Abstract
Background
Dementia with Lewy bodies (DLB) is a common cause of neurodegenerative dementia of old age. Its accurate recognition can be important in clinical management and is essential for the development of disease‐modifying treatments. The current clinical diagnostic criteria are limited particularly by relatively poor sensitivity. Dopamine transporter (DAT) imaging using single‐photon emission computed tomography (SPECT) is the most highly developed supplementary test for DLB, and is now incorporated as a suggestive feature in the consensus diagnostic criteria. However, there is uncertainty about its accuracy and its place in clinical practice. It is most commonly used in people who are already suspected of having DLB.
Objectives
We had two objectives in this review: (A) to estimate the accuracy of DAT imaging for the diagnosis of DLB in people with dementia in secondary care (specialist dementia services), and (B) to estimate the accuracy of DAT imaging for the diagnosis of DLB in people with dementia in secondary care who are already suspected of having DLB on the basis of a prior clinical work‐up.
Search methods
We searched MEDLINE (1946 to February 2013), Embase (1980 to February 2013), BIOSIS Previews (1926 to February 2013), PsycINFO (1806 to February 2013), CINAHL (1982 to February 2013), LILACS (February 2013) and Web of Science and Conference Proceedings (ISI Web of Science) (1945 to February 2013). Several of these sources contain conference abstracts. We also searched four specialised databases containing diagnostic reviews: Meta‐analyses van Diagnostisch Onderzoek (MEDION; February 2013), Database of Abstracts of Reviews of Effects (DARE; February 2013), Health Technology Assessment Database (HTA; February 2013), and Aggressive Research Intelligence Facility (ARIF; February 2013). We checked reference lists of relevant studies and reviews for potential additional studies. Terms for electronic database searching were devised in conjunction with the team at the Cochrane Dementia and Cognitive Improvement Group.
Selection criteria
Study design: We included test accuracy studies with delayed verification, diagnostic case‐control studies, and two‐gate studies with alternative diagnosis controls. Participants: (A) participants with dementia in secondary care, (B) participants in secondary care meeting consensus clinical criteria (other than the DAT imaging criterion) for possible or probable DLB, or both. Index test: SPECT or positron emission tomography (PET) imaging of brain dopamine transporters. Reference standard: Neuropathological diagnosis at autopsy.
Data collection and analysis
Two review authors independently selected studies for inclusion and extracted data. We extracted results into a 2x2 table, showing the binary test results cross‐classified with the binary reference standard. We used this data to calculate sensitivities, specificities, and their 95% confidence intervals. We used the QUADAS‐2 tool plus some additional items to assess methodological quality.
Main results
We included one study that was applicable to our first objective (A). It reported data on 22 participants who met consensus clinical criteria for DLB or National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS‐ADRDA) criteria for Alzheimer's disease, or both (a two‐gate design with alternative diagnosis controls). The index test was SPECT scanning using the ligand 123I‐FP‐CIT. We considered the study to be at high risk of bias in the participant selection and index test domains (QUADAS‐2). 123I‐FP‐CIT SPECT analysed semiquantitatively had a sensitivity of 1.00 (95% confidence interval (CI) 0.66 to 1.00) and a specificity of 0.92 (95% CI 0.64 to 1.00) for the diagnosis of DLB (n = 22, 1 study). Analysed visually, the sensitivity was 0.86 (95% CI 0.42 to 1.00) and the specificity was 0.83 (95% CI 0.52 to 0.98) (n = 19, 1 study).
We considered that the study also provided the best available data to address our second objective (B). At baseline, 15 participants were clinically suspected of having DLB. In this group, 123I‐FP‐CIT SPECT scanning analysed semiquantitatively had a sensitivity of 1.00 (95% CI 0.63 to 1.00) and a specificity of 1.00 (95% CI 0.59 to 1.00) for the diagnosis of DLB (n = 15, 1 study). Analysed visually, accuracy in this group was lower with a sensitivity of 0.83 (95% CI 0.36 to 1.00) and a specificity of 0.71 (95% CI 0.29 to 0.96) (n = 13, 1 study).
Authors' conclusions
Only one study has used a neuropathological reference standard to assess the accuracy of DAT imaging for the diagnosis of DLB. The small size of the included study means that sensitivity and specificity estimates are imprecise. However, data from this study suggest that DAT imaging is more accurate than clinical diagnosis. Clinical diagnosis is therefore unsuitable to use as a reference standard for assessing the accuracy of DAT imaging.
No studies using a neuropathological reference standard have directly addressed the common clinical scenario where the use of DAT imaging is considered as a diagnostic test in a person with possible DLB, or assessed the accuracy of DAT imaging in people with mild dementia. However, the data from the included study suggest that, where there is moderately severe dementia and a strong pre‐existing suspicion of DLB (probable DLB), then a normal (123)I‐FP‐CIT SPECT scan may be an accurate means of excluding the diagnosis.
Semiquantitative ratings of 123I‐FP‐CIT SPECT scans appeared to be more accurate than visual ratings in all analyses.
Plain language summary
Dopamine transporter imaging for the diagnosis of dementia with Lewy bodies
Dementia with Lewy bodies (DLB) is one of the more common causes of dementia in old age. At present, it is diagnosed on the basis of the presence of characteristic symptoms, of which the most important are visual hallucinations, fluctuations in cognition, and Parkinsonism (movement symptoms like those seen in Parkinson's disease). However, many people who have DLB do not have all of these symptoms, and it can be hard to distinguish from other causes of dementia, especially Alzheimer's disease (AD). It is important to diagnose it accurately because people with DLB can have particularly severe side effects if given antipsychotic medication and, in the long term, so that treatments can be improved. Diagnosis of DLB using clinical symptoms alone has proven in some studies not to be very sensitive, that is cases of DLB are often missed.
People with DLB have reduced levels of the dopamine transporter protein (DAT) in a part of the brain known as the corpus striatum. It is possible, using tracers that bind to the dopamine transporter, to identify this reduction on certain brain scans (PET or SPECT scans). This is known as DAT imaging. It has been suggested that DAT imaging may help in the accurate diagnosis of DLB. In this review, we aimed to assess how accurately DAT imaging can identify DLB among all people with dementia seen in specialist dementia services (secondary care). Because DAT scans are expensive, they are usually used in people in whom a doctor already suspects the presence of DLB, so we also aimed to see how accurately DAT imaging identified DLB among these people. We only looked for studies in which the diagnosis was confirmed by examining the person's brain after death.
We found only one study of 22 participants to include in the review. The participants had been selected for the study because they had been diagnosed clinically with DLB or AD. This method of recruiting participants tends to exaggerate the accuracy of a test. The participants in the study had moderately severe dementia when they had the DAT imaging. Overall, the DAT imaging correctly classified 100% of the participants who had DLB and 92% of the participants who did not have DLB at postmortem. When we looked at only the 15 participants whom the doctors thought had DLB at the start of the study, then the DAT imaging correctly classified 100% of the participants who had DLB and 100% of the participants who did not have DLB at postmortem. The small size of the included study meant that we could not be very confident in these estimates of accuracy, and it is still possible that the test is considerably less accurate than this would suggest. We can conclude that DAT imaging is a promising test for diagnosing DLB, but it is important to note that we did not find any studies of participants in whom diagnosis may be more difficult, such as those with only mild dementia or those who have only one symptom to raise a suspicion of DLB.
Summary of findings
Summary of findings'. 'Summary of findings table.
| Title: Dopamine transporter imaging for the diagnosis of dementia with Lewy bodies (DLB) | |||||||
| Index test: Dopamine transporter (DAT) scan, particularly single‐photon emission CT scan with [123I]‐N‐(3‐fluoropropyl)‐2β‐carbomethoxy‐3β‐(4‐iodophenyl) nortropane (123I‐FP‐CIT) | |||||||
| Population: People with dementia in secondary care (objective A) or people with pre‐existing suspicion of DLB on the basis of a clinical work‐up (objective B) | |||||||
| Target condition: DLB | |||||||
| Reference standard: Neuropathological diagnosis at autopsy | |||||||
| Study design: Test accuracy studies with delayed verification or diagnostic case‐control | |||||||
| Objective | Population ‐ index test | Participants (studies) |
Confirmed DLB | Sensitivity (95% CI) | Specificity (95% CI) | Quality | Comment |
| A | DLB & AD; DAT scan (semiquantitative) | 22 (1) | 9 | 100% (66 to 100) | 92% (64 to 100) | Risk of bias was seen in the participant selection and index test QUADAS‐2 domains, with additional risk possibly arising from lack of clarity in the flow and timing domain. The reference standard was a strength of the study. There were no concerns about applicability. |
With the limited information available, DAT scanning provisionally appears to be an accurate test. Further research is essential. Clinical suspicion of DLB in the included study appeared to lack accuracy. |
| A | DLB & AD; DAT scan (rated visually) | 19 (1) | 7 | 86% (42 to 100) | 83% (52 to 98) | ||
| B | Suspected DLB only DAT scan (semiquantitative) | 15 (1) | 8 | 100% (63 to 100) | 100% (59 to 100) | ||
| B | Suspected DLB only DAT scan (rated visually) | 13 (1) | 6 | 83% (36 to 100) | 71% (29 to 96) | ||
Background
Target condition being diagnosed
Dementia with Lewy bodies (DLB) is a common cause of neurodegenerative dementia of old age, with an estimated prevalence of 15% to 25% of cases based on autopsy studies (Perry 1990; Heidebrink 2002). Community prevalence estimates vary more widely, with rates reported of 0% to 5% in the elderly population and 0% to 30.5% among elderly people with dementia (Zaccai 2005).
Diagnosis during life rests on a set of consensus clinical criteria that were adopted in 1996 and revised in 2005 (McKeith 1996; McKeith 2005). We show both the 1996 and 2005 versions in Appendix 1. Probable or possible DLB diagnoses may be made based on the number of core features or suggestive features, or both. The core features of the illness are recurrent visual hallucinations, fluctuating cognition, and Parkinsonism. The consensus criteria state that these features should occur early in the course of the illness.
As for other forms of dementia, the diagnostic gold standard is the neuropathological findings at postmortem. The neuropathological criteria for DLB have also evolved over time and remain a subject of debate. The standards currently considered the most robust combine assessments of Lewy body (LB) pathology and Alzheimer's disease (AD) pathology in order to estimate the probability of an association between the LB pathology and the dementia syndrome. These are either the standards established by McKeith 2005 or a combination of LB pathology assessed according to the method described by Braak 2003 with an accepted AD pathological standard (including as a minimum Braak neurofibrillary tangle staging). Comparisons of pathological and clinical findings suggest that the typical DLB clinical syndrome is seen most clearly in people with 'pure' DLB pathology. In contrast, people who also have significant AD pathology are found to have an attenuated clinical syndrome that is harder to recognise in life.
Changing neuropathological standards for the diagnosis of DLB clearly impact on assessment of the accuracy of the consensus clinical criteria. Several groups have reported sensitivity and specificity using the earlier clinical and neuropathological criteria (McKeith 1996). Overall, a clinical diagnosis of probable DLB in these studies had high specificity but often poor sensitivity (McKeith 2004), although it is likely that overdiagnosis of DLB using older neuropathological standards contributed to poor sensitivity estimates. There are few published data on the accuracy of a diagnosis of possible DLB. Although the possible DLB diagnosis appears to be more sensitive than the probable DLB diagnosis, this is at the cost of a loss of specificity, with the only reported autopsy series finding a specificity of just 28% (Verghese 1999). Nelson, et al. have reported on a very large clinicopathological data set from the National Alzheimer's Disease Coordinating Center (NACC) from 2000 onwards that used current neuropathological methods (Nelson 2010). In line with earlier reports, they found a high specificity of an antemortem diagnosis of DLB (95%), but again low sensitivity (32.1% for pure DLB and 12.1% for DLB plus AD). These authors found that the accuracy of DLB diagnoses in the specialist dementia centres contributing to the NACC database is actually declining with time.
An important but poorly understood issue is how the accuracy of the clinical criteria changes with the stage of the illness. Core features, such as visual hallucinations and Parkinsonian motor symptoms, occur more commonly in other forms of dementia by the moderate to severe stages. Thus, the ability of the criteria to predict the presence of Lewy body pathology declines as dementia progresses (Nelson 2010).
Index test(s)
Brains of people with DLB show a variety of reductions in indices of dopaminergic function both in vitro and in vivo that distinguish them from healthy elderly subjects and from people with AD (Tatsch 2008). Among the distinguishing features is a reduction of pre‐synaptic dopamine transporter (DAT) in the corpus striatum, reflecting degeneration of dopaminergic nigrostriatal neurons. It is possible to image striatal dopamine transporter sites in vivo with single‐photon emission computed tomography (SPECT) or positron emission tomography (PET) using a variety of radioligands. PET offers better image resolution, but SPECT has the advantages of being cheaper and more widely available. Most of the radioligands have been used only for research purposes, but the SPECT ligand [123I]‐N‐(3‐fluoropropyl)‐2β‐carbomethoxy‐3β‐(4‐iodophenyl) nortropane (123I‐FP‐CIT) is commercially available (under the brand name DaTscanTM). We show approved indications for DaTscanTM in Europe and the United States in Appendix 2.
In healthy subjects, 123I‐FP‐CIT SPECT images show the corpus striatum (caudate nucleus and putamen) lit up as a comma‐shaped structure bilaterally, reflecting specific uptake of the DAT ligand. Most experience of abnormal DAT images derives from people with Parkinson's disease. The typical pattern in Parkinson's disease is for the 'comma' to disappear, starting with the tail (the putamen) unilaterally. A similar pattern is seen in the majority of cases of DLB, although the change is usually more symmetrical. In a minority of DLB cases there may be more global loss of specific uptake throughout the corpus striatum from the outset. Images can be rated for the degree of abnormality either visually or using semiquantitative or quantitative methods. Visual ratings may be reported either in a binary fashion as normal or abnormal, or degrees of abnormality (typically two or three) may be reported. Excellent agreement can be reached between experienced visual raters (McKeith 2007). Semiquantitative or quantitative methods use a region of interest technique and derive ratios of ligand uptake in the caudate and in the putamen to nonspecific background uptake, usually in the occipital cortex.
A number of technical and clinical factors are important in image interpretation. The method of image processing can influence the results significantly, so uniformity of method within a study is essential. Particularly in multicentre studies, there is potential for systematic variation in results between centres due to use of different equipment and procedures. A pan‐European initiative recently proposed a framework to address this potential variation (Dickson 2012). This framework has been used in the production of a normative database for 123I‐FP‐CIT SPECT images, addressing the effects of age, gender, and image‐processing method (Varrone 2013), that may be used as a reference resource in future studies. Vascular lesions of the corpus striatum or nigrostriatal pathway can lead to abnormal DAT images, so in order to reduce the risk of false positive results, structural brain images may be necessary to aid interpretation. A small number of prescribed medications or drugs of abuse may affect DAT images. In any large series, a small proportion of images will be hard to classify and should be rated as 'indeterminate'. Guidelines for brain SPECT using 123I‐labelled DAT ligands, which cover patient procedures, image acquisition and processing, image interpretation and reporting, were published in 2010 by the European Association of Nuclear Medicine and in 2012 by the Society of Nuclear Medicine (Darcourt 2010; Djang 2012).
Several studies have investigated the relationship between a clinical diagnosis of DLB and the result of FP‐CIT SPECT imaging. Cross‐sectional case‐control studies have consistently found striatal binding of FP‐CIT to be lower in groups of people with a diagnosis of DLB than in groups of healthy controls or people with a diagnosis of AD (Walker 2002; Ceravolo 2003; O'Brien 2004). The largest study was a multicentre European study of 326 people with dementia, including 151 with probable (two or more core clinical features) or possible DLB (McKeith 2007). An abnormal FP‐CIT SPECT scan had a sensitivity of 77.7% (95% confidence interval (CI) 64.1% to 88.3%) and a specificity of 90.4% (95% CI 82.1% to 95.5%) for probable DLB diagnosed by a consensus panel, indicating a high degree of concordance between clinical and imaging data. Considering the performance of the clinical criteria against neuropathological findings, this result still allows for a wide range of possible performances of the imaging test against neuropathology. For example, there is no way of knowing if the nearly one‐quarter of clinically probable DLB cases whose FP‐CIT SPECT scan was rated as normal were all clinical false positives (although specificity of the clinical criteria in expert hands is generally high) or if SPECT scanning was missing even more true positives than the clinical criteria. Nor is there any way of knowing how many cases of DLB were missed by both the clinical criteria and the SPECT scan. Although AD is generally regarded as the main differential diagnosis for DLB, other diagnoses may also need to be considered, including frontotemporal dementia (FTD). FP‐CIT SPECT imaging may be less accurate at discriminating between DLB and FTD than between DLB and AD (Morgan 2012).
On the basis of studies demonstrating a high degree of concordance between an abnormal FP‐CIT SPECT result and clinical diagnosis, low dopamine transporter uptake on SPECT or PET was added to the consensus clinical criteria at their revision in 2005 as a suggestive feature, that is a diagnosis of probable DLB could be made on the basis of only one core clinical feature if there was also an abnormal dopamine transporter scan. The test consequently became more widely used, initially in Europe. DaTscanTM was not approved for use in the United States until 2011.
Clinical pathway
In current clinical practice, an FP‐CIT SPECT scan is used to confirm or refute a clinical suspicion of DLB, so subjects undergoing the test are likely to be those who already meet other criteria for possible or probable DLB. In most cases an FP‐CIT SPECT scan would be ordered for patients being seen by specialist dementia services after a standard work‐up for dementia, usually including some form of structural imaging and after a specialist physician had determined that a patient met some or all of the core or suggestive clinical features of DLB.
Performing DAT imaging only after applying the clinical diagnostic criteria for DLB means that the estimated accuracy of DAT imaging is limited by the sensitivity of the clinical criteria. This trade‐off of sensitivity against feasibility and cost may be inevitable in clinical practice, but in some research contexts a higher priority may be placed on identifying 'pure' diagnostic groups. Hence, it would also be of value to know the accuracy of DAT imaging for identifying DLB in secondary care patients with dementia who are not selected for any other DLB clinical features.
Currently, DAT imaging is the only special investigation included in the consensus diagnostic criteria (although polysomnography may be used to confirm the presence of rapid eye movement (REM) sleep behaviour disorder, another suggestive feature). Several other biomarker tests, including brain perfusion SPECT, fluorodeoxyglucose PET, I123‐metaiodobenzylguanidine cardiac scintigraphy, and various cerebrospinal fluid and blood biomarkers, have been shown to discriminate between DLB and AD and have been suggested as having diagnostic utility (Aarsland 2008). However, at present none of these have been approved for use in the diagnostic pathway for DLB, and none is yet in regular clinical use.
Rationale
Increasing importance is attached to making precise diagnoses of the subtypes of dementia. Although patients and relatives attach value to accurate diagnosis, its real importance comes when it leads to differences of clinical management that translate into better outcomes for patients. Currently, the most important implication of a DLB diagnosis for patient management is the recognition by clinicians of the high risk of severe adverse reactions to antipsychotic medication. Although antipsychotic use is regarded as a risk factor for mortality among all people with dementia (Schneider 2005), there is evidence that the most severe effects occur largely in people with Lewy body disease, with Aarsland finding severe sensitivity reactions among 8 out of 15 people with DLB and 0 out of 17 with AD (Aarsland 2005). Given the high rate of neuropsychiatric symptoms in DLB, including psychosis, these patients may be at increased risk for antipsychotic exposure, and it is important for clinicians to recognise this risk. The other key reason for being interested in more accurate diagnosis of DLB is the prospect of future treatment. If, as is widely hoped, increasing understanding of the pathophysiology of the dementing illnesses is translated into disease‐specific therapies capable of modifying the course of the disease, then accurate diagnosis at the earliest possible stage of illness will be essential to direct research efforts and subsequently treatments to the correct patients.
In the case of DLB, current diagnostic criteria are particularly limited by relatively poor sensitivity even in specialist research centres. A biologically derived diagnostic test of sufficient accuracy could be a great help. At present, FP‐CIT SPECT is the most highly developed supplementary test for DLB, but there is uncertainty about its accuracy and its place in clinical practice. As the test is expensive, it is in practice likely to be used in a two‐stage diagnostic process, that is after a clinical suspicion of DLB has been established.
This review aimed to provide a rigorous assessment of the accuracy of DAT imaging for DLB diagnosis in order to identify whether and how it can be usefully employed in research and clinical practice.
Objectives
This review had two primary objectives.
Objective A: To estimate the accuracy of DAT imaging for DLB in people with dementia in secondary care (that is patients who have been referred to a specialist dementia service). Objective B: To estimate the accuracy of DAT imaging for the diagnosis of DLB in people with dementia in secondary care who are already suspected of having DLB on the basis of a prior clinical work‐up.
Secondary objectives
The secondary objective was to use subgroup analyses to investigate the following potential sources of heterogeneity.
(a) Age of participants.
(b) Severity of dementia. The performance of the index test itself may vary with dementia severity. For objective B, since the performance of the clinical criteria may also depend on the severity of dementia, the 'added value' to be obtained from the index test may vary significantly.
(c) For objective B, diagnostic status at inclusion (possible or probable DLB, or both).
(d) DAT imaging method (SPECT or PET ligand).
Methods
Criteria for considering studies for this review
Types of studies
(a) Test accuracy studies with delayed verification.
(b) Diagnostic case‐control studies. This applied to objective A only. These may be true case‐control studies, conducted retrospectively, in which participants who underwent DAT imaging in life are included on the basis of the presence or absence of a neuropathological diagnosis of DLB at autopsy. We anticipated that a more common design would be studies in which participants were selected for inclusion on the basis of their clinically defined DLB diagnostic status. 'Cases' would meet clinical criteria (excluding the DAT imaging criterion) for probable DLB; 'controls' would not meet these criteria but would meet clinical criteria for one or more other subtypes of dementia. There is no universally agreed term for these studies, but they have been called 'two‐gate designs with alternative diagnosis controls' (Rutjes 2005). These study designs were eligible for inclusion but would, if possible, be the subject of sensitivity analysis because of the risk of spectrum bias they entail.
Participants
Objective A: Participants with dementia in secondary care settings. Dementia should have been diagnosed using validated clinical criteria (for example ICD, DSM). Studies that specifically excluded subjects meeting criteria for dementias other than DLB or AD (for example vascular dementia) could also be included.
Objective B: Participants meeting clinical criteria (other than the DAT imaging criterion) for possible or probable DLB, or both. We considered this to be the most useful test of utility in clinical practice, where DAT imaging is likely to be used in addition to clinical assessment. A population meeting clinical criteria for probable DLB will already have missed many true DLB cases (because of the relatively poor sensitivity), and the opportunity for adding diagnostic value is limited (because of the already high specificity). Hence, the potential clinical utility of the test is greatest if applied to a population meeting the more sensitive but less specific criteria for possible DLB. However, participants meeting probable DLB criteria could also be included; we would examine this as a potential source of heterogeneity.
We excluded participants with Parkinson's disease or other Parkinsonian syndromes known to be associated with abnormal DAT imaging.
Index tests
SPECT or PET imaging of brain dopamine transporters (DAT).
Target conditions
DLB, with or without co‐morbid Alzheimer's disease (AD).
Reference standards
Neuropathological diagnosis at autopsy using contemporaneously accepted neuropathological criteria.
We did not include in this review any studies in which a clinical diagnosis of probable DLB was the reference standard. There were two reasons for this:
1. Firstly, as discussed above, the accuracy―particularly the sensitivity―of the clinical criteria measured against the neuropathological reference standard is too low for them to constitute a satisfactory proxy. There is a serious possibility that the index test might outperform clinical diagnosis, rendering the latter unsuitable as a reference standard (Reitsma 2009). For some conditions, the correct clinical diagnosis may become more evident over time so that clinical diagnosis at follow‐up may be a better reference standard than the cross‐sectional diagnosis. (It has been used, for example, in studies of DAT imaging in the diagnosis of Parkinson's disease.) However, this strategy is not necessarily suitable for DLB where, as the condition progresses, symptoms converge with other types of dementia. Beyond a certain (undetermined) point, therefore, clinical diagnosis may become less discriminating with time.
2. Secondly, due to their complexity and expense, SPECT scans will probably only ever be useful as an adjunct to clinical assessment. Studies that by design cannot demonstrate an accuracy above that of careful clinical diagnosis alone have limited usefulness.
A difficulty with a neuropathological reference standard (common to all diagnostic studies with delayed verification designs) is that the pathology may change between application of the index test and reference standard. In this context, the most likely scenario is that AD pathology may develop in the interim, reducing the likelihood that the initial clinical syndrome will be correctly classified as due to DLB. This may lead to a systematic bias, underestimating the specificity of the index test.
Search methods for identification of studies
We used a variety of information sources, aiming to retrieve as many relevant studies as possible. We devised terms for electronic database searching in conjunction with the team at the Cochrane Dementia and Cognitive Improvement Group.
Electronic searches
We searched MEDLINE (1946 to February 2013) (Ovid SP); Embase (1980 to February 2013) (Ovid SP); BIOSIS previews (1926 to February 2013)(ISI Web of Science); Web of Science and Conference Proceedings (ISI Web of Science) (1945 to February 2013); PsycINFO (1806 to February 2013) (OvidSP); CINAHL (1982 to February 2013) (EBSCO); and LILACS (February 2013) (BIREME). We present the search strategies, designed using terms appropriate for each database, in Appendix 3. Several databases we searched, including Embase and the Conference Proceedings Citation Indexes, include conference abstracts, which allowed for assessment of this type of grey literature.
We also searched specialised sources containing diagnostic studies and reviews:
Meta‐analyses van Diagnostisch Onderzoek (MEDION database; www.mediondatabase.nl);
Database of Abstracts of Reviews of Effects (DARE; http://www.crd.york.ac.uk/CRDWeb);
Health Technology Assessment Database, The Cochrane Library (HTA Database; http://onlinelibrary.wiley.com/cochranelibrary/search);
Aggressive Research Intelligence Facility (ARIF database; www.arif.bham.ac.uk).
We requested a search of the Cochrane Register of Diagnostic Test Accuracy Studies (maintained and managed by the Cochrane Renal Group).
We applied no language or date restrictions to the electronic searches. We checked the reference lists of relevant studies and reviews in the field for additional potentially relevant studies.
Initial searches were performed by a single researcher with extensive experience of systematic reviewing.
Data collection and analysis
Selection of studies
Two review authors (JMcC, SM) independently examined titles and abstracts of all papers retrieved by the search. We obtained full‐text papers if either review author considered the paper to be potentially relevant. Again working independently, JMcC and SM identified papers for inclusion. We contacted authors of three papers for further information relevant to deciding whether to include or exclude the study.
Data extraction and management
Two review authors (JMcC, CH) extracted the following data.
Bibliographic details of primary paper:
author, study title, year and journal;
reported or potential conflicts of interest.
Basic clinical and demographic details:
number of participants;
age;
gender;
setting;
inclusion and exclusion criteria.
Details of index test:
definition of a positive and negative DAT imaging result.
Reference standard:
neuropathological criteria used to define presence or absence of DLB.
We also extracted data necessary for the assessment of quality (as defined below). We extracted the results of 2x2 tables relating index test results to reference standard results directly into Review Manager (RevMan) tables.
Assessment of methodological quality
We assessed the included study for methodological quality using the QUADAS‐2 tool (Whiting 2011), as recommended in the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (Reitsma 2009), plus additional items in the index test domain, which were tailored to this review. We give the list of additional quality items to be coded and the criteria used to judge each item as 'yes', 'no', or 'unclear' in Appendix 4. We included all quality items in the data extraction form, which required each review author to record, along with a judgement, a description of the evidence supporting that judgement. We discussed disagreements until consensus was reached.
Statistical analysis and data synthesis
We applied the DTA framework for the analysis of a single test and extracted the data from the study into a 2x2 table, showing the binary test results cross‐classified with the binary reference standard.
We used data from the 2x2 tables abstracted from the included study (TP, FN, FP, TN) and entered into RevMan to calculate the sensitivities, specificities, and their 95% confidence intervals. We also presented the study results graphically by plotting estimates of sensitivities and specificities in forest plots.
Due to lack of data, we conducted no meta‐analyses.
Investigations of heterogeneity
We were able to include only one study, and so issues of heterogeneity did not arise.
Sensitivity analyses
There were insufficient data to permit any sensitivity analyses.
Assessment of reporting bias
We did not investigate reporting bias.
Results
Results of the search
The 'raw' search returned 9125 references. After de‐duplication and first assessment by Anna Noel‐Storr, Cochrane Dementia and Cognitive Improvement Group Trials Search Co‐ordinator, 199 references were passed to the review authors for further consideration. One or both review authors considered 51 references to need closer scrutiny, and the full text of the paper or conference abstract was obtained. We identified one paper from a reference list, and also examined the full text of this. We could rapidly exclude the majority as review papers or on the basis of the index test (not dopamine transporter imaging) or the reference standard (not neuropathology). We identified five studies as possibly including data that fulfilled our inclusion criteria. Of these, we included one and excluded one on the basis of published data. We sought further information from the authors of the other three; we subsequently excluded two and classified one as ongoing. We illustrate the process of study selection in Figure 1.
1.
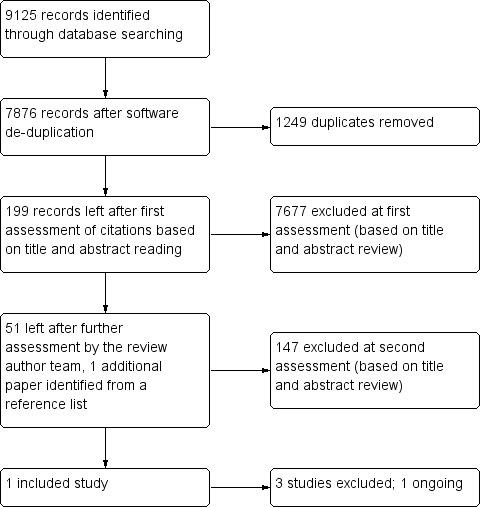
Flow diagram.
Included study
See Characteristics of included studies.
Walker 2009 refers to a study that has been published in several papers as the number of patients coming to autopsy has expanded (2002 paper, n = 10; 2007 paper, n = 20; 2009 paper, n = 23). It was conducted in a secondary care setting in London, United Kingdom. Participants were recruited as part of a cohort of 45 people with dementia who were diagnosed clinically with DLB, AD or, in one case, corticobasal degeneration (CBD); 16 healthy elderly controls; and 19 people with clinical diagnoses of Parkinson's disease (PD). Neither the healthy controls nor the people with PD were eligible for inclusion in this review, but the controls were used to define a normal scan result. All participants had 123I‐FP‐CIT SPECT scans and were followed prospectively. The most recent publication reported on 23 people with dementia who had died and for whom neuropathological data were available (Walker 2009). This included the person with a baseline clinical diagnosis of CBD who was also not eligible for inclusion in this review; our analysis therefore included outcome data on 22 participants. Demographic data were available on the 20 participants reported in the 2007 paper. They were predominantly female (14 out of 20), had a mean age over 75 years, and had moderately severe dementia at the time of the index test.
SPECT scans were assessed both semiquantitatively and by visual rating. The 16 healthy controls described in Walker 2002 were used to define a normal scan result. The semiquantitative analysis was performed by an expert manually drawing regions of interest through the reconstructed SPECT transverse slices that demonstrated the most intense striatal uptake (caudate nucleus, anterior and posterior putamen) and dividing this specific uptake by a background region of interest within the occipital cortex. An abnormal scan was defined prospectively in one of two ways: (a) as one with a ratio of uptake (binding ratio) between the worst affected posterior putamen and the occiptal cortex that was greater than 2 standard deviations (SDs) below the mean of the ratios in controls, or (b) as one in which the binding ratio in both right and left posterior putamen was greater than 2 SDs below the mean binding ratio in the controls. Visual ratings were performed by three independent raters, each of whom rated scans as 0 (normal uptake), 1 (slightly reduced uptake), or 2 (significantly reduced uptake) in any of right or left caudate, or right or left putamen. Scores of 0 or 1 were combined to define a normal scan; a score of 2 defined an abnormal scan.
All participants had a detailed neuropathological examination that included a CERAD (Consortium to Establish a Registry for Alzheimer’s Disease) score and diagnosis, Braak stage, and NIA‐RI (National Institute on Aging and Reagan Institute) AD diagnosis.There was additional staging of neocortical neurofibrillary tangle pathology. DLB diagnoses were made using the neuropathological diagnostic criteria recommended by the third report of the DLB Consortium (McKeith 2005).
The mean interval between index test and reference standard was 42 months (range 6 to 106 months).
Potential conflicts of interest were noted, with financial support for the study being provided by the manufacturer of the SPECT ligand.
Excluded studies
Perju‐Dumbrava 2009 described a series of 25 people with Parkinsonian disorders who had undergone DAT SPECT imaging and for whom autopsy data were available. The series included only two people with DLB and no people with other dementias, other than two with dementia in Parkinson's disease (W. Pirker, July 2013, correspondence).
Lim 2009 studied 14 participants with probable DLB and 10 with probable AD (clinical diagnoses). The purpose of the study was “to optimize visual and quantitative interpretation of 18F‐FDG PET images in differentiating DLB from AD and compare these results with DAT‐based SPECT in the same patient cohort.” All participants had 123I‐β‐CIT scans. There was no predefined threshold for classifying participants, although the authors derived a cutoff in the course of the study which they reported had 100% sensitivity and specificity for distinguishing clinically diagnosed probable DLB from AD in this context. Only 5 out of 24 participants (4 DLB, 1 AD) had had autopsies at the time of publication, and results for the autopsied participants were not separately reported. The authors confirmed that no further patients had come to autopsy and that they had no plans to expand the autopsy series (C. Rowe, July 2013, correspondence).
Gilman 2004 examined striatal presynaptic monoamine terminals using a PET ligand, 11C‐dihydrotetrabenazine, in 20 participants with clinically diagnosed DLB or Parkinson's disease dementia, 25 with AD, and 19 healthy controls. The study was exploratory with no predefined threshold for classifying normal or abnormal scans. Only 3 participants (antemortem diagnoses: 1 DLB, 2 AD) had autopsy confirmation of diagnosis.
Ongoing studies
Colloby 2012 reported detailed neuropathological assessment of 23 cases who had undergone antemortem 123I‐FP‐CIT SPECT scanning. At postmortem, 4 participants had AD, 7 had DLB, and 12 had Parkinson's disease dementia. The main purpose of the study was to investigate the relationship between in vivo imaging and loss or dysfunction of nigrostriatal dopaminergic neurons. The participants had been part of a prospective longitudinal study during which all had antemortem clinical diagnoses and a SPECT scan classification (normal/abnormal). These data were not available in the published report, although the authors note that one included patient with a clinical diagnosis of possible DLB had a 'normal' SPECT scan on visual rating and a subsequent neuropathological diagnosis of AD. The relevant data on the other DLB and AD participants who had reached autopsy were not available to us for this review. The authors confirmed that other participants from the longitudinal study may undergo autopsy in future and that further results, including antemortem scans results and neuropathological findings, may be published. We considered this best classified as an ongoing study; although it is not designed specifically to be a study of the diagnostic accuracy of 123I‐FP‐CIT SPECT scanning, it may lead to data that could be included in this review in future.
Methodological quality of included studies
We rated the included study (Walker 2009) using the QUADAS‐2 tool plus additional items tailored to this review. The study had a two‐gate design with alternative diagnosis controls (Rutjes 2005), including secondary care patients on the basis of their meeting consensus clinical criteria for DLB or National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS‐ADRDA) criteria for Alzheimer's disease, or both. This design has an intrinsically high risk of spectrum bias, hence we rated the study as being at high risk of bias on the patient selection domain. We also considered that there was a high risk of the interpretation of the index test introducing bias because SPECT scans were not interpreted in conjunction with structural images (one of the additional items). This risks false positive classifications on the index test and can therefore lead to underestimates of test specificity. We judged the study to be at low risk of bias in the reference standard domain. We labelled the risk of bias in the flow and timing domain 'unclear'. In studies of this nature, the application of the reference standard is inevitably delayed until patient death. In this case the mean interval was 42 months (range 6 to 106 months), during which additional pathology could have developed. There is some risk of bias associated with this, but it is unavoidable and hard to quantify.
Findings
The most recent publication in 2009 reported data on 22 eligible participants with dementia and clinical diagnoses of DLB or AD. In this group, using the autopsy findings as the reference standard, the consensus clinical criteria for DLB had a sensitivity of 0.89 and a specificity of 0.46 for the discrimination of DLB from non‐DLB (data taken from table 2, Walker 2009). Of 15 participants who were given an initial clinical diagnosis of DLB, 7 were false positives.
We considered first the complete group of 22 participants. Using semiquantitative analysis of the scans, with an abnormal scan defined as worst‐affected posterior putamen to occipital cortex binding ratio greater than 2 SDs below controls, 123I‐FP‐CIT SPECT had a sensitivity of 1.00 (95% CI 0.66 to 1.00) and a specificity of 0.92 (95% CI 0.64 to 1.00) for the diagnosis of DLB (Data table 1, Figure 2). Only 1 of 22 cases had discordant imaging and neuropathology. This was a false positive―an abnormal index scan in a patient who at autopsy was found to have AD and cerebrovascular disease that affected the substantia nigra and putamen. The 2009 paper did not report visual ratings, but the 2007 paper reported visual ratings for 19 eligible participants. In this case, 123I‐FP‐CIT SPECT had a sensitivity of 0.86 (95% CI 0.42 to 1.00) and a specificity of 0.83 (95% CI 0.52 to 0.98) (Data table 2, Figure 3).
1. Test.

123I‐FP‐CIT SPECT scan, analysed semi‐quantitatively, in patients with dementia meeting clinical criteria for AD or DLB or both.
2.

Forest plot of 1 123I‐FP‐CIT SPECT scan, analysed semiquantitatively, in participants with dementia meeting clinical criteria for AD or DLB, or both.
2. Test.

123I‐FP‐CIT SPECT scan, rated visually, in patients with dementia meeting clinical criteria for AD or DLB or both.
3.

Forest plot of 2 123I‐FP‐CIT SPECT scan, rated visually, in participants with dementia meeting clinical criteria for AD or DLB, or both.
The intention of the study was to address the discrimination of DLB from AD (our objective A) rather than primarily to estimate the value of DAT imaging in clinical practice (objective B), but we considered that it did provide the best evidence available to assess the accuracy of DAT imaging among people identified clinically as having possible or probable DLB. We therefore also considered only the 15 participants who met consensus clinical criteria for DLB at baseline. The 2007 publication described 13 of these in more detail. Twelve of these 13 had 2 or 3 core features of DLB and could be classified as having 'probable DLB'; the remaining participant had only 1 core feature of DLB and may have met criteria for 'possible DLB' (suggestive features not reported). The clinical features of the two additional participants in the 2009 paper were not described.
In this group of 15 participants, 123I‐FP‐CIT SPECT scanning, analysed semiquantitatively with an abnormal scan defined as a worst‐affected posterior putamen to occipital cortex binding ratio greater than 2 SDs below controls, had a sensitivity of 1.00 (95% CI 0.63 to 1.00) and a specificity of 1.00 (95% CI 0.59 to 1.00) for the diagnosis of DLB (Data table 3, Figure 4). Visual scan ratings were also available for the 13 participants in the 2007 paper. Rated visually, accuracy in this group was lower with a sensitivity of 0.83 (95% CI 0.36 to 1.00) and a specificity of 0.71 (95% CI 0.29 to 0.96) (Data table 4, Figure 5). One participant had a scan rated visually as normal but had DLB diagnosed at autopsy; two participants had scans rated visually as abnormal but had diagnoses of AD plus cerebrovascular disease and frontotemporal lobar degeneration at autopsy.
3. Test.

123I‐FP‐CIT SPECT scan, analysed semi‐quantitatively, in patients with clinically diagnosed DLB.
4.

Forest plot of 3 123I‐FP‐CIT SPECT scan, analysed semiquantitatively, in participants with clinically diagnosed DLB.
4. Test.

123I‐FP‐CIT SPECT scan, rated visually, in patients with clinically diagnosed DLB.
5.

Forest plot of 4 123I‐FP‐CIT SPECT scan, rated visually, in participants with clinically diagnosed DLB.
Discussion
Summary of main results
Objective A. We included only one study in the review. This study addressed the accuracy of 123I‐FP‐CIT SPECT scanning for the discrimination of DLB from AD in secondary care. We found 123I‐FP‐CIT SPECT, analysed semiquantitatively, to have a sensitivity of 100% and a specificity of 92% for the diagnosis of DLB at autopsy among secondary care patients with moderately severe dementia selected on the basis of a clinical diagnosis of either DLB or AD. Due to the small size of the study, the confidence intervals for both estimates were wide. No participant who had a normal SPECT scan at presentation was found to have DLB at autopsy. One participant with a SPECT scan classified as abnormal did not have neuropathologically confirmed DLB, the reduced ligand uptake being due to focal cerebrovascular disease. The study's authors acknowledged that this error would have been avoided if the SPECT images had been interpreted in conjunction with high‐quality structural images. The specificity is therefore likely to be an underestimate in comparison with standard practice. Visual rating of SPECT scans was slightly less accurate, with a sensitivity of 86% and a specificity of 83%, again with wide confidence intervals.
Objective B. Using data from the same study, we found that among 15 participants with initial clinical diagnoses of DLB (mostly probable) made by expert clinicians, 123I‐FP‐CIT SPECT, analysed semiquantitatively, had a sensitivity of 100% and a specificity of 100% for confirmed DLB at autopsy. Again this result was imprecise; the data are also compatible with much lower sensitivity and specificity.
Strengths and weaknesses of the review
Despite an extensive literature search, we were able to include only one study with 22 eligible participants, hence our accuracy estimates were relatively imprecise. This paucity of evidence reflects the very significant challenges inherent in conducting long‐term prospective studies of well‐characterised participants, followed up to the point of autopsy. However, we consider that neuropathological diagnosis is the only reference standard that will lead to accurate estimates of the diagnostic accuracy of DAT imaging. The high level of accuracy in the one included study strongly reinforces the possibility that DAT imaging is more accurate than clinical diagnosis of DLB and, therefore, that clinical diagnosis cannot serve as a reference standard for estimating the diagnostic accuracy of DAT imaging.
The one included study did have significant methodological limitations that weaken confidence in the findings of the review. The study had a case‐control‐type design (more strictly, a two‐gate design with alternative diagnosis controls (Rutjes 2005)). This study design tends to exclude patients who may present the most diagnostic challenge and is therefore at high risk of spectrum bias. The interpretation of the index test, which was done without reference to structural imaging, also risked introducing bias (underestimating specificity) and, in fact, this risk was realised in the study. The delayed verification design is associated with an unavoidable risk of the development of additional pathology between the index test and the reference standard, which may also lead to underestimates of specificity.
A strength of the study, and hence of the review, is that the neuropathological diagnoses were made according to rigorous standards.
Applicability of findings to the review question
The included patients were recruited from standard secondary care settings in the United Kingdom and met clinical criteria for DLB or for AD, the major differential diagnosis for DLB. The index test used employed the only DAT imaging ligand licensed for clinical use, and the reference standard used was likely to have classified patients as well as possible. The study was applicable to our first review objective (objective A).
We intended our second review objective (objective B) to be more applicable to clinical practice. It asked how accurately DAT imaging identifies DLB in people suspected of having DLB after a clinical work‐up. We found no studies specifically designed to answer this question. However, data from the included study do suggest that DAT imaging is highly accurate, at least among people with moderately severe dementia and clinically probable DLB. It is important to note that the study did not include participants who might present the greatest diagnostic challenge, such as those with very mild dementia or those with only one core or suggestive DLB feature. DAT imaging in this context cannot add to the sensitivity of the clinical assessment (false negatives, or missed DLB diagnoses, on clinical assessment cannot be 'corrected'), but these data suggest that it can enhance specificity, that is a normal scan in this context can accurately exclude DLB. The specificity of the initial clinical diagnosis of DLB in the included study was only 46%, which is unusually low in comparison with most published reports of the accuracy of clinical criteria (McKeith 2004; Nelson 2010). This might exaggerate the apparent value added by SPECT imaging; on the other hand, it may be that this level of specificity is common in routine clinical settings.
Authors' conclusions
Implications for practice.
There is a very limited amount of data using a neuropathological reference standard to assess the accuracy of DAT imaging for the diagnosis of DLB. There are no such data that directly address the common clinical scenario where the use of DAT imaging is considered as a diagnostic test in a patient with possible DLB. There are also no data assessing the accuracy of DAT imaging in people with mild or very mild dementia. Data from one study suggest that, where there is a strong pre‐existing suspicion of DLB (probable DLB) in a person with moderately severe dementia, then a normal 123I‐FP‐CIT SPECT scan may be an accurate means of excluding the diagnosis.
The included study found semiquantitative ratings of 123I‐FP‐CIT SPECT scans to be more accurate than visual ratings in all analyses, although the 95% confidence intervals overlapped.
Implications for research.
The evidence reviewed suggests that, among people with moderately severe dementia who meet criteria for probable DLB or probable AD, 123I‐FP‐CIT SPECT imaging is more accurate than the consensus clinical criteria for discriminating DLB from AD. This supports the position that, despite the challenges in long‐term follow‐up to autopsy, neuropathology is currently the only reference standard that can yield accurate estimates of the diagnostic accuracy of DAT imaging.
If DLB is to be diagnosed accurately early in the course of dementia―a prerequisite for studying potential disease‐modifying treatments―then further studies are needed in which possible biomarkers of DLB are assessed against neuropathological diagnoses. Given the prevalence of DLB, researchers collecting any cohort of patients with mild cognitive impairment or mild dementia whom they intend to follow to autopsy might usefully consider the addition of potential DLB biomarkers, including DAT imaging, to the baseline work‐up.
There is a need for pragmatic research that addresses how a test is likely to be used in clinical practice. In this context, in order to maximise both the overall sensitivity and specificity of the diagnostic pathway, it would be most useful to study the accuracy of DAT imaging in people who present clinically with only one core feature or only suggestive features of DLB (possible DLB).
Acknowledgements
We should like to thank Jennifer Ware for her work on the initial screening of search results.
We are grateful to the authors of included and excluded studies who responded to our requests for additional information.
Appendices
Appendix 1. Consensus criteria for the clinical diagnosis of possible and probable dementia with Lewy bodies
| Criteria | Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. McKeith 1996. | Revised criteria: Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. McKeith 2005. |
| Central feature | Progressive cognitive decline of sufficient magnitude to interfere with normal social or occupational function. Prominent or persistent memory impairment may not necessarily occur in the early stages but is usually evident with progression. Deficits on tests of attention and of frontal‐subcortical skills and visuospatial ability may be especially prominent. | Progressive cognitive decline of sufficient magnitude to interfere with normal social or occupational function. Prominent or persistent memory impairment may not necessarily occur in the early stages but is usually evident with progression. Deficits on tests of attention, executive function, and visuospatial ability may be especially prominent. |
| Core features (probable DLB ‐ 2; possible DLB ‐ 1) |
|
|
| Suggestive features (2005 criteria only) | ― | If one or more of these is present together with 1 or more core features, a diagnosis of probable DLB can be made. In the absence of any core features, 1 or more suggestive features is sufficient for possible DLB. Probable DLB should not be diagnosed on the basis of suggestive features alone.
|
| Supportive features |
|
|
| A diagnosis of DLB is less likely |
|
|
| Temporal sequence of symptoms | ― | DLB should be diagnosed when dementia occurs before or concurrently with parkinsonism (if it is present). The term Parkinson's disease dementia should be used to describe dementia that occurs in the context of well‐established Parkinson's disease. In a practice setting the term that is most appropriate to the clinical situation should be used; generic terms such as Lewy body disease are often helpful. In research studies in which a distinction needs to be made between DLB and Parkinson's disease dementia, the existing 1‐year rule between the onset of dementia and parkinsonism DLB continues to be recommended. Adoption of other time periods will simply confound data pooling or comparison between studies. In other research settings that may include clinicopathologic studies and clinical trials, both clinical phenotypes may be considered collectively under categories such as Lewy body disease or alpha‐synucleinopathy. |
Appendix 2. Marketing approvals for DaTscanTM
European Union
First marketing authorisation in 2000.
Currently approved for the following therapeutic indications:
This medicinal product is for diagnostic use only.
DaTscan is indicated for detecting loss of functional dopaminergic neuron terminals in the striatum:
In patients with clinically uncertain Parkinsonian Syndromes, in order to help differentiate Essential Tremor from Parkinsonian Syndromes related to idiopathic Parkinson's Disease, Multiple System Atrophy and Progressive Supranuclear Palsy. DaTscan is unable to discriminate between Parkinson's Disease, Multiple System Atrophy and Progressive Supranuclear Palsy.
To help differentiate probable dementia with Lewy bodies from Alzheimer's disease. DaTscan is unable to discriminate between dementia with Lewy bodies and Parkinson's disease dementia.
United States
FDA approval in 2011 for the following indications and usage:
DaTscan (Ioflupane I 123 Injection) is a radiopharmaceutical indicated for striatal dopamine transporter visualization using single photon emission computed tomography (SPECT) brain imaging to assist in the evaluation of adult patients with suspected Parkinsonian syndromes (PS). In these patients, DaTscan may be used to help differentiate essential tremor from tremor due to PS (idiopathic Parkinson's disease, multiple system atrophy and progressive supranuclear palsy). DaTscan is an adjunct to other diagnostic evaluations.
Appendix 3. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| 1. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1946 to present (OvidSP) [searched 12 February 2013] |
1. Lewy Bodies/ 2. dement*.ti,ab. 3. (LDB or DLB or LBD).ti,ab. 4. (lewy* adj2 bod*).ti,ab. 5. Lewy Body Disease/ 6. or/1‐5 7. DAT scan*.ti,ab. 8. Dopamine Plasma Membrane Transport Proteins/ 9. "dopamine transporter".ti,ab. 10. Dopaminergic Neurons/ 11. FP‐CIT.ti,ab. 12. DaTSCAN.ti,ab. 13. ("123I‐β‐CIT" or "[123I]β‐CIT" or "[123I]beta‐CIT").ti,ab. 14. "123I‐FP‐CIT".ti,ab. 15. SPECT.ti,ab. 16. "[99mTc]TRODAT‐1".ti,ab. 17. "[123I]PE2I".ti,ab. 18. "[123I]altropane".ti,ab. 19. "[11C]cocaine".ti,ab. 20. "[3H]WIN".ti,ab. 21. "[11C]altropane".ti,ab. 22. ("[11C]/[18F]beta‐CFT" or "[11C]/[18F]β‐CFT").ti,ab. 23. "[11C]FE‐CIT".ti,ab. 24. "[11C]dMP".ti,ab. 25. Tomography, Emission‐Computed, Single‐Photon/ 26. SPET.ti,ab. 27. "single photon emission tomography".ti,ab. 28. "single photon emission computed tomography".ti,ab. 29. or/7‐28 30. 6 and 29 |
1586 |
| 2. Embase 1980 to 11 February 2013 (OvidSP) [searched 12 February 2013] |
1. Lewy body/ 2. dement*.ti,ab. 3. (LDB or DLB or LBD).ti,ab. 4. (lewy* adj2 bod*).ti,ab. 5. diffuse Lewy body disease/ 6. or/1‐5 7. DAT scan*.ti,ab. 8. dopamine transporter/ 9. "dopamine transporter".ti,ab. 10. dopaminergic nerve cell/ 11. FP‐CIT.ti,ab. 12. DaTSCAN.ti,ab. 13. ("123I‐β‐CIT" or "[123I]β‐CIT" or "[123I]beta‐CIT").ti,ab. 14. "123I‐FP‐CIT".ti,ab. 15. SPECT.ti,ab. 16. "[99mTc]TRODAT‐1".ti,ab. 17. "[123I]PE2I".ti,ab. 18. "[123I]altropane".ti,ab. 19. "[11C]cocaine".ti,ab. 20. "[3H]WIN".ti,ab. 21. "[11C]altropane".ti,ab. 22. ("[11C]/[18F]beta‐CFT" or "[11C]/[18F]β‐CFT").ti,ab. 23. "[11C]FE‐CIT".ti,ab. 24. "[11C]dMP".ti,ab. 25. single photon emission computer tomography/ 26. SPET.ti,ab. 27. "single photon emission tomography".ti,ab. 28. "single photon emission computed tomography".ti,ab. 29. or/7‐28 30. 6 and 29 |
3293 |
| 3. PsycINFO 1806 to February week 2 2013 (Ovid SP) [searched 12 February 2013] |
1. exp Dementia with Lewy Bodies/ 2. dement*.ti,ab. 3. (LDB or DLB or LBD).ti,ab. 4. (lewy* adj2 bod*).ti,ab. 5. or/1‐4 6. DAT scan*.ti,ab. 7. exp Neurotransmitter Transporters/ 8. "dopamine transporter".ti,ab. 9. exp Dopamine/ 10. FP‐CIT.ti,ab. 11. DaTSCAN*.ti,ab. 12. ("123I‐β‐CIT" or "[123I]β‐CIT" or "[123I]beta‐CIT").ti,ab. 13. "123I‐FP‐CIT".ti,ab. 14. SPECT.ti,ab. 15. "[99mTc]TRODAT‐1".ti,ab. 16. "[123I]PE2I".ti,ab. 17. "[123I]altropane".ti,ab. 18. "[11C]cocaine".ti,ab. 19. "[3H]WIN".ti,ab. 20. "[11C]altropane".ti,ab. 21. ("[11C]/[18F]beta‐CFT" or "[11C]/[18F]β‐CFT").ti,ab. 22. "[11C]FE‐CIT".ti,ab. 23. "[11C]dMP".ti,ab. 24. exp Single Photon Emission Computed Tomography/ 25. SPET.ti,ab. 26. "single photon emission tomography".ti,ab. 27. "single photon emission computed tomography".ti,ab. 28. or/6‐27 29. 5 and 28 |
688 |
| 4. BIOSIS Previews (ISI Web of Knowledge) (1926 to present) [searched 12 February 2013] |
Topic=("lewy bod*" OR dement* OR DLB OR LBD) AND Topic=("DAT scan*" OR "dopamine transporter" OR FP‐CIT OR DaTSCAN OR "123I‐β‐CIT" OR "[123I]β‐CIT" OR "[123I]beta‐CIT" OR "123I‐FP‐CIT" OR SPECT OR "[99mTc]TRODAT‐1" OR "[123I]PE2I" OR "[123I]altropane" OR "[11C]cocaine" OR "[3H]WIN" OR "[11C]altropane" OR "[11C]/[18F]beta‐CFT" OR "[11C]/[18F]β‐CFT" OR "[11C]FE‐CIT" OR "[11C]dMP" OR SPET OR "single photon emission tomography" OR "single photon emission computed tomography") Timespan=All Years. Search language=English |
1241 |
| 5. Web of Science and Conference Proceedings (1945 to present) [searched 12 February 2013] |
Topic=("lewy bod*" OR dement* OR DLB OR LBD) AND Topic=("DAT scan*" OR "dopamine transporter" OR FP‐CIT OR DaTSCAN OR "123I‐β‐CIT" OR "[123I]β‐CIT" OR "[123I]beta‐CIT" OR "123I‐FP‐CIT" OR SPECT OR "[99mTc]TRODAT‐1" OR "[123I]PE2I" OR "[123I]altropane" OR "[11C]cocaine" OR "[3H]WIN" OR "[11C]altropane" OR "[11C]/[18F]beta‐CFT" OR "[11C]/[18F]β‐CFT" OR "[11C]FE‐CIT" OR "[11C]dMP" OR SPET OR "single photon emission tomography" OR "single photon emission computed tomography") Timespan=All Years. Search language=English |
2112 |
| 6. LILACS (BIREME) [searched 12 February 2013] |
lewy body OR lewy bodies OR DLB OR LBD OR "demencia con cuerpos de Lewy" OR "enfermedad con cuerpos de Lewy" [Words] | 32 |
| 7. CINAHL (EBSCOhost) [searched 12 February 2013] |
S1 (MH "Lewy Body Disease") S2 TX dement* S3 TX DLB OR LBD S4 TX "lewy* bod*" S5 S1 OR S2 OR S3 OR S4 S6 TX "DAT scan*" S7 TX "dopamine transporter" S8 (MH "Dopamine Plasma Membrane Transport Proteins") S9 (MH "Dopamine Neuron Agents") S10 TX "FP‐CIT" S11 TX "DaTSCAN" S12 TX "123I‐β‐CIT" OR "[123I]β‐CIT" OR "[123I]beta‐CIT" S13 TX "123I‐FP‐CIT" S14 TX SPECT S15 TX "[99mTc]TRODAT‐1" S16 TX "[123I]PE2I" S17 TX "[123I]altropane" S18 TX "[11C]cocaine" S19 TX "[3H]WIN" S20 TX "[11C]altropane" S21 TX "[11C]/[18F]beta‐CFT" or "[11C]/[18F]β‐CFT" S22 TX "[11C]FE‐CIT" S23 TX "[11C]dMP" S24 (MH "Tomography, Emission‐Computed, Single‐Photon") S25 TX SPET S26 TX "single photon emission tomography" S27 TX "single photon emission computed tomography" S28 S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 S29 S5 AND S28 |
173 |
| TOTAL before de‐duplication | 9125 | |
| TOTAL after de‐dupe and first‐assess | 199 | |
Appendix 4. Additional items for assessing methodological quality in index test domain
Question 1: Were uninterpretable or intermediate test results reported? Question 2: Were structural brain images available for comparison? Question 3: Was the method of image reconstruction consistent throughout the study? Question 4: Had test operators had appropriate training? Question 5: Were data on observer variation in DAT image interpretation reported and within an acceptable range?
| Question | Judgement | Criteria |
| 1: Were uninterpretable or intermediate test results reported? | Yes | The number or proportion of uninterpretable or intermediate test results is reported. |
| No | Uninterpretable or intermediate test results arose but the number or proportion is not reported. | |
| Unclear | It is not possible to tell whether or not there were any uninterpretable or intermediate test results. | |
| 2: Were structural brain images available for comparison? | Yes | Structural brain images taken within 6 months of the DAT images were available to aid interpretation. |
| No | No structural brain images (± 6 months) were available to aid image interpretation. | |
| Unclear | Insufficient information to make a judgement. | |
| 3: Was the method of image reconstruction consistent throughout the study? | Yes | The method of image reconstruction is stated and was the same for all participants in the study. |
| No | The method of image reconstruction varied within the study. | |
| Unclear | Insufficient information to make a judgement. | |
| 4: Had test operators had appropriate training? | Yes | DAT image interpreters were fully qualified or certified nuclear medicine specialists with prior experience of the technique. |
| No | DAT image interpreters lacked this training or experience. | |
| Unclear | Insufficient information to make a judgement. | |
| 5: Were data on observer variation in DAT image interpretation reported and within an acceptable range? | Yes | Data on intra‐ and inter‐observer variation in DAT image interpretation are reported and agreement is good (kappa > 0.6). |
| No | Observer variation is not reported or agreement was poor (kappa < 0.6). | |
| Unclear | It is not clear whether observer variation was measured. |
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Walker 2009.
| Study characteristics | |||
| Patient sampling | Participants were recruited from a memory clinic, psychiatry and neurology outpatient clinics, and hospital wards in London, UK. They were included on the basis of clinical diagnosis, fulfilling either consensus clinical criteria for DLB or NINCDS‐ADRDA criteria for AD, or both. (One participant included on the basis of a clinical diagnosis of corticobasal degeneration was not eligible for this review; outcome data on this participant was excluded.) No further details of sampling were given. The 22 included patients were those who had died and whose relatives had given permission for autopsy by the time of publication. | ||
| Patient characteristics and setting | Participants were recruited from secondary care settings in London, UK. There were 22 eligible participants in the latest paper in 2009. 15 met consensus clinical criteria for DLB at inclusion. 7 did not meet these criteria but did meet NINCDS‐ADRDA criteria for AD. Demographic data are available on 20 participants in the 2007 paper, divided according to a final neuropathological diagnosis of DLB or non‐DLB. These data include the participant with a baseline clinical diagnosis of corticobasal degeneration who is otherwise excluded from this review. No demographic data are available for the 3 additional participants reported in the 2009 publication.
|
||
| Index tests | All participants had an 123I‐FP‐CIT SPECT scan between 1996 and 1999 at the Institute of Nuclear Medicine, University College London Medical School using a Strichman Medical Equipment 810 camera. Scanning took place 3 to 4 hours after injection of DaTscanTM. All scans were subject to a semiquantitative analysis, interpreted by a specialist in nuclear medicine, and to visual rating by 3 suitably experienced independent raters. Image reconstruction was automatic (no further details). An abnormal scan on semiquantitative analysis was defined as having binding > 2 SDs below that of healthy controls in the posterior putamen on 1 or both sides. An abnormal scan on visual rating had 'significantly reduced' uptake in any of 4 regions (right and left caudate, right and left putamen). Visual raters disagreed on the classification of 3/20 cases; in these 3 cases the majority result was reported. In all cases, structural brain images were not used to aid interpretation of the scans. |
||
| Target condition and reference standard(s) | Target condition: DLB Reference standard: The neuropathological diagnostic criteria employed for DLB were those recommended by the third report of the DLB Consortium (McKeith 2005). The neuropathological diagnostic criteria employed for AD included the following: CERAD score and diagnosis, Braak stage, and NIA‐RI AD diagnosis. In addition, neurofibrillary tangles were counted in the temporal, frontal, parietal, and occipital neocortices, and their densities were used to stage the neocortical neurofibrillary tangle pathology. Neuropathological examinations were conducted in Newcastle, UK. |
||
| Flow and timing | For 23 participants (including the participant with a clinical diagnosis of corticobasal degeneration), the mean interval between index test and reference standard was 42 months (median 30 months, range 6 to 106 months). By the time of the 2007 paper, a further 8 participants had died, but relatives had refused permission for autopsy. By the time of the last paper in 2009, 22 potentially eligible participants had not reached autopsy. 8 of these were known to have died by the time of the 2007 paper, but the relatives had refused permission for autopsy. The 2009 paper did not report the status of the remaining 14. |
||
| Comparative | |||
| Notes | We judged the study to be at high risk of bias in the index test domain because of the failure to perform a structural scan alongside the DAT scan. We noted potential conflicts of interest. Several study authors had received consultancy fees from the manufacturer of DaTscanTM. The manufacturer also supplied the ligand for the study. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
CAMCOG: Cambridge Cognitive Examination CDR: Clinical Dementia Rating CERAD: Consortium to Establish a Registry for Alzheimer’s Disease MMSE: mini mental state examination NIA‐RI: National Institute on Aging and Reagan Institute NINCDS‐ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gilman 2004 | Included participants with clinical diagnoses of DLB or AD. 3/45 patients (1 DLB, 2 AD) had neuropathological confirmation of diagnosis. |
| Lim 2009 | Included participants with clinical diagnoses of DLB or AD. 5/24 patients (4 DLB, 1 AD) had neuropathological confirmation of diagnosis. Results for these 5 not separately available. |
| Perju‐Dumbrava 2009 | Case series of 25 people with Parkinsonian syndromes (2 had DLB). |
Characteristics of ongoing studies [ordered by study ID]
Colloby 2012.
| Trial name or title | (See Ongoing studies above. This study relates to a prospective longitudinal study from which data relevant to this review may become available in future). |
| Target condition and reference standard(s) | |
| Index and comparator tests | |
| Starting date | |
| Contact information | |
| Notes |
Differences between protocol and review
None.
Contributions of authors
JMcC and SM selected studies.
JMcC and CH extracted data.
All authors contributed through writing or comments to the texts of the protocol and review.
Declarations of interest
JMcC has acted as a local investigator in a trial sponsored by GE Healthcare, manufacturer of DaTscanTM, for which her institution received some financial benefit.
SM has received research support (free ligand) from GE Healthcare.
KMB. AHN‐S, OA, CH: none known
New
References
References to studies included in this review
Walker 2009 {published data only}
- Walker RWH, Walker Z. Dopamine transporter single photon emission computerized tomography in the diagnosis of dementia with Lewy bodies. Movement Disorders 2009;24(Suppl2):S754‐9. [DOI] [PubMed] [Google Scholar]
- Walker Z, Costa DC, Walker RWH, Shaw K, Gacinovic S, Stevens T, et al. Differentiation of dementia with Lewy bodies from Alzheimer’s disease using a dopaminergic presynaptic ligand. Journal of Neurology, Neurosurgery, and Psychiatry 2002;73(2):134‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker Z, Jaros E, Walker RWH, Lee L, Costa DC, Livingston G, et al. Dementia with Lewy bodies: a comparison of clinical diagnosis, FP‐CIT single photon emission computed tomography imaging and autopsy. Journal of Neurology, Neurosurgery, and Psychiatry 2007;78(11):1176‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Gilman 2004 {published data only}
- Gilman S, Koeppe RA, Little R, An H, Junck L, Giordani B, et al. Striatal monoamine terminals in Lewy body dementia and Alzheimer's disease. Annals of Neurology 2004;55(6):774‐80. [DOI] [PubMed] [Google Scholar]
Lim 2009 {published data only}
- Lim SM, Katsifis A, Villemagne VL, Best R, Jones G, Saling M, et al. The 18F‐FDG PET cingulate island sign and comparison to 123I‐β‐CIT SPECT for diagnosis of dementia with Lewy bodies. Journal of Nuclear Medicine 2009;50(10):1638‐45. [DOI] [PubMed] [Google Scholar]
Perju‐Dumbrava 2009 {published data only}
- Perju‐Dumbrava L, Kovacs GG, Jellinger K, Schmidbauer M, Hoffmann M, Asenbaum S, et al. DAT SPECT in post mortem‐confirmed Parkinsonian disorders. European Journal of Neurology Conference: 13th Congress of the EFNS. Florence, 2009.
References to ongoing studies
Colloby 2012 {published data only}
- Colloby SJ, McParland S, O'Brien JT, Attems J. Neuropathological correlates of dopaminergic imaging in Alzheimer's disease and Lewy body dementias. Brain 2012;135:2798‐808. [DOI] [PubMed] [Google Scholar]
Additional references
Aarsland 2005
- Aarsland D, Perry R, Larsen JP, McKeith IG, O'Brien JT, Perry EK, et al. Neuroleptic sensitivity in Parkinson's disease and Parkinsonian dementias. Journal of Clinical Psychiatry 2005;66(5):633‐7. [PubMed] [Google Scholar]
Aarsland 2008
- Aarsland D. Early discriminatory diagnosis of dementia with Lewy bodies: The emerging role of CSF and imaging biomarkers. Dementia and Geriatric Cognitive Disorders 2008;25:195‐205. [DOI] [PubMed] [Google Scholar]
Braak 2003
- Braak H, Tredici K, Rüb U, Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiology of Aging 2003;24(2):197‐211. [DOI] [PubMed] [Google Scholar]
Ceravolo 2003
- Ceravolo R, Volterrani D, Gambaccini G, Rossi C, Logi C, Manca G, et al. Dopaminergic degeneration and perfusional impairment in Lewy body dementia and Alzheimer's disease. Neurological Sciences 2003;24:162‐3. [DOI] [PubMed] [Google Scholar]
Darcourt 2010
- Darcourt J, Booij J, Tatsch K, Varrone A, Vander Borght T, Kapucu OL, et al. EANM procedure guidelines for brain neurotransmission SPECT using 123I‐labelled dopamine transporter ligands, version 2. European Journal of Nuclear Medicine & Molecular Imaging 2010;37(2):443‐50. [DOI] [PubMed] [Google Scholar]
Dickson 2012
- Dickson JC, Tossici‐Bolt L, Sera T, Nijs R, Booij J, Bagnara MC, et al. Proposal for the standardisation of multi‐centre trials in nuclear medicine imaging: prerequisites for a European 123I‐FP‐CIT SPECT database. European Journal of Nuclear Medicine & Molecular Imaging 2012;39(1):188‐97. [DOI] [PubMed] [Google Scholar]
Djang 2012
- Djang DS, Janssen MJ, Bohnen N, Booij J, Henderson T, Herholz K, et al. SNM practice guideline for dopamine transporter imaging with 123I‐Ioflupane SPECT. Journal of Nuclear Medicine 2012;53(1):154‐63. [DOI] [PubMed] [Google Scholar]
Heidebrink 2002
- Heidebrink JL. Is dementia with Lewy bodies the second most common cause of dementia?. Journal of Geriatric Psychiatry and Neurology 2002;15:182‐7. [DOI] [PubMed] [Google Scholar]
McKeith 1996
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the consortium on DLB international workshop. Neurology 1996;47:1113‐24. [DOI] [PubMed] [Google Scholar]
McKeith 2004
- McKeith I, Mintzer J, Aarsland D, Burn D, Chiu H, Cohen‐Mansfield J, et al. Dementia with Lewy bodies. Lancet Neurology 2004;3:19‐28. [DOI] [PubMed] [Google Scholar]
McKeith 2005
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863‐72. [DOI] [PubMed] [Google Scholar]
McKeith 2007
- McKeith I, O'Brien J, Walker Z, Tatsch K, Booji J, Darcourt J, et al. Sensitivity and specificity of dopamine transporter imaging with 123I‐FP‐SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurology 2007;6:305‐13. [DOI] [PubMed] [Google Scholar]
Morgan 2012
- Morgan S, Kemp P, Booij J, Costa DC, Padayachee S, Lee L, et al. Differentiation of frontotemporal dementia from dementia with Lewy bodies using FP‐CIT SPECT. Journal of Neurology, Neurosurgery, and Psychiatry 2012;83(11):1063‐70. [DOI] [PubMed] [Google Scholar]
Nelson 2010
- Nelson PT, Jicha GA, Kryscio RJ, Abner EL, Schmitt FA, Cooper G, et al. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. Journal of Neurology 2010;257:359‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brien 2004
- O'Brien JT, Colloby S, Fenwick J, WIlliams ED, Firbank M, Burn D, et al. Dopamine transporter loss visualised with FP‐CIT SPECT in the differential diagnosis of dementia with Lewy bodies. Archives of Neurology 2004;61(6):919‐25. [DOI] [PubMed] [Google Scholar]
Perry 1990
- Perry RH, Irving D, Blessed G, Fairbairn A, Perry EK. Senile dementia of Lewy body type. A clinically and neuropathologically distinct form of Lewy body dementia in the elderly. Journal of Neurological Sciences 1990;95:119‐39. [DOI] [PubMed] [Google Scholar]
Reitsma 2009
- Reitsma JB, Rutjes AWS, Whiting P, Vlassov VV, Leeflang MMG, Deeks JJ. Chapter 9: Assessing methodological quality. In: Deeks JJ, Bossuyt PM, Gatsonis C (editors), Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy, Version 1.0.0. The Cochrane Collaboration, 2009. Available from: http://srdta.cochrane.org/.
Rutjes 2005
- Rutjes AWS, Reitsma JB, Vandenbroucke JP, Glas AS, Bossuyt PMM. Case‐control and two‐gate designs in diagnostic accuracy studies. Clinical Chemistry 2005;51(8):1335‐41. [DOI] [PubMed] [Google Scholar]
Schneider 2005
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta‐analysis of randomized placebo‐controlled trials. JAMA 2005;294:1934‐43. [DOI] [PubMed] [Google Scholar]
Tatsch 2008
- Tatsch K. Imaging of the dopaminergic system in differential diagnosis of dementia. European Journal of Nuclear Medicine and Molecular Imaging 2008;35 Suppl 1:S51‐7. [DOI] [PubMed] [Google Scholar]
Varrone 2013
- Varrone A, Dickson JC, Tossici‐Bolt L, Sera T, Asenbaum S, Booij J, et al. European multicentre database of healthy controls for [123I]FP‐CIT SPECT (ENC‐DAT): age‐related effects, gender differences and evaluation of different methods of analysis. European Journal of Nuclear Medicine and Molecular Imaging 2013;40(2):213‐27. [DOI] [PubMed] [Google Scholar]
Verghese 1999
- Verghese J, Crystal HA, Dickson DW, Lipton RB. Validity of clinical criteria for the diagnosis of dementia with Lewy bodies. Neurology 1999;53:1974‐82. [DOI] [PubMed] [Google Scholar]
Walker 2002
- Walker Z, Costa DC, Walker RW, Shaw K, Gacinovic S, Stevens T, et al. Differentiation of dementia with Lewy bodies from Alzheimer's disease using a dopaminergic presynaptic ligand. Journal of Neurology, Neurosurgery, and Psychiatry 2002;73:134‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: A revised tool for the quality assessment of diagnostic test accuracy studies. Annals of Internal Medicine 2011;155:529‐36. [DOI] [PubMed] [Google Scholar]
Zaccai 2005
- Zaccai J, McCracken C, Brayne C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age and Ageing 2005;34:561‐6. [DOI] [PubMed] [Google Scholar]


