Abstract
One of the goals of biomaterials science is to reverse engineer aspects of human and nonhuman physiology. Similar to the body’s regulatory mechanisms, such devices must transduce changes in the physiological environment or the presence of an external stimulus into a detectable or therapeutic response. This review is a comprehensive evaluation and critical analysis of the design and fabrication of environmentally responsive cell-material constructs for bioinspired machinery and biomimetic devices. In a bottom-up analysis, we begin by reviewing fundamental principles that explain materials’ responses to chemical gradients, biomarkers, electromagnetic fields, light, and temperature. Strategies for fabricating highly ordered assemblies of material components at the nano to macro-scales via directed assembly, lithography, 3D printing and 4D printing are also presented. We conclude with an account of contemporary material-tissue interfaces within bioinspired and biomimetic devices for peptide delivery, cancer theranostics, biomonitoring, neuroprosthetics, soft robotics, and biological machines.
Keywords: Cell-material constructs, biofabrication, bioprinting, biomimetic materials, intelligent hydrogels, molecular machines, drug delivery, theranostics, tissue engineering
I. Introduction
1.1. Rationale and Relevant Definitions for Bioinspired Actuators and Biomimetic Devices
Recent advances in the rational design and synthesis of intelligent materials, especially polymers, for medical, biological and other applications have led to systems that are uniquely capable of responding to a dynamic surrounding environment such as a biological or physiological fluid. Research on formulating precise chemical architectures that recognize target molecules or ions from an ensemble of closely related entities in the surrounding environment has resulted in new classes of intelligent polymers for numerous applications. These environmental interactions can be general (e.g. temperature, pH, ionic strength, thermodynamic compatibility of the solvent, nature of co-analyte) or specific (e.g. molecular recognition) in nature. The composition and supramolecular assembly of material components drives the sensitivity and physical nature of a response.
A major research goal is to engineer new formulations and chemical architectures of polymeric or biohybrid material that ‘communicate’ with their surrounding environment. By ‘communication’, we refer to the ability of a material to act as an intelligent actuator – integrating biological stimuli and transducing a response (i.e. a mechanical output). Here, we define an ‘actuator’ as a control system component that converts a stimulus into an output. Biomedical actuators convert biological stimuli into useful outputs. Biomedical applications that require intelligent polymeric actuators include biosensors, intelligent and/or triggerable drug delivery systems, closed-loop devices that respond to physiological stimuli with therapeutic intervention, and scaffolds for biological machinery.
Such systems can be adapted to respond to environmental conditions by emitting a signal or intervening therapeutically. Closed-loop devices integrate biochemical stimuli and therapeutic intervention through controller function, which is either a component of a complex actuation system or a property of the actuator material itself. Intelligent materials that transduce perturbations in the environment into mechanical outputs are particularly useful as auto-control elements, which deliver drugs, monitor physiological conditions, or modulate embedded cells.
Researchers are also integrating cellular and molecular components with intelligent material scaffolds to construct novel biohybrid machines that solve elusive medical and biological problems. These applications include continuous monitoring of analytes for diagnostic purposes, identification of tumors in vivo, regeneration of diseased or damaged tissue (e.g. skeletal muscle, cardiac muscle), and recapitulation of highly-ordered biological activity within advanced devices (i.e. biomimetic design). The integration of biomedical material actuators with native (or non-native) tissue offers promise to restore disease-induced, aberrant control systems in the body. Creative constructs of intelligent biomaterial and embedded cells have found utility as a class of ‘biomimetic machines’ that perform unique mechanical functions beyond the capability of purely mechanical elements, for medical and non-medical purposes.
1.2. A Framework for the Design, Fabrication, and Evaluation of Biomedical Actuators
A schematic capturing the lineages of design inquiry for bioinspired therapeutic devices and biomimetic machines is presented in Figure 1.1. This schematic provides not only a useful guide for organizing one’s critical evaluation of a new biomedical actuator (as we will throughout this manuscript) but also a framework for designing new bioinspired and biomimetic devices for medical purposes. We therefore hope that you will find it useful for contextualizing existing work and informing innovative designs.
Figure 1.1:
Intelligent polymers can serve as actuators to transduce a signal from the biological environment into a mechanical output or detectable signal. These advanced material platforms function as novel biosensors, vehicles for protein and peptide delivery, and scaffolds for tissue growth. When interfacing novel biomaterials with embedded and/or genetically engineered cells, cell-laden hybrids can mimic the activity and function of a biological system.
Starting with the biomaterial, engineering intervention is informed by a desired function, or outcome. In a typical therapeutic case, in order to design a useful biomaterial actuator, you must first identify an area of deficiency, disease, or need; determine the stimuli or signals that characterize the disease; and select a corrective action that provides benefit to the patient.
By the example of type I diabetes, the area of deficiency is insufficient insulin production. This deficiency manifests as an elevated blood glucose level, which can cause significant detriment for the patient if untreated. However, overcorrection (hypoglycemia) also presents a significant danger. Therefore, the deficiency is insulin presentation to tissue, the relevant biological signal is blood glucose level according to specified thresholds, and the necessary corrective action is insulin release. A suitable engineering intervention must turn the biological signal of glucose level into a switch or trigger for tunable and calibrated insulin release.
Synthesis, selection, fabrication, and processing of intelligent materials is necessary to achieve the complex structure, network, or assembly capable of transducing the biological signal (e.g. glucose) into a therapeutic response (e.g. insulin release). These responsive biomaterials can be natural or synthetic in origin, possess pH, ion, or otherwise responsive chemical moieties, have labile functional groups or crosslinks, and interact with differential affinity to biomacromolecules. Co-polymerization, blending, and interconnectivity can yield combinations of, and interactions between, material-environment interactions. Precise supramolecular and macroscopic assembly of these intelligent materials results in the formation of dynamic, bioactive materials. We provide a fundamental analysis and critique of environmentally responsive materials in Chapter 2, and a review of material fabrication and bioprinting methods in Chapter 3.
Again using the example of continuous glucose monitoring, we can use advanced materials science and fabrication to generate glucose-responsive networks. An example of a glucose-responsive network is poly(methacrylic acid) (P(MAA)) with orthogonal, conjugated, enzymatic units (i.e. glucose oxidase), and insulin. Glucose oxidase (GOx) catalyzes the conversion of glucose to gluconic acid, resulting in a dip in the local pH that corresponds to the pKa of MAA. The polymer network responds by collapsing, facilitating insulin release. The combination of P(MAA) and GOx achieves molecular recognition (i.e. signal detection) and mechanical response (i.e. pH-responsive swelling). Consequently, the rational assembly of the two, applied in the context of type I diabetes, is a biomedical actuator. Therapeutic and theranostic applications of environmentally responsive polymers are presented in Chapter 4.1. Additional medical devices for continuous analyte monitoring are discussed in Chapter 4.2.
Actuation:
In the bolded box, we present the types of intelligent responses typically exhibited by biomedical actuators. The most common biomaterial responses to physiological stimuli are swelling, assembly, or disassembly. Molecular mobility and electrical conductivity are also possible, and are discussed in the later sections of this report. As was the case in the glucose biosensor example, many biomedical actuators couple their physical output with a molecular recognition event (e.g. antibody-antigen, enzyme-substrate, or other highly specific interaction).
The initial physical response of biomedical actuators can frequently accomplish the entirety of the initial design specification. This is the case in most diffusion-controlled drug delivery systems and biosensors. However, in scaffolds for tissue regeneration or biomimetic machines, biomedical material actuators must additionally integrate with cellular components to produce complex behavior.
Cellular Actuators provide additional activity, responsiveness, and design potential. Certain cell lineages, such as cardiac muscle, will respond to an electrical stimulus with contraction and can therefore convert an external signal (i.e. delivered by a conducting polymer) into mechanical work. The incorporation of cells as actuators into implants and devices requires an additional engineering intervention (i.e. selection and differentiation of cells, fabrication of scaffolds that achieve necessary cell density and alignment, modification of genome and gene expression, etc.). The major advantage of generating cell-laden devices is to achieve complex environmentally responsive behavior that non-living elements cannot generate.
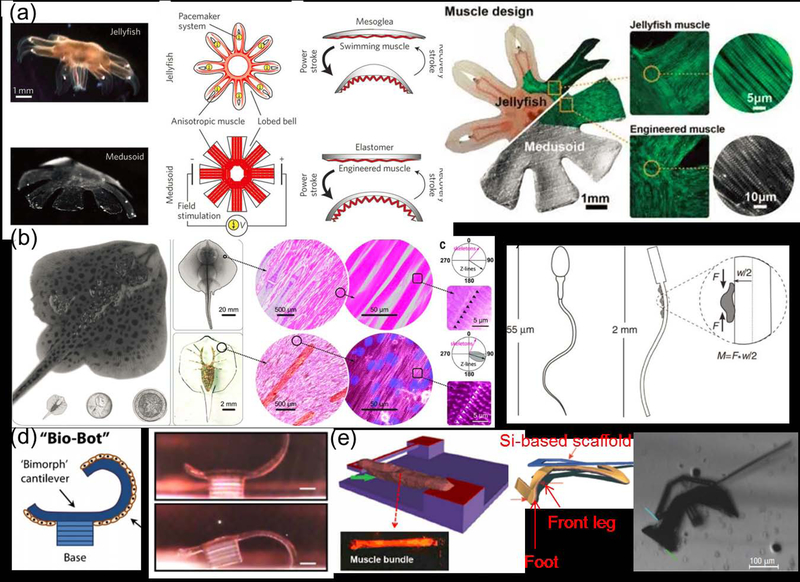
By example, in Chapter 4.3 we describe a jellyfish bioinspired machine. The material for this hybrid bioactuator was fabricated with precise geometric constraints to capture the jellyfish geometry and facilitate the necessary alignment of cardiomyocytes. The research team modified the cardiomyocytes genetically (an additional level of engineering control) so that stimulation with visual light (blue light at 10 mW) would activate the muscle cells. Upon final preparation of the cell-laden construct, the biomimetic jellyfish machine could swim with the characteristic undulations of the native fish, with precise external control over the swim trajectory (i.e. through light). We discuss this example, and many other novel biological machines, in Chapter 4.3.
As illustrated in these brief examples, researchers are using biologically responsive polymers to construct therapeutic devices, biosensors, and biological machines. Recent efforts in the field have addressed fundamental scientific inquiry and the development of translational technology. Fundamental advancements have included refined thermodynamic theories to explain the behavior of biopolymers in physiological solutions; new chemistries for synthesizing diverse, environmentally responsive polymers; nano- and micro-fabrication technology for forming ordered assemblies of natural and synthetic device components; and bioprinting technology for generating cell-laden constructs. Recent contributions to translational science include novel controlled release devices that deliver drugs, peptides, and proteins in response to changing physiological conditions; new diagnostic systems that minimize patient discomfort and maximize efficacy; and implantable cardiovascular devices.
1.3. Intelligence of Molecules, Materials, and Biomedical Devices: Relevancy across Molecular Complexity, Length Scale, and Application Space
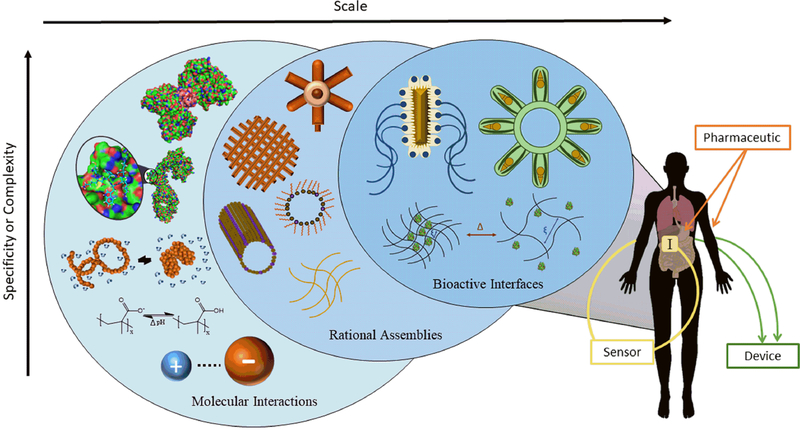
A pictorial representation of this review’s scope, sequence, and structure is presented in Figure 1.2. The image has three interlocking, cascading circles, where each circle represents a subsection of our review. Within each circle exists a continuum of scale and complexity. All three seemingly disparate units coincide in the design of advanced pharmaceutical formulations, biosensors, and biomimetic devices that interface with, or enhance, human physiology. Analyzing the graphical depiction in Figure 1.2 in a bottom-up manner, we start with molecular interactions.
Figure 1.2:
Next-generation material-tissue interfaces function through the synergy of multiple material-environment interactions. These interactions range from simple (anion-cation) to complex (protein-protein). Biomaterials are fabricated into rational assemblies by chemical crosslinking, self-assembly, patterning, or printing. The resulting cell-material or material-tissue interfaces can navigate the physiological environment and/or mimic native bioactivity as components of pharmaceutical formulations, biosensors, and bioinspired machinery.
Molecular interactions span along a continuum from general to specific. At the most general, there is the attraction or repulsion between two atoms or molecules (e.g. an anion and cation, as shown). The strength of these general interactions depends on a number of parameters, including the identity of the molecules, distance or molecular orientation, and solvent condition. These interactions are ‘general’ because they exist between classes or groups of molecules, rather than single, specific ones (e.g. anions and cations exhibit attractive forces for one another, Na+ attracts Cl−, but Na+ does not exclusively interact with Cl−). At increased scales, these intramolecular forces govern the conformational state of a polymer and its interactions with solutes in the environment. Biopolymers with specific sequences and three-dimensional (3D) structure can achieve specific interactions (i.e. orders of magnitude greater affinity for a single analyte or solute) because of multiple complementary, spatially oriented functional moieties.
Intermolecular forces, chemical equilibria, and thermodynamic laws govern the dynamic polymer-environment interactions necessary for actuation. Therefore, in order to design next-generation bio-actuators for bioinspired and biomimetic devices, one must first appreciate and understand the corresponding fundamental principles. This critical background, as well as relevant examples from research in materials engineering and biomaterials science, are given in Chapter 2.
Rational Assemblies:
Certain materials possess chemical properties that enable the formation of stable 3D architectures. At the general level, crosslinking creates network polymers. Networks are insoluble, but can respond to environmental conditions with swelling conditions and/or degrade in response to biological stimuli in a formulation-dependent manner. At higher orders of engineering control, self-assembled structures (i.e. micelles, vesicles, tubes) form out of chemically ordered molecules (i.e. lipids, peptides, surfactants) under certain environmental conditions. Lithographic techniques, 3D printing, and bioprinting enable the generation of amenable materials with precise geometric control.
Intermolecular forces, and polymer-solvent compatibility drive the formation (i.e. self-assembled structures) and environmental responsiveness (self-assembled and crosslinked structures) of rationally assembled materials. Photoinitiated chemistries enable the formation of precise and repeatable microstructures by two-photon lithography and PRINT. This is why it was critical to analyze molecular interactions first. Molecular interactions explain the behavior of polymers in physiological solutions. Therefore, one cannot design rationally assembled polymeric materials without first addressing the fundamentals of intermolecular forces and environmental response. Rational and/or creative assemblies of such biopolymers translate fundamental findings in materials chemistry into supramolecular biomaterials that improve human health. These fabrication strategies are discussed in detail in Chapter 3.
Bioactive interfaces involve the interaction between natural, synthetic, or hybrid materials and biological systems (i.e. bioactive proteins, cells, tissues, organs). In most cases, bioactive interfaces either recapitulate biology (i.e. biomimetic devices and technology) or respond to biological cues (i.e. bioinspired pharmaceuticals or sensors). In Chapter 4, we review diverse applications of bioactive interfaces in the design of next-generation vehicles for oral and transdermal drug delivery, theranostic systems for cancer therapy, closed-loop sensors for glucose monitoring, wearable electronics, neural prostheses, bacteriobots, and skeletal muscle-laden devices.
Proper molecular architectures are required to interface material systems with biological components productively. For example in drug delivery, an application may require environmentally responsive, pulsatile, or sustained elution of a payload. To achieve the desired release profile, engineers must employ the combination of supramolecular assemblies or optimized networks. In constructing a bioactuator with cardiac muscle, proper cell alignment is crucial to generating coordinated, cyclic contraction. Bioconjugation and biofabrication technology allow for presentation of adhesion ligands to promote the adhesion of cardiomyocytes. Additionally, 3D printing and bioprinting technologies enable the fabrication of materials with proper geometric constraints for mimicking muscle fibers.
Interactions between chemical moieties, biological fluids, and solutes drive the environmental sensitivity of biomaterials. Creative and rational assemblies of biomaterials are necessary in bioactive interfaces. Furthermore, as we discuss throughout this review, spatiotemporal distributions of cues (i.e. mechanical, chemical) significantly alter the behavior of embedded cells, and subsequently the function of the cell-material construct. While a fundamental understanding of materials chemistry was necessary for generating new complex biomaterial constructs, a knowledge of biofabrication or bioprinting is a prerequisite for engineering bioinspired and biomimetic devices.
Returning to Figure 1.2, we now see why molecular interactions, rational assemblies, and bioactive interfaces interlock and progress in sequence. The current and future outcomes of synergy in all three research areas are novel pharmaceutical formulations, biosensors, and devices that solve pressing medical issues. After all, addressing these problems, designing devices and products as solutions, and improving the quality-of-life of patients are the motivations for our work.
1.4. Intelligent Materials for Next-Generation Material-Tissue Interfaces:
In this introduction, we have provided a useful engineering framework for the conceptualization and critique of biomimetic and bioinspired medical devices. Using the illustrative examples of closed loop glucose monitoring and cardiac muscle-laden cell actuators, we discussed the cascading structure-function relationships at the molecular, material, and device scales.
In the following sections, we highlight fundamental concepts and recent progress in the areas of environmentally responsive materials, biofabrication, and biomimetic/bioinspired device development. This comprehensive analysis highlights both the recent progress and opportunities for further development in each area.
2. Advanced Functional Materials for Dynamic and Intelligent Systems
Response to a stimulus is one of the most basic processes found in living systems. The desire to engineer dynamic and functional materials is becoming more prevalent in an effort to precisely detect changes in an environment, which can be transduced through an actuator into a signal or therapeutic intervention. Environmentally responsive biomaterials are unique because each material response is purposefully engineered at the molecular level to communicate with physiological processes through complex structure-property relationships and produce a macroscopic functional behavior. When joined together via modular or hierarchical assembly, these responsive components form intelligent devices that simultaneously respond to multiple stimuli and perform complex functions.
Functional intelligent materials recognize a specific environmental stimulus and respond in a predetermined and predictable manner. The degree of response is controlled by the intensity of applied stimuli, and these materials return to the original state when the stimuli is discontinued. To engineer environmentally responsive biomaterials for various applications, researchers have looked to nature for inspiration. By biomimetic design, they have reverse engineered the molecular responsiveness and complex structure-property relationships necessary to transduce biological stimuli into useful mechanical outputs.
Environmental stimuli are divided into three main categories: chemical, physical, and biological. Chemical stimuli are those that stimulate intermolecular interactions such as pH, solvent, and ionic strength. Physical cues include mechanics (stress/strain), temperature, light, magnetic fields, and electric fields. Biological stimuli include enzymatic reactions, membrane permeating peptides, and receptor-ligand recognition. A response can take many forms, from a simple shift in solubility to a series of complex biochemical reactions that transduce minute signals into an optical output. The choice of stimuli and response is driven by the device’s therapeutic or diagnostic application, which will be covered in a later section (Chapter 4).
Understanding the intricacies of a stimulus-response relationship is essential to achieve kinetic, thermodynamic, and spatial control in a useful biomaterial actuator within the device-tissue interface. The following section provides the reader with a fundamental basis for how materials and stimuli interact, and offers engineering considerations to enable the rational design of next-generation materials.
2.1. Thermodynamics of Polymer-Solvent and Polymer-Solute Interactions:
Dynamic material-tissue interfaces rely on materials that recognize shifts or perturbations in the surrounding environment. At the molecular level, this means that a shift in the environmental conditions (e.g., pH, temperature, presence of an analyte) must alter the polymer-polymer, polymer-solvent, or polymer-solute interactions with sufficient vigor to alter the bulk materials’ conformation and/or bioactivity. In this section, we highlight the relevant fundamental principles that explain dynamic material interfaces in physiological buffers. A robust understanding of these principles is crucial to engineering novel biomimetic and bioinspired materials.
2.1.1. Polymer-Solvent Interactions within Hydrogel Biomaterials
In dilute solutions, polymers act as large macromolecular chains and can display a wide variety of possible conformation states (1–3). Typically, they exhibit a random coil or globular conformation to varying degrees rather than existing in an elongated or fully stretched state. A polymer will present itself in a more elongated form in a good solvent, as there is a significant increase in the flexibility of the polymer chains. In non-solvents, there is a loss of chain flexibility and a polymer will aggregate or shrink to varying degrees.
The polymer-solvent affinity determines how energetically preferred polymer-solvent interactions are over polymer-polymer interactions (1–4). This particular behavior allows attractive forces between polymer segments to become stronger or weaker than polymer-solvent interactions. Stronger attractive forces between polymer segments result in random coil or globular states, and weaker forces result in elongated or stretched conformation states. A greater polymer-solvent affinity corresponds to a lower chemical potential within the polymer aggregate or matrix network than that of the surrounding environment. In turn, the polymer-solvent mixing pulls the surrounding fluid into the matrix network and leads to an increase in the osmotic pressure,.
The change in total Gibbs energy (ΔGtotal) and the energy associated with mixing (ΔGmixing) at constant temperature and pressure are governed by the thermodynamic formulas described here:
In the first expression, the total Gibbs energy of the system is presented as the sum of the free energy associated with stretching or compressing chemical bonds (i.e. elastic) and the energy associated with mixing two compatible phases (i.e. mixing). The changes in enthalpy and entropy associated with the mixing process are represented in the second equation by ΔHmixing and ΔSmixing, respectively. T represents the absolute temperature in Kelvin. It is important to note that Flory-Rehner theory, presented here, is valid for nonionic gels and that direct use it to explain the behavior of ionic hydrogels is incorrect (3). In this case, the theory must be adapted to account for additional ionic interactions and forces within the hydrogel network and between the hydrogel and surrounding environment.
As the vital studies of Flory and Huggins have demonstrated, the contribution of the polymer-solvent affinity to the free energy (ΔGmixing) depends on several factors including: (i) the polarity of the solvent, and (ii) the hydrophobic-hydrophilic character of the groups attached to the polymer backbone (1). This is detailed by the Flory-Huggins solution theory:
where the total moles of the solvent and polymer are represented by n1 and n2, respectively, and the respective volume fractions are represented by v1 and v2, respectively. The introduction of a mixing parameter, χ12, is present to account for the increase of energy due to the interspersing and contacts between polymer and solvent molecules. T represents the absolute temperature in Kelvin, and R is the universal gas constant. It is important to consider that the polymer equilibrium properties are highly dependent upon several key physicochemical parameters, including the type of solvent, pH, type of salts present, total ionic strength of the surrounding solution, and temperature (3, 5).
Similarly, the change in osmotic pressure from polymer and solvent mixing can calculated using the formula below:
In the equation, the molar volume of the solvent is represented as V1. The formula was further simplified using the known identity:
One relevant example of dynamic biomaterial-solvent interaction is that of a non-ionic hydrogel and water. Hydrogels are 3D, insoluble water-swollen structures composed of hydrophilic homopolymers or co-polymers. They are crosslinked either chemically or physically and swell in aqueous solutions (5, 6). They can absorb as little as 20%, and up to 90% water by total weight when submerged in biological fluids or water. Hydrogels represent a very important and broad class of biomaterials, can be prepared using a wide variety of techniques, and can exhibit a range of behaviors. Due to the significant water content, hydrogels offer a degree of flexibility akin to natural tissue, which has garnered significant attention in biomedical applications and tissue engineering.
The 3D polymeric networks can be prepared using chemical or physical crosslinking techniques (5, 6). Chemical crosslinks entail covalent attachment of the polymer chains and are stable mechanically and thermally. Physical crosslinks may be incorporated into the network through molecular entanglement of the polymer chains or crystalline segments. These hydrogels are not homogeneous, but rather are clusters of polymer chain entanglements or clustered domains governed by attractive hydrophobic forces.
A non-ionic hydrogel will start to swell when in contact with water due to the favorable thermodynamic state of the polymer chains and solvent molecules. This phenomenon has been studied extensively by Peppas et al., and is the subject of many comprehensive reviews (5–9). Swelling in non-ionic hydrogel systems is driven primarily by a gain in entropy of the system through the mixing of the solvent molecules and polymer chains (1, 2, 5, 6). The elastic, retractile forces imparted by the polymer crosslinks counterbalance the forces of fluid intake and network expansion. Hydrogel swelling reaches an equilibrium condition when the two opposing forces are equal.
In order to define the structure of a hydrogel network, three important parameters should be considered: (i) the polymer volume fraction in the swollen state, (ii) the number average molecular weight between crosslinks, and (iii) the corresponding network mesh size (5, 6). The equilibrium volume fraction of the polymer phase is calculated as the ratio of polymer volume to the volume of the fully swollen hydrogel network. It is also the reciprocal of the polymer network volume ratio between the collapsed and fully swollen states, which can be determined experimentally through equilibrium swelling studies.
As mentioned above, the swelling behavior can be influenced by environmental (pH, temperature, ionic strength, etc.) and internal factors (polymer composition, hydrophobicity, etc.). Additionally, the swelling behavior is significantly affected by the nature of the crosslinks (covalent vs. physical) and crosslinking density (5, 6). The density directly influences the network mesh size and should be considered significantly depending on the application. For example in drug delivery applications, network porosity is a critical characteristic in defining the structure as it directly correlates to the ability of an entrapped solute or molecule to diffuse out through the network (5, 6). With higher crosslinking densities, the permeability and ability to diffuse out drastically decreases. This is especially true in the case of high molecular weight solutes such as growth factors, proteins, and antibodies.
2.1.2. Dynamics of Material-Solute Interfaces:
It is critical to understand the magnitude, length scale, and governing properties of biomolecule-biomaterial interactions in order to discuss their implications in bioactive devices and interfaces. In particular, electrostatic interactions, hydrogen bonding, and hydrophobic interactions are critical to the affinity of a material surface for biomolecules of all sizes.
Electrostatic Interactions:
A simple approximation of the force of electrostatic interaction between charged biomolecules and biomaterials according to Coulomb’s law(10, 11):
where ε0 is the permittivity of free space, D is the dielectric constant, q1 and q2 are the charges of the two interacting molecules, and r is the distance separating them. In water, this expression can be simplified to:
Electrostatic interactions are extremely enthalpically favorable. Additionally, even though the length of an electrostatic interaction is shielded in physiological salt solutions, this length is much greater than that of other intermolecular interactions (i.e. hydrogen bonding, van der Waals forces). Considering the charged state of nearly all biomacromolecules (DNA, RNA, protein), electrostatic interactions are critical in natural biomolecule interactions, are common targets in the development of inhibitory compounds, and, in the greatest relevancy to this review, are a critical design criterion in synthesizing bio-interfacial materials(12).
Electrostatic Interactions in Physiological Solutions:
In typical biological solutions, such as those present in vitro or in vivo, however, the presence other ionic species (salts, etc.) results in a charge-screening cloud. At a characteristic length, called the Debye length, the charged species and screening cloud together are effectively neutral. Therefore, electrostatic protein-material interactions occur at a closer proximity than the Debye length, which is calculated:
where kB is the Boltzmann constant, T is temperature, z is the valency of the ion, e is the elementary charge, and cinf is the counter-ion concentration in the screening cloud(11). A reasonable simplifying assumption is that this concentration is not significantly different from that of the solution at equilibrium. A typical Debye length in biological solutions is approximately 0.7 nm.(13)
Hydrogen Bonding:
Hydrogen bonding, another critical intermolecular interaction between biomolecules, materials, ligands, and water, is far more dependent on distance and orientation than electrostatic interactions (14). The average distance between a hydrogen bond donor and acceptor varies depending on the identity of each species, but ranges from approximately 1.54 to 2.79 angstroms(15). Hydrogen bonding interactions are extremely favorable when the spatial orientation of the species is appropriate. However, at distances that are closer than optimum, hydrogen bond interactions act in a repulsive manner, and at distances greater than the optimum the interaction strength declines rapidly (Figure 2.1). As shown in the figure, the repulsion force between hydrogen bond donors and acceptors increases sharply (scales with r−12) as they are brought in closer proximity than the optimal position. As the donor and acceptor are separated from their ideal orientation, the bond energy decreases (scales with r−6)(14).
Figure 2.1:
Distance-dependent bond potential of a hydrogen bond. Hydrogen bonds can be stabilizing or destabilizing, depending on the donor and acceptor. Few hydrogen bonds deviate substantially from the optimal distance Reprinted with permission from (14) published by Wiley.
Hydrogen bonding is critical to proper biological function, as it is responsible for complementary base pair formation in DNA and plays a significant role in the 3D folding of proteins (16). The precise spatial constraints of hydrogen bonding make it ideal for maintaining specific biomolecule interactions, and thus it is a commonly exploited molecular interaction in the design of biomimetic devices.
Hydrophobic Interactions:
Hydrophobic interactions are widely believed to be the driving thermodynamic forces behind high affinity ligand-receptor interactions in vivo(17). Hydrophobic ligand-receptor interactions are thermodynamically favorable in a number of ways. In an unbound state, hydrophobic binding pockets within a macromolecular receptor must interact with the solvent environment (water). As this interaction between hydrophobic residues and water is unfavorable, immediate water molecules will assemble into fixed orientations that maximize hydrogen bonding between the fixed water molecules and minimize contact with the hydrophobic material. This coordinated orientation is very entropically unfavorable(18, 19).
Proper ligands, which engage in hydrophobic interactions with the receptor’s binding pocket, liberate the fixed water molecules from this bound conformation. These water molecules, now expelled from the binding cavity, can then engage in hydrogen bonding with neighboring water in solution, but can also explore many spatial orientations, resulting in a higher entropy state(20).
As applied in a molecular and materials engineering context, properly aligned hydrophobic interactions are a critical tool. Modulation of hydrophilicity, evidenced through water contact angle, was one of the early methods for increasing the biocompatibility of medical implants(21). To this day, coating biomaterials with hydrophilic polymers such as poly(ethylene glycol) (PEG) is the primary approach for ‘passivation’ or prevention of immune recognition of adsorbed molecules(22). In drug delivery, regenerative medicine, and biosensing, proper design is necessary to provide complementary hydrophobic interactions between material carriers and tissue biomarkers, loaded therapeutic cargo, or regenerative factors. These interactions are used to impart material-protein affinity leading to lower dissociation constants, increased loading capacities, extended drug retention, and ideal release profiles(23).
2.1.3. Molecular Recognition:
A molecular recognition event occurs when a multiplicity of intermolecular interactions coincide productively to generate a binding environment with specificity for a particular analyte. Nature has mastered the art of molecular recognition, with antibody-antigen binding as a classic example of high affinity and specificity recognition. The engineering of biomaterials with molecular recognition properties has recently emerged as a materials science research area, particular for bio-separations, molecular sensing, and pharmaceutical applications. In the following section, we review the thermodynamic principles that explain molecular recognition in natural biological systems, biomaterials, and at the interfaces of the two.
Free Energy of Binding:
Minimization of the relevant systems’ free energy state drives material-protein and protein-ligand interactions. The first relevant contributor to free energy is the enthalpic gain or loss attributed to protein-material-solvent interactions. Electrostatic interactions, hydrogen bonds, and van der Waals forces each contribute directly to the systems’ enthalpy:
Here, ΔH represents the enthalpy off the system, which is the sum of the enthalpic contributions (favorable and deleterious) of electrostatic interactions, hydrogen bonding, van der Waals forces, and other intermolecular forces (i.e. polar interactions) between molecules within the system (i.e. ligand, receptor, and solvent).
The second major contributor to free energy state is entropic gain or cost, associated with the number of physical arrangements the material-ligand-solvent system can sample. By argument of statistical mechanics, the entropy is calculated by:
where kB is the Boltzmann constant and W is the number of equally-probably states (i.e. orientations and arrangements) that the system can explore(24). The following relation approximates the change in entropy of a ligand-receptor interaction.
Extending this expression to a protein-material-solvent system:
where ΔS is the change in entropy for either the system, solvent (i.e. bound or liberated molecules), the molecular ligands/receptors, and other species (i.e. associated or liberated ions, physiological molecules).
The Gibbs free energy expression, therefore, contains the above contributions:
where the system’s enthalpic and entropic contributions are the sum of all components, and the temperature is expressed in absolute units (Kelvin)(25). As applied to recognitive material systems, these expressions describe a favorable binding event as one that leads to additional enthalpically favorable molecular interactions, so long as the enthalpic gain overcomes the entropic loss of a fixed conformation. However, binding events are not always entropically unfavorable, as protein-material interactions can also liberate alternative ligands, molecules of solvent, or adsorbed ions. These outcomes can subsequently increase the total system entropy.
Equilibrium Adsorption Constants:
The equilibrium adsorption constant expression takes into account the binding free energy:
The standard binding free energy (ΔG°) is an expression that describes the free energy of association under an idealized system (i.e. 1M ligand and receptor concentration, 1 atmosphere of pressure, 298K). In the expression, R is the universal gas constant, T is the absolute temperature, and ka is the equilibrium adsorption constant. Frequently dissociation constants (kd), rather than association constants, are employed to compare the affinity of different ligand-receptor systems:
where [L] and [R] describe the concentration of ligand and receptor, and [LR] describes the concentration of the bound ligand-receptor complex. It is standard practice in the field to quantify affinity interactions by presenting the magnitude of the dissociation constant (e.g., micromolar). Many typical dissociation constants for protein-protein interactions are in the micromolar range, although high-affinity or super-affinity partners (e.g., antibody-antigen interactions, avidin-biotin binding) can reach nanomolar, picomolar, or even femtomolar affinity.
Upon deviation from an equilibrium condition, the Gibbs free energy of the system can be described by the following relation;
taking note that as the system approaches an equilibrium condition (ΔG→0) the expression simplifies back to the standard binding energy (17).
Multiplicity:
High affinity protein-protein and protein-material interactions are achieved through the productive sum of multiple intermolecular interactions. The important thermodynamic aspect of this reality is the concept of multiplicity(26). If a receptor can productively engage a ligand at two epitopes that provide similar enthalpic gains, for example, the total enthalpic gain of the ligand-receptor interaction will effectively double. As the ligand-receptor complex is held in a fixed orientation from the first interaction, there is minimal entropic loss from engagement in a second epitope-binding event. This will decrease the Gibbs free energy of binding significantly, shifting the equilibrium further toward the bound complex. Antibodies achieve high functional affinity (or avidity) for their antigen through multiplicity, and mimicking multiplicity within synthetic biomaterials using spatial composition control has been a research goal for several decades(27).
Super Affinity:
Molecular dynamic simulations have been utilized to explore the concept of ‘super affinity’, which is a term commonly used to describe affinity between binding proteins and other biomolecules (e.g., other proteins, peptides, or sugars) that are higher than anticipated based on the magnitude of individual intermolecular interactions(28, 29). Super affinity appears to emerge where the formation of hydrophobic contacts or enclosures coordinate with hydrogen bonding. These super affinity interactions are unique to certain properties of the protein receptor, as the specific hydrophobic ligand cavity must be naturally present, but nonetheless demonstrate the incredible specificity that can be achieved by synergistic intermolecular forces in confined geometries(30). Super affinity interactions are of particular interest to engineers designing next-generation biosensors, as well as scaffolds for tissue engineering that retain necessary growth factors.
2.2. Engineering Materials that Respond to Chemical Stimuli
With a fundamental understanding of the principles underlying polymer-polymer, polymer-solvent, and polymer-solute interactions, it is possible to design material systems that respond predictably to biological stimuli. To achieve the design goal of engineering devices that respond to dynamic physiological environments, researchers have employed a diverse repertoire of chemically-active natural and synthetic materials.
It is most practical to guide the design of advanced biomaterials that recognize and respond to changes in the physiological environment by characteristics of native physiology or a pathological state. The medical application and necessary transformation (e.g., change in protonation state, interaction with solvent, adsorption events, or cleavage of crosslinks) will guide researchers’ selection of material architecture and chemical stimulus. The following section details recent developments, challenges, and limitations in biomaterials that respond to several chemical stimuli, including pH, salts, ionic strength, oxidation, and reduction.
2.2.1. Switchable Polymer Brushes
In recent years, there has been great interest in engineering materials with solvent-responsive surface properties. This has been important in generating advanced devices and implants, such as those with anti-fouling and self-healing properties to more complex signaling systems that adaptively control the permeability and flow through nano/micro-pores. To this extent, there have been extensive studies on polymer films with switchable properties through a change the conformation of surface grafted chains (31–35).
Polymers such as poly(methyl methacrylate) (PMMA), poly(styrene) (PS), PEG, poly(butyl acrylate), and poly(dimethylaminoethyl methacrylate) (PDMAEMA) have been extensively researched in the fabrication of solvent responsive polymers (31–34). When treated with different solvents, the polymer brushes can exhibit a degree of deformation or change in orientation. As described in the previous section, the exact response is dependent upon the solvent environment and the thermodynamic nature of the polymer segments (i.e. the relative strength of the polymer/polymer vs. polymer/solvent interactions). Good solvent environments will yield an extended, stretched brush-like regime due to swelling, maximized solvent contacts, and increased flexibility of the macromolecular chains. When exposed to poor or non-solvent environments, the polymer brush deforms to yield more aggregated mushroom-like regimes.
Mixed polymer brush systems are those consisting of two or more homopolymer segments with different characteristics (32). The system are more complex, but have generated a lot of interest as they can display an amplified response when exposed to external solvents or proteins. This is due to the combination of a change in the network conformational state with the immiscibility and subsequent phase separation of the specific network domains.
For example, a mixed polymer brush can be comprised of a distinct hydrophilic homopolymer segment and hydrophobic homopolymer segment. If the polymer brush is in an aqueous environment, polymer/solvent interactions with the hydrophilic segment are more energetically favorable. Thus, the hydrophilic portion orients itself outward, and the surface becomes hydrophilic. If exposed to a hydrophobic environment, the same polymer system will instead preferentially segregate the hydrophobic homopolymer outward.
Control of the polymer brush architecture and morphology is critical for determining its performance in the end application. To this extent, nanoscale patterning of solventresponsive polymer brushes has opened up the ability to generate films or particle surfaces of varying controlled morphology, as illustrated in Figure 2.2. The structure and the surface roughness of the brushes can be controlled by varying the solvent quality during sequential surface reactions. The length scale between neighboring chains (low or high density grafting) influences the type of brush produced, and the brushes will naturally stretch away from the surface at high densities in order to reduce neighboring interactions (i.e. steric repulsion). Self-healing films can be generated by designing a system with hidden tethers that are exposed upon the rearrangement of externally grafted polymers at the interface. In more complex systems, direct patterning after grafting the polymer brushes has been demonstrated to fabricate nanochannels or pores in the system via electron beam lithography (36).
Figure 2.2.
Polymer brushes can be utilized to synthesize responsive films or surfaces. (a) Simple methods can be utilized to generate polymer brush surfaces with smooth or rough nanoscale morphology. Here, the degree of roughness can be controlled by varying the solvent quality during subsequent polymerizations. Reprinted with permission from (33) published by Elsevier. (b) The morphology and design can impart switchable anti-fouling and self-healing properties. Here, the additional self-healing aspect of the antifouling film is due to the rearrangement of internally grafted polymers to the interface (marked as dark blue chains). Reprinted with permission from (37) published by Wiley.
The reversible deformation with a change in solvent has been of particular importance in designing switchable surfaces for electronics (microactuators), microfluidic devices (lab on a chip, organ on a chip), and biosurfaces (biosensors and implants). Further, it has opened up many new possibilities in surface engineering concepts for improved wettability and adhesion at biological interfaces, mechanical actuation, chemical sensing, cell growth and separation, and micro- and nano-fluidics (31–33, 37). These applications will be covered in detail in Chapter 4.
2.2.2. pH-Responsive Polymers
pH-responsive polymers, commonly known as polyelectrolytes, contain functional moieties that are capable of accepting or donating protons as a response to pH changes in the surrounding environment (5, 6). The groups are often utilized as pendant groups or in side chains, but may be present in the polymer backbone. The extent of ionization depends on the environment pH. When a homopolymer is in an ionized state, the moieties carry a charge (all positively or negatively) and the chains repel one another due to electrostatic repulsion. The change ionization state may also cause the polymer to alternate between hydrophobic and hydrophilic behavior. Within the class of charged materials, polymers can be divided and characterized as anionic or cationic. Both anionic and cationic polymers can have a characteristic pKa between pH 1 and 14, and the underlying mechanism of the environmental response is the same for both species.
Anionic polymers, commonly known as polyacids, are polymers containing acidic functional moieties. Function groups used typically include carboxylic acid (−COOH), sulfonic acid (−SO3H), boronic acid (−B(OH)2), and phosphonic acid (−PO(OH)2). Under acidic conditions (below the pKa), the functional groups on polyacids will accept protons to yield a neutral polymer. The protonation enables hydrogen bonds to form with accepting groups in the polymer network and contributes to structural deformation or the formation of complexes (5, 6). At neutral or basic conditions (above the pKa value), the moieties release protons to form a negatively charged polymer. The charged state results in electrostatic repulsion, an increased solubility of the polymer, and maximized solvent contacts to yield an extended or stretched conformation. If in a crosslinked network, the Coulomb repulsion will result in a swelling or expansion.
Cationic polymers, on the other hand, contain basic functional groups and display an inverse behavior to anionic polymers. Common examples include tertiary amines (−NR2), pyridine (−C5H4N), pyrrolidine (−C4H8N), imidazole (−C3H3N2), and piperazines (−C4H4N2R). As with polyacids, cationic polymers will accept protons under acidic conditions below the pKa (5, 6). However, the functional moieties will now carry a positive charge. The positive charge results in the same electrostatic repulsion and conformation change as described above. At higher pH values (above the pKa value), the groups release the protons to yield a neutral polymer.
Amphiphilic polymers, or polyampholytes, are comprised of both acidic and basic functional groups (5, 6). The groups used to create amphiphilic polymers are the same as described above. Specific examples may include naturally occurring polymers (e.g., gelatin), synthetic polymers (e.g., P(MAA)-b-P(DMAEMA)), and hybrid semi-synthetic or modified natural polymers (e.g., modifications of chitosan, cellulose esters, alginate). As a significant portion of research has focused on creating biomimetic materials, these polymers have generated great interest. A portion of the increased interest stems from the discovery that many biomacromolecules are amphoteric in nature, including amino acids and nearly all proteins. Polyampholytes can also contain a hydrophilic and hydrophobic domain simultaneously across a range of solvents and pH values, making them very useful as surfactants.
Compared to simply anionic and cationic homopolymers, polyampholytes display a much more complex behavior to changes in pH due to competition between the acid-base equilibria of each unit. With weak acidic and basic groups, the polymer will likely have a net charge a certain pH values. However, it is possible for both groups to be charged throughout the entire pH range, yielding a net zero charge on the polymer. This is highly dependent upon the nature of the functional moieties used. Further, the conformation state in solution is highly dependent upon the charge distribution and location (not only the average composition). The acidic and basic moieties are commonly located in separate monomer units, but may be located on the same chain. For example, polybetaines are a subclass of polyampholytes that carry both a positive and negative species in each monomer unit.
The “pH critical point” of a polymer is a term used to represent the pH value where the polymer displays an inflection in the ionization state and/or solvent interaction (5, 6). As described above, a pH-responsive polymer typically displays a more compact, folded state in the unionized state. When the polymer is ionized, the moieties carry a charge and the chains move away from one another due to the Coulomb repulsion. However, it is important to note that the ionization process and subsequent structural change reflects deformation of the polymer or a change in the conformation state. The process does not involve breaking of bonds and is reversible.
The interplay between hydrophobicity and electrostatic repulsion dictates the extent and character of pH-responsive behavior. Repulsion between proximal anions or cations results in an increase in the hydrodynamic volume of the polymer. Additionally, osmotic pressure exerted by mobile counterions neutralizing the network charges explain polymer chain transition from a coiled to an expanded state. To this extent, network swelling is influenced by any condition that modifies the ionization state or electrostatic repulsion (e.g., type of counter ions, ionic strength, temperature) (5).
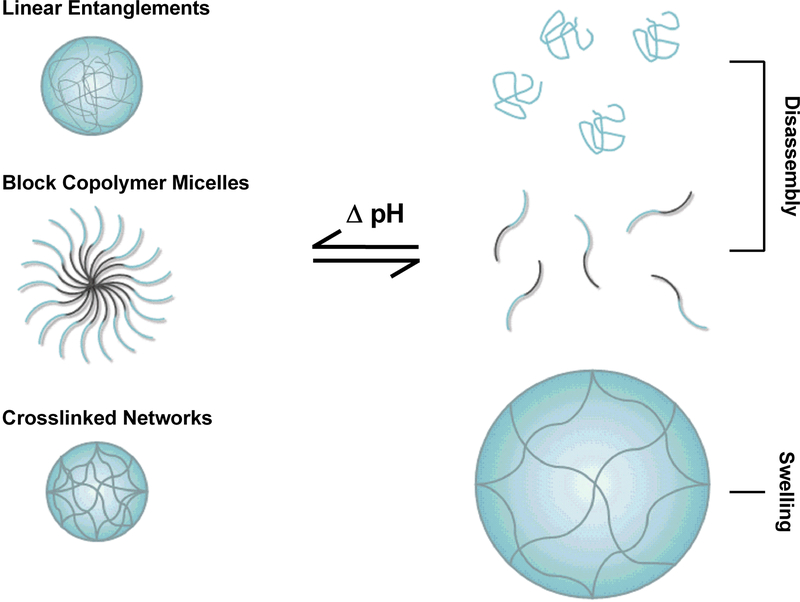
As detailed above, swelling in hydrogel networks is driven by a balance of thermodynamic and physical forces. In the case of simple linear or complexation polymers, the pH-dependent hydrogen bonding and electrostatic interactions result in a transition from an aggregated state to complete or partial dissociation (Figure 2.3). With crosslinked polymer networks, the swelling behavior is still governed by hydrogen bonding and electrostatics. However, the osmotic pressure of the counterions results in a swelling or deswelling behavior with the crosslinks serving as tie points to maintain 3D network structure.
Figure 2.3.
pH-responsive polymers may be comprised from linear entanglements, block copolymer micelles, and crosslinked networks.
A well-studied, pH-responsive hydrogel is crosslinked poly(acrylic acid), or P(AA) (5, 6). The solubility of P(AA) as a function of pH defines the conformation of the 3-dimensional network in an environment. At pH values below the pKa, the ionizable carboxylic acid pendant groups are neutral (−COOH). Electrostatic repulsion is absent, hydrogen bonding is favorable, and the polymer network is in a collapsed conformation state. At high pH (above the pKa of the carboxylic group), the functional groups are deprotonated and carry a negative charge (−COO−). In this salt form, there is a rapid influx of mobile counterions, the polymer network adsorbs water, and it rapidly swells.
In addition to P(AA), synthetic monomers commonly used to impart pH-responsive behavior include those with carboxylic acid (−COOH), sulfonic acid (−SO3H), boronic acid (−B(OH)2), amine (−NH2), and imidazole (−C3N2H4) groups. For example, MAA, itaconic acid (IA), ethylene imine (EI), DMAEMA, and diethylaminoethyl methacrylate (DEAEMA), and β-amino esters are widely studied in the literature (5, 6, 38–40). In certain cases, a polymer may also undergo multiple ionization processes. For example, a polymer comprised with phosphonates or phosphonic acid (H3PO3) functional groups will pass through each ionization state and gradually reach a fully dissociated form.
There are also a breadth of natural polymers that display reversible pH-responsive behavior. Common natural polyacids and polybases studied in the biomedical literature include chitosan, alginate, hyaluronic acid, and dextran (38, 41, 42). Natural amphoteric molecules are also prevalent in the literature and include collagen and albumin (43–46). Natural polymers are appealing because they offer low antigenicity and high biocompatibility - making them an ideal choice for biomedical and tissue engineering applications. Further, natural polymers are often derived from renewable sources and can display biomimetic properties (e.g., hydroxyapatite, collagen).
However, natural polymers have several disadvantages, which may be significant depending on the desired application (5, 6). Natural polymers are often derived using methods that can result in inconsistent production, batch-to-batch variability, and potential contamination. Additionally, they may degrade too rapidly in vivo or lack sufficient mechanical strength for a specific site in the body. In that regard, synthetic polymers may offer increased control over the structure-property relationship. A summary of common pH-responsive natural, synthetic, and hybrid biomedical polymers is given in Figure 2.4.
Figure 2.4.
Schematic with representative structures for common synthetic, hybrid semi-synthetic and natural pH-responsive polymers.
Modulation of pH Critical Point:
The pH range of the network phase transition can be modified using two common strategies. In a first strategy, the pKa of the ionizable group is selected to closely match the desired pH range in the ultimate application (6, 7, 47). This strategy requires careful selection of the acidic and/or basic functional groups with respect to the characteristic pH range of and the biocompatibility necessary for the intended application. By definition, the pKa of pH-responsive polymers is a function of the ionizable groups. To this extent, changing the characteristic nature of the functional groups (e.g., electronegativity, steric hindrance, hybridization, induction, resonance) can efficiently modulate the pKa. Thus, the pH-induced, reversible transition can be adapted to meet requirements of the desired application.
Alternatively, copolymers can be designed and synthesized to precisely tailor the pKa (6, 7, 47). Statistical copolymers comprised of multiple ionizable monomers can exhibit a pKa between those of the individual homopolymers, and fine-tuning can be achieved through variation of the monomer feed ratio. Hydrophobic moieties may also be incorporated directly into the polymer backbone. In an environment where ionizable groups are near neutral, electrostatic repulsion forces disappear within the polymer network and hydrophobic attractions dominate. The hydrophobic moiety can be varied in terms of both chain length and steric bulk. As the hydrophobic content in the network increases, greater proton activity is required to promote polymer/solvent interactions (required for dissolution or swelling) over the polymer/polymer attractive forces. For example, the enteric polymer hydroxypropyl methylcellulose acetate succinate (HPMCAS) can be tailored within a wide pKa range by changing the ratios of either the hydroxypropyl, methoxyl, acetyl, and/or succinoyl groups within the polymer functional groups. This strategy enables precise control over the structure (i.e. via the comonomer type, ratio, and distribution), and typically offers a wider variety of analogs to probe the relationship between structure and functionality.
The versatility of pH-sensitive chemistries offers many opportunities for exploiting pH variations found in native biological systems or characteristic physiological changes of diseased states (5–9, 39, 47). While the primary response to a change in pH is swelling or collapse, other possible responses are possible. pH-modulation can also induce degradation via acid-liable linkages, a sol-gel transition (from a solution to a gel), and/or the formation of micelles. These behaviors will be discussed further in Chapters 3.1 and 4.1.
2.2.3. Ion-Responsive Polymers
An ion is an atom or group of atoms carrying an electrical charge formed by the gain or loss of one or more electrons (48). In solution, ions originally present within the crystal lattice of a solid dissociate into both negatively and positively charged species (anions and cations, respectively). The species are present as electrically neutral combinations with the ability to migrate nearly independently throughout the solution. Ions in electrolyte solutions consisting of only a single atom are termed monatomic (e.g., Na+, Cl−), and those consisting of a few atoms or larger form molecular ions or polyatomic ions (e.g., NH4+, CH3COO−).
The underlying mechanism of ion-responsive polymers is that the disassociated, charged ion species will interact with the polymer or copolymers, reducing the electrostatic interactions between segments, chains, and other molecules in the surrounding environment (48). As detailed earlier in our discussion of electrostatic interactions in physiological fluids, this behavior is due to charge screening, in which the forces between any charged groups in the polymer are lowered.
Polymers containing a large number of ionizable functional groups will demonstrate increased solubility in environments where the groups carry a charge. However, the charge shielding seen with addition of salts may alter the conformation state of the polymer into a contracted coil or globular form. As a result, ionic strength and type of ion can show a strong effect on the solution behavior of many responsive polymers (e.g., pH, temperature) (48–57).
The pivotal work of Frank Hofmeister can be used to describe how ion type and concentration can alter the stability of macromolecules in aqueous solutions (58). As ranked in Hofmeister series, the order for the ability of anionic species to decrease hydrogen bond interactions between water and strengthening surface tension is generally:
and the order for cationic species is generally:
although the effect of cationic species has generally been shown to be less pronounced (59, 60). With hydrophilic macromolecules, the effect of ions on the left-hand side is characterized as decreasing the solubility or having a “salting-out effect.” In contrast, the ions to the right-hand side will increase solubility or have a “salting-in effect.” The exact order of the ion species listed should be considered qualitative (59, 60). It has been can vary with respect to the nature and structure of macromolecule being studied. Often, the effect of neighboring ions can change order. In certain cases, the effect can actually follow a reverse order of the classic Hofmeister series (53).
While experimentally useful, the fundamental mechanism of the Hofmeister series still remains poorly understood. Numerous theories to explain the effects have been suggested in literature (51, 52, 55, 60). Of those suggested, many hypothesize the ability of the different ion species to affect water structure, dividing the nature of the salts into water structure “makers” (kosmotropes) and “breakers” (chaotropes). Kosmotropes are strongly hydrated and increase the local order of water. This effect can lead to increased surface tension and increased stability of hydrophobic aggregates in solution. Using an example of a hydrophobic pH-responsive polymer, this effect can result in an apparent decrease of the pKa due to the decreased solubility of hydrophobic segments. Conversely, chaotropes are weakly hydrated anions that can break down hydrogen bonds and disrupt the local water structure. This can destabilize hydrophobic aggregates and lead to a decreased surface tension and increased solubility in solution.
In responsive materials, ion exchange describes a reversible trade between the material and its surrounding environment (48, 49, 60). Ion exchange is used in a wide variety of technical applications, including water softening, wastewater treatment, environmental remediation, chemical synthesis, and purification (61–65). In the medical field, ion exchange is well-studied for application in drug delivery, diagnostics, sensors, and a variety of other areas (44, 48, 52, 66–69).
Specifically in delivery applications, ion exchange resins are often used a drug reservoirs to enable taste-masking and sustained drug release as a function of time or exposure to counterions in the body. A variety of polymer matrices with the ability to control drug release have been reported in the literature (52, 70–77). The functional groups typically employed to generate the ion-responsive materials include quaternary ammonium, tertiary amine substitutes, sulfonic acid, and carboxylic acid.
The process and rate of ion exchange involves diffusion of the counterions through the material matrix. To this extent, porosity and particle size are critical attributes of the material. These attributes can be controlled through careful variation of the polymerization conditions and manufacturing technologies (48, 49). With polymer particles, the diffusion rate is typically under kinetic control at high concentrations. Larger length scales are slow, and smaller particle sizes typically result in improved efficiency. Conversely, the diffusion rate is kinetically controlled at low concentrations in polymer films. With both particle and film diffusion, lower polydispersity enables a more consistent kinetic performance than highly polydisperse polymers.
Outside of therapeutic delivery, the usefulness of ion exchange in the biotechnology and biomedical field is often correlated with the ability to regenerate the material for continuous use. The reaction should be reversible with no permanent change or loss of efficiency of the material as a result of ion exchange or regeneration process (48). To this extent, a variety of responsive materials have been reported in the literature for use in diagnostic sensors, self-healing hydrogels, and chromatographic separations of many biotechnology and fermentation processes such as the isolation and purification of monoclonal antibodies, proteins, and peptides (69, 78–85).
2.2.4. Oxidation – Reduction Polymers
Oxidation-reduction responsive polymers respond to environmental stimuli that can provoke a change in the oxidation state of redox sensitive groups (86). In inorganic chemistry, transition metals have historically been used to design this type of responsive material. The discovery of free radicals in biological systems occurred in 1954 (87). Over the last few decades, continued advances in our knowledge of and ability to mimic biological systems have stimulated great interest in redox-responsive polymers for potential applications in biomedical fields.
Many biomedical materials take advantage of reactive oxygen species (ROS) and reactive nitrogen species (RNS) found in the body, either by natural processes or abnormalities of diseased states (46, 88–90). Both species are characterized by a highly reactive nature, resulting as a byproduct of their unpaired electron. Common ROS include hydrogen peroxide (H2O2), hypochlorous acid (HOCl−), superoxide (O2•−), and subsequent derivatives including hydroxyl radical (•OH), hydroperoxyl radical (HO2•), peroxyl radicals (RO2•), alkoxyl radicals (RO•), and singlet oxygen. Common RNS include nitric oxide (NO•) and its derived reactive species including peroxynitrite (ONOO−), nitroxyl anion (NO−), nitroxyl cation (NO+), and nitrogen dioxide (NO2•).
Both species are produced at low levels naturally, as byproducts of healthy activity and metabolism in the human body. They can be beneficial at these low levels and are physiologically significant for complex functions such as muscle contraction, metabolism of nutrients, energy production, blood pressure regulation, and cognitive function. Additionally, they play critical roles in biological processes that are necessary to regulate life, such as cell proliferation and growth, normal apoptosis, regulatory mediators in cell signaling, and innate immunity (86, 91, 92). For example, superoxide is a natural byproduct of the immune system in response to oxygen-dependent killing mechanisms of foreign microorganisms or pathogens. Nitric acid is commonly formed as a result of the enzymatic activity of various nitric oxide synthases (NOS) with oxygen, NADPH oxidase, and L-arginine.
Overproduction of both radical species may also be induced by either external environmental stimuli or internal stimuli as a result of disease. Environmental triggers that provoke ROS/RNS production in the body can include tobacco, radiation, and air pollutants from car emissions, fossil fuels, and smoke. Internally, inflammation and inflammatory responses are associated with abnormal enzyme activity resulting in high concentrations of oxidizing agents and radical species. This state of oxidative stress is commonly associated with aging, many metabolic syndromes, and diseases such as Type 2 diabetes, cancer, muscle and tissue injuries, neurodegenerative diseases, coronary heart disease, cardiovascular disease, and hypertension. When overproduced, the stress can lead to mitochondrial and cellular dysfunction through the damage of key proteins, DNA, and RNA. This dysfunction often results in dysregulation or deactivation of certain enzymes and of polyunsaturated fatty acids in lipids (lipid peroxidation). In some cases, long term accumulation of damage from oxidative stress may lead to organism death (90).
In biomedical applications, redox-responsive materials typically use similar design principles to those described in pH-responsive materials that undergo an acid-labile cleavage or degradation. Molecularly, materials are commonly designed with functional groups with multiple oxidation states (e.g., iron, selenium, sulfur) and disulfide, diselenide, and ditellurium linkages (39, 86, 91, 92). Table 2.1 illustrates several common responsive moieties and details their oxidative states.
Table 2.1.
Commonly studied oxidation-reduction responsive moieties and their respective oxidative states. Reprinted by permission from (91) published by Wiley.
| H2O2-responsive materials | Chemical structure and oxidation |
|---|---|
| Part 1: H2O2-induced change | |
| Thioether containing materials | |
| Selenium containing materials | |
| Tellurium containing materials | |
| Phenylboronic acid/ester containing materials |  |
| Part 2; H2O2 induced degradation | |
| Diselenide groups containing materials | |
| Thioketal containing materials | |
| Phenylboronic ester capped materials | |
| Aryl oxalate ester containing materials | |
As shown in Figure 2.5, the ultimate goal of the design is typically to provoke the production of a current, controlled degradation, chain cleavage, or a conformational change in overall material structure (39, 92). This can be accomplished through either a specific bond cleavage or solubility changes at the molecular level. These responses can be useful for both sequestration/release or as bottom-up assembly approaches to nanoscale arrays and protein patterning for biosensors, bioactuators, and miniaturized diagnostic assays. Recent developments in both the manufacturing and application of these responses are reviewed in detail in Chapters 3 and 4.
Figure 2.5.
Illustration of common ROS-responsive systems utilized in biomedical applications as (a) drug delivery vehicles, (b) molecular sensors, (c)electrochromic devices, and (d) mechanical actuators Adapted with permission from (532) published by Royal Society of Chemistry.
2.3. Advanced Materials that Respond to External Signals:
In the previous section, we reviewed diverse materials that respond to changes in the physiological environment (e.g., through protonation state, interaction with solvent, adsorption events, or cleavage of crosslinks). Each of these responsive or intelligent materials transduces a biological or molecular signal into a mechanical (i.e. swelling, polymer chain extension) or conformational (i.e. assembly/disassembly) output.
In the design of next-generation biomaterials for machine-tissue interfaces, it is often productive to apply an external signal (e.g., electrical field, magnetic field, light), which the material transduces into a physical output (e.g., swelling, heat) that is productive for a diagnostic or therapeutic purpose. In the following section, we will review recent developments in biomaterials that respond to temperature, magnetic fields, electrical stimulation, or light. Each of these classes of materials will become useful for fabricating advanced molecular structures (Chapter 3) for machine-tissue interface applications (Chapter 4) in diagnostic and therapeutic medicine.
2.3.1. Temperature Responsive Materials:
Temperature-responsive polymers are an active area of research within biomedical materials, as gradients in temperature can both be natural (e.g., deviation from a normothermia in a disease state) or induced (e.g., ablation therapy)(93). Most commonly, copolymers of n-isopropyl acrylamide (NIPAAm) are applied as thermoresponsive biomaterials, as they possess a lower critical solution temperature (LCST) phase transition near body temperature (LCST ~ 32°C for the homopolymer)(94, 95). Copolymerization with alternate functional monomers can shift the LCST, so that polymer collapse coincides with a slightly hyperthermic condition(96). In addition to NIPAAm copolymers, other alkyl-substituted acrylamides have been studied as LCST thermoresponsive biomaterials (e.g., poly(N’N’-diethyl acrylamide)) (97). Poly(ethylene oxide) and poly(2-oxazoline) copolymers can also exhibit useful temperature-responsive properties, particularly upon copolymerization or end-group functionalization with hydrophobic or ionizable groups (98, 99). Many natural and synthetic polymers that exhibit LSCT-behavior, including polysaccharides (i.e. cellulose, chitosan, and dextran derivatives), polypeptides (gelatin, and elastin like peptides), synthetic homopolymers (alkyl-substituted acrylamides, Polyorganophosphazenes) and synthetic block copolymers (pluronic) (100, 101). In this section, we focus primarily on PNIPAAm gels and hybrid systems thereof to exemplify the fundamental characteristics and biomedical applications of thermoresponsive polymers.
Recent reviews have nicely summarized alternate temperature-responsive LCST hydrogels for biomedical applications(101, 102). The mechanism of swelling and/or collapse for these systems involves their unique polymer-solvent interactions (103). At low temperatures, the entropic energetic cost of holding water molecules in an arranged conformation around the hydrophobic alkyl-containing monomers is not as significant as the enthalpic gain of polymer-solvent interaction (hydrogen bonding between water and pendant amides, as well as functional groups provided by co-monomers). As temperature rises above the LCST, the entropic cost becomes too great, and a collapsed conformation that liberates the previously arranged solvent molecules becomes favorable (Figure 2.6). (103)
Figure 2.6:
P(NIPAAm) copolymers exhibit temperature behavior, where as a polymer solution is heated above the LCST, the polymeric chains collapse from a coil to a globule conformation. This collapse liberates water molecules that had adopted an ordered conformation around the alkyl-substituted amide moieties. Reprinted by permission from (103) published by Elsevier.
In contrasts with LCST-exhibiting biomaterials, a limited number of upper critical solution temperature (UCST) gels have been developed. While LCST gels collapse upon heating through the LCST, UCST materials swell with heating through the critical temperature. UCST behavior is driven by hydrogen bonding between functional moieties in the polymer chain. At temperatures above the UCST, polymer-solvent interaction breaks polymer-polymer hydrogen bonds, enables greater polymer chain mobility, and thus a more disordered polymeric state. Conversely, at temperatures below the UCST, the formation of enthalpically favorable hydrogen bonds between polymer chains achieves the lowest energy state. UCST-exhibiting biomaterials are less common in research applications than LCST gels because they are highly affected by charged impurities in the polymer backbone, or ionic species within biological media(104).
There is vibrant translational research in the area of composite systems containing thermally responsive polymers(93, 105). Such composite systems couple thermoresponsive polymers and conductive materials. For example, p(NIPAAm) copolymer shells can be synthesized on the surface of metal nanoparticles, so that heating the system via an external signal (e.g., light or magnetic stimulation) is transduced mechanically through NIPAAm collapse (106, 107). Specific processing techniques and fabrication processes for composite material systems are reviewed in Chapter 3.1. Biomedical applications of these composite systems, within biosensing and drug delivery, are reviewed in Chapter 4.1 and 4.2.
2.3.2. Electrically and Magnetically Active Materials:
Materials that respond to magnetic or electrical stimulation have found extensive applications in biosensing and tissue engineering applications. Within diagnostic applications, conductive polymers are a valuable component of next-generation sensors, including wearable electronics(108). For tissue engineering and medical device applications, composite systems that contain conductive polymers are useful for delivering electric stimuli to biological components(109).
The most common conductive polymers for biomedical applications are polypyrrole, polythiophene, polyaniline, and poly(3,4-ethylenedioxythiophene)(110). Each of these polymers has a conjugated backbone that can conduct and transduce an electrical stimulus. Because most conductive homopolymers are brittle in nature, composite systems that contain conductive polymer components are most common for proper mechanical function in biomedical applications. To diversify the bioactivity of conductive polymers, numerous research groups have adsorbed, entrapped, or covalently attached active biomolecules to conductive biomaterials for the purpose of tissue integration or drug delivery. Within tissue engineering, conductive polymers have found extensive utility for delivering electrical stimuli to neurons(111) and mimicking the mechanical activity of muscle(112, 113). These types of intelligent and/or bioactive cell-material constructs are a major focus of Chapter 5.
Hybrid biomaterials containing iron oxide nanomaterials are commonly used as magnetic field-responsive systems for sensing, drug delivery, and regenerative medicine. For example, nanomaterials that contain an iron oxide core are being researched extensively for cancer theranostics, to simultaneously enhance contrast for magnetic resonance imaging and delivery a chemotherapeutic payload(106). Within tissue engineering, and bone tissue engineering in particular, hydrogels that contain iron oxide nanomaterials have been utilized to exert physiologically relevant forces on embedded cells to support the adhesion and proliferation of bone marrow stem cells(114).
2.3.3. Photo Responsive Materials:
Photo-responsive materials are categorized by transduction mechanism (i.e. heat, bond alteration, bond cleavage) and application space. Recently, materials that respond to light have found significant research applications in theranostics and tissue engineering(115–117). For example within theranostics, nano or micro-gels containing gold nanomaterials are used to simultaneously image and deliver chemotherapy(118), or image and enable photothermal ablation therapy to a tumor(119). Within tissue engineering, scaffolds with photo-responsive crosslinks facilitate dynamic modulation of mechanical properties(120) and growth factor release(121) to mimic a native tissue environment or promote differentiation to specific cell lineages.
Heating can be therapeutic itself, or can induce a mechanical change in a composite system with a thermally responsive polymer. Gold nanomaterials (e.g., nanoparticles, nanorods, and nanoshells) exhibit localized surface plasmon resonance (LSPR) behavior, where light irradiation at the LSPR wavelength is transduced efficiently and rapidly to heating of the surrounding matrix (e.g., NIPAAm copolymer) or medium (e.g., water, extracellular matrix (ECM), cell cytosol)(122, 123). In ablation therapy, targeted heating of tissue surrounding the gold nanomaterial or nanomaterial-polymer hybrid for the purpose of killing tumors or bacteria via hyperthermia (124, 125). As described above, thermally responsive drug delivery with pNIPAAm copolymers involves heating the gel matrix above the LCST via a nanomaterial transducer, leading to gel collapse and triggered convective release.
Photoinitiated and photomediated chemistries have garnered substantial interest for tissue engineering applications(126, 127). By recent example, DeForest and Tirrell generated PEG scaffolds with NPPOC-photocaged alkoxyamines(128). Upon exposure to light at either 365nm or 740nm (which can be patterned within the scaffold bulk), now the liberated alkoxyamines react with active proteins through an aldehyde-terminated, light-sensitive linker molecule (n-hydroxysuccinimide - o-nitrobenzyl ester - aldehyde) (Figure 2.7). The linker chemistry enables not only photo-triggerable protein release, but also cyclic photo-release and ligation. The sequential retention and release of active proteins could be used to dynamically present cell adhesion molecules, or predictably deliver bioactive factors to modulate cell behavior.
Figure 2.7:
Sequential photosensitive chemistries enable the sequential patterning and release of bioactive proteins from synthetic matrices. Here, a protein is patterned within a gel matrix through a light-activated reaction between a liberated alkoxyamine (in the gel) and a free aldehyde (attached to protein via bioconjugation). Future light irradiation leads to cleavage of the linker, and triggered protein release. This process of protein conjugation and release can be repeated cyclically. Adapted by permission from (128) published by Springer Nature.
Alternately, within tissue engineering, photo-responsive materials can be employed as dynamically stiffening and softening systems. For example hydrogels containing β-cyclodextrin and azobenzene-modified hyaluronic acids exhibit photo-switchable mechanical properties upon irradiation with 420 nm light (forms guest-host pair, stiffening) or irradiation at 365 nm (azo units isomerize to the cis conformation, breaking the guest-host pair, and softening the gel)(129). By alternate approach, alginate matrices have been engineered with photo-dynamic mechanical properties through co-encapsulation of calcium chloride or diethylenetriaminepentaacetic acid (DTPA)-containing liposome-gold nanorod hybrids. Release of calcium chloride (via laser irradiation at 808nm, causing heat-induced gel-to-fluid phase transition of the liposome) stiffened the gels, while the converse DTPA-system softened the alginate matrix (by chelating calcium ions)(130).
As shown through these illustrative examples, photo-responsive material systems have a high degree of spatial tunability, particularly for directing the loading and release of biomarkers or adjusting mechanical properties. This makes light-sensitive materials, and advanced material systems containing photo-responsive moieties, promising candidates as components of next-generation cell-laden or bioactive devices.
2.4. Materials that respond to biological molecules:
One of the many unique characteristics of biological systems is their dynamic presentation of specific and active molecules. In particular, the expression level of an array of proteins characterizes the transition between physiological environments at the tissue, cellular, and subcellular levels(131). Protein biomarkers are as diverse as they are numerous, range widely in molecular weight and isoelectric point, and can be freely soluble or associated with membrane structures. These proteins, in addition to performing necessary functions for the biological host, present an array of markers that clinicians, scientists, and engineers can exploit for diagnostic or therapeutic purposes(132).
In addition to protein expression and presentation, living systems rely on numerous small molecules, namely sugars, steroids, nucleotides, lipids, vitamins, salts, and peptides. These molecules perform critical functions in their own right, regulating the body’s energy balance, altering cellular and organelle membrane properties, and mediating communication between cells, to name a few. From a medical and engineering perspective, these molecules are also immensely useful as they provide insight into a patients’ state in transient or cyclical processes (i.e. circadian rhythm, metabolism)(133). More so, deviation of cytokine levels from normal concentrations can indicate the need for therapeutic intervention (134). Therefore, materials that recognize cytokine levels and transduce a useful output (i.e. mechanical, electrical) can have extensive utility as components of therapeutic systems and medical devices.
Material systems that facilitate communication of devices and tissue must act as functional biosensors. In the following sections, we highlight recent progress in the detection and transduction of biological signals with material systems.
2.4.1. Polymer-Based Bio-Recognition Elements:
When designing a material-based biosensor, it is critical to determine the sensitivity and selectivity that is necessary for proper function. The ideal biosensor would be entirely specific (i.e. binds the desired analyte, and only that analyte) and highly sensitive (i.e. small changes in the analyte level lead to the transduction of a significant signal). Such a system, while ideal, is neither possible nor necessary for biomedical engineering applications. Instead, in the design of material systems that interface with biological systems, bioengineers must balance tradeoffs in sensitivity and selectivity with other factors such as cost, stability during manufacture, storage, and usage, and time scale of signal transduction(132).
In Chapter 2.2, we reviewed a pH biosensor in the simplest sense. Polymers with pendant acidic or basic moieties, after all, can exchange protons in an environmentally dependent manner, resulting in the transduction of a mechanical signal (gel swelling). Similar polymers with charged moieties can attract ionic species in a diagnostic assay in vitro or for continual monitoring in vivo, but suffer from significant cross-reactivity due to the numerous competing ionic species in biological fluids, as well as the inherent non-specificity of columbic interactions. From a polymer engineering point-of-view, these charged polymers can be immensely useful in spite of their cross-reactivity(135). For example, crosslinked polymers with a range of mesh sizes and a multitude of ionic species with differing dissociation constants will exhibit diverse size exclusion and affinity characteristics as a function of buffer and analyte properties. This concept is essential to size-exclusion and affinity chromatography, and has recently emerged in the biomedical domain, particularly in differential sensing for diagnostic purposes(136).
In a typical free radical polymerization, monomer identity and synthesis conditions dictate the incorporation and orientation molecules within the final material. Controlled or living polymerization techniques are continually increasing in sophistication, and can generate uniform or block structures with consistent molecular weight distributions. Alternatively, sequential click reactions can reliably generate repeating polymers that mimic repeating nucleic acid sequences. Inclusion of biomolecule template molecules (molecular imprinting) or can impart some unique supramolecular structure within network polymers, and porogens can serve to enhance their porosity to enable ligand diffusion and adsorption(137, 138). These advances in polymer materials science are significant, and have utility within and beyond the medical device industry. However, the current state-of-the-art in synthetic materials for molecular recognition is limited in compared to that achieved by biological systems. Nature has mastered the art of generating complex molecules with reproducible and specific 3-D structure, achieving the multiplicity of intermolecular interactions necessary for specific affinity described above. Therefore, molecular engineering and synthetic biology approaches have a key place in the development of next-generation devices that productively and specifically interface with human tissue.
2.4.2. Hybrid Systems for Molecular Recognition:
A repertoire of approaches are available for scientists and engineers that want to leverage the synthetic precision of biological systems for medical applications. One particularly relevant example of molecular engineering is the generation of therapeutic proteins and antibodies.
Therapeutic antibodies have become increasingly prevalent in the pharmaceutical space for the treatment of a number of these conditions including cancer, arthritis, and autoimmune disease(139). Rational design with synthetic biology approaches allow us to achieve desirable pharmacokinetics, optimize in vivo bioactivity, and minimize adverse immune responses for patients. Therapeutic antibodies have specific bioactivity, which ranges from neutralizing undesirable cytokines, to inhibiting the function of membrane-bound proteins, and enabling antibody-mediated cell dependent cytotoxicity. A binding event mediates each of these specific in vivo functions, and all of the molecular interactions discussed previously play an important role. Many years of success, both in the laboratory and the clinic, have demonstrated that the specific functions of therapeutic antibodies are capable of productively intertwining with those of a patients’ living system, correcting chemical imbalances(140) or augmenting natural surveillance processes(141).
There is a great deal of synergy between intelligent polymers and biological molecules for molecular recognition. Enzymes and antibodies function optimally in a narrow range of physical conditions (i.e. temperature, pH, ionic strength), while synthetic biomaterials are typically environmentally robust. Our ability to specify the sequence of synthetic materials at the molecular level to engage in multiple complementary intermolecular attractions with biological analytes is rather limited, while natural ligands have a specific 3D structure determined by natural selection. Consequently, a logical conjecture that has manifested in next-generation biomaterials are hybrid materials of natural and synthetic origin for dynamic and intelligent systems.
A key characteristic of hybrid systems is the method with which the synthetic and natural materials are integrated. The application of interest, as well as nature of the system, largely determine the integration strategy. Macromolecules can be physically incorporated within a synthetic matrix through equilibrium partitioning, adsorption, or encapsulation. Physical incorporation has multiple advantages, namely maintenance of the macromolecular structure and avoidance of bioactivity alteration during conjugation reactions. However, a potential shortcoming of these systems is insufficient retention of the natural molecules. In contrast to physical incorporation, another common strategy is to conjugate macromolecules to biomaterials.
Biomacromolecules such as nucleic acids and proteins naturally possess functional groups that are amenable to conjugation reactions. While bioconjugation through pendant thiols (cysteine) and amines (n-terminal and lysine residues) is most prevalent, reactions with carboxylic acid (aspartic and glutamic acid, c-terminus) are also possible (142, 143). Typically, a short spacer enables mobility of the biomacromolecule on the surface or within the bulk of the final biomaterial. While bifunctional PEG spacers are most common due to their versatility, availability, and biocompatibility, multi-armed spacers allowing the conjugation of multiple ligands and non-PEG linkers are also available. These non-PEG linkers, which can be natural or synthetic in origin, in contrast to PEG are frequently biodegradable and can be especially useful for responsive ligand revelation or shedding (144).
2.4.3. Polymers that Recognize and Respond to Enzymes:
Using the bioconjugation reactions described above, a common biomaterials strategy is to immobilize active proteins onto the surface, or within the bulk, of an otherwise inert material. These biomacromolecules can subsequently engage extracellular receptors on the surface of target cells, or perform their native catalytic function within the gel microenvironment. These bioconjugate material-protein hybrids are particularly popular within drug delivery research in the treatment of cancer and diabetes, respectively.
Enzymatically active hydrogels have garnered substantial interest within the field of intelligent hydrogels, particularly for their applications in drug delivery. For example, Knipe et al. recently developed trypsin-labile networks of poly(methacrylic acid-co-N-vinyl pyrrolidone) (P(MAA-co-NVP) with peptide crosslinkers for oral delivery applications. Upon swelling in simulated intestinal fluid, the peptide crosslinkers were exposed and trypsin, which was naturally present, was able to penetrate and cleave crosslinks(145). The advantage of using an enzymatically responsive system, in this case, was that the degradation behavior was specific for trypsin and was generally unresponsive to alternate proteolytic enzymes such as pepsin. This responsiveness was much more specific than degradation and/or dissolution in response to general environmental parameters such as pH or temperature (146).
Biodegradable nanogels with enzyme-labile crosslinks are also popular as targeted agents for cancer nanotherapeutics. Matrix metalloproteinase (MMP)-labile peptides, used within cancer pro-drugs or targeted nanoparticles, exhibit preferential degradation in tumors due to upregulated MMP activity. In a recent example, Jiang et al. used a peptide that was broken down by MMP-2 or MMP-9 (sequence: XPLGLAG) as a part of a theranostic construct for cancer. The construct was taken up 3.1 times greater by fibrosarcoma cells in vivo, relative to control peptide linked nanoparticles (sequence scrambled) (147).
2.4.4. Bioactive Polymeric Scaffolds:
Bioactive scaffolds enable the adhesion, infiltration, and/or proliferation of cells. Advanced bioactive scaffolds may also, possess macroporous architecture, have dynamic mechanical properties, facilitate cellular alignment, deliver growth factors to drive differentiation processes, or degrade in response to cellular cues (23).
Scientists and engineers have used to facilitate cell adhesion to biomaterials for purposes ranging from promoting implant integration to fabricating cell-laden bioactuators. Certain natural polypeptide materials (e.g. fibrin, elastin, collagen) and polysaccharides (e.g. hyaluronic acid, chitosan, alginate) naturally facilitate cell adhesion by presenting adhesion ligands (148). Other synthetic biomaterials (e.g. polyethylene or silicone) promote the nonspecific adsorption of serum proteins (e.g. fibronectin, vitronectin, laminin), which offer adhesive sequences (149). A final, alternate approach is to assemble a coating, on a material or surface, comprised of native ECM. Cells secrete ECM, or it can be purchased commercially (e.g. matrigel, cultrex) (150, 151).
As a hybrid material approach, one can instead synthesize polymeric gels that contain peptide sequences derived from ECM proteins. The most common example of this approach is conjugating otherwise inert gels with RGD peptides, which subsequently engage integrins on the cell surface (152). Other adhesion peptides, derived from ECM proteins, have also been identified (149). Lithographic approaches, as will be discussed in the next chapter, are particularly useful for ligand patterning, which can direct specific patterns of cell adhesion on a substrate.
Polymeric scaffolds will possess differential affinity for proteins that are native to the physiological environment (adsorption) or pre-loaded by partitioning. Therefore, they can be used to sequester and delivery modulatory factors that influence cell behavior. Physical cues, such as stiffness or topography, also alter cell activity and can be tuned to optimize cell alignment, differentiation, and/or proliferation (23).
2.4.5. Accelerating or Simulating Natural Selection for Ligand Identification:
Numerous laboratory-scale procedures are available to researchers for identifying ligands that possess specific affinity characteristics. Two of the most popular options are the Systematic Evolution of Ligands by Exponential Enrichment (SELEX) process for aptamer generation and phage display for peptide epitope selection. In each case, nucleic acids or peptides from a random library are screened for desired bioactivity and are cyclically reproduced and amplified until a limited number of high affinity ligand options remain. In this section, we review the novelty of each method, along with some key applications in biomaterial design.
Two independent groups first introduced SELEX in 1990, and the process can be used to generate single stranded DNA (ssDNA) or RNA aptamers(153, 154). As illustrated in Figure 2.6, a random library of aptamers is screened by depleting non-specific aptamers through an initial incubation step with competing molecules bound to a matrix, prior to incubation with the target analyte. This target analyte could be a soluble protein (e.g., VEGF), a material surface, or specific cells (e.g., tumors). Following the binding step, the aptamers are eluted using aqueous buffers that contain salts that outcompete aptamer-analyte interactions. Following elution, a polymerase chain reaction (PCR) or reverse transcription-PCR (RT-PCR) are used for DNA and RNA aptamers respectively to amplify aptamers that bound specifically. Following several cycles of SELEX, researchers can isolate a limited number of promising aptamers, as they will increase in prevalence in the amplified library(155).
Phage display, invented in 1985, is a biochemical technique similar in concept to SELEX, which identifies promising peptide ligands for a variety of applications(156). In phage display, engineered bacteriophages express a random epitope library (typically 7 or 12 amino acids in length) within their coat protein. Following screening against competitive and target analytes, high-affinity phages are amplified by a bacterial host (E. coli) and returned for subsequent cycles of screening. While phage display can be employed to screen random libraries for high-affinity peptide ligands, another major application of the technique is to engineer phages to express antibody libraries or protein epitopes(157). These custom phage libraries can be immensely useful in the evolution of therapeutic antibodies, as described in the previous section, or as a research tool to understand protein function and inhibition. In vivo techniques have also been developed to leverage phage display technology to identify suitable homing or targeting peptides for drug delivery devices(158).
Both phage display and SELEX can be simulated in silico for rapid, low-cost screening. To date, these simulations have been used to provide insight into the docked conformation of peptides and/or aptamers with protein surfaces, as well as to inform the identity of libraries for experimental screening(159).
In the previous sections, we discussed the fundamental principles underlying intermolecular attraction and highlighted some examples of how both synthetic materials and natural elements are capable of molecular recognition. Both SELEX and phage display are useful techniques when, as the scientist or engineer, you are aware of a desired ligand property but not of the existence of a suitable molecule. In the final sections of this chapter, we will highlight how natural molecules – existing and to be identified in the future – are incorporated into intelligent material systems to interface with biological systems in a predictable and productive manner.
2.5. Medical Device Applications of Hybrid Materials:
We have reviewed the extensive repertoire of intelligent and responsive biomedical materials that are available to scientists and engineers when designing novel tissue-material interfaces. These materials can respond reversibly or irreversibly to a physiological cue, transduce biological signals into electrical or mechanical outputs, and select target biomarkers from complex solutions. We particularly emphasized the fundamental relationships that explain the behavior of biomaterials and biomaterial-biomolecule systems in a various media.
It is rarely sufficient, when designing machine-tissue material interfaces, to capture a single responsive or recognitive behavior. Rather, systems that perform multiple functions are more typical.
By example, a drug delivery carrier for cancer treatment must have the proper surface chemistry and recognitive properties to evade immune recognition and accumulate in tumors. The carrier must have a loading and release mechanism to encapsulate therapeutic cargo and expel the drugs in the tumor cell cytoplasm. How can we achieve such complex behavior, which includes aspects of molecular recognition, spatial constraint, and environmental response?
In an alternate application, we want to generate scaffolds that will facilitate the growth of cardiomyocytes to make a device that mimics the bioactivity of cardiac muscle. The ideal material for this case must enable the specific spatial adhesion of cardiomyocytes, aid in synchronous cellular contractions via an electrical stimulus, and degrade in a cell-responsive manner with reproducible kinetics. How can we fabricate such hybrid systems that respond to diverse external cues and produce necessary anisotropic behavior?
Chapter 3 will address these fundamental design and fabrication questions. Each section will highlight a different biofabrication technique, which scientists and engineers have invented to produce materials with the necessary supramolecular structure, spatial resolution, or dynamic composition for advanced and productive device-tissue interfaces.
3. Advanced Fabrication of Bio-Hybrid Interfaces
3.1. Self-Assembling Structures
Self-assembly is a process where molecules align or aggregate in a well-defined manner. Self-assembly is important within biology and materials science for forming micro and macro-structures from vesicles to molecular machines (160). Moreover, fundamental concepts within biology, such as the stability and molecular dynamics of lipid membranes, reflect the vitality of self-assembly in bioprocesses. The research applications and commercial products based on self-assembled structures are diverse and rapidly increasing. Contemporary applications of self-assembly in medical research include the development of novel nanostructures (e.g. those with self-healing, multicomponent, biomimetic properties) and soft robotics (161–166).
Self-assembly involves multiple molecular features (e.g. shape complementarity) and non-covalent interactions that enable alignment into a well-defined structure (167). Surface properties, forces (e.g. capillary, shear, centrifugal, electrostatic), and external fields (e.g. electric, magnetic) affect self-assembly at the nano- and mesoscales (168, 169). Engineering principles for micro- and nano-fabrication and their respective applications to biology can be learned by understanding molecular self-assembly phenomena in nature. Some work has already been done in this direction and numerous self-assembling systems have already been developed for various applications in tissue engineering, medicine, and biology (170). These range from block copolymers biomaterials, to scaffolds for 3D cell culture, DNA-based structures, and models to study protein folding.
3.1.1. Fundamentals of Self-Assembly
Self-assembly has shown immense potential in modern molecular, biologic, and material sciences. The spontaneous assembly of simple building blocks into complex nano- and micro-sized structures underpins major developmental progress in a wide range of technologies that are at the forefront of biomedical engineering. For this review, we will be looking at self-assembly from the perspective of its applications in the biomedical field.
Self-assembly implies spontaneity that causes a structure to be built into an ordered whole from its disordered units. In this assembly, there might be involvement of external energy, depending upon the scale of the self-assembly and the forces that are at play. Generally, the interactions that drive units together to form a self-assembled structure are different from the stronger bonding forces within the units themselves (i.e. covalent bonds). The shape, size, and pattern of individual units is what dictates a self-assembly process, making precise control over these parameters a prime design goal. It is imperative to synthesis building blocks (i.e. block copolymers, polypeptides, or lipids) with accurate dimensions so that they will form integrated chemical, biological or physical self-assembled structures and properly perform a desired function (171). Electrostatic, hydrophobic, hydrophilic, or other chemical interactions define the surface properties of the material itself.
Depending on the level and scale of self-assembly, energy will dissipate from, or be input from and external source, to the system. This gives rise to two types of self-assembly: static and dynamic. Static self-assembly generally happens spontaneously. The formation of a static ordered state may, very rarely, require energy input in different forms such as stirring, or sonication (160).
Static self-assembled systems are at local or global equilibria, resulting in stable, ordered structures (164). Examples of static self-assembled structures include chemical crystals, self-assembled monolayers, globular proteins, and some nanomaterials. Dynamic assembly requires external influences to prevail and adjust to the local or global environment. This external input maintains an energetic minimum by adding energy into the system. The integrity of such a system is subservient to the energy that flows into the system. The dynamic self-assembly system ceases to exist in the absence of external energy. Examples include smaller assembly systems like some chemical reactions and bacterial colonies all the way up to weather patterns, solar systems, and galaxies (160).
The self-assembly process can be roughly subdivided into three more categories, which apply to both static and dynamic self-assembly: co-assembly, directed self-assembly, and hierarchical self-assembly (Figure 3.1).
Figure 3.1.
Graphical rendition of static and dynamic self-assembly and how they relate to co-assembly, hierarchical assembly, and directed assembly. Reprinted with permission from (533) published by Wiley.
Co-assembly is defined as a situation within a single system when different building blocks form a synergistic structure that would not have been otherwise possible by self-assembly of the individual blocks. An example of this is surfactant micelle templating of individual silicate blocks that leads to the formation of periodic mesoporous silica (172). When a single building block organizes in such a way that the resulting structure exists at multiple length scales, the process of such an assembly is called hierarchical.
In the hierarchical assembly, the initial building blocks form what is referred to as a ‘first order’ assembly, which in turn combines with similar assemblies to give rise to a larger ‘second order’ structure, and so on (164). Living systems are a perfect example of hierarchical assembly; organelles build cells; cells build tissues; tissues build organs; then organ systems and finally an organism. Eventually, organisms may form further higher orders as families and colonies.
An example of an artificial hierarchical system is mesostructured siloxane-organic hybrid films with ordered macropores by templated self-assembly (173). A recent study used polystyrene opal films as a template. Siloxane precursors bearing alkyl chains self-assembled, giving rise to a two-dimensional (2D) hexagonal structure or a lamellar structure. Alkyl chain length (n=10 or n=16) of the precursors dictated whether a 2D hexagonal or a lamellar structure is formed. In both of the cases, the mesostructures oriented along the spherical surface of the template and template removal did not result in structural loss. Upon calcination, the 2D hexagonal hybrid produced ordered porous silica with both macro- and micro-porosities. The lamellar hybrid film, on the other hand, exhibited a unique property of pocketing alcohols with an expansion of the interlayer spacing.
When any self-assembly is directed by external forces that have been placed voluntarily, the process is called directed assembly. An example of a directed assembly is the self-assembly of colloids from a solution on a substrate with the help of a lithographic pattern to drive the process (174).
One classification of self-assembly, that takes into account most or all of the previously discussed processes, is stimuli-responsive self-assembly. Stimuli responsive materials have been at the center of immense research interest over the past decade (166, 175–179) Stimulus responsive nanostructures arrange, organize, or disassemble in adaptation or reaction to the surrounding environment. Depending on the desired application, these nanostructures may regulate molecular transport, release biomolecules, affect material surface properties, change their wettability and adhesion, or transduce a biochemical signal (i.e. into optical, thermal, mechanical, or electrical signals and vice-versa).
The applications of self-assembly are far and wide, from molecular electronics to smart materials and drug delivery devices. The ability to control and exploit the properties of materials that are built through self-assembly requires a proper knowledge of thermodynamics, as structures are formed from monomers that are held together by forces ranging from hydrophobic interactions to van der Waal’s forces and hydrogen bonding. In the next section, we will briefly discuss the thermodynamic basis of self-assembly.
3.1.2. Thermodynamics
As discussed in the previous section, self-assembly of molecular structures into much bigger colloidal or nano- and macro-sized aggregates has many technical applications and as such the product base of such materials is ever increasing. In fundamental life processes, self-assembly is at display at many levels (e.g. lipid bilayer membranes around cells and organelles, interactions of proteins with each other to form protein complexes or with DNA and RNA to drive gene expression, replication, and protein synthesis). In this part of the chapter, we will discuss important aspects of thermodynamics that drive these molecular entities to self-assemble into more complex motifs or machines.
Since self-assembly is a process that builds on both attractive and repulsive forces between its constituents, it makes a more logical argument to discuss the thermodynamic fundamentals of those molecules that contain both hydrophobic as well as hydrophilic parts – amphiphilic molecules. Such molecules are technically and biologically important. In the process of assembling molecules, there is an increase in the order of the system, which is not favorable from an entropic point of view. In the absence of driving forces or additional interactions, molecules that interact favorably with a solvent will dissolve as a single phase. In contrast, a sufficiently strong enthalpy of association will overcome the unfavorable entropy and result in spontaneous self-assembly.
Surfactants consist of a hydrophilic head and a hydrophobic tail. The head either is charged (i.e. ionic moieties) or uncharged (i.e. non-ionic surfactants). In an ideal case, mixing N amphiphilic molecules with NS solvent molecules into a single phase, the system entropy is given by this general expression, which describes the mixing of differential solvent and solute volumes (180, 181):
where kb is the Boltzmann’s constant, and υ1 and υ2 are the solvent and solute volume fractions, respectively.
For the self-assembly of a single structure, N amphiphilic molecules assemble into an aggregate or layer. In such situations, there is a particular order in which the individual amphiphilic molecules assemble, resulting in a considerable change in the entropy of the system. The entropy of mixing resulting from self-assembly into a single structure can be obtained from the expression;
where υ2,SA is the volume or mole fraction of the single aggregate. The entropy change for such a process is obtained by the difference of the two expressions, giving:
Generally, the volume fractions of the single aggregate and the starting fraction of the single amphiphilic molecules are in the same order of magnitude so that the entropy change is approximated to:
The above equation is valid when only a single molecular species is involved in the formation of a single self-assembled structure. For the case where N≫ 1, ΔSSA will be less than zero, meaning a reduction in the overall entropy and hence an ordered structure. What this implies is that the molecules that can interact to a certain level with the solvent will not form any assembly and instead remain as free molecules as is the case in an ideal solution. Additionally, it is quite clear from the above equation that the entropic driving force of dissociating molecules will increase as the solute concentration (υ2) increases. It is this entropic contribution that is of utmost importance in determining the equilibrium of the self-assembled interfaces.
Since we are discussing amphiphilic molecules, the hydrophobic effect together with some contributing forces to the micelle formation can be represented as a single free energy parameter ΔGt (182). In a thermodynamic sense, a spontaneous self-assembly process can be considered a set of reactions, one for each value of N, meaning:
The free energy of forming a micelle will be NΔGt and the entropically unfavorable free energy of the self-assembling amphiphilic molecules will be
Which will be greater than zero. T is the absolute temperature. The total free energy ΔGf of all the processes can then be written as the addition of the two free energies, i.e.
What follows from this expression is that ΔGt < 0 and is a necessary condition for the process to overcome the unfavorable entropy of self-assembly (ΔSSA < 0). The above equation can now be written as:
The equilibrium conditions can be written by simplifying the expression for total free energy.
This expression is an important thermodynamic relationship that defines the spontaneous and reversible self-assembly process in case of an amphiphilic entity. Thermodynamics lays down the guidelines under which self-assembly can take place.
In this section, we discussed self-assembly and the various factors that affect it. Thermodynamic guidelines help in designing new materials that have the tendency to self-assemble into different moieties of interest. More research into the field will provide us further insights into the nuances that go into the science and ‘art’ of self-assembly and thus provide us better materials with unique qualities tailored for materials and biologic research. In the next section, we discuss some of the self-assembly structures of interest in the biomedical field.
3.1.3. Peptide and Lipid Self-Assembly
Different molecules of natural or synthetic origin have been used for biomedical advancements through the exploitation of their tendency to self-assemble. In this section, we will shed some light on two important classes of such materials, peptides and lipids.
Peptide self-assembly: In the previous section, we talked about the thermodynamics of self-assembly of amphiphilic molecules. The basic unit of peptides and proteins is an amino acid, which can have a positive, negative, polar, non-polar, or aromatic pendant group. These molecular characteristics, and the interactions formed between them, are what make the amino acid form assemblies, such as α-helices, β-sheets, and protein complexes. Synergistic non-covalent interactions, including but not limited to, hydrophobic, electrostatic, hydrogen bonding, and van der Waals interactions drive peptide self-assembly.
A large body of research focusses on fabricating self-assembling peptides and proteins for a variety of biomaterials applications. The most important advantages for self-assembling peptides include availability and cost-effectiveness. Constituent polypeptides can be synthesized in the lab. Amino acids are flexible in structural programmability and have versatile functionality (183, 184). Peptides, and peptide-based self-assembled constructs, can be precisely tuned to respond to temperature, ionic environments, pH, differential concentration of metabolites, and the solvent environment (185–188).
Given these advantages, peptide self-assembly has been used for creating various architectures (Figure 3.2a) including peptide nanoparticles, nanotubes, fibers, and hydrogels (189). The fact that structural assemblies from nanotubes to fibers existing at different length scales can be made puts self-assembled peptides in a sweet spot for creating complex functional systems or devices for different applications (e.g. nanotechnology and energy) (165, 190, 191). This means such devices can be used not only at the cellular level but also at organ system levels and beyond.
Figure 3.2.
Peptide (a) and lipid (b) self-assembly into different nanostructures. Reprinted with permission from (534) and (535) published by the Royal Society of Chemistry and Elsevier, respectively.
Peptide self-assembly into nano-sized structures is generally achieved through three methods: solid phase peptide synthesis, ring opening polymerization, and protein engineering (192). While solid-phase synthesis is used for the high precision synthesis of peptides, the limitation of this method is that only very small peptides with less than 100 amino acid units can be synthesized (193). The peptides are synthesized by conjugating individual amino acids using standard and well-established peptide synthesis methods utilizing tert-butyloxycarbonyl (t-Boc) and 9-fluorenylmethoxycarbonyl (Fmoc) protecting groups (194, 195). This method is used for the synthesis of those peptides which cannot otherwise be synthesized in bacterial or yeast systems. For the synthesis of larger peptides and proteins, protein engineering has been used (196, 197). On an industrial scale, ring-opening polymerization is the method of choice, where cyclic monomers are introduced to the end of the synthesized amino acid sequences to form a longer peptide (192). Self-assembly of different types of peptides leads to the formation of aromatic dipeptides, amphiphilic peptides, polypeptides, and amyloid-relevant peptides (189).
Many peptide self-assembly structures have been used for drug delivery and vaccine development in the form of injectable hydrogels (198, 199). Some of these assemblies have been used to trap anticancer drugs to be delivered at tumor sites (200–203). Nanotubes from self-assembled peptides have also been used for gene-drug delivery through the transformation of nanotubes to nanovesicles during the endocytosis process (204). Many gene-peptide conjugate self-assembled nanostructures have already been developed for effective gene-drug delivery systems with minimal side-effects (163). Peptides have also shown a lot of promise in controlled drug delivery for other disease conditions through stimuli-responsive properties (198).
The other self-assembly structures that have found immense applications are self-assembled monolayers (SAMs), lipid-based vesicles, and micelles. In the year 1980, the formation of organo-silicon monolayers was studied on hydroxylated silicon oxide surfaces (205). A couple of years later, gold surfaces were used as a substrate for alkyl disulfides to form close-packed monolayers of chemisorbed alkane-thiolate molecules (206). These two studies laid the foundation of SAMs and are still considered the prototypical SAM models. They are among the simplest forms of self-assembled materials. SAMs are generally formed from small amphiphilic aliphatic organic molecules through the interaction of intermolecular weak forces (207). The assembly is mainly driven by adsorption on different liquid or solid surfaces by the segregation of molecules (208). This association is thermodynamically favored and results in a phase boundary on the surface of the substrate on which the SAM is formed (209). The best-defined materials that are currently available for research and study are the SAMs formed by various sulfur-based organic compounds, especially alkanethiols, on metal surfaces such as those formed by gold, silver, palladium, copper, and other metals (210). The interest in these materials has increased greatly in recent years as a direct response to the needs of technology. Preventing corrosion, controlling adhesion at interfaces, developing alternate approaches for manufacturing electronic devices through micropatterning, or studying molecular recognition for different biologic applications through selective adsorption or avoidance of proteins or lipids to the surface are some of the applications of the SAMs (211, 212). These are just some of the diverse interests which have stimulated significant research into this field (213).
Due to the advances that have been made in nanotechnology, especially in the development of tools for their characterization, it has been possible to understand not only the mechanisms leading to SAM formation but also better understand the correlation between their structure and their properties. Major insights have been made in the study of the kinetics of SAM formation, along with the necessary conditions for 2D long-range order to be achieved from very small individual one-dimensional molecular constituents (207, 214, 215). Parameters that control self-assembly include chain length, concentration and nature of the SAM-forming monomers, temperature, solvent, the incubation time, and the preparation of the substrate surface (207).
As mentioned before, SAMs have applications from electronics to biotechnology. Discussions on their applications in the semiconductor and electronics (213) industry are beyond the scope of this review. SAMs are actively used in nanotechnology, and for biomedical applications. They are used to promote selective interactions (adherence or the lack of it) of proteins and cell membranes, and thus cells, at a surface or a phase boundary (211, 212). Such characteristics of SAMs have been exploited in bioelectronics through tethering of a biomolecule to a semi-conductive substrate, leading to a change in potential difference or electric current (216). Additionally, SAMs have been specifically made with antifouling properties that could be used for biomedical devices such as contact lenses, prostheses, implantable devices, and catheters (217–221). Similar applications have been reported for cell biology (222), tissue engineering (223), drug delivery (224), and microfluidics (225). SAMs have also found tremendous application in various lithography techniques for patterning micro- and nanostructures. Among the most famous of these techniques are dip-pen (226), constructive (227), and micro-contact printing (228). In the dip-pen technique, an AFM tip is coated with a material that can form a SAM. This material is then released to the substrate by diffusion of the molecules through the water meniscus formed between the AFM tip and the substrate. Constructive lithography is similar to dip-pen in the sense that both of these techniques build on AFM. In this method, the terminal group of a SAM-forming molecule on native silicon is patterned by means of a conductive stamp. The nature of the terminal group determines whether a local anodic oxidation or a cathodic reduction is preferred, and this reaction is completed by flowing a current across the stamp/SAM/silicon junction. Micro-contact printing is different from the other two techniques. It relies on a poly(dimethylsiloxane) (PDMS) stamp that contains SAM-forming molecules. When the stamp contacts a metal substrate that has a metal film (i.e. gold), the SAM rapidly forms at the substrate-stamp contact. As a result, there is a positive transfer of the stamp pattern to the surface.
SAMs represent the bottom-up approach of nanotechnology for the ability to be built up from singular moieties into architectures that are integrated at multiple length scales. Because of their ease of processing and modular design, they are versatile. Their ability to affect properties at interfacial surfaces imparts them a unique robustness for very rare and important applications (213).
Lipid-based self-assembly:
Another important class of molecules for self-assembly applications is lipids. In addition to proteins, lipids form basic building blocks in every living organism. The fact that cell membranes are comprised of self-assembled phospholipid molecules positions them at the center of physical interactions with other cells or materials. This imparts their utilization for pharmaceutical and biomedical applications a certain advantage from a biocompatibility and bio-adaptability point of view (229). They are inherently amphiphilic and can be synthesized artificially as well (230).
A well-known example is liposomes (lipids arranged in lamellar structures) which, together with other lipid-based structures such as exosomes and micelles (Figure 3.2b), have been extensively used for drug delivery, diagnostic, and theranostic purposes (231–234). In addition to these applications, they also provide a medium for studying basic biological phenomena such as the flip-flop motion within lipid bilayers (235) or membrane fusion (233, 236, 237).
Use of natural or artificial lipid-based self-assembled structures has led to the mimicry of various biologically important functions such as proton gradient formation (238), ion-channeling (239), and photosynthetic processes (240). Liposomes can be synthesized via various methods, such as evaporation of the organic solvent of a lipid solution, dispersion of lipids in an aqueous medium, sonication, and mechanical extrusion (231). Lipids can also be used to synthesize bigger and more-complex structures through layer-by-layer assembly (241).
Other artificial systems such as vesicle-based rechargeable batteries have also been proposed (242). Based on the number of bilayers and size, liposomes are mainly classified into two categories: (a) multilamellar vesicles (MLV) and (b) unilamellar vesicles. Unilamellar vesicles can also be classified into two categories: (a) large unilamellar vesicles (LUV) and (b) small unilamellar vesicles (SUV) (243). There are additional examples of unique mesophasic structures of lipids formed as a result of lipid polymorphisms, which include micellar, cubic-, hexagonal- or sponge like structures (172, 244, 245). These structures provide the advantages of stability and production feasibility similar to those of liposomes. Lipids also exist as supramolecular nanostructures for different applications in medicine. Cubosomes, which exist in a cubic structure, have improved stability, bioadhesive capacity and biocompatibility (246). Similarly, hexagonal phases or hexosomes, as the name suggests, exhibit hexagonal arrangements of constituent lipids and can be used in drug delivery through encapsulation of different drugs with high stability (247). Apart from these structures, lipids also form tubules and ribbons that are utilized in different biomedical applications (248).
Immune stimulating complexes are nanocage-like structures have been utilized for gene, vaccine and drug delivery (249). Similar to liposomes, another class of lipid self-assembly is micelles. They are nanoscopic core/shell structures formed by amphiphilic lipids or block copolymers (250). Similar to liposomes, micelles have been particularly useful in drug delivery applications (234). They are formed when the concentration of amphiphilic molecules reaches a threshold, referred to as the critical micelle concentration (CMC), resulting in a change in surface tension, thereby triggering self-assembly of the amphiphilic monomer constituents (251).
Another important class of self-assembled lipid vesicles is exosomes. They are cell-derived vesicles that are present in many, perhaps all, eukaryotic fluids, including urine, blood, and spent medium of cell cultures (252). Exosomes have garnered a lot of research attention lately and are being recognized as potential therapeutics due to their ability to trigger potent cellular responses in vitro and in vivo (253, 254). Exosomes are unique compared to liposomes or micelles as they contain various molecular constituents of their cell of origin, including proteins, RNA, cell-surface markers, ion channels, and membrane proteins which they use to mediate regenerative outcomes in injury and disease that recapitulate observed bioactivity of stem cell populations (254, 255).
As the research on lipid-based self-assembly continues to grow, the structures that have already come to the fore have shown enormous potential as dynamic materials, ranging from artificial lipid membranes, liposomes, and vesicles, to exosomes and cell membranes. Their applications span from controlled drug delivery to regenerative medicine to biosensing, and such work has resulted in numerous pharmaceutical formulations and novel food products.
3.1.4. Nanomaterials
With the latest developments in the field of nanotechnology, its applications have expanded to electronics, catalysis, medicine, and more (256–258). With the tremendous applications they offer in manipulating at a molecular level, they have evoked a great amount of attention for improving disease prevention, diagnosis, and treatment (257). Nanomaterials in medicine and carbon-based materials are discussed in detail elsewhere (257, 259). As such, this section will not be exhaustive and will give instead a general overview of nanomaterials and their various biomedical applications.
Nanomaterials can be made from a variety of materials including polymers, metals, organic matter, lipids, ceramics, and composites thereof. Nanomaterials include nanoparticles, nanocrystals, nanotubes, nanowires, nanorods, nanoclusters, nanofibers, nanofilms, etc. Various top-down and bottom-up nanofabrication technologies have been used for the synthesis of different types of nanomaterials. These include, but are not limited to, chemical reduction in aqueous media, emulsification, phase separation, electrospinning, thin film and chemical vapor deposition, chemical etching, nano-imprinting, and photo- and electron beam lithography (260–263). Nanomaterial properties such as increased surface to volume ratio and enhanced surface roughness lead to superior physiochemical properties (e.g. increased mechanical strength, enhanced electrical and magnetic properties, non-linear optical effects, increased catalysis, etc.) (264–267).
Nanomaterials have been investigated for a wide range of biomedical applications as well, particularly in drug delivery, diagnostics, and regenerative medicine (116, 268, 269). Since natural tissues often exist in the nanoscale, and cells directly interact with and create nanostructured ECM, the biomimetic features and excellent physiochemical properties of engineered nanomaterials play a key role in stimulating cell growth as well as guiding tissue regeneration. This biomimicry is mainly achieved with nanoporous hydrogels that simulate the ECM mechanical niche or by using nanomaterials that elicit a desired biochemical reaction (270, 271).
Currently, research on nanomaterials for tissue engineering is focused on the fabrication of cytocompatible biomimetic nanomaterial scaffolds for cell encapsulation. Material structures under investigation include nanoscale fibers, meshes, cages or hydrogels that give researchers the freedom to mimic biologic systems in the lab (270, 272).
Nanoparticles have been used extensively as components of scaffolds, for the purpose of increasing cellular proliferation rates. For a new implanted cellularized scaffold, it is very important to make sure that the proliferation rate is fast enough to achieve integrity with the host tissue. Two types of nanoparticles, gold and ceramic oxide, enhance cell proliferation in bone and cardiac tissue implants, respectively. In addition to increased osteogenic differentiation of an osteoblast precursor cell line MC3T3-E1 (273), gold NPs have been shown to influence osteoclast formation and have protective effects on mitochondrial dysfunction of osteoblastic cells (274). Owing to their excellent conductive properties, gold nanowires are used alongside otherwise electrically inert material scaffolds to enhance electric coupling between cardiac tissue engineering. Cardiac muscle cells have been grown successfully within 3D porous scaffolds containing gold nanomaterials, resulting in improved synapse propagation (275). Furthermore, nanomaterials have been shown to direct stem cell differentiation(276).
Given the biocompatibility and targeting ability of some nanomaterials, efforts in the therapeutic realm include engineering nanomaterial vehicles for tissue ablation, immunomodulation, drug elution, and gene delivery (277). Nanomaterials can mitigate bacterial infection during reconstructive bone surgery or nosocomial infections. Inclusion of silver nanoparticles in the implanted scaffolds enhances protection against infectivity and hence malfunctioning implants resulting in rejection. Antibacterial nano-scaffolds containing silver nanoparticles in alginate and hydroxyapatite demonstrate enhanced antimicrobial properties that improve prosthesis implementation (278). In addition to therapeutic applications such as tumor ablation through phototherapy and radiotherapy using gold and magnetic NPs, nanomaterials have been used in gene delivery applications. Magnetic nanoparticles were used as a non-viral DNA transfection method, magnetofection. It was used to increase the gene expression levels in endothelial cells (279) and embryonic stem cells (280), which are otherwise resistant to transfection. Similarly, in fibroblasts and keratinocytes the transfection efficiencies using magentofection were 36- and 10- times higher than those achieved by lipofection with cationic liposomes (281).
Novel nanotherapeutics have garnered much interest over the last few decades for excellent delivery of not only small molecule drugs, but of proteins and genetic materials as well. Numerous methods have evolved over time for improving the successful clinical translation of these systems for use in a plethora of diseases ranging from cancer to genetic disorders using uniquely tailored nanomaterial platforms. With the latest developments in synthetic approach, multifunctional nanomaterials are not out of reach.
Patterning of materials
In order for the materials to be used for biomedical applications, it is important to modify their properties such as geometry, shape, etc. in a proper way to help in their efficient use. Lithography has been extensively used for this purpose. In this section, we will discuss micropatterning lithography techniques, their fundamentals, uses, and make a comparison for precision and resolution. Bioprinting techniques will be discussed in section 3.3.
Micropatterning of materials
To have a better control for shaping and reshaping of materials to be able to execute unique functions, techniques have been developed to affect their shape and mold them in desired architectures. Recent micropatterning techniques that create micrometer-scale structures and scaffolds at high fidelity are anticipated to address many of the challenges in medicine (282, 283). The most used micropatterning technique is based on lithography. Lithography literally means ‘writing’ or printing. In the context of this review, we will discuss lithography from the perspective of microfabrication where a geometric pattern is made or transferred from a photomask to a light-sensitive material on a substrate. Among the various fabrication techniques, rapid prototyping seems promising for generating desired structures by a programmed positioning of relevant materials and/or living cells (284, 285). Using state-of-the-art printing technology, rapid prototyping techniques produce complex objects from 3D computer-aided design (CAD) files by digitally slicing the shape of each object into a series of parallel, 2D frames and rendering these frames one layer at a time into the 3D structures. While the classical 3D printing techniques (i.e., shaping a thermoplastic or photocurable polymer layer-by-layer (286)) have been used to create custom-made implants in biomedical engineering, fixtures, and surgical tools (287), recent advances in 3D printing has allowed for the building tissue constructs by using cell-laden hydrogels or bioinks (288). Key benefits of bioprinting not only include highly controlled 3D patterning of cells but also cost-effectiveness compared to other biofabrication and microfabrication techniques (289). Bioprinting will be discussed in detail in the next section. Here we discuss the fundamentals and features of some important lithography techniques. We will discuss briefly the different types of printing techniques such as inkjet and extrusion printing, photolithography, and x-ray lithography.
Inkjet printing emerged in the 1950s and has since then been used for creating digital ‘images’ by using droplets of ink on paper, plastic or other substrates (290). With the progress in the technology, inkjet printing has been extended from photography and art applications to creating “ink” that comprises of living cells and can thus be used for applications in tissue engineering and creation of biosensors (291). There are two main technologies in use in contemporary inkjet printers: continuous (CIJ) and Drop-on-demand (DOD). In DOD printing, the desired ink is pushed under pressure through a small orifice causing the resulting jet to break up into droplets through Rayleigh instability.
Two subtypes of DOD bioprinters, including those based on thermal and piezoelectric print heads, have been used for bioprinting of molecules onto desired surfaces (292). For thermal print heads, a micro-resistor near the nozzle is heated to temperatures of 200–3000 °C by electric pulses that are delivered within microseconds, to rapidly form bioink vapor bubbles and thus create a pressure pulse to eject droplets (292). In some CIJ printers, a micro-heater is used to heat up a small volume of the ink, while an acoustic pressure pulse is used to form vapor bubbles (284, 293).
Most of the present-day 3D printer systems are extrusion based. The process of extrusion consists of simultaneously melting a ribbon of material or flowing under pressure (as in the case of bioprinters) and adding on different layers to the construct through a computer controlled nozzle deposition unit. A constant pressure ensures the material flows at a constant rate (294). A major factor in the physical integrity of a 3D object can be directly linked to the continuity in the flow of the ink that is used. Hence the pneumatic pumps and pistons used in extrusion-based technologies can be more beneficial in printing structures with superior integrity in the vertical orientation when compared to inkjet-based methods. Using extrusion technique different types of materials ranging from ceramics (295), polymers (296, 297), metals (298–300), and cell-based bioinks (294, 301, 302). The biggest advantage of the extrusion-based printing is that one can build small architectures of any shape, which might not be possible by any other contemporary methods (294).
In both these types of printing technologies, light is not used for curing of materials and the resolution of the printed structures is practically dictated by the nozzle size that dispenses the material (e.g., ink) and geometry of the printed construct (e.g., length, shape, and the print orientation). On the other hand, light-assisted photolithography printing uses a light source to either selectively crosslink photocurable inks onto a movable stage or eject the ink droplets from a light-absorbing layer onto a substrate. The printing resolution in light-assisted technologies is controlled by characteristics of the light and optical properties of the bioink such as refractive index (303). In addition, a sophisticated photocrosslinking process using nonlinear laser absorption (i.e., two-photon induced luminescence (TPL)) has been employed for potential applications in fabricating small-scale constructs (304). This review aims to identify key parameters in controlling resolution in bioprinting, with outlooks in improving bioink properties and in designing new bioinks. This knowledge is anticipated to contribute to the continued success of the bioprinting field.
X-ray lithography, LIGA, and Electron-Beam Lithography
Proposed in 1973, X-ray lithography makes use of high energy X-rays to make a pattern on a substrate (305). Following the initial exposure, a series of chemical treatments make sure that the produced pattern is engraved into the chosen material. Originally used to produce microprocessors in the semiconductor industry, X-ray lithography overcame the diffraction limit of the other photolithography techniques due to the use of shorter wavelengths than those used in other techniques (306, 307). A comparison of printing speed, resolution, and flexibility for X-ray lithography, as compared to other printing techniques, is given in Table 3.1.
Table 3.1.
An overview of existing printing techniques for materials.
| Mechanism | Speed | Resolution (μm) | Flexibility |
|---|---|---|---|
| Inkjet | Medium | 100–500 | Low |
| Extrusion | Low | 100–500 | High |
| Light-assisted (UV) | High | 20–100 | High |
| Two-photon | Low | 0.1–10 | Medium |
| X-ray | High | 0.001–0.01 | Medium |
Contrary to other photolithographic techniques, X-ray lithography does not involve optical components like lenses or mirrors and as such involves the use of what is called as proximity printing. It uses a very thin, a few microns in thickness, a transparent mask that has patterns drawn on it with X-ray absorbing material, typically of metals like gold (308) or tungsten (309) that is put in close proximity of a resist-coated wafer (Figure 3.3a) (306). The resolution that is reached by this technique is about 15–30nm. X-ray lithography being a high-resolution technique has garnered a lot of interest mainly from electronics and semiconductor based industry.
Figure 3.3.
Schematic illustration of the unique printing techniques; a.) X-ray lithography, b.) Two-photon lithography, and c.) PRINT technology. (b) Reprinted with permission from (536) and (c) reprinted with permission from (537), published by American Institute of Physics and Future Medicine, respectively.
Most recently it was used for to pattern nanocrystal films by inhibiting cation exchange (310) for applications in organic electronic structures. Additionally, it has found applications in spectroscopy for the superior mask-feature pattern in bow-tie structures for surface-enhanced Raman spectroscopy (SERS) (306). The dose distribution of x-rays on the substrate can also be achieved through double-exposure x-ray lithography. In this technique, a two-step dose distribution of x-rays is controlled by the combination of x-ray exposures with and without an x-ray mask. Such an updated and modified x-ray lithography technique has shown immense potential to create sharply pointed microneedles that can be used for drug delivery to diagnostics and real-time monitoring of metabolites (311).
Pushing the resolution limits of the x-ray photolithography, high aspect-ratio structures are possible through the use of high energy x-rays from a synchrotron source. The technique is known as x-ray LIGA, where LIGA is a German acronym for ‘Lithographie, Gavanoformung, Abformung’. The technique was developed in the 1980s and is used to create smooth walls for optical mirrors with a roughness average (Ra) of approximately 10 nm, mini structural heights spanning micro- to millimeter and all the way up to centimeter length scales (312). In microfabrication research, the technique is used to create multi-step and cylindrical microstructures with both positive and negative sidewalls creating unique MEMS for applications in electronics and biomedical technologies.
As a maskless technique, Electron-beam lithography (EBL) is used for patterning surfaces with nanoscale features. Conventionally, a focused beam of electrons is used to etch custom shapes on a surface with the help of a photoresist. The biggest advantage of this technique is the direct writing ability of features in the nanoscale domain (313). EBL offers the ability to write patterns with lateral dimensions varying from 10 nm – 1 mm with small inter-feature spacing. Additionally, EBL is used to generate features of arbitrary shape and can iteratively pattern surfaces with different chemistries by immobilizing multiple molecules on a single substrate.
For biomolecules, EBL has been employed to pattern features and structures. It is used to locally change the hydrophobicity or functionality of conventional lithographic polymer resists, thereby affecting the crosslinking ability at user defined surfaces in a scaffold. Once the pattern is ready, biomolecules are immobilized on either the pattern itself or the background via crosslinking chemistries or hydrophobic interactions. In one such example, EBL was utilized facilitate the formation of self-assembled monolayers (SAMs) by preparing gold features with a traditional lift off process. These features then served as sites for formation of SAMs capable of immobilizing biomolecules (314). EBL is also used for add-on purposes. By example, Christman et al. fabricated a protein-resistant oligo(ethylene glycol) (OEG)- terminated SAM, and then selectively removed it to form protein-adhesive regions. Likewise, regions of protein monolayers can be degraded for subsequent adsorption of a second protein. In this case, the monolayer functioned as a positive resist. (315).
EBL has many advantages, and is often also used in conjunction with other techniques such as nanoimprint and capillary lithography for the purpose of improved resolution. It suffers from some inherent limitations, however, notably cost and speed. EBL writing instruments are very expensive and often need to be installed in cleanroom facilities. EBL suffers from slower patterning speeds in comparison to photolithography, stamping, or self-assembly methods. As such, EBL is most amenable to applications for which fewer substrates, high resolutions, and/ or small inter-feature spacing is required, and for which the ability to prepare substrates with a variety of different patterns is critical.
Focused Ion Beam Lithography (FIBL) is similar to EBL in the sense that instead of electrons, it uses a low energy focused beam of ions to scan a surface in a patterned fashion. It has many advantages over EBL, such as its low diffraction and short exposure times. FIBL can be used for physical sputtering to mill away atoms and/or deposit material with sub-10 nm resolution (316). An important aspect of FIBL is that it can be used for patterning without the use of photo resists. It is frequently used to write directly on hard surfaces, such as silicon glass and metals. Potential tissue engineering applications of FIBL include topographical modification of PDMS (317). PDMS substrates were patterned with uncommon wrinkled patterns along specified paths by controlling the relative motion of the ion beam and the substrate. Wrinkled surface patterns, combined with amorphous carbon film deposition, were biologically compatible and non-thrombogenic (318). Such materials are useful for applications in medical devices and artificial organs.
Non-radiation patterning methods
Site-specific immobilization of biomolecules such as proteins and peptides with nanometer resolution plays an important role for subsequent processes like recognition and adhesion of cells and organization of tissues.
Nanoimprint lithography (NIL) is a novel solution for wafer-scale, low-cost nanopatterning, with resolution down to 2 nm. Mechanical deformation of a photoresist or a mold, depending upon the curing method, lies at the heart of NIL. NIL offers low-cost and high throughput, and is considered one of the most promising lithography techniques for applications in biosensors (319). In a standard NIL process, a physical pattern is made on a thin polymer layer using an imprint mold or master. The polymer layer is pre-spun on a silicon or glass wafer substrate. This polymer layer can itself be a functional material, or can act as an etching mask for transferring the imprinted pattern into the underlying substrate (320). The imprint process, thanks to its high fidelity, transfers all features, including defects and surface roughness to the substrate. This makes ensuring the initial mold quality critical. Master molds have to be made by precise techniques such as electron or ion beam lithography. However, the massive replication property by NIL justifies this initial high cost. NIL has been used to study the effect of surface morphology, topography, and cell-cell interactions on patterned polystyrene (321). On similar lines, NIL was used to study the effect of surface morphology of a pattern on cellular extensions, adherence, and contact guidance in osteoblasts, smooth muscle cells, and neurons, respectively (322–324). While NIL has some excellent characteristics in terms of resolution and high-throughput, most NIL processes are radiation based. Technologies like PRINT provide an alternative to such processes where the patterning is non-radiation based.
Chemical patterning or colloidal lithography (CL) for protein and cell patterning is relatively new. In general, CL templating prior to protein and cell immobilization is carried out by either depositing colloids electrostatically onto various substrates in a geometrically random manner with defined separation distance or by the self-assembly of colloids into a two-dimensional hexagonal close packed structure. For example, CL was used to create nanopillars of dodecyl phosphate treated protein adherent titanium oxide, while silica was made protein resistant by grafting a block copolymer. Streptavidin was patterned on the nanopillars with a 50 nm feature size over a large area. In another example, human α-thrombin binding aptamer and platelet derived growth factor (PDGF) binding aptamers were incorporated into a DNA template and the thrombin and PDGF were subsequently patterned (325).
Gradients of surface chemistry within porous 3D objects can be used to pattern a 3D scaffold. This property has been exploited to control fibroblast adhesion in a porous poly(lactic acid) tissue scaffold formed by super critical CO2 processing (326). Such a chemical gradient affected fibroblast density and counteracted the tendency of the fibroblasts to adhere to the periphery.
Replica molding (REM) is a form of soft lithography, and utilizes materials such as PDMS to create microfluidic devices through duplication of shape and structure of a mold.
Typically, a mold pattern with features on a nano-scale (typically ≤100 nm in dimension) is used to print replicates. These dimension ranges are difficult to replicate through other forms of molding, such as injection molding. PDMS is the most commonly used polymer in tissue engineering applications of REM. One of the biggest advantages of REM, similar to NIL, is the consistency of features in the printed structures.
In tissue engineering, REM being used to generate PDMS channels and make hydrogel molds. The hydrogels can facilitate the growth and patterning of cells for different research applications. For example, if a cell grows in an oval shaped channel, it is physically confined and forced to form the shape of the channel. A cardiopatch platform for 3D culture and maturation of human induced pluripotent stem cell-derived cardiomyocytes was recently developed that, after 5 weeks of differentiation, showed robust electromechanical coupling (327). This replica molding strategy depended on photolithography and CL to generate the initial mold pattern.
Two-photon lithography
Two-photon lithography is one of the many new additions to the 3D printing technologies that offer the finest resolution for the fabrication of 3D scaffolds (328–331). As is the case with most of the printing technologies, this technique does not rely on the use of complex optical systems or photomasks to print in a photosensitive material. This method involves the use of a laser, mainly a near-infrared ultrafast femtosecond laser as compared to more conventional UV light sources used in stereolithographic printers. At the heart of this technique lies a multi-photon absorption process in a material that is transparent and does not absorb at the wavelength of the laser used for creating the pattern. The freedom that laser systems offer in terms scanning and tightly modulating the energies at a certain point of interest, submicron features can be easily printed. The beam can be controlled to create an arbitrary 3D periodic or non-periodic pattern, leading to fine structures to be print rapidly with utmost precision (Figure 3.3b). The precision of printing using two-photon lithography scales with the square of the intensity of the incident light. The maximum absorption occurs at the focal point of laser-beam and is given by:
Where β is referred to as the two-photon absorption coefficient and dz is the thickness of the medium component through which the light passes. The transition rate of the two-photon process per unit volume is given by:
Where ℏ is the Dirac constant, ω is the photon frequency, I is the irradiance, and is the transition rate of the two-photon process per unit volume.
Given a better resolution than UV lithography, the two-photon technique has been used to create extremely small 3D structures in the nanometer range with extremely detailed features (332). Even though two-photon lithography has been around for almost two decades, it has been used for bioprinting very recently (333–335). In 2002, Watanabe et al introduced the two-photon lithography compatible optically controllable and movable 3D gels using acryloylacetone, acrylamide, and N,N-methylene bisacrylamide (336, 337). This was followed by Ovsianikov et al, who produced highly porous 3D scaffolds of polyethylene glycol diacrylate (PEGDA) and seeded with cells by means of laser-induced forward transfer (LIFT) (338, 339). Since then many two-photon lithography responsive hydrogels have been introduced (127, 340–342).
Two-photon lithography is a promising 3D printing technology to create 3D tissue scaffolds with submicron resolution, which could otherwise not be achieved with the conventional tools. The field is very new. Even though some companies have come up with specific resins for this technique, we have to wait until we see its full potential.
An acronym for particle replication in non-wetting templates, PRINT is a continuous and high-resolution molding technology essentially dedicated to the precise synthesis of micro- and nanoparticles. The technology is a conjunction that builds on the lithographic techniques from the microelectronics research and the roll-to-roll processes from the photographic film industry. The outcome of such a unique union and association enables researchers to have an unprecedented control over the different characteristics of particles on an individual level, such as particle size, shape, surface properties, modulus, and chemical composition.
Beginning with the synthesis of a new elastomer perfluoropolyether (PFPE) in early 2000, the technology built of the photo responsiveness of the fluoropolymer. PFPE and other fluoropolymers seemed to fill the gap that was created by the swelling issues of PDMS microfluidic channels when in contact with certain organic solvents. PFPEs showed an excellent resistance to such issues from the low-molecular-weight organic molecules (343). This property, in turn, meant the fabrication of less than 100 nm high-resolution features with high fidelity (344). Particularly the attributes that make these polymers stand out are an extremely low surface energy, high gas permeability, low modulus, and low toxicity (345). The PRINT technology uses PFPEs to fabricate monodisperse particles and unlike other micro- and nanofabrication techniques, it allows complete control over the physicochemical characteristics of the particle. Starting with a PFPE mold that is made from an etched silicon master template with different features raised, a liquid precursor is used to fill the micro- and nano-cavities in the PFPE mold through capillary flow.
While filling the mold with the liquid precursor (pre-particle solution), a high surface energy counter sheet is used to remove any excess material during the filling process. The filled in precursor material in the PFPE mold cavities is then solidified through various means such as UV light crosslinking, lyophilization or thermal heating. Sacrificial harvesting is then used to remove the solidified particles from the mold cavities by laminating the mold with using a sacrificial film made of a polymer that can then be dissolved in a particle safe solvent(Figure 3.3c).
The PRINT platform technology has demonstrated the potential to impact both the biomedical and material sciences for applications in drug delivery, electronics, optics, sensing, and imaging (345–347). The uniqueness of PRINT lies in its ability to prepare anisotropic particles with a wide variety of sizes and shapes. Particles of disc, rod, and boomerang-shape have been prepared which have been used to study how colloidal self-assemblies take place (348). As an example, PRINT was used to improve the encapsulation of drugs that showed an increased potency of the released drug compared to the same dosage as from a particle that was prepared through a routine encapsulation method (349). PRINT has also been used for RNA therapeutics for genetic manipulation at nucleic acid level (350). In material science PRINT has been used in studying colloidal self-assembly, advancement of micro-lens for solar cell research (351), non-symmetric and anisotropic particles for photonic applications (352), etc. PRINT is comparatively a new technology and continues to grow in different fields of application. It is envisioned that PRINT will help designing smart particles to achieve high selectivity and extremely tunable drug release. Material science will gain from the possibility of designing and production of lightweight and high tensile structural components.
In this section, we threw some light on different patterning methods that are in practice for different materials. Different printing methods have been developed and are constantly improved upon for different applications in medicine and materials science. It has been possible to push the limits of resolution beyond the diffraction limit for patterning of extremely intricate designs and structures. Newer patterning technologies such as PRINT have only helped in opening more avenues for non-conventional materials in terms of shape and anisotropy. With the advances in photonics and artificial intelligence, we see some exciting technologies developing in the next decade, which will help designing and production of materials with an ‘intelligent’ sense for interaction and adaptation to the needs, without any human interference.
3D and 4D Printing technologies for fabrication of next-generation bio-hybrid machines.
Recent microfabrication techniques that create micrometer-scale acellular or cellular architectures are anticipated to address many of the challenges in biological applications. Over the past few decades, bioengineers have developed various microfabrication methods such as micromolding, biotextiles, photolithography, stereolithography, and bioprinting for engineering highly organized and functional 3D complex constructs (353–357). Among different microfabrication techniques, rapid prototyping methods such as bioprinting offer significant potential to recapitulate the architectural complexity of nature and critical features of in vivo tissue and organ physiology systems. 3D printing approaches allow for the precise positioning of biomaterials or biological reagents with motorized operations on demand, facilitating the formation of complex of hybrid constructs.
Various printing techniques such as microextrusion (358, 359), inkjet (360, 361), magnetic (362) and laser printing (363, 364) according to the different technologies for deposition and patterning of bioinks have been developed and used in various research fields until now. Despite significant advantages of conventional printing technologies and their successful application in multiple areas, there are still possess many limitations to fabricate complex constructs which consist of multiple biomaterials and biological components which are mainly required to generate next-generation bio-hybrid machines. To overcome the barriers of conventional printing technique, researchers have employed, adapted, and invented a wide range of technologies from the entirely different field. Recently, several innovative technologies have developed and integrated on the conventional 3D printing modality resulting in multiple-nozzle printing (365), digitally controlled multiple-inlet printing (366), embedded printing (367), sacrificial printing (368), and microfluidic combined printing system (369), etc.
To improve the function of engineered bio-hybrid machines further, conventional or advanced 3D bioprinting techniques have challenges when creating 3D constructs to replicate behaviors of a native organism such as shape transformation and repeatable movement. Recently, a four-dimensional (4D) printing technology, which is based on 3D printing but with the embedded ability of shape transformation and sensing using smart materials, have been introduced by several research groups (370, 371). In this strategy, advanced functional elements for dynamic and intelligent systems as mentioned in chapter II can be integrated via 3D printing technology to bring more freedom of shape transformation and intelligent sensing to mimic the dynamics and intelligence system of the living organism. Therefore, using the advanced printing technologies and materials, we can successfully create next-generation bio-hybrid machines or devices with high performance, which can have potential to solve significant challenges in the biomedical area. In this chapter, we introduced current state-of-art in 3D and 4D printing technologies along with their associated problems. Besides, we presented several pioneering studies and highlight their applications to build bio-hybrid machines.
Inkjet-based bioprinting
Inkjet-based bioprinting which is originated from conventional 2D inject printing is the one of the earliest bioprinting technology. Droplets consisting of various biomaterials, cells, biomolecules (growth factors, DNA, etc.), or nanoparticles are ejected from the printer head to the substrate by thermal or piezoelectric actuation system during the electronically controlled printing process. Depends on the physical properties of droplets, thermal or piezoelectric actuators will be selected to manage the size of droplets and deposition speed. Regarding thermal actuator assisted technique, a heating element in the printer head can increase the temperature around 200~300 °C to eject the droplets. However, the ejection process is quite fast (~ few μsec) so the minimal damage of printed cell or biomolecules have observed after finishing the printing process. For piezoelectric technique, an applied voltage with high frequencies (15–25 kHz) induces a shape change of the piezoelectric material that generates droplets of ink from the nozzle.
Droplet shape and size can be adjusted by tuning the applied voltage and frequency to the inks. One of the advantages of the piezoelectric actuator is that it allows a broader selection of biomaterials compared with thermal actuator assisted technique. There are not specific required components (i.e. a volatile or toxic solvent to decrease the viscosity of ink and coagulation process). Additionally, multi-jets methods have been developed to build complex tissue and organ prototypes by using multiple materials and multiple cell types (360). Therefore, this bioprinting has various advantages including rapid fabrication process, highly repeatable patterns, high printing resolution (< ~50 μm), and relatively high cellular viability (> 80%). However, there still have several limitations such as restriction on material viscosity (< 0.01 Pa·s) and low cell density (< 10 million cells/mL) to avoid nozzle clogging and high shear stress.
Extrusion-based bioprinting
Extrusion-based bioprinting dispenses continuous hydrogel filaments through a micro-nozzle by pressure or plunger-actuated dispenser and precisely deposits the filaments on the substrate following digitally defined the scaffold design by an automated x-y-z positioning robotic system. This digitally tunable continuous deposition can provide better structural integrity during rapid fabrication. The printing resolution can be controlled by the dispensing procedure and rate, moving speed, nozzle diameter, and the displacement of the cartridge. In addition, this bioprinting technique allows printing a wide range of viscous materials from 30 to > 6×107 mPa·s resulting in a more comprehensive selection of biomaterials such as natural polymers, synthetic biopolymers, ECM, nanocomposite, shear-thinning materials, etc. Therefore, these extrusion-based bioprinting techniques have been used in the various biomedical field for quite a long time due to efficiently provide a higher printing fidelity to fabricate the different tissue constructs using bioinks at the expense of reduced spatial resolution (~ few hundred μm) (372–375). To further expand the capability of conventional extrusion bioprinting technologies to create complex 3D constructs, several researchers have developed various techniques including the temperature-controlled nozzle, self-healing hydrogel, independently controlled multiple nozzles, multiple direction-controlled nozzles, sacrificial material, and coaxial nozzle systems, among others. For example, these multi-material extrusion bioprinters have significantly improved since the inception of concept (Fig.1 (a)) (376). Since the initial demonstration of the multi-nozzle system was capable of fabricating hydrogel constructs from multiple materials (377, 378). The multi-nozzle bioprinter now can simultaneously or sequentially extrude biomaterials such as polycaprolactone (PCL), polylactic acid (PLA), and cell-laden hydrogel bioinks to create functional tissue constructs (Fig. 3.4 (b)). A recent demonstration by Kang and co-workers proved that, by rationally integrating these different aspects into a bioprinting system, functional tissues at relatively large scales with vascularization, such as the mandible bone, calvarial bone, ear cartilage, and skeletal muscle could obtained. Another similar approach is a programmable and continuous multi-materials bioprinting technology by using a single print head consisting of a bundled of seven capillaries and programmed motorized stage (Fig. 3.4 (c)). Each capillary connected to bioink reservoirs that individually actuated by digitally controlled pneumatic pressure. So this novel printing technique allows rapid and smooth deposition of multiple bioinks in a continuously manner along with quickly switching among different bioinks. Finally, this printer successfully fabricated human organ-like constructs (Fig. 3.4 (c)).
Figure 3.4.
(a–b) Multi-material extrusion-based bioprinting for simultaneous deposition of scaffolding polymer, tissue bioinks, and sacrificial bioinks for vascularization. Adapted by permission from (365) published by Springer Nature. (c) Schematic diagram of the seven-channel print head connected digitally tunable multilaterals extrusion bioprinter. Photograph showing the printed microfiber and human organ-like constructs (brain, lung, heart, liver, kidneys, pancreas, stomach, small/large intestines, bladder, and prostate). Adapted by permission from (366) published by Wiley. (d) Schematic diagram of embedded bioprinting technique. (d-i) Photograph of injection tip and (d-ii) printed a continuous knot. (d-iii) A continuous network of hollow vessels with features spanning several orders of magnitude in diameter and aspect ratio (insets: confocal cross sections). (d-iv) A dark-field image of the 3D printed heart with internal structure visible through the translucent heart wall. Adapted from (381). (e) Schematic diagram of the microfluidic print head with two microchannels for inlet integrated bioprinting. Adapted by permission from (369) published by Wiley. (f) Photograph of the microfluidic print head with many microchannels for the outlet. Adapted by permission from (538) published by Wiley.
However, the primary drawback of conventional or multi-materials extrusion bioprinting technologies lies in its inability to produce freeform objects due to the gravitational force that would deform the mechanically weak hydrogel structures. It is difficult to precisely control materials position in Z directions to create freestanding hydrogel architectures. To address these challenges, several individual groups recently developed an embedded extrusion bioprinting technique using a self-healing or shear-thinning supporting hydrogel bath to allow fabrication of self-sustained complex 3D architectures which consisted of deposition of bioinks in the supporting bath (Fig. 3.4 (d)) (379–381). During the embedded bioprinting procedure, the generated scratch in the supporting bath after nozzle movements automatically heals and interlocks by strong interactions (ea. physical crosslinking) between polymeric chains, which composed the supporting bath, with the biological component or reagents in the bioink to stabilize the printed shape. The unique properties of embedded bioprinting allow for the first time the direct fabrication of 3D freestanding constructs without any structural collapse. For example, 3D multi-scale hollow tube networks were successfully fabricated within supporting bath using embedded printing method which will be very useful to mimic native vascularized tissue construct (Fig. 3.4 (d-ii and iii)). In addition, a trabeculated embryonic hearts have successfully fabricated that emulated a complex 3D internal and external anatomical architecture and still maintained mechanically robust 3D architectures after removing supporting bath (Fig. 3.4 (d-iv)). While embedded bioprinting has solved the one of the significant limitation of extrusion bioprinting by providing physical supporting to deposit bioink everywhere using a self-healing supporting bath. Furthermore, the combination of multi-materials and embedded printing strategies allows bioengineers to develop multifunctional bio-hybrid machines along with highly complex architectures in a rapid manner.
Another advanced method is a microfluidic strategy combined a 3D extrusion printing system. Colosi et al. have developed a microfluidic print head incorporated bioprinting approach (382), could be expanded to multiples channel. For example, two inlets could be merged into a single outlet covered by crosslinking reagents, thus enabling alternative or simultaneous bioprinting of two bioinks (Fig. 3.4 (e)). A similar device without the sheath flow was used by Colosi et al. to extrude inks to the multiples microchannel extrusion system (383) (Fig. 3.4 (f)). Such the bioprinting process was unprecedented and enabled direct deposition of different and gradient bioinks with large-scale constructs within short printing process.
Stereolithography and digital micromirror device (DMD) bioprinting
Conventional stereolithography printing, reported by Hull et al. in 1986, was the first 3D printing technology to create a 3D structure with relatively high resolution and fast printing speed compared with extrusion-based printing techniques [42]. The main advantage of the printing method is that can spatially control the x-y-z position of irradiation of light or laser in the bath consisted of photopolymerization materials that allow to selectively solidify the materials and later create 2D or 3D constructs with a predefined pattern in a layer-by-layer manner (384). After finishing printing process, the uncured materials can be easily removed and then we obtained freestanding 3D constructs. The resolution of printing construct controlled by photo-initiator, light scattering property of each material, and irradiant exposure conditions to change the size of the laser spot, exposure time, power, etc. Recently, to improve the resolution, the micro-stereolithography techniques have been developed that achieve the high resolution with ~5 μm in the x-y directions and ~10 μm in the z-direction (385, 386). In addition, the optically projected digital mask system as a digital micromirror device (DMD) has been used to significantly improve the printing resolution (few tens of micrometers) and speed. DMD system, which has digitally controlled each mirror up to millions of mirrors, can simultaneously solidify the desired pattern in an ultrafast manner, thus allowing for dynamic projection of patterns with a higher speed and versatility, which is difficult to achieve by using other bioprinting techniques. For example, it can directly fabricate 3D microstructures using biocompatible hydrogel with various shapes (e.g., flower, square-shaped microwells, domes, and pyramid, etc.) (387). In addition, using a series of digital masks, which generated from vascular CT image from animal or human, the DMD printer successfully fabricated branched vascular and capillary networks. Therefore, this technique has potential to fabricate personalized tissue construct. Also, a multi-material printing approach can easily achieve by changing bioinks. For example, Ma et al. have developed the biomimetic hepatic construct with hexagonal lobule structure by using sequentially evolving the two different bioinks inside printing chamber (Fig. 3.5 (a)) (388).
Figure 3.5.
(a) Schematic diagram of multi-material stereolithography bioprinting approach to creating vascularized hepatic lobule structure (Scale bars: 500 μm). Reprinted from (388). (b) Schematic diagram of continuous microfluidic bioprinting designs for the formation of mosaic hydrogels and (c) dynamically encoded letters using cell-laden bioink in planar hydrogel sheet (Scale bars: 2 mm). Reprinted with permission from (389) published by Wiley.
4D printing to create next-generation bio-hybrid machines
In a recent work, an exciting bioprinting approach has developed that can continuously code and tessellate the biomaterials by using microfluidic devices (389). In addition, this system can employ a range of biomaterials and different cell types (Fig. 3.5 (b)). They have demonstrated the ability of consistently writing and coding within hydrogel sheet using cell-laden bioink (Fig. 3.5 (c)). The printing strategy can establish a fully automated and continuous format for culturing cells in physiologically relevant microenvironments.
4D printing technology has been achieved by integration of various stimuli-responsive biomaterials into a pre-defined 3D structure using bioprinting approaches. This printed 3D construct shows shape transformation and changing their function with time upon desired stimulation such as temperature, light, pressure, electric, magnetic and humidity, etc. To successfully create transformative 3D constructs which allow reflecting the dynamics of the living organisms, a careful design from the understanding of living organisms, material programmability, and advanced 3D printing techniques (as mentioned in section 2.1) are primary requirements. Furthermore, selection and design of stimuli-responsive materials are the essential parameters to achieve robust and accurate transformation ability. The sensitivity of stimuli-responsive materials usually consisted of natural or synthetic polymers can be easily tuned by chemical modification of their functional group using different classes of function group, oligomers, or polymer, etc. resulting in possessing programmable ability under specific conditions. Incorporation of the programmed stimuli-responsive materials into predefined substrates consisted of no- or opposite-responsive materials by using 3D printer can achieve prescribed shape-transformation by desired stimulation.
Several pioneering studies have demonstrated to show a potential ability of 4D bioprinting technologies to fabricate next generation of bio-hybrid machines, biosensors, prosthesis biomedical devices, and soft robotics, among others. For example, Lewis et al. first introduced 4D biomimetic printing technology to mimic nastic plant motions such as flowers, leaves, and bracts which responded by changing natural environment conditions (humidity, mechanical stress, or light) (370). The transformation principle of plants is internal turgor within anisotropic microstructural arrangement by environmental stimuli (Fig. 3.6 (a)). Nanocellulose fibrils embedded with bioink, which can control the swelling behavior by the alignment of cellulose fibrils, were fabricated by extrusion-based bioprinting technique. The obtained plant-inspired constructs showed complex 3D shape transformation with folding and helix structures (Fig. 3.6 (b) and (c)). Another 4D printing approach was developing magnetically or chemically guided microfish swimmers using magnetic/catalytic nanoparticles (platinum and iron oxide) incorporated bioink (Fig. 3.6 (d)) (390). The deposition of three different types of nanoparticles in biomimetic microfish structure was achieved by rapid DMD printing method (Fig. 3.6 (e)). The microfish swimmers showed magnetically guided propulsion by iron oxide nanoparticles and chemically guided propulsion behavior using platinum nanoparticles (Fig. 3.6 (f)). Cells, and their traction forces, are important biologically responsive materials for inducing shape transformations in cell origami. Therefore, this 4D printing strategy has the potentially significant impact on improving designs of bio-hybrid machines for reflecting the dynamics of the living organisms.
Figure 3.6.
(a) Schematic diagram of shape transformation mechanism using anisotropic stiffness (E) and swelling ratio (α) of the shear-induced alignment of cellulose fibrils. (b) Printed a flower structure and their shape transformation with the dendrobium helix after swelling (Scale bars: 5mm). (c) Printed a flower with −45°/45° bilayers and each petal’s shape change with time-lapse sequences after swelling (Scale bars: 5mm, inset:2.5mm). (d) Schematic diagram of the process of nanoparticle incorporated a microfish. (e) EDX spectroscopy images of a PEGDA microfish body with the iron-oxide head and platinum tail (Scale bar: 50 μm). (f) Track lines of chemically guided propulsion behavior of a micro manta ray after treatment of hydrogen peroxide for 3 sec. (a–c) Reproduced by permission from (370) published by Springer Nature. (d–f) Reproduced by permission from (390) published by Wiley.
4. Next Generation Devices for Biomedical Applications
4.1. Micro- and nano-devices for intelligent drug delivery:
A primary clinical goal is to couple the delivery of a therapeutic payload to the recognition of relevant biomarkers. Biomarkers can be highly specific to a given condition or disease (i.e. an overexpressed cell receptor or secreted protein), spatially variable (i.e. concentration gradients of a marker from extracellular to intracellular environments), or temporally variable (i.e. cyclical changes in temperature or pH). Micro and nano-scale materials that respond to physiological stimuli can expand the therapeutic window of existing drugs, interact predictably with cells and tissues, and enable new routes of patient-friendly administration. In the following sections, we will highlight recent applied materials research where researchers have applied advanced materials science and device fabrication to generate devices that interface with patients’ physiology.
4.1.1. Micro and nano-materials that enable patient-friendly drug administration routes:
Typically, micro and nano-scale drug delivery devices are administered via injection. However, some novel platforms are designed specifically to enable alternate routes of administration.
Systems for the Oral Delivery of Therapeutic Macromolecules:
The gastrointestinal tract presents several barriers to the delivery of viable therapeutic proteins to the bloodstream. First, delicate protein therapeutics will denature in the acidic gut conditions. Second, proteolytic enzymes in the oral cavity, gut, and intestine will degrade unprotected protein. Finally, systems must facilitate therapeutic protein transport across the intestinal epithelium in order to reach circulation. Polymeric carriers for oral protein delivery enable doctors and engineers to overcome these barriers(391, 392). In the following section, we highlight recent biomaterial platforms that interact with patients’ physiology and therapeutic payloads to overcome transport barriers in the gastrointestinal tract.
Synthetic co-polymers containing P(MAA) are especially applicable for protein protection through the gut. Other synthetic acidic polymers (e.g. P(IA), PLA, PAA) and natural acidic polymers (e.g. alginate, dextran) have also been employed in a similar manner(7). MAA is protonated in the gastric environment, and hence collapses in acidic physiological environments. In the gut, pendant acid moieties from the MAA backbone engage in hydrogen bonding with a hydrogen acceptor (of another MAA, or of a grafted copolymer such as PEG). In such collapsed state, entrapped protein payloads are protected, and their bioactivity conserved, while upon pH-responsive swelling in intestinal environments, the viable therapeutic is released(393, 394).
By illustrative example, Horava et al. developed a P(MAA)-g-PEG microparticulate system, with optimal crosslinking for bulk loading and pH-responsive, oral delivery of hematological factor IX (Figure 4.1)(395). When short-length PEG crosslinking agents were used, the primary mechanism of factor IX loading was adsorption onto the carrier surface, whereas with longer PEG crosslinkers (MW = 1,000), factor IX became entrapped within the carrier bulk. Subsequently, when the protein-loaded carriers were subjected sequentially to gastric and intestinal conditions, the bulk-loaded protein was released in viable form, whereas the surface-loaded factor IX lost most of its bioactivity. This system offers a platform to deliver hematological factor IX to the intestine for hemophilia treatment. The study’s results demonstrate quantitatively the deleterious effects of gastric conditions on therapeutic protein payloads if they are not properly protected by a carrier.
Figure 4.1:
(a) Schematic illustration of P(MAA-g-PEG) microcarriers’ pH responsiveness, protein protection, and release mechanism. (b) Release kinetics of factor IX payloads in simulated gastric and intestinal conditions, as a function of crosslinker identity. (c) Surface and bulk loading of factor IX in hydrogel microcarrier, visualized by confocal microscopy. (d) Bioactivity of released factor IX. Adapted by permission from (395) published by Springer Nature.
Similar copolymer systems have been employed for the oral delivery of insulin, salmon calcitonin, interferon-β, and monoclonal antibodies(394, 396, 397). Primarily, studies have demonstrated that microparticle carriers (and conjugates thereof) can protect protein payloads through the gut, facilitate delivery in the upper small intestine, extend residence time in the small intestine, and facilitate transport across the intestinal epithelium.
Research groups have also applied a multitude of natural and synthetic self-assembled or biodegradable polymers to overcome biological barriers to the oral delivery of bioactive macromolecules. By example, Ball et al. recently reported the use of lipid nanoparticles for siRNA delivery to treat intestinal diseases(398). By deconstructing the gastrointestinal tract into iterative in vitro and in vivo tests (i.e. pH stability, mucus interactions, biodistribution) they were able to quantify the regionally relevant material-environment and material-tissue interactions responsible for the platform’s therapeutic efficacy. Such self-assembled systems have the potential to deliver active pharmaceuticals to target regions within the gut, while degrading into biocompatible and clearable material byproducts.
Systems for Transdermal Drug Delivery:
Transdermal drug delivery is a patient-friendly route of drug administration that is particularly advantageous for molecules that readily permeate the skin. However, for macromolecules and hydrophilic small molecules, traversing the skin epithelium and passing to the bloodstream is challenging, and requires the aid of chemical and/or material systems. Advanced material design and fabrication techniques enable the delivery of many bioactive molecules, which could make substantial clinical impacts in the future(399). There are many approaches to transdermal drug delivery that combine biomaterials and external interventions (i.e. iontophoresis, electroporation, sonophoresis, or ablation), which are outside of the scope of this report(400). Here, we focus our research synthesis and highlight on applications of chemicals, materials, and polymer conjugates to improve therapeutic delivery through the skin.
Transdermal patches have been used for years to delivery small molecule therapeutics, but such approaches fail to deliver peptides and proteins through the skin’s outer stratum corneum. Recently, researchers have achieved promising results using ionic liquids and deep eutectic solvents to enhance active proteins’ permeation. In a recent report, Banerjee et al. used choline and geranate (CAGE) to deliver active proteins through porcine skin(401). While phosphate buffered saline and two common chemical permeation enhancers (diethylene glycol monoethyl ether, ethanol) failed to enhance the transdermal transport of three proteins (BSA, insulin, ovalbumin), the protein-CAGE formulations successfully facilitated transport (Figure 4.2) without carrier-associated irritation or toxicity. The bioactivity of insulin was preserved during and following transdermal delivery with this platform, as demonstrated by circular dichroism and glucose reduction in nondiabetic rats. Relevant to our discussion of device-tissue interfaces, the CAGE platform works by productively extracting lipid from the drug-skin interface.
Figure 4.2:
CAGE successfully facilitated the transport of BSA, insulin, and ovalbumin across the porcine across the stratum corneum and into the dermis and epidermis. The insulin that passed through the epithelium maintained its structure (circular dichroism) and activity (glucose level reduction in rats). Adapted by permission from (401) published by Wiley.
Skin penetrating peptides have also been identified, for co-administration with topical drugs for transdermal delivery(402). Both experimental and computational approaches have been applied to identify peptides with high-affinity domains for both a drug of interest and skin keratin. One particular skin penetrating and cell entering (SPACE) peptide was identified using phage display techniques, and successfully facilitated the transport of conjugated small molecules, proteins, and siRNA across the stratum corneum into the dermis and epidermis(402).
A final transdermal drug delivery approach is to puncture the stratum corneum with a microneedle patch array, facilitating direct delivery of drugs to the underlying cells (and subsequent circulation). Microneedles can create micropores in the skin for subsequent therapeutic delivery, be coated in an active therapeutic for skin release, or can encapsulate and deliver drugs from within their pores with diffusive kinetics(403). Lithographic techniques (discussed in detail in Chapter 3) are crucial to generate the proper geometry of microneedle arrays(404). Current research in this area focusses on extending microneedle technology for the transdermal delivery of vaccines, protein drugs, local anesthetics, and other drugs.
4.1.2. Nanodevices for Combination Therapy and Theranostics
Theranostic nanodevices are constructs containing therapeutic payloads and agents for enhanced imaging (i.e. contrast agents, fluorophores) for the simultaneous detection and treatment of disease. While theranostic devices have been researched for infectious(405) and cardiovascular diseases(406), their most common application is in the simultaneous monitoring and treatment of cancer.
Novel polymeric and hybrid nanomaterials enable combinatorial treatment regimens for cancer. For example, from the perspective of medical imaging, colloidal gold (and other gold nanostructures) enhance contrast in optical coherence tomography (OCT) and computed tomography (CT)(407). Iron oxide nanoparticles enhance MRI contrast, whereas quantum dots enable fluorescence imaging (118, 408). From a therapeutic angle, similar metal nanomaterials (gold, iron oxide) can induce hyperthermia under light irradiation and alternating magnetic field conditions. Nanoparticles and nanogels with tailored composition and supramolecular structure can deliver chemotherapeutic agents, immunomodulatory factors, and gene therapies. Materials science and device engineering for cancer theranostics is a vibrant research area, and the subject of multiple recent reviews (409–412). In this section, we will highlight a few recent examples from the literature, where advanced materials fabrication (i.e. novel formulations, structures, and assemblies) enabled an intelligent and therapeutic function at the biological interface.
For a first illustrative example, Song et al. developed hydrogel nanocarriers for the co-delivery of chemotherapeutics (paclitaxel) and immunomodulatory agents (interleukin-2) to treat melanoma(413). The research team fabricated a UV-crosslinked blend of natural polymer derivatives (methacrylamide N-carboxyethyl chitosan (CECm), N-(2-hydroxy)propyl-3-trimethylammonium chitosan chloride (HTCCm), and 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) (5:1:1 molar ratio) at the nanoscale (308±8.4 nm) which efficiently loaded both paclitaxel and interleukin-2. These nanogels were further modified, to increase circulation time, with a red blood cell membrane (taken from collected murine whole blood) (Figure 4.3). Due to the intelligent, pH-responsiveness of the nanogel formulation, the hybrid nanomaterials exhibited rapid dissolution in acidic environments (pH < 6.6) resulting in the release of both paclitaxel and interleukin-2. Mice treated with the hybrid nanogel (gel coated in red blood cell membrane) had a significant reduction in tumor volume, and extended lifetime as compared to mice treated with the uncoated drug-loaded carrier or free drugs. This example illustrates the synergistic application of advanced materials science (i.e. pH-responsive polymers for tumor responsiveness, hydrophobic-moiety containing polymers for enhanced drug partitioning) and biomimicry (i.e. red blood cell mimicry for enhanced circulation) to enable a novel therapeutic regimen.
Figure 4.3:
Hybrid nanomaterials comprised of a biodegradable nanogel and red blood cell coating effectively responded to the acidic tumor environment, modulated an anti-cancer immune response, and delivered chemotherapy to treat melanoma in mice. Adapted by permission from (413) published by the American Chemical Society.
In a recent example of advanced biofabrication enabling cancer theranostics (photothermal therapy and CT imaging), Tang et al. treated breast tumors with platinum nanodots fabricated by albumin-coordinated assembly(119). They were also able to decorate the surface of the nanostructure with Cy7 or Cy5 to enable fluorescent imaging of cell cultures (Figure 4.4). The platinum-albumin nanostructures were uptaken by breast tumor cells in vitro by clathrin-mediated endocytosis and exhibited effective killing of the 4T1 breast tumor cell line under irradiation (24 hour uptake, followed by irradiation at 785nm, 1.5 W/cm2, 5 min). In vivo, the nanocomposite platform enhanced both the photoacoustic and CT signals at the tumor (as well as fluorescent signal, due to the fluorescent tag). Thermal ablation (24-hour post-injection, 785 nm, 1.5 W/cm2, 5 min) of mice treated with 10 to 15μmol/kg of the nanocomposite led to complete tumor ablation.
Figure 4.4:
Albumin-coordinated platinum nanodots, with fluorescent coatings, were used for the simultaneous photothermal treatment and monitoring (photoacoustic, CT, and fluorescent imaging) of tumors in mice. The combination of nanoparticle treatment with 5 minutes of light irradiation completed ablated the breast tumor (4T1 cells). Adapted by permission from (119) published by Elsevier.
While the last two examples highlighted research on advanced nanoparticle development for co-delivery or multi-functionality, an alternate approach is to employ advanced devices (in this case, a hydrogel patch) to deliver multiple, unique therapeutic nanoparticles. In a recent account, Conde et al. engineered an adhesive dendrimer-dextran hydrogel containing drug-doped and rationally decorated gold nanomaterials(414). Specifically, they engineered PEGylated gold nanorods coated with a cocktail of fluorescent markers (Alexa Fluor 555), therapeutic antibodies (Avastin) targeting peptides (TCP-1) and HA1 peptides (for endosomal escape). They also fabricated and co-delivered PEGylated gold nanospheres coated in anti-KRAS siRNA, targeting peptides, and endosomal escape peptides. This complex, hybrid platform uniquely treated colorectal tumors locally through many therapeutic mechanisms (i.e. thermal ablation, chemotherapy, gene therapy). Relevant to this review, their approach also combined multiple material transduction mechanisms (i.e. molecular recognition, light-heat) and intelligent material-tissue interfaces in multiple cell compartments (i.e. cell membrane, endosome, and cytoplasm). While mice with colorectal tumors that received any one therapy (i.e. thermal, chemo, or gene therapy alone) experience tumor recurrence, mice that received all three therapies in combination demonstrated complete remission.
As shown in the following series of illustrative examples, advanced materials that interact with and/or respond to physiological environments are useful for therapeutic and diagnostic purposes. In particular, each example uniquely demonstrated how biomaterial transducers convert biological (e.g. receptor expression) and external (e.g. light) stimuli into therapeutic outcomes, and/or how biomaterial composites enhance the utility of existing imaging modalities (i.e. MRI, CT). In the following section, we will transition and detail how material transducers are being applied to couple diagnostic sensing and therapeutic intervention within advanced medical devices.
4.2. Diagnostic Implants and Wearable Technology
In recent years, there have been significant advancement in the areas of implants and wearable technology for biomedical applications. This is especially true for devices to monitor and measure physiological responses in vivo via in-situ communication with organs and tissue.
Inventions in this area have been driven heavily by growing knowledge of human genetics and the motor neuron system. Advances of semiconductor technology dovetailed with novel responsive materials and advanced architectures have radically expanded our capability to interface with living tissues at the micro- or nano-scale. As discussed earlier, intelligent materials can sense changes in the molecular composition of the physiological environment. Further, these materials have demonstrated the potential to simulate biological processes – moving closer to the ultimate goal of engineering continuous, closed systems with both sensing and treatment functions.
Next-generation implantable and wearable medical technologies are made possible through the combination of responsive materials and advanced manufacturing techniques. Here, we present a brief overview of some of recent developments in this area, and examine key remaining challenges toward personalized healthcare and diagnostic technologies.
4.2.1. General Requirements and Characteristics
All materials in implantable or wearable medical devices must meet a set of strict chemical, mechanical, and manufacturing requirements in order to be used in contact with the body or tissues. For the duration of the intended application, the device must be compatible, not induce an adverse reaction, be chemically stable and resistant to corrosion, be capable of withstanding considerable stress/strain forces (appropriate to the organ or tissue where the device will be), and be flexible or capable of in situ deformation to any geometrical and physiological constraints. (415, 416)
To this extent, it is critical to consider the characteristic of the environment where the device will be located and the duration of the application (416). In many applications, the biomechanical demands of implantable and wearable devices are significant in contrast to the shorter-term drug delivery applications discussed in the prior section. The device design must consider tensile strength, fatigue resistance, and flow stress (415). Since these properties can potentially be at odds with the desired function, long-term biomedical implants and wearable devices are often comprised from multiple classes of materials (415, 416).
Implantable biomaterials can belong to one of a number of categories, depending on the nature and degree of their interaction with the surrounding tissue. Bioinert materials are characterized by their low toxicity and low reactivity, and are well tolerated for implantation in the body. These materials are often used in applications where long term implantation is desired and there is no desire for the materials to change as a function of its environment. Materials typically included are titanium, aluminum, zirconium oxide, tantalum, magnesium, alloys, and carbon.
Biodegradable or bioabsorbable materials have the capability to degrade, solubilize, or absorb after a specified period of time. Any resulting byproducts must also be non-toxic or readily eliminated via ordinary mechanisms. This family of materials covers a broad range of natural and synthetic polymers (e.g., containing ester, amide, or ether bonds).
Bioactive materials in implantable devices make chemical bonds with tissue allowing cells to grow freely on the material surface. Typical materials in this family may include hydroxyapatite, tricalcium phosphate, and calcium phosphate. Responsive materials are able to adapt to their environment and undergo a predictable change. These materials can also be joined together to simultaneously respond to multiple stimuli and perform complex functions.
Responsive materials have opened up new horizons for the creation of smart and multifunctional diagnostic devices (416). Importantly, innovations in each of the material families mutually favor one another since they are often used in combination. This development of unique material combinations has led to the successful realization of a large number of active implantable devices, enabling monitoring and treatment for a large number of previously unmet medical conditions.
4.2.2. Continuous Glucose Biosensors
Glucose biosensing devices have been crucial in the management of diabetes for many patients worldwide. Although many different systems have been developed over the last 50 years, the fundamental principles used in continuous glucose monitoring biosensors still remain relatively unchanged. The majority of modern continuous monitoring devices utilize subcutaneous, amperometric, needle-like electrodes to sense and report blood glucose level in the skin interstitial fluid (417, 418). The amperometric electrodes monitor small changes in the fluid based on electric current when glucose exchanges electrons with the sensor.
The glucose biosensor is comprised of three main parts with individual functions (417, 418). First, the responsive material differentiates and recognizes a target molecule in a competitive environment. To accomplish this, the material is designed to include a pH-responsive moiety, an oxidative-reductive moiety, or glucose-specific receptors, enzymes, or antibodies. Next, the sensing event is converted to a measurable and distinct signal by a transducer. The transducers utilized are typically either electrochemical, piezoelectric, optical, thermometric, or magnetic (417). Subsequently, the signal is transformed into a user-friendly output by a signal processing system.
For the recognition mechanism, enzymatic detection is by far the most studied and commercially available technology to date (417, 418). Oxidation of glucose is energetically favorable, allowing enzymes to react rapidly with circulating glucose in fluid. Two of the common enzymes studied include GOx and glucose-1-dehydrogenase (GDH). Comparably, GOx offers a higher selectivity for glucose and enhanced stability within the range of normal variation in bodily fluids (e.g., temperature, pH, and ionic strength). In addition to being inexpensive relative to GDH, these properties have led to the wide adoption of GOx–based sensors.
GOx is immobilized to a nonporous PDMS layer and subsequently catalyzes the oxidization of β-D-glucose (417, 418). This reaction yields both hydrogen peroxide and gluconic acid. At the advent of this technology, sensors either monitored the oxygen consumed during the reaction or the amount of hydrogen peroxide produced (Figure 4.5). However, many of the sensors lacked sufficient sensitivity due to side reactions with compounds in the body (e.g., lactic acid, ascorbic acid, and acetaminophen).
Figure 4.5.
Schematic illustration of the generations and recent developments in sensing principles of enzymatic GOx-based glucose sensors. Adapted by permission from (539) published by Wiley.
To overcome the sensitivity issues, next-generation GOx-based sensors focused on the use of redox mediators such as ferrocene. This improved their sensitivity, but garnered much concern for the potential toxicity of leaching organometal. More recently, researchers have used genetic engineering to create enzymes with modified structures. These modifications support direct electron exchange without the need for additional redox mediators. Additionally, advances in carbon nanotube (CNT) manufacturing techniques have led to nanostructured electrodes. The nano-electrodes can be coupled to the enzyme, further enabling direct electron transfer.
In addition to enzymatic detection, implants and transdermal microneedle patches made from pH-responsive polymers have been studied for the detection of glucose and coupled delivery of insulin (419–430). Glucose is oxidized by GOx to form gluconic acid, which locally decreases the environment from pH 7.4 to pH 5.8. Extensive studies have been done using P(MAA), P(DEAEMA), and more complex structures.
Non-invasive, continuous glucose monitoring has long been a goal in the field as it holds promise to be painless, risk-free, and relatively low-cost for patients. One popular and extensively studied approach is dermal sensing. The first FDA-approved transdermal glucose sensor was a wearable and flexible skin-worn device coined the GlucoWatch® Automatic Glucose Biographer (Cygnus, Inc., Redwood City, CA, USA) (417, 431). The device used reverse iontophoresis to extract dermal interstitial fluid. However, the technology had many flaws including inaccuracy and skin irritation, and was withdrawn from the market in 2008 (431).
Recently, researchers have focused on using multiple non-invasive methods in combination for glucose sensing (432). Techniques have included combinations of pH measurements, heat conduction, infrared absorption spectroscopy, photo acoustics, impedance spectroscopy, Raman spectroscopy, optical coherence tomography, and polarimetry. For example, researchers recently designed a flexible, noninvasive skin patch that incorporated detection and drug delivery in one device (433). As shown in Figure 4.6, the patch is a graphene-based electrochemical device with thermo-responsive epidermal microneedles. Multiple methods are used to monitor the patient’s sweat, including temperature, humidity, enzyme-based glucose, and pH sensors. A bilayer of gold-doped graphene and gold mesh provides the electrochemical signal transduction. A signal is sent to a battery when sufficient glucose is detected, which heats thermally-activated polymeric microneedles to release and deliver insulin transcutaneously.
Figure 4.6.
A thin-film, wearable diabetes monitoring and therapeutic delivery system. The system monitors the pH and glucose levels in sweat, and enables transcutaneous drug delivery using a bioabsorbable, thermoresponsive microneedle patch. (a, b) The skin-mounted graphene-hybrid electrochemical device array is connected to a portable electrochemical analyzer via Bluetooth. (c) Relative humidity measurement data from the device. (d) Example pH variation between two human subjects, and (e) consistent glucose concentrations of sweat vs. blood. (f, g) Before and after correction using the measured pH, and sensitivity after multiple reuses. (h) Microneedle illustration. (i) Drug release via microneedle patch as a function of temperature and (j) stepwise drug release. (k) Photograph illustrating the stepwise microneedle dissolution. (l) Heater integrated with microneedles, laminated on the skin near the mouse abdomen. (m) Mouse skin stained to visualize the micro-sized holes made by the penetration of the microneedles. (n) Images of the patch with the thermal actuation. (o) Blood glucose concentrations of mice between the treated (red) and the non-treated group (blue and green). Adapted by permission from (433) published by Springer Nature.
4.2.3. Electro-Active Lenses
Smart contact lenses and electro-active lenses have gained much recent attention for their potential to non-invasively gather and monitor information real-time on analytes in eye and tear fluid (434, 435). Previous designs were limited to opaque and brittle materials carrying a high risk of eye damage. Additionally, the need for time-consuming fabrication processes, bulky power supplies, and large transducers restricted the realization of many designs. Recently, researchers have investigated new materials and unconventional approaches to develop optical glucose sensors using signals in the anterior chamber of the eye.
As shown in Figure 4.7, Park et al. reported the design and fabrication of a soft lens incorporating a glucose sensor and wireless power transfer circuits (434). The device was developed to measure glucose concentration in the tears of diabetic patients using an optical polymer and a thermal oxide layer with immobilized GOx on graphene via a pyrene linker and π-π stacking interaction. The sensor resistance reduces with an increasing concentration of glucose. Efficient and continuous wireless monitoring of increasing glucose concentrations was demonstrated using an in vivo rabbit model. Further, their design integrated transparent and stretchable nanostructures to display sensing signals in real time without interfering with a clear view.
Figure 4.7.
‘A stretchable, transparent smart contact lens system. (a) Schematic of the soft, smart contact lens. The lens is composed of a hybrid substrate, functional devices (rectifier, LED, and glucose sensor), and a transparent, stretchable conductor (for antenna and interconnects). (b) Circuit diagram. (c) Electric power is wirelessly transmitted to the lens through the antenna. This power activates the LED pixel and the glucose sensor. After detecting the glucose level in tear fluid above the threshold, the pixel turns off.’ Figure and caption adapted from (434).
In another study, Elsherif et al. developed a wearable contact lens to measure glucose using an optical sensor (435). The device uses a glucose-responsive hydrogel film functionalized with phenylboronic acid. On the film, a photonic microstructure was printed with a periodicity of 1.6 μm. When present in high concentrations, glucose binds to the hydrogel film. The binding causes the microstructure to swell and the periodicity constant to change, which is subsequently measured via a change in the Bragg diffraction. The authors demonstrated the ability to measure glucose between 0–50 mM with a sensitivity of 12 nm mM−1. Further, the authors incorporated a smartphone camera readout into the sensor and successfully showed a continuous monitoring mode when integrated with a commercial contact lens.
Smart lenses have also recently been developed for multi-detection. For example, Badugu et al. developed a system to monitor dry eye disease by analyzing individual ion concentrations in tears in situ using a pH- and chloride-sensitive silicone hydrogel lens (436). The authors utilized hydrophobic carbon-18 chains attached to water-soluble fluorescent probes, and subsequently bound ion-sensitive fluorophores to the lens. Studies demonstrated that the individual probes could be detected independently of total intensity by wavelength-ratiometric measurement. Further, the system can be utilized with many ion-sensitive fluorescent probes, expanding the applicability of the approach.
4.2.4. Smart Tattoos
Smart tattoos have gained a lot of attention for their use as diagnostic devices and therapeutic platforms. Fundamentally, the material requirements for smart tattoos can be demanding, as they must be flexible, stretchable, adhesive, and durable for everyday wear. Researchers have focused on two classes on materials (i.e. stretchable structures and stretchable materials) to achieve the necessary material properties. Many extensive reviews have been published in this area (437–440).
It is important to carefully consider the patterning and thickness of the materials. Bending and stiffness become a significant issue with thicker materials. The flexibility of a material or micro/nano-structure has been shown to scale with the cube of its thickness, and the propensity to break from stress/strain decreases linearly (437, 438, 441).
Conductive polymers offer a versatile route to designing thin, stretchable, responsive materials (440, 442–444). Polymers such as hydrogels and ionic nanogels are highly biocompatible, can be optically transparent, and offer great flexibility in compositional design and biomechanical properties (e.g., stretching, self-healing, shape memory). Additionally, the incorporation of 1-D metallic nanowires and patterned CNTs into a polymer matrix have been recently studied (437, 445, 446). Nanowires and CNTs are able to enhance the conductivity of the polymer-based system and increase the overall mechanical durability to bending and strain. In addition to wires, silver, copper, and gold nanoparticles have had recent success in the literature as they can be easily manufactured and incorporated into polymer matrices (437, 439, 445).
For example, Rogers et al. designed a temporary tattoo that included a flat, flexible electronic sensor that can be printed directly onto skin (447). This sensor design used ultrathin microelectronics (thinner than a human hair) incorporated into water-soluble polyvinyl alcohol sheets and applied to the skin with water. The polymer film was then dissolved, leaving behind a device attached to the skin via van der Waals forces. This fabrication process yields a lightweight, functional material that can hold up to the mechanical requirements of everyday wear (wrinkling and twisting of the skin) (Figure 4.8). The tattoo receives power from incorporated solar cells, and includes a wireless transmitter for data analysis. Future applications of similar tattoos are far-reaching, from monitoring electric signals produced by the heart (ECG) and brain (EEG) to stimulating nerves and muscle.
Figure 4.8.
‘(a) A platform for multifunctional electronics with physical properties matched to the epidermis. Mounting this device on a sacrificial, water-soluble film of PVA, placing the entire structure against the skin, with electronics facing down, and then dissolving the PVA leaves the device conformably attached to the skin through van der Waals forces alone, in a format that imposes negligible mass or mechanical loading effects on the skin. (b) EES partially (top) and fully (bottom) peeled away from the skin. (Inset) A representative cross-sectional illustration of the structure, with the neutral mechanical plane (NMP) defined by a red dashed line. (c) Multifunctional EES on skin: without deformation (left), compressed (middle), and stretched (right). (d) A commercial temporary transfer tattoo provides an alternative to polyester/PVA for the substrate; in this case, the system includes an adhesive to improve bonding to the skin. Images are of the backside of a tattoo (far left), electronics integrated onto this surface (middle left), and attached to skin with electronics facing down in initial (middle right) and compressed (far right) states.’ Figure and caption adapted from (447).
Recently, researchers developed a large-scale, high resolution ‘living tattoo’ to visually monitor multiple programmable biomarkers (448). As shown in Figure 4.9, the design incorporates a new method and material system capable of 3D-printing flexible hydrogel inks with encapsulated bacteria. The 3D-printing process takes places in two steps. First, Pluronic F127 diacrylate micelles are printed as inks along with various cells and a photoinitiator (Irgacure 2959). Subsequently, the polymer micelles are covalently crosslinked into 3D constructs via exposure to ultraviolet light. The engineered bacteria included in the tattoo are programmed to detect chemical analytes in the skin, efficiently process the desired signals, and fluoresce in response. Ultimately, the authors demonstrate this new paradigm can be used to produce smart tattoos with ‘living’ properties for a variety of applications.
Figure 4.9.
‘Design and 3D printing of large-scale, high-resolution living responsive materials and devices. (a) Schematic workflow of living material design, which can be achieved by combined genetic circuit design in the encapsulated cells and structural design of living materials. The responses of living materials, including chemical diffusion and cell induction, can be predicted by simulation, which can provide feedback for living material design. (b) Schematic illustration shows direct writing of hydrogel inks. The packing of Pluronic F127-DA micelles in the ink leads to a physically crosslinked hydrogel, which is fluidized by shear force during ink extrusion and recovers to the packing state right after printing. (c) Schematic illustration shows that covalent crosslinks form among micelles after UV crosslinking. The fabrication processes result in a large-scale, high-resolution, and multi-ink living material.’ Figure and caption reprinted with permission from (448) published by Wiley.
4.2.5. Neural Interfaces and Implants
Neural implants are designed to regenerate, restore, or supplement motor functions and stimulation after loss from injury or neuronal diseases (449, 450). The interface between the implants and neural tissue is delicate with many challenges. Over the last 50 years, advanced materials have played an integral role in the rehabilitation strategies and have led to new treatment options.
Polymers have been a widely used class of materials in this area, and have led to a large number of the neural interfaces and implants that exist today (449, 451, 452). Polymers have a long history of use as a substrate and insulation with metal wires for electrical stimulation. In this context, PDMS has the most widely used. However, polymer substrates with metallic wires suffer from significant drawbacks. For example, they often suffer from delamination at the connection surface after a short period of time. Additionally, it is difficult to precisely form insulating polymers in the desired patterns (453).
Conductive polymer hydrogels have been advancing as a promising class of materials to address some of these limitations (449, 451–463). Conductive polymers are formed into specific patterns using precision manufacturing techniques, laser structuring, and micromachining. They also offer easy conjugation and functionalization routes in order to improve biocompatibility and tissue interfaces. Poly(3,4-ethylene dioxythiophene) (PEDOT) is a relatively new conductive hydrogel material, but has quickly dominated the literature in this area over the last decade, with its lack of toxicity, chemical stability, and efficient electrical conductivity.
Recently, Kleber et al. developed a conductive polymer hydrogel system for the surface of neural interfaces. Their hybrid system was comprised of PEDOT with a dimethylacrylamide, sodium 4-styrenesulfonate, and the UV-reactive 4-methacryloyloxy benzophenone copolymer. The system can be covalently attached and forms an interpenetrating network, while demonstrating high quality electrical and mechanical properties. It can be easily patterned through a structured mask using ultraviolet curing. The hybrid system demonstrated a charge storage capacity 2.5 times higher than the hydrogel alone. Additionally, the hybrid system exhibited a reduced impedance and proper electrochemical stability over 1000 oxidative-reductive cycles.
Researchers also developed a flexible and foldable design incorporating a multiplexed, high-density electrode array in order to achieve in vivo mapping of brain activity (464). The ultrathin electrodes were constructed from flexible silicon nanomembrane transducers, and subsequent interconnect layers were placed into insulating and encapsulating layers of polyimide and polyimide with epoxy, respectively (Figure 4.10). The 360-channel, high-density array overcame the limitations of previous designs by maintaining high spatial resolution in large areas of the brain, while using fewer wires. Feasibility of the design was successfully demonstrated in vivo using a cat brain model. The authors found that it was possible to record several different types of neural recordings, including electrographic seizures, sleep spindles, and evoked responses by presenting visual stimuli.
Figure 4.10.
A novel flexible and foldable design incorporating a multiplexed, high-density electrode array in order to achieve in vivo mapping of brain activity. (a) Schematic exploded view of each layer: doped silicon nanoribbons (bottom), first and second metal layers (second from bottom), after waterproof encapsulation (third from bottom), and after platinum electrode deposition (top). (b) Bending stiffness of the electrode array as a function of epoxy thickness and polyimide substrate thickness. (c) In vivo studies using a feline model. The flexible array was place on the visual cortex. (d) Visual evoked response was studied and analyzed in the in vivo study. Analysis is shown as 64-color maps, each showing the response of an entire high-density, 360-channel array. Adapted with permission from (464) published by Springer Nature.
4.3. Biomimetic Machines or Devices
Biological devices or actuators, which are composed of organic components and artificial platforms and possess the ability to dynamically deform and sense physiological environments, have generated significant interest for various in vitro applications, including drug screening, artificial muscles formation, and biosensing (465, 466). Sensing, and responding with dynamic control to, the performance of material components is essential for biological devices to accomplish their functional goal. Many engineered cells, of mammalian and bacterial origin, have been used as biological actuators in this manner and are reviewed in this section.
Artificial platforms, such as material scaffolds, ensure the mechanical stability of biomimetic, cell-laden devices and improve the performance of living components in the native or device environment without toxicity. The integration of dynamic control systems into artificial platforms allows for the external manipulation of actuation performance as well as for the direction and speed of the biomimetic device.
Despite recent advancement, autonomously controlled motion, life-like movement, and force production are still considerable limitations of biomimetic devices. These shortcomings have hampered the development of more advanced and useful biological devices. Creating a new class of fully functional, autonomous biological devices is a significant challenge. Nevertheless, overcoming existing limitations and engineering next-generation biological devices has the potential to address pressing medical needs and enable new research applications.
Bioengineers have taken inspiration from nature and living organisms and developed innovative strategies for combining cell and material components into new bioactuators. The rationale for using cells as force-producing elements is that living organisms quickly and efficiently scale-up force production from piconewtons (pN) up to kilonewtons (kN) as a result of their intricacy and metabolic efficiency (467). Leveraging the actuating performance of cells embedded in biomimetic platforms with proper material and geometric constraints, researchers can mimic the performance of functioning organisms. In this section, we introduce several pioneering studies on bioinspired devices that recapitulate or leverage the physical or biological functionality of diverse cell lineages. We also highlight strategies for autonomous control and bioinspired design to control bioactuator performance reproducibly and precisely.
4.3.1. Cells and Tissues as Next-Generation Biological Actuators.
Actuators must generate, in response to a stimulus, either pumping, contracting, folding, stretching, climbing, growing or morphing behaviors. To achieve functional actuation, traditional actuators are fabricated out of shape memory alloys or electroactive polymers (EAPs). However, these actuators have many drawbacks, as compared with biological devices, when applied to solve biomedical problems. Electromagnetic actuators, for example, are heavy, have a low energy density, and demand a sizeable contractile stroke. Other actuators, such as pneumatic and hydraulic actuators, require a cumbersome activation system when trying to miniaturize. Some EAPs need very high driving voltages and have slow actuation response. Most abiotic actuators are challenging to scale-down to the microscale without loss of force production and controllability, meriting the development of miniaturized biological actuators.
However, other critical properties of the non-biological actuators, such as strong force-generating properties and effective scale-up are currently unmatched by living organisms. Biological components, molecular motors based on DNA, proteins, and synthetic molecules are all exciting biological actuators, but their success is limited by force and torque generation. In addition, biological systems present issues for system integration, external control, and upscaling.
In spite of these limitations, there is still substantial unmet need in the area of biomimetic devices that biological actuators must address. This need, specifically, is in the area of bioinspired devices and soft robotics. By example, most of the performance characteristics of muscles are difficult to replicate using solely synthetic materials and existing actuators. Existing machines (even soft and collaborative ones) are not able to reproduce the lifelike movement that is characteristic of animals. Therefore, the existing options are insufficient. High-performance, flexible actuators that are able to recapitulate or even outperform natural muscle functions are needed.
One potential solution is to use muscle cells or tissues to create actuators. This approach has generated significant interest as the potential future of biorobotics. Coordinated movements of molecular motors only a few nanometers in size, which are organized hierarchically to produce macroscopic contractile muscle tissues, drive animals’ movement. Therefore, engineered muscle constructs can potentially generate, as a driving force for biological devices, the powerful and efficient muscle contractions that living systems use to move.
Engineered muscles control their stiffness by recruiting a variable number of myofibers, which are highly scalable, self-healing, and eco-compatible. Biohybrid actuators (i.e. environmentally responsive, force-generating cell-material constructs) require the integration of artificial, engineered structures and living systems. This approach exploits the unique characteristics of biological cells and tissues, which evolution refined. Here, we introduce the advantages and limitations of devices formed out of motile cells, muscle tissue, and biomaterials.
4.3.2. Motile Cell-Based Biological Devices
Various bacteria such as Salmonella typhimurium (S. typhimurium), Escherichia coli (E. coli), Magnetotactic bacteria (MTB) strains, and Serratia marcescens (S. marcescens) have been used to develop fully-powered microactuators due to their high-speed mobility from flagellar motors (Speed: > 100×body length/sec) (467). Flagellar motors directly generate mechanical energy through a chemical reaction in the biological environment with high conversion efficiency (~100%). The behavior of flagellar motors can be controlled by external or environmental stimuli, including chemical gradients, magnetic fields, electric fields, light, temperature, and pH because of each bacterium’s unique sensory mechanisms. Recently, bacteria-based microdevices for targeted drug delivery have been developed (468, 469). For example, Park et al. developed a bacteria-based microrobot (bacteriobot) to target solid tumors (470). To develop a bacteriobot, an attenuated S. typhimurium (green) is immobilized on a polystyrene microbead via biotin-streptavidin affinity (Figure 4.11 (a) and (b)). The microbead contains, and can deliver, high amounts of therapeutic agents. The research team genetically modified a high-mobility strain of the S. typhimurium to swim toward tumors. During in vivo assessment with a CT-26 tumor containing mouse model, the Cy5.5 signal on the bacteriobots was detected strongly within the tumor site. (Figure 4.11 (c)). In this study, the bacteriobots showed chemotactic mobility, with higher migration velocity and tumor targeting ability, according to the concentration gradient of tumor cell lysates.
Figure 4.11:
(a) Schematic diagram of bacteriobots. (b) Confocal microscope image of S. typhimurium (green) immobilized the polystyrene microbead (red). (c) In-vivo bioluminescence imaging of bacteriobots in mouse tumor models. (d) Schematic diagram of the motile sperm cell conjugated a magnetic microtube as the microrobot. (e) Controlled locomotion of the microrobot by the magnetic field at different time points. (a–c) Adapted from (470). (d–e) Adapted by permission from (471) published by Wiley.
It is challenging to build a macro size bacteriobots because single and swarms of bacteria generate a small magnitude of power. It is also difficult to control the direction of bacteria’s movement. Furthermore, toxicity and immune responses associated with bacteria must be addressed for bacteriobots to be used it for in vivo applications. Many researchers have devoted their effort to addressing these limitations, so that the bacteria-based microrobot will be a safe and effective microdevice in the future.
Non-pathogenic, motile cells such as macrophages and sperm cells have been proposed as cellular options to overcome the toxicity and immunogenicity issues associated with bacteria-based devices. When combined with smart materials, these motile mammalian cells can respond to external stimuli such as magnetic or electric signals (471). Schmidt et al. developed a sperm-flagella-driven microrobot by integrating sperm cells inside of magnetic microtubes. The sperm cells acted as a biological power source, inducing the propelling movement of the microrobot. Simultaneously, the magnetic microtubes served as a controller, directing the microrobot using an external magnetic field (Figure 4.11 (d) and (e)).
4.3.3. Skeletal Muscle Biological Devices
Among contractile muscular tissues (e.g. skeletal, smooth and cardiac muscles), skeletal muscle is the primary contractile actuator in the body that allows humans to perform physical work (472, 473). Skeletal muscles have a densely packed structure composed of parallel bundles of muscle fibers generated via myogenesis. Motor neurons precisely control skeletal muscle contraction. Therefore, skeletal muscles do not show spontaneous contraction behavior, which differs significantly from the bioactivity of smooth and cardiac muscles. Non-cyclic contractions enable precise control of skeletal muscle actuation by an external stimulus (e.g. electrical or neural signal).
Regarding muscle function, isotonic muscular contractions can sufficiently generate uniaxial force and move a load. Muscle contraction maintains relatively constant tension against the weight for a while. Furthermore, with tissue engineering approaches, structure and actuation function of engineered skeletal muscle are tuned by advanced microfabrication technologies and biomaterials. Therefore, skeletal muscle is a useful biological component for producing controllable next-generation biological actuators, with complete on-off controllability, which are amenable to modular design.
Research teams have developed bioactuators or soft robots using 2D or 3D engineered skeletal muscle tissues derived from myoblasts (474). In 2013, Sun et al. fabricated contractile actuators using aligned C2C12 myoblasts on micropatterned PDMS films after the differentiation process (Peak twitch stress: ~10 kPa) (475). These 2D skeletal muscle tissues were used to build cantilever or bridge devices as biosensors of environment toxicity.
To move or shift a heavier load or cargo, an actuator has to generate an intense muscle contraction force. To achieve this, it is necessary to fabricate dense or thick muscle constructs. Millimeter-scale 3D skeletal muscle constructs can produce strong, active tension forces (476). To create thick muscle tissue mimetics, various microfabrication techniques such as micromolding, textile method, electrospinning, photolithography, and bioprinting have been used in vitro.
Dennis and Konsink have developed a 3D contractile muscle strip (called “myooids”) using primary rat myogenic precursor cells without material scaffolds (113). The myooids generate contraction forces from 3 to 30 μN, and can be used as biohybrid robots. Later, several groups modified their fabrication method to improve the productivity and alignment of myooids using micropatterned PDMS substrates. This fabrication technique still has many limitations, however, such as contraction reproducibility and imperfect biomimesis following of scale-up.
To overcome these limitations, Cvetkovic et al. developed a stereolithographic 3D printing technique to create biohybrid robots as “bio-bots” (Figure 4.12 (a)) (476). A PEGDA hydrogel was used as a printable material and a biomimetic scaffold for the articulating joint. The bio-bots’ printed mold consisted of two stiff pillars and was fabricated using a modified stereolithography apparatus. C2C12 myoblast cell-laden ECM solution was injected into the bio-bot mold to form muscle tissue around the pillars (Figure 4.12 (b)). The obtained bio-bot produced an actuation motion that generated a passive tension force of up to 1 mN, active tension force around 200 mN, and fast-moving speeds around 156 mm/s following electrical stimulation (Figure 4.12 (c)).
Figure 4.12.
(a) Stereolithography printing method to fabricate a 3D muscle tissue using biomaterials. Biological characterization of 3D muscle tissue with (b) longitudinal fibrin muscle strip slice at day 9 (Scale bar: 500 μm. Scale bar of inset image: 200 μm) and longitudinal collagen muscle strip slice at day 14 (Scale bar: 500 μm). (c) Top-view time-lapse images of the asymmetric bio-bot’s movement (Scale bars: 1 mm). (d) Schematic diagram of designed fiber bundle structure for muscle organization. (e) Confocal microscope image of the 3D printed muscle organization with immunostaining for myosin heavy chain (green) after day 7 differentiation. (f) Photograph of 3D printed construct before (left) and after (right) removing the sacrificial material (Pluronic F127). (a–c) Adapted from (476). (d–f) Adapted by permission from (525) published by Springer Nature.
Another 3D printing approach, proposed by Kang et al., produced well-organized skeletal muscle tissues with cell-laden fiber bundle structures using their integrated tissue–organ printer (ITOP) system (Figure 4.12 (d–f)) (365). The encapsulated myoblasts aligned and matured along the longitudinal direction of the bundle fibers.
4.3.4. Cardiomyocyte Biological Devices
Self-actuating cardiomyocytes are a great cellular component for various bioactuators. Cardiomyocyte-laden bioactuators are comprised of striated muscle cells and electrically integrated syncytium that contract with one another. They generate autonomous contraction behavior without the need for external stimulation.
There have been significant improvements in cardiac tissue engineering using microfabricated biomaterials. These advanced cardiac tissue mimetics enable the formation of cardiomyocyte bioactuator systems and complex biological devices. At the cellular level, Yoon et al. developed a simple microfabrication technique for fabricating poly(D,L-lactide-co-glycolide) microcylinders. Clusters of cardiomyocytes coupled onto the surface of ECM-coated microcylinders, and were able to bend the cell-material construct cyclically (Figure 4.13 (a)).
Figure 4.13.
(a) Selective patterning of cardiomyocytes on PLGA/PLCL micro-cylinders actuator. Adapted by permission from (540) published by Wiley. (b) Muscular thin film actuators using micropatterned PDMS elastomer. 2D myocardium showing well-aligned morphology (nuclei (blue), F-actin (green) and sarcomeric α-actinin (red)). Adapted by permission from (477) published by the American Association for the Advancement of Science. (c) Engineering cardiac tissue constructs using carbon-nanotube embedded hydrogels and their bioactuators. SEM images showing porous surfaces of CNT-GelMA thin film. The phenotype of cardiac cells on CNT-GelMA hydrogels with immunostaining of sarcomeric α-actinin (green), nuclei (blue), and Cx-43 (red). Adapted by permission from (541) published by the American Chemical Society. (d) Bioprinted 3D cardiac tissue construct using gold nanocomposite bioink. Adapted by permission from (480) published by Wiley.
Cardiomyocyte-based actuators were developed using microcontact-printed (μCP) PDMS thin films (477). After culturing cardiomyocytes on flexible micropatterned substrates coated with ECM proteins, the resulting anisotropic 2D myocardium had aligned highly aligned cell morphology. The obtained hybrid muscular thin film with centimeter-scale constructs spontaneously adopted various 3D shapes when the direction of tissue alignment was altered (Figure 4.13 (b)). These centimeter-scale 3D constructs gripped, pumped, walked, and swam, while generating stresses up to four mN/mm2.
Recently, as an extension of this work on micropatterned, biomimetic, cardiomyocyte-laden constructs, jellyfish and skate shaped soft-robots were fabricated (466, 478). To improve the muscle maturation and contraction behavior, novel ECM-based biomaterials with electrically conductive CNTs were developed (Figure 4.13 (c)). The electrically conductive CNT-containing hydrogels facilitated cardiac cell adhesion, organization, and coupling. The bio-hybrid thin films spontaneously adopted a 3D conformation through strong cell traction forces. These centimeter-scale constructs pumped and swam controllably in response to electrical stimulation.
To engineer a 3D mimetic with native cardiac-tissue morphology, Zhu et al. developed a gold nanorod (GNR)/Gelatin methacryloyl (GelMA) bioink for cardiac tissue bioprinting. Their constructs addressed delays in beating propagation, which was a major shortcoming of cardiac-like constructs fabricated with conventional polymeric biomaterials (Figure 4.13 (d)) (479, 480). The authors achieved high printing resolution with their nanocomposite bioink and their printed cardiac tissue construct exhibited synchronized beating behavior. Cardiomyocytes derived from human induced pluripotent stem (iPS) cells also showed strong contractile behavior inside a micropatterned ECM matrix (481).
4.3.5. Advanced Geometric Design and Device Fabrication.
As we discussed previously, various bioinspired actuators or devices have been invented, such as jellyfish- and ray-inspired robots using muscular thin film based bio-hybrid actuators (477, 482). Microorganism mimicked self-propelled swimming robots have also been engineered for therapeutic purposes (483). “Biological bimorph” bio-bots (484, 485), which walk through the deflection of an anchor beam, also have been engineered to exhibit lifelike motion.
The unique shape and movement of various biological organisms have evolved for the purpose of survival. Natural movements are productive and efficient, in terms of both function and energy cost, which are essential for both living organisms and biological devices (486). Recently, robotics engineers have taken inspiration from the geometric constraints and hierarchical structure of biological organisms to design autonomous robots that mimic fish, octopi, snakes, microorganisms, and insects.
Parker et al. developed two soft robots using the previously developed contractile muscle thin film technique in combination with bioinspired architecture for the purpose of mimicking the swimming mechanism of living organisms. For example, jellyfish medusa have radially symmetric structures that are composed of motor neurons and striated muscle (Figure 4.14 (a)). Their repetitive swimming movement is generated by fast muscle contractions and slow muscle relaxations that embedded pacemakers systems control (466, 487, 488). Symmetric structures and contraction behaviors are vital to building the jellyfish-mimetic soft robot, which properly imitates the organism’s propulsion and feeding performances.
Figure 4.14.
(a) Jellyfish mimicking bioactuator. Adapted by permission from (478) published by Springer Nature. (b) Ray fish mimicking swimming robot. Adapted by permission from (466) published by the American Association for the Advancement of Science. (c) Microorganism mimicking biohybrid swimmer. Adapted by permission from (483) published by Springer Nature. (d) Cantilever mimicking “biological bimorph” bio-bots. Adapted from (484). (e) Anchor beam-mimicking microdevice. Adapted by permission from (490) published by Springer Nature.
Parker et al. succeeded in developing their soft robot via a systematic design strategy comprised of simulations and experiments to capture the propulsion and feeding performance of jellyfish. To create the anisotropic muscle structure of jellyfish, micropatterned PDMS thin films with jellyfish shapes were fabricated and neonatal rat cardiomyocytes were seeded and cultured to produce a bi-layer construct. The organized cell-material construct generally replicated the swimming action of jellyfish. However, the swimming motion did not perfectly capture the jellyfish’s stereotypic undulations due to insufficient control over local muscle contraction.
To overcome this shortcoming, they developed an advanced bio-inspired robot with an optogenetic control system. With this revised system, light induced sequential muscle activation facilitated mimicry of skate or stingray swimming behavior (Figure 4.14 (b)). The ray fish are cartilaginous fish with flattened bodies composed of multi-layered muscle tissue and cartilage; and tissue engineered constructs can mimic this biological structure. To achieve undulatory swimming behavior, the researchers fabricated a fish-shaped construct by patterning cardiomyocytes on a PDMS multi-layered material that encapsulated a microfabricated gold skeleton. The cardiomyocytes were modified genetically to express a light-activated cation channel, which allowed sequential muscle activation by optical stimulation (blue light: ~ 10mW). This optogenetic system enabled externally guided and steered swimming, as well as engineering control over the constructs’ velocity (i.e. by modulating light frequency and independently eliciting the right and left fins).
In another biohybrid application, Williams et al. developed a self-propelled synthetic flagellar swimmer out of a PDMS filament (short head and long tail geometry) where a few cardiomyocytes were adhered selectively (Figure 4.14 (c)). During cardiomyocyte contraction, the filaments swam at velocities from five to 10 μm/s. Wehner et al. also recently introduced an octopus-mimetic, abiotic, soft robot (489). An embedded microfluidic controller controls the “octobot” autonomously, while monopropellant decomposition acts as a power supply.
Several researchers are interested in designing biological machines that mimic non-biological materials such as cantilevers and anchor beams. The cantilever is a traditional actuator structure, and can act a biosensor by deflecting in response to external conditions. Many cantilevers, derived from DNA, bacteria, chemicals, and mammalian cells have been developed.
Chan et al. produced biological machines that mimic cantilever actuation by 3D printing (Figure 4.14 (d)) (484). Their bio-bot consisted of a ‘biological bimorph’ cantilever structure that contractile cardiomyocytes powered and a stiff, asymmetric base structure directed. The bio-bot moved at a maximum speed of 236 mm/s and had an average beating frequency of 1.5 Hz. A similar study by Xi et al. developed silicon microdevices based on a micromechanical structure that mimicked anchor beams (Figure 4.14 (e)) (490). Their microdevice design facilitated free movement, which was powered by cardiac muscle bundles seeded between two device legs.
4.3.6. Integration of On-Board Systems for Autonomous Control
A control system has to be integrated into a biological actuator to achieve reproducible and precise actuation within the physiological environment (477, 491). However, integration of control systems that respond to external stimuli (i.e. light, electrical signal, chemical reactions) is challenging because of biocompatibility and hardware concerns. Recently, ontogenetic cells, flexible electrodes, and monopropellant fuels have emerged as promising components of control systems for biological actuators. Because controllability is a primary requirement for any robot design, many recent efforts have focused on directing the contractile behavior of biological actuators.
One interesting approach is genetically modify cardiomyocytes or skeletal muscle cells using microbial agents or viruses so that they express light-responsive proteins. Natural photo-responsive effectors and their microbial sources are Channelrhododopsin-2 (ChR2) from Chlamydomonas reinhardtii, VChR1 from Volvox carteri and NpHR from Natronomonas pharaonic (Figure 4.15 (a–d)) ChR2 can be expressed stably and safely in electroactive cells such as neurons, cardiomyocytes, and skeletal muscle cells, and can drive cellular depolarization. Several pioneering studies have exploited ChR2 expressing cellular constructs as skeletal muscle-powered adaptive biological machines (492), 3D skeletal muscle microtissues (493), and ray inspired swimming devices (466).
Figure 4.15.
(a) Light-responsive effector and microbial source of ChR2. Adapted by permission from (542) published by Springer Nature. (b) Schematic diagram of blue-light sensitive cation channel (ChR2) encoded C2C12 and their engineered muscle ring. (c) Directional locomotion of optogenetic muscle ring powered bio-bots. (d) Comparison of bio-bot speed controlled by optical and electrical stimulations at 1 Hz. (b–d) Adapted from (518). (e) Incorporation of CNT forest microelectrode array into engineering bio-hybrid tissue actuators and their controllable actuation behavior. SEM images of vertically aligned CNT forests. The phenotype of cardiac cells with immunostaining of sarcomeric α-actinin (green), nuclei (blue), and Cx-43 (red). Displacement of the bio-hybrid actuator under electrical stimulation (1.2 V/cm) at various frequencies. Adapted by permission from (275) published by Wiley.
Asada et al. reported the first study that developed a light-sensitive 3D skeletal muscle bioactuator using optogenetically encoded skeletal muscle myoblasts that expressed ChR2 (493). Blue light stimulation locally activated the densely arrayed 3D muscle tissues. Raman et al. also created a modular light-controlled skeletal muscle-powered bioactuator by 3D printing that generated up to 300 μN of active tension force and moved at up to 310 μm/s in response to an optical stimulus. Parker et al. developed a light-guided soft-robotic ray using optogenetic control of cardiomyocytes cultured on an elastomeric material such as PDMS. Their construct replicated the batoid fish’s morphology and swimming behaviors (undulatory locomotion) (466).
As an alternative to optogenetic techniques, flexible microelectrodes are promising candidates for integration into muscle tissue mimetics to control contraction (477, 491). These flexible microelectrodes have several advantages such as long-term stimulation ability, durability, simplicity of fabrication, and standard integration processes (477, 491). In addition, microelectrode arrays can provide local stimulation with low electrical potentials. Microelectrodes can be fabricated on flexible substrates with precisely defined positions by using a printer or other microfabrication technique, subsequently generating a reproducible and well-quantified local electric field (494–496). Flexible microelectrodes reduce significant problems that occur between stiff electrodes and biological samples, such as hydrolysis under higher electric potential (> ± 1V), bubble formation, localized pH gradient, joule heating, and electrode corrosion (112).
Despite the various advantages of flexible microelectrodes, few studies have integrated them into biological machines. Shin et al. addressed this shortcoming by developing aligned CNT microelectrode arrays and incorporating them into cardiac tissue constructs for electrical stimulation (Figure 4.15 (e)). CNTs were selected for their electrical and mechanical properties, chemical stability, biocompatibility, adhesiveness, and flexibility (497, 498). Aligned CNTs, which have anisotropic electrical conductivity, were embedded successfully into a bio-hybrid actuator. This microelectrode was biocompatibility toward cardiomyocytes and created a well-organized tissue structure with improved cell-to-cell coupling on top of the microelectrode incorporated hydrogels. Furthermore, integrating the CNT microelectrode arrays within the hydrogel construct successfully controlled the beating frequencies of the biohybrid actuator.
5. Conclusions and Future Perspectives
In this review, we highlighted the underlying fundamentals and recent applications of bioinspired and biomimetic materials, constructs, and devices. A graphical depiction of our analysis is given in Figure 5.1.
Figure 5.1.
Graphical overview of the topics discussed in this review. Each sequence of connected concepts illustrates how intelligent materials systems are combined rationally, through advanced manufacturing techniques, to advance biomedical therapeutics, biosensing, and tissue engineering.
First, we discussed the fundamental characteristics and biomedical applications of intelligent polymers that recognize, and respond to, changes in the physiological environment. Chemical, electrical, and biomolecular gradients facilitate communication within biological systems. Consequently, biomimetic systems must contain material components that are capable of actuating biological signals. Herein, we discussed the underlying thermodynamics and practical applications of biomaterials that respond to the solvent environment, pH, ions, oxidation-reduction states, temperature, electrical stimulation, magnetic fields, light, and biomolecules (i.e. small molecules, proteins, and nucleic acids).
Next, we detailed techniques for modular and/or additive biofabrication. These techniques, individually or in tandem, are necessary for building novel material constructs that integrate with tissues, interface with biomolecules, and/or modulate cell behavior. Each technique offers unique advantages, which scientists and engineers leverage, for the design and development of bioinspired and biomimetic devices.
Finally, we described a number of contemporary applications of bioinspired or biomimetic systems. In each application, environmentally responsive materials interact with embedded cells, external impulses, and/or native physiological environments for advanced functionality. Therapeutic applications include, but are not limited to pH-responsive systems that protect delicate therapeutic agents through the gastrointestinal tract, degradable nanomaterials that recognize membrane biomarkers for cell-targeted drug delivery, multi-responsive devices for closed loop disease monitoring, and biohybrid systems for the active transport of encapsulated payloads. Diagnostic and rehabilitative applications include deformable, stimulus-responsive systems for biomonitoring and conductive arrays for recording neural activity. In tissue engineering, constructs of skeletal muscle cells and/or cardiomyocytes, manipulated by micropatterned, stimulus-responsive substrates, mimic the physical behavior of complex organisms. These biohybrid devices could function as diagnostic and/or therapeutic biological actuators in the future.
The most noteworthy aspect of the figure, however, is the interconnected network of ideas and technologies that join our three focus areas. These connections illustrate how fundamental advancements in materials science and novel fabrication strategies enable the invention of biomedical technology. Alternately, when motivated by a specific unmet biomedical need, there are a repertoire of intelligent materials and biofabrication strategies in your toolbox, which you can integrate in new ways to build novel biohybrid machines.
5.1. Considerations for the Translation of Advanced Material Designs:
In the United States, products intended for human use are regulated by the Food and Drug Administration (FDA) according to the “mode of action” (MOA) or “primary mode of action” (PMOA) for a combination product. The biomedical regulatory framework under the FDA is illustrated in Figure 5.2. A product’s MOA is “defined as the means by which a product achieves its intended therapeutic effect or action” and includes categories such as drug, biologic, or device (499). A large portion of recent biomedical products are more complex designs, often being comprised of a combination of regulated components – drug and device, biologic and device, drug and biologic, or a drug, biologic, and device.
Figure 5.2.
Biomedical regulatory framework in the United States under the Food and Drug Administration (FDA). (A) Organization of the centers and offices of the FDA. (B) Path to the clinic for drugs and biologics. (C) Path to the clinic for devices follows either Premarket Approval (PMA) or the 510(k) process. Adapted by permission from {Pashuck, 2012 #379} published by the American Association for the Advancement of Science.
Devices are governed by the Center for Devices and Radiological Health (CDRH) and must not achieve their primary intended purpose (i.e. diagnosis, treatment, cure, or prevention) through chemical action. In contrast, drugs, which achieve their primary therapeutic effect via chemical action and are not dependent on metabolism, are governed by the Center for Drug Evaluation and Research (CDER). Biologics achieve their intended purpose through biologically-derived materials (e.g., blood, serums, viruses, vaccines, etc.) and are governed by a separate entity, the Center for Biologic Evaluation and Research (CBER).
The time to market, financial costs, and regulatory and commercialization hurdles progressively increase with each stage of moving a product through to commercialization. This can increase exponentially depending on the complexity of the product (Figure 5.3), potentially leading to a significantly reduced commercial viability (500–508). Often, it is easier to engineer a more complex device out of FDA-approved materials rather than starting the regulatory process from the beginning with the use of new materials (508–510).
Figure 5.3.
Representative schematic illustrating the increasing commercial and regulatory difficulties associated with more advanced biomaterial therapeutic designs and devices.
In the case of combination products, the PMOA will determine the regulatory submission strategy and product development framework (499, 503, 508, 511). The decision on PMOA for a combination product can be challenging. Items that need to be considered include the knowledge and risk assessment of the various modes of action. Other considerations include the product’s primary indication (excluding the desire to expand indications post-market), and previous FDA assignment history for comparable products (505, 508, 512). Of the MOA categories, medical devices are the fastest and most resource-sparing way to reach the market (507, 513). This can lead to a significant reduction in time and financial investment.
Drug and Biological Biomaterial Systems:
In terms of clinical translation, the product development framework for drugs and biological systems is often a very expensive and time-consuming process, with many routes for failure along the way (504–506). Despite significant innovation in academic research, the translation of products to clinical trials and marketed products is slow. Regulatory guidelines can also lag behind product innovation. In new frontiers, this can lead to a constantly evolving regulatory framework, adding unanticipated complications and roadblocks to the process (508, 509).
Successful translation from the laboratory to the patient will need to consider risks and potential challenges upfront, during discovery and preclinical testing. As biomedical products increase in complexity, the Chemistry, Manufacturing, and Controls (CMC) section of regulatory submissions plays a pivotal role (508). Challenges in the drug product space include establishing the physicochemical properties (e.g., formulation design, stability, solubility), designing scale-up and GMP production technology (e.g., reproducibility, infrastructure, expertise, cost), and developing quality control assays for characterization (e.g., in-line process analytical technologies) (506, 508, 512). To simplify development pathways from invention to commercialization, researchers often utilize a Quality by Design (QbD) approach to minimize both time and resources. QbD is a scientific, risk-based tool to define a target product profile (TPP) and understand product critical quality attributes (CQAs) upfront, streamlining efforts from discovery through commercialization (508, 512). It emphasizes process control and early risk assessment, which can enable faster reaction as clinical data is obtained.
Biomedical Devices:
Despite substantial advances in material and design innovation, de novo medical devices still face a number of translational, regulatory, manufacturing, and customer-facing challenges. Of these, significant challenges still include balancing power requirements and lightweight device production (especially true for implantable biomedical electronic systems), biocompatibility and implant associated infections, and big data storage and analytics (502, 503, 505, 507, 510, 511, 513). Connection to the patient has been a growing focus of medical device technology. With increased innovation in technology and apps across all aspects of life, there is a strong trend and ever increasing desire for connectivity and patients being more involved in their healthcare decisions. This has led to growing post-regulatory concerns for the data being collected, analyzed, and stored. In terms of big data, this comes with an increasing concern on how to deal with storage of patient data and potential leaking of confidential information.
For device production, a strong patient-focused trend has emerged to produce devices of smaller volume and weight to enhance the comfort and reduce interference with normal activity (503, 510, 511). Devices are now typically required to weigh less than two percent of the total patient body weight (511). In terms of implantable and long-term systems, this can represent a significant challenge to find lightweight alternatives to meet the necessary power requirements. Batteries typically have a limited lifetime and can contribute a significant portion of the overall size and weight of a device (511). As discussed in the previous chapters, there have been significant advances in micro/nanotechnology and novel biomaterials capable of generating sources of energy produced by the physiological environment, motion, or artificial power sources near the patient. Future research will need to focus on further miniaturization and efficiency of power harvesting for high-energy applications such as cardiac pacemakers and deep brain stimulators (510).
5.2. Perspective on Topics for Further Investigation
We believe that this is a vibrant field, with many extensions of future investigation. As we conclude, we offer a perspective on topics within related fields that merit future investigation.
Intelligent and Bioactive Polymers:
Researchers in the fields of intelligent and bioactive polymers have made substantial advancements, particularly on materials that respond reversibly to chemical stimuli. Copolymers with varying degrees of responsiveness to pH, temperature, and solvent compatibility are numerous and have been applied as components of pharmaceutical formulations (102, 514, 515), cosmetic products (402, 516, 517), and medical devices (518–520). Future frontiers in the area of environmentally responsive materials include the design of higher-order structures with intelligent components via 3D and 4D printing technology, as well as the development of hierarchical structures that respond sequentially to multiple chemical stimuli.
We believe that there is also room for substantial research progress in the development of bioactive polymers and polymers for molecular recognition. Future bioactive polymers will interact chemically with nutrients, hormones, and proteins in a mechanism similar to cells and biomacromolecules. These advancements will result in advanced controlled release systems, sensors, and scaffolds for cell programming and proliferation. In particular, the development of nanomaterials and coatings that degrade in response to macromolecular stimuli, such as tissue or disease-specific proteins, will catalyze substantial advancement in bioactive implants and devices.
In this review, we defined molecular recognition as an event where a multiplicity of intermolecular interactions coincide to generate a binding environment with specificity for an analyte. Currently, intelligent polymers are useful for separating biomolecules according to general parameters (hydrophobicity, charge, size) but have varying degrees of cross reactivity when attempting to isolate one particular analyte from a solution of many molecules with a high degree of structural similarity (132, 135, 521, 522). Further advancement in engineering sequence-specific and uniform polymers, networks, blends, and hybrid structures will enable scientists and engineers to better mimic the complex but precise epitopes that proteins employ in natural molecular recognition events.
Lastly, as synthetic biology continues to advance, there will be more opportunity for intersections of molecular engineering and materials science to solve biomedical problems. In particular, hybrid structures of natural and synthetic origin may be generated that will recognize and/or interact with native proteins, provide therapeutic interventions, and respond to external stimuli.
Bioprinting:
On the one hand, the integration of various devices in state-of-the-art bioprinting platforms has advanced tremendously (523–525). On the other hand, however, the process has become complicated with the costs of biofabrication growing exponentially. High-resolution bioprinting is the key for future biofabrication, (i.e. smaller feature size of constructs). Different factors such as advanced extrusion techniques and light-assisted bioprinting together with optimized bioink will be the main factors that will see a major improvement for increasing the overall resolution of bioprinters to print scaffolds with clinical relevance.
Another important area of focus is going to be print speed. Even though some light-assisted printing modalities have shown a substantial improvement in the printing speed of scaffolds, we anticipate a major improvement in this department in the years to come.
One remarkable frontier to look for in the future of applied bioprinting research is the replacement of human organs. Different techniques are underway to print organs like liver, kidney, lungs, and skin. There is a possibility that the technology could alleviate the organ replacement shortage. Other attractive applications in research and industry where bioprinting will play a major role will be in personalized or precision medicine. These platforms, which will enable the mimicry of a single patient or diseased population’s ailment in a dish or fluidic device, may reduce the number of animal and human subjects needed for experimental studies.
The key to all these applications is being able to print of thick tissues with high resolution. A combination of materials science, cell biology, and biophysics is necessary to take on this challenge to move bioprinting from the realm of research laboratories into real-world biomedical applications.
Device Development and Cell Modulation:
To mimic the native tissue environment, novel biomaterial components must support and/or modulate subcellular processes that regulate cellular adhesion, migration, proliferation, differentiation, and signaling of embedded cells(526, 527). It is possible to design appropriately controlled materials that act directly on cells and modulate signaling and phenotype. Stem and progenitor cells can initiate signaling cascades and unique differentiation in response to biomimetic matrices that contain either chemical or biomechanical cues (528, 529). In these applications, the biologically responsive materials act upon the cells through providing a stimulus and triggering cellular response cascades.
Future research in state-of-the art biomaterials chemistry must integrate biosensors and other instrumentation with cell-material constructs to quantify and draw new insights into the biophysical and biochemical aspects of cell-biomaterial interactions as they relate to system/device development. These findings will inform the design of next-generation materials for biosensing and response, targeted drug delivery, and tissue engineering.
Ultimately, the second-generation application of smart materials will be for screening and detecting secreted protein levels, while in a controllable and predictable manner eluting growth factors or nutrients that enhance the bioprocess (i.e. production of a pharmaceutical, differentiation to desired cell lineage, alteration of proliferation). These materials may be able to sense a deleterious cell population (i.e. one that lacks of production capacity or has differentiated in a non-ideal manner) and respond with apoptotic signaling molecules. These biotechnological applications will require, as previously described, environmentally responsive materials, rationally designed assemblies, and bioactive interfaces.
Biomimicry for Medical Devices:
There has been a tremendous progress toward developing dynamic and intelligent bio-hybrid machines and integrating these cell-laden devices with existing medical technology to enhance their utilities. We presented examples in this report of how intelligent bio-hybrid machines can dynamically deform their shape, sense biological signals, or release pharmaceutical compounds on demand. Engineered bio-hybrid machines have been developed through a combination of advanced functional materials and nano-/micro-fabrication methods that in synergy mimic the complex architectures and physiological properties of living organisms.
Consequently, biomimetic actuators and devices have been used in research applications to repair damaged tissues, replace organs, and treat diseases (520, 530, 531). However, recently developed bio-hybrid machines fall short of solving some major clinical challenges. For improved performance of bio-hybrid machines, they have to accurately predict or monitor responses of the medical devices in the human body. As a result, the engineered bio-hybrid machines have to control biological signals or messengers such as action potentials, neurotransmitter signals, and hormones in an intelligent manner.
Most of the present-engineered intelligent machines suffer an improper or insufficient interface between machine and the human body. To address this problem, future studies must borrow inspiration from nature and concepts from materials/engineering community. For example, direct integration of neural networks or bioreceptors into engineered bio-hybrid machines will be required to sense electrophysiological, chemical or biological cues. Intelligent, bioresponsive materials have to be designed and properly incorporated into the engineered platforms. Electronic-based controlling systems such as flexible electronic devices, implantable biosensors, and microactuators are another option for achieving higher-order engineering control.
All of these developments will require nano- or micro-fabrication techniques using biocompatible and intelligent materials to integrate biomimetic systems with the human body (i.e. for biomimetic devices to communicate with tissues and organs). In the future electronic-based controlling systems can adopt deep learning algorithms for further enhanced prediction and communication. A combination of multidisciplinary approaches is strongly required to develop intelligent medical devices of the future, which can solve major problems in challenging clinical settings.
5.3. Conclusions
As we summarize and conclude this report, it is useful to reconsider the framework for design and evaluation of bioinspired and biomimetic systems that was presented in Chapter 1. In the introduction, we stepped through each stage of inquiry and investigation. We described how a material’s composition and fabrication dictates its interaction with the physiological environment. This ‘interaction’ can include an actuation step, where a biomaterial scaffold converts a biological into a physical output. Mechanical outputs, such as swelling or disassembly, can serve as a signal indicator or induce a therapeutic intervention in a wearable, implantable, or injectable device. As illustration, we highlighted closed-loop insulin delivery for the treatment of diabetes.
Adding another degree of intelligence or responsiveness, as well as engineering intervention, we described how material transducers serve as scaffolds for cell modulation and tissue mimesis. The scaffold can deliver a chemical or mechanical cue that drives cellular behavior, present ligands that enable cell adhesion, infiltration, alignment, and/or proliferation, and/or contain conductive components that deliver electrical signals. Modular or hierarchical cell-material constructs are fabricated by synthesis, assembly, or printing in the presence or absence of biological components (i.e. proteins, lipids, cells). Optogenetics, and other genetic engineering techniques, enable enhanced control over cellular activity or function.
The majority of this review progressed, in bottom-up analysis, through material-biological communication (i.e. environmental responsiveness of biomaterials), material processing and fabrication (i.e. engineering useful constructs out of environmentally responsive components) and biologically inspired devices (i.e. using materials science, micro/nano fabrication, and cellular engineering to solve medical problems). We felt that this style and analytical sequence was logical. It progressed from fundamental principles to applied science; from molecules, to biomaterials, to complex machinery; from well-known thermodynamic laws to novel work-in-progress devices.
The future of bioinspired therapeutics, biomimetic actuators, and biological machinery, however, is top-down. Inspired by sophisticated bioprocess and motivated by unmet medical needs, scientists and engineers will be able to invent solutions using rational combinations of synthetic materials, natural molecules, and cells. As the field develops solutions, we will also uncover new trends, principles, and/or relationships between chemistry, structure, and function. As a result, our cumulative understanding of engineering fundamentals will strengthen.
We believe that the future of bioinspired and biomimetic devices will be vibrant, with substantial growth in basic science and translational research. It is our hope that this review will serve as an organized resource for the field, documenting the current knowledge and engineering approaches for building biohybrid devices and bioactive interfaces.
Figure 2.8:
In SELEX, aptamer libraries are filtered for specific binding to an analyte or surface, and are then amplified by RT-PCR. Following amplification, the process can be iterated (i.e. to arrive on consensus high-affinity ligands), or sequenced (i.e. to characterize the output) Reprinted by permission from (155) published by Elsevier.
Acknowledgements:
The authors gratefully acknowledge Matthew Miller (UT Austin) for his critical review of this manuscript.
J.R.C., A.M.W. and N.A.P gratefully acknowledge funding from the National Institutes of Health (NIH) (EB000246, EB012726, EB022025, GM 56321), the NIH/NCI Center for Oncophysics (Grant CT O PSOC U54-CA-143837), the National Science Foundation (DGE-03–33080) and the UT-Portugal Collaborative Research Program. A.K., S.R.S., and S.H. gratefully acknowledge funding from the National Institutes of Health (NIH) (EB012597, AR057837, DE021468, HL099073, R56AI105024, EB021857). J.R.C. and A.M.W. were supported by National Science Foundation Graduate Research Fellowships (DGE-1610403). A.M.W. would like to acknowledge the S.E.S.H.A. Endowed Graduate Fellowship in Engineering and the Philanthropic Educational Organization Scholar Award. S.R.S. would like to acknowledge Brigham and Women’s Hospital President Betsy Nabel, MD, and the Reny family, for the Stepping Strong Innovator Award through their generous funding, and Air Force Office of Sponsored Research under award (FA9550-15-1-0273). S.H. would like to acknowledge Swiss National Science Foundation (SNSF) for funding. N.A.P. acknowledges financial support from the Cockrell Regent’s Family Chair in Engineering (UT Austin).
List of Acronyms (in order of appearance):
- P(MAA)
Polymethacrylic acid
- GOx
Glucose oxidase
- PEG
Polyethylene glycol
- PMMA
Poly(methyl methacrylate)
- PS
Poly(styrene)
- PDMAEMA
Poly(dimethylaminoethyl methacrylate)
- PAA
Poly(acrylic acid)
- IA
Itaconic acid
- EI
Ethylene imine
- DEAEMA
Diethylaminoethyl methacrylate
- HPMCAS
Hydroxypropyl methylcellulose acetate succinate
- ROS
Reactive oxygen species
- RNS
Reactive nitrogen species
- NOS
Nitric oxide synthases
- NIPAAm
N-isopropyl acrylamide
- LCST
Lower critical solution temperature
- UCST
Upper critical solution temperature
- LSPR
Localized surface plasmon resonance
- ECM
Extracellular matrix
- DTPA
Diethylenetriaminepentaacetic acid
- NVP
N-vinyl pyrrolidone
- MMP
Matrix metalloproteinase
- SELEX
Systematic evolution of ligands by exponential enrichment
- ssDNA
Single stranded DNA
- VEGF
Vascular endothelial growth factor
- RT-PCR
Reverse transcription polymerase chain reaction
- t-Boc
tert-butyloxycarbonyl
- Fmoc
9-fluorenylmethyoxycarbonyl
- SAM
Self-assembled monolayer
- AFM
Atomic force microscopy
- PDMS
Poly(dimethylsiloxane)
- MLV
Multilamellar vesicle
- LUV
Large unilamellar vesicle
- SUV
Small unilamellar vesicle
- CAD
Computer Aided Design
- CIJ
Continuous inkjet
- DOD
Drop on demand
- TPL
Two-photon-induced luminescence
- SERS
Surface enhanced Raman spectroscopy
- LIGA
Lithographie Gavanoformung Abformung
- Ra
Roughness average
- MEMS
Micro-electro-mechanical systems
- EBL
Electron Beam Lithography
- PCL
Polycaprolactone
- FIBL
Focused Ion Beam Lithography
- SNOM
Scanning Near Field Optical Microscopy
- NIL
Nanoimprint Lithography
- CL
Colloidal Lithography
- REM
Replica Molding
- PEGDA
Poly(ethylene glycol) diacrylate
- LIFT
Laser-induced forward transfer
Particle replication in non-wetting templates
- PFPE
Perfluoropolyether
- PLA
Polylactic acid
- DMD
Digital micromirror device
- CAGE
Choline and geranate
- BSA
Bovine serum albumin
- SPACE
Skin penetrating and cell entering
- siRNA
Small interfering RNA
- OCT
Optical coherence tomography
- CT
Computed tomography
- CECm
Methacrylamide N-carboxyethyl chitosan
- HTCCm
N-(2-hydroxy)propyl-3-trimethylammonium chitosan chloride
- HP-β-CD
2-hydroxypropyl-β-cyclodextrin
- MRI
Magnetic resonance imaging
- GDH
Glucose-1-dehydrogenase
- CNT
Carbon nanotube
- ECG
Electrocardiography
- EEG
Electroencephalogram
- PEDOT
Poly(3,4-ethylene dioxythiophene)
- EAP
Electroactive polymers
- S. typhimurium
Salmonella typhimurium
- E. coli
Escherichia co
- MTB
Magnetotactic bacteria
- S. marcescens
Serratia marcescens
- ITOP
Integrated tissue-organ printer
- GNR
Gold nanorod
- GelMA
Gelatin methacryloyl
- iPS
Induced pluripotent stem
- ChR2
Channelrhododopsin-2
- FDA
Food and Drug Administration
- PMOA
Primary Mode of Action
- MOA
Mode of Action
- CDRH
Center for Devices and Radiological Health
- CDER
Center for Drug Evaluation and Research
- CBER
Center for Biologic Evaluation and Research
- CMC
Chemistry, Manufacturing, and Controls
- GMP
Good Manufacturing Practice
- QbD
Quality by Design
- TPP
Target Product Profile
- CQA
Critical Quality Attributes
Biographies

John R. Clegg is a Ph.D. candidate and NSF Graduate Research Fellow in the Department of Biomedical Engineering at the University of Texas at Austin. John received a BS in biomedical engineering with honors from the University of South Carolina in 2014, an MSE in biomedical engineering from the UT Austin in 2016, and an MA in Science, Technology, Engineering, and Mathematics Education from UT Austin in 2018. John’s dissertation focusses on biomimetic hydrogels for drug delivery applications and predictive models for protein adsorption to biomaterials. His research interests include polymer engineering, molecular recognition, drug delivery, and engineering education.

Angela M. Wagner is a Ph.D. candidate and National Science Foundation Graduate Research Fellow in the McKetta Department of Chemical Engineering at The University of Texas at Austin, working under the guidance of Professor Nicholas Peppas. She received her B.S. in Chemical Engineering at The Pennsylvania State University, and earned her M.S.E. in Chemical Engineering at The University of Texas at Austin. Angela’s current research focuses on developing multi-responsive hydrogel systems as treatment agents for ovarian cancer, and her research interests include drug delivery, immunotherapy, and vaccine development.

Su Ryon Shin is the Instructor of Medicine, Division of Engineering in Medicine at the Harvard Medical School. She is one of the most innovative and productive young faculty in regenerative medicine and biomedical engineering, with a growing international reputation for her accomplishments. Dr. Shin is fully committed to address major challenges head-on by using an interdisciplinary approach at the interface between engineering, nanomaterials science, and biology for both therapeutic purposes and in vitro studies. She is interested developing multifunctional scaffolds, biosensors, bioactuators, and organs-on-chip systems using microscale biomaterials and engineering (https://www.suryonshin.com/).

Shabir Hassan completed his bachelor’s in chemistry, biology and English from University of Kashmir and his master’s in biotechnology from University of Pune. After a year of research in molecular biology through LSZGS of UZH/ETH Zurich, he did his PhD in ultrafast laser spectroscopy to study protein folding, aggregation and photothermal effects in proteins in real time. After his PhD, he won the Swiss National Science Foundation (SNSF) Early and Advanced awards in 2016 and 2018, respectively to carry out his research at Harvard Medical School and HST-MIT in drug and metabolite delivery targeting diseases and other medical conditions.

Ali Khademhosseini is the Levi Knight Professor of Bioengineering, Chemical Engineering and Radiology at the University of California-Los Angeles (UCLA). He is the Founding Director of the Center for Minimally Invasive Therapeutics (C-MIT) at UCLA and the Associate Director of the California NanoSystems Institute. Previously, he was a Professor of Medicine at Harvard Medical School. He is recognized as a leader in combining micro- and nano-engineering approaches with advanced biomaterials for regenerative medicine applications. He is interested in developing ‘personalized’ solutions that utilize micro- and nanoscale technologies to enable a range of therapies for organ failure, cardiovascular disease and cancer. In enabling this vision, he works closely with clinicians including interventional radiologists, cardiologists and surgeons. Read more at: http://www.tissueeng.net/.

Nicholas A. Peppas’ research spans a career of 40 years and has concentrated on the elucidation of transport phenomena (notably diffusion and convection problems) in biological and chemical problems with emphasis on the integration of mathematical models and engineering design with cellular processes. He is particularly recognized for his pioneering work on polymer networks and hydrogels. He received his Dipl. Eng. at the National Technical University of Athens in 1971, Greece, and received his Sc.D. at the Massachusetts Institute Technology in 1973. He was professor at Purdue University from 1976–2002, and has been a professor at the University of Texas at Austin since 2003. He is a member of the U.S. NAM, NAE, NAI, IOM, AAAS, the National Academy of France, the Academy of Athens, the Royal Academy of Spain, the Chinese Academy of Medical Sciences, and the Academy of Medicine, Engineering and Sciences of Texas. He has been recognized with more than 120 national and international awards. He is the author of over 1,450 refereed publications and 37 patents, and he has been cited over 102,000 times with an H-index of 149.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Huggins ML. Some Properties of Solutions of Long-chain Compounds. The Journal of Physical Chemistry. 1942;46(1):151–8. [Google Scholar]
- 2.Flory PJ. Thermodynamics of High Polymer Solutions. The Journal of Chemical Physics. 1942;10(1):51–61. [Google Scholar]
- 3.Flory PJ Jr. JR. Statistical Mechanics of Cross-Linked Polymer Networks II. Swelling. The Journal of Chemical Physics. 1943;11(11):521–6. [Google Scholar]
- 4.Polyelectrolyte Intelligent Gels: Design and Applications. Ionic Interactions in Natural and Synthetic Macromolecules. [Google Scholar]
- 5.Koetting MC, Peters JT, Steichen SD, Peppas NA. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Materials Science and Engineering: R: Reports. 2015;93:1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liechty WB, Kryscio DR, Slaughter BV, Peppas NA. Polymers for Drug Delivery Systems. Annu Rev Chem Biomol Eng. 2010;1(1):149–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharpe LA, Daily AM, Horava SD, Peppas NA. Therapeutic applications of hydrogels in oral drug delivery. Expert Opinion on Drug Delivery. 2014;11(6):901–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peppas NA, Van Blarcom DS. Hydrogel-based biosensors and sensing devices for drug delivery. Journal of Controlled Release. [DOI] [PubMed] [Google Scholar]
- 9.Fisher OZ, Khademhosseini A, Peppas NA. Drug Delivery: Nanoscale Devices. Encyclopedia of Materials: Science and Technology: Elsevier; 2010. p. 1–9. [Google Scholar]
- 10.Sharp KA, Honig B. Electrostatic interactions in macromolecules: theory and applications. Annual review of biophysics and biophysical chemistry. 1990;19(1):301–32. [DOI] [PubMed] [Google Scholar]
- 11.Phillips R, Kondev J, Theriot J, Garcia H. Physical biology of the cell: Garland Science; 2012. [Google Scholar]
- 12.Honig B, Nicholls A. Classical electrostatics in biology and chemistry. Science. 1995;268(5214):1144–9. [DOI] [PubMed] [Google Scholar]
- 13.Seyrek E, Dubin PL, Tribet C, Gamble EA. Ionic strength dependence of proteinpolyelectrolyte interactions. Biomacromolecules. 2003;4(2):273–82. [DOI] [PubMed] [Google Scholar]
- 14.Steiner T The hydrogen bond in the solid state. Angewandte Chemie International Edition. 2002;41(1):48–76. [DOI] [PubMed] [Google Scholar]
- 15.Wagner G, Pardi A, Wuethrich K. Hydrogen bond length and proton NMR chemical shifts in proteins. Journal of the American Chemical Society. 1983;105(18):5948–9. [Google Scholar]
- 16.Kortemme T, Morozov AV, Baker D. An orientation-dependent hydrogen bonding potential improves prediction of specificity and structure for proteins and protein–protein complexes. Journal of molecular biology. 2003;326(4):1239–59. [DOI] [PubMed] [Google Scholar]
- 17.Chothia C, Janin J. Principles of protein–protein recognition. Nature. 1975;256(5520):705. [DOI] [PubMed] [Google Scholar]
- 18.Chothia C Hydrophobic bonding and accessible surface area in proteins. Nature. 1974;248(5446):338. [DOI] [PubMed] [Google Scholar]
- 19.Némethy G, Scheraga HA. The structure of water and hydrophobic bonding in proteins. iii. The thermodynamic properties of hydrophobic bonds in proteins1, 2. The Journal of Physical Chemistry. 1962;66(10):1773–89. [Google Scholar]
- 20.Kauzmann W Some Factors in the Interpretation of Protein Denaturation1 Advances in protein chemistry. 14: Elsevier; 1959. p. 1–63. [DOI] [PubMed] [Google Scholar]
- 21.Menzies KL, Jones L. The impact of contact angle on the biocompatibility of biomaterials. Optometry and Vision Science. 2010;87(6):387–99. [DOI] [PubMed] [Google Scholar]
- 22.Pozzi D, Colapicchioni V, Caracciolo G, Piovesana S, Capriotti AL, Palchetti S, et al. Effect of polyethyleneglycol (PEG) chain length on the bio–nano-interactions between PEGylated lipid nanoparticles and biological fluids: from nanostructure to uptake in cancer cells. Nanoscale. 2014;6(5):2782–92. [DOI] [PubMed] [Google Scholar]
- 23.Clegg JR, Wechsler ME, Peppas NA. Vision for Functionally Decorated and Molecularly Imprinted Polymers in Regenerative Engineering. Regenerative Engineering and Translational Medicine. 2017;3(3):166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feynman RP. Statistical mechanics: a set of lectures. 2018. [Google Scholar]
- 25.Erickson HP. Co-operativity in protein-protein association: The structure and stability of the actin filament. Journal of molecular biology. 1989;206(3):465–74. [DOI] [PubMed] [Google Scholar]
- 26.Doolittle RF. The multiplicity of domains in proteins. Annual review of biochemistry. 1995;64(1):287–314. [DOI] [PubMed] [Google Scholar]
- 27.Verheyen E, Schillemans JP, van Wijk M, Demeniex M-A, Hennink WE, van Nostrum CF. Challenges for the effective molecular imprinting of proteins. Biomaterials. 2011;32(11):3008–20. [DOI] [PubMed] [Google Scholar]
- 28.Martino MM, Briquez PS, Güç E, Tortelli F, Kilarski WW, Metzger S, et al. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science. 2014;343(6173):885–8. [DOI] [PubMed] [Google Scholar]
- 29.Wiedemann U, Boisguerin P, Leben R, Leitner D, Krause G, Moelling K, et al. Quantification of PDZ domain specificity, prediction of ligand affinity and rational design of super-binding peptides. Journal of molecular biology. 2004;343(3):703–18. [DOI] [PubMed] [Google Scholar]
- 30.Young T, Abel R, Kim B, Berne BJ, Friesner RA. Motifs for molecular recognition exploiting hydrophobic enclosure in protein–ligand binding. Proceedings of the National Academy of Sciences. 2007;104(3):808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christau S, Thurandt S, Yenice Z, von Klitzing R. Stimuli-Responsive Polyelectrolyte Brushes As a Matrix for the Attachment of Gold Nanoparticles: The Effect of Brush Thickness on Particle Distribution. Polymers. 2014;6(7):1877. [Google Scholar]
- 32.Merlitz H, He G-L, Sommer J-U, Wu C-X. Reversibly Switchable Polymer Brushes with Hydrophobic/Hydrophilic Behavior: A Langevin Dynamics Study. Macromolecules. 2009;42(1):445–51. [Google Scholar]
- 33.Ferhan AR, Zainol N, Kim D-H. A facile method towards rough morphology polymer brush for increased mobility of embedded nanoparticles. Polymer. 2015;75:57–63. [Google Scholar]
- 34.Advincula RC. Polymer brushes : synthesis, characterization, applications. Weinheim: Wiley-VCH; 2004. xxiii, 483 p. p. [Google Scholar]
- 35.Adiga SP, Brenner DW. Stimuli-Responsive Polymer Brushes for Flow Control through Nanopores. Journal of Functional Biomaterials. 2012;3(2):239–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elizabeth WM K OC. Responsive and patterned polymer brushes. J Polym Sci, Part B: Polym Phys. 2013;51(20):1457–72. [Google Scholar]
- 37.Hidenori K, Ihor T, Dmytro N, Ekaterina Z, Sergiy M. Stimuli-Responsive Materials with Self-Healing Antifouling Surface via 3D Polymer Grafting. Adv Funct Mater. 2013;23(36):4593–600. [Google Scholar]
- 38.Wagner AM, Gran MP, Peppas NA. Designing the new generation of intelligent biocompatible carriers for protein and peptide delivery. Acta Pharmaceutica Sinica B. 2018;8(2):147–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WA M, SD S, PN A. Advanced architectures in the design of responsive polymers for cancer nanomedicine. Journal of Applied Polymer Science. 2018;135(24):46154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bazban-Shotorbani S, Hasani-Sadrabadi MM, Karkhaneh A, Serpooshan V, Jacob KI, Moshaverinia A, et al. Revisiting structure-property relationship of pH-responsive polymers for drug delivery applications. Journal of Controlled Release. 2017;253:46–63. [DOI] [PubMed] [Google Scholar]
- 41.Monleón Pradas M, Vicent MJ. Polymers in regenerative medicine : biomedical applications from nano- to macro-structures. Hoboken, New Jersey: Wiley; 2015. xx, 393 pages p. [Google Scholar]
- 42.Kocak G, Tuncer C, Butun V. pH-Responsive polymers. Polym Chem. 2017;8(1):144–76. [Google Scholar]
- 43.Reddy N, Reddy R, Jiang Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015;33(6):362–9. [DOI] [PubMed] [Google Scholar]
- 44.Yadav P, Yadav H, Shah VG, Shah G, Dhaka G. Biomedical Biopolymers, their Origin and Evolution in Biomedical Sciences: A Systematic Review. Journal of Clinical and Diagnostic Research : JCDR. 2015;9(9):ZE21–ZE5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Falde EJ, Yohe ST, Colson YL, Grinstaff MW. Superhydrophobic materials for biomedical applications. Biomaterials. 2016;104:87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raja STK, Thiruselvi T, Mandal AB, Gnanamani A. pH and redox sensitive albumin hydrogel: A self-derived biomaterial. Scientific Reports. 2015;5:15977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phillips MA, Gran ML, Peppas NA. Targeted nanodelivery of drugs and diagnostics. Nano Today. 2010;5(2):143–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshida T, Shakushiro K, Sako K. Ion-Responsive Drug Delivery Systems. Curr Drug Targets. 2018;19(3):225–38. [DOI] [PubMed] [Google Scholar]
- 49.Terefe NS, Glagovskaia O, De Silva K, Stockmann R. Application of stimuli responsive polymers for sustainable ion exchange chromatography. Food Bioprod Process. 2014;92(2):208–25. [Google Scholar]
- 50.Schroffenegger M, Zirbs R, Kurzhals S, Reimhult E. The Role of Chain Molecular Weight and Hofmeister Series Ions in Thermal Aggregation of Poly(2-Isopropyl-2-Oxazoline) Grafted Nanoparticles. Polymers. 2018;10(4):451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willott JD, Murdoch TJ, Humphreys BA, Edmondson S, Wanless EJ, Webber GB. Anion-Specific Effects on the Behavior of pH-Sensitive Polybasic Brushes. Langmuir : the ACS journal of surfaces and colloids. 2015;31(12):3707–17. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida T, Lai TC, Kwon GS, Sako K. pH- and ion-sensitive polymers for drug delivery. Expert opinion on drug delivery. 2013;10(11):1497–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swann JMG, Bras W, Topham PD, Howse JR, Ryan AJ. Effect of the Hofmeister Anions upon the Swelling of a Self-Assembled pH-Responsive Hydrogel. Langmuir : the ACS journal of surfaces and colloids. 2010;26(12):10191–7. [DOI] [PubMed] [Google Scholar]
- 54.Magnusson JP, Khan A, Pasparakis G, Saeed AO, Wang W, Alexander C. Ion-Sensitive “Isothermal” Responsive Polymers Prepared in Water. J Am Chem Soc. 2008;130(33):10852–3. [DOI] [PubMed] [Google Scholar]
- 55.Li Y, Wang Z, Wei Q, Luo M, Huang G, Sumer BD, et al. Non-covalent interactions in controlling pH-responsive behaviors of self-assembled nanosystems. Polym Chem. 2016;7(38):5949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang YJ, Furyk S, Bergbreiter DE, Cremer PS. Specific ion effects on the water solubility of macromolecules: PNIPAM and the Hofmeister series. J Am Chem Soc. 2005;127(41):14505–10. [DOI] [PubMed] [Google Scholar]
- 57.Gibb CLD, Oertling EE, Velaga S, Gibb BC. Thermodynamic Profiles of Salt Effects on a Host–Guest System: New Insight into the Hofmeister Effect. The Journal of Physical Chemistry B. 2015;119(17):5624–38. [DOI] [PubMed] [Google Scholar]
- 58.Hofmeister F Zur Lehre von der Wirkung der Salze. Archiv für experimentelle Pathologie und Pharmakologie. 1888;24(4):247–60. [Google Scholar]
- 59.Cacace MG, Landau EM, Ramsden JJ. The Hofmeister series: salt and solvent effects on interfacial phenomena. Q Rev Biophys. 1997;30(3):241–77. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, Cremer PS. Interactions between macromolecules and ions: the Hofmeister series. Curr Opin Chem Biol. 2006;10(6):658–63. [DOI] [PubMed] [Google Scholar]
- 61.Wang JL, Chen C. Biosorbents for heavy metals removal and their future. Biotechnol Adv. 2009;27(2):195–226. [DOI] [PubMed] [Google Scholar]
- 62.Gadd GM. Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. J Chem Technol Biotechnol. 2009;84(1):13–28. [Google Scholar]
- 63.Cui JR, Zhang LF. Metallurgical recovery of metals from electronic waste: A review. J Hazard Mater. 2008;158(2–3):228–56. [DOI] [PubMed] [Google Scholar]
- 64.Nagarale RK, Gohil GS, Shahi VK. Recent developments on ion-exchange membranes and electro-membrane processes. Adv Colloid Interface Sci. 2006;119(2–3):97–130. [DOI] [PubMed] [Google Scholar]
- 65.Volesky B Detoxification of metal-bearing effluents: biosorption for the next century. Hydrometallurgy. 2001;59(2–3):203–16. [Google Scholar]
- 66.Fox JM, Kang K, Sherman W, Héroux A, Sastry GM, Baghbanzadeh M, et al. Interactions between Hofmeister Anions and the Binding Pocket of a Protein. J Am Chem Soc. 2015;137(11):3859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lutter JC, Wu T-y, Zhang Y. Hydration of Cations: A Key to Understanding of Specific Cation Effects on Aggregation Behaviors of PEO-PPO-PEO Triblock Copolymers. The Journal of Physical Chemistry B. 2013;117(35):10132–41. [DOI] [PubMed] [Google Scholar]
- 68.Price C, Carroll J, Clare TL. Chemoresistive and photonic hydrogel sensors of transition metal ions via Hofmeister series principles. Sensors Actuators B: Chem. 2018;256:870–7. [Google Scholar]
- 69.Lin Q, Liu L, Liu J, Zheng F, Zhang YM, Yao H, et al. An efficient iodide ion chemosensor and a rewritable dual-channel security display material based on an ion responsive supramolecular gel. Rsc Advances. 2017;7(61):38210–5. [Google Scholar]
- 70.Tang S, Tang L, Lu X, Liu H, Moore JS. Programmable Payload Release from Transient Polymer Microcapsules Triggered by a Specific Ion Coactivation Effect. J Am Chem Soc. 2018;140(1):94–7. [DOI] [PubMed] [Google Scholar]
- 71.Long-Hai W, Zi-Dan Z, Chun-Yan H, Xue-Hao H, Wei Y, Ye-Zi Y. Anion–Dipole Interactions Make the Homopolymers Self-Assemble into Multiple Nanostructures. Adv Mater. 2015;27(20):3202–7. [DOI] [PubMed] [Google Scholar]
- 72.del Mercato LL, Ferraro MM, Baldassarre F, Mancarella S, Greco V, Rinaldi R, et al. Biological applications of LbL multilayer capsules: From drug delivery to sensing. Adv Colloid Interface Sci. 2014;207:139–54. [DOI] [PubMed] [Google Scholar]
- 73.Lomora M, Garni M, Itel F, Tanner P, Spulber M, Palivan CG. Polymersomes with engineered ion selective permeability as stimuli-responsive nanocompartments with preserved architecture. Biomaterials. 2015;53:406–14. [DOI] [PubMed] [Google Scholar]
- 74.Chonkar A, Nayak U, Udupa N. Smart Polymers in Nasal Drug Delivery. Indian Journal of Pharmaceutical Sciences. 2015;77(4):367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou SZ, Bismarck A, Steinke JHG. Ion-responsive alginate based macroporous injectable hydrogel scaffolds prepared by emulsion templating. Journal of Materials Chemistry B. 2013;1(37):4736–45. [DOI] [PubMed] [Google Scholar]
- 76.Theogarajan L, Li HS, Busse K, Desai S, Kressler J, Scholz C. Self-assembly of ABA triblock copolymers based on functionalized polydimethylsiloxane and polymethyloxazoline. Polym Int. 2010;59(9):1191–8. [Google Scholar]
- 77.Kazakov S, Kaholek M, Levon K. Nanometer scale ionic reservoir based on ion-responsive hydrogels In: BarCohen Y, editor. Smart Structures and Materials 2002: Electroactive Polymer Actuators and Devices. Proceedings of the Society of Photo-Optical Instrumentation Engineers (Spie) 46952002. p. 42–51. [Google Scholar]
- 78.Saxena A, Tripathi BP, Kumar M, Shahi VK. Membrane-based techniques for the separation and purification of proteins: An overview. Adv Colloid Interface Sci. 2009;145(1–2):1–22. [DOI] [PubMed] [Google Scholar]
- 79.Magalhaes PO, Lopes AM, Mazzola PG, Rangel-Yagui C, Penna TCV, Pessoa A. Methods of endotoxin removal from biological preparations: a review. J Pharm Pharm Sci. 2007;10(3):388–404. [PubMed] [Google Scholar]
- 80.Hubbuch J, Kula MR. Isolation and purification of biotechnological products. J Non-Equilib Thermodyn. 2007;32(2):99–127. [Google Scholar]
- 81.Leblanc Y, Ramon C, Bihoreau N, Chevreux G. Charge variants characterization of a monoclonal antibody by ion exchange chromatography coupled on-line to native mass spectrometry: Case study after a long-term storage at+5 degrees C. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences. 2017;1048:130–9. [DOI] [PubMed] [Google Scholar]
- 82.Walter JG, Tappe A, Kasper C, Zeidler R, Reif OW, Scheper T. Human growth hormone (HGH) purification from CHO-cell culture supernatant utilizing macroporous chromatographic media. Smith R, editor2007. 635–+ p. [Google Scholar]
- 83.Ottens M, Houwing J, Van Hateren SH, Van Baalenz T, Van Der Wielen LAM. Multi-component fractionation in SMB chromatography for the purification of active fractions from protein hydrolysates. Food Bioprod Process. 2006;84(C1):59–71. [Google Scholar]
- 84.Shao H, Wang CF, Zhang J, Chen S. Fabrication of Reversible Phase Transition Polymer Gels toward Metal Ion Sensing. Macromolecules. 2014;47(5):1875–81. [Google Scholar]
- 85.Jiang Zheng W, Gao J, Wei Z, Zhou J, Chen Y. Facile fabrication of self-healing carboxymethyl cellulose hydrogels 2015. [Google Scholar]
- 86.Tao W, He Z. ROS-responsive drug delivery systems for biomedical applications. Asian Journal of Pharmaceutical Sciences. 2018;13(2):101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Commoner B, Townsend J, Pake GE. Free radicals in biological materials. Nature. 1954;174(4432):689–91. [DOI] [PubMed] [Google Scholar]
- 88.Tsarevsky NV, Matyjaszewski K. Combining atom transfer radical polymerization and disulfide/thiol redox chemistry: A route to well-defined (bio)degradable polymeric materials. Macromolecules. 2005;38(8):3087–92. [Google Scholar]
- 89.Tsarevsky NV, Matyjaszewski K. Reversible redox cleavage/coupling of polystyrene with disulfide or thiol groups prepared by atom transfer radical polymerization. Macromolecules. 2002;35(24):9009–14. [Google Scholar]
- 90.Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Medicine and Cellular Longevity. 2016;2016:1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jinqiang W, Yuqi Z, Edikan A, S. LF, Zhen G Leveraging H2O2 Levels for Biomedical Applications. Advanced Biosystems. 2017;1(9):1700084. [DOI] [PubMed] [Google Scholar]
- 92.Hyun LS, K. GM, Beum BJ, Hojae B, Hak-Joon S. Current Progress in Reactive Oxygen Species (ROS)-Responsive Materials for Biomedical Applications. Advanced Healthcare Materials. 2013;2(6):908–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Akimoto J, Nakayama M, Okano T. Temperature-responsive polymeric micelles for optimizing drug targeting to solid tumors. Journal of Controlled Release. 2014;193:2–8. [DOI] [PubMed] [Google Scholar]
- 94.Liu L, Ghaemi A, Gekle S, Agarwal S. One-Component Dual Actuation: Poly (NIPAM) Can Actuate to Stable 3D Forms with Reversible Size Change. Advanced Materials. 2016;28(44):9792–6. [DOI] [PubMed] [Google Scholar]
- 95.Halperin A, Kröger M, Winnik FM. Poly (N-isopropylacrylamide) phase diagrams: fifty years of research. Angewandte Chemie International Edition. 2015;54(51):15342–67. [DOI] [PubMed] [Google Scholar]
- 96.Koetting MC, Peters JT, Steichen SD, Peppas NA. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Materials Science and Engineering: R: Reports. 2015;93(Supplement C):1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Plamper FA, Ballauff M, Müller AH. Tuning the thermoresponsiveness of weak polyelectrolytes by pH and light: lower and upper critical-solution temperature of poly (N, N-dimethylaminoethyl methacrylate). Journal of the American Chemical Society. 2007;129(47):14538–9. [DOI] [PubMed] [Google Scholar]
- 98.Bloksma MM, Bakker DJ, Weber C, Hoogenboom R, Schubert US. The Effect of Hofmeister Salts on the LCST Transition of Poly (2-oxazoline) s with Varying Hydrophilicity. Macromolecular rapid communications. 2010;31(8):724–8. [DOI] [PubMed] [Google Scholar]
- 99.Schlaad H, Diehl C, Gress A, Meyer M, Demirel AL, Nur Y, et al. Poly (2-oxazoline) s as Smart Bioinspired Polymers. Macromolecular rapid communications. 2010;31(6):511–25. [DOI] [PubMed] [Google Scholar]
- 100.Lu Y, Aimetti AA, Langer R, Gu Z. Bioresponsive materials. Nature Reviews Materials. 2017;2(1):16075. [Google Scholar]
- 101.Klouda L Thermoresponsive hydrogels in biomedical applications: A seven-year update. European Journal of Pharmaceutics and Biopharmaceutics. 2015;97:338–49. [DOI] [PubMed] [Google Scholar]
- 102.Gandhi A, Paul A, Sen SO, Sen KK. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. asian journal of pharmaceutical sciences. 2015;10(2):99–107. [Google Scholar]
- 103.Weber C, Hoogenboom R, Schubert US. Temperature responsive bio-compatible polymers based on poly (ethylene oxide) and poly (2-oxazoline) s. Progress in Polymer Science. 2012;37(5):686–714. [Google Scholar]
- 104.Seuring J, Agarwal S. Polymers with upper critical solution temperature in aqueous solution. Macromolecular rapid communications. 2012;33(22):1898–920. [DOI] [PubMed] [Google Scholar]
- 105.Ge Z, Liu S. Functional block copolymer assemblies responsive to tumor and intracellular microenvironments for site-specific drug delivery and enhanced imaging performance. Chemical Society Reviews. 2013;42(17):7289–325. [DOI] [PubMed] [Google Scholar]
- 106.Regmi R, Bhattarai SR, Sudakar C, Wani AS, Cunningham R, Vaishnava PP, et al. Hyperthermia controlled rapid drug release from thermosensitive magnetic microgels. Journal of Materials Chemistry. 2010;20(29):6158–63. [Google Scholar]
- 107.Strong LE, West JL. Thermally responsive polymer–nanoparticle composites for biomedical applications. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2011;3(3):307–17. [DOI] [PubMed] [Google Scholar]
- 108.Kaur G, Adhikari R, Cass P, Bown M, Gunatillake P. Electrically conductive polymers and composites for biomedical applications. RSC Advances. 2015;5(47):37553–67. [Google Scholar]
- 109.Balint R, Cassidy NJ, Cartmell SH. Conductive polymers: towards a smart biomaterial for tissue engineering. Acta biomaterialia. 2014;10(6):2341–53. [DOI] [PubMed] [Google Scholar]
- 110.Guimard NK, Gomez N, Schmidt CE. Conducting polymers in biomedical engineering. Progress in polymer science. 2007;32(8–9):876–921. [Google Scholar]
- 111.Schmidt CE, Shastri VR, Vacanti JP, Langer R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proceedings of the National Academy of Sciences. 1997;94(17):8948–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ahadian S, Ramon-Azcon J, Ostrovidov S, Camci-Unal G, Kaji H, Ino K, et al. A contactless electrical stimulator: application to fabricate functional skeletal muscle tissue. Biomedical microdevices. 2013;15(1):109–15. [DOI] [PubMed] [Google Scholar]
- 113.Dennis RG, Kosnik PE, 2nd. Excitability and isometric contractile properties of mammalian skeletal muscle constructs engineered in vitro. In vitro cellular & developmental biology Animal. 2000;36(5):327–35. [DOI] [PubMed] [Google Scholar]
- 114.Bock N, Riminucci A, Dionigi C, Russo A, Tampieri A, Landi E, et al. A novel route in bone tissue engineering: magnetic biomimetic scaffolds. Acta Biomaterialia. 2010;6(3):786–96. [DOI] [PubMed] [Google Scholar]
- 115.Bardhan R, Lal S, Joshi A, Halas NJ. Theranostic Nanoshells: From Probe Design to Imaging and Treatment of Cancer. Accounts of Chemical Research. 2011;44(10):936–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen F, Ehlerding EB, Cai W. Theranostic Nanoparticles. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014;55(12):1919–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Torgersen J, Ovsianikov A, Mironov V, Pucher N, Qin X, Li Z, et al. Photo-sensitive hydrogels for three-dimensional laser microfabrication in the presence of whole organisms. Journal of biomedical optics. 2012;17(10):105008. [DOI] [PubMed] [Google Scholar]
- 118.Liu T-M, Conde J, Lipiński T, Bednarkiewicz A, Huang C-C. Smart NIR linear and nonlinear optical nanomaterials for cancer theranostics: Prospects in photomedicine. Progress in Materials Science. 2017;88:89–135. [Google Scholar]
- 119.Ya Tang, Yang T, Wang Q, Lv X, Song X, Ke H, et al. Albumin-coordinated assembly of clearable platinum nanodots for photo-induced cancer theranostics. Biomaterials. 2018;154:248–60. [DOI] [PubMed] [Google Scholar]
- 120.Rosales AM, Anseth KS. The design of reversible hydrogels to capture extracellular matrix dynamics. Nature Reviews Materials. 2016;1(2):15012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.DC A, AK S. Photoreversible Patterning of Biomolecules within Click-Based Hydrogels. Angewandte Chemie. 2012;124(8):1852–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huschka R, Zuloaga J, Knight MW, Brown LV, Nordlander P, Halas NJ. Light-Induced Release of DNA from Gold Nanoparticles: Nanoshells and Nanorods. Journal of the American Chemical Society. 2011;133(31):12247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roper DK, Ahn W, Hoepfner M. Microscale heat transfer transduced by surface plasmon resonant gold nanoparticles. The Journal of Physical Chemistry C. 2007;111(9):3636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ju E, Dong K, Liu Z, Pu F, Ren J, Qu X. Tumor microenvironment activated photothermal strategy for precisely controlled ablation of solid tumors upon NIR irradiation. Advanced Functional Materials. 2015;25(10):1574–80. [Google Scholar]
- 125.Kim SH, Kang EB, Jeong CJ, Sharker SM, In I, Park SY. Light controllable surface coating for effective photothermal killing of bacteria. ACS applied materials & interfaces. 2015;7(28):15600–6. [DOI] [PubMed] [Google Scholar]
- 126.DeForest CA, Polizzotti BD, Anseth KS. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nature materials. 2009;8(8):659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Culver JC, Hoffmann JC, Poché RA, Slater JH, West JL, Dickinson ME. Three-Dimensional Biomimetic Patterning in Hydrogels to Guide Cellular Organization. Advanced Materials. 2012;24(17):2344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.DeForest CA, Tirrell DA. A photoreversible protein-patterning approach for guiding stem cell fate in three-dimensional gels. Nature materials. 2015;14(5):523. [DOI] [PubMed] [Google Scholar]
- 129.Rosales AM, Rodell CB, Chen MH, Morrow MG, Anseth KS, Burdick JA. Reversible Control of Network Properties in Azobenzene-Containing Hyaluronic Acid-Based Hydrogels. Bioconjugate Chemistry. 2018;29(4):905–13. [DOI] [PubMed] [Google Scholar]
- 130.Stowers RS, Allen SC, Suggs LJ. Dynamic phototuning of 3D hydrogel stiffness. Proceedings of the National Academy of Sciences. 2015;112(7):1953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Melé M, Ferreira PG, Reverter F, DeLuca DS, Monlong J, Sammeth M, et al. The human transcriptome across tissues and individuals. Science. 2015;348(6235):660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Culver HR, Clegg JR, Peppas NA. Analyte-responsive hydrogels: intelligent materials for biosensing and drug delivery. Accounts of chemical research. 2017;50(2):170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gibney MJ, Walsh M, Brennan L, Roche HM, German B, Van Ommen B. Metabolomics in human nutrition: opportunities and challenges–. The American journal of clinical nutrition. 2005;82(3):497–503. [DOI] [PubMed] [Google Scholar]
- 134.Peppas NA, Clegg JR. The challenge to improve the response of biomaterials to the physiological environment. Regenerative Biomaterials. 2016;3(2):67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Culver HR, Sharma I, Wechsler ME, Anslyn EV, Peppas NA. Charged poly (N-isopropylacrylamide) nanogels for use as differential protein receptors in a turbidimetric sensor array. Analyst. 2017;142(17):3183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Umali AP, Anslyn EV. A general approach to differential sensing using synthetic molecular receptors. Current opinion in chemical biology. 2010;14(6):685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shi H, Tsai W-B, Garrison MD, Ferrari S, Ratner BD. Template-imprinted nanostructured surfaces for protein recognition. Nature. 1999;398(6728):593. [DOI] [PubMed] [Google Scholar]
- 138.Byrne ME, Oral E, Zachary Hilt J, Peppas NA. Networks for recognition of biomolecules: molecular imprinting and micropatterning poly (ethylene glycol)-Containing films. Polymers for Advanced Technologies. 2002;13(10–12):798–816. [Google Scholar]
- 139.Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nature Reviews Immunology. 2010;10(5):301. [DOI] [PubMed] [Google Scholar]
- 140.Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nature reviews immunology. 2010;10(5):345. [DOI] [PubMed] [Google Scholar]
- 141.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer. 2012;12(4):252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cobo I, Li M, Sumerlin BS, Perrier S. Smart hybrid materials by conjugation of responsive polymers to biomacromolecules. Nature materials. 2015;14(2):143. [DOI] [PubMed] [Google Scholar]
- 143.Biju V Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chemical Society Reviews. 2014;43(3):744–64. [DOI] [PubMed] [Google Scholar]
- 144.Pelegri-O’Day EM, Lin E-W, Maynard HD. Therapeutic protein–polymer conjugates: advancing beyond PEGylation. Journal of the American Chemical Society. 2014;136(41):14323–32. [DOI] [PubMed] [Google Scholar]
- 145.Knipe JM, Strong LE, Peppas NA. Enzyme-and pH-responsive microencapsulated nanogels for oral delivery of siRNA to induce TNF-α knockdown in the intestine. Biomacromolecules. 2016;17(3):788–97. [DOI] [PubMed] [Google Scholar]
- 146.Knipe JM, Chen F, Peppas NA. Enzymatic biodegradation of hydrogels for protein delivery targeted to the small intestine. Biomacromolecules. 2015;16(3):962–72. [DOI] [PubMed] [Google Scholar]
- 147.Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(51):17867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kumbar S, Laurencin C, Deng M. Natural and synthetic biomedical polymers: Newnes; 2014. [Google Scholar]
- 149.Hubbell JA. Bioactive biomaterials. Current opinion in biotechnology. 1999;10(2):123–9. [DOI] [PubMed] [Google Scholar]
- 150.Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10(9):1886–90. [DOI] [PubMed] [Google Scholar]
- 151.Schmidt CE, Baier JM. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000;21(22):2215–31. [DOI] [PubMed] [Google Scholar]
- 152.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24(24):4385–415. [DOI] [PubMed] [Google Scholar]
- 153.Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. nature. 1990;346(6287):818. [DOI] [PubMed] [Google Scholar]
- 154.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. science. 1990;249(4968):505–10. [DOI] [PubMed] [Google Scholar]
- 155.Darmostuk M, Rimpelova S, Gbelcova H, Ruml T. Current approaches in SELEX: An update to aptamer selection technology. Biotechnology advances. 2015;33(6):1141–61. [DOI] [PubMed] [Google Scholar]
- 156.Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228(4705):1315–7. [DOI] [PubMed] [Google Scholar]
- 157.Winter G, Griffiths AD, Hawkins RE, Hoogenboom HR. Making antibodies by phage display technology. Annual review of immunology. 1994;12(1):433–55. [DOI] [PubMed] [Google Scholar]
- 158.Hyvönen M, Laakkonen P. Identification and characterization of homing peptides using in vivo peptide phage display Cell-Penetrating Peptides: Springer; 2015. p. 205–22. [DOI] [PubMed] [Google Scholar]
- 159.Savory N, Lednor D, Tsukakoshi K, Abe K, Yoshida W, Ferri S, et al. In silico maturation of binding-specificity of DNA aptamers against Proteus mirabilis. Biotechnology and bioengineering. 2013;110(10):2573–80. [DOI] [PubMed] [Google Scholar]
- 160.Whitesides GM, Grzybowski B. Self-Assembly at All Scales. Science. 2002;295(5564):2418. [DOI] [PubMed] [Google Scholar]
- 161.Doll TAPF, Raman S, Dey R, Burkhard P. Nanoscale assemblies and their biomedical applications. Journal of the Royal Society Interface. 2013;10(80):20120740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kopeček J, Yang J. Smart Self-Assembled Hybrid Hydrogel Biomaterials. Angewandte Chemie International Edition. 2012;51(30):7396–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.McCarthy HO, McCaffrey J, McCrudden CM, Zholobenko A, Ali AA, McBride JW, et al. Development and characterization of self-assembling nanoparticles using a bio-inspired amphipathic peptide for gene delivery. Journal of Controlled Release. 2014;189(Supplement C):141–9. [DOI] [PubMed] [Google Scholar]
- 164.Ozin GA, Hou K, Lotsch BV, Cademartiri L, Puzzo DP, Scotognella F, et al. Nanofabrication by self-assembly. Materials Today. 2009;12(5):12–23. [Google Scholar]
- 165.Rajagopal K, Schneider JP. Self-assembling peptides and proteins for nanotechnological applications. Current Opinion in Structural Biology. 2004;14(4):480–6. [DOI] [PubMed] [Google Scholar]
- 166.Wang A, Shi W, Huang J, Yan Y. Adaptive soft molecular self-assemblies. Soft Matter. 2016;12(2):337–57. [DOI] [PubMed] [Google Scholar]
- 167.Bishop KJM, Wilmer CE, Soh S, Grzybowski BA. Nanoscale Forces and Their Uses in Self-Assembly. Small. 2009;5(14):1600–30. [DOI] [PubMed] [Google Scholar]
- 168.Min Y, Akbulut M, Kristiansen K, Golan Y, Israelachvili J. The role of interparticle and external forces in nanoparticle assembly. Nature Materials. 2008;7:527. [DOI] [PubMed] [Google Scholar]
- 169.Yu J, Wang Q, Zhang X. Effects of external force fields on peptide self-assembly and biomimetic silica synthesis. Applied Surface Science. 2014;311(Supplement C):799–807. [Google Scholar]
- 170.Varner VD, Nelson CM. Toward the Directed Self-Assembly of Engineered Tissues. Annual Review of Chemical and Biomolecular Engineering. 2014;5(1):507–26. [DOI] [PubMed] [Google Scholar]
- 171.Ariga K, Hill JP, Lee MV, Vinu A, Charvet R, Acharya S. Challenges and breakthroughs in recent research on self-assembly. Science and Technology of Advanced Materials. 2008;9(1):014109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature. 1992;359:710. [Google Scholar]
- 173.Sakurai M, Shimojima A, Heishi M, Kuroda K. Preparation of Mesostructured Siloxane−Organic Hybrid Films with Ordered Macropores by Templated Self-Assembly. Langmuir. 2007;23(21):10788–92. [DOI] [PubMed] [Google Scholar]
- 174.Aizenberg J, Braun PV, Wiltzius P. Patterned Colloidal Deposition Controlled by Electrostatic and Capillary Forces. Physical Review Letters. 2000;84(13):2997–3000. [DOI] [PubMed] [Google Scholar]
- 175.Yao H, Qi M, Liu Y, Tian W. Host–Guest Binding-Site-Tunable Self-Assembly of Stimuli-Responsive Supramolecular Polymers. Chemistry – A European Journal. 2016;22(25):8508–19. [DOI] [PubMed] [Google Scholar]
- 176.Yuan C, Xu Y, Zeng B, Luo W, Chen G, Mao J, et al. Supramolecular Assembly and Stimuli-Responsive Behavior of Multielement Hybrid Copolymers In: Kilislioğlu A, Karakuş S, editors. Molecular Self-assembly in Nanoscience and Nanotechnology. Rijeka: InTech; 2017. p. Ch. 05. [Google Scholar]
- 177.Mitomo H, Niikura K, Ijiro K. Stimuli-Responsive Structure Control of Self-Assembled Gold Nanoparticles In: Kawai T, Hashizume M, editors. Stimuli-Responsive Interfaces: Fabrication and Application. Singapore: Springer Singapore; 2017. p. 127–45. [Google Scholar]
- 178.Tao L, Wenhong P, Shenmin Z, Di Z. Bio-inspired fabrication of stimuli-responsive photonic crystals with hierarchical structures and their applications. Nanotechnology. 2016;27(12):122001. [DOI] [PubMed] [Google Scholar]
- 179.Stuart MAC, Huck WTS, Genzer J, Müller M, Ober C, Stamm M, et al. Emerging applications of stimuli-responsive polymer materials. Nature Materials. 2010;9:101. [DOI] [PubMed] [Google Scholar]
- 180.Guggenheim EA . Mixtures; the theory of the equilibrium properties of some simple classes of mixtures, solutions and alloys. Oxford: Clarendon Press; 1952. [Google Scholar]
- 181.Flory PJ. Introductory lecture. Faraday Discussions of the Chemical Society. 1974;57(0):7–18. [Google Scholar]
- 182.Gruen DWR. A model for the chains in amphiphilic aggregates. 2. Thermodynamic and experimental comparisons for aggregates of different shape and size. The Journal of Physical Chemistry. 1985;89(1):153–63. [Google Scholar]
- 183.Chen C, Liu K, Li J, Yan X. Functional architectures based on self-assembly of bio-inspired dipeptides: Structure modulation and its photoelectronic applications. Advances in Colloid and Interface Science. 2015;225(Supplement C):177–93. [DOI] [PubMed] [Google Scholar]
- 184.Smith KH, Tejeda-Montes E, Poch M, Mata A. Integrating top-down and self-assembly in the fabrication of peptide and protein-based biomedical materials. Chemical Society Reviews. 2011;40(9):4563–77. [DOI] [PubMed] [Google Scholar]
- 185.Aggeli A, Bell M, Carrick LM, Fishwick CWG, Harding R, Mawer PJ, et al. pH as a Trigger of Peptide β-Sheet Self-Assembly and Reversible Switching between Nematic and Isotropic Phases. Journal of the American Chemical Society. 2003;125(32):9619–28. [DOI] [PubMed] [Google Scholar]
- 186.Chockalingam K, Blenner M, Banta S. Design and application of stimulus-responsive peptide systems. Protein Engineering, Design and Selection. 2007;20(4):155–61. [DOI] [PubMed] [Google Scholar]
- 187.Selegård R, Aronsson C, Brommesson C, Dånmark S, Aili D. Folding driven self-assembly of a stimuli-responsive peptide-hyaluronan hybrid hydrogel. Scientific Reports. 2017;7(1):7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lowik DWPM Leunissen EHP, van den Heuvel M, Hansen MB, van Hest JCM. Stimulus responsive peptide based materials. Chemical Society Reviews. 2010;39(9):3394–412. [DOI] [PubMed] [Google Scholar]
- 189.Wang J, Liu K, Xing R, Yan X. Peptide self-assembly: thermodynamics and kinetics. Chemical Society Reviews. 2016;45(20):5589–604. [DOI] [PubMed] [Google Scholar]
- 190.Hauser CAE, Zhang S. Peptides as biological semiconductors. Nature. 2010;468:516. [DOI] [PubMed] [Google Scholar]
- 191.Ulijn RV, Woolfson DN. Peptide and protein based materials in 2010: from design and structure to function and application. Chemical Society Reviews. 2010;39(9):3349–50. [DOI] [PubMed] [Google Scholar]
- 192.Klok H-A. Protein-Inspired Materials: Synthetic Concepts and Potential Applications. Angewandte Chemie International Edition. 2002;41(9):1509–13. [DOI] [PubMed] [Google Scholar]
- 193.Amblard M, Fehrentz JA, Martinez J, Subra G. Methods and protocols of modern solid phase Peptide synthesis. Molecular biotechnology. 2006;33(3):239–54. [DOI] [PubMed] [Google Scholar]
- 194.Merrifield RB. Solid-Phase Peptide Synthesis. III. An Improved Synthesis of Bradykinin*. Biochemistry. 1964;3(9):1385–90. [DOI] [PubMed] [Google Scholar]
- 195.Sieber P A new acid-labile anchor group for the solid-phase synthesis of C-terminal peptide amides by the Fmoc method. Tetrahedron Letters. 1987;28(19):2107–10. [Google Scholar]
- 196.Huang Y, Wang Y-J, Wang Y, Yi S, Fan Z, Sun L, et al. Exploring naturally occurring ivy nanoparticles as an alternative biomaterial. Acta Biomaterialia. 2015;25(Supplement C):268–83. [DOI] [PubMed] [Google Scholar]
- 197.Li Z, Zheng Z, Yang Y, Fang G, Yao J, Shao Z, et al. Robust Protein Hydrogels from Silkworm Silk. ACS Sustainable Chemistry & Engineering. 2016;4(3):1500–6. [Google Scholar]
- 198.Habibi N, Kamaly N, Memic A, Shafiee H. Self-assembled peptide-based nanostructures: Smart nanomaterials toward targeted drug delivery. Nano today. 2016;11(1):41–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Eskandari S, Guerin T, Toth I, Stephenson RJ. Recent advances in self-assembled peptides: Implications for targeted drug delivery and vaccine engineering. Advanced Drug Delivery Reviews. 2017;110–111(Supplement C):169–87. [DOI] [PubMed] [Google Scholar]
- 200.Silva RF, Araújo DR, Silva ER, Ando RA, Alves WA. l-Diphenylalanine Microtubes As a Potential Drug-Delivery System: Characterization, Release Kinetics, and Cytotoxicity. Langmuir. 2013;29(32):10205–12. [DOI] [PubMed] [Google Scholar]
- 201.Lu S, Wang H, Sheng Y, Liu M, Chen P. Molecular binding of self-assembling peptide EAK16-II with anticancer agent EPT and its implication in cancer cell inhibition. Journal of Controlled Release. 2012;160(1):33–40. [DOI] [PubMed] [Google Scholar]
- 202.Li N, Li N, Yi Q, Luo K, Guo C, Pan D, et al. Amphiphilic peptide dendritic copolymer-doxorubicin nanoscale conjugate self-assembled to enzyme-responsive anti-cancer agent. Biomaterials. 2014;35(35):9529–45. [DOI] [PubMed] [Google Scholar]
- 203.Yishay-Safranchik E, Golan M, David A. Controlled release of doxorubicin and Smac-derived pro-apoptotic peptide from self-assembled KLD-based peptide hydrogels. Polymers for Advanced Technologies. 2014;25(5):539–44. [Google Scholar]
- 204.Yan X, He Q, Wang K, Duan L, Cui Y, Li J. Transition of Cationic Dipeptide Nanotubes into Vesicles and Oligonucleotide Delivery. Angewandte Chemie International Edition. 2007;46(14):2431–4. [DOI] [PubMed] [Google Scholar]
- 205.Sagiv J Organized monolayers by adsorption. 1. Formation and structure of oleophobic mixed monolayers on solid surfaces. Journal of the American Chemical Society. 1980;102(1):92–8. [Google Scholar]
- 206.Nuzzo RG, Allara DL. Adsorption of bifunctional organic disulfides on gold surfaces. Journal of the American Chemical Society. 1983;105(13):4481–3. [Google Scholar]
- 207.Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chemical Reviews. 2005;105(4):1103–70. [DOI] [PubMed] [Google Scholar]
- 208.Elemans JAAW, Lei S, De Feyter S. Molecular and Supramolecular Networks on Surfaces: From Two-Dimensional Crystal Engineering to Reactivity. Angewandte Chemie International Edition. 2009;48(40):7298–332. [DOI] [PubMed] [Google Scholar]
- 209.Schwartz DK. MECHANISMS AND KINETICS OF SELF-ASSEMBLED MONOLAYER FORMATION. Annual Review of Physical Chemistry. 2001;52(1):107–37. [DOI] [PubMed] [Google Scholar]
- 210.Sung IH, Kim DE. Surface Damage Characteristics of Self-Assembled Monolayers of Alkanethiols on Metal Surfaces. Tribology Letters. 2004;17(4):835–44. [Google Scholar]
- 211.Castner DG, Ratner BD. Biomedical surface science: Foundations to frontiers. Surface Science. 2002;500(1):28–60. [Google Scholar]
- 212.Harder P, Grunze M, Dahint R, Whitesides GM, Laibinis PE. Molecular Conformation in Oligo(ethylene glycol)-Terminated Self-Assembled Monolayers on Gold and Silver Surfaces Determines Their Ability To Resist Protein Adsorption. The Journal of Physical Chemistry B. 1998;102(2):426–36. [Google Scholar]
- 213.Casalini S, Bortolotti CA, Leonardi F, Biscarini F. Self-assembled monolayers in organic electronics. Chemical Society Reviews. 2017;46(1):40–71. [DOI] [PubMed] [Google Scholar]
- 214.Poirier GE. Characterization of Organosulfur Molecular Monolayers on Au(111) using Scanning Tunneling Microscopy. Chemical Reviews. 1997;97(4):1117–28. [DOI] [PubMed] [Google Scholar]
- 215.Hofer R, Textor M, Spencer ND. Alkyl Phosphate Monolayers, Self-Assembled from Aqueous Solution onto Metal Oxide Surfaces. Langmuir. 2001;17(13):4014–20. [Google Scholar]
- 216.Willner I, Katz E. Integration of Layered Redox Proteins and Conductive Supports for Bioelectronic Applications. Angewandte Chemie International Edition. 2000;39(7):1180–218. [DOI] [PubMed] [Google Scholar]
- 217.Madigan MC, Holden BA. Reduced epithelial adhesion after extended contact lens wear correlates with reduced hemidesmosome density in cat cornea. Investigative Ophthalmology & Visual Science. 1992;33(2):314–23. [PubMed] [Google Scholar]
- 218.Fukuda M, Fullard RJ, Willcox MD, Baleriola-Lucas C, Bestawros F, Sweeney D, et al. Fibronectin in the tear film. Investigative Ophthalmology & Visual Science. 1996;37(2):459–67. [PubMed] [Google Scholar]
- 219.Ostuni E, Chapman RG, Holmlin RE, Takayama S, Whitesides GM. A Survey of Structure−Property Relationships of Surfaces that Resist the Adsorption of Protein. Langmuir. 2001;17(18):5605–20. [DOI] [PubMed] [Google Scholar]
- 220.Ratner BD. The engineering of biomaterials exhibiting recognition and specificity. Journal of Molecular Recognition. 1996;9(5–6):617–25. [DOI] [PubMed] [Google Scholar]
- 221.Wallace A, Albadawi H, Patel N, Khademhosseini A, Zhang Y, Naidu S, et al. Anti-fouling strategies for central venous catheters 2017 S246–S57 p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Blümmel J, Perschmann N, Aydin D, Drinjakovic J, Surrey T, Lopez-Garcia M, et al. Protein repellent properties of covalently attached PEG coatings on nanostructured SiO2-based interfaces. Biomaterials. 2007;28(32):4739–47. [DOI] [PubMed] [Google Scholar]
- 223.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, et al. Functional Arteries Grown in Vitro. Science. 1999;284(5413):489–93. [DOI] [PubMed] [Google Scholar]
- 224.Fu K, Klibanov AM, Langer R. Protein stability in controlled-release systems. Nature Biotechnology. 2000;18:24. [DOI] [PubMed] [Google Scholar]
- 225.Shirtcliffe NJ, Roach P. Superhydrophobicity for Antifouling Microfluidic Surfaces In: Jenkins G, Mansfield CD, editors. Microfluidic Diagnostics: Methods and Protocols. Totowa, NJ: Humana Press; 2013. p. 269–81. [DOI] [PubMed] [Google Scholar]
- 226.Ginger DS, Zhang H, Mirkin CA. The Evolution of Dip-Pen Nanolithography. Angewandte Chemie International Edition. 2004;43(1):30–45. [DOI] [PubMed] [Google Scholar]
- 227.Zeira A, Berson J, Feldman I, Maoz R, Sagiv J. A Bipolar Electrochemical Approach to Constructive Lithography: Metal/Monolayer Patterns via Consecutive Site-Defined Oxidation and Reduction. Langmuir. 2011;27(13):8562–75. [DOI] [PubMed] [Google Scholar]
- 228.Jackman R, Wilbur J, Whitesides G. Fabrication of submicrometer features on curved substrates by microcontact printing. Science. 1995;269(5224):664–6. [DOI] [PubMed] [Google Scholar]
- 229.Pizzol CD, Filippin-Monteiro FB, Restrepo JAS, Pittella F, Silva AH, de Souza PA, et al. Influence of Surfactant and Lipid Type on the Physicochemical Properties and Biocompatibility of Solid Lipid Nanoparticles. International Journal of Environmental Research and Public Health. 2014;11(8):8581–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ide T, Ichikawa T. A novel method for artificial lipid-bilayer formation. Biosensors and Bioelectronics. 2005;21(4):672–7. [DOI] [PubMed] [Google Scholar]
- 231.Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, et al. Liposome: classification, preparation, and applications. Nanoscale Research Letters. 2013;8(1):102–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Allen TM, Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews. 2013;65(1):36–48. [DOI] [PubMed] [Google Scholar]
- 233.Sato YT, Umezaki K, Sawada S, Mukai S-a, Sasaki Y, Harada N, et al. Engineering hybrid exosomes by membrane fusion with liposomes. Scientific Reports. 2016;6:21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Movassaghian S, Merkel OM, Torchilin VP. Applications of polymer micelles for imaging and drug delivery. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2015;7(5):691–707. [DOI] [PubMed] [Google Scholar]
- 235.Yaroslavov AA, Melik-Nubarov NS, Menger FM. Polymer-induced flip-flop in biomembranes. Accounts of chemical research. 2006;39(10):702–10. [DOI] [PubMed] [Google Scholar]
- 236.Gong Y, Luo Y, Bong D. Membrane Activation: Selective Vesicle Fusion via Small Molecule Recognition. Journal of the American Chemical Society. 2006;128(45):14430–1. [DOI] [PubMed] [Google Scholar]
- 237.Stengel G, Zahn R, Höök F. DNA-Induced Programmable Fusion of Phospholipid Vesicles. Journal of the American Chemical Society. 2007;129(31):9584–5. [DOI] [PubMed] [Google Scholar]
- 238.Bhosale S, Sisson AL, Talukdar P, Fürstenberg A, Banerji N, Vauthey E, et al. Photoproduction of Proton Gradients with π-Stacked Fluorophore Scaffolds in Lipid Bilayers. Science. 2006;313(5783):84–6. [DOI] [PubMed] [Google Scholar]
- 239.Cazacu A, Tong C, van der Lee A, Fyles TM, Barboiu M. Columnar Self-Assembled Ureido Crown Ethers: An Example of Ion-Channel Organization in Lipid Bilayers. Journal of the American Chemical Society. 2006;128(29):9541–8. [DOI] [PubMed] [Google Scholar]
- 240.Gust D, Moore TA, Moore AL. Mimicking Photosynthetic Solar Energy Transduction. Accounts of chemical research. 2001;34(1):40–8. [DOI] [PubMed] [Google Scholar]
- 241.Ariga K, Lvov YM, Kawakami K, Ji Q, Hill JP. Layer-by-layer self-assembled shells for drug delivery. Advanced Drug Delivery Reviews. 2011;63(9):762–71. [DOI] [PubMed] [Google Scholar]
- 242.Stanish I, Lowy DA, Hung CW, Singh A. Vesicle-Based Rechargeable Batteries. Advanced Materials. 2005;17(9):1194–8. [Google Scholar]
- 243.Sharma A, Sharma US. Liposomes in drug delivery: Progress and limitations. International Journal of Pharmaceutics. 1997;154(2):123–40. [Google Scholar]
- 244.Vartuli JC, Schmitt KD, Kresge CT, Roth WJ, Leonowicz ME, McCullen SB, et al. Effect of Surfactant/Silica Molar Ratios on the Formation of Mesoporous Molecular Sieves: Inorganic Mimicry of Surfactant Liquid-Crystal Phases and Mechanistic Implications. Chemistry of Materials. 1994;6(12):2317–26. [Google Scholar]
- 245.Valldeperas M, Wiśniewska M, Ram-On M, Kesselman E, Danino D, Nylander T, et al. Sponge Phases and Nanoparticle Dispersions in Aqueous Mixtures of Mono- and Diglycerides. Langmuir. 2016;32(34):8650–9. [DOI] [PubMed] [Google Scholar]
- 246.Karami Z, Hamidi M. Cubosomes: remarkable drug delivery potential. Drug Discovery Today. 2016;21(5):789–801. [DOI] [PubMed] [Google Scholar]
- 247.Cubosomes and hexosomes as versatile platforms for drug delivery. Therapeutic Delivery. 2015;6(12):1347–64. [DOI] [PubMed] [Google Scholar]
- 248.Zastavker YV, Asherie N, Lomakin A, Pande J, Donovan JM, Schnur JM, et al. Self-assembly of helical ribbons. Proceedings of the National Academy of Sciences. 1999;96(14):7883–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Emeje MO, Obidike IC, Akpabio EI, Ofoefule SI. Nanotechnology in Drug Delivery In: Sezer AD, editor. Recent Advances in Novel Drug Carrier Systems. Rijeka: InTech; 2012. p. Ch. 04. [Google Scholar]
- 250.Croy SR, Kwon GS. Polymeric Micelles for Drug Delivery. Current Pharmaceutical Design. 2006;12(36):4669–84. [DOI] [PubMed] [Google Scholar]
- 251.Al-Soufi W, Piñeiro L, Novo M. A model for monomer and micellar concentrations in surfactant solutions: Application to conductivity, NMR, diffusion, and surface tension data. Journal of Colloid and Interface Science. 2012;370(1):102–10. [DOI] [PubMed] [Google Scholar]
- 252.van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, Functions, and Clinical Relevance of Extracellular Vesicles. Pharmacological Reviews. 2012;64(3):676–705. [DOI] [PubMed] [Google Scholar]
- 253.Di Rocco G, Baldari S, Toietta G. Towards Therapeutic Delivery of Extracellular Vesicles: Strategies for In Vivo Tracking and Biodistribution Analysis. Stem Cells International. 2016;2016:5029619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Han C, Sun X, Liu L, Jiang H, Shen Y, Xu X, et al. Exosomes and Their Therapeutic Potentials of Stem Cells. Stem Cells International. 2016;2016:7653489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Basu J, Ludlow JW. Exosomes for repair, regeneration and rejuvenation. Expert Opinion on Biological Therapy. 2016;16(4):489–506. [DOI] [PubMed] [Google Scholar]
- 256.Ledbetter N, Goswami T. A Review of Nanotechnology Applications. [Google Scholar]
- 257.Hassan S, Prakash G, Ozturk A, Saghazadeh S, Sohail MF, Seo J, et al. Evolution and Clinical Translation of Drug Delivery Nanomaterials. Nano Today. 2017;15:91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Karim W, Spreafico C, Kleibert A, Gobrecht J, VandeVondele J, Ekinci Y, et al. Catalyst support effects on hydrogen spillover. Nature. 2017;541:68. [DOI] [PubMed] [Google Scholar]
- 259.Shin SR, Li Y-C, Jang HL, Khoshakhlagh P, Akbari M, Nasajpour A, et al. Graphene-based materials for tissue engineering. Advanced Drug Delivery Reviews. 2016;105(Part B):255–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Chen VJ, Ma PX. Nano-fibrous poly(l-lactic acid) scaffolds with interconnected spherical macropores. Biomaterials. 2004;25(11):2065–73. [DOI] [PubMed] [Google Scholar]
- 261.Hata K, Futaba DN, Mizuno K, Namai T, Yumura M, Iijima S. Water-Assisted Highly Efficient Synthesis of Impurity-Free Single-Walled Carbon Nanotubes. Science. 2004;306(5700):1362–4. [DOI] [PubMed] [Google Scholar]
- 262.Sun S, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, et al. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. Journal of the American Chemical Society. 2004;126(1):273–9. [DOI] [PubMed] [Google Scholar]
- 263.Venugopal JR, Low S, Choon AT, Kumar AB, Ramakrishna S. Nanobioengineered Electrospun Composite Nanofibers and Osteoblasts for Bone Regeneration. Artificial Organs. 2008;32(5):388–97. [DOI] [PubMed] [Google Scholar]
- 264.Dan G, Guoxin X, Jianbin L. Mechanical properties of nanoparticles: basics and applications. Journal of Physics D: Applied Physics. 2014;47(1):013001. [Google Scholar]
- 265.Yurkov GY, Fionov AS, Koksharov YA, Koleso VV, Gubin SP. Electrical and magnetic properties of nanomaterials containing iron or cobalt nanoparticles. Inorganic Materials. 2007;43(8):834–44. [Google Scholar]
- 266.Zhang Y-x, Wang Y-h. Nonlinear optical properties of metal nanoparticles: a review. RSC Advances. 2017;7(71):45129–44. [Google Scholar]
- 267.Yang F, Deng D, Pan X, Fu Q, Bao X. Understanding nano effects in catalysis. National Science Review. 2015;2(2):183–201. [Google Scholar]
- 268.De Jong WH, Borm PJA. Drug delivery and nanoparticles: Applications and hazards. International Journal of Nanomedicine. 2008;3(2):133–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Nanotechnology for regenerative medicine: nanomaterials for stem cell imaging. Nanomedicine. 2008;3(4):567–78. [DOI] [PubMed] [Google Scholar]
- 270.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Advanced Materials. 2006;18(11):1345–60. [Google Scholar]
- 271.Mendes PM. Cellular nanotechnology: making biological interfaces smarter. Chemical Society Reviews. 2013;42(24):9207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ishihara K, Chen W, Liu Y, Tsukamoto Y, Inoue Y. Cytocompatible and multifunctional polymeric nanoparticles for transportation of bioactive molecules into and within cells. Science and Technology of Advanced Materials. 2016;17(1):300–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chemical Society reviews. 2009;38(6):1759–82. [DOI] [PubMed] [Google Scholar]
- 274.Ko W-K, Heo DN, Moon H-J, Lee SJ, Bae MS, Lee JB, et al. The effect of gold nanoparticle size on osteogenic differentiation of adipose-derived stem cells. Journal of Colloid and Interface Science. 2015;438(0):68–76. [DOI] [PubMed] [Google Scholar]
- 275.Shin SR, Shin C, Memic A, Shadmehr S, Miscuglio M, Jung HY, et al. Aligned carbon nanotube–based flexible gel substrates for engineering biohybrid tissue actuators. Advanced functional materials. 2015;25(28):4486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Jaconi M Nanomedicine: Gold nanowires to mend a heart. Nature nanotechnology. 2011;6(11):692–3. [DOI] [PubMed] [Google Scholar]
- 277.Wong JKL, Mohseni R, Hamidieh AA, MacLaren RE, Habib N, Seifalian AM. Will Nanotechnology Bring New Hope for Gene Delivery? Trends in Biotechnology.35(5):434–51. [DOI] [PubMed] [Google Scholar]
- 278.Marsich E, Bellomo F, Turco G, Travan A, Donati I, Paoletti S. Nano-composite scaffolds for bone tissue engineering containing silver nanoparticles: preparation, characterization and biological properties. J Mater Sci: Mater Med. 2013;24(7):1799–807. [DOI] [PubMed] [Google Scholar]
- 279.Krotz F, Sohn HY, Gloe T, Plank C, Pohl U. Magnetofection potentiates gene delivery to cultured endothelial cells. Journal of vascular research. 2003;40(5):425–34. [DOI] [PubMed] [Google Scholar]
- 280.Lee CH, Kim EY, Jeon K, Tae JC, Lee KS, Kim YO, et al. Simple, efficient, and reproducible gene transfection of mouse embryonic stem cells by magnetofection. Stem cells and development. 2008;17(1):133–41. [DOI] [PubMed] [Google Scholar]
- 281.Ino K, Kawasumi T, Ito A, Honda H. Plasmid DNA transfection using magnetite cationic liposomes for construction of multilayered gene-engineered cell sheet. Biotechnology and bioengineering. 2008;100(1):168–76. [DOI] [PubMed] [Google Scholar]
- 282.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(8):2480–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Yeong W-Y, Chua C-K, Leong K-F, Chandrasekaran M. Rapid prototyping in tissue engineering: challenges and potential. Trends in biotechnology. 2004;22(12):643–52. [DOI] [PubMed] [Google Scholar]
- 284.Derby B Bioprinting: inkjet printing proteins and hybrid cell-containing materials and structures. Journal of Materials Chemistry. 2008;18(47):5717–21. [Google Scholar]
- 285.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nature biotechnology. 2014;32(8):773–85. [DOI] [PubMed] [Google Scholar]
- 286.Conner BP, Manogharan GP, Martof AN, Rodomsky LM, Rodomsky CM, Jordan DC, et al. Making sense of 3-D printing: Creating a map of additive manufacturing products and services. Additive Manufacturing. 2014;1–4:64–76. [Google Scholar]
- 287.Ventola CL. Medical applications for 3D printing: current and projected uses. PT. 2014;39(10):704–11. [PMC free article] [PubMed] [Google Scholar]
- 288.Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotech. 2016;34(3):312–9. [DOI] [PubMed] [Google Scholar]
- 289.Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: Tissue spheroids as building blocks. Biomaterials. 2009;30(12):2164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Singh M, Haverinen HM, Dhagat P, Jabbour GE. Inkjet Printing—Process and Its Applications. Advanced Materials. 2010;22(6):673–85. [DOI] [PubMed] [Google Scholar]
- 291.Cornelissen D-J, Faulkner-Jones A, Shu W. Current developments in 3D bioprinting for tissue engineering. Current Opinion in Biomedical Engineering. 2017;2(Supplement C):76–82. [Google Scholar]
- 292.Hasenbank MS, Edwards T, Fu E, Garzon R, Kosar TF, Look M, et al. Demonstration of multi-analyte patterning using piezoelectric inkjet printing of multiple layers. Analytica chimica acta. 2008;611(1):80–8. [DOI] [PubMed] [Google Scholar]
- 293.Zhang Y, Tse C, Rouholamin D, Smith P. Scaffolds for tissue engineering produced by inkjet printing. Open Engineering. 2012;2(3):325–35. [Google Scholar]
- 294.Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76(Supplement C):321–43. [DOI] [PubMed] [Google Scholar]
- 295.Faes M, Valkenaers H, Vogeler F, Vleugels J, Ferraris E. Extrusion-based 3D Printing of Ceramic Components. Procedia CIRP. 2015;28(Supplement C):76–81. [Google Scholar]
- 296.Trachtenberg JE, Placone JK, Smith BT, Piard CM, Santoro M, Scott DW, et al. Extrusion-Based 3D Printing of Poly(propylene fumarate) in a Full-Factorial Design. ACS Biomaterials Science & Engineering. 2016;2(10):1771–80. [DOI] [PubMed] [Google Scholar]
- 297.Gkartzou E, Koumoulos EP, Charitidis CA. Production and 3D printing processing of bio-based thermoplastic filament. Manufacturing Rev. 2017;4:1. [Google Scholar]
- 298.Wu G A. Langrana N, Sadanji R, Danforth S. Solid freeform fabrication of metal components using fused deposition of metals. Materials & Design. 2002;23(1):97–105. [Google Scholar]
- 299.Hong S, Sanchez C, Du H, Kim N. Fabrication of 3D Printed Metal Structures by Use of High-Viscosity Cu Paste and a Screw Extruder. Journal of Electronic Materials. 2015;44(3):836–41. [Google Scholar]
- 300.Ren L, Zhou X, Song Z, Zhao C, Liu Q, Xue J, et al. Process Parameter Optimization of Extrusion-Based 3D Metal Printing Utilizing PW–LDPE–SA Binder System. Materials. 2017;10(3):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Tan YJ, Tan X, Yeong WY, Tor SB. Hybrid microscaffold-based 3D bioprinting of multi-cellular constructs with high compressive strength: A new biofabrication strategy. Scientific Reports. 2016;6:39140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab on a Chip. 2014;14(13):2202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Wang S, Lee JM, Yeong WY. Smart hydrogels for 3D bioprinting. International Journal of Bioprinting. 2015;1(1). [Google Scholar]
- 304.Skylar-Scott MA, Liu M-C, Wu Y, Dixit A, Yanik MF. Guided Homing of Cells in Multi-Photon Microfabricated Bioscaffolds. Advanced Healthcare Materials. 2016;5(10):1233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Smith HI, Spears DL, Bernacki SE. X-Ray Lithography: A Complementary Technique to Electron Beam Lithography. Journal of Vacuum Science and Technology. 1973;10(6):913–7. [Google Scholar]
- 306.Maldonado JR, Peckerar M. X-ray lithography: Some history, current status and future prospects. Microelectronic Engineering. 2016;161(Supplement C):87–93. [Google Scholar]
- 307.Yuli V, Antony B, Olga V, Wenlong J, Quinn L. Demagnification in proximity x-ray lithography and extensibility to 25 nm by optimizing Fresnel diffraction. Journal of Physics D: Applied Physics. 1999;32(22):L114. [Google Scholar]
- 308.Georgiou GE, Jankoski CA, Palumbo TA, editors. Dc Electroplating Of Sub-Micron Gold Patterns On X-Ray Masks 1984 Microlithography Conferences; 1984: SPIE. [Google Scholar]
- 309.Cummings KD, Resnick DJ, Frackoviak J, Kola RR, Trimble LE, Grant B, et al. Study of electron beam patterning of resist on tungsten x-ray masks. Journal of Vacuum Science & Technology B: Microelectronics and Nanometer Structures Processing, Measurement, and Phenomena. 1993;11(6):2872–5. [Google Scholar]
- 310.Miszta K, Greullet F, Marras S, Prato M, Toma A, Arciniegas M, et al. Nanocrystal film patterning by inhibiting cation exchange via electron-beam or X-ray lithography. Nano letters. 2014;14(4):2116–22. [DOI] [PubMed] [Google Scholar]
- 311.Naoki M, Yoshikazu H, Osamu T. A novel fabrication process of 3D microstructures by double exposure in deep x-ray lithography (D 2 XRL). Journal of Micromechanics and Microengineering. 2005;15(11):2056. [Google Scholar]
- 312.Saile V Introduction: LIGA and Its Applications LIGA and Its Applications: Wiley-VCH Verlag GmbH & Co. KGaA; 2009. p. 1–10. [Google Scholar]
- 313.Brooks MJ, McCord MA. Electron Beam Lithography In: Rai-Choudhury P, editor. Handbook of Microlithography, Micromachining, and Microfabrication. 1: SPIE; 1997. [Google Scholar]
- 314.Senaratne W, Sengupta P, Jakubek V, Holowka D, Ober CK, Baird B. Functionalized Surface Arrays for Spatial Targeting of Immune Cell Signaling. Journal of the American Chemical Society. 2006;128(17):5594–5. [DOI] [PubMed] [Google Scholar]
- 315.Christman KL, Schopf E, Broyer RM, Li RC, Chen Y, Maynard HD. Positioning Multiple Proteins at the Nanoscale with Electron Beam Cross-Linked Functional Polymers. Journal of the American Chemical Society. 2009;131(2):521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Wanzenboeck H, Waid S. Focused Ion Beam Lithography. 802011. [Google Scholar]
- 317.Hasebe T, Nagashima S, Yoshimoto Y, Hotta A, Suzuki T. Tailoring surface topographies of polymers by using ion beam: Recent advances and the potential applications in biomedical and tissue engineering. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 2012;282:134–6. [Google Scholar]
- 318.Ahmed SF, Rho G-H, Lee K-R, Vaziri A, Moon M-W. High aspect ratio wrinkles on a soft polymer. Soft Matter. 2010;6(22):5709–14. [Google Scholar]
- 319.Cattoni A, Ghenuche P, Haghiri-Gosnet A-M, Decanini D, Chen J, Pelouard J-L, et al. λ3/1000 Plasmonic Nanocavities for Biosensing Fabricated by Soft UV Nanoimprint Lithography. Nano Letters. 2011;11(9):3557–63. [DOI] [PubMed] [Google Scholar]
- 320.Schift H Nanoimprint lithography: An old story in modern times? A review. Journal of Vacuum Science & Technology B: Microelectronics and Nanometer Structures Processing, Measurement, and Phenomena. 2008;26(2):458–80. [Google Scholar]
- 321.Guillemette MD, Cui B, Roy E, Gauvin R, Giasson CJ, Esch MB, et al. Surface topography induces 3D self-orientation of cells and extracellular matrix resulting in improved tissue function. Integrative biology : quantitative biosciences from nano to macro. 2009;1(2):196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Matsuzaka K, Frank Walboomers X, Yoshinari M, Inoue T, Jansen JA. The attachment and growth behavior of osteoblast-like cells on microtextured surfaces. Biomaterials. 2003;24(16):2711–9. [DOI] [PubMed] [Google Scholar]
- 323.Hu W, Yim EKF, Reano RM, Leong KW, Pang SW. Effects of nanoimprinted patterns in tissue-culture polystyrene on cell behavior. Journal of vacuum science & technology A, Vacuum, surfaces, and films : an official journal of the American Vacuum Society. 2005;23(6):2984–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Bremus-Koebberling EA, Beckemper S, Koch B, Gillner A. Nano structures via laser interference patterning for guided cell growth of neuronal cells. Journal of Laser Applications. 2012;24(4):042013. [Google Scholar]
- 325.Chhabra R, Sharma J, Ke Y, Liu Y, Rinker S, Lindsay S, et al. Spatially Addressable Multiprotein Nanoarrays Templated by Aptamer-Tagged DNA Nanoarchitectures. Journal of the American Chemical Society. 2007;129(34):10304–5. [DOI] [PubMed] [Google Scholar]
- 326.Barry JJA, Howard D, Shakesheff KM, Howdle SM, Alexander MR. Using a Core–Sheath Distribution of Surface Chemistry through 3D Tissue Engineering Scaffolds to Control Cell Ingress. Advanced Materials. 2006;18(11):1406–10. [Google Scholar]
- 327.Shadrin IY, Allen BW, Qian Y, Jackman CP, Carlson AL, Juhas ME, et al. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nature Communications. 2017;8(1):1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Deubel M, von Freymann G, Wegener M, Pereira S, Busch K, Soukoulis CM. Direct laser writing of three-dimensional photonic-crystal templates for telecommunications. Nat Mater. 2004;3(7):444–7. [DOI] [PubMed] [Google Scholar]
- 329.Farsari M, Chichkov BN. Materials processing: Two-photon fabrication. Nat Photon. 2009;3(8):450–2. [Google Scholar]
- 330.Gissibl T, Thiele S, Herkommer A, Giessen H. Two-photon direct laser writing of ultracompact multi-lens objectives. Nat Photon. 2016;10(8):554–60. [Google Scholar]
- 331.Cumpston BH, Ananthavel SP, Barlow S, Dyer DL, Ehrlich JE, Erskine LL, et al. Two-photon polymerization initiators for three-dimensional optical data storage and microfabrication. Nature. 1999;398(6722):51–4. [Google Scholar]
- 332.Maruo S, Nakamura O, Kawata S. Three-dimensional microfabrication with two-photon-absorbed photopolymerization. Opt Lett. 1997;22(2):132–4. [DOI] [PubMed] [Google Scholar]
- 333.Applegate MB, Coburn J, Partlow BP, Moreau JE, Mondia JP, Marelli B, et al. Laser-based three-dimensional multiscale micropatterning of biocompatible hydrogels for customized tissue engineering scaffolds. Proceedings of the National Academy of Sciences. 2015;112(39):12052–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Li L, Gattass RR, Gershgoren E, Hwang H, Fourkas JT. Achieving λ/20 Resolution by One-Color Initiation and Deactivation of Polymerization. Science. 2009;324(5929):910–3. [DOI] [PubMed] [Google Scholar]
- 335.Xing JF, Zheng ML, Duan XM. Two-photon polymerization microfabrication of hydrogels: an advanced 3D printing technology for tissue engineering and drug delivery. Chem Soc Rev. 2015;44(15):5031–9. [DOI] [PubMed] [Google Scholar]
- 336.Watanabe T, Akiyama M, Totani K, Kuebler SM, Stellacci F, Wenseleers W, et al. Photoresponsive Hydrogel Microstructure Fabricated by Two-Photon Initiated Polymerization. Advanced Functional Materials. 2002;12(9):611–4. [Google Scholar]
- 337.Tormen M, Businaro L, Altissimo M, Romanato F, Cabrini S, Perennes F, et al. 3D patterning by means of nanoimprinting, X-ray and two-photon lithography. Microelectronic Engineering. 2004;73–74(Supplement C):535–41. [Google Scholar]
- 338.Ovsianikov A, Malinauskas M, Schlie S, Chichkov B, Gittard S, Narayan R, et al. Three-dimensional laser micro- and nano-structuring of acrylated poly(ethylene glycol) materials and evaluation of their cytoxicity for tissue engineering applications. Acta Biomaterialia. 2011;7(3):967–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Ovsianikov A, Gruene M, Pflaum M, Koch L, Maiorana F, Wilhelmi M, et al. Laser printing of cells into 3D scaffolds. Biofabrication. 2010;2(1):014104. [DOI] [PubMed] [Google Scholar]
- 340.Torgersen J, Ovsianikov A, Mironov V, Pucher N, Qin X, Li Z, et al. Photo-sensitive hydrogels for three-dimensional laser microfabrication in the presence of whole organisms. BIOMEDO. 2012;17(10):105008–. [DOI] [PubMed] [Google Scholar]
- 341.Xiong Z, Zheng M-L, Dong X-Z, Chen W-Q, Jin F, Zhao Z-S, et al. Asymmetric microstructure of hydrogel: two-photon microfabrication and stimuli-responsive behavior. Soft Matter. 2011;7(21):10353–9. [Google Scholar]
- 342.Xing J, Liu J, Zhang T, Zhang L, Zheng M, Duan X. A water soluble initiator prepared through host-guest chemical interaction for microfabrication of 3D hydrogels via two-photon polymerization. Journal of Materials Chemistry B. 2014;2(27):4318–23. [DOI] [PubMed] [Google Scholar]
- 343.Liu K, Nie Z, Zhao N, Li W, Rubinstein M, Kumacheva E. Step-Growth Polymerization of Inorganic Nanoparticles. Science. 2010;329(5988):197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Rolland JP, Hagberg EC, Denison GM, Carter KR, De Simone JM. High-Resolution Soft Lithography: Enabling Materials for Nanotechnologies. Angewandte Chemie International Edition. 2004;43(43):5796–9. [DOI] [PubMed] [Google Scholar]
- 345.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. Direct Fabrication and Harvesting of Monodisperse, Shape-Specific Nanobiomaterials. Journal of the American Chemical Society. 2005;127(28):10096–100. [DOI] [PubMed] [Google Scholar]
- 346.Guan J, Ferrell N, James Lee L, Hansford DJ. Fabrication of polymeric microparticles for drug delivery by soft lithography. Biomaterials. 2006;27(21):4034–41. [DOI] [PubMed] [Google Scholar]
- 347.Glangchai LC, Caldorera-Moore M, Shi L, Roy K. Nanoimprint lithography based fabrication of shape-specific, enzymatically-triggered smart nanoparticles. Journal of Controlled Release. 2008;125(3):263–72. [DOI] [PubMed] [Google Scholar]
- 348.Herlihy KP, Nunes J, DeSimone JM. Electrically Driven Alignment and Crystallization of Unique Anisotropic Polymer Particles. Langmuir. 2008;24(16):8421–6. [DOI] [PubMed] [Google Scholar]
- 349.Enlow EM, Luft JC, Napier ME, DeSimone JM. Potent Engineered PLGA Nanoparticles by Virtue of Exceptionally High Chemotherapeutic Loadings. Nano letters. 2011;11(2):808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Xu J, Wong DHC, Byrne JD, Chen K, Bowerman C, DeSimone JM. Future of the Particle Replication in Nonwetting Templates (PRINT) Technology. Angewandte Chemie (International ed in English). 2013;52(26):6580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Ko D-H, Tumbleston JR, Henderson KJ, Euliss LE, DeSimone JM, Lopez R, et al. Biomimetic microlens array with antireflective “moth-eye” surface. Soft Matter. 2011;7(14):6404–7. [Google Scholar]
- 352.Glotzer SC, Solomon MJ. Anisotropy of building blocks and their assembly into complex structures. Nature Materials. 2007;6:557. [DOI] [PubMed] [Google Scholar]
- 353.Akbari M, Tamayol A, Laforte V, Annabi N, Najafabadi AH, Khademhosseini A, et al. Composite living fibers for creating tissue constructs using textile techniques. Advanced Functional Materials. 2014;24(26):4060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Mironov V, Boland T, Trusk T, Forgacs G, Markwald RR. Organ printing: computer-aided jet-based 3D tissue engineering. Trends in biotechnology. 2003;21(4):157–61. [DOI] [PubMed] [Google Scholar]
- 355.Soman P, Kelber JA, Lee JW, Wright TN, Vecchio KS, Klemke RL, et al. Cancer cell migration within 3D layer-by-layer microfabricated photocrosslinked PEG scaffolds with tunable stiffness. Biomaterials. 2012;33(29):7064–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Cui X, Boland T, D’Lima DD, Lotz MK. Thermal Inkjet Printing in Tissue Engineering and Regenerative Medicine. Recent patents on drug delivery & formulation. 2012;6(2):149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM. Evaluation of 3D Printing and Its Potential Impact on Biotechnology and the Chemical Sciences. Analytical Chemistry. 2014;86(7):3240–53. [DOI] [PubMed] [Google Scholar]
- 358.Chang CC, Boland ED, Williams SK, Hoying JB. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. Journal of biomedical materials research Part B, Applied biomaterials. 2011;98(1):160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Duan B, Hockaday LA, Kang KH, Butcher JT. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. Journal of biomedical materials research Part A. 2013;101(5):1255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Xu T, Zhao W, Zhu JM, Albanna MZ, Yoo JJ, Atala A. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials. 2013;34(1):130–9. [DOI] [PubMed] [Google Scholar]
- 361.Christensen K, Xu C, Chai W, Zhang Z, Fu J, Huang Y. Freeform inkjet printing of cellular structures with bifurcations. Biotechnology and bioengineering. 2015;112(5):1047–55. [DOI] [PubMed] [Google Scholar]
- 362.Whatley BR, Li X, Zhang N, Wen X. Magnetic-directed patterning of cell spheroids. Journal of biomedical materials research Part A. 2014;102(5):1537–47. [DOI] [PubMed] [Google Scholar]
- 363.Koch L, Gruene M, Unger C, Chichkov B. Laser assisted cell printing. Current pharmaceutical biotechnology. 2013;14(1):91–7. [PubMed] [Google Scholar]
- 364.Guillotin B, Souquet A, Catros S, Duocastella M, Pippenger B, Bellance S, et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials. 2010;31(28):7250–6. [DOI] [PubMed] [Google Scholar]
- 365.Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016;34(3):312–9. [DOI] [PubMed] [Google Scholar]
- 366.Liu W, Zhang YS, Heinrich MA, De Ferrari F, Jang HL, Bakht SM, et al. Rapid Continuous Multimaterial Extrusion Bioprinting. Adv Mater. 2017;29(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Highley CB, Rodell CB, Burdick JA. Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv Mater. 2015;27(34):5075–9. [DOI] [PubMed] [Google Scholar]
- 368.Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A. 2016;113(12):3179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Cristina C, Su Ryon S, Vijayan M, Solange M, Marco C, Andrea B, et al. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low viscosity bioink. Advanced Materials. 2015;Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Gladman AS, Matsumoto EA, Nuzzo RG, Mahadevan L, Lewis JA. Biomimetic 4D printing. Nat Mater. 2016;15(4):413–8. [DOI] [PubMed] [Google Scholar]
- 371.Li YC, Zhang YS, Akpek A, Shin SR, Khademhosseini A. 4D bioprinting: the next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication. 2016;9(1):012001. [DOI] [PubMed] [Google Scholar]
- 372.Malda J, Visser J, Melchels FP, Jüngst T, Hennink WE, Dhert WJ, et al. 25th anniversary article: engineering hydrogels for biofabrication. Adv Mater. 2013;25(36):5011–28. [DOI] [PubMed] [Google Scholar]
- 373.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotech. 2014;32(8):773–85. [DOI] [PubMed] [Google Scholar]
- 374.Zhang YS, Duchamp M, Oklu R, Ellisen LW, Langer R, Khademhosseini A. Bioprinting the Cancer Microenvironment. ACS Biomaterials Science & Engineering. 2016. 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Pati F, Jang J, Ha DH, Won Kim S, Rhie JW, Shim JH, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nature communications. 2014;5:3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016;34(3):312–9. [DOI] [PubMed] [Google Scholar]
- 377.Khalil S, Nam J, Sun W. Multi-nozzle deposition for construction of 3D biopolymer tissue scaffolds. Rapid Prototyping Journal. 2005;11(1):9–17. [Google Scholar]
- 378.Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv Mater. 2014;26(19):3124–30. [DOI] [PubMed] [Google Scholar]
- 379.Highley CB, Rodell CB, Burdick JA. Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv Mater. 2015;27(34):5075–9. [DOI] [PubMed] [Google Scholar]
- 380.Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue HJ, et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv. 2015;1(9):e1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Bhattacharjee T, Zehnder SM, Rowe KG, Jain S, Nixon RM, Sawyer WG, et al. Writing in the granular gel medium. Sci Adv. 2015;1(8):e1500655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Colosi C, Shin SR, Manoharan V, Massa S, Constantini M, Barbetta A, et al. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low viscosity bioink. Adv Mater. 2015;28(4):677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Hardin JO, Ober TJ, Valentine AD, Lewis JA. Microfluidic Printheads for Multimaterial 3D Printing of Viscoelastic Inks. Adv Mater. 2015;27(21):3279–84. [DOI] [PubMed] [Google Scholar]
- 384.Dhariwala B, Hunt E, Boland T. Rapid prototyping of tissue-engineering constructs, using photopolymerizable hydrogels and stereolithography. Tissue engineering. 2004;10(9–10):1316–22. [DOI] [PubMed] [Google Scholar]
- 385.Park JH, Hong JM, Ju YM, Jung JW, Kang HW, Lee SJ, et al. A novel tissue-engineered trachea with a mechanical behavior similar to native trachea. Biomaterials. 2015;62:106–15. [DOI] [PubMed] [Google Scholar]
- 386.Raman R, Bhaduri B, Mir M, Shkumatov A, Lee MK, Popescu G, et al. High-Resolution Projection Microstereolithography for Patterning of Neovasculature. Adv Healthc Mater. 2016;5(5):610–9. [DOI] [PubMed] [Google Scholar]
- 387.Zhang AP, Qu X, Soman P, Hribar KC, Lee JW, Chen S, et al. Rapid fabrication of complex 3D extracellular microenvironments by dynamic optical projection stereolithography. Adv Mater. 2012;24(31):4266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Ma X, Qu X, Zhu W, Li Y-S, Yuan S, Zhang H, et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proceedings of the National Academy of Sciences. 2016;113(8):2206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Leng L, McAllister A, Zhang B, Radisic M, Gunther A. Mosaic hydrogels: one-step formation of multiscale soft materials. Adv Mater. 2012;24(27):3650–8. [DOI] [PubMed] [Google Scholar]
- 390.Zhu W, Li J, Leong YJ, Rozen I, Qu X, Dong R, et al. 3D-Printed Artificial Microfish. Adv Mater. 2015. [DOI] [PubMed] [Google Scholar]
- 391.Banerjee A, Qi J, Gogoi R, Wong J, Mitragotri S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. Journal of Controlled Release. 2016;238:176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Wagner AM, Gran MP, Peppas NA. Designing the new generation of intelligent biocompatible carriers for protein and peptide delivery. Acta pharmaceutica sinica B. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Steichen S, O’Connor C, Peppas NA. Development of a P ((MAA-co-NVP)-g-EG) Hydrogel Platform for Oral Protein Delivery: Effects of Hydrogel Composition on Environmental Response and Protein Partitioning. Macromolecular bioscience. 2017;17(1). [DOI] [PubMed] [Google Scholar]
- 394.Carrillo-Conde BR, Brewer E, Lowman A, Peppas NA. Complexation Hydrogels as Oral Delivery Vehicles of Therapeutic Antibodies: An in Vitro and ex Vivo Evaluation of Antibody Stability and Bioactivity. Industrial & engineering chemistry research. 2015;54(42):10197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Horava SD, Peppas NA. Design of pH-responsive biomaterials to enable the oral route of hematological factor IX. Annals of biomedical engineering. 2016;44(6):1970–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Nakamura K, Murray RJ, Joseph JI, Peppas NA, Morishita M, Lowman AM. Oral insulin delivery using P (MAA-g-EG) hydrogels: effects of network morphology on insulin delivery characteristics. Journal of Controlled Release. 2004;95(3):589–99. [DOI] [PubMed] [Google Scholar]
- 397.Kamei N, Morishita M, Chiba H, Kavimandan NJ, Peppas NA, Takayama K. Complexation hydrogels for intestinal delivery of interferon β and calcitonin. Journal of controlled release. 2009;134(2):98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Ball RL, Bajaj P, Whitehead KA. Oral delivery of siRNA lipid nanoparticles: Fate in the GI tract. Scientific reports. 2018;8(1):2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Wiedersberg S, Guy RH. Transdermal drug delivery: 30+ years of war and still fighting! Journal of controlled Release. 2014;190:150–6. [DOI] [PubMed] [Google Scholar]
- 400.Marwah H, Garg T, Goyal AK, Rath G. Permeation enhancer strategies in transdermal drug delivery. Drug Delivery. 2016;23(2):564–78. [DOI] [PubMed] [Google Scholar]
- 401.Banerjee A, Ibsen K, Iwao Y, Zakrewsky M, Mitragotri S. Transdermal Protein Delivery Using Choline and Geranate (CAGE) Deep Eutectic Solvent. Advanced healthcare materials. 2017;6(15). [DOI] [PubMed] [Google Scholar]
- 402.Stefano M, Michael Z, Sunny K, Sanchez DOJ, A. MJ, Samir M. De Novo Design of Skin-Penetrating Peptides for Enhanced Transdermal Delivery of Peptide Drugs. Advanced Healthcare Materials. 2016;5(5):602–9. [DOI] [PubMed] [Google Scholar]
- 403.Prausnitz MR. Engineering microneedle patches for vaccination and drug delivery to skin. Annual review of chemical and biomolecular engineering. 2017;8:177–200. [DOI] [PubMed] [Google Scholar]
- 404.Dardano P, Caliò A, Di Palma V, Bevilacqua MF, Di Matteo A, De Stefano L. A photolithographic approach to polymeric microneedles array fabrication. Materials. 2015;8(12):8661–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Zhao Z, Yan R, Yi X, Li J, Rao J, Guo Z, et al. Bacteria-activated theranostic nanoprobes against methicillin-resistant Staphylococcus aureus infection. ACS nano. 2017;11(5):4428–38. [DOI] [PubMed] [Google Scholar]
- 406.Tang J, Lobatto ME, Read JC, Mieszawska AJ, Fayad ZA, Mulder WJ. Nanomedical theranostics in cardiovascular disease. Current cardiovascular imaging reports. 2012;5(1):19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Chen W, Zhang S, Yu Y, Zhang H, He Q. Structural-Engineering Rationales of Gold Nanoparticles for Cancer Theranostics. Advanced Materials. 2016;28(39):8567–85. [DOI] [PubMed] [Google Scholar]
- 408.Gobbo OL, Sjaastad K, Radomski MW, Volkov Y, Prina-Mello A. Magnetic nanoparticles in cancer theranostics. Theranostics. 2015;5(11):1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Spencer DS, Puranik AS, Peppas NA. Intelligent nanoparticles for advanced drug delivery in cancer treatment. Current opinion in chemical engineering. 2015;7:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Wagner AM, Spencer DS, Peppas NA. Advanced architectures in the design of responsive polymers for cancer nanomedicine. Journal of Applied Polymer Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Caldorera-Moore ME, Liechty WB, Peppas NA. Responsive theranostic systems: integration of diagnostic imaging agents and responsive controlled release drug delivery carriers. Accounts of chemical research. 2011;44(10):1061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Melancon MP, Zhou M, Li C. Cancer theranostics with near-infrared light-activatable multimodal nanoparticles. Accounts of chemical research. 2011;44(10):947–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Song Q, Yin Y, Shang L, Wu T, Zhang D, Kong M, et al. Tumor Microenvironment Responsive Nanogel for the Combinatorial Antitumor Effect of Chemotherapy and Immunotherapy. Nano Letters. 2017;17(10):6366–75. [DOI] [PubMed] [Google Scholar]
- 414.Conde J, Oliva N, Zhang Y, Artzi N. Local triple-combination therapy results in tumour regression and prevents recurrence in a colon cancer model. Nature materials. 2016;15(10):1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Diaz Lantada A Handbook on advanced design and manufacturing technologies for biomedical devices. New York: Springer; 2013. [Google Scholar]
- 416.Andreu-Perez J, Leff DR, Ip HMD, Yang GZ. From Wearable Sensors to Smart Implants-–Toward Pervasive and Personalized Healthcare. IEEE Trans Biomed Eng. 2015;62(12):2750–62. [DOI] [PubMed] [Google Scholar]
- 417.Yoo E-H, Lee S-Y. Glucose Biosensors: An Overview of Use in Clinical Practice. Sensors (Basel, Switzerland). 2010;10(5):4558–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Kim J, Campbell AS, Wang J. Wearable non-invasive epidermal glucose sensors: A review. Talanta. 2018;177:163–70. [DOI] [PubMed] [Google Scholar]
- 419.Yang T, Ji R, Deng XX, Du FS, Li ZC. Glucose-responsive hydrogels based on dynamic covalent chemistry and inclusion complexation. Soft Matter. 2014;10(15):2671–8. [DOI] [PubMed] [Google Scholar]
- 420.Dong Y, Wang W, Veiseh O, Appel EA, Xue K, Webber MJ, et al. Injectable and Glucose-Responsive Hydrogels Based on Boronic Acid-Glucose Complexation. Langmuir : the ACS journal of surfaces and colloids. 2016;32(34):8743–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Parker RS, Doyle FJ 3rd, Peppas NA. The intravenous route to blood glucose control. IEEE Eng Med Biol Mag. 2001;20(1):65–73. [DOI] [PubMed] [Google Scholar]
- 422.Podual K, Doyle FJ 3rd, Peppas NA. Dynamic behavior of glucose oxidase-containing microparticles of poly(ethylene glycol)-grafted cationic hydrogels in an environment of changing pH. Biomaterials. 2000;21(14):1439–50. [DOI] [PubMed] [Google Scholar]
- 423.Podual K, Doyle FJ 3rd, Peppas NA. Glucose-sensitivity of glucose oxidase-containing cationic copolymer hydrogels having poly(ethylene glycol) grafts. J Control Release. 2000;67(1):9–17. [DOI] [PubMed] [Google Scholar]
- 424.Lee J, Ko JH, Mansfield KM, Nauka PC, Bat E, Maynard HD. Glucose-Responsive Trehalose Hydrogel for Insulin Stabilization and Delivery. Macromolecular bioscience. 2018;18(5):e1700372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Li X, Fu M, Wu J, Zhang C, Deng X, Dhinakar A, et al. pH-sensitive peptide hydrogel for glucose-responsive insulin delivery. Acta Biomater. 2017;51:294–303. [DOI] [PubMed] [Google Scholar]
- 426.Matsumoto A, Tanaka M, Matsumoto H, Ochi K, Moro-Oka Y, Kuwata H, et al. Synthetic “smart gel” provides glucose-responsive insulin delivery in diabetic mice. Sci Adv. 2017;3(11):eaaq0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Rigla Cros M Glucose-responsive insulin delivery systems. Endocrinol Nutr. 2016;63(4):143–4. [DOI] [PubMed] [Google Scholar]
- 428.Tong Z, Zhou J, Zhong J, Tang Q, Lei Z, Luo H, et al. Glucose- and H2O2-Responsive Polymeric Vesicles Integrated with Microneedle Patches for Glucose-Sensitive Transcutaneous Delivery of Insulin in Diabetic Rats. ACS Appl Mater Interfaces. 2018. [DOI] [PubMed] [Google Scholar]
- 429.Wu W, Zhou S. Responsive materials for self-regulated insulin delivery. Macromolecular bioscience. 2013;13(11):1464–77. [DOI] [PubMed] [Google Scholar]
- 430.Yu J, Zhang Y, Gu Z. Glucose-Responsive Insulin Delivery by Microneedle-Array Patches Loaded with Hypoxia-Sensitive Vesicles. Methods Mol Biol. 2017;1570:251–9. [DOI] [PubMed] [Google Scholar]
- 431.Vashist SK. Non-invasive glucose monitoring technology in diabetes management: a review. Anal Chim Acta. 2012;750:16–27. [DOI] [PubMed] [Google Scholar]
- 432.Yadav J, Rani A, Singh V, Murari B. Prospects and limitations of non-invasive blood glucose monitoring using near-infrared spectroscopy 2015. [Google Scholar]
- 433.Lee H, Choi TK, Lee YB, Cho HR, Ghaffari R, Wang L, et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nature nanotechnology. 2016;11:566. [DOI] [PubMed] [Google Scholar]
- 434.Park J, Kim J, Kim S-Y, Cheong WH, Jang J, Park Y-G, et al. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Science Advances. 2018;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Elsherif M, Hassan MU, Yetisen AK, Butt H. Wearable Contact Lens Biosensors for Continuous Glucose Monitoring Using Smartphones. ACS nano. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Badugu R, Jeng BH, Reece EA, Lakowicz JR. Contact lens to measure individual ion concentrations in tears and applications to dry eye disease. Anal Biochem. 2018;542:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Rogers JA, Someya T, Huang Y. Materials and Mechanics for Stretchable Electronics. Science. 2010;327(5973):1603–7. [DOI] [PubMed] [Google Scholar]
- 438.Huanyu C, Ning Y. Dissolvable tattoo sensors: from science fiction to a viable technology. Phys Scr. 2017;92(1):013001. [Google Scholar]
- 439.Yao S, Zhu Y. Nanomaterial-Enabled Stretchable Conductors: Strategies, Materials and Devices. Adv Mater. 2015;27(9):1480–511. [DOI] [PubMed] [Google Scholar]
- 440.Liu Y, Pharr M, Salvatore GA. Lab-on-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring. ACS nano. 2017;11(10):9614–35. [DOI] [PubMed] [Google Scholar]
- 441.Rogers JA, Lagally MG, Nuzzo RG. Synthesis, assembly and applications of semiconductor nanomembranes. Nature. 2011;477:45. [DOI] [PubMed] [Google Scholar]
- 442.Das S, Ghosh A. Effect of plasticizers on ionic conductivity and dielectric relaxation of PEO-LiClO4 polymer electrolyte. Electrochim Acta. 2015;171:59–65. [Google Scholar]
- 443.Jaehong L, Hyukho K, Jungmok S, Sera S, Hoon KJ, Changhyun P, et al. Conductive Fiber-Based Ultrasensitive Textile Pressure Sensor for Wearable Electronics. Adv Mater. 2015;27(15):2433–9. [DOI] [PubMed] [Google Scholar]
- 444.Liu W, Liu N, Sun J, Hsu P-C, Li Y, Lee H-W, et al. Ionic Conductivity Enhancement of Polymer Electrolytes with Ceramic Nanowire Fillers. Nano Lett. 2015;15(4):2740–5. [DOI] [PubMed] [Google Scholar]
- 445.Bandodkar AJ, Jeerapan I, Wang J. Wearable Chemical Sensors: Present Challenges and Future Prospects. ACS Sensors. 2016;1(5):464–82. [Google Scholar]
- 446.Bandodkar AJ, Wang J. Non-invasive wearable electrochemical sensors: a review. Trends Biotechnol. 2014;32(7):363–71. [DOI] [PubMed] [Google Scholar]
- 447.Kim D-H, Lu N, Ma R, Kim Y-S, Kim R-H, Wang S, et al. Epidermal Electronics. Science. 2011;333(6044):838–43. [DOI] [PubMed] [Google Scholar]
- 448.Liu X, Yuk H, Lin S, Parada German A, Tang TC, Tham E, et al. 3D Printing of Living Responsive Materials and Devices. Adv Mater. 2017;30(4):1704821. [DOI] [PubMed] [Google Scholar]
- 449.Green R, Abidian MR. Conducting Polymers for Neural Prosthetic and Neural Interface Applications. Advanced materials (Deerfield Beach, Fla). 2015;27(46):7620–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Norton JJS, Lee DS, Lee JW, Lee W, Kwon O, Won P, et al. Soft, curved electrode systems capable of integration on the auricle as a persistent brain–computer interface. Proceedings of the National Academy of Sciences. 2015;112(13):3920–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Rylie G, Reza AM. Conducting Polymers for Neural Prosthetic and Neural Interface Applications. Adv Mater. 2015;27(46):7620–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Canales A, Jia X, Froriep UP, Koppes RA, Tringides CM, Selvidge J, et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat Biotechnol. 2015;33:277. [DOI] [PubMed] [Google Scholar]
- 453.Ravichandran R, Sundarrajan S, Venugopal JR, Mukherjee S, Ramakrishna S. Applications of conducting polymers and their issues in biomedical engineering. Journal of the Royal Society, Interface. 2010;7 Suppl 5:S559–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Norton JJ, Lee DS, Lee JW, Lee W, Kwon O, Won P, et al. Soft, curved electrode systems capable of integration on the auricle as a persistent brain-computer interface. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(13):3920–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Abidian MR, Ludwig KA, Marzullo TC, Martin DC, Kipke DR. Interfacing Conducting Polymer Nanotubes with the Central Nervous System: Chronic Neural Recording using Poly(3,4-ethylenedioxythiophene) Nanotubes. Adv Mater. 2009;21(37):3764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Nyberg T, Shimada A, Torimitsu K. Ion conducting polymer microelectrodes for interfacing with neural networks. J Neurosci Methods. 2007;160(1):16–25. [DOI] [PubMed] [Google Scholar]
- 457.Green RA, Lovell NH, Poole-Warren LA. Impact of co-incorporating laminin peptide dopants and neurotrophic growth factors on conducting polymer properties. Acta Biomater. 2010;6(1):63–71. [DOI] [PubMed] [Google Scholar]
- 458.Abidian MR, Martin DC. Experimental and theoretical characterization of implantable neural microelectrodes modified with conducting polymer nanotubes. Biomaterials. 2008;29(9):1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Mario Cheong GL, Lim KS, Jakubowicz A, Martens PJ, Poole-Warren LA, Green RA. Conductive hydrogels with tailored bioactivity for implantable electrode coatings. Acta Biomater. 2014;10(3):1216–26. [DOI] [PubMed] [Google Scholar]
- 460.Reza AM C MD. Multifunctional Nanobiomaterials for Neural Interfaces. Adv Funct Mater. 2009;19(4):573–85. [Google Scholar]
- 461.Ludwig KA, Uram JD, Yang J, Martin DC, Kipke DR. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. Journal of neural engineering. 2006;3(1):59–70. [DOI] [PubMed] [Google Scholar]
- 462.Green RA, Matteucci PB, Hassarati RT, Giraud B, Dodds CW, Chen S, et al. Performance of conducting polymer electrodes for stimulating neuroprosthetics. Journal of neural engineering. 2013;10(1):016009. [DOI] [PubMed] [Google Scholar]
- 463.Loffler S, Melican K, Nilsson KPR, Richter-Dahlfors A. Organic bioelectronics in medicine. Journal of internal medicine. 2017;282(1):24–36. [DOI] [PubMed] [Google Scholar]
- 464.Viventi J, Kim D-H, Vigeland L, Frechette ES, Blanco JA, Kim Y-S, et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat Neurosci. 2011;14:1599.+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Holley MT, Nagarajan N, Danielson C, Zorlutuna P, Park K. Development and characterization of muscle-based actuators for self-stabilizing swimming biorobots. Lab Chip. 2016;16(18):3473–84. [DOI] [PubMed] [Google Scholar]
- 466.Park SJ, Gazzola M, Park KS, Park S, Di Santo V, Blevins EL, et al. Phototactic guidance of a tissue-engineered soft-robotic ray. Science. 2016;353(6295):158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Chan V, Asada HH, Bashir R. Utilization and control of bioactuators across multiple length scales. Lab on a chip. 2014;14(4):653–70. [DOI] [PubMed] [Google Scholar]
- 468.Felfoul O, Mohammadi M, Taherkhani S, de Lanauze D, Zhong Xu Y, Loghin D, et al. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nature nanotechnology. 2016;11(11):941–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Park BW, Zhuang J, Yasa O, Sitti M. Multifunctional Bacteria-Driven Microswimmers for Targeted Active Drug Delivery. ACS nano. 2017;11(9):8910–23. [DOI] [PubMed] [Google Scholar]
- 470.Park SJ, Park SH, Cho S, Kim DM, Lee Y, Ko SY, et al. New paradigm for tumor theranostic methodology using bacteria-based microrobot. Sci Rep. 2013;3:3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Magdanz V, Sanchez S, Schmidt OG. Development of a sperm-flagella driven microbio-robot. Advanced materials. 2013;25(45):6581–8. [DOI] [PubMed] [Google Scholar]
- 472.Herr H, Dennis RG. A swimming robot actuated by living muscle tissue. J Neuroeng Rehabil. 2004;1(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Akiyama Y, Sakuma T, Funakoshi K, Hoshino T, Iwabuchi K, Morishima K. Atmospheric-operable bioactuator powered by insect muscle packaged with medium. Lab Chip. 2013;13(24):4870–80. [DOI] [PubMed] [Google Scholar]
- 474.Duffy RM, Feinberg AW. Engineered skeletal muscle tissue for soft robotics: fabrication strategies, current applications, and future challenges. Wiley interdisciplinary reviews Nanomedicine and nanobiotechnology. 2014;6(2):178–95. [DOI] [PubMed] [Google Scholar]
- 475.Sun Y, Duffy R, Lee A, Feinberg AW. Optimizing the structure and contractility of engineered skeletal muscle thin films. Acta Biomater. 2013;9(8):7885–94. [DOI] [PubMed] [Google Scholar]
- 476.Cvetkovic C, Raman R, Chan V, Williams BJ, Tolish M, Bajaj P, et al. Three-dimensionally printed biological machines powered by skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(28):10125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Feinberg AW, Feigel A, Shevkoplyas SS, Sheehy S, Whitesides GM, Parker KK. Muscular thin films for building actuators and powering devices. Science. 2007;317(5843):1366–70. [DOI] [PubMed] [Google Scholar]
- 478.Nawroth JC, Lee H, Feinberg AW, Ripplinger CM, McCain ML, Grosberg A, et al. A tissue-engineered jellyfish with biomimetic propulsion. Nature Biotechnology. 2012:792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Dvir T, Timko BP, Brigham MD, Naik SR, Karajanagi SS, Levy O, et al. Nanowired three-dimensional cardiac patches. Nat Nanotechnol. 2011;6(11):720–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Zhu K, Shin SR, Kempen Tv, Li Y-C, Ponraj V, Nasajpour A, et al. Gold Nanocomposite Bioink for Printing 3D Cardiac Constructs. Advanced Funcional Materials. 2017;27(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Zhang D, Shadrin IY, Lam J, Xian HQ, Snodgrass HR, Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials. 2013;34(23):5813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Nawroth JC, Lee H, Feinberg AW, Ripplinger CM, McCain ML, Grosberg A, et al. A tissue-engineered jellyfish with biomimetic propulsion. Nat Biotechnol. 2012;30(8):792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Williams BJ, Anand SV, Rajagopalan J, Saif MT. A self-propelled biohybrid swimmer at low Reynolds number. Nat Commun. 2014;5:3081. [DOI] [PubMed] [Google Scholar]
- 484.Chan V, Park K, Collens MB, Kong H, Saif TA, Bashir R. Development of miniaturized walking biological machines. Sci Rep. 2012;2:857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Chan V, Jeong JH, Bajaj P, Collens M, Saif T, Kong H, et al. Multi-material bio-fabrication of hydrogel cantilevers and actuators with stereolithography. Lab Chip. 2012;12(1):88–98. [DOI] [PubMed] [Google Scholar]
- 486.Rus D, Tolley MT. Design, fabrication and control of soft robots. Nature. 2015;521(7553):467–75. [DOI] [PubMed] [Google Scholar]
- 487.Russo RS, Blemker SS, Fish FE, Bart-Smith H. Biomechanical model of batoid (skates and rays) pectoral fins predicts the influence of skeletal structure on fin kinematics: implications for bio-inspired design. Bioinspiration & biomimetics. 2015;10(4):046002. [DOI] [PubMed] [Google Scholar]
- 488.Li T, Li G, Liang Y, Cheng T, Dai J, Yang X, et al. Fast-moving soft electronic fish. Science advances. 2017;3(4):e1602045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Wehner M, Truby RL, Fitzgerald DJ, Mosadegh B, Whitesides GM, Lewis JA, et al. An integrated design and fabrication strategy for entirely soft, autonomous robots. Nature. 2016;536(7617):451–5. [DOI] [PubMed] [Google Scholar]
- 490.Xi J, Schmidt JJ, Montemagno CD. Self-assembled microdevices driven by muscle. Nat Mater. 2005;4(2):180–4. [DOI] [PubMed] [Google Scholar]
- 491.Jia Z, Valiunas V, Lu Z, Bien H, Liu H, Wang HZ, et al. Stimulating cardiac muscle by light: cardiac optogenetics by cell delivery. Circ Arrhythm Electrophysiol. 2011;4(5):753–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Raman R, Cvetkovic C, Uzel SG, Platt RJ, Sengupta P, Kamm RD, et al. Optogenetic skeletal muscle-powered adaptive biological machines. Proc Natl Acad Sci U S A. 2016;113(13):3497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Sakar MS, Neal D, Boudou T, Borochin MA, Li Y, Weiss R, et al. Formation and optogenetic control of engineered 3D skeletal muscle bioactuators. Lab on a chip. 2012;12(23):4976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Tian B, Liu J, Dvir T, Jin L, Tsui JH, Qing Q, et al. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat Mater. 2012;11(11):986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Kim DH, Ghaffari R, Lu N, Rogers JA. Flexible and stretchable electronics for biointegrated devices. Annu Rev Biomed Eng. 2012;14:113–28. [DOI] [PubMed] [Google Scholar]
- 496.Kim DH, Lu N, Ma R, Kim YS, Kim RH, Wang S, et al. Epidermal electronics. Science. 2011;333(6044):838–43. [DOI] [PubMed] [Google Scholar]
- 497.Cellot G, Cilia E, Cipollone S, Rancic V, Sucapane A, Giordani S, et al. Carbon nanotubes might improve neuronal performance by favouring electrical shortcus. Nature Nanotechnology. 2008;4:126–33. [DOI] [PubMed] [Google Scholar]
- 498.Bareket-Keren L, Hanein Y. Carbon nanotube-based multi electrode arrays for neuronal interfacing: progress and prospects. Frontiers in neural circuits. 2012;6:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Food and Drug Administration. 21 CFR Part 3: Definition of Primary Mode of Action of a Combination Product. Federal Register. 2005;70(164):49848–62. [PubMed] [Google Scholar]
- 500.Pashuck ET, Stevens MM. Designing Regenerative Biomaterial Therapies for the Clinic. Science Translational Medicine. 2012;4(160):160sr4–sr4. [DOI] [PubMed] [Google Scholar]
- 501.Ferber G, Glimm E. Chapter 6 - Translational Science Biostatistics In: Wehling M, editor. Principles of Translational Science in Medicine (Second Edition). Boston: Academic Press; 2015. p. 267–80. [Google Scholar]
- 502.Horwitz EM. Advancing Regenerative Medicine the Translational Way. Science Translational Medicine. 2013;5(177):177fs9–fs9. [DOI] [PubMed] [Google Scholar]
- 503.Linehan JH, Chaney A. Academic/Industry Challenges for Medical Device Development. Science Translational Medicine. 2010;2(63):63mr6–mr6. [DOI] [PubMed] [Google Scholar]
- 504.Moreno PG, Joly Y. Chapter 7 - Intellectual Property and Innovation in Translational Medicine. In: Wehling M, editor. Principles of Translational Science in Medicine (Second Edition). Boston: Academic Press; 2015. p. 281–97. [Google Scholar]
- 505.Serban MA. Translational biomaterials—the journey from the bench to the market—think ‘product’. Curr Opin Biotechnol. 2016;40:31–4. [DOI] [PubMed] [Google Scholar]
- 506.Van Norman GA. Drugs, Devices, and the FDA: Part 1: An Overview of Approval Processes for Drugs. JACC: Basic to Translational Science. 2016;1(3):170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Van Norman GA. Drugs, Devices, and the FDA: Part 2: An Overview of Approval Processes: FDA Approval of Medical Devices. JACC: Basic to Translational Science. 2016;1(4):277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Wehling M Principles of Translational Science in Medicine (Second Edition). Wehling M, editor. Boston: Academic Press; 2015. 2015/01/01/. 1–12 p. [Google Scholar]
- 509.Bayon Y, Bohner M, Eglin D, Procter P, Richards RG, Weber J, et al. Innovating in the medical device industry - challenges & opportunities ESB 2015 translational research symposium. J Mater Sci Mater Med. 2016;27(9):144. [DOI] [PubMed] [Google Scholar]
- 510.Ben Amar A, Kouki AB, Cao H. Power Approaches for Implantable Medical Devices. Sensors (Basel, Switzerland). 2015;15(11):28889–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Ko WH. Early History and Challenges of Implantable Electronics. ACM journal on emerging technologies in computing systems. 2012;8(2):8–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Van Norman GA, Eisenkot R. Technology Transfer: From the Research Bench to Commercialization: Part 2: The Commercialization Process. JACC: Basic to Translational Science. 2017;2(2):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Van Norman GA. Drugs and Devices: Comparison of European and U.S. Approval Processes. JACC: Basic to Translational Science. 2016;1(5):399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Koetting MC, Guido JF, Gupta M, Zhang A, Peppas NA. pH-responsive and enzymatically-responsive hydrogel microparticles for the oral delivery of therapeutic proteins: Effects of protein size, crosslinking density, and hydrogel degradation on protein delivery. Journal of Controlled Release. 2016;221:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, McCullough J. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomedicine: nanotechnology, biology and medicine. 2013;9(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Martins IM, Barreiro MF, Coelho M, Rodrigues AE. Microencapsulation of essential oils with biodegradable polymeric carriers for cosmetic applications. Chemical Engineering Journal. 2014;245:191–200. [Google Scholar]
- 517.Ammala A Biodegradable polymers as encapsulation materials for cosmetics and personal care markets. International journal of cosmetic science. 2013;35(2):113–24. [DOI] [PubMed] [Google Scholar]
- 518.Raman R, Cvetkovic C, Uzel SG, Platt RJ, Sengupta P, Kamm RD, et al. Optogenetic skeletal muscle-powered adaptive biological machines. Proceedings of the National Academy of Sciences. 2016;113(13):3497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Duffy RM, Feinberg AW. Engineered skeletal muscle tissue for soft robotics: fabrication strategies, current applications, and future challenges. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2014;6(2):178–95. [DOI] [PubMed] [Google Scholar]
- 520.Salimath AS, García AJ. Biofunctional hydrogels for skeletal muscle constructs. Journal of tissue engineering and regenerative medicine. 2016;10(11):967–76. [DOI] [PubMed] [Google Scholar]
- 521.Clegg JR, Zhong JX, Irani AS, Gu J, Spencer D, Peppas N. Characterization of protein interactions with molecularly imprinted hydrogels that possess engineered affinity for high isoelectric point biomarkers. J Biomed Mater Res Part A A. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Culver HR, Peppas NA. Protein-Imprinted Polymers: The Shape of Things to Come? Chemistry of Materials. 2017;29(14):5753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proceedings of the National Academy of Sciences. 2016;113(12):3179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–43. [DOI] [PubMed] [Google Scholar]
- 525.Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nature biotechnology. 2016;34(3):312. [DOI] [PubMed] [Google Scholar]
- 526.Sridharan R, Cameron AR, Kelly DJ, Kearney CJ, O’Brien FJ. Biomaterial based modulation of macrophage polarization: a review and suggested design principles. Materials Today. 2015;18(6):313–25. [Google Scholar]
- 527.Duan B, Yin Z, Kang LH, Magin RL, Butcher JT. Active tissue stiffness modulation controls valve interstitial cell phenotype and osteogenic potential in 3D culture. Acta biomaterialia. 2016;36:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Ma H, Killaars AR, DelRio FW, Yang C, Anseth KS. Myofibroblastic activation of valvular interstitial cells is modulated by spatial variations in matrix elasticity and its organization. Biomaterials. 2017;131:131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Kobolak J, Dinnyes A, Memic A, Khademhosseini A, Mobasheri A. Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods. 2016;99:62–8. [DOI] [PubMed] [Google Scholar]
- 530.Zhang YS, Khademhosseini A. Seeking the right context for evaluating nanomedicine: from tissue models in petri dishes to microfluidic organs-on-a-chip. Nanomedicine. 2015;10(5):685–8. [DOI] [PubMed] [Google Scholar]
- 531.Barbolosi D, Ciccolini J, Lacarelle B, Barlési F, André N. Computational oncology—mathematical modelling of drug regimens for precision medicine. Nature reviews Clinical oncology. 2016;13(4):242. [DOI] [PubMed] [Google Scholar]
- 532.Gracia R, Mecerreyes D. Polymers with redox properties: materials for batteries, biosensors and more. Polym Chem. 2013;4(7):2206–14. [Google Scholar]
- 533.Cademartiri L, Ozin GA. Concepts of Nanochemistry: Wiley-VCH; 2009. 282 p. [Google Scholar]
- 534.Panda JJ, Chauhan VS. Short peptide based self-assembled nanostructures: implications in drug delivery and tissue engineering. Polymer Chemistry. 2014;5(15):4418–36. [Google Scholar]
- 535.Lasic DD. Novel applications of liposomes. Trends in Biotechnology. 1998;16(7):307–21. [DOI] [PubMed] [Google Scholar]
- 536.Paz VF, Emons M, Obata K, Ovsianikov A, Peterhänsel S, Frenner K, et al. Development of functional sub-100 nm structures with 3D two-photon polymerization technique and optical methods for characterization. Journal of Laser Applications. 2012;24(4):042004. [Google Scholar]
- 537.Challenging nature’s monopoly on the creation of well-defined nanoparticles. Nanomedicine. 2010;5(4):633–9. [DOI] [PubMed] [Google Scholar]
- 538.Hansen CJ, Saksena R, Kolesky DB, Vericella JJ, Kranz SJ, Muldowney GP, et al. High-Throughput Printing via Microvascular Multinozzle Arrays. Advanced Materials. 2013;25(1):96–102. [DOI] [PubMed] [Google Scholar]
- 539.Hyunjae L, Joseph HY, Seungmin B, Taeghwan H, Dae-Hyeong K. Enzyme-Based Glucose Sensor: From Invasive to Wearable Device. Advanced Healthcare Materials. 2018;7(8):1701150. [DOI] [PubMed] [Google Scholar]
- 540.Yoon J, Eyster TW, Misra AC, Lahann J. Cardiomyocyte-driven actuation in biohybrid microcylinders. Advanced Materials. 2015;27(30):4509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Shin SR, Jung SM, Zalabany M, Kim K, Zorlutuna P, Kim Sb, et al. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS nano. 2013;7(3):2369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, De Lecea L, et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nature protocols. 2010;5(3):439. [DOI] [PMC free article] [PubMed] [Google Scholar]