Abstract
The aim of this study was to explore the predictive implications of the composition of immune cell populations prior to lenalidomide plus high-dose dexamethasone (Len-Dex) initiation for the occurrence of infections. We prospectively examined immune cell populations in peripheral blood taken at baseline of lenalidomide plus low-dose dexamethasone (Len-dex) therapy and reviewed clinical and microbiology records in 90 patients with refractory/relapsed multiple myeloma (RRMM). Risk factors for infection were analyzed using logistic regression. During a median of 11 cycles of Len-dex treatment, 52 (57.8%) patients experienced at least 1 infection episode. Of a total of 92 episodes of infection, 58 (63%) episodes were clinically defined, 29 (31.5%) episodes were microbiologically defined, and 5 (5.4%) episodes were fever of unknown origin. Severe episodes were more frequently observed during the first 3 cycles. After adjusting for risk factors for infection based on univariate analyses, multivariate analyses showed that lower Hb (< 10 g/dL) was a clinically independent factor associated with occurrence of infections. Lower frequency (P = 0.044) and absolute count (P = 0.014) of circulating CD3+CD4+CD161+ cells prior to Len-dex treatment were also associated with the occurrence of infection, especially during the first 3 cycles of Len-dex therapy. In addition to several clinical predictive factors, we found that CD3+CD4+CD161+ cells may provide additional information for predicting the occurrence of infection in the early period of Len-dex therapy.
Electronic supplementary material
The online version of this article (10.1007/s00277-018-3401-y) contains supplementary material, which is available to authorized users.
Keywords: Multiple myeloma, Lenalidomide, Low-dose dexamethasone, Infection, CD4+CD161+ T cells
Introduction
Multiple myeloma (MM) is a clonal plasma cell neoplasm that presents variable clinical manifestations often referred to as CRAB symptoms (hyper-Calcemia, Renal insufficiency, Anemia, and/or Bone lesions) [1]. In addition to CRAB symptoms, infection is the leading cause of morbidity and mortality in MM; 26–45% of early deaths are attributable to infection [2, 3].
The increased risk of infection seen in MM is due to disease-related immune deficits involving humoral immunity and various immune system components, including T cells [4–6], dendritic cells [7], and natural killer cells [8]. Treatment-related toxicity and the use of high-dose corticosteroid therapy also increase susceptibility to infection. A large longitudinal cohort study that The et al. conducted in patients with MM receiving current standard treatment with proteasome inhibitors (PIs), immune-modulatory drugs (IMiDs), and autologous stem cell transplantation revealed high-risk factors for infection such as late disease, multiple treatments, intensive combination systemic chemotherapy, and corticosteroid dose [9].
Despite advances in the treatment of MM, most MM patients will nonetheless ultimately relapse or become refractory to current treatment, and so appropriate therapeutic options for patients with relapsed-refractory MM (RRMM) remain a focus of research. The treatment paradigm has shifted with the use of small biologic molecules (PIs, IMiDs and HDAC inhibitors). The effects of these drugs on the immune system are distinct from those of conventional anti-myeloma agents, resulting in changes in the types and patterns of infections. In particular, the combination of lenalidomide and high-dose dexamethasone (Len-Dex) demonstrated efficacy and safety in patients with relapsed/refractory MM in two large, well-designed pivotal clinical trials [10, 11]. After that, lenalidomide plus low-dose dexamethasone (Len-dex) led to better overall survival and lower toxicity than Len-Dex in patients with newly diagnosed MM [12] and also remains the standard of care in the relapsed or refractory setting. Therefore, we analyzed the patterns and risks of infection in patients with RRMM during Len-dex treatment and sought to identify immune cell subtypes related to the occurrence of infection using multicolor flow cytometric analysis of fresh peripheral blood mononuclear cells (PBMCs) isolated from RRMM patients prior to Len-dex initiation.
Patients and methods
Patients and treatment procedures
Patients with RRMM who received salvage treatment with Len-dex at our institution were eligible for this study. The therapy regimen consisted of lenalidomide 25 mg once daily orally on days 1–21 of each 28-day cycle plus low-dose dexamethasone 40 mg per day weekly, and dose modification was performed according to recommendations [13]. Thrombosis prophylaxis with low-dose aspirin was used in all patients. Antibacterial prophylaxis (trimethoprim/sulfamethoxazole) was administered, but prophylactic antifungal and antiviral agents were not used. To identify novel immune cell biomarkers predictive for infections, we prospectively obtained peripheral blood samples at baseline prior to Len-dex treatment. All clinical data were prospectively collected at the time of Len-dex initiation except for cytogenetic and international staging system findings, which were taken from the data established at the time of diagnosis. Written informed consent was obtained from each patient before participation in this study. This study was approved by the Institutional Review Board of the Catholic University of Korea and was conducted in accordance with the Declaration of Helsinki.
Isolation of mononuclear cells and flow cytometric analysis
Blood samples for cell population analysis were collected immediately before Len-dex initiation [14]. PBMCs were isolated from whole blood samples (10 mL) collected in EDTA-coated tubes by density centrifugation using Ficoll-Paque. PBMCs were processed immediately for analysis. Flow cytometry was used to evaluate the percentages of CD3+, CD4+CD161+, and CD8+CD161+ T cells, natural killer (NK) cells (CD16+CD56+), and myeloid-derived suppressor cells (MDSCs) [Lin−HLA-DR−CD11b+CD33+ (granulocytic) and HLA-DR−CD14+ (monocytic)]. Anti-CD3-allophycocyanin (APC), anti-CD4-fluorescein isothiocyanate (FITC), anti-CD8-phycoerythrin (PE), anti-CD161-PerCP-Cy5.5, anti-CD16-FITC, anti-CD56-PE, and anti-CD14-APC monoclonal antibodies (mAbs) were purchased from eBioscience (San Diego, CA, USA). Anti-lineage cocktail 1 (Lin 1)-FITC, anti-HLA-DR-PerCP, rat anti-mouse CD11b-APC-Cy™7, and mouse anti-human CD33-V450 (BD BioSciences) mAbs were purchased from BD Biosciences (San Jose, CA). CD4+CD161+ and CD8+CD161+ T cells were gated on CD3+ cells and are expressed as percentages of lymphocytes. The frequency of HLA-DR−Lin−CD11b+CD33+ and HLA-DR−CD14+ MDSCs is expressed as the percentage of total PBMCs. Flow cytometry was performed using a FACS LSR Fortessa (BD Biosciences).
Definitions
We investigated infectious complications within every cycle (1 cycle = 28 days) after Len-dex treatment, and the data from that period were analyzed. Infections were classified as microbiologically defined, clinically defined and fever of unknown origin (FUO) as proposed by the Immunocompromised Host Society [15, 16]. Infection types were assessed according to a previous report [9]. Severity of infections was graded using the National Cancer Institute Common Toxicity Criteria grading scheme and grade 1–2 infections were defined as low severity and grade 3–5 infections as high severity. Mortality was defined as infection-related death without refractory or progressive disease. Treatment responses were assessed according to the criteria from the International Myeloma Working Group (IMWG) [17].
Statistical analysis
The study objectives were to determine the infection nature, including severity, type, and timing, as well as clinical predictors of infection in RRMM patients receiving Len-dex treatment. In addition, the predictive implications of the composition of immune cell populations prior to Len-dex initiation were explored. The two-tailed Student’s t test was used to analyze continuous variables between two groups. Potential risk factors for the occurrence of infections (≥ grade 3) during the first 3 cycles in RRMM patients receiving Len-dex treatment were assessed using logistic regression analysis. Optimal cutoffs for continuous variables were identified using receiver operating characteristic (ROC) curve analysis. To investigate whether the identified markers were independent predictors, covariates with a P value of less than 0.1 in univariate analyses were included in multivariate analysis.
Results
Patient characteristics
A total of 90 RRMM patients (48 men and 42 women) with a median age of 61 years (range, 29–84 years) who were given Len-dex between January 2014 and February 2015 were analyzed. Patient characteristics are summarized in Table 1. Median time from diagnosis to Len-dex treatment was 3.2 years (0.5–9.5), and the median number of previous treatment lines was 2.0 (1–7) with 41% having received more than two previous treatment lines. All of the patients were previously exposed to bortezomib, with 54 (60%) patients receiving both bortezomib and thalidomide. Among all patients, 54% had undergone prior autologous stem cell transplantation. With a median administered cycle number of 11 (range, 1–24 cycles), the overall response rate was 80%, and very good partial response or greater occurred in 44% of all 90 patients. Two patients were not evaluable: one was lost to follow-up and the other discontinued Len-dex because of adverse events before evaluation. With a median follow-up of 19.9 months (range, 0.4–24.1 months) for survivors, median progression-free survival and overall survival were 11.7 months and not reached, respectively. The most common cause of discontinuation of treatment was disease progression (44%), followed by adverse events (17%) (Table 2).
Table 1.
Characteristics of patients
| Characteristics | All patients (N = 90) (%) |
|---|---|
| Age, years; median (range) | 61 (29–84) |
| Sex (M/F) | 48 (53)/42 (47) |
| Serum M-protein | |
| IgG | 47 (52) |
| IgA | 22 (24) |
| Light chain, kappa | 7 (8) |
| Light chain, lambda | 11 (12) |
| Others | 3(3) |
| Durie-Salmon stage | |
| I/II/III | 7 (8)/5 (5)/78 (87) |
| ISS stage | |
| I/II/III/NA | 21 (23)/27 (30)/29 (32)/13 (14) |
| Cytogeneticsa | |
| Standard risk/high risk/NA | 61 (68)/20 (22)/9 (10) |
| Myeloma bone disease on plain radiographs, yes/no | 74 (82)/16 (18) |
| Time since diagnosis, years; median (range) | 3.2 (0.5–9.5) |
| Previous number of therapiesb, median (range) | 2 (1–7) |
| ≤ 2 | 53 (59) |
| > 2 | 37 (41) |
| Previous ASCT | 49 (54) |
| Previous therapy before Len-dex | |
| Bortezomib-based regimens | 36 (40) |
| Both bortezomib- and thalidomide-based regimens | 54 (60) |
| Laboratory data | |
| Serum M-proteinc, g/dL, median (range) | 2.36 (1.02–6.06) |
| Difference between serum iFLC and uninvolved FLC, median (range) | 237.1 (5.2–34,151.9) |
| Hb, g/dL, median (range) | 11.0 (6.8–15.3) |
| Ca, mg/dL, median (range) | 9.0 (7.2–12.1) |
| LDH, U/L, median (range) | 395.5 (153–1078) |
ASCT autologous stem cell transplantation, iFLC involved free light chain, Len-dex lenalidomide and low-dose dexamethasone, NA not available, LDH lactate dehydrogenase
aHigh-risk cytogenetics is defined as hypodiploid or deletion of chr13 on conventional cytogenetics or presence of t(4;14), t(14;16), and -17p on fluorescent in situ hybridization and/or conventional cytogenetics. All other cytogenetic abnormalities were considered standard risk
bInduction + ASCT was considered one therapeutic line
cPatients with measurable serum M protein of at least 1 g per 100 mL were included
Table 2.
Response to lenalidomide and low-dose dexamethasone
| All patients (N = 90) (%) | |
|---|---|
| Continuing treatment | 20 (22) |
| Discontinued treatment | |
| Failure to achieve ≥ PR after 4 cycles | 6 (7) |
| Progression | 40 (44) |
| Drug-related adverse event | 15 (17) |
| Death | 4 (4) |
| Follow-up loss | 3 (3) |
| ASCT | 2 (2) |
| Response | |
| Overall response | 72 (80) |
| VGPR or greater | 40 (44) |
| CR | 22 (24) |
| VGPR | 18 (20) |
| PR | 32 (36) |
| SD | 7 (8) |
| PD | 9 (10) |
| NA | 2 (2) |
Administered cycles, median (range): 11 cycles (1–24)
ASCT autologous stem cell transplantation, CR complete response, NA not available, PD progressive disease, PR partial response, SD stable disease, VGPR very good partial response
Profile of infection episodes
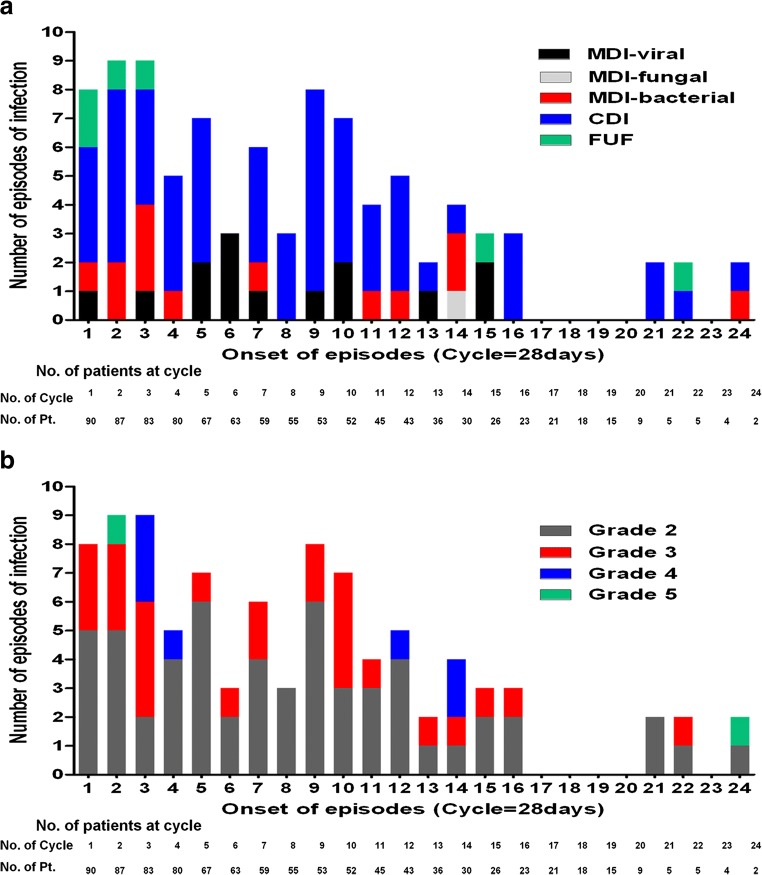
Of the 90 patients, there were 52 patients with at least 1 episode of infection (≥ grade 2); 30 patients with 1 episode, 13 with 2, 1 with 3, 7 with 4, and 1 with 5; and 38 patients had no episodes of infection. High severity infections (grade 3–5) developed in 30 (33.3%) patients, including 14 patients who experienced severe infections during the first 3 cycles.
Next, a total of 92 episodes of infection during Len-dex treatment were evaluated. Of these, 29 (31.5%) were microbiologically defined, 58 (63%) clinically defined, and 5 (5.4%) categorized as FUO. Of 29 microbiologically defined infections, 48.3, 3.4, and 48.3% were bacterial, fungal, and viral in origin, respectively. Variable pathogens were identified in 14 episodes of bacterial origin, including 5 episodes caused by multiple organisms; Escherichia coli (n = 5) was the most frequently isolated organism followed by Pseudomonas aeruginosa (n = 4), and Streptococcus pneumonia (n = 4), Klebsiella pneumonia (n = 2). Micrococcus species (n = 1), Staphylococcus aureus (n = 1), Staphylococcus hominis (n = 1), Streptococcus viridans (n = 1), Clostridium difficile (n = 1), Acinetobacter baumannii (n = 1) were isolated. Of 14 microbiologically defined viral infection episodes, 5 episodes were herpes zoster and 9 episodes occurred due to rhinovirus (n = 3), influenza (n = 3), parainfluenza (n = 2), and coronavirus (n = 1). One episode was caused by Pneumocystis jirovecii which was classified as a fungal infection. The respiratory tract (n = 71) was the most common site of infection, followed by skin and soft tissue infection (n = 7). Multiple site infection (n = 7) was also frequently observed, including bacteremia (n = 4). The majority of episodes (57 of 92) were of grade 2 severity, but 35 episodes were of high severity. Admission to the intensive care unit was required in 9.8% of infection episodes, of which two infection episodes resulted in death. Characteristics of episodes of infection are described in Table 3.
Table 3.
Characteristics of episodes of infection
| Characteristics of episodes of infection | Number of infections = 92 (%) |
|---|---|
| Categories of infection | |
| Microbiologically defined—virala | 14 (15.2) |
| Microbiologically defined—fungal | 1 (1.1) |
| Microbiologically defined—bacterial | 14 (15.2) |
| Clinically defined | 58 (63.0) |
| Fever of unknown focus | 5 (5.4) |
| Severity of infection | |
| Grade 2 | 57 (62.0) |
| Grade 3 | 26 (28.3) |
| Grade 4 | 7 (7.6) |
| Grade 5 | 2 (2.2) |
| Site of infection | |
| Respiratory tract | 71 (77.2) |
| Urinary tract | 2 (2.2) |
| Gastrointestinal tract | 1 (1.1) |
| Skin and soft tissue | 7 (7.6) |
| Multipleb | 7 (7.6) |
| Unknown | 4 (4.3) |
| Intensive care unit admission | |
| Yes | 9 (9.8) |
| No | 83 (90.2) |
| Mortality by episode of infection2 (2.2) | 2 (2.2) |
aFive patients who developed Herpes zoster were included
bFour patients had bacteremia
During the first 3 cycles, microbiologically defined infections (n = 8), clinically defined infections (n = 14), and FUO (n = 4) occurred. Fourteen (53.8%) of 26 episodes of infection occurring during the first 3 cycles were of high severity (Fig. 1).
Fig. 1.
Type (a) and severity (b) of infections across Len-dex cycles
Relationship between immune cell populations and high severity infections
Through longitudinal observation of the epidemiology of infection in RRMM patients receiving Len-dex treatment, we observed that the majority of high severity infections occurred in the first 3 cycles. Accordingly, we compared the frequency and absolute count of various immune cell populations in patients who developed infection (≥ grade 3) during the first 3 cycles of Len-dex treatment with those who did not (Table 4). The frequency of CD3+CD4+CD161+ T cells tended to decrease from 4.00 ± 0.28% at baseline to 2.86 ± 0.26% (P = 0.005) and 2.72 ± 0.29% (P = 0.004) after the 2nd and 3rd cycles of therapy, respectively (Fig. 2). The frequency and absolute count of CD3+CD4+CD161+ cells were lower in patients who developed infection (≥ grade 3) during the first 3 cycles (4.26 ± 0.31% vs. 2.82 ± 0.47%, P = 0.047 for the frequency, and 83.78 ± 6.96 vs. 42.41 ± 6.49 cells/μL, P < 0.001 for the absolute count). Patients with infection (≥ grade 3) during the first 3 cycles tended to have a lower absolute count of CD3+ cells (1092.49 ± 67.87 vs. 810.15 ± 128.35 cells/μL, P = 0.078). In contrast, the frequency of CD3+ cells was not associated with infection (≥ grade 3) during the first 3 cycles.
Table 4.
Comparison of cell populations according to the occurrence of infection (≥ grade 3) during the first 3 cycles of Len-dex treatment
| Parameters | Infection (≥ grade 3) during early 3 cycles | P value | |
|---|---|---|---|
| No (n = 76) | Yes (n = 14) | ||
| Frequency (%), mean ± SE | |||
| CD3+ | 52.39 ± 1.82 | 48.23 ± 5.58 | 0.378 |
| CD4+ | 22.79 ± 1.44 | 21.09 ± 2.99 | 0.619 |
| CD8+ | 28.70 ± 1.48 | 25.66 ± 3.39 | 0.395 |
| CD3+CD4+CD161+ (on lymphocyte) | 4.26 ± 0.31 | 2.82 ± 0.47 | 0.047 |
| CD3+CD8+CD161+ (on lymphocyte) | 9.98 ± 0.72 | 9.58 ± 1.25 | 0.812 |
| NK cell (CD16+CD56+) | 11.35 ± 0.87 | 0.93 ± 1.41 | 0.418 |
| G-MDSC | 0.11 ± 0.01 | 0.13 ± 0.04 | 0.582 |
| M-MDSC | 0.32 ± 0.05 | 0.46 ± 0.11 | 0.231 |
| Absolute count, cells/μL, mean ± SE | |||
| CD3+ | 1092.49 ± 67.87 | 810.15 ± 128.35 | 0.078 |
| CD4+ | 468.61 ± 38.34 | 349.14 ± 60.52 | 0.176 |
| CD8+ | 605.57 ± 45.43 | 436.87 ± 81.43 | 0.113 |
| CD3+CD4+CD161+ | 83.78 ± 6.96 | 42.41 ± 6.49 | < 0.001 |
| CD3+CD8+CD161+ | 200.23 ± 17.10 | 151.05 ± 21.52 | 0.203 |
| NK cell (CD16+CD56+) | 226.46 ± 19.89 | 157.47 ± 22.00 | 0.123 |
| G-MDSC | 2.02 ± 0.20 | 2.16 ± 0.63 | 0.796 |
| M-MDSC | 6.26 ± 0.87 | 7.38 ± 1.83 | 0.586 |
G granulocytic, Len-dex lenalidomide and low-dose dexamethasone, M monocytic, MDSC myeloid-derived suppressor cell, NDMM newly diagnosed multiple myeloma, NK natural killer, RRMM refractory/relapsed multiple myeloma, SE standard error
Fig. 2.
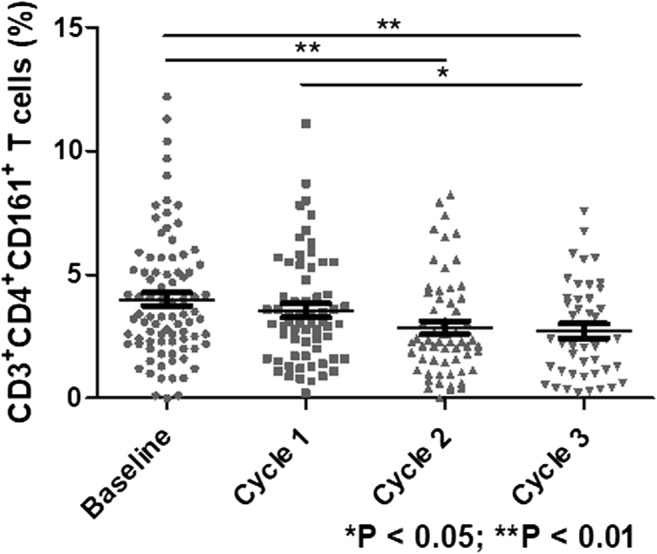
Serial changes in CD3+CD4+CD161+ T cells according to Len-dex therapy. The frequency of CD3+CD4+CD161+ T cells at the time of Len-dex initiation (baseline, n = 90) and after completion of 1st, 2nd, and 3th cycles of administration (n = 64, 59, and 45, respectively) was analyzed. At baseline, the CD3+CD4+CD161+ T cell frequency was 4.00 ± 0.28%, which significantly decreased to 2.86 ± 0.26% (P = 0.005) and 2.72 ± 0.29% (P = 0.004) after the 2nd and 3rd cycles of therapy, respectively. Data are presented as mean ± SEM and t tests were used to compare the continuous variables
Risk factors for the occurrence of high severity infections
To identify risk factors for the occurrence of high severity infections in 90 patients, we evaluated clinical and myeloma-associated variables in univariate analyses (Supplementary Table 1). Higher levels of serum creatinine (≥ 2 mg/dL; compared to < 2 mg/dL, P = 0.037) and lower levels of hemoglobin (P < 0.001) and serum albumin (P = 0.061) were associated with the occurrence of high severity infections. These potential risk factors were entered into multivariate logistic regression models with immune parameters, including the frequency and absolute count of CD3+CD4+CD161+ cells and the absolute count of CD3+ cells (Table 5). High severity infection during the first 3 cycles of Len-dex treatment was significantly predicted by a lower frequency (RR 0.18, P = 0.044) and absolute count (RR 0.13, P = 0.014) of CD3+CD4+CD161+ cells.
Table 5.
Multivariate analyses of predictive factors for the occurrence of infection (≥ grade 3) during the first 3 cycles of Len-dex treatment
| Multivariate variables | Model I | Model II | Model III | |||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| Frequency of CD3+CD4+CD161+ (%) | ||||||
| < 3.2 | 1 | |||||
| ≥ 3.2 | 0.18 (0.04–0.96) | 0.044 | – | – | – | – |
| Absolute count of CD3+CD4+CD161+ (cells/μL) | ||||||
| < 43 | – | – | 1 | – | – | |
| ≥ 43 | – | – | 0.13 (0.03–0.66) | 0.014 | – | – |
| Absolute count of CD3+ (cells/μL) | ||||||
| < 640 | – | – | – | – | 1 | |
| ≥ 640 | – | – | – | – | 0.66 (0.16–2.75) | 0.564 |
| Hb (g/dL), (≥ 10 vs. < 10) | 0.02 (0.00–0.17) | < 0.001 | 0.04 (0.01–0.26) | 0.001 | 0.05 (0.01–0.27) | 0.001 |
| Albumin (mg/dL), (≥ 3.5 vs. < 3.5) | 2.89 (0.43–19.34) | 0.247 | 3.97 (0.55–28.82) | 0.172 | 2.13 (0.37–12.33) | 0.399 |
| Creatinine (mg/dL), (≥ 2 vs. < 2) | 2.12 (0.31–14.62) | 0.446 | 1.78 (0.22–14.21) | 0.586 | 3.64 (0.62–21.17) | 0.151 |
CI confidence interval
Discussion
Although the efficacy and toxicity of lenalidomide for patients with RRMM are well documented, infection remains a leading complication. The results from two pivotal clinical trials found that the incidence rate of infection (≥ grade 3) was approximately 10 to 20% in patients with RRMM receiving Len-Dex [10, 11]. Although the use of low-dose dexamethasone has reduced toxicity while preserving the efficacy of Len-Dex [12], Kim et al. reported incidence rates (≥ grade 3) of febrile neutropenia, sepsis, and pneumonia of 4.6, 2.7, and 9.1%, respectively, in 110 Korean patients with RRMM in which dexamethasone 160 mg per cycle was most commonly used [18]. Moreover, the patterns and risks of infection in patients with RRMM during Len-dex treatment remain unclear.
Herein, we demonstrated that 52 (57.8%) out of 90 patients experienced at least 1 infection episode during Len-dex treatment, and severe episodes were frequently observed during the first 3 cycles. Our observations of an increased risk of infections in the early treatment period are consistent with findings from previous studies. Many studies, which were conducted before the development of the novel agents, have demonstrated an increased risk of infections in early courses, particularly within 4–6 months following treatment, and a decreased risk of infections after achieving a favorable response to treatment [19–21]. In patients with MM receiving bortezomib-based treatment, early course therapy was associated with the development of severe bacterial infections [22]. In addition, considering the disease-related and treatment-related immune deficits in patients with RRMM, we explored immune cell subsets in association with the occurrence of infection in order to evaluate their emerging potential as cell biomarkers. In this study, lower Hb (< 10 g/dL) was a clinically independent factor associated with the occurrence of infections, and lower frequency (P = 0.044) and absolute count (P = 0.014) of CD3+CD4+CD161+ cells in the peripheral blood prior to Len-dex were associated with the occurrence of infection, especially during the first 3 cycles of Len-dex therapy.
Recent studies have reported an increased risk of infections with the use of novel agents, suggesting the need for further exploration of these relationships [3, 23]. Kleber et al. showed a risk of varicella zoster virus infection in lenalidomide-treated MM patients as well as bortezomib treatment or transplants and demonstrated risk factors including suppressed lymphocyte subsets, substantial cell-mediated immune defects, and compromised humoral immune response [23]. Our observations provide specific data on the type of infection and factors affecting infectious complications in patients treated with Len-dex. While the lower frequency and absolute count of CD3+CD4+CD161+ cells were associated with an increased risk of infection during the early period of therapy, CD3+, CD4+, CD8+, NK cells, and MDSCs were not associated with an increased risk of infection. Among various clinical parameters, serum creatinine, Hb, and serum albumin levels were identified as potential factors related to the occurrence of infection.
A unique finding in our study was the suggestion of an immune subtype as a predictive biomarker for the occurrence of infection, especially during the early period of therapy. CD161 is a type II transmembrane glycoprotein with characteristics of the C-type lectin superfamily. This receptor is expressed on NK cells, 25% of all adult peripheral T cells, more than 90% of all peripheral blood monocytes, and on in vitro-derived dendritic cells [24]. We previously evaluated the association of CD161-expressing T cells with the development of acute graft-versus-host disease (AGVHD) after allogeneic SCT and found that a low proportion of CD8+CD161+ cells and a high ratio of CD4+CD161+ cells to CD8+CD161+ cells from peripheral blood at engraftment were associated with the occurrence of AGVHD, with evidence of higher expression of the Th17 transcription factor RORγT in CD4+CD161+ T cells rather than CD8+CD161+ T cells [25]. Within the CD4+ subset, CD161 expression is associated with IL-17 production [26], but other IL-17-producing T cells, such as CD8+ and CD4−CD8− double-negative T cells also, express CD161 [27]. Until recently, there has been an agreement that CD161 receptors on NK cells play an inhibitory role [28–30], whereas the role on T cells lacks consensus. Recently, we reported that the proportion of pretransplant circulating CD3+CD4+CD161+ cells in the allogeneic stem cell transplant setting was associated with the occurrence of neutropenic infections, which suggested the importance of CD3+CD4+CD161+ cells in the rapid immune response to microorganisms before establishment of the post-transplant immune system [31]. The relation between pretransplant CD3+CD4+CD161+ cells and the occurrence of early complications was also seen in autologous stem cell transplant setting [32]. In this study, we found that the presence of preexisting CD3+CD4+CD161+ cells may play an important role in decreasing the risk of severe infection in patients with RRMM during Len-dex therapy.
Several studies reported lymphopenia as a marker of bacterial infection [22, 33] and viral and fungal infections [34] in MM patients receiving bortezomib therapy, suggesting that lymphopenia indicates a preexisting immunosuppressed condition. However, there is no data on immune subtypes as a predictive factor for infection in RRMM patients treated with Len-dex. Evidence about the prophylactic use of antibiotics in non-neutropenic MM patients is still inconclusive [35], and prophylactic antibiotics raise the concern of the emergence of antibiotic resistance [36]. Therefore, the prophylactic use of antibiotics may not be needed for all MM patients, but rather only by patients at high risk for the occurrence of infection. Our results can provide useful information for identifying such high-risk patients who may need the prophylactic use of antiviral and antibiotic agents, especially early during the treatment course for their malignancy.
In conclusion, we found that both patient- and treatment-related factors affected the severity, type, and timing of infection in patients with RRMM receiving Len-dex treatment. In particular, a lower frequency and absolute count of CD3+CD4+CD161+ cells were associated with the occurrence of infection in the early period of Len-dex therapy. Taking into account risk factors for the occurrence of infection could provide significant information to guide the prophylactic use of antibiotics or increased monitoring for developing infections during Len-dex treatment.
Electronic supplementary material
(DOCX 17.7 kb)
Acknowledgements
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (#HI16C0047).
Compliance with ethical standards
Written informed consent was obtained from each patient before participation in this study. This study was approved by the Institutional Review Board of the Catholic University of Korea and was conducted in accordance with the Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 2.Augustson BM, Begum G, Dunn JA, Barth NJ, Davies F, Morgan G, Behrens J, Smith A, Child JA, Drayson MT. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002—Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23:9219–9226. doi: 10.1200/JCO.2005.03.2086. [DOI] [PubMed] [Google Scholar]
- 3.Blimark C, Holmberg E, Mellqvist UH, Landgren O, Bjorkholm M, Hultcrantz M, Kjellander C, Turesson I, Kristinsson SY. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica. 2015;100:107–113. doi: 10.3324/haematol.2014.107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mills KH, Cawley JC. Abnormal monoclonal antibody-defined helper/suppressor T-cell subpopulations in multiple myeloma: relationship to treatment and clinical stage. Br J Haematol. 1983;53:271–275. doi: 10.1111/j.1365-2141.1983.tb02021.x. [DOI] [PubMed] [Google Scholar]
- 5.Ogawara H, Handa H, Yamazaki T, Toda T, Yoshida K, Nishimoto N, Al-ma'Quol WH, Kaneko Y, Matsushima T, Tsukamoto N, Nojima Y, Matsumoto M, Sawamura M, Murakami H. High Th1/Th2 ratio in patients with multiple myeloma. Leuk Res. 2005;29:135–140. doi: 10.1016/j.leukres.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Andres M, Almeida J, Martin-Ayuso M, Moro MJ, Martin-Nunez G, Galende J, Hernandez J, Mateo G, San Miguel JF, Orfao A. Characterization of bone marrow T cells in monoclonal gammopathy of undetermined significance, multiple myeloma, and plasma cell leukemia demonstrates increased infiltration by cytotoxic/Th1 T cells demonstrating a squed TCR-Vbeta repertoire. Cancer. 2006;106:1296–1305. doi: 10.1002/cncr.21746. [DOI] [PubMed] [Google Scholar]
- 7.Brown RD, Pope B, Murray A, Esdale W, Sze DM, Gibson J, Ho PJ, Hart D, Joshua D. Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7-1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-beta1 and interleukin-10. Blood. 2001;98:2992–2998. doi: 10.1182/blood.V98.10.2992. [DOI] [PubMed] [Google Scholar]
- 8.Jurisic V, Srdic T, Konjevic G, Markovic O, Colovic M. Clinical stage-depending decrease of NK cell activity in multiple myeloma patients. Med Oncol. 2007;24:312–317. doi: 10.1007/s12032-007-0007-y. [DOI] [PubMed] [Google Scholar]
- 9.Teh BW, Harrison SJ, Worth LJ, Spelman T, Thursky KA, Slavin MA. Risks, severity and timing of infections in patients with multiple myeloma: a longitudinal cohort study in the era of immunomodulatory drug therapy. Br J Haematol. 2015;171:100–108. doi: 10.1111/bjh.13532. [DOI] [PubMed] [Google Scholar]
- 10.Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, Siegel D, Borrello I, Rajkumar SV, Chanan-Khan AA, Lonial S, Yu Z, Patin J, Olesnyckyj M, Zeldis JB, Knight RD. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 11.Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A, San Miguel J, Hellmann A, Facon T, Foa R, Corso A, Masliak Z, Olesnyckyj M, Yu Z, Patin J, Zeldis JB, Knight RD. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 12.Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, Abonour R, Siegel DS, Katz M, Greipp PR. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11:29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimopoulos MA, Palumbo A, Attal M, Beksac M, Davies FE, Delforge M, Einsele H, Hajek R, Harousseau JL, da Costa FL, Ludwig H, Mellqvist UH, Morgan GJ, San-Miguel JF, Zweegman S, Sonneveld P. Optimizing the use of lenalidomide in relapsed or refractory multiple myeloma: consensus statement. Leukemia. 2011;25:749–760. doi: 10.1038/leu.2011.3. [DOI] [PubMed] [Google Scholar]
- 14.Lee SE, Lim JY, Ryu DB, Kim TW, Yoon JH, Cho BS, Eom KS, Kim YJ, Kim HJ, Lee S, Cho SG, Kim DW, Lee JW, Min WS, Kim M, Min CK. Circulating immune cell phenotype can predict the outcome of lenalidomide plus low-dose dexamethasone treatment in patients with refractory/relapsed multiple myeloma. Cancer Immunol Immunother. 2016;65:983–994. doi: 10.1007/s00262-016-1861-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Straka C, Sandherr M, Salwender H, Wandt H, Metzner B, Hubel K, Silling G, Hentrich M, Franke D, Schwerdtfeger R, Freund M, Sezer O, Giagounidis A, Ehninger G, Grimminger W, Engert A, Schlimok G, Scheid C, Hellmann P, Heinisch H, Einsele H, Hinke A, Emmerich B. Testing G-CSF responsiveness predicts the individual susceptibility to infection and consecutive treatment in recipients of high-dose chemotherapy. Blood. 2011;117:2121–2128. doi: 10.1182/blood-2010-06-290080. [DOI] [PubMed] [Google Scholar]
- 16.From the Immunocompromised Host Society. The design, analysis, and reporting of clinical trials on the empirical antibiotic management of the neutropenic patient. Report of a consensus panel (1990). J Infect Dis 161:397–401 [DOI] [PubMed]
- 17.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim K, Kim SJ, Voelter V, Suh C, Yoon SS, Lee JJ, Kwak JY, Ryoo HM, Kim YS, Moon JH, Park SK, Kim SH, Mun YC, Kim JS, Eom HS, Jo DY, Jun HJ, Kim KH, Lee JO, Lee JH, Min CK. Lenalidomide with dexamethasone treatment for relapsed/refractory myeloma patients in Korea-experience from 110 patients. Ann Hematol. 2014;93:113–121. doi: 10.1007/s00277-013-1893-z. [DOI] [PubMed] [Google Scholar]
- 19.Cesana C, Nosari AM, Klersy C, Miqueleiz S, Rossi V, Ferrando P, Valentini M, Barbarano L, Morra E. Risk factors for the development of bacterial infections in multiple myeloma treated with two different vincristine-adriamycin-dexamethasone schedules. Haematologica. 2003;88:1022–1028. [PubMed] [Google Scholar]
- 20.Perri RT, Hebbel RP, Oken MM. Influence of treatment and response status on infection risk in multiple myeloma. Am J Med. 1981;71:935–940. doi: 10.1016/0002-9343(81)90303-X. [DOI] [PubMed] [Google Scholar]
- 21.Mainwaring CJ, Williams MA, Singer CR, Lush RJ, Smith JG, Haynes CL, Kelsey SM. Monocyte dysfunction in patients with multiple myeloma and lymphoplasmacytic disorders is related to serum paraprotein levels. Br J Haematol. 1999;105:948–954. doi: 10.1046/j.1365-2141.1999.01455.x. [DOI] [PubMed] [Google Scholar]
- 22.Hyun SY, Han SH, Kim SJ, Jang JE, Kim Y, Cho H, Lee JY, Cheong JW, Min YH, Song JW, Kim JS. Pretreatment lymphopenia, poor performance status, and early courses of therapy are risk factors for severe bacterial infection in patients with multiple myeloma during treatment with bortezomib-based regimens. J Korean Med Sci. 2016;31:510–518. doi: 10.3346/jkms.2016.31.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konig C, Kleber M, Reinhardt H, Knop S, Wasch R, Engelhardt M. Incidence, risk factors, and implemented prophylaxis of varicella zoster virus infection, including complicated varicella zoster virus and herpes simplex virus infections, in lenalidomide-treated multiple myeloma patients. Ann Hematol. 2014;93:479–484. doi: 10.1007/s00277-013-1951-6. [DOI] [PubMed] [Google Scholar]
- 24.Iliopoulou EG, Karamouzis MV, Missitzis I, Ardavanis A, Sotiriadou NN, Baxevanis CN, Rigatos G, Papamichail M, Perez SA. Increased frequency of CD4+ cells expressing CD161 in cancer patients. Clin Cancer Res. 2006;12:6901–6909. doi: 10.1158/1078-0432.CCR-06-0977. [DOI] [PubMed] [Google Scholar]
- 25.Lee SE, Lim JY, Yoon JH, Shin SH, Cho BS, Eom KS, Kim YJ, Kim HJ, Lee S, Cho SG, Kim DW, Lee JW, Min WS, Min CK. CD161(+) T cells as predictive markers for acute graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21:421–428. doi: 10.1016/j.bbmt.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 26.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, Berrino L, Fambrini M, Caproni M, Tonelli F, Lazzeri E, Parronchi P, Liotta F, Maggi E, Romagnani S, Annunziato F. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maggi L, Santarlasci V, Capone M, Peired A, Frosali F, Crome SQ, Querci V, Fambrini M, Liotta F, Levings MK, Maggi E, Cosmi L, Romagnani S, Annunziato F. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur J Immunol. 2010;40:2174–2181. doi: 10.1002/eji.200940257. [DOI] [PubMed] [Google Scholar]
- 28.Lanier LL, Chang C, Phillips JH. Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes. J Immunol. 1994;153:2417–2428. [PubMed] [Google Scholar]
- 29.Aldemir H, Prod'homme V, Dumaurier MJ, Retiere C, Poupon G, Cazareth J, Bihl F, Braud VM. Cutting edge: lectin-like transcript 1 is a ligand for the CD161 receptor. J Immunol. 2005;175:7791–7795. doi: 10.4049/jimmunol.175.12.7791. [DOI] [PubMed] [Google Scholar]
- 30.Rosen DB, Bettadapura J, Alsharifi M, Mathew PA, Warren HS, Lanier LL. Cutting edge: lectin-like transcript-1 is a ligand for the inhibitory human NKR-P1A receptor. J Immunol. 2005;175:7796–7799. doi: 10.4049/jimmunol.175.12.7796. [DOI] [PubMed] [Google Scholar]
- 31.Kim Tae Woo, Lee Sung-Eun, Lim Ji-Young, Ryu Da-Bin, Jeon Young-Woo, Yoon Jae-Ho, Cho Byung-Sik, Eom Ki-Seong, Kim Yoo-Jin, Kim Hee-Je, Lee Seok, Cho Seok-Goo, Kim Dong-Wook, Lee Jong Wook, Min Woo-Sung, Min Chang-Ki. Clinical significance of pre-transplant circulating CD3+CD4+CD161+cell frequency on the occurrence of neutropenic infections after allogeneic stem cell transplantation. Transplant Infectious Disease. 2017;19(1):e12643. doi: 10.1111/tid.12643. [DOI] [PubMed] [Google Scholar]
- 32.Lee SE, Lim JY, Ryu DB, Kim TW, Jeon YW, Yoon JH, Cho BS, Eom KS, Kim YJ, Kim HJ, Lee S, Cho SG, Kim DW, Lee JW, Min WS, Min CK. Circulating CD3+CD4+CD161+ cells are associated with early complications after autologous stem cell transplantation in multiple myeloma. BioMed Research Int. 2018;2018:1–8. doi: 10.1155/2018/5097325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung SH, Bae SY, Ahn JS, Kang SJ, Yang DH, Kim YK, Kim HJ, Lee JJ. Lymphocytopenia is associated with an increased risk of severe infections in patients with multiple myeloma treated with bortezomib-based regimens. Int J Hematol. 2013;97:382–387. doi: 10.1007/s12185-013-1270-7. [DOI] [PubMed] [Google Scholar]
- 34.Yi YS, Chung JS, Song MK, Shin HJ, Seol YM, Choi YJ, Cho GJ, Lee GW, Moon JH, Hwang IH, Ahn KH, Lee HS, Shin KH, Hwang JM. The risk factors for herpes zoster in bortezomib treatment in patients with multiple myeloma. Korean J Hematol. 2010;45:188–192. doi: 10.5045/kjh.2010.45.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vesole DH, Oken MM, Heckler C, Greipp PR, Katz MS, Jacobus S, Morrow GR. Oral antibiotic prophylaxis of early infection in multiple myeloma: a URCC/ECOG randomized phase III study. Leukemia. 2012;26:2517–2520. doi: 10.1038/leu.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wittekamp BH, Bonten MJ. Antibiotic prophylaxis in the era of multidrug-resistant bacteria. Expert Opin Investig Drugs. 2012;21:767–772. doi: 10.1517/13543784.2012.681642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 17.7 kb)