Abstract
Objective
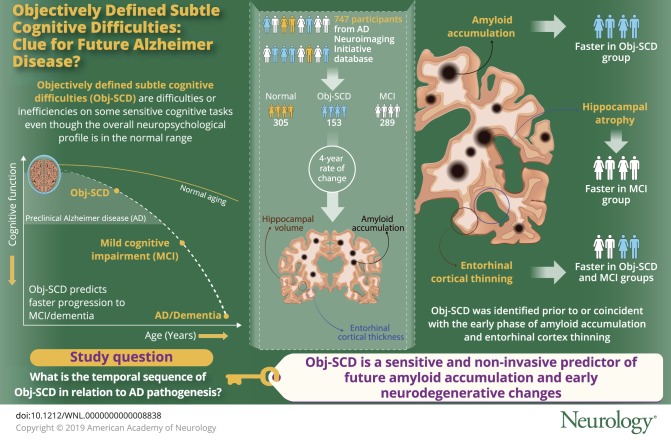
To determine the temporal sequence of objectively defined subtle cognitive difficulties (Obj-SCD) in relation to amyloidosis and neurodegeneration, the current study examined the trajectories of amyloid PET and medial temporal neurodegeneration in participants with Obj-SCD relative to cognitively normal (CN) and mild cognitive impairment (MCI) groups.
Method
A total of 747 Alzheimer's Disease Neuroimaging Initiative participants (305 CN, 153 Obj-SCD, 289 MCI) underwent neuropsychological testing and serial amyloid PET and structural MRI examinations. Linear mixed effects models examined 4-year rate of change in cortical 18F-florbetapir PET, entorhinal cortex thickness, and hippocampal volume in those classified as Obj-SCD and MCI relative to CN.
Result
Amyloid accumulation was faster in the Obj-SCD group than in the CN group; the MCI and CN groups did not significantly differ from each other. The Obj-SCD and MCI groups both demonstrated faster entorhinal cortical thinning relative to the CN group; only the MCI group exhibited faster hippocampal atrophy than CN participants.
Conclusion
Relative to CN participants, Obj-SCD was associated with faster amyloid accumulation and selective vulnerability of entorhinal cortical thinning, whereas MCI was associated with faster entorhinal and hippocampal atrophy. Findings suggest that Obj-SCD, operationally defined using sensitive neuropsychological measures, can be identified prior to or during the preclinical stage of amyloid deposition. Further, consistent with the Braak neurofibrillary staging scheme, Obj-SCD status may track with early entorhinal pathologic changes, whereas MCI may track with more widespread medial temporal change. Thus, Obj-SCD may be a sensitive and noninvasive predictor of encroaching amyloidosis and neurodegeneration, prior to frank cognitive impairment associated with MCI.
The National Institute on Aging–Alzheimer's Association (NIA-AA) research criteria for preclinical Alzheimer disease (AD) proposed that subtle cognitive decline appears after amyloidosis and neurodegeneration, but prior to mild cognitive impairment (MCI).1 Although some investigators recommend using subjective report of cognitive decline to define subtle cognitive decline,2,3 other work has identified objectively defined subtle cognitive difficulties (Obj-SCD) using neuropsychological assessment.4–8 One method that uses neuropsychological assessment incorporates both total scores and sensitive process scores that are consistent with an overall memory profile of early AD.5
Neuropsychological process scores are the quantification of errors or an individual's approach to completing a task such as a neuropsychological test, irrespective of whether the overall total score was within the normal range.9 For example, one may recall an average number of correct words on a list-learning test, yet simultaneously produce extra-list intrusion errors. Process score analyses of memory tests have demonstrated that flattened learning slope, increased susceptibility to interference, and more intrusion errors may be sensitive to early AD-related changes.10–18 Our recent work that operationally defined Obj-SCD via integration of these process scores showed associations with CSF AD markers and predicted faster progression to MCI/dementia when compared to cognitively normal (CN) participants.5
Given these findings, we aimed to determine whether Obj-SCD appears after amyloidosis and neurodegeneration or, instead, predicts future amyloid accumulation and medial temporal lobe (MTL) neurodegeneration. If, according to the NIA-AA criteria1 and amyloid cascade model,2,17 amyloid does invariably accumulate (stage 1) prior to neurodegeneration (stage 2) and detectable cognitive changes (stage 3), we expect that Obj-SCD, at best, would be associated with future MTL degeneration, but not increasing amyloid pathology.
Methods
Data used in the preparation of this study were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public–private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial MRI, PET, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD.
Standard protocol approvals, registrations, and patient consents
This study was approved by the institutional review boards at each of the participating institutions, and written informed consent was obtained from all participants or authorized representatives at each site.
Participants
Specific enrollment criteria for ADNI have been described previously in detail elsewhere.19 Briefly, participants from ADNI were between 55 and 90 years old, had at least 6 years of education or work history equivalent, had a Geriatric Depression Scale score <6, had a Hachinski Ischemia Scale score <5, had adequate vision and hearing in order to perform neuropsychological tests, were in generally good health and without significant head trauma or neurologic disease, were stable on permitted medications, had a reliable study partner, and were fluent in either English or Spanish. Participants included 747 older adults without dementia from ADNI who had baseline florbetapir amyloid PET imaging with processed data. Available PET and MRI data were included at baseline and 12-, 24-, 36-, and 48-month follow-up visits.
Cognitive groups
Cognitive status of CN or MCI was determined using Jak/Bondi actuarial neuropsychological criteria, which define MCI by performance >1 SD below the demographically adjusted (age, education, sex) mean on (1) 2 neuropsychological measures within the same cognitive domain or (2) at least 1 measure across all 3 sampled cognitive domains or (3) a score of 6 or higher on the Functional Activities Questionnaire.20,21
Consistent with previous work, 6 neuropsychological test scores were used in the Jak/Bondi diagnostic criteria for MCI.21,22 There were 2 measures in 3 different cognitive domains (memory, language, attention/executive function). The memory domain included the Rey Auditory Verbal Learning Test delayed free recall correct responses and AVLT recognition (hits minus false-positives). The language domain included the 30-item Boston Naming Test total correct and Animal Fluency total score. The attention/executive function domain included Trail-Making Test parts A and B times to completion.
Participants without dementia who did not meet Jak/Bondi criteria for MCI were considered for either the CN or Obj-SCD groups. Consistent with our previous work, Obj-SCD status was determined by the following criteria: (1) 1 impaired total test score (>1 SD below demographically adjusted mean) in 2 different cognitive domains (memory, language, attention/executive) or (2) 2 impaired neuropsychological process scores (>1 SD below demographically adjusted mean) from the AVLT (learning slope, retroactive interference, intrusion errors) or (3) 1 impaired total test score and 1 impaired process score.5 Total test scores were the 6 neuropsychological variables described above for determining MCI classification. Three process scores derived from the AVLT were also used in the classification of Obj-SCD. The AVLT is a word-list learning and memory test of 15 semantically unrelated words and includes 5 learning trials (list A, trials 1–5), an interference list trial (list B), a short delay free recall trial (list A, trial 6), a long delay free recall trial (list A, trial 7), and delayed recognition. Process scores used in the Obj-SCD criteria included learning slope ([list A trial 5–list A trial 1]/5), retroactive interference (list A trial 6/list A trial 5), and total intrusion errors (total number of extra-list intrusion errors across all recall trials). These scores were previously shown to differ between CN individuals who remained stable and those who progressed to MCI within 5 years in ADNI.16 For both the neuropsychological total scores and process scores, the demographically adjusted (age, sex, education) z scores for the neuropsychological measures were based on regression coefficients derived from a sample of CN participants in ADNI who did not progress to MCI for the duration of their study participation (i.e., robust controls; n = 385).22,23
Florbetapir PET
PET imaging using the 18F-florbetapir AV-45 tracer was used to quantify amyloid burden. The details of data acquisition and processing of ADNI florbetapir PET data are available on the ADNI website (loni.usc.edu). Briefly, florbetapir scans collected at baseline and follow-up visits were coregistered, averaged, reoriented into a standard 160 × 160 × 96 voxel image grid with 1.5 mm cubic voxels, and smoothed to a uniform isotropic resolution of 8 mm full width at half maximum. Structural MRIs (see below for method details) were skull-stripped, segmented, and parcellated using FreeSurfer (version 5.1). This structural image was coregistered to the first florbetapir image for each participant. A summary standardized uptake value ratio (SUVR) was then calculated by dividing the mean florbetapir uptake across 4 main cortical regions (i.e., frontal, anterior/posterior cingulate, lateral parietal, and lateral temporal cortices) by whole cerebellar (white and gray matter) florbetapir uptake. Greater retention of florbetapir is thought to reflect a greater cortical amyloid load. A recommended threshold of 1.11 for cross-sectional descriptive florbetapir analyses, using cerebellum as the reference region, was used to determine amyloid positivity.24–27
T1-weighted anatomical MRI
The details of ADNI MRI data acquisition and processing can be found on the ADNI website (loni.usc.edu). Briefly, structural scans collected at baseline and follow-up visits were motion corrected, skull-stripped, segmented, and parcellated using FreeSurfer (version 5.1).28,29 FreeSurfer-derived entorhinal cortical thickness and hippocampal volume were a priori dependent variables given their implication in early stages of AD (e.g., Braak stages I/II). Normalized hippocampal volume, which was used in the analysis, was created by dividing absolute hippocampal volume by FreeSurfer-derived estimated total intracranial volume and then multiplying the resulting value by 100.
Statistical analyses
Analysis of variance, Kruskal-Wallis tests, or χ2 tests examined baseline differences in demographic and clinical characteristics by group (CN, Obj-SCD, MCI). Pairwise comparisons were Bonferroni-corrected for 3 groups. Proportions of participants who progressed to MCI and dementia at each visit by group are also described.
Multivariable linear mixed effects (LME) modeling was used to examine the 48-month trajectories of change in amyloid burden (as measured by florbetapir PET SUVR), entorhinal cortex thickness, and hippocampal volume as a function of cognitive group status (CN, Obj-SCD, or MCI). All models adjusted for age, education, sex, APOE ε4 allele frequency, and the baseline summary amyloid PET SUVR. The longitudinal amyloid model was run both with and without the inclusion of baseline amyloid PET as a covariate; the pattern of the cognitive group × time interaction did not differ between models, so the model with baseline amyloid PET as a covariate is reported to be consistent with the covariates in the models of neurodegeneration. Time was mean-centered and treated as a continuous variable. Random intercept and slope were included. CN status was used as the reference group for the primary analyses; secondary analysis of the models was then run with MCI as the reference group to ascertain each comparison. Full information maximum likelihood was used to allow all available data to be included,30,31 in order to reduce biases relative to other methods (e.g., list-wise deletion).
Differences in demographic (age, education, sex) and clinical characteristics (e.g., APOE ε4 status, ischemic risk measured by the modified Hachinski Ischemia Scale, depressive symptoms measured by the Geriatric Depression Scale) between participants who were missing (n = 424) or nonmissing (n = 323) at the 48-month follow-up visit were examined. Analyses revealed that only age differed between the missing (mean age 72.88 years, SD 6.89) and nonmissing groups (mean age 71.33 years, SD 7.06) (t[745] = 3.01; p = 0.003).
Data availability
ADNI data were obtained from adni.loni.usc.edu and are available to investigators in the scientific community who have been approved by the ADNI Data Sharing and Publications Committee and who agree to the terms of the ADNI Data Use Agreement for purposes of replicating procedures and results. Anonymized ADNI participant identification numbers used in this article are available by request from any qualified investigator.
Results
Participant characteristics
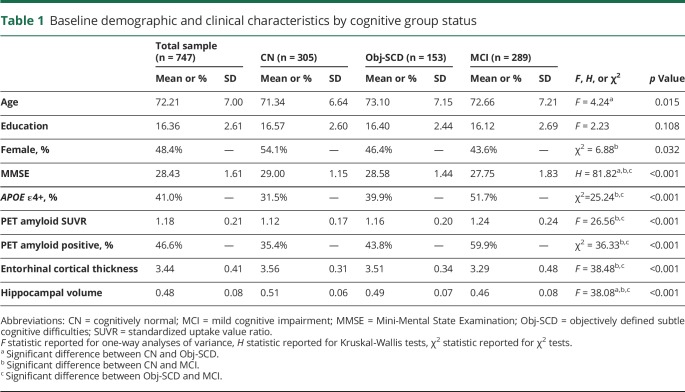
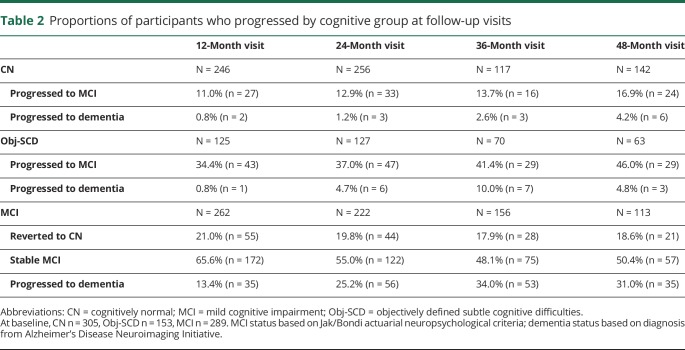
Table 1 shows the baseline demographic and clinical characteristics of participants by cognitive status (CN: n = 305, Obj-SCD: n = 153, MCI: n = 289). There were group differences such that the Obj-SCD group was older, had lower Mini-Mental State Examination (MMSE) scale scores, and had smaller hippocampal volumes than CN participants. Participants with Obj-SCD had slightly higher levels of amyloid at baseline than did CN participants; however, this was not a statistically significant difference. Relative to participants with MCI, participants with Obj-SCD had higher MMSE scores, lower proportion of APOE ε4 carriers, lower levels of amyloid, smaller proportion of amyloid-positive participants, greater entorhinal cortical thickness, and larger hippocampal volumes. Compared to CN participants, participants with MCI were less likely to be female and had lower MMSE scores, a higher proportion of APOE ε4 carriers, higher levels of amyloid, greater proportion of amyloid-positive participants, lower entorhinal cortical thickness, and smaller hippocampal volumes. The proportions of participants from each group who progressed to MCI and dementia at each follow-up visit are shown in table 2. The Obj-SCD group had nearly 3 times the proportion of those who are later classified as MCI (46.0%) at 48 months compared to CN participants (16.9%).
Table 1.
Baseline demographic and clinical characteristics by cognitive group status
Table 2.
Proportions of participants who progressed by cognitive group at follow-up visits
Florbetapir PET trajectories
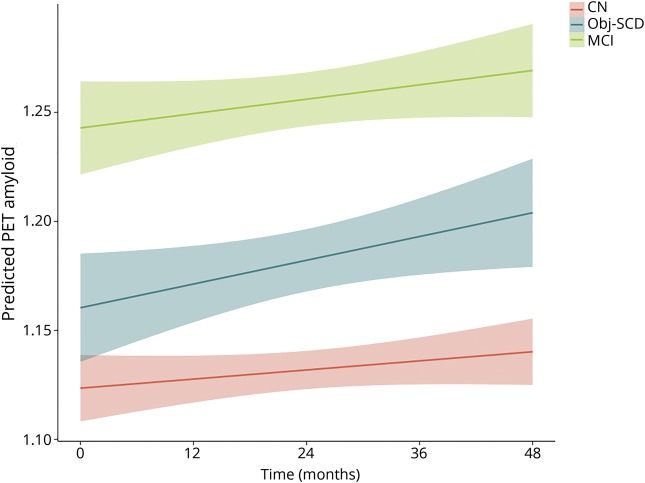
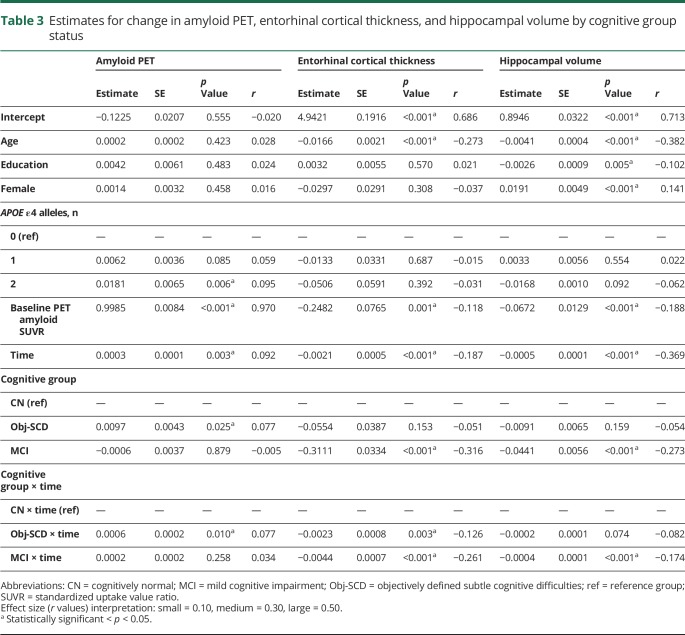
LME models, adjusting for baseline age, education, sex, APOE ε4 frequency, and baseline amyloid PET SUVR, examined whether cognitive group predicted increased rate of amyloid accumulation over 48 months. Figure 1 depicts the trajectories of amyloid PET by group and table 3 shows the model estimates. There was a significant interaction between cognitive group and time such that, relative to CN participants, participants with Obj-SCD had a faster increase in amyloid PET SUVR (t[1109.08] = 2.58, p = 0.010, r = 0.077). Participants with MCI did not differ from CN participants (t[1133.51] = 1.13, p = 0.258, r = 0.034) or participants with Obj-SCD (t[1173.95] = 1.59, p = 0.113, r = 0.046) in the rate of amyloid accumulation over 48 months.
Figure 1. Trajectories of amyloid PET by cognitive group.
Model-predicted values adjusted for age, education, sex, APOE ε4 allele frequency, and baseline amyloid PET summary standardized uptake value ratio. CN = cognitively normal; MCI = mild cognitive impairment; Obj-SCD = objectively defined subtle cognitive difficulties. Shaded area represents 95% confidence intervals.
Table 3.
Estimates for change in amyloid PET, entorhinal cortical thickness, and hippocampal volume by cognitive group status
Entorhinal cortex thickness trajectories
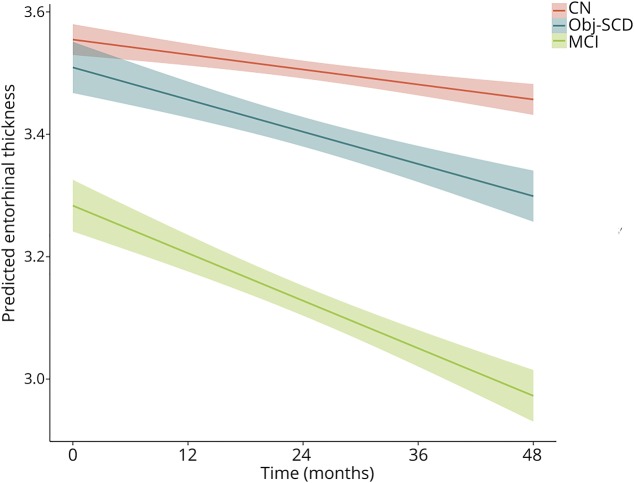
Next, LME models, adjusting for age, education, sex, and baseline amyloid PET SUVR, examined whether cognitive group predicted entorhinal cortex thinning and hippocampal volume loss over 48 months. Figure 2 shows the trajectories of entorhinal cortex thinning by cognitive group and table 3 shows the model estimates. There was a significant interaction between cognitive group and time such that, relative to CN participants, Obj-SCD (t[540.45] = −2.95, p = 0.003, r = −0.126) and MCI (t[590.28] = −6.57, p < 0.001, r = −0.261) groups had faster entorhinal cortex thinning over 48 months. Relative to participants with MCI, those with Obj-SCD had a slower rate of entorhinal cortex thinning (t[571.96] = 2.56, p = 0.011, r = 0.107).
Figure 2. Trajectories of entorhinal cortex thinning by cognitive group.
Model-predicted values adjusted for age, education, sex, APOE ε4 allele frequency, and baseline amyloid PET summary standardized uptake value ratio. CN = cognitively normal; MCI = mild cognitive impairment; Obj-SCD = objectively defined subtle cognitive difficulties. Shaded area represents 95% confidence intervals.
Hippocampal volume trajectories
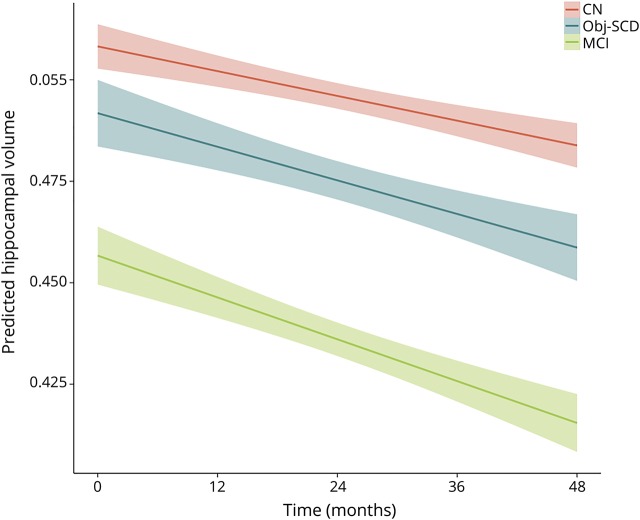
Figure 3 shows the trajectories of hippocampal volume loss by cognitive group and table 3 shows the model estimates. There was a significant interaction between cognitive group and time such that, relative to CN participants, participants with MCI (t[525.68] = −4.06, p < 0.001, r = 0.174) had a faster rate of hippocampal volume loss over 48 months. The rate of volume loss for participants with Obj-SCD did not statistically differ from that of CN participants (t[478.21] = −1.79, p = 0.074, r = −0.082) or participants with MCI (t[511.81] = 1.62, p = 0.105, r = 0.071).
Figure 3. Trajectories of hippocampal atrophy by cognitive group.
Model-predicted values adjusted for age, education, sex, APOE ε4 allele frequency, and baseline amyloid PET summary standardized uptake value ratio. CN = cognitively normal; MCI = mild cognitive impairment; Obj-SCD = objectively defined subtle cognitive difficulties. Shaded area represents 95% confidence intervals.
Discussion
The amyloid cascade model and NIA-AA research criteria for preclinical AD both depict amyloid deposition and early neurodegenerative changes as occurring prior to the onset of cognitive symptoms.1,3,32 This study examined associations of Obj-SCD with amyloid PET and MTL atrophy trajectories. Results show that participants with Obj-SCD and CN participants did not statistically differ from one another at baseline on levels of amyloid deposition, though, qualitatively, participants with Obj-SCD had slightly higher amyloid levels. Thus, this nonsignificant difference in baseline amyloid alone does not rule out the possibility that there are small effects of amyloid contributing to subtle cognitive inefficiencies. However, the nonsignificant baseline finding, in combination with the result that Obj-SCD was associated with a faster rate of amyloid accumulation even after adjusting for baseline amyloid levels, provides more support that Obj-SCD was identified prior to or coincident with the early phase of amyloid accumulation, rather than after amyloid deposition presumably levels off. Prior work has also demonstrated that subtle impairments on sensitive neuropsychological measures can in fact precede or emerge in tandem with amyloid positivity in participants who later progress to MCI and AD.4,16,33 However, this is the first study, to our knowledge, to investigate the relationship between Obj-SCD, defined using sensitive neuropsychological measures, and the trajectory of amyloid PET changes. In addition to participants with Obj-SCD showing faster rates of amyloid accumulation, our results also demonstrated that relative to the CN group, the Obj-SCD group had faster thinning of the entorhinal cortex and nearly 3 times the proportion who are later classified as MCI over 48 months, whereas the MCI group showed faster thinning of the entorhinal cortex and hippocampal atrophy over 48 months.
Related to neurodegeneration, the finding that the Obj-SCD group had faster entorhinal cortical thinning, but only trend-level changes in hippocampal volume relative to the CN group, may suggest that Obj-SCD captures individuals very early in the neurodegenerative process. Indeed, this pattern of atrophy, first of the entorhinal cortex and then the hippocampus, parallels the very early Braak staging of tau pathology (i.e., Braak stage I to II).34–36 These findings are also consistent with recent work by Bangen et al.,37 who found that intraindividual variability of neuropsychological performance, thought to be a sensitive marker of early cognitive difficulties,38 was related to longitudinal entorhinal and hippocampal atrophy in participants with MCI, but only entorhinal cortex thinning in CN participants.
Given evidence that tau pathology is more strongly related to cognition than amyloid pathology,39–42 examination of the cross-sectional and longitudinal relationships between Obj-SCD and tau is needed to determine if early tau deposition, first in the entorhinal cortex (i.e., Braak stage I) and then in the hippocampus (i.e., Braak stage II), may be causing the subtle cognitive difficulties we observed in this Obj-SCD group. Specifically, it is plausible that early tau accumulation may be related to Obj-SCD status since this would closely track with the spatial temporal relationship between Obj-SCD and selective entorhinal cortex thinning that we observed. We hypothesize that Obj-SCD is capturing those individuals with early tau pathology, leading to the MTL atrophy43 that was observed in these analyses. There are also known associations between white matter hyperintensity volume and cognitive decline,44,45 which may be mediated by MTL thickness/volume.46 Indeed, one recent study demonstrated that, although AD was the most frequent (65%) pathology in an autopsy study of 1,079 individuals, it rarely occurred in isolation (9%) and, remarkably, more than 230 different neuropathologic combinations were observed.47 Therefore, future work, particularly as more longitudinal tau PET imaging becomes available within ADNI, will examine the associations of Obj-SCD status, tau, white matter, and other pathologies.
The findings of Obj-SCD being a predictor of entorhinal cortex thinning were largely in line with our hypotheses. However, given evidence that those with Obj-SCD progress to MCI/AD faster than CN participants,5 the fact that Obj-SCD predicts future cortical amyloid accumulation and was not significantly related to cross-sectional PET amyloid is in stark contrast with the biomarker only model of AD.3 Our findings add to previous work within ADNI showing that amyloid is not always the first marker to emerge in preclinical AD. In fact, previous work by Edmonds et al.4 showed that neurodegeneration was the most common marker to emerge first among those who are known to progress to MCI/AD. Further, simply the number of abnormal markers (amyloid, neurodegeneration, cognition), regardless of the temporal sequence in which they appear, was shown to be just as predictive of future progression to MCI/AD as the traditional NIA-AA criteria1 that invariably require amyloid to emerge first. Accumulating evidence of inconsistency in the temporal sequence of AD pathogenesis, in combination with a string of clinical trials that have successfully cleared amyloid but did not affect the clinical trajectory of the cognitive symptoms,48,49 continues to call into question the accuracy and utility of the persistent focus on amyloid in AD.
ADNI data were used in this study, which allowed for a large, well-characterized sample with longitudinal neuropsychological testing and neuroimaging. However, this sample is limited in that it is highly educated, mostly white, and generally very healthy. Therefore, these findings need to be replicated in more generalizable community-based samples with increased diversity. This study includes a 48-month follow-up period, which may be a relatively short period of time to detect changes in brain structure, so future work should continue to investigate the temporal sequence of amyloid, neurodegeneration, and subtle cognitive changes as more participants have longer follow-up durations. In addition, as described above, there were no significant differences in baseline amyloid levels between the CN and Obj-SCD groups and adjusting for baseline amyloid in the longitudinal did not weaken the relationship between Obj-SCD and future amyloid accumulation; however, we cannot rule out the possibility that amyloid had already begun to accumulate at a faster rate in the participants with Obj-SCD. Thus, future work should investigate the transition from CN to Obj-SCD and determine the associated longitudinal changes in amyloid, tau, neurodegeneration, and white matter.
The Obj-SCD classification and neuropsychological process scores have demonstrated value for classifying individuals at risk and predicting future cognitive impairment.5,16 These past findings in combination with the current associations with pathologic changes suggest that Obj-SCD classification may be a particularly useful tool for research to recruit participants at risk for future disease progression. Compared to PET or lumbar puncture, Obj-SCD is a relatively inexpensive and noninvasive method for identifying those at greater risk for progression, and has the potential to be a marker of risk for AD in those who may not have access to or are medically unable to complete more invasive biomarker testing.
We applied a previously described operational definition of Obj-SCD that considers both neuropsychological total scores and sensitive neuropsychological process/error scores and balances the sensitivity of the >1 SD cutoff for impairment on a particular measure with the reliability of the requisite of at least 2 impaired scores to be present. This operational definition of Obj-SCD that incorporates neuropsychological process scores has previously predicted progression to MCI and AD.5 The current findings suggest that Obj-SCD is also a sensitive and noninvasive predictor of future amyloid accumulation and early neurodegenerative changes, prior to frank cognitive impairment consistent with MCI.
Glossary
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- CN
cognitively normal
- LME
linear mixed effects
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- MTL
medial temporal lobe
- NIA-AA
National Institute on Aging–Alzheimer's Association
- Obj-SCD
objectively defined subtle cognitive difficulties
- SUVR
standardized uptake value ratio
Appendix. Authors
Footnotes
Editorial, page 151
Study funding
This work was supported by NIH grants (R01 AG049810 and K24 AG026431 to M.W.B.), the Alzheimer's Association (AARF-17-528918 to K.R.T., AARG-18566254 to K.J.B., AARG-17-500358 to E.C.E.), and the US Department of Veterans Affairs Clinical Sciences Research and Development Service (Career Development Award–2 1IK2CX001865 to K.R.T., 1IK2CX000938 to K.J.B. and 1IK2CX001415 to E.C.E.). Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (NIH grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through contributions from the following: AbbVie; Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern CA. ADNI data are disseminated by the Laboratory for NeuroImaging at the University of Southern California.
Disclosure
K.R. Thomas, K.J. Bangen, A.J. Weigand, E.C. Edmonds, C.G. Wong, S. Cooper, and L. Delano-Wood report no disclosures relevant to the manuscript. M.W. Bondi receives royalties from Oxford University Press and serves as a consultant for Eisai, Novartis, and Roche Pharmaceutical. Go to Neurology.org/N for full disclosures.
References
- 1.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement 2014;10:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW. Subtle cognitive decline and biomarker staging in preclinical Alzheimer's disease. J Alzheimer's Dis 2015;47:231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas KR, Edmonds EC, Eppig J, Salmon DP, Bondi MW; Alzheimer's Disease Neuroimaging Initiative. Using neuropsychological process scores to identify subtle cognitive decline and predict progression to mild cognitive impairment. J Alzheimer's Dis 2018;64:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vos SJ, Xiong C, Visser PJ, et al. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol 2013;12:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knopman DS, Jack CR, Wiste HJ, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology 2012;78:1576–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toledo JB, Weiner MW, Wolk DA, et al. Neuronal injury biomarkers and prognosis in ADNI subjects with normal cognition. Acta Neuropathol Commun 2014;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan E. The process approach to neuropsychological assessment. Aphasiology 1988;2:309–311. [DOI] [PubMed] [Google Scholar]
- 10.Delis DC, Massman PJ, Butters N, et al. Profiles of demented and amnesic patients on the California Verbal Learning Test: implications for the assessment of memory disorders. Psychol Assess 1991;3:19–26. [Google Scholar]
- 11.Welsh K, Butters N, Hughes J, Mohs R, Heyman A. Detection of abnormal memory decline in mild cases of Alzheimer's disease using CERAD neuropsychological measures. Arch Neurol 1991;48:278–281. [DOI] [PubMed] [Google Scholar]
- 12.Loewenstein DA, Acevedo A, Luis C, Crum T, Barker WW, Duara R. Semantic interference deficits and the detection of mild Alzheimer's disease and mild cognitive impairment without dementia. J Int Neuropsychol Soc 2004;10:91–100. [DOI] [PubMed] [Google Scholar]
- 13.Libon DJ, Bondi MW, Price CC, et al. Verbal serial list learning in mild cognitive impairment: a profile analysis of interference, forgetting, and errors. J Int Neuropsychol Soc 2011;17:905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salmon DP, Bondi MW. Neuropsychological assessment of dementia. Annu Rev Psychol 2009;60:257–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodard JL, Dunlosky J, Salthouse TA. Task decomposition analysis of intertrial free recall performance on the Rey Auditory Verbal Learning Test in normal aging and Alzheimer's disease. J Clin Exp Neuropsychol 1999;21:666–676. [DOI] [PubMed] [Google Scholar]
- 16.Thomas KR, Eppig J, Edmonds EC, et al. Word-list intrusion errors predict progression to mild cognitive impairment. Neuropsychology 2018;32:235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loewenstein DA, Curiel RE, DeKosky S, et al. Utilizing semantic intrusions to identify amyloid positivity in mild cognitive impairment. Neurology 2018;91:e976–e984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bondi MW, Salmon DP, Galasko D, Thomas RG, Thal LJ. Neuropsychological function and apolipoprotein E genotype in the preclinical detection of Alzheimer's disease. Psychol Aging 1999;14:295–303. [DOI] [PubMed] [Google Scholar]
- 19.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 2010;74:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jak AJ, Bondi MW, Delano-Wood L, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry 2009;17:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bondi MW, Edmonds EC, Jak AJ, et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimer's Dis 2014;42:275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edmonds EC, Delano-Wood L, Clark LR, et al. Susceptibility of the conventional criteria for mild cognitive impairment to false-positive diagnostic errors. Alzheimers Dement 2015;11:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas KR, Edmonds EC, Delano-Wood L, Bondi MW. Longitudinal trajectories of informant-reported daily functioning in empirically defined subtypes of mild cognitive impairment. J Int Neuropsychol Soc 2017;23:521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landau SM, Breault C, Joshi AD, et al. Amyloid-β imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J Nucl Med 2013;54:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landau SM, Thomas BA, Thurfjell L, et al. Amyloid PET imaging in Alzheimer's disease: a comparison of three radiotracers. Eur J Nucl Med Mol Imaging 2014;41:1398–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joshi AD, Pontecorvo MJ, Clark CM, et al. Performance characteristics of amyloid PET with florbetapir F 18 in patients with Alzheimer's disease and cognitively normal subjects. J Nucl Med 2012;53:378–384. [DOI] [PubMed] [Google Scholar]
- 27.Clark CM, Pontecorvo MJ, Beach TG, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol 2012;11:669–678. [DOI] [PubMed] [Google Scholar]
- 28.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–355. [DOI] [PubMed] [Google Scholar]
- 29.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex 2004;14:11–22. [DOI] [PubMed] [Google Scholar]
- 30.Woodard JL. A quarter century of advances in the statistical analysis of longitudinal neuropsychological data. Neuropsychology 2017;31:1020–1035. [DOI] [PubMed] [Google Scholar]
- 31.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods 2002;7:147–177. [PubMed] [Google Scholar]
- 32.Jack CR, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 2010;9:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roe CM, Ances BM, Head D, et al. Incident cognitive impairment: longitudinal changes in molecular, structural and cognitive biomarkers. Brain 2018;141:3233–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 2006;112:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 2011;70:960–969. [DOI] [PubMed] [Google Scholar]
- 36.Braak H, Del Tredici K. The preclinical phase of the pathological process underlying sporadic Alzheimer's disease. Brain 2015;138:2814–2833. [DOI] [PubMed] [Google Scholar]
- 37.Bangen KJ, Weigand AJ, Thomas KR, et al. Cognitive dispersion is a sensitive marker for early neurodegenerative changes and functional decline in nondemented older adults. Neuropsychology 2019;33:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gleason CE, Norton D, Anderson ED, et al. Cognitive variability predicts incident Alzheimer's disease and mild cognitive impairment comparable to a cerebrospinal fluid biomarker. J Alzheimer's Dis 2017;61:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aschenbrenner AJ, Gordon BA, Benzinger TLS, Morris JC, Hassenstab JJ. Influence of tau PET, amyloid PET, and hippocampal volume on cognition in Alzheimer disease. Neurology 2018;91:e859–e866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol Am Med Assoc 2004;61:378. [DOI] [PubMed] [Google Scholar]
- 41.Brier MR, Gordon B, Friedrichsen K, et al. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer's disease. Sci Transl Med 2016;8:338ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maass A, Lockhart SN, Harrison TM, et al. Entorhinal tau pathology, episodic memory decline, and neurodegeneration in aging. J Neurosci 2018;38:530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison TM, La Joie R, Maass A, et al. Longitudinal tau accumulation and atrophy in aging and Alzheimer disease. Ann Neurol 2019;85:229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brickman AM, Zahodne LB, Guzman VA, et al. Reconsidering harbingers of dementia: progression of parietal lobe white matter hyperintensities predicts Alzheimer's disease incidence. Neurobiol Aging 2015;36:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee S, Viqar F, Zimmerman ME, et al. White matter hyperintensities are a core feature of Alzheimer's disease: evidence from the dominantly inherited Alzheimer network. Ann Neurol 2016;79:929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rizvi B, Narkhede A, Last BS, et al. The effect of white matter hyperintensities on cognition is mediated by cortical atrophy. Neurobiol Aging 2018;64:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyle PA, Yu L, Leurgans SE, et al. Attributable risk of Alzheimer's dementia attributed to age-related neuropathologies. Ann Neurol 2019;85:114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cummings JL, Morstorf T, Zhong K. Alzheimer's disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther 2014;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Honig LS, Vellas B, Woodward M, et al. Trial of solanezumab for mild dementia due to Alzheimer's disease. N Engl J Med 2018;378:321–330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
ADNI data were obtained from adni.loni.usc.edu and are available to investigators in the scientific community who have been approved by the ADNI Data Sharing and Publications Committee and who agree to the terms of the ADNI Data Use Agreement for purposes of replicating procedures and results. Anonymized ADNI participant identification numbers used in this article are available by request from any qualified investigator.