Abstract
Within the recent years, RNA interference (RNAi) has become an almost-standard method for in vitro knockdown of any target gene of interest. Now, one major focus is to further explore its potential in vivo, including the development of novel therapeutic strategies. From the mechanism, it becomes clear that small interfering RNAs (siRNAs) play a pivotal role in triggering RNAi. Thus, the efficient delivery of target gene-specific siRNAs is one major challenge in the establishment of therapeutic RNAi. Numerous studies, based on different modes of administration and various siRNA formulations and/or modifications, have already accumulated promising results. This applies to various animal models covering viral infections, cancer and multiple other diseases. Continuing efforts will lead to the development of efficient and “double-specific” drugs, comprising of siRNAs with high target gene specificity and of nanoparticles enhancing siRNA delivery and target organ specificity.
Keywords: RNA interference, RNAi, siRNA, Gene-targeting, Gene knockdown, Nonviral siRNA delivery, Nanoplexes
Introduction
After antisense technologies and ribozymes, in the late 1990s a novel mechanism for gene-targeting was discovered: RNA interference (RNAi). It soon became clear that RNAi represents a particularly efficient and—at least in vitro—easy-to-use method for the knockdown of the expression of a selected target gene. Consequently, RNAi is now a well-established method for high-throughput analyses as well as for functional studies in vitro, including mammalian cells.
Many pathological conditions rely on the aberrant expression of endogenous normal or mutant genes causing, e.g., alterations in signal transduction pathways, cellular proliferation, apoptosis, or resistance toward external factors. Additionally, the infection of an organism can lead to the introduction and expression of foreign genes. While the inhibition of the activity of (aberrant) gene products, e.g., through small molecule inhibitors or inhibitory antibodies is one major focus in therapy, much attention has now shifted to an earlier step, i.e., the initial knockdown of the specific gene of interest through RNAi. However, for the in vivo application of RNAi in mammals as a therapeutic approach for reversing a pathological condition as well as for the study of particular gene functions, sophisticated strategies for the induction of RNAi are needed.
Mechanism and induction of RNAi
RNAi is a naturally occurring, sequence-specific mechanism for gene silencing. Its discovery in the nematode C. elegans (Fire et al. 1998) was awarded the 2006 Nobel prize for physiology or medicine. However, soon it became obvious that RNAi, although somewhat more complicated, also exists in higher organisms including mammals. RNAi relies on an intracellular multistep process, which can roughly be divided into the initiation phase (see below) and the subsequent effector phase. In the effector phase (Fig. 1, left), which represents the actual RNAi mechanism, small, 21–23 bp double stranded RNA molecules (small interfering RNAs, siRNAs), are incorporated into the RNA-induced silencing complex (RISC; Hammond et al. 2000). Upon adenosine triphosphate (ATP)-dependent unwinding of the double-stranded siRNA molecule through an RNA helicase activity into a single-stranded, so-called guidance RNA (Nykanen et al. 2001), the now activated RISC (RISC*) binds to its target mRNA molecule (Martinez et al. 2002; Nykanen et al. 2001). This process is mediated through the sequence-specific hybridization of the guidance RNA to the mRNA target site and brings RISC into close proximity to its target mRNA molecule, which is then cleaved by the RISC nuclease Argonaute 2 (Ago 2) and rapidly degraded due to its now unprotected ends (Liu et al. 2004; Rand et al. 2004; Rivas et al. 2005). Since RISC is recovered for subsequent rounds, this represents a catalytical process leading to the selective reduction in specific mRNA molecules and thus resulting in decreased expression of the targeted gene. The mechanism also demonstrates the pivotal role of siRNA molecules in initiating RNAi and established the delivery of siRNA molecules as sufficient for RNAi induction (Elbashir et al. 2001a, b).
Fig. 1.
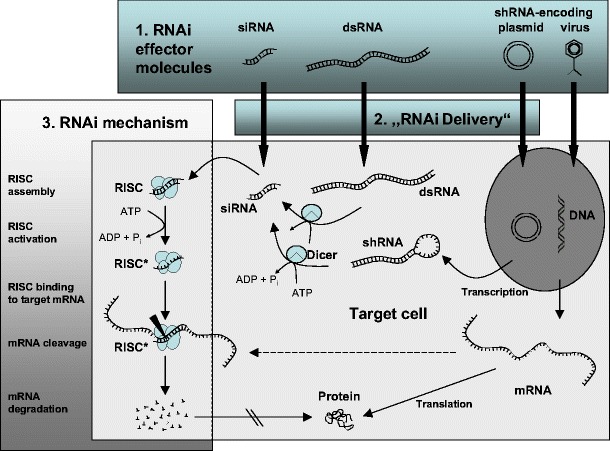
RNA interference (RNAi) is an intracellular mechanism which can be triggered through different effector molecules (upper part). Upon their incorporation into the RNA-induced silencing complex (RISC), siRNAs are unwinded, and, as single-stranded so-called guidance RNAs, mediate the hybridization of the now activated complex (RISC*) to its target mRNA. This results in target mRNA cleavage and subsequent degradation, thus emphasizing the pivotal role of siRNAs in the process (left). The exploration of RNAi in vivo requires strategies for the intracellular delivery of siRNAs or “upstream” initiation molecules, i.e., molecules that lead to the intracellular formation of siRNAs (center)
Induction of RNAi: the initiation phase
In a natural, experimental or therapeutical setting, siRNAs can be directly or as precursor molecules introduced into a target cell through different strategies (Fig. 1, upper part). This includes viral or nonviral delivery of DNAs, which are transcribed into long, double-stranded RNA molecules. In the so-called initiation phase, these dsRNAs are cleaved into siRNAs by the multiprotein complex “Dicer”, which contains an N-terminal helicase domain, an RNA-binding so-called Piwi/Argonaute/Zwille (PAZ) domain, two RNAse III domains and a double-stranded RNA binding domain (Bernstein et al. 2001; Collins and Cheng 2005). Commercially available systems explore this mechanism by providing DNA vector constructs coding for short hairpin RNAs (shRNAs): The double-stranded region of the shRNA is formed through hairpin formation and intramolecular hybridization and is recognized by Dicer, leading to the formation of siRNAs homologous to the target gene of interest. Alternatively, shRNA molecules can be directly introduced into the cell. However, one major disadvantage of long double-stranded RNA molecules, either directly introduced or intracellularly transcribed, is the induction of a cellular immune response through activation of the interferon system. The direct delivery of siRNA molecules into the target cell strategy largely avoids this problem, although some interferon-stimulating sequences are known as well. Furthermore, it does not require the action of Dicer (Bridge et al. 2003; Hornung et al. 2005; Sledz et al. 2003).
Systematic studies on targeting efficacies have shown that optimal siRNAs can be deduced according to certain selection rules. This includes an optimal length of 19–25 bp and a guanine–cytosine content between 30 and 52%, symmetric two nucleotide 3′ overhangs as well as specific nucleotides at certain positions (see, e.g., Dykxhoorn and Lieberman 2006 for review). Based on these already established criteria, several computer-based algorithms allow the identification of optimal siRNA sequences for any given gene of interest. One example is the siRNA Design Software (SDS) by the University of Hong Kong which combines algorithms from different companies and is accessible through the internet (http://i.cs.hku.hk/~sirna/software/sirna.php). Nevertheless, any presumably optimal siRNA still requires extensive testing. This refers to a high targeting efficacy, which is, among others, also determined by variations in the accessibility of the target mRNA at different positions, as well as to the absence of any unwanted side effects. In fact, nonspecific silencing of genes due to only partial sequence homology has been described (Jackson et al. 2003). Furthermore, in vivo some siRNA sequences, as well as longer dsRNA molecules, have been shown to activate the innate immune system leading to nonspecific effects due to the stimulation of inflammatory responses (Heil et al. 2004; Judge et al. 2005; Sioud 2005; Sledz et al. 2003). This phenomenon seems to depend on the presence of GU-rich sequences as well as on the formulation and amount of siRNAs (Heidel et al. 2004; Ma et al. 2005; Sioud and Sorensen 2003), and these aspects need to be considered for any therapeutic siRNA application in vivo.
Use of siRNA molecules in vivo
Since the discovery of the pivotal role of siRNAs for inducing RNAi (Elbashir et al. 2001a, b), the direct application of siRNA molecules has been explored in vitro and in vivo. In vitro, several transfection reagents allow the delivery of siRNAs in mammalian cells in the presence or absence of serum. The in vivo application of siRNAs, however, requires the development of more sophisticated formulations and/or the identification of optimal modes of administration (see below).
Several proof-of-principle studies have shown the delivery of fluorophor-labeled siRNA molecules into various organs (see, e.g., Bradley et al. 2005a, b; Pirollo et al. 2006; Sioud and Sorensen 2003). Beyond that, the specific in vivo knockdown of artificially introduced reporter genes like GFP or luciferase, or various endogenous target genes, has been described. The target organ was often the liver, but gene targeting in other organs, in other parts of the body, or in tumor xenografts has been reported as well (Table 1). Taken together, these studies provide valuable insights into the delivery and efficacy of siRNAs for the induction of RNAi.
Table 1.
Overview on studies employing siRNA-mediated gene-targeting in vivo
| Location/target organ/target mechanism/aim | Targeted gene product | Reference |
|---|---|---|
| Proof-of-principle | ||
| Reporter genes | ||
| Peritoneal cavity | GFP | de Jonge et al. 2005 |
| Developing vascular network of chicken embryo | GFP | Bollerot et al. 2006 |
| S.c. HeLa xenograft | GFP | Bertrand et al. 2002 |
| Liver | GFP | Lewis et al. 2002 |
| Muscle | GFP | Golzio et al. 2005 |
| Bronchiole epithelial cells | EGFP | Howard et al. 2006 |
| Liver and limb grafts | DsRed2, GFP | Sato et al. 2005 |
| Liver | Luciferase | McCaffrey et al. 2002 |
| Brain | Luciferase | Hassani et al. 2005 |
| S.c. melanoma xenografts/hepatic metastases | Luciferase | Takahashi et al. 2005 |
| Endogenous genes | ||
| Liver | Fas | Heidel et al. 2004 |
| Pancreas | Ins2 | Bradley et al. 2005a, b |
| Liver | mdr1a/1b | Matsui et al. 2005 |
| Liver | APOB | Zimmermann et al. 2006 |
| Hypothalamus | TRβ1 + 2 | Guissouma et al. 2006 |
| Vasculature | CD31, Tie2 | Santel et al. 2006a, b |
| Cancer | ||
| Tumor growth inhibition | ||
| Pancreatic adenocarcinoma xenografts | CEACAM6 | Duxbury et al. 2004 |
| Fibrosarcoma xenografts | VEGF | Filleur et al. 2003 |
| S.c. pancreatic carcinoma xenografts | bcl-2 | Ocker et al. 2005 |
| Bladder cancer xenografts | Survivin | Hou et al. 2006 |
| Peritoneal cavity | β-catenin | Verma et al. 2003 |
| Bladder cancer | PLK-1 | Nogawa et al. 2005 |
| S.c. prostate carcinoma xenografts | bcl-2 | Yano et al. 2004 |
| Prostate cancer xenografts | Raf-1 | Pal et al. 2005 |
| S.c. breast cancer xenografts | c-raf | Chien et al. 2005 |
| Ovarian cancer xenografts | FAK | Halder et al. 2006 |
| Liver tumor xenografts | PTEN, CD31 | Santel et al. 2006a, b |
| Breast tumor xenografts | Raf-1 | Leng and Mixson 2005 |
| S.c. prostate carcinoma xenografts | VEGF | Takei et al. 2004 |
| Orthotopic germ cell tumor xenografts (testes) | HST-1/FGF-4 | Minakuchi et al. 2004 |
| S.c. melanoma xenografts | c-myc, MDM2, VEGF | Song et al. 2005 |
| S.c. ovarian carcinoma xenografts | HER-2 | Urban-Klein et al. 2005 |
| S.c. N2A neuroblastoma xenografts | VEGF R2 | Schiffelers et al. 2004 |
| S.c. breast cancer xenografts | RhoA | Pille et al. 2006 |
| S.c. pancreatic carcinoma xenografts | Mutant K-ras | Zhu et al. 2006 |
| Cervical cancer xenografts | HPV E6 + E7 | Fujii et al. 2006 |
| Melanoma xenografts | SOCS1 | Yang et al. 2006 |
| Blockage of cancer metastasis | ||
| Metastatic breast cancer cells | CXCR4 | Liang et al. 2005 |
| Lung metastasis | Tissue factor | Amarzguioui et al. 2006 |
| Liver metastasis | bcl-2 | Yano et al. 2004 |
| Bone-metastatic prostate cancer | EZH2 | Takeshita et al. 2005 |
| Others | ||
| Cancer vaccine potency (antigen-presenting cells) | Bak, Bax | Kim et al. 2005 |
| Breast cancer xenografts, induction of tumor apoptosis | HER-2 | Hogrefe et al. 2006 |
| S.c. HeLa xenografts, enhancement of cisplatin effect | Rad51 | Ito et al. 2005 |
| Vein grafts, attenuation of intimal hyperplasia | Midkine | Banno et al. 2006 |
| Viral infections | ||
| Inhibition of HBV replication | HBsAg | Giladi et al. 2003; Klein et al. 2003 |
| Coxsackieviral cytopathogenicity | CVB 2A | Merl et al. 2005 |
| Influenza virus infections | Nucleoprotein, acidic polymerase | Tompkins et al. 2004 |
| Respiratory viral diseases | RSV-P, PIV-P | Bitko et al. 2005 |
| Reduction of plasma viremia levels | ZEBOV L | Geisbert et al. 2006 |
| Reduced serum HBV DNA | HBV, HBsAg | Morrissey et al. 2005a, b |
| Influenza virus infections | Influenza virus genes | Ge et al. 2004 |
| Respiratory viral diseases | RSV-P, PIV-P | Bitko et al. 2005 |
| Organ-specific effects | ||
| Liver | ||
| Fas-mediated apoptosis/acute liver failure | Caspase-8 | Zender et al. 2003 |
| Fulminant hepatitis | Fas | Song et al. 2003 |
| Kidney | ||
| Renal ischemia-reperfusion injury | Fas | Hamar 2004 no. 829 |
| Glomerulonephritis | TGF-β1 | Takabatake, 2005 no. 8273 |
| Lung | ||
| Hemorrhagic shock and sepsis (lung) | Fas | Perl et al. 2005 |
| Acute lung injury | KC, MIP-2 | Lomas-Neira et al. 2005 |
| Functional analysis in lung ischemia-reperfusion injury | HO-1 | Zhang et al. 2004a, b |
| Increase in lung vascular permeability | Caveolin-1 | Miyawaki-Shimizu et al. 2005 |
| Decreased formation of obstructive bronchiolitis | MIF | Fukuyama et al. 2005 |
| CNS | ||
| Reduction of brain-to-blood transport | Organic anion transporter 3 | Hino et al. 2006 |
| Chronic neuropathic pain/decreased hyperanalgesia | Pain-related cation channel P2X3 | Dorn et al. 2004 |
| Temporal hyperlocomoter response | Dopamine transporter | Thakker et al. 2004 |
| Antidepressant-related behavioural response | Serotonin transporter | Thakker et al. 2005 |
| Antinociception | Delta opioid receptor DELT | Luo et al. 2005 |
| Modulation of pain | NMDA receptor NR2B | Tan et al. 2005 |
| Eye | ||
| Antiapoptosis in retinal ganglion cells | c-Jun, Bax, Apaf-1 | Lingor et al. 2005 |
| Ocular neovascularization | VEGF | Reich et al. 2003 |
| Alterations of synaptic function (retina) | APP/APLP2 | Herard et al. 2005 |
| Others | ||
| Induction of hypoglycemia and hypertriglyceridemia | PPARα | De Souza et al. 2006 |
| Attenuation of morbidity and mortality in sepsis | Fas, caspase-8 | Wesche-Soldato et al. 2005 |
| Collagen-induced arthritis | TNFα | Schiffelers et al. 2005 |
| Cure of collagen-induced arthritis | TNFα | Khoury et al. 2006 |
| Role of V2R in water/sodium homeostasis | V2R | Hassan et al. 2005 |
| Increased metabolic rate/decreased body weight | Agouti-related peptide | Makimura et al. 2002 |
| Inflammation (peritoneum) | IL-12p40 | Flynn et al. 2004 |
| Sepsis after lipopolysaccharide injection | TNF-α | Sorensen et al. 2003 |
| Reduction of apoB and total cholesterol | ApoB | Soutschek et al. 2004 |
| Abrogation of HSF-induced cardioprotection | Heat shock factor 1 | Yin et al. 2005 |
| Hearing loss | GJBR75W | Maeda et al. 2005 |
Therapeutic siRNA molecules
Beyond the detection of the downregulation of an endogenous target gene, the siRNA-mediated RNAi for therapeutical purposes has been explored. Target organs include liver, kidney, lung, eye, ear, heart, pancreas, tumors, blood, as well as the central nervous system, and the peritoneum (see Table 1 for an overview), using locally or systemically administered siRNAs in various formulations.
Cancer
A large body of studies refers to the treatment of cancer with the primary goals being the inhibition of tumor growth. Target molecules usually represent genes that have been shown previously to be relevant or rate-limiting for tumor growth, including growth factors and receptors as well as antiapoptotic or downstream signal transduction proteins in tumor cells. Typically, these studies involve subcutaneous or orthotopic xenografts of different tumor entities in mice, and employ various strategies for local or systemic administration of a wide variety of siRNA formulations (see below). In some cases, the antiangiogenic effect after siRNA delivery to tumor endothelial cells, rather than an inhibitory effect on tumor cells, was explored (Santel et al. 2006a, b), or simultaneous targeting of tumor growth and tumor angiogenesis both contributed to the antitumorigenic effects observed (Grzelinski et al. 2006). Additionally, in some studies the blockage of cancer metastasis, e.g., to the lung, liver or bone has been achieved. Taking into consideration that a large number of cancer patients die from metastases rather than the primary tumor, this represents another very relevant approach in cancer therapy.
Antiviral treatment
Several studies focus on viral gene products, taking into consideration that options for protection or therapy through current antiviral drugs or vaccination strategies are rather limited. Thus, novel RNAi-based approaches to battle viral infections rely on the specific siRNA-mediated knockdown of virus-specific genes. Animal models infected with various viruses including hepatitis virus, influenza virus, respiratory syncytial virus (RSV), SARS corona viruses, or ebola virus have been employed, and primary goals were the reduction in virus titers and protective effects including, when lethal doses were applied, prolonged survival rates upon specific siRNA treatment.
Other targets
Beyond studies related to cancer or viral infection, several target molecules with proven relevance in other pathologies have been selected. Examples include Fas in hepatitis, vascular endothelial growth factor (VEGF) in macular degeneration due to extensive ocular neovascularization or tumor necrosis factor alpha (TNF-α) in arthritis, and these studies aim at the establishment of novel, improved therapeutic avenues through siRNA-mediated gene silencing. As it can be seen from Table 1, some target genes are relevant in more than one pathology (e.g., Fas in fulminant hepatitis, in renal ischemia-reperfusion injury or in hemorrhagic shock and sepsis in the lung; VEGF in age-related macular degeneration or in tumor growth/tumor angiogenesis; caspase-8 in liver failure or in sepsis), and thus, specific siRNAs may represent drugs applicable for the treatment of different diseases. Some studies also compare siRNA-based therapeutic strategies with already established drugs and discuss decreased side effects and/or higher specificity, or additive effects upon combination of both.
Other papers rather aim at the further elucidation of physiological processes through in vivo knockdown of a certain target gene. Examples include OATC3 in blood-brain barrier transport, V2R in water and sodium homeostasis, or HO-1 in lung ischemia-reperfusion injury (Table 1).
Clinical trials
It should be noted that, although the therapeutic potential of siRNAs has only been explored in the recent years, first siRNA-based drugs are already in clinical trials. This includes ALN-RSV01 (Alnylam Pharmaceuticals) for targeting the human RSV after viral infection, which is the first example of an antivirus siRNA-based therapeutic in a phase I clinical study. Other companies aiming at the development of RNAi therapeutics for viral diseases include Nastech/Galenea, which are expected to start clinical trials in 2007. Benitec, in collaboration with City of Hope in Duarte, California, has developed a multi-RNA therapeutic for treatment of AIDS lymphoma. Furthermore, age-related macular degeneration (AMD) was treated with Sirna-027 (Sirna Therapeutics) targeting the VEGF receptor VEGFR1 (Shen et al. 2006), and resulted in stabilization or even improvement of visual acuity (Whelan 2005). A 24-month phase II study to evaluate multiple doses of Sirna-027 (also termed AGN211745) in the treatment of subfoveal choroidal neovascularization associated with AMD is currently recruiting patients. Targeting the ligand (VEGF) rather than its receptor, Cand5 (Acuity Pharmaceuticals) has been employed for treatment of the same disease, and in the so-called C.A.R.E™ trial showed no adverse effects related to the drug. Cand5, which is now named Bevasiranib, was the first siRNA to enter both phase I and II clinical trials. Recently, SR Pharma plc announced the start of a phase I clinical trial with RTP-801i, an siRNA therapeutic licensed from its subsidiary Atugen AG that targets a gene product involved in the progression of AMD. The same company has most recently announced that the FDA has approved an investigational new drug (IND) application for a second siRNA therapeutic, AKIi-5 being developed for the treatment of acute kidney injury. AKIi-5 is expected to reduce the frequency of postsurgery acute kidney injury in high-risk patients undergoing major cardiovascular surgery. Finally, after successful completion of experiments demonstrating its therapeutic efficacy in animal models of pancreatic cancer, Atu027 is scheduled to enter human clinical trials in 2007. In addition, several other pharmaceutical or biotechnology companies are pursuing collaborative or internal projects for the development of drugs based on RNAi (see, e.g., Behlke 2006 for review).
Strategies for in vivo siRNA delivery
Advantages of the direct application of siRNAs, rather than DNA-based constructs coding for long dsRNA, include the relatively easy chemical synthesis of small RNA molecules, the lower probability of nonspecific side effects (see above) and the safety due to the fact that siRNA delivery is based on nonviral transfer strategies and siRNAs cannot integrate into the genome. On the other hand, successful siRNA-based gene targeting relies on several preconditions: protection of the rather instable siRNA molecules from nucleolytic degradation by serum nucleases, efficient cellular uptake and subsequent intracellular release into the cytoplasm, as well as the absence of intracellular immune responses, in vivo toxicity or rapid elimination in liver or kidney.
Strategies for the in vivo application of siRNA molecules include local as well as systemic modes of administration as detailed in Table 2. However, many studies rely on the use of relatively high amounts of siRNAs. Bearing in mind that intracellular immune responses have been shown to be concentration-dependent, this may increase the risk of nonspecific effects in addition to other side effects and cost considerations. When siRNAs are administered locally, lower doses are sufficient since nonspecific delivery to other organs as well as renal or hepatic elimination are reduced. This approach, however, is invasive and limited to tissues that are sufficiently accessible. With regard to systemic application, several studies rely on the hydrodynamic transfection of siRNAs, i.e., the rapid (∼20 s) high-pressure injection of large volumes (up to 2 ml) of siRNA-containing solution. Hydrodynamic injection has led to the efficient induction of RNAi in liver as well as in kidney, lung, pancreas, and spleen and is probably due to the transient enhancement of membrane-permeability. However, in animals, side effects have been observed (Zhang et al. 2004a, b), and in man, this method is not applicable at all.
Table 2.
In vivo application of siRNAs for the induction of RNAi: modes of administration of naked or formulated siRNAs
| Modes of administration | Example references |
|---|---|
| Hydrodynamic transfection | Bradley et al. 2005a, b; Duxbury et al. 2004; Giladi et al. 2003; Hamar et al. 2004; Heidel et al. 2004; Hino et al. 2006; Klein et al. 2003; Lewis et al. 2002; Liang et al. 2005; Matsui et al. 2005; Merl et al. 2005; Sato et al. 2005; Song et al. 2003; Tompkins et al. 2004; Zender et al. 2003 |
| Intravenous (without high pressure) | Bradley et al. 2005a, b; Chien et al. 2005; Ge et al. 2004; Hassan et al. 2005; Miyawaki-Shimizu et al. 2005; Morrissey et al. 2005a, b; Schiffelers et al. 2004; Soutschek et al. 2004; Yano et al. 2004; Takeshita et al. 2005 |
| Intraperitoneal | Filleur et al. 2003; Flynn et al. 2004; de Jonge et al. 2005; Ocker et al. 2005; Sorensen et al. 2003; Verma et al. 2003; Urban-Klein et al. 2005; Yin et al. 2005 |
| Intramuscular | Golzio et al. 2005 |
| Intratracheal | Lomas-Neira et al. 2005; Perl et al. 2005 |
| Intranasal | Bitko et al. 2005; Zhang et al. 2004a, b |
| Subretinal | Reich et al. 2003 |
| Intraocular | Herard et al. 2005 |
| Intradermal | Kim et al. 2005 |
| Subcutaneous | Yano et al. 2004 |
| Intrathecal | Dorn et al. 2004; Luo et al. 2005 |
| Stereotactic injection to hypothalamus | Makimura et al. 2002 |
| Infusion into the ventricular system (brain) | Hassani et al. 2005; Thakker et al. 2004, 2005 |
| Intrathecal infusion using mini-osmotic pump | Dorn et al. 2004 |
| In situ perfusion/intravenous (pancreatic islet) | Bradley et al. 2005a, b |
| Intracardiac | Bollerot et al. 2006 |
| Intratumoral | Bertrand et al. 2002; Ito et al. 2005; Leng and Mixson 2005; Takei et al. 2004 |
| Intratumoral + electroporation | Takahashi et al. 2005 |
| Renal artery and electroporation | Takabatake et al. 2005 |
| Transurethral (bladder cancer) | Nogawa et al. 2005 |
| Local (ear, tracheal grafts, liver, optic nerve stump) | Fukuyama et al. 2005; Lingor et al. 2005; Maeda et al. 2005; McCaffrey et al. 2002 |
| Local injection and electroporation (mouse joint) | Schiffelers et al. 2005 |
Many groups have employed different approaches for the formulation of siRNAs in carrier systems (Table 3), which deliver their siRNA “payload” into the target tissue and target cell, some of them already being known as DNA delivery techniques in gene therapy or antisense targeting.
Table 3.
In vivo application of siRNAs for the induction of RNAi: formulations of siRNAs
| Formulation | Example references |
|---|---|
| Unmodified siRNAs, naked | Bradley et al. 2005a, b; Duxbury et al. 2004; Giladi et al. 2003; Heidel et al. 2004; Hino et al. 2006; Klein et al. 2003; Lewis et al. 2002; Liang et al. 2005; Matsui et al. 2005; Merl et al. 2005; Sato et al. 2005; Song et al. 2003; Tompkins et al. 2004; Zender et al. 2003 |
| Chemically modified, naked | Braasch et al. 2004; Elmen et al. 2005; Soutschek et al. 2004 |
| Chemically modified + lipid encapsulation | Morrissey et al. 2005a, b |
| Coupling to cholesterol | Soutschek et al. 2004 |
| Liposomes | Flynn et al. 2004; Fukuyama et al. 2005; Hassan et al. 2005; Maeda et al. 2005; Miyawaki-Shimizu et al. 2005; Nogawa et al. 2005; Sioud and Sorensen 2003; Sorensen et al. 2003; Verma et al. 2003; Yano et al. 2004 |
| Liposome RPR209120/DOPE | Khoury et al. 2006 |
| Cationic cardiolipin lipsomes | Pal et al. 2005 |
| Cationic cardiolipin analogue | Chien et al. 2005 |
| Cationic lipid (i-Fect) | Luo et al. 2005 |
| Cytofectin GSV | Bertrand et al. 2002 |
| JetSI (+ DOPE) | Hassani et al. 2005 |
| Dioleoylphosphatidylcholine (DOPC) | Landen et al. 2005; Landen et al. 2006 |
| N-[1-(2,3-Dioleoyloxy)]-N,N,N-trimethylammonium propane (DOTAP) | Hassan et al. 2005 |
| Stable nucleic acid lipid particles (SNALP) | Geisbert et al. 2006; Morrissey et al. 2005a, b; Zimmermann et al. 2006 |
| Hybrid siRNA in TfRscFv (anti-transferrin receptor single-chain antibody fragment)-liposome | Hogrefe et al. 2006; Pirollo et al. 2006 |
| Mixture of cationic and fusogenic lipids | Santel et al. 2006a, b |
| Histidine-lysine complex | Leng and Mixson 2005 |
| Inactivated HVJ (hemagglutinating virus of Japan) suspension | Ito et al. 2005 |
| Protamin-antibody fusion protein | Song et al. 2005 |
| TransIT-TKO (polyamine) | Bitko et al. 2005 |
| Virosomes + cationic lipds | de Jonge et al. 2005 |
| Chitosan/chitosan-coated polyisohexylcyanoacrylate | Maksimenko et al. 2005; Pille et al. 2006 |
| Single-walled carbon nanotubes (SWNTs) | Yang et al. 2006 |
| Atelocollagen | Minakuchi et al. 2004; Takei et al. 2004; Takeshita et al. 2005 |
| Polyamines | Yin et al. 2005 |
| Polyethylenimine (PEI) complexation | Ge et al. 2004; Geisbert et al. 2006; Grzelinski et al. 2006; Urban-Klein et al. 2005 |
| In vivo jetPEI | Hassani et al. 2005 |
| PEI-based nanoplexes (RGD-PEG-PEI) | Schiffelers et al. 2004 |
Liposomal formulations
Various liposomes/cationic lipids can be considered as examples of nonviral envelopes that protect siRNAs, thus increasing serum stability, reducing renal excretion and mediating siRNA uptake into the cells through endocytosis. The comparison of neutral versus cationic liposomes also reveals that the biodistribution as well as the uptake into macrophage seems to be dependent on their charge (Landen et al. 2005; Miller et al. 1998), thus emphasizing the need for the further development and analysis of different liposomal particles. SNALPs (stable nucleic acid lipid particles) have been used for siRNA-mediated targeting of an Ebola-virus-specific gene (Geisbert et al. 2006) or ApoB. This is also the first study that describes the systemic efficacy of formulated siRNAs in a nonrodent species (Zimmermann et al. 2006).
Nanoparticles
Another strategy allowing the protection and cellular delivery of siRNAs is the formation of nanoparticles with positively charged macromolecules. Based on electrostatic interactions, complexes are formed with atelocollagen (Banno et al. 2006; Minakuchi et al. 2004; Takei et al. 2004; Takeshita et al. 2005), chitosan (Pille et al. 2006), or polyethylenimine (PEI).
Polyethylenimine
PEIs are synthetic linear or branched polymers available in a wide range of molecular weights (Godbey et al. 1999; Tang and Szoka 1997). Due to the presence of a protonable amino group in every third position, leading to a high cationic charge density at physiological pH, PEIs are able to form noncovalent complexes with DNA as well as small RNA molecules like siRNAs (Urban-Klein et al. 2005) or ribozymes (Aigner et al. 2002). This siRNA complexation results in the complete protection against degradation in the presence of serum or RNase A and allows the efficient cellular uptake of the PEI/siRNA complexes through endocytosis. For any siRNA formulation, the release from endosomes is critical for siRNA delivery. In the case of PEI, the so-called “proton-sponge effect” postulates improved transgene delivery by cationic complexes, which contain H+-buffering polyamines, based on enhanced endosomal Cl− accumulation and subsequent osmotic swelling and lysis (Behr 1997; Boussif et al. 1995). This effect may also apply for PEI-mediated siRNA delivery into the cytoplasm. Additionally, to further enhance the efficacy of PEI complexes through membrane-destabilization, the conjugation of melittin analogs to PEI has been described (Boeckle et al. 2005, 2006; Shir et al. 2006). It should be noted, however, that by far not all PEIs are suitable for the transport of nucleic acids like siRNAs (Hassani et al. 2005; Werth et al. 2006).
In vivo studies in xenografted mice have demonstrated that the i.p. injection of PEI-complexed siRNAs, but not of naked siRNAs, resulted in the delivery of intact siRNA molecules into the subcutaneous tumors, leading to antitumorigenic effects when targeting for example the receptor HER-2 in s.c. ovarian carcinoma xenografts (Urban-Klein et al. 2005) or the growth factors pleiotrophin (PTN, s.c. glioblastoma) (Grzelinski et al. 2006) or VEGF (s.c. prostate carcinoma; Hobel et al., unpublished data). Likewise, intrathecal delivery of PEI-complexed specific siRNAs led to successful PTN targeting and tumor inhibition in an ortotopic glioblastoma mouse model (Grzelinski et al. 2006), or to the knockdown of the N-methyl-d-aspartate (NMDA) receptor subunit protein NR2B in a rat model (Tan et al. 2005). PEI complexation of siRNAs has also been employed in antiviral therapy studies (Ge et al. 2004; Geisbert et al. 2006). Furthermore, PEI/siRNA complexes are a good example for the introduction of chemical modifications to enhance tissue specificity and in vivo biocompatibility, to reduce immunogenicity and toxicity and to increase siRNA delivery through improved endocytosis and intracellular siRNA release. This includes full deacetylation of PEI (Thomas et al. 2005), the introduction of novel low molecular weight PEIs (Werth et al. 2006), and the coupling of PEI to other macromolecules like polyethylene glycol (PEG), either alone (Mao et al. 2006) or in combination with a ligand for tissue-specific targeting (RGD peptide for the recognition of tumor vasculature; Schiffelers et al. 2004).
Chemical siRNA modifications
Alternatively, the goals of increased systemic siRNA stability and serum half-life have also been achieved through extensive chemical modifications of the siRNA strands including the introduction of phosphorothioate (Braasch et al. 2003, 2004), 4′ thioribose (Dande et al. 2006) or methylene linkages between positions 2′ and 4′ (locked nucleic acids, LNAs; Braasch et al. 2003; Elmen et al. 2005), or multiple 2′ modifications (Amarzguioui et al. 2003; Harborth et al. 2003; Holen et al. 2002, 2003). Additionally, chemical conjugation of siRNAs, e.g., to cholesterol (Soutschek et al. 2004) or to a protamin-antibody fusion protein (Song et al. 2005) led to enhanced efficacy and specificity in tissue uptake. For other strategies, refer to Table 3.
Conclusion and outlook
RNAi has already proven to be a very efficient and specific method for the knockdown of physiologically or pathologically relevant genes of interest. Notably, this also applies to so-called “nondruggable” genes, thus opening new therapeutic avenues. Still, the therapeutic applicability and success of siRNAs will largely depend on their efficient and safe in vivo delivery avoiding unwanted side effects. Reflecting the high relevance of RNAi, many studies have been published or are ongoing, which will finally allow to identify optimal strategies based on already promising results. This also refers to the first clinical trials, which are completed or ongoing. Most likely, one major advantage of formulated, modified, or unmodified siRNAs for gene knockdown will be their “double specificity”, i.e., the combination of a high target gene specificity through optimal siRNA sequences and an at least somewhat increased target organ specificity through sophisticated delivery vehicles like liganded nanocarriers.
Acknowledgments
The author’s work reported herein was supported by grants from the Deutsche Forschungsgemeinschaft (AI 24/5-1 and Forschergruppe Nanohale, AI 24/6-1) and by the Deutsche Krebshilfe.
References
- Aigner A, Fischer D, Merdan T, Brus C, Kissel T, Czubayko F. Delivery of unmodified bioactive ribozymes by an RNA-stabilizing polyethylenimine (LMW-PEI) efficiently down-regulates gene expression. Gene Ther. 2002;9:1700–1707. doi: 10.1038/sj.gt.3301839. [DOI] [PubMed] [Google Scholar]
- Amarzguioui M, Holen T, Babaie E, Prydz H. Tolerance for mutations and chemical modifications in a siRNA. Nucleic Acids Res. 2003;31:589–595. doi: 10.1093/nar/gkg147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarzguioui M, Peng Q, Wiiger MT, Vasovic V, Babaie E, Holen T, Nesland JM, Prydz H. Ex vivo and in vivo delivery of anti-tissue factor short interfering RNA inhibits mouse pulmonary metastasis of B16 melanoma cells. Clin Cancer Res. 2006;12:4055–4061. doi: 10.1158/1078-0432.CCR-05-2482. [DOI] [PubMed] [Google Scholar]
- Banno H, Takei Y, Muramatsu T, Komori K, Kadomatsu K. Controlled release of small interfering RNA targeting midkine attenuates intimal hyperplasia in vein grafts. J Vasc Surg. 2006;44:633–641. doi: 10.1016/j.jvs.2006.04.044. [DOI] [PubMed] [Google Scholar]
- Behlke MA. Progress towards in vivo use of siRNAs. Mol Ther. 2006;13:644–670. doi: 10.1016/j.ymthe.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr JP. The proton sponge: a trick to enter cells the viruses did not exploit. Chimia. 1997;51:34–36. [Google Scholar]
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- Bertrand JR, Pottier M, Vekris A, Opolon P, Maksimenko A, Malvy C. Comparison of antisense oligonucleotides and siRNAs in cell culture and in vivo. Biochem Biophys Res Commun. 2002;296:1000–1004. doi: 10.1016/s0006-291x(02)02013-2. [DOI] [PubMed] [Google Scholar]
- Bitko V, Musiyenko A, Shulyayeva O, Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nat Med. 2005;11:50–55. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]
- Boeckle S, Wagner E, Ogris M. C- versus N-terminally linked melittin-polyethylenimine conjugates: the site of linkage strongly influences activity of DNA polyplexes. J Gene Med. 2005;7:1335–1347. doi: 10.1002/jgm.783. [DOI] [PubMed] [Google Scholar]
- Boeckle S, Fahrmeir J, Roedl W, Ogris M, Wagner E. Melittin analogs with high lytic activity at endosomal pH enhance transfection with purified targeted PEI polyplexes. J Control Release. 2006;112:240–248. doi: 10.1016/j.jconrel.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Bollerot K, Sugiyama D, Escriou V, Gautier R, Tozer S, Scherman D, Jaffredo T. Widespread lipoplex-mediated gene transfer to vascular endothelial cells and hemangioblasts in the vertebrate embryo. Dev Dyn. 2006;235:105–114. doi: 10.1002/dvdy.20579. [DOI] [PubMed] [Google Scholar]
- Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braasch DA, Jensen S, Liu Y, Kaur K, Arar K, White MA, Corey DR. RNA interference in mammalian cells by chemically-modified RNA. Biochemistry. 2003;42:7967–7975. doi: 10.1021/bi0343774. [DOI] [PubMed] [Google Scholar]
- Braasch DA, Paroo Z, Constantinescu A, Ren G, Oz OK, Mason RP, Corey DR. Biodistribution of phosphodiester and phosphorothioate siRNA. Bioorg Med Chem Lett. 2004;14:1139–1143. doi: 10.1016/j.bmcl.2003.12.074. [DOI] [PubMed] [Google Scholar]
- Bradley SP, Kowalik TF, Rastellini C, da Costa MA, Bloomenthal AB, Cicalese L, Basadonna GP, Uknis ME. Successful incorporation of short-interfering RNA into islet cells by in situ perfusion. Transplant Proc. 2005;37:233–236. doi: 10.1016/j.transproceed.2004.12.181. [DOI] [PubMed] [Google Scholar]
- Bradley SP, Rastellini C, da Costa MA, Kowalik TF, Bloomenthal AB, Brown M, Cicalese L, Basadonna GP, Uknis ME. Gene silencing in the endocrine pancreas mediated by short-interfering RNA. Pancreas. 2005;31:373–379. doi: 10.1097/01.mpa.0000179730.69081.64. [DOI] [PubMed] [Google Scholar]
- Bridge AJ, Pebernard S, Ducraux A, Nicoulaz AL, Iggo R. Induction of an interferon response by RNAi vectors in mammalian cells. Nat Genet. 2003;34:263–264. doi: 10.1038/ng1173. [DOI] [PubMed] [Google Scholar]
- Chien PY, Wang J, Carbonaro D, Lei S, Miller B, Sheikh S, Ali SM, Ahmad MU, Ahmad I. Novel cationic cardiolipin analogue-based liposome for efficient DNA and small interfering RNA delivery in vitro and in vivo. Cancer Gene Ther. 2005;12:321–328. doi: 10.1038/sj.cgt.7700793. [DOI] [PubMed] [Google Scholar]
- Collins RE, Cheng X. Structural domains in RNAi. FEBS Lett. 2005;579:5841–5849. doi: 10.1016/j.febslet.2005.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dande P, Prakash TP, Sioufi N, Gaus H, Jarres R, Berdeja A, Swayze EE, Griffey RH, Bhat B. Improving RNA interference in mammalian cells by 4′-thio-modified small interfering RNA (siRNA): effect on siRNA activity and nuclease stability when used in combination with 2′-O-alkyl modifications. J Med Chem. 2006;49:1624–1634. doi: 10.1021/jm050822c. [DOI] [PubMed] [Google Scholar]
- de Jonge J, Holtrop M, Wilschut J, Huckriede A. Reconstituted influenza virus envelopes as an efficient carrier system for cellular delivery of small-interfering RNAs. Gene Ther. 2006;13:400–411. doi: 10.1038/sj.gt.3302673. [DOI] [PubMed] [Google Scholar]
- De Souza AT, Dai X, Spencer AG, Reppen T, Menzie A, Roesch PL, He Y, Caguyong MJ, Bloomer S, Herweijer H, et al. Transcriptional and phenotypic comparisons of Ppara knockout and siRNA knockdown mice. Nucleic Acids Res. 2006;34:4486–4494. doi: 10.1093/nar/gkl609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn G, Patel S, Wotherspoon G, Hemmings-Mieszczak M, Barclay J, Natt FJ, Martin P, Bevan S, Fox A, Ganju P, et al. siRNA relieves chronic neuropathic pain. Nucleic Acids Res. 2004;32:e49. doi: 10.1093/nar/gnh044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duxbury MS, Matros E, Ito H, Zinner MJ, Ashley SW, Whang EE. Systemic siRNA-mediated gene silencing: a new approach to targeted therapy of cancer. Ann Surg. 2004;240:667–674. doi: 10.1097/01.sla.0000140755.97224.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykxhoorn DM, Lieberman J. Running interference: prospects and obstacles to using small interfering RNAs as small molecule drugs. Annu Rev Biomed Eng. 2006;8:377–402. doi: 10.1146/annurev.bioeng.8.061505.095848. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Elmen J, Thonberg H, Ljungberg K, Frieden M, Westergaard M, Xu Y, Wahren B, Liang Z, Orum H, Koch T, et al. Locked nucleic acid (LNA) mediated improvements in siRNA stability and functionality. Nucleic Acids Res. 2005;33:439–447. doi: 10.1093/nar/gki193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filleur S, Courtin A, Ait-Si-Ali S, Guglielmi J, Merle C, Harel-Bellan A, Clezardin P, Cabon F. SiRNA-mediated inhibition of vascular endothelial growth factor severely limits tumor resistance to antiangiogenic thrombospondin-1 and slows tumor vascularization and growth. Cancer Res. 2003;63:3919–3922. [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Flynn MA, Casey DG, Todryk SM, Mahon BP. Efficient delivery of small interfering RNA for inhibition of IL-12p40 expression in vivo. J Inflam (Lond) 2004;1:4. doi: 10.1186/1476-9255-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Saito M, Iwasaki E, Ochiya T, Takei Y, Hayashi S, Ono A, Hirao N, Nakamura M, Kubushiro K, et al. Intratumor injection of small interfering RNA-targeting human papillomavirus 18 E6 and E7 successfully inhibits the growth of cervical cancer. Int J Oncol. 2006;29:541–548. [PubMed] [Google Scholar]
- Fukuyama S, Yoshino I, Yamaguchi M, Osoegawa A, Kameyama T, Tagawa T, Maehara Y. Blockage of the macrophage migration inhibitory factor expression by short interference RNA inhibited the rejection of an allogeneic tracheal graft. Transpl Int. 2005;18:1203–1209. doi: 10.1111/j.1432-2277.2005.00197.x. [DOI] [PubMed] [Google Scholar]
- Ge Q, Filip L, Bai A, Nguyen T, Eisen HN, Chen J. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc Natl Acad Sci USA. 2004;101:8676–8681. doi: 10.1073/pnas.0402486101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert TW, Hensley LE, Kagan E, Yu EZ, Geisbert JB, Daddario-DiCaprio K, Fritz EA, Jahrling PB, McClintock K, Phelps JR, et al. Postexposure protection of guinea pigs against a lethal ebola virus challenge is conferred by RNA interference. J Infect Dis. 2006;193:1650–1657. doi: 10.1086/504267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi H, Ketzinel-Gilad M, Rivkin L, Felig Y, Nussbaum O, Galun E. Small interfering RNA inhibits hepatitis B virus replication in mice. Mol Ther. 2003;8:769–776. doi: 10.1016/s1525-0016(03)00244-2. [DOI] [PubMed] [Google Scholar]
- Godbey WT, Wu KK, Mikos AG. Size matters: molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J Biomed Mater Res. 1999;45:268–275. doi: 10.1002/(sici)1097-4636(19990605)45:3<268::aid-jbm15>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Golzio M, Mazzolini L, Moller P, Rols MP, Teissie J. Inhibition of gene expression in mice muscle by in vivo electrically mediated siRNA delivery. Gene Ther. 2005;12:246–251. doi: 10.1038/sj.gt.3302405. [DOI] [PubMed] [Google Scholar]
- Grzelinski M, Urban-Klein B, Martens T, Lamszus K, Bakowsky U, Hobel S, Czubayko F, Aigner A. RNA interference-mediated gene silencing of pleiotrophin through polyethylenimine-complexed small interfering RNAs in vivo exerts antitumoral effects in glioblastoma xenografts. Hum Gene Ther. 2006;17:751–766. doi: 10.1089/hum.2006.17.751. [DOI] [PubMed] [Google Scholar]
- Guissouma H, Froidevaux MS, Hassani Z, Demeneix BA. In vivo siRNA delivery to the mouse hypothalamus confirms distinct roles of TR beta isoforms in regulating TRH transcription. Neurosci Lett. 2006;406:240–243. doi: 10.1016/j.neulet.2006.07.041. [DOI] [PubMed] [Google Scholar]
- Halder J, Kamat AA, Landen CN, Han LY, Lutgendorf SK, Lin YG, Merritt WM, Jennings NB, Chavez-Reyes A, Coleman RL, et al. Focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy. Clin Cancer Res. 2006;12:4916–4924. doi: 10.1158/1078-0432.CCR-06-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamar P, Song E, Kokeny G, Chen A, Ouyang N, Lieberman J. Small interfering RNA targeting Fas protects mice against renal ischemia reperfusion injury. Proc Natl Acad Sci USA. 2004;101(41):14883–14888. doi: 10.1073/pnas.0406421101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SM, Bernstein E, Beach D, Hannon GJ. An RNA-directed nuclease mediates posttranscriptional gene silencing in Drosophila cells. Nature. 2000;404:293–296. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- Harborth J, Elbashir SM, Vandenburgh K, Manninga H, Scaringe SA, Weber K, Tuschl T. Sequence, chemical, and structural variation of small interfering RNAs and short hairpin RNAs and the effect on mammalian gene silencing. Antisense Nucleic Acid Drug Dev. 2003;13:83–105. doi: 10.1089/108729003321629638. [DOI] [PubMed] [Google Scholar]
- Hassan A, Tian Y, Zheng W, Ji H, Sandberg K, Verbalis JG. Small interfering RNA-mediated functional silencing of vasopressin V2 receptors in the mouse kidney. Physiol Genomics. 2005;21:382–388. doi: 10.1152/physiolgenomics.00147.2004. [DOI] [PubMed] [Google Scholar]
- Hassani Z, Lemkine GF, Erbacher P, Palmier K, Alfama G, Giovannangeli C, Behr JP, Demeneix BA. Lipid-mediated siRNA delivery down-regulates exogenous gene expression in the mouse brain at picomolar levels. J Gene Med. 2005;7:198–207. doi: 10.1002/jgm.659. [DOI] [PubMed] [Google Scholar]
- Heidel JD, Hu S, Liu XF, Triche TJ, Davis ME. Lack of interferon response in animals to naked siRNAs. Nat Biotechnol. 2004;22:1579–1582. doi: 10.1038/nbt1038. [DOI] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Herard AS, Besret L, Dubois A, Dauguet J, Delzescaux T, Hantraye P, Bonvento G, Moya KL. siRNA targeted against amyloid precursor protein impairs synaptic activity in vivo. Neurobiol Aging. 2006;27:1740–1750. doi: 10.1016/j.neurobiolaging.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Hino T, Yokota T, Ito S, Nishina K, Kang YS, Mori S, Hori S, Kanda T, Terasaki T, Mizusawa H. In vivo delivery of small interfering RNA targeting brain capillary endothelial cells. Biochem Biophys Res Commun. 2006;340:263–267. doi: 10.1016/j.bbrc.2005.11.173. [DOI] [PubMed] [Google Scholar]
- Hogrefe RI, Lebedev AV, Zon G, Pirollo KF, Rait A, Zhou Q, Yu W, Chang EH. Chemically modified short interfering hybrids (siHYBRIDS): nanoimmunoliposome delivery in vitro and in vivo for RNAi of HER-2. Nucleosides Nucleotides Nucleic Acids. 2006;25:889–907. doi: 10.1080/15257770600793885. [DOI] [PubMed] [Google Scholar]
- Holen T, Amarzguioui M, Wiiger MT, Babaie E, Prydz H. Positional effects of short interfering RNAs targeting the human coagulation trigger tissue factor. Nucleic Acids Res. 2002;30:1757–1766. doi: 10.1093/nar/30.8.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holen T, Amarzguioui M, Babaie E, Prydz H. Similar behaviour of single-strand and double-strand siRNAs suggests they act through a common RNAi pathway. Nucleic Acids Res. 2003;31:2401–2407. doi: 10.1093/nar/gkg338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, Noronha A, Manoharan M, Akira S, de Fougerolles A, et al. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11:263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- Hou JQ, He J, Wang XL, Wen DG, Chen ZX. Effect of small interfering RNA targeting survivin gene on biological behaviour of bladder cancer. Chin Med J (Engl) 2006;119:1734–1739. [PubMed] [Google Scholar]
- Howard KA, Rahbek UL, Liu X, Damgaard CK, Glud SZ, Andersen MO, Hovgaard MB, Schmitz A, Nyengaard JR, Besenbacher F, et al. RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol Ther. 2006;14:476–484. doi: 10.1016/j.ymthe.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Ito M, Yamamoto S, Nimura K, Hiraoka K, Tamai K, Kaneda Y. Rad51 siRNA delivered by HVJ envelope vector enhances the anticancer effect of cisplatin. J Gene Med. 2005;7:1044–1052. doi: 10.1002/jgm.753. [DOI] [PubMed] [Google Scholar]
- Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS. Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- Khoury M, Louis-Plence P, Escriou V, Noel D, Largeau C, Cantos C, Scherman D, Jorgensen C, Apparailly F. Efficient new cationic liposome formulation for systemic delivery of small interfering RNA silencing tumor necrosis factor alpha in experimental arthritis. Arthritis Rheum. 2006;54:1867–1877. doi: 10.1002/art.21876. [DOI] [PubMed] [Google Scholar]
- Kim TW, Lee JH, He L, Boyd DA, Hardwick JM, Hung CF, Wu TC. Modification of professional antigen-presenting cells with small interfering RNA in vivo to enhance cancer vaccine potency. Cancer Res. 2005;65:309–316. [PubMed] [Google Scholar]
- Klein C, Bock CT, Wedemeyer H, Wustefeld T, Locarnini S, Dienes HP, Kubicka S, Manns MP, Trautwein C. Inhibition of hepatitis B virus replication in vivo by nucleoside analogues and siRNA. Gastroenterology. 2003;125:9–18. doi: 10.1016/s0016-5085(03)00720-0. [DOI] [PubMed] [Google Scholar]
- Landen CN, Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, Sood AK. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65:6910–6918. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- Landen CN, Merritt WM, Mangala LS, Sanguino AM, Bucana C, Lu C, Lin YG, Han LY, Kamat AA, Schmandt R, Coleman RL, Gershenson DM, LOpez-Berestein G, Sood AK. Intraperitoneal delivery of liposomal siRNA for therapy of advanced ovarian cancer. Cancer Biol Ther. 2006;5(12):1708–1713. doi: 10.4161/cbt.5.12.3468. [DOI] [PubMed] [Google Scholar]
- Leng Q, Mixson AJ (2005) Small interfering RNA targeting Raf-1 inhibits tumor growth in vitro and in vivo. Cancer Gene Ther [DOI] [PubMed]
- Lewis DL, Hagstrom JE, Loomis AG, Wolff JA, Herweijer H. Efficient delivery of siRNA for inhibition of gene expression in postnatal mice. Nat Genet. 2002;32:107–108. doi: 10.1038/ng944. [DOI] [PubMed] [Google Scholar]
- Liang Z, Yoon Y, Votaw J, Goodman MM, Williams L, Shim H. Silencing of CXCR4 blocks breast cancer metastasis. Cancer Res. 2005;65:967–971. [PMC free article] [PubMed] [Google Scholar]
- Lingor P, Koeberle P, Kugler S, Bahr M. Down-regulation of apoptosis mediators by RNAi inhibits axotomy-induced retinal ganglion cell death in vivo. Brain. 2005;128:550–558. doi: 10.1093/brain/awh382. [DOI] [PubMed] [Google Scholar]
- Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- Lomas-Neira JL, Chung CS, Wesche DE, Perl M, Ayala A. In vivo gene silencing (with siRNA) of pulmonary expression of MIP-2 versus KC results in divergent effects on hemorrhage-induced, neutrophil-mediated septic acute lung injury. J Leukoc Biol. 2005;77:846–853. doi: 10.1189/jlb.1004617. [DOI] [PubMed] [Google Scholar]
- Luo MC, Zhang DQ, Ma SW, Huang YY, Shuster SJ, Porreca F, Lai J. An efficient intrathecal delivery of small interfering RNA to the spinal cord and peripheral neurons. Mol Pain. 2005;1:29. doi: 10.1186/1744-8069-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Li J, He F, Wilson A, Pitt B, Li S. Cationic lipids enhance siRNA-mediated interferon response in mice. Biochem Biophys Res Commun. 2005;330:755–759. doi: 10.1016/j.bbrc.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Fukushima K, Nishizaki K, Smith RJ. In vitro and in vivo suppression of GJB2 expression by RNA interference. Hum Mol Genet. 2005;14:1641–1650. doi: 10.1093/hmg/ddi172. [DOI] [PubMed] [Google Scholar]
- Makimura H, Mizuno TM, Mastaitis JW, Agami R, Mobbs CV. Reducing hypothalamic AGRP by RNA interference increases metabolic rate and decreases body weight without influencing food intake. BMC Neurosci. 2002;3:18. doi: 10.1186/1471-2202-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimenko A, Polard V, Vellemueur M, Elhamess H, Couvreur P, Bertrand JR, Aboubakar M, Gottikh M, Malvy C. In vivo potentialities of EWS-fli-1 targeted antisense aligonucleotides-nanospheres complexes. Ann NY Acad Sci. 2005;1058:52–61. doi: 10.1196/annals.1359.010. [DOI] [PubMed] [Google Scholar]
- Mao S, Neu M, Germershaus O, Merkel O, Sitterberg J, Bakowsky U, Kissel T. Influence of polyethylene glycol chain length on the physicochemical and biological properties of poly(ethylene imine)-graft-poly(ethylene glycol) block copolymer/SiRNA polyplexes. Bioconjug Chem. 2006;17:1209–1218. doi: 10.1021/bc060129j. [DOI] [PubMed] [Google Scholar]
- Martinez J, Patkaniowska A, Urlaub H, Luhrmann R, Tuschl T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell. 2002;110:563–574. doi: 10.1016/s0092-8674(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Kobayashi N, Nishikawa M, Takakura Y. Sequence-specific suppression of mdr1a/1b expression in mice via RNA interference. Pharm Res. 2005;22:2091–2098. doi: 10.1007/s11095-005-8178-8. [DOI] [PubMed] [Google Scholar]
- McCaffrey AP, Meuse L, Pham TT, Conklin DS, Hannon GJ, Kay MA. RNA interference in adult mice. Nature. 2002;418:38–39. doi: 10.1038/418038a. [DOI] [PubMed] [Google Scholar]
- Merl S, Michaelis C, Jaschke B, Vorpahl M, Seidl S, Wessely R. Targeting 2A protease by RNA interference attenuates coxsackieviral cytopathogenicity and promotes survival in highly susceptible mice. Circulation. 2005;111:1583–1592. doi: 10.1161/01.CIR.0000160360.02040.AB. [DOI] [PubMed] [Google Scholar]
- Miller CR, Bondurant B, McLean SD, McGovern KA, O’Brien DF. Liposome-cell interactions in vitro: effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry. 1998;37:12875–12883. doi: 10.1021/bi980096y. [DOI] [PubMed] [Google Scholar]
- Minakuchi Y, Takeshita F, Kosaka N, Sasaki H, Yamamoto Y, Kouno M, Honma K, Nagahara S, Hanai K, Sano A, et al. Atelocollagen-mediated synthetic small interfering RNA delivery for effective gene silencing in vitro and in vivo. Nucleic Acids Res. 2004;32:e109. doi: 10.1093/nar/gnh093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki-Shimizu K, Predescu D, Shimizu J, Broman M, Predescu S, Malik AB. siRNA-induced caveolin-1 knock-down in mice increases lung vascular permeability via the junctional pathway. Am J Physiol Lung Cell Mol Physiol. 2005;290:L405–L413. doi: 10.1152/ajplung.00292.2005. [DOI] [PubMed] [Google Scholar]
- Morrissey DV, Blanchard K, Shaw L, Jensen K, Lockridge JA, Dickinson B, McSwiggen JA, Vargeese C, Bowman K, Shaffer CS, et al. Activity of stabilized short interfering RNA in a mouse model of hepatitis B virus replication. Hepatology. 2005;41:1349–1356. doi: 10.1002/hep.20702. [DOI] [PubMed] [Google Scholar]
- Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, Hartsough K, Machemer L, Radka S, Jadhav V, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23:1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- Nogawa M, Yuasa T, Kimura S, Tanaka M, Kuroda J, Sato K, Yokota A, Segawa H, Toda Y, Kageyama S, et al. Intravesical administration of small interfering RNA targeting PLK-1 successfully prevents the growth of bladder cancer. J Clin Invest. 2005;115:978–985. doi: 10.1172/JCI23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykanen A, Haley B, Zamore PD. ATP requirements and small interfering RNA structure in the RNA interference pathway. Cell. 2001;107:309–321. doi: 10.1016/s0092-8674(01)00547-5. [DOI] [PubMed] [Google Scholar]
- Ocker M, Neureiter D, Lueders M, Zopf S, Ganslmayer M, Hahn EG, Herold C, Schuppan D. Variants of bcl-2 specific siRNA for silencing antiapoptotic bcl-2 in pancreatic cancer. Gut. 2005;54:1298–1308. doi: 10.1136/gut.2004.056192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A, Ahmad A, Khan S, Sakabe I, Zhang C, Kasid UN, Ahmad I. Systemic delivery of RafsiRNA using cationic cardiolipin liposomes silences Raf-1 expression and inhibits tumor growth in xenograft model of human prostate cancer. Int J Oncol. 2005;26:1087–1091. [PubMed] [Google Scholar]
- Perl M, Chung CS, Lomas-Neira J, Rachel TM, Biffl WL, Cioffi WG, Ayala A. Silencing of fas, but not caspase-8, in lung epithelial cells ameliorates pulmonary apoptosis, inflammation, and neutrophil influx after hemorrhagic shock and sepsis. Am J Pathol. 2005;167:1545–1559. doi: 10.1016/S0002-9440(10)61240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pille JY, Li H, Blot E, Bertrand JR, Pritchard LL, Opolon P, Maksimenko A, Lu H, Vannier JP, Soria J, et al. Intravenous delivery of antiRhoA small interfering RNA loaded in nanoparticles of chitosan in mice: safety and efficacy in xenografted aggressive breast cancer. Hum Gene Ther. 2006;17:1019–1026. doi: 10.1089/hum.2006.17.1019. [DOI] [PubMed] [Google Scholar]
- Pirollo KF, Zon G, Rait A, Zhou Q, Yu W, Hogrefe R, Chang EH. Tumor-targeting nanoimmunoliposome complex for short interfering RNA delivery. Hum Gene Ther. 2006;17:117–124. doi: 10.1089/hum.2006.17.117. [DOI] [PubMed] [Google Scholar]
- Rand TA, Ginalski K, Grishin NV, Wang X. Biochemical identification of Argonaute 2 as the sole protein required for RNA-induced silencing complex activity. Proc Natl Acad Sci USA. 2004;101:14385–14389. doi: 10.1073/pnas.0405913101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich SJ, Fosnot J, Kuroki A, Tang W, Yang X, Maguire AM, Bennett J, Tolentino MJ. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol Vis. 2003;9:210–216. [PubMed] [Google Scholar]
- Rivas FV, Tolia NH, Song JJ, Aragon JP, Liu J, Hannon GJ, Joshua-Tor L. Purified Argonaute2 and an siRNA form recombinant human RISC. Nat Struct Mol Biol. 2005;12:340–349. doi: 10.1038/nsmb918. [DOI] [PubMed] [Google Scholar]
- Santel A, Aleku M, Keil O, Endruschat J, Esche V, Fisch G, Dames S, Loffler K, Fechtner M, Arnold W, et al. A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene Therapy. 2006;13:1222–1234. doi: 10.1038/sj.gt.3302777. [DOI] [PubMed] [Google Scholar]
- Santel A, Aleku M, Keil O, Endruschat J, Esche V, Durieux B, Loffler K, Fechtner M, Rohl T, Fisch G, et al. RNA interference in the mouse vascular endothelium by systemic administration of siRNA-lipoplexes for cancer therapy. Gene Ther. 2006;13:1360–1370. doi: 10.1038/sj.gt.3302778. [DOI] [PubMed] [Google Scholar]
- Sato Y, Ajiki T, Inoue S, Fujishiro J, Yoshino H, Igarashi Y, Hakamata Y, Kaneko T, Murakamid T, Kobayashi E. Gene silencing in rat-liver and limb grafts by rapid injection of small interference RNA. Transplantation. 2005;79:240–243. doi: 10.1097/01.tp.0000147786.52502.2f. [DOI] [PubMed] [Google Scholar]
- Schiffelers RM, Ansari A, Xu J, Zhou Q, Tang Q, Storm G, Molema G, Lu PY, Scaria PV, Woodle MC. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 2004;32:e149. doi: 10.1093/nar/gnh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffelers RM, Xu J, Storm G, Woodle MC, Scaria PV. Effects of treatment with small interfering RNA on joint inflammation in mice with collagen-induced arthritis. Arthritis Rheum. 2005;52:1314–1318. doi: 10.1002/art.20975. [DOI] [PubMed] [Google Scholar]
- Shen J, Samul R, Silva RL, Akiyama H, Liu H, Saishin Y, Hackett SF, Zinnen S, Kossen K, Fosnaugh K, et al. Suppression of ocular neovascularization with siRNA targeting VEGF receptor 1. Gene Ther. 2006;3:225–234. doi: 10.1038/sj.gt.3302641. [DOI] [PubMed] [Google Scholar]
- Shir A, Ogris M, Wagner E, Levitzki A. EGF receptor-targeted synthetic double-stranded RNA eliminates glioblastoma, breast cancer, and adenocarcinoma tumors in mice. PLoS Med. 2006;3:e6. doi: 10.1371/journal.pmed.0030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioud M. Induction of inflammatory cytokines and interferon responses by double-stranded and single-stranded siRNAs is sequence-dependent and requires endosomal localization. J Mol Biol. 2005;348:1079–1090. doi: 10.1016/j.jmb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Sioud M, Sorensen DR. Cationic liposome-mediated delivery of siRNAs in adult mice. Biochem Biophys Res Commun. 2003;312:1220–1225. doi: 10.1016/j.bbrc.2003.11.057. [DOI] [PubMed] [Google Scholar]
- Sledz CA, Holko M, de Veer MJ, Silverman RH, Williams BR. Activation of the interferon system by short-interfering RNAs. Nat Cell Biol. 2003;5:834–839. doi: 10.1038/ncb1038. [DOI] [PubMed] [Google Scholar]
- Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, Chen J, Shankar P, Lieberman J. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med. 2003;9:347–351. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM, Feng Y, Palliser D, Weiner DB, Shankar P, et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- Sorensen DR, Leirdal M, Sioud M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J Mol Biol. 2003;327:761–766. doi: 10.1016/s0022-2836(03)00181-5. [DOI] [PubMed] [Google Scholar]
- Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- Takabatake Y, Isaka Y, Mizui M, Kawachi H, Shimizu F, Ito T, Hori M, Imai E. Exploring RNA interference as a therapeutic strategy for renal disease. . Gene Thef. 2005;12(12):965–973. doi: 10.1038/sj.gt.3302480. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Nishikawa M, Kobayashi N, Takakura Y. Gene silencing in primary and metastatic tumors by small interfering RNA delivery in mice: quantitative analysis using melanoma cells expressing firefly and sea pansy luciferases. J Control Release. 2005;105:332–343. doi: 10.1016/j.jconrel.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Takei Y, Kadomatsu K, Yuzawa Y, Matsuo S, Muramatsu T. A small interfering RNA targeting vascular endothelial growth factor as cancer therapeutics. Cancer Res. 2004;64:3365–3370. doi: 10.1158/0008-5472.CAN-03-2682. [DOI] [PubMed] [Google Scholar]
- Takeshita F, Minakuchi Y, Nagahara S, Honma K, Sasaki H, Hirai K, Teratani T, Namatame N, Yamamoto Y, Hanai K, et al. Efficient delivery of small interfering RNA to bone-metastatic tumors by using atelocollagen in vivo. Proc Natl Acad Sci USA. 2005;102:12177–12182. doi: 10.1073/pnas.0501753102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan PH, Yang LC, Shih HC, Lan KC, Cheng JT. Gene knockdown with intrathecal siRNA of NMDA receptor NR2B subunit reduces formalin-induced nociception in the rat. Gene Ther. 2005;12:59–66. doi: 10.1038/sj.gt.3302376. [DOI] [PubMed] [Google Scholar]
- Tang MX, Szoka FC. The influence of polymer structure on the interactions of cationic polymers with DNA and morphology of the resulting complexes. Gene Ther. 1997;4:823–832. doi: 10.1038/sj.gt.3300454. [DOI] [PubMed] [Google Scholar]
- Thakker DR, Natt F, Husken D, Maier R, Muller M, van der Putten H, Hoyer D, Cryan JF. Neurochemical and behavioral consequences of widespread gene knockdown in the adult mouse brain by using nonviral RNA interference. Proc Natl Acad Sci USA. 2004;101:17270–17275. doi: 10.1073/pnas.0406214101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker DR, Natt F, Husken D, van der Putten H, Maier R, Hoyer D, Cryan JF. siRNA-mediated knockdown of the serotonin transporter in the adult mouse brain. Mol Psychiatry. 2005;10:714. doi: 10.1038/sj.mp.4001687. [DOI] [PubMed] [Google Scholar]
- Thomas M, Lu JJ, Ge Q, Zhang C, Chen J, Klibanov AM. Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc Natl Acad Sci USA. 2005;102:5679–5684. doi: 10.1073/pnas.0502067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins SM, Lo CY, Tumpey TM, Epstein SL. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc Natl Acad Sci USA. 2004;101:8682–8686. doi: 10.1073/pnas.0402630101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban-Klein B, Werth S, Abuharbeid S, Czubayko F, Aigner A. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2005;12:461–466. doi: 10.1038/sj.gt.3302425. [DOI] [PubMed] [Google Scholar]
- Verma UN, Surabhi RM, Schmaltieg A, Becerra C, Gaynor RB. Small interfering RNAs directed against beta-catenin inhibit the in vitro and in vivo growth of colon cancer cells. Clin Cancer Res. 2003;9:1291–1300. [PubMed] [Google Scholar]
- Werth S, Urban-Klein B, Dai L, Hobel S, Grzelinski M, Bakowsky U, Czubayko F, Aigner A. A low molecular weight fraction of polyethylenimine (PEI) displays increased transfection efficiency of DNA and siRNA in fresh or lyophilized complexes. J Control Release. 2006;112:257–270. doi: 10.1016/j.jconrel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Wesche-Soldato DE, Chung CS, Lomas-Neira J, Doughty LA, Gregory SH, Ayala A. In vivo delivery of caspase-8 or Fas siRNA improves the survival of septic mice. Blood. 2005;106:2295–2301. doi: 10.1182/blood-2004-10-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan J. First clinical data on RNAi. Drug Discov Today. 2005;10:1014–1015. doi: 10.1016/S1359-6446(05)03547-6. [DOI] [PubMed] [Google Scholar]
- Yang R, Yang X, Zhang Z, Zhang Y, Wang S, Cai Z, Jia Y, Ma Y, Zheng C, Lu Y, et al. Single-walled carbon nanotubes-mediated in vivo and in vitro delivery of siRNA into antigen-presenting cells. Gene Therapy. 2006;13:1714–1723. doi: 10.1038/sj.gt.3302808. [DOI] [PubMed] [Google Scholar]
- Yano J, Hirabayashi K, Nakagawa S, Yamaguchi T, Nogawa M, Kashimori I, Naito H, Kitagawa H, Ishiyama K, Ohgi T, et al. Antitumor activity of small interfering RNA/cationic liposome complex in mouse models of cancer. Clin Cancer Res. 2004;10:7721–7726. doi: 10.1158/1078-0432.CCR-04-1049. [DOI] [PubMed] [Google Scholar]
- Yin C, Xi L, Wang X, Eapen M, Kukreja RC. Silencing heat shock factor 1 by small interfering RNA abrogates heat shock-induced cardioprotection against ischemia-reperfusion injury in mice. J Mol Cell Cardiol. 2005;39:681–689. doi: 10.1016/j.yjmcc.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Zender L, Hutker S, Liedtke C, Tillmann HL, Zender S, Mundt B, Waltemathe M, Gosling T, Flemming P, Malek NP, et al. Caspase 8 small interfering RNA prevents acute liver failure in mice. Proc Natl Acad Sci USA. 2003;100:7797–7802. doi: 10.1073/pnas.1330920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Gao X, Song YK, Vollmer R, Stolz DB, Gasiorowski JZ, Dean DA, Liu D. Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther. 2004;11:675–682. doi: 10.1038/sj.gt.3302210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Shan P, Jiang D, Noble PW, Abraham NG, Kappas A, Lee PJ. Small interfering RNA targeting heme oxygenase-1 enhances ischemia-reperfusion-induced lung apoptosis. J Biol Chem. 2004;279:10677–10684. doi: 10.1074/jbc.M312941200. [DOI] [PubMed] [Google Scholar]
- Zhu H, Liang ZY, Ren XY, Liu TH. Small interfering RNAs targeting mutant K-ras inhibit human pancreatic carcinoma cells growth in vitro and in vivo. Cancer Biol Ther. 2006;5:1693–1698. doi: 10.4161/cbt.5.12.3466. [DOI] [PubMed] [Google Scholar]
- Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, et al. RNAi-mediated gene silencing in nonhuman primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]


