Abstract
Membrane fusion, one of the most essential processes in the life of eukaryotes, occurs when two separate lipid bilayers merge into a continuous bilayer and internal contents of two separated membranes mingle. There is a certain class of proteins that assist the binding of the viral envelope to the target host cell and catalyzing fusion. All class I viral fusion proteins contain a highly conserved 20–25 amino-acid amphipathic peptide at the N-terminus, which is essential for fusion activity and is termed as the ‘fusion peptide’. It has been shown that insertion of fusion peptides into the host membrane and the perturbation in the membrane generated thereby is crucial for membrane fusion. Significant efforts have been given in the last couple of decades to understand the lipid-dependence of structure and function of the fusion peptide in membranes to understand the role of lipid compositions in membrane fusion. In addition, the lipid compositions further change the membrane physical properties and alter the mechanism and extent of membrane fusion. Therefore, lipid compositions modulate membrane fusion by changing membrane physical properties and altering structure of the fusion peptide.
Keywords: Membrane fusion, Fusion peptide, Non-lamellar intermediate, Lipid composition
Introduction
The merging of two membranes into one is an occasion contributing to fertilization, intracellular trafficking, neurotransmission, endocrine hormone secretion and viral infection (Mohler et al. 2002; Jahn et al. 2003; Earp et al. 2005). On a molecular level, membrane fusion leads to amalgamation of lipid and protein components of the two membranes and mixing of volumes enclosed by them. This has been hypothesized that membrane fusion takes place by one of the three following mechanism. The first model, ‘stalk-pore hypothesis’ proposes that the expansion of stalk brings the distal monolayers of two membranes together into a single bilayer and forms hemifusion diaphragm. Eventually, opening of pore occurs at the hemifusion diaphragm leading to completion of the fusion process (Kozlov et al. 1989; Chernomordik et al. 1987; Chernomordik et al. 1995). The second model suggests direct pore formation from the very beginning of a bilayer connection between membranes (Kuzmin et al. 2001; Siegel 1993). The third model is based on the Brownian dynamics of bilayer fusion (Noguchi and Takasu 2001; Muller et al. 2003). In spite of these models, we have a poor molecular understanding of two key steps in the process; initial intermediate and final pore formation. Both the steps involve transient and unfavorable fluctuations in lipid/water arrangement, which is difficult to probe experimentally (Chakraborty et al. 2012). This has been conceptualized that these rearrangement of lipids through water is the foremost contributor to the activation barrier for membrane fusion. In biological scenario it has been broadly accepted that fusion proteins or peptides act as machines that use stored conformational energy to assemble closely apposed lipid bilayers and take them through an arrays of non-lamellar intermediate structures leading to fusion pore formation (Lentz et al. 2000). Generally, three kinds of proteins orchestrate the entire event of the membrane fusion such as docking proteins select which membranes to fuse, regulatory proteins control the process and fusion proteins make it to happen (Lentz et al. 2000). The ideal interplay of these proteins with the membrane is the key of successful fusion events. Our knowledge on membrane fusion mechanism essentially originates from study of the viral entry into a host cell and fusion of synaptic vesicles with the pre-synaptic membrane in neurons. The comprehensive studies on hemagglutinin (HA) and gp41-induced fusion of influenza and human immunodeficiency virus (HIV), respectively, demonstrate that certain structural features of the fusion peptide and transmembrane domain are important for stabilizing or destabilizing an intermediate for driving a lipid arrangement that leads to the formation of a stable pore. The formation of fusion pore is dependent on intricate balance of lipid–protein interaction that leads to restricted pore formation rather than unrestricted fusion (Chernomordik and Kozlov 2005). Even the surface density of the protein alters the rate and extent of membrane fusion. The leakage during the membrane fusion is sometimes being correlated with the lipid rearrangement. Therefore, lipid composition of the membrane turns out to be crucial to minimize the spontaneous leakage by maximizing content mixing.
The enveloped viruses enter into the host cell either by fusing with the plasma membrane or taken up by the receptor-mediated endocytosis followed by fusion with the endosomal membrane triggered by chemical signal such as pH (Earp et al. 2005; Skehel and Wiley 2000). However, in both cases there are specific proteins in the viruses that orchestrate the fusion process and hence they are called fusion proteins. The major glycoprotein on the influenza virus surface is HA that binds to sialic acid residues on the host cell surface and undergoes a dramatic conformational change when pH drops below 5.3 in the endosome. The conformational change involves shredding of larger subunit of HA, HA1, from the smaller subunit HA2 and the exposure of N-terminal hydrophobic peptide thereby making it available for the interaction with the endosomal membrane. The HIV virus is enveloped by a lipid bilayer, which docks the integral membrane glycoprotein gp120/gp41. The gp120 subunit recognizes the host cell surface CD4 receptor, whereas the N-terminus of gp41 subunit binds with the host cell membranes. The significance of N-terminal region in membrane fusion has been shown by mutational study and has been termed as ‘fusion peptide’ (Gething et al. 1986). Though the fusion peptide is crucial for fusion, there is no structural and conformational similarity among the fusion peptides from different viral fusion proteins. Moreover, conformation and efficiency of the fusion peptide depend on the composition of the fusing membranes (Lai et al. 2012; Lai and Freed 2014).
In this review, we have elaborately discussed the mechanistic role of lipid composition on the membrane fusion. We have mentioned about the relevance of inverted cone shaped lipid molecules in stabilizing different non-lamellar intermediates. Moreover, we have discussed the effect of lipid composition on the structure and function of the fusion peptides. We have mainly restricted the review on the structure and function of three important class-I fusion peptides, such as gp41 of HIV, HA of influenza and spike glycoprotein of severe acute respiratory syndrome coronavirus (SARS-CoV) in lipid membranes of various compositions.
Direct Effect of Lipid Composition on Membrane Fusion
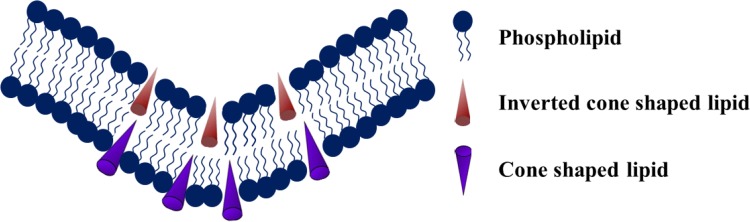
Membrane composition and curvature are two important parameters that modulate the fusogenic property of the membrane. There are handful of literature on the effects of lipids that preferentially generate either positive or negative curvature on membrane fusion (Chernomordik and Kozlov 2003; Haque et al. 2001). The most well-studied lipid is phosphatidylethanolamine (PE), which is known to produce intrinsic negative curvature to the membrane. The ratio of phosphatidylcholine (PC) and phosphatidylethanolamine was monitored for plasma membrane in fusogenic contexts, and it has been found that the ratio varies from 0.9 to 2.0 (Cotman et al. 1969; Breckenridge et al. 1973; Aloia et al. 1993). The importance of PE in the fusogenic membrane is generally linked to its inverted cone-shaped molecular structure, which is assumed to promote spontaneous negative curvature to the membrane (shown in Fig. 1) (Haque et al. 2001). In addition, theoretical treatments of proposed fusion intermediates and x-ray diffraction results demonstrate that PE helps in reducing the energy for the stalk-like state formation, which is believed to be an early fusion intermediate (Chernomordik 1996; Katsov et al. 2004; Kozlovsky et al. 2002, 2004; Kozlovsky and Kozlov 2002; Siegel and Epand 1997). The molecular dynamics simulation results of small unilamellar vesicular fusion (15 nm diameter) with varying PC/PE ratio have shown that the rate constant of stalk formation reduced remarkably with increasing PC/PE ratio (Kasson and Pande 2007). The rate constant of stalk formation in pure POPE vesicles is ~ 3.4 × 105 s−1, whereas the rate decreases to 6.7 × 104 s−1 in 1:1 POPC/POPE vesicles and 3.0 × 103 s−1 in 2:1 POPC/POPE vesicles (Kasson and Pande 2007). However, the effect of PE is not so substantial in the rate constant of hemifusion and fused state formation.
Fig. 1.
Schematic representation of spontaneous negative curvature formation in presence of inverted conical lipids like phosphatidylethanolamine, phosphatidic acid
Lentz group studied polyethylene glycol induced membrane fusion of small unilamellar vesicles of different lipid compositions and showed that though presence of PE enhances rate of lipid mixing and content mixing in any lipid composition, the extent of leakage is controlled by the other lipids present in the membrane (Haque et al. 2001).
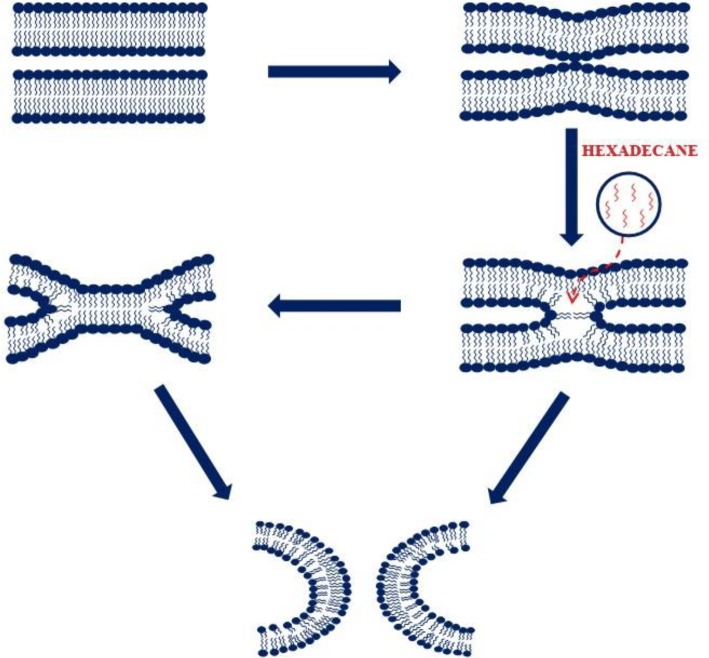
As we know an ideal fusogenic lipid mixing should show high lipid and content mixing with very low content leakage. Therefore, adding more PE alone does not result to a good fusogenic membrane system. Generally, it is assumed that the bilayer structure undergoes series of non-lamellar structural intermediates to form the fusion pore. Thus, the mechanical stability of the lipid membrane is of immense important for the stabilization of the non-lamellar intermediates. It is known that cholesterol (CH) enhances stability and reduces permeability of the membrane in addition to its other roles in biological membranes. Cholesterol is present in all eukaryotic cells with different extent in various organelles (Raffy and Teissie 1999). Cholesterol forms a stoichiometric (1:1) complex with sphingomyelin (SM), and they are thought to be involved in domain formation in the membrane (Needham and Nunn 1990; McIntosh et al. 1992; Xu and London 2000; Lentz et al. 1981). This has been found that a lipid mixture of DOPC/DOPE/SM/CH in a unique mol ratio of 35/30/15/20 provides a membrane that shows high lipid and content mixing with minimum content leakage (Haque et al. 2001). Apart from the role of cholesterol in providing mechanical stability to the membrane, it impacts the structure and dynamics of membrane proteins in lipid bilayer (Epand 2008). The effect of cholesterol could be imparted in two different ways, either by its influence in general membrane properties or specific interaction of cholesterol with the proteins/peptides. Cholesterol enhances the order of the acyl chain region of the lipid bilayers by filling up the free volume (Falck et al. 2004). The altered free volume in the membrane changes the structure and dynamics of membrane proteins. Taken together, cholesterol has immense significance in the mechanical properties of the membrane and the structure and dynamics of membrane proteins. Therefore, the role of cholesterol is important in the context of membrane fusion in multiple ways. A recent review of ‘role of cholesterol in membrane fusion’ described various general and specific role of cholesterol in membrane fusion (Yang et al. 2016). The promotion of membrane fusion by filling up free volume of the membrane has been validated by the addition of hydrophobic molecules like hexadecane and dioleoylglycerol in the membrane. It has been shown that both dioleoylglycerol and hexadecane promote stalk, hemifusion and fusion pore formation in Ca2+ and polyethylene glycol-mediated fusion of lipid vesicles (Chakraborty et al. 2012; Walter et al. 1994). The reduction in free volume in the membrane stabilizes the non-lamellar intermediates and therefore eases the formation of fusion pore (shown in Fig. 2). Hexadecane is known to compete with fusion peptide and transmembrane domain of several fusion proteins in promoting membrane fusion, which establishes the membrane-free volume filling property of fusion peptide and transmembrane domain (Chakraborty et al. 2013; Sengupta et al. 2014). Lipid composition affects the bilayer structure and physical properties of the bilayer through several other pathways such as bilayer dehydration (Wilschut et al. 1985), imperfect lipid packing (Lee and Lentz 1997; Wu et al. 1996), local alteration in bilayer curvature (Lentz et al. 1987; Nir et al. 1982), outer leaflet packing defects (Lee and Lentz 1997), elastic-free energy (Leikin et al. 1996), changes in membrane fluidity (Wilschut et al. 1985; Duzgunes et al. 1987) and locally induced monolayer phases (Ellens et al. 1989).
Fig. 2.
Schematic representation of reorganizations of lipid bilayer presumed to be involved in different non-lamellar intermediates in the progression of the membrane fusion. The long chain hydrocarbon, hexadecane (shown in red color), can fill the void volumes in the non-bilayer intermediates to promote stability and therefore enhance the membrane fusion process
The effect of membrane curvature in promoting fusion was documented earlier for model membranes (Talbot et al. 1997) and is presently well accepted for the evaluation of membrane fusion in vivo. Lipid molecules such as phosphatidylethanolamine and phosphatidic acid (PA) that induce spontaneous negative curvature (as shown in Fig. 1) favor the formation of highly curved fusion intermediates like stalk and transmembrane contact. The stability of these two intermediates assures the pore formation between two lipid bilayers. It is known that significant stress in the outer leaflet promotes membrane fusion (Lee and Lentz 1997; Talbot et al. 1997). Interestingly, removal of lipids from outer leaflet of relatively less curved vesicles also generates outer leaflet stress and enhance fusion (Lee and Lentz 1997). From these results, it is hypothesized that excess stress on the leaflet leads to formation of packing defects, and these defects could be the nucleation point for two membranes to be joined (Chakraborty et al. 2012). Because of the intrinsic negative curvature of PE, it further generates stress in the bilayer leaflet and this excess stress leads to the formation of membrane defects and promotes joining of two adjacent membranes and stalk formation. It has been shown that the addition of wedge-shaped molecules that generate intrinsic positive curvature on the membrane inhibit infectivity of enveloped viruses (St Vincent et al. 2010), and it has been proposed that these molecules could be used to develop general viral inhibitors (Melikyan 2010). In addition to the headgroup chemistry, the unsaturation in the acyl chain influences the lipid geometry. Presence of unsaturation in the acyl chain induces kink in the chain and occupy more space compared to that for their saturated counterpart. The ratio of size between headgroup and acyl chain describes the overall geometry of the lipid (Pinot et al. 2014; Vanni et al. 2014). Therefore, the chemistry of acyl chain is also relevant in the context of fusogenic lipid composition. Moreover, the shape of the lipid and membrane organization are essential for membrane fusion, fission, local tethering and enzyme reaction, which concurs the importance of shape in biology (Shibata et al. 2009; McMahon and Gallop 2005).
Effect of Membrane Composition on Fusion Peptide Structure and Function
Apart from the direct impact of the lipid composition on the membrane physical properties, lipid composition influences the structures of viral fusion peptides. In the following sections, we have discussed about gp41 fusion peptide from HIV, HA fusion peptide from Influenza virus and S2 fusion peptide from SARS-CoV.
gp41 Fusion Peptide from Human Immune Deficiency Virus
The human immune deficiency virus (HIV) enters into the host cell via receptor-mediated endocytosis process. The HIV expresses a membrane glycoprotein, gp160 on its surface, which is proteolytically cleaved into two subunits. The mutagenesis experiment established that the dissociation of gp160 is essential for the entry of HIV (Blumenthal et al. 2012). The larger subunit gp120 contains the binding site for the host cell surface receptor, CD4, whereas the smaller subunit gp41 contains a transmembrane anchoring highly hydrophobic C-terminal sequence and an N-terminal hydrophobic fusion peptide (Skehel and Wiley 2000) (Fig. 3). The N-terminal peptide gets exposed due to the conformational change induced by the receptor binding of the gp120 subunit and makes it available for host cell membrane binding.
Fig. 3.
Schematic representation of gp41 protein. The fusion peptide is situated at the N-terminal of the protein
The sequence of the N-terminal peptide is highly conserved for HIV, but there is no similarity of the sequence with fusion peptides from other viruses. The HIV fusion peptide is comprised of 15 highly hydrophobic residues followed by 8 moderately hydrophobic residues (Li and Tamm 2007). Because of the hydrophobic nature of the peptide, it is prone to self-association. It has been shown that the structure of the fusion peptide depends on the size and lipid composition of the membrane and thereby imparts differential effect on membrane fusion depending on the lipid composition of the membrane. In addition, the binding efficiency of the peptide to the membrane depends on the lipid composition. High-resolution NMR structure of the peptide in detergent micelles shows α-helical conformation (Li and Tamm 2007; Jaroniec et al. 2005). However, the micellar environment is very much different from the bilayer environment and hence the bilayer structure might be different from its structure in micellar medium. Most structural elucidation of gp41 fusion peptide in lipid bilayers was carried out using solid-state NMR, which provides strong evidence of β-sheet structure (Schmick and Weliky 2010; Qiang et al. 2008; Yang et al. 2001). Nevertheless, solid-state NMR method requires high sample concentration and low temperature to obtain decent signal-to-noise ratio, therefore vouching the relevance of the structure in physiological condition. The gp41 fusion peptide of LAV1a and LAVmal strains of HIV binds to the membrane containing negatively charged lipids and promotes lipid mixing and content leakage (Rafalski et al. 1990). The circular dichroism (CD) and infrared spectroscopy (IR) of the gp41 fusion peptide in low peptide-to-lipid ratio (1/200) in the presence of negatively charged membrane show mainly α-helical conformation. However, the α-helix turns into β-conformation when the peptide-to-lipid ratio is relatively higher (1/30). It has been further shown that the wild variety of the gp41 fusion peptide binds to the large unilamellar vesicles and shows an oblique orientation in the membrane, whereas non-fusogenic variants remain in parallel to the plane of the membrane (Martin et al. 1996). There are other evidences to validate the importance of proper peptide–lipid interaction for the successful membrane fusion (Mobley et al. 1999). It has been found from the CD and Fourier transformed IR studies of the peptide bound to planar POPC/POPG (4/1 mol fraction) are predominantly α-helical at lower peptide concentration, whereas it assumes anti-parallel β-sheet structure at higher peptide concentration (Li and Tamm 2007). Increasing peptide concentration could lead to membrane-assisted self-association of the peptide. The self-association of fusion peptide has shown in other studies, where β-sheet structures have been reported for HIV fusion peptide.
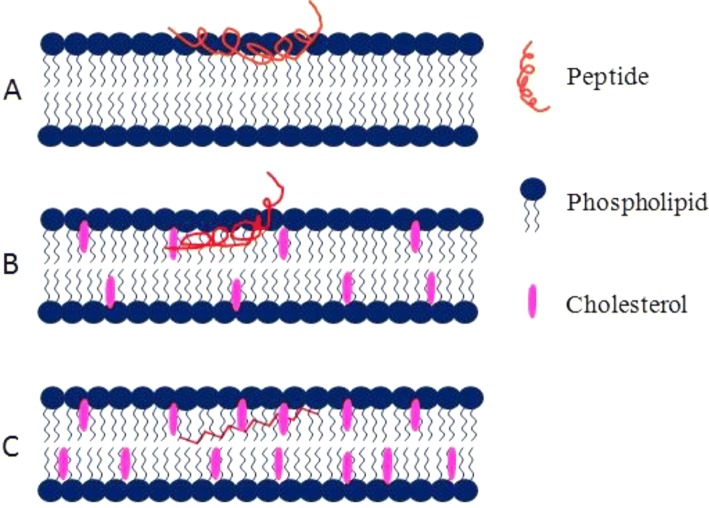
Tamm group has shown that cholesterol plays a crucial role in the structure and function of gp41 fusion peptide (Lai et al. 2012). The peptide shows α-helical conformation in the membrane containing less than 30 mol% cholesterol, whereas the peptide adopts majorly β-sheet conformation in the presence of 30 mol% or more cholesterol content in the membrane (shown in Fig. 4). However, certain amount of α-helical structure is being retained at the high cholesterol concentration (Lai et al. 2012). Therefore, the key factor that determines the secondary structure of the peptide is cholesterol and we know that the distribution of cholesterol is not uniform in cell membrane. There are cholesterol-rich lipid domains in the membrane where the peptide will show β-sheet conformation, whereas in the region of low cholesterol the peptide will be helical in nature and raises the question that which forms is more active in promoting membrane fusion. Though the exact answer of this question pertaining to the physiological condition is yet to be obtained, it has been shown that both α-helix and β-sheet conformations induce membrane fusion. Nonetheless, the depth of membrane penetration depends on the secondary structure of the peptide, in the cholesterol-deficient membrane the α-helical conformation traverses much deeper in the hydrophobic region of the membrane and imparts order to the bilayer. The results suggest that the fusion peptide is extremely plastic in nature, and its secondary structure and depth of penetration are sensitive to the lipid composition of the membrane. The electron spin resonance (ESR) measurements show that the fusion peptide orders the lipid membrane. However, the characteristic of order induction depends on the membrane composition. In the membrane containing less than 30 mol% cholesterol, where peptide is helical in nature, peptide induces concentration-dependent ordering of the interfacial or upper region of the membrane, whereas the order is being induced in the hydrophobic region of the membrane while it contains more than 30 mol% of cholesterol (peptide is presumably β-sheet) (Lai and Freed 2014). It is hypothesized that the peptide-induced membrane ordering is the pre-requisite for any peptide to be fusogenic. However, there is no consensus on the extent and region of ordering that is important for the peptide to be fusogenic.
Fig. 4.
Schematic representation of gp41 fusion peptide (of HIV) insertion into the lipid bilayer in a the absence of cholesterol, b less than 30 mol% cholesterol and c more than 30 mol% cholesterol. The gp41 fusion peptide (red lines) assumes various secondary structures in membranes of different lipid compositions. The pink structures represent the cholesterol molecules, whereas the blue structures are phospholipids. The figure has been adapted and modified from Lai et al. (2012)
Though there is an agreement on the helical structure of gp41 fusion peptide in presence of negatively charged lipids and less than 30 mol% cholesterol (Rafalski et al. 1990; Bodner et al. 2004; Nieva et al. 1994), there is a disagreement on the peptide structure in the absence of negative lipid. Some report suggests helical conformation of the peptide (Curtain et al. 1999), whereas most find β-sheet structure of the peptide in the neutral membrane (Rafalski et al. 1990; Bodner et al. 2004; Pereira et al. 1997). Lentz group has shown that the gp41 fusion peptide assumes compact antiparallel β-sheet in neutral membrane in relatively higher peptide-to-lipid ratio (peptide/lipid ratio of 1:50 to 1:10) (Haque et al. 2005); however, this does not rule out the surface concentration-dependent switchover of peptide secondary structure. The NMR structure of the peptide in micellar medium further supports the β-sheet conformation of the peptide (Qiang et al. 2009) and demonstrate that there is a strong correlation between depth of insertion of the peptide and its fusogenicity.
Taken together, it is evident that the structure of the peptide and therefore its effect on the membrane fusion is extremely dependent on the lipid composition and surface concentration of the peptide.
Hemagglutinin Fusion Peptide from Influenza Virus
Hemagglutinin is the major glycoprotein of influenza, which is responsible for the fusion of influenza virus with the host cell. The hemagglutinin is being synthesized as a precursor protein HA0, which is being cleaved as HA1 and HA2. The HA1 is mainly responsible for receptor binding, whereas HA2 is crucial for the fusion between virus and the host cell (Tamm 2003; Han et al. 2001). The sialic acid binding domain of HA1 binds with the sialic acid present on the target cell surface, which promotes the sticking of the virus particle to the host cell (Skehel and Wiley 2000). The schematic representation of HA2 is shown in Fig. 5.
Fig. 5.
Schematic representation of HA2 protein. The fusion peptide is situated at the N-terminal of the protein
The N-terminal fusion peptide of HA2 is generally buried in the hydrophobic core of the protein, and it is being exposed due to the conformational changes induced by the endosomal low pH (pH ~ 5.3) (White 1990; Durell et al. 1997). The putative role of the fusion peptide has been studied extensively in vitro and in vivo (Kozlovsky and Kozlov 2002; Chakraborty et al. 2013; Haque et al. 2011; Epand et al. 1994) and suggested that fusion peptide could promote fusion by changing bending modulus (Tristram-Nagle and Nagle 2007), filling void volume (Malinin and Lentz 2004), promoting positive curvature (Chakraborty et al. 2013) or negative (Ge and Freed 2009) intrinsic curvature to contacting bilayers and reducing Gaussian energy (Chang et al. 2000). The NMR structure of the HA fusion peptide was solved in dodecylphosphocholine (DPC) micelles, and ESR measurements were taken using different depth-dependent spin probes in vesicular system, and the structure of membrane-bound HA fusion peptide was modelled from NMR and ESR results (Han et al. 2001; Lai et al. 2006). It was proposed that the N-terminal α-helix extends from Leu2 to Ile10 followed by a turn, which is stabilized by hydrogen bonds between NH of Glu11 and Asn12 to the carbonyl of Gly8 and Phe9 and then a short 310-helix at the C-terminal (Han et al. 2001). The close proximity of N and C termini led to the formation of a spring-loaded boomerang like structure at pH 5. This boomerang like structure of the fusion peptide is assumed to be extremely important for the fusogenicity of hemagglutinin. Interestingly, this boomerang-like structure disappears when W14 is being mutated by alanine (W14A mutant) and the mutant is inactive in promoting membrane fusion (Lai et al. 2006). In addition, the boomerang like structure is also missing at pH 7 (Tamm 2003), where the peptide lacks fusion promoting property. Thereby, it is hypothesized that the spring-loaded boomerang structure of hemagglutinin fusion peptide is crucial for its fusogenic property. We have recently shown that the W14 anchors at the interfacial region of the sodium dodecyl sulfate (SDS) micelles and stabilizes the boomerang like structure of the fusion peptide (shown in Fig. 6) (Meher and Chakraborty 2017). The interfacial location of tryptophan and its slow rotational dynamics might be crucial to form bent in between two helices in HA fusion peptide. Chang et al. have shown from their NMR results of 25-amino acid long HA fusion peptide in SDS micelles that segments 2-13 and 17-24 show α-helical character with a much weaker α-helical nature in the 14-16 segment (Chang et al. 2000). They have carried out secondary structural analysis of the 25 amino acid long fusion peptide using circular dichroism, and it has revealed that about 45% helical content is present in the peptide at pH 5. The results of fluorescence intensity quenching of tryptophan by acrylamide and NMR experiments established that the W14 is inside the vesicular interior and residues 16–18 are at the micellar aqueous boundary. The enhancement of fluorescence intensity of the N-terminal labelled fusion peptide in the presence of SDS micelles indicates that the N-terminal is located at the hydrophobic region of the membrane. However, they did not notice any remarkable change in peptide insertion between pH 5 and 7. A 23-amino acid long HA fusion peptide shows helical-hairpin structure, which is stabilized by interactions between residues Trp21-Gly23 and the N-terminal residues of the fusion peptide (Lorieau et al. 2011). There are certain differences in the peptide structure solved by different groups. However, there is no confusion about helical structure of the fusion peptide in the membrane. The subtle differences in the structure of the fusion peptide could be attributed either to the structural plasticity of the peptide or the different membrane mimetic systems that they have used. The structural plasticity is a unique property of the fusion peptide, as the membrane geometry changes continuously during the course of the fusion process and the peptide requires accommodating itself in the membrane. The structure of the hemagglutinin fusion peptide was documented in small unilamellar vesicles in a more biologically relevant lipid composition using circular dichroism and polarized-attenuated total-internal reflection Fourier transform infrared (PATIR-FTIR) spectroscopy. These measurements further confirm that the wild-type HA fusion peptide forms an inverted V-shaped structure as proposed earlier (Han et al. 2001; Haque et al. 2011). Similar measurements have also showed that the fusion inefficient G13L mutant adopts a less helical conformation, and N-terminal of the peptide is closer to the bilayer interface, thereby disrupting the inverted V-shape conformation.
Fig. 6.

Schematic diagram showing interfacial location and confined dynamics of Trp 14 in SDS micelles, which is crucial for assuming inverted V-shaped structure of HA fusion peptide at pH 5. The figure has been adapted from Meher and Chakraborty (2017) with appropriate permission
It has been recently shown that the influenza virus fusion depends on the host cell lipid composition. Two different lipid compositions could produce identical curvature; however, their compressibility and lysis tension space would be different, resulting in diverse extent of fusion (Haldar et al. 2018). The lysolipids are known to have ‘cone’-shaped structure and reported to inhibit membrane fusion by hindering the formation of highly curved intermediates (Chernomordik et al. 1993). This has been found that lysophosphatidylcholine (LPC) inhibits fusion of influenza virus, while LPC was added from external stock solution. However, LPC does not inhibit fusion of influenza virus when it is present uniformly in both the leaflets (Gunther-Ausborn et al. 1995). Recently, Domanska et al. tested the role of cholesterol on the fusion of influenza virus. Interestingly, variation of cholesterol content in the viral membrane showed different effect on fusion compared with similar changes to the target membrane (Domanska et al. 2013). The increase in cholesterol content in the target membrane facilitates viral fusion, whereas severe depletion of cholesterol inhibits the process. In contrast, moderate cholesterol depletion from the viral membrane enhances the kinetics of the membrane fusion while severe depletion retarded the process.
Recently, we have made an effort to understand the fusogenic property of hemagglutinin fusion peptide and its several non-fusogenic mutants by studying depth-dependent membrane order and heterogeneity using several depth-dependent fluorescence probes (Chakraborty et al. 2017). Interestingly, the wild-type fusion peptide shows a unique depth-dependent membrane ordering and heterogeneity pattern compared to its non-fusogenic mutants. Wild-type peptide orders the interfacial region the most with its minimum impact on the ordering of the hydrophobic region. A fusion inefficient mutant G1S orders the interfacial region like the wild-type peptide, but unlike wild-type peptide it orders the hydrophobic region of the membrane. Our results suggest a correlation between the depth-dependent membrane ordering and the fusogenic properties of the hemagglutinin fusion peptide and its mutants.
S2 Fusion Peptide from Severe Acute Respiratory Syndrome (SARS) Coronavirus
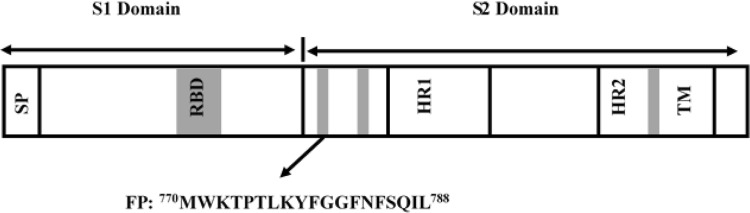
Severe acute respiratory syndrome (SARS) is a viral respiratory disease caused by the SARS coronavirus (SARS-CoV). The infection by SARS-CoV is being achieved via fusion between viral envelope and the host cell membrane. Coronaviruses are having positive stranded RNA protein with 3–4 protein embedded in its envelope. The spike (S) glycoprotein plays the crucial role in the fusion between viral and host cell membranes. The surface glycoprotein, S, binds to the cellular receptors ACE2 and CD209L and induces membrane fusion (Jeffers et al. 2004; Li et al. 2003). The protease cleavage in protein S results to the formation of two non-covalently associated subunits S1 and S2; however, the cleavage is not an absolute requirement for inducing membrane fusion (Masters 2006). The S2 subunit contains two highly conserved heptad region (HR1 and HR2) (Ingallinella et al. 2004; Xu et al. 2004; Howard et al. 2006), N-terminal fusion peptide and C-terminal transmembrane domain like other class 1 virus fusion proteins such as gp41, HA, Ebola virus glycoprotein and paramyxovirus F protein (Fig. 7) (Chambers et al. 1990; Gallaher 1996). The receptor binding of S1 subunit leads to conformational change in S2 subunit that exposes the fusion peptide, which interacts with the target host cell (Epand 2003). It is known that the fusion peptide not only initiates the membrane fusion but also plays a crucial role in transmembrane contact and fusion pore formation, so that the viral genetic materials are being transferred to the host cells (Siegel and Epand 1997; Colotto and Epand 1997; Aranda et al. 2003).
Fig. 7.
Schematic representation of Spike glycoprotein of SARS-CoV, which is composed of two domains S1 and S2. The fusion peptide is situated at the N-terminus of S2 protein
It has been shown that the fusion peptide of SARS-CoV binds to the membrane in a lipid-dependent manner with a preference for the negatively charged lipid in the membrane. The quenching experiments further showed that the tryptophan in the fusion peptide is relatively more buried in the hydrophobic region in the negatively charged membranes compared to that in neutral membranes (Guillen et al. 2008). The SARS-CoV fusion peptide is also capable of altering membrane stability that was evaluated by the leakage of the content. The fusion peptide induces leakage in zwitterionic and negatively charged membranes with higher impact in case of negatively charged membranes (Guillen et al. 2008). The negatively charged lipid dependency on peptide binding and fusion has been schematically shown in Fig. 8. The insertion of the fusion peptide reduces the membrane dipole potential of the negatively charged membranes, and the effect is more pronounced when cholesterol is present in the membrane (Guillen et al. 2008). It has further been shown that the membrane perturbing effect of the fusion peptide is dependent on the Ca2+ ion concentration (Lai et al. 2017). The differential scanning calorimetry (DSC) measurements showed that the peptide strongly perturbs the structural integrity of negatively charged membranes and supports the hypothesis that peptide generates opposing curvature stress on phosphatidylethanolamine membrane that stabilizes the highly curved fusion intermediates (Basso et al. 2016). The ESR measurements showed that the peptide improves lipid packing and headgroup ordering (Basso et al. 2016). The circular dichroism measurements of the fusion peptide showed that the peptide forms a β-sheet structure in the membrane containing phosphatidylcholine and phosphatidylinositol (Sainz et al. 2005); however, it shows α-helical structure in trifluoroethanol (Madu et al. 2009). Trifluoroethanol is known to promote α-helicity in the peptides and proteins (Luidens et al. 1996); therefore, secondary structure of the fusion peptide in trifluoroethanol does not carry much significance in the context of the membrane-bound structure of the fusion peptide. Recently, the atomistic structure of SARS-CoV fusion peptide has been solved by NMR in dodecylphosphocholine (DPC) micelles (Mahajan and Bhattacharjya 2015). Zwitterionic DPC micelles act as a better membrane mimetic environment and support tertiary and quaternary packing of short peptides (Saravanan and Bhattacharjya 2011; Porcelli et al. 2006). The NMR structure showed that fusion peptide is helical in nature and assumes a ‘V-shaped’ bent conformation in DPC micelles. However, the N-terminal of the peptide (M1–W2–L3) does not have a definite structure. The kink of the V-shaped helical conformation of fusion peptide is located in G11–G12 including F10 residue.
Fig. 8.
The schematic representation of lipid-dependence on the S2 fusion peptide (of SARS-CoV)-induced membrane fusion. The peptide is capable of inducing membrane fusion only when the membrane contains certain amount of anionic lipids
The discrepancy in the secondary structure of SARS-CoV fusion peptide obtained from circular dichroism and NMR could be due to their differential membrane environment. However, the actual structure of the peptide in physiologically relevant condition is still elusive. It has been observed from various studies that a negatively charged lipid is necessary for the binding and function of the fusion peptide and therefore, high-resolution structure of the peptide in the presence of negatively charged lipid would provide a more physiologically relevance to the structure.
Concluding Remark
Membrane fusion is one of the most important processes in the life of eukaryotes. Although there is a vast literature on the mechanism of membrane fusion and the role of fusion proteins and peptides in promoting membrane fusion, there is no consensus on its mechanistic details. People have been tried to decipher the role of lipid composition on the membrane fusion, and it is assumed that inverted cone-shaped lipid promotes membrane fusion by stabilizing highly curved fusion intermediates in a protein-free system. However, the response of these lipids in the presence of proteins and peptides is not so straight forward. The secondary and tertiary structures of the fusion peptides are extremely sensitive to the lipid composition of the membrane. It has been found that membrane fusion is being promoted by a specific structure of the peptide and the fusion efficiency changes with the change in the structure of the peptide. Therefore, the lipid composition of the membrane imparts either synergistic or opposing effect on the membrane fusion. In this review, we have highlighted the direct role of lipid composition on the membrane fusion and then we have described the structural dynamism of three different fusion peptides namely, gp41, HA and S2 in various membrane environment. Moreover, the structure determination of the membrane bound peptide is not simple as for soluble peptides. Consequently, the proposed secondary structure of the membrane bound fusion peptides depends on the technique that has been used to evaluate the structure of the peptide. The existing literature majorly discussed the role of lipid composition on the peptide structure and membrane fusion. However, the composition-dependent peptide dynamics is extremely important and the plasticity of the peptide is crucial for the accommodation of the peptide in the highly curved intermediates like stalk and transmembrane contact. Therefore, measurement of static structure of the peptide in equilibrium condition might not be the exclusive idea to interpret the role of lipid composition in membrane fusion. This review provides the current status of the direct effect of lipid composition in membrane fusion, and the indirect effect of lipid composition in modulating the structure of the fusion peptide, thereby altering membrane fusion.
Acknowledgement
This work was supported by research grant from Science and Engineering Research Board, Department of Science and Technology (SERB-DST), New Delhi (File No. ECR/2015/000195). H. C. and G. M. thank the University Grants Commission for UGC-Assistant Professor position and UGC-BSR Research Fellowship, respectively. We acknowledge Department of Science and Technology, New Delhi, and UGC for providing instrument facility to the School of Chemistry, Sambalpur University under the FIST and DRS programs, respectively. We gratefully acknowledge the critical comments and discussions by Dr. S. N. Sahu and the members of Chakraborty laboratory.
Compliance with Ethical Standards
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aloia RC, Tian H, Jensen FC. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc Natl Acad Sci USA. 1993;90:5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda FJ, Teruel JA, Ortiz A. Interaction of a synthetic peptide corresponding to the N-terminus of canine distemper virus fusion protein with phospholipid vesicles: a biophysical study. Biochim Biophys Acta. 2003;1618:51–58. doi: 10.1016/j.bbamem.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Basso LG, Vicente EF, Crusca E, Jr, Cilli EM, Costa-Filho AJ. SARS-CoV fusion peptides induce membrane surface ordering and curvature. Sci Rep. 2016;6:37131. doi: 10.1038/srep37131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal R, Durell S, Viard M. HIV entry and envelope glycoprotein-mediated fusion. J Biol Chem. 2012;287:40841–40849. doi: 10.1074/jbc.R112.406272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodner ML, Gabrys CM, Parkanzky PD, Yang J, Duskin CA, Weliky DP. Temperature dependence and resonance assignment of 13C NMR spectra of selectively and uniformly labeled fusion peptides associated with membranes. Magn Reson Chem. 2004;42:187–194. doi: 10.1002/mrc.1331. [DOI] [PubMed] [Google Scholar]
- Breckenridge WC, Morgan IG, Zanetta JP, Vincendon G. Adult rat brain synaptic vesicles II. Lipid composition. Biochim Biophys Acta. 1973;320:681–686. doi: 10.1016/0304-4165(73)90148-7. [DOI] [PubMed] [Google Scholar]
- Chakraborty H, Tarafdar PK, Bruno MJ, Sengupta T, Lentz BR. Activation thermodynamics of poly(ethylene glycol)-mediated model membrane fusion support mechanistic models of stalk and pore formation. Biophys J. 2012;102:2751–2760. doi: 10.1016/j.bpj.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty H, Tarafdar PK, Klapper DG, Lentz BR. Wild-type and mutant hemagglutinin fusion peptides alter bilayer structure as well as kinetics and activation thermodynamics of stalk and pore formation differently: mechanistic implications. Biophys J. 2013;105:2495–2506. doi: 10.1016/j.bpj.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty H, Lentz BR, Kombrabail M, Krishnamoorthy G, Chattopadhyay A. Depth-dependent membrane ordering by hemagglutinin fusion peptide promotes fusion. J Phys Chem B. 2017;121:1640–1648. doi: 10.1021/acs.jpcb.7b00684. [DOI] [PubMed] [Google Scholar]
- Chambers P, Pringle CR, Easton AJ. Heptad repeat sequences are located adjacent to hydrophobic regions in several types of virus fusion glycoproteins. J Gen Virol. 1990;71:3075–3080. doi: 10.1099/0022-1317-71-12-3075. [DOI] [PubMed] [Google Scholar]
- Chang DK, Cheng SF, Deo Trivedi V, Yang SH. The amino-terminal region of the fusion peptide of influenza virus hemagglutinin HA2 inserts into sodium dodecyl sulfate micelle with residues 16-18 at the aqueous boundary at acidic pH. Oligomerization and the conformational flexibility. J Biol Chem. 2000;275:19150–19158. doi: 10.1074/jbc.M907148199. [DOI] [PubMed] [Google Scholar]
- Chernomordik L. Non-bilayer lipids and biological fusion intermediates. Chem Phys Lipids. 1996;81:203–213. doi: 10.1016/0009-3084(96)02583-2. [DOI] [PubMed] [Google Scholar]
- Chernomordik LV, Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annu Rev Biochem. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
- Chernomordik LV, Kozlov MM. Membrane hemifusion: crossing a chasm in two leaps. Cell. 2005;123:375–382. doi: 10.1016/j.cell.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Chernomordik LV, Melikyan GB, Chizmadzhev YA. Biomembrane fusion: a new concept derived from model studies using two interacting planar lipid bilayers. Biochim Biophys Acta. 1987;906:309–352. doi: 10.1016/0304-4157(87)90016-5. [DOI] [PubMed] [Google Scholar]
- Chernomordik LV, Vogel SS, Sokoloff A, Onaran HO, Leikina EA, Zimmerberg J. Lysolipids reversibly inhibit Ca(2+)-, GTP- and pH-dependent fusion of biological membranes. FEBS Lett. 1993;318:71–76. doi: 10.1016/0014-5793(93)81330-3. [DOI] [PubMed] [Google Scholar]
- Chernomordik L, Kozlov MM, Zimmerberg J. Lipids in biological membrane fusion. J Membr Biol. 1995;146:1–14. doi: 10.1007/BF00232676. [DOI] [PubMed] [Google Scholar]
- Colotto A, Epand RM. Structural study of the relationship between the rate of membrane fusion and the ability of the fusion peptide of influenza virus to perturb bilayers. Biochemistry. 1997;36:7644–7651. doi: 10.1021/bi970382u. [DOI] [PubMed] [Google Scholar]
- Cotman C, Blank ML, Moehl A, Snyder F. Lipid composition of synaptic plasma membranes isolated from rat brain by zonal centrifugation. Biochemistry. 1969;8:4606–4612. doi: 10.1021/bi00839a056. [DOI] [PubMed] [Google Scholar]
- Curtain C, Separovic F, Nielsen K, Craik D, Zhong Y, Kirkpatrick A. The interactions of the N-terminal fusogenic peptide of HIV-1 gp41 with neutral phospholipids. Eur Biophys J. 1999;28:427–436. doi: 10.1007/s002490050225. [DOI] [PubMed] [Google Scholar]
- Domanska MK, Wrona D, Kasson PM. Multiphasic effects of cholesterol on influenza fusion kinetics reflect multiple mechanistic roles. Biophys J. 2013;105:1383–1387. doi: 10.1016/j.bpj.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durell SR, Martin I, Ruysschaert JM, Shai Y, Blumenthal R. What studies of fusion peptides tell us about viral envelope glycoprotein-mediated membrane fusion. Mol Membr Biol. 1997;14:97–112. doi: 10.3109/09687689709048170. [DOI] [PubMed] [Google Scholar]
- Duzgunes N, Allen TM, Fedor J, Papahadjopoulos D. Lipid mixing during membrane aggregation and fusion: why fusion assays disagree. Biochemistry. 1987;26:8435–8442. doi: 10.1021/bi00399a061. [DOI] [PubMed] [Google Scholar]
- Earp LJ, Delos SE, Park HE, White JM. The many mechanisms of viral membrane fusion proteins. Curr Top Microbiol Immunol. 2005;285:25–66. doi: 10.1007/3-540-26764-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellens H, Siegel DP, Alford D, Yeagle PL, Boni L, Lis LJ, Quinn PJ, Bentz J. Membrane fusion and inverted phases. Biochemistry. 1989;28:3692–3703. doi: 10.1021/bi00435a011. [DOI] [PubMed] [Google Scholar]
- Epand RM. Fusion peptides and the mechanism of viral fusion. Biochim Biophys Acta. 2003;1614:116–121. doi: 10.1016/s0005-2736(03)00169-x. [DOI] [PubMed] [Google Scholar]
- Epand RM. Proteins and cholesterol-rich domains. Biochim Biophys Acta. 2008;1778:1576–1582. doi: 10.1016/j.bbamem.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Epand RF, Martin I, Ruysschaert JM, Epand RM. Membrane orientation of the SIV fusion peptide determines its effect on bilayer stability and ability to promote membrane fusion. Biochem Biophys Res Commun. 1994;205:1938–1943. doi: 10.1006/bbrc.1994.2897. [DOI] [PubMed] [Google Scholar]
- Falck E, Patra M, Karttunen M, Hyvonen MT, Vattulainen I. Impact of cholesterol on voids in phospholipid membranes. J Chem Phys. 2004;121:12676–12689. doi: 10.1063/1.1824033. [DOI] [PubMed] [Google Scholar]
- Gallaher WR. Similar structural models of the transmembrane proteins of Ebola and avian sarcoma viruses. Cell. 1996;85:477–478. doi: 10.1016/s0092-8674(00)81248-9. [DOI] [PubMed] [Google Scholar]
- Ge M, Freed JH. Fusion peptide from influenza hemagglutinin increases membrane surface order: an electron-spin resonance study. Biophys J. 2009;96:4925–4934. doi: 10.1016/j.bpj.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething MJ, Doms RW, York D, White J. Studies on the mechanism of membrane fusion: site-specific mutagenesis of the hemagglutinin of influenza virus. J Cell Biol. 1986;102:11–23. doi: 10.1083/jcb.102.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillen J, de Almeida RF, Prieto M, Villalain J. Structural and dynamic characterization of the interaction of the putative fusion peptide of the S2 SARS-CoV virus protein with lipid membranes. J Phys Chem B. 2008;112:6997–7007. doi: 10.1021/jp7118229. [DOI] [PubMed] [Google Scholar]
- Guillen J, Kinnunen PK, Villalain J. Membrane insertion of the three main membranotropic sequences from SARS-CoV S2 glycoprotein. Biochim Biophys Acta. 2008;1778:2765–2774. doi: 10.1016/j.bbamem.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther-Ausborn S, Praetor A, Stegmann T. Inhibition of influenza-induced membrane fusion by lysophosphatidylcholine. J Biol Chem. 1995;270:29279–29285. doi: 10.1074/jbc.270.49.29279. [DOI] [PubMed] [Google Scholar]
- Haldar S, Mekhedov E, McCormick CD, Blank PS, Zimmerberg J. Lipid-dependence of target membrane stability during influenza viral fusion. J Cell Sci. 2018;132:218321. doi: 10.1242/jcs.218321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Bushweller JH, Cafiso DS, Tamm LK. Membrane structure and fusion-triggering conformational change of the fusion domain from influenza hemagglutinin. Nat Struct Biol. 2001;8:715–720. doi: 10.1038/90434. [DOI] [PubMed] [Google Scholar]
- Haque ME, McIntosh TJ, Lentz BR. Influence of lipid composition on physical properties and peg-mediated fusion of curved and uncurved model membrane vesicles: “nature’s own” fusogenic lipid bilayer. Biochemistry. 2001;40:4340–4348. doi: 10.1021/bi002030k. [DOI] [PubMed] [Google Scholar]
- Haque ME, Koppaka V, Axelsen PH, Lentz BR. Properties and structures of the influenza and HIV fusion peptides on lipid membranes: implications for a role in fusion. Biophys J. 2005;89:3183–3194. doi: 10.1529/biophysj.105.063032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque ME, Chakraborty H, Koklic T, Komatsu H, Axelsen PH, Lentz BR. Hemagglutinin fusion peptide mutants in model membranes: structural properties, membrane physical properties, and PEG-mediated fusion. Biophys J. 2011;101:1095–1104. doi: 10.1016/j.bpj.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, Tripet B, Jobling MG, Holmes RK, Holmes KV, Hodges RS. Dissection of the fusion machine of SARS-coronavirus. Adv Exp Med Biol. 2006;581:319–322. doi: 10.1007/978-0-387-33012-9_56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingallinella P, Bianchi E, Finotto M, Cantoni G, Eckert DM, Supekar VM, Bruckmann C, Carfi A, Pessi A. Structural characterization of the fusion-active complex of severe acute respiratory syndrome (SARS) coronavirus. Proc Natl Acad Sci USA. 2004;101:8709–8714. doi: 10.1073/pnas.0402753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Jaroniec CP, Kaufman JD, Stahl SJ, Viard M, Blumenthal R, Wingfield PT, Bax A. Structure and dynamics of micelle-associated human immunodeficiency virus gp41 fusion domain. Biochemistry. 2005;44:16167–16180. doi: 10.1021/bi051672a. [DOI] [PubMed] [Google Scholar]
- Jeffers SA, Tusell SM, Gillim-Ross L, Hemmila EM, Achenbach JE, Babcock GJ, Thomas WD, Jr, Thackray LB, Young MD, Mason RJ, et al. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc Natl Acad Sci USA. 2004;101:15748–15753. doi: 10.1073/pnas.0403812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasson PM, Pande VS. Control of membrane fusion mechanism by lipid composition: predictions from ensemble molecular dynamics. PLoS Comput Biol. 2007;3:e220. doi: 10.1371/journal.pcbi.0030220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsov K, Muller M, Schick M. Field theoretic study of bilayer membrane fusion I. Hemifusion mechanism. Biophys J. 2004;87:3277–3290. doi: 10.1529/biophysj.103.038943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov MM, Leikin SL, Chernomordik LV, Markin VS, Chizmadzhev YA. Stalk mechanism of vesicle fusion. Intermixing of aqueous contents. Eur Biophys J. 1989;17:121–129. doi: 10.1007/BF00254765. [DOI] [PubMed] [Google Scholar]
- Kozlovsky Y, Kozlov MM. Stalk model of membrane fusion: solution of energy crisis. Biophys J. 2002;82:882–895. doi: 10.1016/S0006-3495(02)75450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlovsky Y, Chernomordik LV, Kozlov MM. Lipid intermediates in membrane fusion: formation, structure, and decay of hemifusion diaphragm. Biophys J. 2002;83:2634–2651. doi: 10.1016/S0006-3495(02)75274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlovsky Y, Efrat A, Siegel DP, Kozlov MM. Stalk phase formation: effects of dehydration and saddle splay modulus. Biophys J. 2004;87:2508–2521. doi: 10.1529/biophysj.103.038075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin PI, Zimmerberg J, Chizmadzhev YA, Cohen FS. A quantitative model for membrane fusion based on low-energy intermediates. Proc Natl Acad Sci USA. 2001;98:7235–7240. doi: 10.1073/pnas.121191898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AL, Freed JH. HIV gp41 fusion peptide increases membrane ordering in a cholesterol-dependent fashion. Biophys J. 2014;106:172–181. doi: 10.1016/j.bpj.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AL, Park H, White JM, Tamm LK. Fusion peptide of influenza hemagglutinin requires a fixed angle boomerang structure for activity. J Biol Chem. 2006;281:5760–5770. doi: 10.1074/jbc.M512280200. [DOI] [PubMed] [Google Scholar]
- Lai AL, Moorthy AE, Li Y, Tamm LK. Fusion activity of HIV gp41 fusion domain is related to its secondary structure and depth of membrane insertion in a cholesterol-dependent fashion. J Mol Biol. 2012;418:3–15. doi: 10.1016/j.jmb.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AL, Millet JK, Daniel S, Freed JH, Whittaker GR. The SARS-CoV fusion peptide forms an extended bipartite fusion platform that perturbs membrane order in a calcium-dependent manner. J Mol Biol. 2017;429:3875–3892. doi: 10.1016/j.jmb.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Lentz BR. Outer leaflet-packing defects promote poly(ethylene glycol)-mediated fusion of large unilamellar vesicles. Biochemistry. 1997;36:421–431. doi: 10.1021/bi9622332. [DOI] [PubMed] [Google Scholar]
- Leikin S, Kozlov MM, Fuller NL, Rand RP. Measured effects of diacylglycerol on structural and elastic properties of phospholipid membranes. Biophys J. 1996;71:2623–2632. doi: 10.1016/S0006-3495(96)79454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz BR, Hoechli M, Barenholz Y. Acyl chain order and lateral domain formation in mixed phosphatidylcholine–sphingomyelin multilamellar and unilamellar vesicles. Biochemistry. 1981;20:6803–6809. doi: 10.1021/bi00527a010. [DOI] [PubMed] [Google Scholar]
- Lentz BR, Carpenter TJ, Alford DR. Spontaneous fusion of phosphatidylcholine small unilamellar vesicles in the fluid phase. Biochemistry. 1987;26:5389–5397. doi: 10.1021/bi00391a026. [DOI] [PubMed] [Google Scholar]
- Lentz BR, Malinin V, Haque ME, Evans K. Protein machines and lipid assemblies: current views of cell membrane fusion. Curr Opin Struct Biol. 2000;10:607–615. doi: 10.1016/s0959-440x(00)00138-x. [DOI] [PubMed] [Google Scholar]
- Li Y, Tamm LK. Structure and plasticity of the human immunodeficiency virus gp41 fusion domain in lipid micelles and bilayers. Biophys J. 2007;93:876–885. doi: 10.1529/biophysj.106.102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorieau JL, Louis JM, Bax A. Helical hairpin structure of influenza hemagglutinin fusion peptide stabilized by charge-dipole interactions between the N-terminal amino group and the second helix. J Am Chem Soc. 2011;133:2824–2827. doi: 10.1021/ja1099775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luidens MK, Figge J, Breese K, Vajda S. Predicted and trifluoroethanol-induced alpha-helicity of polypeptides. Biopolymers. 1996;39:367–376. doi: 10.1002/(SICI)1097-0282(199609)39:3%3C367::AID-BIP8%3E3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Madu IG, Roth SL, Belouzard S, Whittaker GR. Characterization of a highly conserved domain within the severe acute respiratory syndrome coronavirus spike protein S2 domain with characteristics of a viral fusion peptide. J Virol. 2009;83:7411–7421. doi: 10.1128/JVI.00079-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan M, Bhattacharjya S. NMR structures and localization of the potential fusion peptides and the pre-transmembrane region of SARS-CoV: implications in membrane fusion. Biochim Biophys Acta. 2015;1848:721–730. doi: 10.1016/j.bbamem.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinin VS, Lentz BR. Energetics of vesicle fusion intermediates: comparison of calculations with observed effects of osmotic and curvature stresses. Biophys J. 2004;86:2951–2964. doi: 10.1016/S0006-3495(04)74346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I, Schaal H, Scheid A, Ruysschaert JM. Lipid membrane fusion induced by the human immunodeficiency virus type 1 gp41 N-terminal extremity is determined by its orientation in the lipid bilayer. J Virol. 1996;70:298–304. doi: 10.1128/jvi.70.1.298-304.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters PS. The molecular biology of coronaviruses. Adv Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh TJ, Simon SA, Needham D, Huang CH. Structure and cohesive properties of sphingomyelin/cholesterol bilayers. Biochemistry. 1992;31:2012–2020. doi: 10.1021/bi00122a017. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- Meher G, Chakraborty H. Organization and dynamics of Trp14 of hemagglutinin fusion peptide in membrane mimetic environment. Chem Phys Lipids. 2017;205:48–54. doi: 10.1016/j.chemphyslip.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Melikyan GB. Driving a wedge between viral lipids blocks infection. Proc Natl Acad Sci USA. 2010;107:17069–17070. doi: 10.1073/pnas.1012748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley PW, Waring AJ, Sherman MA, Gordon LM. Membrane interactions of the synthetic N-terminal peptide of HIV-1 gp41 and its structural analogs. Biochim Biophys Acta. 1999;1418:1–18. doi: 10.1016/s0005-2736(99)00014-0. [DOI] [PubMed] [Google Scholar]
- Mohler WA, Shemer G, del Campo JJ, Valansi C, Opoku-Serebuoh E, Scranton V, Assaf N, White JG, Podbilewicz B. The type I membrane protein EFF-1 is essential for developmental cell fusion. Dev Cell. 2002;2:355–362. doi: 10.1016/s1534-5807(02)00129-6. [DOI] [PubMed] [Google Scholar]
- Muller M, Katsov K, Schick M. A new mechanism of model membrane fusion determined from Monte Carlo simulation. Biophys J. 2003;85:1611–1623. doi: 10.1016/S0006-3495(03)74592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham D, Nunn RS. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys J. 1990;58:997–1009. doi: 10.1016/S0006-3495(90)82444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieva JL, Nir S, Muga A, Goni FM, Wilschut J. Interaction of the HIV-1 fusion peptide with phospholipid vesicles: different structural requirements for fusion and leakage. Biochemistry. 1994;33:3201–3209. doi: 10.1021/bi00177a009. [DOI] [PubMed] [Google Scholar]
- Nir S, Wilschut J, Bentz J. The rate of fusion of phospholipid vesicles and the role of bilayer curvature. Biochim Biophys Acta. 1982;688:275–278. doi: 10.1016/0005-2736(82)90604-6. [DOI] [PubMed] [Google Scholar]
- Noguchi H, Takasu M. Self-assembly of amphiphiles into vesicles: a Brownian dynamics simulation. Phys Rev E. 2001;64:041913. doi: 10.1103/PhysRevE.64.041913. [DOI] [PubMed] [Google Scholar]
- Pereira FB, Goni FM, Muga A, Nieva JL. Permeabilization and fusion of uncharged lipid vesicles induced by the HIV-1 fusion peptide adopting an extended conformation: dose and sequence effects. Biophys J. 1997;73:1977–1986. doi: 10.1016/S0006-3495(97)78228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinot M, Vanni S, Pagnotta S, Lacas-Gervais S, Payet LA, Ferreira T, Gautier R, Goud B, Antonny B, Barelli H. Lipid cell biology. Polyunsaturated phospholipids facilitate membrane deformation and fission by endocytic proteins. Science. 2014;345:693–697. doi: 10.1126/science.1255288. [DOI] [PubMed] [Google Scholar]
- Porcelli F, Buck-Koehntop BA, Thennarasu S, Ramamoorthy A, Veglia G. Structures of the dimeric and monomeric variants of magainin antimicrobial peptides (MSI-78 and MSI-594) in micelles and bilayers, determined by NMR spectroscopy. Biochemistry. 2006;45:5793–5799. doi: 10.1021/bi0601813. [DOI] [PubMed] [Google Scholar]
- Qiang W, Bodner ML, Weliky DP. Solid-state NMR spectroscopy of human immunodeficiency virus fusion peptides associated with host-cell-like membranes: 2D correlation spectra and distance measurements support a fully extended conformation and models for specific antiparallel strand registries. J Am Chem Soc. 2008;130:5459–5471. doi: 10.1021/ja077302m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang W, Sun Y, Weliky DP. A strong correlation between fusogenicity and membrane insertion depth of the HIV fusion peptide. Proc Natl Acad Sci USA. 2009;106:15314–15319. doi: 10.1073/pnas.0907360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafalski M, Lear JD, DeGrado WF. Phospholipid interactions of synthetic peptides representing the N-terminus of HIV gp41. Biochemistry. 1990;29:7917–7922. doi: 10.1021/bi00486a020. [DOI] [PubMed] [Google Scholar]
- Raffy S, Teissie J. Control of lipid membrane stability by cholesterol content. Biophys J. 1999;76:2072–2080. doi: 10.1016/S0006-3495(99)77363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz B, Jr, Rausch JM, Gallaher WR, Garry RF, Wimley WC. Identification and characterization of the putative fusion peptide of the severe acute respiratory syndrome-associated coronavirus spike protein. J Virol. 2005;79:7195–7206. doi: 10.1128/JVI.79.11.7195-7206.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanan R, Bhattacharjya S. Oligomeric structure of a cathelicidin antimicrobial peptide in dodecylphosphocholine micelle determined by NMR spectroscopy. Biochim Biophys Acta. 2011;1808:369–381. doi: 10.1016/j.bbamem.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Schmick SD, Weliky DP. Major antiparallel and minor parallel beta sheet populations detected in the membrane-associated human immunodeficiency virus fusion peptide. Biochemistry. 2010;49:10623–10635. doi: 10.1021/bi101389r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta T, Chakraborty H, Lentz BR. The transmembrane domain peptide of vesicular stomatitis virus promotes both intermediate and pore formation during PEG-mediated vesicle fusion. Biophys J. 2014;107:1318–1326. doi: 10.1016/j.bpj.2014.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Hu J, Kozlov MM, Rapoport TA. Mechanisms shaping the membranes of cellular organelles. Annu Rev Cell Dev Biol. 2009;25:329–354. doi: 10.1146/annurev.cellbio.042308.113324. [DOI] [PubMed] [Google Scholar]
- Siegel DP. Energetics of intermediates in membrane fusion: comparison of stalk and inverted micellar intermediate mechanisms. Biophys J. 1993;65:2124–2140. doi: 10.1016/S0006-3495(93)81256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel DP, Epand RM. The mechanism of lamellar-to-inverted hexagonal phase transitions in phosphatidylethanolamine: implications for membrane fusion mechanisms. Biophys J. 1997;73:3089–3111. doi: 10.1016/S0006-3495(97)78336-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- St Vincent MR, Colpitts CC, Ustinov AV, Muqadas M, Joyce MA, Barsby NL, Epand RF, Epand RM, Khramyshev SA, Valueva OA, et al. Rigid amphipathic fusion inhibitors, small molecule antiviral compounds against enveloped viruses. Proc Natl Acad Sci USA. 2010;107:17339–17344. doi: 10.1073/pnas.1010026107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot WA, Zheng LX, Lentz BR. Acyl chain unsaturation and vesicle curvature alter outer leaflet packing and promote poly(ethylene glycol)-mediated membrane fusion. Biochemistry. 1997;36:5827–5836. doi: 10.1021/bi962437i. [DOI] [PubMed] [Google Scholar]
- Tamm LK. Hypothesis: spring-loaded boomerang mechanism of influenza hemagglutinin-mediated membrane fusion. Biochim Biophys Acta. 2003;1614:14–23. doi: 10.1016/s0005-2736(03)00159-7. [DOI] [PubMed] [Google Scholar]
- Tristram-Nagle S, Nagle JF. HIV-1 fusion peptide decreases bending energy and promotes curved fusion intermediates. Biophys J. 2007;93:2048–2055. doi: 10.1529/biophysj.107.109181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanni S, Hirose H, Barelli H, Antonny B, Gautier R. A sub-nanometre view of how membrane curvature and composition modulate lipid packing and protein recruitment. Nat Commun. 2014;5:4916. doi: 10.1038/ncomms5916. [DOI] [PubMed] [Google Scholar]
- Walter A, Yeagle PL, Siegel DP. Diacylglycerol and hexadecane increase divalent cation-induced lipid mixing rates between phosphatidylserine large unilamellar vesicles. Biophys J. 1994;66:366–376. doi: 10.1016/s0006-3495(94)80786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JM. Viral and cellular membrane fusion proteins. Annu Rev Physiol. 1990;52:675–697. doi: 10.1146/annurev.ph.52.030190.003331. [DOI] [PubMed] [Google Scholar]
- Wilschut J, Duzgunes N, Hoekstra D, Papahadjopoulos D. Modulation of membrane fusion by membrane fluidity: temperature dependence of divalent cation induced fusion of phosphatidylserine vesicles. Biochemistry. 1985;24:8–14. doi: 10.1021/bi00322a002. [DOI] [PubMed] [Google Scholar]
- Wu H, Zheng L, Lentz BR. A slight asymmetry in the transbilayer distribution of lysophosphatidylcholine alters the surface properties and poly(ethylene glycol)-mediated fusion of dipalmitoylphosphatidylcholine large unilamellar vesicles. Biochemistry. 1996;35:12602–12611. doi: 10.1021/bi960168q. [DOI] [PubMed] [Google Scholar]
- Xu X, London E. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry. 2000;39:843–849. doi: 10.1021/bi992543v. [DOI] [PubMed] [Google Scholar]
- Xu Y, Zhu J, Liu Y, Lou Z, Yuan F, Cole DK, Ni L, Su N, Qin L, Li X, et al. Characterization of the heptad repeat regions, HR1 and HR2, and design of a fusion core structure model of the spike protein from severe acute respiratory syndrome (SARS) coronavirus. Biochemistry. 2004;43:14064–14071. doi: 10.1021/bi049101q. [DOI] [PubMed] [Google Scholar]
- Yang J, Gabrys CM, Weliky DP. Solid-state nuclear magnetic resonance evidence for an extended beta strand conformation of the membrane-bound HIV-1 fusion peptide. Biochemistry. 2001;40:8126–8137. doi: 10.1021/bi0100283. [DOI] [PubMed] [Google Scholar]
- Yang ST, Kreutzberger AJB, Lee J, Kiessling V, Tamm LK. The role of cholesterol in membrane fusion. Chem Phys Lipids. 2016;199:136–143. doi: 10.1016/j.chemphyslip.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]