Abstract
Nucleic acid separation is an increasingly important tool for molecular biology. Before modern technologies could be used, nucleic acid separation had been a time- and work-consuming process based on several extraction and centrifugation steps, often limited by small yields and low purities of the separation products, and not suited for automation and up-scaling. During the last few years, specifically functionalised magnetic particles were developed. Together with an appropriate buffer system, they allow for the quick and efficient purification directly after their extraction from crude cell extracts. Centrifugation steps were avoided. In addition, the new approach provided for an easy automation of the entire process and the isolation of nucleic acids from larger sample volumes. This review describes traditional methods and methods based on magnetic particles for nucleic acid purification. The synthesis of a variety of magnetic particles is presented in more detail. Various suppliers of magnetic particles for nucleic acid separation as well as suppliers offering particle-based kits for a variety of different sample materials are listed. Furthermore, commercially available manual magnetic separators and automated systems for magnetic particle handling and liquid handling are mentioned.
Keywords: Magnetic particles, Nucleic acid, Separators, Automation
Introduction
Magnetic separation is an emerging technology that uses magnetism for the efficient separation of micrometre-sized para- and ferromagnetic particles from chemical or biological suspensions. Enrichment of low-grade iron ore, removal of ferromagnetic impurities from large volumes of boiler water in both conventional and nuclear power plants, or the removal of weakly magnetic coloured impurities from kaolin clay are typical examples of magnetic separation in traditional industries. The application of these techniques in biosciences had been restricted and of limited use up to the 1970s. The idea of using magnetic separation techniques to purify biologically active compounds (nucleic acids, proteins, etc.), cells, and cell organelles led to a regrowing interest over the last decade. New magnetic particles with improved properties were developed for the partly complicated separation processes in these fields [see reviews: Olsvik et al. 1994; Safarik and Safarikova 1999; Franzreb et al. 2006].
Magnetic separation of nucleic acids has several advantages compared to other techniques used for the same purpose. Nucleic acids can be isolated directly from crude sample materials such as blood, tissue homogenates, cultivation media, water, etc. The particles are used in batch processes where there are hardly any restrictions with respect to the sample volumes. Due to the possibility of adjusting the magnetic properties of the solid materials, they can be removed relatively easily and selectively even from viscous sample suspensions. In fact, magnetic separation is the only feasible method for the recovery of small particles (diameter approx. 0.05–1 μm) in the presence of biological debris and other fouling material of similar size. Furthermore, the efficiency of magnetic separation is especially suited for large-scale purifications (Safarik et al. 2001; Franzreb et al. 2006).
These upcoming separation techniques also serve as a basis of various automated low- to high-throughput procedures that allow to save time and money. Centrifugation steps can be avoided and the risk of cross-contamination when using traditional methods is no longer encountered. Various types of magnetic particles are commercially available for nucleic acid purification, magnetic separators working in the manual and automated mode are offered. A short description of traditional and magnetic separation methods for nucleic acid isolation, together with a short overview of batch and automated separators, will be given below.
Nucleic acid purification methods
The isolation of DNA or RNA is an important step before many biochemical and diagnostic processes. Many downstream applications such as detection, cloning, sequencing, amplification, hybridisation, cDNA synthesis, etc. cannot be carried out with the crude sample material. The presence of large amounts of cellular or other contaminating materials, e.g. proteins or carbohydrates, in such complex mixtures often impedes many of the subsequent reactions and techniques. In addition, DNA may contaminate RNA preparations and vice versa. Thus, methods for the efficient, reliable and reproducible isolation of nucleic acids from complex mixtures are needed for many methods that are used today and rely on the identification of DNA or RNA, e.g. diagnosis of microbial infections, forensic science, tissue and blood typing, detection of genetic variations, etc.
Traditional non-magnetic methods
Fluid phase
A range of methods are known for the isolation of nucleic acids in the fluid phase, but they are generally based on complex series of precipitation and washing steps and are time-consuming and laborious to perform. Thus, classical methods for the isolation of nucleic acids from complex starting materials such as blood or tissues, involve the lysis of the biological material by a detergent or chaotropic substance, possibly in the presence of protein-degrading enzymes, followed by several processing steps applying organic solvents such as phenol and/or chloroform or ethanol, which in general are highly toxic and require special and, hence, expensive disposal. For example, the complete removal of proteins from nucleic acids can be achieved by the addition of sodium perchlorate (Wilcockson 1973). The separation of RNA from DNA requires selective precipitation steps with LiCl or a specific nuclease-free isolation with guanidinium hydrochloride or guanidinium thiocyanate, combined with phenol extraction and ethanol precipitation (Bowtell 1987). Such methods are not only cumbersome and time-consuming, but the relatively large number of steps required increases the risk of degradation, sample loss or cross-contamination of samples especially when several samples are processed simultaneously. In the case of RNA isolation, the risk of DNA contamination is comparatively high.
Solid phase
Apart from laborious and time-consuming traditional methods, alternative separation techniques have been developed. Sorption processes based on (a) hydrogen-binding interaction with an underivatised hydrophilic matrix, typically silica, under chaotropic conditions, (b) ionic exchange under aqueous conditions by means of an anion exchanger, (c) affinity and (d) size exclusion mechanisms were used for DNA purification. Solid-phase systems which adsorb DNA—silica-based particles (Vogelstein and Gillespie 1979; Boom et al. 1990, 1999; Melzak et al. 1996; Tian et al. 2000; Breadmore et al. 2003), glass fibres, and anion-exchange carriers (Ferreira et al. 2000; Endres et al. 2003; Teeters et al. 2003)–are used in chromatographic separation columns [e.g. DE 41 43 639 C2 (Qiagen GmbH)] for example.
These carriers are applied for DNA isolation or purification together with highly concentrated chaotropic salt solutions (e.g. sodium iodide, sodium perchlorate, guanidinium thiocyanate). In US 5,075,430 (BioRad), for instance, usage of diatomaceous earth as a carrier material is described. Again, bonding takes place in the presence of a chaotropic salt. Other approaches are based on detergence together with a nucleic-acid-binding material (EP 0 796 327 B1, Dynal) or on the usage of a solid carrier with DNA-binding functional groups combined with polyethylene glycol and salts at high concentrations (WO/1999/058664, Whitehead Institute for Biomedical Research).
Magnetic separation
The increasing use of magnetic solid carriers in biochemical and molecular biology processes has many advantages compared to other non-magnetic separation processes. The term ‘magnetic’ means that the support obtains a magnetic moment when placed in a magnetic field. Thus, it can be displaced. In other words, particles having a magnetic moment may be removed readily by the application of a magnetic field, e.g. by using a permanent magnet. This is a quick, simple and efficient way to separate the particles after the nucleic binding or elution step (see Fig. 1) and a far less rigorous method than traditional techniques, such as centrifugation, that generate shear forces which may lead to the degradation of the nucleic acids. It is also possible to isolate components of the cell lysate, which inhibit for example the DNA polymerase of a following PCR reaction like polysaccharides, phenolic compounds or humic substances (Demeke and Adams 1992; Watson and Blackwell 2000).
Fig. 1.
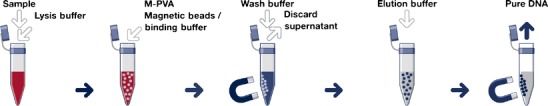
Schematic procedure for nucleic acid purification by magnetic bead technology (illustration by chemagen Biopolymer-Technology AG, Germany)
Usually, it is sufficient to apply a magnet to the side of the vessel containing the sample mixture for aggregating the particles near the wall of the vessel and pouring away the remainder of the sample (see Fig. 1).
Magnetic carriers with immobilised affinity ligands or prepared from a biopolymer exhibiting affinity to the target nucleic acid are used for the isolation process. Many magnetic carriers are commercially available and can also be prepared in the laboratory. Such materials are magnetic particles produced from different synthetic polymers, biopolymers, porous glass, or magnetic particles based on inorganic magnetic materials such as surface-modified iron oxide. Especially suited are superparamagnetic particles, which do not interact among each other in the absence of a magnetic field. These particles will magnetise under a strong magnetic field, but retain no permanent magnetism once the field is removed. When magnetic aggregation and clumping of the particles are prevented during the reaction, easy suspension of the particles and uniform nucleic acid extraction are ensured.
The diameter of the particles is approximately between 0.5 and 10 μm. Materials with a large surface area are preferred for binding the nucleic acids. Without going into theoretical details, the nucleic-acid-binding process may be assisted by the nucleic acid ‘wrapping around’ the support. Such supports generally have an irregular surface and may be porous for example. Particulate materials, e.g. beads and in particular polymer beads, are generally preferred due to their larger binding capacity. Conveniently, a particulate solid support used will comprise spherical beads.
Monodisperse particles (particles of mainly uniform size) have the advantage of providing for a very uniform reproducibility of magnetic separation.
Preparation of magnetic particles for nucleic acid separation
In the laboratory, colloidal magnetite Fe3O4 (or similar magnetic material such as maghemite γFe2O3 or ferrites) particles usually are surface-modified by silanisation. Naked iron oxide (Fe3O4) has the capacity of adsorbing DNA (Davies et al. 1998), but aggregates due to attractive forces reduce the surface area that can be used for adsorption. Silane compounds coupled to magnetite derivatised with carboxyl groups are known to have a DNA extraction ability in solutions containing PEG (Hawkins et al. 1994). Modified bacterial magnetite particles in the presence of amino silane compounds and hyperbranched polyamidoamine dendrimer are used for DNA extraction by Yoza et al. (2002, 2003). Modified magnetic cobalt ferrite particles have been investigated for DNA isolation under high sodium chloride and PEG concentrations by Prodelalova et al. (2004).
Surface modification of magnetic nanoparticles with alkoxysilanes (Bruce et al. 2004; Tan et al. 2004; Bruce and Sen 2005) or polyethyleneimine (Chiang et al. 2005; Veyret et al. 2005) is also useful. The above-mentioned magnetic colloids are not easy to separate using classical magnets. This is due to a small particle size, at which Brownian motion forces are higher than the exerted magnetic force. To enhance phase separation, various magnetic latexes that may interact with nucleic acids were prepared.
Magnetic micro-beads can be prepared in a number of ways, but usually magnetically susceptible particles (e.g. iron oxide) are coated with synthetic or biological polymers. Elaissari et al. (2003) describe the interaction of nucleic acids and different polymers. Biopolymers such as agarose, chitosan, κ-carrageenan, and alginate, can be prepared easily in a magnetic form (Levison et al. 1998; Prodelalova et al. 2004). In the simplest case, the biopolymer solution is mixed with magnetic particles and, after bulk gel formation, the magnetic gel formed is broken into fine particles. Alternatively, the biopolymer solution containing dispersed magnetite is dropped into a mixed hardening solution or a water-in-oil suspension technique is used to prepare spherical particles. Basically, the same process can be used to prepare magnetic particles for nucleic separation from synthetic polymers such as hydrophobic polystyrene (Ugelstad et al. 1992) and hydrophilic polyacryl amide (Elaissari et al. 2001) or poly(vinyl alcohol) (Oster et al. 2001). Genomic DNA was also successfully isolated from cell lysate on weak acid derivatives of magnetic P(HEMA-co-EDMA) and P(HEMA-co-GMA) microparticles in the presence of PEG and sodium chloride (Horak et al. 2005).
The first approach to synthesising micro-sized particles was published by Ugelstad et al. They developed an interesting methodology leading to monosized polystyrene magnetic microspheres, which were studied in various biomedical applications (Ugelstad et al. 1993). These particles have an excellent size distribution and spherical shape, but their surface is very hydrophobic and results in a high amount of unspecific protein binding on the particle surface.
Another possibility consists in combining different polymer matrix materials with silica components (Grüttner et al. 2001; Müller-Schulte et al. 2005) that specifically interact with the nucleic acids.
Depending on the support and the nature of the subsequent processing required, it may or may not be desirable to release the nucleic acid from the support. The direct use of magnetic beads, e.g. in PCR or other amplifications, without eluting the nucleic acid from the surface is not trivial. The enzymatic detection and amplification methods will be inhibited by the magnetic beads, their stabilisers, or their metal oxides (Spanova et al. 2004), which decrease PCR sensitivity or lead to false negative PCR results. For many DNA detections or identification methods, elution is not necessary. Although the DNA may be randomly in contact with the bead surface and bound at a number of points by hydrogen binding or ionic or other forces, there generally will be sufficient lengths of DNA available for hybridisation to oligonucleotides and for amplification. If desired, however, elution of the nucleic acid may be achieved using known methods, e.g. higher ionic strength, heating or pH changes.
Commercially available magnetic particles
Commercially available magnetic particles that are suited for nucleic acid separation can be obtained from a variety of companies. Mostly, the matrixes are based on silica, porous glass, cellulose, agarose, polystyrene and silane (see Tables 1 and 2). Moreover, some important patents exist that describe the synthesis of magnetic carriers not only for nucleic separation:
Table 1.
Selection of commercially available magnetic particles used (or suitable) for DNA, RNA and pDNA separation
| Product | Diameter (μm) | Composition | Surface area (m2 g−1) | Kind of nucleic acid | Manufacturer/supplier |
|---|---|---|---|---|---|
| AGOWA® maga | 5–10 | n. k. | n. k. | DNA, RNA | AGOWA, Berlin, Gemany |
| Dynabeads®DNAa | 1.05, 2.80 | Polystyrene | 1–10 | DNA | Dynal, Oslo, Norway (now Invitrogen) |
| GenoPrep™ DNA magnetic beadsa | n. k. | Silica surface | n. k. | DNA | GenoVision, Oslo, Norway (a Qiagen Company) |
| MagaZorb® a | 1–10 | Cellulose | Porous | DNA/RNA pDNA | Cortex Biochem San Leandro, CA, USA |
| MagneSila | 5–8.5 | Silanisation of iron oxide | 27 | DNA/RNA pDNA | Promega, Madison, WI, USA |
| MagPrep®Silica particlesb | ~1 | Silanisation of iron oxide | 14–25 | DNA | Merck KgaA, Germany |
| MagSib | 1, 2, 5 | n. k. | n. k. | DNA/RNA | MagnaMedics, Aachen, Germany |
| MGPc | n. k. | Pore-free glass shell | Non-porous | DNA/RNA | Roche Diagnostic |
| M-PVAa | 0.5–1, 1–3, 5–8 | Polyvinyl alcohol | Non-porous | DNA/RNA pDNA | chemagen Biopolymer Technology, Baesweiler, Germany |
| Sicastar®-Mb | 1.5, 6 | Sterene-maleic acid copolymer | Non-porous | DNA | Micromod Partikeltechnologie, Rostock, Germany |
| SiMAGa | 0.5, 0.75, 1 | Silanisation of iron oxide | 100 | DNA/RNA pDNA | Chemicell, Berlin, Germany |
| SPHERO magnetic particlesb | 1–2 | Polystyrene | Non-porous | DNA | Spherotech, Libertyville, IL, USA |
aKits and protocols available
bNo kits and protocols available
cOnly kit available without any information
n. k. not known
Table 2.
Selection of commercially available magnetic particles used (or suitable) for mRNA separation
| Product | Diameter (μm) | Polymer composition / surface modification | Surface area (m2 g−1) | Manufacturer/supplier |
|---|---|---|---|---|
| BcMag® mRNA | 1 or 5 | Silanisation of iron oxide | n. k. | Bioclone, San Diego, CA, USA |
| BioMag® oligo (dT)20 | ∼1 | Silanisation of iron oxide | 100 | Bangs Lab., Fisher, IN, USA or Polysciences, USA |
| Dynabeads® oligo (dT)25 | 1.05, 2.8 μm | Polystyrene | 1–10 | Dynal, Oslo, Norway (now Invitrogen) |
| μMACS oligo-dT | 0.05 | Dextran | Non-porous | Miltenyi Biotech, Bergisch Gladbach, Germany |
| MagaCell™ oligo-dT30 | 1–10 | Cellulose | Porous | Cortex Biochem, San Leandro, CA, USA |
| MagneSphere® | ∼1 | Streptavidin-coated magnetite | 100–150 | Promega, Madison, WI, USA |
| MPG® streptavidin oligo (dT)25 | ∼5 | Porous borosilicate glass | 60 | PureBiotech, Middlesex, NJ, USA |
| M-PVA-oligo (dT)30 | Various (0.5–1; 1–3; 5–8) | Polyvinyl alcohol | Non-porous | chemagen AG, Baesweiler, Germany |
| mRNA-cellulose | 1–10 | Cellulose | n. k. | Scipac Ltd., Sittingbourne, UK |
| Nucleo-Adembeads | 0.1–0.5 | Polymer | 100 | Ademtech, Pessac, France |
| Scigen M100 oligo (dT)30 | ∼3.5 | Cellulose | 10 | Vector Lab., Burlingame, USA |
| Sera-Mag oligo (dT)30 | 1 | Polystyrene | Non-porous | Seradyn, Indianapolis, IN, USA |
n. k. not known
One of the first patents for particle synthesis is the ‘Ugelstad polymerization process’, which is described, for example, in EP 0 003 905 B2, US 5,459,378, and US 4,530,956 (SINTEF). It leads to monodisperse magnetic particles by several swelling and polymerisation steps. WO/1992/016581 (Cornell Research Foundation) also describes the preparation of monodisperse particles, particularly macroporous polymer beads. The process proposed uses a three-phase emulsion containing soluble polymer particles, a monomer phase and water. Nucleic acid separation using magnetic beads is described in (Alderton et al. 1992) and in WO/1991/012079 as well as in US 5,523,231 (Amersham). These magnetic beads are able to absorb the nucleic acid after a salt-ethanol precipitation. The approaches are not nucleic-acid-specific, i.e. the magnetic beads adsorb other bio-substances in parallel. Of course, this is a drawback of these approaches.
In the declaration WO/1996/041811 (Boehringer; Roche) mainly non-porous glass particles comprising mica and magnetite particles are described (Bartl et al. 1998). During their production, magnetic particles and a surrounding glass coating are superimposed on a mica core. The disadvantage of these products is their affinity to sedimentation. Furthermore, the production process is time-consuming and based on a complex spray process. Another approach to the production of particles from spherical magnetite kernels with a surface coating of silicon dioxide is covered by the European patent application EP 1 468 430 A1.
Monodisperse magnetic beads are described in WO/1998/012717 (Merck). They consist of a SiO2 core, which is given magnetic properties by a ferric-oxide coating. After a subsequent silanisation of the ferric-oxide coating, the particles can bind nucleic acids.
Many patents concerning nucleic acid separation are from the Dynal company. They developed monodisperse polymer magnetic particles with different sizes (coefficient of variation less than 5%) (see EP 0 796 327 B1), which are sold with a polystyrene matrix under the name of Dynabeads®. The small-size distribution ensures reproducible separation properties. Protocols for nucleic acid separation with these particles are described by EP 0 512 439 B1 and with oligonucleotide-linked particles for specific nucleic acid separation in US 5,512,439.
Magnetic beads based on mica or polystyrene and coated by a magnetic oxide reach a high specific density, which leads to a fast sedimentation. Thus, additional mechanical mixing is necessary. The main drawback of the coated particles consists in the fact that the metal oxides may be in direct contact with the analytical solutions despite silanisation. All state-of-the-art approaches to the production of magnetic beads are laborious; the production process time amounts to several hours. To overcome this problem, the US patents 6,204,033 and 6,514,688 (chemagen Biopolymer Technologie AG) describe spherical, magnetic polymer particles based on polyvinyl alcohol particles, which can be produced in short terms using inverse suspension polymerisation. The polymer particles contain reactive hydroxyl groups to which other molecules can be coupled. Due to their hydrophilic surface, the particles exhibit small unspecific bindings only. Together with an at least partly silanised surface (DE 100 13 955 A1 and EP 1 274 745 A1) or a germanium-containing compound (DE 101 03 652 A1), they can be used for specific nucleic acid separation.
The inverse suspension process for the separation of nano- and micro-sized silica particles is suggested in WO/2002/009125 (Fraunhofer-Gesellschaft). The main idea is the dispersion of aqueous silica-sole containing magnetic colloids, which are hardened to spherical hydrophilic gel particles by adding a suited base. These particles can be used for nucleic acid separation with high binding capacities (WO/2005/50 52 581 A3, MagnaMedics GmbH).
Total DNA/RNA
Both total DNA and RNA are separated by the same magnetic beads. For the purpose of removing RNA from DNA, the RNA is destroyed before the DNA separation step. Adding of an RNAse or an alkali such as NaOH is an appropriate process. Vice versa, RNA can be separated if the DNA is degraded with DNAse.
Plasmid DNA
The primary method considered for plasmid purification is the separation of plasmid DNA (pDNA) from the chromosomal DNA and cellular RNA of the host bacteria. Stadler et al. (2004) show that even in the case of a high copy plasmid, pDNA represents not more than 3% of the cleared lysate and that most of the critical contaminants are negatively charged (RNA, cDNA, endotoxin) and similar in size (cDNA, endotoxins) and hydrophobicity (endotoxins). A number of methods have been developed to generate a cleared lysate, but they are not able to remove proteins and lipids. Alkaline lysis of harvested bacterial cells with a subsequent neutralisation, as originally described by Birnboim and Doly (1979), is the process of choice. Cleared lysate protocols may vary slightly from each other as regards salt concentrations, volume, pH, temperature, and process step durations (Hirt 1967; Holmes and Quigley 1981; Birnboim 1983). These techniques make use of the differences in denaturation and renaturation characteristics of covalently closed circular plasmid DNA and chromosomal DNA fragments.
For example, superparamagnetic nano particles modified by multivalent cationic polyethyleneimine are used to isolate pDNA from cleared bacterial lysate (Chiang et al. 2005).
Table 1 shows some commercially available magnetic particles used for DNA, RNA and pDNA isolation. Many magnetic particles are available with optimised buffers and protocols for small lab scale and automated systems. There are also some companies offering particles for nucleic acid purification without any further information.
Affinity capture of RNA and DNA
The magnetic carrier is provided with binding solutions to assist in the selective capture of nucleic acids. For example, complementary DNA or RNA sequences (Satokari et al. 2005) or DNA-binding proteins may be used as well as viral proteins binding to viral nucleic acids. In this review, a short overview of eukaryotic mRNA and viral DNA/RNA will be given.
There are several companies (see Table 2) offering oligodeoxythymidine immobilised with magnetic particles, which can be used effectively for the rapid isolation of highly purified mRNA from eukaryotic cell cultures or total RNA preparations (Jacobsen et al. 2004). These procedures are based on the hybridisation of the oligonucleotide dT sequence with the stable polyadenylated 3 termini of the eukaryotic mRNA. The length of the complementary sequence differs between 20 and 30 oligonucleotides. This sequence is directly bound covalently to the particle surface or indirectly by biotinylated oligonucleotides and the interaction of streptavidin-coated particles. CPG and Dynal (now Invitrogen) offer MPG® and Dynabeads® with already immobilised biotinylated oligonucleotide, but also other companies offer streptavidin-modified particles, which can be used for mRNA isolation, as described, e.g. by the mRNA isolation kit with MagneSphere® from Promega. Nearly all magnetic particles (except for MagaCell™ oligo-dT30 and Sera-Mag oligo-(dT)30) are available together with an optimised buffer system and helpful protocols.
Automated extraction of viral RNA and DNA from the plasma mini-pool is performed by the chemagic Viral DNA/RNA Kit and chemagic Magnetic Separation Module I (Hourfar et al. 2005a,b; Pichl et al. 2005).
A rapid diagnosis of enterovirus infection by magnetic bead extraction has been established by Muir et al. (1993). Enterovirus RNA can be separated from large-volume water samples using the NucliSens miniMAG System (Rutjes et al. 2005). Hei and Cai (2005) developed a system for purifying SARS coronavirus RNA by a hybridisation of a specific oligonucleotide sequence, which is immobilised on the magnetic bead surface.
Magnetic separators
A variety of magnetic separators are available on the market, ranging from very simple concentrators for one tube to complicated fully automated devices. The isolation of nucleic acids is mostly performed in the batch mode using commercially available lab-scale magnetic separators (particle concentrators). Separators are usually made of strong rare-earth permanent magnets designed to hold various amounts of micro-tubes or tubes.
Particles with a diameter larger than 1 μm can be separated easily using simple magnetic separators, while separation of smaller particles (magnetic colloids with a particle size ranging from ten to hundreds of nanometres) may require the use of high-gradient magnetic separators.
Many different companies that mostly offer magnetic particles also have batch separators in their portfolio. A selection of commercially available batch-type magnetic separators is given in Table 3.
Table 3.
Selection of commercially available batch-type magnetic separators
| Company | Separator | Description |
|---|---|---|
| Ademtech | Adem-Mag96 | 96-well plate |
| AutoMag | 12 × 1.5 ml reaction tubes (mixing and separation) | |
| AGOWA GmbH | AGOWA®Sep 9600 | 96-well plate (temperature can be adjusted, pc-controllable) |
| AGOWA®Sep 7200 | 48-well plate | |
| AGOWA®maxisepPLUS 2400 | 24-well plate (5 ml volume) | |
| Bangs Laboratories, Inc. | BioMag®multi-6microcentrifuge Tube separator | 96-well plate |
| BioMag®96-well Plate separator | ||
| Bioclone, Inc. | Bc®Mag magnetic separator-2 | 2 × 1.5 ml tubes |
| Bc®Mag magnetic separator-6 | 6 × 1.5 ml tubes | |
| Bc®Mag magnetic separator-24 | 24 × 1.5 ml or 2 ml tubes | |
| Bc®Mag magnetic separator-50 | 1 × 50 ml + 1 × 15 ml tubes | |
| chemagen Biopolymer-Technologie AG | chemagen stand 2 × 12 | 12 × 1.5 ml + 12 × 2 ml tubes |
| chemagen stand 96 | 96-well plate | |
| chemagen stand 50 k Type A | 10–50 ml total volume | |
| chemagen stand 50 k Type B | max. 15 ml volume | |
| chemagen stand MultiTube | 16 × 50 ml (overturn) | |
| Chemicell GmbH | MagnetoPure | 2 × 1.5 ml + 2 × 2 ml tubes |
| Cortex Biochem | 96-well plate magnetic separator | bottom pull and side pull |
| CPG, Inc. | 3-in-1 MPS® | 8 × 1.5 ml or 1 × 15 ml + 1 × 1.5 ml or 1 × 50 ml + 1 × 1.5 ml |
| 96-Well MPS® | 96-well plate | |
| Kisker GmbH | Magna separation rack | 5 × 1.5/2 ml for separation plus 5 × 1.5/2 ml for storage |
| VariMag separation rack | Three different combinations | |
| UltraMag separation rack | 96-well plate | |
| Miltenyi Biotech GmbH | MACS™ separators | Different models |
| Promega | MagnaBot®96 magnetic separation device | 96-well plate |
The racks are designed to hold various amounts of micro-tubes or tubes. Test tube magnetic separators allow to separate magnetic particles from volumes between approximately 5 μl and 50 ml. There are many combinations with other features like a mixing function (Ademtech) or a possibility to turn the separator over for the removal of the supernatant (chemagen Biopolymer-Technologie AG). Other devices are applied for the separation of magnetic particles from the wells of standard micro-titration plates. In some of them the temperature can be pc-controlled (AGOWA), other devices may be inserted into automated separation devices.
Laboratory automation is increasingly important in molecular biology and biotechnology. Constantly increasing numbers of analyses of different sources and sample volumes have resulted in an enormous importance of flexible robots or automated systems. Automation is also required for handling a large number of samples without human errors.
Many instruments have been developed to automate PCR amplification, the sequencing reaction and the detection of nucleic acids, but automating DNA extraction by traditional methods with centrifugation and vacuum steps still is difficult. A complete separation of the solid carrier matrix by centrifugation is not possible. Supports filled with carrier materials cannot be used, as the ineluctable dead volumes of the support lead to sample material loss in case of small amounts of sample materials. Another drawback is the danger of mutual contamination of different biological samples, especially if directly neighbouring supports are emptied by the vacuum. However, the last decade shows that DNA purification using magnetic bead technology is suitable for automation systems, and several automated instruments for handling magnetic beads have been developed (Alderton et al. 1992; Wahlberg et al. 1992; Rolfs and Weber 1994; Fangan et al. 1999; Obata et al. 2001; Akutsu et al. 2004; Vuosku et al. 2004).
More and more vendors offer commercially automated devices for the handling of magnetic particles, e.g. for the purification of nucleic acid (see Table 4). Most systems are offered together with system-specific optimised particles, buffer systems and protocols.
Table 4.
Selection of commercially available automated magnetic separators
| Company | Device | Samples in parallel | Samples per run | Sample volumea |
|---|---|---|---|---|
| High throughput | ||||
| chemagen Biopolymer-Technologie AG | chemagic MSM I (96 rod head) | 96 | 96 | 10–400 μl |
| Qiagen GmbH | BioSprint®96 | 96 | 96 | 100 μl or 200 μl |
| Thermo Labsystems | KingFisher®96 | See BioSprint®96 (Qiagen GmbH) | ||
| Low and medium throughput; large volumes | ||||
| chemagen Biopolymer-Technologie AG | Chemagic MSM I (12 rod head) | 12 | 12 | 1–10 ml |
| Promega | Maxwell™ 16 | 16 | 16 | 10–400 μl |
| PSS Bio Instruments | Magtration®System 8Lx | 8 | 8 | 7 ml |
| Qiagen GmbH | BioRobot®M48 | 6 | 48 | max. 200 μl |
| BioRobot®EZ1 | 6 | 6 | max. 200 μl | |
| BioRobot®15 | 15 | 15 | 50–1,000 μl | |
| Roche Diagnostics GmbH | MagNa Pure Compact | 8 | 8 | 100–1,000 μl |
| MagNa Pure LC | 8 | 32 | 100–1,000 μl | |
| Cobas®AmpliPrep | 1 | 72 | 250–1,500 μl | |
| Themo Labsystems | KingFisher®ML | See BioRobot®15 (Qiagen GmbH) | ||
| GenoVision | GenoM™-6 | See BioRobot®EZ1 (Qiagen GmbH) | ||
aWhole blood; other applications available
The devices are able to process between six and 96 samples in parallel and commonly customised for small buffer volumes. For larger volumes, the chemagic Magnetic Separation Module I (<10 ml) (see Fig. 2) or the Magtration® System 8 l × (7 ml) can be used.
Fig. 2.

chemagic Magnetic Separation Module I consisting of (A) separation head with magnetizable rods [here 12-well format for large (50 ml) volumes; 96-well format for MTPs also available], (B) electro magnet, (C) chemagic dispenser for parallel filling of all required buffer solutions (accessory) and (D) tracking unit. The principle functionality regarding separation and resuspension of magnetic beads is shown in the scheme
Conclusion
The present review has shown that the separation of nucleic acid is a highly dynamic field of research and development. An increasing number of commercial vendors offer magnetic particles, also in the form of a kit that is optimally suited for the application desired. The increasing number of publications shows that magnetic particles of higher potential are currently under research. Materials with more specific-binding properties and a better separability are promising approaches. A higher degree of automation leads to systems analysing a larger number of samples and higher sample volumes at the same time.
Up to now, however, no real scale-up for the purification of large volumes (in the scale of litres) has been realised.
References
- Akutsu J-I, Tojo Y, Okochi M, Yohda M, Segawa O, Obata K, Tajima H. Development of an integrated automation system with a magnetic bead-mediated nucleic acid purification device for genetic analysis and gene manipulation. Biotechnol Bioeng. 2004;86:667–671. doi: 10.1002/bit.20049. [DOI] [PubMed] [Google Scholar]
- Alderton RP, Eccleston LM, Howe RP, Read CA, Reeve MA, Beck S. Magnetic bead purification of M13 DNA sequencing templates. Anal Biochem. 1992;201:166–169. doi: 10.1016/0003-2697(92)90190-I. [DOI] [PubMed] [Google Scholar]
- Bartl K, Wenzig P, Kleiber J. Simple and broadly applicable sample preparation by use of magnetic glass particles. Clin Chem Labor Med. 1998;36:557–559. doi: 10.1515/CCLM.1998.095. [DOI] [PubMed] [Google Scholar]
- Birnboim HC. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Birnboim HC, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acid Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boom R, Sol C, Beld M, Weel J, Goudsmit J, Wertheim-van Dillen P. Improved silica-guanidinium thiocyanate DNA isolation procedure based on selective binding of bovine alpha-casein to silica particles. J Clin Microbiol. 1999;37:615–619. doi: 10.1128/jcm.37.3.615-619.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowtell DD. Rapid isolation of eukaryotic DNA. Anal Biochem. 1987;162:463–465. doi: 10.1016/0003-2697(87)90421-0. [DOI] [PubMed] [Google Scholar]
- Breadmore MC, Wolfe KA, Arcibal IG, Leung WK, Dickson D, Giordano BC, Power ME, Ferrance JP, Feldman SH, Norris PM, Landers JP. Microchip-based purification of DNA from biological samples. Anal Chem. 2003;75:1880–1886. doi: 10.1021/ac0204855. [DOI] [PubMed] [Google Scholar]
- Bruce IJ, Sen T. Surface modification of magnetic nanoparticles with alkoxy-silanes and their application in magnetic bioseparations. Langmuir. 2005;21:7029–7035. doi: 10.1021/la050553t. [DOI] [PubMed] [Google Scholar]
- Bruce IJ, Taylor J, Todd M, Davies MJ, Borioni E, Sangregorio C, Sen T. Synthesis, characterisation and application of silica-magnetite nanocomposites. J Magn Magn Mater. 2004;284:145–160. doi: 10.1016/j.jmmm.2004.06.032. [DOI] [Google Scholar]
- Chiang C-L, Sung C-S, Wu T-F, Chen C-Y, Hsu C-Y. Application of superparamagnetic nanoparticles in purification of plasmid DNA from bacterial cells. J Chromatogr B. 2005;822:54–60. doi: 10.1016/j.jchromb.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Taylor JI, Sachsinger N, Bruce IJ. Isolation of plasmid DNA using magnetite as a solid-phase adsorbent. Anal Biochem. 1998;262:92–94. doi: 10.1006/abio.1998.2743. [DOI] [PubMed] [Google Scholar]
- Demeke T, Adams RP. The effects of plant polysaccharides and buffer additives on PCR. Biotechniques. 1992;12:332–334. [PubMed] [Google Scholar]
- Elaissari A, Rodrigue M, Meunier F, Herve C. Hydrophilic magnetic latex for nucleic acid extraction, purification and concentration. J Magn Magn Mater. 2001;225:127–133. doi: 10.1016/S0304-8853(00)01240-3. [DOI] [Google Scholar]
- Elaissari A, Ganachaud F, Pichot C. Biorelevant latexes and microgels for the interaction with nucleic acids. Top Curr Chem. 2003;227:169–193. doi: 10.1007/3-540-36412-9_7. [DOI] [Google Scholar]
- Endres HN, Johnson JA, Ross CA, Welp JK, Etzel MR. Evaluation of an ion-exchange membrane for the purification of plasmid DNA. Biotechnol Appl Biochem. 2003;37:259–266. doi: 10.1042/BA20030025. [DOI] [PubMed] [Google Scholar]
- Fangan BM, Dahlberg OJ, Deggerdal AH, Bosnes M, Larsen F. Automated system for purification of dye-terminator sequencing products eliminates up-stream purification of templates. Biotechniques. 1999;26:980–983. doi: 10.2144/99265pf01. [DOI] [PubMed] [Google Scholar]
- Ferreira GNM, Cabral JMS, Prazeres DMF. Studies on the batch adsorption of plasmid DNA onto anion-exchange chromatographic supports. Biotechnol Prog. 2000;16:416–424. doi: 10.1021/bp0000196. [DOI] [PubMed] [Google Scholar]
- Franzreb M, Siemann-Herzberg M, Hobley TJ, Thomas ORT. Protein purification using magnetic adsorbent particles. Appl Microbiol Biotechnol. 2006;70:505–516. doi: 10.1007/s00253-006-0344-3. [DOI] [PubMed] [Google Scholar]
- Grüttner C, Rudershausen S, Teller J. Improved properties of magnetic particles by combination of different polymer materials as particle matrix. J Magn Magn Mater. 2001;225:1–7. doi: 10.1016/S0304-8853(00)01220-8. [DOI] [Google Scholar]
- Hawkins TL, O’Connor-Morin T, Roy A, Santillan C. DNA purification and isolation using a solid-phase. Nucleic Acids Res. 1994;22:4543–4544. doi: 10.1093/nar/22.21.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hei AL, Cai JP. Development of a method for concentrating and purifying SARS coronavirus RNA by a magnetic bead capture system. DNA and Cell Biol. 2005;24:479–484. doi: 10.1089/dna.2005.24.479. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Holmes DS, Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981;114:193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Horak D, Rittich B, Spanova A, Benes MJ. Magnetic microparticulate carriers with immobilized selective ligands in DNA diagnostics. Polymer. 2005;46:1245–1255. doi: 10.1016/j.polymer.2004.11.049. [DOI] [Google Scholar]
- Hourfar MK, Michelsen U, Schmidt M, Berger A, Seifried E, Roth WK. High-throughput purification of viral RNA based on novel aqueous chemistry for nucleic acid isolation. Clinic Chem. 2005;51:1217–1222. doi: 10.1373/clinchem.2005.048603. [DOI] [PubMed] [Google Scholar]
- Hourfar MK, Schmidt M, Seifried E, Roth WK. Evaluation of an automated high-volume extraction method for viral nucleic acids in comparison to a manual procedure with preceding enrichment. Vox Sang. 2005;89:71–76. doi: 10.1111/j.1423-0410.2005.00649.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen N, Nielsen PS, Jeffares DC, Eriksen J, Ohlsson H, Arctander P, Kauppinen S. Direct isolation of poly(A)(+) RNA from 4 M guanidine thiocyanate-lysed cell extracts using locked nucleic acid-oligo(T) capture. Nucleic Acids Res. 2004;32:e64. doi: 10.1093/nar/gnh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison PR, Badger SE, Dennis J, Hathi P, Davies MJ, Bruce IJ, Schimkat D. Recent developments of magnetic beads for use in nucleic acid purification. J Chromatogr A. 1998;816:107–111. doi: 10.1016/S0021-9673(98)00064-8. [DOI] [PubMed] [Google Scholar]
- Melzak KA, Sherwood CS, Turner RFB, Haynes CA. Driving forces for DNA adsorption to silica in perchlorate solutions. J Colloid Interface Sci. 1996;181:635–644. doi: 10.1006/jcis.1996.0421. [DOI] [Google Scholar]
- Muir P, Nicholson F, Jhetman M, Neogi S, Banatvala JE. Rapid diagnosis of enterovirus infection by magnetic bead extraction and polymerase chain-reaction detection of enterovirus RNA in clinical specimes. J Clin Microbiol. 1993;31:31–38. doi: 10.1128/jcm.31.1.31-38.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Schulte D, Schmitz-Rode T, Born P. Ultra-fast synthesis of magnetic and luminescent silica beads for versatile bíoanalytical applications. J Magn Magn Mater. 2005;293:135–143. doi: 10.1016/j.jmmm.2005.01.088. [DOI] [Google Scholar]
- Obata K, Segawa O, Yakabe M, Ishida Y, Kuroita T, Ikeda K, Kawakami B, Kawamura Y, Yohda M, Matsunaga T, Tajima H. Development of a novel method for operating magnetic particles, Magtration Technology, and its use for automating nucleic acid purification. J Biosci Bioeng. 2001;91:500–503. doi: 10.1263/jbb.91.500. [DOI] [PubMed] [Google Scholar]
- Olsvik Ø, Popovic T, Skjerve E, Cudjoe KS, Hornes E, Ugelstad J, Uhlén M. Magnetic separation techniques in diagnostic microbiology. Clin Microbiol Rev. 1994;7:43–54. doi: 10.1128/cmr.7.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster J, Parker J, Brassard LÀ. Polyvinyl-alcohol-based magnetic beads for rapid and efficient separation of specific or unspecific nucleic acid sequences. J Magn Magn Mater. 2001;225:145–150. doi: 10.1016/S0304-8853(00)01243-9. [DOI] [Google Scholar]
- Pichl L, Heitmann A, Herzog P, Oster J, Smets H, Schottstedt V. Magnetic bead technology in viral RNA and DNA extraction from plasma minipools. Transfusion. 2005;45:1106–1110. doi: 10.1111/j.1537-2995.2005.04356.x. [DOI] [PubMed] [Google Scholar]
- Prodelalova J, Rittich B, Spanova A, Petrova K, Benes MJ. Isolation of genomic DNA using magnetic cobalt ferrite and silica particles. J Chromatogr A. 2004;1056:43–48. doi: 10.1016/j.chroma.2004.08.090. [DOI] [PubMed] [Google Scholar]
- Rolfs A, Weber I. Fully-automated, nonradioactive solid-phase sequencing of genomic DNA obtained from PCR. Biotechniques. 1994;17:782–787. [PubMed] [Google Scholar]
- Rutjes SA, Italiaander R, van den Berg HHJL, Lodder WJ, de Roda Husman AM. Isolation and detection of enterovirus RNA from large-volume water samples by using the nucliSens miniMAG System and real-time nucleic acid sequence-based amplification. Appl Environ Microbiol. 2005;71:3734–3740. doi: 10.1128/AEM.71.7.3734-3740.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safarik I, Safarikova M. Use of magnetic techniques for the isolation of cells. J Chromatogr B. 1999;722:33–53. [PubMed] [Google Scholar]
- Safarik I, Ptackova L, Safarikova M. Large-scale separation of magnetic bioaffinity adsorbents. Biotechnol Lett. 2001;23:1953–1956. doi: 10.1023/A:1013742002533. [DOI] [Google Scholar]
- Satokari RM, Kataja K, Soderlund H. Multiplexed quantification of bacterial 16S rRNA by solution hybridization with oligonucleotide probes and affinity capture. Microb Ecol. 2005;50:120–127. doi: 10.1007/s00248-004-0136-1. [DOI] [PubMed] [Google Scholar]
- Spanova A, Horak D, Soudkova E, Rittich B. Magnetic hydrophilic methacrylate-based polymer microspheres designed for polymerase chain reactions applications. J Chromatogr B. 2004;800:27–32. doi: 10.1016/j.jchromb.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Stadler J, Lemmens R, Nyhammar T. Plasmid DNA purification. J Gene Med. 2004;6:S54–S66. doi: 10.1002/jgm.512. [DOI] [PubMed] [Google Scholar]
- Tan W, Wang K, He X, Zhao XJ, Drake T, Wang L, Bagwe RP. Bionanotechnology based on silica nanoparticles. Med Res Rev. 2004;24:621–638. doi: 10.1002/med.20003. [DOI] [PubMed] [Google Scholar]
- Teeters MA, Conrardy SE, Thomas BL, Root TW, Lightfoot EN. Adsorptive membrane chromatography for purification of plasmid DNA. J Chromatogr A. 2003;989:165–173. doi: 10.1016/S0021-9673(03)00027-X. [DOI] [PubMed] [Google Scholar]
- Tian H, Huhmer AFR, Landers JP. Evaluation of silica resins for direct and efficient extraction of DNA from complex biological matrices in a miniaturized format. Anal Biochem. 2000;283:175–191. doi: 10.1006/abio.2000.4577. [DOI] [PubMed] [Google Scholar]
- Ugelstad J, Berge A, Ellingsen T, Schmid R, Nilsen T-N, Sienstad P, Mørk PC, Hornes E, Olsvik Ø. Preparation and application of new monosized polymer particles. Prog Polym Sci. 1992;17:87–161. doi: 10.1016/0079-6700(92)90017-S. [DOI] [Google Scholar]
- Ugelstad J, Mork PC, Schmid R, Ellingsen T, Berge A. Preparation and biochemical and biomedical applications of new monosized polymer particles. Polym Intern. 1993;30:157–168. [Google Scholar]
- Veyret R, Delair T, Pichot C, Elaissari A. Amino-containing magnetic nanoemulsions: elaboration and nucleic acid extraction. J Magn Magn Mater. 2005;295:155–163. doi: 10.1016/j.jmmm.2005.01.008. [DOI] [Google Scholar]
- Vogelstein B, Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci USA. 1979;76:615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuosku J, Jaakola L, Jokipii S, Karppinen K, Kamarainen T, Pelkonen VP, Jokela A, Sarjala T, Hohtola A, Haggman H. Does extraction of DNA and RNA by magnetic fishing work for diverse plant species? Mol Biotechnol. 2004;27:209–215. doi: 10.1385/MB:27:3:209. [DOI] [PubMed] [Google Scholar]
- Wahlberg J, Holmberg A, Bergh S, Hultman T, Uhlen M. Automated magnetic preparation of DNA templates for solid phase sequencing. Electrophoresis. 1992;13:547–551. doi: 10.1002/elps.11501301112. [DOI] [PubMed] [Google Scholar]
- Watson RJ, Blackwell B. Purification and characterization of a common soil component which inhibits the polymerase chain reaction. Can J Microbiol. 2000;46:633–642. doi: 10.1139/cjm-46-7-633. [DOI] [PubMed] [Google Scholar]
- Wilcockson J. The use of sodium perchlorate in deproteinization during the preparation of nucleic acids. Biochem J. 1973;135:559–561. doi: 10.1042/bj1350559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoza B, Matsumoto M, Matsunaga T. DNA extraction using modified bacterial magnetic particles in the presence of amino silane compound. J Biotechnol. 2002;94:217–224. doi: 10.1016/S0168-1656(01)00427-8. [DOI] [PubMed] [Google Scholar]
- Yoza B, Arakaki A, Matsunaga T. DNA extraction using bacterial magnetic particles modified with hyperbranched polyamidoamine dendrimer. J Biotechnol. 2003;101:219–228. doi: 10.1016/S0168-1656(02)00342-5. [DOI] [PubMed] [Google Scholar]
Patent specifications and applications
- DE 100 13 995 A1 / EP 1 274 745 A1 Magnetische, silanisierte Trägermaterialien auf Basis von Polyvinylalkohol (2001) Parker J, Oster J, Brassard LÀ; chemagen Biopolymer-Technologie AG, Germany
- DE 101 03 652 A1 Magnetische Polyvinylalkoholpartikel mit modifizierter Oberfläche zur Isolierung und Reinigung von Nukleinsäuren (2002) Brassard LÀ, Parker J, Smets H, Oster J; chemagen Biopolymer-Technologie AG, Germany
- DE 41 43 639 C2 Verfahren zur Isolierung und Reinigung von Nukleinsäuren (1991) Colpan M; Qiagen GmbH, Germany
- DE 41 27 657 A1 Perlförmige Polyvinylalkoholgele für die Aufreinigung und Auftrennung biologischer Flüssigkeiten, Verfahren zu ihrer Herstellung und Verwendung (1991) Müller-Schulte D; Germany
- EP 0 003 905 B2 Process for preparing an aqueous emulsion or dispersion of a partly water-soluble material (1979) Ugelstad J; SINTEF, Norway
- EP 0 728 198 B1 Isolation of nucleic acid (1994) Breivik J, Gaudernack G, Spurkland A; Qiagen GmbH, Germany
- EP 0 796 327 B1 Isolation of nucleic acid (1995) Deggerdal AH, Larsen F; Dynal A/S
- EP 1 468 430 A1 Siliziumhaltige Magnetpartikel: Verfahren zu deren Herstellung und Verwendung der Partikel (2003) Hennig G, Hildenbrand K; Bayer AG, Germany
- US 4,336,173 Process for preparing an aqueous emulsion or dispersion of a partly water-soluble material, and optionally further conversion of the prepared dispersion or emulsion to a polymer dispersion when the partly water-soluble material is a polymerizable monomer (1980) Ugelstad J; SINTEF, Norway
- US 4,530,956 Process for the preparation of aqueous dispersions of organic materials and possible further conversion to a polymer dispersion when the organic material is a polymerizable monomer (1985) Ugelstad J, Berge A; SINTEF Norway
- US 4,459,378 Monodisperse polymer particles and dispersions thereof (1982) Ugelstad J; SINTEF, Norway
- US 5,075,430 Process for purification of DNA on diatomaceous earth (1990) Little MC.; Bio-Rad Laboratories
- US 5,512,439 Oligonucleotide-linked magnetic particles and uses thereof (1994) Hornes E, Korsnes L; Dynal AS, Oslo, Norway
- US 5,523,231 Method to isolate macromolecules using magnetically attractable beads which do not specifically bind the macromolecules (1996) Reeve MA; Amersham International PLC, GB
- US 5,234,809 and EP 0 389 063 B1 Process for isolating nucleic acid (1991) Boom WR, Adriaanse HMA, Kievits T, Lens PF; Akzo N.V., the Netherlands
- US 6,204,033 Preparation of polyvinyl alcohol-based magnetic particles for binding biomolecules (1998) Müller-Schulte D; Germany
- US 6,514,688 Separating, detecting or quantifying biological materials using magnetic cross-linked polyvinyl alcohol particles (2000) Müller-Schultem D; chemagen Biopolymer-Technologie AG, Germany
- WO/1991/012079 Method to isolate macromolecules using magnetically attractable beads which do not specifically bind the macromolecules (1991) Reeve MA; Amersham International PLC
- WO/1996/041811 Magnetic pigment (1996) Kleiber J, Walter T, Harttig H, Lesniak C, Mennig M, Riedling M, Schmidt H; Boehringer Mannheim GmbH, Germany
- WO/1998/012717 Spherical magnetic particles (1996) Anselmann R, Pellatt MG; Merck Patent GmbH, Germany
- WO/2002/009125 Spherical, magnetic SiO2 particles with an adjustable particle and pore size and an adjustable magnetic content. Method for producing them and use of SiO2 particles of this type (2001) Müller-Schulte D, Fischer R; Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V. Germany
- WO/2005/50581 A3 Sphärische, magnetische Silicagel-Träger mit vergrößerter Oberfläche für die Aufreinigung von Nukleinsäuren (2003) Müller-Schulte D; MagnaMedics GmbH, Germany


