Abstract
A white-rot basidiomycete Ganoderma spp. has long been used as a medicinal mushroom in Asia, and it has an array of pharmacological properties for immunomodulatory activity. There have been many reports about the bioactive components and their pharmacological properties. In order to analyze the current status of Ganoderma products, the detailed process of cultivation of Ganoderma spp. and development of their products are restated in this review article. These include the breeding, cultivating, extracting bioactive component, and processing Ganoderma products, etc. This article will expand people’s common knowledge on Ganoderma, and provide a beneficial reference for research and industrial production.
Keywords: Ganoderma spp. biotechnology, Breeding and cultivation, Development and utilizations, Ganoderma-based products, Quality control
Introduction
Ganoderma lucidum (Fr.) Karst (named as Lingzhi in China), a species of basidiomycetes which belongs to Polyporaceae (or Ganodermaceae) of Aphyllophorales, is one of the most popular medicinal mushrooms in China, Japan, Korea, and other Asian countries. It has been under modern biochemical and pharmacological research during the last 30 years (Gao et al. 2006). However, in the earliest Chinese literatures, this medicinal mushroom is not called “Lingzhi”, instead of “Rui Cao” (means auspicious herbs) or “Zhi”. After the Han dynasty, the name of Lingzhi started to appear in the ancient Chinese literatures. It should be noted that Lingzhi mentioned in ancient Chinese literatures is different from the Ganoderma described in fungal classification today. Besides the Ganoderma and its relatives, Lingzhi described in ancient Chinese literatures also included some fungi belonging to Polyporales and Agaricales, etc., especially in Taoism history books (Zhou and Lin 1999).
In the opinion of modern traditional Chinese medicine (TCM), Lingzhi presents three characters for prevention or treatment of diseases. Firstly, the usage of Lingzhi is without any toxicity and apparent absence of side effects; secondly, it has no pertinence on a special organ; and the last one is its improvement effects on normalization of the organ function. With the development of biotechnology, many researchers have intensively studied the bioactive components of Lingzhi and many Lingzhi-based products. Modern pharmacological and clinical trials have demonstrated that Lingzhi showed a significant effect on the prevention and treatment of various diseases. For example, the anti-cancer effects of Lingzhi were associated with triterpenes, polysaccharides, and fungal immunomodulatory proteins (FIPs) by the mechanisms of DNA polymerase inhibition, post-translation modification inhibition of the Ras oncoprotein, or cytokine production stimulation (Sliva 2006; Ding et al. 2009a, b; Ogbe et al. 2011). Nowadays, there is an increasing public interest on the secondary metabolites of Lingzhi for exploring new drugs or leading compounds. Therefore, a number of bioactive constituents have been isolated from Lingzhi, including small molecule compounds, polysaccharides, proteins, enzyme, polysaccharide–protein complexes, etc. (Zhong and Xiao 2009; Xu et al. 2010a, b; Ferreira et al. 2010; Xu et al. 2011). Because of the unique pharmacological function and apparent absence of side effects, it has attained a reputation in the East and some African countries as the ultimate herbal substance. Now Lingzhi has been added not only to the Chinese Pharmacopoeia (Zhou et al. 2007a) but also to the American Herbal Pharmacopoeia and Therapeutic Compendium (Sanodiya et al. 2009).
In an overview of previous literatures, there was a larger collection of papers on Lingzhi’s bioactive components and their pharmacological properties, and a number of reviews had appeared on these aspects (Luo and Lin 2002; Shiao 2003; Yuen and Gohel 2005; Zhou et al. 2007a, b; Sanodiya et al. 2009; Olaku and White 2011; Xu et al. 2011). The reason why Lingzhi draws so much attention is because it is a potential pharmacological macrofungi (Sanodiya et al. 2009) and it plays an important role in disease prevention and treatment in folk medicine; meanwhile, modern pharmacological tests have also demonstrated some actions and properties of Lingzhi, including immunomodulating, inducing cytokine production, anti-allergic, anti-radiation, anti-tumor, anti-inflammatory, anti-parasitic, anti-oxidant, benefiting on the cardiovascular system, respiratory system, endocrine and metabolic systems, etc. (Wasser 2002; Gao et al. 2004; Hong et al. 2004; Zhou et al. 2007a; Mahajna et al. 2009). However, Lingzhi products processed by bioactive components depend on the upstream cultivation, such as the fruiting bodies, mycelia, and culture broths; meanwhile, Lingzhi-based products also rely on the downstream process. In summary, the qualitative and quantitative differences in the chemical composition of Lingzhi products are dependent on the strain, origin, extracting process, and cultivation conditions (Mizuno 1995; Zhou and Lin 1999; Zhou et al. 2008a; McKenna et al. 2002). In this review article, the cultivation methods and conditions of Lingzhi have been overviewed. Subsequently, the current status of products process would be addressed. Finally, problems and prospects were analyzed and viewed. This review is beneficial to researchers and producers.
Cultivation of Ganoderma species
Wild Lingzhi is difficult to collect and to control its quality. In 1970, a Chinese technician used “spore separation cultivation method” to successfully cultivate Lingzhi. From then on, artificial cultivation of Lingzhi has been available in China.
Breeding of Lingzhi
The good quality of Lingzhi strain is the precondition or key for Lingzhi production. The strain quality has an effect not only on the yield of Lingzhi-based product but also its quality. Therefore, selection of a good Lingzhi strain is a very important task. At any time, high production and good quality are always the principal goals for agriculturally important crops, without the exception of medicinal mushrooms. There are a lot of breeding methods, such as mass selection, programmed mutation, cross-breeding and transgenic breeding, etc. Up to now, with the development of modern biotechnology, the protoplast fusion techniques widely applied on mushroom breeding have made greater progress. Transgenic engineering techniques applied on the medicinal mushroom breeding are a technological innovation on the molecular level. However, the selection and transgenic breeding are more objective and promising, and have made a lot of progress from theory to practice in the last 20 years. The breeding strategy of Lingzhi is summarized in Fig. 1.
Fig. 1.
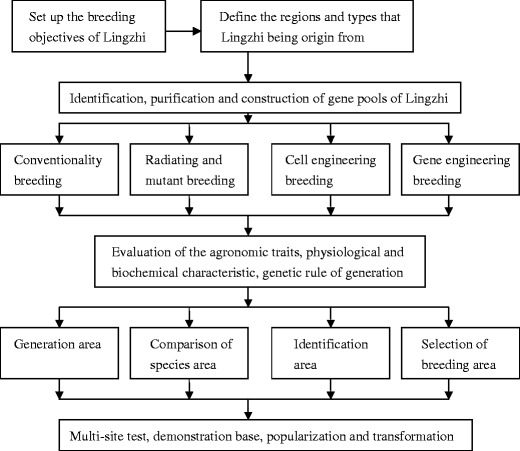
The breeding strategy of Ganoderma species
Artificial selection
Artificial selection, also known as selective breeding, is a primitive breeding method, which uses artificial means to choose superior strains from nature, for biologically obtaining the new species and reproducing it selectively. The basic methods of artificial selection were the tissue separation and spore separation methods for obtaining the pure strain, followed by optimization of this strain, and then the required strain is obtained (Chen and Su 2008). In fact, during the procedure of mushroom production, tissue separation method is often employed in getting the strain because Lingzhi spore is hard to germinate (Lin and Zhou 1999a). Artificial selection is more commonly used in the breeding of other edible mushrooms than in Lingzhi.
Mutation breeding
Mutation breeding is a new and more effective method compared to artificial selection method, which changes genes of the strains, and achieves genic recombination. A general procedure of mutation breeding involves the following steps: selection of original strain → preparation of spore (or protoplast) suspension liquid → viable count and mutagenizing → spreading plate for cultivation → picking up strain and inoculation → initial screening → slope culture → re-screening → selection of superior strain. The protoplast is usually chosen for mutation breeding (Li et al. 2001), and the increased bioactive components, such as polysaccharides (Gao et al. 2008), triterpenoids (Li et al. 2001), and organic germanium (Dong et al. 2009), are regarded as the breeding objectives. Mutation breeding could not only increase strain mutation rate through simple operation but also provide genetic markers for further cross-breeding and cell fusion breeding. However, it also has some disadvantages. For example, the mutation generation is random and the work on selecting the mutant is complicated, etc.
Cross-breeding
Cross-breeding technology is the most widely used and effective breeding method in breeding the new species of edible or medicinal mushroom. The principle of cross-breeding is to achieve genetic recombination through haploid mating, and then strains from generation with parent’s good traits are selected. After the 1980s, cross-breeding technology was widely used in the breeding researches of edible fungi in China and other Asian countries (Zhao and Chang 1993; Chiu et al. 2005). The Lingzhi spores are difficult to germinate under artificial conditions, so the monokaryotic strains cannot be obtained, which is necessary for the breeding of the Lingzhi. As a result, the hybridization process of Lingzhi is restricted. Therefore, the protoplast monokaryogenesis method to obtain the new strain are used in practice (Wu et al. 2009a). There have been some reports about artificial cross-breeding where most of them were selected using protoplast as materials (Chiu et al. 2005).
Cell fusion breeding
Cell fusion breeding is an important part of modern biotechnology and is also a significant leap on genetic breeding. In the fusion of cell protoplasts, the different genotypes of protoplasts from various organisms are fused with each other, which is induced by the fusion agent after breaking the cell wall. In that case, the fusions could make the cell genomes from different genus mix effectively, which produces a whole set of genetic exchange and restructures to generate a new individual (Tan et al. 2005). A common method of cell fusion engineering breeding involves the following procedure: selecting parent strain → ensuring genetic markers of parent strain → isolating protoplast from parent strain → regenerating and culturing protoplast → fusing protoplast → regenerating and culturing the fusion → detecting and selecting fusion. In the 1970s, this method has been widely applied in basidiomycetes breeding (Ferenczy et al. 1974). At the beginning of the 1980s, it has been used in edible mushroom breeding in some Asian countries. Initially, most of the researches focused on isolation and preparation of Lingzhi protoplast (Choi et al. 1987; Li and Li 1999; Chen et al. 2007a, b), and then it was gradually applied in breeding the new strains by fusing intragenus protoplast (Park et al. 1988) and intergeneric protoplast (Yoo et al. 2002). Some researchers also successfully obtained the new variety of Lingzhi by protoplast monokaryogenesis method (Wu et al. 2009a).
Genetic engineering breeding
Genetic engineering is a technological innovation in the area of molecular biology. Using this technology, a DNA sequence from one species can be isolated and then transferred to another. Meanwhile, the good characters of donor strain can be expressed in host strain, which will become a high-production and good-quality strain. This novel breeding method provides a new breeding solution for edible or medicinal mushroom, especially for those limited by conventional breeding means. The general procedure of genetic engineering breeding is presented as follows: selecting donor strains → separating gene → genes reconstruction in vitro → transfer the gene into the recipient cell → reproduction and expression of recombinant DNA → selection of new individual. Transformation is the key to Lingzhi genetic engineering breeding. Up to now, six kinds of transformation methods have been applied for filamentous fungi, which includes protoplast-mediated transformation (PMT), agrobacterium-mediated transformation, electroporation, biolistic transformation, restriction enzyme-mediated integration (REMI), and lithium acetate, etc. (Zhou et al. 2010). Most of these methods have successfully worked in breeding of Lingzhi (Park et al. 1991; Sun et al. 2001a; Kim et al. 2004a).
For example, Li et al. (2004) constructed fungal expression plasmid pAN7-1 (6.7 kb), which carried the promoters of hph (hygromycin phosphate dehydrogenase) gene from Escherichia coli and the gpd (glyceraldehyde-p-dehydrogenase) gene from Aspergillus nidulans. The plasmid could express hygromycin B resistance in fungal culture and achieve the transformation of Lingzhi protoplasts with 60% polyethylene glycol (PEG) 4000. So it provided a foundation for transforming Lingzhi protoplast by the PMT method (Li et al. 2004). Kim et al. (2004a, b) reported the studies on genetic transformation and mutant isolation based on REMI technology in G. lucidum. They constructed a plasmid pJS205-1(6.5 kb) carrying the resistance gene of geneticin (geneticin, an aminoglycoside antibiotic) and phosphinothricin. After using restriction enzyme EcoRV, NotI, and XhoI, plasmid pJS205-1 was transformed into the Lingzhi protoplasts. Then a series of mutants were obtained and its preliminary identification on biochemical characteristics was observed (Kim et al. 2004a). For example, Li and Chen (2002) used a Ti plasmid vector containing an exogenous gene to transform the exogenous gene into the fungus protoplasts and made it stably replicated and expressed (Li and Chen 2002). Zhang et al. (2011) over-expressed rice OsUgp2 gene in Ganoderma sinensis and increased the content of intracellular and extracellular polysaccharide (EPS) in G. sinensis (Zhang et al. 2011). In all, gene engineering techniques could contribute to the new vigor for using secondary engineering to breed Lingzhi.
Setting up the fingerprint of good quality species
It is crucial for both growers and researchers to understand the features and qualities of the best strains. The choice of a proper strain can determine success or failure. Therefore, the analysis and evaluation of the strain qualities have always been the focus of the producer and researcher of medicinal mushroom. Up to now, in China, the evaluated content of medicinal mushroom (including Lingzhi) includes six major indices of determinations and assays, which are the appearance of mycelia or strains, microorganism examination, microbe testing, determination of the growing speed, the esterase isozyme analysis, and the cultivated character. The abovementioned cultivated character includes the shape of fruit bodies, mycelia growing speed, vegetative stage (and reproductive stages), yield, etc.
The existing knowledge and technique for identification of strain qualities are based on the cultivation test, determination, and assay of agronomic traits in the course of cultivation. In the developed countries and developing countries, the effects of mushroom breeding rely on the amount of cultivation test for verification. In any case, from the viewpoint of the study level of mushroom genetics, the method of DNA special analysis cannot be applied directly to explain and decide on the detection of strain quality and other problems relative to the strain quality (Zhang et al. 2005a, b). Today, the quality standards of Lingzhi strains have been addressed in some provinces in China where the Lingzhi could be produced on a large scale (Fujian Bureau of Quality and Technical Supervision 2002; Anhui Bureau of Quality and Technical Supervision 2004). The local standards were used for the evaluation of Lingzhi strains.
In different opinions, TCM fingerprint generates from different names, such as chemical fingerprints and biological fingerprint, spectral fingerprint and chromatographic fingerprint, etc. From the view of modern biotechnology analysis, it includes the DNA fingerprint, protein fingerprint, and chemical fingerprints. At present, there are many studies about chemical fingerprints of Lingzhi. However, chromatography methods were popular and were studied in depth during the past decade (Xing et al. 2004; Huang et al. 2004a, b; Zhang et al. 2009; Chen et al. 2010a, b; Dejaegher and Heyden 2010). With the development of molecular biology technology, DNA fingerprint technology was firstly introduced to fungi to research genetic diversity related to the genus, species, strain, etc. (Anderson et al. 1987; Hwang and Kim 1995). Subsequently, it was introduced to variety identification and authentication in Lingzhi (Hseu et al. 1996; Shi et al. 2008; Wu et al. 2009b; Zheng et al. 2009). Some scholars tried to use the function gene such as FIPs to identify Lingzhi (Zhou et al. 2008b). However, there are many different opinions about how to build an effective fingerprint (Gottlieb et al. 2000; Zhou et al. 2005). The total strategies are shown in Fig. 2 (Zhou et al. 2006).
Fig. 2.
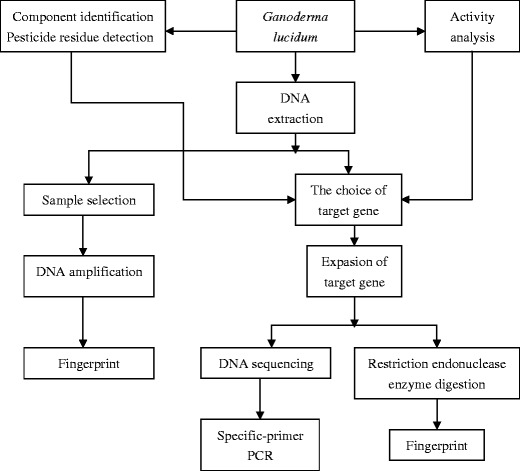
Formation strategy of DNA fingerprint of Ganoderma (revised from Zhou et al. 2006)
DNA fingerprint has two main purposes: identification of the TCM authenticity and analysis of the TCM quality. The DNA molecular marker technology, which DNA fingerprint depends on, can be divided into three groups: (1) restriction fragment length polymorphism (RFLP) and other technologies, in which the core is based on the electrophoretic techniques and molecular hybridization techniques (Tanksley et al. 1989; Deragon and Landry 1992); (2) DNA fingerprints and DNA sequencing technology such as random amplified polymorphic DNA (RAPD) (Bardakci 2001), simple sequence repeats (SSR), arbitrarily primed polymerase chain reaction (AP-PCR), etc., which depend on the electrophoretic techniques and PCR techniques; and (3) amplified fragment length polymorphism (AFLP) (Vos et al. 1995), sequence characterized amplified regions (SCAR), direct amplification of length polymorphism (DALP), RFLP-PCR, RAPD-PCR, and the latest gene chip diagnostic technique, which combine the two abovementioned techniques. Of course, all kinds of molecular marker methods have its advantages and disadvantages (Jones et al. 1997). Any kind of marker technologies cannot overall analyze all the medicinal materials. The selection of methods must lie on the actual need, different features, and advantages of the molecular marking techniques. However, the fingerprint techniques are better or advanced, but not exact. When selecting the specific technologies, the popularity of the technology and the cost of samples analysis also should be taken into consideration (Wu et al. 2009b; Zhou et al. 2006; Blundeoll 2006; Urbanelli et al. 2007).
Cultivation procedures
The methods of Lingzhi cultivation can be divided into two major types, including liquid-state cultivation (LSC) and solid-state cultivation (SSC). The SSC also can be divided into two methods based on the used raw materials, which are called as the log (or basswood) cultivation and substituted cultivation. At the beginning of the artificial cultivation of this valuable medicinal mushroom, only four species was used for trials, which included G. lucidum (Leyss. Ex. Fr.) Karst, G. lucidum (Leyss. Ex. Fr) Karst vat., G. japonicum (Fr.) Lloyd, and G. capense (Lloyd) Teng (Zhou and Lin 1999). Through more than 40 years of development, the cultivation techniques have achieved significant progress. Currently, the various methods are widely used for commercial production (Hou and Liao 2009; Zhou et al. 2010).
Artificial cultivation of Lingzhi fruit bodies
It is necessary to meet the requirement of Lingzhi fruit body growth, which includes the nutrients components and the environmental conditions. Meanwhile, we must also master the culture methods.
The nutrition conditions of Lingzhi growth mainly include carbon resources and nitrogen resources, together with inorganic salts and growing factors. The Lingzhi growth generally uses organic carbon resources such as sugar, starch, cellulose, hemicelluloses, and lignin. The glucose and sucrose are usually used in culture of mycelia, the sawdust of broad-leaved tree (Jiang 2001; Xia et al. 2003; Liao and Xiao 2006), and the agricultural by-products, such as cotton seed husk, straw, and corn cob, in cultivation of fruit bodies (Wei et al. 2005; Yan 2000). Some small molecular weight compounds, such as amino acid, urea, nitrogen, etc., can be utilized by Lingzhi mycelia. During the process of Lingzhi cultivation, yeast powder and peptone are usually added in the media of culturing mycelia, while wheat bran, corn powder, coarse powders of rice bran, ammonium sulfate, urea, etc. are used in the media of culturing fruit bodies. When the rate of carbon to nitrogen is 15–45:1 in the substrate, the Lingzhi mycelia may grow well. The appropriate rate of carbon to nitrogen on the substrate for cultivation of fruit bodies is 30–40:1 (Lin and Zhou 1999b; Han et al. 2003; Wu et al. 2008).
Besides carbon and nitrogen resources, Lingzhi growth also needs inorganic salts and other inorganic elements, including kalium, natrium, calcium, magnesium, phosphorus, sulfur, zinc, etc. Among them, phosphorus, kalium, and magnesium are the three main nutrition elements. Their appropriate concentration in the media is 100–150 mg/L. Although some of the elements have existed in the raw materials, the substrates are still added the CaSO4, KH2PO4, and MgSO4, especially the amount of usage CaSO4 is at most up to 1% of total substrate weight. It is a significant reason that calcium sulfate may adjust the pH value of substrate, change the substrate porosity, increase the air flow, fix the nitrogen, and enhance the mounts of calcium and sulfur elements. In the development process of Lingzhi, the growth factors are the necessary components, including vitamin B1, B6, and biotin, which are related to Lingzhi metabolism. Because of these growth factors existing in the natural substrates, they do not necessarily need to be added into the substrates. In addition, clean well water should be used in large-scale cultivation of Lingzhi. It is necessary that the water is not contaminated with undesirable pollutants. The appropriate content of water should be 65–70% (Zhou and Lin 1999; Han et al. 2003).
Lingzhi is found more frequently in subtropical regions than in the temperate zones. It is an annual mushroom, growing on a wide variety of dead and dying trees. The growth parameters (Lin 1996; Chang and Miles 2004) are summarized mainly to include four aspects: temperature, humidity, air, and light. Firstly, temperature for mycelia growth ranges from 15 to 35 °C and the optimum temperature is 25 to 30 °C; for primordial initiation, it ranges from 18 to 25 °C; and for fruiting body development, the range is from 24 to 28 °C. Under the condition of 20 °C or lower, the fruit body would become yellow and stops growing. In the procedure of fruit body development, it is actually not necessary to be stimulated with temperature difference. The higher temperature differences easily cause macrocephalic Lingzhi. Secondly, humidity is a term for the amount of water vapor in the air or in the substrates. During the process of Lingzhi cultivation, the water content in substrates should be maintained at 60% to 65% level. Relative humidity for the mycelial running is within 60% to 70% level, the primordial initiation is within 85% to 90% level, and the fruit body development is with 85% to 95% humidity level in the environmental air. Finally, in the different development periods, Lingzhi have the different requirements on the rate of oxygen and carbon dioxide. Lingzhi mycelial running do not need oxygen. But during the fructification period, good ventilation is necessary. When the concentration of CO2 is below 0.1%, the fruit body would be abnormally developed. When the concentration of CO2 is above 0.1%, the fruit body would be normally developed.
Similar to other mushrooms, Lingzhi do not require the various types and amount of light to grow. Because of the light for Lingzhi mycelial running having an inhibiting effect, colonizing substrate should be kept under dark conditions to make sure the substrate does not pin prematurely. During primordia initiation of Lingzhi growth, the light level is required at 500 to 1,000 lx; for fruit body development, it is at 3,000 to 50,000 lx. At the condition of light level about 3,000–10,000 lx, the stip and cap (pileus) shape are normal. The optimum light level is at 15,000 to 50,000 lx. The optimum pH value for mycelial running is 5.0 to 5.5 (Zhou and Lin 1999; Hou and Liao 2009).
Artificial cultivation of Lingzhi has a history of more than 40 years. Many methods of cultivating Lingzhi derive from the name of cultivation raw materials (log cultivation, sawdust cultivation, substituted cultivation, etc.), the packaging forms of raw materials (bag or bottle), or cultivation location (outdoor cultivation, indoor cultivation, bionics wild cultivation, etc.). For example, based on the cultivation raw materials, basswood cultivation technology is actually designed to culture mushroom using basswood as raw materials (Zhang et al. 2004). The substituted cultivation technology was defined as using the sawdust of hardwood, cotton seed husk, or foot materials of farm crops to cultivate mushroom (Zhang and Wang 2010). The word “cultivation technology” may mean different things, in different people and countries. However, regarding the choice of what kind of method or raw materials to use, the production of the basic process is roughly the same. Similarly, as to other cultivated edible mushrooms, the process for producing Lingzhi fruiting bodies can be divided into two major stages. The first stage is the preparation of various raw materials, which involves the selection of mother spawn, cultivation method, and cultivation relative to materials. The second stage is the performance of cultivation, including the planting spawn, stock culture, fruiting culture, and utilization of the growth substrates for mushroom production. Currently, the methods adopted for commercial production shows a variety of forms, which mainly include the wood log, short basswood segment, tree stump, sawdust bag, and bottle procedures (Mayzumi et al. 1997; Lin and Zhou 1999a; Erkel 2009; Chen 1999; Han et al. 2008).
Because some methods are not beneficial for the protection of the ecological system, Lingzhi cultivation using wood log is not adopted in production practice. Here, we only restate the basic procedure related to substituted cultivation. In the research of substituted cultivation of Lingzhi, the research content often focuses on the following aspects: selection of substrate and its formulation, cultivation models (bag or bottle, inside or outside), sterilization and inoculation, cultivation and administration of mycelia, and administration of the stage of fruit body development. Briefly, spawn is transferred onto a sterile solid-state substrate, which frequently consists of a mixture of hardwood sawdust, wheat bran, and other supplemental substances, and is incubated under dark conditions until colonization of substrate is achieved. After this, incubation under the weak light condition and at increased oxygen level is carried out to develop the primordial initiation. Once the desired primordial initiation is achieved, the conditions are again altered to aid in the fruiting body development (shown in Fig. 3). The entire growth of Lingzhi from spawn running to cropping in artificial cultivation is different from the method of cultivation used. In general, it should take on average approximately 90–150 days.
Fig. 3.
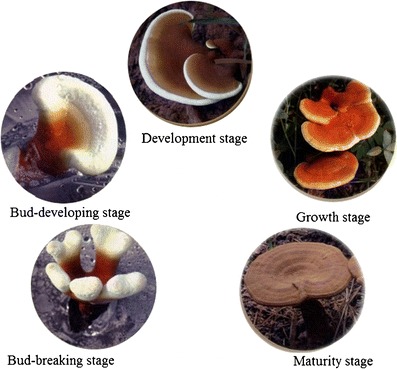
The developmental process of Lingzhi fruit body
It directly affects the Lingzhi product’s quality to administer the development process of fruit body. In the overview of previous publications, the cultivation procedures were roughly the same; the difference was only diverse from different cultivation methods or raw materials (Chen 1999; Chi 2005; Erkel 2009; Hossain et al. 2009; Xiao et al. 2010). Up to now, in the Lingzhi production practice in China, the preparation of substrate, including substrate formulations, making the synthetic logs in bag cultivation and substrate sterilization etc., have appeared in the textbook of some vocational schools or popular science books (Zhang 1998; Liu and Jiang 2007). Meanwhile, the cultivation processes of Lingzhi have also been described in these books. Such questions regarding the crucial stages in Lingzhi cultivation, how to speed up the spawn running, how to control the environmental factors, and how to produce Lingzhi with caps have been introduced in various media, including magazines, popular science books, DVD video, TV etc. Growers will benefit from concentrating on the step-by-step instruction on how to cultivate it.
Artificial cultivation of Lingzhi mycelia
Both the liquid-state fermentation and solid-state fermentation are popular for the production of Lingzhi mycelia.
Liquid-state (submerged) fermentation of Lingzhi mycelia
Liquid-state fermentation (LSF) of mushroom, also known as liquid culture or submerged fermentation, is a process of culturing microorganism in liquid media, but not on the surface of liquid media. Preparation of mushroom mycelium in submerged culture was initially developed during the 1970s. At that time, only lower fungi (fungi that are not from basidiocarps) were successfully cultured in fermentors for economical production of various natural products (Yang and Liau 1998). The main principle is that the liquid medium is added to the fermentation tank or flask. When sterile air is led to reactor, it will increase the dissolved oxygen content in the medium to improve respiratory metabolism of the mushroom mycelium. At the same time, the mushroom mycelium is agitated or oscillated, and appropriate external conditions are controlled so that mushroom cells grow in the liquid depths of breeding, producing a lot of the mycelium or metabolites. Another review indicated that there were few investigations into the development of high fungi (basidiomycetes) bioprocesses (Fang and Zhong 2002a, b).
Compared with the artificial cultivation of Lingzhi fruit bodies, LSF holds obvious advantages. Firstly, the advantage of LSF over the traditional, multinational cultivation of fruit body is the reduction in the time spent to obtain the product of interest (Mshandete and Mgonja 2009). The production of fruit bodies takes at least 3–5 months, while reasonable amounts of ganoderic acids (GAs) and polysaccharides can be obtained by submerged fermentation after 3–5 weeks only (Wagner et al. 2003). Secondly, the mycelium grown in LSF holds high stability and standardization in which the environmental conditions (temperature, dissolved oxygen, pH, etc.) are easier to control. This is important not only for producing the desired product but also might be beneficial for producing mushroom-based medicines and nutraceuticals. The products obtained by this method are easy to achieve, with higher quality standards and safety (Wasser and Weis 1999). Thirdly, advantage of mycelia culturing in LSF is to provide a new method for scalable production and increased yields of biologically active compounds. By LSF method, the producer can increase the yield of protein rich in essential amino acids and vitamins serving as functional foods compared with the yield of these components in carpophores of the standard fruiting basidiomycetes (Friel and McLoughlin 2000). Thus, production of fungus metabolites through LSF method has received a lot of attention because of short time cultivation, high productivity, fewer chances of contamination, and easy recovery of producing metabolites (Huang and Liu 2008; Kim et al. 2007).
In general, the methods of artificial cultivation typically in liquid media involve five stages: (1) selection of Lingzhi strain; (2) preparation of culture maintenance medium for different culture phases; (3) inoculation; (4) cultivation of strain in Erlenmeyer flasks, seeding tank, and fermentor, respectively; and (5) harvest of Lingzhi production. In the process of Lingzhi mycelia LSF, it is very important to select and control the appropriate fermentation conditions, such as strain, amount of inoculation, temperature, pH, air flow, stirring rate, etc. The determination of the indices of the filamentous morphology, concentration, nutrients consumption, and the outward appearance and viscosity of broth could be used as the final quality standard of control fermentation (Zhao 2002; Sánchez 2004; Wang et al. 2007a, b, c).
However, a multi-objective analysis and research was presented in the previous study of the Lingzhi LSF. Some aimed simply to produce biomass, with no concern for its composition. Others aimed to maximize the production of either GAs or polysaccharides, and to understand how different variables affect their production. Among these studies, many researchers studied Lingzhi LSF to obtain substances and special metabolic products (food, medicine or industrial enzyme, etc.) (Fang and Zhong 2002a; Yang 2005; Chen et al. 2008; Songulashvili et al. 2007). Also, other researchers focused on the parameters in Lingzhi LSF including culture media (Sun et al. 2000), initial pH (Fang and Zhong 2002b), culture times and temperature, etc. (Wei et al. 2007a, b; Xia et al. 2007; Zhang et al. 2008a, b; Liang 2011). In summary, in the process of culturing Lingzhi by LSF method, the suitable carbon and nitrogen resources are corn flour and soybean meal with concentrations of 3% and 2.5%, respectively. The optimum cultivation conditions were as follows: initial pH 4.5–5.0, rotation speeds 120–150 rpm, 100–120 mL medium/500 mL Erlenmeyer flask at 25 °C. When the diameter of mycelium pellet was 0.85–0.9 mm in the process of culturing with Erlenmeyer flask, the biomass of Lingzhi mycelia is the highest (Dong et al. 2004; Zhu et al. 2009).
In spite of having achieved significant progress in Lingzhi LSF, the applications of LSF techniques have an interval to industrialization production. In other words, the successful example in large-scale culture Lingzhi by LSF is extraordinary. In the previous review articles, the researchers greatly summarized the published data on submerged fermentation with Lingzhi (Wagner et al. 2003; Sanodiya et al. 2009). In this review article, only one report of a large-scale fermentation was described in which Ganoderma tsugae was cultivated in tanks with a volume of 20 m3. All other reported studies of LSF with Lingzhi were done only in volumes of 10 L or less. By estimating results generally, half the studies were undertaken in Erlenmeyer flasks and others were in fermentors. However, the best yields reported to date are 22.1 g/L for biomass, 1.71 g/L for EPS, 2.49 g/L for intercellular polysaccharides (IPS), and 582 mg/L for GA. In recent years, Chinese scientists have reported that they produced G. lucidum 730 mycelia in a 500-L automatic stainless steel fermentor. The results showed that after a fermentation time of 70 h, there was no obvious germination and lock-like concentration on the mycelia wall. There was a little mycelium dissolved with no other bacteria. The consistence of G. lucidum clump is about 30%. EPS and IPS is 3.5 g/L and 4.8 g/L, respectively (Wei et al. 2007a, b). Other reported studies of fermentation with G. lucidum were carried out in volumes of 10 m3 with the fermentation liquid 7.5 m3, the average production cycle 150 h, and pH reduced from 6.5 to 3.5. The results showed that after the mycelia fermented, the average weight of dry powders in every fermentor is 66.1 kg by spray drying, the average recovery 8.76 kg/m3, the content of pure polysaccharide 68.5 g/kg in the dry powders, and the weight of the pure polysaccharide 4.225 kg in every fermentor (Hu 2006).
In addition, the cost–benefit ratio of liquid culture and solid culture technologies for spawn production were investigated by some researchers. The results demonstrated that liquid culture technology for spawn production is obviously more advantageous, which is reflected in better spawn quality, reduction of contamination, increased efficiency, reduction of cost, and increased production stability. For this technology to be broadly used, it is key to improve the incubator shakers, fermentation tanks, and inoculation equipment. Efforts to facilitate researches according to the demand of a dynamic market would promote the application of this technology in the fast-growing mushroom spawn production industry (Guo and Liu 2011). Based on the character of Lingzhi mycelium having bio-enrichment of mineral nutrition, some researchers also focused their interest on the accumulation of some minerals by submerged fermentation, which include the selenium-enriched Lingzhi (Xie et al. 1996; Shen and Yu 2008; Ling et al. 2008), the calcium-enriched Lingzhi (Gao et al. 2007), the zinc-enriched Lingzhi (Liu et al. 2005; Wei et al. 2010), the iron-enriched Lingzhi (Miao and Lv 2007), and so on. The abovementioned studies provide a foundation of development of functional food using mineral nutrition enrichment Lingzhi as raw materials (Wang et al. 2001; Mao and Ma 2009).
Solid-state fermentation of Lingzhi mycelia
In contrast to LSF, solid-state fermentation (SSF), also known as SSC, is the cultivation of microorganisms under controlled conditions in the absence of free water. The production examples of SSF include the industrial enzymes (Wu et al. 2000), biofuels, biopesticide and nutrient-enriched animal feeds, etc. (Habijanic and Berovic 2000; Sun et al. 2007; Chen et al. 2010b). In recent years, the SSC of mycelia has led to a wide range of applications at the laboratory scale because information from SSC can be applied to more commonly used liquid-state cultivation (Maldonado and Strasser de Saad 1998; Mahapatra and Banerjee 2009). SSC has also been frequently utilized in preliminary tests for cultivating microorganisms under experimental conditions because it requires less time and labor than LSF.
Though there is very little information about the production of Lingzhi in SSF, it is not difficult to find some basic researches under experimental conditions, such as substrate components, pH, and temperature, etc. Among these studies, the most praiseworthy work is an experiment conducted by Habijanic and Berovic. They carried out the Lingzhi fermentation in a horizontal stirred tank reactor with a total volume of 30 L with suitable conditions (Habijanic and Berovic 2000). Their eminent work showed a positive aspect for satisfactory rates of growth and exopolysaccharide production by favorable conditions. In their study, the substrate consisted of beech sawdust, olive oil, (NH4)2SO4, KH2PO4, CaCl2·2H2O, MgSO4·7H2O, FeSO4·7H2O, and distilled water, which provide the optimal substrates for the production of immunostimulatory animal feed supplements because the whole fermented substrate is used as the product. In other previous reports, Hsieh and Yang (2004) used soy residue for the Lingzhi SSF. In their experiment, the SSF was conducted in three types of containers: test tube, 500-mL flask, and sterilizable polypropylene plastic bag. The highest rate of mycelial growth of 6 mm/day was observed in the medium of carbon/nitrogen (C/N) ratio of 80 using test tubes. However, a growth rate of 7.5 mm/day was found at the C/N ratio of 70–80 in the 500-mL flasks (Hsieh and Yang 2004). Tea waste (TW) is used as a new supplement for substrate mixtures in Lingzhi cultivation. Peksen and Yakupoglu determined the effects of sawdust (S)-based substrates supplemented with TW at the various levels (75S:25TW, 80S:20TW, 85S:15TW, and 90S:10TW) and G. lucidum strains on the yield, biological efficiency (BE), and the chemical composition of fruiting bodies in SSF. The result showed that the significant differences were found among substrates regarding yield and BE, while yield and BE of the strains were not different (Peksen and Yakupoglu 2009).
The combination of modern biotechnology and traditional fermentation technology of Chinese materia medica (CMM) provides a broad space for the rapid development of fermentation technology of CMM. It is interesting that a new type of fermentation technique, known as the bidirectional SSF technology, has emerged in China after the 1990s. Along with the theoretical and technical development of the engineering science, it was considered as the new turning point of modern biological technology and the new channel of the new drug R & D of TCM (Zhuang et al. 2007). Based on inventor’s opinion, the bidirectional SSF has two characteristics. The used nutrient substance contains the medicinal herbs with multiple active compositions, instead of the one that consists of only farming byproducts. The products formed by this technique are fungous substance containing herbs which was produced by a medicinal fungous substance (Zhuang 1991, 1995, 2002; Zhuang et al. 2007).What is the medicinal fungi bidirectional SSF technology? The key of this technology is that the medicinal mushroom strains are cultured in the special substrate, which consisted of CMM or medicine slag as medicinal substrate instead of the traditional nutritious substrate cultured. The fermentation products are known as the medicinal fungal substance. In the fermentation process, while the medicinal substrate provided nutrients for fungal growth, it was also affected by the enzyme produced from fungi. Hence, the tissues and components of this medicinal substrate would be changed and produce new functional components, so the biochemical process of medicinal fungi and herbs in fermentation substrate hold “bidirectional”, which present the perfect combination with medicinal fungi and CMM (Zhang et al. 2005a, b; Zhuang and Hong 2006; Zhuang et al. 2004).
Since the invention of the technique, Lingzhi is the most preferred species to study by Chinese scientists. For example, based on the purpose of drug research and development, Chen and Chen reported their research results. In their experiment, SSF was applied for medicinal fungi by fermenting G. lucidum with Radix astragali containing medium. G. lucidum was fermented in ordinary medium, CMM-containing medium (containing Radix astragali), and selenium-rich CMM-containing medium, respectively. The polysaccharide contents of fermentation products from the three kinds of culture media were investigated at different times, and the changes were compared. The results showed that the polysaccharide contents of fermentation products from the three kinds of culture media were 4.65%, 3.76%, and 4.50%, respectively, and their relative standard deviation were 1.61%, 1.99%, and 1.86%, respectively. By observing the changes of the contents of polysaccharide, protein, and total saponin in fermentation products from the CMM-containing medium at different times, it was found that the 28th fermentation day was the time when secondary metabolism was the most active, and it should be the fermented terminal point (Chen and Chen 2004). For the same purpose, many combinations of Lingzhi and other medicinal herbs, such as Lingzhi with Astragalus membranaceu (Huang qi) (Zhu et al. 2010), Radix glycyrrhizaze (Gan cao) (Zhu et al. 2009), and Radix astragali (also called as Huang qi in Chinese) (He 2010), etc., containing medium have been investigated in the SSF (Gu et al. 2005).
In other reports, based on the purpose of functional food research and development, Lingzhi production by SSF using corn substrate was investigated, and the fermentation conditions were optimized through single factor and orthogonal tests. Meanwhile, the content of polysaccharide reducing sugar, protein, and amino acid was determined. The results showed that the size of crushed granule, strain, time, temperature, and inoculum size had a significant effect on the content of polysaccharide, reducing sugar, protein, and amino acid. The optimum fermentation conditions were 10 mesh granularity, 12% inoculum size, 28 °C fermentation temperature, and 20-day fermentation time. The content of polysaccharide was 21.97 mg/g under the optimized cultivation conditions (Gao 2007). In addition, more than ten beans as substrate supplements were applied for SSF of Lingzhi. These researches provided a foundation for further development of health food (Zhang et al. 2006; Wei et al. 2009; You 2009). In addition, Lingzhi (mushroom) cultivation can also help to convert agricultural and forest wastes into useful matter and reduce pollution in the environment.
Development of Ganoderma-based products
There has been a recent upsurge of interest in Lingzhi, which is not only a health food rich in polysaccharides and triterpenoids but also a source of biologically active compounds of medicinal value. Many of Lingzhi or its extracts may be processed as the complementary medicine/dietary supplements (DSs) for anti-cancer, anti-viral, immunopotentiating, hypocholesterolemic, and hepatoprotective agents (Paterson 2006; Zheng 2011). The bioactive compounds are extractable from either the Lingzhi mycelium or fruiting body and represent important components of the expanding Lingzhi biotechnology industry.
Extraction and purification of bioactive components
Lingzhi contains numerous bioactive components, such as polysaccharides, ergosterols, various proteins, unsaturated fatty acids, vitamins, and minerals (Zhou et al. 2007a, b). In previous publications, numerous studies all focused on the following three kinds of biactiove components, i.e., polysaccharides, triterpenoids, and functional proteins.
Polysaccharides
Lingzhi polysaccharide is one type of bioactive components isolated from Lingzhi and has a wide range of physicochemical properties. The bioactive polysaccharides (including protein/peptide bound polysaccharides) as pharmaceuticals have a long history and have received considerable attention in recent years. Importantly, previous research reports discovered that the water-soluble polysaccharides from Lingzhi characterized more than 20 types and strongly inhibited tumor growth (Zhou et al. 2007a). The major biologically active polysaccharides from Lingzhi are glucans, whose basic structure is β-1-3 d-glucopyronan with 1 to 15 U of β-1-6 monoglucosyl side chains and with (1→3)-β, (1→4)-β, and/or (1→6)-β linkages. The bioactive polysaccharides differ greatly in their composition and consequently in chemical structure, and one common feature is their relatively high molecular weight which has an average molecular weight of 104–6 Da (Huie and Di 2004; Chang and Lu 2004; Zhong et al. 2009).
The basic extraction methods for Lingzhi polysaccharides are the water extract–alcohol precipitation method. So many researches focused their attention on some parameters related to extraction such as extraction of temperature, time, and rate of raw materials to water (Song et al. 2008; Chen et al. 2009). However, the water extraction–alcohol precipitation methods have some disadvantages, such as long extraction time, low extraction rate, high extraction temperature, etc., and so previous researchers have done a lot of work on comparing the different extracting methods. Based on different cell-wall broken methods, the extraction methods can be divided into three ways: ultrasonic extraction method (Zhang et al. 2007, 2010), microwave extraction methods, and enzymatic method (Zhu et al. 2004; Lu 2009; Huang and Ning 2010). A previous review article presented a standard methodology involved in the extraction of Lingzhi polysaccharide (Huie and Di 2004). Actually, this is one of the extraction methods of water-insoluble polysaccharides from “Lingzhi”. This method is based on the attack of the enzyme on the polysaccharide substrates, composed of cellulose and lignin. For information, it has been reported that enzyme hydrolysis reaction could be enhanced by ultrasonic waves.
Triterpenoids/triterpenes
Other main bioactive components from Lingzhi are triterpenoids/triterpenes of which pharmacological effects have been demonstrated that are well known as antioxidative, immunomodulating and anti-tumor, etc. Major triterpenoids isolated from Lingzhi are different types of GA. There are dozens of GAs that have been isolated and characterized. Among them, GA-A, GA-B, and GA-C are the best representatives. The bitterness of Lingzhi mainly originated from GAs. Now, there are more than 130 oxygenated triterpenes (mostly lanostane-type triterpenes) that have been isolated from the fruiting bodies, spores, mycelia, and broths. The basic chemical structure is based on the ground structure of lanosterol, which is an important intermediate in the biosynthetic for steroid and triterpene in microorganisms and animals (Chang and Buswell 1999). According to the number of carbon atoms and the structural and functional groups, various triterpenes could be divided into three groups: C30, C27, and C24 compounds (Huie and Di 2004; Luo and Lin 2002). They have been demonstrated as GAs R, T, U, V, W, X, Y, and Z; lucidimol A and B; ganodermanondiol; ganoderiol F, etc. Importantly, many of them may also be a potential useful chemotherapeutical agent due to their biological activity which has been exhibited against actively growing tumors in vitro (Sliva 2003; Tang et al. 2006a, b; Zhang et al. 2008a, b). Up to 2010, a report presented that 43 triterpenoids was isolated from Lingzhi, and six of them are hitherto unknown. All of the compounds were assayed for their inhibitory activities against human HeLa cervical cancer cell lines (Cheng et al. 2010).
The different extraction methods of Lingzhi triterpenes mainly include (1) extraction with methanol or ethanol as the solvent, followed by direct separation of the extracts; (2) extraction by methanol or ethanol, followed by separation of the total acid portion by alkali treatment and separation of Lingzhi triterpene; and (3) extraction of the total acid portion by ether, followed by diazomethane methylation and then separation. Extraction of Lingzhi triterpene using ethanol is the easiest approach to maintain the activity of the extracts and to scale up its production (Gao et al. 2011). In addition, in order to enhance the rate of extraction of triterpenes and shorten the extraction time, the ultrasonic technique was often used for treatment of raw materials (fruit body or spore) in the production, which would destroy the dense structure of Lingzhi cells. After the abovementioned treatment, the Lingzhi triterpene was extracted by employing solvent (Huang et al. 2005).
The extraction and separation of triterpene components from Lingzhi have been reported in several studies. Ma et al.(2003) analyzed four medicinal triterpene components (ganosporeic acid A, lucidenic acid A, GA-B, GA-C) in the Lingzhi fruiting body of different origins by high performance liquid chromatography (HPLC) (Ma et al. 2003). Another report showed that they obtained three types of triterpenes through separation and purification from the Ganoderma fruiting body by chromatographic extraction, and the structures of the separated products were analyzed using ultraviolet–visible spectrophotometry (UV–vis), electrospray ionization (ESI), and proton nuclear magnetic resonance (1H NMR) spectroscopy (Ma 2008). Gu and his colleagues extracted the Lingzhi triterpene from the dry cells by means of solvent extraction within 14 days, and the maximum concentration of Lingzhi triterpene was 2.7 mg/100 mg (dry cells). When using 5% NaHCO3 solution as solvent, under the condition of ultrasonic treatment at 3 h, the concentration of Lingzhi triterpene was 2.63 mg/100 mg (dry cells). It reached 97% of maximum concentration of Lingzhi triterpene (Gu 2002). Huang et al. (2004a, b) added a step of ultrasonic treatment in the process of extraction of Lingzhi triterpene based on traditional extraction methods; meanwhile, the amount of solvent was reduced, the extraction time was shortened, and the extraction rate of Lingzhi triterpene also increased to 40% (Huang et al. 2004a, b).
In recent years, the supercritical CO2 extraction technology has been widely used in several food and pharmaceutical processing applications. Its characters with green and safety make it a desirable option compared with traditional organic solvent extractions. Traditional processing procedures often require additional steps, such as distillation, solvent extraction and maceration, etc. They are usually inferior to supercritical fluid extraction with respect to selectivity. Because the physical and chemical properties of carbon dioxide are safe, non-toxic, non-combustible, and is inexpensive with low critical temperature and pressure (31 °C, 1.38 MPa), the supercritical carbon dioxide to extract natural compounds from raw materials soon became popular for producing the nutraceuticals and functional foods and application in the pharmaceutical industry (Boumghar et al. 2009). The application methods for extraction of triterpene from Lingzhi fruit body were emergent in 2001 (Hsu et al. 2001), but the Chinese scientists are superior to researchers from other countries. For example, in 1998, Ma et al. (1998) studied impact factors of using supercritical CO2 extraction technology for extraction of the total triterpenoids from G. lucidum fruit body. Their experimental results showed that the better process parameters are extraction temperature 55 °C, methyl alcohol 10% (v/v), filling density of extractant 0.40 g/mL (it is 0.80 g/mL without methyl alcohol), equilibrium time 3 min, and fluid flow rate 3.5 mL/min (Ma et al. 1998). Yang et al. (2008) studied and optimized the process conditions of the supercritical CO2 extraction of the total triterpenoids of G. lucidum by response surface methods. The results showed that optimization of extraction conditions were: extraction pressure 30 MPa, extraction temperature 51 °C, and entrainment dosage of 2.2 mL/g (Yang et al. 2008). These studies provided a theoretical foundation for further extraction of bioactive components from Lingzhi fruit body by supercritical CO2 extraction technology. Up to now, many departments can use the technology to extract triterpenoid from Lingzhi. Chinese Academy of Sciences has extracted the triterpenoid from Lingzhi spore powder, named as “Lingzhi spore oil”, where the amount of extraction is superior to 20%. The large-scale productions of Lingzhi triterpene products have been successfully achieved.
Proteins/peptides
Except for Lingzhi polysaccharides and triterpenoids, Lingzhi also contains a large number of proteins and peptides with interesting biological activities, such as lectins, FIPs, ribosome inactivating proteins (RIP), antimicrobial proteins, ribonucleases, and laccases. They are not only important for life activity but also possess special immunogenicity so they showed immunomodulatory and anti-tumor effects (Zhou et al. 2007a, b; Xu et al. 2011). Among these proteins, many researches paid more attention to the FIPs which play important roles in anti-tumor activities, anti-allergy activities, promoting proliferation of lymphocytes, inducing the expression of cytokines, and anti-transplant rejection activities (Zhou et al. 2007a, b; Sun and Wang 2009; Li et al. 2011a, b). Since the first FIP was isolated and purified in 1989 (Kino et al. 1989), up to now, seven FIPs have been identified, i.e., LZ-8 or FIP-glu (from G. lucidum), FIP-gts (G. tsugae), FIP-fve (Flammulina velutipes), FIP-vvo (Volvariella volvacea), FIP-gja (GenBank accession no. AY987805, G. japoncium), FIP-gmi (G. microsporum), and FIP-gsi (G. sinense) (Li et al. 2011a, b).
FIP, one molecule of which weighs approximately 13 kDa, is composed of 110 to 114 amino acids, but it lacks His, Cys, and Met while it is rich in Asp and Val. In the N-terminal, the amino acid was acetylated. The original studies have demonstrated that the LZ-8 (FIP-glu) contained the carbohydrate in a low level (about 1.3%) (Kino et al. 1989). Subsequently, the other three FIPs (FIP-fve, FIP-vvo, and FIP-gts) were isolated and identified, respectively, and their physical and chemical characteristics presented that these proteins were the pure protein without carbohydrate. All of them have great similarity with immunoglobulin super family in the aspects of structure and function, and they have many pharmaceutical activities such as anti-tumor and anti-allergy, stimulating immune cells to produce a variety of cytokines (Li et al. 2011a, b). All of these promise a good clinical application prospect and the value of medicinal care.
Because the lower content of FIPs exists in Lingzhi, which becomes the key restrictive factors for developing and utilizing FIPs, how to get an amount of protein has become the major concern. With the development of the molecular biology and bioengineering technology, the utilization of engineering strain for the industrial production of medicinal proteins has been made possible. Therefore, scientists worldwide focused their interests on utilization of genetic engineering technology to develop FIP products, such as cloning the FIP genes (Murasugi et al. 1991; Lin et al. 1997; Ko et al. 1997; Tsai 2007; Zhou et al. 2009), expression of the genes in prokaryotic and eukaryotic cells (Huang et al. 2008; Bai et al. 2006; Li et al. 2009; Xu 2009; Li et al. 2010a, b; Li et al. 2011b), and how to enhance the bioactivities of FIPs (Ko et al. 2009; Lin 2009a, b; Zhou et al. 2011a, b).
Along with the fast development of urban industrialization and aggravation of environmental pollution, a variety of new diseases, especially those caused by virus, have been emergent, such as the severe acute respiratory syndrome (SARS), avian influenza, H1N1 influenza virus, etc., which seriously threaten our health and even life. Though we could prevent some diseases by means of vaccines, the development of vaccine does not always catch up with the speed of virus mutation. Therefore, the most fundamental way is to improve the human body’s immune system. Furthermore, biotechnology gives a chance and challenge to the study of fungal immunomodulatory proteins.
Quality control of Ganoderma-based product
The quality control of Lingzhi is a complicated and continuous process used to maintain standards in products in services. A good quality control system of Lingzhi production includes the selection of strain, cultivation, harvest, processing, packaging, and transportation. Thus, the quality control of Lingzhi must begin to carry out from the cultivation, and the determining techniques and performance standards of each section are fundamental for the quality control of Lingzhi products.
Firstly, we should establish a standard production technology system of Lingzhi raw materials. After large-scale cultivation of Lingzhi had been performed, the standard production technology system was set up gradually, which was divided into two stages or two cultivation patterns.
The first stage of cultivation pattern was to culture the Lingzhi under a big awning from 1988 to 1990. The raw materials for the cultivation of Lingzhi are mainly short basswood. At that time, 10,100 stere (m3) short basswood were used for testing to culture Lingzhi in Fujian (China); the Lingzhi yields reached 25–30 kg per stere short basswood. The pileus (caps) of over 8 cm was at the rate of 60% of the total yield, which could be designed in the grade degree for international export. During the 3 years, 250 tons of Lingzhi were produced in Fujian province, and the quality of products reached the international level of similar products (Chen and Wu 2005). Subsequently, in order to obtain more spore powders, some producers deteriorated the quality of Lingzhi fruit bodies. Therefore, how to ensure the standard production technology system should not be ignored.
The second stage of cultivation pattern was to set up the Lingzhi culture base. At that time, a series of laws were issued for TCM. For example, the Good Agricultural Practice (GAP) Guideline for CMM was issued by the State Drug Administration in July 2002. The GAP Certification Examination Evaluation Standards for CMM (for trial implementation) and the Management Methods (for trial implementation) of this file were declared in September 2003. From then on, the GAP Guidelines served as the basic principles for the production and quality management of CMM, and these guidelines were applicable to the whole production process, transportation, and all major quality-managing procedures of CMM (including medicinal mushroom). The major contents included selecting the strains, ecological environment of production site, cultivation management, harvest and primary processing, packaging, transportation and storage, quality management, personnel and facilities, documentation, etc. The GAP Guidelines also referred to monitor the air, water, and soil quality, and analyze the heavy metals and pesticide residues, etc. (Luo et al. 2005; Lin et al. 2005a, b).
Secondly, the determination and control techniques of bioactive components from Lingzhi should be confirmed. As mentioned above, polysaccharides and triterpenoids are major components for developing various products in which the content determination methods of those bioactive components could be divided into two types: UV spectrophotometry and HPLC. Using these techniques, Lingzhi and its products could be better in quality.
UV spectrophotometry was previously used to determine polysaccharides in China, and now it has become a simple and reliable method with a mature technology. In the process of polysaccharides content determination, glucose is often used as the standard substance. The phenol–vitriolic colorimetric method and anthrone–sulfuric acid colorimetric method were usually employed (Ning 2006; Wang et al. 2007a, b, c; Guo et al. 2010). Now beta glucans is often used as the standard for examining the polysaccharide content (Lin et al. 2005a, b), and the HPLC method for polysaccharide determination is also developed gradually (Han et al. 2009).
The triterpenoid determination often uses oleanolic acid, ursolic acid, or GA-B as the standard substance. When using oleanolic acid and ursolic acid as the standard substance, the triterpenoid content determination exists in the high deviations. When using GA-B as the standard substance, the determining data is close to the actual content in products. It is noteworthy that the oleanolic acid and ursolic acid are neither bioactive components from Lingzhi nor the specificity ingredients of Lingzhi. Using these substances as standards for the triterpenoid contents determination from Lingzhi has been in dispute in the academic circles (Wasser 2011). Which bioactive components should be used for evaluation of the quality of Lingzhi-based products? What standard substance should be used for determination of polysaccharides or triterpenoid from Lingzhi? In our opinion, the effective components are different due to different research or producing purpose, and the selection of bioactive component or standard substance should be based on the usage of the products and the demand of customers. The reports of bioactive component should state the standard used in the determining process.
A simple, rapid, and accurate HPLC method allowing the quantification of GA in different kinds of Lingzhi and their products was developed and validated (Huie and Di 2004; Tang et al. 2006a, b; Wang et al. 2007a, b, c; Keypour et al. 2010). So setting up the standard fingerprint and analysis methods of Lingzhi, and controlling the Lingzhi and its product quality have attracted attention in the academic circles worldwide (Gu and Weng 2008; Ding et al. 2009a, b; Adamec et al. 2009). Determination of triterpenoid content usually uses the GA-B as the standard. The methods of the determination have undergone a rapid development, which may provide multiple choices. Modern hyphenated techniques, such as GC–MS, HPLC–MS, HPLC–MS–MS, and HPLC–NMR, have been rapidly developed, which can provide useful structural information online on Lingzhi triterpene metabolites and allowed the rapid structural determination of known organism constituents with only a small amount of materials. However, these techniques have not been applied in the research and development of Lingzhi (Tang et al. 2006a, b). It is worthwhile to apply modern hyphenated chromatographic techniques to the characterization and determination of a variety of components in Lingzhi extracts.
Actually, the quality control of Lingzhi medicinal materials and its extracts is a complicated engineering system. Besides the cultivation of Lingzhi that is based on the GAP Guidelines, we should also pay much attention to some key technologies, such as extracting technique, determining technique, and other engineering techniques. The extraction and purification of Lingzhi bioactive components are based on international standards. The Lingzhi producers producing the materials and preparation should also adopt the international standards. If we do so, Lingzhi and its products would be further promoted in the international markets (Lin 2009a, b).
Development products
As mentioned above, Lingzhi has been reported to possess a number of pharmacological effects. Lingzhi has now become recognized as an alternative adjuvant in the treatment of leukemia, carcinoma, hepatitis, and diabetes. As an immune system enhancer and modulator with health benefits, Lingzhi is generally safe for long-term use. In the last decade, clinical trials on the use of Lingzhi preparations to treat cancer and other diseases have been reported in international peer-reviewed journals. A summary of the therapeutic effects and bioactive components of Lingzhi reported has been presented in a recent review article (Sanodiya et al. 2009). Lingzhi is now consumed worldwide as a health tonic and as a DS. Millions of people take it every day to enhance their energy, to improve their digestion, and to sleep better. Lingzhi is used also both for the prevention and for the treatment of a number of health problems that require a balanced immunoresponse system and also a healthy cardiovascular system (Chang and Miles 2004). Lingzhi-based products have attracted a great deal of attention during the last 20 years not only in some Asian countries but also in North America and Europe, and they are generally divided into three types of products (Xie et al. 2002).
Developmental products based on Lingzhi fruit bodies
About 80–85% of all Lingzhi products are derived from the fruit bodies, which have been either commercially cultured or collected from the wild. Lingzhi has beneficial effects not only as drugs but also as a novel class of products as follows: DS, functional foods, nutraceuticals, mycopharmaceuticals, and designer foods that produce healthy benefits through everyday use as part of a healthful diet (Wasser 2011). Based on the forms of Lingzhi preparation, Lingzhi products could be divided into the single form of preparation and the complex form of preparation. The single form of preparation mainly includes Lingzhi slices, form of pill, tablet, powder, granule, etc., and complex form of preparation refers to the Lingzhi produce contained in the Lingzhi and other components from Chinese herb medicine (Wu and Zeng 2005). Here, in order to state conveniently, we present the fruit-based products relative to its processing detail level. According to this opinion, the process forms of utilizing Lingzhi fruit body involve the following three aspects.
Firstly, the fruit body is directly crushed for processing products. Breaking up the fruit body is generally realized by using a breaking machine. In the process of crushing fruit body, the Lingzhi hairs are easy to produce, which are difficult to go through the filter screen. So someone added cool water to Lingzhi powder and worked the mixture into a paste. This mixture was aired or dried under a lower temperature and then crushed again (Xu and Xu 1999). This method is usually used for processing Lingzhi health wine. For example, Kim et al. studied how the Lingzhi fruit body affected the function of traditional rice wine (Yakju). The results showed that the Lingzhi Yakju has a higher angiotensin-converting enzyme inhibitory activities and similar to the superoxide dismutase activities. In other words, Lingzhi Yakju was a new functional rice wine for anti-hypertension (Kim et al. 2004b). Till now, a series of wine or liquor, such as the ginseng G. lucidum Sihe liquor (Liang et al. 2003), the healthy wine of germanium (Ge)-enriched Ganoderma and Codycepes (Wang 1998, 2005) etc., have been developed (Leskosek-Cukalovic et al. 2010).
Secondly, the developing product is based on the crude extracts from Lingzhi. As mentioned above, many extracting methods were usually applied in the Lingzhi fruit body. The different methods result from the component differences, and the product development often relies on the different extracting methods for different purposes. Till now, the Lingzhi fruit body-based products are often adopted in the crude extracts in many cases. For example, numbers of Lingzhi-based products consist of the crude extracts from Lingzhi, such as Ge Quan-yuan oral liquid exploited by Japan, Dong-fang Lingzhi Bao, Shuang-ling Gu-ben San, etc.
Thirdly, the bioactive components are the materials for Lingzhi-based products. The main bioactive components from Lingzhi are triterpenes and polysaccharides. The products-based triterpenes are from Lingzhi spore, while products-based polysaccharides are from fruit body. The bioactive components extracted from Lingzhi should be processed into various forms of products, such as tablet, capsule or soft capsule, injections, etc. The developing products-based fruit body was also summarized in some references (Xiao et al. 2006; Sun et al. 2001b).
The increasing interest in traditional remedies for various physiological disorders and the recognition of numerous biological activities of Lingzhi products have led to the coining of the term “Lingzhi Bao”, which should not be confused with nutraceuticals, functional foods, and pharmaceuticals. For example, China Ganoderma lucidum essence (CGLE), a famous Lingzhi product in China, consists of Lingzhi powders and Lingzhi spores, which contained the rich polysaccharides, proteins, amino acids, triterpenes, organic germanium, etc., and the producer declares that the product holds broad bioactive functions, such as regulating and nourishing the body, strengthening and consolidating the body, tranquilizing and allaying excitement, etc. The scientists of Shanghai Institute of Materia Medica Chinese Academy of Sciences studied the inhibitory effects of CGLE on DNA topoisomerases and its ability to induce apoptosis of K562 cells. They found that CGLE could markedly inhibit the activities of topoisomerase I and II, and promote the relaxing and breaking of DNA (Jiang et al. 1999). Shi et al. studied the determination methods of Lingzhi polysaccharides of CGLE based on the description methods in Chinese Pharmacopoeia and presented the quality standard of this product (Shi et al. 2002). However, the CGLE is only a nutraceutical but not a pharmaceutical. So it is difficult to define whether a Lingzhi product is a drug or nutraceutical, which is consumed in the form of capsules or tablets as a DS (not a food) and has potentially therapeutic applications.
Developmental products based on Lingzhi mycelia
Except for the products based on fruit bodies, only 15–20% of all products are based on extracts from Lingzhi mycelia. A smaller percent of Lingzhi-based products are obtained from culture filtrates. But as the above-mentioned advantage, cultivation of Lingzhi mycelia is the main method for large-scale production of Lingzhi metabolism substances in the industry. Utilization of substances by LSF of Lingzhi was divided into three aspects.
Firstly, the fermentation broth used for processing products was directly utilized. Someone inoculated Lingzhi mycelia into the liquid media with high protein and deleting fat. After fermentation for 3 days, milk powder and stabilizer were added into the fermentation broth, and then the fermentation broth was homogenized and sterilized to further develop a new nutraceutical drink, which was named Lingzhi protein milk. This procedure utilized all Lingzhi mycelia for a drink, which increased the content of free amino acid and removed the beany flavor (He 2000).
Secondly, Lingzhi fermentation broth is combined with other bioactive components for use in developing new products. In the process of Lingzhi fermentation, the enzyme excreted from mycelia affects the components of fermentation broth to produce a novel bioactive component. Meanwhile, the metabolism substances produced could be released into the fermentation broth. So Lingzhi fermentation broth may directly be used for producing nutraceutical wine, drink, or beer (Leskosek-Cukalovic et al. 2010) such as the Lingzhi tea and Lingzhi drink produced by Korea and other countries. In China, after filtering off the mycelia, Lingzhi fermentation broth was inoculated into the beer yeast for further fermentation, and then the complex was developed as “double fungi” fermentation drink (Pan and Li 1997). Qin and Gao initially researched to manufacture the orally taken liquid of mythic fungus dark chicken, which was related to the technical process and correlated parameter (Qin and Gao 2002)
Thirdly, the bioactive components extracted from Lingzhi mycelia are used for developing the products. In industry, the extraction of the bioactive components from Lingzhi mycelia is the same as the extraction from fruit bodies. Till now, the developing products include the Leishi Pai Antai Lingzhi mycelia capsule produced by Shanghai Ley’s Pharmaceutical Co., Ltd., and the Dahan Lingzhi mycelia capsule produced by Shanghai Dahan Lingzhi nutraceuticale Co., Ltd. In addition, Lingzhi tablet, Jisheng injection, Bozhi glycopeptide injection, Bozhi injection, etc., were all produced by Lingzhi mycelia (Hong 1992). Also, some complex nutraceutical that was manufactured by Lingzhi mycelia adding other natural materials, for instance, Lingzhi-Yiner oral liquid, consisted of the extracts from Lingzhi mycelia, adding other natural materials from Tremella fuciformis, Liriope platyphylla Wang et Tang, Codonopsis pilosula, Glycyrrhiza uralensis, etc. (Xiao 2002).
Developmental products based on Lingzhi spore powder
For processing the Lingzhi spore, the main performances included the following: (1) after sterilizing, Lingzhi spore powders are directly used for developing the decoction or capsule; (2) after breaking the cell wall of Lingzhi spore, it was directly processed as a series of products, such as Lingzhi spore capsule, tablet, granular infusion, etc.; (3) extracting the bioactive components manufacture as the intensely processed products (Xie et al. 2002). For example, Lingzhi spore oils have been proven to be the useful bioactivity components for blood-fat lowering (Li et al. 2006; Liu et al. 2007) and anti-tumor products (Bian et al. 2008), so many famous Lingzhi products, such as Zhongke Lingzhi spore oils soft capsule manufactured by Nanjing Zhongke Group Corp., Ltd., mainly consist of Lingzhi spore oils.
It is estimated that there are more than 100 research institutes that specialize in the study of medicinal mushroom Lingzhi, and more than 200 factories engage in the production of drugs and nutraceuticals in China. Meanwhile, many patented products have emerged which include the preparation of anti-tumor, liver function accelerant, lowering of blood pressure, hypoglycemic activity, lowering of cholesterol levels, treatment of chronic bronchitis, immunomodulator, lysozyme as antibiotic and shampoo, body shampoo, etc. (Xie et al. 2002).
Problems and prospects
Early in 1980s, there are some Lingzhi-based products in the international market, such as Lingzhi decoction, syrup, tablet, and injection liquid, etc. Nowadays, the types of Lingzhi products are already completed, including over 20 kinds of Lingzhi drugs (capsule, tablet, granule, oral liquid and tincture, etc.), 20 kinds of Lingzhi health liquor, over 30 kinds of Lingzhi DSs, and more than 10 kinds of cosmetology products. However, these products could neither meet the demand of consumer nor achieve the goals of development not only in the technology content but also in the product quality. And so the development of Lingzhi-based product needs further in-depth study.
Problems
First, it is critical to improve and enhance the classification and identification level of Lingzhi species. Lingzhi encompasses several Ganoderma species, which are widely used for medicinal purposes, e.g., G. lucidum (Leyss. ex Fr.) Kars; G. sinensis Zhao, Xu et Zhang, G. japonicum (Fr.) Lloyd, G. tsugae Murr., G. atrum, G. applanatum (Pers.) Pat., and G. tenue Zhao, Xu and Zhang. According to the famous ancient Chinese plant medical books, i.e., Shen Nong Ben Cao Jing and Ben Cao Gang Mu, six Lingzhi varieties were known in China at that time. In other words, Lingzhi are classified into six types of Lingzhi based on such colors as purple, red, white, black, green, and yellow. Actually, this classification has no scientific basis at all and should not be used in scientific literatures. However, many scientific reports still refer to these names. Now more than 100 Lingzhi species have been reported in China, and over 250 Lingzhi species have been described in the world (Wasser and Weis 1997; Zhou and Lin 1999).
In possession of the data resource of fungi, the classification and identification of fungi are mostly based on the morphologic characters, ecological environment characters, physiological and biochemical characteristics, culture characteristics, etc. In recent years, because of the necessity of investigating and developing medicinal mushroom, taming strain and cross-breeding, it is necessary to reasonably determine and exactly identify the medicinal mushroom species. However, the techniques only relying on the traditional classification have exposed some disadvantages and difficulties. The results based on traditional classification methods often cause the errors or could not be capable to analyze the similar strains (Ryvarden 1991; Dai and Yuan 2010). However, in therapeutic practices and literature citations, Lingzhi usually refers to the species of G. lucidum.
Second, we must improve and consummate the quality standard of the fungus. Setting up the quality management system of Lingzhi products includes the identification and control of the bioactive components, and the hazardous and noxious substances such as heavy metals and residual pesticide, etc., which can promote the realization of large-scale and standardization production.
The bioactive components are a significant index for quality evaluation of Lingzhi products. However, the amount of bioactive components is large and the type of bioactive components is various from Lingzhi. Thus, only relying on crude compounds for controlling the product’s quality does not represent the bioactivity of Lingzhi. It is necessary to deeply study the bioactive components from different Lingzhi, i.e., rapidly isolate the bioactive components and identify their structures and study their affecting mechanisms. Then, based on the chemistry and pharmacodynamics research, the new control standard and production process of Lingzhi products should be set up.
The safety evaluation, especially for heavy metals and residual pesticide, is an indispensable work to control the quality of Lingzhi products. Nowadays, every country has adjusted the content of heavy metals. For example, the European Union has carried out the new laws, EC629/2008 setting the maximum levels for certain contaminants in foodstuffs, in which the lead and cadmium contents of some mushroom are adjusted to 0.3 mg/kg and 0.2 mg/kg, respectively. But in Chinese national standard for edible fungi (GB7096-2003), the maximum level of lead is 1.0 mg/kg, but the cadmium content has not been illustrated. Therefore, the improvement of edible fungus quality standard makes the Lingzhi products conform to worldwide hygienic requirement so that the Lingzhi products move towards the world market.
Finally, we should carry out the study of quality control while we develop the prescription product based on the compound from Lingzhi. Lingzhi contains a huge quantity of metabolite (Paterson 2006), and many scholars devote their time in the isolation and purification of fungal metabolites, and are expected to obtain the bioactive compound with a specific structure as the pro-drug. But it is noteworthy that not all medicinal mushrooms could be developed for a single structure drug (Chang 2009). Of course, Lingzhi is also not an exception. Various components from Lingzhi need to mix with the compounds, which can hold good curative effects for some diseases. Based on previous literatures, TCM components and Lingzhi metabolites affect each other by enhancing their curative effects (Huang et al. 2006; Xu et al. 2010a, b). It is true that the TCM is a crystallization of the wisdom of our Chinese nation, and is the major component of excellent culture of Chinese nation and the world traditional medicine. However, the bioactive components and the mechanism of their actions are more complex, which give a challenge for mushroom researchers. What is the quality standard? How do you control the product quality? A series of problems await our further study.
Prospects
Lingzhi have been cultivated for more than 50 years, and it is expected that their production will increase further in the future due to market demand. With the development of modern biotechnology, especially genetic engineering technique, molecular manipulations have been added to mutational techniques as means of increasing titers and yields of microbial processes. The breeding of a mushroom species with great quality would become capable for production. For example, the breeding of new Lingzhi strains will improve the development of strains with high yield and resistance to diseases, increasing productivity and diminishing the use of chemicals for pest control. In addition, the improvement and development of modern engineering technologies, such as computerized control systems to control environmental parameters, techniques for the production of mushrooms in a new substrate, and new methods for substrate sterilization and spawn preparation, will increase the productivity of mushroom culture. All these aspects will be crucial in the production of Lingzhi with better appearance, texture, nutritional qualities, and medicinal properties at low cost.
LSF techniques have been widely developed for most of the main medicinal mushrooms (including Lingzhi) and used in the propagation of mycelium for three main applications, viz. (1) liquid spawn for solid substrate fruit-body production, (2) biomass that can be used for food and dietary supplements, and (3) biomass and/or extruded metabolites especially exopolysaccharides as raw materials for pharmaceutical studies. Comparing SSF with the LSF, in the process of SSF, the cultivation of Lingzhi mycelia used agricultural byproducts, in which the contents of polysaccharide from mycelia are several times larger than natural or artificial cultivation of Lingzhi fruit body (Sheng and Ma 2004; Chen et al. 2007a, b). So using the SSF technique to produce polysaccharides could reduce the cost of production of polysaccharide from mycelia. But the quality controls of SSF are really worthwhile to study further. In addition, the bidirectional SSF technique is a new technology with developmental prospect, and using these methods could probably be helpful to find some novel drugs or pro-drugs, but the study regarding the control of fermentation process and the analysis and determination of the metabolites from bidirectional SSF process should be emphasized.
Environmental pollution is one of the problems threatening human existence. Lingzhi cultivation and research is beneficial for the prevention and control of environmental pollution. On the one hand, zero pollution should be regarded as the goals of development and utilization of Lingzhi. The production of Lingzhi generates a large amount of spent substrate, which can be used as animal feed, soil conditioner, for mushroom re-cultivation, and for bioremediation, among other applications. On the other hand, resource utilization of cellulose has become a hot topic in recent years. Due to less damage to the environment and realization of resource recycling, microorganism, as a way of degrading cellulose, has been given more and more attention. Lingzhi is one of the most potential genera. Medicinal mushroom culture is a biotechnological process that recycles lignocellulosic wastes since these are converted to a food for human consumption and the spent mushroom substrate could be used in several ways. In addition, mushroom cultivation could also help to convert agricultural and forest wastes into useful matter and reduce pollution in the environment. Therefore, mushroom cultivation at least has three important contributions: production of health food, manufacture of nutraceuticals, and reduction of environmental pollution. Based on the existing international research progress about medicinal mushroom, there are both opportunities and challenges for the Lingzhi development.
Acknowledgments
This research is financially supported by the Natural Science Foundation of China, Shanghai Science and Technology Committee and Shanghai Leading Academic Discipline Project (Project Number B209).
Contributor Information
Xuan-Wei Zhou, Phone: +86-21-34205778, FAX: +86-21-65642425, Email: xuanweizhou@sjtu.edu.cn.
Yong-Ming Zhang, Phone: +86-21-64321071, FAX: +86-21-64321071, Email: zhym@shnu.edu.cn.
References
- Adamec J, Jannasch A, Dudhgaonkar S, Jedinak A, Sedlak M, Sliva D. Development of a new method for improved identification and relative quantification of unknown metabolites in complex samples: determination of a triterpenoid metabolic fingerprint for the in situ characterization of Ganoderma bioactive compounds. J Separ Sci. 2009;32:4052–4058. doi: 10.1002/jssc.200900496. [DOI] [PubMed] [Google Scholar]
- Anderson JB, Petsche DM, Smith ML. Restriction fragment polymorphisms in biological species of Armillaria mellea. Mycologia. 1987;79:69–76. [Google Scholar]
- Anhui Bureau of Quality and Technical Supervision (2004) Anhui province local standards—Ganoderma in Dabie Mountain (strain). DB34/T 482-2004 (In Chinese)
- Bai JY, Zeng L, Liu Y, Li YF, Lin ZP, Hu YL. Expression of LZ-8 from Ganoderma lucidum in transgenic tobacco and primary study on characteristic of recombinant LZ-8. Mol Plant Breed. 2006;4:645–649. [Google Scholar]
- Bardakci F. Random amplified polymorphic DNA (RAPD) Turk J Biol. 2001;25:185–196. [Google Scholar]
- Bian S, Xu L, Fang TH, Jia M, Chen CH. Simple explanation of the function on Ganoderma lucidum spore oil in mouse T lymphocyte. J Practical Tradit Chin Intern Med. 2008;22:47–48. [Google Scholar]
- Blundeoll V. DNA fingerprinting—review paper. J Appl Sci Res. 2006;1:136–140. [Google Scholar]
- Boumghar Y, Benali M, El-Mehdi N, Martel AC (2009) Supercritical fluid extraction of triterpenoids from powdery white birch barks. The 8th World Congress of Chemical Engineering. Aug 23–27; Montreal, Quebec, Canada
- Chang ST (2009) Medicinal mushroom products: nutriceuticals and/or pharmaceutics. Proceedings of the 5th International Medicinal Mushroom Conference; Sep 3–12; Nantong, China
- Chang ST, Buswell JA. Ganoderma lucidum (Curt.: Fr.) P. Karst. (Aphyllophoromycetideae): a mushrooming medicinal mushroom. Int J Med Mush. 1999;1:139–146. [Google Scholar]
- Chang YW, Lu TJ. Molecular characterization of polysaccharides in hot-water extracts of Ganoderma lucidum fruiting bodies. J Food Drug Anal. 2004;12:59–67. [Google Scholar]
- Chang ST, Miles PG. Mushrooms cultivation, nutritional value, medicinal effect, and environmental impact. Boca Raton: CRC; 2004. pp. 357–372. [Google Scholar]
- Chen AW. Cultivation of the medicinal mushroom Ganoderma lucidum (Curt.: Fr.) P. Karst. (Reishi) in North America. Int J Med Mushrooms. 1999;1:263–282. [Google Scholar]
- Chen HZ, Chen JW. A preliminary report on solid-state fermentation of Ganoderma lucidum with Radix astragali containing medium. Chin J Integr Med. 2004;2:216–218. doi: 10.3736/jcim20040320. [DOI] [PubMed] [Google Scholar]
- Chen J, Su KM. Proceeding of heredity, breeding and identification of edible fungi. Edible Fungi Chin. 2008;27:3–8. [Google Scholar]
- Chen TQ, Wu JZ. Study of log-cultivated Ganoderma in Fujian. Fujian: Xiamen University Press; 2005. [Google Scholar]
- Chen H, Hu YH, Qin JZ, Gu SR. Study on conditions of Ganoderma protoplasts fusion. Food Sci. 2007;28:173–176. [Google Scholar]
- Chen Y, Liang YX, Hu YM, Ge XY. Extraction and component analysis of polysaccharides from Ganoderma lucidum mycelia. Edible Fungi Chin. 2007;26:34–37. [Google Scholar]
- Chen SD, Hsieh MC, Chiou MT, Lai YS, Chenga YH. Effects of fermentation products of Ganoderma lucidum on growth performance and immunocompetence in weanling pigs. Arch Anim Nutr. 2008;62:22–32. doi: 10.1080/17450390701780201. [DOI] [PubMed] [Google Scholar]
- Chen J, Lei LS, Chang HL. Study on extraction of active polysaccharides from Ganoderma lucidum. J Guangdong Pharmaceut Coll. 2009;25:348–351. [Google Scholar]
- Chen Y, Bicker W, Wu J, Xie MY, Lindner W. Ganoderma species discrimination by dual-mode chromatographic fingerprinting: a study on stationary phase effects in hydrophilic interaction chromatography and reduction of sample misclassification rate by additional use of reversed-phase chromatography. J Chrom. 2010;1217:1255–1265. doi: 10.1016/j.chroma.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Chen ZH, Yu ZH, Zeng WB, Yang JY, Yuan J, Chen YJ. Solid fermentation technique for cordycepin production by Cordyceps nigrella strain cnig -56. Acta Edulis Fungi. 2010;17:80–82. [Google Scholar]
- Cheng CR, Yue QX, Wu ZY, Song XY, Tao SJ, Wu XH, Xu PP, Liu X, Guan SH, Guo DA. Cytotoxic triterpenoids from Ganoderma lucidum. Phytochemistry. 2010;71:1579–1585. doi: 10.1016/j.phytochem.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Chi XM. Research on Ganoderma cultivation techniques in China. Lishizhen Med Mater Med Res. 2005;16:791–792. [Google Scholar]
- Chiu SW, Luk VY, Yu S, Lee P, Wai N, Fu P, Cheung KW. Artificial hybridization of Ganoderma lucidum and G. tsugae Murrill by protoplast fusion for sustainability. Int J Med Mushrooms. 2005;7:263–280. [Google Scholar]
- Choi SH, Kim BK, Kim HW, Kwak JH, Choi EC, Kim YC, Yoo YB, Park YH. Studies on protoplast formation and regeneration of Ganoderma lucidum. Arch Pharm Res. 1987;10:158–164. [Google Scholar]
- Dai YC, Yuan HS. Type studies on polypores described by J. D. Zhao. Ann Bot Fennici. 2010;47:113–117. [Google Scholar]
- Dejaegher B, Heyden YV. HILIC methods in pharmaceutical analysis. J Separ Sci. 2010;33(6-7):698–715. doi: 10.1002/jssc.200900742. [DOI] [PubMed] [Google Scholar]
- Deragon JM, Landry BS. RAPD and other PCR-based analysis of plant genomes using DNA extracted from small leaf disks. Genome Res. 1992;1:175–180. doi: 10.1101/gr.1.3.175. [DOI] [PubMed] [Google Scholar]
- Ding P, Qiu J, Liang Y, Wang H. Chromatographic fingerprints of triterpenoid constituents of Ganoderma lucidum. Chin J Chin Mater Med. 2009;34:2356–2359. [PubMed] [Google Scholar]
- Ding Y, Seow SV, Huang CH, Liew LM, Lim YC, Kuo IC, Chua KY. Coadministration of the fungal immunomodulatory protein FIP-Fve and a tumour-associated antigen enhanced antitumour immunity. Immunology. 2009;128:e881–e894. doi: 10.1111/j.1365-2567.2009.03099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HX, Liang HX, Qu HJ. Study on submerged culture characteristics of Ganoderma lucidum. Jiangsu Agr Sci. 2004;4:88–89. [Google Scholar]
- Dong YW, Miao JZ, Cao ZH, Gao MX, Lv ZQ. UV mutagenization of protoplast for screening and breeding of high-yield organo germanium-producing strains of Ganoderma lucidum. Food Sci. 2009;30:188–192. [Google Scholar]
- Erkel EI. The effect of different substrate mediums on yield of Ganoderma lucidum (Fr.) Karst. J Food Agr Environ. 2009;7:841–844. [Google Scholar]
- Fang OH, Zhong JJ. Submerged fermentation of high fungus Ganoderma lucidum for production of valuable bioactive metabolites—ganoderic acid and polysaccharides. Biochem Eng J. 2002;10:61–65. [Google Scholar]
- Fang QH, Zhong JJ. Effect of initial pH on production of ganoderic acid and polysaccharide by submerged fermentation of Ganoderma lucidum. Process Biochem. 2002;37:769–774. [Google Scholar]
- Ferenczy L, Kevei F, Zsolt J. Fusion of fungal protoplasts. Nature. 1974;248:793–794. doi: 10.1038/248793a0. [DOI] [PubMed] [Google Scholar]
- Ferreira IC, Vaz JA, Vasconcelos MH, Martins A. Compounds from wild mushrooms with antitumor potential. Anticancer Agents Med Chem. 2010;10:424–436. doi: 10.2174/1871520611009050424. [DOI] [PubMed] [Google Scholar]
- Friel MT, McLoughlin AJ. Production of a liquid inoculum/spawn of Agaricus bisporus. Biotechnol Lett. 2000;22:351–354. [Google Scholar]
- Fujian Bureau of Quality and Technical Supervision (2002) Study of log-cultivated Ganoderma in Pucheng (strain); DB35/T 163.2-2002. (In Chinese)
- Gao WG. The production of Ganoderma lucidum by solid-state fermentation using corn as substrate. Chin Agr Sci Bull. 2007;23:422–427. [Google Scholar]
- Gao YH, Lan J, Dai XH, Ye JX, Zhou SH. A phase I/II study of Ling Zhi mushroom Ganoderma lucidum. (W. Curt.: Fr.) Lloyd (Aphyllophoromycetideae) extract in patients with type II diabetes mellitus. Int J Med Mushrooms. 2004;6:33–39. [Google Scholar]
- Gao JJ, Hirakawa A, Min BS, Nakamura N, Hattori M. In vivo antitumor effects of bitter principles from the antlered form of fruiting bodies of Ganoderma lucidum. J Nat Med. 2006;60:42–48. [Google Scholar]
- Gao MX, Qin WD, Miao JZ, Tu BJ, Lv ZQ. Research on deep cultivation of calcium-enriched Ganoderma lucidum. Biotechnol Lett. 2007;18:641–643. [Google Scholar]
- Gao MX, Miao JZ, Cao ZH, Dong YW, Lv ZQ. Study on breeding of Ganoderma strains producing high polysaccharides by protoplast electrofusion technique. Jiangsu Agr Sci. 2008;6:89–91. [Google Scholar]
- Gao Y, Zhang RH, Zhang J, Gao S, Gao WX, Zhang HF, Wang HT, Han B. Study of the extraction process and in vivo inhibitory effect of Ganoderma triterpenes in oral mucosa cancer. Molecules. 2011;16:5315–5332. doi: 10.3390/molecules16075315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb AM, Ferrer E, Wright JE. rDNA analysis as an aid to the taxonomy of species of Ganoderma. Mycol Res. 2000;104:3033–3045. [Google Scholar]
- Gu J. Extraction of ganoderic acid from Ganoderma lucidum by using ultrasonic assistance. J Nantong Inst Tech. 2002;3:13–16. [Google Scholar]
- Gu ZY, Weng XC. RP-HPLC fingerprint for quality assessment of Ganoderma lucidum. J Shanghai Univ (Nat Sci) 2008;14:652–655. [Google Scholar]
- Gu SR, Qin JZ, Chen H. New solid fermentation of 14 Ganoderma strains and the study on polysaccharose content of the fermentation product. Lishizhen Med Mater Med Res. 2005;16:313–314. [Google Scholar]
- Guo JL, Liu X. Comparison between liquid culture and solid culture technologies for edible mushroom spawn production and analysis on their economic benefits. Sci Agr Sin. 2011;44:835–841. [Google Scholar]
- Guo XL, Zhu SC, Zhai XF, Wang HY, Bao L. Comparison of methods in determination of polysaccharide in Ganoderma lucidum. Chin Archiv Tradit Chin Med. 2010;28:2000–2002. [Google Scholar]
- Habijanic J, Berovic M. The relevance of solid-state substrate moisturing on Ganoderma lucidum biomass cultivation. Food Tech Biotechnol. 2000;38:225–228. [Google Scholar]
- Han XH, Wang MC, Wang HZ, He B. A preliminary study on nutritional conditions for the strain mycelium growth of several cultivars of Ganoderma. J Hainan Nor Univ (Nat Sci) 2003;16:88–92. [Google Scholar]
- Han SH, Su CA, Fan AL, Xu SS. Cultivation utilization and development of edible fungi in China. Edible Fungi Chin. 2008;27:3–5. [Google Scholar]
- Han ZT, Zhao YJ, Liu HW, Li RH. Determination of Ganoderma lucidum polysaccharide by reversed-phase high performance liquid chromatography. J Agr Sci Tech. 2009;11:65–67. [Google Scholar]
- He H. Research advances and prospect on submerged fermentation technology of Ganoderma lucidum. Prim J Chin Mater Med. 2000;14:48–49. [Google Scholar]
- He B (2010) Study on solid fermentation between Ganoderma lucidum and Salvia miltiorrhiza bge. Dissertation. Chengdu: Sichuan Agriculture University; Sichuan, China (In Chinese)
- Hong Z. Edible and medicinal fungi fermentation and experimental techniques. Beijing: China Agricultural Science and Technology Press; 1992. [Google Scholar]
- Hong KJ, Dunn DM, Shen CL, Pence BC. Effects of Ganoderma lucidum on apoptotic and anti-inflammatory function in HT-29 human colonic carcinoma cells. Phytother Res. 2004;18:768–770. doi: 10.1002/ptr.1495. [DOI] [PubMed] [Google Scholar]
- Hossain K, Sarker NC, Kakon AJ, Khan AS, Ahmed S. Cultivation of Reishi mushroom (Ganoderma lucidum) on sawdust of different tree species. Bangladesh J Mushroom. 2009;3:1–5. [Google Scholar]
- Hou RH, Liao ST. Research development of the artificial cultivation of Ganoderma lucidum in China. Guangdong Agr Sci. 2009;11:29–32. [Google Scholar]
- Hseu RS, Wang HH, Wang HF, Moncalvo JM. Differentiation and grouping of isolates of the Ganoderma lucidum complex by random amplified polymorphic DNA-PCR compared with grouping on the basis of internal transcribed spacer sequences. Appl Environ Microbiol. 1996;62:1354–1363. doi: 10.1128/aem.62.4.1354-1363.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C, Yang FC. Reusing soy residue for the solid-state fermentation of Ganoderma lucidum. Bioresour Technol. 2004;91:105–109. doi: 10.1016/s0960-8524(03)00157-3. [DOI] [PubMed] [Google Scholar]
- Hsu RC, Lin BH, Chen CW. The study of super-critical carbon dioxide extraction for Ganoderma lucidum. Ind Eng Chem Res. 2001;40:4478–4481. [Google Scholar]
- Hu HR. Study on commercial production from deep submerged fermentation of Ganoderma lucidum mycelia. Food Sci. 2006;27:196–198. [Google Scholar]
- Huang HC, Liu YC. Enhancement of polysaccharide production by optimization of culture conditions in shake flask submerged cultivation of Grifola umbellata. J Chin Inst Chem Eng. 2008;39:307–311. [Google Scholar]
- Huang SQ, Ning ZX. Extraction of polysaccharide from Ganoderma lucidum and its immune enhancement activity. Int J Biol Macromol. 2010;47:336–341. doi: 10.1016/j.ijbiomac.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Huang SM, Yang XL, Huang J, Wu JH, Xu JL, Zhu HS. HPLC fingerprinting of triterpenoids from Ganoderma lucidum. J Beijing Inst Tech. 2004;24:458–461. [Google Scholar]
- Huang SM, Yang XL, Zhang JL, Xu JL, Zhu HS. Study on ultrasonic circulation technique to extraction of triterpenoids from Ganoderma lucidum. Chin Tradit Herbal Drugs. 2004;35:508–510. [Google Scholar]
- Huang XQ, Zheng LY, Peng WH. Advance in the study of extraction and purification techniques of polysaccharides and triterpenoids from Ganoderma spp. Acta Edulis Fungi. 2005;12:56–62. [Google Scholar]
- Huang JS, Chang HC, Li E, Huang TM, Su YH, Wang KC. Enhancement of hepatoprotective efficacy of Antrodia camphorata by Chinese traditional medicine. J Gastroen. 2006;21:A234. [Google Scholar]
- Huang J, Zhang LX, Hu SN, Yu L. Optimizing expression of LZ-8 protein and primary assaying of its immunomodulatory activity. J Zhejiang Univ (Agr & Life Sci) 2008;34:7–12. [Google Scholar]
- Huie CW, Di X. Chromatographic and electrophoretic methods for Lingzhi pharmacologically active components. J Chrom B. 2004;812:241–257. doi: 10.1016/j.jchromb.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Hwang SK, Kim JG. Nuclear sequence analysis of the 5S ribosomal RNA gene of the mushroom Tricholoma matsutake. Microbiol. 1995;3:136–141. [Google Scholar]
- Jiang DH. Effects of medium formulae and cultivating ways on yield and quality of Ganoderma lucidum. Nat Sci J Hainan Univ. 2001;19:76–79. [Google Scholar]
- Jiang C, Qing C, Meng LH, Ding J. Inhibitory effects of China Ganoderma lucid essence (CGLE) on DNA topoisomerases and its ability to induce apoptosis of K562 cells. Chin J Canc. 1999;18:661–663. [Google Scholar]
- Jones N, Ougham H, Thomas H. Markers and mapping: we are all geneticists now. New Phytol. 1997;137:165–177. [Google Scholar]
- Keypour S, Rafati H, Riahi H, Mirzajani F, Moradali MF. Qualitative analysis of ganoderic acids in Ganoderma lucidum from Iran and China by RP-HPLC and electrospray ionisation–mass spectrometry (ESI–MS) Food Chem. 2010;119:1704–1708. [Google Scholar]
- Kim JH, Lee DH, Lee SH, Choi SY, Lee JS. Effect of Ganoderma lucidum on the quality and functionality of Korean traditional rice wine, Yakju. J Biosci Bioeng. 2004;97:24–28. doi: 10.1016/S1389-1723(04)70160-7. [DOI] [PubMed] [Google Scholar]
- Kim S, Song J, Choi HT. Genetic transformation and mutant isolation in Ganoderma lucidum by restriction enzyme-mediated integration. FEMS Microbiol Lett. 2004;233:201–204. doi: 10.1016/j.femsle.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Kim SW, Hwang HJ, Lee BC, Yun JW. Submerged production and characterization of Grifola frondosa polysaccharides—a new application to cosmeceuticals. Food Tech Biotechnol. 2007;45:295–305. [Google Scholar]
- Kino K, Yamashita A, Yamaoka K, Watanabe J, Tanaka S, Ko K, Shimizu K, Tsunoo H. Isolated and characterization of a new immunomodulatory protein Ling Zhi-8 (LZ-8), from Ganoderma lucidum. J Biol Chem. 1989;264:472–478. [PubMed] [Google Scholar]
- Ko JL, Lin SJ, Hsu CI, Kao CL, Lin JY. Molecular cloning and expression of a fungal immunomodulatory protein, FIP-fve, from Flammulina velutipes. J Formos Med Assoc. 1997;96:517–524. [PubMed] [Google Scholar]
- Ko JL, Huang YL, Chen TC, Huang HW, Jiang HL, Hu CL, Kuan CC, Thou HJ (2009) Fungal immunomodulatory protein (FIP) prepared by microorganism and uses thereof. United States patent US 20090042776A1
- Leskosek-Cukalovic I, Despotovic S, Lakic N, Niksic M, Nedovic V, Tesevic V. Ganoderma lucidum—medical mushroom as a raw material for beer with enhanced functional properties. Food Res Int. 2010;43:2262–2269. [Google Scholar]
- Li BJ, Chen D (2002) Agrobacterium-mediated transformation method in Ganoderma lucidum. Chinese Patent CN: 1342752A
- Li G, Li BJ. Study on the separation and regeneration of Ganoderma protoplast. Mycosystema. 1999;18:79–88. [Google Scholar]
- Li G, Yang F, Li R, Xu Z, Li B. A study on the breeding of new Ganoderma varieties by UV induced mutagenesis. Acta Microbiol Sin. 2001;41:229–233. [PubMed] [Google Scholar]
- Li G, Wang Q, Liu QY, Li BJ. Establishment of a transformation system of Ganoderma lucidum using PEG method. Mycosystema. 2004;23:255–261. [Google Scholar]
- Li SZ, Xie YZ, Zhou JW, Luo B. Analysis on bioactive ingredients in Ganoderma lucidum spore oil and its effects on reducing serum lipid in vivo. Edible Fungi Chin. 2006;25:40–53. [Google Scholar]
- Li QZ, Huang L, Xie MQ, Zhou XW (2009) Cloning, expression, and purification of a fungal immunomodulatory protein from Ganoderma lucidum. The 5th International Medicinal Mushroom Conference; 2009 Sep 5–8; Nantong, Jiangsu, China. p. 338–346
- Li QZ, Wang XF, Chen YY, Lin J, Zhou XW. Cytokines expression induced by Ganoderma sinensis fungal immunomodulatory protein (FIP-gsi) in mouse spleen cells. Appl Biochem Biotech. 2010;162:1403–1413. doi: 10.1007/s12010-010-8916-1. [DOI] [PubMed] [Google Scholar]
- Li QZ, Wang XF, Bao TW, Ran L, Lin J, Zhou XW. In vitro synthesis of a recombinant fungal immunomodulatory protein from Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (W. Curt.: Fr.) P. Karst. (Aphyllophoromycetideae) and analysis of its immunomodulatory activity. Int J Med Mushrooms. 2010;12:347–358. [Google Scholar]
- Li QZ, Wang XF, Zhou XW. Recent status and prospects of the fungal immunomodulatory protein family. Crit Rev Biotechnol. 2011;31:365–375. doi: 10.3109/07388551.2010.543967. [DOI] [PubMed] [Google Scholar]
- Li QZ, Huang L, Wang XF, Li XS, Wu SQ, Zhou XW. Fungal immunomodulatory protein from Flammulina velutipes induces cytokine gene expression in mouse spleen cells. Curr Top Nutraceut R. 2011b;9:111–118. [Google Scholar]
- Liang S. Parameters study on deep bed liquid fermentation of edible fungus. Food Sci. 2011;22:38–41. [Google Scholar]
- Liang M, Ren GH, Zou DH. Development of Ginseng Ganoderma lucidum Si He liquor. Liquor Mak. 2003;30:91–92. [Google Scholar]
- Liao ST, Xiao GS. Innovative use of sericulture resources. Beijing: China Agricultural Science and Technology Press; 2006. [Google Scholar]
- Lin ZB. Physiological effects of Ganoderma lucidum. In: Lin ZB, editor. Modern research on Ganoderma lucidum. Beijing: Beijing Medical University; 1996. pp. 148–177. [Google Scholar]
- Lin SQ (2009) Quality control and assessment of Lingzhi and its products. The 9th National Medicinal Mushroom Conference and Annual Meeting Celebrating 30 Years of Establishing the Medicinal Fungi Professional Group; Wuyishan, Fujian, China
- Lin TL (2009) Immunomodulatory protein cloned from Ganoderma microsporum. United States patent US 7601808B2
- Lin J, Zhou XW. Artificial cultivation of organizational separation of Ganoderma lucidum. Edible Fungi. 1999;2:10–11. [Google Scholar]
- Lin J, Zhou XW. Comparison test of four strains of Ganoderma species. Edible Fungi Chin. 1999;18:6–9. [Google Scholar]
- Lin WH, Hung CH, Hsu CI, Lin JY. Dimerization of the n-terminal amphipathic alpha-helix domain of the fungal immunomodulatory protein from Ganoderma tsugae (Fip-gts) defined by a yeast two-hybrid system and site-directed mutagenesis. J Biol Chem. 1997;272:20044–20048. doi: 10.1074/jbc.272.32.20044. [DOI] [PubMed] [Google Scholar]
- Lin SH, Xie D, Yu NC. Research on polysaccharides extraction and determination in Ganoderma lucidum and its preparation. Lishizhen Med Mater Med Res. 2005;16:346–347. [Google Scholar]
- Lin SQ, LiuYK QYA, Wang SZ, Lin SG, Liu B. Research of Ling-Zhi GAP organic plant and its environment quality. Strait Pharmaceut J. 2005;17:7l–73l. [Google Scholar]
- Ling HT, Song B, Lin QY, Li TH, Zeng ZJ. Advances in selenium-enriched edible fungi study. J Microbiol. 2008;28:78–84. [Google Scholar]
- Liu XL, Jiang ZH. Cultivation of Ganoderma lucidum. Changchun: Jilin Science and Technology Press; 2007. [Google Scholar]
- Liu K, Ding CY, Wang YH, Zhang KC. Study on the capacity of Zn-accumulating of Ganoderma lucidum, Grifola frondosa, Agaricus blazei Murill. Food Res Dev. 2005;26:29–33. [Google Scholar]
- Liu GQ, Bao HR, Jiang YY. Progress of study on antihyperlipidaemia of fungi with both food and medicine function. Food Sci. 2007;28:626–630. [Google Scholar]
- Lu HL. The review of the extraction and purification methods of polysaccharides in Ganoderma lucidum. Inform Sci Tech. 2009;27:31–32. [Google Scholar]
- Luo J, Lin ZB. Advances of pharmacological effects of triterpenes from Ganoderma lucidum. Acta Pharm Sin. 2002;37:574–758. [PubMed] [Google Scholar]
- Luo LZ, Lin SQ, Xie BG, Lin ZB. DNA fingerprinting analysis of Ganoderma strains. Acta Edulis Fungi. 2005;12:7–13. [Google Scholar]
- Ma JY. Ganoderma triterpenoids purification and structural identification. Liaoning Chem Ind. 2008;37:355–357. [Google Scholar]
- Ma LJ, Yao SH, Huang ZR. Supercritical fluid extraction of triterpines from Ganoderma lucidum. J Southwest Chin Agr Univ. 1998;19:107–110. [Google Scholar]
- Ma L, Wu F, Chen R. Analysis of triterpenoids from Ganoderma lucidum. Acta Pharm Sin. 2003;1:50–52. [Google Scholar]
- Mahajna J, Dotan N, Zaidman BZ, Petrova RD, Wasser SP. Pharmacological values of medicinal mushrooms for prostate cancer therapy: the case of Ganoderma lucidum. Nutr Cancer. 2009;61:16–26. doi: 10.1080/01635580802379323. [DOI] [PubMed] [Google Scholar]
- Mahapatra S, Banerjee D. Extracellular tannase production by endophytic Hyalopus sp. J Gen Appl Microbiol. 2009;55:255–259. doi: 10.2323/jgam.55.255. [DOI] [PubMed] [Google Scholar]
- Maldonado MC, Strasser de Saad AM. Production of pectinesterase and polygalacturonase by Aspergillus niger in submerged and solid state systems. J Indust Microbiol Biotechnol. 1998;20:34–38. doi: 10.1038/sj.jim.2900470. [DOI] [PubMed] [Google Scholar]
- Mao J, Ma HL. Development of selenium-enriched Ganoderma lucidum yogurt. Food Sci. 2009;30:406–411. [Google Scholar]
- Mayzumi F, Okamoto H, Mizuno T. Cultivation of Reishi (Ganoderma lucidum) Food Rev Int. 1997;13:365–382. [Google Scholar]
- McKenna DJ, Jones K, Hughes K. Reishi botanical medicines. The desk reference for major herbal supplements. 2. New York: The Haworth Herbal Press; 2002. [Google Scholar]
- Miao JZ, Lv ZQ. The study on the bioenrichment of iron in G. Lucidum by deep-submerged fermentation. Xuzhou Inst Tech. 2007;22:36–39. [Google Scholar]
- Mizuno T. Reishi, Ganoderma lucidum and Ganoderma tsugae: bioactive substances and medicinal effects. Food Rev Int. 1995;11:151–166. [Google Scholar]
- Mshandete AM, Mgonja JR. Submerged liquid fermentation of some Tanzanian basidiomycetes for the production of mycelial biomass, exopolysaccharides and mycelium protein using wastes peels media. Arpn J Agr Biol Sci. 2009;4:1–13. [Google Scholar]
- Murasugi A, Tanaka S, Komiyama N, Iwata N, Kino K, Tsunoo H, Sakuma S. Molecular cloning of a cDNA and a gene encoding an immunomodulatory protein, LingZhi-8, from a fungus, Ganoderma lucidum. Anal Bioanal Chem. 1991;266:2486–2493. [PubMed] [Google Scholar]
- Ning HQ. The research of Ganoderma polysaccharides’s determination. J Taiyuan Normal Univ (Nat & Sci) 2006;5:105–107. [Google Scholar]
- Ogbe AO, Efenu P, Nicholas U, Pam A, Abarshi A, Banyigyi S, Odugbo M. Response to treatment of skin ailments in animal patients using aqueous Ganoderma extract. Electron J Environ Agr Food Chem. 2011;10:1816–1820. [Google Scholar]
- Olaku O, White JD. Herbal therapy use by cancer patients: a literature review on case reports. Eur J Cancer. 2011;47:508–514. doi: 10.1016/j.ejca.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JH, Li F. Preparation of a “bifungi” beverage fermented with Ganoderma lucidum and Saccharomyces cerevisiae. Food Sci. 1997;18:22–24. [Google Scholar]
- Park YD, Lee JS, Yoo YB, Shin PG, You CH, Cha DY, Park YH. Interspecific protoplast fusion of Ganoderma applanatum and Ganoderma lucidum and fruit body formation of the fusants. Kor J Mycol. 1988;16:79–86. [Google Scholar]
- Park SH, Choi EC, Kim BK. Studies on intergeneric protoplast fusion and nuclear transfer between Ganoderma lucidum and Corilus versicolor. Arch Pharm Res. 1991;14:282–283. [Google Scholar]
- Paterson RM. Ganoderma—a therapeutic fungal biofactory. Phytochemistry. 2006;67:1985–2001. doi: 10.1016/j.phytochem.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Peksen A, Yakupoglu G. Tea waste as a supplement for the cultivation of Ganoderma lucidum. World J Microbiol Biotechnol. 2009;25:611–618. [Google Scholar]
- Qin WD, Gao MX. Research on healthy beverage with Ganoderma and black-bone chicken. Jiangsu Food Ferment. 2002;2:4–6. [Google Scholar]
- Ryvarden L. Genera of polypores: nomenclature and taxonomy. Synop Fungorum. 1991;5:1–363. [Google Scholar]
- Sánchez C. Modern aspects of mushroom culture technology. Appl Microbiol Biotechnol. 2004;64:756–762. doi: 10.1007/s00253-004-1569-7. [DOI] [PubMed] [Google Scholar]
- Sanodiya BS, Thakur GS, Baghel RK, Prasad GB, Bisen PS. Ganoderma lucidum: a potent pharmacological macrofungus. Curr Pharmaceut Biotechnol. 2009;10:717–742. doi: 10.2174/138920109789978757. [DOI] [PubMed] [Google Scholar]
- Shen X, Yu SG. Research on organic selenium accumulation of Ganoderma lucidum cultured in lanthanide-containing liquid. Southwest Chin J Agr Sci. 2008;21:1654–1657. [Google Scholar]
- Sheng DM, Ma Y. Selection of optimal medium of Ganoderma lucidum polysaccharide produced by the way of solid fermentation. Guangzhou Food Sci Tech. 2004;20:18–20. [Google Scholar]
- Shi J, Wang L, Wang YX. Determination of polysaccharide in Zhonghua Lingzhibao capsules. Chin Tradit Patent Med. 2002;24:388–389. [Google Scholar]
- Shi XM, Zhang JS, Tang QJ, Yang Y, Hao RX, Pan YJ. Fingerprint analysis of Lingzhi (Ganoderma) strains by high-performance liquid chromatography coupled with chemometric methods. World J Microbiol Biotechnol. 2008;24:2443–2450. [Google Scholar]
- Shiao MS. Natural products of the medicinal fungus Ganoderma lucidum: occurrence, biological activities, and pharmacological functions. Chem Recover. 2003;3:172–180. doi: 10.1002/tcr.10058. [DOI] [PubMed] [Google Scholar]
- Sliva D. Ganoderma lucidum (Reishi) in cancer treatment. Integr Cancer Ther. 2003;2:358–364. doi: 10.1177/1534735403259066. [DOI] [PubMed] [Google Scholar]
- Sliva D. Ganoderma lucidum in cancer research. Leuk Res. 2006;30:767–768. doi: 10.1016/j.leukres.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Song JF, Liu CQ, Li DQ, Jin BQ. Optimization of extraction process of Ganoderma lucidum fruit body polysaccharides by response surface methodology. Food Sci. 2008;29:176–180. [Google Scholar]
- Songulashvili G, Elisashvili V, Wasser SP, Nevo E, Hadar Y. Basidiomycetes laccase and manganese peroxidase activity in submerged fermentation of food industry wastes. Enzym Microb Tech. 2007;41:57–61. [Google Scholar]
- Sun ZX, Wang RX. Present status and future prospects of bioactive proteins from edible fungi. Acta Edulis Fungi. 2009;12:85–90. [Google Scholar]
- Sun DP, Pan F, Shi XL, Yang SL. Selection of optimal medium and extraction and purification of Ganoderma lucidum extracellular polysaccharide. Chin Tradit Herbal Drugs. 2000;31:341–394. [Google Scholar]
- Sun L, Cai HQ, Xu WH, Hu YL, Gao Y, Lin ZP. Efficient transformation of the medicinal mushroom Ganoderma lucidum. Plant Mol Biol Rep. 2001;19:383a–383j. [Google Scholar]
- Sun SQ, Du DG, Liang XY, Yang XR. A rapid method for distinguishing the different Ganoderma lucidum products by Fourier transform infrared spectroscopy. Chin J Anal Chem. 2001;29:309–312. [Google Scholar]
- Sun S, Song JM, Zhang CS. The current research and application of the solid-state fermentation technology. Chin Food Addit. 2007;4:54–58. [Google Scholar]
- Tan ZJ, Yang HJ, Xiao QM, Lin S, Wu T. Protoplast fusion technology and microbial breeding. Acta Agri Nucleatae Sin. 2005;19:75–79. [Google Scholar]
- Tang W, Gu TY, Zhong JJ. Separation of targeted ganoderic acids from Ganoderma lucidum by reversed phase liquid chromatography with ultraviolet and mass spectrometry detections. Biochem Eng J. 2006;32:205–210. [Google Scholar]
- Tang W, Liu JW, Zhao WM, Wei DZ, Zhong JJ. Ganoderma acid T from Ganoderma lucidum mycelia induces mitochondria mediated apoptosis in lung cancer cells. Life Sci. 2006;80:205–211. doi: 10.1016/j.lfs.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Tanksley SD, Young ND, Paterson AH, Bonierbale MW. RFLP mapping in plant breeding: new tools for an old science. Nat Biotechnol. 1989;7:257–264. [Google Scholar]
- Tsai LL (2007) Immunomodulatory protein cloned from Ganoderma microsporum. United States patent US 7601808 B2
- Urbanelli S, Della Rosa V, Punelli F, Porretta D, Reverberi M, Fabbri AA, Fanelli C. DNA-fingerprinting (AFLP and RFLP) for genotypic identification in species of the Pleurotus eryngii complex. Appl Genet Mol Biotechnol. 2007;74:592–600. doi: 10.1007/s00253-006-0684-z. [DOI] [PubMed] [Google Scholar]
- Vos P, Hogers R, Bleeker M, Reijans M, Lee VDH, Hornes M, Friters A, Pot J, Paleman J, Kuiper M, Zabeau M. AFLP: a new technique for DNA fingerprinting. Nucl Acids Res. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R, Mitchell DA, Sassaki GL, Amazonas MALA, Berovi M. Current techniques for the cultivation of Ganoderma lucidum for the production of biomass, ganoderic acid and polysaccharides. Food Tech Biotechnol. 2003;41:371–382. [Google Scholar]
- Wang T. The processing technique of cordyceps sinensis and high-Ge Ganoderma lucidum nourishing wine. Edible Fungi Chin. 1998;14:39–41. [Google Scholar]
- Wang M. Research on the preparation technique of healthy wine made from Ge-enriched Ganoderma lucidum herb. Chin Brewing. 2005;7:58–59. [Google Scholar]
- Wang GL, Shang DJ, Yang WX. Study on the nutritional components and antioxidant of Ganoderma lucidum rich in selenium. Acta Nutr Sin. 2001;23:73–75. [Google Scholar]
- Wang SF, Bu QM, Liu LD, Dong HX, Ying QQ. Study on fermentation conditions of wild Ganoderma lucidum of Mountain Kunyu. Food Sci. 2007;28:364–368. [Google Scholar]
- Wang XM, Guan SH, Liu RX, Sun JH, Liang Y, Yang M, Wang W, Bi KS, Guo DA. HPLC determination of four triterpenoids in rat urine after oral administration of total triterpenoids from Ganoderma lucidum. J Pharmaceut Biomed Anal. 2007;43:1185–1190. doi: 10.1016/j.jpba.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhou K, Qi ML. Content determination of Ganoderma polysaccharide in Lingzhi pill. Drug Stand Chin. 2007;8:42–44. [Google Scholar]
- Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002;60:258–274. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- Wasser SP. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl Microbiol Biotechnol. 2011;89:1323–1332. doi: 10.1007/s00253-010-3067-4. [DOI] [PubMed] [Google Scholar]
- Wasser SP, Weis AL. In: Medicinal mushrooms. Ganoderma lucidum (Curtis: Fr.) Karst P, Nevo E, editors. Haifa: Peledfus; 1997. p. 39. [Google Scholar]
- Wasser SP, Weis AL. Medicinal properties of substances occurring in higher Basidiomycetes mushrooms: current perspectives (Review) Int J Med Mushrooms. 1999;1:31–62. [PubMed] [Google Scholar]
- Wei HP, Liu ZY, Tan YM. Experimental study on Ganoderma cultivating with crop stalks. Chin Agr Sci Bull. 2005;21:146–147. [Google Scholar]
- Wei J, Li X, Liu FZ. The research of Ganoderma lucidum optimal liquid ferment conditions. Food Res Dev. 2007;28:18–21. [Google Scholar]
- Wei SJ, Xu XR, Huang L, Tu GQ. Study on the production of polysaccharide from Ganoderma lucidum mycelia in deep-liquid fermentation. Jiangxi Sci. 2007;25:292–294,301. [Google Scholar]
- Wei SJ, Fu XQ, Xiong XR, Li KT. Optimization for the solid-state fermentation conditions of Ganoderma lucidum mycelium. Guangdong Agr Sci. 2009;10:122–124. [Google Scholar]
- Wei SJ, Cheng X, Tu XR. Study on zinc enrichment by liquid fermentation of Ganoderma lucidum. Jiangsu Agr Sci. 2010;1:242–244. [Google Scholar]
- Wu Y, Zeng LK. The development and utilization of Ganoderma lucidum. Food Nutr Chin. 2005;5:24–26. [Google Scholar]
- Wu DZ, Zhang LX, Xu R, Zhang KC. Production of bacterial alpha-amylase by solid-state fermentation. J Wuxi Univ Light Ind. 2000;19:54–57. [Google Scholar]
- Wu BF, Liu LL, Fang ZH, Liu XY. Effects of nutrition factors on mycelium growth of 51427 in Ganoderma lucidum. Anhui Agr Sci Bull. 2008;14:57–58. [Google Scholar]
- Wu SQ, Guo XB, Zhou X, Li XS, Chen YY, Lin J. AFLP analysis of genetic diversity in main cultivated strains of Ganoderma spp. Afr J Biotechnol. 2009;8:3448–3454. [Google Scholar]
- Wu XP, Liu F, Xie YR, Wang ZL, He QL, Xie BG. Hybridization of Ganoderma lucidum by protoplast monokaryogenesis method. Chin Agr Sci Bull. 2009;25:64–69. [Google Scholar]
- Xia ZN, Jiang JA, He CZ, Liu MY, Liu DY. Preliminary researches on high-yield cultivation techniques of Ganoderma. Hunan Agr Sci. 2003;6:56–58. [Google Scholar]
- Xia ZL, Yu TS, Zhou LY, Liu YM. Submerged fermentation condition optimization of Ganoderma lucidum. J Microbiol. 2007;27:10–15. [Google Scholar]
- Xiao GP. The development of health liquid production from Ganoderma lucidum and Tremella fuciformis. Food Ferment Ind. 2002;28:54–58. [Google Scholar]
- Xiao ZJ, Wang JJ, Lian B. Actuality of the exploitation and research on production of Ganoderma lucidum. Food Sci. 2006;27:838–842. [Google Scholar]
- Xiao ZT, Tan ZY, Ye YC, He HQ. Comparative study on the yield of different Ganoderma lucidum strains. Guangdong Agr Sci. 2010;37:60–62. [Google Scholar]
- Xie BF, Lin L, Shi QQ, Zhang FJ. Study on accumulation Se in Ganoderma by submerged culture. Food Ferment Ind. 1996;4:54–57. [Google Scholar]
- Xie YZ, Zhang Z, Li SZ, Li C. Recent advances in the development and processing works of the Ling-zhi fungus. J Microbiol. 2002;22:43–45. [Google Scholar]
- Xing ZT, You QH, Zhang JS, Pan YJ. Comparative study on triterpenesin in different Ganoderma species. J Chin Med Mater. 2004;27:575–576. [PubMed] [Google Scholar]
- Xu GX (2009) The Elementary study of recombinant expression and biological characteristics of fungal immunomodulatory protein from Flammulina velutipes. Dissertation. Changchun: Northeast Normal University (In Chinese)
- Xu GY, Xu ZY. Improvement on Ganoderma lucidum crushing methods. Chin Tradit Patent Med. 1999;21:614. [Google Scholar]
- Xu JW, Zhao W, Zhong JJ. Biotechnological production and application of ganoderic acids. Appl Microbiol Biotechnol. 2010;87:457–466. doi: 10.1007/s00253-010-2576-5. [DOI] [PubMed] [Google Scholar]
- Xu ZH, Lu ZM, Xu HY. Status and trend of R&D of medicinal mushroom in China from the view point of 5th International Medicinal Mushroom Conference. Food Sci Biotechnol. 2010;29:491–495. [Google Scholar]
- Xu XF, Yan HD, Chen J, Zhang XW. Bioactive proteins from mushrooms. Biotechnol Adv. 2011;29:667–674. doi: 10.1016/j.biotechadv.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Yan MH. Cultivation Ganoderma lucidum using oak leaf and waste tea. Edible Fungi. 2000;2:22–23. [Google Scholar]
- Yang HL. Ganoderic acid produced from submerged culture of Ganoderma lucidum induces cell cycle arrest and cytotoxicity in human hepatoma cell line BEL7402. Biotechnol Lett. 2005;27:835–838. doi: 10.1007/s10529-005-6191-y. [DOI] [PubMed] [Google Scholar]
- Yang FC, Liau CB. Effects of cultivation conditions on the mycelial growth of Ganoderma lucidum in submerged flask cultures. Bioprocess Eng. 1998;19:233–236. [Google Scholar]
- Yang J, Yu DS, Luo J, Luo XR. Study on the supercritical carbon dioxide extraction process of total triterpenoids from Ganoderma lucidum. Lishizhen Med Mater Med Res. 2008;19:536–540. [Google Scholar]
- Yoo YB, Lee KH, Kim BG. Characterization of somatic hybrids with compatible and incompatible species by protoplast fusion in genera Pleurotus (Fr.) P. Karst. and Ganoderma P. Karst. by RAPD-PCR analysis. Int J Med Mush. 2002;4:147–157. [Google Scholar]
- You JJ. The present status and future aspect of medicinal fungi in China. Edible Fungi Chin. 2009;28:3–5. [Google Scholar]
- Yuen JW, Gohel MD. Anticancer effects of Ganoderma lucidum: a review of scientific evidence. Nutr Cancer. 2005;53:11–17. doi: 10.1207/s15327914nc5301_2. [DOI] [PubMed] [Google Scholar]
- Zhang GZ. High yield cultivation and processing technology of Ganoderma lucidum. Shengyang: Liaoning Science and Technology Press; 1998. [Google Scholar]
- Zhang LH, Wang SX. Study on the binding and packing cultivation technology of the G. lucidum’s artificial alternative compost. Agr Tech Serv. 2010;27:516–517. [Google Scholar]
- Zhang HJ, Cao LS, Ye SQ. Measures of log-cultivated Ganoderma lucidum for high-yielding and quality. J Agr Sci. 2004;3:136–138. [Google Scholar]
- Zhang JX, Huang CY, Hu QX. The protective technology of strain identification and strain power. Edible Fungi Chin. 2005;24:14–16. [Google Scholar]
- Zhang LY, Zhou YF, Yu WY. The study on medicinal fungi fermentation substance to regulate endogenous hormone secretion and immunity in broiler chicken. J Yangzhou Univ (Agr Life Sci Ed) 2005;26:16–38. [Google Scholar]
- Zhang WG, Liu X, Chen YQ. Research of nutritious & healthy Ganoderma polysaccharide oral liquid by solid fermentation. Food Res Dev. 2006;27:94–96. [Google Scholar]
- Zhang CJ, Yuan FY, Shao HB. Application of extraction of polysaccharide compound by ultrasonic method. Chem Ind Times. 2007;21:54–56. [Google Scholar]
- Zhang XQ, Pang GJ, Cheng Y, Wang Y, Ye WC. Chemical constituents of the spores of Ganoderma lucidum. J Chin Med Mater. 2008;31:41–43. [PubMed] [Google Scholar]
- Zhang ZJ, Luo Y, Li SF, Yang LW, Wang WZ. Effect of culture time on fermentation of liquid strain of Ganoderma lucidum. Chin Med Biotechnol. 2008;3:289–292. [Google Scholar]
- Zhang JL, Luo X, Zheng LY, Xu XY, Ye LM. Discrimination of Ganoderma based on high performance liquid chromatographic fingerprints combined with chemometrics methods. Chin J Chrom. 2009;27:776–780. [PubMed] [Google Scholar]
- Zhang L, Cheng YL, Sun CY, Zhang MP, Wang Y. Optimization of ultrasonic extraction on the main active ingredient of Ganoderma lucidum. J Beihua Univ (Nat & Sci) 2010;11:140–144. [Google Scholar]
- Zhang F, Zhong W, Mu H, Li G. Increasing polysaccharide yield by over-expression of OsUgp2 in Ganoderma sinense. Mycosystema. 2011;30:442–452. [Google Scholar]
- Zhao P. The effects of medium ingredients and thickening agent on the submerged culture of Ganoderma lucidum. Food Sci. 2002;23:88–91. [Google Scholar]
- Zhao J, Chang ST. Monokaryotization by protoplasting heterothallic species of edible mushrooms. World J Microbiol Biotechnol. 1993;9:538–554. doi: 10.1007/BF00386290. [DOI] [PubMed] [Google Scholar]
- Zheng WF. Drug discovery from fungal metabolites: a review of the papers in this monographical issue of mycosystema concerned with the natural resources, problems and strategies. Mycosystema. 2011;30:151–157. [Google Scholar]
- Zheng LY, Jia DH, Fei XF, Luo X, Yang ZY. An assessment of the genetic diversity within Ganoderma strains with AFLP and ITS PCR-RFLP. Microbiol Res. 2009;164:312–321. doi: 10.1016/j.micres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Zhong JJ, Xiao JH. Secondary metabolites from higher fungi: discovery, bioactivity, and bioproduction. Adv Biochem Eng Biotechnol. 2009;113:79–150. doi: 10.1007/10_2008_26. [DOI] [PubMed] [Google Scholar]
- Zhong X, Zhou SM, Wang Q. Advances in pharmaceutical fungi polysaccharides. Sci Tech Rev. 2009;27:97–101. [Google Scholar]
- Zhou XW, Lin J. Resources of wild Ganoderma spp. in China and their development and utilization. Acta Edulis Fungi. 1999;6:58–64. [Google Scholar]
- Zhou XW, Chen WQ, Deng BW, Wang Z, Peng H, Lin J. Application of biotechnology to exploitation and preservation of medicinal fungi. Chin Tradit Herbal Drugs. 2005;36(3):451–455. [Google Scholar]
- Zhou XW, Lin J, Sun XF, Tang KX. Fingerprint of Chinese materia medica and research methods of quality for Ganoderma spp. Chin Tradit Herb drugs. 2006;37(8):1121–1124. [Google Scholar]
- Zhou XW, Lin J, Li QZ, Yin YZ, Sun XF, Tang KX. Study progress on bioactive proteins from Ganoderma spp. Nat Prod Res Dev. 2007;19:916–924. [Google Scholar]
- Zhou XW, Lin J, Yin YZ, Zhao JY, Sun XF, Tang KX. Ganodermataceae: natural products and their related pharmacological functions. Am J Chin Med. 2007;35:559–574. doi: 10.1142/S0192415X07005065. [DOI] [PubMed] [Google Scholar]
- Zhou XW, Li QZ, Yin YZ, Chen YY, Lin J. Identification of medicinal Ganoderma species based on PCR with specific primers and PCR-RFLP. Planta Med. 2008;74:197–200. doi: 10.1055/s-2008-1034289. [DOI] [PubMed] [Google Scholar]
- Zhou XW, Lin J, Zhou L. Determine and analysis on primary nutritional components of Ganoderma spp. J Shaanxi Normal Univ (Nat Sci Ed) 2008;26:214–217. [Google Scholar]
- Zhou XW, Xie MQ, Hong F, Li QZ. Genomic cloning and characterization of a FIP-gsi gene encoding a fungal immunomodulatory protein from Ganoderma sinensis Zhao et al (Aphyllophoromycetideae) Int J Med Mushrooms. 2009;11:77–86. [Google Scholar]
- Zhou M, Yang D, Cheng W, Du X, Gao H, Shi DF, Li L, He JJ, Ye LX. Feasibility study on cotton stalks instead of wood for cultivating wood-decay fungi Ganoderma lucidum. Hubei Agr Sci. 2010;49:2771–2774. [Google Scholar]
- Zhou XW, Wang XF, Li QZ, Su KQ, Cong WR (2011a) The gene sequence recombined from intrageneric species, the gen encoding protein and its application. Chinese Patent Application No. 201110068813.0 (in Chinese)
- Zhou XW, Wang XF, Li QZ, Su KQ, Cong WR (2011b) The gene sequence recombined from intergeneric species, the gen encoding protein and its application. Chinese Patent Application No. 201110068761.7 (in Chinese)
- Zhu JJ, Zhang SQ, Wang CZ, Wu H. Study on comprehensive method to extract polysaccharides in Ganoderma lucidum spore. Trans Chin Soc Agr Mach. 2004;35:184–185. [Google Scholar]
- Zhu Q, Xiong XH, Wang F. Effects of radix glycyrrhizae on production of Ganoderma lucidum polysaccharide. Chin Brewing. 2009;2:86–88. [Google Scholar]
- Zhu Q, Xia YQ, Dong KK, Yang CF, Wang ZJ. Optimization of Ganoderma lucidum medicinal solid fermentation medium by response surface test. Chin Biotechnol. 2010;30:75–79. [Google Scholar]
- Zhuang Y. Solid-state fermentation of medicinal fungi. Chin J Chin Mater Med. 1991;26:80–82. [Google Scholar]
- Zhuang Y. Some suggestions on development of Chinese medicine new drugs using solid fermentation engineering technology. Tradit Chin Drug Res Clin Pharm. 1995;6:41–42. [Google Scholar]
- Zhuang Y. New type (two-way pattern) solid fermentation engineering in medicinal mushrooms. Edible Fungi Chin. 2002;21:3–6. [Google Scholar]
- Zhuang Y, Hong J. Two-way pattern solid fermentation engineering in medicinal mushrooms and further development of Chinese medicine residue. Chin J Chin Mater Med. 2006;31:1918–1919. [Google Scholar]
- Zhuang Y, Chi YM, Chen SB, Ding RN, Min ZD. Preparation of medicinal fungal new type bi-directional solid fermentation engineering and Huai Qi fungal substance (F) Chin Pharm J. 2004;39:175–178. [Google Scholar]
- Zhuang Y, Pan Y, Xie XM, Zhang LY. The origin, development and its advantage and potential of “the bi-directional solid fermentation” for medicinal fungi. Edible Fungi Chin. 2007;26:3–6. [Google Scholar]


