Abstract
Background
The use of an effective contraceptive may be necessary after an abortion. Insertion of an intrauterine device (IUD) may be done the same day or later. Immediate IUD insertion is an option since the woman is not pregnant, pain of insertion is less because the cervical os is open, and her motivation to use contraception may be high. However, insertion of an IUD immediately after a pregnancy ends carries risks, such as spontaneous expulsion.
Objectives
To assess the safety and efficacy of IUD insertion immediately after spontaneous or induced abortion.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, POPLINE, ClinicalTrials.gov, and ICTRP in January 27, 2014. We also contacted investigators to identify other trials.
Selection criteria
We sought all randomised controlled trials (RCTs) with at least one treatment arm that involved IUD insertion immediately after an induced abortion or after curettage for spontaneous abortion.
Data collection and analysis
We evaluated the methodological quality of each report and abstracted the data. We focused on discontinuation rates for accidental pregnancy, perforation, expulsion, and pelvic inflammatory disease. We computed the weighted average of the rate ratios. We computed risk ratios (RRs) with 95% Confidence Intervals (CIs). We performed an intention‐to‐treat (ITT) analysis by including all randomised participants in the analysis according to the Cochrane Handbook for Systematic Reviews of Interventions.
Main results
We identified 12 trials most of which are of moderate risk of bias involving 7,119 participants which described random assignment. Five trials randomised to either immediate or delayed insertion of IUD. One of them randomised to immediate versus delayed insertion of Copper 7 showed immediate insertion of the Copper 7 was associated with a higher risk of expulsion than was delayed insertion (RR 11.98, 95% CI 1.61 to 89.35,1 study, 259 participants); the quality of evidence was moderate. Moderate quality of evidence also suggests that use and expulsion of levonorgestrel‐releasing intrauterine system or CuT380A was more likely for immediate compared to delayed insertion risk ratio (RR) 1.40 (95% CI 1.24 to 1.58; 3 studies; 878 participants) and RR 2.64 ( 95% CI 1.16 to 6.00; 3 studies; 878 participants) respectively. Another trial randomised to the levonorgestrel IUD or Nova T showed discontinuation rates due to pregnancy were likely to be higher for women in the Nova T group. (MD 8.70, 95% CI 3.92 to 13.48;1 study; 438 participants); moderate quality evidence.
Seven trials examined immediate insertion of IUD only. From meta‐analysis of two multicentre trials, pregnancy was less likely for the TCu 220C versus the Lippes Loop (RR 0.43, 95% CI 0.24 to 0.75; 2 studies; 2257 participants ) as was expulsion (RR 0.61, 95% CI 0.46 to 0.81; 2 studies; 2257 participants). Estimates for the TCu 220 versus the Copper 7 were RR 0.42 ( 95% CI 0.23 to 0.77; 2 studies, 2,274 participants) and RR 0.68, (95% CI 0.51 to 0.91); 2 studies, 2,274 participants), respectively. In other work, adding copper sleeves to the Lippes Loop improved efficacy (RR 3.40, 95% CI 1.28 to 9.04, 1 study, 400 participants) and reduced expulsion (RR 3.00, 95% CI 1.51 to 5.97; 1 study, 400 participants).
Authors' conclusions
Moderate quality evidence shows that insertion of an IUD immediately after abortion is safe and practical. IUD expulsion rates appear higher immediately after abortions compared to delayed insertions. However, at six months postabortion, IUD use is higher following immediate insertion compared to delayed insertion.
Plain language summary
Inserting an IUD right after abortion or miscarriage versus at a later time
Inserting an intrauterine device (IUD) right after an abortion or miscarriage can be good for many reasons. The woman is not pregnant and may be thinking about birth control, and the time and place are convenient for the woman. If asked to delay IUD insertion, many women do not return to get the device. However, the IUD might be more likely to come out on its own if put in right after abortion or miscarriage. This review looked at how safe it was to insert an IUD right after abortion or miscarriage. We also looked at whether the IUD stayed in.
We did computer searches for randomised trials of IUDs inserted right after abortion or miscarriage. We also wrote to researchers to find more studies. Trials could compare types of IUDs or times for insertion. We found 12 studies to include.
Four trials randomised women to an IUD inserted right away or at a later time. One had no major difference. Three recent trials (of levonorgestrel intrauterine system or CuT380A) showed use was greater at six months for an IUD inserted right away compared to one inserted later. Another trial assigned women to the levonorgestrel IUD or Nova T; more women with the Nova T stopped use due to pregnancy. A subanalysis showed more IUDs came out when inserted right after abortion or miscarriage rather than later.
Seven trials looked at inserting the IUD right away. From two large trials, the TCu 220C was better than the Lippes Loop and the Copper 7 for preventing pregnancy and staying in. The IUD was more likely to come out on its own when inserted after a mid‐pregnancy abortion than after an earlier one. In other work, when the Lippes Loop had copper arms added, fewer women got pregnant and the IUD stayed in more often.
Moderate level evidence shows that inserting an IUD right after an abortion or miscarriage is safe and practical. However, the IUD is more likely to come out when inserted right away rather than at a later time. Women are more likely to use an IUD at six months if they had it inserted right away compared to some weeks after the abortion or miscarriage.
Summary of findings
Summary of findings for the main comparison. Immediate versus delayed insertion (LNG‐IUS or CuT380A IUD) for.
| Immediate versus delayed insertion (LNG‐IUS or CuT380A IUD) for | ||||||
| Patient or population: patients with Settings: Intervention: Immediate versus delayed insertion (LNG‐IUS or CuT380A IUD) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Immediate versus delayed insertion (LNG‐IUS or CuT380A IUD) | |||||
| Expulsion by 6 months Rate in percentage Follow‐up: mean 6 months | Study population | RR 2.9 (1.25 to 6.71) | 878 (3 studies) | ⊕⊕⊕⊝ moderate1,2 | ||
| 15 per 1000 | 43 per 1000 (19 to 100) | |||||
| Moderate | ||||||
| 19 per 1000 | 55 per 1000 (24 to 127) | |||||
| Removal by 6 months Rate in percentage Follow‐up: mean 6 months | Study population | RR 2.01 (0.99 to 4.06) | 790 (2 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 28 per 1000 | 56 per 1000 (28 to 114) | |||||
| Moderate | ||||||
| 22 per 1000 | 44 per 1000 (22 to 89) | |||||
| Use at 6 months Rate in percentage Follow‐up: mean 6 months | Study population | RR 1.4 (1.24 to 1.58) | 878 (3 studies) | ⊕⊕⊕⊝ moderate4 | ||
| 464 per 1000 | 650 per 1000 (575 to 733) | |||||
| Moderate | ||||||
| 386 per 1000 | 540 per 1000 (479 to 610) | |||||
| Pregnancy at six months Rate in percentage | Study population | RR 0.37 (0.12 to 1.14) | 878 (3 studies) | ⊕⊕⊕⊝ moderate5 | ||
| 23 per 1000 | 9 per 1000 (3 to 27) | |||||
| Moderate | ||||||
| 23 per 1000 | 9 per 1000 (3 to 26) | |||||
| upper genital tract infection Rate in percentage | Study population | OR 1 (0.32 to 3.14) | 878 (3 studies) | ⊕⊕⊕⊝ moderate5 | ||
| 13 per 1000 | 13 per 1000 (4 to 39) | |||||
| Moderate | ||||||
| 16 per 1000 | 16 per 1000 (5 to 49) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Cremer 2011 stated that IUD was not always confirmed to be present by a provider so but self‐reported by perticipants so it is not possible to be certain of the true continuation rates or expulsion rates. 2 Two of the included studies did not blind participants nor providers. 3 Attrition too high in the three studies analysed for this outcome 4 Presence of IUD was not always confirmed by a provider but self‐reported by participants 5 Number lost to follow up in all 3 studies too high.
Summary of findings 2. TCu 220C compared to Lippes Loop for immediate insertion post‐abortion.
| TCu 220C compared to Lippes Loop for immediate insertion post‐abortion | ||||||
| Patient or population: patients with immediate insertion post‐abortion Settings: Clinic Intervention: TCu 220C Comparison: Lippes Loop | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Lippes Loop | TCu 220C | |||||
| Discontinuation due to perforation (750 days) Tietze‐Potter gross rate (Discontinuation in percentages Follow‐up: mean 750 days | Study population | RR 0.92 (0.13 to 6.6) | 2269 (2 studies) | ⊕⊕⊕⊝ moderate1,2 | ||
| 1 per 1000 | 1 per 1000 (0 to 6) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Discontinuation due to pelvic inflammatory disease (750 days) Follow‐up: mean 750 days | Study population | RR 1.17 (0.29 to 4.71) | 2269 (2 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 3 per 1000 | 3 per 1000 (1 to 13) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Discontinuation rate due to pregnancy (750days) Follow‐up: mean 750 days | Study population | RR 0.38 (0.2 to 0.72) | 2269 (2 studies) | ⊕⊕⊕⊝ moderate4 | ||
| 35 per 1000 | 13 per 1000 (7 to 25) | |||||
| Moderate | ||||||
| Discontinuation rate due to expulsion (750 days) Tietze‐Potter gross rate (Discontinuation in percentages) Follow‐up: mean 750 days | Study population | RR 0.51 (0.3 to 0.88) | 2269 (2 studies) | ⊕⊕⊕⊝ moderate4 | ||
| 100 per 1000 | 51 per 1000 (30 to 88) | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 WHO 1983b‐ did not state whether sealed envelopes were opaque or sequentially‐numbered 2 There was no blinding in the two WHO studies. 3 No explanation was provided 4 No blinding in the two studies
Summary of findings 3. TCu 220C compared to Copper 7 for Immediate insertion Post abortion.
| TCu 220C compared to Copper 7 for Immediate insertion Post abortion | ||||||
| Patient or population: patients with Immediate insertion Post abortion Settings: Intervention: TCu 220C Comparison: Copper 7 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Copper 7 | TCu 220C | |||||
| Discontinuation due to pregnancy (750 days) Follow‐up: mean 750 days | Study population | RR 0.44 (0.04 to 4.83) | 2274 (2 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 31 per 1000 | 14 per 1000 (1 to 149) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Discontinuation due to expulsion (750 days) Follow‐up: mean 750 days | Study population | RR 0.46 (0.14 to 1.5) | 2274 (2 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 91 per 1000 | 42 per 1000 (13 to 137) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Discontinuation due to pelvic inflammation Follow‐up: mean 750 days | Study population | Not estimable | 2274 (2 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 7 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| Discontinuation due to perforation Follow‐up: mean 750 days | Study population | Not estimable | 2274 (2 studies) | ⊕⊕⊕⊝ moderate4 | ||
| 2 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 No blinding 2 No blinding 3 No blinding 4 No blinding
Background
Abortion is the termination of pregnancy prior to viability. It could be due to a spontaneous miscarriage or be medically or surgically induced. Abortion and its complications are a major cause of maternal morbidity and mortality, especially, in low and middle income countries.
Description of the condition
The termination of pregnancy prior to viability could occur spontaneously. Spontaneous abortion is also known as spontaneous miscarriage. The true incidence of spontaneous abortion is unknown but 15% of clinically evident pregnancies and 60% of chemically diagnosed pregnancies end in spontaneous abortion (Uzelac 2007). There are different classifications of abortion.
Threathened abortion occurs when there is uterine bleeding with or without lower abdominal pain and the cervical os is closed. In missed abortion, there is retention of the dead embryo or fetus prior to viability. It presents with slight uterine bleeding, regression of pregnancy symptoms and therefore mimics threatened abortion. However, ultrasonography would confirm a missed abortion when fetal heart activities are not demonstrated. Ineveitable abortion is diagnosed when there is dilatation of the cervix and intrauterine bleeding without expulsion of any products of conception (Uzelac 2007).
Occassionally, spontaneous miscarriage could be incomplete. In these instances, uterine bleeding is usually associated with lower abdominal pains, the cervical os is open with partial expulsion of products of conception (Uzelac 2007). It may be possible to see products of conception at the cervical os.Complete spontaneous miscarriage occurs when all the products of conception are expelled and the cervical os is closed. Due to the closed os, it may be difficult to diagnose but, ultrasonography may aid diagnosis.
Intentional termination of pregnancy is known as induced abortion. Induced abortion may be medical or surgical, and medications used to induce abortion include Mefipristone and Misoprostol. Surgically‐induced abortion could be done with the manual vacuum or electric aspirator.
The cause of most cases of spontaneous abortion is unknown. However, abnormal karyotype account for about 50% of first trimester spontaneous abortions (Uzelac 2007). Other causes of spontaneous abortion are infection, anatomic defects, endocrine factors, immunologic factors and maternal systemic diseases.
The impact abortion has on health systems depends on how liberal the legislation on abortion is. In settings with restrictive abortion laws, abortion takes a large toll on health systems due to the morbidity and mortality that complicate clandestine induced abortions. Abortion‐related complications accounted for 5.4% of maternal deaths (Okusanya 2013) and 11.4% of severe maternal outcomes in the cluster of facilities involved in the World Health Organization (WHO) Multicountry Survey (Souza 2013).
By contrast, abortion is procured in health facilities of countries with liberal legislation on abortion. In the United States, 1.3 million women have abortions annually and half of these are repeat procedures (Goodman 2008). In these settings, family planning counselling and provision of contraceptive products such as the intrauterine contraceptive device (IUD) are offered to avert repeat, unintended pregnancies.
Description of the intervention
Postabortion IUD insertion could be immediate or at an interval (otherwise known as delayed). When an IUD is inserted the same day after an induced abortion or complete spontaneous miscarriage, it is termed 'immediate IUD insertion'. Insertion of an IUD immediately after an abortion has several potential advantages. The woman is known not to be pregnant (a major concern for clinicians) and it avoids repeat unintended pregnancies despite return to sexual activity and of ovulation. For example, many clinicians refuse to insert an IUD in a woman who is not menstruating (Stanback 1997). More so, after induced abortion, a woman's motivation to use contraception may be high, and for women who have limited access to a clinician, abortion care may provide a unique opportunity to address a woman's need for contraception (Mahomed 1997; McLaurin 1993; Wolf 1994). In addition, insertion of an IUD immediately after abortion may avoid discomfort related to insertion, and any bleeding from the insertion will be disguised by the expected bleeding after abortion. Less than a third of women who intend to have an IUD after abortion may actually have one inserted, and many prefer to have the option of immediate insertion (Stanek 2009). Interval postabortion IUD insertion allows time for the woman to come to terms with the loss of a wanted pregnancy after a spontaneous miscarriage and affords a woman who had an induced abortion the opportunity to be sure of her choice of contraceptive since a visit is required postabortion. However, observational studies have reported that 40% of clients do not return for insertion after opting for delayed IUD insertion (Goodman 2008), and that the additional visit was a barrier to delayed IUD insertion (McNicholas 2012).
Insertion of an IUD immediately after a pregnancy ends carries potential risks as well. There are concerns over expulsion of the IUD due to dilated cervix and risk of perforation may be increased due to softening of the myometrium. Another potential concern is pelvic inflammatory disease (PID), particularly when postabortion IUD insertion is done after a clandestine or unsafe abortion which increases the risk of upper genital tract infection compared with interval insertion.
How the intervention might work
Intrauterine contraceptive devices act locally on the endometrium causing inflammatory reaction. In addition, progestin‐impregnated devices cause daily release of hormones into maternal circulation.
Copper‐containing IUDs are thought to have spermicidal actions, and their effect on the endometrium interferes with normal development of ova or fertilization of ova. It also promotes phagocytosis of sperm cells and inhibits the movement of sperm cells from the vagina to the fallopian tubes where fertilization occurs (Burkman 2007). It is also thought that the inflammatory changes of the endometrium may prevent implantation of the embryo should fertilization occur.
Hormone‐impregnated IUDs release the progestin into the maternal circulation daily. They act primarily by thickening the cervical mucus, thereby impeding the ascent of sperm cells and its migration. They also inhibit ovulation, especially following insertion when the woman's serum concentration is relatively high. For instance, levonorgestrel‐20 (LNG‐20) IUD causes anovulation in approximately 10‐15% of cycles and changes the endometrium to reduce the likelihood of implantation (Burkman 2007).
While the mechanism of action of IUDs is irrespective of whether insertion is immediate or delayed, immediate IUD insertion would afford the woman an opportunity to have an effective contraceptive method earlier, before resumption of sexual activities. This would prevent an unplanned pregnancy and the need for a repeat induced abortion, or space the interval between a spontaneous miscarriage and the next planned pregnancy.
Why it is important to do this review
An effective contraceptive is required postabortion. The return of fertility after an abortion is good and 83% of women who had an abortion ovulate during the first menstrual cycle following the abortion (Saav 2012), and ovulation may occur as early as eight to 10 days after an induced abortion (Saav 2012). This, coupled with return to sexual activity by most women within two weeks of an induced abortion make the use of postabortion contraceptives necessary. A postabortion contraceptive method should be effective, long‐lasting and convenient to use. The IUDs, being similar to sterilisation in terms of contraceptive efficacy meet these criteria (Goodman 2008; Grimes 2008), yet they are simpler, less expensive, and promptly reversible.
The early return of ovulation, the likelihood of the woman not returning for a delayed IUD insertion and return to sexual activities make the concept of immediate insertion of intrauterine contraceptive devices worthy of a systematic review to assess the evidence for its effectiveness over delayed insertion.
Objectives
This review assesses the safety and efficacy of immediate IUD insertion after induced abortion or uterine evacuation for completion of a spontaneous incomplete abortion.
Methods
Criteria for considering studies for this review
Types of studies
This review includes only RCTs using at least one IUD intervention arm. We included studies of both induced and spontaneous abortion. Studies could have randomised participants to immediate or delayed insertion. They could also be two‐ or three‐arm comparisons of different types of IUDs using immediate insertion.
Types of participants
Trials included women of any age or gravidity who received an IUD immediately after induced abortion or uterine evacuation for spontaneous incomplete abortion.
Types of interventions
We included any type of IUD, regardless of its current availability.
Types of outcome measures
The principal outcome measures were accidental pregnancy, spontaneous expulsion, uterine perforation, and upper genital tract infection.
Search methods for identification of studies
Electronic searches
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) part of The Cochrane Library, www.thecochranelibrary.com (accessed 27 January 2014), MEDLINE via Pubmed (April 2010 to January 2014), EMBASE (2010 to January 2014) and POPLINE (2010 to January 2014) for trials of postabortal IUD insertions. We also searched for current trials via ClinicalTrials.gov (accessed 28 January 2014) and the International Clinical Trials Registry Program (ICTRP) (accessed 8 January 2014). Details of previous searches are in Appendix 1. The search strategies are given below.
MEDLINE via PubMed
(iud* OR iucd* OR intrauterine devices) AND insert* AND (postabort* OR post‐abort* OR abortion) limited to: Publication date from 2010/04/01 to 2014/01/31 Results:38 references
CENTRAL
strategy 1 Title, Abstract, Keywords ‐ (post‐abort* OR postabort* OR abort*) AND (IUD* OR intrauterine device*) Results: 50 references strategy 2 Title, Abstract, Keywords‐(intrauterine device OR IUD) AND ((delayed OR immediate) AND insertion) Results: 30 references
POPLINE
(Popline changed software so that’s why needed to use “OR” instead of “/” and “AND” instead of “&”
All fields‐ (iud* OR iucd* OR intrauterine device* OR intrauterine contraceptive device*) AND (postabortal OR postabortion OR post‐abortion OR abortion) AND insert* AND years‐ 2010 to 2014 Results: 9 references
EMBASE
(directly from Elsevier) iud* OR iucd* OR 'intrauterine'/exp AND device* AND insert* AND (postabort* OR postpartum OR 'puerperium'/exp OR 'abortion'/exp) AND [embase]/lim AND ('clinical study'/de OR 'clinical trial'/de OR 'comparative study'/de OR 'controlled clinical trial'/de OR 'controlled study'/de OR 'major clinical study'/de OR 'multicenter study'/de OR 'randomized controlled trial'/de) limited to 2010‐2014 Results: 33 references
ClinicalTrials.gov
strategy 1 (intrauterine device OR IUD) AND ((delayed OR immediate) AND insertion) Results: 37 references strategy 2 post‐abort* OR postabort* OR abort* AND IUD* OR intrauterine device* Results: 182 references
WHO, International Clinical Trials Registry Platform (ICTRP) Strategy 1 intrauterine device OR IUD AND ((immediate OR delayed) AND insertion) Results: 18 references Strategy 2 (truncation is with % rather than *) (post‐abort% OR postabort% OR abort%) AND (IUD% OR intrauterine device%) Results: 17 references
Searching other resources
For the initial review, we used several comprehensive review articles to begin our search (PIP 1995; WHO 1987). We also contacted investigators in the field to find studies we might have missed, including unpublished reports.
Data collection and analysis
Selection of studies
Two review authors (OO, EE) independently screened search results for eligible studies based on a priori inclusion criteria. Authors resolved disagreements by consensus.
Data extraction and management
One author (OO) entered the data into Review Manager (RevMan 2012), and another author (BO) checked the entries for accuracy. The same two review authors (OO, BO) resolved disagreements by consensus.
Assessment of risk of bias in included studies
Two authors (OO, BO) determined the risk of bias for the included trials by assessing random sequence generation, allocation concealment, blinding, selective reporting, and other sources of bias, and recorded the assessment in the 'Risk of bias' table. We ranked the studies as low risk, unclear risk and high risk of bias, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)
Measures of treatment effect
Although authors in previous update abstracted the life‐table rates and standard errors for meta‐analysis of WHO 1983a and WHO 1983b. We performed all meta‐analysis using number of events and number of participants assigned to each group (Higgins 2011).These outcomes included discontinuations due to pregnancy, expulsion, total medical events, perforation and pelvic inflammatory disease (PID). Where number of events were not reported, we calculated the standard error (SE) from reported p‐values in Pakarinen 2003 and used the inverse variance method for analysis (RevMan 2012).
Unit of analysis issues
Not applicable
Dealing with missing data
Authors of previous update contacted Gillett 1980; Lim 1985; McCarthy 1985; and Pakarinen 2003 for method of allocation and concealment because it was not described in these studies.We performed an intention‐to‐treat (ITT) analysis by including all randomised participants in the analysis according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
We considered heterogeneity statistically significant when the I2 statistic was 50% or more. We will use the random‐effects model for meta‐analysis if the I2 statistic is > 50%. We did not use the random effects model for meta‐analysis because the I2 statistic was 0% in all meta‐analysis.
Assessment of reporting biases
We did not test asymmetry with a funnel plot because the number of studies included in each meta‐analysis were too few.
Data synthesis
For this update, we retrieved all studies that were previously included and re‐analysed some data where necessary. Two authors abstracted information onto the data collection forms, and resolved any discrepancies by discussion or consultation with a third author. We attempted to contact several researchers by mail for supplemental information. One author entered the data into Review Manager (RevMan 2012), and another author checked the entries for accuracy.
Most studies could not be aggregated into a meta‐analysis due to having different interventions.We extracted the estimates of effects (MDs) and SE of the mean for some outcomes and calculated 95% CIs of the MDs using generic inverse variance. Where the SE was not reported, we calculated the SE using the P‐values reported. We estimated the (RR) and 95% confidence interval (CI) for most of the trials using the crude number of events for dichotomous outcomes, the Mantel‐Haenszel odds ratio (OR) with 95% CI was calculated using a fixed‐effect model. An example is the proportion of women with spontaneous expulsion. Fixed‐ and random‐effects give the same result if no heterogeneity exists, as when a comparison includes only one study. The Peto OR can be used when a study arm has no events, e.g. pregnancy; the Peto OR does not require correction for zero events (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
We did not aggregate most studies into a meta‐analysis because they have different interventions; therefore investigation of heterogeneity was not feasible. We used a random‐effects model instead of a fixed‐effect model to address any possible heterogeneity.
Sensitivity analysis
We did not perform a sensitivity analysis because we only included very few studies per meta‐analysis.
Results
Description of studies
Results of the search
Our updated 2014 search yielded 414 studies. Only five met the inclusion criteria; three (Bednarek 2011; Cremer 2011;Hohmann 2012) were included in the analysis, although during the last update (Grimes 2010), preliminary data from Cremer 2011 and Hohmann 2012 were included in the analysis. We have now included the complete data for these studies in this update. The remaining two studies NCT00877344 and NCT00877344) are on‐ going.
Included studies
Twelve trials; involving 7119 participants met our inclusion criteria. Nine were published more than 10 years ago while the other three studies were published in the last three years; two of which had their preliminary results included in the last update of this review, see Characteristics of included studies.
Five trials examined immediate versus delayed insertion.
Five trials randomised participants to immediate or delayed insertion of the following devices: Copper 7 (Gillett 1980); levonorgestrel‐releasing intrauterine system (LNG‐IUS) (Hohmann 2012); LNG‐IUS or CuT380A IUD (Bednarek 2011); CuT380A IUD (Cremer 2011).
Pakarinen 2003 randomised to LNG‐IUS or Nova T, but a subgroup analysis examined timing of insertion (immediate or delayed).
Seven trials studied immediate insertion of different IUDs or modifications of IUDs.
Two large international trials studied immediate insertion. WHO 1983b examined insertions of the Lippes Loop, Copper TCu 220C, and the Copper 7 immediately after spontaneous abortion. The other trial (WHO 1983a) studied the same three devices after induced abortion.
Three trials were two‐arm comparisons of immediate insertion of different IUDs. Nielsen 1984 compared the Nova T to the Copper T 200, while McCarthy 1985 compared the Nova T to the Multiload 250. Lim 1985 compared the Multiload 375 versus Multiload 250.
Two trials examined immediate insertion with modifications of an IUD. In Randic 1991, copper sleeves were added to a Lippes Loop D. In Randic 1983, topical hydrogel was applied to a spring coil.
Excluded studies
One trial proved not to be randomised (Querido 1985). The researchers used alternate assignment of patients, and so we excluded this trial from subsequent analysis.
Two trials had a sham IUD insertion arm without the knowledge of the women involved. Chowdhury 1979 stated that "Although all of the women thought that they had insertion of device, in fact one group received Lippes loop (Group B), one group Cu T (Group C), and the other group did not receive any device (Group A) in immediate postabortal period." The researchers did not disclose when or if they informed the 100 participants in Group A that they had a sham insertion. Similarly, Goldsmith 1972 randomised 584 women to receive either a Lippes Loop or a sham insertion. The design was double blind, and the blinding ended after 30 days of observation, when the women without contraception were provided an IUD. In an addendum to the published report, the researchers acknowledged that women in the sham insertion group "were exposed to a risk of pregnancy albeit an extremely small one." They reasoned that this "risk was more than justified." In both studies, women lost to follow‐up may have incorrectly assumed they were using an IUD when they were not. We excluded both trials because of unethical research conduct.
Risk of bias in included studies
Allocation
The WHO trials (WHO 1983a; WHO 1983b) were of moderate quality (Table 2 and Table 3). Both were large studies, and used a computer‐generated random sequence and sealed envelopes for allocation concealment. Communication with the researchers indicated that the envelopes were sequentially numbered and opaque. Also randomisation sequence in Bednarek 2011 and Hohmann 2012 was generated by a statistician not affiliated with the study. Participants and investigators were blinded to the intervention given according to the sequence generated by the statistician. Randic 1983 and Randic 1991 reported that investigators were blinded to intervention arms of participants and randomisation was computer generated and concealed in opaque envelopes.
Several studies did not give sufficient information on the methods of randomisation or allocation concealment. Communication with researchers (Gillett 1980; Lim 1985; McCarthy 1985; Suvisaari from Pakarinen 2003) confirmed that computer‐generated randomisation had been done, with allocation concealment by sealed envelopes. Whether the envelopes were opaque and sequentially numbered is unknown Figure 1.
1.
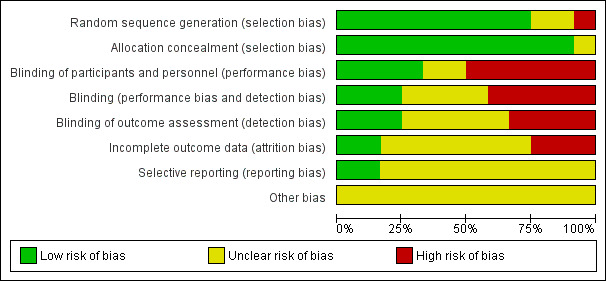
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Blinding
The baseline characteristics did not differ significantly between treatment groups in the included studies. In Bednarek 2011; and Hohmann 2012 , the study personnel and participants were blinded to IUD inserted into each participants. Randic 1983 and Randic 1991 reported that investigators were blinded to the IUD assigned to study participants because only the patients order form was inserted in the patients file during follow‐up visits. However, It was unclear whether blinding was done by McCarthy 1985 and Nielsen 1984 because it was not reported. The rest of the included studies, Gillett 1980,WHO 1983a, WHO 1983b, Lim 1985, Cremer 2011,Pakarinen 2003 reported that they did not blind participants and investigators.
Incomplete outcome data
Both WHO trials (WHO 1983a; WHO 1983b) excluded from analysis patients who had problems within 48 hours of insertion. While the number of excluded participants was small (12 and one, respectively), these exclusions were improper and led to an underestimation of discontinuation rates. While all the included studies accounted for the number of participants lost to follow‐up, none of them included them in the final analysis.
Selective reporting
We could not ascertain whether there was selective reporting in the included studies as we had no access to the study protocols of the trials.
Other potential sources of bias
Cremer 2011 had a limitation in that continuation or expulsion rate of IUD was not confirmed by the providers but was self‐reported by participants.
Effects of interventions
See: Table 1; Table 2; Table 3
Immediate versus delayed insertion
In Gillett 1980, low quality evidence suggests that immediate insertion of the Copper 7 was associated with a higher risk of expulsion than was delayed insertion for three to five weeks. (RR 11.98.; 95% CI 1.61 to 89.35; 1 study; 259 participants) (Analysis 5.2). No other significant differences emerged. However, 42% of women assigned to delayed insertion did not return for IUD insertion.
5.2. Analysis.
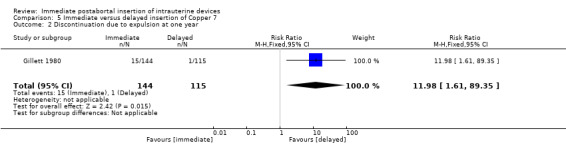
Comparison 5 Immediate versus delayed insertion of Copper 7, Outcome 2 Discontinuation due to expulsion at one year.
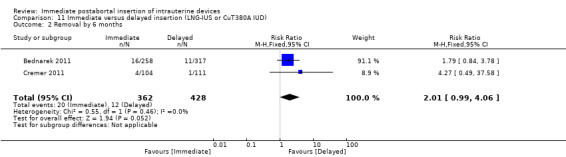
Three recent studies randomised women to immediate or delayed insertion (Bednarek 2011; Cremer 2011; Hohmann 2012). Hohmann 2012 used the LNG‐IUS; Cremer 2011 used Cu T80A and Bednarek 2011 offered either LNG‐IUS or Cu T380A (Table 1). When the trials were combined in a meta‐analysis, moderate level evidence showed that expulsion by six months was more likely for the group assigned to immediate insertion than delayed insertion (RR 2.64, 95% CI 1.16 to 6.00; 3 studies; 878 participants) (Analysis 11.1). Also low quality evidence shows that use at six months was greater in the immediate insertion group compared to the delayed insertion group (RR 1.40; 95% CI 1.24 to 1.58; 3 studies; 878 participants) (Analysis 11.3). Although there was more than a three‐fold increase in risk of pregnancy in the delayed group when compared to the group who had immediate IUD insertion; there was no statistical difference between the two (RR 0.37; 95% CI 0.12 to 1.14; 3 studies; 878 participants (Analysis 11.4). However, infection of the upper genital tract was similar for the groups assigned to immediate and delayed insertion of IUD (Analysis 11.5), quality of evidence was moderate.
11.1. Analysis.
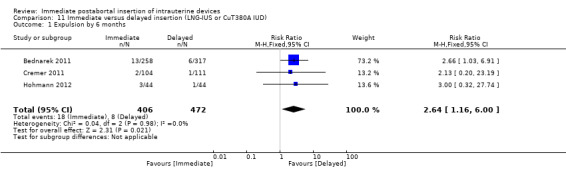
Comparison 11 Immediate versus delayed insertion (LNG‐IUS or CuT380A IUD), Outcome 1 Expulsion by 6 months.
11.3. Analysis.
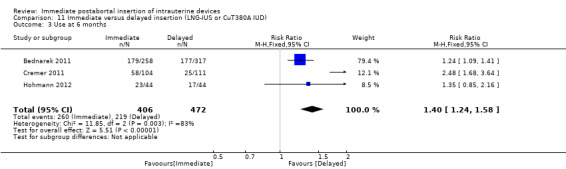
Comparison 11 Immediate versus delayed insertion (LNG‐IUS or CuT380A IUD), Outcome 3 Use at 6 months.
11.4. Analysis.
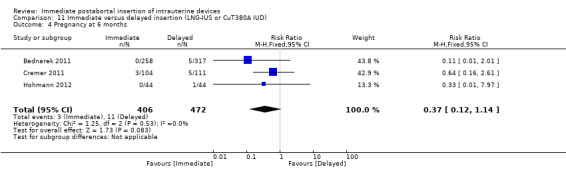
Comparison 11 Immediate versus delayed insertion (LNG‐IUS or CuT380A IUD), Outcome 4 Pregnancy at 6 months.
11.5. Analysis.
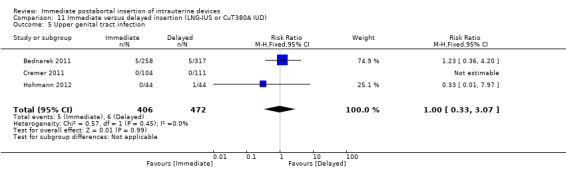
Comparison 11 Immediate versus delayed insertion (LNG‐IUS or CuT380A IUD), Outcome 5 Upper genital tract infection.
From the trial comparing the Nova T and levonorgestrel‐releasing device (Luukkainen et al, 1987, see Pakarinen 2003), two subgroup analyses were published, only Pakarinen 2003 met our inclusion criteria. Pakarinen 2003 analysed 438 immediate postabortal insertions, with 305 women randomised to the LNG system (LNG‐IUS) and 133 to the Nova T. Over five years of use, pregnancies were significantly less common with the LNG‐IUS than with the Nova T;(MD 8.70, 95% CI 3.92 to 13.48; 1 study; 438 participants) the gross discontinuation rate was 0.8 versus 9.5 per 100 women (Analysis 8.1). A moderate level of evidence suggests that five‐year cumulative discontinuation rates for hormonal reasons were higher with the LNG‐IUS (MD ‐12.00, 95% CI ‐20.39 to ‐3.61; 1 study; 438 participants) (Analysis 8.1). Expulsion rate between the two IUDs was no different (MD 4.90, 95% CI ‐5.99 to 15.79; 1 study; 438 participants). The Nova T was reportedly associated with more total days of bleeding and episodes of bleeding (MD 8.30, 95% CI ‐2.03 to 18.63; 1 study; 438 participants). On the other hand, amenorrhoea was reported as more common with the LNG‐IUS (MD ‐2.10, 95% CI ‐5.02 to 0.82; 1 study; 438 participants). There is however no statistically significant different between Nova T and LNG‐IUS for the two outcomes respectively.
8.1. Analysis.

Comparison 8 Nova T versus levonorgestrel IUS, Outcome 1 Discontinuation rates (5‐year) per 100 women due to pregnancy.
Immediate insertion of different IUDs or IUD modifications
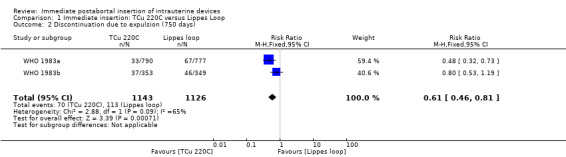
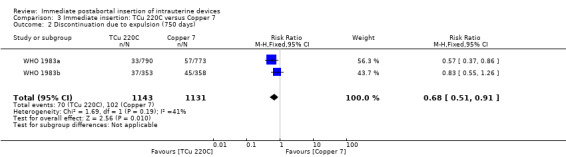
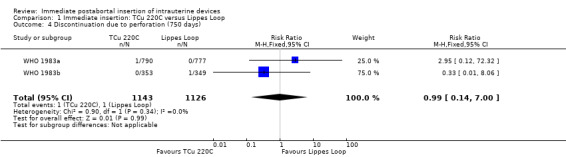
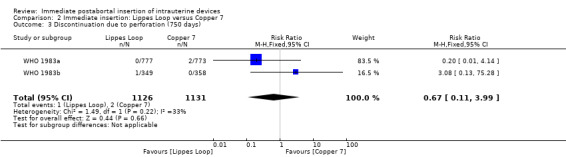
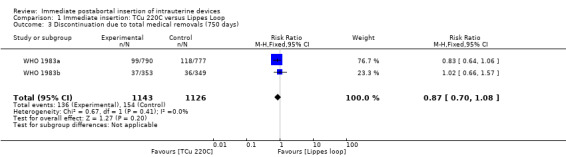
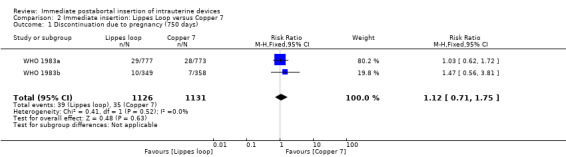
In two trials that compared three different IUDs (WHO 1983a; WHO 1983b), the TCu 220C proved to be superior to the Lippes Loop D and the Copper 7. The Lippes Loop and Copper 7 did not differ significantly. When data from both trials were combined, moderate level evidence suggests that accidental pregnancy was more likely amongst women assigned to Lippes loop than TCu 220C (RR 0.43, 95% CI 0.24 to 0.75, 2 studies, 2,269 participants) (Analysis 1.1). Compared with the Copper 7, the effect was RR 0.42 ( 95% CI 0.23 to 0.77; 2 studies; 2274 participants) (Analysis 3.1). Expulsions were also significantly less frequent with the TCu 220C than with either of the other two IUDs. The estimate for the TCu 200C compared to the Lippes Loop was RR 0.61, (95% CI 0.46 to 0.81; 2studies; 2269 participants) (Analysis 1.2), and compared to the Copper 7 it was RR 0.68, ( 95% CI 0.51 to 0.91; 2 studies; 2274 participants) (Analysis 3.2). Uterine perforations were uncommon; one woman in the TCu 220C group, two in the Copper 7 group, and one in the Lippes loop group respectively; these figures are not statistically different (Analysis 1.4; Analysis 2.3 and; Analysis 3.4 ). Pelvic inflammatory disease was also rare with IUD use after both induced and spontaneous abortion. Eight women in the Copper 7 group had PID, three in the Lippes loop group and four in the TCu 220C group; this difference however is not statistically significant (Analysis 1.5;Analysis 2.5; and Analysis 3.5) .
1.1. Analysis.
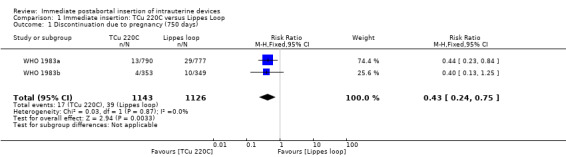
Comparison 1 Immediate insertion: TCu 220C versus Lippes Loop, Outcome 1 Discontinuation due to pregnancy (750 days).
3.1. Analysis.
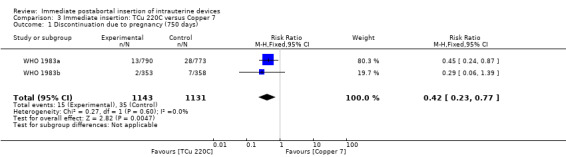
Comparison 3 Immediate insertion: TCu 220C versus Copper 7, Outcome 1 Discontinuation due to pregnancy (750 days).
1.2. Analysis.
Comparison 1 Immediate insertion: TCu 220C versus Lippes Loop, Outcome 2 Discontinuation due to expulsion (750 days).
3.2. Analysis.
Comparison 3 Immediate insertion: TCu 220C versus Copper 7, Outcome 2 Discontinuation due to expulsion (750 days).
1.4. Analysis.
Comparison 1 Immediate insertion: TCu 220C versus Lippes Loop, Outcome 4 Discontinuation due to perforation (750 days).
2.3. Analysis.
Comparison 2 Immediate insertion: Lippes Loop versus Copper 7, Outcome 3 Discontinuation due to perforation (750 days).
3.4. Analysis.
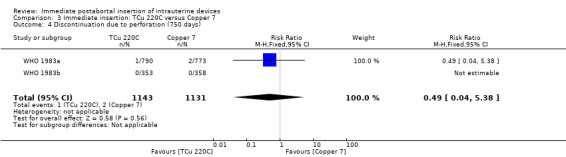
Comparison 3 Immediate insertion: TCu 220C versus Copper 7, Outcome 4 Discontinuation due to perforation (750 days).
1.5. Analysis.

Comparison 1 Immediate insertion: TCu 220C versus Lippes Loop, Outcome 5 Discontinuation due to pelvic inflammatory disease (750 days).
2.5. Analysis.
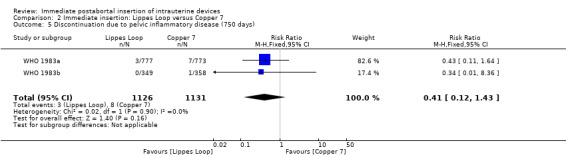
Comparison 2 Immediate insertion: Lippes Loop versus Copper 7, Outcome 5 Discontinuation due to pelvic inflammatory disease (750 days).
3.5. Analysis.
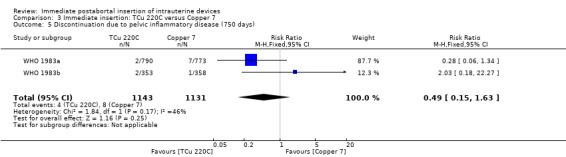
Comparison 3 Immediate insertion: TCu 220C versus Copper 7, Outcome 5 Discontinuation due to pelvic inflammatory disease (750 days).
Furthermore, the WHO trials reported that IUDs inserted after second‐trimester abortions had higher expulsion rates than did IUDs inserted after earlier abortions. In the trial of induced abortion (WHO 1983a), this difference was statistically significant for all three IUDs. For example, after abortions at less than 13 weeks' gestation, the cumulative net probability of expulsion at 120 days was 1.9 for the TCu 220C, 4.8 for the Lippes Loop, and 4.5 for the Copper 7. The corresponding figures after abortions at 13 to 20 weeks' gestation were 19.5, 48.8, and 21.3, respectively. Although this trend was also evident after spontaneous abortion, not all of the differences reached statistical significance (WHO 1983b). Neither the type of induced abortion procedure (sharp versus suction curettage) nor the use of oxytocic drugs significantly influenced outcomes.
The Nova T offered less protection against pregnancy than did the MLCu 250 (McCarthy 1985). The RR of a failure with the Nova T was 6.00 (95% CI 0.73 to 49.39, 1 study, 400 participants). compared with the MLCu 250 (Analysis 4.1). Other differences between these two IUDs were not significant either. The trial comparing the MLCu 250 and MLCu 375 (Lim 1985) found no significant differences between them.
4.1. Analysis.
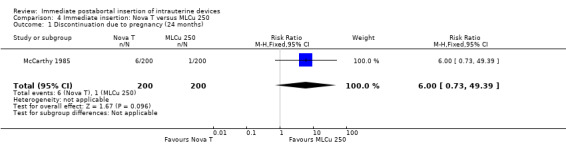
Comparison 4 Immediate insertion: Nova T versus MLCu 250, Outcome 1 Discontinuation due to pregnancy (24 months).
In contrast, in Nielsen 1984, the Nova T was superior to the Copper T 200 in contraceptive efficacy, (RR 0.21; 95% CI 0.05 to 0.94, 1 study, 331 participants), quality of evidence was moderate. (Analysis 7.1). Expulsions were also somewhat higher for the Nova T but it was not significantly different (RR 1.64; 95% CI 0.86 to 3.12, 1 study, 331 participants) (Analysis 7.2). No other important differences emerged between these two devices.
7.1. Analysis.
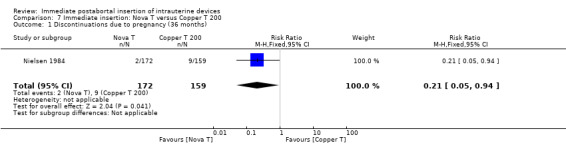
Comparison 7 Immediate insertion: Nova T versus Copper T 200, Outcome 1 Discontinuations due to pregnancy (36 months).
7.2. Analysis.
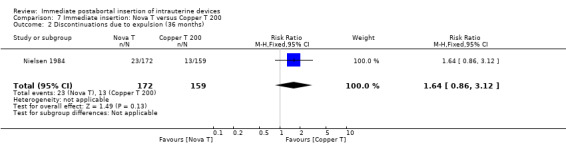
Comparison 7 Immediate insertion: Nova T versus Copper T 200, Outcome 2 Discontinuations due to expulsion (36 months).
In Randic 1991 moderate quality of evidence also suggest that addition of copper sleeves significantly improved the efficacy of the Lippes Loop D (RR 3.40; 95% CI 1.28 to 9.04, 1 study, 400 participants) (Analysis 9.1). This modification also significantly reduced the likelihood of expulsion or displacement (RR 3.00; 95% CI 1.51 to 5.97, 1 study, 400 participants) (Analysis 9.2). In contrast, addition of a hydrogel (Randic 1983) to the surface of a spring coil IUD did not improve tolerance of this device (Analysis 10.1 and Analysis 10.2).
9.1. Analysis.
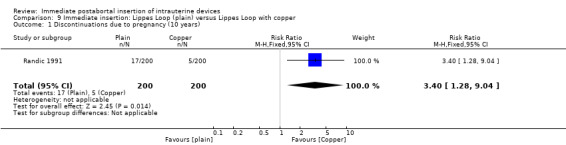
Comparison 9 Immediate insertion: Lippes Loop (plain) versus Lippes Loop with copper, Outcome 1 Discontinuations due to pregnancy (10 years).
9.2. Analysis.
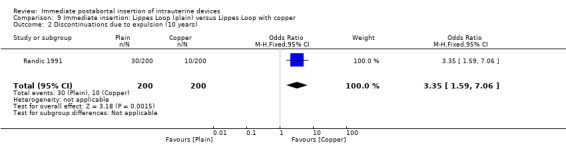
Comparison 9 Immediate insertion: Lippes Loop (plain) versus Lippes Loop with copper, Outcome 2 Discontinuations due to expulsion (10 years).
10.1. Analysis.

Comparison 10 Immediate insertion: Spring coil (plain) versus spring coil with hydrogel, Outcome 1 Discontinuation due to pregnancy per 100 women at 24 months.
10.2. Analysis.
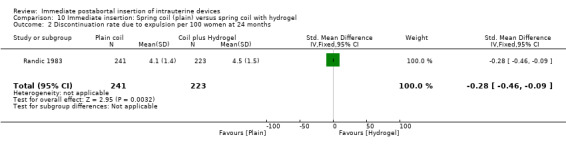
Comparison 10 Immediate insertion: Spring coil (plain) versus spring coil with hydrogel, Outcome 2 Discontinuation rate due to expulsion per 100 women at 24 months.
Discussion
Summary of main results
Several trials compared immediate and delayed insertion of the same IUD(s). Four randomised to the time of insertion. Meta‐analysis of three recent trials showed expulsion to be more likely in the immediate insertion group than the delayed insertion group; the quality of evidence was moderate. Moderate level evidence shows that IUD use at six months was also greater in the immediate insertion group. An older RCT showed a non‐significant difference in expulsion, with the immediate group having a slightly higher frequency than the delayed group. The high drop‐out rate with delayed insertion underscores a major public health point: many women who desire an IUD do not return if the insertion is delayed (Stanek 2009). The increased risk of spontaneous expulsion with immediate postabortal insertion needs to be balanced against the high rate of loss to follow‐up. While some women who were lost to follow‐up may have adopted other contraceptive methods, an unknown proportion remained unprotected against unintended pregnancy. A fifth trial randomised to different IUDs, but a subanalysis examined immediate versus delayed insertion. Both the Nova T and the LNG‐IUS had higher expulsion rates with postabortal insertion than with delayed insertion; this difference was also not statistically significant (Analysis 8.2).
8.2. Analysis.
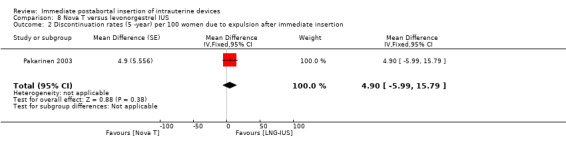
Comparison 8 Nova T versus levonorgestrel IUS, Outcome 2 Discontinuation rates (5 ‐year) per 100 women due to expulsion after immediate insertion.
RCTs comparing different IUDs found immediate postabortal insertion to be safe and effective. Perforations were rare with the devices studied, despite pregnancy‐related changes in the myometrium. Postabortal IUD insertion appears to carry a perforation risk similar to that of interval insertions (Sivin 1981). PID was also uncommon. Although populations may not be directly comparable, PID rates in these trials appear similar to those reported with interval insertions (Farley 1992; Sinei 1990; Walsh 1998). Pregnancy rates were low, although some significant differences emerged between devices. For example, the levonorgestrel‐releasing device was significantly more effective than the Nova T.
The configuration of the IUD influenced the risk of spontaneous expulsion. IUDs shaped like the letter T fared better than did alternative IUDs, such as the Lippes Loop or Copper 7. However, evidence is inadequate to determine which currently available IUD is best for immediate postabortal insertion. Rates of expulsion were higher after second‐trimester abortion than after earlier abortion. Based on this observation, the WHO researchers (WHO 1983a) recommended against IUD insertion immediately after second‐trimester abortion. This advice is likely inappropriate, given more recent evidence (Hohmann 2012).
While addition of copper sleeves to the Lippes Loop D improved the contraceptive efficacy of the device, this modification is not commercially available. The explanation for the benefit seen in terms of expulsions and displacements is unclear, although the researchers speculate that it may relate to an effect of copper on uterine motility. Further research with topical applications of hydrogel appears unwarranted.
Overall completeness and applicability of evidence
This review included 12 trials; nine (Gillett 1980; Randic 1983; WHO 1983a; WHO 1983b; Nielsen 1984; Lim 1985; McCarthy 1985; Randic 1991; Pakarinen 2003) of which were conducted over ten year ago; two of these nine studies (WHO 1983a and WHO 1983b) are large trials. Few reports had a sample size calculation, and several had little power to detect differences. Newer data are available on this topic. This review extracted full data from the published reports of three recent studies; two (Bednarek 2011; Hohmann 2012) of which provided preliminary results included in the last update, and the third (Cremer 2011) was ongoing at that time. Two trials (NCT00877344 and[ISRCTN: 19506752]) are ongoing. All should further inform the field in the near future WHO 1983b.
Quality of the evidence
Only four of the included studies (Hohmann 2012,Bednarek 2011,Randic 1983, and Randic 1991) described the blinding process and who was blinded. It was unclear whether McCarthy 1985 and Nielsen 1984 performed any blinding. The remaining six studies (Cremer 2011, Pakarinen 2003, Lim 1985, WHO 1983a; WHO 1983b and Gillett 1980 ) were of high risk of bias as they did not blind the study participants or the investigators. However, allocation was adequately concealed in all studies included; this was not described in (Gillett 1980; Lim 1985; McCarthy 1985; and Pakarinen 2003) but communication with them confirmed that computer‐generated randomisation had been done, with allocation concealment by sealed envelopes.
Potential biases in the review process
None
Agreements and disagreements with other studies or reviews
Outcomes with interval or immediate IUD insertions may also be useful for comparison. In an international trial (WHO 1994), 3655 healthy parous women were randomly allocated to receive either a Multiload 375 or a TCu 380A. The gross cumulative discontinuation rates with the Multiload 375 at one year were 1.2% for pregnancy and 3.6% for expulsion; 89% were continuing with the device. At three years, these figures were 2.9%, 6.4%, and 78%, respectively. The corresponding figures for the TCu 380A at one year were 0.8% for pregnancy and 3.8% for expulsion; 88% were continuing with the device. At three years, these figures were 1.6%, 5.2%, and 78%, respectively. In a US study of immediate insertion, 256 women were contacted six weeks after insertion (Drey 2009). The study showed 7.4% discontinuation; 1.9% had expulsions and 2.7% had suspected PID. Women with immediate insertion after first trimester abortions had slightly lower rates than those with second trimester abortions.
Insertion of an IUD at the time of abortion has several benefits compared with later insertion. After an unintended pregnancy, a woman may be highly motivated to avoid a recurrence (Mahomed 1997; McLaurin 1993; Wolf 1994). IUD insertion after abortion ensures effective contraception by the time ovulation resumes, and it eliminates the need for another visit for IUD insertion. Concerns about uterine perforation and PID, however, have limited postabortal IUD insertions. These concerns appear unwarranted.
Authors' conclusions
Implications for practice.
Moderate level evidence suggests that immediate insertion of an IUD after abortion is both safe and effective. This was true for both induced and reported 'spontaneous' abortions, many of which may have been induced under clandestine circumstances (WHO 1983b). IUD use is higher at six months with immediate insertion than with delayed insertion, though expulsion of IUD at six months may also be higher for immediate insertion.
Guidelines and package labelling that argue against postabortal insertions lack a scientific foundation. With immediate postabortal insertions, contraceptive efficacy is high, and PID and perforations are rare. While the risk of spontaneous expulsion of an IUD appears to be greater in this setting than with interval insertions, this potential disadvantage may be outweighed by provision of highly effective contraception with one procedure. The one‐month follow‐up visit (after the next menses) may be especially important for identifying unsuspected complete or partial expulsions. IUD insertion immediately after second‐trimester abortion carries a higher risk of spontaneous expulsion than insertion after first‐trimester abortion.
Implications for research.
Newer data are available on immediate versus delayed insertion. Addition of full reports of three recent studies in this review has shown that expulsion of IUD is more likely after immediate insertion than delayed insertion of IUD. Two ongoing trials are also directly comparing the time of insertion. Those trials should help inform the field in the near future.
Many trials compared different IUDs for immediate postabortal insertion. Hence, these trials cannot address the comparative safety and efficacy of immediate insertion versus insertion at a later time. Also, many of the reports were of suboptimal quality, and communication with researchers was needed for supplementary information. Few reports had a sample size calculation, and several had little power to detect differences. Some IUDs reviewed here are no longer widely used.
What's new
| Date | Event | Description |
|---|---|---|
| 12 July 2014 | New citation required and conclusions have changed | Published report of Bednarek 2011; Hohmann 2012 and Cremer 2011 obtained and included in the analysis. 'Summary of findings' table included |
| 29 January 2014 | New search has been performed | Searches were updated and converted to new reporting format |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 15 April 2008 | Amended | Converted to new review format |
| 3 June 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Carol Manion of FHI 360 assisted with the literature search for the initial review and updates through 2010, and for this update and Clare Dooley for the copy editing.
Appendices
Appendix 1. Previous search strategy
MEDLINE via PubMed
(iud* OR iucd* OR intrauterine devices) AND insert* AND (postabort* OR post‐abort* OR abortion)
CENTRAL
1) post‐abort* OR postabort* OR abort* in Title, Abstract or Keywords AND IUD* OR intrauterine device* in Title, Abstract or Keywords
2) (intrauterine device OR IUD) AND ((delayed OR immediate) AND insertion) in Title, Abstract or Keywords
POPLINE
(iud*/iucd*/intrauterine device*/intrauterine contraceptive device*) & (postabortal/postabortion/post‐abortion/abortion) & insert*
EMBASE
(iud?(3n)insertion? or iucd?(3n)insertion? or intrauterine(w)device?(3n)insertion?) and (postabort? or postpartum or puerperium or abortion) and clinical trial or study
ClinicalTrials.gov
(intrauterine device OR IUD) AND ((delayed OR immediate) AND insertion)
ICTRP
(intrauterine device OR IUD) AND ((delayed OR immediate) AND insertion)
Data and analyses
Comparison 1. Immediate insertion: TCu 220C versus Lippes Loop.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuation due to pregnancy (750 days) | 2 | 2269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.24, 0.75] |
| 2 Discontinuation due to expulsion (750 days) | 2 | 2269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.46, 0.81] |
| 3 Discontinuation due to total medical removals (750 days) | 2 | 2269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.70, 1.08] |
| 4 Discontinuation due to perforation (750 days) | 2 | 2269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.14, 7.00] |
| 5 Discontinuation due to pelvic inflammatory disease (750 days) | 2 | 2269 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.31, 5.11] |
1.3. Analysis.
Comparison 1 Immediate insertion: TCu 220C versus Lippes Loop, Outcome 3 Discontinuation due to total medical removals (750 days).
Comparison 2. Immediate insertion: Lippes Loop versus Copper 7.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuation due to pregnancy (750 days) | 2 | 2257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.71, 1.75] |
| 2 Discontinuation due to expulsion (750 days) | 2 | 2257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.87, 1.44] |
| 3 Discontinuation due to perforation (750 days) | 2 | 2257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.99] |
| 4 Discontinuation due to total medical removals (750 days) | 2 | 2257 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.01, 1.67] |
| 5 Discontinuation due to pelvic inflammatory disease (750 days) | 2 | 2257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.12, 1.43] |
2.1. Analysis.
Comparison 2 Immediate insertion: Lippes Loop versus Copper 7, Outcome 1 Discontinuation due to pregnancy (750 days).
2.2. Analysis.
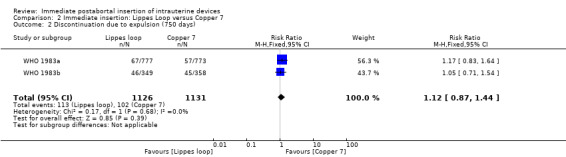
Comparison 2 Immediate insertion: Lippes Loop versus Copper 7, Outcome 2 Discontinuation due to expulsion (750 days).
2.4. Analysis.
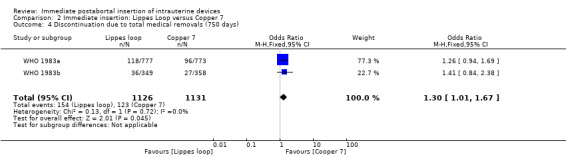
Comparison 2 Immediate insertion: Lippes Loop versus Copper 7, Outcome 4 Discontinuation due to total medical removals (750 days).
Comparison 3. Immediate insertion: TCu 220C versus Copper 7.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuation due to pregnancy (750 days) | 2 | 2274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.23, 0.77] |
| 2 Discontinuation due to expulsion (750 days) | 2 | 2274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.51, 0.91] |
| 3 Discontinuation due to total medical removals (750 days) | 2 | 2274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.87, 1.37] |
| 4 Discontinuation due to perforation (750 days) | 2 | 2274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.04, 5.38] |
| 5 Discontinuation due to pelvic inflammatory disease (750 days) | 2 | 2274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.15, 1.63] |
3.3. Analysis.
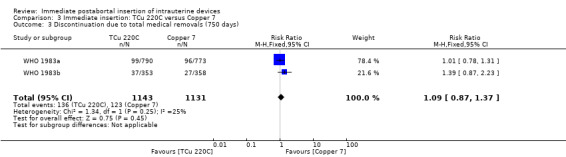
Comparison 3 Immediate insertion: TCu 220C versus Copper 7, Outcome 3 Discontinuation due to total medical removals (750 days).
Comparison 4. Immediate insertion: Nova T versus MLCu 250.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuation due to pregnancy (24 months) | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.73, 49.39] |
| 2 Discontinuation due to expulsion (24 months) | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.55, 2.46] |
4.2. Analysis.
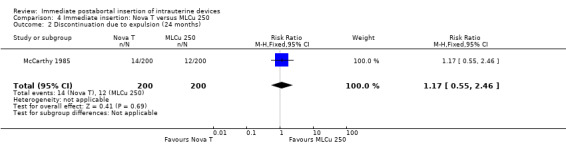
Comparison 4 Immediate insertion: Nova T versus MLCu 250, Outcome 2 Discontinuation due to expulsion (24 months).
Comparison 5. Immediate versus delayed insertion of Copper 7.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuation due to pregnancy at one year | 1 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.19, 82.50] |
| 2 Discontinuation due to expulsion at one year | 1 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.98 [1.61, 89.35] |
| 3 Discontinuation due to pelvic inflammatory disease at one year | 1 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.6 [0.29, 107.33] |
| 4 Discontinuation rates (5‐year) per 100 women due to hormonal reasons after immediate insertion | 1 | Mean Difference (Fixed, 95% CI) | ‐12.0 [‐20.39, ‐3.61] |
5.1. Analysis.
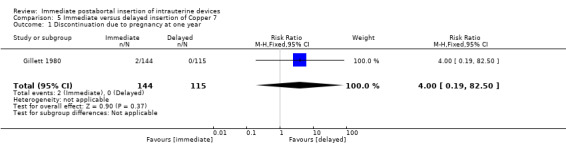
Comparison 5 Immediate versus delayed insertion of Copper 7, Outcome 1 Discontinuation due to pregnancy at one year.
5.3. Analysis.

Comparison 5 Immediate versus delayed insertion of Copper 7, Outcome 3 Discontinuation due to pelvic inflammatory disease at one year.
5.4. Analysis.

Comparison 5 Immediate versus delayed insertion of Copper 7, Outcome 4 Discontinuation rates (5‐year) per 100 women due to hormonal reasons after immediate insertion.
Comparison 6. Immediate insertion: MLCu 250 versus MLCu 375.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuation due to pregnancy (24 months) | 1 | 549 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.22, 2.96] |
| 2 Discontinuation due to expulsion (24 months) | 1 | 549 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.52, 5.93] |
6.1. Analysis.
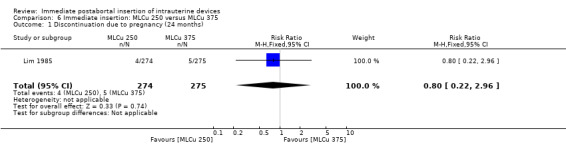
Comparison 6 Immediate insertion: MLCu 250 versus MLCu 375, Outcome 1 Discontinuation due to pregnancy (24 months).
6.2. Analysis.
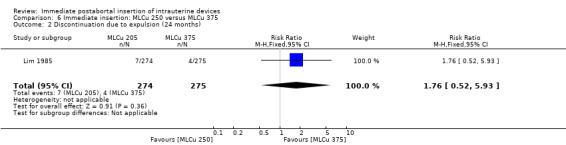
Comparison 6 Immediate insertion: MLCu 250 versus MLCu 375, Outcome 2 Discontinuation due to expulsion (24 months).
Comparison 7. Immediate insertion: Nova T versus Copper T 200.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuations due to pregnancy (36 months) | 1 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.05, 0.94] |
| 2 Discontinuations due to expulsion (36 months) | 1 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.86, 3.12] |
| 3 Discontinuations due to infection (36 months) | 1 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.58, 3.30] |
7.3. Analysis.
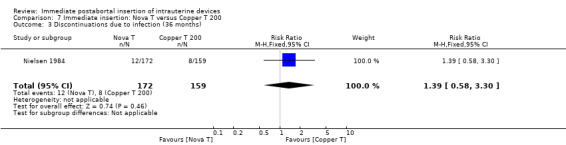
Comparison 7 Immediate insertion: Nova T versus Copper T 200, Outcome 3 Discontinuations due to infection (36 months).
Comparison 8. Nova T versus levonorgestrel IUS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuation rates (5‐year) per 100 women due to pregnancy | 1 | Mean Difference (Fixed, 95% CI) | 8.7 [3.92, 13.48] | |
| 2 Discontinuation rates (5 ‐year) per 100 women due to expulsion after immediate insertion | 1 | Mean Difference (Fixed, 95% CI) | 4.9 [‐5.99, 15.79] | |
| 3 Discontinuation rate (5‐ years) due to bleeding problems | 1 | Mean Difference (Fixed, 95% CI) | 8.3 [‐2.03, 18.63] | |
| 4 Discontinuation rate (5‐years) due to Amenorrhea | 1 | Mean Difference (Fixed, 95% CI) | ‐2.1 [‐5.02, 0.82] |
8.3. Analysis.

Comparison 8 Nova T versus levonorgestrel IUS, Outcome 3 Discontinuation rate (5‐ years) due to bleeding problems.
8.4. Analysis.

Comparison 8 Nova T versus levonorgestrel IUS, Outcome 4 Discontinuation rate (5‐years) due to Amenorrhea.
Comparison 9. Immediate insertion: Lippes Loop (plain) versus Lippes Loop with copper.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuations due to pregnancy (10 years) | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.4 [1.28, 9.04] |
| 2 Discontinuations due to expulsion (10 years) | 1 | 400 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.35 [1.59, 7.06] |
Comparison 10. Immediate insertion: Spring coil (plain) versus spring coil with hydrogel.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuation due to pregnancy per 100 women at 24 months | 1 | 464 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.48, ‐0.12] |
| 2 Discontinuation rate due to expulsion per 100 women at 24 months | 1 | 464 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.46, ‐0.09] |
Comparison 11. Immediate versus delayed insertion (LNG‐IUS or CuT380A IUD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Expulsion by 6 months | 3 | 878 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [1.16, 6.00] |
| 2 Removal by 6 months | 2 | 790 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.99, 4.06] |
| 3 Use at 6 months | 3 | 878 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.24, 1.58] |
| 4 Pregnancy at 6 months | 3 | 878 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.12, 1.14] |
| 5 Upper genital tract infection | 3 | 878 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.33, 3.07] |
11.2. Analysis.
Comparison 11 Immediate versus delayed insertion (LNG‐IUS or CuT380A IUD), Outcome 2 Removal by 6 months.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bednarek 2011.
| Methods | Randomised, blinded (subject, caregiver, investigator), safety/efficacy study. Randomisation in 5:6 ratio | |
| Participants | 575 women, 18 years or older, requesting suction aspiration for spontaneous or induced abortion. Inclusion criteria: intrauterine pregnancy documented with ultrasound with gestational age >= 5 weeks but <= 12 weeks; desiring intrauterine contraception; in general good health. Exclusion criteria: evidence of active cervicitis or PID, PID or STI in past 3 months, history of actinomycosis, unexplained vaginal bleeding, uterine anomaly, leiomyomata, complete molar pregnancy, ectopic pregnancy, AIDS without treatment, prior surgical aspiration during this current pregnancy, use of osmotic dilators or misoprostol during aspiration procedure; allergy to polyethylene, levonorgestrel (for LNG‐containing IUS), or copper (for copper T380A IUD); Wilson's disease (for copper T380A IUD) | |
| Interventions | Insertion of intrauterine device (IUD) immediately after a suction aspiration procedure (within minutes) (n = 258) compared to inserting the IUD 2 to 6 weeks after the procedure (n = 317). Women could choose LNG‐IUS or CuT380A IUD | |
| Outcomes | Primary: expulsion by 6 months Secondary: continuation, adverse events, and satisfaction by 6 months | |
| Notes | This update has included all 575 randomised participants because the final result has been published | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation in 5:6 ratio was computer generated. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque envelopes containing cards with computer ‐ generated assignments were used. Envelopes were opened only after the aspiration procedure had been completed and the investigator had determined that immediate IUD was feasible |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Subject, caregiver, and investigator were blinded to the intervention given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not all randomised participants were included in the final analysis. Participants lost to follow‐up were not included in the analysis of important outcomes |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
Cremer 2011.
| Methods | Open labelled randomised controlled trial. Randomisation was done using computer generated table of random numbers | |
| Participants | 215 women, 16 years or older. Inclusion criteria: intrauterine pregnancy > 14 weeks gestation, desires abortion, desires IUD for contraception, no contraindication for dilation and evacuation abortion Exclusion criteria: uterine anomaly including fibroids if they distort the uterine cavity, acute PID, uterine or cervical neoplasia or unresolved abnormal PAP smear, untreated acute cervicitis or vaginitis, chlamydia or gonorrhoea infection in past 90 days, acute liver disease or liver tumour, woman or partner currently with multiple sexual partners, history of Wilson's disease, hypersensitivity to any component of Copper T IUD |
|
| Interventions | Copper T 380A IUD: Immediate insertion ‐ IUD inserted within 15 minutes after delivery of the placenta immediately following abortion Delayed insertion ‐ IUD inserted at the post‐operative visit 2 to 4 weeks after the abortion | |
| Outcomes | Primary: use at 6 months Secondary: satisfaction, expulsion by 6 months, use of other contraceptives, infection and repeat abortion | |
| Notes | This study was previously listed under ongoing studies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using computer generated table of random numbers |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque sealed envelopes were opened on the day of abortion. Randomisation was not known until final day of procedure if abortion was a two‐day procedure |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | assessment was done by participants and not by providers |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not all randomised participants were included in the final analysis. Participants lost to follow‐up were not included in the analysis of important outcomes |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Not all randomised participants were included in the final analysis |
Gillett 1980.
| Methods | Randomised controlled trial without blinding. Method of randomisation listed only as 'balanced.' Communication with authors indicated a computer‐generated randomisation sequence and allocation concealment by use of sealed envelopes | |
| Participants | 259 women at 3 sites in Canada having vacuum aspiration abortion. The gestational ages were not described | |
| Interventions | Copper 7 inserted immediately versus Copper 7 inserted 3 to 5 weeks after the abortion | |
| Outcomes | Primary outcome measures included pregnancy, expulsion, and removal for bleeding/pain, or other medical reason | |
| Notes | 46 women (31.9%) and 27 (42.9) allocated to immediate and delayed insertion respectively failed to return for IUD insertion. The report provided no a priori hypothesis or sample size and power calculation. Denominators for rates were woman‐days of use | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Communication with authors indicated a computer‐generated randomisation sequence. allocation concealment by use of sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment by use of sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 46 women (31.9%) and 27 (42.9) allocated to immediate and delayed insertion respectively were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to study protocol, so it is unclear |
| Other bias | Unclear risk | The report provided no a priori hypothesis or sample size and power calculation. Denominators for rates were woman‐days of use |
Hohmann 2012.
| Methods | Randomised, open label, safety study | |
| Participants | 88 women, 18 years or older were randomised. Inclusion criteria: gestational age 15 weeks to 23 weeks 6 days, already consented to an induced abortion, desires to use the LNG‐IUS for contraception for 12 months or more; lives in specified counties of Pennsylvania Exclusion criteria: allergy to either polyethylene or levonorgestrel, urgent need for abortion (active bleeding or infection), exposure to or treatment for gonorrhoea or Chlamydia in past 90 days, PID in past year, leiomyomata > 3 cm diameter, uterine anomaly (other than repaired septate uterus) Post‐enrollment pre‐randomisation exclusion criteria (assessed at D&E completion): uterine perforation; haemorrhage as defined by (1) need for transfusion, (2) estimated blood loss > 500 cc, (3) intrauterine placement of a Foley catheter, or (4) use of >= 3 doses of uterotonic medications; infection at time of D&E, including fever (temperature >= 38°C) or pus at the cervical os; subject no longer desires a LNG‐IUS |
|
| Interventions | Insertion of LNG‐IUS immediately following dilation & evacuation compared to delayed insertion (3 to 6 weeks post‐abortion) | |
| Outcomes | Primary: use at 6 months Secondary: uptake, expulsion, continuation, acceptability, utility of ultrasound in predicting expulsion | |
| Notes | This study was completed and data in this update was obtained from the published study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Subjects were stratified into two strata by parity (parous or nulliparous) with random block sizes of 2,4 and 6 using computer generated sequence |
| Allocation concealment (selection bias) | Low risk | Eligible subjects were randomised by opening the next sequentially numbered sealed opaque envelope in the operating room |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A statistician not affiliated to the study prepared the envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study staff for these evaluations were blinded to subjects' randomisation assignments |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study staff for these evaluations were blinded to subjects' randomisation assignments |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not all randomised participants were included in the final analysis. Participants lost to follow‐up were not included in the analysis of important outcomes Number of participants lost to follow‐up was high. 17 participants were lost to follow‐up in each group. |
| Selective reporting (reporting bias) | Unclear risk | unclear |
| Other bias | Unclear risk | unclear |
Lim 1985.
| Methods | Randomised controlled trial without blinding. Communication with authors indicated use of computer‐generated randomisation sequence | |
| Participants | 549 women aged 18 to 40 years in Singapore who were having induced abortions | |
| Interventions | Immediate insertion of Multiload 250 or Multiload 375 | |
| Outcomes | Principal outcome measures included pregnancy, expulsions, removal for bleeding/pain, and other medical reasons | |
| Notes | The report had no a priori hypothesis or sample size and power calculation. Denominators for rates were woman‐months of use | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Communication with authors indicated use of computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Pre‐sealed envelopes used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No blinding |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | The report had no a priori hypothesis or sample size and power calculation. Denominators for rates were woman‐months of use |
McCarthy 1985.
| Methods | Randomised controlled trial at one hospital among women having induced abortion. Report does not describe the method of randomisation or allocation concealment. Communication with authors indicated computer‐generated randomisation sequence | |
| Participants | 400 women in Singapore between the ages of 16 and 40 years. Demographic information was not provided. The report does not describe the abortion procedures | |
| Interventions | Immediate insertion of Nova T or Multiload Cu250 | |
| Outcomes | Principal outcomes included pregnancy, expulsion, pain/bleeding, and other medical reasons. Only the first two outcomes are included because of their objective nature | |
| Notes | The report did not contain an a priori hypothesis or sample size and power calculation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Communication with authors indicated computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes according to communication with authors |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | The report did not contain an a priori hypothesis or sample size and power calculation |
Nielsen 1984.
| Methods | Randomised controlled trial without blinding among women after first‐trimester induced abortion. Report does not describe method of randomisation or allocation concealment | |
| Participants | 331 women in Denmark and Finland. More than 96% of participants had abortions at <= 12 weeks' gestation. Report did not provide demographic information about participants | |
| Interventions | Immediate insertion of Nova T or Copper T 200 | |
| Outcomes | Principal outcomes included pregnancy; expulsion; and medical removals for bleeding and pain, infection, and other | |
| Notes | Report provided no a priori hypothesis or sample size and power calculations. Denominators for rates were woman‐months of use. Report provided both gross and net continuation rates to 36 months. This report is a subgroup analysis of a larger trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report does not describe method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Report does not describe method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
Pakarinen 2003.
| Methods | Randomised controlled international trial without blinding among women after induced abortion at less than 12 weeks' gestation; computer‐generated randomisation. Randomisation was 2:1 (LNG‐IUS:Nova T) | |
| Participants | 438 Women in Finland, Sweden, Denmark, Norway, and Hungary. Participants were 18 to 38 years old, healthy and had to have had at least one prior pregnancy. Exclusion criteria included history of ectopic pregnancy, current breastfeeding, recent injectable contraception, anaemia, or acute cervicitis or vaginitis | |
| Interventions | Immediate insertion of Nova T copper IUD versus Mirena levonorgestrel intrauterine system | |
| Outcomes | Principal outcomes included pregnancy, expulsion, bleeding problems, pain, salpingitis, amenorrhoea, hormonal problems, and overall discontinuation | |
| Notes | This report was from a large trial (Luukkainen 1987). Some of the same Finnish participants are included in Pakarinen 2003. A subgroup analysis appears in Suvisaari 1996. Sample size calculation provided for overall trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised controlled international trial |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No Blinding |
| Blinding (performance bias and detection bias) All outcomes | High risk | No Blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No Blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
Randic 1983.
| Methods | Randomised controlled trial with blinding. Computer‐generated random number sequence | |
| Participants | 464 women in Rijeka, Yugoslavia, immediately after induced first‐trimester abortion by dilation and curettage | |
| Interventions | Immediate insertion of Hydron‐coated Spring Coil versus Spring Coil. Hydron is a biocompatible hydrogel intended to decrease adverse endometrial response and improve tolerance of IUDs | |
| Outcomes | Principal outcomes included pregnancy, expulsion, removals for bleeding/pain, and continuation | |
| Notes | Raw data not provided, only rates | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | Labels in sealed, opaque, sequentially‐numbered envelopes opened at the time of insertion |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The type of IUD inserted was not known to the investigators, only the patients order number was known by the investigators so that they are unaware of what participants were assigned to. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The type of IUD inserted was not known to the investigators, only the patients order number was known by the investigators so that they are unaware of what participants were assigned to. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The type of IUD inserted was not known to the investigators, only the patients order number was known by the investigators so that they are unaware of what participants were assigned to. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
Randic 1991.
| Methods | Randomised controlled trial with blinding. Computer‐generated random number sequence | |
| Participants | 400 women in Rijeka, Yugoslavia, immediately after medical abortion of first‐trimester pregnancy | |
| Interventions | Immediate insertion of Lippes Loop D or Lippes Loop D with addition of copper sleeves containing 200 square millimetres of copper | |
| Outcomes | Principal outcomes included pregnancy, expulsion/displacement, and removals for bleeding/pain or other medical reasons | |
| Notes | The report provided no a priori hypothesis or sample size calculation, although the latter is moot given the significant differences found Denominators for rates were woman‐months of use. Details of allocation concealment missing from report were obtained from investigator | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | 'Allocation card' opened prior to IUD insertion |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | .The type of IUD inserted was not known to the investigators, only the patients order number was known by the investigators so that they are unaware of what participants were assigned to. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The type of IUD inserted was not known to the investigators, only the patients order number was known by the investigators so that they are unaware of what participants were assigned to. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The type of IUD inserted was not known to the investigators, only the patients order number was known by the investigators so that they are unaware of what participants were assigned to. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants lost to follow up were accounted for. |
| Selective reporting (reporting bias) | Unclear risk | Not detectable |
| Other bias | Unclear risk | The report provided no a priori hypothesis or sample size calculation |
WHO 1983a.
| Methods | Randomised controlled trial without blinding conducted at 8 centres. Randomisation performed by computer‐generated table of numbers and random permuted blocks. Twelve participants who had problems within 48 hr of insertion were excluded from analysis | |
| Participants | 2340 women who had an induced abortion at the participating centres. Study sites included Cuba, Yugoslavia, United Kingdom, Zambia, India, Korea, Singapore, and Hungary. Suction or sharp curettage was used for most of the abortions; prostaglandin use was rare. About 96% of the abortions took place at <=12 weeks' gestation | |
| Interventions | One of three different devices was inserted immediately after the abortion: T Cu 220C, Lippes Loop D, or Copper 7. Prophylactic antibiotics were not used | |
| Outcomes | Pregnancy (includes ectopic pregnancies in this review), uterine perforation, expulsion, total medical removals (further broken down into pelvic inflammatory disease, pain alone, bleeding alone, pain/bleeding, and other) | |
| Notes | Report provided no a priori hypothesis or sample size and power calculation. Non‐medical removals (such as desire for pregnancy) and other discontinuations are not included in this review. No subjects who had an expulsion or removal of the device were readmitted to the study, thus analysis was based on first‐segment event rates. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation performed by computer‐generated table of numbers and random permuted blocks |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, sequentially‐numbered envelopes with a method indicator card |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Low risk | Not detected |
| Other bias | Unclear risk | No blinding |
WHO 1983b.
| Methods | Randomised controlled trial without blinding. Randomisation by computer‐generated random number table. One participant who had a problem within 48 hr of insertion was excluded from analysis | |
| Participants | 1060 women at 6 hospitals (in Egypt, United Kingdom, Zambia, Philippines, Chile, and Singapore) who were admitted for care of spontaneous abortions. Nearly all had sharp curettage for completion; suction curettage was rare. From 18% to 25% of the participants were 13‐20 weeks pregnant at the time of spontaneous abortion | |
| Interventions | Randomly assigned to one of three different IUDs: T Cu 220C, Lippes Loop D, or Copper 7 (all immediate insertion). Prophylactic antibiotics were not used | |
| Outcomes | Pregnancy (none of which was ectopic), uterine perforation, expulsion, and total medical removals (further broken down as pelvic inflammatory disease, pain alone, bleeding alone, pain/bleeding, and other) | |
| Notes | The report provides no a priori hypothesis or sample size and power calculation. Given the high proportion of abortions after 12 weeks and that legal abortion is unavailable or inaccessible in several of these countries, many of these "spontaneous" abortions were likely induced, possibly by unsafe methods. Thus, the risk of infection may be increased in this population. Non‐medical and other reasons for discontinuation (such a desire for pregnancy) are not included in this review. No subjects who had an expulsion or removal of the device were readmitted to the study, thus analysis was based on first‐segment event rates. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer‐generated random number table |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes; commuictions with authors confirmed that envelopes were opaque and sequentially‐numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Low risk | Not detected |
| Other bias | Unclear risk | Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chowdhury 1979 | Violation of informed consent: sham IUD insertion arm |
| Goldsmith 1972 | Violation of informed consent: sham IUD insertion arm |
| NCT00737178 | Immediate was defined as one week |
| Querido 1985 | Although reported to be randomised assignment, methods reveal alternate allocation. Allocation concealment not possible. |
Characteristics of ongoing studies [ordered by study ID]
[ISRCTN: 19506752].
| Trial name or title | immediate vs delayed insertion of intrauterine contraception after second trimester abortion: study protocol for a randomised controlled trial |
| Methods | RCT |
| Participants | 716 consenting women choosing to use intrauterine contraception after abortion for a pregnancy for a pregnancy of 12 to 24 weeks will be randomised |
| Interventions | Levonorgestrel‐releasing IUC or CuT380‐IUC. insertion timing groups either immediately (experimental intervention) or four week (recommended care) post abortion |
| Outcomes | primary outcome: pregnancy rate at one year; secondary outcomes:costs and cost effectiveness, cumulative annual pregnancy rate, device insertion rate, loss to follow‐up, continuation of methods, infection, perforation, expulsion |
| Starting date | May 2010 |
| Contact information | wvnorman@interchange.ubc.ca; Contraception and Abortion Research Team, Women's Health Research Institute, Vancouver, British Columbia V6H, 1g3, Canada |
| Notes | Expected to complete in late 2011 and data on one year analysis will be available in 2014 |
NCT00877344.
| Trial name or title | Better contraceptive choices: immediate or delayed insertion of IUD after second trimester abortion |
| Methods | RCT |
| Participants | 1372 women, 18 years or older. Inclusion criteria: completed informed consent for abortion at gestation 12 weeks to 23 weeks 6 days, choosing an IUD for contraception postabortion, residents of British Columbia. Exclusion criteria: contraindications to IUD use (current untreated PID, chlamydia or gonorrhoea; uterine cavity anomalies including fibroids > 5 cm, excluding repaired uterine septum; hypersensitivity to copper or polyethylene or Wilson's Disease; intend to move from British Columbia or to conceive in 1 year. Post randomisation exclusion: uterine perforation at the time of abortion, bleeding > 500 cc during abortion or use of non‐routine uterotonic agents to manage haemorrhage during abortion or prior to discharge |
| Interventions | Immediate or delayed timing of insertion for a copper T380A IUD after an abortion. Immediate insertion to occur during visit for abortion immediately after abortion is complete; delayed insertions scheduled for 2 to 4 weeks after abortion |
| Outcomes | Primary: pregnancy at 1 year |
| Starting date | Jun 2009; estimated completion of data collection for primary outcome, Dec 2011 |
| Contact information | Wendy Norman, MD; 604‐918‐1134, wvnorman@interchange.ubc.ca |
| Notes |
Contributions of authors
For the 2014 update, authorship differed from earlier versions. Olabisi Oduwole (OO) and Emmanuel Effa (EE) reviewed literature searches for this update and selected studies for inclusion. Babasola Okusanya (BO) and OO extracted data and analysed data. BO drafted the background of the new update and all authors read and agreed to the final draft.
Sources of support
Internal sources
No sources of support supplied
External sources
-
U.S. Agency for International Development, USA.
Support for conducting the review and updates at FHI 360 (through 2010)
-
National Institute of Child Health and Human Development, USA.
Support for conducting the review and updates at FHI 360 (through 2010)
Declarations of interest
None known for this update.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Bednarek 2011 {published data only}
- Bednarek PA, Creinin MD, Reeves MF, Cwiak CA, Espey E, Jensen JT, Post‐Aspiration IUD Randomization (PAIR) Study Trial Group. Immediate versus delayed IUD insertion after uterine aspiration. The New England Journal of Medicine 2011;364(23):2208‐17. [NCT00562276] [DOI] [PubMed] [Google Scholar]
Cremer 2011 {published data only}
- Cremer M, Bullard KA, Mosley RM, Weiselberg C, Molaei M, Lerner V, et al. Immediate vs. delayed insertion of Copper T 380A IUD after termination of pregnancy over 12‐weeks gestation. Contraception 2011;83:522‐7. [DOI: 10.1016/j.contraception.2010.10.005] [DOI] [PubMed] [Google Scholar]
Gillett 1980 {published and unpublished data}
- Gillett PG, Lee NH, Yuzpe AA, Cerskus I. A comparison of the efficacy and acceptability of the Copper‐7 intrauterine device following immediate or delayed insertion after first‐trimester therapeutic abortion. Fertility and Sterility 1980;34(2):121‐4. [DOI] [PubMed] [Google Scholar]
Hohmann 2012 {published data only}
- Hohmann HL, Reeves MF, Chen BA, Perriera L, Hayes JL, Creinin MD. Immediate versus delayed insertion of the levonorgestrel‐releasing intrauterine device following dilation and evacuation: a randomized controlled trial. Contraception 2012;85(3):240‐5. [DOI: 10.1016/j.contraception.2011.08.002; NCT00475228] [DOI] [PubMed] [Google Scholar]
Lim 1985 {published and unpublished data}
- Lim LS, McCarthy T, Yong YM, Ratnam SS. Post‐abortion insertion of MLCu 250 and MLCu 375 ‐ a comparative trial. Contraception 1985;31(5):471‐7. [DOI] [PubMed] [Google Scholar]
McCarthy 1985 {published and unpublished data}
- McCarthy T, Ramachandran L, Huang HS, Ratnam S. Postabortion insertion of the Nova T and MLCu250: preliminary results of a comparative study. Advances in Contraception 1985;1(2):161‐5. [DOI] [PubMed] [Google Scholar]
Nielsen 1984 {published data only}
- Nielsen NC, Nygren K‐G, Allonen H. Three years of experience after post‐abortal insertion of Nova‐T and Copper‐T‐200. Acta Obstetricia et Gynecologica Scandinavica 1984;63(3):261‐4. [DOI] [PubMed] [Google Scholar]
Pakarinen 2003 {published and unpublished data}
- Luukkainen T, Allonen H, Haukkamaa M, Holma P, Pyorala T, Terho J, et al. Effective contraception with the levonorgestrel‐releasing intrauterine device: 12‐month report of a European multicenter study. Contraception 1987;36(2):169‐79. [DOI] [PubMed] [Google Scholar]
- Pakarinen P, Toivonen J, Luukkainen T. Randomized comparison of levonorgestrel‐ and copper‐releasing intrauterine systems immediately after abortion, with 5 years' follow‐up. Contraception 2003;68(1):31‐4. [DOI] [PubMed] [Google Scholar]
- Suvisaari J, Lahteenmaki P. Detailed analysis of menstrual bleeding patterns after postmenstrual and postabortal insertion of a copper IUD or a levonorgestrel‐releasing intrauterine system. Contraception 1996;54(4):201‐8. [DOI] [PubMed] [Google Scholar]
Randic 1983 {published and unpublished data}
- Randic L, Balogh SA. The effect of hydrogel on the reduction of bleeding associated with IUD use: a comparative study of the plain and Hydron‐coated Spring Coil. Contraceptive Delivery Systems 1983;4(4):301‐10. [PubMed] [Google Scholar]
Randic 1991 {published and unpublished data}
- Randic L, Haller H, Perovic M, Farr G. The effect of adding copper onto Lippes Loop IUDs: results from a ten‐year study in Yugoslavia. Contraception 1991;43(3):229‐39. [DOI] [PubMed] [Google Scholar]
WHO 1983a {published and unpublished data}
- World Health Organization Task Force on Intrauterine Devices for Fertility Regulation. IUD insertion following termination of pregnancy: a clinical trial of the TCu 220C, Lippes Loop D, and Copper 7. Studies in Family Planning 1983;14(4):99‐108. [PubMed] [Google Scholar]
WHO 1983b {published data only}
- World Health Organization Task Force on Intrauterine Devices for Fertility Regulation. IUD insertion following spontaneous abortion: a clinical trial of the TCu 220C, Lippes Loop D, and Copper 7. Studies in Family Planning 1983;14(4):109‐14. [PubMed] [Google Scholar]
References to studies excluded from this review
Chowdhury 1979 {published data only}
- Chowdhury NNR, Mandal GS, Das M. Comparative study of Lippes Loop and CuT inserted in immediate post‐abortal period. Journal of Obstetrics and Gynaecology of India 1979;29(2):239‐44. [PubMed] [Google Scholar]
Goldsmith 1972 {published data only}
- Goldsmith A, Goldberg R, Eyzaguirre H, Lizana L. Immediate postabortal intrauterine contraceptive device insertion: a double‐blind study. American Journal of Obstetrics and Gynecology 1972;112(7):957‐62. [DOI] [PubMed] [Google Scholar]
NCT00737178 {published data only}
- Davis A. Immediate versus delayed insertion of the copper intrauterine device (IUD) after medication abortion. www.clinicaltrials.gov/ct2/show/NCT00737178 (accessed 03 Feb 2010).
Querido 1985 {published data only}
- Querido L, Ketting E, Haspels AA. IUD insertion following induced abortion. Contraception 1985;31(6):603‐10. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
[ISRCTN: 19506752] {published data only}
- Norman WV, Kaczorowski J, Soon JA, Brant R, Bryan S, Trouton KJ, et al. Immediate vs. delayed insertion of intrauterine contraception after second trimester abortion: study protocol for a randomized controlled trial. Trials 2011;12:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00877344 {published data only}
- Norman WV. Better contraceptive choices: immediate or delayed insertion of IUD after second trimester abortion. www.clinicaltrials.gov/ct2/show/NCT00877344 (accessed 03 Feb 2010).
Additional references
Burkman 2007
- Burkman RT. Contraception & Family Planning. In: Decherney AH, Nathan L, Goodwin TM, Laufer N editor(s). Current Diagnosis & Treatment. Obstetrics & Gynecology. 10th Edition. New York, USA: McGraw‐Hill Companies, 2007:579‐597. [Google Scholar]
Drey 2009
- Drey EA, Reeves MF, Ogawa DD, Sokoloff A, Darney PD, Steinauer JE. Insertion of intrauterine contraceptives immediately following first‐ and second‐trimester abortions. Contraception 2009;79(5):397‐402. [DOI] [PubMed] [Google Scholar]
Farley 1992
- Farley TMM, Rowe PJ, Meirik O, Rosenberg MJ, Chen J‐H. IUDs and pelvic inflammatory disease. Lancet 1992;340(8813):248‐9. [DOI] [PubMed] [Google Scholar]
Goodman 2008
- Goodman S, Hendlish SK, Reeves MF, Foster‐Rosales A. Impact of immediate postabortal insertion of intrauterine contraception on repeat abortion. Contraception 2008;78(2):143‐8. [DOI] [PubMed] [Google Scholar]
Grimes 2008
- Grimes DA, Mishell DR. Intrauterine contraception as an alternative to interval tubal sterilization. Contraception 2008;77(1):6‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration. Available from www.cochrane‐handbook.org [updated March 2011]; Vol. Version 5.1.0.
Mahomed 1997
- Mahomed K, Healy J, Tandom S. Family planning counselling ‐ a priority for post abortion care. Central African Journal of Medicine 1997;43(7):205‐7. [PubMed] [Google Scholar]
McLaurin 1993
- McLaurin KE, Senanayake P, Toubia N, Ladipo OA. Post‐abortion family planning: reversing a legacy of neglect. Lancet 1993;342(8879):1099‐100. [DOI] [PubMed] [Google Scholar]
McNicholas 2012
- McNicholas C, Hotchkiss T, Madden T, Zhao Q, Allsworth J, Peipert JF. Immediate postabortion intrauterine device insertion: continuation and satisfaction. Womens Health issues 2012;22(4):e365‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okusanya 2013
PIP 1995
- Population Information Program. IUDs ‐ an update. Population Reports 1995;Series B(6):1‐35. [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Saav 2012
- Saav I, Stephansson O, Gemzell‐Danielsson K. Early versus delayed insertion of intrauterine contraception after medical abortion ‐ a randomized controlled trial. PLoS ONE 2012;7(11):e48948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinei 1990
- Sinei SK, Schulz KF, Lamptey PR, Grimes DA, Mati JK, Rosenthal SM, et al. Preventing IUCD‐related pelvic infection: the efficacy of prophylactic doxycycline at insertion. British Journal of Obstetrics and Gynaecology 1990;97(5):412‐9. [DOI] [PubMed] [Google Scholar]
Sivin 1981
- Sivin I, Tatum HJ. Four years of experience with the TCu 380A intrauterine contraceptive device. Fertility and Sterility 1981;36(2):159‐63. [PubMed] [Google Scholar]
Souza 2013
- Souza JP, Gülmezoglu AM, Vogel J, Carroli G, Lumbiganon P, Qureshi Z, et al. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross‐sectional study. Lancet 2013;381(9879):1747‐55. [DOI] [PubMed] [Google Scholar]
Stanback 1997
- Stanback J, Thompson A, Hardee K, Janowitz B. Menstruation requirements: a significant barrier to contraceptive access in developing countries. Studies in Family Planning 1997;28(3):245‐50. [PubMed] [Google Scholar]
Stanek 2009
- Stanek AM, Bednarek PH, Nichols MD, Jensen JT, Edelman AB. Barriers associated with the failure to return for intrauterine device insertion following first‐trimester abortion. Contraception 2009;79(3):216‐20. [DOI] [PubMed] [Google Scholar]
Uzelac 2007
- Uzelac PS, Garmel SH. Early pregnancy risks. In: Decherney AH, Nathan L, Goodwin TM, Laufer N editor(s). Current Diagnosis & Treatment. Obstetrics and Gynecology. 10th Edition. New York, USA: The McGraw‐Hill Companies, 2007:259‐272. [Google Scholar]
Walsh 1998
- Walsh T, Grimes D, Frezieres R, Nelson A, Bernstein L, Coulson A, et al. Randomised controlled trial of prophylactic antibiotics before insertion of intrauterine devices. Lancet 1998;351(9108):1005‐8. [DOI] [PubMed] [Google Scholar]
WHO 1987
- World Health Organization. Mechanism of action, safety and efficacy of intrauterine devices. Technical Report Series 753. Geneva: World Health Organization, 1987. [PubMed] [Google Scholar]
WHO 1994
- World Health Organization. A randomized multicentre trial of the Multiload 375 and TCu380A IUDs in parous women: three‐year results. Contraception 1994;49(6):543‐9. [PubMed] [Google Scholar]
Wolf 1994
- Wolf M, Benson J. Meeting women's needs for post‐abortion family planning. Report of a Bellagio Technical Working Group. International Journal of Gynaecology and Obstetrics 1994;45(Suppl):S1‐S23. [PubMed] [Google Scholar]
References to other published versions of this review
Grimes 2010
- Grimes DA, Lopez LM, Schulz KF, Stanwood NL. Immediate postabortal insertion of intrauterine devices. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD001777.pub3] [DOI] [PubMed] [Google Scholar]


