Abstract
A series of 5-substituted 2-amino-4,6-dihydroxypyrimidines were prepared by a modified condensation of the corresponding monosubstituted malonic acid diesters with guanidine in an excess of sodium ethoxide. The optimized procedure using Vilsmeier–Haack–Arnold reagent, followed by immediate deprotection of the (dimethylamino)methylene protecting groups, has been developed to convert the 2-amino-4,6-dihydroxypyrimidine analogs to novel 5-substituted 2-amino-4,6-dichloropyrimidines in high yields. Pilot screening for biological properties of the prepared compounds was done in mouse peritoneal cells using the in vitro nitric oxide (NO) assay. Irrespective of the substituent at the 5 position, 2-amino-4,6-dichloropyrimidines inhibited immune-activated NO production. The most effective was 5-fluoro-2-amino-4,6-dichloropyrimidine with an IC 50 of 2 µM (higher activity than the most potent reference compound) while the IC 50s of other derivatives were within the range of 9–36 µM. The 2-amino-4,6-dihydroxypyrimidine counterparts were devoid of any NO-inhibitory activity. The compounds had no suppressive effects on the viability of cells. The Mechanism of action remains to be elucidated.
Keywords: Pyrimidine, Nitric oxide, NO, Anti-inflammatory
Introduction
This work was initially motivated by the finding that 2-amino-4,6-dichloropyrimidine inhibits replication of a broad range of viruses such as members of the Herpes, Picorna, and Pox groups (Marcialis et al., 1973, 1974; La Colla et al., 1975). It has been reported that maturation of viral particles was prevented as viral proteins synthesized in the presence of this compound were not assembled into new virions (Flore et al., 1977; La Colla et al., 1977; Marcialis et al., 1979). This represents a unique and very attractive mechanism of action for new antiviral drugs, especially in combination with other antiviral agents with the aim to suppress resistance development. The pyrimidine heterocycle is an elemental structural motif of several essential natural products (Undheim and Benneche, 1996; Ralevic and Burnstock, 1998; Lagoja, 2005) and a number of synthetic drugs. It was also reported that 2,4-diamino-6-hydroxypyrimidine suppresses NO production in chicken macrophages (Sung et al., 1994).
There is a large class of pharmacologically important pyrimidine derivatives that act as dihydrofolate reductase (DHFR) inhibitors (Hitchings, 1989), as well as compounds with anti-HIV (Holý, 2003; De Clercq and Holý, 2005), anti-adenovirus (Naesens et al., 2005), and anti-HBV activities (Ying et al., 2005), inhibitors of tetrahydrobiopterin synthesis (Bogdan et al., 1995; Kolinsky and Gross, 2004), regulators of pain sensitivity and persistence (Tegeder et al., 2006), antidepressants (Arvanitis et al., 1999), compounds which suppress accumulation of cytokine-induced NF-κB (Ikemoto et al., 2008), inhibitors of EGFR and Her-2 tyrosine kinases (Suzuki et al., 2012) and cyclin-dependent kinases as potential drug candidates for cancer therapy (Beattie et al., 2003; Breault et al., 2003). Substitution at position 5 of the pyrimidine moiety has been used in the past to improve the biological property of several pharmacologically interesting pyrimidine derivatives with anti-HIV (Hocková et al., 2003, 2004) or anti-influenza activity (Jansa et al., 2012), with inhibitory activity against human thymidine phosphorylase (Nencka et al., 2007) or with antioxidative activity (Procházková et al., 2012a, b).
The aim of the present study was the preparation of 5-substituted 2-amino-4,6-dihydroxypyrimidines and 2-amino-4,6-dichloropyrimidines, and the pilot screening of their intrinsic biological potential. Substituents at position 5 of the pyrimidine ring were designed to probe this position with a standard homologous series of alkyl substituents to study steric requirements in this position (e.g., length, branching, saturation, and desaturation). Possible antiviral activity of compounds and their influence on the immune-activated high output nitric oxide (NO) production were analyzed. The NO assay has been employed for its capacity to indicate both positive and negative interferences of compounds with cell immune responses including secretion of cytokines and cytotoxicity (Kmoníčková et al., 2007; Harmatha et al., 2013).
Chemistry
At first, 2-amino-4,6-dihydroxypyrimidines A2–A11 (Scheme 1) were prepared by the condensation of the properly substituted malonic acid diesters with guanidine under basic conditions. Despite the fact that this reaction is well-known in the literature (Rembold and Schramm, 1963; Baraldi et al., 2003; Patel et al., 2007; Rostom et al., 2009), optimization of the reaction conditions in our case had a crucial impact on the isolation, yield, and purity of the products especially in multi-gram scales (for details see experimental). The following is our key findings: methanol or ethanol is required as the solvent to achieve full conversion and the clean reaction profile (alcohols are able to at least partially dissolve the product which otherwise precipitates in quantities that quickly precludes stirring); addition of water after the reaction (which dissolves the sodium salt of the product) and subsequent neutralization by acetic acid is required to keep excess of guanidine in solution while the product quantitatively precipitates from the neutralized solution.
Scheme 1.
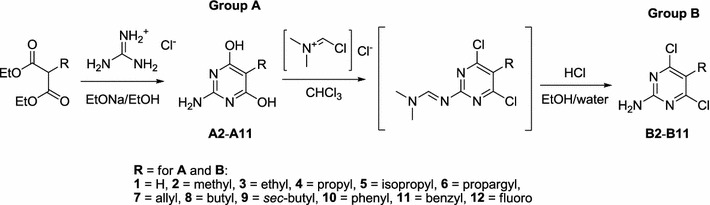
General synthesis of 5-substituted 2-amino-4,6-dihydroxypyrimidines (a) and 2-amino-4,6-dichloropyrimidines (b). For the source of A1, B1, A12, and B12 see experimental
The second step in the synthesis of the targeted 5-substituted 2-amino-4,6-dichloropyrimidines B2–B11 (Scheme 1) consists of the transformation of the 4,6-dihydroxypyrimidine moiety into the 4,6-dichloropyrimidine residue. For such reactions, chlorides of mineral acids such as POCl3, PCl5, SOCl2, or COCl2 with diverse additives like DMF, pyridine, 2-methylpyridine, diphenylamine, or triethylamine are generally used (Altenbach et al., 2008; Jang et al., 2011). Nevertheless, these classical procedures turned out to be unsuitable for the preparation of the desired 5-substituted 2-amino-4,6-dichloropyrimidines B2–B11, owing to their complicated isolations and very low isolated yields (max. 30 %). This synthetic issue was resolved by application of a modified synthetic procedure for the preparation of 4,6-dichloro-2,5-bis{[(dimethylamino)methylene]amino}pyrimidine (Daluge et al., 2000). The use of the Vilsmeier–Haack–Arnold reagent (Reichardt, 1999), followed by immediate deprotection of the (dimethylamino)methylene protecting groups, gave final compounds B2–B11 (Scheme 1). The final step was carried out according to the method described in the literature (Daluge et al., 2000), which was modified in order to obtain B2–B11 directly from the reaction mixture by simple precipitation and filtration (for details see experimental).
Biological properties
Antiviral
None of the compounds A1–A12 or B1–B12 showed any antiviral activity (EC50 > 100 μg/mL) toward the following viruses: HIV-1, HIV-2 in CEM cells, HCMV, HSV-1, HSV-2, vaccinia virus and vesicular stomatitis virus (VSV) in HeLa cells, Feline herpes virus and Feline corona virus (FIPV) in CRFK cells, VSV, Coxsackie virus B4 and respiratory syncytial virus (RSV) in HeLa cells, Parainfluenza 3-virus, Reovirus-1, Coxsackie B4 virus and Punta Toro virus in MDCK cells. The parent 2-amino-4,6-dichloropyrimidine (B1) was also tested and no antiviral activity was observed (EC50 > 100 μg/mL). It is important to mention that antiviral activities described in the literature for this compound required relatively high concentrations in the range of the 100 μg/mL (Marcialis et al., 1973, 1974, La Colla et al., 1975).
NO production
The in vitro stimulation of mouse peritoneal cells with LPS plus IFN-γ substantially enhanced NO biosynthesis. The concentration of nitrites varied from 53.30 ± 0.90 µM to 74.00 ± 3.30 µM (mean ± SEM) in various experiments. The spontaneous production of NO by control cells was negligible, ranging from 0.80 ± 0.30 µM to 1.70 ± 1.60 µM (mean ± SEM) in individual experiments.
None of the 5-substituted 2-amino-4,6-dihydroxypyrimidines A1–A12 at 50 µM concentrations significantly influenced (F/12, 39/= 0.85, p = 0.61) the IFN/LPS-activated NO biosynthesis (Fig. 1a). On average, the formation of nitrites dropped to 91.6 % of control values. The compounds remained ineffective even at fourfold higher (i.e., 200 µM) concentrations (data not shown).
Fig. 1.
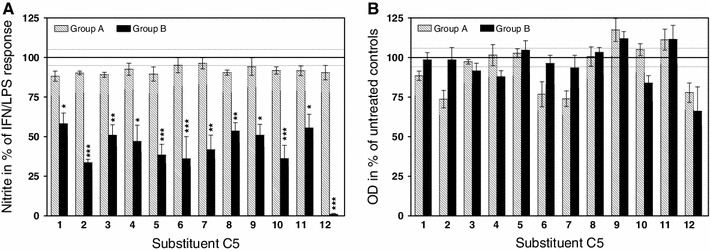
Effects of 5-substituted 2-amino-4,6-dihydroxypyrimidines A1–A12 and 2-amino-4,6-dichloropyrimidines B1–B12 on the production of NO by mouse peritoneal cells (a) and their viability (b). The compounds were applied at a concentration of 50 µM. The NO data are means obtained from at least four experiments. The viability data were averaged from two to four experiments. All columns are mean ± SEM. Dotted lines denote 95 % confidential intervals of control values. * p < 0.05, ** p < 0.01, *** p < 0.001
In contrast, all 5-substituted 2-amino-4,6-dichloropyrimidines B1–B12, applied at 50 µM concentrations, suppressed NO production by at least 55 % (Fig. 1a). The effects were highly statistically significant (F/12, 66/= 6.04, p < 0.0001). The most effective was 5-fluoro-2-amino-4,6-dichloropyrimidine (B12).
The suppression of NO production by 5-substituted 2-amino-4,6-dichloropyrimidines B1–B12 was strictly dose-dependent (Fig. 2). The effects of B6 and B12 were already apparent at concentrations of 2.5 µM. The IC 50s estimates (Table 1) ranged between 2 µM and 36 µM (B12 and B8, respectively).
Fig. 2.
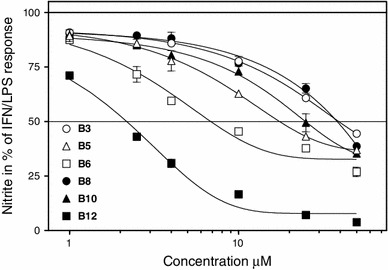
Dose-dependent inhibitory effects of 5-substituted 2-amino-4,6-dichloropyrimidines B1–B12 on in vitro NO production. The points are mean ± SEM. The data represent one of two identical experiments. The concentration of nitrites following the LPS/IFN stimulus (i.e. 100 % response) was 67.90 ± 1.50 µM. It was 1.80 ± 0.30 µM in untreated controls
Table 1.
The NO-inhibitory IC 50s
| IC 50 (µM) | 95 % Limits of confidence | |
|---|---|---|
| 5-Substituted 2-amino-4,6-dichloropyrimidines | ||
| B3 | 35.7 | 26.78–47.59 |
| B5 | 19.64 | 15.76–24.48 |
| B6 | 9 | 6.26–12.93 |
| B8 | 36.23 | 22.83–57.49 |
| B10 | 24.23 | 18.18–32.31 |
| B12 | 1.99 | 1.81–2.19 |
| Reference NO-inhibitors | ||
| Aminoguanidine | 77.83 | 52.82–114.70 |
| L-NMMA | 35.13 | 20.70–59.63 |
| L-NIL | 8.14 | 5.83–11.36 |
| 1,400 W | 3.87 | 2.94–5.11 |
The NO-inhibitory effects of tested pyrimidines were compared to the effects of recognized NO-inhibitors (Fig. 3). The most effective proved to be 1,400 W; the least effective was aminoguanidine. The corresponding IC 50s are shown in Table 1. They are in good agreement with activities reported previously (Stenger et al., 1995; Xu and Krukoff, 2005).
Fig. 3.
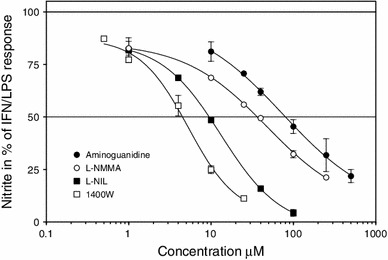
Dose-dependent inhibitory effects of standard NO-inhibitors on in vitro NO production. The points are mean ± SEM. The data represent one of two identical experiments. The concentration of nitrites following the LPS/IFN stimulus (i.e. 100 % response) was 64.10 ± 1.50 µM. It was 0.30 ± 0.20 µM in untreated controls. L-NMMA, N G-monomethyl-L-arginine; L-NIL, L-N 6-(1-iminoethyl)lysine; 1,400 W, N-(3-aminomethyl)-benzylacetamide
Cell viability
Applied at 50 µM concentrations, the compounds had no statistically significant influence on the viability of cells (Fig. 1b). No difference between the two groups of pyrimidine derivatives was observed (F/1, 35/= 0.25, p = 0.62).
Conclusions
An optimized synthetic procedure for the preparation of 5-substituted 2-amino-4,6-dichloropyrimidines B1–B12 starting from the monosubstituted malonic acid diesters has been developed. Final compounds B1–B12 inhibit NO production in mouse peritoneal cells. Compounds B5, B6, B10, and B12 are more potent than the most frequently used inhibitor of the NO production (N G-monomethyl-l-arginine). The most effective was compound B12 bearing the fluorine atom at position 5 of the pyrimidine ring, which nearly completely suppressed the production of NO and which exhibited higher activity than the most potent reference compound (1,400 W).
This interesting discovery of the inhibitory effect of the 5-substituted 2-amino-4,6-dichloropyrimidines on NO production in vitro represents an initial hit in our development of potent compounds with anti-inflammatory activity (patent pending). Compounds of the next generations and further in vitro and in vivo data will be published elsewhere.
Experimental
General
Unless stated otherwise, solvents were evaporated at 40 °C/2 kPa and compounds were dried at 13 Pa. Analytical TLC was performed on silica gel 60 F254 plates (Merck KGaA, Darmstadt, Germany). The NMR spectra were measured on an FT NMR spectrometer (Bruker Avance II 500) in DMSO-d 6 (1H at 500 MHz and 13C at 125.7 MHz), referenced to the residual solvent signal, chemical shift are expressed in parts per million, ppm, and interaction constants J in Hz. GC/MS analyses were measured using a 6,890 N gas chromatograph (Agilent, Santa Clara, CA, USA) attached to a quadrupole mass detector. A HP-5 ms capillary (30 m × 0.25 mm; 0.25 μm; Agilent) was used for the analyses. The carrier gas was helium with a flow rate of 1 mL/min. The EI mass spectra were measured on a GCT Premier (Waters) OA-TOF GC mass spectrometer. The elemental composition of the prepared compounds was determined using a PE 2400 Series II CHNS/O Elemental Analyzer (Perkin Elmer, USA, 1999). Melting points were determined on a Stuart SMP3 Melting Point Apparatus and are uncorrected. Compounds A12 and B12 were prepared according to the literature (Schostarez, 1992). Compounds A1 and B1 were purchased from Sigma-Aldrich.
General procedure for the preparation of 5-substituted 2-amino-4,6-dihydroxypyrimidines A2–A11
Metallic sodium (12.9 g, 0.56 mol) was dissolved in absolute ethanol (300 mL) under argon while being intensively stirred with a mechanical stirrer. The reaction flask was equipped with a reflux condenser with a chlorocalcium tube. After all the sodium was dissolved and the reaction mixture was cooled to room temperature; guanidine hydrochloride (21.02 g, 0.22 mol) was added under intensive stirring, followed by the corresponding monosubstituted malonic acid diester (0.2 mol). The reaction mixture was further intensively stirred due to the production of the solid product, which is so massive that after 2 h it already practically precludes stirring. After another 2 h, absolute ethanol (200 mL) was added and the reaction mixture was refluxed for 1 h while being stirred. Afterward, ethanol (ca 200–300 mL) was evaporated on a vacuum rotary evaporator and water (500 mL) was added to the reaction mixture. After stirring, the product (in the form of sodium salt) was almost dissolved. The obtained mixture was subsequently neutralized by dropwise addition of acetic acid, resulting in immediate and quantitative precipitation of the desired product in the form of a fine solid. This mixture was subsequently heated under reflux for 10 min and then cooled to laboratory temperature. This heating and cooling was repeated twice to get a well-filterable solid product. The solid product was filtered off, washed with water (2 × 50 mL), ethanol (2 × 50 mL), and acetone (2 × 50 mL). The product was dried under high vacuum at 60 °C for 2 days. The obtained purity of the product prepared in this manner is sufficient for the following reaction and based on analyses contains only crystalline water.
2-Amino-5-methylpyrimidine-4,6-diol (A2)
It was obtained as a white solid; yield: 30.86 g (91 %); m.p. >250 °C; 1H NMR (DMSO-d 6): δ = 10.70 (2H, bs, 2× OH), 6.88 (2H, bs, NH2), 1.57 (3H, s, H-1′); 13C NMR (DMSO-d 6): δ = 164.97 (C-4 and 6), 152.53 (C-2), 84.06 (C-5), 8.11 (C-1′); Anal. Calcd. for C5H7N3O2 + 1.6 H2O: C, 35.34; H, 6.05; N, 24.72. Found: C, 35.57; H, 6.15; N, 24.59.
2-Amino-5-ethylpyrimidine-4,6-diol (A3)
It was obtained as a white solid; yield: 32.54 g (88 %); m.p. >250 °C; 1H NMR (DMSO-d 6): δ = 10.30 (2H, bs, 2× OH), 6.30 (2H, bs, NH2), 2.14 (2H, q, J(1′,2′) = 7.3, H-1′), 0.88 (3H, t, J(2′,1′) = 7.3, H-2′); 13C NMR (DMSO-d 6): δ = 164.47 (C-4 and 6), 152.54 (C-2), 91.88 (C-5), 15.62 (C-1′), 13.89 (C-2′); Anal. Calcd. for C6H9N3O2 + 1.7 H2O: C, 38.79; H, 6.73; N, 22.62. Found: C, 38.83; H, 7.90; N, 22.41.
2-Amino-5-propylpyrimidine-4,6-diol (A4)
It was obtained as a white solid; yield: 33.86 g (94 %); m.p. >250 °C; 1H NMR (DMSO-d 6): δ = 10.30 (2H, bs, 2× OH), 6.31 (2H, bs, NH2), 2.10 (2H, t, J(1′,2′) = 7.5, H-1′), 1.32 (2H, m, H-2′), 0.80 (3H, t, J(3,2′) = 7.4, H-3′); 13C NMR (DMSO-d 6): δ = 164.75 (C-4 and 6), 152.57 (C-2), 90.20 (C-5), 22.44 (C-1′), 21.89 (C-2′), 14.17 (C-3′); Anal. Calcd. for C7H11N3O2 + 0.6 H2O: C, 46.71; H, 6.83; N, 23.35. Found: C, 46.80; H, 6.79; N, 23.32.
2-Amino-5-isopropylpyrimidine-4,6-diol (A5)
It was obtained as a white solid; yield: 32.36 g (93 %); m.p. >250 °C; 1H NMR (DMSO-d 6): δ = 10.45 (2H, bs, 2× OH), 6.62 (2H, bs, NH2), 2.96 (1H, sept, J(CH,CH3) = 7.1, H-CH), 1.08 (6H, d, J(CH3,CH) = 7.1, 2× CH3); 13C NMR (DMSO-d 6): δ = 164.19 (C-4 and 6), 152.40 (C-2), 94.80 (C-5), 22.94 (CH), 20.96 (CH3); Anal. Calcd. for C7H11N3O2 + 0.3 H2O: C, 48.16; H, 6.70; N, 24.07. Found: C, 48.11; H, 7.62; N, 23.98.
2-Amino-5-(prop-2-yn-1-yl)pyrimidine-4,6-diol (A6)
It was obtained as a white solid; yield: 33.47 g (96 %); m.p. >250 °C; 1H NMR (DMSO-d 6): δ = 10.55 (2H, bs, 2× OH), 6.79 (2H, bs, NH2), 2.95 (2H, d, J(1,3′) = 2.6, H-1′), 2.43 (1H, t, J(3′,1′) = 2.6, H-3′); 13C NMR (DMSO-d 6): δ = 163.69 (C-4 and 6), 152.69 (C-2), 89.05 (C-2′), 84.97 (C-5), 68.02 (C-3′), 12.06 (C-1′); Anal. Calcd. for C7H7N3O2 + 0.5 H2O: C, 48.28; H, 4.63; N, 24.13. Found: C, 48.09; H, 4.33; N, 24.23.
5-Allyl-2-aminopyrimidine-4,6-diol (A7)
It was obtained as a white solid; yield: 38.57 g (95 %); m.p. >250 °C; 1H NMR (DMSO-d 6): δ = 10.35 (2H, bs, 2× OH), 6.40 (2H, bs, NH2), 5.73 (1H, ddt, J(2′,1′) = 6.1, J(2′,3′cis) = 10.0, J(2′,3′trans) = 17.2, H-2′), 4.87 (1H, ddt, J(3′trans,1′) = 1.6, J(gem) = 2.3, J(3′trans,2′) = 17.2, H-3′trans), 4.79 (1H, ddt, J(3′cis,1′) = 1.6, J(gem) = 2.3, J(3′cis,2′) = 10.0, H-3′cis), 2.85 (2H, dt, J(1′,3′) = 1.6, J(1′,2′) = 6.1, H-1′); 13C NMR (DMSO-d 6): δ = 164.36 (C-4 and 6), 152.71 (C-2), 137.73 (C-2′), 113.32 (C-3′), 87.73 (C-5), 26.67 (C-1′); Anal. Calcd. for C7H9N3O2 + 2 H2O: C, 41.38; H, 6.45; N, 20.68. Found: C, 41.44; H, 6.17; N, 20.47.
2-Amino-5-butylpyrimidine-4,6-diol (A8)
It was obtained as a white solid; yield: 39.61 g (97 %); m.p. >250 °C; 1H NMR (DMSO-d 6): δ = 10.30 (2H, bs, 2× OH), 6.32 (2H, bs, NH2), 2.12 (2H, t, J(1′,2′) = 7.1, H-1′), 1.28 and 1.23 (2× 2H, 2× m, H-2′ and H-3′), 0.85 (3H, t, J(4′,3′) = 7.2, H-4′); 13C NMR (DMSO-d 6): δ = 164.67 (C-4 and 6), 152.54 (C-2), 90.36 (C-5), 31.04 (C-1′), 22.32 (C-2′), 21.98 (C-3′), 14.22 (C-4′); Anal. Calcd. for C8H13N3O2 + 1.2 H2O: C, 46.91; H, 7.58; N, 20.52. Found: C, 47.15; H, 7.69; N, 20.36.
2-Amino-5-(sec-butyl)pyrimidine-4,6-diol (A9)
It was obtained as a white solid; yield: 36.89 g (93 %); m.p. >250 °C; 1H NMR (DMSO-d 6): δ = 10.20 (2H, bs, 2× OH), 6.31 (2H, bs, NH2), 2.70 (1H, m, H-1′), 1.65 and 1.40 (2× 1H, 2× m, H-2′), 1.06 (3H, d, J(1″,1′) = 7.0, H-1″), 0.72 (3H, t, J(3′,2′) = 7.4, H-3′); 13C NMR (DMSO-d 6): δ = 164.46 (C-4 and 6), 152.47 (C-2), 93.61 (C-5), 30.08 (C-1′), 27.09 (C-2′), 19.00 (C-1″), 13.03 (C-3′); Anal. Calcd. for C8H13N3O2 + 0.8 H2O: C, 48.62; H, 7.45; N, 21.26. Found: C, 48.57; H, 7.47; N, 21.18.
2-Amino-5-phenylpyrimidine-4,6-diol (A10)
It was obtained as a white solid; yield: 41.43 g (94 %); m.p. >250 °C; 1H NMR (DMSO-d 6): δ = 10.60 (2H, bs, 2× OH), 7.50 (2H, d, phenyl), 7.19 (2H, t, phenyl), 7.02 (1H, t, phenyl), 6.74 (2H, bs, NH2); 13C NMR (DMSO-d 6): δ = 162.84 (C-4 and 6), 152.02 (C-2), 135.40, 130.26, 126.84 and 124.19 (C-phenyl), 106.11 (C-5); Anal. Calcd. for C10H9N3O2 + 1 H2O: C, 54.29; H, 5.01; N, 19.00. Found: C, 54.17; H, 5.19; N, 18.82.
2-Amino-5-benzylpyrimidine-4,6-diol (A11)
It was obtained as a white solid; yield: 43.21 g (91 %); m.p. >250 °C; 1H NMR (DMSO-d 6): δ = 10.42 (2H, bs, 2× OH), 7.18 (4H, m, phenyl), 7.07 (1H, m, phenyl), 6.46 (2H, bs, NH2), 3.44 (2H, s, CH2); 13C NMR (DMSO-d 6): δ = 164.47 (C-4 and 6), 152.68 (C-2), 143.10, 128.31, 127.90 and 125.20 (C-phenyl), 94.51 (C-5), 28.12 (CH2); Anal. Calcd. for C11H11N3O2 + 1.1 H2O: C, 55.74; H, 5.61; N, 17.73. Found: C, 55.71; H, 5.54; N, 17.60.
General procedure for the preparation of 5-substituted 2-amino-4,6-dichloropyrimidines B2–B11
Prior to the reaction, the starting 5-substituted 2-amino-4,6-dihydroxypyrimidine A2–A11 was dried in a vacuum drier at 80 °C and under 0.1 mbar for 1 day, because crystalline water increases the amount of the Vilsmeier–Haack–Arnold reagent required for full conversion. Subsequently, 5-substituted 2-amino-4,6-dihydroxypyrimidine (10 mmol) was suspended under inert atmosphere in a 2 M solution of the Vilsmeier–Haack–Arnold reagent (80 mmol, 40 mL) in chloroform. The reaction mixture was subsequently heated at reflux for 4 h, during which the starting material was completely dissolved. The reaction mixture was cooled to the room temperature, poured onto ice and rapidly neutralized with a saturated aqueous NaHCO3 solution. The obtained mixture was quickly transferred into a separatory funnel and immediately extracted with chloroform (3 × 20 mL). The organic fractions were combined together, dried over MgSO4, filtered through a 0.5 cm layer of silica gel and concentrated down on a rotary evaporator. This crude residue of 5-substituted 4,6-dichloro-2-{[(dimethylamino)methylene]amino}pyrimidine was dissolved in the mixture of 99 % ethanol (20 mL) and 37 % aqueous HCl (2 mL). The reaction mixture was heated at 50 °C for 2 h, during which a crystalline product began to precipitate directly from the reaction mixture. After that, water (30 mL) was added and the reaction mixture was intensively stirred for 10 min. The precipitated product was filtered off and washed with a water/ethanol mixture (1/1, 2 × 10 mL), 5 % aqueous solution of NaHCO3 (10 mL) and a water/ethanol mixture (1/1, 10 mL). The product was subsequently recrystallized from aqueous ethanol, filtered off, washed with a water/ethanol mixture (1/1, 10 mL) and dried under high vacuum.
4,6-Dichloro-5-methylpyrimidin-2-amine (B2)
It was obtained as a white solid; yield: 1.26 g (71 %); m.p. 189–190 °C; 1H NMR (DMSO-d 6): δ = 7.26 (2H, bs, NH2), 2.17 (3H, s, H-1′); 13C NMR (DMSO-d 6): δ = 161.01 (C-4 and 6), 160.78 (C-2), 113.60 (C-5), 14.93 (C-1′); Anal. Calcd. for C5H5Cl2N3: C, 33.73; H, 2.83; Cl, 39.83; N, 23.60. Found: C, 33.53; H, 2.78, Cl, 40.02; N, 23.42; MS (EI), m/z (%): 177 and 179 [M+] (100); MS (ESI +), m/z (%): 178 and 180 [M+H+] (100).
4,6-Dichloro-5-ethylpyrimidin-2-amine (B3)
It was obtained as a white solid; yield: 1.58 g (82 %); m.p. 183–185 °C; 1H NMR (DMSO-d 6): δ = 7.32 (2H, bs, NH2), 2.61 (2H, q, J(1′,2′) = 7.4, H-1′), 1.07 (3H, t, J(2′,1′) = 7.4, H-2′); 13C NMR (DMSO-d 6): δ = 160.83 (C-2), 160.76 (C-4 and 6), 118.90 (C-5), 22.41 (C-1′), 12.92 (C-2′); Anal. Calcd. for C6H7Cl2N3: C, 37.52; H, 3.67; Cl, 36.92; N, 21.88. Found: C, 37.59; H, 3.73; Cl, 36.76; N, 21.77; MS (EI), m/z (%): 191 and 193 [M+] (100).
4,6-Dichloro-5-propylpyrimidin-2-amine (B4)
It was obtained as a white solid; yield: 1.60 g (78 %); m.p. 182–183 °C; 1H NMR (DMSO-d 6): δ = 7.52 (2H, bs, NH2), 2.69 (2H, t, J(1′,2′) = 7.6, H-1′), 1.52 (2H, m, H-2′), 0.91 (3H, t, J(3′0.2′) = 7.2, H-3′); 13C NMR (DMSO-d 6): δ = 160.78 (C-2), 160.69 (C-4 and 6), 119.20 (C-5), 31.12 (C-1′), 20.86 (C-2′), 13.75 (C-3′); Anal. Calcd. for C7H9Cl2N3: C, 40.80; H, 4.40; Cl, 34.41; N, 20.39. Found: C, 40.79; H, 4.32; Cl, 34.15; N, 20.19; MS (EI), m/z (%): 205 and 207 [M+] (100).
4,6-Dichloro-5-isopropylpyrimidin-2-amine (B5)
It was obtained as a white solid; yield: 1.43 g (69 %); m.p. 175–176 °C; 1H NMR (DMSO-d 6): δ = 7.31 (2H, bs, NH2), 3.46 (1H, sept, J(CH,CH3) = 7.2, CH), 1.28 (6H, d, J(CH3,CH) = 7.2, 2× CH3); 13C NMR (DMSO-d 6): δ = 160.62 (C-2), 160.32 (C-4 and 6), 121.65 (C-5), 28.57 (CH), 19.82 (CH3); Anal. Calcd. for C7H9Cl2N3: C, 40.80; H, 4.40; Cl, 34.41; N, 20.39. Found: C, 40.57; H, 4.54; Cl, 34.69; N, 20.67; MS (EI), m/z (%): 205 and 207 [M+] (100).
4,6-Dichloro-5-(prop-2-yn-1-yl)pyrimidin-2-amine (B6)
It was obtained as a white solid; yield: 1.63 g (81 %); m.p. 159–161 °C; 1H NMR (DMSO-d 6): δ = 7.50 (2H, bs, NH2), 3.52 (2H, d, J(1′,3′) = 2.7, H-1′), 2.96 (1H, t, J(3′,1′) = 2.7, H-3′); 13C NMR (DMSO-d 6): δ = 161.20 (C-2), 160.84 (C-4 and 6), 113.30 (C-5), 79.86 (C-2′), 71.96 (C-3′), 19.03 (C-1′); Anal. Calcd. for C7H5Cl2N3: C, 41.61; H, 2.49; Cl, 35.09; N, 20.80. Found: C, 41.41; H, 2.48; Cl, 34.96; N, 20.55; MS (EI), m/z (%): 201 and 203 [M+] (100).
5-Allyl-4,6-dichloropyrimidin-2-amine (B7)
It was obtained as a white solid; yield: 1.50 g (74 %); m.p. 175–177 °C; 1H NMR (DMSO-d 6): δ = 7.40 (2H, bs, NH-2), 5.83 (1H, ddt, J(2′,1′) = 5.8, J(2′,3′cis) = 10.1, J(2′,3′trans) = 17.1, H-2′), 5.06 (1H, dq, J(3′cis,1′) = J(gem) = 1.6, J(3′cis,2′) = 10.1, H-3′cis), 4.96 (1H, dq, J(3′trans,1′) = J(gem) = 1.7, J(3′trans,2′) = 17.1, H-3′trans), 3.36 (2H, dt, J(1′,3′) = 1.7, J(1′,2′) = 5.8, H-1′); 13C NMR (DMSO-d 6): δ = 161.33 (C-4 and 6), 161.08 (C-2), 133.57 (C-2′), 116.28 (C-3′), 115.01 (C-5), 32.70 (C-1′); Anal. Calcd. for C7H7Cl2N3: C, 41.20; H, 3.46; Cl, 34.75; N, 20.59. Found: C, 41.12; H, 3.37; Cl, 34.54; N, 20.56; MS (EI), m/z (%): 203 and 205 [M+] (100).
5-Butyl-4,6-dichloropyrimidin-2-amine (B8)
It was obtained as a white solid; yield: 1.92 g (87 %); m.p. 169–170 °C; 1H NMR (DMSO-d 6): δ = 7.29 (2H, bs, NH2), 2.59 (2H, m, CH2), 1.45 (2H, m, CH2), 1.33 (2H, m, CH2), 0.91 (3H, t, J(4′,3′) = 7.3, H-4′); 13C NMR (DMSO-d 6): δ = 160.78 (C-2), 160.76 (C-4 and 6), 117.71 (C-5), 30.37, 28.46 and 22.04 (C-1′, 2′ and 3′), 13.81 (C-4′); Anal. Calcd. for C8H11Cl2N3: C, 43.66; H, 5.04; Cl, 32.22; N, 19.09. Found: C, 43.70; H, 4.93; Cl, 32.24; N, 18.87; GC/MS-EI (R T 16.03 min), m/z (%): 219 and 221 [M+] (18), 176 and 178 [M+-Pr] (100), min. 99.5 % purity.
5-(Sec-butyl)-4,6-dichloropyrimidin-2-amine (B9)
It was obtained as a white solid; yield: 1.66 g (75 %); m.p. 159–160 °C; 1H NMR (DMSO-d 6): δ = 7.33 (2H, s, NH-2), 3.23 (1H, m, H-1′), 1.83 and 1.64 (2× 1H, 2× m, H-2′), 1.25 (3H, d, J(1″,1′) = 6.2, H-1″), 0.77 (3H, t, J(3′,2′) = 7.4, H-3′); 13C NMR (DMSO-d 6): δ = 163.64 (C-2), C-4 and C-6 not found, 120.12 (C-5), 35.72 (C-1′), 26.67 (C-2′), 18.13 (C-1″), 12.53 (C-3′); Anal. Calcd. for C8H11Cl2N3: C, 43.66; H, 5.04; Cl, 32.22; N, 19.09. Found: C, 43.63; H, 4.82; Cl, 32.17; N, 18.86; MS (EI), m/z (%): 219 and 221 [M+] (100).
4,6-Dichloro-5-phenylpyrimidin-2-amine (B10)
It was obtained as a white solid; yield: 1.71 g (71 %); m.p. 193–195 °C; 1H NMR (DMSO-d 6): δ = 7.60 (2H, bs, NH2), 7.44 (2H, t, phenyl), 7.40 (1H, t, phenyl), 7.30 (2H, d, phenyl); 13C NMR (DMSO-d 6): δ = 161.46 (C-2), 160.26 (C-4 and 6), 134.24, 130.47, 128.55 and 128.46 (phenyl), 119.71 (C-5); Anal. Calcd. for C10H7Cl2N3: C, 50.03; H, 2.94; Cl, 29.53; N, 17.50. Found: C, 49.86; H, 2.82; Cl, 38.44; N, 17.23; MS (EI), m/z (%): 239 and 241 [M+] (100).
5-Benzyl-4,6-dichloropyrimidin-2-amine (B11)
It was obtained as a white solid; yield: 2.02 g (80 %); m.p. 196–197 °C; 1H NMR (DMSO-d 6): δ = 7.46 (2H, bs, NH2), 7.29 (2H, t, phenyl), 7.20 (1H, t, phenyl), 7.15 (2H, d, phenyl), 4.01 (2H, s, CH2); 13C NMR (DMSO-d 6): δ = 161.75 (C-4 and 6), 161.15 (C-2), 138.16, 128.72, 127.92 and 126.56 (C-phenyl), 116.29 (C-5), 34.15 (CH2); Anal. Calcd. for C11H9Cl2N3: C, 51.99; H, 3.57; Cl, 27.90; N, 16.54. Found: C, 51.92; H, 3.66; Cl, 28.02; N, 16.68; MS (EI), m/z (%): 253 and 255 [M+] (100).
Preparation of the compound solutions for biological assays
The 200 mM stock solutions of compounds were prepared in DMSO. They were further diluted to working concentrations in complete RPMI-1640 culture medium (described below). In order to exclude any possible interference of DMSO with NO production and viability of cells, appropriately diluted DMSO was included in all experiments. It was found ineffective in the assays (data not shown). Standard inhibitors of NO production, i.e., N G-monomethyl-l-arginine (L-NMMA), aminoguanidine (AG), L-N 6-(1-iminoethyl)lysine (L-NIL), and N-(3-aminomethyl)benzylacetamide (1,400 W) were bought from Sigma-Aldrich. Stock solutions (5 mM) were prepared in apyrogenic distilled water.
Animals; isolation and cultivation of cells
Female mice of the inbred strain C57BL/6, 8- to 10-week-old, were purchased from Charles River Deutschland (Sulzfeld, Germany). They were kept in transparent plastic cages in groups of ten, and maintained in an Independent Environmental Air Flow Animal Cabinet (ESI Flufrance, Wissous, France). Lighting was set on 06–18 h, temperature at 22 °C.
Animals, killed by cervical dislocation, were intraperitoneally injected with 8 mL of sterile saline. Pooled peritoneal cells collected from mice (n = 4–10 in individual experiments) were washed in sterile saline, resuspended in culture medium, and seeded into 96-well round-bottom microplates (Costar). The final amount of cells was 2 × 106 cells/mL. The cultures were maintained at 37 °C, 5 % CO2 in humidified Heraeus incubator.
Complete RPMI-1640 culture medium (Sigma-Aldrich) contained 10 % heat-inactivated fetal bovine serum, 2 mM l-glutamine, 50 μg/mL gentamicin, and 5 × 10−5 M 2-mercaptoethanol (all Sigma).
All protocols were approved by the institutional ethics committee.
Nitric oxide assay
The cells were cultured 24 h in presence of the test compounds. They were applied either alone or concomitantly with the NO-enhancing stimulus provided by combination of murine recombinant interferon-γ (IFN-γ, 0.5 ng/mL; R&D Systems, Minneapolis, MN) and lipopolysaccharide (LPS from E. coli 055: B5, 0.1 ng/mL; Sigma). All variants were run in duplicate.
The concentration of nitrites in supernatants of cells was taken as a measure of NO production (Marletta et al., 1988). It was detected in individual, cell-free samples (50 μL) incubated 5 min at ambient temperature with an aliquot of a Griess reagent (1 % sulphanilamide/0.1 % naphtylendiamine/2.5 % H3PO4). The absorbance at 540 nm was recorded using a microplate spectrophotometer (Tecan, Austria). A nitrite calibration curve was used to convert absorbance to μM nitrite.
Cell viability assay
Viability of cells was determined using a colorimetric assay based on cleavage of the tetrazolium salt WST-1 (Roche Diagnostics, Mannheim, Germany) by mitochondrial dehydrogenases in viable cells. The cells were cultured quadruplicate, as described above. After the 24 h culture, the WST-1 was added and the cells were kept in the Heraeus incubator at 37 °C for additional 3 h. Optical density at 450/690 nm was evaluated.
Statistical evaluation
Analysis of variance (ANOVA) with subsequent Bonferroni´s multiple comparison test and graphical presentation of data was done using the Prism program (GraphPad Software, San Diego, CA). Several experiments were performed. The concentration of nitrites as well as optical density, the measure of viability of cells, varied among experiments. In order to amalgamate the data from various experiments, they were expressed as a percentage of control values.
Acknowledgments
The research was supported by grant CZ: GA ČR:303/12/0172 from the Grant Agency of the Czech Republic. The antiviral testing provided by the group of prof. Jan Balzarini, Prof. Lieve Naesens, Prof. Johan Neyts, Prof. Robert Snoeck and Prof. Graciela Andrei (Rega Institute for Medical Research, Katholieke Universiteit Leuven, Minderbroedersstraat 10, B-3000 Leuven, Belgium) is highly appreciated.
Footnotes
Antonín Holý: Deceased.
Contributor Information
Petr Jansa, Email: jansa@uochb.cas.cz, jansap@email.cz.
Zdeněk Zídek, Email: zidekz@biomed.cas.cz.
References
- Altenbach RJ, Adair RM, Bettencourt BM, Black LA, Fix-Stenzel SR, Gopalakrishnan SM, Hsieh GC, Liu HQ, Marsh KC, McPherson MJ, Milicic I, Miller TR, Vortherms TA, Warrior U, Wetter JM, Wishart N, Witte DG, Honore P, Esbenshade TA, Hancock AA, Brioni JD, Cowart MD. Structure-activity studies on a series of a 2-aminopyrimidine-containing histamine H-4 receptor ligands. J Med Chem. 2008;51:6571–6580. doi: 10.1021/jm8005959. [DOI] [PubMed] [Google Scholar]
- Arvanitis AG, Gilligan PJ, Chorvat RJ, Cheeseman RS, Christos TE, Bakthavatchalam R, Beck JP, Cocuzza AJ, Hobbs FW, Wilde RG, Arnold C, Chidester D, Curry M, He L, Hollis A, Klaczkiewicz J, Krenitsky PJ, Rescinito JP, Scholfield E, Culp S, De Souza EB, Fitzgerald L, Grigoriadis D, Tam SW, Shen HL. Non-peptide corticotropin-releasing hormone antagonists: syntheses and structure-activity relationships of 2-anilinopyrimidines and -triazines. J Med Chem. 1999;42:805–818. doi: 10.1021/jm980222w. [DOI] [PubMed] [Google Scholar]
- Baraldi PG, Bovero A, Fruttarolo F, Romagnoli R, Tabrizi MA, Preti D, Varani K, Borea PA, Moorman AR. New strategies for the synthesis of A(3) adenosine receptor antagonists. Bioorg Med Chem. 2003;11:4161–4169. doi: 10.1016/S0968-0896(03)00484-X. [DOI] [PubMed] [Google Scholar]
- Beattie JF, Breault GA, Ellston RP, Green S, Jewsbury PJ, Midgley CJ, Naven RT, Minshull CA, Pauptit RA, Tucker JA, Pease JE. Cyclin-dependent kinase 4 inhibitors as a treatment for cancer. Part 1: identification and optimisation of substituted 4,6-bis anilino pyrimidines. Bioorg Med Chem Lett. 2003;13:2955–2960. doi: 10.1016/S0960-894X(03)00202-6. [DOI] [PubMed] [Google Scholar]
- Bogdan C, Werner E, Stenger S, Wachter H, Röllinghoff M, Werner-Felmayer G. 2,4-Diamino-6-hydroxypyrimidine, an inhibitor of tetrahydrobiopterin synthesis, downregulates the expression of iNOS protein and mRNA in primary murine macrophages. FEBS Lett. 1995;363:69–74. doi: 10.1016/0014-5793(95)00284-G. [DOI] [PubMed] [Google Scholar]
- Breault GA, Ellston RP, Green S, James SR, Jewsbury PJ, Midgley CJ, Pauptit RA, Minshull CA, Tucker JA, Pease JE. Cyclin-dependent kinase 4 inhibitors as a treatment for cancer. Part 2: identification and optimisation of substituted 2,4-bis anilino pyrimidines. Bioorg Med Chem Lett. 2003;13:2961–2966. doi: 10.1016/S0960-894X(03)00203-8. [DOI] [PubMed] [Google Scholar]
- Daluge SM, Martin MT, Sickles BR, Livingston DA. An efficient, scalable synthesis of the HIV reverse transcriptase inhibitor Ziagen (1592U89) Nucleos Nucleot Nuc Acids. 2000;19:297–327. doi: 10.1080/15257770008033011. [DOI] [PubMed] [Google Scholar]
- De Clercq E, Holý A. Acyclic nucleoside phosphonates: a key class of antiviral drugs. Nature Rev Drug Disc. 2005;4:928–940. doi: 10.1038/nrd1877. [DOI] [PubMed] [Google Scholar]
- Flore O, Marcialis MA, Marongiu ME, Pompei R, La Colla P, Loddo B. Dichloropyrimidines: specific inhibitors of virus growth. Experientia. 1977;33:1155–1157. doi: 10.1007/BF01922299. [DOI] [PubMed] [Google Scholar]
- Harmatha J, Buděšínský M, Vokáč K, Kostecká P, Kmoníčková E, Zídek Z. Trilobolide and related sesquiterpene lactones from Laser trilobum possessing immunobiological properties. Fitoterapia. 2013;89:157–166. doi: 10.1016/j.fitote.2013.05.025. [DOI] [PubMed] [Google Scholar]
- Hitchings GH. Selective inhibitors of dihydrofolate reductase (Nobel Lecture) Angew Chem Int Edit Eng. 1989;28:879–888. doi: 10.1002/anie.198908791. [DOI] [PubMed] [Google Scholar]
- Hocková D, Holý A, Masojidková M, Andrei G, Snoeck R, De Clercq E, Balzarini J. 5-Substituted-2,4-diamino-6-2-(phosphonomethoxy)ethoxylpyrimidines acyclic nucleoside phosphonate analogues with antiviral activity. J Med Chem. 2003;46:5064–5073. doi: 10.1021/jm030932o. [DOI] [PubMed] [Google Scholar]
- Hocková D, Holý A, Masojidková M, Andrei G, Snoeck R, De Clercq E, Balzarini J. Synthesis and antiviral activity of 2,4-diamino-5-cyano-6-2-(phosphonomethoxy)ethoxy pyrimidine and related compounds. Bioorg Med Chem. 2004;12:3197–3202. doi: 10.1016/j.bmc.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Holý A. Phosphonomethoxyalkyl analogs of nucleotides. Curr Pharm Des. 2003;9:2567–2592. doi: 10.2174/1381612033453668. [DOI] [PubMed] [Google Scholar]
- Ikemoto K, Matsumoto T, Ohtsuki M, Itoh M, Tada S, Udagawa Y, Sumi-Ichinose C, Kondo K, Nomura T. 2,4-Diamino-6-hydroxypyrimidine (DAHP) suppresses cytokine-induced VCAM-1 expression on the cell surface of human umbilical vein endothelial cells in a BH(4)-independent manner. Biochim Biophys Acta. 2008;1780:960–965. doi: 10.1016/j.bbagen.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Jang MY, Lin YA, De Jonghe S, Gao LJ, Vanderhoydonck B, Froeyen M, Rozenski J, Herman J, Louat T, Van Belle K, Waer M, Herdewijn P. Discovery of 7-N-piperazinylthiazolo 5,4-d pyrimidine analogues as a novel class of immunosuppressive agents with in vivo biological activity. J Med Chem. 2011;54:655–668. doi: 10.1021/jm101254z. [DOI] [PubMed] [Google Scholar]
- Jansa P, Hradil O, Baszczyňski O, Dračínský M, Klepetářová B, Holý A, Balzarini J, Janeba Z. An efficient microwave-assisted synthesis and biological properties of polysubstituted pyrimidinyl- and 1,3,5-triazinylphosphonic acids. Tetrahedron. 2012;68:865–871. doi: 10.1016/j.tet.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmoníčková E, Melkusová P, Farghali H, Holý A, Zídek Z. Nitric oxide production in mouse and rat macrophages: a rapid and efficient assay for screening of drugs immunostimulatory effects in human cells. Nitric Oxide. 2007;17:160–169. doi: 10.1016/j.niox.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Kolinsky MA, Gross SS. The mechanism of potent GTP cyclohydrolase I inhibition by 2,4-diamino-6-hydroxypyrimidine: requirement of the GTP cyclohydrolase I feedback regulatory protein. J Biol Chem. 2004;279:40677–40682. doi: 10.1074/jbc.M405370200. [DOI] [PubMed] [Google Scholar]
- La Colla P, Marcialis MA, Flore O, Schivo ML, Garzia A, Loddo B. Irreversible inactivation by 2-amino-4,6-dichloropyrimidine of certain structural proteins of poliovirus. Experientia. 1975;31:797–798. doi: 10.1007/BF01938471. [DOI] [PubMed] [Google Scholar]
- La Colla P, Marcialis MA, Flore O, Sau M, Garzia A, Loddo B. Specific inhibition of virus multiplication by bichlorinated pyrimidines. Ann N Y Acad Sci USA. 1977;284:294–304. doi: 10.1111/j.1749-6632.1977.tb21964.x. [DOI] [PubMed] [Google Scholar]
- Lagoja IM. Pyrimidine as constituent of natural biologically active compounds. Chem Biodivers. 2005;2:1–50. doi: 10.1002/cbdv.200490173. [DOI] [PubMed] [Google Scholar]
- Marcialis MA, Schivo ML, Atzeni A, Garzia A, Loddo B. On the mechanism of the inhibitory action of 2-amino-4,6-dichloropyrimidine on poliovirus growth. Experientia. 1973;29:1559–1561. doi: 10.1007/BF01943917. [DOI] [PubMed] [Google Scholar]
- Marcialis MA, Flore O, Firinu A, La Colla P, Garzia A, Loddo B. In vitro and in vivo inhibitory action of 2-amino-4,6-dichloropyrimidine on polio and herpes virus. Experientia. 1974;30:1272–1273. doi: 10.1007/BF01945180. [DOI] [PubMed] [Google Scholar]
- Marcialis MA, Flore O, Marongiu ME, Pompei R, Pani A, Manconi PE. On the inhibitory effect of 2-amino-4,6-dichloropyrimidine on growth of vaccinia virus. Experientia. 1979;35:321–322. doi: 10.1007/BF01964326. [DOI] [PubMed] [Google Scholar]
- Marletta MA, Yoon PS, Iyengar R, Leaf CD, Wishnok JS. Macrophage oxidation of l-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988;27:8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Naesens L, Lenaerts L, Andrei G, Snoeck R, Van Beers D, Holý A, Balzarini J, De Clercq E. Antiadenovirus activities of several classes of nucleoside and nucleotide analogues. Antimicrob Agents Chem. 2005;49:1010–1016. doi: 10.1128/AAC.49.3.1010-1016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nencka R, Votruba I, Hřebabecký H, Jansa P, Tloust’ová E, Horská K, Masojídková M, Holý A. Discovery of 5-substituted-6-chlorouracils as efficient inhibitors of human thymidine phosphorylase. J Med Chem. 2007;50:6016–6023. doi: 10.1021/jm070644i. [DOI] [PubMed] [Google Scholar]
- Patel PR, Ramalingan C, Park YT. Synthesis and antimicrobial evaluation of guanylsulfonamides. Bioorg Med Chem Lett. 2007;17:6610–6614. doi: 10.1016/j.bmcl.2007.09.060. [DOI] [PubMed] [Google Scholar]
- Procházková E, Jansa P, Březinová A, Čechová L, Mertlíková-Kaiserová H, Holý A, Dračínský M. Compound instability in dimethyl sulphoxide, case studies with 5-aminopyrimidines and the implications for compound storage and screening. Bioorg Med Chem Lett. 2012;22:6405–6409. doi: 10.1016/j.bmcl.2012.08.065. [DOI] [PubMed] [Google Scholar]
- Procházková E, Jansa P, Dračínský M, Holý A, Mertliková-Kaiserová H. Determination of the antioxidative activity of substituted 5-aminopyrimidines. Free Radical Res. 2012;46:61–67. doi: 10.3109/10715762.2011.638292. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Reichardt C. Vilsmeier–Haack–Arnold formylations of aliphatic substrates with N-chloromethylene-N, N-dimethylammonium salts. J Prakt Chem. 1999;341:609–615. doi: 10.1002/(SICI)1521-3897(199910)341:7<609::AID-PRAC609>3.0.CO;2-0. [DOI] [Google Scholar]
- Rembold H, Schramm HJ. Kondensation des 2.4-diamino-6-hydroxy-pyrimidins mit Aldosen. Chem Ber. 1963;96:2786–2797. doi: 10.1002/cber.19630961038. [DOI] [Google Scholar]
- Rostom SAF, Ashour HMA, El Razik HAA. Synthesis and biological evaluation of some novel polysubstituted pyrimidine derivatives as potential antimicrobial and anticancer agents. Arch Pharm. 2009;342:299–310. doi: 10.1002/ardp.200800223. [DOI] [PubMed] [Google Scholar]
- Schostarez HJ (1992) A process for the preparation of 5-fluoro-6-(1-piperidinyl)-2,4-pyrimidinediamine 3-oxide (5-fluorominoxidil) and is use as hair growth agent and antihypertensive. WO9208705A1, p 19
- Stenger S, Thuring H, Rollinghoff M, Manning P, Bogdan C. L-N6-(1-iminoethyl)-lysine potently inhibits inducible nitric oxide synthase and is superior to NG-monomethyl-arginine in vitro and in vivo. Eur J Pharmacol. 1995;294:703–712. doi: 10.1016/0014-2999(95)00618-4. [DOI] [PubMed] [Google Scholar]
- Sung YJ, Hotchkiss JH, Dietert RR. 2,4-Diamino-6-hydroxypyrimidine, an inhibitor of GTP cyclohydrolase I, suppresses nitric oxide production by chicken macrophages. Int J Immunopharmacol. 1994;16:101–108. doi: 10.1016/0192-0561(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Shiota T, Watanabe F, Haga N, Murashi T, Ohara T, Matsuo K, Omori N, Yari H, Dohi K, Inoue M, Iguchi M, Sentou J, Wada T. Discovery of novel 5-alkynyl-4-anilinopyrimidines as potent, orally active dual inhibitors of EGFR and Her-2 tyrosine kinases. Bioorg Med Chem Lett. 2012;22:456–460. doi: 10.1016/j.bmcl.2011.10.103. [DOI] [PubMed] [Google Scholar]
- Tegeder I, Costigan M, Griffin RS, Abele A, Belfer I, Schmidt H, Ehnert C, Nejim J, Marian C, Scholz J, Wu T, Allchorne A, Diatchenko L, Binshtok AM, Goldman D, Adolph J, Sama S, Atlas SJ, Carlezon WA, Parsegian A, Lötsch J, Fillingim RB, Maixner W, Geisslinger G, Max MB, Woolf CJ. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269–1277. doi: 10.1038/nm1490. [DOI] [PubMed] [Google Scholar]
- Undheim K, Benneche T (1996) 6.02—Pyrimidines and their benzo derivatives. In: Katritzky AR, Rees CW, Scriven EFV (eds) Comprehensive heterocyclic chemistry II; 602—pyrimidines and their benzo derivatives. Pergamon, Oxford, pp 93–231
- Xu Y, Krukoff TL. Adrenomedullin stimulates nitric oxide release from SK-N-SH human neuroblastoma cells by modulating intracellular calcium mobilization. Endocrinology. 2005;146:2295–2305. doi: 10.1210/en.2004-1354. [DOI] [PubMed] [Google Scholar]
- Ying C, Holý A, Hocková D, Havlas Z, De Clercq E, Neyts J. Novel acyclic nucleoside phosphonate analogues with potent anti-hepatitis B virus activities. Antimicrob Agents Chem. 2005;49:1177–1180. doi: 10.1128/AAC.49.3.1177-1180.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


