Abstract
Various diseases in the pediatric age group can present as an intrathoracic rounded opacity on a chest radiograph. The purpose of this pictorial essay is to emphasize the imaging appearance of round pneumonia, an entity that occurs especially in the pediatric population. Additional pathologies with similar chest radiographic appearances are also presented. The diagnosis of round pneumonia should be made in children who have the typical clinical presentation along with chest radiographs demonstrating the characteristic findings.
Keywords: Round pneumonia, Children, Mimickers, Chest radiograph
Introduction
One of the various imaging manifestations of community-acquired pneumonia in the pediatric age group is a round opacity on a chest radiograph [1]. This appearance is termed “round pneumonia” and is most commonly caused by bacteria. A round opacity in adults is suspicious for malignancy, whereas in children it is likely to represent a benign process [2]. In a child with symptoms and signs of pneumonia and a round opacity on the chest radiographs, the diagnosis is most likely round pneumonia. However, one has to be aware of other pathologies that can present a similar imaging appearance in children [3]. In this essay, we discuss the etiology and clinical and radiographic features of round pneumonia, as well as its less common mimickers. Awareness of radiographic mimickers can prevent diagnostic pitfalls, unnecessary tests and radiation, as well as assist in improving patient care.
Clinical features
Round Pneumonias initially present with tachypnea, cough and generalized malaise followed by an acute febrile illness. Less common symptoms are vomiting, abdominal pain and chest pain.
In a series of 109 children with round pneumonia described by Kim and Donnelly [4], 75% were younger than 8 years of age and 90% were younger than 12 years. The mean age was 5 years. Older children are less susceptible to round pneumonia because they have more developed pathways of collateral ventilation and larger alveoli. When a round opacity is seen in older age groups, atypical microorganisms, immunodeficiencies, underlying lesions and other rare etiologies, such as a primary malignancy, must be considered.
Pathophysiology
Smaller alveoli, closely apposed septa, and underdeveloped pores of Kohn and channels of Lambert predispose young children to round pneumonia. Pores of Kohn are openings that develop between adjacent alveoli (interalveolar connections). The channels of Lambert are bronchoalveolar channels that involve the terminal bronchioles opening into alveolar sacs. These structures function as collateral circulation and allow the passage of fluid or bacteria. With developed collaterals, the infection can spread easily into adjacent alveolar sacs and throughout the lobe. With less developed collaterals, there is centrifugal spread, and the consolidation is spherical with sharp margins. These factors result a slow progression of the disease and a higher chance of detecting the infection in children. There is a preferential posterior and lower lobe distribution of round pneumonia, likely resulting from gravity and a supine position while sleeping [4, 5].
Etiology
Streptococcus pneumoniae is the most common organism (90%) causing round pneumonia. Other organisms, such as Klebsiella pneumoniae and Haemophilus influenzae, are rare. In older children, particularly those with underlying primary or secondary immunodeficiencies, atypical infectious agents such as fungi and mycobacterium are occasionally encountered [5, 6].
Imaging appearance
Chest radiographs are frequently obtained in children presenting with respiratory tract infection. In most cases, round pneumonia presents as a solitary lesion with a predilection for the posterior segment of the lower lobe and is commonly in contact with the pleura, hilum or pulmonary fissure (Fig. 1). Chest radiographs typically reveal a circumscribed, homogeneous spherical lesion greater than 3 cm (range 1–12 cm). The definition of the margins of the round opacity decreases with increasing age, indicating more developed collateral circulation (Fig. 2). Satellite lesions are sometimes present, and air bronchograms can be seen in up to 20% of cases. Round pneumonia does not characteristically demonstrate calcifications, cavitations, lymphadenopathy or pleural effusions. Multiple round pneumonias (Fig. 3) are rare and account for approximately 1% of children with round pneumonia [4].
Fig. 1.
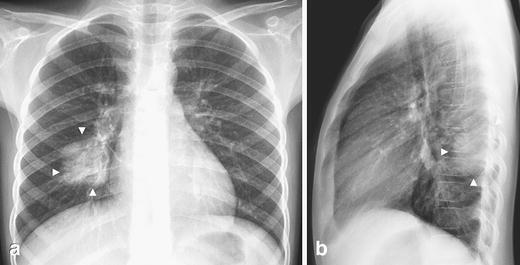
Typical round pneumonia in a 7-year-old boy with cough and fever. a Frontal and (b) lateral chest radiographs show the appearance of a typical round pneumonia (arrowheads)—a well-defined round opacity in the right lower lobe touching the pleura posteriorly
Fig. 2.
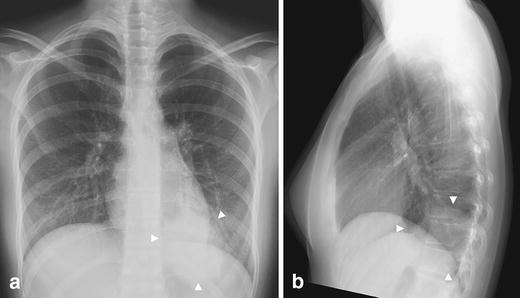
Round pneumonia in a 16-year-old girl with fever and cough. a Frontal and (b) lateral chest radiographs demonstrate a round opacity in the basal segment of the left lower lobe with poorly defined margins (arrowheads). In older children, the margins of the lesions are less sharp because of maturation of the mechanisms of collateral air drift. Follow-up study obtained at 6 weeks revealed complete resolution (not shown)
Fig. 3.
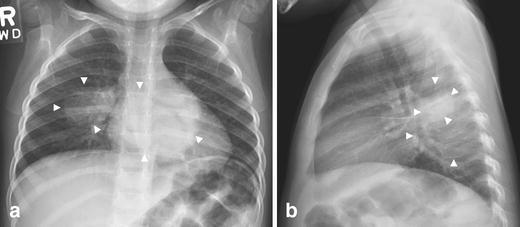
Multiple round pneumonias in a 3-year-old boy with fever and vomiting. Chest radiographs (a=frontal; b=lateral) show round opacities (arrowheads) in the superior segment of right lower lobe and in the left lower lobe posteriorly. Follow-up radiographs demonstrated complete resolution (images not shown)
Imaging workup
The main goal in imaging pulmonary infections is to obtain an early diagnosis, start adequate treatment and to possibly prevent complications. Chest radiographs are sufficiently sensitive and highly specific for the diagnosis of community-acquired pneumonia and evaluation for possible complications. CT can provide more information than plain radiographs, but must be reserved for specific situations such as complicated pulmonary infections and immunocompromised patients [7, 8]. In the appropriate clinical setting, a chest radiograph with typical findings is sufficient to diagnose round pneumonia. After appropriate treatment, a follow-up radiograph is useful to ensure resolution. There are no evidence-based guidelines regarding the appropriate radiographic follow-up of children with round pneumonia. However, the literature on round pneumonia suggests a follow-up chest radiograph 10–14 days after appropriate antibiotic therapy. Rapid loss of spherical shape or resolution of the pneumonia should be seen, confirming the diagnosis; no further imaging is necessary in such cases.
Indications for cross-sectional imaging
When dealing with a round opacity on a chest radiograph of a child, the importance of correlation with the clinical symptoms cannot be overemphasized. In a child younger than 8 years with clinical symptoms and signs of pneumonia and a typical radiographic appearance, the diagnosis is almost always round pneumonia. Likewise, in a child with clinical symptoms and signs of pneumonia, even if the round opacity has atypical features on the chest radiographs, round pneumonia is still the likely diagnosis. However, one has to be aware of other pathologies that present a similar imaging appearance in children [3, 9–12]. CT can provide more detailed information than plain radiographs, but the principles of the “Image Gently” campaign must always be kept in mind.
Cross-sectional imaging with CT should be considered in the presence of a round opacity on chest radiographs in the following instances:
If the clinical features are not consistent with a pneumonic process
If the round opacity does not resolve after appropriate antibiotic treatment
If there are radiographic signs of a non-pulmonary origin on chest radiograph
Even in the not-so-typical cases, a wait and watch approach with a follow-up chest radiograph after appropriate antibiotic treatment might be all that is needed (Fig. 4). An underlying process such as a developmental lung malformation that has become infected could be obscured in a CT scan of the chest performed too soon.
Fig. 4.
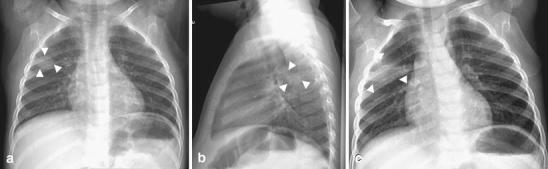
Evolution of an atypical round pneumonia in a 2-year-old with fever and cough. a-b Chest radiograph shows a round opacity in the posterior segment of the right upper lobe. c Follow-up radiograph obtained a few days later demonstrates rapid change. A radiograph obtained several weeks later after antibiotic treatment showed complete resolution (not shown). Note that on the first exam the lesion is in the right upper lobe and surrounded by lung. The clinical presentation and the age of the child suggested round pneumonia as the first possibility. The atypical features prompted the follow-up evaluation
Round pneumonia mimics in children
One of the goals of this article is to outline the classic features of round pneumonia. The purpose in doing so is to prevent misdiagnoses that can lead to unwarranted additional imaging, which causes unnecessary radiation, cost and parent/patient stress. With the typical clinical presentation and radiographic appearance, misdiagnosis of round pneumonia is unlikely. However, the typical radiographic presentation of a solitary round lesion located in the posterior lower lobe was seen in only 63% of the patients in the series by Kim and Donnelly [4]. Round opacities in chest radiographs can be the manifestation of other conditions in children, such as inflammatory masses, congenital anomalies, neoplasms and pseudomasses (Table 1).
Table 1.
Characteristic features of round pneumonia and mimics
| Round pneumonia | 2–8 years old with typical symptoms of pneumonia such as fever, cough and tachypnea |
| CXR—parenchymal round opacity in posterior lower lobe, touching the fissures, ± air bronchograms (Figs. 1, 2, 3 and 4) | |
| Fungal infection | Immunocompromised. Variable appearances. On CXR can present as a round homogeneous opacity that cavitates as the child’s immune system recovers; on CT presents as the halo sign, crescent sign (indicates fungal ball) (Fig. 5), cavitation |
| Lung abscess | Bacterial etiology. On CXR, round opacity with air-fluid level (similar dimensions in both views); CT can help to better characterize and to evaluate relationship with adjacent structures in case drainage is needed and to identify concomitant empyema, lung infarction (Fig. 6) |
| Tuberculosis | Immigrant population, immunocompromised, low socioeconomic groups |
| CXR—any kind of parenchymal opacity + hilar adenopathy (Fig. 7) ± effusion | |
| Developmental pulmonary malformations—sequestration | Can present as a lung mass in infants, clinically/radiographically presents as pneumonia when infected, located in lower lobes (left >right); CT angiography shows diagnostic, systemic arterial supply, systemic venous (extralobar sequestration ) versus pulmonary venous (intralobar sequestration) drainage (Fig. 8) |
| Developmental pulmonary malformations—CPAM (CCAM) | Can present as a lung mass / acute respiratory distress in a newborn, clinically/radiographically presents as pneumonia when infected; CT (ideally after clearance of infection ) appearance depends on the type, no associated systemic vessels or tracheobronchial communication (Fig. 9) |
| Developmental pulmonary malformations—bronchogenic cyst | When intrapulmonary, appears as a well-defined opacity on CXR; CT angiography provides optimal evaluation to exclude abnormal vascular supply and for surgical planning |
| Neoplastic lesions | Clinical presentation is not typical of pneumonia |
| Lymphoma—adolescents, isolated parenchymal opacity uncommon (Fig. 10), usually with adenopathy | |
| Neuroblastoma—posterior mediastinal mass on CXR (Fig. 11), frequently with calcifications, CT/MRI/PET/MIBG required for evaluation | |
| Chest wall lesions—“extrapleural sign,” obtuse angles with chest wall on CXR, ±rib involvement (Fig. 12) | |
| Diaphragmatic hernia/eventration | Clinical/imaging presentation—depends on site, size, herniating contents, pulmonary compromise; commonly in costophrenic recess on CXR, air-fluid levels suggest bowel content; CT/MRI provide optimal demonstration (Fig. 13) |
Pulmonary infections
The etiology of round pneumonia is almost always bacterial, and Streptococcus pneumoniae is the most common agent. Infectious agents such as fungi and mycobacteria are rare, but can cause a round-pneumonia-like appearance both clinically and radiographically. This has implications in treatment, as these are more commonly seen in immunocompromised children. Some of these agents present as multifocal, round pulmonary lesions on radiographs. Kosut et al. [13] and Shetty et al. [14] reported cases of round pneumonias in patients with chronic granulomatous disease. The case reported by Kosut was that of a newborn with multilobar round pneumonias. The age of the patient and the multilobar presentation were unusual for the typical round pneumonia. The etiology in this case was Aspergillus. The case reported by Shetty was that of a 15-year-old, also an unusual age for typical round pneumonia. The etiology in this case was Nocardia.
Invasive pulmonary aspergillosis occurs in immunocompromised children. Children are usually asymptomatic when immunocompromised and develop symptoms as their immunity recovers. Initially, these children present with lobar consolidation, peripheral nodular masses or small pulmonary nodules without cavitation [15]. In cases of secondary immunosuppression, the classic radiological signs of a peripheral halo, crescent sign and cavitation are not seen until the immune system improves (Fig. 5). In cases of chronic granulomatous disease, these signs are not typically seen. Aspergillosis is the most common cause of fungal pneumonia in children with chronic granulomatous disease. When fungal infection is suspected, CT is the imaging modality of choice in suggesting the diagnosis and evaluating the extent of the disease [16].
Fig. 5.
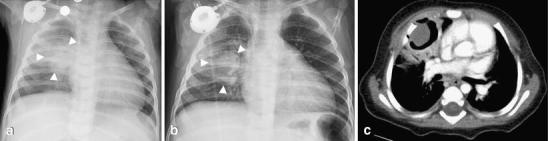
Aspergilloma in a 10-month-old child with leukemia who presented with a fever. a Initial chest radiograph demonstrates a round opacity (arrowheads) in the right parahilar region. Note the presence of a right chest Port-A-Cath. b Frontal chest radiograph obtained several days later shows a well-defined cavitary lesion (arrowheads) in the right upper lobe surrounded by air. c Contrast-enhanced CT on the same day shows an intracavitary lesion consistent with a fungal ball. No other lesions were present. The final diagnosis was an aspergilloma by cultures and histological analysis after surgical removal
Pulmonary abscess can occur in children because of untreated pneumonitis or by hematogenous spread. In the absence of an air-fluid level, pulmonary abscesses can mimic round pneumonia on radiographs (Fig. 6). There is a resurgence of childhood pulmonary TB in developed countries because of HIV infection, overcrowding and immigration [17]. Although mediastinal and hilar adenopathy is common in children with tuberculosis, parenchymal opacities occur in the form of consolidation. Tuberculosis can present as any type of parenchymal opacity, such as round (Fig. 7), patchy, linear or mass-like. Ghon complex (a.k.a. Ranke complex) refers to the parenchymal lesion of primary pulmonary tuberculosis associated with a corresponding lymph node. Even though the primary complex of tuberculosis can involve any part of the lung, the middle lobe is the least involved [18]. Radiologists must look carefully for adenopathy, a distinguishing feature that is very common in pediatric tuberculosis. The right hilum is more commonly involved because of the pattern of lymphatic circulation through the lungs. A right-side parenchymal lesion leads to right hilar adenopathy. On the other hand, a left-side lesion can lead to bilateral hilar adenopathy [19].
Fig. 6.
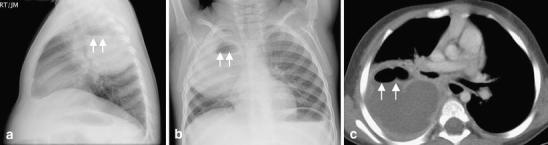
Lung abscess in a 2-year-old boy with fever and cough. a Lateral and (b) frontal chest radiographs reveal a large round opacity (arrowheads) with an air-fluid level (arrowheads) located posteriorly in the right upper lobe. c Contrast-enhanced CT scan obtained later shows a large rim-enhancing lesion with an air-fluid (arrowheads) level consistent with a lung abscess
Fig. 7.
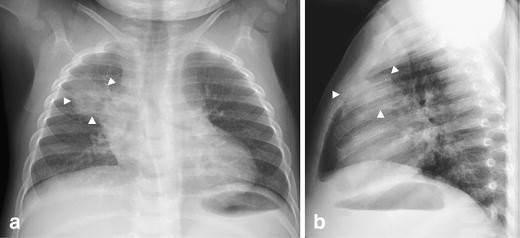
Tuberculosis mimicking a round pneumonia in a 13-month-old asymptomatic child with a positive PPD test. Chest radiographs (a, b) demonstrate a round opacity (arrowheads) in the anterior segment of the right upper lobe. Chest radiographs obtained a year later following anti-tuberculous treatment revealed only a residual fibrotic scar (not shown). Note the upper lobe location and indistinct margins
Viruses and proteobacteria have been implicated in causing round pneumonia in adolescents and young adults. During the Severe Acute Respiratory Syndrome (SARS) epidemic in 2003, Wan et al. [6] reported eight cases of SARS presenting as round pneumonias. Likewise, there are reports of Q fever presenting in adolescents and young adults as multiple round pneumonias [20–23].
Developmental pulmonary malformations
Developmental pulmonary malformations such as sequestration, congenital lobar emphysema (a.k.a. infantile lobar emphysema, congenital lobar hyperinflation), bronchogenic cysts, arteriovenous malformations, and congenital pulmonary airway malformation (CPAM) can present as a round opacity and be mistaken for round pneumonia.
CPAM, previously known as congenital cystic adenomatoid malformation (CCAM), refers to the disorganized adenomatoid and hamartomatous proliferation of bronchioles that are in communication with the bronchial tree [24]. The entity has been classified into five types (types 0–4) based on the location or stage of development of the abnormality involving the tracheobronchial airway [24, 25]. They are often found in association with sequestrations and can have overlapping features. However, by definition, there is no systemic arterial supply in a CPAM, whereas sequestrations have a systemic arterial supply [26]. CPAM, sequestrations and congenital lobar hyperinflation can appear as round opacities on chest radiographs on a newborn due to the lack of focal reabsorption of the parenchymal fluid within the lesion. In this period, age is a differentiating feature, as round pneumonias and even lobar pneumonias are exceedingly rare in the first few months of life. CPAM Type 3, because of its microcystic nature, typically presents as a round, solid mass most similar to a round pneumonia.
Sequestrations are commonly seen at the lung bases, which are also a common location for round pneumonia. In older children and adolescents, developmental lung malformations may present as round opacities because of superimposed infection or lack of connection with the tracheobronchial tree, precluding aeration of the lesion (Figs. 8 and 9).
Fig. 8.
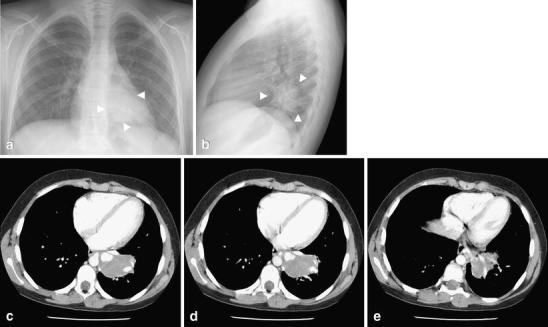
Intralobar sequestration in a 12-year-old girl with chronic cough. a Frontal and (b) lateral chest radiographs show a round opacity (arrowheads) with well-defined margins in the left lower lobe. c–e Contrast-enhanced CT demonstrates a round soft-tissue mass (arrows) in the posteromedial left lower lobe without tracheobronchial airway communication, but with arterial supply by a branch from the descending thoracic aorta and a draining pulmonary vein into the left atrium. The age of the girl and the symptoms should alert the radiologist about a possible underlying lesion. Compare these images with those in Fig. 2 (round pneumonia)
Fig. 9.
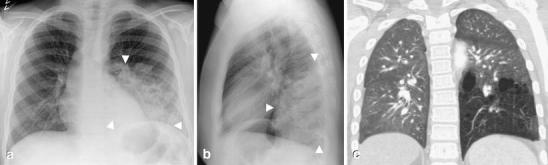
Congenital pulmonary airway malformation (CPAM) in a 13-year-old boy with recurrent respiratory tract infections. a Frontal and (b) lateral chest radiographs show a large opacity (arrowheads) in the left lower lobe. On the frontal projection, the margins are indistinct. However, on the lateral radiograph, the lesion has well-defined margins anteriorly and superiorly. c Because of the boy’s age, the clinical history and the radiographic appearance, a CT angiogram was obtained; showing a large lesion with multiple air-filled cystic spaces and no systemic arterial supply. The diagnosis of CPAM was confirmed after surgery
Bronchogenic cysts present as round masses that contain fluid and frequently do not communicate with the esophagus. Approximately one-third of bronchogenic cysts are intraparenchymal and perihilar, which is a location shared with round pneumonia [27]. When the child presents with clinical features of pneumonia and there is suspicion of a bronchogenic cyst on chest radiographs, a follow-up radiograph after a course of antibiotic treatment is suggested. If the opacity persists or is suggestive of an underlying pulmonary developmental malformation, CT angiography is the imaging modality of choice [28].
Malignancies
Neoplastic processes such as lymphoma or metastasis can present as round pulmonary opacities. In a very small percentage of cases, pulmonary involvement is the first manifestation of lymphoma (Fig. 10) [29]. Further investigation should be prompted if a process different from round pneumonia is suggested by the clinical presentation and careful scrutinization of the radiographs. For example, an apparent round pneumonia in a posteromedial location with erosion of adjacent bony structures is a neuroblastoma until proved otherwise (Fig. 11) [30]. Thoracic neuroblastomas are typically posterior mediastinal in location, have associated calcification (50%) and rib destruction, and occur within the age range of round pneumonia [31]. Other pleural-based or even chest wall lesions, when large, can be diagnostic dilemmas on radiographs. Basic radiological signs are very helpful in deciding whether the lesion is parenchymal, pleural, mediastinal or within the chest wall (Fig. 12). Lesions in each of these locations can appear as round opacities, but careful evaluation of the radiographs can in most cases, determine the correct location.
Fig. 10.
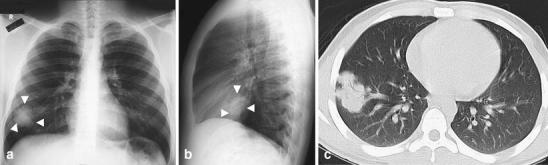
Lymphoma presenting as a round opacity in a 17-year-old boy with malaise, fatigue and cervical adenopathy. a Frontal and (b) lateral chest radiographs show a round lobulated opacity (arrowheads) in the right lower lobe. c Contrast-enhanced CT shows the lesion as a well-defined lobulated opacity with air bronchograms within and barely touching the pleura. Multifocal adenopathy was also present, though not in the mediastinum (not shown). Pathological diagnosis of lymphoma was made after cervical surgical lymph node resection
Fig. 11.
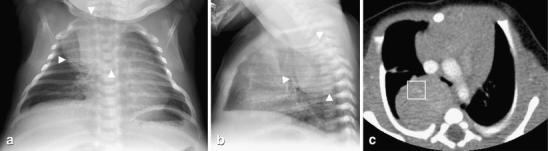
Neuroblastoma in a 1-month-old with shortness of breath and stridor. a Frontal and (b) lateral chest radiographs show a large, round well-defined right posterior mediastinal mass (arrowheads) with mass effect upon the airway. c Contrast-enhanced CT obtained the same day shows a well-defined heterogeneously enhancing posterior mediastinal mass with faint calcifications (within square box) and mass effect on the carina. The pathological diagnosis of neuroblastoma was made by surgical resection
Fig. 12.
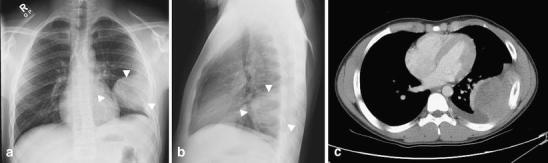
PNET of the chest wall in a 15-year-old boy with left chest wall pain for several weeks. a Frontal and (b) lateral chest radiographs show a large round opacity (arrowheads) with well-defined smooth medial and anterior borders but with obtuse borders at the pleural margins. Subtle widening of the left 7th rib with associated permeative destruction and periosteal reaction are seen. These findings are suggestive of a chest wall mass. c Corresponding CT study shows a large heterogeneously enhancing mass arising from the chest wall. The left 7th rib is expanded with periosteal reaction. The final pathological diagnosis was a peripheral PNET
Miscellaneous
Rarely, diaphragmatic hernias and eventrations can mimic round pneumonia (Fig. 13). When small, hernias can be overlooked in the neonatal period and found incidentally in children imaged for upper respiratory infections. In these cases, attention to radiographic signs will usually suggest a typical anterior or posterior location, as in the case of Morgagni and Bochdalek hernias, respectively. Small hernias can also present as a solitary pulmonary nodule [32]. The presence of air-fluid-containing structures, such as bowel or very sharp margins, can help distinguish against round pneumonia. The fluid-filled or collapsed bowel loops within the hemithorax have a homogeneously opaque appearance. Less commonly, bowel becomes entrapped in a small hernia, producing a large pleural effusion. Sonography can be useful in rendering a diagnosis [33].
Fig. 13.
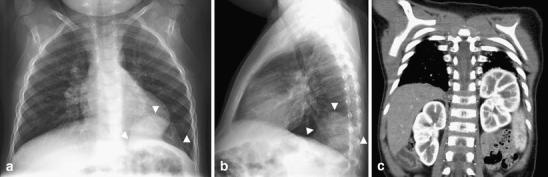
Diaphragmatic eventration in a 22-month-old with fever and cough. a Frontal and (b) lateral chest radiographs show an opacity (arrowheads) in the left lower lobe with well-defined superior margins. The opacity remained unchanged on radiographs obtained 6 weeks later when the child had clinically shown improvement (not shown). c Contrast-enhanced CT study reveals eventration of the left hemidiaphragm posteromedially with the left kidney at a high ectopic location
Conclusion
Attention to the basic principles of chest radiography leads to a more accurate diagnosis of round pneumonia and prevents unnecessary, expensive exams and radiation. In a child younger than 8 years of age with clinical symptoms and signs of pneumonia and a typical radiographic appearance, the diagnosis is almost always round pneumonia. This is different from adult imaging, in which malignant processes predominate. With appropriate antibiotic therapy, a follow-up frontal radiograph to ensure improvement or resolution can confirm the round pneumonia diagnosis. In a child with clinical symptoms and signs of pneumonia, even if the round opacity has atypical features on chest radiographs, round pneumonia is still the likely diagnosis. One needs to recognize the clinical scenario and the mimics of round pneumonia. Although uncommon, knowledge of these red herrings in imaging, although uncommon can help to avoid misdiagnosis and optimize further imaging and investigations as necessary for cost-effective patient care.
References
- 1.Rose RW, Ward BH. Spherical pneumonias in children simulating pulmonary and mediastinal masses. Radiology. 1973;106:179–182. doi: 10.1148/106.1.176. [DOI] [PubMed] [Google Scholar]
- 2.Wagner AL, Szabunio M, Hazlett KS, et al. Radiologic manifestations of round pneumonia in adults. AJR. 1998;170:723–726. doi: 10.2214/ajr.170.3.9490962. [DOI] [PubMed] [Google Scholar]
- 3.Yikilmaz A, Lee EY. CT imaging of mass-like nonvascular pulmonary lesions in children. Pediatr Radiol. 2007;37:1253–1263. doi: 10.1007/s00247-007-0637-4. [DOI] [PubMed] [Google Scholar]
- 4.Kim Y, Donnelly LF. Round pneumonia: imaging findings in a large series of children. Pediatr Radiol. 2007;37:1235–1240. doi: 10.1007/s00247-007-0654-3. [DOI] [PubMed] [Google Scholar]
- 5.McLennan MK. Radiology rounds. Round pneumonia. Can Fam Physician. 1998;44:757–759. [PMC free article] [PubMed] [Google Scholar]
- 6.Wan YL, Kuo HP, Tsai YH, et al. Eight cases of severe acute respiratory syndrome presenting as round pneumonia. AJR. 2004;182:1567–1570. doi: 10.2214/ajr.182.6.1821567. [DOI] [PubMed] [Google Scholar]
- 7.Medina SL, Applegate KE, Blackmore CC. Evidence-based imaging in pediatrics: optimizing imaging in pediatric patient care. 1. New York: Springer; 2009. pp. 401–418. [Google Scholar]
- 8.Donnelly LF. Imaging in immunocompetent children with pneumonia. Radiol Clin North Am. 2005;43:253–265. doi: 10.1016/j.rcl.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Shady K, Siegel MJ, Glazer HS. CT of focal pulmonary masses in childhood. Radiographics. 1992;12:505–514. doi: 10.1148/radiographics.12.3.1609141. [DOI] [PubMed] [Google Scholar]
- 10.Eggli KD, Newman B. Nodules, masses, and pseudomasses in the pediatric lung. Radiol Clin North Am. 1993;31:651–666. [PubMed] [Google Scholar]
- 11.Daltro P, Fricke BL, Kuroki I, et al. CT of congenital lung lesions in pediatric patients. AJR. 2004;183:1497–1506. doi: 10.2214/ajr.183.5.1831497. [DOI] [PubMed] [Google Scholar]
- 12.Hernanz-Schulman M. Cysts and cystlike lesions of the lung. Radiol Clin North Am. 1993;31:631–649. [PubMed] [Google Scholar]
- 13.Kosut JS, Kamani NR, Jantausch BA. One-month-old infant with multilobar round pneumonias. Pediatr Infect Dis J. 2006;25:95–97. doi: 10.1097/01.inf.0000195717.40250.8b. [DOI] [PubMed] [Google Scholar]
- 14.Shetty AK, Arvin AM, Gutierrez KM. Nocardia farcinica pneumonia in chronic granulomatous disease. Pediatrics. 1999;104:961–964. doi: 10.1542/peds.104.4.961. [DOI] [PubMed] [Google Scholar]
- 15.Greene RE, Schlamm HT, Oestmann JW, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007;44:373–379. doi: 10.1086/509917. [DOI] [PubMed] [Google Scholar]
- 16.Antachopoulos C, Walsh TJ, Roilides E. Fungal infections in primary immunodeficiencies. Eur J Pediatr. 2007;166:1099–1117. doi: 10.1007/s00431-007-0527-7. [DOI] [PubMed] [Google Scholar]
- 17.Shingadia D, Novelli V. Diagnosis and treatment of tuberculosis in children. Lancet Infect Dis. 2003;10:624–632. doi: 10.1016/S1473-3099(03)00771-0. [DOI] [PubMed] [Google Scholar]
- 18.Inselman LS. Tuberculosis in children: an update. Pediatr Pulmonol. 1996;21:101–120. doi: 10.1002/(SICI)1099-0496(199602)21:2<101::AID-PPUL6>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 19.Starke JR. Tuberculosis in infants and children. In: Schlossberg D, editor. Tuberculous and nontuberculous mycobacterial infections. 4. Philadelphia: Saunders; 1999. pp. 303–324. [Google Scholar]
- 20.Pickworth FE, El-Soussi M, Wells IP, et al. The radiological appearances of Q fever pneumonia. Clin Radiol. 1991;44:150–153. doi: 10.1016/S0009-9260(05)80857-8. [DOI] [PubMed] [Google Scholar]
- 21.Gordon JD, MacKeen AD, Marrie TJ, et al. The radiographic features of epidemic and sporadic Q fever pneumonia. J Can Assoc Radiol. 1984;35:293–296. [PubMed] [Google Scholar]
- 22.Millar JK. The chest film findings in Q fever: a series of 35 cases. Clin Radiol. 1978;29:371–375. doi: 10.1016/S0009-9260(78)80092-0. [DOI] [PubMed] [Google Scholar]
- 23.Anton E. A frequent error in etiology of round pneumonia. Chest. 2004;125:1592–1593. doi: 10.1378/chest.125.4.1592. [DOI] [PubMed] [Google Scholar]
- 24.Stocker JT, Madewell JE, Drake RM. Congenital cystic adenomatoid malformation of the lung: classification and morphologic spectrum. Hum Pathol. 1977;8:155–171. doi: 10.1016/S0046-8177(77)80078-6. [DOI] [PubMed] [Google Scholar]
- 25.Stocker J. Congenital pulmonary airway malformation: a new name and expanded classification of congenital cystic adenomatoid malformations of the lung. Histopathology. 2002;41(Suppl 2):424–458. [Google Scholar]
- 26.Lee EY, Siegel MJ, Sierra LM, et al. Evaluation of angioarchitecture of pulmonary sequestration in pediatric patients using 3D MDCT angiography. AJR. 2004;183:183–188. doi: 10.2214/ajr.183.1.1830183. [DOI] [PubMed] [Google Scholar]
- 27.Kocaoglu M, Frush DP, Ugurel MS, et al. Bronchopulmonary foregut malformations presenting as mass lesions in children: spectrum of imaging findings. Diagn Interv Radiol. 2010;16:153–161. doi: 10.4261/1305-3825.DIR.2063-08.2. [DOI] [PubMed] [Google Scholar]
- 28.Newman B. Congenital bronchopulmonary foregut malformations: concepts and controversies. Pediatr Radiol. 2006;36:773–791. doi: 10.1007/s00247-006-0115-4. [DOI] [PubMed] [Google Scholar]
- 29.Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252–260. doi: 10.1002/1097-0142(197201)29:1<252::AID-CNCR2820290138>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Kirks DR. Practical pediatric imaging: diagnostic radiology of infants and children. 3. Philadelphia: Lippincott-Raven; 1998. pp. 639–642. [Google Scholar]
- 31.Papaioannou G, McHugh K. Neuroblastoma in childhood: review and radiological findings. Cancer Imaging. 2005;5:116–127. doi: 10.1102/1470-7330.2005.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katsenos S, Kokkonouzis I, Lachanis S, et al. Right-sided Bochdalek hernia presenting as a solitary pulmonary nodule. Radiology Case Reports. 2008;3:1–5. doi: 10.2484/rcr.v3i2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alford BA, McIlhenny J, Jones JE, et al. Asymmetric radiographic findings in the pediatric chest: approach to early diagnosis. RadioGraphics. 1993;13:77–93. doi: 10.1148/radiographics.13.1.8426938. [DOI] [PubMed] [Google Scholar]


