Abstract
Background
Asthma is the most common chronic childhood illness and is a leading cause for paediatric admission to hospital. Asthma management for children results in substantial costs. There is evidence to suggest that hospital admissions could be reduced with effective education for parents and children about asthma and its management.
Objectives
To conduct a systematic review of the literature and update the previous review as to whether asthma education leads to improved health outcomes in children who have attended the emergency room for asthma.
Search methods
We searched the Cochrane Airways Group Trials Register, including the MEDLINE, EMBASE and CINAHL databases, and reference lists of trials and review articles (last search May 2008).
Selection criteria
We included randomised controlled trials of asthma education for children who had attended the emergency department for asthma, with or without hospitalisation, within the previous 12 months.
Data collection and analysis
Two authors independently assessed trial quality and extracted data. We contacted study authors for additional information. We pooled dichotomous data with a fixed‐effect risk ratio. We used a random‐effects risk ratio for sensitivity analysis of heterogenous data.
Main results
A total of 38 studies involving 7843 children were included. Following educational intervention delivered to children, their parents or both, there was a significantly reduced risk of subsequent emergency department visits (RR 0.73, 95% CI 0.65 to 0.81, N = 3008) and hospital admissions (RR 0.79, 95% CI 0.69 to 0.92, N = 4019) compared with control. There were also fewer unscheduled doctor visits (RR 0.68, 95% CI 0.57 to 0.81, N = 1009). Very few data were available for other outcomes (FEV1, PEF, rescue medication use, quality of life or symptoms) and there was no statistically significant difference between education and control.
Authors' conclusions
Asthma education aimed at children and their carers who present to the emergency department for acute exacerbations can result in lower risk of future emergency department presentation and hospital admission. There remains uncertainty as to the long‐term effect of education on other markers of asthma morbidity such as quality of life, symptoms and lung function. It remains unclear as to what type, duration and intensity of educational packages are the most effective in reducing acute care utilisation.
Plain language summary
What are the effects of educational interventions delivered to children and/or their families, who have experienced an emergency department visit with their asthma within the previous 12 months?
Asthma care for children in our society is common and costly. There is now evidence that educational intervention for children who have attended the emergency department for asthma lowers the risk of the need for future emergency department visits and hospital admissions. This review looked at studies which compared usual care for asthma to more intensive educational programmes and the results showed a statistically significant reduction in the treatment groups needing subsequent emergency department visits or hospital admissions. We were not able to determine the most effective type, duration or intensity of education that should be offered to children to offer the best asthma outcomes.
Background
Throughout many western countries, asthma now ranks as the most common chronic disease of childhood (AIHW 2005). In children, asthma is a frequent cause of visits to hospital emergency departments and admissions to hospital. There is epidemiological evidence to suggest that the prevalence of asthma and hospital admission rates for asthma in children have increased over the past two decades (Lukacs 2002). The direct and indirect costs to the community due to asthma are substantial and the largest portion of the cost for asthma health care is due to hospitalisations (Castro 2003; McPherson 2001). Hospital admissions are also a strong marker of asthma severity, increased risk of readmission and death (Martin 1995; Mitchell 1994). However, there is evidence to suggest that many hospital admissions could be prevented if children and their parents were given and used an individualised asthma management plan, had greater general knowledge of asthma, complied with their preventive treatment, commenced appropriate medication early during an asthma attack and sought local medical assistance early if their condition was not improving (Ordonez 1998).
There is a widespread view that education is an essential component of asthma therapy and should be offered to all patients (CMAJ 2005; SIGN 2003). Educational interventions may be of particular benefit in patients who have a history of emergency department visits as these patients are likely to have severe asthma and poor asthma management skills, representing an appropriate group to target for asthma education (Gibson 2002b). Although educational programmes for children with asthma have been in use for decades, many hospitals do not have a routine approach for the education of children and their families about appropriate asthma management (McPherson 2001). One reason for this could be the lack of a systematic evaluation of the evidence base in this area, since the results of single studies have not consistently demonstrated reduced asthma morbidity or hospital re‐attendances following education.
Wolf 2002 looked at various self‐management programmes in children with chronic asthma. The primary outcome measures were lung function, days absent from school, self‐efficacy and emergency department visits. With self‐management educational programs there was a moderate improvement in airflow and self‐efficacy and modest reduction in school absenteeism, days of restricted activities, emergency department visits and nights disturbed by asthma. The authors concluded that self‐management education directed to the prevention and management of attacks should be incorporated into routine asthma care.
Although an earlier meta‐analysis showed that asthma education was not effective in reducing morbidity due to asthma, it was limited by low statistical power and heterogeneity of outcome measurement (Bernard‐Bonnin 1995). Other work in adults suggests that limited asthma education can reduce emergency room visits (Gibson 2002b), and that education delivered following recent emergency department presentation can reduce subsequent hospital admission (Tapp 2007). These findings have yet to be replicated in the paediatric population. One can hypothesise that during an emergency room visit for asthma related symptoms there is greater potential for behaviour change and/or increased receptiveness of the children and their parents to asthma education.
This is an update of a previous review (Haby 2001), which did not find firm evidence supporting the use of asthma educational interventions in children who have attended the emergency department for asthma. There is still intense interest in this field as new studies have been conducted in continued attempts to improve health outcomes for children with asthma and to assess cost effectiveness of educational programmes.
Objectives
To conduct a systematic review of controlled trials to identify whether asthma education leads to improved health outcomes in children who have attended the emergency department for asthma (with or without hospitalisation). A secondary aim is to identify the characteristics of the asthma education programmes that had the greatest positive effect on health outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs). Quasi‐randomised controlled trials (e.g. participants allocated by day of week or hospital number) were eligible.
Types of participants
Children (0 to 18 years of age) who have attended the emergency room for asthma, as defined by doctor's diagnosis or objective criteria for asthma symptoms and severity, within the previous 12 months.
Types of interventions
Any educational intervention targeted at children, their parents or both, individually or as a group. The educational intervention may take place in the emergency room, the hospital, at home or in the community. The intervention could involve a nurse, a pharmacist, educator or health or medical practitioner associated with the hospital or referred to by the hospital. The intervention may include information administered in a range of formats, counselling, the use of home peak flow or symptom monitoring or a written action plan. A change in therapy with appropriate education will also be considered.
We excluded studies where the primary intervention was environmental remediation alone (i.e. where educational intervention was absent, or was provided in conjunction with significant environmental changes in the home). Studies which delivered education to families on environmental triggers such as tobacco smoke, house dust mite antigen or mould were eligible for inclusion provided that the focus of the intervention remained effecting behavioural change.
The main comparison for this review was:
Education of any type versus control.
The control group could be usual care, waiting list or lower intensity education.
Types of outcome measures
Primary outcomes
The primary outcome assessed was subsequent emergency department visits.
Secondary outcomes
Hospital admissions for asthma.
Duration of hospital admissions.
Unscheduled health care professional visits (GP/Paediatrician/Asthma Nurse).
Use of oral steroids.
Use of inhaler medications.
Symptom frequency and severity.
Lung function: FEV1, PEFR.
Quality of life, functional health status.
Days home sick (lost from school, childcare).
Cost.
Duration of symptoms.
Withdrawals from intervention or usual care.
We opted to include hospital admission and unscheduled doctor visits as key secondary outcomes, and performed subgroup analysis on these endpoints in the review.
Search methods for identification of studies
Electronic searches
Trials were identified using the Cochrane Airways Group Trials Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see the Airways Group Module for further details). All records in the Trials Register coded as 'asthma' were searched using the following terms:
(educat* or self‐manag* or "self manag*" or self‐car* or "self car*" or train* or instruct* or "patient cent*" or patient‐cent* or patient‐focus* or "patient focus*") and (child* or paediat* or pediat* or adolesc* or infan* or toddler* or bab* or young* or preschool* or "pre school*" or pre‐school* or newborn* or "new born*" or new‐born* or neo‐nat* or neonat*)
The most recent search was carried out in May 2008.
Searching other resources
We also searched the reference lists of all available primary studies and review articles for additional studies. We contacted authors of included studies to identify other published and unpublished studies. In addition, we made personal contact with colleagues, collaborators and other trialists working in the field of asthma to identify potentially relevant studies.
Data collection and analysis
Selection of studies
MB and TL coded the studies identified by the above search strategy into three categories based on the title, abstract and key words (see below).
Include: definitely a RCT, subjects 0 to 18 years and recruited following emergency room attendance and intervention is asthma education.
Possible: appears to fit inclusion criteria but need full methods to verify.
Exclude: definitely not a RCT, subjects not 0 to 18 years or not recruited following emergency room attendance, or intervention is not asthma education.
Two independent review authors (MB and TL) retrieved full text copies for all studies in categories 1 and 2 and assessed these against the review eligibility criteria. We calculated a Kappa statistic to measure the amount of agreement between the authors in their initial selection of studies. Disagreement regarding the inclusion of studies was settled by a third author (MM) through adjudication.
Data extraction and management
MB and TL extracted data from each study. They identified and extracted characteristics of the included studies (study design and eligibility criteria, baseline severity of asthma and demographic details of study participants, type of educational intervention and control group, study outcomes), and also numerical results for eligible study outcomes. Differences in data extracted by the authors were discussed and MM adjudicated where necessary. TL entered data into the Cochrane Collaboration software (Review Manager) (RevMan 2008) with random checks on accuracy by MB.
Assessment of risk of bias in included studies
MB and TL independently assessed the design of included studies. We assessed the risk of bias for each study according to concealment of allocation and completeness of follow up (see Appendix 1). Blinding of participants and investigators would not be possible for usual care controlled trials; we are uncertain as to the impact of open label trials on the primary outcome of our review. We tabulated our judgements of the risk of bias for each study.
Dealing with missing data
We contacted authors of included studies where we were unable to extract data from clinical trial reports.
Assessment of heterogeneity
We assessed the degree of statistical variation in the primary outcome with the I2 statistic (Higgins 2003). We explored possible reasons for this statistical variation when this level exceeded 50%.
Data synthesis
For continuous outcomes, we used the weighted mean difference (WMD) or standardised mean difference (SMD) to estimate pooled effect sizes, with 95% confidence intervals (CI). For dichotomous outcomes, we used the risk ratio (RR) with 95% CIs.
For emergency department attendance and hospital admission we restricted the analysis to binary data on patients with one or more attendances or admissions, since the means and SDs collected showed evidence of skew (see Table 9). Where the binary data were not available or could not be extracted from information presented, we contacted trialists for the relevant information.
1. ED visits and hospital admissions (continuous data).
| Outcome | Study ID | Units | When measured | Intervention | Control | Comments |
| ED visits | Alexander 1988 | Mean no. (SD) | During 12‐month intervention | 0.6 (0.9) | 2.4 (2.1) | |
| Agrawal 2005 | Mean no. (SD) | During follow up | 0.5 (0.71) | 1 (0.61) | ||
| Homer 2000 | Mean no. | During 12‐month follow up | 0.86 | 0.73 | ||
| Karnick 2007 | Mean no. | During follow up | Group 1: 0.54 Group 2: 0.55 |
0.89 | ||
| Khan 2004 | Median | During follow up | 1 | 0 | ||
| McNabb 1985 | Mean no. | For 12 months after intervention | 1.9 | 7.4 | SD not available | |
| NCICAS | Mean no. (SD) | 2‐year rate post‐randomisation | 1.99 (2.97) | 1.89 (2.79) | ||
| Talabere 1993 | Mean no. (SD) | For 12 weeks after intervention | 0.44 (0.77) | 1.08 (1.32) | ||
| Hospital admissions | Karnick 2007 | Mean no. | During follow up | Group 1: 0.19 Group 2: 0.15 |
0.24 | |
| Khan 2004 | Median | During follow up | 0 | 0 | ||
| Mitchell 1986 | Mean no. (SD) | For 12 months after intervention | 0.81 (1.65) | 0.25 (0.65) | Data for Europeans | |
| Mitchell 1986 | Mean no. (SD) | For 12 months after intervention | 0.69 (1.34) | 0.57 (1.10) | Data for Polynesians | |
| Talabere 1993 | Mean no. (SD), adjusted for 12‐week period 1 year prior to study | For 12 weeks after intervention | 0.08 (0.28) | 0.12 (0.33) |
We pooled data with a fixed‐effect model. Random‐effects modelling was also applied in the presence of statistical heterogeneity (see above). We calculated a number needed to treat (NNT) for the primary outcome using the pooled odds ratio and different baseline risks (Cates 2007).
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses on key variables regarding patient characteristics, intervention and control types in order to estimate the magnitude of these effects.
Age of subjects (1 to 5, 6 to 12, 13 to 18 years) ‐ does the age of the child at the time of educational intervention influence outcome?
Type of intervention ‐ what type of education was delivered (comprehensive programme, information only or education with environmental remediation).
Person delivering intervention ‐ does the status of the person delivering intervention affect the outcome?
Timing of the intervention in relation to the emergency department attendance. Educational interventions delivered after a prolonged time interval after the index attendance may be more or less effective as implementing or recruiting for the intervention immediately after the emergency department visit. Studies recruiting participants at different intervals after index attendance were separated according to whether they intervened 1 to 4 weeks post‐emergency department visit and greater than four weeks after.
Type of control ‐ usual care (may involve a degree of education), waiting list control or lower intensity educational intervention.
Timing of outcome assessment (1 to 4 weeks; > 4 to 12 weeks; > 12 to 24 weeks; > 24 to 52 weeks; > 52 weeks) ‐ do the effects of intervention diminish with time?
We tested the difference between subgroups with a test for interaction (Altman 2003).
Sensitivity analysis
We performed sensitivity analyses to determine the robustness of findings on the basis of the risk of bias. We removed studies with a high risk of bias from the analyses to ascertain whether this affected the size and direction of the pooled treatment effect.
Results
Description of studies
Results of the search
All years searches to May 2008 identified 583 citations. We included 30 new studies for the update of the review, generating a total of 38 eligible studies when combined with eight studies from the initial review (Figure 1). Agreement on inclusion/exclusion was good (Kappa: 0.8). The source of disagreement on inclusion related to intervention type or recruitment of participants. Disagreement was resolved by third party adjudication, which led to the inclusion of four studies (Brown 2002; Cicutto 2005; Clark 1986; Warschburger 2003), and the exclusion of six (Bryant‐Stephens 2004; Guendelman 2002; La Roche 2006; Levy 2006; Porter 2006; Williams 2006).
1.
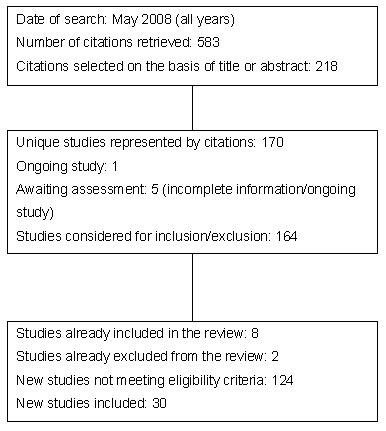
Flow diagram of literature search for 2008 update.
Included studies
Participants
A total of 7843 children were randomised in the 38 studies. We have opted to retain Garrett 1994 in this review as an eligible study, but we have excluded outcome data from this trial since we do not have available paediatric data as a subgroup of the study population, which ranged in age from 2 to 55 years.
In 21 studies, subjects were recruited at the time of the emergency department visit or hospital admission for asthma (Brown 2006; Couriel 1999; Cowie 2002; Farber 2004; Garrett 1994; Gorelick 2006; Harish 2001; Karnick 2007; Khan 2004; Kinlow 2001; Madge 1997; Mitchell 1986; Ng 2006; Smith 2004; Smith 2006; Sockrider 2006; Stevens 2002; Talabere 1993; Teach 2006; Warschburger 2003; Wesseldine 1999). Charlton 1994 and NCICAS recruited some subjects during the admission and some within 12 months of the admission. In the remaining studies subjects were recruited within 12 months of the emergency department visit or hospital admission for asthma.
Interventions
Type and delivery
A variety of educational interventions were tested. All included interactive transfer of information. Six trials included self‐monitoring of symptoms and/or PEFR (Alexander 1988; Charlton 1994; Garrett 1994; Madge 1997; McNabb 1985; Wesseldine 1999); in five trials, medical therapy was assessed or modified as a part of the intervention (Alexander 1988; Charlton 1994; Garrett 1994; Madge 1997; McNabb 1985) and in six trials, participants received an individualised written action plan (Charlton 1994; Couriel 1999; Garrett 1994; Madge 1997; McNabb 1985; Wesseldine 1999). In four studies a component of the intervention included education about environmental asthma triggers, or the provision of materials aimed at encouraging care givers to undertake environmental remediation (Harish 2001; NCICAS; Teach 2006; Wilson 2001). We excluded two studies which involved education and environmental change, since they primarily involved direct environmental remediation rather than behavioural modification (ICAS; SKCHHP).
There was some variation between the studies in the delivery of intervention. Nurses delivered, or were strongly involved in the delivery of the intervention in 16 studies (Alexander 1988; Brown 2002; Butz 2006; Charlton 1994; Couriel 1999; Garrett 1994; Harish 2001; Kelly 2000; Madge 1997; McNabb 1985; Mitchell 1986; Ng 2006; Stevens 2002; Talabere 1993; Walders 2006; Wesseldine 1999; Wilson 2001). Trained health educators were involved in the delivery of intervention in 10 studies (Becker 2003; Brown 2006; Cicutto 2005; Clark 1986; Cowie 2002; Greineder 1999; Khan 2004; NCICAS; Sockrider 2006; Teach 2006). Social workers delivered the intervention in three studies (Ghosh 1998; Smith 2004; Smith 2006), and a case manager delivered the intervention in three trials (Gorelick 2006; Karnick 2007; Shames 2004). The delivery of intervention in Farber 2004 was described as being made by trained staff. One study assessed an educational intervention delivered via a computer game (Homer 2000). In two studies the intervention was described in terms of its content (Agrawal 2005; Warschburger 2003), but not the mode of delivery. One study, presented as a conference abstract, did not enable us to ascertain this information and follow up with study authors was not successful (Kinlow 2001).
Setting
The setting of the intervention was a hospital (seven studies: Alexander 1988; Charlton 1994; Ghosh 1998; Homer 2000; Smith 2006; Warschburger 2003; Wesseldine 1999), community education centre (three studies: Agrawal 2005; Becker 2003; Cowie 2002 ) the home (10 studies: Brown 2002; Brown 2006; Butz 2006; Couriel 1999; Gorelick 2006; Khan 2004; Mitchell 1986; NCICAS; Shames 2004; Smith 2004 ), school (one study: Cicutto 2005); an outpatient clinic (six studies: Clark 1986; Greineder 1999; Harish 2001; McNabb 1985; Walders 2006; Wilson 2001), a combination of the hospital/clinic and home (eight studies: Farber 2004; Karnick 2007; Kelly 2000; Madge 1997; Ng 2006; Sockrider 2006; Talabere 1993; Teach 2006), hospital and outpatient clinic (Stevens 2002) or the home and community education centre (Garrett 1994). In one study the setting of the intervention was not clear and could not be verified (Kinlow 2001). The duration of the intervention ranged from a single 20‐minute session (Wesseldine 1999) at time of discharge, to a programme of visits or reinforcement over 12 months (Alexander 1988; Charlton 1994; Greineder 1999).
Control
Sixteen studies described control group treatment as lower intensity, basic or routine asthma education (Becker 2003; Butz 2006; Charlton 1994; Couriel 1999; Cowie 2002; Farber 2004; Gorelick 2006; Greineder 1999; ICAS; Karnick 2007; Khan 2004; Ng 2006; Teach 2006; Walders 2006; Warschburger 2003; Wilson 2001). These interventions ranged in intensity between provision of leaflets/short booklets only to provision of a written action plan and follow up.
Trials were categorised according to the difference between the intervention and control groups (see Table 10).
2. Components of intervention.
| Study ID | Information | Self‐monitoring | Medication adjusted | Action plan | Control | Intervention |
| Agrawal 2005 | Yes | No | No | Yes | Usual care | Individualised written home management plan |
| Alexander 1988 | Yes | Yes | Yes | Unclear | Usual care | Consistency of care |
| Becker 2003 | Yes | Not stated | Not stated | Not stated | Basic information | 4 weekly sessions with health educator; regular personalised correspondence |
| Brown 2002 | Yes | Yes | Yes | Yes | Usual care | Action plan, information, asthma trigger awareness delivered in home setting |
| Brown 2006 | Yes | Yes | Yes | Yes | Usual care (including written discharge instructions and review of inhaler devices technique) | Comprehensive nurse‐led education including optimisation of medical therapy, action management plan and follow‐up visits. Assessment of home environment made. |
| Butz 2006 | Yes | Not stated | No | Yes | Basic education | Adapted wee wheezers programme with information and emphasis on action plan |
| Charlton 1994 | Yes | Yes | Yes | Yes | Lower intensity | Information, medication, action plan, different diary used for self‐monitoring, letters suggesting GP review |
| Cicutto 2005 | Yes | No | No | No | Usual care | Group session with content aimed at building awareness of symptoms, correct inhaler device technique |
| Clark 1986 | Yes | Yes | No | No | Usual care | Awareness of symptoms, communication with treating physicians and performance at school |
| Couriel 1999 | Yes | No | No | Yes | Usual care | Education delivered over 3 sessions and action plan |
| Cowie 2002 | Yes | No | No | Yes | Advice on inhaler technique | Young adult asthma programme with emphasis on maintenance ICS and bronchodilator therapy |
| Farber 2004 | Yes | No | No | Yes | Brief education | Inhaler device instruction and self‐management plan |
| Garrett 1994 | Yes | Yes | Yes | Yes | Usual care | Information, self‐monitoring, referred to GP for medication, action plan |
| Ghosh 1998 | Yes | Yes | No | Yes | Usual care | 4 sessions of self‐management training and written instruction on managing symptoms |
| Gorelick 2006 | Yes | No | Yes | Yes | Basic education | Education given in ED followed up by intensive primary care linkage; provision of care plan |
| Greineder 1999 | Yes | No | Yes | No | Educational intervention as for treatment group | Nursing outreach reinforcing educational components conveyed during teaching sessions |
| Harish 2001 | Yes | No | Yes | No | Usual care | Review of medications, inhaler technique assessment, provision of allergen impermeable mattresses and encouragement to use telephone line |
| Homer 2000 | Yes | No | Yes | No | Usual care | Interactive computer programme emphasising importance of regular medication, symptom recognition and awareness of allergens |
| Karnick 2007 | Yes | No | Yes | No | Basic education | Reinforcement of education in control group with follow‐up contact from trained educators |
| Kelly 2000 | Yes | No | Not stated | Yes | Usual care | Information and management plan delivered by outreach nurse |
| Khan 2004 | Yes | No | Yes | Yes | Usual care plus action plan | Telephone consultation with experienced educator; advice given to parents at discharge was reinforced |
| Kinlow 2001 | Yes | Not stated | Not stated | Not stated | Usual care | Starbright ‐ interactive computer programme including education & peer support |
| Madge 1997 | Yes | Yes | Yes | Yes | Usual care | Information, self‐monitoring, oral steroids, action plan, review, telephone advice |
| McNabb 1985 | Yes | Yes | Yes | Yes | Usual care | Information, self‐monitoring, medication assessed but generally not changed, action plan |
| Mitchell 1986 | Yes | No | No | No | Usual care | Information, encouraged to attend GP for review |
| NCICAS | Yes | No | No | Yes | Usual care | Education programme aimed at encouraging environmental remediation |
| Ng 2006 | Yes | Yes | No | Yes | Basic education intervention | Education programme delivered by nurse |
| Shames 2004 | Yes | No | No | No | Usual care | Case manager and interactive computer package. |
| Smith 2004 | Yes | No | No | No | Usual care | Telephone call to emphasise importance of primary care follow up, including identification of barriers; monetary incentive |
| Smith 2006 | Yes | No | No | No | Usual care | Discussion with parents during ED visit of primary care follow‐up, including identification of barriers |
| Sockrider 2006 | Yes | Yes | Yes | Yes | Usual care | ED based computer package with follow up and availability of telephone line |
| Stevens 2002 | Yes | No | No | Yes | Usual care | Two interviews with trained nurse; action plan and booklet given to child and parent(s) |
| Talabere 1993 | Yes | No | No | No | Usual care | Information |
| Teach 2006 | Yes | Yes | No | Yes | Basic education | Education aimed at improving self‐management and primary care linkage; provision of house dust mite mattress |
| Walders 2006 | Yes | Yes | Not stated | Yes | Action plan and lower intensity education | Action plan, peak flow meter and education regarding triggers and physiology of asthma. Access to helpline. |
| Warschburger 2003 | Yes | Yes | No | No | Lower intensity education | BASE ‐ Bremer Asthma Training for Parents delivered over 6 sessions |
| Wesseldine 1999 | Yes | Yes | No | Yes | Usual care | Information, self‐monitoring, action plan |
| Wilson 2001 | Yes | No | Yes | No | Medication adjustment | Parental intervention to reduce tobacco smoke exposure |
Outcomes
The primary outcome, subsequent emergency department visits, was available for our analyses as dichotomous data (i.e. proportions of participants) in 17 studies (45% included studies), representing 38% randomised children.
Other outcomes reported and suitable for meta‐analysis were:
Hospital admissions (18 studies).
Unscheduled doctor visits (seven studies).
Study withdrawal (11 studies).
Lung function: PEFR (one study); FEV1 (two studies); symptoms (one study); rescue medication (one study).
Quality of life, functional health status (three studies, two of which measured this with the AQLQ).
Days home sick (seven studies) ‐ reported as a dichotomous outcome (% of patients with at least one day lost from work or school) in one study, an event rate (number of days over number of participants in a specific period of time) in 2 studies, and as a median number of days off school in two studies. In the remaining studies where this was available there was evidence of skew.
In one study (NCICAS), hospital admission data were reported for year one and year two as separate follow‐up periods. We have extracted data from year one since this represents a complete set of data collected from the outset of the study.
Excluded studies
A total of 126 studies failed to meet the eligibility criteria of the review. The reasons for their exclusion are listed in 'Characteristics of excluded studies'.
Risk of bias in included studies
The authors assessed domains of study design according to a revised protocol for this update of the review which took account of recently formulated recommendations regarding the assessment of the risk of bias in reviews (Handbook 2008).
Information for each domain of our risk of bias assessment are given in 'Characteristics of included studies', and a plot of these judgements is shown in Figure 2.
2.
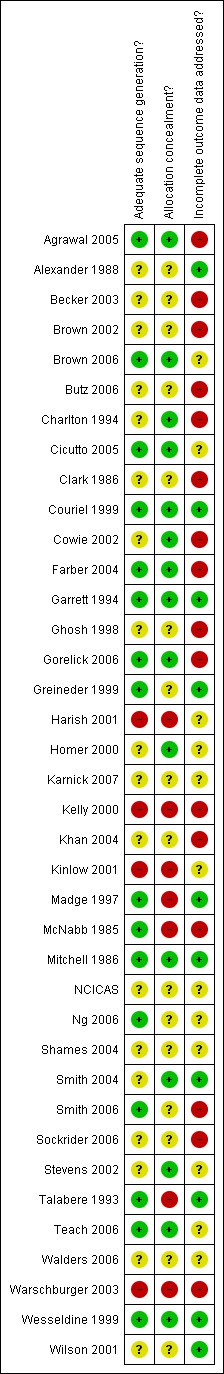
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Sufficient information was available to judge the generation of allocation sequences in 20 studies. The generation of allocation sequence was adequately performed to minimise selection bias in 16 studies. In 15 studies this process had been adequately concealed. In four studies this was inadequate, both in terms of the sequence generation and concealment of allocation.
Blinding
Although none of the trials could be reasonably expected to mask participants to treatment, in 17 trials the outcome assessors were blinded to treatment group assignment.
Incomplete outcome data
Follow up of participants for our hospital contact outcomes was generally poorly described, or at risk of bias with only available case populations analysed. Nine studies reported data as complete sets, or used audit checks or medical record verification in order to collect hospitalisation data. Low attrition rate in Couriel 1999 (< 5%), with low numbers of losses to follow up in each group, meant that the risk of bias posed by incomplete data was low in this study.
Effects of interventions
Primary outcome: emergency department visits
Following education, there was a statistically significant reduction in the risk of an emergency department visit compared with control (17 studies (N = 3008); RR 0.73, 95% CI 0.65 to 0.81 Figure 3). The control group event rates ranged from seven to 67%, with corresponding NNTs ranging from 53 to 7 (Table 11). Follow up was conducted from 12 weeks to a maximum of two years post‐intervention. The I2 statistic indicated that there was a moderate level of statistical heterogeneity between the results of the studies (55%). Random‐effects modelling gave a very similar result to the fixed‐effect estimate (RR 0.73, 95% CI 0.6 to 0.88).
3.
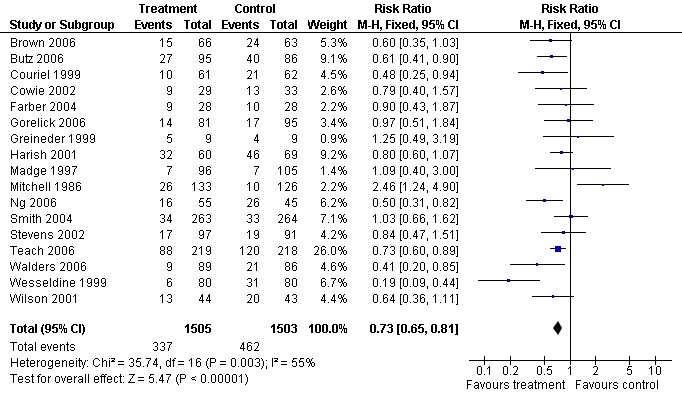
Forest plot of comparison: 1 Education (any type) versus control, outcome: 1.1 ED visits (% subjects).
3. NNTs.
| Study ID | CER (%) | Endpoint (weeks) | NNT |
| Brown 2006 | 38 | 24 | 10 |
| Butz 2006 | 47 | 52 | 8 |
| Cowie 2002 | 39 | 52 | 10 |
| Couriel 1999 | 33.3 | 52 | 12 |
| Farber 2004 | 36 | 24 | 11 |
| Gorelick 2006 | 18 | 24 | 21 |
| Greineder 1999 | 44 | 52 | 9 |
| Harish 2001 | 67 | 104 | 6 |
| Madge 1997 | 7 | 48 | 53 |
| Mitchell 1986 | 8 | 52 | 47 |
| Ng 2006 | 58 | 12 | 7 |
| Smith 2004 | 13 | 24 | 29 |
| Stevens 2002 | 21 | 52 | 18 |
| Teach 2006 | 55 | 24 | 7 |
| Walders 2006 | 24 | 52 | 16 |
| Wesseldine 1999 | 39 | 24 | 10 |
| Wilson 2001 | 47 | 52 | 8 |
We performed two sensitivity analyses by risk of bias: restricting the analysis to studies adjudged to be at a low risk of bias based on our assessment of the allocation sequence generation (selection bias), and those studies where we judged the completeness of follow up to be at a low risk of bias (attrition bias). Sensitivity analysis by low risk of selection bias gave a similar result to our primary analysis (Analysis 8.1). For the majority of studies we excluded from this outcome, information regarding the allocation process was missing. Sensitivity analysis by low risk of attrition bias gave a similar point estimate, but the upper confidence limit that was closer to 'no difference': RR 0.74, 95% CI 0.59 to 0.93 (Analysis 8.2).
8.1. Analysis.

Comparison 8 Sensitivity analysis by risk of bias, Outcome 1 ED visits (allocation bias).
8.2. Analysis.
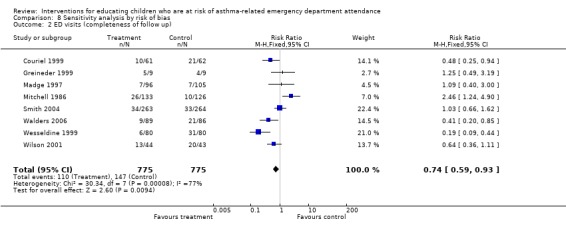
Comparison 8 Sensitivity analysis by risk of bias, Outcome 2 ED visits (completeness of follow up).
Eight studies involving 2179 participants reported data as means with standard deviations. Of these, three studies reported statistically significant reductions in emergency department visits following intervention (Alexander 1988; Kelly 2000; Talabere 1993). In two studies (Garrett 1994; Ghosh 1998) data were complete but the adult and paediatric populations could not be separated. The data were incomplete for two studies (McNabb 1985; Sockrider 2006). Becker 2003 reported significant reductions in emergency department visits in the education groups, without sufficient information to use the data in our analyses.
Secondary outcomes
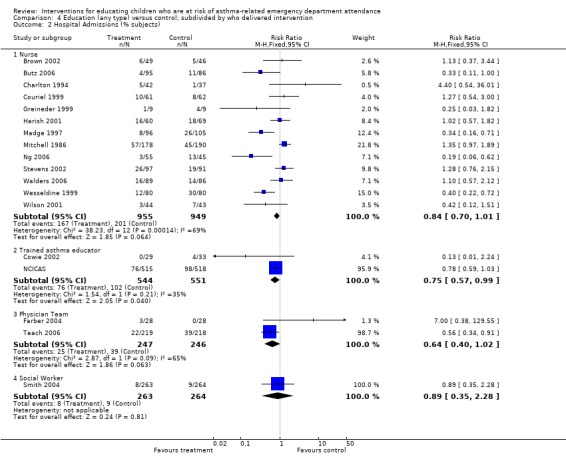
Hospital admission
There was a statistically significant reduction in hospital admissions following education compared with control (18 studies, RR 0.79, 95% CI 0.69 to 0.92, Analysis 1.2). The level of statistical heterogeneity was high (I2 62%). The pooled effect estimate with random‐effects modelling gave a slightly lower relative risk following treatment compared with the fixed‐effect, but the confidence interval also suggested that the true effect under this model may not be different from control: RR 0.75, 95% 0.56 to 1.
1.2. Analysis.
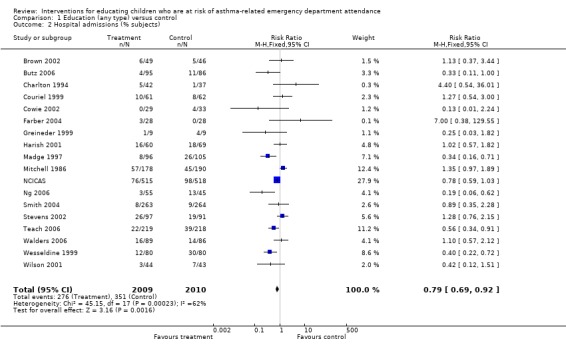
Comparison 1 Education (any type) versus control, Outcome 2 Hospital admissions (% subjects).
Unscheduled doctor visits
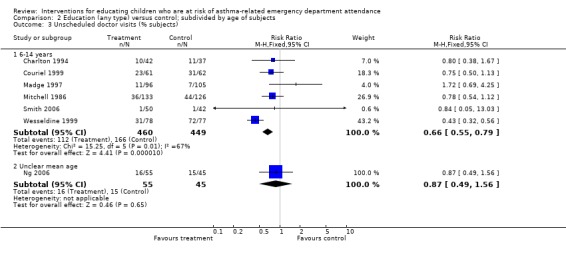
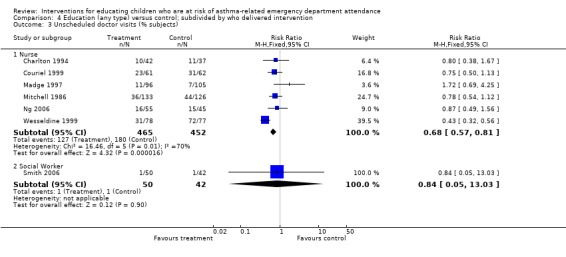
There was a lower risk of unscheduled doctor visits following education (seven studies, RR 0.68, 95% CI 0.57 to 0.81, Analysis 1.3). As with hospital admission the level of statistical heterogeneity between the study effect sizes was high (I2 64%). Applying random‐effects modelling to the result gave a smaller effect that was not statistically significant (RR 0.74, 95% CI 0.53 to 1.04).
1.3. Analysis.

Comparison 1 Education (any type) versus control, Outcome 3 Unscheduled doctor visits (% subjects).
Other secondary outcomes
The remaining secondary outcomes did not reach statistical significance: FEV1 predicted (two studies, 0.24%; 95% confidence interval ‐5.25 to 5.73) or Quality of Life scores (two studies, WMD 0.13, 95% 0.73 to 0.99).
There was no evidence of increased withdrawal/loss to follow up with education or usual care (12 studies, RR 0.95, 95% CI 0.83 to 1.09).
Subgroup analyses
We undertook six subgroup analyses, in an attempt to explore the heterogeneity amongst studies. We restricted subgroup analysis to emergency department visits, admission to hospital and unscheduled doctor visits.
The results of subgroup analysis do not throw any light on whether type and timing of education or control group intervention, timing of outcome assessment or the age of participants influence the results of the studies, as considerable heterogeneity remains within the subgroups. Even where subgroup differences reached statistical significance, such as in Analysis 6.1 where the pooled effect of actively controlled trials (provision of verbal, written or audiovisual information) was almost twice as large as that of trials without a standardised control group intervention (RRR: 0.58 95% CI 0.44 to 0.78, P = 0.0003), the subgroups of studies were themselves heterogeneous. Moreover, the findings from emergency department visits were not replicated in hospital admissions (Analysis 6.2) or unscheduled doctor visits (Analysis 6.3). In many instances the subgroup estimates were similar to each other, and the overlap of the confidence intervals between the subgroups does not rule out similar effects.
6.1. Analysis.

Comparison 6 Education (any type) versus control; subdivided by intensity of control intervention, Outcome 1 ED visits (% subjects).
6.2. Analysis.
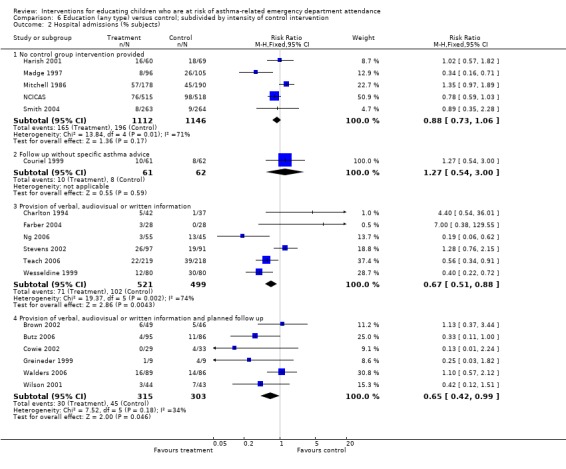
Comparison 6 Education (any type) versus control; subdivided by intensity of control intervention, Outcome 2 Hospital admissions (% subjects).
6.3. Analysis.
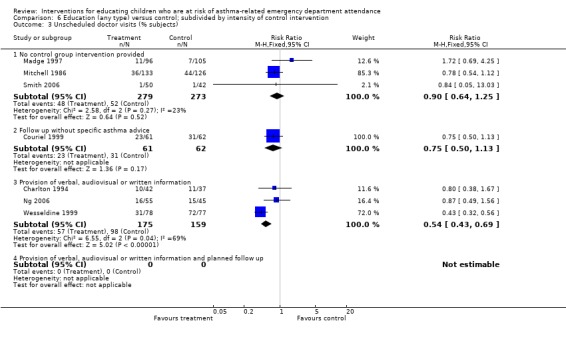
Comparison 6 Education (any type) versus control; subdivided by intensity of control intervention, Outcome 3 Unscheduled doctor visits (% subjects).
Discussion
Summary of results
We have reviewed 38 studies involving 7843 children who attended the emergency department for asthma. Our findings are supportive of an educational package for them, their parents or both in order to reduce subsequent emergency department visits and hospital admissions. The risk of subsequent emergency department visits following educational intervention was reduced by just over a quarter. Based on variation in control group risk between the study populations, this effect translates to a number needed to treat (NNT) of between 55 and 7 to prevent one child experiencing an emergency department visit (Table 11). The reduction in the relative risk of hospital admission and unscheduled doctor visits also favoured children exposed to education. We could not find evidence of statistically significant effects on measures of FEV1, PEF, rescue medication use, quality of life or symptoms; very few studies contributed data to these outcomes and interpreting this apparent lack of findings is difficult. Withdrawal rates did not differ significantly between control and intervention groups, indicating that education following an acute exacerbation of asthma is no more or less acceptable for children and their carers compared with usual follow up. The nature and delivery of educational intervention varied between the studies, and we have not been able to identify the exact characteristics of educational interventions which are most closely associated with a successful outcome.
Although statistical variation between individual study results for our primary outcome suggested that the trials collectively estimated more than one related effect, applying a random‐effects model did not alter the pooled risk ratio. Neither sensitivity analysis by selection bias nor attrition bias changed the direction of our pooled effect estimate. Nevertheless, the populations recruited, the intensity and type of intervention provided to the trial populations, and the timing of outcome measurement all varied between the studies, and may influence our results. Indeed, the results for hospital admission and unscheduled doctor visits exhibited sufficient levels of statistical heterogeneity to bring the size and direction of the result pooled with a fixed‐effect model into question. We shall consider how these different aspects of the studies could influence the results of this review.
Impact of age, socio‐economic status and access to primary care
The majority of the studies we included recruited children younger than 10 years of age. Given the likelihood of parental involvement with the administration of maintenance therapies with children of this age (Orrell‐Valente 2008), involving caregivers may have enhanced asthma management. The challenges associated with managing adolescent asthma remain (Jones 2008): one study exclusively recruited adolescents (Cowie 2002; mean age 17 years), and the validity of the results of this study are affected by its high attrition rate (52%). This may reflect wider difficulties associated with how adolescents perceive and adhere with treatment regimens prescribed for their asthma (Buston 2000).
Fundamental differences in the way that children from low‐income families access acute asthma care under different healthcare systems (i.e. government run versus private) may also explain different responses to treatment (Sun 2003). A considerable number of studies recruited children from low‐income, inner‐city or disadvantaged families, particularly in North America (Brown 2002; Butz 2006; Clark 1986; Farber 2004; Garrett 1994; Gorelick 2006; Harish 2001; Karnick 2007; Kelly 2000; McNabb 1985; Mitchell 1986; NCICAS; Shames 2004; Smith 2004; Smith 2006; Teach 2006; Wilson 2001). Our subgroups did not test for differences between study results based on socio‐economic status, coverage and type of health insurance, or level of primary care available locally. Even within the disadvantaged populations recruited to the studies, variation in treatment effect may not be random: household income, severity of asthma, admission history, access to health insurance, primary care provision, and race and ethnicity, have all been shown to influence emergency department presentation and subsequent asthma morbidity (Boudreaux 2003; Séguin 2005; Sharma 2007; Szilagyi 2006). Differences between the studies in these characteristics may have increased the levels of heterogeneity in our analysis.
An unexpected finding was the presence of one outlying study result suggesting that educational intervention increased emergency department visits (Mitchell 1986). The study investigators hypothesised that families exposed to educational intervention were more inclined to present to emergency care settings if the child's asthma was not responsive to medication and access to primary care was limited. When this study was removed from the primary outcome, the I2 statistic reduced from 55% to 37%. It is noteworthy that this trial featured in a subgroup of studies with dispersed effects, where participants received information only (Analysis 3.1). Whilst statistical analysis of the subgroup differences did not indicate significantly different estimates between this and other net interventions, it is reasonable to anticipate variable treatment effects if access to primary care is limited, since routine management is unlikely to be maintained effectively in this context (Halterman 2007).
3.1. Analysis.
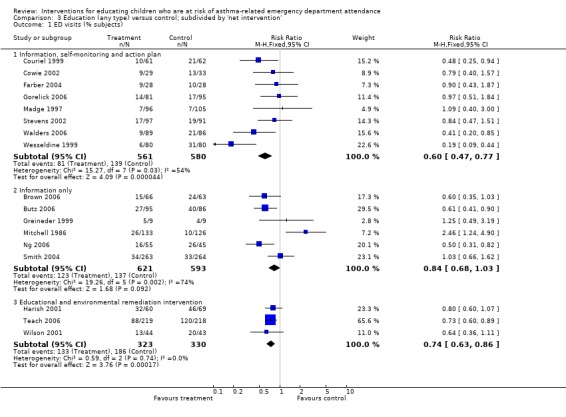
Comparison 3 Education (any type) versus control; subdivided by 'net intervention', Outcome 1 ED visits (% subjects).
Variation in components of intervention, usual care and timing of outcome assessment
The studies we included varied in terms of the delivery and content of education conveyed to study participants and additional components of treatment (Table 10). Indeed, the inclusion of Smith 2004 and Smith 2006, where intervention consisted of reinforcement and emphasis of primary care follow up, might perhaps be more suited to an assessment of a supportive intervention, rather than explicit transfer of information.
Evidence of the relationship between asthma symptoms and the environment suggests that the home is one of a potential number of sources of asthma triggers (Smith 2005). In low‐income urban households, such as those represented by many of the trial populations in our analyses, concentrations of mite and cockroach antigens in addition to other environmental triggers such as damp and extraneous tobacco smoke, are likely to increase the risk of asthma exacerbations (Shapiro 2002). We included four studies where part of the educational intervention included promotion of changes to the home environment (Harish 2001; NCICAS; Teach 2006; Wilson 2001). Whether better understanding of asthma and enhanced routine management, or reduced exposure to asthma triggers (including the provision of mattress casings or smoking cessation advice) moderate asthma control is not easy to discern. Emphasising the importance of asthma triggers in the home environment as part of a behavioural approach to asthma management is likely to standardise the focus of education and deliver consistent, targeted content.
In 11 trials contributing to the primary endpoint, intervention was delivered by a nurse. Research assessing the effect of physician and other allied health teams (such as peers, health educators, case managers and social workers) is not well represented in our analyses. Future work in this area should focus on whether there are important differences between teams delivering intervention.
It is reasonable to anticipate that a more intensive and standardised control group intervention would have led to smaller effect sizes in our subgroup analyses. In fact the contrary was the case. We are uncertain whether this is because of study misclassification (reported control group interventions inadequately conveying the true nature of usual care), whether the interventions assessed in the subgroups of trials with active controls were more likely to be multifaceted, or a combination of these factors.
Timing of intervention (early versus delayed) and the timing of outcome assessment (short, medium and long‐term) were other sources of variation, but these variables did not provide a reliable basis for explaining the statistical heterogeneity between the study results.
Agreements and disagreements with other studies or reviews
A recently published meta‐analysis of studies conducted in the USA found similar results to our own analysis of emergency department visits (odds ratio of 0.78, although the confidence intervals included unity, Coffman 2008). A subset of these studies feature in our review, although there are some differences in eligibility criteria which might partly explain different levels of statistical significance. We did not exclude studies on the basis of geographical location, and we note that a number of studies included in Coffman 2008 recruited participants without an index emergency department visit.
Our findings are somewhat concordant with recent work in both children (Smith 2005) and adults (Gibson 2002a; Tapp 2007). Smith 2005 undertook a review of studies looking at psycho‐education interventions which indicated that hospital admission was significantly reduced following intervention. Tapp 2007 showed a reduction in hospital admission, although not emergency department presentation. Written asthma plans, education on symptoms and triggers of asthma and follow‐up sessions delivered by specialists featured commonly in adult trials. Similar findings were reported by Gibson 2002a, with reduced emergency department and hospitalisation following taught asthma self‐management skills. They concluded that self‐management education that involves a written action plan, self‐monitoring and regular medical review should be offered to adults with asthma. Less intensive interventions, particularly those without a written action plan were less efficacious. Direct head to head comparisons of different intensities and type of educational material would help to elucidate whether specific educational strategies determine successful outcome in children.
Limitations of the review and potential biases
There was significant heterogeneity between the results of eligible studies which is attributable to several plausible causes including different levels of background care available to study populations and intervention types. Subgroup analyses were used in an attempt to explore statistical heterogeneity, but these did not indicate that the differences between study results could be explained in terms of our pre‐defined subgroups.
Many of the outcomes of interest were not reported in the trials, or the data could not be used in our meta‐analyses. Where outcomes are measured in further trials, better reporting of data would help to improve our analyses. For example, the event rates for emergency department visits and hospital admissions, which had skewed distributions, could have been combined in the meta‐analysis if the original data were available as rate ratios, or made available as dichotomous data (Table 9).
Follow up was generally undertaken by chart review for the primary outcome. Concerns have been raised as to the accuracy and completeness of outcome data relating to emergency care episodes, although asthma‐related visits represent one of the more reliable categorisations available to research teams (Gorelick 2007).
Some studies were available in abstract form only, reported incomplete follow‐up data, or did not separate paediatric and adult data. The funnel plot for our primary outcome was not sufficiently asymmetrical to suggest an absence of negative studies (Figure 4). Whilst the search methods used to find suitable studies were thorough, obtaining data in a format for our meta‐analysis often required correspondence with study investigators, and our analyses may be affected by censored availability of relevant outcome data. Our stipulated eligibility criterion led to the exclusion of studies where previous emergency department visits occurred in a subset of the population sampled, but where stratified data were not available to us.
4.
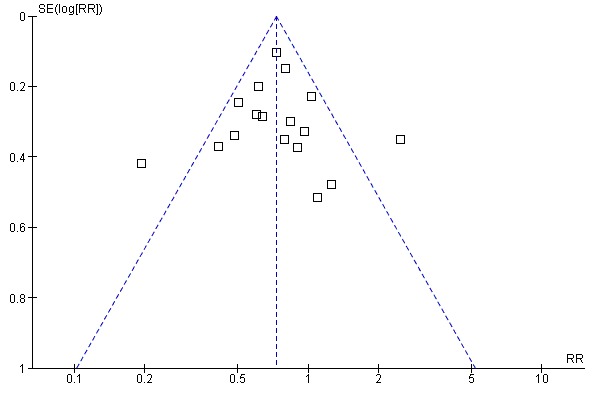
Funnel plot of comparison: 1 Education (any type) versus control, outcome: 1.1 ED visits (% subjects).
Authors' conclusions
Implications for practice.
Asthma education aimed at children and their carers who present to the emergency department for acute exacerbations can result in lower risk of future emergency department presentation and hospital admission. There remains uncertainty as to the long‐term effect of education on other markers of asthma morbidity such as quality of life, symptoms and lung function. It remains unclear as to what type, duration and intensity of educational packages are the most effective in reducing acute care utilisation.
Implications for research.
We remain uncertain as to what characterises the essential characteristics of effective interventions.
Specific issues that should be addressed in future research include:
Whether educational interventions delivered, or supported, by the child's own doctor or other medical practitioners are more effective than other forms of education.
Control for possible non‐specific effects of an educational intervention such as additional contact with a clinician.
Interventions which target adolescents with asthma require development and assessment in clinical trials.
Defining intention‐to‐treat populations in terms of how missing data are handled (e.g. worst case scenario, imputation), and indicating where chart reviews have been performed to identify emergency department visit or hospitalisation.
Measuring and reporting all important outcomes (e.g. days off school, quality of life), regardless of statistical significance, in units suitable for meta‐analysis.
Head to head comparisons of different types and intensities of educational intervention.
What's new
| Date | Event | Description |
|---|---|---|
| 15 May 2009 | Amended | Study previously listed as awaiting assessment moved to 'Excluded studies' (Augustin 2003). |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 3, 2000
| Date | Event | Description |
|---|---|---|
| 19 March 2009 | Amended | Correction to appendix |
| 6 November 2008 | New citation required and conclusions have changed | 30 studies added to the review; primary outcome substantially changed by addition of new data. |
| 29 May 2008 | New search has been performed | New search run. |
| 1 May 2008 | Amended | Converted to new review format. |
| 21 September 2000 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
We are grateful for the assistance provided by members of the Cochrane Airways Review Group who helped with protocol development, database searches, obtaining studies, translating studies from languages other than English, and contacting authors (Steve Milan, Jane Dennis, Anna Bara, Karen Blackhall, Liz Arnold). We thank Jennifer Roberts for help in various aspects of conducting the review, including protocol development and preparation of data extraction forms. We also thank the Airways Review Group editorial board (Paul Jones, Chris Cates).
We thank the following authors for providing information about their trials:
Included studies Dr I Charlton ‐ provided details about subject selection, study methods and the intervention.
Dr J Couriel ‐ provided an unpublished manuscript for the study.
Dr J Garrett ‐ provided details about the study methods.
Prof R Henry ‐ attempted to obtain unpublished data from his study.
Ms P Madge ‐ provided details about the study methods and the intervention.
Dr Margellos ‐ attempted to obtain unpublished data from her study.
Dr W McNabb ‐ provided details about the study methods, the intervention and supplied some additional data.
Dr EA Mitchell ‐ provided details about the study methods and the intervention.
Dr L Talabere ‐ provided details about the study methods and the intervention.
Prof S Teach ‐ provided unpublished data from his study.
Ms L Wesseldine ‐ provided details about the study methods and the intervention.
Dr N Walders ‐ provided data for emergency department visits and admissions.
Excluded studies Dr U Brook ‐ provided details about subject selection.
Dr J Dahl Olerud ‐ provided details about subject selection.
Dr S Wilson ‐ provided details about the study methods and the intervention.
Appendices
Appendix 1. Criteria for risk of bias
Generation of random allocation sequence
Yes (if the method used was described and the resulting sequences were unpredictable); Unclear (if the method was not described); No (for sequences such as alternate allocation).
Allocation concealment
Yes (if participants and the investigators enrolling participants could not foresee assignment); Unclear (method not described); No (if investigators enrolling participants could foresee next assignment).
Incomplete data
Yes (no or minimal attrition: all randomised participants contributed to data analysis); Unclear (information not available); No (analysis based on available cases).
Data and analyses
Comparison 1. Education (any type) versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ED visits (% subjects) | 17 | 3008 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.65, 0.81] |
| 2 Hospital admissions (% subjects) | 18 | 4019 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.69, 0.92] |
| 3 Unscheduled doctor visits (% subjects) | 7 | 1009 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.57, 0.81] |
| 4 Withdrawal | 12 | 2445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.83, 1.09] |
| 5 Mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 FEV1 predicted | 2 | % (Fixed, 95% CI) | 0.24 [‐5.25, 5.73] | |
| 7 PEF | 1 | L/min (Fixed, 95% CI) | Totals not selected | |
| 8 Rescue medication use (puffs/d) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Quality of life (AQLQ) | 2 | 224 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.35, 0.34] |
| 10 Symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.

Comparison 1 Education (any type) versus control, Outcome 1 ED visits (% subjects).
1.4. Analysis.

Comparison 1 Education (any type) versus control, Outcome 4 Withdrawal.
1.5. Analysis.
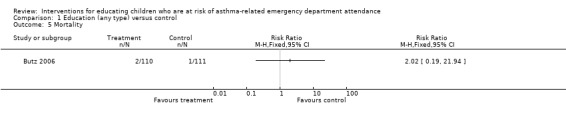
Comparison 1 Education (any type) versus control, Outcome 5 Mortality.
1.6. Analysis.

Comparison 1 Education (any type) versus control, Outcome 6 FEV1 predicted.
1.7. Analysis.

Comparison 1 Education (any type) versus control, Outcome 7 PEF.
1.8. Analysis.
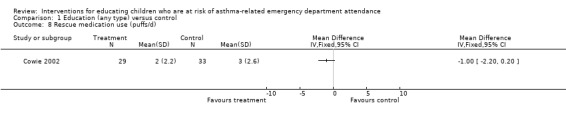
Comparison 1 Education (any type) versus control, Outcome 8 Rescue medication use (puffs/d).
1.9. Analysis.
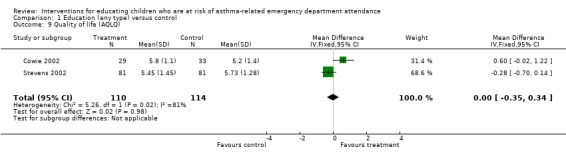
Comparison 1 Education (any type) versus control, Outcome 9 Quality of life (AQLQ).
1.10. Analysis.

Comparison 1 Education (any type) versus control, Outcome 10 Symptoms.
Comparison 2. Education (any type) versus control; subdivided by age of subjects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ED visits (% subjects) | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 1‐5 years | 3 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.53, 0.98] |
| 1.2 6‐14 years | 9 | 1764 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.62, 0.94] |
| 1.3 > 15 years | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.40, 1.57] |
| 1.4 Mean age not available | 4 | 795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.60, 0.81] |
| 2 Hospital admissions (% subjects) | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 1‐5 years | 4 | 482 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.59, 1.33] |
| 2.2 6‐14 years | 10 | 2809 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.72, 1.01] |
| 2.3 > 15 years | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.24] |
| 2.4 Mean age not available | 3 | 666 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.42, 0.84] |
| 3 Unscheduled doctor visits (% subjects) | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6‐14 years | 6 | 909 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.55, 0.79] |
| 3.2 Unclear mean age | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.49, 1.56] |
2.1. Analysis.

Comparison 2 Education (any type) versus control; subdivided by age of subjects, Outcome 1 ED visits (% subjects).
2.2. Analysis.

Comparison 2 Education (any type) versus control; subdivided by age of subjects, Outcome 2 Hospital admissions (% subjects).
2.3. Analysis.
Comparison 2 Education (any type) versus control; subdivided by age of subjects, Outcome 3 Unscheduled doctor visits (% subjects).
Comparison 3. Education (any type) versus control; subdivided by 'net intervention'.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ED visits (% subjects) | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Information, self‐monitoring and action plan | 8 | 1141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.47, 0.77] |
| 1.2 Information only | 6 | 1214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.68, 1.03] |
| 1.3 Educational and environmental remediation intervention | 3 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.63, 0.86] |
| 2 Hospital admissions (% subjects) | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Information, self‐monitoring and action plan | 8 | 1044 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.60, 1.02] |
| 2.2 Information only | 6 | 1289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.70, 1.20] |
| 2.3 Educational and environmental remediation intervention | 4 | 1686 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.59, 0.91] |
| 3 Unscheduled doctor visits (% subjects) | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
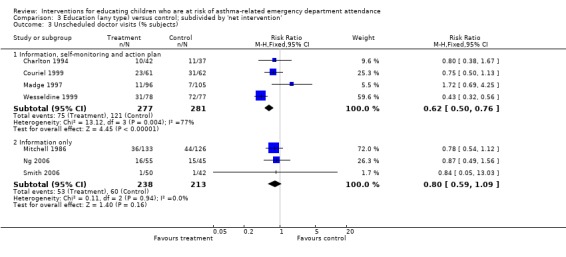
| 3.1 Information, self‐monitoring and action plan | 4 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.50, 0.76] |
| 3.2 Information only | 3 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.59, 1.09] |
3.2. Analysis.

Comparison 3 Education (any type) versus control; subdivided by 'net intervention', Outcome 2 Hospital admissions (% subjects).
3.3. Analysis.
Comparison 3 Education (any type) versus control; subdivided by 'net intervention', Outcome 3 Unscheduled doctor visits (% subjects).
Comparison 4. Education (any type) versus control; subdivided by who delivered intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ED visits (% subjects) | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Nurse | 11 | 1621 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.57, 0.79] |
| 1.2 Trained asthma educator | 2 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.43, 1.01] |
| 1.3 Physician team | 2 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.61, 0.90] |
| 1.4 Social Worker | 1 | 527 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.66, 1.62] |
| 1.5 Case Manager | 1 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.51, 1.84] |
| 2 Hospital Admissions (% subjects) | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nurse | 13 | 1904 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.70, 1.01] |
| 2.2 Trained asthma educator | 2 | 1095 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.57, 0.99] |
| 2.3 Physician Team | 2 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.40, 1.02] |
| 2.4 Social Worker | 1 | 527 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.35, 2.28] |
| 3 Unscheduled doctor visits (% subjects) | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Nurse | 6 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.57, 0.81] |
| 3.2 Social Worker | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.05, 13.03] |
4.1. Analysis.

Comparison 4 Education (any type) versus control; subdivided by who delivered intervention, Outcome 1 ED visits (% subjects).
4.2. Analysis.
Comparison 4 Education (any type) versus control; subdivided by who delivered intervention, Outcome 2 Hospital Admissions (% subjects).
4.3. Analysis.
Comparison 4 Education (any type) versus control; subdivided by who delivered intervention, Outcome 3 Unscheduled doctor visits (% subjects).
Comparison 5. Education (any type) versus control; subdivided by timing of intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ED visits (% subjects) | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Early intervention | 13 | 2547 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.67, 0.86] |
| 1.2 Delayed intervention | 4 | 461 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.45, 0.79] |
| 2 Hospital admissions (% subjects) | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Early intervention | 12 | 2446 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.68, 0.97] |
| 2.2 Delayed intervention | 6 | 1573 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.61, 0.97] |
| 3 Unscheduled doctor visits (% subjects) | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Early intervention | 6 | 930 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.56, 0.80] |
| 3.2 Delayed intervention | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.38, 1.67] |
5.1. Analysis.
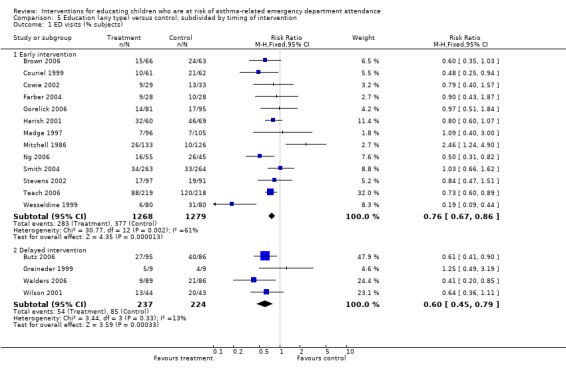
Comparison 5 Education (any type) versus control; subdivided by timing of intervention, Outcome 1 ED visits (% subjects).
5.2. Analysis.

Comparison 5 Education (any type) versus control; subdivided by timing of intervention, Outcome 2 Hospital admissions (% subjects).
5.3. Analysis.
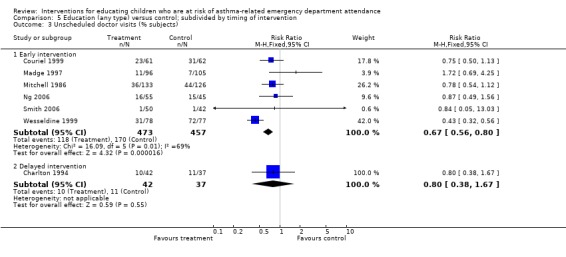
Comparison 5 Education (any type) versus control; subdivided by timing of intervention, Outcome 3 Unscheduled doctor visits (% subjects).
Comparison 6. Education (any type) versus control; subdivided by intensity of control intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ED visits (% subjects) | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 No control group intervention provided | 4 | 1116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.86, 1.38] |
| 1.2 Follow up without specific asthma advice | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.25, 0.94] |
| 1.3 Provision of verbal, audiovisual or written information | 5 | 941 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.54, 0.76] |
| 1.4 Provision of verbal, audiovisual or written information and planned follow up | 7 | 828 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.53, 0.82] |
| 2 Hospital admissions (% subjects) | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 No control group intervention provided | 5 | 2258 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.73, 1.06] |
| 2.2 Follow up without specific asthma advice | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.54, 3.00] |
| 2.3 Provision of verbal, audiovisual or written information | 6 | 1020 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.51, 0.88] |
| 2.4 Provision of verbal, audiovisual or written information and planned follow up | 6 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.42, 0.99] |
| 3 Unscheduled doctor visits (% subjects) | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 No control group intervention provided | 3 | 552 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.64, 1.25] |
| 3.2 Follow up without specific asthma advice | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.50, 1.13] |
| 3.3 Provision of verbal, audiovisual or written information | 3 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.43, 0.69] |
| 3.4 Provision of verbal, audiovisual or written information and planned follow up | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Education (any type) versus control; subdivided by timing of outcome assessment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ED visits (% subjects) | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Less than 6 months | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.31, 0.82] |
| 1.2 Between 6 months and less than 12 months | 6 | 1485 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.60, 0.84] |
| 1.3 12 months or longer | 10 | 1423 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.65, 0.92] |
| 2 Hospital admissions (% subjects) | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Less than 6 months | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.06, 0.62] |
| 2.2 Between 6 months and 12 months | 6 | 1627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.70, 1.11] |
| 2.3 12 months or longer | 11 | 2292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.65, 0.94] |
| 3 Unscheduled doctor visits (%subjects) | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Less than 6 months | 2 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.49, 1.54] |
| 3.2 Between 6 months and less than 12 months | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.69, 4.25] |
| 3.3 12 months or longer | 4 | 616 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.51, 0.74] |
7.1. Analysis.
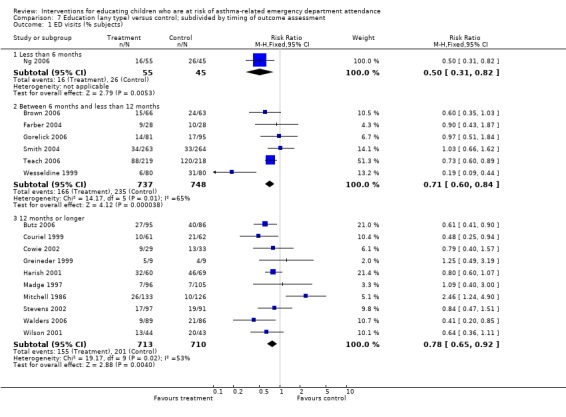
Comparison 7 Education (any type) versus control; subdivided by timing of outcome assessment, Outcome 1 ED visits (% subjects).
7.2. Analysis.
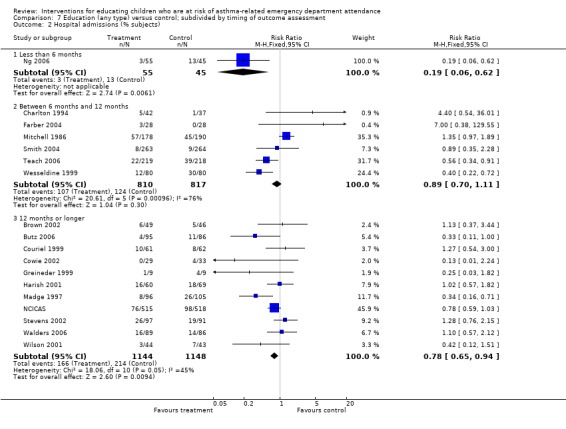
Comparison 7 Education (any type) versus control; subdivided by timing of outcome assessment, Outcome 2 Hospital admissions (% subjects).
7.3. Analysis.
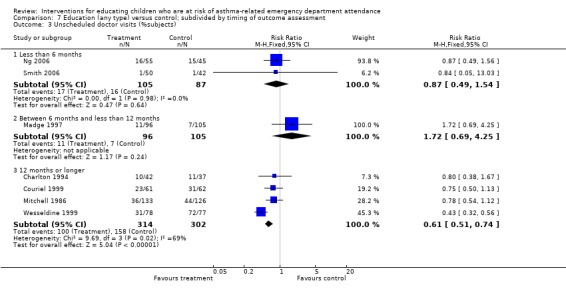
Comparison 7 Education (any type) versus control; subdivided by timing of outcome assessment, Outcome 3 Unscheduled doctor visits (%subjects).
Comparison 8. Sensitivity analysis by risk of bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ED visits (allocation bias) | 7 | 1340 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.61, 0.85] |
| 2 ED visits (completeness of follow up) | 8 | 1550 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.59, 0.93] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agrawal 2005.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: Single centre in India
DURATION OF STUDY: 4 months No blinding of outcome assessor |
|
| Participants | N SCREENED: Not reported N RANDOMISED: 68 (treatment: 35; control: 33) N COMPLETED: 60 M = Not reported F = Not reported MEAN AGE: 8 years BASELINE DETAILS: Mean ER visits per child in previous year: 1; PEF 76% predicted; all children received steroids (BUD or FP) INCLUSION CRITERIA: 5 to 12 years; physician‐diagnosed moderate persistent asthma (NHLBI guidelines); moderate dose of inhaled corticosteroids with as needed beta‐2 agonist when required EXCLUSION CRITERIA: Uncontrolled medical conditions besides asthma | |
| Interventions | EDUCATION GROUP: Individualised written home management plan Setting: Community CONTROL GROUP: No plan At enrolment, children and parent were given a basic education course instructing them on asthma and its causes TREATMENT PERIOD: Not applicable FOLLOW‐UP PERIOD: 4 months |
|
| Outcomes | Acute asthma events; school absence; symptoms; withdrawal | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random sequence |
| Allocation concealment? | Low risk | Sealed cover technique |
| Incomplete outcome data addressed? All outcomes | High risk | Data analysed for available cases |
Alexander 1988.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: Single centre in USA
DURATION OF STUDY: 12 months No blinding of outcome assessor |
|
| Participants | N SCREENED: Not reported N RANDOMISED: 21 (treatment: 11; control: 10) N COMPLETED: 21 M = Not reported F = Not reported MEAN AGE: Range 15 months to 13 years BASELINE DETAILS: Mean ER visits per child in previous year: 2.5 INCLUSION CRITERIA: Presentation at ED with acute asthma in previous 12 months; no primary care contact for asthma within previous 12 months EXCLUSION CRITERIA: Not stated | |
| Interventions | EDUCATION GROUP: Allocation of an individual Clinical Nurse Specialist to provide management and review over a 12‐month period. The nurse worked within the General Paediatric Clinic. Children and family included; intervention began within one year of ER visit. There were 3 visits scheduled over 12 months plus phone contact; actual: 2.8 visits plus 3.5 phone contacts CONTROL: Usual care (follow up with Paediatric Residents) Duration: 3 visits over 12 months; actual: only 5/10 returned for first follow‐up visit and 1/10 thereafter TREATMENT PERIOD: 12 months (3 visits) FOLLOW‐UP PERIOD: 12 months |
|
| Outcomes | ED visits ‐ measured for 12 months from beginning to end of intervention, i.e. DURING intervention | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Incomplete outcome data addressed? All outcomes | Low risk | Complete set (no withdrawals) |
Becker 2003.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: Canada
DURATION OF STUDY: 12 months Blinding of outcome assessor could not be obtained |
|
| Participants | N SCREENED: Not reported N RANDOMISED: 398 (intervention: 200; control: 198) N COMPLETED: 300 (intervention: 171; control: 129) M = Not reported F = Not reported MEAN AGE: Not reported BASELINE DETAILS: Not reported INCLUSION CRITERIA: 3 to 16 years; ED visit or hospitalisation with asthma EXCLUSION: Not reported | |
| Interventions | EDUCATION GROUP: 4 x weekly education sessions by trained health educator & personalised letters at 2, 4, 6 and 12 months post‐enrolment Setting: Community CONTROL GROUP: Asthma information booklet and usual care TREATMENT PERIOD: 4 weeks FOLLOW‐UP PERIOD: 12 months |
|
| Outcomes | Exacerbations (hospital re‐presentation; requirement for additional medical treatment) | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Incomplete outcome data addressed? All outcomes | High risk | Data analysed as available case (assumed) |
Brown 2002.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: Atlanta, USA; 3 asthma clinics and several primary care paediatricians in low‐income areas
DURATION OF STUDY: 12 months Outcome assessors blinded to treatment group allocation |
|
| Participants | N SCREENED: 144 N RANDOMISED: 95 (intervention: 49; control: 46) N COMPLETED: 95 M = 59 F = 36 MEAN AGE: 4 years BASELINE DETAILS: African American: 90%, European American: 7%, Other 3%; Medicaid: 82%; Severity of asthma: mild asthma: 75%; moderate: 21%; severe: 4%; Mean acute asthma presentations in preceding 12 months: 5 INCLUSION CRITERIA: 1 to 7 years of age; healthcare visit for asthma in previous year; prescribed daily medication; primary care giver spoke English EXCLUSION: Primary care giver had known involvement with illegal drugs | |
| Interventions | EDUCATION GROUP: Adapted wee wheezers at home programme, with handouts tailored to family needs. 8 x 90 minute sessions at weekly intervals. Home visits conducted by trained nurses. Setting: Home CONTROL GROUP: Usual care (families in this group were offered one home visit following completion of study) TREATMENT PERIOD: 8 weeks FOLLOW‐UP PERIOD: 12 months |
|
| Outcomes | Symptoms; exacerbations; care giver quality of life; cough scores; changes in environmental risk factors | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Incomplete outcome data addressed? All outcomes | High risk | Data analysed as available case (assumed) |
Brown 2006.
| Methods | STUDY DESIGN: Parallel group
LOCATION, NUMBER OF CENTRES: USA, 1 centre
DURATION OF STUDY: 6 months No blinding of outcome assessor |
|
| Participants | N SCREENED: 771 N RANDOMISED: 129 M = Not reported F = Not reported BASELINE DETAILS: Primary care physician: 87%; Asthma action plan: 23%; Spacer: 57%; ICS: 78%; PEF meter: 44%; 37% were African American, 56% had moderate‐to‐severe persistent asthma, 78% on ICS at baseline INCLUSION CRITERIA: Children or adults; asthma exacerbation presenting on ED visit, have had asthma symptoms in the prior 2 weeks, or a previous hospitalisation or ED visit in the past year EXCLUSION CRITERIA: Not described | |
| Interventions | EDUCATION GROUP: Conducted by trained asthma educators and included a facilitated office visit with patient and primary care provider within 2 to 4 weeks of enrolment, a home‐visit 2 to 4 weeks thereafter Setting: Home CONTROL GROUP: Usual care, including instructions in inhaler device technique, written discharge instructions and planned follow up TREATMENT PERIOD: 2 visits up to 8 weeks post‐enrolment FOLLOW‐UP PERIOD: 6 months |
|
| Outcomes | Urgent asthma visit; treatment compliance; withdrawals | |
| Notes | 39% in intervention group did not comply with any aspect of planned educational programme | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random number sequences |
| Allocation concealment? | Low risk | Sealed envelopes |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Described as intention‐to‐treat; no explicit description of how this population was composed |
Butz 2006.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: USA, 2 large urban hospitals and affiliated practices
DURATION OF STUDY: 12 months No blinding of outcome assessor |
|
| Participants | N SCREENED: 513 N RANDOMISED: 221 N COMPLETED: 181 M = 145 F = 76 MEAN AGE: 4.5 years BASELINE DETAILS: African American: 89%; Medicaid: 90%; mild asthma: 65%, moderate asthma: 21%, severe asthma: 14%; mean ED visits in previous 12 months: 2 INCLUSION CRITERIA: 2 to 9 years; diagnosis of asthma; symptom frequency at least 2 or more times a week in last month; night‐time asthma symptom frequency at least 2 or more times in last month; use of a nebuliser in last month 30 days, resident of Baltimore, and 1 or more ED visits for asthma within the past 12 months or hospitalisation for asthma in the past 12 months EXCLUSION: Not reported | |
| Interventions | EDUCATION GROUP: Home‐based education programme (based on 3 programmes: wee wheezer programme; A+ asthma club programme & nebulizer therapy recommendations). Parents of children received 6 one‐hour sessions. Delivered by trained nurses. Setting: Home CONTROL GROUP: Usual asthma education ‐ 3 visits incorporating information on dose of maintenance therapies, asthma care plan TREATMENT PERIOD: 6 months FOLLOW‐UP PERIOD: 12 months |
|
| Outcomes | ED visits, medication prescriptions, withdrawal, death | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Incomplete outcome data addressed? All outcomes | High risk | Available case (assumed) |
Charlton 1994.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: Single centre in Australia
DURATION OF STUDY: 2 years Outcome assessors were blinded to treatment group allocation |
|
| Participants | SCREENED: Not reported N RANDOMISED: 91 (treatment: 48; control 43) N COMPLETED: 79 (treatment: 42; control 37) M = 52 F = 39 MEAN AGE: 6.8 BASELINE DETAILS: 55% had hospital admission, 34% ED visit, 59% GP home visit in previous 6 months INCLUSION CRITERIA: Admission for asthma or attended outpatients department for asthma at time of recruitment; hospital admission for asthma in previous 12 months EXCLUSION CRITERIA: Not stated | |
| Interventions | EDUCATION GROUP: Nurse run asthma clinic; information; self‐monitoring of symptoms, PEF and medications; written action plan allowing self adjustment of medications based on symptoms or PEF; reminders sent for regular medical review with own GP; medication modified if necessary (on consultation with hospital doctor) Parents and children included; delivered at time of visit or admission. Initial interview lasted 45 minutes; follow‐up letters sent every 3 months for 12 months reminding patients to have asthma reviewed by their GP or nurse CONTROL GROUP: Lower intensity education consisting of self‐monitoring of symptoms, PEF and medications (different diary to intervention group). This involved an interview of about 15 minutes only. TREATMENT PERIOD: 12 months FOLLOW‐UP PERIOD: 12 months |
|
| Outcomes | Hospital admissions and home visits by GP ‐ measured for the 6 to 12 month period from beginning to end of the intervention, i.e. DURING intervention. Skills (response to an acute attack), daily PEF, day and night wheeze scores, daily puffs of bronchodilator and inhaled steroids, days of oral steroids, days lost from school, daily activity restriction score ‐ measured for 12 months from beginning to end of the intervention, i.e. DURING intervention. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Information not available |
| Allocation concealment? | Low risk | Sealed envelope |
| Incomplete outcome data addressed? All outcomes | High risk | Available case |
Cicutto 2005.
| Methods | STUDY DESIGN: Cluster‐randomised controlled trial
LOCATION, NUMBER OF CENTRES: Canada, 26 schools
DURATION OF STUDY: 12 months Outcome assessors were blinded to treatment group allocation |
|
| Participants | N SCREENED: Not reported N RANDOMISED: 129 children from 256 randomised had experienced an ED visit within previous year. Demographics taken from total cohort (treatment: 132; control: 124) N COMPLETED: 239 (treatment: 121; control: 118) M = 151 F = 105 MEAN AGE: 8.6 BASELINE DETAILS: 70% children had mild asthma INCLUSION CRITERIA: Enrolled in Grade 2 to 5, spoke English, given consent/assent, report of physician diagnosed asthma, asthma medication use, asthma symptoms 3 or more times in past year EXCLUSION: Presence of 2nd major chronic illness with pulmonary component | |
| Interventions | EDUCATION GROUP: Six 60‐minute group sessions based on Roaring Adventures of Puff (RAP). The sessions include the following: (1) getting to know each other, goal setting, use of a peak flowmeter and diary monitoring; (2) trigger identification, control and avoidance, and basic pathophysiology; (3) medications and the proper use of inhalers; (4) symptom recognition and action plan use; (5) lifestyle, exercise and managing an asthma episode; and (6) sharing asthma information with teachers and parents. Teaching strategies include puppetry, games, role playing, model building, discussions and asthma diary recordings. Parental involvement is encouraged through the use of asthma‐related homework activities for the family during the weekly intervals. Intervention delivered by health educators. Setting: School CONTROL GROUP: Usual care TREATMENT PERIOD: 6 weeks FOLLOW‐UP PERIOD: 12 months |
|
| Outcomes | Quality of life; school absence; parental work absence; health services use | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random numbers schedule |
| Allocation concealment? | Low risk | Centralised system |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Follow up based on extreme case scenario |
Clark 1986.
| Methods | STUDY DESIGN: Parallel group randomised trial
LOCATION, NUMBER OF CENTRES: USA, paediatric allergy clinics in deprived area of New York
DURATION OF STUDY: 52 weeks No blinding of outcome assessor |
|
| Participants | N SCREENED: Not reported N RANDOMISED: 35 (treatment: 19; control: 16); this number taken only from subgroup of children who experienced hospitalisation visits in previous year. Remaining demographic details taken from study cohort (N = 256) N COMPLETED: Not reported M= Not clear F= Not clear MEAN AGE: Not reported BASELINE DETAILS: Mean ED visit rate 2.8 INCLUSION CRITERIA: Physician diagnosed asthma; >/= 1 clinic visits in previous year; >/=1 episodes of wheezing in previous year; 4 to 17 years of age EXCLUSION: Handicap that would prevent participation in education programme | |
| Interventions | EDUCATION GROUP: Asthma management instruction taken by the child with asthma and the child's parents, delivered via training sessions developed after collection of initial interview data. 6 hour long sessions were offered monthly in English and Spanish. Sessions were conducted on a group level with 10 to 15 families present. The sessions consisted of discussion and problem solving led by a health educator. Emphasis was placed on managing asthma exacerbations, exercise, controlling asthma and asthma triggers, communication with treating physician and improving performance at school. Setting: Outpatient clinic CONTROL GROUP: Usual care TREATMENT DURATION: 24 weeks FOLLOW‐UP PERIOD: 52 weeks |
|
| Outcomes | ED visits; hospitalisation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Incomplete outcome data addressed? All outcomes | High risk | Available case |
Couriel 1999.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: UK, A&E department
DURATION OF STUDY: 12 months Blinding of personnel involved in data collection and ongoing care; outcome assessor blinding could not be ascertained |
|
| Participants | N SCREENED: Not reported N RANDOMISED: 128 (intervention: 65; control: 63) N COMPLETED: 123 M= 75 F= 53 MEAN AGE: 9.8 years BASELINE DETAILS: Hospital in previous six months: 23%; school absence in previous six months: 6.75 INCLUSION CRITERIA: 6 to 16 years; attending A&E without requirement for admission EXCLUSION: Not reported | |
| Interventions | EDUCATION GROUP: Structured education programme of 3 home visits at 2 weeks, one month and 3 months after enrolment. Principal aims were to enable recognition of early signs of worsening asthma and commencing appropriate treatment based on individualised written self‐management plan. Peak flow meter and inhaler technique instruction given to child and a parent. Advice given on trigger avoidance and managing asthma in school, on holidays and with exercise. Participants encouraged to discuss concerns about asthma. A work book was designed to reinforce the sessions, and children encouraged to personalise this and use as a record, and a way of identifying their objectives. Each child given written self‐management plan. The plan was reviewed and reinforced at follow‐up sessions. Telephone support was available for children in the intervention group. Setting: Community/home CONTROL GROUP: Children visited at home by a research nurses within 2 weeks of the baseline visit and 3, 6 and 12 months post. No specific advice about managing asthma offered by the research nurse. TREATMENT PERIOD: 3 months FOLLOW‐UP PERIOD: 12 months post baseline |
|
| Outcomes | A&E attendance; admission to hospital with asthma symptoms | |
| Notes | Data available on request from study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "The randomisation schedule was developed by computer in blocks of six" |
| Allocation concealment? | Low risk | "As eligible subjects were identified, a sealed numbered envelope allocating subjects to one of the groups was opened by a single person who was not otherwise involved with the study" |
| Incomplete outcome data addressed? All outcomes | Low risk | Data available for 96% of trial population at end of follow up |
Cowie 2002.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: Canada, ED records from hospitals in Alberta
DURATION OF STUDY: 12 months No blinding of outcome assessor |
|
| Participants | N SCREENED: 254 N RANDOMISED: 130 (of which 93 attended initial assessment); 3‐month data reported for 79 participants (intervention: 32; control: 47) N COMPLETED: 62 M = 18 F = 44 MEAN AGE: 17 years BASELINE DETAILS: ICS use: 75%; mean SABA use per day: 4 puffs; FEV1 predicted: 81% INCLUSION CRITERIA: 15 to 20 years; attendance at ED with asthma; EXCLUSION CRITERIA: Not reported | |
| Interventions | EDUCATION GROUP: YAAP ‐ Young Adult Asthma Programme (one‐off visit to central site where therapists assessed inhaler device technique, information provided on asthma, emphasis on ICS & bronchodilators; exposure to risk factors +/‐ action plan Setting: Community CONTROL GROUP: Control: basic advice on inhaler technique delivered at some site as intervention but scheduled at different times TREATMENT PERIOD: 90 to 120 minute session FOLLOW‐UP PERIOD: 12 months |
|
| Outcomes | ED use; hospital admission; use of maintenance therapy; quality of life; withdrawal | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Computer‐generated randomisation schedule |
| Allocation concealment? | Low risk | Consecutively numbered sealed envelopes |
| Incomplete outcome data addressed? All outcomes | High risk | Available case |
Farber 2004.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: USA, inner‐city ED
DURATION OF STUDY: 6 months Outcome assessors were blinded to treatment group allocation |
|
| Participants | N SCREENED: Not reported N RANDOMISED: 56 (intervention: 28; control group: 28) N COMPLETED: 46 M = Not clear F = Not clear MEAN AGE: 7.5 years BASELINE DETAILS: ICS use: 25%; exposure to passive smoke: 57%; N in household where income < 15000$: 82%. INCLUSION CRITERIA: Presentation in ED; 2 to 18 years; Medicaid insurance; home telephone; history of asthma EXCLUSION CRITERIA: Intubation/mechanical ventilation for asthma | |
| Interventions | EDUCATION GROUP: Educational intervention delivered during ED visit/hospital admission by trained staff. Education consisted of inhaler device instruction and action plans. Follow‐up phone calls made 1 to 2 weeks, 4 to 6 weeks and 3 months post‐enrolment Setting: ED & home CONTROL GROUP: Brief education routinely used in ED as normal procedure TREATMENT PERIOD: 1 session (plus phone calls) FOLLOW‐UP PERIOD: 6 months |
|
| Outcomes | ED visits; medication use | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated block randomisation |
| Allocation concealment? | Low risk | Schedule generated by third party |
| Incomplete outcome data addressed? All outcomes | High risk | Available case |
Garrett 1994.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: New Zealand, deprived area of Auckland.
DURATION OF STUDY: 9 months Outcome assessors and the child's doctor were blinded |
|
| Participants | N SCREENED: 980 N RANDOMISED: 500 (treatment: 251; control: 249) N COMPLETED: 451 (500 for hospital data) M = 210 F = 290 MEAN AGE: Range 2 to 55 years BASELINE DETAILS: 11% had hospital admission, 28% ED visit, and 41% had an acute attack requiring GP care in previous 9 months INCLUSION CRITERIA: 2 to 55 years, attending ED for treatment of acute asthma and lived within catchment area of hospital, able to answer questionnaire in English, intended to reside in South Auckland for next 9 months, and could be contacted within 5 days of ED attendance EXCLUSION CRITERIA: Not stated | |
| Interventions | EDUCATION GROUP: Community education centre run by a nurse and 3 community health workers; information; self‐management skills; patients referred to their GP if changes in medication required and/or to obtain a written action plan if they didn't have one. Patient's social, financial needs and cultural beliefs assessed and addressed within programme.
Patient plus other members of household included if possible; delivered as soon as possible after attendance at ED
Duration: when all education topics completed, median number of interactions was 3 (range 1 to 10). Time period not stated. CONTROL GROUP: Usual care TREATMENT PERIOD: Not stated FOLLOW‐UP PERIOD: 9 months |
|
| Outcomes | Hospital admissions, ED visits, acute attacks requiring GP care, and days lost from work or school ‐ measured for 9 months from beginning of intervention. Cough during day (for 2 to 14 year olds), PEF variability, breathlessness with exercise, night awakenings ‐ measured for 1 week before 9 month interview. Knowledge, inhaler technique, quality of life (data not given) ‐ measured at 9 months after beginning of intervention. Time period of intervention not stated so not sure about overlap between intervention and measurement of outcomes. | |
| Notes | About 50% to 60% of data refers to children | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random numbers schedule |
| Allocation concealment? | Low risk | Centrally prepared by person not involved in recruiting participants |
| Incomplete outcome data addressed? All outcomes | Low risk | Complete set of data for hospital contact outcomes |
Ghosh 1998.
| Methods | STUDY DESIGN: Parallel group
LOCATION, NUMBER OF CENTRES: Single outpatient clinic in India
DURATION OF STUDY: 12 months
CONCEALMENT OF ALLOCATION: Unclear
DESCRIBED AS RANDOMISED: Yes
METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not described
DESCRIPTION OF WITHDRAWALS/DROPOUTS: Not stated
TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): No blinding of outcome assessor |
|
| Participants | N SCREENED: Not reported N RANDOMISED: 83 (intervention: 45; control: 38) N COMPLETED: Not reported M = Not clear F = Not clear MEAN AGE: Not available BASELINE DETAILS: Not available INCLUSION CRITERIA: 10 to 45 years; > 15% improvement in FEV1 predicted post‐SABA; diurnal variation in PEFR > 20%; 1 or more hospitalisations/emergency room visits in year prior to the study; drug therapy for at least 50% of days in month EXCLUSION CRITERIA: Chronic respiratory infections; COPD; multisystem disorders, smoking history | |
| Interventions | EDUCATION GROUP: Self‐management training (SMT). 4 sessions (2 hours duration) of asthma SMT education & training sessions during first month following the baseline interview. Training delivered by social scientist under guidance of a physician. Participants trained to adjust treatment depending on severity of disease. CONTROL GROUP: Usual care TREATMENT PERIOD: 4 weeks FOLLOW‐UP PERIOD: 12 months |
|
| Outcomes | PEF; hospitalisations/ER visits; cost | |
| Notes | Age 10 to 45; unable to separate out data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Incomplete outcome data addressed? All outcomes | High risk | Available case (assumed) |
Gorelick 2006.
| Methods | STUDY DESIGN: Parallel group 3‐arm study.
LOCATION, NUMBER OF CENTRES: Single centre in Milwaukee, USA
DURATION OF STUDY: 6 months Outcome assessors were blinded to treatment group allocation |
|
| Participants | N SCREENED: 617 N RANDOMISED: 352 N COMPLETED: 275 (baseline presented for completers: PCP group: 95; case manager group: 81; usual care: 99) M = 180 F = 95 MEAN AGE: 6.8 years BASELINE DETAILS: 69% African‐American; Median hospitalisations in past year: 2; 40% live in household with a smoker; 60% have public insurance INCLUSION CRITERIA: 2 to 18 years; treated at Children's Hospital of Wisconsin ED for acute asthma EXCLUSION CRITERIA: Families in which none of the primary care givers were English‐speaking; other lung disease; presence of tracheostomy; previous treatment with case manager | |
| Interventions | EDUCATION GROUP 1 (PCP group): Educational intervention comprising: videotape shown during ED visit, teaching of proper use of peak‐flow meter & inhaler technique instruction, provision of acute asthma medications, instruction to follow‐up with primary care provider (PCP) within 1 week, written asthma care plan; 2. Intensive primary care linkage: copy of the ED chart & letter recommending asthma care plan, sent to primary care provider's (PCP) office; PCP contacted to establish whether follow‐up appointment had been made. Contact made with participants to ask whether appointment had been scheduled and assistance offered if this had not been done; follow‐up calls repeated until appointment had been reported. Visit verified with PCP; final contact made at 14 days to establish that PCP visit had taken place. In absence of PCP, parents instructed to contact insurance company for approved PCP or where no insurance/Medicaid contact recommended with clinics accepting new patients in the area. EDUCATION GROUP 2 (Case manager group): Same interventions as listed for 1 and 2 above, plus: 3. Assignment to case manager who made home visits & telephone calls during the 6‐month follow‐up period. During these visits and calls, the case manager assessed asthma needs; instigated personalised care plan for all the family; provided asthma education by using a pack of educational materials and made onwards referrals as appropriate. CONTROL GROUP: Usual care including educational intervention and discharge planning as detailed in PCP and 1 TREATMENT PERIOD: For PCP group: 14 days; for case manager group: 6 months. FOLLOW‐UP PERIOD: 6 months post‐ED visit |
|
| Outcomes | ED visits; quality of life | |
| Notes | Average visits 4 per patient in case manager group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated list |
| Allocation concealment? | Low risk | Sequentially number opaque sealed envelope |
| Incomplete outcome data addressed? All outcomes | High risk | Available case |
Greineder 1999.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: USA, Hospitals in New England
DURATION OF STUDY: 24 months No blinding of outcome assessor |
|
| Participants | N SCREENED: Not reported N RANDOMISED: 57 (18 of which were identified from index hospitalisation: intervention: 9; control: 9) N COMPLETED: 18 M = 8 F = 10 MEAN AGE: 4 years BASELINE DETAILS: Not available for hospitalised participants INCLUSION CRITERIA: Hospitalisation within one year of study enrolment EXCLUSION CRITERIA: Not reported | |
| Interventions | EDUCATION GROUP: Child and family received educational programme with advice on triggers, warning signs and maintenance medication in an initial session. Outreach follow up was by specialist nurse care over 12‐month period with educational and reinforcement components. Setting: Outpatient clinic CONTROL GROUP: Child and family had the same educational session as described above, but no contact from outreach nurse. TREATMENT PERIOD: 12 months FOLLOW‐UP PERIOD: 12 months |
|
| Outcomes | Hospitalisation; cost | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Paired randomisation sequence from random numbers table |
| Allocation concealment? | Unclear risk | Information not available |
| Incomplete outcome data addressed? All outcomes | Low risk | All participants completed |
Harish 2001.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: Paediatric ED at urban hospital USA
DURATION OF STUDY: 24 months Outcome assessors blinded to treatment group allocation |
|
| Participants | N SCREENED: 300 N RANDOMISED: 298 (NB 129 analysed). N COMPLETED: 129 M= Not reported F= Not reported MEAN AGE: Not reported BASELINE DETAILS: Not reported INCLUSION CRITERIA: 2 to 17 years; ED attendance with acute asthma EXCLUSION CRITERIA: Not reported | |
| Interventions | EDUCATION GROUP: 3 x 1 hour visits 2 weeks apart, including a review of treatment regimens, inhaler technique, use of PEF meter, skin‐prick test and provision of allergen control measures; encouragement to telephone specialist centre for advice regarding symptoms. Education delivered by nurses. Setting: Outpatient clinic CONTROL GROUP: Usual care TREATMENT PERIOD: 6 weeks FOLLOW‐UP PERIOD: 12 months and 24 months |
|
| Outcomes | ED visits; hospitalisations | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Date of birth |
| Allocation concealment? | High risk | Date of birth; even date of birth ‐ intervention; odd date of birth ‐ control |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Available case |
Homer 2000.
| Methods | STUDY DESIGN: Parallel group
LOCATION, NUMBER OF CENTRES: USA; primary care clinic at Children's Hospital & affiliated local health centre
DURATION OF STUDY: 10 months No blinding of outcome assessor |
|
| Participants | N SCREENED: 471 approached N RANDOMISED: 137 (treatment: 76; control: 61) N COMPLETED: 106 M = 95 F = 42 MEAN AGE: 7.4 years BASELINE DETAILS: African American: 61%; private health insurance: 13.3% INCLUSION CRITERIA: 3 to 12 years; any outpatient visits, emergency department visits, or inpatient admissions for asthma during the year prior to enrolment EXCLUSION CRITERIA: Significant co‐morbid lung disease; residence outside of Boston/surrounding communities; involvement in other clinical research in asthma | |
| Interventions | EDUCATION GROUP: Interactive educational computer programme imparting knowledge of symptom recognition, identification of allergens, medication use, appropriate use of health services & normal activity. Children exposed to computer programme over 3 visits. Setting: Hospital CONTROL GROUP: Follow up on 3 occasions (usual clinical assessment) TREATMENT PERIOD: 3 visits FOLLOW‐UP PERIOD: 10 months |
|
| Outcomes | Emergency visits; knowledge; withdrawals; availability of PEF meter | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Computer‐generated randomisation lists at each site; within each site children stratified on age (above or below 7 years of age) |
| Allocation concealment? | Low risk | Study assignment contained in sealed, opaque envelope |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Available case (assumed) |
Karnick 2007.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: USA, Mount Sinai Hospital ED & referrals to paediatric chest unit
DURATION OF STUDY: 9 months No blinding of outcome assessor |
|
| Participants | N SCREENED: Not reported N RANDOMISED: 212 (intervention i: 68; intervention ii: 70; control: 74) N COMPLETED: 165 M = 127 F = 85 MEAN AGE: 4 years BASELINE DETAILS: Medicaid: 89%; mean ED visits (baseline year): 1.87; hospital admissions: 1.04; unscheduled clinic visits: 2.84 INCLUSION CRITERIA: 1 to 16 years; recruitment through Mount Sinai Hospital ED or referral to specialist paediatric chest unit EXCLUSION CRITERIA: Other significant chronic disease | |
| Interventions | EDUCATION GROUP 1: Reinforced education ‐ 20 to 30‐minute session followed up by regular telephone contact. Participating families were encouraged to call educator. EDUCATION GROUP 2: Reinforced education & case management ‐ 20 to 30‐minute session followed up by regular telephone contact. Participating families were encouraged to call health educator. Case manager/nurse practitioner worked with family on action plan. Called upon if necessary by health educator. CONTROL GROUP: Basic asthma education ‐ 20 to 30‐minute session TREATMENT PERIOD: 9 months FOLLOW‐UP PERIOD: 9 months |
|
| Outcomes | ED visits; hospitalisations; length of hospital stay; cost | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Available case |
Kelly 2000.
| Methods | STUDY DESIGN: Parallel group alternate allocation trial
LOCATION, NUMBER OF CENTRES: USA, Children's hospital
DURATION OF STUDY: 12 months No blinding of outcome assessor |
|
| Participants | N SCREENED: 102 families N RANDOMISED: 80 (baseline reported for 78 children who completed) N COMPLETED: 78 M = 54 F = 24 MEAN AGE: 2 to 5 years: 32; 6 to 10 years: 26; 11 to 15: 20 BASELINE DETAILS: 94% African American; all had Medicaid insurance; regular maintenance therapy: 47%; smoker in household: 48% INCLUSION CRITERIA: 2 to 16 years; ED presentation 2 or more times/hospitalised at least once in previous year; insurance coverage through Medicaid; primary care received in hospital outpatient clinic; not evaluated by an asthma specialist in preceding 2 years EXCLUSION CRITERIA: Not reported | |
| Interventions | EDUCATION GROUP: One‐on‐one session with physician and outreach nurse including emphasis on regular medication use, action plan. Education reinforced during follow‐up by physician and outreach nurse. Outreach nurse followed up with families by phone (or left messages with friends/neighbours where no phone access was possible). Setting: Outpatient clinic and home CONTROL GROUP: Usual care as provided by primary care provider TREATMENT PERIOD: 1 session and subsequent phone calls during data collection (12 months) FOLLOW‐UP PERIOD: 12 months |
|
| Outcomes | ED visits, hospitalisation, quality of life, cost | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Alternate allocation |
| Allocation concealment? | High risk | Alternate allocation |
| Incomplete outcome data addressed? All outcomes | High risk | Available case |
Khan 2004.
| Methods | STUDY DESIGN: Parallel group, single‐blind study
LOCATION, NUMBER OF CENTRES: ED treated children from Sydney Children's Hospital
DURATION OF STUDY: 6 months No blinding of outcome assessor |
|
| Participants | N SCREENED: Not reported N RANDOMISED: 310 (intervention: 155; control: 155) N COMPLETED: 236 M = 178 F = 99 MEAN AGE: 5 years BASELINE DETAILS: ED visits in 6 months prior to study: 1.5; ICS therapy: 34% INCLUSION CRITERIA: Seen and discharged from ED of Sydney Children's Hospital with asthma EXCLUSION CRITERIA: Not reported | |
| Interventions | EDUCATION GROUP: Telephone consultation with experienced asthma educator <2 weeks of return of initial questionnaires by parents; intervention aimed to empower family & reinforce advice given to parents at ED discharge. Emphasis made on importance of regular maintenance therapy. Setting: Home CONTROL GROUP: Usual care + WAP Both groups received written action plan TREATMENT PERIOD: 1 phone call of between 5 and 44 minutes duration FOLLOW‐UP PERIOD: 6 months |
|
| Outcomes | ED visits; hospitalisation; symptoms | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Incomplete outcome data addressed? All outcomes | High risk | Available case |
Kinlow 2001.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: Unclear
DURATION OF STUDY: Not reported Blinding of outcome assessor could not be ascertained |
|
| Participants | N SCREENED: Not reported N RANDOMISED: 47 (distribution between intervention and control groups not clear) N COMPLETED: Not clear M = Not clear F = Not clear MEAN AGE: Not reported BASELINE DETAILS: 98% African American INCLUSION CRITERIA: 8 to 18 years EXCLUSION CRITERIA: Not reported | |
| Interventions | EDUCATION GROUP: STARBRIGHT an interactive computer assisted programme including education and peer support Setting: Not clear CONTROL GROUP: Usual care TREATMENT PERIOD: Not reported FOLLOW‐UP PERIOD: Not reported |
|
| Outcomes | Knowledge scores; satisfaction with intervention | |
| Notes | Abstract only Asthma & sickle cell disease | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Randomisation according to time of hospitalisation |
| Allocation concealment? | High risk | See above |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Information not available |
Madge 1997.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: Single centre in urban area of UK
DURATION OF STUDY: Not reported Blinding of outcome assessor not described |
|
| Participants | N SCREENED: 201 N RANDOMISED: 201 (treatment: 96; control: 105) N COMPLETED: 201 (hospital data); 129 (questionnaire) M = 124 F = 77 MEDIAN AGE: 5 BASELINE DETAILS: Median (range) number of previous admissions: intervention 2 (0 to 8) control 2 (0 to 19) INCLUSION CRITERIA: >= 2 years admitted to a children's hospital for acute asthma EXCLUSION CRITERIA: Children admitted on a weekend | |
| Interventions | EDUCATION GROUP: Type: asthma management training programme by specialist asthma nurse: information (written and interactive); instruction in self‐monitoring of PEF (> 5 years) and/or symptoms; short course of oral steroids with guidance on when to start them; written action plan; 1 review session at nurse‐run asthma clinic and telephone advice after discharge Parents and children included; delivered during admission and continued at home Duration: about 45 minutes over 2 to 3 meetings, plus 1 follow‐up clinic visit and telephone advice as required CONTROL GROUP: Usual care TREATMENT PERIOD: 2 to 3 weeks FOLLOW‐UP PERIOD: 14 months |
|
| Outcomes | Hospital admissions, ED visits ‐ measured for between 2 and 14 months from discharge, i.e. AFTER intervention completed. Urgent GP visit within 3 to 4 weeks from discharge. Day and night morbidity scores, disability score ‐ measured at 3 to 4 weeks following discharge, i.e.. AFTER intervention completed. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Drawing cards to allocate each sequential future admission to intervention or control |
| Allocation concealment? | High risk | Open list |
| Incomplete outcome data addressed? All outcomes | Low risk | Complete for hospital data; available case for other endpoints |
McNabb 1985.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: Single centre in urban area of UK
DURATION OF STUDY: Not reported Outcome assessors were blinded |
|
| Participants | N SCREENED: 16 N RANDOMISED: 16 (treatment: 8; control: 8) N COMPLETED: 14 M = 11 F = 3 MEAN AGE: 10.5 BASELINE DETAILS: Not stated INCLUSION CRITERIA: Children with asthma, 9 to 13 years, >= 1 emergency treatment for asthma in previous year, using bronchodilators, recruited from 2 allergy clinics. Author confirmed that all had ED visit within previous 12 months. EXCLUSION CRITERIA: Known developmental or behavioural problems | |
| Interventions | EDUCATION GROUP: 30‐minute diagnostic interview followed by 4 individually tailored educational sessions on the self‐management of asthma (4 weekly sessions): included information, goal setting, self‐evaluation and self‐monitoring. The programme (known as AIR WISE) also included: assessment of medications (although generally not changed) and an action plan. Interviews conducted in allergy clinic. Children were the focus but the child's parents and physician included in the process. CONTROL GROUP: Usual care TREATMENT PERIOD: 4 weeks FOLLOW‐UP PERIOD: 12 months |
|
| Outcomes | Knowledge (no data given), emergency treatments for asthma, asthma drug regimen (no data given), dollars saved by reduced number of emergency treatments minus cost of programme ‐ measured for 12 months AFTER intervention completed | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Randomised by coin toss |
| Allocation concealment? | High risk | External experimenter; patients matched on: clinic where enrolled, number of emergency treatments for asthma in previous 12 months, asthma medication regimen and age |
| Incomplete outcome data addressed? All outcomes | High risk | Available case |
Mitchell 1986.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: Single centre in multiethnic area of New Zealand
DURATION OF STUDY: Not reported Outcome assessors were blinded |
|
| Participants | N SCREENED: Not reported N RANDOMISED: 368 N COMPLETED: 368 (hospital data); 259 (questionnaire) M = Not stated (ratio of M:F given for Europeans 1.4:1 and Polynesians: 1.6:1) F = Not stated MEAN AGE: 6 BASELINE DETAILS: Not stated INCLUSION CRITERIA: 2 to 14 years, admitted to hospital for asthma EXCLUSION CRITERIA: Lived outside catchment area of hospital, previous life threatening attack of asthma; known developmental or behavioural problems | |
| Interventions | EDUCATION GROUP: Monthly home visits by a community child health nurse; information only, including encouragement to attend GP or clinic follow up visits and to consult GP for asthma attacks rather than going to the ED. Children and their families included; delivered following hospital admission at home. 6 visits were made over 6 months ‐ duration of visit not specified. About 50% to 70% of patients had all 6 visits. CONTROL GROUP: Usual care TREATMENT PERIOD: 6 months FOLLOW‐UP PERIOD: 12 months post‐intervention |
|
| Outcomes | Hospital readmissions ‐ measured for the 6‐month period of the intervention (data not used in this review) and for 12 months AFTER intervention completed. Urgent treatment for asthma attack, days off school ‐ measured DURING the 6‐month period of the intervention. Knowledge, current asthma drug treatment (sympathomimetics, oral steroids, inhaled steroids, cromoglycate) ‐ measured at the end of the intervention. Data stratified by ethnicity (Polynesian, European) but combined for the meta‐analysis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random numbers, first stratified by ethnicity (Polynesian, European) |
| Allocation concealment? | Low risk | Done without knowledge of patient details |
| Incomplete outcome data addressed? All outcomes | Low risk | Complete for hospital data. Available case for questionnaire. |
NCICAS.
| Methods | STUDY DESIGN: Parallel group
LOCATION, NUMBER OF CENTRES: 8 sites located in inner city American conurbations
DURATION OF STUDY: 2 years Outcome assessors were blinded |
|
| Participants | N SCREENED: 2847 N RANDOMISED: 1033 (treatment: 515; control: 518) N COMPLETED: Not clear M = 661 F = 372 MEAN AGE: 7.7 BASELINE DETAILS: African American: 75%; caretaker smokes: 42%; hospitalisation in previous month: 4.5% INCLUSION CRITERIA: English/Spanish‐speaking; 5 to 11 years; physician‐diagnosed asthma; resident in inner city; use 2 or more medications for asthma, asthma hospitalisation and one unscheduled visit for asthma in 6 months prior to study. Alternatively child had to have symptoms for more than 2 days/sleep disruption for more than 2 nights during 2 weeks prior to study entry EXCLUSION CRITERIA: Not stated | |
| Interventions | EDUCATION GROUP: Intervention delivered to caretaker of child by counsellor who encouraged better communication between family and physician. Primary care physician sent asthma care plan, a spacer, a peak flow meter, and asthma guidelines. Caretakers invited to attend 2 group sessions and individual meeting with their counsellor during 2 months after baseline. Group sessions covered triggers, environmental controls, asthma physiology, strategies for problem solving, and communicating with their child's physician. Children participated in group sessions during following 2‐month period. Additionally, bedding provided to families in intervention group & encouraged to minimise exposure to environmental triggers (tobacco and pet exposure). Counsellor maintained contact with families via telephone every 2 months, tailoring contact based on risk assessment (allergen and trigger exposure, access to care, adherence) Setting: Home CONTROL GROUP: Usual care Arrangements made to assign a primary care physician for participants in both the intervention and control groups without one TREATMENT PERIOD: 4 months FOLLOW‐UP PERIOD: 2 years |
|
| Outcomes | Symptoms; ED visits; hospitalisation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Block randomisation within site |
| Allocation concealment? | Unclear risk | Information not available |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Intention‐to‐treat analysis; no explicit description of how data were analysed for hospital contact outcomes |
Ng 2006.
| Methods | STUDY DESIGN: Parallel group
LOCATION, NUMBER OF CENTRES: Single centre in Hong Kong.
DURATION OF STUDY: 3 months Outcome assessors were blinded |
|
| Participants | N SCREENED: Not clear N RANDOMISED: 100 (treatment: 45; control: 55) N COMPLETED: 100 M = 74 F = 26 MEAN AGE: 2 to 5 years: 68; 6 to 9 years: 24; 10 to 15 years: 8 BASELINE DETAILS: Mild and mild to moderate asthma INCLUSION CRITERIA: 2 to 15 years; admitted with an acute asthmatic attack EXCLUSION CRITERIA: Children with severe acute asthma requiring intensive care; non‐Chinese speakers | |
| Interventions | EDUCATION GROUP: 6 components: (i) contact with Asthma Nurse < 24 hours post‐admission; ii) booklet with same information & action plan with modified cartoon figures. Asthma diary given to parents (iii) video intervention; (iv) 30‐minute teaching & discussion session; v) assessment of inhaler technique & reinforcement of knowledge of asthma prior to discharge; (vi) telephone follow up 1 week after discharge Setting: Hospital & home CONTROL GROUP: 3 components (i) Asthma Nurse acted 1 to 2 days after admission; (ii) information sheet describing nature of asthma, avoidance of triggers, usage of medication, & steps to be take in acute asthmatic attack. Asthma diary given to parents; (iii) 30‐minute teaching & discussion session. TREATMENT PERIOD: 1 to 2 days (in hospital) FOLLOW‐UP PERIOD: 3 months |
|
| Outcomes | ED visits; hospitalisation; compliance; school absence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated random‐number table |
| Allocation concealment? | Unclear risk | Information not available |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Information not available |
Shames 2004.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: 3 centres serving low‐income families in San Francisco, USA
DURATION OF STUDY: 12 months
CONCEALMENT OF ALLOCATION: Unclear
DESCRIBED AS RANDOMISED: Yes
METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE:
DESCRIPTION OF WITHDRAWALS/DROPOUTS: Stated
TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT No blinding of outcome assessor |
|
| Participants | N SCREENED: Not reported N RANDOMISED: 119 (intervention: 59; control: 60) N COMPLETED: 97 M = 69 F = 50 MEAN AGE: 8 years BASELINE DETAILS: Hispanic: 57%; African American: 21%; Medicaid: 71.5%; INCLUSION CRITERIA: Moderate‐severe asthma; low‐income family; 5 to 12 years; covered by state health insurance or eligible for state insurance; history of asthma > 6 months; hospitalisation or > 2 ED visits for asthma in previous year EXCLUSION CRITERIA: Children under the care of allergist/pulmonary specialist | |
| Interventions | EDUCATION GROUP: Disease management programme including assignment to a case manager who delivered a 3‐session course. Case manager also maintained dialogue over 32 weeks of study. Participants also given computer game aimed to improve asthma; 2 visits to specialist; telephone advice line staffed 18 hours/day by specialists. Setting: Home CONTROL GROUP: Usual care and non‐violent computer game TREATMENT PERIOD: 32 weeks (duration of availability of case manager) FOLLOW‐UP PERIOD: 12 months |
|
| Outcomes | ED visits; symptoms; lung function; quality of life; knowledge scores | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Block randomisation to generate balance between younger and older children |
| Allocation concealment? | Unclear risk | Information not available |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Described as intention‐to‐treat; no explicit description of how this population was composed |
Smith 2004.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: Urban ED in USA
DURATION OF STUDY: 6 months Outcome assessors were blinded |
|
| Participants | N SCREENED: 702 N RANDOMISED: 543 (of which 527 enrolled) N COMPLETED: 302 M = 349 F = 178 MEAN AGE: 6.4 years BASELINE DETAILS: 92% African American; 92% Medicaid; INCLUSION CRITERIA: 2 to 12 years; Medicaid or no medical insurance EXCLUSION CRITERIA: Admission to hospital during index ED visit; chronic illness other than asthma; no working telephone in the home; participation in another asthma study; no primary care physician; parents unable to communicate effectively in English | |
| Interventions | EDUCATION GROUP: 2 follow‐up phone calls and monetary incentive delivered by health educator. Call on day 2 (2‐day call) and the other on day 5 (5‐day call) post‐index ED visit. Coach reinforced importance of PCP follow up and discussed advantages of seeking follow‐up care with child's PCP. Strategies to overcome barriers to follow‐up care mentioned by the parents also discussed. Setting: Home CONTROL GROUP: Usual care TREATMENT PERIOD: 5 days FOLLOW‐UP PERIOD: 6 months |
|
| Outcomes | ED visit; scheduled attendance with primary care provider | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Information not available |
| Allocation concealment? | Low risk | Information not available |
| Incomplete outcome data addressed? All outcomes | Low risk | All participants analysed from audit checks |
Smith 2006.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: Urban ED in USA
DURATION OF STUDY: 2 weeks Outcome assessors were blinded |
|
| Participants | N SCREENED: Not reported N RANDOMISED: 92 N COMPLETED: 86 M = 54 F = 38 MEAN AGE: 6.5 years BASELINE DETAILS: 90% African American; 97% Medicaid INCLUSION CRITERIA: 2 to 12 years of age; Medicaid or no insurance cover; presenting to ED requiring bronchodilator therapy for acute asthma EXCLUSION CRITERIA: Admission to hospital during index ED visit; chronic illness other than asthma; no working telephone in the home; participation in another asthma study; no primary care physician; parents unable to communicate effectively in English | |
| Interventions | EDUCATION GROUP: Parental coaching during ED visit and monetary incentive. Coach asked questions of parent regarding perceptions of ED visit and discussed advantages of follow up with PCP. Coach included discussion of barriers to follow up. Setting: Hospital CONTROL GROUP: Usual care TREATMENT PERIOD: In ED. Both groups were reminded of importance of follow‐up with PCP. FOLLOW‐UP PERIOD: 2 weeks |
|
| Outcomes | Scheduled attendance at PCP; unscheduled attendance at PCP office with acute asthma | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomisation schedule. |
| Allocation concealment? | Unclear risk | Information not available |
| Incomplete outcome data addressed? All outcomes | High risk | Available case |
Sockrider 2006.
| Methods | STUDY DESIGN: Parallel group
LOCATION, NUMBER OF CENTRES: 4 clinical sites in Texas, USA
DURATION OF STUDY: 12 months No blinding of outcome assessors |
|
| Participants | N SCREENED: Not reported N RANDOMISED: 464 (intervention: 263; control: 201) N COMPLETED: 218 M = 294 F = 170 MEAN AGE: 6.56 years BASELINE DETAILS: African American: 54.7%; Hispanic: 28.7%; insured/uninsured: 85.3/14.7% INCLUSION CRITERIA: Presentation to ED with acute asthma; 1 to 18 years; diagnosed asthma; care giver should have been able to speak English EXCLUSION CRITERIA: Diagnosis of another chronic lung or cardiovascular disease | |
| Interventions | EDUCATION GROUP: ED self‐management intervention focusing individualised content based around triggers and therapy regimens. Delivered in ED as a computer‐based programme, with follow‐up telephone call 1 to 2 weeks after the visit by educator. Follow‐up phone call made by trained educator who also constructs a written action plan for the child. All materials are available in English and Spanish. Telephone advice line set up for participants in intervention group. Setting: Hospital & home CONTROL GROUP: Usual care TREATMENT PERIOD: 1 to 2 weeks post‐discharge FOLLOW‐UP PERIOD: 12 months (data reported for 9 month outcome) |
|
| Outcomes | Quality of life; ED visits; hospitalisation | |
| Notes | Data incomplete ‐ study presented as preliminary analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Incomplete outcome data addressed? All outcomes | High risk | Available case: "Medical chart reviews of health care utilization were unavailable from community hospitals not participating in the network, and therefore it was not possible to discern possible underreporting by caregivers" |
Stevens 2002.
| Methods | STUDY DESIGN: Prospective, randomised, partly blinded, controlled trial
LOCATION, NUMBER OF CENTRES: UK; Children's Hospital, Leicester Royal Infirmary, Booth Hall Children's Hospital, Manchester
DURATION OF STUDY: 12 months Outcome assessors were blinded |
|
| Participants | N SCREENED: 595 N RANDOMISED: 200 (101 intervention; 99 control) N COMPLETED: Intervention ‐ successful follow up at 3 months = 82, 6 months = 88, 12 months = 90. Control at 3 months = 83, 6 months = 82, 12 months = 87 M = 134 F = 66 MEAN AGE: 32 months (2.7 years) BASELINE DETAILS: Previous hospital admissions, pattern & severity of asthma symptoms, atopic disease, precipitating factors for wheeze, medication on discharge; parent's recall of information delivered about asthma on discharge, who delivered, how long it took, written or verbal, its usefulness. INCLUSION CRITERIA: 18 months to 5 years, recruited on admission to hospital or presentation to ED or Children's Assessment Unit (CAU) for acute severe asthma or wheeze EXCLUSION CRITERIA: Not stated | |
| Interventions | EDUCATION GROUP: Given by nurse specialist (1) a general education booklet about asthma in pre‐school children; (2) a written guided self‐management plan; (3) 2 20‐minute structured educational sessions given on a one to one basis to the parent(s) and child. Setting: Hospital and clinic CONTROL GROUP: Usual care, range of advice TREATMENT PERIOD: Inpatients received the first session on the ward on the day of discharge and returned 1 month later for the second session. Children recruited from A&E/CAU received their initial education session in the outpatient clinic within 2 weeks of attendance at A&E/CAU and returned 1 month later for their second visit. FOLLOW‐UP PERIOD: 12 months |
|
| Outcomes | Outcomes were measured at 3, 6 and 12 months. Primary outcomes: GP consultation rates, hospital readmissions, attendances at A&E or CAU. Secondary outcome measures included the child's asthma symptoms and consequent level of disability and quality of life. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Information not available |
| Allocation concealment? | Low risk | Numerical codes in random blocks of 10, delivered in sealed envelopes |
| Incomplete outcome data addressed? All outcomes | Unclear risk | N analysed for hospital contact data outcomes > N completing the study. Information on whether audit checks picked up missing data not explained. |
Talabere 1993.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: Single centre in Spain
DURATION OF STUDY: 12 weeks No blinding of outcome assessors |
|
| Participants | N SCREENED: Not reported N RANDOMISED: 50 (treatment: 25; control: 25) N COMPLETED: 50 M = Not reported F = Not reported MEAN AGE: 32 months (2.7 years) BASELINE DETAILS: Mean (SD) number of emergency health care visits in 12 weeks prior to study: intervention 1.5 (0.8), control 2.0 (1.0) INCLUSION CRITERIA: 8 to 12 years, recent ED visit or admission (to inpatient unit that offered the Asthma Education Program) for asthma at the participating hospital EXCLUSION CRITERIA: Additional chronic health problems, needed a community health nurse referral for post‐discharge follow up, or were participating in a concurrent asthma education programme | |
| Interventions | EDUCATION GROUP: Asthma education programme; conducted by nurses after training from researcher plus previous experience, or by the researcher (who was also a nurse); information only (written and interactive). Parents and children included; delivered at earliest mutually convenient time (for those admitted, it was done during the hospitalisation). Intervevention delivered over 2 1‐hour sessions. CONTROL GROUP: Usual care TREATMENT PERIOD: Not stated (2 x 1 hour sessions) FOLLOW‐UP PERIOD: 12 weeks |
|
| Outcomes | Hospital admissions, emergency health care visits, altered breathing episodes, medication use (no data given), school absences ‐ measured for 12 weeks AFTER intervention completed. Child asthma knowledge ‐ measured at 12 weeks AFTER intervention completed. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Coin toss |
| Allocation concealment? | High risk | Blocking to control for gender, race and age; allocation by coin toss in presence of investigator and family |
| Incomplete outcome data addressed? All outcomes | Low risk | Complete set of data |
Teach 2006.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: USA, Children's National Medical Centre, Washington DC
DURATION OF STUDY: 6 months
CONCEALMENT OF ALLOCATION: Adequate
DESCRIBED AS RANDOMISED: Yes
METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Stated
DESCRIPTION OF WITHDRAWALS/DROPOUTS: Not clear
TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT Outcome assessors blinded |
|
| Participants | N SCREENED: 2791
N RANDOMISED: 490 (244 intervention, 244 control)
N COMPLETED: 437 (219 intervention, 218 control)
M = 63.9%
F = 36.1% MEAN AGE: Not available BASELINE DETAILS: 86% African‐American; 43% households annual income <30000 USD; 52% participants used ED > 3 times in previous year INCLUSION CRITERIA: age 1 to 17 years, prior physician diagnosed asthma; >/= 1 unscheduled visits for acute asthma last 6 months or 1 or > admission to hospital last 12 months; a parent or guardian available; residence in Washington, DC; requirement for 3 or more doses of nebulised albuterol in the ED at the time of enrolment EXCLUSION CRITERIA: significant medical conditions of CVS/RESP system; specialist visit in last 6 months; 2 or more of the following: a current written asthma medical action plan, current use of more than 1 controller medication, or a scheduled visit for asthma care with their PCP in the prior 2 weeks; enrolment in another asthma research study; unavailability for telephone follow up; unable to peak English or Spanish |
|
| Interventions | EDUCATION GROUP: Asthma self‐monitoring & management, environmental modification & trigger control, links/referrals to PCP (follow up with PCP arranged within 3 weeks, hypoallergenic mattress casing given, phone follow up at 1, 3 and 6 months), delivered by health educator Setting: Clinic & home CONTROL GROUP: Asthma education book, no follow up TREATMENT PERIOD: Single visit to IMPACT DC asthma clinic located in ED 60 to 90 minutes 2 to 15 days after ED visit FOLLOW‐UP PERIOD: 6 months |
|
| Outcomes | Unscheduled visits for acute asthma; secondary ‐ hospital admissions, scheduled PCP visits, asthma medication and device use, efforts to control asthma triggers in the home, linkages to care providers, asthma classification by NHLBI criteria, current asthma symptoms, and asthma QOL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Each batch of 30 envelopes was then exhaustively shuffled and numbered with participant identification numbers. During enrolment, the research assistants opened each sequential envelope after informed consent and assent was obtained and after the baseline interview was conducted" |
| Allocation concealment? | Low risk | Opaque, sealed envelopes |
| Incomplete outcome data addressed? All outcomes | Unclear risk | "All outcomes were analyzed among those completing follow‐up for the relevant period using an intention‐to‐treat paradigm" |
Walders 2006.
| Methods | STUDY DESIGN: Parallel group randomised controlled trial
LOCATION, NUMBER OF CENTRES: 1 ‐ USA, Cleveland, Ohio
DURATION OF STUDY: 12 months
CONCEALMENT OF ALLOCATION: Not clear
DESCRIBED AS RANDOMISED: Yes
METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE:
DESCRIPTION OF WITHDRAWALS/DROPOUTS: Not stated/not clear
TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): Permuted block randomisation scheme according to age Outcome assessors blinded |
|
| Participants | N SCREENED: Not clear (327 eligible families asked to participate, 216 attended baseline visit) N RANDOMISED: 175 (89 intervention, 86 control) N COMPLETED: 83 of 89 in intervention group M = 126 F = 49 MEAN AGE: 7.3 BASELINE DETAILS: English speaking children, 4 to 12 years, physician diagnosed asthma > 3months INCLUSION CRITERIA: (1) 2 or more emergency department visits for asthma in the past year and/or (2) 1 or more asthma hospitalisations in the past year; and (3) the lack of an asthma treatment plan EXCLUSION CRITERIA: Under specialist care, near fatal asthma, co‐morbid conditions | |
| Interventions | EDUCATION GROUP: WAP, PFM, spacer device, treatment group also 1‐hour education on asthma (pathophysiology, triggers, treatment). Intervention group visit 3 1 week later for problem‐solving session based on ARP (asthma risk profile), access to 24‐hour nurse run helpline Setting: Clinic CONTROL GROUP: WAP, PFM, spacer education in visit 2 TREATMENT PERIOD: Baseline visit ‐ info gathering, 2‐week run‐in period then visit 2 for education/PFM and spacer device training. 3 weeks in total for 3 visits. FOLLOW‐UP PERIOD: 12 months (telephone at 2, 4, 8, 10 months, clinic visit at 6 & 12 months) |
|
| Outcomes | Primary ‐ asthma symptom reports; secondary ‐ health care utilisation & QOL | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Permuted block randomisation scheme according to age"' |
| Allocation concealment? | Unclear risk | Information not available |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Described as intention‐to‐treat analysis; explicit description of how this population was defined is not provided |
Warschburger 2003.
| Methods | STUDY DESIGN: Parallel group design
LOCATION, NUMBER OF CENTRES: Germany; 4 inpatient rehabilitation units
DURATION OF STUDY: 24 weeks No blinding of outcome assessors |
|
| Participants | N SCREENED: 242 N RANDOMISED: 185 (treatment: 85; control: 100) N COMPLETED: 140 M = 128 F = 57 MEAN AGE: 4.4 BASELINE DETAILS: Age, gender, functional severity, asthma severity, duration of symptoms, care giver demographics INCLUSION CRITERIA: Parents with at least 1 child under the age of 8 and diagnosed with asthma. For inclusion in the study, the care givers had to: (1) have asthma management responsibilities for their child, and (2) have not previously participated in a formal asthma health education | |
| Interventions | EXPERIMENTAL GROUP: The intensified BASE‐program (''Bremer asthma training for parents'') comprises 6 sessions of 90 minutes, including training in perception of early warning signs; trigger identification; medication delivery; and non‐pharmacological techniques for handling asthma symptoms, as well as management of stress Setting: Hospital CONTROL GROUP: Information‐centered standard programme= 2 x 90‐minute sessions of educational material. The main focus lies in improving the asthma‐specific knowledge of the parents. Teaching methods through modelling & persuasive communication. TREATMENT PERIOD: 3 to 4 weeks FOLLOW‐UP PERIOD: 24 weeks |
|
| Outcomes | Parental knowledge; parental QOL; functional severity of the children | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Participants allocated on basis of arrival date |
| Allocation concealment? | High risk | Open list |
| Incomplete outcome data addressed? All outcomes | High risk | Available case |
Wesseldine 1999.
| Methods | STUDY DESIGN: Parallel design, controlled trial
LOCATION, NUMBER OF CENTRES ‐ Leicester UK, Children's hospital
DURATION OF STUDY:18 months Outcome assessors blinded |
|
| Participants | N SCREENED: Not reported N RANDOMISED: 160 (treatment: 80; control: 80) N COMPLETED: 160 M = 98 F = 62 MEAN AGE: Range: 2 to 16 years BASELINE DETAILS: Previous ED visit: intervention 23%, control 19%; hospital admission in previous 6 months: intervention 20%, control 24% INCLUSION CRITERIA: 2 to 16 years, admitted to a children's hospital for asthma during 1996 EXCLUSION CRITERIA: Not reported | |
| Interventions | EDUCATION GROUP: Type: structured discharge package by trained children's asthma nurse, consisting of information (written and interactive); instruction in self‐management; individual written action plan, which allowed medication to be adjusted according to symptoms and peak flow (for children over 7 to 8 years) Children and families included; delivered at time of discharge Setting: hospital Duration: 20 minutes; actual mean (SD): 23 (2.9) minutes CONTROL GROUP: Usual care TREATMENT PERIOD: Delivered at discharge FOLLOW‐UP PERIOD: 6 months |
|
| Outcomes | Hospital admissions, ED visits, GP consultations for problematic asthma, and school days lost for any medical illness ‐ measured for 6 months after discharge, i.e. AFTER intervention completed Nocturnal symptoms, activity restrictions also measured but data not given | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated numerical codes in blocks of 10 |
| Allocation concealment? | Low risk | Opaque, sealed envelopes, opened after consent obtained |
| Incomplete outcome data addressed? All outcomes | Low risk | Complete set of data |
Wilson 2001.
| Methods | STUDY DESIGN: Parallel design, controlled trial
LOCATION, NUMBER OF CENTRES: 1 ‐ USA Valley Children's Hospital, California
DURATION OF STUDY: 12 months No blinding of outcomes from assessors |
|
| Participants | N SCREENED: 867 families contacted N RANDOMISED: 87 (44 intervention, 43 control) N COMPLETED: 60 (intervention: 32 of 44 attended all 3 sessions, 2 x 2 sessions, 5 x 1 session, 5 x 0 sessions) M = 44 F = 43 MEAN AGE: 7.2 intervention, 7.5 control BASELINE DETAILS: family demographics, asthma hx, current symptoms, activity limitations, environmental triggers, medications, detailed smoking hx (what, how much, degree of exposure, limitations to smoking around the child) INCLUSION CRITERIA: 3 to 12 years, seen for urgent asthma visit in ED/urgent clinic (PedsPlus) and/or hospital in past 12 months, Medicaid eligible, exposed to ETS, spoke English/Spanish EXCLUSION: Not stated | |
| Interventions | EDUCATION GROUP: Counselling to parents in home where children were exposed to environmental tobacco smoke. 3 behaviourally based education sessions on effects of smoking on asthma & strategies to quit/reduce ETS exposure. Examination/asthma hx & PFT review by pulmonologist. Medications altered to reach national guidelines. Urine cotinine at baseline & 12 months. Pre & post‐bronchodilator PFT at baseline & 12 months. Setting: Clinic CONTROL GROUP: Examination/asthma hx & PFT review by pulmonologist. Medications altered to reach national guidelines. Urine cotinine at baseline & 12 months. Pre & post‐bronchodilator PFT at baseline & 12 months. Basic verbal information about asthma. TRETAMENT PERIOD: 5 weeks (3 sessions) FOLLOW‐UP PERIOD: 12 months |
|
| Outcomes | Emergency/urgent health care utilisation for asthma, ETS exposure by CCR (urine cotinine/creatinine ratio) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomisation in blocks of 4 |
| Allocation concealment? | Unclear risk | Information not available |
| Incomplete outcome data addressed? All outcomes | Low risk | All participants passively observed through their medical records for hospital contact outcomes |
In all studies numbers refer to intervention and control groups, respectively; ARP = asthma risk profile; ATS ‐ American Thoracic Society; ED ‐ Emergency Department; ETS: Envinronmental tobacco smoke; GP ‐ General Practitioner; HMO ‐ Health Maintenance Organisation; hx = history; ITT = intention‐to‐treat; PEF ‐ Peak Expiratory Flow; PCP = primary care provider; QOL = quality of life; RCT ‐ Randomised Controlled Trial; SD ‐ Standard Deviation; SE ‐ Standard Error
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adams 2004 | Sample recruited from ambulatory setting |
| Amirav 1995 | Intervention targeted at physicians |
| Augustin 2003 | Participants with recent ED visits in the treatment groups very low (N = 7). |
| Baren 2001 | Patient population older than that intended for review |
| Baren 2006 | Ways to improve follow up rather than education intervention |
| Bartholomew 2000 | Review article |
| Bartholomew 2006 | Sample recruited from ambulatory setting |
| Bobb 2003 | Patient population older than that intended for review |
| Bonner 2002 | Sample recruited from ambulatory setting |
| Boone 2002 | Sample recruited from ambulatory setting |
| Brook 1993 | Subjects not recruited following ED attendance and not all had an ED visit within previous 12 months (personal correspondence with author) |
| Bryant‐Stephens 2004 | Primarily concerned with environmental remediation |
| Burkhart 2002 | About adherence to peak flow device, clinic based & not about impact of education on asthma control |
| Bynum 2001 | Sample recruited from ambulatory setting |
| Cabana 2005 | Intervention targeted at physicians |
| Caliguiri 2002 | Not randomised |
| Callahan 2003 | Not randomised |
| Cano‐Garcia 2007 | Not recruited from a population with index ED visit |
| Charlton 1990 | Urgent asthma visits restricted to primary care |
| Chen 2004 | Sample recruited from ambulatory setting |
| Clark 2005 | Sample recruited from ambulatory setting |
| Claus 2004 | Not randomised |
| Cohen 1979 | Unable to determine eligibility criteria |
| Cojocaru 2006 | Not randomised |
| Colland 1993 | Sample recruited from ambulatory setting |
| Colland 2004 | Sample recruited from ambulatory setting |
| Cowie 1997 | Patient population older than that intended for review |
| Cunningham 2008 | Assessment of integrated care pathway |
| Dahl 1990 | An ED visit within previous 12 months was not a criteria for entrance into the study. Unable to confirm with author that all subjects had ED visit within previous 12 months |
| Deaves 1993 | Not randomised |
| Delaronde 2005 | Not recruited from ED, not required to go to ED in prior 12 months, mostly adults, some data 13 to 20 years |
| Dolinar 2000 | Sample recruited from ambulatory setting |
| Eggleston 2005 | Environmental intervention |
| Evans 1997 | Intervention targeted at physicians |
| Fireman 1981 | Not randomised |
| Gardida 2002 | Sample recruited from ambulatory setting |
| Gebert 1998 | Non‐randomised design |
| Gerald 2006 | Intervention targeted at school staff and children |
| Gillies 1996 | Sample recruited from ambulatory setting |
| Gonzalez 2003 | Not required to attend ED in past 12 months, clinic based study |
| Guendelman 2002 | Two active interventions |
| Heard 1999 | GP setting with no requirement for ED visit prior to study |
| Hederos 2005 | Not an ED intervention, not required to attend ED for entry criteria |
| Hill 1991 | 3rd party intervention |
| Hockemeyer 2002 | Patient population older than that intended for review |
| Holzheimer 1998 | Sample recruited from ambulatory setting |
| Hughes 1991 | Subjects recruited from hospital admission data but this was within previous 5 years |
| Hung 2002 | Not randomised |
| Huss 2003 | Some recruited from hospital records but not stated what the contact with hospital was for |
| ICAS | Intervention primarily concerned with environmental intervention |
| Irvine 1999 | Smoking cessation intervention for parents. No ED requirement. |
| Jan 2007 | Population drawn from ambulatory setting |
| Jones 1995 | Study conducted in adults |
| Joseph 2005 | Intervention targeted at physicians |
| Jospeh 2007 | ED visit not sole entry criterion; mean baseline ED visits indicated some skew with a number experiencing 0 ED visits |
| Kamps 2003 | Intervention under assessment not educational in nature |
| Klein 1981 | Sample recruited from ambulatory setting |
| Klinnert 2004 | Not asthma |
| Kojima 2005 | Asthma camp, not sure where recruited from |
| Krishna 2003 | Outpatient setting, education intervention not related to ED |
| Krishna 2006 | Sample recruited from ambulatory setting |
| La Roche 2006 | Two different interventions |
| Langhammer 1999 | Sample recruited from ambulatory setting |
| Lans 1997 | Sample recruited from ambulatory setting |
| LeBaron 1985 | Sample recruited from ambulatory setting |
| Letz 2004 | Two active interventions |
| Levy 2006 | At risk children, rather than those with definite attendances |
| Lewis 1984 | Sample recruited from ambulatory setting |
| Lewis 2005 | Sample recruited from ambulatory setting |
| Lirsac 1991 | Adults |
| Liu 2001 | Non‐randomised comparison between treatment groups and control |
| Lukacs 2002 | Not randomised |
| Marks 1999 | Although recruited from hospital the study looks at improvements in communication with the GP and not the impact this has on asthma morbidity |
| Maslennikova 1998 | Different interventions given to Rx group |
| McCann 2006 | Sample recruited from ambulatory setting |
| McCarthy 2002 | Not randomised |
| McConnell 2005 | Cockroach allergen avoidance setting not related to ED visits or recruitment |
| McGhan 2003 | Sample recruited from ambulatory setting |
| McMullen 2002 | Sample recruited from ambulatory setting |
| McPherson 2006 | Sample recruited from ambulatory setting |
| Mesters 1995 | Intervention targeted at physicians |
| Nishioka 2006 | Sample recruited from ambulatory setting |
| PAC PORT | Intervention targeted at physicians |
| Patterson 2005 | Sample recruited from ambulatory setting |
| Perez 1999 | Sample recruited from ambulatory setting |
| Perrin 1992 | Sample recruited from ambulatory setting |
| Perry 2000 | Not randomised |
| Persaud 1996 | An ED visit within previous 12 months was not a criteria for entrance into the study. Unable to confirm with author that all subjects had ED visit within previous 12 months. |
| Phillips 2005 | Not an educational intervention |
| Ploska 1999 | Not randomised |
| Porter 2006 | Not randomised |
| Rakos 1985 | Sample recruited from ambulatory setting |
| Ronchetti 1997 | Sample recruited from ambulatory setting |
| Rubin 1986 | An ED visit within previous 12 months was not a criteria for entrance into the study. Unable to confirm with author that all subjects had ED visit within previous 12 months. |
| Salisbury 2002 | Sample recruited from ambulatory setting |
| Scarfone 2002 | Not randomised |
| Schatz 2006 | Study conducted in adults |
| Schmidt 1993 | Sample recruited from ambulatory setting |
| Schmidt 2002 | Sample recruited from ambulatory setting |
| Shah 2001 | Sample recruited from ambulatory setting |
| Shegog 2001 | Sample recruited from ambulatory setting |
| Shields 1990 | All subjects had ED visit or had been admitted to hospital but this was within the previous 4 years |
| Shields 2004 | Not randomised |
| SKCHHP | Intervention primarily concerned with environmental remediation; education intervention provided to both treatment groups |
| Smith 1986 | Sample recruited from ambulatory setting |
| Splett 2006 | Sample recruited from ambulatory setting |
| Stergachis 2002 | Intervention targeted at pharmacists |
| Sulaiman 2004 | Intervention targeted at physicians |
| Tanyeli 2001 | Study conducted in adults |
| Turgeon 1996 | Two active interventions |
| Valery 2007 | No index ED visit (correspondence with B Masters) |
| Velsor‐Friedrich 2004 | Not randomised |
| Vilozni 2001 | Sample recruited from ambulatory setting |
| Volovitz 2003 | Not randomised |
| Wakefield 2002 | Sample recruited from ambulatory setting |
| Wensley 2004 | Two active interventions |
| Whitman 1985 | Not randomised |
| Willems 2004 | Sample recruited from ambulatory setting |
| Williams 2006 | Environmental remediation |
| Wilson 1996 | An ED visit in the previous 12 months was not a criteria for entrance into the study (personal correspondence with author) |
| Wong 2001 | Sample recruited from ambulatory setting |
| Yang 2005 | Not randomised |
| Yawn 2000 | Sample recruited from ambulatory setting |
| Yilmaz 2002 | Adults |
| Yoon 2004 | Sample recruited from ambulatory setting |
| Zorc 2003 | Intervention does not appear to be educational ‐ supportive |
Differences between protocol and review
We added a subgroup analysis by timing of outcome assessment. The time limits for the subgroup categorisations were based on distinctions made in a Health Technology Assessment (Smith 2005; short‐term (< 6 months), medium‐term (≥ 6 to < 12 months) and long‐term ≥ 12 months).
We have adopted the 'Risk of bias' assessment tool as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2008).
Contributions of authors
Michelle Boyd: Lead author of 2008 update; assessment of studies, data extraction, write‐up
Toby Lasserson: Author on 2008 update; assessment of studies, data extraction, data analysis, write‐up
Mike McKean: Author on 2008 update; development of discussion; write‐up
Michelle Haby: Lead author of 2001 review; advice on data extraction in 2008 update
Francine Ducharme: Editorial support; write‐up
Peter Gibson: Editorial support; write‐up
Previous authors: Colin Robertson: write‐up; Elizabeth Waters: write‐up
Sources of support
Internal sources
St George's, University of London, UK.
External sources
Victorian Government Department of Human Services ‐ Public Health Division, Australia.
NHS Research and Development, UK.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Agrawal 2005 {published data only}
- Agrawal SK, Singh M, Mathew JL, Malhi P. Efficacy of an individualized written home‐management plan in the control of moderate persistent asthma: A randomized, controlled trial. Acta Paediatrica 2005;94(12):1742‐6. [DOI] [PubMed] [Google Scholar]
Alexander 1988 {published data only}
- Alexander JS, Younger RE, Cohen RM, Crawford LV. Effectiveness of a nurse‐managed program for children with chronic asthma. Journal of Pediatric Nursing 1988;3(5):312‐7. [PubMed] [Google Scholar]
Becker 2003 {published data only}
- Becker AB, Whitters D, Gillespie CA, Filuk SE, McColm JE, Thomas NJ, et al. Impact of a randomized asthma education program on asthma control in children. Journal of Allergy and Clinical Immunology 2003;111(Suppl 2):212s. [Google Scholar]
Brown 2002 {published data only}
- Brown JV, Bakeman R, Celano MP, Demi AS, Kobrynski L, Wilson SR. Home‐based asthma education of young low‐income children and their families. Journal of Pediatric Psychology 2002;27(8):677‐8. [DOI] [PubMed] [Google Scholar]
- Brown JV, Demi AD, Wilson SR, Lee SY, Bakeman R, Celano M, et al. A home‐based asthma education program for low‐income families and their young asthmatic children [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A902. [Google Scholar]
Brown 2006 {published data only}
- Brown MD, Reeves MJ, Meyerson K, Korzeniewski SJ. Randomized trial of a comprehensive asthma education program after an emergency department visit. Annals of Allergy, Asthma and Immunology 2006;97(1):44‐51. [DOI] [PubMed] [Google Scholar]
- Reeves MJ, Brown MD, Meyerson K, Korzeniewski S. A randomized controlled trial of an asthma education program following an emergency department (ED) visit for asthma in children and adults. American Thoracic Society 2005 International Conference. 2005:A22.
Butz 2006 {published data only}
- Butz AM, Syron L, Johnson B, Spaulding J, Walker M, Bollinger ME. Home‐based asthma self‐management education for inner city children. Public Health Nursing 2005;22(3):189‐99. [DOI] [PubMed] [Google Scholar]
- Butz AM, Tsoukleris MG, Donithan M, Hsu VD, Zuckerman I, Mudd KE. Effectiveness of nebulizer use‐targeted asthma education on underserved children with asthma. Archives of Pediatrics & Adolescent Medicine 2006;160(6):622‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd K, Bollinger ME, Doren H, Manning A, Tsoukleris MG, Butz A. Concordance of Medicaid and pharmacy record data in inner‐city children with asthma. Contemporary Clinical Trials 2007; Vol. ePub. [DOI: 10.1016/j.cct.2007.05.002] [DOI] [PubMed]
Charlton 1994 {published data only}
- Charlton I, Antonio AG, Atkinson J, Campbell MJ, Chapman E, Mackintosh T, et al. Asthma at the interface: bridging the gap between general practice and a district general hospital. Archives of Disease In Childhood 1994;70:313‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cicutto 2005 {published data only}
- Cicutto L, Murphy S, Coutts D, O'Rourke J, Lang G, Chapman C, et al. Breaking the access barrier: evaluating an asthma center's efforts to provide education to children with asthma in schools. Chest 2005;128(4):1928‐35. [DOI] [PubMed] [Google Scholar]
Clark 1986 {published data only}
- Clark NM, Feldman CH, Evans D, Duzey O, Levison MJ, Wasilewski Y, et al. Managing better: children, parents, and asthma. Patient Education and Counseling 1986;8(1):27‐38. [DOI] [PubMed] [Google Scholar]
- Clark NM, Feldman CH, Evans D, Levison MJ, Wasilewski Y, Mellins RB. The impact of health education on frequency and cost of health care use by low income children with asthma. Journal of Allergy & Clinical Immunology 1986;78(1):108‐15. [DOI] [PubMed] [Google Scholar]
Couriel 1999 {published data only}
- Couriel J, Littleton V, Milnes L, Barow S. Patient education for children attending an accident and emergency (a & e) department with acute asthma ‐ a randomised controlled study. European Respiratory Society; Oct 9‐13; Madrid, Spain 1999:P1756.
Cowie 2002 {published data only}
- Cowie RL, Underwood MF, Little CB, Mitchell I, Spier S, Ford GT. Asthma in adolescents: a randomized, controlled trial of an asthma program for adolescents and young adults with severe asthma. Canadian Respiratory Journal 2002;9(4):253‐9. [DOI] [PubMed] [Google Scholar]
Farber 2004 {published data only}
- Farber HJ, Oliveria L. Can provision of patient education and a written management plan as part of an emergency room er visit improve outcomes for inner city children with asthma?. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23. 2001.
- Farber HJ, Oliveria L. Trial of an asthma education program in an inner‐city pediatric emergency department. Pediatric Asthma Allergy & Immunology 2004;17(2):107‐15. [Google Scholar]
Garrett 1994 {published data only}
- Garrett J, Fenwick JM, Taylor G, Mitchell E, Rea H. Peak expiratory flow meters (PEFMs) ‐ who uses them and how and does education affect the pattern of utilisation?. Australian and New Zealand Journal of Medicine 1994;24:521‐9. [DOI] [PubMed] [Google Scholar]
- Garrett J, Fenwick JM, Taylor G, Mitchell E, Stewart J, Rea H. Prospective controlled evaluation of the effect of a community based asthma education centre in a multiracial working class neighbourhood. Thorax 1994;49:976‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghosh 1998 {published data only}
- Ghosh CS, Ravindran P, Joshi M, Stearns SC. Reductions in hospital use from self management training for chronic asthmatics. Social Science & Medicine 1998;46(8):1087‐93. [DOI] [PubMed] [Google Scholar]
Gorelick 2006 {published data only}
- Gorelick MH, Meurer JR, Walsh‐Kelly CM, Brousseau DC, Grabowski L, Cohn J, et al. Emergency department allies: a controlled trial of two emergency department‐based follow‐up interventions to improve asthma outcomes in children. Pediatrics 2006;117(4):S127‐34. [DOI] [PubMed] [Google Scholar]
Greineder 1999 {published data only}
- Greineder DK, Loane KC, Parks P. A randomized controlled trial of a pediatric asthma outreach program. Journal of Allergy & Clinical Immunology 1999;103(3):436‐40. [DOI] [PubMed] [Google Scholar]
Harish 2001 {published data only}
- Harish Z, Bregante AC, Morgan C, Fann CS, Callaghan CM, Witt MA, et al. A comprehensive inner‐city asthma program reduces hospital and emergency room utilization. Annals of Allergy, Asthma and Immunology 2001;86(2):185‐9. [DOI] [PubMed] [Google Scholar]
Homer 2000 {published data only}
- Homer C, Susskind O, Alpert HR, Owusu M, Schneider L, Rappaport LA, et al. An evaluation of an innovative multimedia educational software program for asthma management: report of a randomized, controlled trial. Pediatrics 2000;106(1):210‐5. [PubMed] [Google Scholar]
Karnick 2007 {published data only}
- Johnson D, Karnick P, Seals G, Margellos H, Silva A, Whitman S, et al. Randomized study of the impact of intensive asthma education (IAE), with/without case management (CM), on the health of inner city asthmatic children: preliminary results of the Chicago Asthma Initiative. Pediatric Research 2002;51(4):212a. [Google Scholar]
- Karnick P, Margellos‐Anast H, Seals G, Whitman S, Aljadeff G, Johnson D. The pediatric asthma intervention: a comprehensive cost‐effective approach to asthma management in a disadvantaged inner‐city community. Journal of Asthma 2007;44(1):39‐44. [DOI] [PubMed] [Google Scholar]
Kelly 2000 {published data only}
- Kelly CS, Morrow AL, Shults J, Nakas N, Strope GL, Adelman RD. Outcomes evaluation of a comprehensive intervention program for asthmatic children enrolled in medicaid. Pediatrics 2000;105(5):1029‐35. [DOI] [PubMed] [Google Scholar]
Khan 2004 {published data only}
- Khan MSR, O'Meara M, Stevermuer TL, Henry RL. Randomized controlled trial of asthma education after discharge from an emergency department. Journal of Paediatrics & Child Health 2004;40(12):674‐7. [DOI] [PubMed] [Google Scholar]
- Khan MSR, O’Meara M, Henry RL. Background severity of asthma in children discharged from the emergency department. Journal of Paediatrics & Child Health 2003;39(6):432‐5. [DOI] [PubMed] [Google Scholar]
Kinlow 2001 {published data only}
- Kinlow M, Hazzard A, Adams F, Batts B. Computer based interactive education programs for hospitalized children with asthma and sickle cell disease. Respiratory Care 2001;46(10):1112. [Google Scholar]
Madge 1997 {published data only}
- Callery P. A nurse led home management training programme reduced readmissions to the hospital in children with acute asthma commentary on Madge P, McColl J, Paton J. Impact of a nurse‐led home management training programme in children admitted to hospital with acute asthma: a randomised controlled study. Thorax 1997 Mar;52:223‐8. Evidence‐Based Nursing 1998;1(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madge P, McColl J, Paton J. Impact of a nurse‐led home management training programme in children admitted to hospital with acute asthma: a randomised controlled study. Thorax 1997;52(3):223‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
McNabb 1985 {published data only}
- McNabb W. Self‐management of childhood asthma. Doctoral dissertation. Denver: University of Denver, 1984. [Google Scholar]
- McNabb WL, Wilson‐Pessano SR, Hughes GW, Scamagas P. Self‐management education of children with asthma: AIR WISE. American Journal Of Public Health 1985;75(10):1219‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 1986 {published data only}
- Mitchell EA, Ferguson V, Norwood M. Asthma education by community child health nurses. Archives of Disease In Childhood 1986;61:1184‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCICAS {published data only}
- Evans R, Gergen PJ, Mitchell H, Kattan M, Kercsmar C, Crain E, et al. A randomized clinical trial to reduce asthma morbidity among inner‐city children: results of the National Cooperative Inner‐City Asthma Study. Journal of Pediatrics 1999;135(3):332‐8. [DOI] [PubMed] [Google Scholar]
- Gergen PJ, Mortimer KM, Eggleston PA, Rosenstreich D, Mitchell H, Ownby D, et al. Results of the National Cooperative Inner‐City Asthma Study (NCICAS) environmental intervention to reduce cockroach allergen exposure in inner‐city homes. Journal of Allergy and Clinical Immunology 1999;103(3):501‐6. [DOI] [PubMed] [Google Scholar]
- Kattan M, Mitchell H, Eggleston P, Gergen P, Crain E, Redline S, et al. Characteristics of inner‐city children with asthma: the National Cooperative Inner‐City Asthma Study. Pediatric Pulmonology 1997;24(4):253‐62. [DOI] [PubMed] [Google Scholar]
- Mitchell H, Senturia Y, Gergen P, Baker D, Joseph C, McNiff‐Mortimer K, et al. Design and methods of the National Cooperative Inner‐City Asthma Study. Pediatric Pulmonology 1997;24(4):237‐52. [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Weiss KB, Lynn H, Mitchell H, Kattan M, Gergen PJ, et al. The cost‐effectiveness of an inner‐city asthma intervention for children. Journal of Allergy and Clinical Immunology 2002;110(4):576‐81. [DOI] [PubMed] [Google Scholar]
- Wade S, Weil C, Holden G, Mitchell H, Evans R, Kruszon‐Moran D, et al. Psychosocial characteristics of inner‐city children with asthma: a description of the NCICAS psychosocial protocol. National Cooperative Inner‐City Asthma Study. Pediatric Pulmonology 1998;24(4):263‐76. [DOI] [PubMed] [Google Scholar]
Ng 2006 {published data only}
- Ng DK, Chow PY, Lai WP, Chan KC, And BLT, So HY. Effect of a structured asthma education program on hospitalized asthmatic children: a randomized controlled study. Pediatrics International 2006;48(2):158‐62. [DOI] [PubMed] [Google Scholar]
Shames 2004 {published data only}
- Shames RS, Sharek P, Mayer M, Robinson TN, Hoyte EG, Gonzalez‐Hensley F, et al. Effectiveness of a multi‐component self‐management program in at‐risk, school‐aged children with asthma. Annals of Allergy, Asthma, & Immunology 2004;92(6):611‐8. [DOI] [PubMed] [Google Scholar]
Smith 2004 {published data only}
- Smith SR, Jaffe DM, Fisher EB Jr, Trinkaus KM, Highstein G, Strunk RC. Improving follow‐up for children with asthma after an acute Emergency Department visit. Journal of Pediatrics 2004;145:772‐7. [DOI] [PubMed] [Google Scholar]
- Smith SR, Jaffe DM, Petty M, Worthy V, Banks P, Strunk RC. Recruitment into a long‐term pediatric asthma study during emergency department visits. Journal of Asthma 2004;41(4):477‐84. [DOI] [PubMed] [Google Scholar]
Smith 2006 {published data only}
- Smith SR, Jaffe DM, Highstein G, Fisher EB, Trinkaus KM, Strunk RC. Asthma coaching in the pediatric Emergency Department. Academic Emergency Medicine 2006;13(8):835‐9. [DOI] [PubMed] [Google Scholar]
Sockrider 2006 {published data only}
- Sockrider MM, Abramson S, Brooks E, Caviness AC, Pilney S, Koerner C, et al. Delivering tailored asthma family education in a pediatric emergency department setting: a pilot study. Pediatrics 2006;117(4):135s‐44. [DOI] [PubMed] [Google Scholar]
Stevens 2002 {published data only}
- Martin J. Parental education does not reduce morbidity in pre‐school children with asthma: commentary. Australian Journal of Physiotherapy 2003;49(3):222. [PubMed] [Google Scholar]
- Stevens CA, Wesseldine LJ, Couriel JM, Dyer AJ, Osman LM, Silverman M. Parental education and guided self‐management of asthma and wheezing in the pre‐school child: a randomised controlled trial. Thorax 2002;57(1):39‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Talabere 1993 {published data only}
- Talabere LR. The effects of an asthma education program on selected health behaviors of school‐age children who have recently experienced an acute asthma episode. Ohio State University, 1990. [Google Scholar]
- Talabere LR. The effects of an asthma education program on selected health behaviors of school‐aged children with asthma. In: Tornquist EM, Champagne MT, Funk SG editor(s). Key aspects of caring for the chronically ill: hospital and home. New York: Springer, 1993:319‐30. [Google Scholar]
Teach 2006 {published data only}
- Teach SJ, Crain EF, Quint DM, Hylan ML, Joseph JG. Improved asthma outcomes in a high‐morbidity pediatric population: results of an emergency department‐based randomized clinical trial. Archives of Pediatrics & Adolescent Medicine 2006;160(5):535‐41. [DOI] [PubMed] [Google Scholar]
Walders 2006 {published data only}
- Walders N, Kercsmar C, Schluchter M, Redline S, Kirchner HL, Drotar D. An interdisciplinary intervention for under‐treated pediatric asthma. Chest 2006;129(2):292‐9. [DOI] [PubMed] [Google Scholar]
- Zebracki K, Drotar D, Kirchner HL, Schluchter M, Redline S, Kercsmar C, et al. Predicting attrition in a pediatric asthma intervention study. Journal of Pediatric Psychology 2003;28:519‐28. [DOI] [PubMed] [Google Scholar]
Warschburger 2003 {published data only}
- Warschburger P, Schwerin AD, Buchholz HT, Petermann F. An educational program for parents of asthmatic preschool children: short‐ and medium‐term effects. Patient Education and Counseling 2003;51:83–91. [DOI] [PubMed] [Google Scholar]
Wesseldine 1999 {published data only}
- Wesseldine LJ, McCarthy P, Silverman M. Structured discharge procedure for children admitted to hospital with acute asthma: a randomised controlled trial of nursing practice. Archives of Disease in Childhood 1999;80:110‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2001 {published data only}
- Wilson SR, Yamada EG, Sudhakar R, Roberto L, Mannino D, Mejia C, et al. A controlled trial of an environmental tobacco smoke reduction intervention in low‐income children with asthma. Chest 2001;120(5):1709‐22. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adams 2004 {published data only}
- Adams CD, Joseph KE, MacLaren JE, DeMore M, Koven L, Detweiler MF, et al. Parent‐youth teamwork in pediatric asthma management [Abstract]. Journal of Allergy and Clinical Immunology 2004;113(Suppl 2):S159. [Google Scholar]
Amirav 1995 {published data only}
- Amirav I, Goren A, Kravitz RM, Pawlowski NA. Physician‐targeted program on inhaled therapy for childhood asthma. Journal Allergy and Clinical Immunology 1995;95(4):818‐23. [DOI] [PubMed] [Google Scholar]
Augustin 2003 {published data only}
- Augustin J. An intensive asthma intervention in a school‐based clinic. Rush University 2003.
Baren 2001 {published data only}
- Baren JM, Shofer FS, Ivey B, Reinhard S, DeGeus J, Stahmer SA, et al. A randomized, controlled trial of a simple emergency department intervention to improve the rate of primary care follow‐up for patients with acute asthma exacerbations. Annals of Emergency Medicine 2001;38(2):115‐22. [DOI] [PubMed] [Google Scholar]
Baren 2006 {published data only}
- Baren JM, Boudreaux ED, Brenner BE, Cydulka RK, Rowe BH, Clark S, et al. Randomized controlled trial of emergency department interventions to improve primary care follow‐up for patients with acute asthma. Chest 2006;129(2):257‐65. [DOI] [PubMed] [Google Scholar]
Bartholomew 2000 {published data only}
- Bartholomew LK, Gold RS, Parcel GS, Czyzewski DI, Sockrider MM, Fernandez M, et al. Watch, Discover, Think, and Act: evaluation of computer‐assisted instruction to improve asthma self‐management in inner‐city children. Patient Education & Counseling 2000;39(2‐3):269‐80. [DOI] [PubMed] [Google Scholar]
Bartholomew 2006 {published data only}
- Bartholomew LK, Sockrider M, Abramson SL, Swank PR, Czyzewski DI, Tortolero SR, et al. Partners in school asthma management: evaluation of a self‐management program for children with asthma. Journal of School Health 2006;76(6):283‐90. [DOI] [PubMed] [Google Scholar]
Bobb 2003 {published data only}
- Bobb C, Ritz T. Do asthma patients in general practice profit from a structured allergy evaluation and skin testing? A pilot study. Respiratory Medicine 2003;97(11):1180‐7. [DOI] [PubMed] [Google Scholar]
Bonner 2002 {published data only}
- Bonner S, Zimmerman BJ, Evans D, Irigoyen M, Resnick D, Mellins RB. An individualized intervention to improve asthma management among urban Latino and African‐American families. Journal of Asthma 2002;39(2):167‐79. [DOI] [PubMed] [Google Scholar]
Boone 2002 {published data only}
- Boone D, Counsell P, Dobson C, Baker EH. Effect of computer‐assisted education on inhaler technique and knowledge in children with asthma [abstract]. European Respiratory Society Annual Congress. 2002:P2062.
Brook 1993 {published data only}
- Brook U, Mendelberg A, Heim M. Increasing parental knowledge of asthma decreases the hospitalization of the child: a pilot study. Journal of Asthma 1993;30:45‐9. [DOI] [PubMed] [Google Scholar]
Bryant‐Stephens 2004 {published data only}
- Bryant‐Stephens T. Asthma and environmental control: the results of a randomized, controlled study to remove asthma triggers from the bedrooms of children with persistent asthma [Abstract]. Chest 2004;126(Suppl 4):761s. [Google Scholar]
- Bryant‐Stephens T. Reducing asthma triggers in the asthmatic child's bedroom: a randomized, controlled study using lay home visitors. Annual meeting of the American Public Health Association, Philadelphia (October 22). 2001.
Burkhart 2002 {published data only}
- Burkhart PV, Dunbar‐Jacob JM, Fireman P, Rohay J. Children's adherence to recommended asthma self‐management. Pediatric Nursing 2002;28(4):409‐14. [PubMed] [Google Scholar]
- Burkhart PV, Rayens MK. Self‐concept and health locus of control: factors related to children's adherence to recommended asthma regimen. Pediatric Nursing 2005;31(5):404‐9. [PubMed] [Google Scholar]
- Burkhart PV, Ward HJ. Children's self‐reports of characteristics of their asthma episodes. Journal of Asthma 2003;40(8):909‐16. [DOI] [PubMed] [Google Scholar]
Bynum 2001 {published data only}
- Bynum A, Hopkins D, Thomas A, Copeland N, Irwin C. The effect of telepharmacy counselling on metered‐dose inhaler technique among adolescents with asthma in rural Arkansas. Telemedicine Journal and E‐Health 2001;7(3):207‐17. [DOI] [PubMed] [Google Scholar]
Cabana 2005 {published data only}
- Cabana D, Slish K, Mellins R, Evans D, Brown R, Clark M. Impact of provider education on the quality of pediatric asthma care: results of a controlled trial [Abstract]. European Respiratory Journal 2004;24(Suppl 48):382s. [Google Scholar]
- Cabana MD, Birk NA, Slish KK, Yoon EY, Pace K, Nan B, et al. Exposure to tobacco smoke and chronic asthma symptoms. Pediatric Asthma Allergy & Immunology 2005;18(4):180‐8. [Google Scholar]
Caliguiri 2002 {published data only}
- Caliguiri LA, Light WC, Green RL, Corder L, Gallagher PE, Kilby J. Asthma education for pediatric patients and their caregivers improves outcomes. Journal of Allergy and Clinical Immunology 2002;109(1):95s. [Google Scholar]
Callahan 2003 {published data only}
- Callahan KA, Eggleston PA, Rand CS, Kanchanaraksa S, Swartz LJ, Wood RA. Knowledge and practice of dust mite control by specialty care. Annals of Allergy, Asthma, & Immunology 2003;90(3):302‐7. [DOI] [PubMed] [Google Scholar]
Cano‐Garcia 2007 {published data only}
- Cano‐Garcinuno A, Diaz‐Vazquez C, Carvajal‐Uruena I, Praena‐Crespo M, Gatti‐Vinoly A, Garcia‐Guerra I. Group education on asthma for children and caregivers: a randomized, controlled trial addressing effects on morbidity and quality of life. Journal of Investigational Allergology and Clinical Immunology 2007;17(4):216‐26. [PubMed] [Google Scholar]
Charlton 1990 {published data only}
- Charlton I, Charlton G, Broomfield J, Mullee MA. Evaluation of peak flow and symptoms only self management plans for control of asthma in general practice. BMJ 1990;301(6765):1355‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2004 {published data only}
- Chen S‐H, Yeh K‐W, Chen S‐H, Yen D‐C, Yin TJC, Huang J‐L. The development and establishment of a care map in children with asthma in Taiwan. Journal of Asthma 2004;41(8):855‐61. [DOI] [PubMed] [Google Scholar]
Clark 2005 {published data only}
- Clark NM, Gong M, Kaciroti N, Yu J, Wu G, Zeng Z, et al. A trial of asthma self‐management in Beijing schools. Chronic Illness 2005;1(1):31‐8. [DOI] [PubMed] [Google Scholar]
- Clark NM, Gong MZ, Kaciroti N, Yu J, Zeng Z, Wu G, et al. Effect of self management education on school children with asthma in Beijing, China [Abstract]. European Respiratory Journal 2003;22(Suppl 45):Abstract No: P2637. [Google Scholar]
Claus 2004 {published data only}
- Claus R, Michael H, Josef L, Jan‐Torsten T, Marion S. Internet based patient education evaluation of a new tool for young asthmatics [Abstract]. European Respiratory Journal 2004;24(Suppl 48):383s. [Google Scholar]
Cohen 1979 {published data only}
- Cohen HI, Harris C, Green HW, Goodriend‐Resnik S. Cost‐benefit analysis of asthma self‐management educational program in children. Journal of Allergy and Clinical Immunology 1979;63(3):155‐6. [Google Scholar]
Cojocaru 2006 {published data only}
- Cojocaru B, Blic J, Scheinmann P, Cheron G. Prospective comparison of child asthma education in the emergency department and at scheduled follow‐up consultation. Archives de Pediatrie 2006;13(8):1112‐7. [DOI] [PubMed] [Google Scholar]
Colland 1993 {published data only}
- Colland VT. Learning to cope with asthma: a behavioural self‐management program for children. Patient Education & Counseling 1993;22(3):141‐52. [DOI] [PubMed] [Google Scholar]
Colland 2004 {published data only}
- Colland VT, Essen‐Zandvliet LEMV, Lans C, Denteneer A, Westers P, Brackel HJL. Poor adherence to self‐medication instructions in children with asthma and their parents. Patient Education & Counseling 2004;55(3):416‐21. [DOI] [PubMed] [Google Scholar]
Cowie 1997 {published data only}
- Cowie RL, Revitt SG, Underwood MF, Field SK. The effect of a peak flow‐based action plan in the prevention of exacerbations of asthma. Chest 1997;112(6):1534‐8. [DOI] [PubMed] [Google Scholar]
Cunningham 2008 {published data only}
- Cunningham S, Logan C, Lockerbie L, Dunn MJ, McMurray A, Prescott RJ. Effect of an integrated care pathway on acute asthma/wheeze in children attending hospital: cluster randomized trial. Journal of Pediatrics 2008;152(3):315‐20. [DOI] [PubMed] [Google Scholar]
Dahl 1990 {published data only}
- Dahl J, Gustafsson D, Melin L. Effects of a behavioral treatment program on children with asthma. Journal of Asthma 1990;27(1):41‐6. [DOI] [PubMed] [Google Scholar]
Deaves 1993 {published data only}
- Deaves DM. An assessment of the value of health education in the prevention of childhood asthma. Journal of Advanced Nursing 1993;18(3):354‐63. [DOI] [PubMed] [Google Scholar]
Delaronde 2005 {published data only}
- Delaronde S, Peruccio DL, Bauer BJ. Improving asthma treatment in a managed care population. American Journal of Managed Care 2005;11(6):361‐8. [PubMed] [Google Scholar]
Dolinar 2000 {published data only}
- Dolinar RE. Influence of a home‐based asthma health education program on quality of life and coping in parents of children with asthma. University of Ottawa 1997.
- Dolinar RM, Kumar V, Coutu‐Wakulczyk G, Rowe BH. Pilot study of a home‐based asthma health education program. Patient Education & Counseling 2000;40(1):93‐102. [DOI] [PubMed] [Google Scholar]
Eggleston 2005 {published data only}
- Eggleston PA, Butz A, Rand C, Curtin‐Brosnan J, Kanchanaraksa S, Swartz L, et al. Home environmental intervention in inner‐city asthma: a randomized controlled clinical trial. Annals of Allergy, Asthma, & Immunology 2005;95(6):518‐24. [DOI] [PubMed] [Google Scholar]
Evans 1997 {published data only}
- Evans D, Mellins R, Lobach K, Ramos Bonoan C, Pinkett Heller M, Wiesemann S, et al. Improving care for minority children with asthma: professional education in public health clinics. Pediatrics 1997;99(2):157‐64. [DOI] [PubMed] [Google Scholar]
Fireman 1981 {published data only}
- Fireman P, Friday GA, Gira C, Vierthaler WA, Michaels L, et al. Teaching self‐management skills to asthmatic children and their parents in an ambulatory care setting. Pediatrics 1981;68(3):341‐8. [PubMed] [Google Scholar]
Gardida 2002 {published data only}
- Gardida A, Rojas M, Tavera C, Catalan M. Evaluation of an educational program to control asthma in school age children in the Morelos state, Mexico [Evaluación de un panorama educativo para el control del asma en niños de edad escolar en el estado de Morelos, México]. Revista del Instituto Nacional de Enfermedades Respiratorias 2002;15(1):27‐30. [Google Scholar]
Gebert 1998 {published data only}
- Gebert N, Hummelink R, Konning J, Staab D, Schmidt S, Szczepanski R, et al. Efficacy of a self‐management program for childhood asthma ‐ a prospective controlled study. Patient Education & Counseling 1998;35(3):213‐20. [DOI] [PubMed] [Google Scholar]
Gerald 2006 {published data only}
- Gerald LB, Redden D, Wittich AR, Hains C, Turner‐Henson A, Hemstreet MP, et al. Outcomes for a comprehensive school‐based asthma management program. Journal of School Health 2006;76(6):291‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillies 1996 {published data only}
- Gillies J, Barry D, Crane J, Jones D, MacLennan L, Pearce N, et al. A community trial of a written self management plan for children with asthma. New Zealand Medical Journal 1996;109(1015):30‐3. [PubMed] [Google Scholar]
Gonzalez 2003 {published data only}
- Gonzalez‐Martin G, Joo I, Sanchez I. Evaluation of the impact of a pharmaceutical care program in children with asthma. Patient Education & Counseling 2003;49(1):13‐8. [DOI] [PubMed] [Google Scholar]
Guendelman 2002 {published data only}
- Guendelman S, Meade K, Benson M, Chen YQ, Samuels S. Improving asthma outcomes and self‐management behaviors of inner‐city children: a randomized trial of the Health Buddy interactive device and an asthma diary. Archives of Pediatrics & Adolescent Medicine 2002;156(2):114‐20. [DOI] [PubMed] [Google Scholar]
- Guendelman S, Meade K, Chen YQ, Benson M. Asthma control and hospitalizations among inner‐city children: Results of a randomized trial. Telemedicine Journal & E‐Health 2004; Vol. 10, issue Suppl 2. [PubMed]
Heard 1999 {published data only}
- Heard AR, Richards IJ, Alpers JH, Pilotto LS, Smith BJ, Black JA. Randomised controlled trial of general practice based asthma clinics. Medical Journal of Australia 1999;171(2):68‐71. [DOI] [PubMed] [Google Scholar]
Hederos 2005 {published data only}
- Hederos CA, Janson S, Hedlin G. Group discussions with parents have long‐term positive effects on the management of asthma with good cost‐benefit. Acta Paediatrica 2005;94(5):602‐8. [DOI] [PubMed] [Google Scholar]
Hill 1991 {published data only}
- Hill R, Williams J, Britton J, Tattersfield A. Can morbidity associated with untreated asthma in primary school children be reduced?: a controlled intervention study. BMJ 1991;303(6811):1169‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hockemeyer 2002 {published data only}
- Hockemeyer J, Smyth J. Evaluating the feasibility and efficacy of a self‐administered manual‐based stress management intervention for individuals with asthma: results from a controlled study. Behavioral Medicine 2002;27(4):161‐72. [DOI] [PubMed] [Google Scholar]
Holzheimer 1998 {published data only}
- Holzheimer L, Mohay H, Masters IB. Educating young children about asthma: comparing the effectiveness of a developmentally appropriate asthma education video tape and picture book. Child: Care, Health & Development 1998;24(1):85‐99. [DOI] [PubMed] [Google Scholar]
Hughes 1991 {published data only}
- Hughes DM, McLeod M, Garner B, Goldbloom RB. Controlled trial of a home and ambulatory program for asthmatic children. Pediatrics 1991;87:54‐61. [PubMed] [Google Scholar]
Hung 2002 {published data only}
- Hung CC, Chen YC, Mao HC, Chiang BL. Effects of systematic nursing instruction of mothers on using medication and on health status of asthmatic children. Journal of Nursing Research 2002;10(1):22‐32. [DOI] [PubMed] [Google Scholar]
Huss 2003 {published data only}
- Huss K, Winkelstein M, Nanda J, Naumann PL, Sloand ED, Huss RW. Computer game for inner‐city children does not improve asthma outcomes. Journal of Pediatric Health Care 2003;17(2):72‐8. [DOI] [PubMed] [Google Scholar]
ICAS {published data only}
- Crain EF, Walter M, O'Connor GT, Mitchell H, Gruchalla RS, Kattan M, et al. Home and allergic characteristics of children with asthma in seven U.S. urban communities and design of an environmental intervention: the Inner‐City Asthma Study. Environmental Health Perspectives 2002;110(9):939‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruchalla RS, Pongracic J, Plaut M, Evans R, Visness CM, Walter M, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. Journal of Allergy and Clinical Immunology 2005;115(3):478‐85. [DOI] [PubMed] [Google Scholar]
- Kattan M, Crain EF, Steinbach S, Visness CM, Walter M, Stout JW, et al. A randomized clinical trial of clinician feedback to improve quality of care for inner‐city children with asthma. Pediatrics 2006;117(6):1095‐103. [DOI] [PubMed] [Google Scholar]
- Morgan WJ, Crain EF, Gruchalla RS, O'Connor GT, Kattan M, Evans R 3rd, et al. Results of a home‐based environmental intervention among urban children with asthma. New England Journal of Medicine 2004;351(11):1068‐80. [DOI] [PubMed] [Google Scholar]
Irvine 1999 {published data only}
- Irvine L, Crombie IK, Clark RA, Slane PW, Feyerabend C, Goodman KE, et al. Advising parents of asthmatic children on passive smoking: randomised controlled trial. BMJ 1999;318(7196):1456‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jan 2007 {published data only}
- Jan R‐L, Wang J‐Y, Huang M‐C, Tseng S‐M, Su H‐J, Liu L‐F. An internet‐based interactive tele‐monitoring system for improving childhood asthma outcomes in Taiwan. Telemedicine Journal and E‐Health 2007;13(3):257‐68. [DOI] [PubMed] [Google Scholar]
Jones 1995 {published data only}
- Jones KP, Mullee MA, Middleton M, Chapman E, Holgate ST. Peak flow based asthma self‐management: a randomised controlled study in general practice. British Thoracic Society Research Committee. Thorax 1995;50(8):851‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joseph 2005 {published data only}
- Joseph CL, Havstad S, Anderson EW, Brown R, Johnson CC, Clark NM. Effect of asthma intervention on children with undiagnosed asthma. Journal of Pediatrics 2005;146(1):96‐104. [DOI] [PubMed] [Google Scholar]
Jospeh 2007 {published data only}
- Joseph CLM, Peterson E, Havstad S, Johnson CC, Hoerauf S, Stringer S, et al. A web‐based, tailored asthma management program for urban African‐American high school students. American Journal of Respiratory & Critical Care Medicine 2007;175(9):888‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamps 2003 {published data only}
- Kamps AWA. Unscheduled care for people with asthma in a multi‐ethnic area is reduced following educational outreach programme by specialist nurses. Evidence‐Based Healthcare 2004;8(4):190‐1. [Google Scholar]
- Kamps AWA, Brand PLP, Kimpen JLL, Maillé AR, Overgoor‐van de Groes AW, Helsdingen‐Peek LCJAM, et al. Outpatient management of childhood asthma by paediatrician or asthma nurse: randomised controlled study with one year follow up. Thorax 2003;58:968‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klein 1981 {published data only}
- Klein GL, Zenk KE. A Medical Allergy Profile MAP card. Annals of Allergy 1981;46(6):328‐30. [PubMed] [Google Scholar]
Klinnert 2004 {published data only}
- Klinnert MD, Liu AH, Price M, Ellison MC, Budhiraja N. Short‐term impact of a multi‐faceted intervention for wheezy infants at risk for asthma. Journal of Allergy and Clinical Immunology 2004;113(Suppl 2):302s. [Google Scholar]
Kojima 2005 {published data only}
- Kojima N, Takeda Y, Akashi M, Kamiya T, Matsumoto M, Ohya Y, et al. Interactive education during summer camp for children with asthma improved adherence of self‐management [Abstract]. Journal of Allergy & Clinical Immunology 2005;115(Suppl 2):115s. [Google Scholar]
Krishna 2003 {published data only}
- Krishna S, Francisco BD, Andrew Balas E, Konig P, Graff GR, Madsen RW. Internet‐enabled interactive multimedia asthma education program: a randomized trial. Pediatrics 2003;111(3):503‐10. [DOI] [PubMed] [Google Scholar]
Krishna 2006 {published data only}
- Krishna S, Balas EA, Francisco BD, Konig P. Effective and sustainable multimedia education for children with asthma: A randomized controlled trial. Children's Health Care 2006;35(1):75‐90. [Google Scholar]
La Roche 2006 {published data only}
- Roche MJ, Koinis‐Mitchell D, Gualdron L. A culturally competent asthma management intervention: a randomized controlled pilot study. Annals of Allergy, Asthma, & Immunology 2006;96(1):80‐5. [DOI] [PubMed] [Google Scholar]
Langhammer 1999 {published data only}
- Langhammer A, Holmen TL, Holmen J. Nurse‐run education program for children with asthma in general practice. European Respiratory Society; Oct 9‐13; Madrid, Spain. 1999:P1763.
Lans 1997 {published data only}
- Lans C, Denteneer A, Colland V, Essen‐Zandvliet E. Are prodromal signs useful in a self‐management programme for children with asthma?. European Respiratory Journal 1997;10(Suppl 25):116s. [Google Scholar]
LeBaron 1985 {published data only}
- LeBaron S, Zeltzer LK, Ratner P, Kniker WT. A controlled study of education for improving compliance with cromolyn sodium (Intal): The importance of physician‐patient communication. Annals of Allergy 1985;55(6):811‐8. [PubMed] [Google Scholar]
Letz 2004 {published data only}
- Letz K, Smits W. A randomized trial comparing peak expiratory flow versus symptom self‐management plans for children with persistent asthma [Abstract]. Journal of Allergy and Clinical Immunology 2004;113(Suppl 2):286s. [Google Scholar]
- Letz KL, Schlie AR, Smits WL. A randomized trial comparing peak expiratory flow versus symptom self‐management plans for children with persistent asthma. Pediatric Asthma Allergy & Immunology 2004;17(3):177‐90. [Google Scholar]
Levy 2006 {published data only}
- Levy M, Heffner B, Stewart T, Beeman G. The efficacy of asthma case management in an urban school district in reducing school absences and hospitalizations for asthma. Journal of School Health 2006;76(6):320‐4. [DOI] [PubMed] [Google Scholar]
Lewis 1984 {published data only}
- Lewis CE, Rachelefsky G, Lewis MA, Sota A, Kaplan M. A randomized trial of A.C.T. asthma care training for kids. Pediatrics 1984;74(4):478‐86. [PubMed] [Google Scholar]
Lewis 2005 {published data only}
- Lewis CJ, Thompson RE, Butz AM, Hill KL, Huss K, Lewis‐Bowyer LL, et al. Asthma education increases knowledge of rural parents and children with asthma and affects parents reports of their child's asthma symptoms [Abstract]. Journal of Allergy & Clinical Immunology 2005;115(Suppl 2):132s. [Google Scholar]
Lirsac 1991 {published data only}
- Lirsac B, Braunstein G. Randomized evaluation of two teaching methods using aerosol dosers. Revue des Maladies Respiratoires 1991;8(6):559‐65. [PubMed] [Google Scholar]
Liu 2001 {published data only}
- Liu CY, Feekery C. Can asthma education improve clinical outcomes? An evaluation of a pediatric asthma education program. Journal of Asthma 2001;38(3):269‐78. [DOI] [PubMed] [Google Scholar]
Lukacs 2002 {published data only}
- Lukacs SL, Francis EK, Baron AE, Crane LA. Effectiveness of an asthma management program for pediatric members of a large health maintenance organization. Archives of Pediatrics and Adolescent Medicine 2002;156(9):872‐6. [DOI] [PubMed] [Google Scholar]
Marks 1999 {published data only}
- Marks MK, Hynson JL, Karabatsos G. Asthma: communication between hospital and general practitioners. Journal of Paediatrics & Child Health 1999;35(3):251‐4. [DOI] [PubMed] [Google Scholar]
Maslennikova 1998 {published data only}
- Maslennikova GY, Morosova ME, Salman NV, Kulikov SM, Oganov RG. Asthma education programme in Russia: educating patients. Patient Education and Counseling 1998;33(2):113‐27. [DOI] [PubMed] [Google Scholar]
McCann 2006 {published data only}
- McCann DC, McWhirter J, Coleman H, Calvert M, Warner JO. A controlled trial of a school‐based intervention to improve asthma management. European Respiratory Journal 2006;27(5):921‐8. [DOI] [PubMed] [Google Scholar]
McCarthy 2002 {published data only}
- McCarthy MJ, Herbert R, Brimacombe M, Hansen J, Wong D, Zelman M. Empowering parents through asthma education. Paediatric Nursing 2002;28(5):465‐73. [PubMed] [Google Scholar]
McConnell 2005 {published data only}
- McConnell R, Milam J, Richardson J, Galvan J, Jones C, Thorne PS, et al. Educational intervention to control cockroach allergen exposure in the homes of Hispanic children in Los Angeles: results of the La Casa study. Clinical and Experimental Allergy 2005;35(4):426‐33. [DOI] [PubMed] [Google Scholar]
McGhan 2003 {published data only}
- McGhan SL, Wong E, Jhangri GS, Wells HM, Michaelchuk DR, Boechler VL, et al. Evaluation of an education program for elementary school children with asthma. Journal of Asthma 2003;40(5):523‐33. [DOI] [PubMed] [Google Scholar]
McMullen 2002 {published data only}
- McMullen AH, Yoos HL, Kitzman H. Peak flow meters in childhood asthma: parent report of use and perceived usefulness. Journal of Pediatric Health Care 2002;16(2):67‐72. [PubMed] [Google Scholar]
McPherson 2006 {published data only}
- Glazebrook C, McPherson A, Forster D, James C, Crook I, Smyth A. The asthma files: randomised controlled trial of interactive multimedia program to promote self‐management skills in children [Abstract]. American Thoracic Society 100th International Conference, May 21‐26, Orlando. 2004:D92 Poster 506.
- McPherson A, Glazebrook C, Smyth S. Effectiveness of a multimedia education program for children with asthma [Abstract]. European Respiratory Journal 2003;22(Suppl 45):Abstract No: P2299. [Google Scholar]
- McPherson AC, Glazebrook C, Forster D, James C, Smyth A. A randomized, controlled trial of an interactive educational computer package for children with asthma. Pediatrics 2006;117(4):1045‐54. [DOI] [PubMed] [Google Scholar]
Mesters 1995 {published data only}
- Mesters I, Nunen M, Crebolder H, Meertens R. Education of parents about paediatric asthma: effects of a protocol on medical consumption. Patient Education and Counseling 1995;25(2):131‐6. [DOI] [PubMed] [Google Scholar]
Nishioka 2006 {published data only}
- Nishioka K, Saito A, Akiyama K, Yasueda H. Effect of home environment control on children with atopic or non‐atopic asthma. Allergology International 2006;55(2):141‐8. [DOI] [PubMed] [Google Scholar]
PAC PORT {published data only}
- Finkelstein JA, Lozano P, Streiff KA, Arduino KE, Sisk CA, Wagner EH, et al. Clinical effectiveness research in managed‐care systems: lessons from the Pediatric Asthma Care PORT. Patient Outcomes Research Team. Health Services Research 2002;37(3):775‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano P, Finkelstein JA, Carey VJ, Fuhlbrigge AL, Inui T, Sullivan SD, et al. A randomized trial of physician education and organizational change in chronic asthma care: health outcomes in the pediatric asthma care PORT Trial. Pediatric Research 2002;51(4):178A. [Google Scholar]
- Lozano P, Finkelstein JA, Carey VJ, Wagner EH, Inui TS, Fuhlbrigge AL, et al. A multisite randomized trial of the effects of physician education and organizational change in chronic‐asthma care: health outcomes of the Pediatric Asthma Care Patient Outcomes Research Team II Study. Archives of Pediatrics & Adolescent Medicine 2004;158(9):875‐83. [DOI] [PubMed] [Google Scholar]
- Sullivan S, Lee T, Blough D, Finkelstein J, Lozano P, Inui T, et al. Cost‐effectiveness of physician peer leader education and practice‐based redesign in managed care: the pediatric asthma care PORT‐II trial. Journal of Allergy and Clinical Immunology 2004;113(2):s339. [Google Scholar]
- Sullivan SD, Lee TA, Blough DK, Finkelstein JA, Lozano P, Inui TS, et al. A multisite randomized trial of the effects of physician education and organizational change in chronic asthma care: cost‐effectiveness analysis of the Pediatric Asthma Care Patient Outcomes Research Team II (PAC‐PORT II). Archives of Pediatric and Adolescent Medicine 2005;159(5):428‐34. [DOI] [PubMed] [Google Scholar]
- Weiss KB, Lozano P, Finkelstein JA, Carey V, Sullivan S, Fuhlbrigge A, et al. A randomized controlled clinical trial to improve asthma care for children through provider education and health systems change: a description of the pediatric asthma care patient outcome research team (PAC‐PORT II) study design. Health Services & Outcomes Research Methodology 2003;4(4):265‐82. [Google Scholar]
Patterson 2005 {published data only}
- Patterson EE, Brennan MP, Linskey KM, Webb DC, Shields MD, Patterson CC. A cluster randomised intervention trial of asthma clubs to improve quality of life in primary school children: the School Care and Asthma Management Project (SCAMP). Archives of Disease in Childhood 2005;90(8):786‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perez 1999 {published data only}
- Perez MG, Feldman L, Caballero F. Effects of a self‐management educational program for the control of childhood asthma. Patient Education & Counseling 1999;36(1):47‐55. [DOI] [PubMed] [Google Scholar]
Perrin 1992 {published data only}
- Perrin JM, MacLean WE Jr, Gortmaker SL, Asher KN. Improving the psychological status of children with asthma: a randomized controlled trial. Journal of Developmental and Behavioral Pediatrics 1992;13(4):241‐7. [PubMed] [Google Scholar]
Perry 2000 {published data only}
- Perry CS, Toole KA. Impact of school nurse case management on asthma control in school‐aged children. Journal of School Health 2000;70(7):303‐4. [DOI] [PubMed] [Google Scholar]
Persaud 1996 {published data only}
- Persaud DI, Barnett SE, Weller SC, Baldwin CD, Niebuhr V, McCormick DP. An asthma self‐management program for children, including instruction in peak flow monitoring by school nurses. Journal of Asthma 1996;33(1):37‐43. [DOI] [PubMed] [Google Scholar]
Phillips 2005 {published data only}
- Phillips CB, Yates R, Glasgow NJ, Ciszek K, Attewell R. Improving response rates to primary and supplementary questionnaires by changing response and instruction burden: Cluster randomised trial. Australian & New Zealand Journal of Public Health 2005;29(5):457‐60. [DOI] [PubMed] [Google Scholar]
Ploska 1999 {published data only}
- Ploska JF. Asthma education nurses in the hospital [Infirmiere educatrice de l'asthme en secteur hospitalier]. Revue de l'Infirmiere 1999;50:35‐40. [PubMed] [Google Scholar]
Porter 2006 {published data only}
- Porter SC, Forbes P, Feldman HA, Goldmann DA. Impact of patient‐centered decision support on quality of asthma care in the emergency department. Pediatrics 2006;117(1):e33‐e42. [DOI] [PubMed] [Google Scholar]
Rakos 1985 {published data only}
- Rakos RF, Grodek MV, Mack KK. The impact of a self‐administered behavioral intervention program on pediatric asthma. Journal of Psychosomatic Research 1985;29(1):101‐8. [DOI] [PubMed] [Google Scholar]
Ronchetti 1997 {published data only}
- Indinnimeo L, Mercuri M, Marolla F, et al. Asthma education program in outpatient children [Programma di educazione sanitaria sull'asma bronchiale nella pratica ambulatoriale in eta pediatrica]. Revista Italia Pediatrica 1997;23(5):873. [Google Scholar]
- Indinnimeo L, Mercuri M, Raponi M, Petrilli MT, Morano S, Frisenda F, et al. Efficacy of a short asthma education program referred to the parents of asthmatic children in an outpatient clinic. European Respiratory Journal 1994;7(Suppl 18):91s. [Google Scholar]
- Ronchetti R, Indinnimeo L, Bonci E, Corrias A, Evans D, Hindi‐Alexander M, et al. Asthma self‐management programmes in a population of Italian children: a multicentric study. Italian Study Group on Asthma Self‐Management Programmes. European Respiratory Journal 1997;10(6):1248‐53. [DOI] [PubMed] [Google Scholar]
Rubin 1986 {published data only}
- Rubin DH, Leventhal JM, Sadock RT, Letovsky E, Schottland P, Clemente I, et al. Educational intervention by computer in childhood asthma: a randomized clinical trial testing the use of a new teaching intervention in childhood asthma. Pediatrics 1986;77:1‐10. [PubMed] [Google Scholar]
Salisbury 2002 {published data only}
- Salisbury C, Francis C, Rogers C, Parry K, Thomas H, Chadwick S, et al. A randomised controlled trial of clinics in secondary schools for adolescents with asthma. British Journal of General Practice 2002;52:988‐96. [PMC free article] [PubMed] [Google Scholar]
Scarfone 2002 {published data only}
- Scarfone RJ, Capraro GA, Zorc JJ, Zhao H. Demonstrated use of metered‐dose inhalers and peak flow meters by children and adolescents with acute asthma exacerbations. Archives of Pediatrics & Adolescent Medicine 2002;156(4):378‐83. [DOI] [PubMed] [Google Scholar]
Schatz 2006 {published data only}
- Schatz M, Gibbons C, Nelle C, Harden K, Zeiger RS. Impact of a care manager on the outcomes of higher risk asthmatic patients: a randomized controlled trial. Journal of Asthma 2006;43(3):225‐9. [DOI] [PubMed] [Google Scholar]
Schmidt 1993 {published data only}
- Schmidt S, Konning J, Szczepanski R, Gebert N, Hummelink R, Wahn U. Asthma training in practice. Evaluation of an integrated care concept including the involvement of a paediatrician in practice. Pediatrische Praxis 1993;45:635‐41. [Google Scholar]
Schmidt 2002 {published data only}
- Schmidt CK. Comparison of three teaching methods on 4‐ through 7‐year‐old children's understanding of the lungs in relation to a peak flow meter in the management of asthma: a pilot study. Journal of Asthma 2002;39(7):641‐8. [DOI] [PubMed] [Google Scholar]
- Schmidt CK. Comparison of three teaching methods on four‐through‐seven‐year‐old children's understanding of the lungs in relation to a peak flow meter in the management of asthma: a pilot study. University of Pittsburgh 1999. [DOI] [PubMed]
Shah 2001 {published data only}
- Shah S, Peat J, Cantwell G, Wang H, Sindusake P, Gibson P. Peer‐led asthma education improves quality of life in adolescents. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A902. [Google Scholar]
- Shah S, Peat J, Wang H, Sindusake P, Henry R, Gibson P. Peer‐led asthma education improves quality of life in adolescents. Journal of Paediatrics & Child Health 2001;37(6):A2. [Google Scholar]
- Shah S, Peat JK, Mazurski EJ, Wang H, Sindhusake D, Bruce C, et al. Effect of peer led programme for asthma education in adolescents: cluster randomised controlled trial. BMJ 2001;322(7286):583‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shegog 2001 {published data only}
- Shegog R, Bartholomew LK, Parcel GS, Sockrider MM, Masse L, Abramson SL. Impact of a computer assisted education program on factors related to asthma self‐management behavior. Journal of the American Medical Informatics Association 2001;8(1):49‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shields 1990 {published data only}
- Shields MC, Griffin KW, McNabb WL. The effect of a patient education program on emergency room use for inner‐city children with asthma. American Journal of Public Health 1990;80:36‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shields 2004 {published data only}
- Shields MD, Patterson EE, Brennan MP, Linskey K, Webb D, Patterson CC. A cluster randomised intervention trial of asthma clubs to improve quality of life in primary school children ‐ the school care and asthma management project (SCAMP). Thorax 2004;59(Suppl ii):ii21. [DOI] [PMC free article] [PubMed] [Google Scholar]
SKCHHP {published data only}
- Krieger J, Takaro TK, Allen C, Song L, Weaver M, Chai S, et al. The Seattle‐King County Healthy Homes Project: implementation of a comprehensive approach to improving indoor environmental quality for low‐income children with asthma. Environmental Health Perspectives 2002;110(Suppl 2):311‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger JW, Song L, Takaro TK, Stout J. Asthma and the home environment of low‐income urban children: preliminary findings from the Seattle‐King County healthy homes project. Journal of Urban Health 2000;77(1):50‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger JW, Takaro TK, Song L, Weaver M. The Seattle‐King County Healthy Homes Project: a randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. American Journal of Public Health 2005;95(4):652‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaro TK, Krieger JW, Song L. Effect of environmental interventions to reduce exposure to asthma triggers in homes of low‐income children in Seattle. Journal of Exposure Analysis and Environmental Epidemiology 2004;14(Suppl 1):S133‐43. [DOI] [PubMed] [Google Scholar]
Smith 1986 {published data only}
- Smith NA, Seale JP, Ley P, Shaw J, Bracs PU. Effects of intervention on medication compliance in children with asthma. Medical Journal of Australia 1986;144(3):119‐22. [DOI] [PubMed] [Google Scholar]
Splett 2006 {published data only}
- Splett PL, Erickson CD, Belseth SB, Jensen C. Evaluation and sustainability of the healthy learners asthma initiative. Journal of School Health 2006;76(6):279‐82. [DOI] [PubMed] [Google Scholar]
Stergachis 2002 {published data only}
- Stergachis A, Gardner JS, Anderson MT, Sullivan SD. Improving pediatric asthma outcomes in the community setting: does pharmaceutical care make a difference?. Journal of the American Pharmaceutical Association 2002;42(5):743‐52. [DOI] [PubMed] [Google Scholar]
Sulaiman 2004 {published data only}
- Sulaiman ND, Barton CA, Abramson MJ, Liaw T, Harris C, Chondros P, et al. Factors associated with ownership and use of written asthma action plans in North‐West Melbourne. Primary Care Respiratory Journal 2004;13(4):211‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanyeli 2001 {published data only}
- Tanyeli GE, Demir R, Ozylimaz S, Kiyan E, Gurses NH. Effects of a comprehensive rehabilitation program and patient education on posture and breathing patterns in asthmatics. European Respiratory Journal 2001;18(Suppl 33):299s. [Google Scholar]
Turgeon 1996 {published data only}
- Turgeon JP, St‐Laurent‐Gagnon T, Chabot G, Allard‐Dansereau C, Gaudreault P, Thivierge RL, et al. Teaching inhalation techniques to asthmatic children: a randomized clinical trial. Ambulatory Child Health 1996;1(3):205‐13. [Google Scholar]
Valery 2007 {published data only}
- Valery PC, Masters IB, Clements V, Taylor B, Laifoo Y, Chang AB. A randomised controlled study on education intervention for childhood asthma by the Aboriginal and Torres Strait Islander health workers in Torres Strait region. Respirology 2007;12(Suppl 4):A193. [Google Scholar]
Velsor‐Friedrich 2004 {published data only}
- Velsor‐Friedrich B, Pigott T, Srof B. A practitioner‐based asthma intervention program with African American inner‐city school children. Journal of Pediatric Health care 2005;19(3):163‐71. [DOI] [PubMed] [Google Scholar]
- Velsor‐Friedrich B, Pigott TD, Louloudes A. The effects of a school‐based intervention on the self‐care and health of African‐American inner‐city children with asthma. Journal of Pediatric Nursing 2004;19(4):247‐56. [DOI] [PubMed] [Google Scholar]
Vilozni 2001 {published data only}
- Vilozni D, Barker M, Jellouschek H, Heimann G, Blau H. An interactive computer‐animated system spiro‐game facilitates spirometry in preschool children. American Journal of Respiratory and Critical Care Medicine 2001;164(12):2200‐5. [DOI] [PubMed] [Google Scholar]
Volovitz 2003 {published data only}
- Volovitz B, Friedman N, Levin S, Kertes J, Iny‐Cordova S, Nussinovitch M, et al. Increasing asthma awareness among physicians: impact on patient management and satisfaction. Journal of Asthma 2003;40(8):901‐8. [DOI] [PubMed] [Google Scholar]
Wakefield 2002 {published data only}
- Wakefield M, Banham D, McCaul K, Martin J, Ruffin R, Badcock N, et al. Effect of feedback regarding urinary cotinine and brief tailored advice on home smoking restrictions among low‐income parents of children with asthma: a controlled trial. Preventive Medicine 2002;34(1):58‐65. [DOI] [PubMed] [Google Scholar]
Wensley 2004 {published data only}
- Wensley D, Silverman M. Peak flow monitoring for guided self‐management in childhood asthma: a randomized controlled trial. American Journal of Respiratory & Critical Care Medicine 2004;170(6):606‐12. [DOI] [PubMed] [Google Scholar]
Whitman 1985 {published data only}
- Whitman N, West D, Brough FK, Welch M. A study of a self‐care rehabilitation program in pediatric asthma. Health Education Quarterly 1985;12(4):333‐42. [DOI] [PubMed] [Google Scholar]
Willems 2004 {published data only}
- Willems DCM, Joore MA, Hendriks HJE, Duurling AH, Wesseling G‐J, Wouters EFM. The cost effectiveness of an ICT supporting self‐management program in children with asthma. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1189. [Google Scholar]
Williams 2006 {published data only}
- Williams SG, Brown CM, Falter KH, Alverson CJ, Gotway‐Crawford C, Homa D, et al. Does a multifaceted environmental intervention alter the impact of asthma on inner‐city children?. Journal of the National Medical Association 2006;98(2):249‐60. [PMC free article] [PubMed] [Google Scholar]
Wilson 1996 {published data only}
- Wilson SR, Latini D, Starr NJ, Fish L, Loes LM, Page A, et al. Education of parents of infants and very young children with asthma: a developmental evaluation of the Wee Wheezers program. Journal of Asthma 1996;33(4):239‐54. [DOI] [PubMed] [Google Scholar]
Wong 2001 {published data only}
- Wong EY, McGhan S, Wells H, Boechler V, Befus D, Majaesic C, et al. Dropouts from childhood asthma education program have more problems. Annual Thoracic Society 97th International Conference; San Francisco CA ,May 18‐23. 2001.
Yang 2005 {published data only}
- Yang BH, Chen YC, Chiang BL, Chang YC. Effects of nursing instruction on asthma knowledge and quality of life in schoolchildren with asthma. Journal of Nursing Research: JNR 2005;13(3):174‐83. [DOI] [PubMed] [Google Scholar]
Yawn 2000 {published data only}
- Yawn BP, Algatt‐Bergstrom PJ, Yawn RA, Wollan P, Greco M, Gleason M, et al. An in‐school CD‐ROM asthma education program. Journal of School Health 2000;70(4):153‐9. [DOI] [PubMed] [Google Scholar]
Yilmaz 2002 {published data only}
- Yilmaz A, Akkaya E. Evaluation of long‐term efficacy of an asthma education programme in an out‐patient clinic. Respiratory Medicine 2002;96(7):519‐24. [DOI] [PubMed] [Google Scholar]
Yoon 2004 {published data only}
- Yoon YM, Suk MH, Oh WO, Park ES. Effects of an asthma camp program on self efficacy and a self care behaviour of asthmatic children. European Respiratory Journal 2004;24(Supp 48):383s. [Google Scholar]
Zorc 2003 {published data only}
- Zorc JJ, Scarfone RJ, Li Y, Hong T, Harmelin M, Grunstein L, et al. Scheduled follow‐up after a pediatric emergency department visit for asthma: a randomized trial. Pediatrics 2003;111(3):495‐502. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Ben‐Noun 1990 {published data only}
- Ben‐Noun L. Use of stethoscope by mothers of asthmatic children ages 1‐5. Harefuah 1990;119(11):362‐4. [PubMed] [Google Scholar]
Bunjaroonsilp 2002 {published data only}
- Bunjaroonsilp N, Bunnag A, Jungsomjatepaisal W, Pongsaranunthakul Y, Maksuwan D. Effectiveness of the nurse‐run asthma self‐management program for sick children of the university hospitals in Bangkok. Thai Journal of Nursing Research 2002;6(3):128‐35. [Google Scholar]
Romàn 1998 {published data only}
- Román JM, Figuerola J, llobera J, Fuster J, Andreu MJ. Efficacy of group education in asthmatic children. European Respiratory Journal 1998;12(Suppl 29):25s. [Google Scholar]
Soh 2001 {published data only}
- Soh JH, Lee JH, Lim JY, Yoon JK, Choung JT. A model of game program for childhood asthma. Pediatric Allergy and Respiratory Disease 2001;11(4):310‐8. [Google Scholar]
Westhus 1998 {published data only}
- Westhus NK. The test of a mnemonic device to help children with asthma learn to use a metered‐dose inhaler. Saint Louis University 1998; Vol. 134.
References to ongoing studies
Singh 2001 {published data only}
- Singh SB, Gorelick MH. Asthma education in the emergency department. Pediatric Research 2001;49(4):79a. [Google Scholar]
Additional references
AIHW 2005
- Australian Institute of Health and Welfare. Chronic respiratory diseases in Australia: their prevalence, consequences and prevention. AIHW Canberra. AIHW, 2005, issue Cat. No. PHE63.
Altman 2003
- Altman DG, Bland JM. Statistics notes: interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernard‐Bonnin 1995
- Bernard‐Bonnin A, Stachenko S, Bonin D, Charette C, Rousseau E. Self‐management teaching programs and morbidity of pediatric asthma: a meta‐analysis. Journal of Allergy & Clinical Immunology 1995;95:34‐41. [DOI] [PubMed] [Google Scholar]
Boudreaux 2003
- Boudreaux ED, Emond SD, Clark S, Camargo CA Jr, Multicenter Airway Research Collaboration Investigators. Race/Ethnicity and asthma among children presenting to the emergency department: differences in disease severity and management. Pediatrics 2003;111(5 pt 1):e615‐21. [DOI] [PubMed] [Google Scholar]
Buston 2000
- Buston KM, Wood SF. Non‐compliance amongst adolescents with asthma: listening to what they tell us about self‐management. Family Practice 2000;17(2):134‐8. [DOI] [PubMed] [Google Scholar]
Castro 2003
- Castro M, Zimmermann NA, Crocker S, Bradley J, Leven C, Schechtman KB. Asthma intervention program prevents readmissions in high healthcare users. American Journal of Respiratory and Critical Care Medicine 2003;168:1095‐9. [DOI] [PubMed] [Google Scholar]
Cates 2007 [Computer program]
- Cates C. Visual Rx. Version 2.0. Cates C, 2007.
CMAJ 2005
- Summary of recommendations from Canadian Asthma Consesus Guidelines, Canadian Pediatric Consensus guidelines. Education and follow‐up. Canadian Medical Association Journal 2005; Vol. 173, issue Suppl 6. [PMC free article] [PubMed]
Coffman 2008
- Coffman JM, Cabana MD, Halpin HA, Yelin EH. Effects of asthma education on children's use of acute care services: a meta‐analysis. Pediatrics 2008;121(3):575‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibson 2002a
- Gibson PG, Coughlan J, Wilson AJ, Abramson M, Bauman A, Hensley MJ, et al. Self‐management education and regular practitioner review for adults with asthma. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD001117] [DOI] [PubMed] [Google Scholar]
Gibson 2002b
- Gibson PG, Powell H, Coughlan J, Wilson AJ, Hensley MJ, Abramson M, et al. Limited (information only) patient education programs for adults with asthma. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD001005] [DOI] [PubMed] [Google Scholar]
Gorelick 2007
- Gorelick MH, Knight S, Alessandrini EA, Stanley RM, Chamberlain JM, Kuppermann N, et al. Lack of agreement in pediatric emergency department discharge diagnoses from clinical and administrative data sources. Academic Emergency Medicine 2007;14(7):646‐52. [DOI] [PubMed] [Google Scholar]
Halterman 2007
- Halterman JS, Auinger P, Conn KM, Lynch K, Yoos HL, Szilagyi PG. Inadequate therapy and poor symptom control among children with asthma: findings from a multistate sample. Ambulatory Pediatrics 2007;7(2):153‐9. [DOI] [PubMed] [Google Scholar]
Handbook 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2008. [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2008
- Jones BL, Kelly KJ. The adolescent with asthma: fostering adherence to optimize therapy. Clinical Pharmacology & Therapeutics 2008;84(6):749‐53. [DOI] [PubMed] [Google Scholar]
Martin 1995
- Martin AJ, Campbell DA, Gluyas PA, Coates JR, Ruffin RE, Roder DM, et al. Characteristics of near‐fatal asthma in childhood. Pediatric Pulmonology 1995;20:1‐8. [DOI] [PubMed] [Google Scholar]
McPherson 2001
- McPherson A, Glazebrook C, Smyth A. Double click for health: the role of multimedia in asthma education. Archives of Diseases in Childhood 2001;85:447‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 1994
- Mitchell EA, Bland JM, Thompson JMD. Risk factors for readmission to hospital for asthma in childhood. Thorax 1994;49:33‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ordonez 1998
- Ordonez GA, Phelan PD, Olinsky A, Robertson CF. Preventable factors in hospital admission for asthma. Archives of Disease in Childhood 1998;78:143‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Orrell‐Valente 2008
- Orrell‐Valente JK, Jarlsberg LG, Hill LG, Cabana MD. At what age do children start taking daily asthma medicines on their own?. Pediatrics 2008;122(6):e1186‐92. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2008.
Shapiro 2002
- Shapiro GG, Stout JW. Childhood asthma in the United States: urban issues. Pediatric Pulmonology 2002;33:47‐55. [DOI] [PubMed] [Google Scholar]
Sharma 2007
- Sharma HP, Matsui EC, Eggleston PA, Hansel NN, Curtin‐Brosnan J, Diette GB. Does current asthma control predict future health care use among black preschool‐aged inner‐city children?. Pediatrics 2007;120(5):e1174‐81. [DOI] [PubMed] [Google Scholar]
SIGN 2003
- British Thoracic Society, Scottish Intercollegiate Guidelines Newtwork (SIGN). British guideline on the management of asthma. Thorax 2003;58(Suppl I):i1‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2005
- Smith JR, Mugford M, Holland R, Candy B, Noble MJ, Harrison BDW, et al. A systematic review to examine the impact of psycho‐educational interventions on health outcomes and costs in adults and children with difficult asthma. Health Technology Assessment 2005;23(19):1‐370. [DOI] [PubMed] [Google Scholar]
Sun 2003
- Sun BC, Burstin HR, Brennan TA. Predictors and outcomes of frequent emergency department users. Academic Emergency Medicine 2003;10(4):320‐8. [DOI] [PubMed] [Google Scholar]
Szilagyi 2006
- Szilagyi PG, Dick AW, Klein JD, Shone LP, Zwanziger J, Bajorska A, et al. Improved asthma care after enrolment in the State Children's Health Insurance Program in New York. Pediatrics 2006;117(2):486‐96. [DOI] [PubMed] [Google Scholar]
Séguin 2005
- Séguin L, Xu Q, Gauvin L, Zunzunegui M, Potvin L, Frohlich K. Understanding the dimensions of socioeconomic status that influence toddlers' health: unique impact of lack of money for basic needs in Quebec's birth cohort. Journal of Epidemiology and Community Health 2005;59(1):42‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tapp 2007
- Tapp S, Lasserson TJ, Rowe BH. Education interventions for adults who attend the emergency room for acute asthma. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD003000.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolf 2002
- Wolf FM, Guevara JP, Grum CM, Clark NM, Cates CJ. Educational interventions for asthma in children. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD000326] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Haby 2001
- Haby MM, Waters E, Robertson CF, Gibson PG, Ducharme FM. Interventions for educating children who have attended the emergency room for asthma. Cochrane Database of Systematic Reviews 2001, Issue 1. [Art. No.: CD001290. DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]


