Abstract
Oxidized phospholipids (OxPL) were originally discovered as by-products and mediators of chronic inflammation such as in atherosclerosis. Over the last years, an increasing body of evidence led to the notion that OxPL not only contribute to the pathogenesis of chronic inflammatory processes but in addition play an integral role as modulators of inflammation during acute infections. Thereby, host defense mechanisms involve the generation of oxygen radicals that oxidize ubiquitously present phospholipids, which in turn act as danger-associated molecular patterns (DAMPs). These OxPL-derived DAMPs can exhibit both pro- and anti-inflammatory functions that ultimately alter the host response to pathogens. In this review, we summarize the currently available data on the role of OxPL in infectious diseases.
Keywords: Oxidized phospholipids, Infectious diseases, Danger-associated molecular patterns, Innate immunity
Introduction
Phospholipids are ubiquitously present as they are major constituents of cell membranes. Oxidation of these phospholipids occurs during physiological processes such as metabolism or in the course of inflammatory responses, notoriously so during chronic inflammatory diseases like atherosclerosis or diabetes. Lipid modification through oxidation renders phospholipids biologically active, hence influencing inflammatory processes. It is generally accepted that oxidation-specific epitopes signal the presence of damage or danger, and these epitopes are, therefore, considered danger-associated molecular patterns (DAMPs) [1] in analogy to pathogen-associated molecular patterns (PAMPs), which signal the presence of infections. To protect tissue homeostasis, soluble and cell-bound receptors that include natural antibodies, complement factor H or scavenger receptors were shown to bind and clear these lipid-derived DAMPs [1, 2]. Any failure to remove these oxidized phospholipid (OxPL)-derived DAMPs is believed to interfere with the restoration of tissue homeostasis and to propagate or even enhance inflammatory conditions such as atherosclerosis [3].
In addition to the mostly pro-inflammatory function of oxidation-specific lipid-modifications during chronic inflammatory diseases, it is increasingly appreciated that oxidized phospholipids strongly impact the function of innate immune cells during acute inflammations that include acute infections. Innate immune cells recognize microbes via pattern-recognition receptors, which sense the presence of conserved microbial structures, such as lipopolysaccharide (LPS), to then induce an inflammatory response. The host response to microbes involves the generation of anti-bacterial oxygen radicals by macrophages and activated neutrophils that are recruited to the site of infection. These oxygen radicals can induce the peroxidation of endogenous lipids, hence altering host structures [4, 5]. Upon oxidation, these phospholipids acquire the ability to alter important functional properties of innate immune cells that include the release of cytokines, phagocytosis, or the respiratory burst [6–8]. Not surprisingly so, there is accumulating evidence demonstrating a role for OxPL in infectious diseases. Here, we will summarize the available data that show a role for OxPL as endogenous modulators of inflammation during infectious diseases.
Generation of OxPL
Phospholipids (PL) are ubiquitously found throughout the body and as such constitute an integral part of the lipid bilayer of cell membranes. Glycerophospholipids comprise a glycerol backbone with three carbon residues. The first two carbon residues are connected to fatty acid chains, which form the hydrophobic tails, and the third carbon residue is linked to a negatively charged phosphate group, forming the polar headgroup. Binding of choline, serine, ethanolamine, or inositol to the polar head group at the sn-3 position of the glycerol backbone differentiates PL into distinct classes. The most abundant phospholipid is phosphatidylcholine (PC), which is located mainly in the extracytosolic leaflet of the plasma membrane, whereas phosphatidylserine, phosphatidylethanolamine, phosphatidylinositol, or phosphatidic acid are found in the cytosolic leaflet [9]. Polyunsaturated fatty acids are highly prone to oxidation due to the presence of methylene groups between the double bonds. Oxidation of PL can occur either enzymatically, by, e.g., 12/15 lipoxygenase, or non-enzymatically by reactive oxygen species (ROS) that are produced by macrophages and neutrophils to aid the anti-bacterial defense [4, 5, 10]. Other sources of non-enzymatic oxidation are air pollution, UV radiation, and smoking [11]. Following the primary peroxidation reaction that can be enzymatic or non-enzymatic, intermediates such as peroxyl radicals and hydroperoxides are formed, which then undergo additional oxidation steps by an enzyme-independent process, leading to the formation of a variety of different PL oxidation products [11].
In this respect, OxPL differ from other lipid mediators generated by oxidation of polyunsaturated fatty acids such as prostaglandins and leukotrienes, which are exclusively formed by enzymatic reactions [12]. The diversity of glycerophospholipids and the different chemical and enzymatic reactions involved give rise to a plethora of OxPL derivatives. A prototypic mixture of OxPL is oxidized 1-palmitoyl-2-arachidonoyl-sn-phosphatidylcholine (OxPAPC), which is generated upon oxidation of 1-palmitoyl-2-arachidonoyl-sn-phosphatidylcholine (PAPC) and contains different oxidized PCs that include 1-palmitoyl-2-(5,6)-epoxyisoprostane E2-sn-glycero-3-phosphocholine (PEIPC) and 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine (POVPC), and smaller chemical fragments [11]. If not stated otherwise, studies cited in this review used a mixture of different phosphatidylcholines, namely OxPAPC, as data on the role of its different compounds is limited. In addition to changing their biological properties, oxidation of PL also induces a conformational shift in cell membranes, whereby the previously hydrophobic fatty acid portions turn from the interior of lipid bilayers to the hydrophilic exterior. Thus, oxidized epitopes on the surface of, e.g., apoptotic cells can be recognized by receptors expressed on, e.g., macrophages and trigger downstream signaling events [13] (see cell signaling section, Fig. 1).
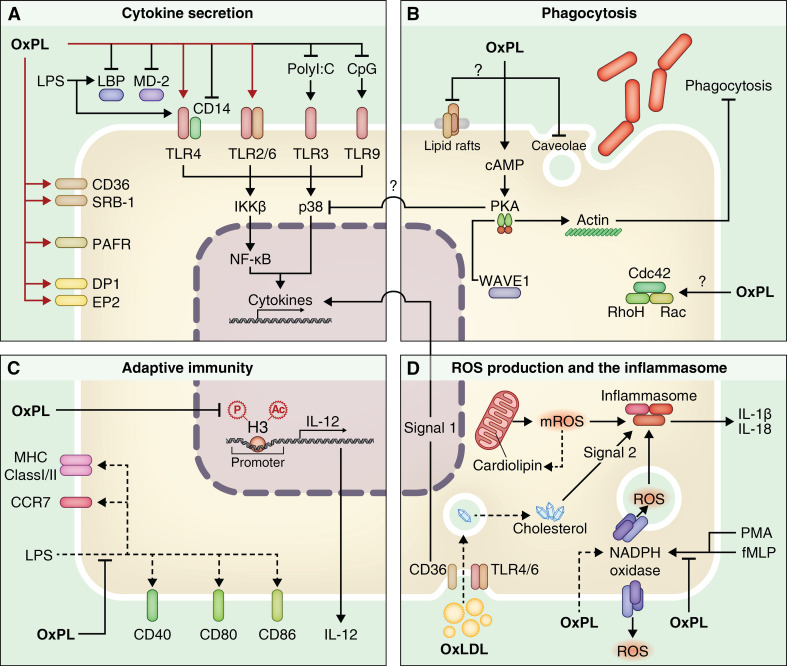
Fig. 1.
The effects of OxPL on immune cell function. OxPL can affect four main aspects of immune cell function indicated in a–d: cytokine secretion (a), phagocytosis (b), adaptive immunity, (c) and ROS production (d). The various receptors for OxPL are highlighted in red and downstream signaling is indicated. Further, cross-talk between these four aspects of immune function is indicated. Question mark indicates possible effects of OxPL on immune cell function. See text for further details
Inactivation of OxPL occurs (1) enzymatically via, e.g., epoxide hydrolase and aldehyde dehydrogenase, (2) by formation of adducts, or (3) by neutralization and scavenging via soluble or cell-bound pattern-recognition molecules such as natural antibodies, C-reactive protein, complement factor H, or scavenger receptors [11]. The biological effects exerted by OxPL during infectious diseases are inevitably dictated by the balance between activation, degradation, and scavenging of these DAMPs.
Detection of OxPL during infections in vivo
Oxidized phospholipids are generated locally, at the site of injury or infection, where they undergo rapid transformation and degradation, which makes the quantitative and qualitative assessment of various OxPL epitopes challenging. Mass spectrometry is the best and most comprehensive technique to analyze the multitude of OxPL derivatives that are generated during infection and a more detailed guide was published recently [11]. Using immunological assays, the generation and presence of OxPL has been established in a variety of organs and inflammatory diseases. As such OxPL were discovered in atherosclerotic lesions [14–16], plasma of patients with coronary artery disease [17], inflamed lung tissue [18–20], diabetic renal glomerulopathy [21], peritoneal dialysate of patients undergoing chronic peritoneal dialysis [7], in hemochromatosis [22], on apoptotic cells [23–25], on cells stimulated with inflammatory agonists [26], in CNS lesions in multiple sclerosis [27] and Alzheimer’s disease [28], but also in healthy mice [24]. Table 1 summarizes current evidence for the generation of OxPL in different infectious diseases, ranging from mycobacterial infections, to sepsis and a variety of viral infections. The majority of these data stems from ELISA and immunohistochemistry techniques that employed a well-defined monoclonal antibody, namely EO6. EO6 was derived from hybridomas from spleens of apoE−/− mice and the antigenic specificity of this antibody is well defined [29]. EO6 is an IgM antibody that specifically recognizes the phosphocholine head group of OxPL, but cannot bind to head groups of unoxidized, native phospholipids [30]. As such, EO6 recognizes a number of oxidized phosphocholine derivatives, including PEIPC and POVPC [30].
Table 1.
Documented generation of OxPL in infectious diseases
| Pathogen | Source | Species | Method | References |
|---|---|---|---|---|
| Mycobacterium leprae (Lepra) | Tissue biopsy | Human | EO6 immunohistochemistry, mass spectrometry | [109] |
| Mycobacterium bovis | Macrophages | Human | Mass spectrometry | [109] |
| E. coli (peritonitis) | Peritoneal lavage fluid; peritoneal macrophages | Mouse | EO6 ELISA | [7] |
| SARS coronavirus | Lung | Human | EO6 immunohistochemistry | [20] |
| Avian influenza virus (H5N1) | Lung | Human, mouse | EO6 immunohistochemistry | [20] |
| Bacillus anthracis (anthrax) | Lung | Rhesus monkey, rabbit | EO6 immunohistochemistry | [20] |
| Yersinia pestis (pneumonic plague) | Lung | Cynomolgus monkey | EO6 immunohistochemistry | [20] |
| Monkeypox (Monkeypox virus) | Lung | Cynomolgus monkey | EO6 immunohistochemistry | [20] |
| Influenza A virus (H1N1) | Lung epithelial cells | Human | Mass spectrometry | [94] |
| Influenza A virus (mouse adapted “PR8”) | Lung | Mouse | Mass spectrometry | [93] |
| Influenza A (H1N1, H3N2) | Lung | Mouse | EO6 immunohistochemistry | [99] |
Effects of OxPL on cell signaling and function in the context of infectious disease
Oxidized phospholipids impact various effector functions of innate immune cells. A detailed description of the effects of OxPL on cell function is beyond the scope of this review and has been extensively reviewed by others [11, 12]. Here, we focus on the cellular functions affected by OxPL relevant to infectious diseases and immune cell functions including cytokine synthesis, phagocytosis, immune cell maturation, respiratory burst, and mitochondrial dysfunction (Fig. 1a–d). These effects are shaped by signaling events initiated by these DAMPs, which cross-talk with signals activated by PAMPS on invading pathogens. The majority of insight in this context is due to studies using “sterile” PAMPs, such as LPS (of Gram-negative bacteria) or poly(I:C), a synthetic dsRNA, that acts as a viral RNA mimetic. So far, only few studies used living bacteria or viruses.
Receptors that recognize OxPL include but are not limited to CD36, SRB-1, platelet activating factor receptor (PAFR), prostaglandin receptors including E2 and D2 receptors, and possibly TLRs [11] (Fig. 1a, indicated in red). Binding of OxPL to CD36 and SRB-1 is blocked by EO6 antibodies, demonstrating the importance of the PC headgroup of OxPL in binding to these scavenger receptors [31, 32]. OxPL compete with PAF for binding to the PAFR in macrophages, suggesting that the PAFR recognizes OxPL [33]. The PEIPC but not the POVPC component of OxPAPC activates the prostaglandin E2 receptor (EP2R) and can compete with the natural ligand of the EP2R, prostaglandin E2 (PGE2), for binding to EP2R [34]. Evidence that TLRs are involved in the recognition of OxPL are provided by functional studies in TLR or TLR-adaptor-deficient mice showing that OxPAPC-mediated lung inflammation is attenuated in TLR4, myeloid differentiation primary response gene 88 (MyD88) and TIR domain-containing adaptor inducing interferon-beta (TRIF) null mice compared to their WT counterparts [20]. OxPAPC-mediated IL-8 production is lowered by TLR4 anti-sense oligonucleotides, suggesting that OxPL have the capacity of inducing inflammation in a TLR4-dependent manner [35]. Furthermore, another report showed that OxPAPC-induced inflammation is reduced in TLR2-deficient bone marrow-derived macrophages, implicating a role for TLR2 in OxPL-mediated signaling [36]. TLR4 and 2 initiate inflammatory responses in collaboration with several co-receptors, including CD14, MD2, TLR6, and CD36 [37–40], which renders the possibility that co-receptors are the actual receptors for OxPL and can explain differences in inflammatory responses in TLR-deficient model systems. The exact contribution of these co-receptors to OxPL-mediated inflammation requires further investigation especially given the potent and well-described anti-inflammatory effects of OxPL on PAMP-mediated cytokine production (Fig. 1a).
Indeed, OxPL exert well-described tissue protective and anti-inflammatory activities in models of inflammation using PAMPs. LPS is the major endotoxin of Gram-negative bacteria and as such capable of inducing a potent inflammatory response. OxPAPC inhibits LPS but not TNF-α or IL-1β induced NF-κB activation and gene expression as OxPAPC prevents binding of LPS to LPS-binding protein and CD14 [6]. The authors of this study demonstrated the significance of the anti-inflammatory effects of OxPAPC in LPS-induced shock as OxPAPC co-administration with LPS improved survival of mice [6]. The anti-inflammatory effects of OxPAPC on LPS-mediated inflammation were later replicated in several studies, and OxPAPC components were also found to interfere with LPS binding to the accessory protein MD2 [41, 42]. These inhibitory effects of OxPAPC were furthermore extended to other TLRs by showing that CpG, a short synthetic oligonucleotide, containing unmethylated CpG dinucleotides, that acts as a bacterial DNA mimetic, elicited inflammation via TLR9 was attenuated following OxPAPC treatment of RAW 264.7 macrophages [43]. Likewise, OxPAPC inhibited the inflammatory response elicited by the TLR2 ligand Pam3CSK4 [41]. These studies demonstrated that OxPAPC could interfere with PAMP-induced NF-κB and p38 signaling, which are critical nodes in TLR-induced signal transduction and pro-inflammatory cytokine production [41, 42, 44, 45].
Aside from OxPAPC binding to LPS-binding protein, MD2 and CD14 and preventing LPS-induced NF-κB and p38 mediated cytokine synthesis, direct activation of signaling cascades by OxPL could also exert anti-inflammatory effects on cytokine synthesis. OxPAPC treatment increases levels of the second messenger cAMP [46, 47]. cAMP has pleiotropic effects on cells, including the inhibition of NF-κB and p38 activation via downstream signaling through PKA [48, 49]. It should be noted that although the aforementioned studies demonstrating OxPAPC-mediated cAMP activation have been carried out in endothelial cells [46, 47], recent data from our own laboratory show that OxPAPC also activates PKA in peritoneal macrophages. The functional consequence of this in the context of bacterial infection is the inhibition of bacterial phagocytosis [7]. Importantly, we could demonstrate that forskolin, a well-known activator of the cAMP/PKA pathway, could not mimic the effects of OxPAPC on macrophages, suggesting that the manner whereby OxPAPC activates PKA in macrophages is different to classical activators [7]. The exact mechanism of PKA activation by OxPAPC and whether this has any effects on the OxPAPC-mediated inhibition of PAMP-induced cytokine synthesis in macrophages will be an interesting area of future investigation, especially given that LPS antagonism cannot explain the effects of OxPAPC on TLR9-mediated cytokine synthesis and NF-κB activation (Fig. 1a, b). However, OxPAPC inhibits CpG-induced IκB-α phosphorylation and degradation, suggesting it is acting at an upstream node of NF-κB signaling, possibly by interfering with IKK activation, a critical mediator of IκB-α phosphorylation [43, 45] (Fig. 1a, b).
Our studies, demonstrating that OxPAPC inhibits bacterial phagocytosis in macrophages [7, 50], are consistent with observations that OxPAPC activates the small GTPases, Rho, Rac, and Cdc42 [11], which play important functions in actin cytoskeletal remodeling [11, 51]. However, pre-treatment of peritoneal macrophages with clostridium toxin B (an inhibitor of small GTPases), could not prevent the inhibition of phagocytosis by OxPAPC suggesting small GTPases are not involved in this process [7]. The exact contribution of small GTPases to OxPAPC-mediated actin remodeling, however, requires further examination and may have some cell type specificity, especially given that OxPAPC-mediated actin and cytoskeletal remodeling in human pulmonary artery endothelial cells (HPAECs) was inhibited by dominant negative Rac and Cdc42 mutants [52] (Fig. 1b). It is also possible that OxPL additionally affects phagocytosis through non-receptor-mediated mechanisms, such as by disrupting caveolae and plasma membrane lipid rafts [11] (Fig. 1b).
Oxidized 1-palmitoyl-2-arachidonoyl-sn-phosphatidylcholine not only inhibits bacterial phagocytosis but also modulates ROS production (Fig. 1d). The major cellular sources of ROS are the membrane-associated nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex and mitochondria. Following phagocytosis, the NADPH oxidase complex assembles at the phagolysosomal membrane and transports electrons across it to oxygen in the phagolysosome, resulting in the generation of ROS and oxidative burst [4]. Treatment of neutrophils with OxPAPC inhibits phorbol-12-myristate-13-acetate (PMA) and formyl-methionyl-leucyl-phenylalanine (fMLP) induced ROS production [8]. The authors of this study did not observe that OxPAPC inhibited PMA-induced p38 phosphorylation in neutrophils, which is in contrast to the previously mentioned studies demonstrating effects of OxPAPC on PAMP-induced p38 MAPK [6, 43]. Differences in stimuli or cell type could explain these different observations. Furthermore, in some cell types such as endothelial cells, OxPAPC treatment induces ROS production, in part via effects on the NADPH oxidase complex [53]. The effects of OxPAPC on oxidative burst are important in the context of infectious diseases as oxidative burst is crucial for optimal bacterial killing [4]. Indeed, patients with defects in the NADPH oxidase complex, exhibit chronic granulomatous disease (CGD), which is associated with recurrent bacterial and fungal infections [54].
Mitochondria produce ROS as a by-product of ATP generation during oxidative phosphorylation. Dysfunction of mitochondria can lead to excessive mitochondrial ROS (mROS) production, which damages mitochondrial DNA, proteins and lipids including the inner mitochondrial membrane phospholipid, cardiolipin [55]. Further, in vitro studies indicate that PazPC modulates the interaction of mitochondrial-associated proteins with their membranes [56]. Interestingly, cardiolipin has recently been shown to be a component of the inflammasome, a multi-protein complex required for the maturation of IL-1β and IL-18 [57]. These cytokines are produced via two signals. The first signal induces the production of their pro-forms in an NF-κB-dependent manner in response to PAMPs. The second signal results in inflammasome generated active caspase 1, which cleaves the pro-forms into the active cytokine [58]. Notably, the OxPL receptor CD36, in co-operation with a TLR4/6 heterodimer, has recently been shown to provide both signals for the inflammasome in response to OxLDL, a process that requires endocytosis and generates cholesterol crystals (Fig. 1d) [59]. It is tempting to hypothesize that oxidiation of cardiolipin and other mitochondrial lipids could impact inflammasome-mediated maturation of these cytokines, influencing outcome during acute infections, especially given the documented role of IL-1β in neutrophil influx following acute challenges such as LPS, Pseudomonas aeruginosa and S. pneumoniae [60–63]. Consistent with a possible role of OxPL in inflammasome activation in immune cells, activation of RAW macrophages with POVPC induces ceramide synthesis, which is an activator of the NLRP3 inflammasome [64, 65]. However, to our knowledge there are no reports of IL-1β maturation in response to OxPL. Further, in line with a potential detrimental role for mitochondrial dysfunction and OxPL generation herein in acute infections, mROS overproduction and mitochondrial dysfunction correlate with poor outcome during sepsis [66, 67].
Oxidized phospholipids can also modulate immunity during infectious diseases by affecting adaptive immunity (Fig. 1c). Poly(I:C) is a viral RNA mimetic, and as such recognized by TLR3 expressed on antigen-presenting cells (macrophages, B cells, and dendritic cells). OxPAPC inhibits LPS and poly(I:C)-induced upregulation of the co-stimulatory molecules CD40, CD80, and CD86 as well as surface expression of both MHC class I and II and the chemokine receptor CCR7 on dendritic cells (DCs). Functionally, this is associated with a decreased T-cell stimulatory capacity and IL-12 production by DCs [68]. This study not only demonstrated the significance of OxPL in adaptive immunity, but also importantly showed that TLR3-stimulated cytokine production could be attenuated by OxPAPC [68]. In a later study, the authors could show that the decreased IL-12 production by OxPAPC-treated DCs was associated with reduced histone H3 phosphorylation and acetylation on the IL-12 promoter [69]. These data suggest that OxPL can modulate immune responses by epigenetic mechanisms. Interestingly, chronic downregulation of IL-12 gene expression, associated with epigenetic modifications on the IL-12 promoter, has been observed in DCs of post-septic mice [70]. These data provide an intriguing possibility that “immuno-paralysis” and impaired leukocyte function observed in patients who survive severe sepsis [71] is associated with epigenetic modifications on the promoters of cytokines. Data from our laboratory demonstrated that OxPL are endogenously produced during bacterial peritonitis [7]; however, we did not examine any potential epigenetic effects in post-septic mice. This will be an interesting area of future investigation.
Altogether, the aforementioned studies demonstrate that different oxidation-specific epitopes of OxPL are recognized by multiple receptors on immune cells that may cross-talk (Fig. 1a–d). A plethora of signaling events then occur ranging from kinase and transcription factor activation, second messenger, ROS production, cytokine transcription, and cytoskeletal remodeling. It should be noted that OxPL can also impact infectious diseases via their effects on non-immune cells, such as endothelial cells, by for instance affecting endothelial barrier function and cytokine production, which will have downstream effects on leukocyte migration. Of note, all signaling studies we are aware of in the context of OxPL were performed with PAMPs, and not with living microorganisms. Thus, a protective role in LPS endotoxemia cannot be extrapolated to Gram-negative infections. Overall in experimental acute infectious disease models, OxPL were associated with detrimental effects, which may aggravate disease progression (as discussed below). Furthermore, it is clear that soluble mediators including CRP, complement factor H and natural antibodies exert housekeeper functions by acting as scavengers for these DAMPS, consequently influencing immunity during infectious disease [2]. In the following sections, we will discuss the various infectious diseases that OxPL have been shown to impact.
How OxPL impact pulmonary inflammation
Surfactant lining the alveolar space consists of 80–90 % of PL, most of which are saturated and protected from oxidation, but a small proportion of unsaturated PL are present. These unsaturated PL are prone to oxidation due to physiologically high concentrations of oxygen, air pollution, or ROS produced by immune cells [72]. Under steady state conditions surfactant is protected by antioxidants like glutathione and surfactant proteins A and D [73, 74]. Oxidative processes within the lungs are implicated in the pathogenesis of a number of chronic inflammatory diseases such as asthma [75], chronic obstructive pulmonary disease [76], and cystic fibrosis [77]. Infections of the respiratory tract were shown to cause surfactant alterations including changes in the composition of pulmonary phospholipids [78, 79]. Thus, a myriad of data points toward a modulation of inflammation by oxidation products in different lung pathologies.
Indeed, although mice treated with 100 % oxygen die after 5 days, survival is significantly improved via overexpression of the antioxidant enzyme Prdx6 [80, 81]. The authors hypothesized this was due to a reduction in the levels of peroxidized phospholipids. One specific oxidized phospholipid (namely 1-palmitoyl-2-(9′-oxo-nonanoyl)-glycerophosphocholine) derived from ozone-treated calf lung surfactant was found to reduce macrophage and epithelial cell viability [82], and to induce IL-8 in lung epithelial cells [83]. The scavenger receptors MARCO and SR-AI/II were shown to protect against pro-inflammatory effects of this specific OxPL in mice [84]. These data demonstrate the detrimental effects of OxPL in the lung and the importance of scavenger-receptor-mediated mechanisms in protecting against these.
Aside from receptor-mediated mechanism, additional receptor-independent mechanisms have been proposed to protect from the detrimental effects of pulmonary OxPL. As such it was shown that the antibody EO6, which binds to OxPL on apoptotic cells, has the ability to block inflammation in vitro [25] and in vivo [20]. In a similar manner, the endogenous acute phase protein C-reactive protein (CRP), which is typically induced during infections such as pneumonia, binds to oxidized phosphorylcholine present in OxLDL and on apoptotic cells [85]. This binding was found to promote the clearance of apoptotic cells and contribute to the resolution of inflammation [86]. It is tempting to speculate that CRP is involved in the neutralization of OxPL in vivo and several lines of evidence point toward a protective role for CRP in bacterial pneumonia. As such, CRP was found to protect mice from pneumococcal infection independent of its role in binding to pneumococcal C-polysaccharide and promoting opsono-phagocytosis [87]. Also, C5a-induced lung injury in rabbits was attenuated by CRP, suggesting that CRP promotes improved outcome during acute respiratory distress syndrome (ARDS) [88, 89]. Further confirming the idea of a protective role for CRP during lung inflammation, a clinical study reported that increased CRP plasma levels were associated with improved outcome in patients suffering from ARDS [90]. However, the precise molecular mechanism of CRP’s protective role and the potential interplay with OxPL still needs to be proven.
Generation of oxygen radicals [91, 92] or pulmonary OxPL—assessed by mass spectrometry or immunohistochemistry (Table 1)—was revealed in various studies of murine influenza virus infection [20, 93], as was the up to 70-fold increased production of superoxide anion [92]. Underscoring the importance of OxPL in influenza-mediated lung pathology, infection of human lung epithelial cells with influenza A virus resulted in the local release and oxidation of PL, as assessed by mass spectrometry [94]. Addition of the ApoA-I mimetic peptide D-4F, a main constituent of HDL, reduced OxPL levels and in parallel attenuated the production of pro-inflammatory cytokines (IL-6, IFN-α, and IFN-β) by lung epithelial cells in vitro [94]. These findings suggest a pro-inflammatory role for OxPL in this setting. The same group investigated the potential interplay between infection and cardiovascular events, and focused on changes in the anti-inflammatory and anti-oxidative properties of HDL upon infection. As such, they discovered that HDL lost its anti-inflammatory properties upon influenza infection in vivo, which was associated with increased oxidation of LDL [95] and enhanced monocyte traffic into arteriosclerotic plaques of LDL-R deficient animals [96]. The authors hypothesized that the infection-triggered alteration in anti-oxidant molecules could explain the increased rate of cardiovascular events following influenza infection. Epidemiological findings support these concepts as respiratory tract infections are associated with an increased risk of cardiovascular events such as stroke and myocardial infarction [97, 98].
Oxidized phospholipids generation in lungs was furthermore discovered by immunohistochemistry (Table 1) following acid aspiration-induced acute lung injury and pulmonary infections such as human SARS, murine H5N1, Anthrax in monkeys and rabbits, and Yersinia pestis or Monkeypox virus-infected monkeys [20]. Mechanistically, the authors of this specific study propose OxPL to be a common denominator that drives lung inflammation and injury in the course of infections. As such, they found that intranasal application of OxPAPC (20 µg/g mouse) followed by mechanical ventilation triggered a TLR4-dependent inflammatory response in the lungs. In a mouse model of acute lung injury using acid aspiration, knockdown of TLR4 or downstream signaling molecules like TRIF tremendously blunted lung damage, as assessed by lung elastance, edema formation, and histopathology [20]. IL-6 produced by alveolar macrophages in vivo and upon OxPAPC challenge ex vivo was found to be the main cytokine driving lung injury as IL-6 knockout mice were protected. Demonstrating the importance of OxPL in this model, the authors could show that administration of EO6 dampened OxPAPC-mediated inflammation in vitro and in vivo [20]. Furthermore, acid aspiration or infection with H5N1 triggered ROS production, upregulated TLR4, and elevated OxPL generation, which was assessed by EO6 immunohistochemistry. Neutrophil cytosolic factor 1 (ncf1)-deficient mice, which display defects in ROS production, showed improved outcome upon challenge with inactivated H5N1 [20]. Lee et al. [99] corroborated the presence and impact of OxPL using different Influenza strains (H1N1 and H3N2) in a murine model: similarly, immunohistochemical staining using the EO6 antibody revealed the presence of OxPL epitopes in infected mice, and ncf1-deficient mice were protected in pneumonia. These data are in accordance with the pro-inflammatory effects of OxPL that possibly occur in a TLR4-dependent manner (Fig. 1). In a similar study performed in our laboratory, we detected elevated—albeit not statistically significant—levels of OxPL in bronchoalveolar lavage fluid in a murine model of acute lung injury caused by acid aspiration [100]. As opposed to Imai et al. [20], we did not ventilate mice after acid administration. These technical differences might be important in this context, as application of oxygen might further increase levels of OxPL and exacerbate lung injury.
These findings were recently extended by Shirey et al., who treated mice and rats in a model of influenza with the synthetic lipid-A analog Eritoran, which inhibits TLR-4/MD2-mediated responses [93]. Strikingly, even if applied 6 days after infection, Eritoran was able to rescue animals from influenza-induced mortality [93]. In accordance with the findings of Imai et al., the authors of this study postulated that OxPLs exert pro-inflammatory effects via TLR4 and as such they discovered reduced OxPL levels and substantially diminished lung inflammation in mice that were treated with Eritoran after influenza virus infection. In addition to TLR4, the authors were able to show that the protective effects of Eritoran also required CD14, as Eritoran failed to rescue not only TLR4-, but also CD14-knockout mice from influenza-associated mortality [93].
Another group corroborated our earlier findings of OxPL-induced inhibition of bacterial phagocytosis [7, 50] in a setting of bacterial pneumonia [101]. They discovered that cigarette smoke induced the generation of OxPL in the broncho-alveolar lavage fluid of mice, and that these OxPL impaired the clearance of Pseudomonas aeruginosa via inhibition of phagocytosis by alveolar macrophages. Of note, in this study different PC moieties were used (POVPC, PGPC, and PaZPC), all of which showed the same overall inhibition of bacterial uptake as the mixture OxPAPC [101].
While the aforementioned studies discovered detrimental effects of OxPL in lung injury, other reports found protective effects. In a rat model of LPS-induced pneumonitis, intravenous administration of OxPL attenuated neutrophil influx into the bronchoalveolar space, edema formation, and pro-inflammatory cytokine production [102]. In vitro, endothelial barrier disruption, and monolayer integrity of HPAECs was found preserved upon OxPL treatment, via OxPL-induced cytoskeletal rearrangements that involved Cdc42 and Rac [52]. This study is in accordance with data demonstrating that barrier disruption caused by IL-6 or thrombin was prevented by OxPL [103]. Furthermore, intravenous administration of OxPL dampened ventilation-induced lung injury in mice [104]. Accordingly, co-administration of LPS or CpG DNA together with OxPL (but not unoxidized phospholipids) intratracheally lowered bronchoalveolar TNF-α levels [43]. These barrier-protective effects were more recently shown to require binding of OxPL to the chaperone protein GRP78 on the surface of endothelial cells [105].
Together, OxPL substantially contribute to lung pathology during infectious and inflammatory lung diseases. The precise nature of OxPL’s contribution remains ambiguous and perhaps depends on the context. It seems that OxPL can protect endothelial barriers in models of mild lung inflammation, whereas during more pronounced inflammatory conditions such as during acute lung injury and ventilation or various viral infections, OxPL importantly contribute and even mediate inflammation and tissue injury (Fig. 1).
The role of OxPL during bacterial peritonitis
At a time when OxPL were still considered to mainly contribute to chronic inflammation via their pro-inflammatory properties [106], Bochkov et al. [6] published a study where they demonstrated that OxPAPC dampen acute inflammation by blocking the interaction of LPS with LPS-binding protein and CD14 (Fig. 1). In this report, the authors revealed that OxPL prevented the activation of MAP kinases and NF-κB in endothelial cells and that this ultimately resulted in reduced leukocyte recruitment in an air pouch model of inflammation and upon peritoneal LPS injection. Ultimately, these inhibitory effects of OxPL led to an improved outcome in a murine LPS-induced shock model [6]. These striking findings prompted us to investigate the impact of OxPL during E. coli peritonitis, i.e., a model with viable bacteria instead of LPS. We thereby discovered that OxPL-treated animals showed an impaired survival, paralleled by an increased inflammatory response and enhanced bacterial outgrowth and dissemination, which we found to be independent of CD14 [50]. Mechanistically, we discovered that OxPAPC inhibited phagocytosis of E. coli by macrophages and neutrophils. Moreover, OxPL inhibited a broad spectrum of different ingestion mechanisms by macrophages, including macropinocytosis or fluid-phase pinocytosis, thus pointing toward a more generalized interference with uptake mechanisms [50]. In subsequent studies, we delineated that OxPL-induced actin polymerization via activation of PKA and that inhibitors of PKA were able to reverse the inhibitory effects of OxPL on macrophage phagocytosis (Fig. 1) [7]. In-depth studies enabled us to reveal that the A-kinase anchoring protein WAVE1 was required for the specific inhibitory effects of OxPL both in vitro and upon infection with E. coli in vivo. Importantly, we not only discovered increased levels of OxPL epitopes in peritoneal lavage fluid of mice infected with E. coli compared to uninfected mice, but also in the peritoneal dialysis fluid of patients undergoing continuous ambulatory peritoneal dialysis, as assessed by ELISA using the EO6 antibody (Table 1). Of potential clinical importance, peritoneal dialysis fluid (once immunoglobulins were depleted) also inhibited phagocytosis of bacteria in a PKA/WAVE1-dependent manner. Importantly, inhibition of phagocytosis was reversed by adding EO6, confirming that the PC moiety is responsible for the described effects.
Lepra
Mycobacterium leprae can cause two distinct clinical syndromes: lepromatous leprae (l-leprae), and tuberculous leprae (t-leprae). Whereas in t-leprae, mycobacteria evoke a potent immune response and thus can be contained locally, in l-leprae the pathogen disseminates [107]. Concomitantly, in l-leprae a Th2-immune response dominates, as compared to t-leprae, where Th1-cytokines are prevailing [108]. In an elegant study, Cruz et al. [109] found that in lesions of patients suffering from l-leprae, host genes regulating lipid metabolism were significantly upregulated as compared to t-leprae patients. Underscoring the importance of OxPL, immunohistochemical staining of l-leprae lesions with EO6 revealed the presence of OxPL in macrophages, similar to that observed in atherosclerosis [109]. In vitro, infection of human macrophages with Mycobacterium bovis led to the generation of OxPL, most notably PEIPC, as assessed by mass spectrometry. Functionally, PEIPC impaired CD1-expression, and resulted in reduced IFN-γ secretion by M. leprae antigen challenged T-lymphocytes. Moreover, PEIPC inhibited IL-12 and enhanced IL-10 production, thereby giving a potential explanation how l-leprae leads to a Th2-immune response. Furthermore, PEIPC inhibited the TLR2/1 triggered induction of cathelicidin via vitamin D maturation and, therefore, prevented the induction of an important antimicrobial molecule [109, 110]. HDL, that has the ability to neutralize OxPL, partly via its enzymatic activity, was tested for its ability to restore the functional properties of DCs in the presence of OxPL. While HDL from healthy volunteers indeed restored CD1b expression during differentiation of DC, HDL from l-leprae patients inhibited CD1b-mediated antigen presentation to T cells. Overall, M. leprae infection triggered the generation of host-derived OxPL, which not only provided an essential substrate for M. leprae, but also attracted monocytes that served as host cells for mycobacteria, and dampened the innate immune response.
Concluding remarks
In atherosclerosis, a role for OxPL as modulators of the inflammatory process is well established. Accumulating evidence for a role of OxPL in infectious diseases has emerged over the last decade. The generation of OxPL was subsequently discovered at sites of infections caused by a variety of bacterial and viral pathogens across different host species. It is tempting to speculate that, due to the omnipresent production of ROS upon inflammation, OxPL derivatives might be ubiquitously present in infectious processes.
Considering the functions OxPL exert during various infectious diseases, the data available so far report both anti- and pro-inflammatory properties of OxPL. Researchers in favor of the anti-inflammatory properties of OxPL argue that these effects serve as negative feedback mechanism to induce resolution and to prevent tissue damage. However, it has to be noticed, that most of the reports about beneficial anti-inflammatory properties are based on models of sterile inflammation such as that induced by LPS. Data on the role of OxPL during bacterial infections are still limited, and the major finding thus far was the observation that OxPL inhibit bacterial phagocytosis by macrophages. At the same time, a body of evidence illustrates the profound pro-inflammatory properties of OxPL, such as in severe acute lung injury or during acute influenza infections. The evolutionary conserved presence of molecules that counteract potential effects of OxPL, which include scavenger receptors, complement factor H, C-reactive protein or natural antibodies support the potentially harmful function of OxPL. One challenge of future research in this field will be to find clinical settings where these counteracting mechanisms are overwhelmed. As mentioned above, there is epidemiological evidence that infections trigger cardiovascular events such as myocardial infarction and stroke [97, 98, 111]. It is tempting to speculate that OxPL generated during respiratory infections like influenza contribute to this higher risk.
Current knowledge is limited in several aspects (1) only few reports studied the role of OxPL in “true” infections with live microorganisms, instead sterile PAMPs such as LPS or Poly(I:C) serve as imitations of bacterial or viral infections, (2) real levels of OxPL in general and infectious diseases in particular are not known, as quantification is technically difficult as huge spatial and temporal variations exist, and (3) little is known about the function of specific OxPL compounds, as in most studies a mixture of OxPLs is used.
We are confident that future research will shed additional light on the biological role of OxPL during infectious diseases and that these studies will provide further insight in the interplay of pathogens and OxPL that might even disclose novel therapeutic targets.
Acknowledgments
Part of this work was funded by a grant provided by the Science Fund of the Austrian National Bank (#14107; to SK).
Abbreviations
- ARDS
Acute respiratory distress syndrome
- cAMP
Cyclic adenosine monophosphate
- CD14
Monocyte differentiation antigen CD14
- Cdc42
Cell division control protein 42 homolog
- CRP
C-reactive protein
- DAMP
Danger-associated molecular pattern
- DCs
Dendritic cells
- EP2R
Prostaglandin E2 receptor
- GTP
Guanosine-5′-triphosphate
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- LPS
Lipopolysaccharide
- MARCO
Macrophage receptor with collagenous structure
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NF-κB
Nuclear factor kappa light chain enhancer in B cells
- NLRP3
NOD-like receptor family, pyrin domain containing 3
- OxLDL
Oxidized low-density lipoprotein
- OxPAPC
Oxidized 1-palmitoyl-2-arachidonoyl-sn-phosphatidylcholine
- OxPL
Oxidized phospholipids
- p38
p38 Map kinase
- PAF(R)
Platelet activating factor (receptor)
- PAMP
Pathogen-associated molecular patterns
- Pam3CSK4
N-Palmitoyl-S-dipalmitoylglyceryl Cys-Ser-(Lys)4
- PAPC
1-Palmitoyl-2-arachidonoyl-sn-phosphatidylcholine
- PazPC
1-Palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine
- PEIPC
1-Palmitoyl-2-(5,6)-epoxyisoprostane E2-sn-glycero-3-phosphocholine
- PGPC
1-Palmitoyl-2-glutaryl-sn-glycero-3-phosphocholine
- PGE2
Prostaglandin E2
- PKA
Protein kinase A
- POVPC
1-Palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine
- PC
Phosphatidylcholine
- PL
Phospholipids
- Rho
Ras homolog
- Rac
Rho-related C3 botulinum toxin substrate
- ROS
Reactive oxygen species
- SRB-1
Scavenger receptor class B, member 1
- TLR
Toll-like receptor
- WAVE1
Wasp family, verprolin homology domain-containing protein 1
Footnotes
U. Matt and O. Sharif contributed equally to this work.
References
- 1.Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108(2):235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weismann D, Binder CJ. The innate immune response to products of phospholipid peroxidation. Biochim Biophys Acta 1818. 2012;10:2465–2475. doi: 10.1016/j.bbamem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S, Birukov KG, Romanoski CE, Springstead JR, Lusis AJ, Berliner JA. Role of phospholipid oxidation products in atherosclerosis. Circ Res. 2012;111(6):778–799. doi: 10.1161/CIRCRESAHA.111.256859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92(9):3007–3017. [PubMed] [Google Scholar]
- 5.Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109(1):33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 6.Bochkov VN, Kadl A, Huber J, Gruber F, Binder BR, Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002;419(6902):77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- 7.Matt U, Sharif O, Martins R, Furtner T, Langeberg L, Gawish R, Elbau I, Zivkovic A, Lakovits K, Oskolkova O, Doninger B, Vychytil A, Perkmann T, Schabbauer G, Binder CJ, Bochkov VN, Scott JD, Knapp S. WAVE1 mediates suppression of phagocytosis by phospholipid-derived DAMPs. J Clin Investig. 2013;123(7):3014–3024. doi: 10.1172/JCI60681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bluml S, Rosc B, Lorincz A, Seyerl M, Kirchberger S, Oskolkova O, Bochkov VN, Majdic O, Ligeti E, Stockl J. The oxidation state of phospholipids controls the oxidative burst in neutrophil granulocytes. J Immunol. 2008;181(6):4347–4353. doi: 10.4049/jimmunol.181.6.4347. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda M, Kihara A, Igarashi Y. Lipid asymmetry of the eukaryotic plasma membrane: functions and related enzymes. Biol Pharm Bull. 2006;29(8):1542–1546. doi: 10.1248/bpb.29.1542. [DOI] [PubMed] [Google Scholar]
- 10.Zhang R, Shen Z, Nauseef WM, Hazen SL. Defects in leukocyte-mediated initiation of lipid peroxidation in plasma as studied in myeloperoxidase-deficient subjects: systematic identification of multiple endogenous diffusible substrates for myeloperoxidase in plasma. Blood. 2002;99(5):1802–1810. [PubMed] [Google Scholar]
- 11.Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stockl J. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal. 2010;12(8):1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bochkov VN. Inflammatory profile of oxidized phospholipids. Thromb Haemost. 2007;97(3):348–354. [PubMed] [Google Scholar]
- 13.Greenberg ME, Li XM, Gugiu BG, Gu X, Qin J, Salomon RG, Hazen SL. The lipid whisker model of the structure of oxidized cell membranes. J Biol Chem. 2008;283(4):2385–2396. doi: 10.1074/jbc.M707348200. [DOI] [PubMed] [Google Scholar]
- 14.Watson AD, Leitinger N, Navab M, Faull KF, Horkko S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, Subbanagounder G, Fogelman AM, Berliner JA. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;272(21):13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 15.Subbanagounder G, Leitinger N, Schwenke DC, Wong JW, Lee H, Rizza C, Watson AD, Faull KF, Fogelman AM, Berliner JA. Determinants of bioactivity of oxidized phospholipids. Specific oxidized fatty acyl groups at the sn-2 position. Arterioscler Thromb Vasc Biol. 2000;20(10):2248–2254. doi: 10.1161/01.atv.20.10.2248. [DOI] [PubMed] [Google Scholar]
- 16.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Febbraio M, Hajjar DP, Silverstein RL, Hoff HF, Salomon RG, Hazen SL. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J Biol Chem. 2002;277(41):38517–38523. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- 17.Tsimikas S, Bergmark C, Beyer RW, Patel R, Pattison J, Miller E, Juliano J, Witztum JL. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J Am Coll Cardiol. 2003;41(3):360–370. doi: 10.1016/s0735-1097(02)02769-9. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimi N, Ikura Y, Sugama Y, Kayo S, Ohsawa M, Yamamoto S, Inoue Y, Hirata K, Itabe H, Yoshikawa J, Ueda M. Oxidized phosphatidylcholine in alveolar macrophages in idiopathic interstitial pneumonias. Lung. 2005;183(2):109–121. doi: 10.1007/s00408-004-2525-0. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T, Henson PM, Murphy RC. Occurrence of oxidized metabolites of arachidonic acid esterified to phospholipids in murine lung tissue. Anal Biochem. 1998;262(1):23–32. doi: 10.1006/abio.1998.2749. [DOI] [PubMed] [Google Scholar]
- 20.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, Penninger JM. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133(2):235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horie K, Miyata T, Maeda K, Miyata S, Sugiyama S, Sakai H, van Ypersole de Strihou C, Monnier VM, Witztum JL, Kurokawa K. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J Clin Investig. 1997;100(12):2995–3004. doi: 10.1172/JCI119853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houglum K, Ramm GA, Crawford DH, Witztum JL, Powell LW, Chojkier M. Excess iron induces hepatic oxidative stress and transforming growth factor beta1 in genetic hemochromatosis. Hepatology. 1997;26(3):605–610. doi: 10.1002/hep.510260311. [DOI] [PubMed] [Google Scholar]
- 23.Huber J, Vales A, Mitulovic G, Blumer M, Schmid R, Witztum JL, Binder BR, Leitinger N. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte-endothelial interactions. Arterioscler Thromb Vasc Biol. 2002;22(1):101–107. doi: 10.1161/hq0102.101525. [DOI] [PubMed] [Google Scholar]
- 24.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Backhed F, Miller YI, Horkko S, Corr M, Witztum JL, Binder CJ. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Investig. 2009;119(5):1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang MK, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, Witztum JL. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J Exp Med. 2004;200(11):1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subbanagounder G, Wong JW, Lee H, Faull KF, Miller E, Witztum JL, Berliner JA. Epoxyisoprostane and epoxycyclopentenone phospholipids regulate monocyte chemotactic protein-1 and interleukin-8 synthesis. Formation of these oxidized phospholipids in response to interleukin-1beta. J Biol Chem. 2002;277(9):7271–7281. doi: 10.1074/jbc.M107602200. [DOI] [PubMed] [Google Scholar]
- 27.Newcombe J, Li H, Cuzner ML. Low density lipoprotein uptake by macrophages in multiple sclerosis plaques: implications for pathogenesis. Neuropathol Appl Neurobiol. 1994;20(2):152–162. doi: 10.1111/j.1365-2990.1994.tb01174.x. [DOI] [PubMed] [Google Scholar]
- 28.Dei R, Takeda A, Niwa H, Li M, Nakagomi Y, Watanabe M, Inagaki T, Washimi Y, Yasuda Y, Horie K, Miyata T, Sobue G. Lipid peroxidation and advanced glycation end products in the brain in normal aging and in Alzheimer’s disease. Acta Neuropathol. 2002;104(2):113–122. doi: 10.1007/s00401-002-0523-y. [DOI] [PubMed] [Google Scholar]
- 29.Palinski W, Horkko S, Miller E, Steinbrecher UP, Powell HC, Curtiss LK, Witztum JL. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Investig. 1996;98(3):800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman P, Horkko S, Steinberg D, Witztum JL, Dennis EA. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol condensation. J Biol Chem. 2002;277(9):7010–7020. doi: 10.1074/jbc.M108860200. [DOI] [PubMed] [Google Scholar]
- 31.Gillotte-Taylor K, Boullier A, Witztum JL, Steinberg D, Quehenberger O. Scavenger receptor class B type I as a receptor for oxidized low density lipoprotein. J Lipid Res. 2001;42(9):1474–1482. [PubMed] [Google Scholar]
- 32.Boullier A, Friedman P, Harkewicz R, Hartvigsen K, Green SR, Almazan F, Dennis EA, Steinberg D, Witztum JL, Quehenberger O. Phosphocholine as a pattern recognition ligand for CD36. J Lipid Res. 2005;46(5):969–976. doi: 10.1194/jlr.M400496-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Pegorier S, Stengel D, Durand H, Croset M, Ninio E. Oxidized phospholipid: POVPC binds to platelet-activating-factor receptor on human macrophages. Implications in atherosclerosis. Atherosclerosis. 2006;188(2):433–443. doi: 10.1016/j.atherosclerosis.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Li R, Mouillesseaux KP, Montoya D, Cruz D, Gharavi N, Dun M, Koroniak L, Berliner JA. Identification of prostaglandin E2 receptor subtype 2 as a receptor activated by OxPAPC. Circ Res. 2006;98(5):642–650. doi: 10.1161/01.RES.0000207394.39249.fc. [DOI] [PubMed] [Google Scholar]
- 35.Walton KA, Hsieh X, Gharavi N, Wang S, Wang G, Yeh M, Cole AL, Berliner JA. Receptors involved in the oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine-mediated synthesis of interleukin-8. A role for Toll-like receptor 4 and a glycosylphosphatidylinositol-anchored protein. J Biol Chem. 2003;278(32):29661–29666. doi: 10.1074/jbc.M300738200. [DOI] [PubMed] [Google Scholar]
- 36.Kadl A, Sharma PR, Chen W, Agrawal R, Meher AK, Rudraiah S, Grubbs N, Sharma R, Leitinger N. Oxidized phospholipid-induced inflammation is mediated by Toll-like receptor 2. Free Radic Biol Med. 2011;51(10):1903–1909. doi: 10.1016/j.freeradbiomed.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monaco C. The tolls and dangers of atherosclerotic disease. Curr Pharm Biotechnol. 2012;13(1):77–87. doi: 10.2174/138920112798868700. [DOI] [PubMed] [Google Scholar]
- 38.Nilsen NJ, Deininger S, Nonstad U, Skjeldal F, Husebye H, Rodionov D, von Aulock S, Hartung T, Lien E, Bakke O, Espevik T. Cellular trafficking of lipoteichoic acid and Toll-like receptor 2 in relation to signaling: role of CD14 and CD36. J Leukoc Biol. 2008;84(1):280–291. doi: 10.1189/jlb.0907656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433(7025):523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 40.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11(2):155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erridge C, Kennedy S, Spickett CM, Webb DJ. Oxidized phospholipid inhibition of toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition. J Biol Chem. 2008;283(36):24748–24759. doi: 10.1074/jbc.M800352200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim MJ, Choi NY, Koo JE, Kim SY, Joung SM, Jeong E, Lee JY. Suppression of Toll-like receptor 4 activation by endogenous oxidized phosphatidylcholine, KOdiA-PC by inhibiting LPS binding to MD2. Inflamm Res. 2013;62(6):571–580. doi: 10.1007/s00011-013-0609-0. [DOI] [PubMed] [Google Scholar]
- 43.Ma Z, Li J, Yang L, Mu Y, Xie W, Pitt B, Li S. Inhibition of LPS- and CpG DNA-induced TNF-alpha response by oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol. 2004;286(4):L808–L816. doi: 10.1152/ajplung.00220.2003. [DOI] [PubMed] [Google Scholar]
- 44.Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13(9):679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 45.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8(1):49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 46.Cole AL, Subbanagounder G, Mukhopadhyay S, Berliner JA, Vora DK. Oxidized phospholipid-induced endothelial cell/monocyte interaction is mediated by a cAMP-dependent R-Ras/PI3-kinase pathway. Arterioscler Thromb Vasc Biol. 2003;23(8):1384–1390. doi: 10.1161/01.ATV.0000081215.45714.71. [DOI] [PubMed] [Google Scholar]
- 47.Kronke G, Bochkov VN, Huber J, Gruber F, Bluml S, Furnkranz A, Kadl A, Binder BR, Leitinger N. Oxidized phospholipids induce expression of human heme oxygenase-1 involving activation of cAMP-responsive element-binding protein. J Biol Chem. 2003;278(51):51006–51014. doi: 10.1074/jbc.M304103200. [DOI] [PubMed] [Google Scholar]
- 48.Parry GC, Mackman N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappaB-mediated transcription. J Immunol. 1997;159(11):5450–5456. [PubMed] [Google Scholar]
- 49.Takahashi N, Tetsuka T, Uranishi H, Okamoto T. Inhibition of the NF-kappaB transcriptional activity by protein kinase A. Eur J Biochem. 2002;269(18):4559–4565. doi: 10.1046/j.1432-1033.2002.03157.x. [DOI] [PubMed] [Google Scholar]
- 50.Knapp S, Matt U, Leitinger N, van der Poll T. Oxidized phospholipids inhibit phagocytosis and impair outcome in gram-negative sepsis in vivo. J Immunol. 2007;178(2):993–1001. doi: 10.4049/jimmunol.178.2.993. [DOI] [PubMed] [Google Scholar]
- 51.Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33(Pt 5):891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 52.Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, Verin AD, Bokoch GM, Leitinger N, Garcia JG. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res. 2004;95(9):892–901. doi: 10.1161/01.RES.0000147310.18962.06. [DOI] [PubMed] [Google Scholar]
- 53.Rouhanizadeh M, Hwang J, Clempus RE, Marcu L, Lassegue B, Sevanian A, Hsiai TK. Oxidized-1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine induces vascular endothelial superoxide production: implication of NADPH oxidase. Free Radic Biol Med. 2005;39(11):1512–1522. doi: 10.1016/j.freeradbiomed.2005.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith RM, Curnutte JT. Molecular basis of chronic granulomatous disease. Blood. 1991;77(4):673–686. [PubMed] [Google Scholar]
- 55.Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292(1):C33–C44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 56.Wallgren M, Lidman M, Pham QD, Cyprych K, Grobner G. The oxidized phospholipid PazePC modulates interactions between Bax and mitochondrial membranes. Biochim Biophys Acta 1818. 2012;11:2718–2724. doi: 10.1016/j.bbamem.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 57.Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, Eisenbarth SC, Nauseef WM, Cassel SL, Sutterwala FS. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39(2):311–323. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 59.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14(8):812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schultz MJ, Rijneveld AW, Florquin S, Edwards CK, Dinarello CA, van der Poll T. Role of interleukin-1 in the pulmonary immune response during Pseudomonas aeruginosa pneumonia. Am J Physiol Lung Cell Mol Physiol. 2002;282(2):L285–L290. doi: 10.1152/ajplung.00461.2000. [DOI] [PubMed] [Google Scholar]
- 61.Mason MJ, Van Epps DE. In vivo neutrophil emigration in response to interleukin-1 and tumor necrosis factor-alpha. J Leukoc Biol. 1989;45(1):62–68. doi: 10.1002/jlb.45.1.62. [DOI] [PubMed] [Google Scholar]
- 62.Cybulsky MI, Colditz IG, Movat HZ. The role of interleukin-1 in neutrophil leukocyte emigration induced by endotoxin. Am J Pathol. 1986;124(3):367–372. [PMC free article] [PubMed] [Google Scholar]
- 63.Rijneveld AW, Florquin S, Branger J, Speelman P, Van Deventer SJ, van der Poll T. TNF-alpha compensates for the impaired host defense of IL-1 type I receptor-deficient mice during pneumococcal pneumonia. J Immunol. 2001;167(9):5240–5246. doi: 10.4049/jimmunol.167.9.5240. [DOI] [PubMed] [Google Scholar]
- 64.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Halasiddappa LM, Koefeler H, Futerman AH, Hermetter A. Oxidized phospholipids induce ceramide accumulation in RAW 264.7 macrophages: role of ceramide synthases. PLoS ONE. 2013;8(7):e70002. doi: 10.1371/journal.pone.0070002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360(9328):219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 67.Ruggieri AJ, Levy RJ, Deutschman CS (2010) Mitochondrial dysfunction and resuscitation in sepsis. Crit Care Clin 26(3):567–575. doi:10.1016/j.ccc.2010.04.007 [DOI] [PMC free article] [PubMed]
- 68.Bluml S, Kirchberger S, Bochkov VN, Kronke G, Stuhlmeier K, Majdic O, Zlabinger GJ, Knapp W, Binder BR, Stockl J, Leitinger N. Oxidized phospholipids negatively regulate dendritic cell maturation induced by TLRs and CD40. J Immunol. 2005;175(1):501–508. doi: 10.4049/jimmunol.175.1.501. [DOI] [PubMed] [Google Scholar]
- 69.Bluml S, Zupkovitz G, Kirchberger S, Seyerl M, Bochkov VN, Stuhlmeier K, Majdic O, Zlabinger GJ, Seiser C, Stockl J. Epigenetic regulation of dendritic cell differentiation and function by oxidized phospholipids. Blood. 2009;114(27):5481–5489. doi: 10.1182/blood-2008-11-191429. [DOI] [PubMed] [Google Scholar]
- 70.Wen H, Dou Y, Hogaboam CM, Kunkel SL. Epigenetic regulation of dendritic cell-derived interleukin-12 facilitates immunosuppression after a severe innate immune response. Blood. 2008;111(4):1797–1804. doi: 10.1182/blood-2007-08-106443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reddy RC, Chen GH, Tekchandani PK, Standiford TJ. Sepsis-induced immunosuppression: from bad to worse. Immunol Res. 2001;24(3):273–287. doi: 10.1385/IR:24:3:273. [DOI] [PubMed] [Google Scholar]
- 72.Chroneos ZC, Sever-Chroneos Z, Shepherd VL. Pulmonary surfactant: an immunological perspective. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2010;25(1):13–26. doi: 10.1159/000272047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuzmenko AI, Wu H, Bridges JP, McCormack FX. Surfactant lipid peroxidation damages surfactant protein A and inhibits interactions with phospholipid vesicles. J Lipid Res. 2004;45(6):1061–1068. doi: 10.1194/jlr.M300360-JLR200. [DOI] [PubMed] [Google Scholar]
- 74.Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J. 2000;16(3):534–554. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]
- 75.Cho YS, Moon HB. The role of oxidative stress in the pathogenesis of asthma. Allergy Asthma Immunol Res. 2010;2(3):183–187. doi: 10.4168/aair.2010.2.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yao H, Rahman I. Current concepts on oxidative/carbonyl stress, inflammation and epigenetics in pathogenesis of chronic obstructive pulmonary disease. Toxicol Appl Pharmacol. 2011;254(2):72–85. doi: 10.1016/j.taap.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Galli F, Battistoni A, Gambari R, Pompella A, Bragonzi A, Pilolli F, Iuliano L, Piroddi M, Dechecchi MC, Cabrini G, Working Group on Inflammation in Cystic Fibrosis Oxidative stress and antioxidant therapy in cystic fibrosis. Biochim Biophys Acta 1822. 2012;5:690–713. doi: 10.1016/j.bbadis.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 78.Gunther A, Siebert C, Schmidt R, Ziegler S, Grimminger F, Yabut M, Temmesfeld B, Walmrath D, Morr H, Seeger W. Surfactant alterations in severe pneumonia, acute respiratory distress syndrome, and cardiogenic lung edema. Am J Respir Crit Care Med. 1996;153(1):176–184. doi: 10.1164/ajrccm.153.1.8542113. [DOI] [PubMed] [Google Scholar]
- 79.Schmidt R, Markart P, Ruppert C, Wygrecka M, Kuchenbuch T, Walmrath D, Seeger W, Guenther A. Time-dependent changes in pulmonary surfactant function and composition in acute respiratory distress syndrome due to pneumonia or aspiration. Respir Res. 2007;8:55. doi: 10.1186/1465-9921-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y, Manevich Y, Feinstein SI, Fisher AB. Adenovirus-mediated transfer of the 1-cys peroxiredoxin gene to mouse lung protects against hyperoxic injury. Am J Physiol Lung Cell Mol Physiol. 2004;286(6):L1188–L1193. doi: 10.1152/ajplung.00288.2003. [DOI] [PubMed] [Google Scholar]
- 81.Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic Biol Med. 2005;38(11):1422–1432. doi: 10.1016/j.freeradbiomed.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 82.Uhlson C, Harrison K, Allen CB, Ahmad S, White CW, Murphy RC. Oxidized phospholipids derived from ozone-treated lung surfactant extract reduce macrophage and epithelial cell viability. Chem Res Toxicol. 2002;15(7):896–906. doi: 10.1021/tx010183i. [DOI] [PubMed] [Google Scholar]
- 83.Kafoury RM, Pryor WA, Squadrito GL, Salgo MG, Zou X, Friedman M. Induction of inflammatory mediators in human airway epithelial cells by lipid ozonation products. Am J Respir Crit Care Med. 1999;160(6):1934–1942. doi: 10.1164/ajrccm.160.6.9902025. [DOI] [PubMed] [Google Scholar]
- 84.Dahl M, Bauer AK, Arredouani M, Soininen R, Tryggvason K, Kleeberger SR, Kobzik L. Protection against inhaled oxidants through scavenging of oxidized lipids by macrophage receptors MARCO and SR-AI/II. J Clin Investig. 2007;117(3):757–764. doi: 10.1172/JCI29968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang MK, Binder CJ, Torzewski M, Witztum JL. C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: phosphorylcholine of oxidized phospholipids. Proc Natl Acad Sci USA. 2002;99(20):13043–13048. doi: 10.1073/pnas.192399699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gershov D, Kim S, Brot N, Elkon KB. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J Exp Med. 2000;192(9):1353–1364. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Suresh MV, Singh SK, Ferguson DA, Jr, Agrawal A. Human C-reactive protein protects mice from Streptococcus pneumoniae infection without binding to pneumococcal C-polysaccharide. Journal of immunology. 2007;178(2):1158–1163. doi: 10.4049/jimmunol.178.2.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heuertz RM, Piquette CA, Webster RO. Rabbits with elevated serum C-reactive protein exhibit diminished neutrophil infiltration and vascular permeability in C5a-induced alveolitis. Am J Pathol. 1993;142(1):319–328. [PMC free article] [PubMed] [Google Scholar]
- 89.Webster RO, Heuertz R, Xia D, Samols D. Attenuation of complement-mediated acute lung injury in rabbits and transgenic mice by C-reactive protein. Chest. 1994;105(3 Suppl):101S. doi: 10.1378/chest.105.3.101s. [DOI] [PubMed] [Google Scholar]
- 90.Bajwa EK, Khan UA, Januzzi JL, Gong MN, Thompson BT, Christiani DC. Plasma C-reactive protein levels are associated with improved outcome in ARDS. Chest. 2009;136(2):471–480. doi: 10.1378/chest.08-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Akaike T, Ando M, Oda T, Doi T, Ijiri S, Araki S, Maeda H. Dependence on O2-generation by xanthine oxidase of pathogenesis of influenza virus infection in mice. J Clin Investig. 1990;85(3):739–745. doi: 10.1172/JCI114499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buffinton GD, Christen S, Peterhans E, Stocker R. Oxidative stress in lungs of mice infected with influenza A virus. Free Radic Res Commun. 1992;16(2):99–110. doi: 10.3109/10715769209049163. [DOI] [PubMed] [Google Scholar]
- 93.Shirey KA, Lai W, Scott AJ, Lipsky M, Mistry P, Pletneva LM, Karp CL, McAlees J, Gioannini TL, Weiss J, Chen WH, Ernst RK, Rossignol DP, Gusovsky F, Blanco JC, Vogel SN. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature. 2013;497(7450):498–502. doi: 10.1038/nature12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hui EK, Nayak DP, Fogelman AM. D-4F, an apolipoprotein A-I mimetic peptide, inhibits the inflammatory response induced by influenza A infection of human type II pneumocytes. Circulation. 2004;110(20):3252–3258. doi: 10.1161/01.CIR.0000147232.75456.B3. [DOI] [PubMed] [Google Scholar]
- 95.Van Lenten BJ, Wagner AC, Nayak DP, Hama S, Navab M, Fogelman AM. High-density lipoprotein loses its anti-inflammatory properties during acute influenza A infection. Circulation. 2001;103(18):2283–2288. doi: 10.1161/01.cir.103.18.2283. [DOI] [PubMed] [Google Scholar]
- 96.Van Lenten BJ, Wagner AC, Anantharamaiah GM, Garber DW, Fishbein MC, Adhikary L, Nayak DP, Hama S, Navab M, Fogelman AM. Influenza infection promotes macrophage traffic into arteries of mice that is prevented by D-4F, an apolipoprotein A-I mimetic peptide. Circulation. 2002;106(9):1127–1132. doi: 10.1161/01.cir.0000030182.35880.3e. [DOI] [PubMed] [Google Scholar]
- 97.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(25):2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 98.Clayton TC, Thompson M, Meade TW. Recent respiratory infection and risk of cardiovascular disease: case-control study through a general practice database. Eur Heart J. 2008;29(1):96–103. doi: 10.1093/eurheartj/ehm516. [DOI] [PubMed] [Google Scholar]
- 99.Lee YH, Lai CL, Hsieh SH, Shieh CC, Huang LM, Wu-Hsieh BA. Influenza A virus induction of oxidative stress and MMP-9 is associated with severe lung pathology in a mouse model. Virus Res. 2013;178(2):411–422. doi: 10.1016/j.virusres.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 100.Matt U, Warszawska JM, Bauer M, Dietl W, Mesteri I, Doninger B, Haslinger I, Schabbauer G, Perkmann T, Binder CJ, Reingruber S, Petzelbauer P, Knapp S. Bbeta(15-42) protects against acid-induced acute lung injury and secondary pseudomonas pneumonia in vivo. Am J Respir Crit Care Med. 2009;180(12):1208–1217. doi: 10.1164/rccm.200904-0626OC. [DOI] [PubMed] [Google Scholar]
- 101.Thimmulappa RK, Gang X, Kim JH, Sussan TE, Witztum JL, Biswal S. Oxidized phospholipids impair pulmonary antibacterial defenses: evidence in mice exposed to cigarette smoke. Biochem Biophys Res Commun. 2012;426(2):253–259. doi: 10.1016/j.bbrc.2012.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nonas S, Miller I, Kawkitinarong K, Chatchavalvanich S, Gorshkova I, Bochkov VN, Leitinger N, Natarajan V, Garcia JG, Birukov KG. Oxidized phospholipids reduce vascular leak and inflammation in rat model of acute lung injury. Am J Respir Crit Care Med. 2006;173(10):1130–1138. doi: 10.1164/rccm.200511-1737OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Birukova AA, Fu P, Chatchavalvanich S, Burdette D, Oskolkova O, Bochkov VN, Birukov KG. Polar head groups are important for barrier-protective effects of oxidized phospholipids on pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2007;292(4):L924–L935. doi: 10.1152/ajplung.00395.2006. [DOI] [PubMed] [Google Scholar]
- 104.Nonas S, Birukova AA, Fu P, Xing J, Chatchavalvanich S, Bochkov VN, Leitinger N, Garcia JG, Birukov KG. Oxidized phospholipids reduce ventilator-induced vascular leak and inflammation in vivo. Crit Care. 2008;12(1):R27. doi: 10.1186/cc6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Birukova AA, Singleton PA, Gawlak G, Tian X, Mirzapoiazova T, Mambetsariev B, Dubrovskyi O, Oskolkova OV, Bochkov VN, Birukov KG (2014) GRP78 is a novel receptor initiating a vascular barrier protective response to oxidized phospholipids. Mol Biol Cell 25(13):2006–2016 [DOI] [PMC free article] [PubMed]
- 106.Lusis AJ. Atherosclerosis. Nature. 2000;407(6801):233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Walker SL, Lockwood DN. Leprosy. Clin Dermatol. 2007;25(2):165–172. doi: 10.1016/j.clindermatol.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 108.Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BR, Modlin RL. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 109.Cruz D, Watson AD, Miller CS, Montoya D, Ochoa MT, Sieling PA, Gutierrez MA, Navab M, Reddy ST, Witztum JL, Fogelman AM, Rea TH, Eisenberg D, Berliner J, Modlin RL. Host-derived oxidized phospholipids and HDL regulate innate immunity in human leprosy. J Clin Investig. 2008;118(8):2917–2928. doi: 10.1172/JCI34189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 111.Bova IY, Bornstein NM, Korczyn AD. Acute infection as a risk factor for ischemic stroke. Stroke. 1996;27(12):2204–2206. doi: 10.1161/01.str.27.12.2204. [DOI] [PubMed] [Google Scholar]