Abstract
Greater stiffness of the large elastic arteries is associated with end-organ damage and dysfunction. At the same time, resistance artery vasoconstrictor responsiveness influences vascular tone and organ blood flow. However, it is unknown if large elastic artery stiffness modulates the responsiveness to vasoconstrictors in resistance arteries of the cerebral or skeletal muscle circulations. We previously described the elastin haploinsufficient (Eln+/−) mouse as a model with greater aortic stiffness, but with similar cerebral and skeletal muscle feed artery stiffness to wildtype (Eln+/+) mice. Here, we utilized this model to examine the relation between large elastic artery stiffness and resistance artery vasoconstrictor responses. In middle cerebral arteries (MCAs), vasoconstriction to angiotensin II (Ang II) was ~40% greater in Eln+/− compared with Eln+/+ mice (p=0.02), and this group difference was ameliorated by losartan, indicating a role for Ang II type 1 receptors (AT1Rs). In gastrocnemius feed arteries (GFAs), Eln+/− and Eln+/+ mice did not differ in the response to Ang II. In addition, the vasoconstrictor responses to norepinephrine, endothelin-1, and potassium chloride were not different between Eln+/− and Eln+/+ mice for either MCAs or GFAs. MCA AT1R gene expression did not differ between groups, while Ang II type 2 receptor gene expression was ~50% lower in MCAs from Eln+/− vs. Eln+/+ (p=0.01). In conclusion, greater large elastic artery stiffness is associated with an exacerbated vasoconstriction response to Ang II in cerebral arteries, but not associated with the responses to other vasoconstrictors in cerebral or skeletal muscle feed arteries.
Keywords: Large artery stiffness, cerebral arteries, vasoconstriction
INTRODUCTION
Stiffening of the large elastic arteries (i.e., aorta and carotid arteries) is a key feature of advancing age (Avolio et al., 1983), and is hypothesized to have detrimental effects on the small arteries and the microvasculature (Mitchell, 2008; Thorin-Trescases et al., 2018). Correlational studies in human subjects find that large elastic artery stiffness is associated with resistance artery dysfunction (Mitchell et al., 2005), lower cerebral blood flow (Tarumi et al., 2011; Jefferson et al., 2018), cognitive impairment (Hanon et al., 2005; Mitchell et al., 2011; Pase et al., 2016; Meyer et al., 2017), and Alzheimer’s disease (Hanon et al., 2005; Oh et al., 2016; Pase et al., 2016). At the same time, advancing age is characterized by resistance artery dysfunction that can manifest as greater sensitivity to vasoconstrictors, leading to increased vascular tone and reduced blood flow (Donato et al., 2005; Van Guilder et al., 2007; Wray et al., 2008). It is unclear, however, if large elastic artery stiffness contributes to the greater vasoconstrictor sensitivity in resistance arteries.
When stiffness of the large elastic arteries is increased, there is less dampening of the pulse by the central arteries, leading to greater pulsatility of blood flow and pressure in resistance arteries (Mitchell, 2008; Thorin-Trescases et al., 2018). This greater pulsatility in resistance arteries may lead to impaired arterial function (Mitchell, 2008; Thorin-Trescases et al., 2018). To study the effect of increased large elastic artery stiffness independent of co-morbidity, we previously described the use of the elastin haploinsufficient (Eln+/−) mouse that has greater aortic stiffness, measured by aortic pulse wave velocity, and lower aortic elastin content compared with wildtype (Eln+/+) mice (Walker et al., 2015). Importantly, the difference in aortic pulse wave velocity between Eln+/− and Eln+/+ mice is similar in magnitude to the difference we find between young and old mice (Eln+/+ and young: ~300 cm/s; Eln+/− and old: ~400 cm/s)(Donato et al., 2013; Walker et al., 2015). Increases in large elastic artery stiffness naturally lead to an increase in systolic blood pressure due to widening of the pulse pressure and a quicker return of the reflected wave, thus Eln+/− have a greater systolic blood pressure compared with Eln+/+ mice, but again this difference is similar to the increases we have shown with aging in mice (systolic blood pressure measured by tail-cuff: Eln+/+ and young: ~115 mmHg; Eln+/− and old: ~135 mmHg)(Donato et al., 2013; Walker et al., 2015). Importantly, we demonstrated that elastin haploinsufficiency does not affect elastin content or stiffness in cerebral or skeletal muscle feed arteries (Walker et al., 2015). Thus, the Eln+/− mouse can be used to study the effects of greater large elastic artery stiffness, similar to that which occurs with aging, on resistance artery function.
We previously found Eln+/− mice have impaired cerebral artery endothelium-dependent dilation due to reduced nitric oxide bioavailability and increased oxidative stress compared to Eln+/+ mice (Walker et al., 2015). Likewise, it was recently demonstrated that cerebral blood flow is lower in Eln+/− compared with Eln+/+ mice (Knutsen et al., 2018). Interestingly, we found that skeletal muscle feed artery endothelial function is similar between Eln+/− and Eln+/+ mice (Walker et al., 2015), indicating that greater large elastic artery stiffness does not affect all vascular beds similarly. Thus, it appears that large elastic artery stiffness is an important modulator of resistance artery endothelial function and blood flow, at least in the cerebral circulation.
In the present studies, we sought to assess the association of greater large elastic artery stiffness and receptor-mediated [angiotensin II (Ang II), norepinephrine (NE), and endothelin-1 (ET-1)] and receptor-independent [potassium chloride (KCl)] vasoconstrictor responses in resistance arteries. We focused on cerebral arteries [middle cerebral artery (MCA)] and skeletal muscle feed arteries [gastrocnemius feed artery (GFA)], as these are known to be dysfunctional with age and have important implications for cognitive and physical function (Woodman et al., 2002; Modrick et al., 2009; Walker et al., 2014). We hypothesized that a mouse model of greater large elastic artery stiffness (Eln+/− mouse) would have greater vasoconstrictor responses to Ang II, NE, ET-1, and KCl in MCAs and GFAs compared with wildtype (Eln+/+) mice. As a follow-up, for any vasoconstrictor responses that were different between Eln+/− and Eln+/+ mice, we sought to determine the receptor(s) responsible. Specifically, related to Ang II, we evaluated the role of Ang II type 1 receptors (AT1Rs) and measured gene expression of AT1R isoforms Agtr1a and Agtr1b and the Ang II type 2 receptor (Agtr2). In addition, we measured the gene expression of the Ang(1-7) receptor Mas and of pro-inflammatory cytokines interleukin-1β (IL1b) and tumor necrosis factor-α (Tnfa) as they are potentially upregulated by Ang II signaling (Marchesi et al., 2008).
MATERIALS AND METHODS
Ethical Approval.
All animal procedures conformed to the Principles and standards for reporting animal experiments in Experimental Physiology (Grundy, 2015) and were approved by Animal Care and Use Committee at the University of Utah (16_02002) and the Salt Lake City VA Medical Center (A16/16).
Animals.
Young male mice with a heterozygote deletion of exon 1 of the elastin gene (Eln+/−) and their wildtype littermates (Eln+/+) were used for our studies (Li et al., 1998). In total, 81 mice were studied, and the specific n’s for each outcome are noted in the figures. All mice were generated from a breeding colony at the Salt Lake City VA Medical Center’s (SLC VAMC) Animal Facility. Mice were housed at the SLC VAMC Animal Facility on a 12:12 light:dark cycle and were provided water and normal rodent chow ad libitum. All mice were 5-8 months of age at the time of study. Mice were euthanized by exsanguination under inhaled isoflurane.
Vasoconstrictor responses.
Isolated MCAs and GFAs were studied ex vivo using methods previously described in detail (Lesniewski et al., 2008; Donato et al., 2014). Briefly, GFAs and MCAs were excised and placed in myograph chambers (DMT Inc.) with physiological salt solution (PSS) containing 145.0 mM NaCl, 4.7 mM KCl, 2.0 mM CaCl2, 1.17 mM MgSO4, 1.2 mM NaH2PO4, 5.0 mM glucose, 2.0 mM pyruvate, 0.02 mM EDTA, 3.0 mM MOPS buffer and 1 g/100 ml BSA, pH 7.4 at 37°C, cannulated onto glass micropipettes and secured with nylon (11-0) sutures. Once cannulated, arteries were warmed to 37°C, pressurized to 50 mmHg and allowed to equilibrate for ~1 h. Changes in luminal diameter were measured in response to Ang II (1×10−11 to 1×10−5 M), NE (1×10−9 to 1×10−4 M), ET-1 (1×10−11 to 1×10−7 M), and KCl (10 to 100 mM). Dose responses to Ang II were also performed in the presence of losartan, a selective AT1R blocker (1×10−5 M, 30 minute pre-incubation). It should be noted that not all dose responses were performed on each artery, as we find arteries do not fully recover from Ang II or ET-1, necessitating the use of multiple artery segments to complete all dose responses.
Gene expression.
mRNA expression for Agtr1a, Agtr1b, Agtr2, Mas, IL1b, and Tnfa was measured in lysed MCAs and GFAs by qRT-PCR using the Quantitect Reverse Transcription kit (Qiagen, Inc.) and FastStart SYBR Green Master (Roche Diagnostics Corporation, Roche Applied Science) or PowerUp SYBR Green Master Mix (Applied Biosystems) according to the manufacturer’s protocols. mRNA expression was calculated using the 2-ΔΔCT method. 18s rRNA (QuantiTect Primer Assay: Qiagen, Inc.) was used as a housekeeping gene transcript to control for tissue concentration in samples. The Eln+/+ group was used as the control group. Values for GFAs and MCAs were further normalized for Eln+/+ MCA expression for that particular gene. Agtr1a primers: fwd-AACAGCTTGGTGATCGTC, rev-CATAGCGGTATAGACAGCCCA. Agtr1b primers: fwd-TGGCTTGCGTAGTTTGCCG, rev-ACCCAGTCCAATGGGGAGT. Agtr2 primers: fwd-GCTTACTTCAGCCTGCATTT, rev-GGACTCATTGGTGCCAGTTG. Mas primers: fwd-AGAAATCCCTTCACGGTCTACA, rev-GTCACCGATAATGTCACGATTGT. IL1b primers: fwd-GCAACTGTTCCTGAACTCAACT, rev-ATCTTTTGGGGTCCGTCAACT. Tnfa primers: fwd- CTGAACTTCGGGGTGATCGG, rev-GGCTTGTCACTCGAATTTTGAGA.
Statistics.
For animal and artery characteristics, maximal constriction/dilation, EC50’s, and gene expression, group differences were determined by t-test for independent samples. Gene expression measures were normalized to the mean of the MCA Eln+/+ group. For dose responses, group differences were determined by repeated measures ANOVA. An LSD post-hoc test was used for preplanned comparisons where appropriate. EC50’s were calculated by fitting each dose response to a 4-parameter logistic equation. EC50’s for the response to Ang II were not calculated given the biphasic nature of the curve. Data are presented as mean±SD. Significance was set at p<0.05.
RESULTS
Animal characteristics
Eln+/+ and Eln+/− mice did not differ in age (6.8±1.2 and 7.4±1.6 months, p=0.23) and body mass (31.2±3.57 and 32.4±2.7 g, p=0.27). We previously characterized Eln+/+ and Eln+/− mice of similar ages and demonstrated that Eln+/− mice have less aortic elastin content and greater aortic stiffness compared with Eln+/+ (Walker et al., 2015). In tail cuff measurement in conscious mice, Eln+/− mice had greater systolic blood pressure compared with Eln+/+ mice (134±16 vs. 112±17 mmHg, p=0.001)(Walker et al., 2015). However, we found that stiffness, elastin content and maximal diameter does not differ in either the MCA or GFA between Eln+/+ and Eln+/− mice (Walker et al., 2015).
Vasoconstriction responses
In MCAs from Eln+/+ and Eln+/− mice, the maximal vasoconstriction, dose-responses, and sensitivity (EC50) to KCl, NE, and ET-1 did not differ between groups (all p>0.05, Figure 1, Table 1). However, MCAs from Eln+/− mice had a ~40% greater maximal vasoconstriction to Ang II compared with MCAs from Eln+/+ mice (p=0.02, dose response p=0.04, Figure 2A). In the second phase of the biphasic response to Ang II, the maximal vasodilation (from the point of maximal constriction) was not different between groups (p=0.53, Figure 2A). Pre-incubation with losartan, an AT1R blocker, blunted the maximal constriction to Ang II in Eln+/− MCAs (p=0.03, Figure 2B). In contrast, pre-incubation with losartan did not change the maximal constriction to Ang II in Eln+/+ MCAs (p=0.40, Figure 2B). For both Eln+/− and Eln+/+ MCAs, losartan shifted the response to Ang II, such that maximal vasoconstriction occurred at a higher dose and vasodilation was not observed. Furthermore, following losartan pre-incubation, there was no difference in the maximal constriction to Ang II between Eln+/+ and Eln+/− MCAs (p>0.05, Figure 2B), indicating that Eln+/− mice had a greater vasoconstriction to Ang II that was mediated by AT1Rs.
Figure 1.
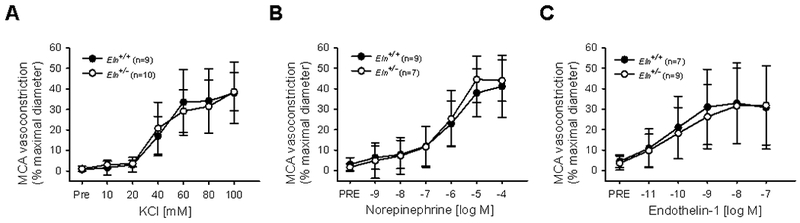
Middle cerebral artery (MCA) vasoconstriction to (A) KCl, (B) norepinephrine, and (C) endothelin-1 in Eln+/+ and Eln+/− mice. Group n’s shown in legend. Values are mean±SD.
TABLE 1.
Vasoconstrictor sensitivities
| MCA | GFA | |||
|---|---|---|---|---|
| Eln+/+ | Eln+/− | Eln+/+ | Eln+/− | |
| KCl EC50, log M | 40.8 ± 11.6 | 37.6 ± 11.6 | 28.3 ± 4.9 |
32.7 ± 6.2 |
| NE EC50, log M | −6.3 ± 0.7 | −6.2 ± 0.7 | −6.0 ± 1.3 | −5.8 ± 1.2 |
| ET-1 EC50, log M | −10.0 ± 0.6 | −10.8 ± 2.5 | −10.2 ± 0.5 | −10.0 ± 0.9 |
MCA, middle cerebral artery; GFA, gastrocnemius feed artery; KCl, potassium chloride; NE, norepinephrine; ET-1, endothelin-1. Data are mean±SD.
Figure 2.
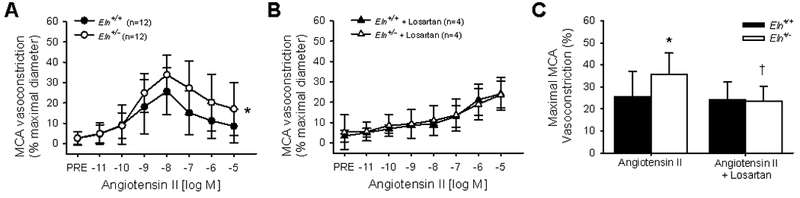
Middle cerebral artery (MCA) vasoconstriction to angiotensin II (A,C) alone and (B,C) in the presence of angiotensin II type 1 receptor blocker losartan in Eln+/+ and Eln+/− mice. The dose response to angiotensin II is shown in A,B and the maximal vasoconstriction in C. Group n’s shown in legend. *p<0.05 vs. Eln+/+ angiotensin II alone, †p<0.05 vs. Eln+/− angiotensin II alone. Values are mean±SD.
In GFAs, the maximal vasoconstriction, dose-responses, and sensitivity to KCl, NE, ET-1, or Ang II did not differ between Eln+/+ and Eln+/− mice (all p>0.05, sensitivity not calculated for biphasic Ang II, Figure 3, Table 1).
Figure 3.
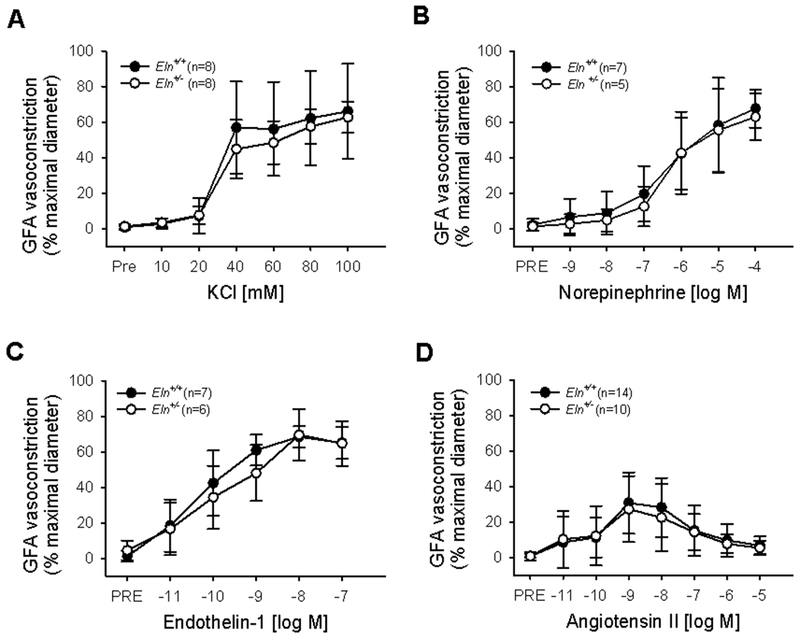
Gastrocnemius feed artery (GFA) vasoconstriction to (A) KCl, (B) norepinephrine, (C) endothelin-1, and (D) angiotensin II in Eln+/+ and Eln+/− mice. Group n’s shown in legend. Values are mean±SD.
Ang receptors and pro-inflammatory cytokine gene expression
In the MCAs, gene expression of Agtr2 and Mas were ~50% less in Eln+/− compared with Eln+/+ mice (p=0.01), but gene expression for AT1Rs (Agtr1a and Agtr1b) did not differ between groups (p>0.05, Figure 4A). In the GFAs, expression of Agtr1a, Agtr1b, Agtr2 and Mas did not differ between groups (p>0.05, Figure 4B). When gene expression for these receptors was compared between MCAs and GFAs for all mice, it was found that the MCAs had a ~10-fold lower expression of Agtr1a (p=0.001) and a ~2-fold greater expression of Agtr2 (p=0.009) and ~1.5-fold greater expression of Mas (p=0.03) than GFAs, while Agtr1b expression was not different between arteries (p>0.05, Figure 4). In the MCAs, gene expression of IL-1β trended to be greater in Eln+/− compared with Eln+/+ mice (p=0.06). while gene expression of TNF-α did not differ between groups (p=0.42, Figure 5).
Figure 4.
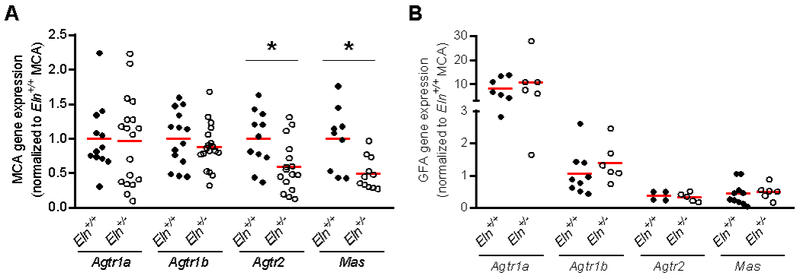
Gene expression of angiotensin II receptors Agtr1a, Agtr1b, and Agtr2 and Ang(1-7) receptor Mas in (A) middle cerebral artery (MCA) and (B) gastrocnemius feed artery (GFA) from Eln+/+ and Eln+/− mice. All values are normalized to MCA expression of the particular gene in Eln+/+ mice. Group means indicated by red line. *p<0.05 vs. Eln+/+.
Figure 5.
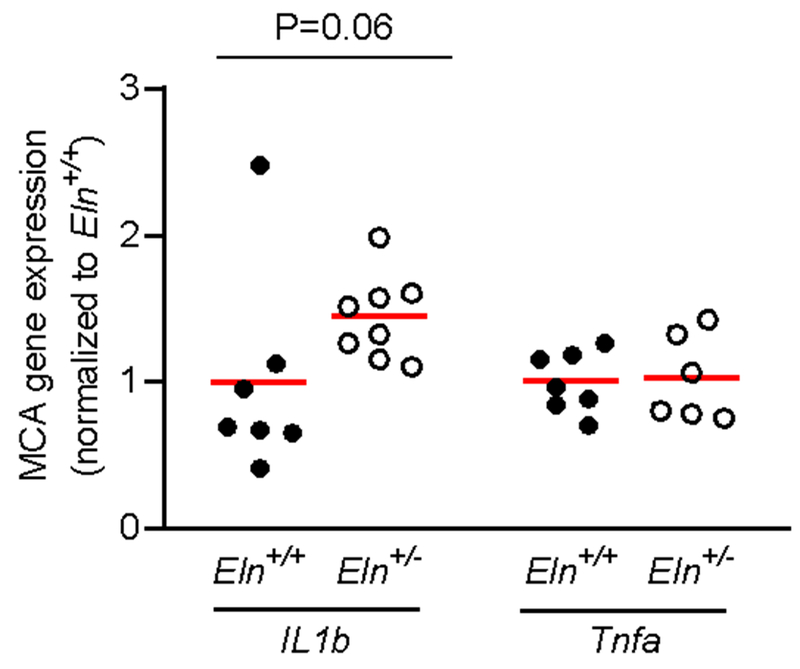
Gene expression of interleukin-1β (IL1b) and tumor necrosis factor-α (Tnfa) in the middle cerebral artery (MCA) from Eln+/+ and Eln+/− mice. All values are normalized to expression of the particular gene in Eln+/+ mice. Group means indicated by red line. P-value vs. Eln+/+.
DISCUSSION
In these studies we utilized the Eln+/− mouse, which was previously characterized as a model with increased large elastic artery stiffness similar to aging, but without co-morbidity except those related to the large elastic artery phenotype (Wagenseil et al., 2005; Pezet et al., 2008; Walker et al., 2015). We found that when compared with Eln+/+ mice, Eln+/− mice have a greater vasoconstriction to Ang II in cerebral arteries, an effect mediated by AT1R. In contrast, there were no differences between Eln+/− and Eln+/+ mice for cerebral artery response to NE, ET-1, or KCl, and no differences between groups in skeletal muscle feed artery responses to any of the above vasoconstrictors. Therefore, these studies indicate that greater large elastic artery stiffness is associated with a greater resistance artery vasoconstriction specific to Ang II in cerebral arteries.
Large elastic artery stiffness and resistance artery Ang II response
The effects of large elastic artery stiffness on resistance artery function are dependent on the specific vascular bed (Walker et al., 2015). While the present study is the first to examine the cerebral artery response to Ang II in Eln mice, a previous study in mesenteric arteries also found a greater vasoconstrictor response to Ang II in Eln+/− that appears to be mediated by Rho kinase activity (Osei-Owusu et al., 2014). However, the physiological consequences of this greater Ang II response is unclear, as the blood pressure response to infusion of Ang II does not differ between Eln+/− and Eln+/+ mice (Faury et al., 2003; Wagenseil et al., 2007), perhaps due to the baroreflex response in intact animals. In addition, similar to our findings in the GFA, it was previously observed that the femoral artery vasoconstrictor response to Ang II is similar between Eln+/− and Eln+/+ mice (Osei-Owusu et al., 2014). Thus, the effects of large elastic artery stiffness on the small artery response to Ang II are not consistent across vascular beds. Similarly, our previous studies in these mice found that greater large elastic artery stiffness led to impaired endothelium-dependent dilation in MCAs, but not GFAs (Walker et al., 2015). The reason for the apparent greater vulnerability of cerebral arteries to increased large elastic artery stiffness is unknown, but potential mediators may include the higher resting blood flow in the brain (Mitchell, 2008), the thinner arterial wall and lack of external elastic lamina in cerebral resistance arteries (Walker et al., 2015; Thorin-Trescases et al., 2018), or the closeness to the heart, at least compared with skeletal muscle feed arteries. It is also important to consider the role of autoregulation by cerebral resistance arteries in maintaining constant cerebral blood flow (Mitchell, 2008). The greater pressure pulsatility associated with increased large elastic artery stiffness may result in myogenic vasoconstriction of cerebral resistance arteries (Raignault et al., 2017), an effect that may be additive with the greater Ang II responsiveness.
Large elastic artery stiffness and resistance artery angiotensin receptors
Losartan normalized MCA vasoconstriction between groups of Eln mice, indicating that AT1Rs mediate the exacerbated vasoconstriction to Ang II in Eln+/− mice. In the presence of losartan, Ang II did induce MCA vasoconstriction, although only at high doses, indicating either an incomplete blockade of the AT1R or vasoconstriction mediated by AT2R. The second phase of the biphasic response to Ang II involves vasodilation or relaxation of the artery, however this second phase was absent in MCAs in the presence of losartan. Thus, AT1R inhibitors appear to neutralize this Ang II mediated vasodilation/relaxation, although we do not know the exact mechanism as the vasodilation/relaxation could result from Ang II mediated vasodilation, likely by increased nitric oxide production (Boulanger et al., 1995; Pueyo et al., 1998), or ATR desensitization or internalization from repeated or high dose Ang II stimulation (Kuttan & Sim, 1993; Kai et al., 1996; Jerez et al., 2001). Nevertheless, there were no differences between Eln+/− and Eln+/+ MCAs in the second phase of the biphasic response to Ang II in the presence of losartan.
However, these functional findings do not align with the MCA gene expression patterns, where AT1R expression did not differ between groups. In contrast, we found less Agtr2 and Mas gene expression in Eln+/− compared with Eln+/+ mice in the MCA. Moreover, MCAs have greater Agtr2 and Mas gene expression than GFAs, indicating a potentially greater importance of these receptors in the cerebral circulation. The functional effects of reduced Agtr2 gene expression in Eln+/− mice is unclear, as AT2Rs typically induce vasodilation (Matavelli & Siragy, 2015), but we did not observe group differences in the vasodilatory response to Ang II in the present study. AT2Rs can cause vasoconstriction, as seen in mesenteric arteries of Eln+/− mice (Osei-Owusu et al., 2014), however this is an unlikely source of the group differences in the MCA, as losartan abolished the differences between Eln+/− and Eln+/+ responses to Ang II. Therefore, the gene expression of both AT1Rs and AT2Rs do not follow the apparent functional activity of the receptors in MCAs from Eln+/− mice. Thus, it is likely that ATR protein expression or activity is modulated by large elastic artery stiffness independent of changes in gene expression. Unfortunately, given the small amount of tissue in a mouse MCA we were unable to assess these factors in our mode. Like AT2R, the Mas receptor is also associated with vasodilation, but in response to Ang(1-7)(Lemos et al., 2005; Schindler et al., 2007) rather than Ang II. Our finding that Eln+/− mice have lower Mas expression suggests that the response to other angiotensin forms may also be affected by large artery stiffness. Future studies will be needed to examine the effects of large artery stiffness on the response to Ang(1-7) in cerebral arteries as well as the functional activity of ATRs in the cerebral circulation.
Large elastic artery stiffness and the resistance artery response to other vasoconstrictors.
The present studies are the first to examine the resistance artery responses to ET-1 and KCl in Eln+/− mice, and there appears to be no influence of large elastic artery stiffness on responses to these vasoconstrictors. While we also found no difference between groups for NE responses, previous studies in Eln+/− mice found a greater vasoconstrictor response to phenylephrine in renal and carotid arteries for a single dose (Faury et al., 2003) and in mesenteric arteries at submaximal (but not maximal) doses (Osei-Owusu et al., 2014). Thus, there may be an effect of large elastic artery stiffness on the vasoconstriction responses mediated by adrenergic receptors in some vascular beds, although these effects appear to be minimal. Similar to findings with greater large elastic artery stiffness in the present studies, aging does not increase the resistance artery vasoconstrictor responses to NE or KCl (Muller-Delp et al., 2002). However, aging does increase the skeletal muscle feed artery vasoconstriction to ET-1 (Donato et al., 2005). Therefore, mechanisms independent of greater large elastic artery stiffness are likely responsible for the increase in resistance artery vasoconstriction to ET-1 with advancing age.
Oxidative stress and inflammation
In addition to modulating vascular tone, Ang II also stimulates oxidative stress and inflammatory pathways (Dikalov et al., 2008; Marchesi et al., 2008). Consistent with our finding of an increased Ang II responsiveness in MCAs from Eln+/− mice in this study, we previously demonstrated that Eln+/− MCAs have greater oxidative stress compared with Eln+/+ MCAs (Walker et al., 2015). In the present study, we also find a trend (p=0.06) for greater IL-β gene expression in Eln+/− MCAs compared with Eln+/+ MCAs. Interestingly, IL-1β has also been demonstrated to increase the vasoconstrictor response to Ang II (Vicaut et al., 1996; Dorrance, 2007). Therefore, it is unclear whether elevated IL-1β or increased Ang II responsiveness comes first in the presence of greater large artery stiffness. Nevertheless, elevated cerebrovascular inflammatory signaling associated with greater large artery stiffness can also induce increased permeability of the blood brain barrier (Blamire et al., 2000). Increased blood brain barrier permeability is associated with aging and Alzheimer’s disease (Farrall & Wardlaw, 2009) and can alter the extracellular environment of the brain leading to neuroinflammation and neuropathology (Zlokovic, 2008). Thus, the greater Ang II responsiveness that is associated with large artery stiffness may have implications beyond vascular tone, particularly contributing to a pro-oxidative stress and pro-inflammatory environment of cerebral arteries.
Limitations
There are a few notable limitations to the current studies: 1) Although our previous studies indicate that elastin haploinsufficiency does not change stiffness or elastin content in MCAs or GFAs (Walker et al., 2015), there still remains a possibility that the genotypic changes in the resistance arteries could directly affect other characteristics. However, we believe the Eln+/− mouse is currently the best model for studying greater large artery stiffness, as it does not have the confounding co-morbidities of studying aging or disease models. Therefore, our results provide important insights into the associations of large artery stiffness and resistance artery function. 2) While the scientific basis for these studies is that increased aortic stiffness causes dysfunction in resistance arteries by increasing pressure pulsatility, we did not directly measure this pulsatility in these mice. Future studies are needed to demonstrate that elastin haploinsufficient mice do indeed have increased resistance artery pulsatility. 3) Increased in systolic blood pressure is a direct effect of large artery stiffening, and thus Eln+/− mice have greater systolic blood pressure compared with Eln+/+ mice. While these group differences in systolic blood pressure could be viewed as a confounding factor, we believe this makes the Eln model more physiologically relevant, as these blood pressure differences are similar to those seen with advancing age. Future studies may involve a normalization of systolic blood pressure in this model to control for this factor. 4) It has been previously demonstrated that old age leads to an increased responsiveness of peripheral arteries to Ang II in humans (Wray et al., 2008; Barrett-O’Keefe et al., 2013), however the effect of aging on the cerebral artery responsiveness to Ang II is unknown. Thus, we cannot conclude that our model of greater large artery stiffness replicates the effects of aging with respect to Ang II responsiveness in cerebral arteries.
Clinical importance of large elastic artery stiffness and the cerebral circulation
The consequences of large elastic artery stiffening are becoming increasingly appreciated, as described in previous reviews (O’Rourke & Safar, 2005; Mitchell, 2008; Thorin-Trescases et al., 2018). In particular, mounting evidence suggests an association between greater large elastic artery stiffness and lower cerebral blood flow as well as cognitive impairment and Alzheimer’s disease (Mitchell et al., 2011; Tarumi et al., 2011; Oh et al., 2016; Pase et al., 2016; Meyer et al., 2017; Jefferson et al., 2018; Knutsen et al., 2018). In light of these associations, one could hypothesize that Ang II responsiveness in cerebral arteries is a potential mediator of the association between large elastic artery stiffness and reduced cerebral blood flow; however, further studies are needed to definitively test this hypothesis.
Conclusions
In the present studies, we utilized the Eln+/− mouse as a model of isolated large elastic artery stiffness. We found greater large elastic artery stiffness was associated with a greater vasoconstrictor response to Ang II in cerebral arteries, mediated by AT1Rs. However, we did not find that large elastic artery stiffness altered the responses to other vasoconstrictors in cerebral arteries or had any effects on vasoconstrictor responses to skeletal muscle feed arteries. As aging is associated with greater large elastic artery stiffness, as well as an increased risk for cognitive impairment and Alzheimer’s disease, these results provide insight into the potential role of Ang II in these outcomes.
NEW FINDINGS.
What is the central question of this study?
Greater large artery stiffness is associated with dysfunctional resistance artery vasodilatory responses, impaired memory and greater Alzheimer’s disease risk. However, it is unknown if stiffer large arteries affect cerebral and skeletal muscle feed artery responses to vasoconstrictors.
What is the main finding and its importance?
In a mouse model with greater large artery stiffness (Eln+/−), we find an exacerbated vasoconstriction response to angiotensin II in cerebral arteries, but not skeletal muscle feed arteries, thus implicating altered cerebral artery angiotensin II responsiveness in the poor brain outcomes associated with greater large artery stiffness.
Acknowledgments
FUNDING
This work was supported in part by awards from the National Institute of Health, AG046326, HL007576, AG045339, AG050238, and AG048366, and by Merit Review Award 1I01BX002151 from the United States (U.S.) Department of Veterans Affairs Biomedical Laboratory Research and Development Service. The contents do not represent the views of the U.S. Department of Veterans Affairs, the National Institutes of Health or the United States Government.
Footnotes
COMPETING INTERESTS
None declared.
REFERENCES
- Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF & O’Rourke MF (1983). Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation 68, 50–58. [DOI] [PubMed] [Google Scholar]
- Barrett-O’Keefe Z, Witman MA, McDaniel J, Fjeldstad AS, Trinity JD, Ives SJ, Conklin JD, Reese V, Runnels S, Morgan DE, Sander M, Richardson RS & Wray DW (2013). Angiotensin II potentiates alpha-adrenergic vasoconstriction in the elderly. Clin Sci (Lond) 124, 413–422. [DOI] [PubMed] [Google Scholar]
- Blamire AM, Anthony DC, Rajagopalan B, Sibson NR, Perry VH & Styles P (2000). Interleukin-1beta -induced changes in blood-brain barrier permeability, apparent diffusion coefficient, and cerebral blood volume in the rat brain: a magnetic resonance study. Journal of Neuroscience 20, 8153–8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger CM, Caputo L & Levy BI (1995). Endothelial AT1-mediated release of nitric oxide decreases angiotensin II contractions in rat carotid artery. Hypertension 26, 752–757. [DOI] [PubMed] [Google Scholar]
- Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG & Griendling KK (2008). Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radical Biology and Medicine 45, 1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Lesniewski LA & Delp MD (2005). The effects of aging and exercise training on endothelin-1 vasoconstrictor responses in rat skeletal muscle arterioles. Cardiovascular Research 66, 393–401. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Lesniewski LA, Stuart D, Walker AE, Henson G, Sorensen L, Li D & Kohan DE (2014). Smooth muscle specific disruption of the endothelin-A receptor in mice reduces arterial pressure, and vascular reactivity and affects vascular development. Life Sciences 118, 238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, Lawson BR, Lesniewski LA & Seals DR (2013). Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell 12, 772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrance AM (2007). Interleukin 1-beta (IL-1beta) enhances contractile responses in endothelium-denuded aorta from hypertensive, but not normotensive, rats. Vascul Pharmacol 47, 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrall AJ & Wardlaw JM (2009). Blood-brain barrier: ageing and microvascular disease--systematic review and meta-analysis. Neurobiology of Aging 30, 337–352. [DOI] [PubMed] [Google Scholar]
- Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, Minkes RK, Blumer KJ, Kovacs A, Kelly DP, Li DY, Starcher B & Mecham RP (2003). Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. Journal of Clinical Investigation 112, 1419–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. Experimental Physiology 100, 755–758. [DOI] [PubMed] [Google Scholar]
- Hanon O, Haulon S, Lenoir H, Seux ML, Rigaud AS, Safar M, Girerd X & Forette F (2005). Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke 36, 2193–2197. [DOI] [PubMed] [Google Scholar]
- Jefferson AL, Cambronero FE, Liu D, Moore EE, Neal JE, Terry JG, Nair S, Pechman KR, Rane S, Davis LT, Gifford KA, Hohman TJ, Bell SP, Wang TJ, Beckman JA & Carr JJ (2018). Higher Aortic Stiffness is Related to Lower Cerebral Blood Flow and Preserved Cerebrovascular Reactivity in Older Adults. Circulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerez S, de Bruno MP & Coviello A (2001). Endothelium-dependent desensitization to angiotensin II in rabbit aorta: the mechanisms involved. Canadian Journal of Physiology and Pharmacology 79, 481–489. [PubMed] [Google Scholar]
- Kai H, Fukui T, Lassegue B, Shah A, Minieri CA & Griendling KK (1996). Prolonged exposure to agonist results in a reduction in the levels of the Gq/G11 alpha subunits in cultured vascular smooth muscle cells. Molecular Pharmacology 49, 96–104. [PubMed] [Google Scholar]
- Knutsen RH, Beeman SC, Broekelmann TJ, Liu D, Tsang KM, Kovacs A, Ye L, Danback JR, Watson A, Wardlaw A, Wagenseil JE, Garbow JR, Shoykhet M & Kozel BA (2018). Minoxidil improves vascular compliance, restores cerebral blood flow, and alters extracellular matrix gene expression in a model of chronic vascular stiffness. Am J Physiol Heart Circ Physiol 315, H18–H32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttan SC & Sim MK (1993). Angiotensin II-induced tachyphylaxis in aortas of normo- and hypertensive rats: changes in receptor affinity. European Journal of Pharmacology 232, 173–180. [DOI] [PubMed] [Google Scholar]
- Lemos VS, Silva DM, Walther T, Alenina N, Bader M & Santos RA (2005). The endothelium-dependent vasodilator effect of the nonpeptide Ang(1-7) mimic AVE 0991 is abolished in the aorta of mas-knockout mice. Journal of Cardiovascular Pharmacology 46, 274–279. [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Donato AJ, Behnke BJ, Woodman CR, Laughlin MH, Ray CA & Delp MD (2008). Decreased NO signaling leads to enhanced vasoconstrictor responsiveness in skeletal muscle arterioles of the ZDF rat prior to overt diabetes and hypertension. Am J Physiol Heart Circ Physiol 294, H1840–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E & Keating MT (1998). Elastin is an essential determinant of arterial morphogenesis. Nature 393, 276–280. [DOI] [PubMed] [Google Scholar]
- Marchesi C, Paradis P & Schiffrin EL (2008). Role of the renin-angiotensin system in vascular inflammation. Trends in Pharmacological Sciences 29, 367–374. [DOI] [PubMed] [Google Scholar]
- Matavelli LC & Siragy HM (2015). AT2 receptor activities and pathophysiological implications. Journal of Cardiovascular Pharmacology 65, 226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer ML, Palta P, Tanaka H, Deal JA, Wright J, Knopman DS, Griswold ME, Mosley TH & Heiss G (2017). Association of Central Arterial Stiffness and Pressure Pulsatility with Mild Cognitive Impairment and Dementia: The Atherosclerosis Risk in Communities Study-Neurocognitive Study (ARIC-NCS). J Alzheimers Dis 57, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF (2008). Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. Journal of Applied Physiology 105, 1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, Garcia M, Aspelund T, Harris TB, Gudnason V & Launer LJ (2011). Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility--Reykjavik study. Brain 134, 3398–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D & Benjamin EJ (2005). Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation 112, 3722–3728. [DOI] [PubMed] [Google Scholar]
- Modrick ML, Didion SP, Sigmund CD & Faraci FM (2009). Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am J Physiol Heart Circ Physiol 296, H1914–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Delp J, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD & Delp MD (2002). Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 282, H1843–1854. [DOI] [PubMed] [Google Scholar]
- O’Rourke MF & Safar ME (2005). Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 46, 200–204. [DOI] [PubMed] [Google Scholar]
- Oh YS, Kim JS, Park JW, An JY, Park SK, Shim YS, Yang DW & Lee KS (2016). Arterial stiffness and impaired renal function in patients with Alzheimer’s disease. Neurol Sci 37, 451–457. [DOI] [PubMed] [Google Scholar]
- Osei-Owusu P, Knutsen RH, Kozel BA, Dietrich HH, Blumer KJ & Mecham RP (2014). Altered reactivity of resistance vasculature contributes to hypertension in elastin insufficiency. Am J Physiol Heart Circ Physiol 306, H654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pase MP, Beiser A, Himali JJ, Tsao C, Satizabal CL, Vasan RS, Seshadri S & Mitchell GF (2016). Aortic Stiffness and the Risk of Incident Mild Cognitive Impairment and Dementia. Stroke 47, 2256–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet M, Jacob MP, Escoubet B, Gheduzzi D, Tillet E, Perret P, Huber P, Quaglino D, Vranckx R, Li DY, Starcher B, Boyle WA, Mecham RP & Faury G (2008). Elastin haploinsufficiency induces alternative aging processes in the aorta. Rejuvenation Res 11, 97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueyo ME, Arnal JF, Rami J & Michel JB (1998). Angiotensin II stimulates the production of NO and peroxynitrite in endothelial cells. American Journal of Physiology 274, C214–220. [DOI] [PubMed] [Google Scholar]
- Raignault A, Bolduc V, Lesage F & Thorin E (2017). Pulse pressure-dependent cerebrovascular eNOS regulation in mice. Journal of Cerebral Blood Flow and Metabolism 37, 413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C, Bramlage P, Kirch W & Ferrario CM (2007). Role of the vasodilator peptide angiotensin-(1-7) in cardiovascular drug therapy. Vasc Health Risk Manag 3, 125–137. [PMC free article] [PubMed] [Google Scholar]
- Tarumi T, Shah F, Tanaka H & Haley AP (2011). Association between central elastic artery stiffness and cerebral perfusion in deep subcortical gray and white matter. American Journal of Hypertension 24, 1108–1113. [DOI] [PubMed] [Google Scholar]
- Thorin-Trescases N, de Montgolfier O, Pincon A, Raignault A, Caland L, Labbe P & Thorin E (2018). Impact of pulse pressure on cerebrovascular events leading to age-related cognitive decline. Am J Physiol Heart Circ Physiol 314, H1214–H1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL & DeSouza CA (2007). Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension 50, 403–409. [DOI] [PubMed] [Google Scholar]
- Vicaut E, Rasetti C & Baudry N (1996). Effects of tumor necrosis factor and interleukin-1 on the constriction induced by angiotensin II in rat aorta. J Appl Physiol (1985) 80, 1891–1897. [DOI] [PubMed] [Google Scholar]
- Wagenseil JE, Knutsen RH, Li DY & Mecham RP (2007). Elastin-insufficient mice show normal cardiovascular remodeling in 2K1C hypertension despite higher baseline pressure and unique cardiovascular architecture. Am J Physiol Heart Circ Physiol 293, H574–582. [DOI] [PubMed] [Google Scholar]
- Wagenseil JE, Nerurkar NL, Knutsen RH, Okamoto RJ, Li DY & Mecham RP (2005). Effects of elastin haploinsufficiency on the mechanical behavior of mouse arteries. Am J Physiol Heart Circ Physiol 289, H1209–1217. [DOI] [PubMed] [Google Scholar]
- Walker AE, Henson GD, Reihl KD, Morgan RG, Dobson PS, Nielson EI, Ling J, Mecham RP, Li DY, Lesniewski LA & Donato AJ (2015). Greater impairments in cerebral artery compared with skeletal muscle feed artery endothelial function in a mouse model of increased large artery stiffness. J Physiol 593, 1931–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE, Henson GD, Reihl KD, Nielson EI, Morgan RG, Lesniewski LA & Donato AJ (2014). Beneficial effects of lifelong caloric restriction on endothelial function are greater in conduit arteries compared to cerebral resistance arteries. Age (Dordr) 36, 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman CR, Price EM & Laughlin MH (2002). Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol (1985) 93, 1685–1690. [DOI] [PubMed] [Google Scholar]
- Wray DW, Nishiyama SK, Harris RA & Richardson RS (2008). Angiotensin II in the elderly: impact of angiotensin II type 1 receptor sensitivity on peripheral hemodynamics. Hypertension 51, 1611–1616. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV (2008). The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201. [DOI] [PubMed] [Google Scholar]


