Abstract
Purpose
To provide recommendations and standard operating procedures for intensive care units and hospital preparedness for an influenza pandemic.
Methods
Based on a literature review and expert opinion, a Delphi process was used to define the essential topics.
Results
Key recommendations include: Hospitals should increase their ICU beds to the maximal extent by expanding ICU capacity and expanding ICUs into other areas. Hospitals should have appropriate beds and monitors for these expansion areas. Establish a management system with control groups at facility, local, regional and/or national levels to exercise authority over resources. Establish a system of communication, coordination and collaboration between the ICU and key interface departments. A plan to access, coordinate and increase labor resources is required with a central inventory of all clinical and non-clinical staff. Delegate duties not within the usual scope of workers’ practice. Ensure that adequate essential medical equipment, pharmaceuticals and supplies are available. Protect patients and staff with infection control practices and supporting occupational health policies. Maintain staff confidence with reassurance plans for legal protection and assistance. Have objective, ethical, transparent triage criteria that are applied equitably and publically disclosed. ICU triage of patients should be based on the likelihood for patients to benefit most or a ‘first come, first served’ basis. Develop protocols for safe performance of high-risk procedures. Train and educate staff.
Conclusions
Mortality, although inevitable during a severe influenza outbreak or disaster, can be reduced by adequate preparation.
Electronic supplementary material
The online version of this article (doi:10.1007/s00134-010-1759-y) contains supplementary material, which is available to authorized users.
Keywords: Recommendations, Intensive care unit, Hospital, Influenza epidemic, Pandemic, H1N1, Mass disaster, Triage
Introduction
In 2007, the European Society of Intensive Care Medicine established a Task Force for Intensive Care Unit (ICU) Triage during an Influenza Epidemic or Mass Disaster to develop recommendations and standard operating procedures (SOPs). At that time worldwide intensive care, infectious disease/microbiology and pulmonary societies sent representatives to participate. Based on a literature review and expert opinion, a Delphi process was used to define the essential topics. This review provides the recommendations and SOPs of the Task Force focusing on the ICU and H1N1. Key points for each of these topics are noted in Table 1. The information should also be helpful for other hospital areas and other mass casualty events (MCEs). Preliminary information regarding H1N1 patients is available. Approximately 8% of H1N1 patients are hospitalized [1, 2] (23 per 100,000 population) [3], and 6.5–25% of these require being in the ICU [1, 3, 4] (28.7 per million inhabitants) [5] for a median of 7–12 days [5, 6] with a peak bed occupancy of 6.3–10.6 per million inhabitants [5]; 65–97% of ICU patients require mechanical ventilation [2, 5–7], with median ventilatory duration for survivors of 7–15 days [4, 6, 7]; 5–22% require renal replacement therapy [5, 6], and 28-day ICU mortality is 14–40% [4, 6, 7]. Search terms used for the literature review are in Appendix Table 1. The authors' first-hand experience with emergency responses is found in Appendix Table 2.
Table 1.
Key points
| 1. Introduction |
| 2. Surge capacity and infrastructure considerations |
| Hospitals should increase their ICU beds to the maximal extent by using monitored, procedure and recovery areas for critical care |
| Hospitals should have appropriate beds and monitors for these expansion areas |
| Ventilators are expensive and difficult to stockpile but contingency plans at the facility and government (local, state, provincial, national) levels should provide for additional ventilators |
| Hospital critical care leadership should develop a phased staffing plan (nursing and physician) for the ICUs that provides for sufficient patient care supervision during these contingency and crisis situations |
| Critical care physicians should provide expert input to the emergency management personnel at the hospital both during planning for surge capacity as well as during response and assure that adequate infrastructure support is present to support critical care activities |
| Designated locations for expansion should be prioritized by expanding existing ICUs, using postanesthesia care units and emergency departments to capacity, then step-down units, large procedure suites, telemetry units and finally hospital wards |
| Prioritization of support services (minimizing tests ordered and restrictions to essential tests) should be developed |
| 3. Coordination and collaboration with interface units |
| Regions should establish an Incident Management System with Emergency Executive Control Groups at facility, local, regional/state or national levels to exercise authority and direction over resources |
| A SOP provides a system of communication, coordination and collaboration between the ICU and key interface departments/units |
| The SOP should identify key functions or processes requiring coordination and collaboration, the most important of these being manpower and resources utilization (surge capacity) and re-allocation of personnel, equipment and physical space |
| The framework provided by the SOP should allow smooth inter-departmental patient transfers |
| Creating systems and guidelines is not sufficient, it is important to identify: |
| The roles and responsibility of key individuals necessary for the implementation of the guidelines |
| Ensure that these individuals are adequately trained and prepared to perform their roles |
| Ensure sufficient equipment, pharmaceuticals and supplies and an adequate physical environment to allow staff to properly implement guidelines |
| Trigger events for determining a crisis should be defined |
| 4. Manpower |
| The number of trained staff is the dominant rate limiting step to increasing surge capacity |
| A plan to access, coordinate and increase labor resources is required for continued and expanded ICU care including increasing critical care specialists and expanded practice for non-critical care personnel |
| Education, preparation and communication are required to ensure a well-protected and prepared workforce and coordinated rapid manpower expansion |
| A central inventory of all clinical and non-clinical staff with their current roles along with possible emergency re-training possibilities should be maintained |
| The Hospital Emergency Executive Control Group coordinates all clinical and non-clinical staffing requirements and determines the hospital’s daily needs including a sick and no-show list together with ICU requirements |
| Only clinical staff should provide care to patients; non-clinical staff should not provide clinical care |
| It may be necessary, under crisis conditions, for staff to undertake duties that are not within their usual scope of practice, supervised and supported by experienced clinicians to ensure patient safety |
| If patient surge exceeds the number of available ICU-trained specialists, intensivists should supervise nonintensivist physicians to expand the workforce |
| 5. Essential equipment, pharmaceuticals and supplies |
| Hospitals should ensure that adequate essential medical equipment (mechanical ventilators, syringe pumps, etc.), pharmaceuticals (antiviral, antibiotic, bronchodilators, sedatives, etc.) and other important supplies are available during a disaster |
| A communication and coordination system between each health care facility and the local/regional/state/country governmental authorities should be developed for the provision of additional support |
| Key personnel within various departments should determine the required resources, order and stockpile adequate numbers of resources, and cautiously distribute them |
| Additional mechanical ventilators should be portable, provide adequate gas exchange for a range of clinical conditions, function with low-flow oxygen and without high pressures, provide volume and pressure control ventilation, be safe for patients (disconnect alarms) and safe for staff (reduce staff time in patients’ rooms) |
| ICUs should be able to provide advanced ventilatory support and most rescue therapies including high levels of inspired oxygen and positive end-expiratory pressure (PEEP), pressure control ventilation, inhaled nitric oxide, high-frequency ventilation, prone positioning ventilation and extracorporeal membrane oxygenation (ECMO) |
| If sufficient medical equipment, pharmaceuticals and supplies are not available for all patients, triage of scarce resources should be based on those who benefit most or on a ‘first come, first served’ basis |
| 6. Protection of patients and staff |
| For clinical risks relating to potential disease transmission, infection control and occupational health policies are essential |
| For clinical risks relating to adequacy of facilities, there should be advanced planning to maximize capacity by increasing essential equipment, drugs, supplies and encouraging staff availability |
| To minimize non-clinical risks and help maintain or escalate essential services, robust systems should be created to maintain staff confidence and safety |
| Handwashing, wearing gloves and gowns and use of N95 respirators reduces the transmission of epidemic respiratory viruses |
| Institutions should prepare formal reassurance plans for legal protection and for the provision of assistance to staff working outside their normal domain |
| Given the medical-legal implications of many decisions, comprehensive documentation is essential |
| 7. Critical care triage |
| Mass casualty events generate many critically ill patients overwhelming resources, and triage is used to guide the prioritization of resources |
| Each region should establish an Incident Management System with Emergency Executive Control Groups at facility, local, regional/state or national levels to exercise authority and direction over resources |
| Developing fair and equitable policies may require restricting ICU services to patients most likely to benefit |
| Usual treatments and standards of practice may be impossible to deliver |
| ICU care and treatments may have to be withheld from patients likely to die even with ICU care and withdrawn after a trial in patients who do not improve or deteriorate |
| Triage criteria should be objective, ethical, transparent, applied equitably and be publically disclosed |
| Critical care triage protocols for mass casualty events should only be triggered when critical care resources across a broad geographic area are or will be overwhelmed despite all reasonable efforts to extend resources or obtain additional resources |
| Triage of patients for ICU should be based on the likelihood for patients to benefit most or a ‘first come, first served’ basis |
| Critically ill patients will be assessed by a triage officer who will apply inclusion and exclusion criteria to determine their qualification for ICU admission |
| When resources permit, emergency triage should cease in a graduated fashion by altering prioritization criteria and then exclusion thresholds |
| 8. Medical procedures |
| Specify high risk procedures (aerosol-generating procedures) |
| Determine if certain procedures will not be performed during a pandemic |
| Develop protocols for safe performance of high-risk procedures that include appropriateness, qualifications of personnel, site, PPE, safe technique and equipment needs |
| Ensure adequate training of personnel in high-risk procedures |
| Procedures should be performed at the bedside whenever possible |
| Ensure safe respiratory therapy practices to avoid aerosols |
| Provide safe respiratory equipment (i.e., adequate filters, closed suctioning, etc.) |
| Determine criteria for cancelling and/or altering elective procedures |
| 9. Educational process |
| Preparation will depend on adequate training and education of ICU, ward staff and those co-opted to perform new roles |
| Training should begin as soon as possible with demonstrations followed by supervised practice |
| The staff should be educated about the disease, its ramifications and treatment |
| The hospital command structure should be trained in crisis management procedures |
| Subjects to be taught include medical management, personal protection techniques, environmental contamination, laboratory specimens, alert lists, training of non-ICU staff pre-determined tasks, ethical issues, dealing with the deceased and families of dying patients and visitors restrictions |
| Mortality, although inevitable during a disaster or influenza outbreak, can be reduced by adequate preparation including education and training |
| The administration should identify the staff to participate in training programs, verify that they participated and evaluate their knowledge annually |
SOP standard operating procedure
Surge capacity and infrastructure considerations
The type of MCE is a major determinant of the demands on a hospital. The proportion of ICU beds occupied by patients with H1N1 varies. In Australia and New Zealand, it peaked at 9–19% [5], but in Mexico they were overwhelmed, and many patients required ventilation outside ICUs [4]. Critical care capacity is a key element of hospital surge capacity planning. Critical care physicians and staff should be involved with the development of SOPs for their institution and understand their roles during a response.
Surge capacity spans a continuum of care across conventional (usual spaces, staff and resources), contingency (functionally equivalent care using non-traditional patient care space, staff and resources) and crisis (sufficiency of care in a scarce resource setting) [8]. The institutional plan should account for the provision of care across this surge capacity spectrum so that the maximum number of patients can be treated during each phased response appropriate to the demands. Hospitals should be able to increase their ICU beds to the maximal extent by expanding ICUs and other areas with appropriate beds and monitors. Increases beyond 25% over usual capacity are unlikely with the current H1N1 virus. Future mutations, outbreaks or MCE may require maximum feasible expansion of capacity. This maximal feasible number will vary between institutions and countries, and be determined by the number of excess ICU patients, the usual ICU bed proportion of the total population and the maximum feasible expansion. One group has recommended a 300% expansion target, but many facilities may not be able to reach this target and should consider phased expansion to double capacity [9].
Designated locations for expansion should be prioritized by expanding existing ICUs, using postanesthesia care units and emergency departments to capacity, then step-down units, large procedure suites, telemetry units and finally hospital wards [10]. Hospitals should balance ICU needs and the potential decreasing benefits of increasing ICU capacity due to excess workload [11] with other hospital needs that may suffer more as services are depleted. During the surge of patients, stable patients may have to be transferred to other facilities [9].
Worksheets and SOPs should reflect the specifics of the three phases to be easily used in an incident. The overall goal should be to place unstable and highly resource-dependent patients in usual critical care areas and move stable and less resource-dependent ICU patients to non-traditional areas until the situation improves or until patients can be transferred to other facilities [12].
Effective management of a “surge” of ICU patients may require mass critical care. This is dependent on the institution having the procedures, training and integrated support of a broad range of stakeholders as well as adequate space, staff and supplies. An appropriate incident management system should be utilized to gather information and make decisions about service provision, set operational objectives and define the resources that need to be obtained or the adaptations that should be made [13, 14]. Decision-makers should communicate with community emergency services and other local hospitals to ensure a coordinated approach to patient transfers, standards of care and resource allocation [15].
Infection control personnel should create a phased plan to accommodate larger numbers of patients with highly infectious diseases. Planning should account for ongoing support for infrastructure protection, power, water, oxygen, suction and compressed air provisions, which are also necessary. Laboratory, radiology, nutrition and other departments should help meet ICU disaster needs and be engaged in prioritization of support services (minimizing tests ordered and restrictions to essential tests).
Coordination and collaboration with interface units
During a MCE ICUs should effectively collaborate with their hospital coordinating structure, other hospitals and regional resource authorities to ensure the best possible patient care. A detailed SOP for coordination and collaboration should be formulated and components tested by simulation during the pre-crisis phase, implemented when the crisis occurs, updated as the crisis evolves and evaluated and improved in the post-crisis phase. While a general SOP can serve as a guide, situational knowledge is key to the preparation of the detailed systems and guidelines.
A communication, coordination and collaboration system should be developed between the ICU and key departments, such as central hospital administration, clinical departments (e.g., internal medicine, surgery, operating rooms, emergency department), nursing, infectious diseases, laboratory services and supporting services such as radiology, physiotherapy, housekeeping and medical supplies (Fig. 1).
Fig. 1.
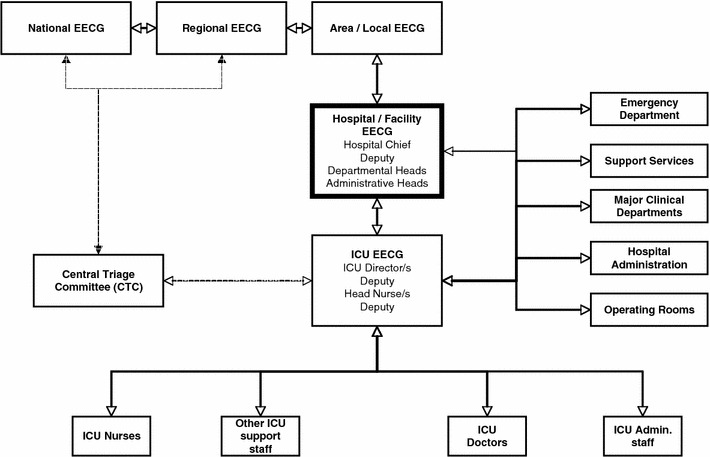
Schematic algorithm describing key lines of authority (command chain) and information flow (bi-directional) during a MCE/crisis. The Hospital Emergency Executive Control Group (HEECG) is the central operations center with “command and control” responsibility for the overall management of the crisis. It should consist of the hospital chief, heads of all major clinical and support departments, and key supply and logistic divisions. The Hospital EECG should determine whether to open new wards, re-deploy staff, suspend or redirect services (e.g., elective operations), prioritize the allocation of hospital supplies (including personal protective equipment), endorse triage policies, and formalize infection control and occupational health policies. The ICU EECG provides the Hospital EECG with information such as ICU functionality, capacity, projected staff and supply requirements, and preferred triage and discharge policies. The ICU EECG ensures that relevant policies agreed upon and endorsed by the HEECG are implemented within the ICU. It is made up of at least the ICU director, a deputy, the head nurse and deputy, and one or more triage officers. In the case of multiple ICUs in a hospital under different administrative authorities, each ICU should have an independent ICU EECG. An additional combined ICUEECG may be considered desirable. Other potentially important interfaces are shown
Each region should establish an Incident Management System (IMS) with Emergency Executive Control Groups at facility, local, regional/state or national levels to exercise authority and direction over resources. Each IMS includes five functional areas—command, operations, planning, logistics and finance/administration [16] (Fig. 2). Within the regional IMS is a Central Triage Committee of experts with broad situational awareness, capacity to develop and modify protocols, monitor outcome and coordinate responses. Cooperation and communication between various levels are essential [17].
Fig. 2.
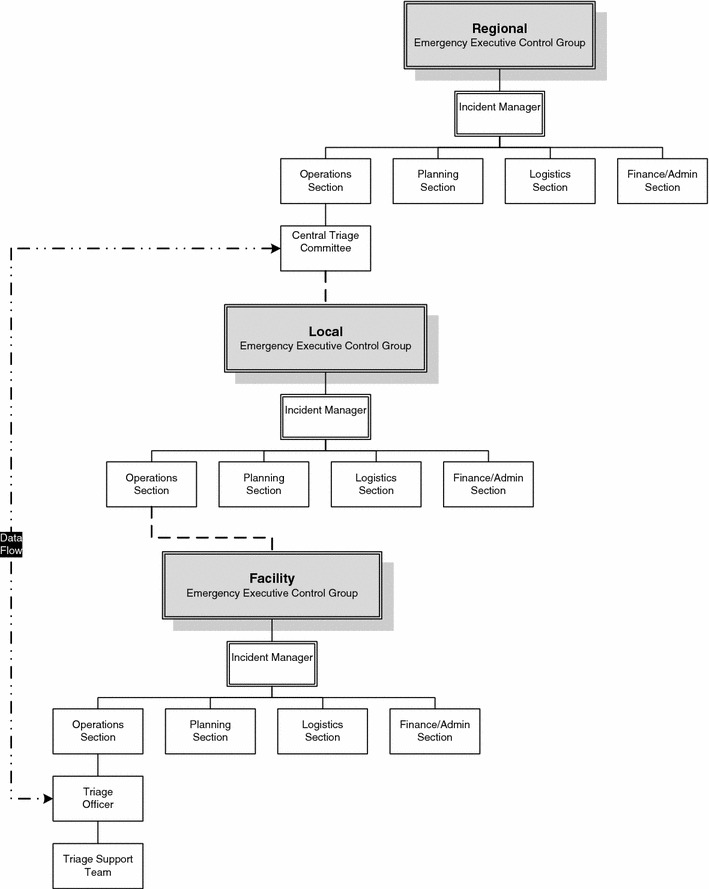
Coordination with interface units: Dashed lines indicate the continuity of the lines of authority for triage from the CTC down through the IMS levels. Two-way communication should flow through this chain. This is not meant to indicate lines of command and control. The dashed and dotted lines indicate the direct data inputs that will flow between (bi-directional) the local triage officer and the CTC
It is important to clearly identify key functions requiring coordination and collaboration. The most important functions are manpower, resource utilization and re-allocation of personnel, equipment and physical space. Clinical information should be shared through a unified hospital database.
Guidelines for the systematic management of patient admission and discharge to the hospital and between hospital departments (particularly the ICU) should be developed. Appropriate personnel to function as inter-departmental contacts, such as an ICU Triage officer, Infection Control Officer, Emergency Department Admissions and/or Patient Transfer Officer should be identified. Inter-departmental contact methods (creating and promulgating master contact lists) should also be developed.
Creating systems and guidelines is not sufficient. The roles and responsibilities of key individuals necessary for the implementation of the guidelines should be defined. These individuals should be properly trained to perform their duties [18–20]. Not only should operational guidelines be developed, but the availability of sufficient equipment should be ensured and an adequate physical environment to allow staff to properly implement the guidelines and function optimally should be implemented. Hospitals should utilize this approach with cooperation at the local, regional and national levels (Fig. 2). As ICU resources are frequently limited and vary in quantity and complexity from hospital to hospital, direct coordination with a regional ICU authority is recommended to share information regarding availability of vital equipment, manpower and pharmaceuticals.
Clinicians should join regional databases with a common global registry of ICU H1N1 patients to gain important, timely information for treating severe H1N1 patients [21]. Information can help to evaluate triage decisions and provide data to areas not yet affected by a pandemic [5]. Randomized controlled trials testing treatment strategies should be expedited with rapid Investigational Review Board approvals [21, 22].
Manpower
During disasters staffing may be limited due to staff absenteeism, illness and closure of child care facilities. Planning to coordinate and increase staff is necessary for continued and expanded ICU care. This includes increasing intensivists and expanded practice for non-critical care personnel. Roles and responsibilities of key individuals expanding the work force should be defined before the disaster. Education, preparation and communication are required to ensure a well-protected and prepared workforce. Coordinated manpower expansion should include adequate psychosocial and family support and adequate rest and support. The number of trained staff is the dominant rate-limiting step to increasing surge capacity.
The following groups may be able to provide staff to work in the ICU: medical and nursing staff, respiratory care practitioners/therapists, pharmacists, administrators, ancillary staff (assistants, transport, social services, clergy, housekeeping, clerks), support therapists (occupational, physical and speech), clinical infectious disease and microbiology laboratory support, radiology, surgical and other equipment specialists, infection control and health care epidemiologists, dieticians, volunteers, retirees and physical and environmental support. The ICU needs should be balanced against other hospital service needs.
The scope of practice for non-critical care personnel should be expanded to provide critical care. These personnel may include hospital-based specialists, primary care physicians, surgical sub-specialists, medical/surgical nurses, respiratory therapists, medical and nursing students, veterinarians, dentists and other health professionals. Only clinical staff should provide care to patients. Credentialing and training should be provided by the hospital in coordination with regulatory authorities.
Manpower needs should be assessed by the operations, logistics and planning sections of the Hospital Emergency Executive Control Group. They coordinate all clinical and non-clinical staffing requirements and determine the ICU and hospital’s daily needs including a sick and no-show list. A central inventory of all clinical and non-clinical staff with their current roles along with potential emergency re-training possibilities should be maintained. Staffing needs (housing, food, family support and childcare) and appropriate protective measures (vaccinations, protective equipment and antivirals) along with the appropriate training should be provided. Staffing ratios may have to be altered to compensate for working in a unfamiliar environment, use of less skilled staff and time to don personal protection equipment (PPE). Once hospital manpower needs are exceeded, the local authority followed by regional or national authorities may provide support for health care facilities.
Recommendations for increasing the labor pool and their functions include:
Care should be provided by the most experienced clinicians available.
Assignments should be based on staff abilities and experience.
It may be necessary, under crisis conditions, for staff to undertake duties that are not within their usual scope of practice, supervised and supported by experienced clinicians to ensure patient safety [10].
If patient surge exceeds the number of available critical care trained specialists, intensivists should supervise nonintensivist physicians [10].
Staffing ratios are altered based on needs and laws. Ideally, the ratio should remain constant and equal throughout ICUs in the hospital and region to provide equitable care.
Essential equipment, pharmaceuticals and supplies
Hospitals should ensure that adequate essential medical equipment, pharmaceuticals and other important supplies hereafter referred to as resources are available during a disaster. As resources are depleted local/regional/state/country authorities may have to provide additional support. They should let hospitals know in advance what resources will be potentially available.
The Hospital Emergency Executive Control Group should liase with key personnel within various departments to determine the required resources, order and stockpile adequate numbers and judiciously distribute them. Depending on sources of supply, which may vary in different countries, ICU, hospital and regional stockpiles may have to be increased by weeks or even months.
Essential medical equipment, pharmaceuticals and supplies are shown in Table 2. Prompt medical treatment should include neuraminidase inhibitors as survivors were more likely to have received treatment, although this was only one study with a low level of evidence [4].
Table 2.
Essential medical equipment, pharmaceuticals and supplies
| 1. Essential medical equipment includes: |
| Mechanical ventilators |
| Monitors: heart rate, blood pressure, respiration, electrocardiography |
| Noninvasive blood pressure cuffs |
| Intravenous pumps |
| Pumps for nutrition |
| Ambu bags |
| Nebulizers (and nebulizers for drug administration via ventilators) |
| ICU beds |
| Dialysis or hemofiltration machines |
| Pulse oximeters |
| Sequential compression devices |
| Suction machines |
| 2. Essential pharmaceuticals include: |
| Anti-virals (especially neuraminidase inhibitors) |
| Antibiotics |
| Vasopressors |
| Bronchodilators |
| Sedatives |
| Analgesics |
| Neuromuscular blocking agents |
| Steroids (although WHO recommendation are that steroids not be administered to patients with H1N1-related ARDS because of increased viral spread [23], many physicians have used them [4, 7]) |
| Thromboembolism prophylaxis |
| Gastrointestinal hemorrhage prophylaxis |
| Fluids for resuscitation |
| 3. Other essential supplies include: |
| Nutrition: enteral and parenteral |
| Masks: Ambu, CPAP, tracheal, oxygen, oxygen + nebulizer, surgical |
| Respirators: N95 respirator, powered air purifying respirators (PAPR) |
| Endotracheal and tracheostomy tubes |
| Catheters: triple, double and single lumen for central lines |
| Catheter: regular peripheral intravenous |
| Catheters: arterial lines |
| Catheters: regular suction, closed-circuit suction, Yankauer suction |
| Catheter: urinary and collection bags |
| Catheter supplies: administration sets, flush, dressings |
| Connector for suction catheter (finger tip) |
| Suction tubing |
| Suction container: wall mounted, disposable |
| Suction trap and hoses |
| Nasogastric or orogastric tubes |
| Oral airway |
| Full face shields; goggles |
| Gloves: sterile and non-sterile |
| Oxygen tubing and regulators |
| Ventilatory circuits |
| Filters including high-efficiency particulate air (HEPA) |
| Humidifiers |
| Respiratory medication delivery systems: metered dose inhaler (MDI) adapters, nebulizers |
| Medical gas: compressed air, compressed oxygen, liquid oxygen |
| T tube |
| Mouth suction piece |
| Syringes: for arterial blood gases, bloods |
| Oxygen regulators and clock |
| Vacuum clock |
| Electrocardiography cables and leads |
| Electrodes |
| Gowns: sterile and nonsterile |
| Nasal prongs |
| Culture bottles |
| Thermometers |
| Needles |
| 4. Other important equipment that may not be present in every hospital |
| Extracorporeal membrane oxygenation (ECMO) |
| Pumpless extracorporeal lung assist (pECLA) |
| High-frequency jet ventilator or oscillator |
| Machines or tanks providing nitric oxide |
During MCEs, hospitals may have to consider restricting interventions that (1) have demonstrated an improved survival and without which death is likely, (2) require extraordinarily expensive equipment and (3) consume extensive staff or hospital resources [24].
Most hospitals cannot double the number of ventilators required during a disaster and will have to attempt to procure new ones [10]. Additional ventilators should have as many of the following attributes as possible: be portable, provide adequate gas exchange for a range of clinical conditions, function with low-flow oxygen without high pressures (important with a loss of high-pressure oxygen supply due to expansion outside conventional hospital settings or failure of delivery), provide volume and pressure control ventilation, be safe for patients (disconnect alarms) and safe for staff (reduce staff time in patients’ rooms) [9, 24]. If sufficient ventilators are not available, manual ventilation is usually not recommended because of operator fatigue, patient hypoventilation and high risk for disease transmission. Each facility should determine whether manual ventilation will be considered based on availability of personnel, equipment and safety for staff. Some H1N1 ICU patients have experienced severe hypoxemia requiring advanced ventilatory support and rescue therapies including high levels of inspired oxygen and positive end-expiratory pressure (PEEP), pressure control ventilation, inhaled nitric oxide, high-frequency ventilation, prone positioning ventilation and ECMO [4, 6, 7, 25]. If hospitals cannot provide such services, they should consider transferring patients with severe disease to regional centers [26].
As resources are depleted:
Pharmacies may need to make drug substitutions, decrease medication frequency, change parenteral to oral or enteral administration, restrict medications, extend drug shelf-life and authorize certain medical personnel to prescribe scarce medications [5].
If sufficient resources are not available for all patients, triage of scarce resources should be based on those who are likely to benefit most [27, 28] or on a ‘first come, first served’ basis [29].
Protection of patients and staff
It is important to clarify the potential safety issues for health care staff during a pandemic. Plans to provide the best achievable care for as many patients as possible will be predominantly dependent on staff availability. Information from major events suggests that advanced preparation for maintaining staff confidence and morale helps to maintain response systems created for such circumstances [9, 30].
Patient and staff protection requirements can be broadly divided into two main areas: clinical and non-clinical risks. Clinical risks relate to potential disease transmission for which infection control and occupational health policies are important as well as adequate equipment and manpower. Non-clinical risks predominantly relate to staff members, the most concerning being those that may undermine confidence. Lack of confidence may influence attendance and willingness to undertake challenging additional responsibilities and hence impact on patient care. Therefore, in the presence of uncertainty, staff protection should start at the highest level and then be gradually reduced. Institutions should prepare formal reassurance plans for legal protection and for assisting staff working outside their normal domain. Debriefing and communication may reduce psychological stress for both staff and patients. Given the medical-legal implications of many decisions, comprehensive documentation is essential. Support of relevant professional organizations and medical/nursing authorities will also benefit members working outside their normal areas of expertise.
Risks include
risks of infection or contamination (work-acquired, family transmission, community/travel acquired),
work implications (compromised care standards, treatment limitations or withdrawal, excessive working time, disagreement on decisions, working outside normal domain)
personal/psychological (anxiety about personal and family risks, distress on triaging, death of family members/friends, potential errors caused by excessive/inappropriate workload, antisocial relatives’ reactions, fatigue-related anxiety, lack of confidence in employer support),
potential litigation (triaging, care compromised by working outside area of expertise, excessive workload)
security (triage decisions may lead to threats or violence)
Handwashing, wearing gloves and gowns and use of N95 respirators reduces the transmission of epidemic respiratory viruses [31]. The low rate of nosocomial transmission among ICU patients during the recent H1N1 flu outbreak [4, 7] may be because of following the robust infection control procedures proven to reduce the risk of contracting severe acute respiratory syndrome (SARS) from patients [32]. For diseases with high rates of transmission, the risk to staff versus the benefit to patients should be weighed. Although wearing surgical masks may provide similar benefits [33], there are still concerns that masks may not be sufficiently protective for the number of aerosol-generating procedures in the ICU. Staff training in PPE use (e.g., fit-testing for N95 respirators, avoiding contamination when placing/removing, environmental cleaning, etc.) is essential [34]. The use of negative pressure isolation rooms with adequate ventilation facilities is also recommended although they may be limited in many ICUs and expanded areas. The possibility of reducing the risk of airborne pathogens by modifying ICU design [35] may also be of benefit.
Critical care triage
MCEs generate many critically ill patients that can overwhelm health care resources [36]. Triage is used to guide the prioritization of limited resources following disasters [37–42]. In severe circumstances insufficient ICU bed availability may result in the occurrence of potentially avoidable deaths, which may be influenced by compulsory triaging decisions. Triage protocols for ICUs have been developed based on the probability that needs during a disaster are greater than availability [9, 17, 24, 43–45]. Ideally triage plans should be developed at a national or regional level.
Developing fair and equitable policies for “the greatest good for the greatest number” of patients [43] may require restricting services to patients likely to benefit from ICU care. Usual treatments and standards of practice may be impossible to deliver. ICU care and treatments may have to be withheld from patients likely to die even with ICU care and withdrawn after a trial in patients who do not improve or who deteriorate [24]. In an influenza pandemic, hospitals should expect the greatest surge of ICU patients approximately 4–6 weeks after the first confirmed winter ICU admission and extra workload and resource use lasting several weeks [5, 7].
Triage criteria should be objective, ethical, transparent, applied equitably and publically disclosed. ICU triage protocols include inclusion criteria that identify patients who may benefit from ICU admission (Table 3) and exclusion criteria that identify patients who are not candidates for ICU admission including patients: (1) with a poor prognosis despite ICU care, (2) requiring resources that cannot be provided, (3) whose underlying illness has a poor prognosis with a high likelihood of death and (4) who are “too well” (Table 4) [17].
Table 3.
Inclusion criteria for admission to critical care during a mass casualty event
| The patient must have one of the following from either category A or B: |
| (A) Requirement for invasive ventilatory support: |
| Refractory hypoxemia (SpO2 <90% on non-rebreather mask/FiO2 >0.85) |
| Respiratory acidosis with pH <7.2 |
| Clinical evidence of impending respiratory failure |
| Inability to protect or maintain airway (altered level of consciousness, significant secretions or other airway issue) |
| (B) Hypotension: |
| Hypotension (SBP <90 mmHg or relative hypotension) with clinical evidence of shock (altered level of consciousness, decreased urine output or other end organ failure) refractory to volume resuscitation requiring vasopressor/inotrope support |
Table 4.
Exclusion criteria from admission to critical care during a mass casualty event
| The patient is excluded from admission to critical care if any of the following are present: |
| A. Severe trauma |
| A Trauma Injury Severity Score (TRISS) with predicted mortality of >80% (see calculator at http://www.sfar.org/scores2/triss2.html) |
| B. Severe burns of patient with any two of the following: |
| Age >60 years |
| >40% of total body surface area affected |
| Inhalation injury |
| C. Cardiac arrest |
| Unwitnessed cardiac arrest |
| Witnessed cardiac arrest, not responsive to electrical therapy (defibrillation or pacing) |
| Recurrent cardiac arrest |
| A second cardiac arrest less than 72 h following return of spontaneous circulation and stabilization following successful electrical therapy for initial malignant arrhythmia |
| D. Severe baseline cognitive impairment |
| A patient who is unable to perform activities of daily living (AODLs) independently due to cognitive impairment OR is institutionalized due to cognitive impairment |
| E. Advanced untreatable neuromuscular disease |
| F. Metastatic malignant disease |
| G. Advanced and irreversible immunocompromised patient |
| Most commonly this will be due to AIDS where there are NO antiviral treatment options available or rarely one of the congenital immunocompromised conditions |
| H. Severe and irreversible neurologic event or condition |
| I. End-stage organ failure meeting the following criteria: |
| 1. Heart |
| NYHA class III or IV heart failure |
| Class I: patients with no limitation of activities; they suffer no symptoms from ordinary activities |
| Class II: patients with slight, mild limitation of activity; they are comfortable with rest or with mild exertion |
| Class III: patients with marked limitation of activity; they are comfortable only at rest |
| Class IV: patients who should be at complete rest, confined to bed or chair; any physical activity brings on discomfort and symptoms occur at rest |
| 2. Lungs |
| COPD with FEV1 < 25% predicted, baseline |
| PaO2 <55 mmHg or secondary pulmonary hypertension |
| Cystic fibrosis with post bronchodilator FEV1 <30% or baseline PaO2 <55 mmHg |
| Pulmonary fibrosis with VC or TLC <60% predicted, baseline PaO2 <55 mm Hg or secondary pulmonary hypertension |
| Primary pulmonary hypertension with NYHA class III or IV heart failure, right atrial pressure >10 mmHg or mean pulmonary arterial pressure >50 mmHg |
| Requirement for home oxygen |
| 3. Liver |
| Child-Pugh score ≥7 |
| 1. Total serum bilirubin |
| 1. Bilirubin <2 mg/dl: 1 point |
| 2. Bilirubin 2–3 mg/dl: 2 points |
| 3. Bilirubin >3 mg/dl: 3 points |
| 2. Serum albumin |
| 1. Albumin >3.5 g/dl: 1 point |
| 2. Albumin 2.8–3.5 g/dl: 2 points |
| 3. Albumin <2.8 g/dl: 3 points |
| 3. INR |
| 1. INR <1.70: 1 point |
| 2. INR 1.71 to 2.20: 2 points |
| 3. INR >2.20: 3 points |
| 4. Ascites |
| 1. No ascites: 1 point |
| 2. Ascites controlled medically: 2 points |
| 3. Ascites poorly controlled: 3 points |
| 5. Encephalopathy |
| 1. No encephalopathy: 1 point |
| 2. Encephalopathy controlled medically: 2 points |
| 3. Encephalopathy poorly controlled: 3 points |
| J. Elective palliative surgery |
| Surgery that is intended for symptomatic relief in a patient with an otherwise terminal condition (i.e., cancer) for which the average 2-year survival is less than 50% |
| K. Patients who are too well |
ICU triage protocols for pandemics should only be triggered when ICU resources across a broad geographic area are or will be overwhelmed despite all reasonable efforts to extend resources or obtain additional resources [44]. The triage of patients for ICU care remains controversial. Experts have recommended accepting patients likely to benefit most from ICU [27, 28] or on a ‘first come, first served’ basis [29]. Each institution should determine its own triage criteria using senior clinicians in a transparent fashion. All critically ill patients will be assessed by a triage officer who should apply inclusion and exclusion criteria together possibly with a prioritization tool to determine qualification for ICU admission [17, 27–29] (Tables 3, 4, 5 are one example to consider adapting to the situation). The Table 5 prioritization tool utilizes the sequential organ failure assessment (SOFA) score [47, 48]. The tool has limitations and has not yet been validated. Although SOFA day-1 scores have been shown to be significantly associated with 28-day and overall mortality in H1N1 patients, little improvement in SOFA scores between admission and day 3 casts doubt on the usefulness of re-assessing patients on day 2 [4, 7]. Patients not meeting inclusion criteria remain on the ward and can be re-evaluated. All ICU patients at the time of the MCE will also be assessed for eligibility based on the same criteria. Patients admitted to the ICU should subsequently be reassessed and re-categorized. When resources permit, emergency triage should cease in a graduated fashion by altering prioritization criteria and then exclusion thresholds.
Table 5.
Triage prioritization tool

Patients not meeting inclusion criteria remain on the ward and can be re-evaluated. Patients who are triaged as ‘red’ are given priority for ICU followed by those triaged as ‘yellow’. Patients categorized as ‘blue/black’ remain on the ward and receive palliative care with active medical therapy at the discretion of the primary care physician with patient and/or family input. Patients admitted to ICU should be reassessed at days 2 and 5 and re-categorized. Decisions beyond ICU day 5 will be dependent upon resource availability. Although this triage procedure is based only on expert opinion [17], an ICU trial with reappraisal at day 5 has proved useful in mechanically ventilated cancer patients [46]. It should be noted, however, that ventilated H1N1 patients had a median ventilatory duration in survivors of 7-15 days [4, 6, 7] so reevaluation may have to be delayed
Medical procedures
Judicious planning for performance of procedures and monitoring during a pandemic is necessary to optimize outcomes in ICU patients. Adequate resources should be made available and appropriate protocols developed to perform procedures safely in patients with and without influenza illness.
Procedures
Procedures that constitute a high risk for disease transmission [aerosol generating procedures (AGPs)] when performed on infected or potentially infected patients should be specified in advance [49] (Table 6). Each facility should determine if certain AGPs will not be performed during a pandemic. Protocols should be developed for safe performance of high-risk procedures that address the following issues [50, 51]: appropriateness of high-risk procedures, qualifications of clinicians performing high-risk procedures, required use of PPE during and following a high-risk procedure, optimal site for performing high-risk procedures, essential personnel and exposure time during high-risk procedures, room entrance and exiting during procedures and safe disposal of or adequate sterilization of utilized equipment. Appropriate equipment needs should be determined, and adequate training of personnel should be provided for high-risk procedures. Procedures should be performed at the bedside whenever possible, and appropriate safety precautions should be taken if patients are transported outside the ICU.
Table 6.
Procedures with potential high risk for disease transmission
| Aerosol humidification |
| Bag-mask ventilation |
| Bronchoscopy |
| Cardiopulmonary resuscitation |
| Disconnection of endotracheal or tracheal tube from ventilator |
| Extubation |
| High-flow oxygen therapy |
| High-frequency oscillatory ventilation |
| Intubation |
| Mechanical ventilation without HEPA filter on exhaust port |
| Nasopharyngeal swabs |
| Nasotracheal or orotracheal suctioning |
| Nebulization of medications |
| Noninvasive positive pressure ventilation |
| Surgical airway |
HEPA high-efficiency particulate air
Respiratory/aerosol issues
Safe practices and safe respiratory equipment are needed to minimize aerosol generation when caring for patients with influenza. These include minimizing disconnecting the ventilator circuit and using bag-mask ventilation, putting the ventilator on ‘stand-by’ mode before disconnecting the patient, and avoiding Venturi masks and nebulized medications. Respiratory equipment optimizing safety includes closed suction systems if available, high-quality bacterial/viral filter attached to the expiratory port of ventilators, high-quality bacterial/viral heat and moisture exchanger and filter (HMEF) attached to the endotracheal tube/tracheostomy tube and a bacterial/viral filter attached to the expiratory port of the bag-mask ventilation device with another filter between the mask and valve. Use of heated humidifiers on ventilators should be avoided.
Elective procedures
Each facility should determine criteria for cancelling and/or altering elective procedures when resources are limited. The safety of areas (environment and equipment) used for elective procedures should be assessed to prevent exposure of uninfected patients to influenza.
Educational process
The quality of health services depends upon an informed, committed and confident staff [44, 52]. Training should begin as soon as possible followed by supervised simulations to ensure optimal use of available facilities and to minimize infections [53]. PP techniques and reduction of environmental contamination should preferably be taught by infection control staff with assistance from ICU directors [54]. Interventions aimed at changing clinical practice show that outreach visits, posted reminders, interactive educational meetings and other multifaceted interventions were effective, but time constraints and potential lethality of the disease were limiting factors [55]. Seminars, on-site demonstrations, problem-based learning and simulations are valuable when time is of the essence. In the SARS epidemic, use of a simulator allowed effective training of 275 workers in 2 weeks [56–58]. Teleconferencing involving clinicians and representatives from public health, infection control, infectious diseases, hospital administration and government together with website dissemination of instructional materials (http://www.eunid.eu/) is a useful tool for updating knowledge during a pandemic [50, 51, 59].
Knowledge and compliance with PP protocols are poor, and consequently knowledge should be re-evaluated frequently [60]. Although an element of coercion is frequently necessary [61, 62], reasons for poor compliance should be addressed. These include availability of appropriate equipment, quality of leadership and an organizational culture that promotes safety [63]. These factors also reduce psychological stress by inspiring confidence [64].
Areas requiring teaching and training are:
Personal protection techniques [33]
Hand washing pre- and post-patient contact, procedures for donning and removing gowns, gloves, protective glasses, hoods, N95 respirators and powered air purifying respirators (including proper facial seal), personal hygiene (protected coughing, avoidance of touching the face, eyes or masks), proper disposal of contaminated materials and correct techniques for high-risk procedures such as mechanical ventilation, intubation, suctioning, tracheostomy and endotracheal tube care.
Drug treatment (anti-virals and other typical drugs with doses and administration), mechanical ventilation and respiratory rescue strategies and palliative care.
-
3.
Environmental contamination
The use of correct specification pleated filters at the catheter mount and exhalation port [67], appropriate and safe disposal of organic and inorganic waste, and decontamination of floors, beds and respiratory equipment.
-
4.
Laboratory specimens
Management and transport of laboratory specimens in cooperation with the laboratory.
-
5.
Training of non-ICU staff
Complete syllabi are available [50]. Tasks should be assigned and taught according to need. Ability to record blood pressure, pulse, respiratory rate, oxygenation, fluid intake and output, suctioning and attention to pressure sites are a minimum requirement. Palliative care and monitoring of noninvasive and mechanical ventilation should be considered.
-
6.
Alert lists
Design and instruction in clinical signs indicating deterioration potentially necessitating transfer to ICU.
-
7.
Ethical issues [68]
The duty to provide care efficiently and with compassion necessitates instruction in triage, including nonbeneficial treatments and allocation of vaccines and antiviral medicines, ventilators and ICU in resource-scarce environments [27–29].
-
8.
Methods to deal with the deceased and families of dying patients
-
9.
Policies for restricting visitors and mechanisms for enforcement
-
10.
Community education
Educational materials to reduce community spread should be available for distribution [CDC (http://www.cdc.gov/H1N1FLU/) and WHO (http://www.who.int/csr/disease/swineflu/en/) websites]. The public should be informed that usual treatments may be impossible to deliver and treatments may have to be triaged but by avoiding unnecessary panic.
Conclusions
These recommendations and SOPs have been developed to provide guidance in the preparation and management of a pandemic. This guidance should be used as a framework to guide the development of detailed systems and processes at a hospital. The detailed guidelines for frontline use should be a product of the SOP, local situational awareness and the specific threat faced. In the H1N1 setting, assumptions based on previous H1N1 data may change because of effective vaccinations, viral mutations and resistance to antiviral drugs [5]. Rapidly evolving data should result in appropriate responses and changes in guidelines. Such changes will be necessary because preparations should occur as soon as possible. “Any deaths from 2009 influenza A (H1N1) will be regrettable, but those that result from insufficient planning and inadequate preparation will be especially tragic [26].”
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors wish to thank Charles Gomersall, Andrew Rhodes and Steve Webb for their helpful and insightful comments in reviewing the manuscript and Renee Bernstein for her technical assistance.
Appendix
ESICM Task Force Members
Canada: Michael D. Christian, University of Toronto, Toronto (Canadian Critical Care Society representative).
Colombia: Ruben Camargo (Colombian Intensive Care Society representative), Daniel Ceraso (Colombian Intensive Care Society representative).
France: Elie Azoulay, Hôpital Saint-Louis, Paris; Alexandre Duguet, Hospital group of Pitié-Salpêtrière, Paris (Société de Pneumologie de Langue Française representative); Benoit Guery, CHU of Lille (French Infectious Disease Society representative).
Germany: Konrad Reinhart, Friedrich-Schiller Universitat, Jena (World Federation of World Intensive and Critical Care Societies representative).
Israel: Bruria Adini, Israeli Ministry of Health, Ben-Gurion University of the Negev, Tel Aviv, Beer Sheva; Yaron Barlavie, Rambam Medical Center, Haifa; Odeda Benin-Goren,Tel Aviv Sourasky Medical Center, Tel Aviv; Robert Cohen, Israeli Ministry of Health, Hebrew University Faculty of Medicine, Tel Aviv, Jerusalem; Motti Klein, Soroka Medical Center, Beer Sheva, Yuval Leoniv, Sheba Medical Center, Tel Hashomer; Gila Margalit, Sheba Medical Center, Tel Hashomer; Bina Rubinovitch, Beilinson Medical Center, Petach Tikva; Moshe Sonnenblick, Shaare Zedek Medical Center, Jerusalem; Charles L. Sprung, Hadassah Hebrew University Medical Center, Jerusalem (European Society of Intensive Care Medicine and Israel Society Critical Care Medicine representative); Avraham Steinberg, Shaare Zedek Medical Center, Jerusalem; Charles Weissman, Hadassah Hebrew University Medical Center, Jerusalem; Donna Wolff, Hadassah Hebrew University Medical Center, Jerusalem.
The Netherlands: Jozef Kesecioglu, University Medical Center Utrecht, Utrecht, Menno de Jong, Academic Medical Center, University of Amsterdam, Amsterdam (European Society of Clinical Microbiology and Infectious Diseases representative).
Portugal: Rui Moreno, Centro Hospitalar de Lisboa Central, Lisbon (Portuguese Society of Intensive Care representative).
PR China: Youzhong An, Peking University People’s Hospital, Beijing (Chinese Critical Care Society representative); Bin Du, Peking University People’s Hospital, Beijing (Chinese Critical Care Society representative); Gavin M. Joynt, The Chinese University of Hong Kong, Sha Tin, Hong Kong (Australia and New Zealand Intensive Care Societies representative).
Scotland: John Colvin, Ninewells Hospital, Dundee, (Scottish Intensive Care Society representative).
Singapore: Shi Loo, Tan Tock Seng Hospital, Singapore (Singapore Critical Care Society representative).
South Africa: Guy Richards, University of the Witwatersrand, Johannesburg (South African Critical Care Society representative).
Spain: Antonio Artigas, Sabadell Hospital, CIBER Enfermedades Respiratorias, Parc Tauli University Institute, Autonomous University of Barcelona, Sabadell.
Switzerland: Jerome Pugin, University Hospital of Geneva, Geneva.
United States: Dennis Amundson, University of California, San Diego (American College of Chest Physicians representative); Asha Devereaux, Coronado, John Beigel, National Institutes of Health, Bethesda (Society Critical Care Medicine representative); Marion Danis, Department of Bioethics at the Clinical Center of the National Institutes of Health, Bethesda; Chris Farmer, Mayo Clinic, Rochester; John L. Hick, Hennepin County Medical Center, Minneapolis; Dennis Maki, University of Wisconsin School of Medicine and Public Health, Madison; Henry Masur, National Institutes of Health, Bethesda (Infectious Diseases Society of America representative); Lewis Rubinson, University of Washington, Seattle (American Thoracic Society representative); Christian Sandrock, University of California at Davis, Sacramento, Daniel Talmor, Beth Israel Deaconess Medical Center, Boston (Society Critical Care Medicine representative); Robert Truog, Harvard Medical School, Boston; Janice Zimmerman, Weill Cornell Medical College, Houston.
United Kingdom: Steve Brett, Imperial College Healthcare NHS, London (United Kingdom Intensive Care Society representative); Hugh Montgomery, University College London, London; Andrew Rhodes, St George's Healthcare NHS trust, London; Frances Sanderson, Imperial College London, London (British Infection Society representative); Bruce Taylor, Portsmouth Hospitals NHS Trust, Portsmouth (United Kingdom Intensive Care Society representative).
Steering Committee: Charles L. Sprung (Chairman), Bruria Adini, Elie Azoulay, Michael D Christian, Robert Cohen, Menno de Jong, Hugh Montgomery, Lewis Rubinson, Christian Sandrock, Moshe Sonnenblick, Daniel Talmor.
Footnotes
The following societies approved the recommendations found in this review: the European Society of Intensive Care Medicine, World Federation of Societies of Intensive and Critical Care Medicine, Australian and New Zealand Intensive Care Society, Chinese Society of Critical Care Medicine, Portuguese Society of Intensive Care, Scottish Intensive Care Society, Society of Intensive Care Medicine (Singapore) and the French Infectious Disease Society.
On behalf of the European Society of Intensive Care Medicine’s Task Force for Intensive Care Unit Triage during an Influenza Epidemic or Mass Disaster.
References
- 1.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, Jasuja S, Deutscher M, Chen S, Walker JD, Duchin JS, Lett S, Soliva S, Wells EV, Swerdlow D, Uyeki TM, Fiore AE, Olsen SJ, Fry AM, Bridges CB, Finelli L. The 2009 Pandemic Influenza A (H1N1) Virus Hospitalizations Investigation Team (2009) Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 2.Echevarría-Zuno S, Mejía-Aranguré JM, Mar-Obeso AJ, Grajales-Muñiz C, Robles-Pérez E, González-León M, Ortega-Alvarez MC, Gonzalez-Bonilla C, Rascón-Pacheco RA, Borja-Aburto VH (2009) Infection and death from Influenza A H1N1 virus in Mexico: a retrospective analysis. Lancet. doi:10.1016/S0140-6736(09)61638-X [DOI] [PubMed]
- 3.Bishop JF, Murnane MP, Owen R (2009) Australia’s Winter with the 2009 Pandemic Influenza A (H1N1) Virus. N Engl J Med. doi:10.1056/NEJMp0910445 [DOI] [PubMed]
- 4.Domínguez-Cherit G, Lapinsky SE, Macias AE, Pinto R, Espinosa-Perez L, de la Torre A, Poblano-Morales M, Baltazar-Torres JA, Bautista E, Martinez A, Martinez MA, Rivero E, Valdez R, Ruiz-Palacios G, Hernández M, Stewart TE, Fowler RA (2009) Critically ill patients with 2009 Influenza A (H1N1) in Mexico. JAMA. doi:10.1001/jama.2009.1536 [DOI] [PubMed]
- 5.The ANZIC Influenza Investigators Critical Care Services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361:1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 6.Rello J, Rodríguez A, Ibañez P, Socias L, Cebrian J, Marques A, Guerrero J, Ruiz-Santana S, Marquez E, Del Nogal-Saez F, Alvarez-Lerma F, Martínez S, Ferrer M, Avellanas M, Granada R, Maraví-Poma E, Albert P, Sierra R, Vidaur L, Ortiz P, Prieto del Portillo I, Galván B, León-Gil C, H1N1 SEMICYUC Working Group Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit Care. 2009;13:R148. doi: 10.1186/cc8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, Turgeon AF, Lapinsky S, Ahern SP, Smith O, Siddiqui F, Jouvet P, Khwaja K, McIntyre L, Menon K, Hutchison J, Hornstein D, Joffe A, Lauzier F, Singh J, Karachi T, Wiebe K, Olafson K, Ramsey C, Sharma S, Dodek P, Meade M, Hall R, Fowler RA, Canadian Critical Care Trials Group H1N1 Collaborative (2009) Critically ill patients with 2009 influenza A (H1N1) infection in Canada. JAMA. doi:10.1001/jama.2009.1496
- 8.Hick JL, Barbera JA, Macintyre AG, Kelen GD. Refining surge capacity: conventional, contingency, and crisis capacity. Disaster Med Public Health Preparedness. 2009;3:S59–S67. doi: 10.1097/DMP.0b013e31819f1ae2. [DOI] [PubMed] [Google Scholar]
- 9.Devereaux A, Christian MD, Dichter JR, Geiling JA, Rubinson L. Summary of suggestions from the Task Force for Mass Critical Care summit, January 26–27, 2007. Chest. 2008;133:1S–7S. doi: 10.1378/chest.08-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubinson L, Hick JL, Curtis JR, Branson RD, Burns S, Christian MD, Devereaux AV, Dichter JR, Talmor D, Erstad B, Medina J, Geiling JA. Definitive care for the critically ill during a disaster: medical resources for surge capacity: from a Task Force for Mass Critical Care summit meeting. Chicago, IL. Chest. 2007;133(5 Suppl):32S–50S. doi: 10.1378/chest.07-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarnow-Mordi WO, Hau C, Warden A, Shearer AJ. Hospital mortality in relation to staff workload: a 4-year study in an adult intensive-care unit. Lancet. 2000;356:185–189. doi: 10.1016/S0140-6736(00)02478-8. [DOI] [PubMed] [Google Scholar]
- 12.Rubinson L, Hick JL, Hanfling DG, Devereaux AV, Dichter JR, Christian MD, Talmor D, Medina J, Curtis JR, Geiling JA. Definitive care for the critically ill during a disaster: a framework for optimizing critical care surge capacity: from a Task Force for Mass Critical Care summit meeting. Chest. 2008;133:26–27. doi: 10.1378/chest.07-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hospital Incident Command System. HICS IV (2006) California Emergency Medical Services Authority
- 14.Federal Emergency Management Agency-Department of Homeland Security (2008) National Incident Management System
- 15.Barbera J, MacIntyre A, Sunday D (2007) Medical surge capacity and capability: a management system for integrating medical and health resources during large-scale emergencies. CNA Corporation, Alexandria (RA 645.5 B276 2004)
- 16.Christian MD, Kollek D, Schwartz B. Emergency preparedness: what every healthcare worker needs to know. Can J Emerg Med. 2005;7:330–337. doi: 10.1017/s1481803500014548. [DOI] [PubMed] [Google Scholar]
- 17.Christian MD, Hawryluck L, Wax RS, Cook T, Lazar NM, Herridge MS, Muller MP, Gowans DR, Fortier W, Burkle FM. Development of a triage protocol for critical care during an influenza pandemic. CMAJ. 2006;175:1377–1381. doi: 10.1503/cmaj.060911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu EB, Thomas TL, Bass EB, Whyne D, Kelen GD, Green GB. Healthcare worker competencies for disaster training. BMC Med Educ. 2006;6:19–28. doi: 10.1186/1472-6920-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu EB, Jenckes MW, Catlett CL, Robinson KA, Feuerstein C, Cosgrove SE, Green GB, Bass EB. Effectiveness of hospital staff mass-casualty incident training methods: a systematic literature review. Prehosp Disaster Med. 2004;19:191–199. doi: 10.1017/s1049023x00001771. [DOI] [PubMed] [Google Scholar]
- 20.Williams J, Nocera M, Casteel C. The effectiveness of disaster training for health care workers: a systematic review. Ann Emerg Med. 2008;52:211–222. doi: 10.1016/j.annemergmed.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 21.Marshall J, on behalf of the InFact Global H1N1 Collaboration (2010) In fact: a global critical care research response to H1N1. Lancet (in press) [DOI] [PubMed]
- 22.Cook D, Burns K, Finfer S, Kissoon N, Bhagwanjee S, Annane D, Sprung C, Fowler R, Latronico N, Marshall J (2010) Clinical research ethics for critically ill patients: a pandemic proposal. Critical Care Med (in press) [DOI] [PubMed]
- 23.Pan American Health Organization and World Health Organization (2009) Considerations and interim recommendations for the clinical management of human infection with the pandemic influenza (H1N1) 2009 virus. PAHO/WHO expert consultation
- 24.Rubinson L, Nuzzo JB, Talmor DS, O’Toole T, Kramer BR, Inglesby TV. Augmentation of hospital critical care capacity after bioterrorist attacks or epidemics: recommendations of the Working Group on Emergency Mass Critical Care. Crit Care Med. 2005;33:E2393–E2403. doi: 10.1097/01.CCM.0000173411.06574.D5. [DOI] [PubMed] [Google Scholar]
- 25.The Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators (2009) Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome JAMA. doi:10.1001/jama.2009.1535 [DOI] [PubMed]
- 26.White DB, Angus DC (2009) Preparing for the sickest patients with 2009 influenza A (H1N1). JAMA. doi:10.1001/jama.2009.1539 [DOI] [PubMed]
- 27.The Society of Critical Care Medicine Ethics Committee Consensus statement on the triage of critically ill patients. JAMA. 1994;271:1200–1203. doi: 10.1001/jama.271.15.1200. [DOI] [PubMed] [Google Scholar]
- 28.Task Force of the American College of Critical Care Medicine, Society of Critical Care Medicine Guidelines for intensive care unit admission, discharge, and triage. Crit Care Med. 1999;27:633–638. doi: 10.1097/00003246-199903000-00048. [DOI] [PubMed] [Google Scholar]
- 29.American Thoracic Society Bioethics Task Force Fair allocation of Intensive Care Unit resources. Am J Respir Crit Care Med. 1997;156:1282–1301. doi: 10.1164/ajrccm.156.4.ats7-97. [DOI] [PubMed] [Google Scholar]
- 30.Peng PW, Wong DT, Bevan D, Gardam M. Infection control and anesthesia: lessons learned from the Toronto SARS outbreak. Can J Anesth. 2003;50:989–997. doi: 10.1007/BF03018361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jefferson T, Del Mar C, Dooley L, Ferroni E, Al-Ansary LA, Bawazeer GA, van Driel ML, Foxlee R, Rivetti A. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ. 2009;339:b3675. doi: 10.1136/bmj.b3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomersall CD, Joynt GM, Ho OM, Ip M, Yap F, Derrick JL, Leung P. Transmission of SARS to healthcare workers: the experience of a Hong Kong ICU. Intensive Care Med. 2006;32:564–569. doi: 10.1007/s00134-006-0081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loeb M, Dafoe N, Mahony J, John M, Sarabia A, Glavin V, Webby R, Smieja M, Earn DJ, Chong S, Webb A, Walter SD. Surgical Mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302:1865–1871. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- 34.Yap FH, Gomersall CD, Fung KS, Ho PL, Ho OM, Lam PK, Lam DT, Lyon DJ, Joynt GM. Increase in methicillin-resistant Staphylococcus aureus acquisition rate and change in pathogen pattern associated with an outbreak of severe acute respiratory syndrome. Clin Infect Dis. 2004;39:511–516. doi: 10.1086/422641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang JW, Li Y, Eames I, Chan PKS, Ridgway GL. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64:100–114. doi: 10.1016/j.jhin.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christian MD, Devereaux AV, Dichter JR, Geiling JA, Rubinson L. Definitive care for the critically ill during a disaster: current capabilities and limitations: from a Task Force for Mass Critical Care summit meeting, January 26–27, 2007, Chicago, IL. Chest. 2008;133(5 Suppl):8S–17S. doi: 10.1378/chest.07-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fauci AS. Pandemic influenza threat and preparedness. Emerg Infect Dis. 2006;12:73–77. doi: 10.3201/eid1201.050983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garner A, Lee A, Harrison K, Schultz CH. Comparative analysis of multiple-casualty incident triage algorithms. Ann Emerg Med. 2001;38:541–548. doi: 10.1067/mem.2001.119053. [DOI] [PubMed] [Google Scholar]
- 39.Romig LE. Pediatric triage: a system to JumpSTART your triage of young patients at MCIs. JEMS. 2002;27:52–53. [PubMed] [Google Scholar]
- 40.Robertson-Steel I. Evolution of triage systems. Emerg Med J. 2006;23:154–155. doi: 10.1136/emj.2005.030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iserson KV, Moskop JC. Triage in medicine, part I: concept, history, and types. Ann Emerg Med. 2007;49:275–281. doi: 10.1016/j.annemergmed.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 42.Moskop JC, Iserson KV. Triage in medicine, part II: underlying values and principles. Ann Emerg Med. 2007;49:282–287. doi: 10.1016/j.annemergmed.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Hick JL, O’Laughlin DT. Concept of operations for triage of mechanical ventilation in an epidemic. Acad Emerg Med. 2006;13:223–229. doi: 10.1111/j.1553-2712.2006.tb01677.x. [DOI] [PubMed] [Google Scholar]
- 44.Devereaux AV, Dichter JR, Christian MD, Dubler NN, Sandrock CE, Hick JL, Powell T, Geiling JA, Amundson DE, Baudendistel TE, Braner DA, Klein MA, Berkowitz KA, Curtis JR, Rubinson L (2008) Definitive care for the critically ill during a disaster: a framework for allocation of scarce resources in mass critical care: from a Task Force for Mass Critical Care summit meeting, January 26–27, 2007, Chicago, IL. Chest 133:51S–66S [DOI] [PubMed]
- 45.Powell T, Christ KC, Birkhead GS. Allocation of ventilators in a public health disaster. Disaster Med Public Health Prep. 2008;2:20–26. doi: 10.1097/DMP.0b013e3181620794. [DOI] [PubMed] [Google Scholar]
- 46.Lecuyer L, Chevret S, Thiery G, Darmon M, Schlemmer B, Azoulay E. The ICU trial: a new admission policy for cancer patients requiring mechanical ventilation. Crit Care Med. 2007;35:808–814. doi: 10.1097/01.CCM.0000256846.27192.7A. [DOI] [PubMed] [Google Scholar]
- 47.Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 48.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The sepsis-related organ failure assessment (SOFA) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 49.Lapinsky SE, Hawryluck L. ICU management of severe acute respiratory syndrome. Intensive Care Med. 2003;29:870–875. doi: 10.1007/s00134-003-1821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gomersall CD, Tai DY, Loo S, Derrick JL, Goh MS, Buckley TA, Chua C, Ho KM, Raghavan GP, Ho OM, Lee LB, Joynt GM. Expanding ICU facilities in an epidemic: recommendations based on experience from the SARS epidemic in Hong Kong and Singapore. Intensive Care Med. 2006;32:1004–1013. doi: 10.1007/s00134-006-0134-5. [DOI] [PubMed] [Google Scholar]
- 51.Mount Sinai Critical Care Unit SARS Resources. http://www.sars.medtau.org/. Accessed 8 Sept 2009
- 52.London DH (2007) Pandemic flu: a national framework for responding to an influenza pandemic. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_080734
- 53.Lau JT, Fung KS, Wong TW, Kim JH, Wong E, Chung S, Ho D, Chan LY, Lui SF, Cheng A. SARS transmission among hospital workers in Hong Kong. Emerg Infect Dis. 2004;10:280–286. doi: 10.3201/eid1002.030534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rebmann T, English JF, Carrico R. Disaster preparedness lessons learned and future directions for education: results from focus groups conducted at the 2006 APIC Conference. Am J Infect Control. 2007;35:374–381. doi: 10.1016/j.ajic.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bero L, Grilli R, Grimshaw J. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote implementation of research findings by health care professionals. BMJ. 1998;317:465–468. doi: 10.1136/bmj.317.7156.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng SM, Melanee EC, Rawson B. Infection prevention and control learning preferences of nurses sampled at a teaching hospital. Can J Infect Control. 2008;165:168–171. [PubMed] [Google Scholar]
- 57.Marshall CS, Yamada S, Inada MK. Using problem-based learning for pandemic preparedness. Kaohsiung J Med Sci. 2008;24(Suppl 3):S39–S45. doi: 10.1016/S1607-551X(08)70093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abrahamson SD, Canzian S, Brunet F. Using simulation for training and to change protocol during the outbreak of severe acute respiratory syndrome. Crit Care. 2006;10:R3. doi: 10.1186/cc3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Booth CM, Stewart TE. Severe acute respiratory syndrome and critical care medicine: the Toronto experience. Crit Care. 2005;33(Suppl):S53–S60. doi: 10.1097/01.CCM.0000150954.88817.6. [DOI] [PubMed] [Google Scholar]
- 60.Wang C, Wei S, Xiang H, Xu Y, Han S, Mkangara OB, Nie S. Evaluating the effectiveness of an emergency preparedness training programme for public health staff in China. Public Health. 2008;122:471–477. doi: 10.1016/j.puhe.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daugherty EL, Perl TM, Needham DM, Rubinson L, Bilderback A, Rand CS. The use of personal protective equipment for control of influenza among critical care clinicians: a survey study. Crit Care Med. 2009;37:1210–1216. doi: 10.1097/CCM.0b013e31819d67b5. [DOI] [PubMed] [Google Scholar]
- 62.Shigayeva A, Green K, Raboud JM, Henry B, Simor AE, Vearncombe M, Zoutman D, Loeb M, McGeer A, SARS Hospital Investigation Team Factors associated with critical-care healthcare workers’ adherence to recommended barrier precautions during the Toronto severe acute respiratory syndrome outbreak. Infect Control Hosp Epidemiol. 2007;28:1275–1283. doi: 10.1086/521661. [DOI] [PubMed] [Google Scholar]
- 63.Yassi A, Lockhart K, Copes R, Kerr M, Corbiere M, Bryce E, Danyluk Q, Keen D, Yu S, Kidd C, Fitzgerald M, Thiessen R, Gamage B, Patrick D, Bigelow P, Saunders S, SARS Study Team Determinants of healthcare workers’ compliance with infection control procedures. Healthc Q. 2007;10:44–52. doi: 10.12927/hcq.2007.18648. [DOI] [PubMed] [Google Scholar]
- 64.Chua SE, Cheung V, Cheung C, McAlonan GM. Psychological effects of the SARS outbreak in Hong Kong on high-risk health care workers. Can J Psych. 2004;49:391–393. doi: 10.1177/070674370404900609. [DOI] [PubMed] [Google Scholar]
- 65.Schünemann HJ, Hill SR, Kakad M, Bellamy R, Uyeki TM, Hayden FG, Yazdanpanah Y, Beigel J, Chotpitayasunondh T, Del Mar C, Farrar J, Tran TH, Ozbay B, Sugaya N, Fukuda K, Shindo N, Stockman L, Vist GE, Croisier A, Nagjdaliyev A, Roth C, Thomson G, Zucker H, Oxman AD, WHO Rapid Advice Guideline Panel on Avian Influenza (2007 WHO Rapid Advice Guidelines for pharmacological management of sporadic human infection with avian influenza A (H5N1) virus. Lancet Infect Dis 7:21–31 [DOI] [PMC free article] [PubMed]
- 66.American Thoracic Society: Salvage Therapies for H1N1-induced ARDS. http://www.thoracic.org/sections/clinical-information/critical-care/salvage-therapies-h1n1/index.html. Accessed 19 Oct 2009
- 67.Anonymous Mechanical ventilation of SARS patients: safety issues involving breathing-circuit filters. Health Devices. 2003;32:220–222. [PubMed] [Google Scholar]
- 68.Thompson AK, Faith K, Gibson JL, Upshur RE. Pandemic influenza preparedness: an ethical framework to guide decision-making. BMC Med Ethics. 2006;7:12. doi: 10.1186/1472-6939-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


