Abstract
We describe two previously healthy children who were hospitalized in the same period in different departments of our University with clinical signs of Kawasaki syndrome, which were treated with intravenous immunoglobulins and acetylsalicylic acid: in both cases, Coxsackie virus infection was concurrently demonstrated by enzyme-linked immunosorbent assay, and complement fixation test identified antibodies to serotype B3. In the acute phase, both patients presented hyperechogenic coronary arteries, but no cardiologic sequels in the mid term. The etiological relationship between Kawasaki syndrome and Coxsackie viruses is only hypothetical; however, the eventual identification of ad hoc environmental triggers is advisable in front of children with Kawasaki syndrome, with the aim of optimizing epidemiological surveillance and understanding the intimate biological events of this condition.
Keywords: Kawasaki syndrome, Coxsackie virus type B3
Introduction
Kawasaki syndrome (KS) is mostly diagnosed in infants and children without having the possibility of identifying its “primum movens”, which remains largely mysterious in almost all clinical settings [1]. Diagnostic assessment of KS derives from the combination of prolonged fever lasting more than 5 days with at least 4 of 5 standard clinical signs: bilateral conjunctival injection, oropharyngitis, polymorphous skin rash, cervical lymphadenopathy and peripheral changes involving extremities and perineum [2, 3]. The lack of a specific diagnostic test contributes to the delay in KS diagnosis, even in populations where the condition is widely recognized [4]. The timely recognition of KS is essential to maximize the prevention of overt coronary artery damage, because treatment beyond 10 days of onset is often associated with a worse outcome and an increased incidence of coronary abnormalities [5]. Specific rational interventions could be developed if the etiopathogenesis of KS was fully understood: many immunologic abnormalities in KS suggest an infectious trigger, but the role of coexisting infections at KS onset has not been adequately investigated [6]. We hereby report two healthy patients with clinical pictures related to KS who revealed to be concurrently infected by Coxsackie virus type B3 (CVB3).
Case histories
Case 1
A 2-year-old healthy male child of Caucasian origin was hospitalized for fever (maximal peaks of 39.0°C) and abdominal pain, which began 3 days before. Cardiac, respiratory and abdominal examination was normal at admission, but tonsillopharyngitis and cervical lymph node enlargement were seen. Laboratory findings revealed hemoglobin 11.3 g/dl, white blood cell count 21.700/mm3, neutrophils 73.3%, lymphocytes 21.5%, platelet count 343.000/mm3, C-reactive protein 70.7 mg/l (CRP, n.v. <3), aspartate transaminase 292 IU/l (AST, n.v. 7–45), alanine transaminase 105 IU/l (ALT, n.v. 7–45), total bilirubin 2.2 mg/dl (n.v. 0.3–1.2), conjugated bilirubin 1.4 mg/dl (n.v. 0.1–0.3), γ-glutamyl transpeptidase 197 IU/l (n.v. 2–25), alkaline phosphatase 1,009 IU/l (n.v. <750) and lactate dehydrogenase 1,054 IU/l (n.v. 230–460); the other laboratory tests, including clotting tests, were normal. During the following 2 days, the patient remained feverish and presented bilateral non-purulent conjunctival hyperemia. At the 6th day of hospitalization, a diffuse erythematous maculopapular rash was noted in association with mild edema of both hands, glossitis and red cracked lips, while fever (of 9-day duration) persisted with hypertransaminasemia. Blood, throat and stool cultures were sterile. An “atypical” KS (due to liver involvement) was diagnosed, and the patient was treated with intravenous immunoglobulins (IVIG, 2 g/kg in a 12-h infusion) along with oral acetylsalicylic acid (50 mg/kg/day). At this time, echocardiography showed a hyperechogenous aspect of coronary arteries and a mild mitral insufficiency. Abdominal ultrasound showed normal dimension and structure of liver and normal gallbladder without signs of lithiasis. Clinical improvement was rapidly obtained on fever, which subsided within 8 h, while hypertransaminasemia regressed on the 12th day of hospitalization (AST 71 IU/l, ALT 52 IU/l). Cutaneous desquamation of fingers and toes became evident after 2 weeks, when C-reactive protein became normal and acetylsalicylic acid was shifted to a single dose of 5 mg/kg/day. Serological tests for Epstein–Barr virus, hepatitis A, B, C and Widal–Wright reaction were negative. An indirect, solid-phase, microplate enzyme-linked immunosorbent assay (ELISA, Inst. Virion-Serion, GmbH, Würzburg, Germany) detected IgG, IgM and IgA to Coxsackie viruses (methods for rapid Enterovirus molecular diagnosis were not available). The further clinical course was uneventful, and the young boy was discharged after 20 days, when echocardiography was completely normal and ELISA confirmed IgG and IgA to Coxsackie viruses. Complement fixation test (CFT) for group A and B Coxsackie viruses (Inst. Virion Ltd, Ruschlikon, Switzerland) was performed 12 weeks after diagnosis and showed antibodies only to CVB3 (1/64). Acetylsalicylic acid was maintained for a period of 6 months and at this time, cardiologic assessment was again within normal limits.
Case 2
A 14-month-old healthy female child, born in the Philippines, was hospitalized in consequence of a 2-day history of fever (with peaks of 39.5°C), poor feeding, itchy erythematous rash (resistant to cetirizine) and slight edema of both feet. Physical examination revealed exudative pharyngitis, while a micropapular rash became diffuse all over her body on the 3rd day, suggesting an infectious nature of the disease. Laboratory tests showed hemoglobin 11.1 g/dl, white blood cell count 16.490/mm3, neutrophils 60.2%, lymphocytes 31.7%, platelet count 423.000/mm3, CRP 59.5 mg/l, with normal serum transaminase level and clotting tests. Echocardiography was performed at the 4th day of fever and was negative. Attempts to culture viruses from throat swabs, cerebrospinal fluid and stools were unsuccessful. On the 8th day of intermittent fever (until 40.2°C), the child presented conjunctival hyperemia. On this day, echocardiography revealed a diffuse hyperechogenicity of the right coronary artery and a normal left coronary artery (Fig. 1), confirming the clinical suspicion of KS. Treatment with IVIG (2 g/kg as a single 12-h infusion) and oral acetylsalicylic acid (50 mg/kg/day) was started and fever disappeared on the following day. Serological tests for Epstein–Barr virus, Cytomegalovirus, Toxoplasma gondii, Herpes virus, Rubella virus, Adenovirus, Parvovirus B19 and rickettsial diseases were negative, but IgM and IgA to Coxsackie viruses were found by ELISA (methods for Enteroviral RNA recognition were unavailable). After 2 weeks, heart ultrasound assessment was completely normal and ELISA revealed IgG to Coxsackie viruses. A mild peeling of the I and II fingers of the right hand was also noted. The further clinical course of this child was regular and she was discharged after an overall period of 17 days. Acetylsalicylic acid was maintained at an antiplatelet dosage (3 mg/kg/day) for a period of 8 weeks. The patient was recalled 13 weeks later for convalescent phase serum sampling, and CFT revealed antibodies to CVB3 (1/64).
Fig. 1.
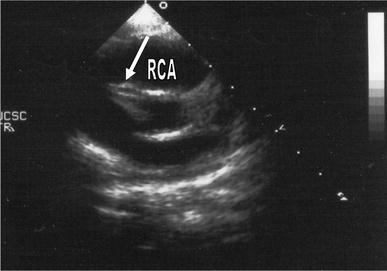
Echocardiogram in patient 2 showing a hyperechogenic aspect (arrow) of the right coronary artery (RCA)
Discussion
Among factors that make KS such a difficult disease to study from the etiological point of view, we have the strict predilection of smaller age in all ethnic groups and the problematic collection of biopsy specimens. It has been long debated whether the etiologic agents of KS might be bacterial or viral in origin [7]: in fact, KS clinical course appears quite similar to scarlet fever and toxic shock syndrome, both caused by bacterial exotoxins and treatable with proper antibiotics, but also to viral illnesses such as adenoviral infection, measles and infectious mononucleosis [8]. The search for a single microbiological cause of KS has been disappointing and many hypothetical etiologic factors have been reported [9–18, Table 1]. The role of superantigens of group A Streptococcus and Staphylococcus aureus that promote T-cell activation leading to an extensive immunologic reaction in genetically susceptible individuals is controversial [19]. The epidemiological characteristics of KS are in conflict with the possibility that newly emerged viruses, such as avian influenza or retroviruses might cause KS [20]. Esper et al. reported the association between the new human coronavirus NL-63 and KS, detecting the virus by reverse transcription-polymerase chain reaction in respiratory samples from 8 of 11 infants with KS [21], though this association was disavowed in further studies [22]. Catalano-Pons et al. have reported 2 infants with incomplete KS and coronary aneurysms during primary Cytomegalovirus infection, in whom intravenous ganciclovir improved both clinical and echocardiographic abnormalities, while IVIG were ineffective [23]. In 2005, Benseler et al. have evaluated retrospectively the rate of concurrent infections at the time of KS diagnosis in a cohort of 129 children during a 2-year period: they found that various infections (tonsillitis with positive throat culture for group A Streptococcus, viral illnesses confirmed by the evidence of serologic positive IgM antibody, chest radiograph-proven pneumonias, urinary tract infections, gastroenteritis and/or sepsis) were present in 33% of patients, though no infection influenced the response to IVIG, similarly to the apparently uninfected patients [24].
Table 1.
Putative etiological agents of Kawasaki syndrome
| Exotoxin-producing streptococci |
| Exotoxin-producing staphylococci |
| Streptococcus viridans |
| Propionibacterium acnes |
| Ehrlichia chaffeensis |
| Bartonella henselae |
| Mycoplasma species |
| Chlamydia pneumoniae |
| Rickettsia species |
| Coxiella burnetii |
| Adenovirus |
| Herpesvirus |
| Epstein–Barr virus |
| Human Coronavirus New Haven |
| Measles virus |
| Rotavirus |
| Dengue virus |
| Retrovirus |
Coxsackie B viruses have been identified as major causes of human viral myocarditis since 1955 and are also thought to play a significant role in the development of dilated cardiomyopathy [25]. In particular, CVB3, one of the 6 Coxsackie B serotypes, is known to cause aseptic meningitis, encephalitis, myocarditis and generalized infections in infants [26]. Although it has been recognized that CVB3 can be cardiotoxic, the mechanisms leading to viral cardiovirulence are still unraveled. The prevalence of Coxsackie virus infections in the developed world is unknown, but outbreaks in many countries have not been rare, even in newborns [27]. The role of Enteroviruses in KS has not been defined and to the best of our knowledge, no association with Coxsackie B viruses has been previously reported.
Our report has described two KS cases: patient 1 presented all of the 5 diagnostic signs (combined with signs of liver disease) and developed hyperechogenicity of coronary arteries; patient 2 presented 4 of 5 criteria and presented transient hyperechogenic right coronary artery, though no cardiologic sequel was observed in both ones. Diagnosis of Coxsackie virus infection in our 2 KS patients was based on ELISA: over the past decade, there has been a trend to replace the CFT with more direct, sensitive and rapid techniques, such as the ELISA. Even so, the CFT remains extremely useful when a definite virus identification and typing are needed [28]: in our patients, in fact, CFT consented to demonstrate a significant antibody serum titer against CVB3 during the convalescence phase. CVB3 might have displayed a trigger role for KS development in these two previously healthy unrelated children, but further investigations are needed to explore the potential relationship between Enteroviruses and KS or establish if an infection caused by specific serotypes of Coxsackie virus is a mere concurrent phenomenon. Clarifying the etiology of KS might disclose gene–environment interactions that are involved in the development of other vascular diseases (such as atherosclerosis), though the lack of a single unifying etiological agent, despite significant research efforts, suggests that KS can follow the exposure to a variety of infectious agents and that a stereotyped immune response might occur in a genetically susceptible host.
References
- 1.Kawasaki T, Kosaki F, Osawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54:271–276. [PubMed] [Google Scholar]
- 2.De Rosa G, Pardeo M, Rigante D. Current recommendations for the pharmacologic therapy in Kawasaki syndrome and management of its cardiovascular complications. Eur Rev Med Pharmacol Sci. 2007;11:301–308. [PubMed] [Google Scholar]
- 3.Ayusawa M, Sonobe T, Uemura S, Ogawa S, Nakamura Y, Kiyosawa N, Ishii M, Harada K. Kawasaki Disease Research Committee. Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition) Pediatr Int. 2005;47:232–234. doi: 10.1111/j.1442-200x.2005.02033.x. [DOI] [PubMed] [Google Scholar]
- 4.Falcini F. Kawasaki disease. Curr Opin Rheumatol. 2006;18:33–38. doi: 10.1097/01.bor.0000197998.50450.f6. [DOI] [PubMed] [Google Scholar]
- 5.Rigante D, Valentini P, Rizzo D, Leo A, De Rosa G, Onesimo R, De Nisco A, Angelone DF, Compagnone A, Delogu AB. Responsiveness to intravenous immunoglobulins and occurrence of coronary artery abnormalities in a single-centre cohort of Italian patients with Kawasaki syndrome. Rheumatol Int. 2010;30:841–846. doi: 10.1007/s00296-009-1337-1. [DOI] [PubMed] [Google Scholar]
- 6.Lee KY, Han JW, Lee JS. Kawasaki disease may be a hyperimmune reaction of genetically susceptible children to variants of normal environmental flora. Med Hypotheses. 2007;69:642–651. doi: 10.1016/j.mehy.2006.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgner D, Harnden A. Kawasaki disease: what is the epidemiology telling us about the etiology? Int J Infect Dis. 2005;9:185–194. doi: 10.1016/j.ijid.2005.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowley AH, Shulman ST. New developments in the search for the etiologic agent of Kawasaki disease. Curr Opin Pediatr. 2007;19:71–74. doi: 10.1097/MOP.0b013e328012720f. [DOI] [PubMed] [Google Scholar]
- 9.Anderson DG, Warner G, Barlow E. Kawasaki disease associated with streptococcal infection within a family. J Paediatr Child Health. 1995;31:355–357. doi: 10.1111/j.1440-1754.1995.tb00827.x. [DOI] [PubMed] [Google Scholar]
- 10.Hall M, Hoyt L, Ferrieri P, Schlievert PM, Jenson HB. Kawasaki syndrome-like illness associated with infection caused by enterotoxin B secreting Staphylococcus aureus. Clin Infect Dis. 1999;29:586–589. doi: 10.1086/598638. [DOI] [PubMed] [Google Scholar]
- 11.Leung DYM, Meissner HC, Fulton DR, Muray DL, Kotzin BL, Schlievert PM. Toxic shock syndrome toxin-secreting Staphylococcus aureus in Kawasaki syndrome. Lancet. 1993;342:1385–1387. doi: 10.1016/0140-6736(93)92752-F. [DOI] [PubMed] [Google Scholar]
- 12.Barton M, Melbourne R, Morais P, Christie C. Kawasaki syndrome associated with group A streptococcal and Epstein-Barr virus coinfections. Ann Trop Paediatr. 2002;22:257–260. doi: 10.1179/027249302125001543. [DOI] [PubMed] [Google Scholar]
- 13.Wang JN, Wang SM, Liu CC, Wu JM. Mycoplasma pneumoniae infection associated with Kawasaki disease. Acta Paediatr. 2001;90:594–595. doi: 10.1111/j.1651-2227.2001.tb00810.x. [DOI] [PubMed] [Google Scholar]
- 14.Strigl S, Kutlin A, Roblin PM, Shulman S, Hammerschlag MR. Is there an association between Kawasaki disease and Chlamydia pneumoniae? J Infect Dis. 2000;181:2103–2105. doi: 10.1086/315526. [DOI] [PubMed] [Google Scholar]
- 15.Konishi N, Baba K, Abe J, Maruko T, Waki K, Takeda N, Tanaka M. A case of Kawasaki disease with coronary artery aneurysms documenting Yersinia pseudotuberculosis infection. Acta Paediatr. 1997;86:661–664. doi: 10.1111/j.1651-2227.1997.tb08952.x. [DOI] [PubMed] [Google Scholar]
- 16.Kato H, Fujimoto T, Inoue O, Kondo M, Koga Y, Yamamoto S, Shingu M, Tominaga K, Sasaguri Y. Variant strain of Propionibacterium acnes: a clue to the etiology of Kawasaki disease. Lancet. 1983;2:1383–1388. doi: 10.1016/S0140-6736(83)90921-2. [DOI] [PubMed] [Google Scholar]
- 17.Nigro G, Zerbini M, Krzysztofiak A, Gentilomi G, Porcaro MA, Mango T, Musiani M. Active or recent parvovirus B19 infection in children with Kawasaki disease. Lancet. 1994;343:1260–1261. doi: 10.1016/S0140-6736(94)92154-7. [DOI] [PubMed] [Google Scholar]
- 18.Usta Guc B, Cengiz N, Yildirim SV, Uslu Y. Cytomegalovirus infection in a patient with atypical Kawasaki disease. Rheumatol Int. 2008;28:387–389. doi: 10.1007/s00296-007-0440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsubara K, Fukaya T. The role of superantigens of group A Streptococcus and Staphylococcus aureus in Kawasaki disease. Curr Opin Infect Dis. 2007;20:298–303. doi: 10.1097/QCO.0b013e3280964d8c. [DOI] [PubMed] [Google Scholar]
- 20.Lee K-Y, Han JW, Lee J-S. Kawasaki disease may be a hyperimmune reaction of genetically susceptible children to variants of normal environmental flora. Med Hypotheses. 2007;69:642–651. doi: 10.1016/j.mehy.2006.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esper F, Shapiro ED, Weibel C, Ferguson D, Landry ML, Kahn JS. Association between novel human coronavirus and Kawasaki disease. J Infect Dis. 2005;191:499–502. doi: 10.1086/428291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dominguez SR, Anderson MS, Glodé MP, Robinson CC, Holmes KV. Blinded case-control study of the relationship between human coronavirus NL63 and Kawasaki syndrome. J Infect Dis. 2006;194:1697–1701. doi: 10.1086/509509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catalano-Pons C, Quartier P, Leruez-Ville M, Kaguelidou F, Gendrel D, Lenoir G, Casanova JL, Bonnet D. Primary cytomegalovirus infection, atypical Kawasaki disease, and coronary aneurysms in 2 infants. Clin Infect Dis. 2005;41:e53–e56. doi: 10.1086/432578. [DOI] [PubMed] [Google Scholar]
- 24.Benseler SM, McCrindle BW, Silverman ED, Tyrrell PN, Wong J, Yeung RS. Infections and Kawasaki disease: implications for coronary artery outcome. Pediatrics. 2005;116:e760–e766. doi: 10.1542/peds.2005-0559. [DOI] [PubMed] [Google Scholar]
- 25.Dalldorf G. The Coxsackie viruses. Annu Rev Microbiol. 1955;9:277–296. doi: 10.1146/annurev.mi.09.100155.001425. [DOI] [PubMed] [Google Scholar]
- 26.Baboonian C, Davies MJ, Booth JC, McKenna WJ. Coxsackie B viruses and human heart disease. In: Tracy S, Chapman NM, Mahy BWJ, editors. The Coxsackie B viruses. Berlin, Germany: Springer; 1997. pp. 31–52. [DOI] [PubMed] [Google Scholar]
- 27.Verma NA, Zheng XT, Harris MU, Cadichon SB, Melin-Aldana H, Khetsuriani N, Oberste MS, Shulman ST. Outbreak of life-threatening Coxsackie virus B1 myocarditis in neonates. Clin Infect Dis. 2009;49:759–763. doi: 10.1086/605089. [DOI] [PubMed] [Google Scholar]
- 28.Alexander JG, Talbot D. Diagnosis of Coxsackie enterovirus infections by serum complement fixation tests. Br J Clin Pract. 1976;30:111–113. [PubMed] [Google Scholar]


