1.16) | 0.12 |
| Urolithin B | 1.23 (0.43, 2.04) | 0.003 | 1.33 (0.47, 2.19) | 0.003 | 1.28 (0.37, 2.20) | 0.007 | 0.48 (0.23, 1.01) | 0.05 | 0.38 (0.16, 0.89) | 0.027 | 0.38 (0.15, 0.92) | 0.032 |
| Urolithin B glucuronide | 0.92 (0.24, 1.61) | 0.009 | 0.94 (0.20, 1.68) | 0.014 | 0.88 (0.11, 1.65) | 0.026 | 0.56 (0.32, 0.98) | 0.041 | 0.50 (0.26, 0.95) | 0.036 | 0.49 (0.25, 0.96) | 0.037 |
| Hydroxyhippuric acid | −0.24 (−1.21, 0.73) | 0.62 | −0.13 (−1.15, 0.90) | 0.81 | 0.11 (−0.98, 1.20) | 0.84 | 0.98 (0.52, 1.87) | 0.96 | 0.85 (0.42, 1.72) | 0.65 | 0.66 (0.30, 1.45) | 0.30 |
| Hydroxyphenylacetic acid | 0.74 (−0.05, 1.53) | 0.06 | 0.65 (−0.14, 1.45) | 0.11 | 0.67 (−0.14, 1.49) | 0.10 | 0.46 (0.26, 0.83) | 0.010 | 0.47 (0.25, 0.86) | 0.015 | 0.45 (0.24, 0.87) | 0.017 |
| Dodecanedioic acid | 0.83 (−0.00, 1.66) | 0.05 | 1.17 (0.30, 2.04) | 0.009 | 1.00 (0.08, 1.93) | 0.033 | 0.69 (0.38, 1.23) | 0.21 | 0.48 (0.25, 0.95) | 0.035 | 0.51 (0.25, 1.02) | 0.06 |
| Dimethylglutaric acid | 1.50 (0.34, 2.66) | 0.011 | 1.75 (0.57, 2.94) | 0.004 | 1.77 (0.56, 2.98) | 0.005 | 0.38 (0.16, 0.94) | 0.036 | 0.29 (0.11, 0.78) | 0.014 | 0.29 (0.10, 0.79) | 0.016 |
| Indole-3-acetic acid-O-glucuronide | −0.39 (−1.30, 0.53) | 0.40 | −0.55 (−1.47, 0.37) | 0.24 | −0.60 (−1.52, 0.32) | 0.20 | 1.43 (0.76, 2.67) | 0.27 | 1.52 (0.79, 2.92) | 0.21 | 1.60 (0.81, 3.15) | 0.17 |
| Indoxyl sulphate | 0.19 (−0.49, 0.87) | 0.59 | 0.22 (−0.50, 0.94) | 0.55 | 0.26 (−0.46, 0.98) | 0.48 | 0.98 (0.62, 1.54) | 0.92 | 0.88 (0.52, 1.47) | 0.62 | 0.85 (0.50, 1.45) | 0.56 |
| Dihydroxy--benzoxazinone | 0.61 (−0.32, 1.54) | 0.20 | 0.91 (−0.05, 1.87) | 0.06 | 1.12 (0.13, 2.10) | 0.027 | 0.46 (0.23, 0.94) | 0.033 | 0.39 (0.18, 0.84) | 0.017 | 0.30 (0.12, 0.74) | 0.009 |
| Multimetabolite urinary biomarker panelsb | ||||||||||||
| Model A | 0.004 (0.001, 0.007) | 0.004 | 0.006 (0.003, 0.008) | <0.001 | 0.005 (0.002, 0.007) | 0.002 | 0.997 (0.995, 0.999) | 0.010 | 0.996 (0.994, 0.999) | 0.002 | 0.996 (0.994, 0.999) | 0.003 |
| Model C | 0.004 (−0.001, 0.009) | 0.11 | 0.006 (0.001, 0.011) | 0.029 | 0.006 (0.001, 0.011) | 0.029 | 0.997 (0.993, 1.00) | 0.056 | 0.996 (0.992, 1.00) | 0.028 | 0.995 (0.991, 0.999) | 0.016 |
| Model B | 0.005 (0.002, 0.009) | 0.003 | 0.007 (0.003, 0.011) | <0.001 | 0.006 (0.002, 0.010) | 0.003 | 0.997 (0.995, 1.00) | 0.026 | 0.996 (0.993, 0.999) | 0.008 | 0.996 (0.993, 0.999) | 0.013 |
| Model D | 0.011 (0.001, 0.021) | 0.028 | 0.015 (0.005, 0.025) | 0.004 | 0.012 (0.002, 0.022) | 0.022 | 0.994 (0.987, 1.00) | 0.07 | 0.992 (0.985, 1.00) | 0.047 | 0.993 (0.985, 1.00) | 0.07 |
Linear and logistic regression models were used, and the following three separate models are presented: model 1, which was adjusted for baseline score of cognitive function, sex and age; model 2, which was further adjusted for BMI, energy intake (only diet), alcohol consumption, education, physical activity and smoking status; and model 3, which was further adjusted for depressive symptoms, stroke, cardiovascular disease, hypertension and diabetes.
Multimetabolite urinary biomarkers models evaluated using Tobit models as follows: Model A) all 13 known identified urinary metabolite; Model B) a selection of 7 identified urinary metabolite biomarkers (urolithin A and its three conjugates, urolithin B and its glucuronide, and dodecanedioic acid; Model C) a reduced panel with 4 metabolite biomarkers from Model A (urolithin A glucuronide, hydroxyhippuric acid, hydroxyphenylacetic acid and dimethylglutaric acid); and Model D) a reduced panel with 2 metabolite biomarkers from Model B (urolithin A and its glucuronide).
Regarding the urinary markers, participants who excreted a higher amount of urolithin A and its three conjugates (urolithin A glucuronide, urolithin A sulphate and urolithin A sulphoglucuronide), urolithin B and its glucuronide, dodecanedioic acid, dimethylglutaric acid and dihydroxy--benzoxazinonealso had better cognitive function. Similar association between hydroxyphenylacetic acid and change in MMSE score was also observed, although the result was not statistically significant in the fully adjusted model. In addition, the four multimetabolite urinary biomarker panels of nut intake were inversely associated with the change in cognition. In the fully adjusted logistic regression models (Model 3), urolithin A, urolithin A sulphate, urolithin B, urolithin B glucuronide, hydroxyphenylacetic acid, dimethylglutaric acid and dihydroxy-benzoxazinone levels were inversely associated with cognitive decline. No statistically significant associations between urolithin A glucuronide, urolithin A sulphoglucuronide and dodecanedioic acid levels and cognitive decline were observed. In the fully adjusted model, multimetabolite urinary biomarker panels of nut intake (Models A, B and C), except for Model D, were also associated with cognitive decline (Table 4).
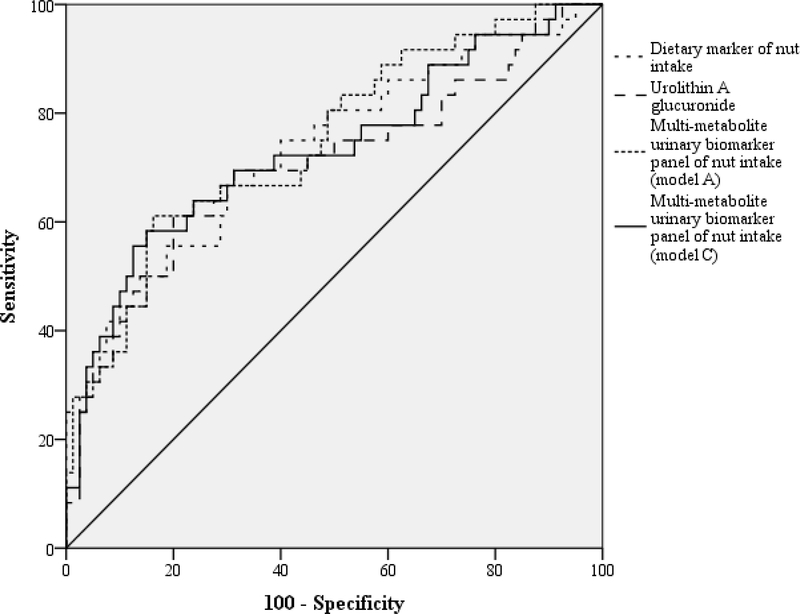
The ROC curves and the corresponding AUC (95% CI), as well as the calculation of the sensitivity and specificity of dietary and urinary (individual and combined) markers of nut intake for the prediction of cognitive decline adjusted for all the potential factors, are shown in Figure 1 and (Supporting Information Table 4). The AUC for the dietary marker and multimetabolite urinary biomarker models (Models A, B, C and D) was 73.2, 74.8, 72.8, 73.1 and 71.3, respectively, and all were statistically significant (P < 0.001). All sensitivity and specificity values from these models were higher than 60 and 70 %, respectively (Supporting Information Table 4).
Figure 1.
ROC curves of the associations between nut exposure and cognitive decline.
4. Discussion
Our prospective study in non-demented older subjects shows that high intake of nuts was associated with better cognitive function and a lower risk of cognitive decline over a 3-year follow-up. To our knowledge, this is the first study using both FFQ and untargeted metabolomics data to investigate the association between nut intake and cognitive decline risk. In addition, our results support that the use of multiple nut biomarkers in epidemiological studies may provide a more accurate assessment of the nut exposure than the use of individual biomarkers and therefore a proper estimation of the associations with health outcomes.
We found an association between high intake of nuts and lower cognitive decline risk whether the nuts exposure is measured based on the traditional FFQ or using a multimetabolite biomarker model. In line with previous prospective studies, nuts consumption was associated with a better cognitive function [32] and with a lower global cognitive decline [33]. Evidence focused on nut exposure alone is limited, but several studies have been conducted on dietary patterns including nuts as a key component. The Mediterranean diet supplemented with nuts was associated with improved cognitive function [34]. In a previous InCHIANTI study, a polyphenol-rich diet intake was related to a lower cognitive decline [35]. The mechanism by which nut intake protects cognitive function during aging is not clearly defined. Oxidative stress, inflammation and reduced cerebral blood flow have been considered to be important mechanisms leading to cognitive decline in older subjects [36]. Thus, nuts, alone or as part of healthy dietary patterns, may exert beneficial effects against the development of cognitive decline due to their high concentrations of antioxidants [37], including polyphenols (e.g. proanthocyanidins in almonds and hazelnuts; ellagitannins in walnuts and hazelnuts), MUFA (e.g. oleic acid in almonds and hazelnuts) [38], PUFA (e.g. α-linolenic and linoleic acid in walnuts) and vitamins [10].
Taking into consideration the whole composition of nuts, a multibiomarker approach to assess overall consumption may be more accurate for predicting nut exposure than the use of a single one [11], as previously described by our group [13, 15].
In the present study, urinary multimetabolite biomarker models of nut exposure (mainly Model C composed by Urolithin A glucuronide, hydroxyhippuric acid, hydroxyphenylacetic acid and dimethylglutaric acid) presented a greater predictive ability as a biomarker of nut intake, than individual markers. The discriminative capacity of Model C, as well as of urolithin A glucuronide, to classify subjects according to cognitive decline was observed. These may be valuable dietary biomarkers of cognitive decline, because of the potential role of polyphenols and polyunsaturated fatty acids in reducing oxidative stress and inflammation [10]. However, no statistically significant differences across nut groups in relation to inflammatory markers were observed (data not tabulated).
Urolithin A was the most discriminant phenolic marker also observed elsewhere [15, 39, 40]. Urolithins are gut microbiota products from ellagic acid and ellagitannins. In the lower gastrointestinal tract, these compounds are converted into urolithins, which are absorbed and metabolized to finally circulate in blood reaching different tissues prior to excretion [41]. Hence, these results represent an important step ahead in the validation of these compounds as biomarkers of nut exposure. Recently, three urolithin phenotypes were observed [42]. A higher percentage of “phenotype B”, which produced isourolithin A and/or urolithin B in addition to urolithin A, was observed in those participants with chronic disease associated with microbial imbalance. In this study, the prevalence of “Phenotype A” (only urolithin A conjugates excreted), “Phenotype B” and “Phenotype 0” (no detected urolithins) with cognitive decline was 20, 20 and 60 %, respectively (P = 0.78). This may be due to the low sample size (n = 47, loss due to phenotypic variation) (data not tabulated). Therefore, further studies are needed to evaluate whether this phenotypic variation could be a biomarker associated with cognitive decline.
The presence of other microbial-derived metabolites including flavan-3-ols (2-hydroxyphenylacetic acid) and procyanidins (hydroxyhippuric acid), from walnuts [13, 39, 43, 44] and almonds [45], respectively and markers of the tryptophan metabolism such as indole-3-acetic-acid-O-glucuronide and indoxyl sulphate/indoxylsulphuric acid, mainly from the consume of walnuts [46] highlight again an interplay between nut intake, gut microbiota and cognitive decline. Currently, the brain-gut-microbiome connection is a hot research topic. Nuts, which are rich in fibre, omega-3 fatty acids and polyphenols, increase healthy gut microbiota and may improve cognition [47–49]. For example, the excretion of urinary indoles reflects a variation in gut microbiota composition in relation to their role in inhibition or promotion of the growth of specific bacterial species, also observed in a number of diseases states [46].
Generally, evidence concerning nut exposure and cognition relies on the measurement of nut exposure using mainly self-reported dietary questionnaires. Consequently, studies using biomarkers of nut exposure are needed to confirm these potential protective effects. Therefore, the use of a metabolomic approach to identify and validate proper and predictive nutritional biomarkers is now highly promising [11].
The main strengths of this study were its longitudinal design and the assessment of habitual nut exposure with the use of a nut-derived metabolites panel as a nutritional biomarker. The combination of different metabolites as a nutritional biomarker provided a more accurate estimation than that provided by only a single biomarker [15]. Another strength was the use of a validated method assessment test to evaluate cognitive decline [21, 22]. Finally, our models were adjusted for the most important confounding variables related to nut exposure and cognitive decline; however, possible residual confounding cannot be excluded.
Nevertheless, this study had some limitations. First, it is important to bear in mind that no type specification of nut consumption was discerned in the administered FFQ, although the percentage consumption of individual tree nuts and peanuts consumed as a whole in Italy (EPIC study) was approximately 60 (where walnuts are more consumed than almonds and hazelnuts) and 32 %, respectively [50]. In addition, the mean ± SD intake of nuts in their study (n = 3961; 1.7 ± 0.2 g/d) was similar to that in our study (n = 119; 1.9 ± 3.2 g/d). However, the present study sample might not be representative of nut consumption in general Italian population, because InCHIANTI was performed in community-dwelling older subjects living in two sites in Tuscany (Italy). Second, the present study population was aged ≥ 65 and therefore may be less accurate in recalling food intake, although demented participants were excluded. Finally, because biomarker assessment was performed only once at baseline, it may not necessarily reflect the participants’ long-term nut consumption. Although this is to be expected with aging [51], there was a decrease in the nut intake during the follow-up, which was 1.9 and 0.9 g/d at baseline and at the 3-year follow-up visit, respectively (data not tabulated). Their r was 0.46 (P < 0.001).
In conclusion, this study showed that a higher habitual exposure to nuts was associated with a lower risk of cognitive decline in a cohort of older individuals. Moreover, this study opens up a large area of research with more reliable and accurate tools for identifying and validating new biomarkers of nut exposure and evaluating their association with cognitive decline.
Supplementary Material
Acknowledgements/funding
The InCHIANTI study was supported in part by a grant from the Italian Ministry of Health (PE-2011-02350413), and by the U.S. Intramural Research Program at the National Institute on Aging. This study was supported by the INC International Nut and Dried Fruit Council by FBG307906, and by the award of from the Generalitat de Catalunya’s Agency GAUR (2017SGR1546). MR and RZ-R would like to thank the “Sara Borrell” (CD16/00157) and “Miguel Servet” (CP15/00100) research contracts, respectively, from the Carlos III Institute of Health and the European Social Fund (ESF). MP-R would like to thank predoctoral fellowship APIF-INSA of University of Barcelona. CA-L gratefully acknowledges the financial support by ICREA under the ICREA Academia programme.
Abbreviations used
- ROC
receiver operating characteristic
- MMSE
Mini-Mental State Examination
- InCHIANTI
Invecchiare in Chianti (Aging in Chianti)
- FFQ
food frequency questionnaire
- NC
non-nut consumers
- OSC-PLS-DA
partial least-squares discriminant analysis with orthogonal signal correction
- RC
regular consumer
- VIP
variable importance projection
- ADL
activities of daily living
- IADL
instrumental activities of daily living
- CES-D
Center for Epidemiological Studies Depression Scale
- IL-6
Interleukin-6
- IL-1ra
Interleukin-1 receptor antagonist
- hsCRP
high-sensitivity C-reactive protein
- TNF-α
tumour necrosis factor-α
Footnotes
Conflict of Interest
The authors have declared no conflict of interest.
5. References
- [1].Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP., The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s Dement. 2013, 9, 63–75.e2. [DOI] [PubMed] [Google Scholar]
- [2].World Health Organization and Alzheimer’s disease International, DEMENTIA A public Helath Priority. World Heal. Organ; 2012, 1–4. [Google Scholar]
- [3].Barnard ND, Bush AI, Ceccarelli A, Cooper J, Celeste A, Erickson KI., de Jager, Fraser G, Kesler S, Levin SM, Lucey B, Morris MC, Squitti R, Dietary and lifestyle guidelines for the prevention of Alzheimer’s disease. Neurobiol. Aging 2014, 35, S74–S78. [DOI] [PubMed] [Google Scholar]
- [4].Daviglus ML, National Institutes of Health State-of-the-Science Conference Statement: Preventing Alzheimer Disease and Cognitive Decline. Ann. Intern. Med 2010, 153, 176. [DOI] [PubMed] [Google Scholar]
- [5].Valls-Pedret C, Sala-Vila A, Serra-Mir M, Corella D, de la Torre R, Martínez-González MA., Martínez-Lapiscina EH, Fitó M, Pérez-Heras A, Salas-Salvadó J, Estruch R, Ros E, Mediterranean Diet and Age-Related Cognitive Decline. JAMA Intern. Med 2015, 175, 1094. [DOI] [PubMed] [Google Scholar]
- [6].Sabaté J, Ros E, Salas-Salvadó J, Nuts: nutrition and health outcomes. Preface. Br. J. Nutr 2006, 96 Suppl 2, S1–2. [DOI] [PubMed] [Google Scholar]
- [7].Afshin A, Micha R, Khatibzadeh S, Mozaffarian D, Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes : a systematic review and meta-analysis. Am. J. Clin. Nutr 2014, 100, 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bao Y, Han J, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS, Association of nut consumption with total and cause-specific mortality. N. Engl. J. Med 2013, 369, 2001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].U.S., U.S.D. of H. and H.S. and D. of A., 2015 – 2020 Dietary Guidelines for Americans. 8th Edition. December 2015., n.d.
- [10].Grosso G, Estruch R, Nut consumption and age-related disease. Maturitas 2016, 84, 11–16. [DOI] [PubMed] [Google Scholar]
- [11].Scalbert A, Brennan L, Manach C, Andres-Lacueva C, Dragsted LO, Draper J, Rappaport SM, van der Hooft J JJ., Wishart, D.S., The food metabolome: a window over dietary exposure. Am. J. Clin. Nutr 2014, 99, 1286–1308. [DOI] [PubMed] [Google Scholar]
- [12].Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM, Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J. Am. Geriatr. Soc 2000, 48, 1618–25. [DOI] [PubMed] [Google Scholar]
- [13].Tulipani S, Llorach R, Jáuregui O, López-Uriarte P, Garcia-Aloy M, Bullo M, Salas-Salvadó J, Andrés-Lacueva C, Metabolomics Unveils Urinary Changes in Subjects with Metabolic Syndrome following 12-Week Nut Consumption. J. Proteome Res. 2011, 10, 5047–5058. [DOI] [PubMed] [Google Scholar]
- [14].Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F, Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int. J. Epidemiol 1997, 26 Suppl 1, S152–60. [DOI] [PubMed] [Google Scholar]
- [15].Garcia-Aloy M, Llorach R, Urpi-Sarda M, Tulipani S, Estruch R, Martínez-González MA, Corella D, Fitó M, Ros E, Salas-Salvadó J, Andres-Lacueva C, Novel multimetabolite prediction of walnut consumption by a urinary biomarker model in a free-living population: The predimed study. J. Proteome Res 2014, 13, 3476–3483. [DOI] [PubMed] [Google Scholar]
- [16].Llorach-Asunción R, Jauregui O, Urpi-Sarda M, Andres-Lacueva C, Methodological aspects for metabolome visualization and characterization: a metabolomic evaluation of the 24 h evolution of human urine after cocoa powder consumption. J. Pharm. Biomed. Anal 2010, 51, 373–81. [DOI] [PubMed] [Google Scholar]
- [17].Wishart DS, Feunang YD, Marcu A, Guo AC, Liang K, Vázquez-Fresno R, Sajed T,Johnson D, Li C, Karu N, Sayeeda Z, Lo E, Assempour N, Berjanskii M, Singhal S, Arndt D, Liang Y, Badran H, Grant J, Serra-Cayuela A, Liu Y, Mandal R, Neveu V, Pon A, Knox C, Wilson M, Manach C, Scalbert A, HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Smith C. a, Maille GO, Want EJ, Qin C, Trauger S, Brandon T, Custodio D, Abagyan R, Siuzdak G, METLIN. Ther. Drug Monit 2005, 27, 747–751. [DOI] [PubMed] [Google Scholar]
- [19].Wolf S, Schmidt S, Müller-Hannemann M, Neumann S, In silico fragmentation for computer assisted identification of metabolite mass spectra. BMC Bioinformatics 2010, 11, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, Fan TW-M, Fiehn O, Goodacre R,Griffin JL, Hankemeier T, Hardy N, Harnly J, Higashi R, Kopka J, Lane AN, Lindon JC, Marriott P, Nicholls AW, Reily MD, Thaden JJ, Viant MR, Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mitchell AJ, A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J. Psychiatr. Res 2009, 43, 411–431. [DOI] [PubMed] [Google Scholar]
- [22].Folstein MF, Folstein SE, McHugh PR, “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res 1975, 12, 189–98. [DOI] [PubMed] [Google Scholar]
- [23].Hensel A, Luck T, Luppa M, Glaesmer H, Angermeyer MC, Riedel-Heller SG, Does a Reliable Decline in Mini Mental State Examination Total Score Predict Dementia? Dement. Geriatr. Cogn. Disord 2009, 27, 50–58. [DOI] [PubMed] [Google Scholar]
- [24].Salvini S, A food composition database for epidemiological studies in Italy. Cancer Lett. 1997, 114, 299–300. [DOI] [PubMed] [Google Scholar]
- [25].Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Montoye HJ, Sallis JF, Paffenbarger RS, Compendium of physical activities: classification of energy costs of human physical activities. Med. Sci. Sports Exerc 1993, 25, 71–80. [DOI] [PubMed] [Google Scholar]
- [26].Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW, Studies of Illness in the Aged: The Index of ADL: A Standardized Measure of Biological and Psychosocial Function. JAMA 1963, 185, 914–9. [DOI] [PubMed] [Google Scholar]
- [27].Lawton MP, Brody EM, Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–86. [PubMed] [Google Scholar]
- [28].Radloff LS, The CES-D scale: A self report depression scale for research in the general population. Appl. Psychol. Meas 1977, 1, 385–401. [Google Scholar]
- [29].Littlejohns TJ, Kos K, Henley WE, Cherubini A, Ferrucci L, Lang IA, Langa KM,Melzer D, Llewellyn DJ, Serum Leptin and Risk of Cognitive Decline in Elderly Italians. J. Alzheimer’s Dis 2015, 44, 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].James T, Estimation of Relationships for Limited Dependent Variables. Econometrica 1958, 26, 24–36. [Google Scholar]
- [31].Rabassa M, Zamora-Ros R, Urpi-Sarda M, Andres-Lacueva C, Resveratrol metabolite profiling in clinical nutrition research--from diet to uncovering disease risk biomarkers: epidemiological evidence. Ann. N. Y. Acad. Sci 2015, 1348, 107–115. [DOI] [PubMed] [Google Scholar]
- [32].O’Brien J, Okereke O, Devore E, Rosner B, Breteler M, Grodstein F, Long-term intake of nuts in relation to cognitive function in older women. J. Nutr. Health Aging 2014, 18, 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nooyens ACJ, Bueno-de-Mesquita HB, van Boxtel MPJ, van Gelder BM, Verhagen H, Verschuren WM, Fruit and vegetable intake and cognitive decline in middle-aged men and women: the Doetinchem Cohort Study. Br. J. Nutr 2011, 106, 752–761. [DOI] [PubMed] [Google Scholar]
- [34].Martínez-Lapiscina EH, Clavero P, Toledo E, Estruch R, Salas-Salvadó J, San Julián B, Sanchez-Tainta A, Ros E, Valls-Pedret C, Martinez-Gonzalez MA, Mediterranean diet improves cognition: The PREDIMED-NAVARRA randomised trial. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1318–1325. [DOI] [PubMed] [Google Scholar]
- [35].Rabassa M, Cherubini A, Zamora-Ros R, Urpi-Sarda M, Bandinelli S, Ferrucci L, Andres-Lacueva C, Low Levels of a Urinary Biomarker of Dietary Polyphenol Are Associated with Substantial Cognitive Decline over a 3-Year Period in Older Adults: The Invecchiare in Chianti Study. J. Am. Geriatr. Soc 2015, 63, 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ownby RL, Neuroinflammation and cognitive aging. Curr. Psychiatry Rep 2010, 12, 39–45. [DOI] [PubMed] [Google Scholar]
- [37].Cherubini A, Martin A, Andres-Lacueva C, Di Iorio A, Lamponi M, Mecocci P, Bartali B, Corsi A, Senin U, Ferrucci L, Vitamin E levels, cognitive impairment and dementia in older persons: the InCHIANTI study. Neurobiol. Aging 2005, 26, 987–994. [DOI] [PubMed] [Google Scholar]
- [38].Cherubini A, Andres-Lacueva C, Martin A, Lauretani F, Bartali B, Corsi A,Bandinelli S, Mattson MP, Ferrucci L, Low plasma N-3 fatty acids and dementia in older persons: the InCHIANTI study. J. Gerontol. A. Biol. Sci. Med. Sci 2007, 62, 1120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mora-Cubillos X, Tulipani S, Garcia-Aloy M, Bulló M, Tinahones FJ, Andres-Lacueva C, Plasma metabolomic biomarkers of mixed nuts exposure inversely correlate with severity of metabolic syndrome. Mol. Nutr. Food Res 2015, 59, 2480–2490. [DOI] [PubMed] [Google Scholar]
- [40].Tulipani S, Urpi-Sarda M, García-Villalba R, Rabassa M, López-Uriarte P, Bulló M, Jáuregui O, Tomás-Barberán F, Salas-Salvadó J, Espín JC, Andrés-Lacueva C, Urolithins Are the Main Urinary Microbial-Derived Phenolic Metabolites Discriminating a Moderate Consumption of Nuts in Free-Living Subjects with Diagnosed Metabolic Syndrome. J. Agric. Food Chem 2012, 60, 8930–8940. [DOI] [PubMed] [Google Scholar]
- [41].Garcia-Muñoz C, Vaillant F, Metabolic fate of ellagitannins: implications for health, and research perspectives for innovative functional foods. Crit. Rev. Food Sci. Nutr 2014, 54, 1584–98. [DOI] [PubMed] [Google Scholar]
- [42].Tomás-Barberán FA, García-Villalba R, González-Sarrías A, Selma MV, Espín J, Ellagic Acid Metabolism by Human Gut Microbiota: Consistent Observation of Three Urolithin Phenotypes in Intervention Trials, Independent of Food Source, Age, and Health Status. J. Agric. Food Chem 2014, 62, 6535–6538. [DOI] [PubMed] [Google Scholar]
- [43].Espín JC, Larrosa M, García-Conesa MT, Tomás-Barberán F, Biological Significance of Urolithins, the Gut Microbial Ellagic Acid-Derived Metabolites: The Evidence So Far. Evidence-Based Complement. Altern. Med 2013, 2013, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Garcia-Aloy M, Rabassa M, Casas-Agustench P, Hidalgo-Liberona N, Llorach R, Andres-Lacueva C, Novel strategies for improving dietary exposure assessment: Multiple- data fusion is a more accurate measure than the traditional single- biomarker approach. Trends Food Sci. Technol 2017, 69, 220–229. [Google Scholar]
- [45].Llorach R, Garrido I, Monagas M, Urpi-Sarda M, Tulipani S, Bartolome B, Andres-Lacueva C, Metabolomics study of human urinary metabolome modifications after intake of almond (Prunus dulcis (Mill.) D.A. Webb) skin polyphenols, 2010. [DOI] [PubMed]
- [46].Keszthelyi D, Troost FJ, Masclee AAM, Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol. Motil 2009, 21, 1239–1249. [DOI] [PubMed] [Google Scholar]
- [47].Robertson RC, Seira Oriach C, Murphy K, Moloney GM, Cryan JF, Dinan TG, Paul Ross R, Stanton C, Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain. Behav. Immun 2017, 59, 21–37. [DOI] [PubMed] [Google Scholar]
- [48].Brickman AM, Khan UA, Provenzano FA, Yeung L-K, Suzuki W, Schroeter H, Wall M, Sloan RP, Small SA, Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat. Neurosci 2014, 17, 1798–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hayek N, Chocolate, gut microbiota, and human health. Front. Pharmacol 2013, 4, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jenab M, Sabaté J, Slimani N, Ferrari P, Mazuir M, Casagrande C, Deharveng G, Tjønneland A, Olsen A, Overvad K, Boutron-Ruault M-C, Clavel-Chapelon F, Boeing H, Weikert C, Linseisen J, Rohrmann S, Trichopoulou A, Naska A, Palli D, Sacerdote C, Tumino R, Mattiello A, Pala V, Bueno-de Mesquita HB, Ocké MC, Peeters PH, Engeset D, Skeie G, Jakszyn P, Ardanaz E, Quirós JR, Chirlaque MD, Martinez C, Amiano P, Berglund G, Palmqvist R, van Guelpen B, Bingham S, Key T, Riboli E, Consumption and portion sizes of tree nuts, peanuts and seeds in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohorts from 10 European countries. Br. J. Nutr 2006, 96 Suppl 2, S12–23. [DOI] [PubMed] [Google Scholar]
- [51].Drewnowski A, Shultz JM, Impact of aging on eating behaviors, food choices, nutrition, and health status. J. Nutr. Health Aging 2001, 5, 75–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.