Abstract
Objective
This study investigates the effects of dipyrithione (PTS2) on the expression of IP-10/CXCL10, which has been observed in a wide variety of chronic inflammatory disorders and autoimmune conditions.
Methods
RAW264.7 cells (a murine macrophage-like cell line) were cultured in the absence or in the presence of PTS2 (3–10 μM) together with or without IFN-γ (10 ng/ml). IP-10/CXCL10 expression was measured by specific enzyme-amplified immunoassays and reverse transcriptase-PCR (RT-PCR). Phosphorylation of JAK1, JAK2 and STAT1 were detected by Western blot analysis.
Results
We found that PTS2 inhibited IFN-γ-induced up-regulation of IP-10/CXCL10 protein level in a dose- and time-dependent manner in RAW264.7 cells. RT-PCR experiments showed that PTS2 suppressed IFN-γ-induced IP-10/CXCL10 expression at mRNA levels. Mechanistically, PTS2 prevented phosphorylation of JAK1, JAK2 and STAT1, but did not interfere with the p38 pathway. Furthermore, the inhibitory effect of PTS2 on IP-10/CXCL10 up-regulation was slightly stronger than JAK2 inhibitor AG490.
Conclusion
PTS2 inhibits IFN-γ-induced IP-10/CXCL10 expression in RAW264.7 cells by targeting the JAK/STAT1 signaling pathway, suggesting that PTS2 could exert anti-inflammatory effects through attenuating the formation of chemokine IP-10/CXCL10.
Keywords: Dipyrithione, Interferon-γ, IP-10/CXCL10, JAK/STAT1 signaling, RAW264.7 cells
Introduction
Mononuclear phagocytes play an essential role in the regulation of host defense and homeostasis, and in the development of acute and chronic inflammatory diseases. Interferon-γ secreted by activated T cells and NK cells is intimately involved in the innate and acquired immune responses [1–3]. As a major macrophage activation factor, IFN-γ binds to its receptor on the macrophage cell surface and reprograms gene expression necessary for the execution of host defense functions by modulating signal transduction pathways. IFN-induced proteins include enzymes, transcription factors, cell surface glycoproteins, cytokines, chemokines and a large number of factors with unknown functions [4].
IP-10/CXCL10 is a non-ELR (Glu-Leu-Arg) CXC chemokine first cloned in 1985 as a protein secreted by peripheral blood mononuclear cells (PBMC), fibroblasts and endothelial cells [5]. IP-10/CXCL10 binds its receptor CXCR3 to play a biological role in stimulation of monocytes, natural killer (NK) and T cell migration, regulation of T cell and bone marrow progenitor maturation, modulation of adhesion molecule expansion and inhibition of angiogenesis [6–13]. The expression of IP-10/CXCL10 has also been observed in a wide variety of chronic inflammatory disorders and auto immune conditions [14–18]. Recently, it is reported that SARS-CoV may instigate an excessive immune response through overproduction of IP-10/CXCL10, MCP-1 and IL-8, resulting in transendothelial infiltration and accumulation of neutrophils, alveolar macrophages and Th1 lymphocytes into lung tissue causing pulmonary inflammation and destruction [19]. Previous studies have shown that IP-10/CXCL10 could be an excellent prognostic marker for SARS disease progression [20, 21]. IP-10/CXCL10 is also induced by hepatitis C virus (HCV) in hepatocytes for recruiting inflammatory cells into the lobules and acts as an important predictor of chronic HCV infection progression [22]. Thus, IP-10/CXCL10 is considered as a predictive marker of successful treatment response in HCV/HIV-coinfected patients [23]. In addition, Kong et al. [24] have reported that IP-10/CXCL10 levels are increased in SLE, and serum IP-10/CXCL10 may represent a more sensitive marker for monitoring disease activity than standard serological tests.
In various cells, including macrophages, the intracellular signaling pathway triggered by IFN-γ receptors involves the rapid and direct activation of Janus tyrosine protein kinases JAKs-STAT signaling pathways [2, 25–28]. Numerous biological actions of IFN-γ have been demonstrated to be dependent on STAT1 signal transduction pathway-associated gene products [29]. Another transcription factor involved in IFN-γ-induced IP-10/CXCL10 expression is nuclear factor κB (NF-κB) [30]. In addition, IFN-γ has also been shown to activate the p38 signaling pathway to enhance IP-10/CXCL10 gene expression in macrophages [31].
Dipyrithione (2,2′-dithiobispyridine-1,1′-dioxide, PTS2) (CAS number: 3696-28-4), a pyrithione (PTO) derivate (Fig. 1), possesses antibacterial and antifungal activity. The effect of skin color on the percutaneous penetration of PTS2 in men has been described [32]. PTO, a monomer of PTS2 which inhibits the growth of fungi, yeast, mold and bacteria, is widely used in cosmetics and shampoo. Recently, we reported that PTS2 induced Hela cells apoptosis through activating MAPKs pathway [33] and inhibited LPS-induced up-regulation of iNOS and COX-2 protein levels and NO production in RAW264.7 cells [34]. Here we show evidence that PTS2 inhibits IFN-γ-induced up-regulation of IP-10/CXCL10 in RAW264.7 cells. We also found that IFN-γ-induced increase of IP-10/CXCL10 mRNA level was suppressed significantly by PTS2 treatment. The mechanism utilized by PTS2 to prevent IP-10/CXCL10 expression is related to its inhibitory effects on JAK/STAT1 signal transduction induced by IFN-γ. These results suggest that PTS2 may affect host immune responses by down-regulating IFN-γ-induced gene expression.
Fig. 1.
Chemical structure of PTS2
Materials and methods
Cell culture
RAW264.7, a murine macrophage-like cell line purchased from the CBCAS (Cell Bank of the Chinese Academic of Sciences, Shanghai, China) was maintained in Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% (v/v) fetal bovine serum (Hyclone) and antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin) (HyClone) at 37°C in an atmosphere of 5% CO2.
Antibodies and reagents
Polyclonal antibodies against JAK1, phospho-JAK1 (Tyr1022/1023), JAK2, phospho-JAK2 (Tyr1007/1008), phospho-STAT1 (Tyr701), p38 MAPK and phospho-p38 MAPK (Thr180/Tyr182) were obtained from Cell Signaling Technology. These antibodies were diluted at the ratio of 1:1,000 according to protocol. Antibody to STAT1 was from Santa Cruz Biotechnology, diluted at the ratio of 1:1,500 according to protocol. All secondary antibodies used for Western blot were purchased from Rockland Immunochemical. Recombinant Murine Interferon-γ (IFN-γ) was purchased from Peprotech. AG490 (a JAK2 inhibitor) was purchased from Calbiochem. PTS2 was purchased from J&K Chemical Ltd.
IP-10/CXCL10 ELISA
RAW264.7 cells were seeded in 24-well plates at 5 × 105 cells/well the day before the experiment. After IFN-γ and PTS2 treatment, media were collected and centrifuged at 10,000 rpm for 5 min. IP-10/CXCL10 level of the media was then determined by a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) using a mouse-specific IP-10/CXCL10 ELISA (R&D Systems) according to the manufacturer’s instructions.
Western blotting
Cells were rinsed twice with ice-cold PBS, and solubilized in lysis buffer containing 20 mM Tris (pH 7.5), 135 mM NaCl, 2 mM EDTA, 2 mM DTT, 25 mM β-glycerophosphate, 2 mM sodium pyrophosphate, 10% glycerol, 1% Triton X-100, 1 mM sodium orthovanadate, 10 mM NaF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride (PMSF) for 30 min on ice. Lysates were centrifuged (15,000×g) at 4°C for 10 min. Equal amounts of the soluble protein were denatured in SDS, electrophoresed on a 12% SDS-polyacrylamide gel, and transferred to nitrocellulose membranes. The immunoblotting was performed as described [35]. The IRdye 800 conjugated IgG secondary antibody antibodies were used against respective primary antibodies. The proteins were visualized using the Odyssey infrared imaging system (LI-COR).
Reverse transcriptase-PCR
Total RNA was extracted with Trizol reagent (Gibco) as described by the manufacturer. Reverse transcriptase-PCR (RT-PCR) was performed by Access RT-PCR System kit (Promega) according to the protocol with indicated primers (IP-10/CXCL10: sense primer 5′-gtcattttctgcctcatcct-3′, antisense primer 5′-gagcccttttagacctttt-3′; Glyceraldehyde-3-phosphate dehydrogenase (GAPDH): sense primer 5′-tgaaggtcggtgtgaacggatttggc-3′, antisense primer 5′-tggttcacacccatcacaaacatgg-3′). PCR was performed for 30 cycles in 25 μl of reaction mixture. PCR products were visualized in 1.2% agarose gels stained with EtBr. GAPDH was utilized as a housekeeping gene where indicated.
Statistics
Analysis of variance (ANOVA) was used to compare the results between the two groups. Individual points were compared using a Student’s t test (STATISTICA, Statsoft Inc., Tulsa, USA) and differences were considered significant for P < 0.01. Data are presented as mean ± SD. Western blotting analysis experiments were repeated three times with similar trends.
Results
PTS2 inhibits IFN-γ-induced up-regulation of IP-10/CXCL10 in RAW 264.7 cells
In order to determine whether PTS2 could affect IFN-γ-induced IP-10/CXCL10 protein up-regulation, we measured IP-10/CXCL10 level in the cultural medium of RAW264.7 cells by ELISA. We pretreated RAW264.7 cells with 0, 3.0, 5.0 or 10.0 μM PTS2 for 2 h, and then stimulated cells with IFN-γ (10 ng/ml) for 24 h followed by medium collection. The result from ELISA assay showed that IFN-γ stimulated IP-10/CXCL10 secretion dramatically and PTS2 dose-dependently inhibited the elevation of IP-10/CXCL10 level with significant reductions at doses of 5.0 and 10.0 μM. PTS2 alone, even at concentration of 10.0 μM, did not influence IP/CXCL10 protein level in normal RAW264.7 cells (Fig. 2a).
Fig. 2.
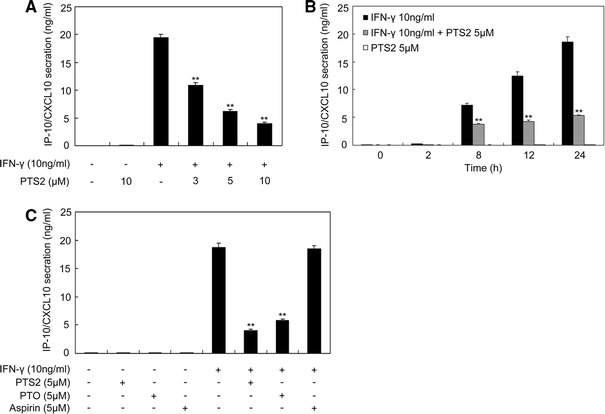
Effect of PTS2 on IFN-γ-induced up-regulation of IP-10/CXCL10 protein level in RAW264.7 cells. a RAW264.7 cells were pretreated with 3.0, 5.0 or 10.0 μM PTS2 for 2 h or not, and then were stimulated with IFN-γ (10 ng/ml) for 24 h. b Cells were pretreated with 5.0 μM PTS2 for 2 h or not, and then stimulated with IFN-γ (10 ng/ml) for 0–24 h, respectively. c Cells were pretreated with 5.0 μM PTS2, PTO or Aspirin for 2 h followed by incubating with IFN-γ (10 ng/ml) for 24 h. Cell culture supernates from a, b and c were subjected to ELISA for measuring IP-10/CXCL10 protein level. Data are presented as mean ± SD. **P < 0.01 compared with the IFN-γ-stimulated cells
Further experiments were performed by treating RAW264.7 cells with 5.0 μM PTS2 for 2 h or not, followed by incubating these cells with IFN-γ (10 ng/ml) for 0–24 h. The cell culture supernates were subjected to ELISA analysis to measure IP-10/CXCL10 protein level. As shown in Fig. 2b, IP-10/CXCL10 protein increased apparently 8, 12 and 24 h after IFN-γ stimulation, whereas PTS2 treatment led to a decrease in IFN-γ-induced IP-10/CXCL10 up-regulation. We also detected the inhibitory ability of PTO, the monomer of PTS2, on IFN-γ-induced IP-10/CXCL10 up-regulation. RAW264.7 cells were treated with PTO (5.0 μM) or PTS2 (5.0 μM) for 2 h and then were stimulated with IFN-γ (10 ng/ml) for 24 h. Comparing PTS2 with PTO, we found that PTS2 was a little more effective in inhibiting IFN-γ-induced IP-10/CXCL10 expression. In addition, aspirin was used as control, which is an acetylated salicylate utilized to treat inflammation and arthritis pain [36]. The data suggested that aspirin did not influence IFN-γ-induced IP-10/CXCL10 up-regulation (Fig. 2c).
PTS2 inhibits IFN-γ-induced increase of IP-10/CXCL10 mRNA level in RAW 264.7 cells
Since the above results indicated that PTS2 inhibited the increase of IP-10/CXCL10 protein level induced by IFN-γ, we then performed RT-PCR to analyze the effect of PTS2 on IFN-γ-induced IP-10/CXCL10 expression at transcription levels. RAW264.7 cells were pretreated with PTS2 (5.0 μM) for 2 h or not, and then were stimulated with IFN-γ (10 ng/ml). Twelve hours after IFN-γ stimulation, total RNA was isolated and mRNA level of IP-10/CXCL10 was determined by RT-PCR. As shown in Fig. 3, IFN-γ stimulation elevated endogenous mRNA levels of IP-10/CXCL10, whereas PTS2 suppressed IFN-γ-induced increase of IP-10/CXCL10 mRNA levels. The housekeeping gene GAPDH did not change with treatment. This result, together with data shown above in Fig. 2, demonstrated that PTS2 inhibited IFN-γ-induced IP-10/CXCL10 expression.
Fig. 3.
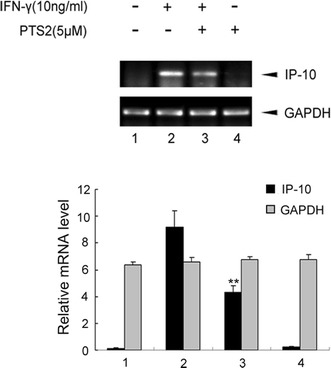
Effect of PTS2 on IFN-γ-induced rise of IP-10/CXCL10 mRNA level in RAW264.7 cells. Cells were pretreated with 5.0 μM PTS2 for 2 h or not, followed by incubating with IFN-γ (10 ng/ml) for 12 h. Total RNA were isolated and IP-10/CXCL10 mRNA were determined by RT-PCR. GAPDH mRNA was used as control. Data are presented as mean ± SD. **P < 0.01 compared with the IFN-γ-stimulated control cells
PTS2 prevents IFN-γ-induced JAK/STAT1 signaling in RAW 264.7 cells
It has been demonstrated that the transcriptional activation of the IP-10/CXCL10 gene is dependent upon IFN-γ-induced JAK/STAT1 signaling activation [29]. We thus evaluated the effects of PTS2 on IFN-γ-induced JAK1, JAK2 and STAT1 activation in RAW264.7 cells. The cells were pretreated with 3.0, 5.0, 10.0 μM of PTS2 for 2 h or not, and then incubated with IFN-γ (10 ng/ml) for 30 min. Western blot analysis showed that JAK1, JAK2 and STAT1 were markedly activated by IFN-γ stimulation, whereas PTS2 treatment led to a reduction in IFN-γ-induced JAK1, JAK2 and STAT1 phosphorylation in a concentration-dependent manner (Fig. 4a). Next, RAW264.7 cells were treated with PTS2 (5.0 μM) for 2 h or not, and then incubated with IFN-γ (10 ng/ml) for 0–60 min. As assessed by Western blotting, IFN-γ rapidly (within 3 min) increased the phosphorylation of tyrosine residues 1022/1023 of JAK1 and 1007/1008 of JAK2, and tyrosine 701 of STAT1 (Fig. 4b). PTS2 decreased the phosphorylation of JAK1, JAK2 and STAT1 in a time-dependent manner (Fig. 4b). When RAW264.7 cells were treated with different concentration of IFN-γ (1–10 ng/ml), a dramatic and concentration-dependent JAK1, JAK2 and STAT1 phosphorylation was observed (Fig. 4c). PTS2 significantly attenuated IFN-γ-induced JAK1, JAK2 and STAT1 phosphorylation (Fig. 4c), whereas the amount of total JAK1, JAK2 and STAT1 proteins in RAW264.7 cells were not affected by both IFN-γ and PTS2 treatment (Fig. 4a–c). Previous reports showed that the selective pharmacological JAK2 inhibitor AG490 could significantly inhibit upregulated chemokine expression induced by IFN-γ in mouse mesangial cells [29]. Therefore, we compared the effects of PTS2 and AG490 on IFN-γ-induced IP-10/CXCL10 expression. RAW264.7 cells were pretreated with PTS2 (5.0 μM) or AG490 (30.0 μM) or both of them, respectively, for 2 h, and then incubated with IFN-γ (10 ng/ml) for 24 h. The cell culture supernates were subjected to ELISA analysis to measure IP-10/CXCL10 protein levels. As shown in Fig. 4d, PTS2, at concentration of 5.0 μM, proved more effective in suppressing IFN-γ-induced IP-10/CXCL10 expression than 30.0 μM of AG490. Furthermore, IFN-γ-induced IP-10/CXCL10 expression in the cells co-incubated with PTS2 and AG490 together was lower than in those treated with either PTS2 or AG490. Taken together, these results suggested that PTS2 inhibited IFN-γ-induced IP-10/CXCL10 expression in RAW 264.7 cells through suppressing activation of JAK/STAT1 signaling pathway.
Fig. 4.
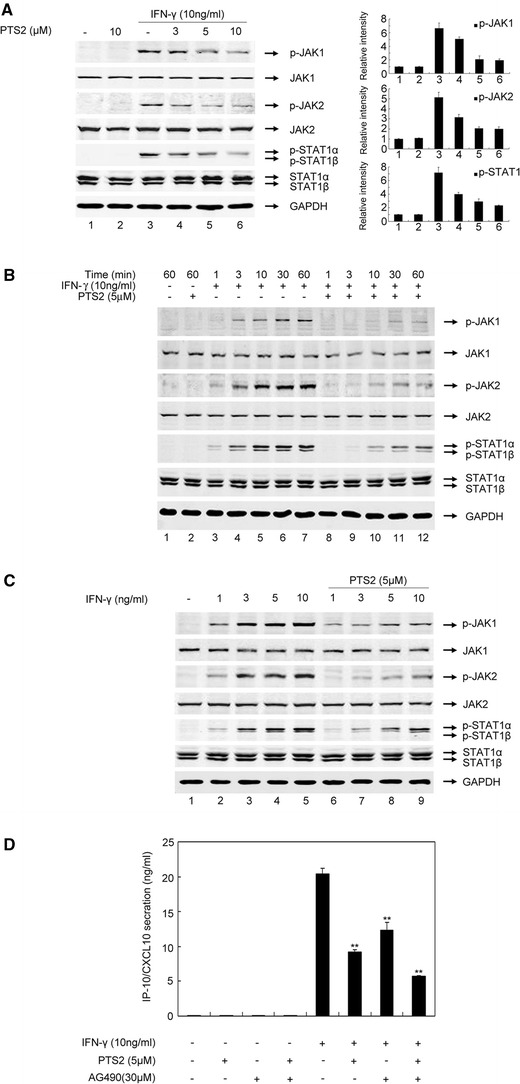
Effect of PTS2 on IFN-γ-induced JAK/STAT1 signaling in RAW 264.7 cells. a RAW264.7 cells were pretreated with 3.0, 5.0 or 10.0 μM PTS2 or not, respectively, for 2 h, and then incubated with IFN-γ (10 ng/ml) for 30 min. Western blot analysis was performed by using antibodies to JAK1, p-JAK1, JAK2, p-JAK2, STAT1, or p-STAT1, respectively. GAPDH was used as control. b Cells were incubated with PTS2 (5.0 μM) for 2 h or not, followed by stimulating with IFN-γ (10 ng/ml) for 0–60 min, respectively. Cell lysates were subjected to Western blot analysis. c Cells were pretreated with 5.0 μM PTS2 for 2 h or not, and then stimulated with different concentration of IFN-γ (1–10 ng/ml). d Cells were pretreated with PTS2 (5.0 μM), AG490 (30.0 μM) or both of them, respectively, for 2 h, and then incubated with IFN-γ (10 ng/ml) for 24 h. The cell culture supernates were subjected to ELISA analysis to measure IP-10/CXCL10 protein level. Data are presented as mean ± SD. **P < 0.01 compared with the IFN-γ-stimulated control cells
PTS2 dose not influence IFN-γ-induced p38 activation
P38 has been reported to play a critical role in regulation of the expression of a number of cytokines and chemokines including IP-10/CXCL10 under IFN-γ stimulation in macrophages [31]. We thus evaluated the effect of PTS2 on IFN-γ-induced p38 activation in RAW264.7 cells. The cells were pretreated with 5.0 μM PTS2 for 2 h or not, and then were incubated with IFN-γ (10 ng/ml) for 0–1.5 h respectively. Figure 5 shows that IFN-γ-induced phosphorylation of p38 reached maximum at 0.5 h after IFN-γ treatment and PTS2 pretreatment did not affect this p38 activation.
Fig. 5.
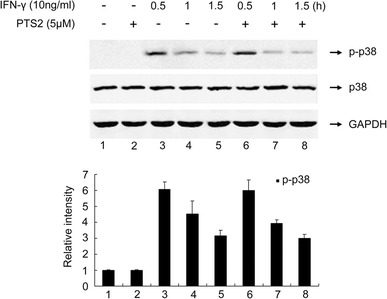
Effect of PTS2 on IFN-γ-induced p38 activation. RAW264.7 cells were pretreated with 5.0 μM PTS2 for 2 h or not, followed by incubating with 10 ng/ml IFN-γ for 0–1.5 h. Western blot was performed by using antibodies to p38 or p-p38. GAPDH was used as control. The results were representative of three independent experiments
Discussion
A number of steroidal agents, such as glucocorticoids, have been used as anti-inflammatory drugs for a long time, but frequent association of serious side effects, such as liver damage, cancers, stroke and growth stoppage, have been a long-standing dilemma in clinical steroid anti-inflammatory therapy. Non-steroidal anti-inflammatory drugs (NSAIDs) represent one of the most widely used drug classes, including aspirin, indomethacin, phenylbutazone, etc. Moreover, new anti-inflammatory drugs are being discovered and developed based on their effects on signal transduction and as anti-cytokine agents [37]. Some pyridine derivates, such as pyroxin and sulfasalazine, have been known for their anti-inflammatory effects. As a member of the pyridinethione compound family, PTS2 has been used as bactericide, pesticide and fungicide for a long time [38]. However, relatively fewer studies have focused on the anti-inflammatory activity and the mechanisms of PTS2.
IFN-γ is a key cytokine involved in the synergistic generation of many inflammatory responses. Activated T lymphocytes and NK cells produce IFN-γ which is the major macrophage activation factor. Activation of JAK1, JAK2 and STAT1, plays a central role in the primary transcriptional response to IFN-γ [2, 28]. It has been well documented that excess IFN-γ release could result in IP-10/CXCL10 overexpression, which regulates almost all stages in the development of inflammation, in particular, the early stage of T cells and NK cells transmigration to the sites of inflammation [2]. Some medicines, such as glucocorticoids, which reduce the incidence of the inflammatory disease process, show inhibitory effects on IP-10/CXCL10 expression, and some chemicals with efficiency of inhibiting IP-10/CXCL10 overexpression are also considered to have the immense potential for treating a broad range of and inflammatory diseases including SARS, chronic hepatitis C, SLE, etc. [20, 39, 40].
In this study we focused on the influence of PTS2 on the expression of IP-10/CXCL10, an IFN-γ inducible chemokine whose transcriptional regulation is mainly dependent on JAK/STAT1 signaling pathway activation. Sakaeda et al. [41] showed that IFN-γ-stimulated IP-10/CXCL10 expression in RAW264.7 cells. In the current study, we demonstrated that IFN-γ-induced up-regulation of IP-10/CXCL10 in RAW264.7 cells was dose and time-dependently inhibited by PTS2. Although IFN-γ induces a rapid tyrosine phosphorylation of JAK1 and JAK2 and activation of STAT1 in RAW264.7 cells, when treating cells with IFN-γ in the presence of PTS2, JAK/STAT1 activation was depressed. Moreover, we found that the inhibitory effect of PTS2 on IP-10/CXCL10 up-regulation was slightly stronger than JAK2 inhibitor AG490 while RAW264.7 cells co-incubated with PTS2 and AG490 showed marked reduction of IP-10/CXCL10, suggesting the synergistic effect of PTS2 and AG490. These results indicate that PTS2 inhibits IFN-γ-induced IP-10/CXCL10 expression through preventing activation of the JAK/STAT1 signaling pathway. A similar result revealed in our recent report indicates that PTS2 inhibits the LPS-induced iNOS expression by depressing phosphorylation of STAT1 in RAW264.7 cells [34]. It has been demonstrated that activation of p38 appears to play a critical role in regulation of the expression of a number of chemokines and cytokines induced by IFN-γ in macrophages [31], but in the present study, PTS2 did not alter IFN-γ-induced p38 phosphorylation. Majumder et al. [42] showed that the induction of IP-10/CXCL10 was dependent on the ISRE (interferon-stimulated response element) and one of the two NF-κB recognition sites. We also tried to study the effect of PTS2 on NF-κB signaling, however, we did not find NF-κB activation by IFN-γ stimulation through detecting ubiquitination of IκBα and IκBβ in RAW264.7 cells (data not shown). The result is consistent with the results that NF-κB can regulate IP-10/CXCL10 expression but IFN-γ did not detectably activate NF-κB in both Myd88 −/− and wild-type macrophages [43]. In addition, our work also indicates that PTS2 has no significant effect on cell viability (data not shown).
In conclusion, our study demonstrates that PTS2 suppresses IFN-γ-induced IP-10/CXCL10 expression via attenuating the activation of JAK/STAT1 signaling pathways. This finding provides a new insight for understanding the anti-inflammatory activities and clinical therapeutic potential of PTS2 and other pyridine derivates.
Acknowledgments
This work was supported by grants from the National Nature Science Foundation of China (Nos. 30770842 and 30771979), Jiangsu Major Nature Science Foundation of High Education (No. 07KJA18026), and the Specialized Research Fund for the Doctoral Program of Higher Education of China (2007104SBJ0152).
Abbreviations
- PTS2
Dipyrithione
- IFN-γ
Interferon gamma
- IP-10
IFN-γ-inducible protein-10
- GAPDH
Glyceralde-hyde-3-phosphate dehydrogenase
- JAK
Janus protein tyrosine kinase
- STAT1
Signal transducers and activators of transcription 1
- NF-κB
Nuclear factor κB
- MTT
3-(4,5 Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
Contributor Information
Lan Luo, Phone: +86-25-83686657, FAX: +86-25-83686657, Email: lanluo@nju.edu.cn.
Zhimin Yin, Phone: +86-25-85891305, FAX: +86-25-85891305, Email: yinzhimin@njnu.edu.cn.
References
- 1.Platanias LC. Interferons: laboratory to clinic investigations. Curr Opin Oncol. 1995;7:560–565. doi: 10.1097/00001622-199511000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 3.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-γ. Ann Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 4.Ohmori Y, Hamilton TA. Regulation of macrophage gene expression by T-cell-derived lymphokine. Pharmacol Ther (Oxford) 1994;63:235–264. doi: 10.1016/0163-7258(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 5.Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- 6.Neville LF, Mathiak G, Bagasra O. The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev. 1997;8:207–219. doi: 10.1016/S1359-6101(97)00015-4. [DOI] [PubMed] [Google Scholar]
- 7.Lazzeri E, Romagnani P. CXCR3-binding chemokines: novel multifunctional therapeutic targets. Curr Drug Targets Immune Endocr Metab Disord. 2005;5:109–118. doi: 10.2174/1568008053174723. [DOI] [PubMed] [Google Scholar]
- 8.Kieseier BC, Tani M, Mahad D, Oka N, Ho T, Woodroofe N, Griffin JW, Toyka KV, Ransohoff RM, Hartung HP. Chemokines and chemokine receptors in inflammatory demyelinating neuropathies: a central role for IP-10. Brain. 2002;125:823–834. doi: 10.1093/brain/awf070. [DOI] [PubMed] [Google Scholar]
- 9.Liu MT, Chen BP, Oertel P, Buchmeier MJ, Armstrong D, Hamilton TA, Lane TE. The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J Immunol. 2000;165:2327–2330. doi: 10.4049/jimmunol.165.5.2327. [DOI] [PubMed] [Google Scholar]
- 10.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am Physiol Soc. 2002;283:7–28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 11.Arai K, Liu ZX, Lane T. Dennert G IP-10 and Mig facilitate accumulation of T cells in the virus-infected liver. Cell Immunol. 2002;219:48–56. doi: 10.1016/S0008-8749(02)00584-1. [DOI] [PubMed] [Google Scholar]
- 12.Wetzel MA, Steele AD, Henderson EE, Rogers TJ. The effect of X4 and R5 HIV-1 on C, CC, and CXC chemokines during the early stages of infection in human PBMCs. Virology. 2002;292:6–15. doi: 10.1006/viro.2001.1249. [DOI] [PubMed] [Google Scholar]
- 13.Agostini C, Facco M, Siviero M, Carollo D, Galvan S, Cattelan AM, Zambello R, Trentin L, Semenzato G. CXC chemokines IP-10 and Mig expression and direct migration of pulmonary CD8+/CXCR3+ T cells in the lungs of patients with HIV infection and T-cell alveolitis. Am J Respir Crit Care Med. 2000;162:1466–1473. doi: 10.1164/ajrccm.162.4.2003130. [DOI] [PubMed] [Google Scholar]
- 14.Rotondi M, Lazzeri E, Romagnani P, Serio M. Role for interferon-gamma inducible chemokines in endocrine autoimmunity: an expanding field. J Endocrinol Investig. 2003;26:177–180. doi: 10.1007/BF03345149. [DOI] [PubMed] [Google Scholar]
- 15.Christen U, Von Herrath MG. IP-10 and type 1 diabetes: a question of time and location. Autoimmunity. 2004;37:273–282. doi: 10.1080/08916930410001713124. [DOI] [PubMed] [Google Scholar]
- 16.Hanaoka R, Kasama T, Muramatsu M, Yajima N, Shiozawa F, Miwa Y, Negishi M, Ide H, Miyaoka H, Uchida H, Adachi M. A novel mechanism for the regulation of IFN-gamma inducible protein-10 expression in rheumatoid arthritis. Arthritis Res Ther. 2003;5:74–81. doi: 10.1186/ar616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azzurri A, Sow OY, Amedei A, Bah B, Diallo S, Peri G, Benagiano M, D’Elios MM, Mantovani A, Del Prete G. IFN-gamma-inducible protein 10 and pentraxin 3 plasmal levels are tools for monitoring inflammation and disease activity in Mycobacterium tuberculosis infection. Microbes Infect. 2005;7:1–8. doi: 10.1016/j.micinf.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Romero AI, Lagging M, Westin J, Dhillon AP, Dustin LB, Pawlotsky JM, Neumann AU, Ferrari C, Missale G, Haagmans BL, Schalm SW, Zeuzem S, Negro F, Hart EV, Hellstrand K. Interferon (IFN)-gamma-inducible protein-10: association with histological results, viral kinetics, and outcome during treatment with pegylated IFN-alpha 2a and ribavirin for chronic hepatitis C virus infection. J Infect Dis. 2006;194:895–903. doi: 10.1086/507307. [DOI] [PubMed] [Google Scholar]
- 19.Huang KJ, Su IJ, Theron M, Wu YC, Lai SK, Liu CC, Lei HY. An interferon-gamma-related cytokine storm in SARS patients. J Med Virol. 2005;75:185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Y, Xu J, Zhou C, Wu Z, Zhong S, Liu J, Luo W, Chen T, Qin Q, Deng P. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am J Respir Crit Care Med. 2005;171:850–857. doi: 10.1164/rccm.200407-857OC. [DOI] [PubMed] [Google Scholar]
- 21.Tang NL, Chan PK, Wong CK, To KF, Wu AK, Sung YM, Hui DS, Sung JJ, Lam CW. Early enhanced expression of interferon-inducible protein-10(CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clin Chem. 2005;51:233–240. doi: 10.1373/clinchem.2005.054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvey CE, Post JJ, Palladinetti P, Freeman AJ, Ffrench RA, Kumar RK, Marinos G, Lloyd AR. Expression of the chemokine IP-10(CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J Leukoc Biol. 2003;74:360–369. doi: 10.1189/jlb.0303093. [DOI] [PubMed] [Google Scholar]
- 23.Zeremski M, Markatou M, Brown QB, Dorante G, Cunningham-Rundles S, Talal AH. A Predictive marker of successful treatment response in hepatitis C virus/HIV-coinfected patients. Acquir Immune Defic Syndr. 2007;45:262–268. doi: 10.1097/QAI.0b013e3180559219. [DOI] [PubMed] [Google Scholar]
- 24.Kong KO, Tan AW, Thong BYH, Lian TY, Cheng YK, Teh CL, Koh ET, Leong KP, Leung BP, Howe HS. Enhanced expression of interferon-inducible protein-10 correlates with disease activity and clinical manifestations in systemic lupus erythematosus. Clin Exp Immunol. 2009;156:134–140. doi: 10.1111/j.1365-2249.2009.03880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shuai K. Interferon-activated signal transduction to the nucleus. Curr Opin Cell Biol. 1994;6:253–259. doi: 10.1016/0955-0674(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 26.Leaman DW, Leung S, Li X, Stark GR. Regulation of STAT-dependent pathways by growth factors and cytokines. FASEB J. 1996;10:1578–1588. [PubMed] [Google Scholar]
- 27.Leonard WJ, O’Shea JJ. JAKS AND STATS: biological implications. Ann Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 28.Ramana CV, Chatterjee-Kishore M, Nguyen H, Stark GR. Complex roles of Stat1 in regulating gene expression. Oncogene. 2000;19:2619–2627. doi: 10.1038/sj.onc.1203525. [DOI] [PubMed] [Google Scholar]
- 29.Panzer U, Zahner G, Wienberg U, Steinmetz OM, Peters A, Turner JE, Paust HJ, Wolf G, Stahl RA, Schneider A. 15-Deoxy-12, 14-prostaglandin J2 inhibits IFN-γ-induced JAK/STAT1 signaling pathway activation and IP-10/CXCL10 expression in mesangial cells. Nephrol Dial Transplant. 2008;23:3776–3785. doi: 10.1093/ndt/gfn361. [DOI] [PubMed] [Google Scholar]
- 30.Deb A, Haque SJ, Mogensen T, Silverman RH, Williams BR. RNA-dependent protein kinase PKR is required for activation of NF-κB by IFN-γ in a STAT1-independent pathway. J Immunol. 2001;166:6170–6180. doi: 10.4049/jimmunol.166.10.6170. [DOI] [PubMed] [Google Scholar]
- 31.Valledor AF, Sánchez-Tilló E, Arpa L, Park JM, Caelles C, Lloberas J, Celada A. Selective roles of MAPKs during the macrophage response to IFN-gamma. J Immunol. 2008;180:4523–4529. doi: 10.4049/jimmunol.180.7.4523. [DOI] [PubMed] [Google Scholar]
- 32.Wedig JH, Maibach HI. Percutaneous penetration of PTS2 in man: effect of skin color (race) J Am Acad Dermatol. 1981;5:433–438. doi: 10.1016/S0190-9622(81)70105-1. [DOI] [PubMed] [Google Scholar]
- 33.Fan YM, Chen H, Qiao B, Luo L, Ma H, Li H, Jiang JH, Niu DZ, Yin ZM. Opposing effects of ERK and p38 MAP kinases on HeLa cell apoptosis induced by dipyrithione. Mol Cell. 2007;23:30–38. [PubMed] [Google Scholar]
- 34.Liu ZW, Fan YM, Wang Y, Han C, Pan Y, Huang H, Ye Y, Luo L, Yin ZM. Dipyrithione inhibits lipopolysaccharide-induced iNOS and COX-2 up-regulation in macrophages and protects against endotoxic shock in mice. FEBS Lett. 2008;582:1643–1650. doi: 10.1016/j.febslet.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 35.Wu YF, Fan YM, Xue B, Luo L, Shen JY, Zhang SY, Jiang Y, Yin ZM. Human glutathione S-transferase P1-1 interacts with TRAF2 and regulates TRAF2–ASK1 signals. Oncogene. 2006;25:5787–5800. doi: 10.1038/sj.onc.1209576. [DOI] [PubMed] [Google Scholar]
- 36.McConkey B, Crockson RA, Crockson AP, Wilkinson AR. The effects of some anti-inflammatory drugs on the acute-phase proteins in rheumatoid arthritis. QJM. 1973;42:785–791. [PubMed] [Google Scholar]
- 37.Rainsford KD. Anti-inflammatory drugs in the 21st century. Subcell Biochem. 2007;42:3–27. doi: 10.1007/1-4020-5688-5_1. [DOI] [PubMed] [Google Scholar]
- 38.Malhotra GG, Zatz JL. Investigation of nail permeation enhancement by chemical modification using water as a probe. J Pharm Sci. 2002;91:312–323. doi: 10.1002/jps.10058. [DOI] [PubMed] [Google Scholar]
- 39.Kelley VR, Rovin BH. Chemokines: therapeutic targets for autoimmune and inflammatory renal disease. Springer Semin Immunopathol. 2003;24:411–421. doi: 10.1007/s00281-003-0124-4. [DOI] [PubMed] [Google Scholar]
- 40.Zeremski M, Petrovic LM, Chiriboga L, Brown QB, Yee HT, Kinkhabwala M, Jacobson IM, Dimova R, Markatou M, Talal AH. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology. 2008;48:1440–1450. doi: 10.1002/hep.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakaeda Y, Hiroi M, Shimojima T, Iguchi M, Kanegae H, Ohmori Y. Sulindac, a nonsteroidal anti-inflammatory drug, selectively inhibits interferon-γ-induced expression of the chemokine CXCL9 gene in mouse macrophages. Biochem Biophys Res Commun. 2006;350:339–344. doi: 10.1016/j.bbrc.2006.09.058. [DOI] [PubMed] [Google Scholar]
- 42.Majumder S, Zhou LZH, Chaturvedi P, Babcock G, Aras S, Ransohoff RM. p48/STAT-1α-containing complexes play a predominant role in induction of IFN-γ-inducible protein, 10 kDa (IP-10) by IFN-γ alone or in synergy with TNF-α. J Immunol. 1998;161:4736–4744. [PubMed] [Google Scholar]
- 43.Sun D, Ding A. MyD88-mediated stabilization of interferon-γ-induced cytokine and chemokine mRNA. Nature Immunol. 2006;7:375–381. doi: 10.1038/ni1308. [DOI] [PubMed] [Google Scholar]


