Abstract
The demand for production of glycoproteins from mammalian cell culture continues with an increased number of approvals as biopharmaceuticals for the treatment of unmet medical needs. This is particularly the case for humanized monoclonal antibodies which are the largest and fastest growing class of therapeutic pharmaceuticals. This demand has fostered efforts to improve the efficiency of production as well as to address the quality of the final product. Chinese hamster ovary cells are the predominant hosts for stable transfection and high efficiency production on a large scale. Specific productivity of recombinant glycoproteins from these cells can be expected to be above 50 pg/cell/day giving rise to culture systems with titers of around 5 g/L if appropriate fed-batch systems are employed. Cell engineering can delay the onset of programmed cell death to ensure prolonged maintenance of productive viable cells. The clinical efficacy and quality of the final product can be improved by strategic metabolic engineering. The best example of this is the targeted production of afucosylated antibodies with enhanced antibody-dependent cell cytotoxicity, an important function for use in cancer therapies. The development of culture media from non-animal sources continues and is important to ensure products of consistent quality and without the potential danger of contamination. Process efficiencies may also be improved by employing disposable bioreactors with the associated minimization of downtime. Finally, advances in downstream processing are needed to handle the increased supply of product from the bioreactor but maintaining the high purity demanded of these biopharmaceuticals.
Keywords: Biopharmaceuticals, CHO cells, Glycosylation, Apoptosis, Antibodies, Vaccines
Introduction
Since an earlier review on the production of biopharmaceuticals from animal cell culture (Butler 2005), there has been a steady increase in the number and demand for the production of this class of drugs for the treatment of human diseases. Biopharmaceuticals (including monoclonal antibodies, Mabs) outstrip all other sectors of the pharmaceutical industry. Global sales of biologics are now reported at US$120 billion per annum with an expected increase to US$150 billion by 2015 (Repligen 2011) and clearly outpace the growth of small molecule therapeutics. This economic success has been dominated by humanized Mabs produced from mammalian cell culture bioprocesses. Despite the global economic downturn, annual growth rate for Mabs is predicted at 9.5 % up to 2015 (Datamonitor 2010). They are the largest and fastest growing class of therapeutic pharmaceuticals with around 28 approved and around 350 in various stages of clinical trials (Reichert 2012). The rate of regulatory approval of Mabs is currently higher than that of small molecule therapeutics and this is likely to continue because of the areas of unmet medical need and lower competitive intensity of novel targets. The global sales of Mabs in 2011 were estimated at US$44.6 billion and are predicted to increase to US$58 billion by 2016 (BCC Research 2012). The domination of Mabs in this market is shown in Fig. 1 as 36 % of the total global value of biologics (Aggarwal 2011). There are presently six blockbuster therapeutic Mab products on the market (>US$1 billion annual sales): Avastin, Herceptin, Remicade, Rituxan, Humira, and Erbitux. In addition, the global value of the human vaccine market has been estimated at US$33 billion (Research and Markets 2011). Advances have been made in new approaches for some of these vaccines, particularly those such as influenza that are still routinely produced from eggs. New vaccines are being developed for some infectious diseases that include dengue fever, West Nile virus, and SARS. The present review seeks to identify recent pertinent issues that concern the production of these biopharmaceuticals from mammalian cell bioprocesses.
Fig. 1.
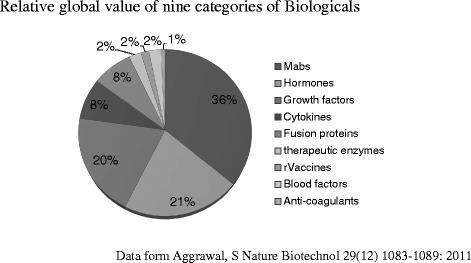
Relative global value of nine categories of biologicals
Cell line selection
Chinese hamster ovary (CHO) cells are the most widely used mammalian cell line for the industrial production of recombinant proteins. This is likely to continue as the cell line is very well characterized and has been used successfully for the production of a range of clinical biopharmaceuticals. Thus, for new bioproducts produced from this cell line, regulatory approval is likely to be more rapid than for a less well-known cell line. Nevertheless, there are a number of alternative cell types that are available. One notable example is the PER.C6 cell line which is derived from human embryonic retina cells transformed by adenovirus E1 (Havenga et al. 2008). There are two potentially important advantages of PER.C6 cells for the production of recombinant proteins. Firstly, they have been shown to produce very high cell densities approaching 108 cells/mL and titers of secreted protein levels of 8 g/L in fed-batch or 25 g/L in perfusion cultures (Kuczewski et al. 2011). Secondly, because they are human cells, the glycosylation profile of the resulting proteins is entirely of a human type. Thus, for example, erythropoietin produced from PER.C6 cells was shown to be free of glycolylneuraminic acid, whereas CHO cells have been shown to have a small but significant amount of Neu5Gc (Diaz et al. 2009). Another human cell line that has found extensive use for the rapid production of research grade recombinant proteins is the HEK293 which is derived from human embryonic kidney cells and transformed by adenovirus. The main use for these cells is in a platform for transient protein expression with a family of expression vectors containing an Epstein–Barr virus origin of replication (Pham et al. 2006). Transient gene expression can result in consistently high specific productivity (around 20 pg/cell/day). Although the perceived batch to batch variability has restricted the technique for adoption in large-scale commercial production, it has been shown that this can be reduced to reasonable limits (Nallet et al. 2012)
For virus production, there is a wider variety of cell types in use depending upon the susceptibility of the cell line to the targeted virus. Genzel and Reichl reviewed a range of continuous cell lines suitable for influenza vaccine production (Genzel and Reichl 2009). Although there are a number of “designer” continuous cell lines that can be grown in suspension with susceptibility to the virus, it is not clear which would be optimal for large-scale production. These cells include PER.C6, HEp-2, a suspension-adapted MDCK cell and cells from duck such as AGE1.CR and EB66. The effectiveness of these cell lines can of course vary depending upon the virus being produced (Houspie et al. 2012). Although all these cell lines are immortalized, it is now established that the tumorigenic risk of the final vaccine is assessed by the dose DNA content (Aunins 2000).
Genomic characteristics of CHO cells
The characterization of the CHO cell line and continued usage over several decades without any clear adverse effects have allowed regulatory approval of over 100 biopharmaceuticals. A “draft” genomic sequence was published recently showing 2.45 Gbases and 24,383 predicted genes (Xu et al. 2011). The advantage of high viral resistance of the CHO cells can be explained by the fact that most known viral entry genes are not expressed in the cells. The ability of the CHO cells to produce products that are suitable as human therapeutics can be explained by the presence of gene homologues for 99 % of human glycosylating enzymes. Although 141 of these genes are not expressed under normal exponential growth conditions, the final glycoprotein products are sufficiently similar to human forms to minimize unwanted immunogenicity.
This genomic sequence is clearly a major breakthrough in characterizing these cells for future genetic engineering in order to enhance their use for high yields of recombinant proteins. However, there is considerable genetic heterogeneity in subpopulations of the CHO cells in general use. The sequence published is that of the CHO-K1 line, which was originally derived by Puck in 1958 from a spontaneously immortalized population of fibroblast cells from cultured ovarian cells from a Chinese hamster (Puck et al. 1958). Subsequently, various subpopulations have been generated—the most notable being the CHO-DXB11/DUKX and CHO-DG44, both of which were independently derived by chemical mutagenesis to produce DHFR deficiency needed for cell selection and transgene amplification using methotrexate (Wurm and Hacker 2011). Early work showing the extent of genetic heterogeneity of the CHO cell line by Giemsa banding patterns indicated that of the 22 chromosomes in normal Chinese hamsters, only eight appeared cytogenetically similar in CHO with the remaining showing multiple deletions and translocations (Deaven and Petersen 1973).
Protein productivity
The titers of recombinant products obtainable from mammalian cell cultures have increased at least 20-fold over the last two decades (De Jesus and Wurm 2011; Lim et al. 2010). This is the result of two factors. Firstly, the ability to isolate high producer cell lines has improved by strategic gene amplification and cell isolation. This has meant that typical specific productivities (QP) of a decade ago of 10 pg/cell/day have now increased to around 50 pg/cell/day. Secondly, the more important factor in improving protein titers is the ability to develop strategic fed-batch protocols to provide minimal quantities of carbon and nitrogen substrates (such as glucose and glutamine) necessary to sustain cell viability to productivity. This enables an efficient cellular metabolism that minimizes the production of metabolic by-products (such as lactate and ammonia) and allows a cell population to be maintained at a high percentage of viability for an extended period of time. This means that a bioprocess that previously would reach a maximum cell yield of around a million cells per milliliter over a 7-day period can now be extended over a time period of around 21 days with a maximum cell density of 10 million cells per mL. The important factor from the standpoint of an enhanced protein titer is the integral of viable cell density (IVCD) over the entire bioprocess and before there is a significant loss of viability. This was previously called the viability index and is determined by integrating the cell growth curve. The overall titer of a bioprocess is a product of these two factors (QP⋅IVCD) and typical values of 100 mg/L obtainable previously have now increased to 1–5 g/L as a result of using improved cell lines in efficient fed-batch processes (De Jesus and Wurm 2011; Lim et al. 2010).
Cellular regulators of recombinant protein production
High cell producers secrete around 2,000 molecules/cell/s which for monoclonal antibodies corresponds to around 45 pg/cell/day. There have been a number of studies aimed at surveying the transcriptome and proteome profiles of such high producers to determine the critical factors that distinguish them from low producers (Doolan et al. 2011; Seth et al. 2007). From microarray profile analysis, clusters of highly expressed genes can be identified with an association to growth and productivity (Clarke et al. 2011). Network analysis indicates that there can be a number of phenotypic characteristic of high production but that there must be a threshold of competency in the series of cellular processes that lead to protein secretion (O'Callaghan et al. 2010). Correlation has been shown between specific antibody production and the measured cellular level of heavy chain messenger RNA in a range of cell lines, but above a certain saturation level of mRNA, the control may shift to posttranslational events (Barnes et al. 2007). This is consistent with findings where chaperones in the endoplasmic reticulum and cytoskeletal proteins show increased abundance with elevated QP (Dinnis et al. 2006). Specific elements of the secretory pathway have been investigated following the identification of X-box binding protein 1 (Ku et al. 2008) and activating transcription factor 4 (Ohya et al. 2008) as key regulators of an enhanced secretory pathway in cells. However, overexpression of these elements does not always result in enhanced cell-specific productivity and may depend on other factors related to the metabolic state of the cells (Ku et al. 2010). Thus, the identification of a key cellular regulator of recombinant protein production continues to prove elusive and is consistent with the previously expressed idea that a true limiting step may be a rare occurrence in biological networks (Fell 1998).
Glycosylation
There is considerable data to show that glycan variants of proteins affect biological activity. These can be analyzed by a variety of analytical methods (Butler and Perreault 2010), some of which have become high throughput (Ruhaak et al. 2010). Enhanced sialylation of recombinant erythropoietin by the introduction of two extra glycan sites results in a hyperglycosylated protein (darbepoetin) with a significantly greater serum half-life and considerable clinical advantages (Kiss et al. 2010). For immunoglobulin antibodies, it is clear that alterations of glycoforms occur under physiological and pathological conditions in vivo. The most prominent circulatory antibody is IgG, which has a conserved glycan site at Asn297 of the Fc region of the heavy chain. The normal profile of 30 or so glycans is subjected to changes during aging, pregnancy, or disease (Anthony et al. 2012). Decreased terminal galactosylation has been shown for patients with rheumatoid arthritis or several forms of autoimmune disease (Parekh et al. 1985). Differential sialylation has been shown to affect the inflammatory properties of IgG and has been proposed as a mechanism of a molecular switch to induce an anti-inflammatory condition (Kaneko et al. 2006). It would seem that these alterations in activity are related to the differential interaction of the Fc domain of the IgG with a series of Fcγ receptors. This interaction is modulated through variable glycan structures and is lost in aglycosylated IgG (Arnold et al. 2007).
The therapeutic activity of many monoclonal antibodies requires recruitment of the patients' immune system via antibody-dependent cell cytotoxicity (ADCC), which is initiated by an interaction of the Fc domain of Mab with an FcγRIII receptor present on the surface of NK cells and macrophages. This interaction is affected by the structure of the Fc glycan. A notable enhancement of activity occurs with the absence of fucose (Shields et al. 2002), which can be up to 100-fold in the complete absence of fucosylated glycans (Yamane-Ohnuki et al. 2004). This important finding has led to a considerable amount of activity to identify alternative methods for producing afucosylated glycoforms of Mabs. Targeted gene deletion of the α1,6-fucosyltransferase gene (FUT8) has been shown to be a particularly efficient method of removal of all fucosylation activity in CHO cells (Malphettes et al. 2010). The removal of fucose from the Mab can be achieved by genetic inactivation of the fucosyl transferase gene (FUT8) by RNA interference (Shen et al. 2007). There is also the possibility of introducing a specific glycosylation inhibitor such as the alkaloid, kifunensine, or sugar analogs into cell cultures to block the action of specific glycosylation enzyme I (Rillahan et al. 2012; Yu et al. 2011). These techniques lead to structures with low fucose which provide a significantly enhanced ADCC activity with minimal effect on the circulatory half-life of the resulting antibody (Zhou et al. 2008).
The extent of glycosylation of any secreted protein in culture is affected by a number of factors including the availability of carbohydrate substrates. This is a pertinent consideration when developing strategies for fed-batch cultures in which the substrate levels are maintained at a low set-point in order to minimize the accumulation of metabolic by-products. The danger is that during the cycle of nutrient feeding, the levels of substrate are reduced to concentrations that could cause a temporary limitation to glycosylation. In one example of this, it was shown that although low glutamine (<0.3 mM) and low glucose (<4 mM) feeding reduced the specific production of ammonia and lactate, lower levels of glutamine (<0.1 mM) and glucose (<0.7 mM) decreased sialylation and led to high mannose structures of secreted gamma interferon (Chee Furng Wong et al. 2005). In subsequent analysis of varying levels of glycosylation gene expression during the progress of a fed-batch culture, the same group demonstrated the need to maintain a threshold of 0.5 mM glutamine in order to ensure consistency of glycosylation gene expression and comparable quality of secreted recombinant glycoprotein (Wong et al. 2010).
Although monoclonal antibodies have now been used extensively as therapeutics, a concern has arisen over hypersensitive reactions by subjects treated with cetuximab, which is a chimeric mouse–human IgG1 against EGFR (Chung et al. 2008). The antibody is produced from SP2/0 murine myeloma cells and contains a Fab-linked glycan that may contain the gal-α1-3-gal antigen. Curiously, the recorded instances of anaphylactic reactions to treatment of the antibody appear to be confined to certain specific states in the USA. One plausible hypothesis is that this region corresponds to the habitat of the tick, Amblyomma americanum, which is a vector for Rocky Mountain fever and results in production of IgE antibodies to the gal-α1-3-gal immunogen. This leaves subjects in danger of excessive exposure to the immunogen and resulting in a hypersensitive reaction (Commins et al. 2011). CHO cells have been thought to be free of the enzyme (N-acetyllactosaminide 3-α-glactosyltransferase) responsible for addition of the antigen to terminal glycans. However, a recent report shows that this is not the case and that even CHO cells have some capability of synthesizing the antigen (Bosques et al. 2010).
Chemically defined media
Although bovine serum was used as a universal supplement for mammalian cell culture medium at one time, it is now not so popular because of the perceived danger of contaminating product biopharmaceuticals with viruses and prions from bovine sources. This has now been extended further with a drive to develop media free of any components of animal origin. Thus, the peptide hydrolysates of plant and microbial origin used widely in the food industry have now become valuable sources of non-animal components to promote mammalian cell growth. Often these have been supplemented to cultures as optimal blends from various sources such as soy, wheat gluten, and yeast designed by statistical design of experiment protocols (Kim and Lee 2009). There has been some concerns over the batch-to-batch variation in the content of these hydrolysates. This can be improved by methods using cocktails of degradative enzymes that ensure a high degree of hydrolysis of the source material followed by ultrafiltration (Siemensma et al. 2010) in an attempt to remove the unwanted high molecular weight components (Chun et al. 2007). The potential lot-to-lot variability of these or other chemically undefined components can be screened by various methods including near-infrared and 2D-fluorescence spectroscopy (Jose et al. 2011).
The chemical identity of the bioactive components in these hydrolysates has been sought after but proves elusive. The consensus is that there may be a combination of nutritive components and growth factors in these hydrolysates (Kim et al. 2011). There are clear advantages in being able to identify the bioactive components because they then could be included in a completely chemically defined media which potentially could be manufactured from pure ingredients to a consistent standard. Although animal component-free, chemically defined culture media formulations do exist, many have inferior growth-promoting qualities.
Programmed cell death
The identification, delay, or elimination of programmed cell death (PCD) has been an important area for increasing productivities in animal cell culture. Since 1990, when apoptosis (programmed cell death type I) was identified in hybridoma cultures (al-Rubeai and Emery 1990), this type of PCD was regarded as the main type of death that takes place in cell cultures under stress variations such as changes in pH, oxygen, osmolarity, and nutrient deficiency (Zhu et al. 2008). To date, there have been three different cellular pathways identified and referred to as apoptosis (extrinsic, intrinsic, and endoplasmic reticulum related). All are characterized by the dependence of ATP; the participation of different initiator and executing caspases as well as other molecules which are interconnected by a biochemical cascade to promote the activation of “suicide” genes such as p53 or Rb and typical morphological aspects such as cell shrinkage, chromatin condensation, and formation of apoptotic bodies among others (Jin and El-Deiry 2005). Many efforts have been made to delay or avoid such a process in mammalian cultures. The most successful involves the overexpression of genes related to the Bcl-2 family (Carlage et al. 2012), which have been demonstrated to increase the productivity of different cell lines without loss of product quality (Majid et al. 2007). The robustness of Bcl-2 transfected cells is also enhanced as evidenced by increased resistance to changes in pH, high concentrations of ammonium and lactate (Dorai et al. 2009), and hyperosmotic media (Han et al. 2011a; Krampe and Al-Rubeai 2010).
Progress in molecular and cellular biology has allowed the identification of other PCD types such as autophagy (cell death type II) (Gozuacik and Kimchi 2007; Jardon et al. 2012). This process was initially described as a cellular survival mode and is responsible for organelle replacement. However, to date, it has been found that macroautophagy can also be triggered as a PCD by different stimuli, mainly the lack of nutrients (Hwang and Lee 2008). This process is directed by complex molecular pathways that involve at least 30 different proteins (Atg's) and characterized morphologically by the formation of autophagosomes (Gump and Thorburn 2011). It has been observed that this process can occur simultaneously with apoptosis; participate in an opposite way (survival promoting cell) or independently of it, so that its identification is important in attempts to prolong cell viability in culture. New cell survival strategies are being established through the manipulation of genes related to autophagosome formation such as the manipulation of participant proteins such as mTOR, beclin, some Atg proteins, as well as Bcl-x(L) (Han et al. 2011a, b).
Finally, necrotic death has been considered a passive event (cell death type III) independent of ATP in which a severe insult could affect the integrity of the plasma membrane by accident. However, recently, it has been discovered that some cell lines present a cell death type that shares characteristics of apoptosis and necrosis; such PCD has been designated “necroptosis” (Dunai et al. 2011) or programmed necrosis. This type of death appears to be related to pathological cellular processes and is dependent on caspase-independent receptors. Its transduction pathway is related to the protein kinases RIP1 and the RIP3 (Wu et al. 2012) and involves Fas/tumor necrosis factor-α death domain receptor activation and inhibition of receptor-interacting protein I kinase (Smith and Yellon 2011). Even though necroptosis has only been observed in some diseases associated with viral infections, it is possible that the viral vaccine production could be optimized by the study of such a PCD and by the manipulation of the necrostatins. These new observations related to the different patterns of PCD suggest that the concept of cell viability should be reconsidered with appropriate analytical techniques implemented to monitor the status of the cell population during a bioprocess (Browne and Al-Rubeai 2011).
Disposable bioreactors
Simple and scalable bioreactor operations as well as decreased downtimes are required to improve bioprocesses for obtaining high productivity in the generation of biopharmaceuticals. Traditional stainless steel bioreactors require validated cleaning and sterilization protocols involving the consumption of high quality steam and water. These protocols can be avoided by the use of disposable bioreactors which have now become widely used in mammalian cell bioprocesses (Singh 1999). Such systems include the use of disposable sterile bags or bottles, with agitation that promotes the homogenization of cells, nutrients, and gases mainly by a rocking device. As in other bioreactors, it is possible to control culture parameters such as pH, DOT, temperature, etc. The versatile size offered by such systems range from 1 mL up to 2,000 L at industrial scale (Fontova et al. 2006). These types of bioreactors have been used to promote the cultivation of various cells including bacteria, yeast, plant cells (Girard et al. 2006), insect cells (Kadwell and Hardwicke 2007), and various mammalian cells (Hahnel et al. 2011; Yuk et al. 2011). In the case of mammalian cells, the application ranges from biomass generation, production of veterinary and human vaccines (Genzel et al. 2010), the generation of recombinant proteins by transient expression (Stettler et al. 2007) or stable production, gene therapy vectors, stem cells, and the generation of organs and tissues (Eibl and Eibl 2009). These types of bioreactors have also proved to be very efficient in the production of cells in suspension as well as in the propagation and cultivation on microcarriers (Singh 1999).
The versatility and ease of operation make such disposable bioreactors a promising tool in the improvement of bioprocesses. The most relevant parameters in wave bioreactors are liquid surface level, liquid velocity, bulk shear stress, and wall shear stress. A study of such parameters has been presented to optimize the performance of the mammalian cultures (Kalmbach et al. 2011). In addition, different mixing devices are used to avoid oxygen limitations such as rocking, stirred, paddle agitation, orbital shakers, and rotating sparging (Oosterhuis and Van den Berg 2011). It has been demonstrated that productivities in this type of bioreactor can be comparable and even better than for conventional stirred tanks, making them a viable option to support the production of commercial cell lines such as CHO cells for various applications in an environment with low shear stress (Stettler et al. 2007; Yuk et al. 2011). Table 1 presents a list of advantages and disadvantages of this type of bioreactor.
Table 1.
Advantages and disadvantages of disposable bioreactors
| Advantages | Disadvantages |
|---|---|
| Low shear stress | Integrity of the bags |
| Disposability of the PET bags after a single use | Possible contamination in the bags |
| Use for suspension or microcarrier systems | Volumes up to 2,000 L |
| Eliminates time- and energy-consuming sterilization process | Gradients of oxygen, pH, temperature, and nutrients at high volumes |
| Avoids cleaning validation studies: not cross-contamination | Some limitations in the control of parameters |
| Lower plant investment than in the case of stainless steel or glass bioreactors | It is necessary to use an adequate mixing device to increase the k La value |
| Uses: production of human and veterinary vaccines, Mabs, proteins, and gene therapy vectors | Perfusion systems are less explored |
Downstream process
A purification process for biopharmaceuticals must ensure that contaminants from different sources such as the bioreactor as well as from the cell are removed from the final product to obtain the desired product quality and satisfy the regulatory requirements. The performance of a downstream process could be improved by a reduced number of stages of purification or the implementation of a single-use system (Laukel et al. 2011). Currently, strategies based on the quality by design, design of experiments (DoE), and process analytical technology have led to increased yields and a better understanding of such processes to allow their optimization (Pieracci et al. 2010; Treier et al. 2012). Different unit operations such as centrifugation, filtration, precipitation, and chromatography may be considered to exploit the physicochemical features of the compound of interest to enable the separation from its impurities. Various types of chromatography (hydrophobic, ion exchange, and affinity) are used in the purification of biopharmaceuticals from cultures to enable the high product purity required (>95 %) from the bioprocess harvest which may yield up to 10 g/L (Pa and Mm 2012). In the specific case of monoclonal antibodies, the consistent physicochemical characteristics enable the use of a platform technology which typically incorporates affinity chromatography with the use of protein A as a resin purification system. This has been the predominant method during the last decade (Liu et al. 2010). This resin promotes binding affinity of the Fc fragment of the antibody. The elution process is easy and rapid and it has been shown that the characteristic activity of the protein is not affected (opsonization capabilities, neutralizing effect, antigen binding, etc.). While this resin (matrix) provides an excellent performance in purity (over 90 %), one of the limitations is its high cost, which in turn is reflected in the cost of the final formulated product.
As there have been substantial gains in the final concentration of product obtainable by cell culture, so the proportional cost of downstream processing has crept upward. This has prompted a search for alternative and cheaper methods of purification. An alternative to protein A affinity is mixed mode chromatography (anion exchange and hydrophobic interaction) that can be optimized by DoE and provide similar yields to those obtained with the protein A but at a lower cost (Toueille et al. 2011). Such methods, along with the use of different types of chromatographic matrix (Chen et al. 2008, 2010), are alternative strategies to obtain high purity Mabs (Conley et al. 2011). The use of other unit operations such as precipitation with various compounds such as polyamines can also help to obtain an acceptable product quality (Liu et al. 2010; Ma et al. 2010). In addition, specific unit operations of chemical molecules, such as crystallization, are starting to be considered as potential operations to reduce costs because they have demonstrated a purity of Mab >90 %. However, there are still some limitations in their performance that avoid its scale-up and use in purification trains (Zang et al. 2011).
Future prospective
The development and demand for biopharmaceuticals are likely to continue in the near future because of their application to unmet medical needs. For monoclonal antibodies, the largest group of such therapeutics, there is likely to be a need for a diversity of structural forms depending upon the mode of clinical action (Reichert 2012). In some cases, the mode of action requires neutralization of a targeted antigen, and in these cases, it may be desirable to use antibody fragments. Full size antibodies are important when effector function is required in the clinical activity. For this, there may be molecular designs, such as the human-camelid fusion antibodies, that have the advantage of small size and therefore enhanced tumor penetration (Bell et al. 2010). A further prospect is the selection of patients to be treated with Mab-based immunotherapies because the success of treatment is dependent on the presence of a specific targeted antigen and also the genetic predisposition of the patient (Campoli et al. 2010). These factors are likely to have a future effect on the levels of individual biopharmaceuticals required for clinical use but the increasing number of regulatory approved biotherapeutics is likely to maintain the need for a high production capacity from mammalian cell bioprocesses.
Acknowledgments
The Natural Science and Engineering Council of Canada is gratefully acknowledged for the financial support for the study of antibody glycosylation through MabNet and a Discovery grant.
Contributor Information
M. Butler, Email: butler@cc.umanitoba.ca
A. Meneses-Acosta, Email: angelica_meneses@uaem.mx
References
- Aggarwal S. What's fueling the biotech engine—2010 to 2011. Nat Biotechnol. 2011;29(12):1083–1089. doi: 10.1038/nbt.2060. [DOI] [PubMed] [Google Scholar]
- Al-Rubeai M, Emery AN. Mechanisms and kinetics of monoclonal antibody synthesis and secretion in synchronous and asynchronous hybridoma cell cultures. J Biotechnol. 1990;16(1–2):67–85. doi: 10.1016/0168-1656(90)90066-K. [DOI] [PubMed] [Google Scholar]
- Anthony RM, Wermeling F, Ravetch JV (2012) Novel roles for the IgG Fc glycan. Ann N Y Acad Sci. doi:10.1111/j.1749-6632.2011.06305.x [DOI] [PubMed]
- Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- Aunins J. Viral vaccine production in cell culture. In: Spier R, editor. Encyclopedia of cell technology. New York: Wiley; 2000. [Google Scholar]
- Barnes LM, Bentley CM, Moy N, Dickson AJ. Molecular analysis of successful cell line selection in transfected GS-NS0 myeloma cells. Biotechnol Bioeng. 2007;96(2):337–348. doi: 10.1002/bit.21119. [DOI] [PubMed] [Google Scholar]
- Bell A, Wang ZJ, Arbabi-Ghahroudi M, Chang TA, Durocher Y, Trojahn U, Baardsnes J, Jaramillo ML, Li S, Baral TN, O’Connor-McCourt M, Mackenzie R, Zhang J. Differential tumor-targeting abilities of three single-domain antibody formats. Cancer Lett. 2010;289(1):81–90. doi: 10.1016/j.canlet.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Bosques CJ, Collins BE, Meador JW, 3rd, Sarvaiya H, Murphy JL, Dellorusso G, Bulik DA, Hsu IH, Washburn N, Sipsey SF, Myette JR, Raman R, Shriver Z, Sasisekharan R, Venkataraman G. Chinese hamster ovary cells can produce galactose-alpha-1,3-galactose antigens on proteins. Nat Biotechnol. 2010;28(11):1153–1156. doi: 10.1038/nbt1110-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne SM, Al-Rubeai M. Defining viability in mammalian cell cultures. Biotechnol Lett. 2011;33(9):1745–1749. doi: 10.1007/s10529-011-0644-2. [DOI] [PubMed] [Google Scholar]
- Butler M. Animal cell cultures: recent achievements and perspectives in the production of biopharmaceuticals. Appl Microbiol Biotechnol. 2005;68(3):283–291. doi: 10.1007/s00253-005-1980-8. [DOI] [PubMed] [Google Scholar]
- Butler M, Perreault H. Approaches and methods for determining glycosylation. In: Flickinger M, editor. Encyclopedia of industrial biotechnology, bioprocess, bioseparation, and cell technology. New York: Wiley; 2010. [Google Scholar]
- Campoli M, Ferris R, Ferrone S, Wang X. Immunotherapy of malignant disease with tumor antigen-specific monoclonal antibodies. Clin Cancer Res: Off J Am Assoc Cancer Res. 2010;16(1):11–20. doi: 10.1158/1078-0432.CCR-09-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlage T, Kshirsagar R, Zang L, Janakiraman V, Hincapie M, Lyubarskaya Y, Weiskopf A, Hancock WS. Analysis of dynamic changes in the proteome of a Bcl-XL overexpressing Chinese hamster ovary cell culture during exponential and stationary phases. Biotechnol Prog. 2012;28(3):814–823. doi: 10.1002/btpr.1534. [DOI] [PubMed] [Google Scholar]
- Chee Furng Wong D, Tin Kam Wong K, Tang Goh L, Kiat Heng C, Gek Sim Yap M. Impact of dynamic online fed-batch strategies on metabolism, productivity and N-glycosylation quality in CHO cell cultures. Biotechnol Bioeng. 2005;89(2):164–177. doi: 10.1002/bit.20317. [DOI] [PubMed] [Google Scholar]
- Chen J, Tetrault J, Ley A. Comparison of standard and new generation hydrophobic interaction chromatography resins in the monoclonal antibody purification process. J Chromatogr A. 2008;1177(2):272–281. doi: 10.1016/j.chroma.2007.07.083. [DOI] [PubMed] [Google Scholar]
- Chen J, Tetrault J, Zhang Y, Wasserman A, Conley G, Dileo M, Haimes E, Nixon AE, Ley A. The distinctive separation attributes of mixed-mode resins and their application in monoclonal antibody downstream purification process. J Chromatogr A. 2010;1217(2):216–224. doi: 10.1016/j.chroma.2009.09.047. [DOI] [PubMed] [Google Scholar]
- Chun BH, Kim JH, Lee HJ, Chung N. Usability of size-excluded fractions of soy protein hydrolysates for growth and viability of Chinese hamster ovary cells in protein-free suspension culture. Bioresour Technol. 2007;98(5):1000–1005. doi: 10.1016/j.biortech.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D, Slebos RJ, Zhou Q, Gold D, Hatley T, Hicklin DJ, Platts-Mills TA. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–1117. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke C, Doolan P, Barron N, Meleady P, O’Sullivan F, Gammell P, Melville M, Leonard M, Clynes M. Large scale microarray profiling and coexpression network analysis of CHO cells identifies transcriptional modules associated with growth and productivity. J Biotechnol. 2011;155(3):350–359. doi: 10.1016/j.jbiotec.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, Kocan KM, Fahy JV, Nganga LW, Ronmark E, Cooper PJ, Platts-Mills TA. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2011;127(5):1286.e6–1293.e6. doi: 10.1016/j.jaci.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley GP, Viswanathan M, Hou Y, Rank DL, Lindberg AP, Cramer SM, Ladner RC, Nixon AE, Chen J. Evaluation of protein engineering and process optimization approaches to enhance antibody drug manufacturability. Biotechnol Bioeng. 2011;108(11):2634–2644. doi: 10.1002/bit.23220. [DOI] [PubMed] [Google Scholar]
- Datamonitor (2010) Monoclonal antibodies; 2010
- De Jesus M, Wurm FM. Manufacturing recombinant proteins in kg-ton quantities using animal cells in bioreactors. Eur J Pharm Biopharm. 2011;78(2):184–188. doi: 10.1016/j.ejpb.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Deaven LL, Petersen DF. The chromosomes of CHO, an aneuploid Chinese hamster cell line: G-band, C-band, and autoradiographic analyses. Chromosoma. 1973;41(2):129–144. doi: 10.1007/BF00319690. [DOI] [PubMed] [Google Scholar]
- Diaz SL, Padler-Karavani V, Ghaderi D, Hurtado-Ziola N, Yu H, Chen X, Brinkman-Van der Linden EC, Varki A, Varki NM. Sensitive and specific detection of the non-human sialic acid N-glycolylneuraminic acid in human tissues and biotherapeutic products. PLoS One. 2009;4(1):e4241. doi: 10.1371/journal.pone.0004241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnis DM, Stansfield SH, Schlatter S, Smales CM, Alete D, Birch JR, Racher AJ, Marshall CT, Nielsen LK, James DC. Functional proteomic analysis of GS-NS0 murine myeloma cell lines with varying recombinant monoclonal antibody production rate. Biotechnol Bioeng. 2006;94(5):830–841. doi: 10.1002/bit.20899. [DOI] [PubMed] [Google Scholar]
- Doolan P, Barron N, Kinsella P, Clarke C, Meleady P, O’Sullivan F, Melville M, Leonard M, Clynes M (2011) Microarray expression profiling identifies genes regulating sustained cell specific productivity (S-Qp) in CHO K1 production cell lines. Biotechnol J. doi:10.1002/biot.201100255 [DOI] [PubMed]
- Dorai H, Kyung YS, Ellis D, Kinney C, Lin C, Jan D, Moore G, Betenbaugh MJ. Expression of anti-apoptosis genes alters lactate metabolism of Chinese hamster ovary cells in culture. Biotechnol Bioeng. 2009;103(3):592–608. doi: 10.1002/bit.22269. [DOI] [PubMed] [Google Scholar]
- Dunai Z, Bauer PI, Mihalik R. Necroptosis: biochemical, physiological and pathological aspects. Pathol Oncol Res: POR. 2011;17(4):791–800. doi: 10.1007/s12253-011-9433-4. [DOI] [PubMed] [Google Scholar]
- Eibl R, Eibl D. Application of disposable bag bioreactors in tissue engineering and for the production of therapeutic agents. Adv Biochem Eng Biotechnol. 2009;112:183–207. doi: 10.1007/978-3-540-69357-4_8. [DOI] [PubMed] [Google Scholar]
- Fell DA. Increasing the flux in metabolic pathways: a metabolic control analysis perspective. Biotechnol Bioeng. 1998;58(2–3):121–124. doi: 10.1002/(SICI)1097-0290(19980420)58:2/3<121::AID-BIT2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Fontova A, Soley A, Galvez J, Sarro E, Lecina M, Rosell J, Riu P, Cairo J, Godia F, Bragos R (2006) Multiple automated minibioreactor system for multifunctional screening in biotechnology. Conference proceedings. Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference, 1:632–635. doi: 10.1109/IEMBS.2006.260628 [DOI] [PubMed]
- Genzel Y, Reichl U. Continuous cell lines as a production system for influenza vaccines. Expert Rev Vaccines. 2009;8(12):1681–1692. doi: 10.1586/erv.09.128. [DOI] [PubMed] [Google Scholar]
- Genzel Y, Dietzsch C, Rapp E, Schwarzer J, Reichl U. MDCK and Vero cells for influenza virus vaccine production: a one-to-one comparison up to lab-scale bioreactor cultivation. Appl Microbiol Biotechnol. 2010;88(2):461–475. doi: 10.1007/s00253-010-2742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard LS, Fabis MJ, Bastin M, Courtois D, Petiard V, Koprowski H. Expression of a human anti-rabies virus monoclonal antibody in tobacco cell culture. Biochem Biophys Res Commun. 2006;345(2):602–607. doi: 10.1016/j.bbrc.2006.03.219. [DOI] [PubMed] [Google Scholar]
- Gozuacik D, Kimchi A. Autophagy and cell death. Curr Top Dev Biol. 2007;78:217–245. doi: 10.1016/S0070-2153(06)78006-1. [DOI] [PubMed] [Google Scholar]
- Gump JM, Thorburn A. Autophagy and apoptosis: what is the connection? Trends Cell Biol. 2011;21(7):387–392. doi: 10.1016/j.tcb.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnel A, Putz B, Iding K, Niediek T, Gudermann F, Lutkemeyer D. Evaluation of a disposable stirred tank bioreactor for cultivation of mammalian cells. BMC Proceed. 2011;5(Suppl 8):54. doi: 10.1186/1753-6561-5-S8-P54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YK, Ha TK, Kim YG, Lee GM. Bcl-x(L) overexpression delays the onset of autophagy and apoptosis in hyperosmotic recombinant Chinese hamster ovary cell cultures. J Biotechnol. 2011;156(1):52–55. doi: 10.1016/j.jbiotec.2011.07.032. [DOI] [PubMed] [Google Scholar]
- Han YK, Ha TK, Lee SJ, Lee JS, Lee GM. Autophagy and apoptosis of recombinant Chinese hamster ovary cells during fed-batch culture: effect of nutrient supplementation. Biotechnol Bioeng. 2011;108(9):2182–2192. doi: 10.1002/bit.23165. [DOI] [PubMed] [Google Scholar]
- Havenga MJ, Holterman L, Melis I, Smits S, Kaspers J, Heemskerk E, van der Vlugt R, Koldijk M, Schouten GJ, Hateboer G, Brouwer K, Vogels R, Goudsmit J. Serum-free transient protein production system based on adenoviral vector and PER.C6 technology: high yield and preserved bioactivity. Biotechnol Bioeng. 2008;100(2):273–283. doi: 10.1002/bit.21757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houspie L, Keyaerts E, Maes P, Van Ranst M. Susceptibility of the PER.C6 cell line for infection with clinical human respiratory syncytial virus isolates. J Virol Methods. 2012;181(1):37–42. doi: 10.1016/j.jviromet.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Hwang SO, Lee GM. Nutrient deprivation induces autophagy as well as apoptosis in Chinese hamster ovary cell culture. Biotechnol Bioeng. 2008;99(3):678–685. doi: 10.1002/bit.21589. [DOI] [PubMed] [Google Scholar]
- Jardon MA, Sattha B, Braasch K, Leung AO, Cote HC, Butler M, Gorski SM, Piret JM. Inhibition of glutamine-dependent autophagy increases t-PA production in CHO cell fed-batch processes. Biotechnol Bioeng. 2012;109(5):1228–1238. doi: 10.1002/bit.24393. [DOI] [PubMed] [Google Scholar]
- Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4(2):139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- Jose GE, Folque F, Menezes JC, Werz S, Strauss U, Hakemeyer C. Predicting Mab product yields from cultivation media components, using near-infrared and 2D-fluorescence spectroscopies. Biotechnol Prog. 2011;27(5):1339–1346. doi: 10.1002/btpr.638. [DOI] [PubMed] [Google Scholar]
- Kadwell SH, Hardwicke PI. Production of baculovirus-expressed recombinant proteins in wave bioreactors. Methods Mol Biol. 2007;388:247–266. doi: 10.1007/978-1-59745-457-5_12. [DOI] [PubMed] [Google Scholar]
- Kalmbach A, Bordas R, Oncul AA, Thevenin D, Genzel Y, Reichl U. Experimental characterization of flow conditions in 2- and 20-L bioreactors with wave-induced motion. Biotechnol Prog. 2011;27(2):402–409. doi: 10.1002/btpr.516. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313(5787):670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- Kim SH, Lee GM. Development of serum-free medium supplemented with hydrolysates for the production of therapeutic antibodies in CHO cell cultures using design of experiments. Appl Microbiol Biotechnol. 2009;83(4):639–648. doi: 10.1007/s00253-009-1903-1. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim YG, Han YK, Choi HS, Kim YH, Lee GM. Proteomic understanding of intracellular responses of recombinant Chinese hamster ovary cells cultivated in serum-free medium supplemented with hydrolysates. Appl Microbiol Biotechnol. 2011;89(6):1917–1928. doi: 10.1007/s00253-011-3106-9. [DOI] [PubMed] [Google Scholar]
- Kiss Z, Elliott S, Jedynasty K, Tesar V, Szegedi J. Discovery and basic pharmacology of erythropoiesis-stimulating agents (ESAs), including the hyperglycosylated ESA, darbepoetin alfa: an update of the rationale and clinical impact. Eur J Clin Pharmacol. 2010;66(4):331–340. doi: 10.1007/s00228-009-0780-y. [DOI] [PubMed] [Google Scholar]
- Krampe B, Al-Rubeai M. Cell death in mammalian cell culture: molecular mechanisms and cell line engineering strategies. Cytotechnology. 2010;62(3):175–188. doi: 10.1007/s10616-010-9274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku SC, Ng DT, Yap MG, Chao SH. Effects of overexpression of X-box binding protein 1 on recombinant protein production in Chinese hamster ovary and NS0 myeloma cells. Biotechnol Bioeng. 2008;99(1):155–164. doi: 10.1002/bit.21562. [DOI] [PubMed] [Google Scholar]
- Ku SC, Toh PC, Lee YY, Chusainow J, Yap MG, Chao SH. Regulation of XBP-1 signaling during transient and stable recombinant protein production in CHO cells. Biotechnol Prog. 2010;26(2):517–526. doi: 10.1002/btpr.322. [DOI] [PubMed] [Google Scholar]
- Kuczewski M, Schirmer E, Lain B, Zarbis-Papastoitsis G. A single-use purification process for the production of a monoclonal antibody produced in a PER.C6 human cell line. Biotechnol J. 2011;6(1):56–65. doi: 10.1002/biot.201000292. [DOI] [PubMed] [Google Scholar]
- Laukel M, Rogge P, Dudziak G. Disposable downstream processing for clinical manufacturing. Bioprocess International. 2011;9(S2):14–21. [Google Scholar]
- Lim Y, Wong NS, Lee YY, Ku SC, Wong DC, Yap MG. Engineering mammalian cells in bioprocessing—current achievements and future perspectives. Biotechnol Appl Biochem. 2010;55(4):175–189. doi: 10.1042/BA20090363. [DOI] [PubMed] [Google Scholar]
- Liu HF, Ma J, Winter C, Bayer R. Recovery and purification process development for monoclonal antibody production. MAbs. 2010;2(5):480–499. doi: 10.4161/mabs.2.5.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Hoang H, Myint T, Peram T, Fahrner R, Chou JH. Using precipitation by polyamines as an alternative to chromatographic separation in antibody purification processes. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(9–10):798–806. doi: 10.1016/j.jchromb.2010.01.044. [DOI] [PubMed] [Google Scholar]
- Majid FA, Butler M, Al-Rubeai M. Glycosylation of an immunoglobulin produced from a murine hybridoma cell line: the effect of culture mode and the anti-apoptotic gene, bcl-2. Biotechnol Bioeng. 2007;97(1):156–169. doi: 10.1002/bit.21207. [DOI] [PubMed] [Google Scholar]
- Malphettes L, Freyvert Y, Chang J, Liu PQ, Chan E, Miller JC, Zhou Z, Nguyen T, Tsai C, Snowden AW, Collingwood TN, Gregory PD, Cost GJ. Highly efficient deletion of FUT8 in CHO cell lines using zinc-finger nucleases yields cells that produce completely nonfucosylated antibodies. Biotechnol Bioeng. 2010;106(5):774–783. doi: 10.1002/bit.22751. [DOI] [PubMed] [Google Scholar]
- Research and Markets (2011) Global vaccine market forecast to 2013
- Nallet S, Fornelli L, Schmitt S, Parra J, Baldi L, Tsybin YO, Wurm FM (2012) Glycan variability on a recombinant IgG antibody transiently produced in HEK-293E cells. New Biotechnol. doi:10.1016/j.nbt.2012.02.003 [DOI] [PubMed]
- O’Callaghan PM, McLeod J, Pybus LP, Lovelady CS, Wilkinson SJ, Racher AJ, Porter A, James DC. Cell line-specific control of recombinant monoclonal antibody production by CHO cells. Biotechnol Bioeng. 2010;106(6):938–951. doi: 10.1002/bit.22769. [DOI] [PubMed] [Google Scholar]
- Ohya T, Hayashi T, Kiyama E, Nishii H, Miki H, Kobayashi K, Honda K, Omasa T, Ohtake H. Improved production of recombinant human antithrombin III in Chinese hamster ovary cells by ATF4 overexpression. Biotechnol Bioeng. 2008;100(2):317–324. doi: 10.1002/bit.21758. [DOI] [PubMed] [Google Scholar]
- Oosterhuis N, Van den Berg H. How multipurpose is a disposable bioreactor? Biopharm Int. 2011;24(3):51–56. [Google Scholar]
- Pa MG, Mm A (2012) State of the art in downstream processing of monoclonal antibodies: process trends in design and validation. Biotechnol Prog. doi:10.1002/btpr.1567 [DOI] [PubMed]
- Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T, Taniguchi T, Matsuta K, Takeuchi F, Nagano Y, Miyamoto T, Kobata A. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316(6027):452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- Pham PL, Kamen A, Durocher Y. Large-scale transfection of mammalian cells for the fast production of recombinant protein. Mol Biotechnol. 2006;34(2):225–237. doi: 10.1385/MB:34:2:225. [DOI] [PubMed] [Google Scholar]
- Pieracci J, Perry L, Conley L. Using partition designs to enhance purification process understanding. Biotechnol Bioeng. 2010;107(5):814–824. doi: 10.1002/bit.22866. [DOI] [PubMed] [Google Scholar]
- Puck TT, Cieciura SJ, Robinson A. Genetics of somatic mammalian cells. III. Long-term cultivation of euploid cells from human and animal subjects. J Exper Med. 1958;108(6):945–956. doi: 10.1084/jem.108.6.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert JM. Which are the antibodies to watch in 2012? MAbs. 2012;4(1):1–3. doi: 10.4161/mabs.4.1.18719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repligen (2011) Annual Report. www.repligen.com. Accessed August 2012
- BCC Research (2012) Antibody drugs: technologies and global markets. Biotechnology
- Rillahan CD, Antonopoulos A, Lefort CT, Sonon R, Azadi P, Ley K, Dell A, Haslam SM, Paulson JC (2012) Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat Chem Biol. doi:10.1038/nchembio.999 [DOI] [PMC free article] [PubMed]
- Ruhaak LR, Hennig R, Huhn C, Borowiak M, Dolhain RJ, Deelder AM, Rapp E, Wuhrer M. Optimized workflow for preparation of APTS-labeled N-glycans allowing high-throughput analysis of human plasma glycomes using 48-channel multiplexed CGE-LIF. J Proteome Res. 2010;9(12):6655–6664. doi: 10.1021/pr100802f. [DOI] [PubMed] [Google Scholar]
- Seth G, Philp RJ, Lau A, Jiun KY, Yap M, Hu WS. Molecular portrait of high productivity in recombinant NS0 cells. Biotechnol Bioeng. 2007;97(4):933–951. doi: 10.1002/bit.21234. [DOI] [PubMed] [Google Scholar]
- Shen A, Ng D, Joly J, Snedecor B, Lu Y, Meng G, Nakamura G, Krummen L. Metabolic engineering to control glycosylation. In: Butler M, editor. Cell culture and upstream processing. Oxford: Bios Scientific; 2007. [Google Scholar]
- Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, Weikert SH, Presta LG. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277(30):26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- Siemensma A, Babcock J, Wilcox C, Huttinga H. Towards an understanding of how protein hydrolysates stimulate more efficient biosynthesis in cultured cells. In: Pasupuleti VK, Demain AL, editors. Protein hydrolysates in biotechnology. Dordrecht: Springer; 2010. pp. 33–54. [Google Scholar]
- Singh V. Disposable bioreactor for cell culture using wave-induced agitation. Cytotechnology. 1999;30(1–3):149–158. doi: 10.1023/A:1008025016272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smelko JP, Wiltberger KR, Hickman EF, Morris BJ, Blackburn TJ, Ryll T. Performance of high intensity fed-batch mammalian cell cultures in disposable bioreactor systems. Biotechnol Prog. 2011;27(5):1358–1364. doi: 10.1002/btpr.634. [DOI] [PubMed] [Google Scholar]
- Smith CC, Yellon DM. Necroptosis, necrostatins and tissue injury. J Cell Mol Med. 2011;15(9):1797–1806. doi: 10.1111/j.1582-4934.2011.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler M, Zhang X, Hacker DL, De Jesus M, Wurm FM. Novel orbital shake bioreactors for transient production of CHO derived IgGs. Biotechnol Prog. 2007;23(6):1340–1346. doi: 10.1021/bp070219i. [DOI] [PubMed] [Google Scholar]
- Toueille M, Uzel A, Depoisier JF, Gantier R. Designing new monoclonal antibody purification processes using mixed-mode chromatography sorbents. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(13–14):836–843. doi: 10.1016/j.jchromb.2011.02.047. [DOI] [PubMed] [Google Scholar]
- Treier K, Berg A, Diederich P, Lang K, Osberghaus A, Dismer F, Hubbuch J (2012) Examination of a genetic algorithm for the application in high-throughput downstream process development. Biotechnol J. doi:10.1002/biot.201200145 [DOI] [PubMed]
- Wong DC, Wong NS, Goh JS, May LM, Yap MG. Profiling of N-glycosylation gene expression in CHO cell fed-batch cultures. Biotechnol Bioeng. 2010;107(3):516–528. doi: 10.1002/bit.22828. [DOI] [PubMed] [Google Scholar]
- Wu W, Liu P, Li J. Necroptosis: an emerging form of programmed cell death. Crit Rev Oncol Hematol. 2012;82(3):249–258. doi: 10.1016/j.critrevonc.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Wurm FM, Hacker D. First CHO genome. Nat Biotechnol. 2011;29(8):718–720. doi: 10.1038/nbt.1943. [DOI] [PubMed] [Google Scholar]
- Xu X, Nagarajan H, Lewis NE, Pan S, Cai Z, Liu X, Chen W, Xie M, Wang W, Hammond S, Andersen MR, Neff N, Passarelli B, Koh W, Fan HC, Wang J, Gui Y, Lee KH, Betenbaugh MJ, Quake SR, Famili I, Palsson BO. The genomic sequence of the Chinese hamster ovary (CHO)-K1 cell line. Nat Biotechnol. 2011;29(8):735–741. doi: 10.1038/nbt.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, Kusunoki M, Iida S, Nakano R, Wakitani M, Niwa R, Sakurada M, Uchida K, Shitara K, Satoh M. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng. 2004;87(5):614–622. doi: 10.1002/bit.20151. [DOI] [PubMed] [Google Scholar]
- Yu C, Crispin M, Sonnen AF, Harvey DJ, Chang VT, Evans EJ, Scanlan CN, Stuart DI, Gilbert RJ, Davis SJ. Use of the alpha-mannosidase I inhibitor kifunensine allows the crystallization of apo CTLA-4 homodimer produced in long-term cultures of Chinese hamster ovary cells. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011;67(Pt 7):785–789. doi: 10.1107/S1744309111017672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuk IH, Baskar D, Duffy PH, Hsiung J, Leung S, Lin AA. Overcoming challenges in WAVE Bioreactors without feedback controls for pH and dissolved oxygen. Biotechnol Prog. 2011;27(5):1397–1406. doi: 10.1002/btpr.659. [DOI] [PubMed] [Google Scholar]
- Zang Y, Kammerer B, Eisenkolb M, Lohr K, Kiefer H. Towards protein crystallization as a process step in downstream processing of therapeutic antibodies: screening and optimization at microbatch scale. PLoS One. 2011;6(9):e25282. doi: 10.1371/journal.pone.0025282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Shankara S, Roy A, Qiu H, Estes S, McVie-Wylie A, Culm-Merdek K, Park A, Pan C, Edmunds T. Development of a simple and rapid method for producing non-fucosylated oligomannose containing antibodies with increased effector function. Biotechnol Bioeng. 2008;99(3):652–665. doi: 10.1002/bit.21598. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Cuenca JV, Zhou W, Varma A. NS0 cell damage by high gas velocity sparging in protein-free and cholesterol-free cultures. Biotechnol Bioeng. 2008;101(4):751–760. doi: 10.1002/bit.21950. [DOI] [PubMed] [Google Scholar]


