Abstract
Purpose
In view of the expected 2009 influenza A(H1N1) pandemic, the Italian Health Authorities set up a national referral network of selected intensive care units (ICU) able to provide advanced respiratory care up to extracorporeal membrane oxygenation (ECMO) for patients with acute respiratory distress syndrome (ARDS). We describe the organization and results of the network, known as ECMOnet.
Methods
The network consisted of 14 ICUs with ECMO capability and a national call center. The network was set up to centralize all severe patients to the ECMOnet centers assuring safe transfer. An ad hoc committee defined criteria for both patient transfer and ECMO institutions.
Results
Between August 2009 and March 2010, 153 critically ill patients (53% referred from other hospitals) were admitted to the ECMOnet ICU with suspected H1N1. Sixty patients (48 of the referred patients, 49 with confirmed H1N1 diagnosis) received ECMO according to ECMOnet criteria. All referred patients were successfully transferred to the ECMOnet centers; 28 were transferred while on ECMO. Survival to hospital discharge in patients receiving ECMO was 68%. Survival of patients receiving ECMO within 7 days from the onset of mechanical ventilation was 77%. The length of mechanical ventilation prior to ECMO was an independent predictor of mortality.
Conclusions
A network organization based on preemptive patient centralization allowed a high survival rate and provided effective and safe referral of patients with severe H1N1-suspected ARDS.
Electronic supplementary material
The online version of this article (doi:10.1007/s00134-011-2301-6) contains supplementary material, which is available to authorized users.
Keywords: Viral infection, Extracorporeal membrane oxygenation, Preparedness plan, ARDS
Introduction
In recent years, health systems worldwide have been repeatedly faced with new epidemic and pandemic infections, including severe acute respiratory syndrome (SARS) [1, 2], avian influenza [3], and influenza A(H1N1) [4–11]. While a lot has been done in terms of preventive and control measures [12], specific plans for the organization of ICU services have received less attention, and only recently the European Society of Intensive Care Medicine task force promulgated a document suggesting a set of standard operating procedures for the ICUs [13].
We report here about the organization of an Italian specialized ICU network designed to cope with the expected high incidence of severe ARDS cases related to H1N1 infection in the winter of 2009–2010. In response to the 2009 H1N1 pandemic, the Italian Ministry of Health instituted a national network of selected ICU centers (Extracorporeal Membrane Oxygenation Network, ECMOnet). The primary aim was to minimize the number of respiratory deaths while making the best use of available resources.
The Italian network was set up to centralize all potentially severe patients and all necessary resources in a limited number of tertiary hospitals to provide advanced treatment options including ECMO, following the report by the Australian and New Zealand ECMO investigators (ANZ ECMO) [14].
The ECMOnet prospectively collected the epidemiological and clinical features, treatment data, and outcomes of 60 patients with ARDS due to confirmed or suspected H1N1 treated with ECMO according to predefined ECMOnet eligibility criteria between August 2009 and March 2010.
Methods
Network organization
The Italian Ministry of Health officially completed the ECMOnet organization by November 5, 2009. The network was based on four key points:
Two clinical experts coordinated the communication between the Health Ministry and the ECMOnet and guided its organization and operation.
Fourteen ICU centers (Supplementary Table 1) were selected based on their (1) experience in treating ARDS patients; (2) experience in respiratory ECMO or presence of a cardiac surgery team expert in ECMO; and (3) territorial distribution. Five of the centers assured interhospital transportation throughout the Italian territory whenever the nearest ECMOnet center could not cope with a case.
A 24 h national ECMOnet Call Center Service sorted all requests from any hospital in Italy and directed them to the closest ECMOnet center, and/or to the transportation ECMO team on call.
Ten sessions of a 3 day ECMO training course, open to physicians, perfusionists, and nurses of the ECMOnet were organized in Milan.
Patient selection and referral
The Italian Ministry of Health issued specific recommendations for patient referral to the ECMOnet. National recommendations for centralization and ECMO eligibility criteria were promulgated by the Ministry through written communication to all local sanitary authorities and to the administration of all Italian hospitals.
Procedures for patient referral are summarized in Fig. 1. Criteria for patient referral and ECMO eligibility criteria are reported in Table 1. These criteria were consistent with those reported by others [15].
Fig. 1.
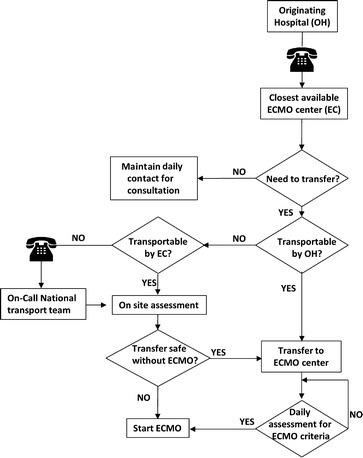
Management algorithm for the referrals to the Italian ECMOnet system
Table 1.
List of recommended national clinical criteria for early patient centralization and for ECMO eligibility
| Recommended criteria for early patient centralization |
| From primary and secondary hospitals to tertiary hospitals with ARDS treatment experience |
| Suspected H1N1 infection with one of the following: |
| 1. Need for invasive mechanical ventilation with PEEP |
| 2. FiO2 > 0.6 |
| From any non-ECMO center to ECMOnet centers |
| Suspected H1N1 infection with one of the following: |
| 1. HbO2 < 85% |
| 2. OI > 25 |
| 3. PaO2/FiO2 < 100 with PEEP ≥ 10 cmH2O |
| 4. Hypercapnia and respiratory acidosis with pH < 7.25 |
| 5. SvO2 or SvcO2 < 65% despite Ht > 30 and administration of vasoactive drugs |
| ECMO criteria |
| Inclusion criteria |
| All adult and pediatric patients with severe ARDS related to suspected influenza A(H1N1) presenting with at least one of the following criteria despite the use of available rescue therapies: |
| 1. OI > 30 |
| 2. PaO2/FiO2 < 70 with PEEP ≥ 15 cmH2O (in patient already admitted to one of the ECMOnet centers) |
| 3. PaO2/FiO2 < 100 with PEEP ≥ 10 cmH2O (in patients still to be transferred) |
| 4. pH < 7.25 for at least 2 h |
| 5. Hemodynamic instability |
| Exclusion criteria |
| Absolute |
| 1. Intracranial bleeding or other major contraindication to anticoagulation |
| 2. Previous severe disability |
| 3. Poor prognosis because of the underlying disease (i.e., unresolved malignancy) |
| Relative |
| 1. MV > 7 days |
PEEP Positive end-expiratory pressure, FiO 2 inspired oxygen fraction, HbO 2 oxygenated hemoglobin, PaO 2 /FiO 2 arterial partial pressure of oxygen to FiO2 ratio, OI oxygenation index (computed as FiO2 × mean airway pressure × 100/PaO2), MV mechanical ventilation
Each center was free to provide ECMO for H1N1 cases without complying with the National Guidelines, however these patients were excluded from the study population. If required, an ECMOnet team travelled to the referring site to take care of the transfer. After an attempt to stabilize/improve the status of the patient, the ECMOnet team would decide to either transport the patient conventionally or establish ECMO at the referring hospital. In consideration of the adjunctive risks due to transportation, we defined less severe criteria for ECMO in patients to be transferred (Table 1). Transportation was carried out via ambulance, helicopter, or fixed-wing aircraft, depending on distance, weather conditions, and ECMOnet center resources.
Proof of H1N1 infection was obtained by polymerase chain reaction, viral culture, or both on upper and lower respiratory tract specimens.
ECMO settings and ventilator management
ECMO was run with the aim of decreasing the ventilator-induced lung injury while assuring adequate gas exchange. The management of the patient’s ventilation and ECMO settings were left to the decision of the local ECMO team.
Data collection
We collected data prospectively by means of electronic Excel-based forms submitted off line to the coordinating center. (See the Electronic Supplementary Material for more information.)
Data analysis
All patients were classified as H1N1 according to demonstration of influenza A(H1N1) infection (ARDSH1N1 vs. ARDSothers). Descriptive statistics include frequency analysis for categorical variables and medians and interquartile ranges (IRQs) for continuous variables. Differences between groups were compared with the Mann-Whitney test for continuous data, and with chi-squared or two-sided Fisher’s exact tests for categorical data. To evaluate the effect of the length of mechanical ventilation (MV) before ECMO upon survival we used a logistic regression analysis. Hosmer-Lemeshow goodness-of-fit test was used to verify adequacy of the model. A P value of less than 0.05 defined statistical significance.
All statistical analyses were performed with SPSS 18 (SPSS, Chicago, IL, USA).
Ethical and legal aspects
For all patients, under emergency conditions, informed consent was waived according to Italian legislation. Families were informed and consent obtained for enrollment in the ECMOnet program. Individual data were anonymized according to the Public Health Ministry mandate for epidemiological and outcome surveillance of the ECMOnet program.
The Italian Ministry of Health allocated all economical, human, and technological resources required for the development and activity of the network.
Results
Patient characteristics
Between August 20, 2009 and March 31, 2010, 153 critically ill patients with suspected H1N1 were admitted to the ICUs of the 14 ECMOnet centers (Table 2). During the same period a total of 685 patients were admitted to all Italian ICUs (personal communication by Doctor Maria G. Pompa, Department of Infective Diseases and International Prophylaxis, Ministry of Health, Italy).
Table 2.
Patient characteristics before ECMO institution: comparison between patients with confirmed with influenza A(H1N1) (ARDSH1N1) and those with other diagnoses (ARDSother)
| ARDSH1N1 (n = 49) | ARDSother (n = 11) | |
|---|---|---|
| Age (years) | 39 (32–46) | 44 (35–55) |
| Male sex | 28 (57) | 8 (73) |
| BMI (kg m−2) | 27.5 (24–35) | 29.4 (26–35) |
| MV days before ECMO | 2 (1–5)* | 8 (1–14) |
| MV ≤ 7 days | 43 (88)* | 5 (45) |
| SAPS II | 30 (23–38) | 30 (24–35) |
| Co-morbidity factors | ||
| Obesity | 19 (39) | 4 (36) |
| Asthma and chronic lung disease | 6 (12) | 1 (9) |
| Pregnancy or postpartum | 4 (8) | 0 (0) |
| Diabetes mellitus | 3 (6) | 2 (18) |
| Respiratory severity and ventilatory parameters before ECMO | ||
| CRX infiltrate, n quadrants | 4 (3–4) | 4 (4–4) |
| PaO2/FiO2 (mmHg) | 63.3 (56–79) | 63 (47–79) |
| PaCO2 (mmHg) | 57 (47.5–71.5) | 59 (53–78) |
| pH | 7.3 (7.22–7.4) | 7.31 (7.21–7.4) |
| FiO2 | 1 (1–1) | 1 (1–1) |
| PEEP (cmH2O) | 16 (14–19) | 16 (14–19) |
| Vt/kg IBW (ml/kg) | 6.2 (4.7–7.1) | 7.19 (4.6–10.1) |
| Respiratory rate (bpm) | 26 (20–33) | 30 (20–33) |
| Peak airway pressure (cmH2O) | 33 (30–39) | 32 (30–36) |
| Plateau airway pressure (cmH2O) | 33 (30–35) | 32 (30–35) |
| Mean airway pressure (cmH2O) | 24 (22–27) | 22 (20–25) |
| Acute lung injury score | 3.75 (3.3–3.75) | 3.5 (3.25–3.75) |
| OI (mmHg cmH2O) | 36.3 (31–50) | 33.9 (28–47.5) |
| Rescue therapies and adjunctive therapies before ECMO | ||
| Recruitment maneuvers | 33 (70) | 7 (64) |
| Prone positioning | 13 (28) | 3 (27) |
| HFOV | 2 (4) | 2 (18) |
| iNO | 7 (15) | 3 (27) |
| Vasoactive drugs | 30 (62) | 7 (64) |
| Steroid therapy | 16 (33) | 4 (36) |
| None | 14 (29) | 4 (36) |
| Nonrespiratory organ function before ECMO | ||
| Heart rate (bpm) | 105 (90–120) | 103 (80–112) |
| Mean arterial pressure (mmHg) | 76 (65–86) | 78 (70–80) |
| SOFA score | 7 (6–9) | 6 (6–10) |
| Ht (%) | 32.5 (30–37.2) | 32.9 (29.5–39) |
| Platelet count (×103/μl) | 153 (109–268) | 145 (110–240) |
| Creatinine (mg/dl) | 0.8 (0.7–1.3) | 0.74 (0.45–1.1) |
| Bilirubin (mg/dl) | 0.9 (0.6–1.5) | 0.8 (0.55–1.2) |
Data presented as n (%) for categorical variables and median (interquartile range) for parametric variables
BMI Body mass index, MV mechanical ventilation, SAPS II simplified acute physiology score, CRX chest X ray, PaO 2 /FiO 2 arterial partial pressure of oxygen to inspired oxygen fraction ratio, PaCO 2 arterial partial pressure of carbon dioxide, PEEP positive end-expiratory pressure, Vt/kg IBW tidal volume indexed by ideal body weight, OI oxygenation index (computed as FiO2 × mean airway pressure × 100/PaO2), HFOV high frequency oscillatory ventilation, iNO inhaled nitric oxide, SOFA sepsis-related organ failure assessment, Ht hematocrit
* P < 0.05 ARDSH1N1 versus ARDSother
Details on patient referral and transportation are reported in Fig. 2. Once the ECMOnet system became operational, all patients requiring referral and fulfilling criteria for centralization were safely transferred to one of the ECMOnet centers. In three patients who were considered not transportable without ECMO support but who also had absolute exclusion criteria for ECMO, referral was declined.
Fig. 2.
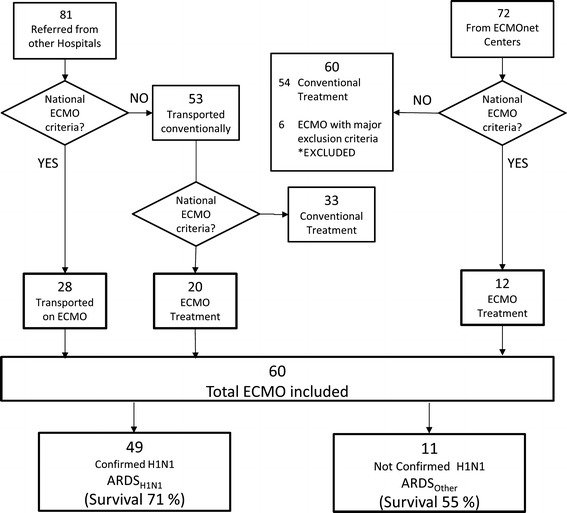
Flow diagram of patients with suspected influenza A(H1N1) admitted to the ICU of the ECMOnet centers. ECMO Extracorporeal membrane oxygenation
Sixty patients received ECMO according to ECMO eligibility criteria, of which 49 had a confirmed H1N1 diagnosis (ARDSH1N1). The remaining 11 patients had the following diagnoses: 1 non-H1N1 influenza A, 1 co-infection of Cytomegalovirus and Mycobacterium tuberculosis, 2 Klebsiella pneumoniae, 1 respiratory syncytial virus, and 6 had an alternative diagnosis. Six of the patients directly admitted to the ECMOnet centers who were excluded from the study because of the presence of absolute exclusion criteria (unresolved hematologic malignancy) received ECMO for compassionate use according to local clinical and ethical protocols and using local resources.
None of the patients had received H1N1 immunization previous to hospital admission. All patients with confirmed H1N1 received oseltamivir 150 mg twice daily, starting 0 to 12 days after hospitalization (median 2, IQR 1–3 days).
One patient was pregnant (22 week of pregnancy). Three patients were in puerperium (<28 days since delivery). All pregnant and puerperal patients had confirmed influenza A(H1N1) infection.
There were no differences in comorbidity factors between the ARDSH1N1 and ARDSother groups.
Severity of illness and treatment before ECMO
Median duration of MV before ECMO was 2 (IRQ 1–5) days (Table 2). All patients fulfilled criteria for ARDS [16]. Ten patients (17%) had PaO2/FiO2 < 50 mmHg, while 55 (92%) had PaO2/FiO2 < 100 mmHg. Before ECMO, forty-two patients (70%) received at least one of the rescue therapies. Duration of MV before ECMO was significantly lower in the ARDSH1N1 group compared to the ARDSother group. There were no statistically significant differences between ARDSH1N1 and ARDSother in terms of severity of respiratory failure, treatment, and nonrespiratory organ function before ECMO.
Indications for ECMO
Main indications for ECMO were refractory hypoxia (in 55 patients) or oxygenation index > 30 despite PaO2/FiO2 ratio > 100 mmHg (in 5 patients). Hypoxia was associated with metabolic acidosis with hemodynamic instability (in 19 patients) or with hypercapnia with life-threatening respiratory acidosis (in 4 patients).
Patient outcomes
Overall survival at hospital discharge was 68.3% (41 patients). Details on patient outcomes are reported in Table 3.
Table 3.
Patient outcomes. Comparison between patients with ARDS who demonstrated influenza A(H1N1) infection (ARDSH1N1) and who did not (ARDSother)
| ARDSH1N1 (n = 49) | ARDSother (n = 11) | |
|---|---|---|
| Survival at ICU discharge | 35 (71) | 6 (54) |
| Survival at hospital discharge | 35 (71) | 6 (54) |
| Tracheostomy | 23 (49) | 6 (55) |
| Infection | 26 (53) | 4 (36) |
| CRRT | 14 (29) | 3 (27) |
| Length of ICU stay | ||
| All patients | 22 (14–37) | 17 (14–32) |
| Surviving patients | 23 (16–37) | 14 (12–32) |
| Length of hospital stay | ||
| All patients | 39 (22–50) | 40 (32–47) |
| Surviving patients | 43 (28–54) | 42 (34–54) |
| Days of MV | ||
| All patients | 18 (14–41) | 21 (13–31) |
| Surviving patients | 19 (15–37) | 18.5 (11–27.5) |
| Days of ECMO | ||
| All patients | 10 (7–17) | 8 (3–21) |
| Surviving patients | 9 (7–15) | 8 (2–15) |
| Cause of death | ||
| MOF due to sepsis | 8 (57.2) | 2 (40) |
| Neurological disorder | 1 (7.2) | 1 (20) |
| Acute liver failure | 1 (7.2) | – |
| Right heart failure | 1 (7.2) | – |
| Septic shock | 3 (21.2) | 2 (40) |
| ECMO-related complications, n (n ECMO complication-related deaths) | ||
| CNS hemorrhage | 1 (1) | – |
| Abdominal bleeding | 2 (0) | – |
| Airways bleeding | 1 (0) | – |
| Cannulation complications | 4 (0) | – |
Data presented as n (%) for categorical variables and median (interquartile range) for parametric variables
CRRT Continuous renal replacement therapy, ICU intensive care unit, MV mechanical ventilation, MOF multiple organ failure, CNS central nervous system
* P < 0.05 ARDSH1N1 versus ARDSother
Most common causes of death were multiple organ failure associated with sepsis in ten patients (17%) and septic shock in five patients (8%).
Comparison between surviving and nonsurviving patients
Overall population
The time on MV before ECMO was lower for survivors than for nonsurvivors. Survivors also showed higher hematocrit, lower SOFA and SAPS II scores, and lower bilirubin levels (Table 4).
Table 4.
Comparison between surviving and nonsurviving patients in the overall population, and in the group of patients with confirmed influenza A(H1N1) infection
| All ECMO, n = 60 (100%) | ARDSH1N1, n = 49 (82%) | |||
|---|---|---|---|---|
| Survivor, n = 41 (68%) | Nonsurvivor, n = 19 (32%) | Survivor, n = 35 (71%) | Nonsurvivor, n = 14 (29%) | |
| Age (years) | 38 (29–48) | 43 (36–50) | 37 (30–46) | 43 (36–47) |
| BMI (kg m−2) | 27 (24–38) | 29 (26–34) | 27 (24–38) | 29 (24–34) |
| MV (days) | 2 (1–4)* | 6 (1–12) | 1 (1–3)* | 5 (1–8) |
| SAPS II | 29 (23–35)* | 35 (30–49) | 29 (23–35)* | 44 (31–52) |
| Co-morbidity factors | ||||
| Obesity | 14 (34) | 9 (47) | 13 (37) | 6 (43) |
| Asthma and chronic lung disease | 4 (10) | 3 (16) | 3 (9) | 3 (22) |
| Pregnancy or postpartum | 4 (10) | 0 (0) | 4 (12) | 0 (0) |
| Diabetes mellitus | 5 (12) | 0 (0) | 3 (9) | 0 (0) |
| Respiratory severity and ventilatory parameters before ECMO | ||||
| PaO2/FiO2 (mmHg) | 63 (54–81) | 61 (54–79) | 63 (55–81) | 61 (53–72) |
| PaCO2 (mmHg) | 57 (48–78) | 60 (50–70) | 57 (50–79) | 62 (48–70) |
| pH | 7.29 (7.23–7.41) | 7.31 (7.20–7.37) | 7.29 (7.23–7.41) | 7.34 (7.20–7.39) |
| PEEP (cmH2O) | 16 (15–20) | 16 (12–19) | 16 (14–20) | 15 (13–18) |
| Vt/kg (ml/kg) | 6.2 (4.8–8.4) | 5.8 (4.5–7.1) | 6.2 (4.7–7.1) | 6.7 (4.9–7.1) |
| Respiratory rate (bpm) | 25 (19–33) | 30 (25–34) | 25 (20–33) | 30 (20–33) |
| Peak airway pressure (cmH2O) | 32 (30–37) | 33 (32–46) | 33 (30–38) | 35 (32–46) |
| Plateau airway pressure (cmH2O) | 33 (30–35) | 33 (30–35) | 33 (30–35) | 34 (31–35) |
| Mean airway pressure (cmH2O) | 24 (22–26) | 24 (21–29) | 24 (22–26) | 25 (22–29) |
| Acute lung injury score | 3.67 (3.25–3.75) | 3.75 (3.25–3.75) | 3.66 (3.25–3.75) | 3.75 (3.45–3.75) |
| OI (mmHg cmH2O) | 36 (30–50) | 35 (30–45) | 36 (29–50) | 38 (34–47) |
| Rescue therapies and adjunctive therapies before ECMO | ||||
| Recruitment maneuvers | 28 (68) | 13 (68) | 24 (69) | 10 (71) |
| Prone positioning | 12 (29) | 4 (21) | 10 (29) | 3 (21) |
| HFOV | 2 (4) | 2 (10) | 1 (3) | 1 (7) |
| iNO | 7 (17) | 3 (16) | 6 (17) | 1 (7) |
| Vasoactive drugs | 25 (61) | 12 (63) | 21 (60) | 9 (64) |
| Steroid therapy | 11 (27) | 10 (53) | 8 (23) | 8 (57) |
| None | 12 (29) | 6 (32) | 10 (29) | 4 (29) |
| Nonrespiratory organ function before ECMO | ||||
| HR (bpm) | 100 (89–120) | 108 (100–120) | 100 (85–120) | 114 (100–120) |
| Mean arterial pressure (mmHg) | 78 (67–90) | 73 (59–81) | 80 (65–92) | 70 (60–81) |
| SOFA score | 7 (5–9)* | 10 (7–11) | 7 (5–9)* | 10 (7–12) |
| Ht (%) | 35 (31–38)* | 30 (26–34) | 35 (31–38)* | 30 (25–35) |
| Platelet count (×103/μl) | 157 (120–259) | 143 (97–330) | 156 (120–239) | 139 (93–335) |
| Creatinine (mg/dl) | 0.8 (0.6–1.1) | 0.9 (0.7–1.9) | 0.8 (0.7–1.1) | 1.1 (0.7–2.1) |
| Bilirubin (mg/dl) | 0.8 (0.6–1.2)* | 1.2 (0.7–1.8) | 0.8 (0.4–1.2)* | 1.4 (1–2.1) |
Data presented as n (%) for categorical variables and median (interquartile range) for parametric variables
BMI Body mass index, MV mechanical ventilation, SAPS II simplified acute physiology score, CRX chest X ray, PaO 2 /FiO 2 arterial partial pressure of oxygen to inspired oxygen fraction ratio, PaCO 2 arterial partial pressure of carbon dioxide, PEEP positive end-expiratory pressure, Vt/kg IBW tidal volume indexed by ideal body weight, OI oxygenation index (computed as FiO2 × mean airway pressure × 100/PaO2), HFOV high frequency oscillatory ventilation, iNO inhaled nitric oxide, SOFA sepsis-related organ failure assessment, Ht hematocrit
* P < 0.05 survivor versus nonsurvivor
Each day of MV before ECMO increased by 29% the log odds of death (OR 1.291, 95 CI 1.092–1.527, P = 0.003) (Fig. 3). The Hosmer-Lemeshow test indicated a good fit of the model (P = 0.818).
Fig. 3.
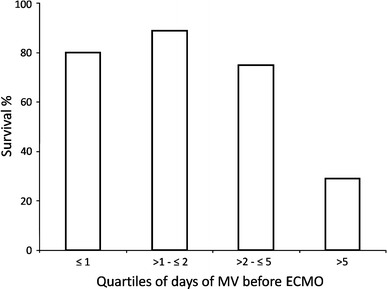
Survival in relation to the days of mechanical ventilation (MV) before ECMO. Results are shown for quartiles according to days of MV before ECMO. Fisher’s exact test P < 0.01
ARDSH1N1 group
The time on MV before ECMO was lower for survivors than for nonsurvivors. Survivors also showed higher hematocrit, lower SOFA and SAPS II scores, and lower bilirubin levels. Each day of delay in starting ECMO increased by 34.8% the log odds of death (OR 1.348, 95 CI 1.071–1.697, P = 0.011). The Hosmer-Lemeshow test indicated a good fit of the model (P = 0.660).
Details of ECMO treatment
ECMO bypass was veno-venous in 59 cases (femoral-jugular in 33 and femoral-femoral in 26 cases), and veno-arterial in one patient. All ECMO circuits were heparin-coated and included centrifugal pumps and polymethylpentene oxygenators of different types and brands. All cannulas were heparin coated. The details of the EMCO treatment are presented in Table 5.
Table 5.
Time course of main respiratory, ventilator, and ECMO-related treatment variables at baseline, at days 1, 7, and 14 after starting ECMO, and within 6 h before stopping ECMO treatment and patient decannulation
| Baseline (n = 60) | Day 1 (n = 60) | Day 7 (n = 50) | Day 14 (n = 30) | Decannulation (n = 41) | |
|---|---|---|---|---|---|
| FiO2 | 1 (1–1)* | 0.6 (0.4–0.8)† | 0.6 (0.5–0.8) | 0.5 (0.45–0.6) | 0.5 (0.4–0.53) |
| PEEP (cmH20) | 16 (14–19) | 16 (14–19)† | 15 (11–18) | 12 (10–15) | 12 (10–15) |
| Vt/kg IBW (ml/kg) | 6.2 (4.7–7.7)* | 4.6 (3–6.3)† | 5 (3.4–7.5) | 6.1 (4.5–8.3) | 8.5 (6.9–10.1) |
| Respiratory rate (bpm) | 28 (20–33)* | 10 (8–12)† | 15 (10–20) | 18 (12–24) | 20 (14–24) |
| Minute volume (l/min) | 10 (6.5–12.3)* | 2.5 (1.7–4)† | 3 (2–8) | 4.6 (2.4–10) | 9.7 (8.3–11.6) |
| Mean airway pressure (cmH20) | 25 (22–28)* | 22 (18–24) | 20 (18–24) | 18 (15–22) | 17 (16–20) |
| Peak airway pressure (cmH2O) | 33 (30–40)* | 29 (26–30) | 28 (25–30) | 27 (23–28) | 26 (24–29) |
| PaO2/FiO2 (mmHg) | 63 (54–79)* | 160 (112–215)† | 156 (96–209) | 166 (131–224) | 225 (179–300) |
| PaCO2 (mmHg) | 58 (50–75)* | 41 (37–47)† | 45 (39–49) | 43 (37–49) | 44 (40–50) |
| pH | 7.31 (7.21–7.40)* | 7.45 (7.40–7.49)† | 7.43 (7.4–7.47) | 7.42 (7.39–7.45) | 7.42 (7.39–7.45) |
| Coagulation function | |||||
| Ht (%) | 33 (30–37) | 32 (28–34) | 30 (26–33) | 31 (27–32) | 29 (27–32) |
| Platelet count | 149 (110–262) | 150 (103–211) | 154 (95–226) | 189 (114–280) | 177 (131–250) |
| aPTT ratio | – | 1.6 (1.3–1.8) | 1.6 (1.3–2.3) | 1.7 (1.3–2.3) | – |
| ACT | – | 180 (170–200) | 185 (163–206) | 176 (161–180) | – |
| ECMO setting | |||||
| BF (l/min) | – | 4 (3–4.8) | 3.8 (2.6–4.9) | 3.5 (2.5–4.3) | – |
| GF (l/min) | – | 4 (4–6) | 6 (4–7.2) | 5 (2.8–7) | – |
| GFoxygen | – | 1 (0.9–1) | 1 (0.9–1) | 0.9 (0.5–1) | – |
Data presented as median (interquartile range)
FiO 2 Inspired oxygen fraction, PEEP positive end-expiratory pressure, Vt/kg IBW tidal volume indexed by ideal body weight, PaO 2 /FiO 2 arterial partial pressure of oxygen to inspired oxygen fraction ratio, PaCO 2 arterial partial pressure of carbon dioxide, Ht hematocrit, ACT activated clotting time, aPTT activated partial thromboplastin time, BF ECMO blood flow, GF ECMO gas flow, GF oxygen ECMO gas flow oxygen concentration
* P < 0.05 baseline versus day 1, † P < 0.05 day 1 versus decannulation
Anticoagulation consisted of continuous heparin infusion (median 248, IRQ 120–370 IU/kg/day) based on activated clotting time (ACT) and/or activated partial thromboplastin time (aPTT).
Sixteen patients had hemorrhagic complications and in ten of them a major bleeding event occurred, requiring blood transfusions and temporary reduction or suspension of heparin administration (Table 3). One patient died of cerebral hemorrhage diagnosed 2 days after cannulation. In none of the other patients did ECMO treatment have to be stopped because of bleeding complications. Blood components were transfused in 47 (78%) patients. The median amount of packed red blood cells transfused per patient during the entire ECMO treatment was 1,500 (400–2,990) ml.
Details of patient transport
Seventy-one patients were transferred by ambulance (19 on ECMO), 8 by helicopter (all on ECMO), and 2 by fixed wing aircraft (1 on ECMO). All patients were transported successfully and without complications to the referral hospital. Survival rate in the patients transported on ECMO was 81%. There were no statistically significant differences between patients transported on ECMO and patients starting ECMO at the ECMOnet center in terms of severity of respiratory failure, treatment, and outcomes.
Discussion
We report here the activity of the Italian national ECMOnet during the 2009 influenza A(H1N1) pandemic.
Assessment of ECMOnet activity
The network achieved its established aims. Once in operation, all patients referred to the network were safely transferred to one of the ECMOnet centers. All patients fulfilling ECMO criteria received ECMO within 24 h of the first call. The ECMOnet teams instituted ECMO at the referring hospital in 28 patients and transfer was carried out safely while on ECMO.
The network was developed to provide centralized, coordinated access to ECMO support throughout the country. Centralization was a key aspect of the network effectiveness.
We assumed that centralization of patients to a few selected, specifically equipped centers could improve patient outcome [16]. The main criticism of this concept is that the risks associated with patient transport may outweigh the benefits of centralization [17]. To minimize these risks we planned two complementary strategies. First, indications for referral were based on a risk anticipation principle: clinical criteria for referral aimed to transfer in advance the largest possible proportion of patients potentially at risk of severe respiratory deterioration. Second, we identified precise criteria to place the patients under the responsibility of expert transportation teams, able to institute ECMO at the referring hospital and provide safe transportation with ECMO.
ECMO and influenza A(H1N1)
In this study, patients receiving ECMO support who had been ventilated for less than 7 days had a 77% survival to hospital discharge. The rate of ECMO-associated major complications was low; survival rate was higher in patients who received ECMO earlier; and ECMO allowed safe transportation of patients otherwise deemed too sick for safe transfer.
During the H1N1 pandemic, hundreds of ARDS patients worldwide received ECMO [18]. Of all reports [14, 18–21], the most relevant is that of the ANZ ECMO investigators, which included 68 patients with suspected H1N1-associated ARDS treated with ECMO [14]. Severity of ARDS, patient characteristics before ECMO, and survival rates observed in our study were similar to those of the ANZ ECMO. Other case series on ECMO in H1N1 patients reported survival rates between 55 and 79% [18–21].
The worldwide acceleration in the use of ECMO in H1N1 patients has caused a debate between ECMO advocates and skeptics [22–27].
Most studies have reported mortality rate of general ICU patient populations, independent of the need for MV or independent of the severity of respiratory illness [4, 10, 18, 21]. There are limited data on outcomes in patients who qualified for ECMO support but did not receive it. For this reason we do not have enough information to estimate an expected mortality against which we could compare our results. To our knowledge, the Argentinian report is the only study stratifying mortality according to PaO2/FiO2 ratio [8]. It reported a 67% mortality in patients with PaO2/FiO2 ratio < 100 mmHg. These patients received rescue therapies with a frequency similar to that in our study but did not receive ECMO treatment. The Italian Group for the Evaluation of Interventions in Intensive care Medicine (GiViTi) prospectively collected data from 152 Italian ICUs on H1N1 patients. The GiViTi H1N1 database included 45 mechanically ventilated ARDS patients whose lowest PaO2/FiO2 ratio was <100 mmHg but did not receive ECMO. Mortality at hospital discharge in this group was 45% (data by GiViTi following formal application to the GiViTi Coordination Center).
A recent report from a single center in Utah [7] demonstrated a 73% survival rate in 30 H1N1 patients with ARDS not receiving any rescue therapy. However, the survival rate decreased to 42% in the subgroup of 14 patients with PEEP ≥ 20 cmH2O.
There were 48 (80%) and 49 (72%) patients referred from other hospitals to ECMO centers in our study and in the ANZ ECMO study [14], respectively. The high survival rate observed in our study and in other studies on ECMO in severe H1N1 ARDS patients echoes the recently published findings of a recent trial comparing MV with ECMO (CESAR) [15] and strongly supports the use of ECMO as an effective means to improve outcome in the most severe H1N1 ARDS patients. However, as most of these studies are descriptive, randomized trials comparing ECMO to conventional treatment are needed.
Occurrence of bleeding remains the most important complication during ECMO support [28]. In the ANZ ECMO report, hemorrhagic complications were the cause of death in 10 of the 14 patients who died. In our study most patients died of multiorgan failure or septic shock, and in only one patient could death be related to a possible direct ECMO complication (cerebral hemorrhage). This result is comparable to that of the CESAR trial in which only one patient died because of an ECMO-related hemorrhagic complication [15]. Notably, in our study the need for ECMO was anticipated (following the ANZ ECMO experience) and planned as part of the critical response plan. Preventing and managing ECMO complications by promoting support from more experienced centers was a primary aim of the network.
Though we aimed to anticipate patient centralization to ECMO centers, given the observed rapid progression of respiratory failure associated with H1N1 pneumonia, in 28 (46%) patients it was necessary to start ECMO on site and provide transportation while on ECMO. All our patients transported on ECMO were successfully transferred to the ECMO centers with no reported complications. Survival rate in these patients was 81%, supporting the effectiveness of ECMO in providing safe transportation of patients with severe respiratory failure. In the CESAR trial 22 of the 90 patients initially allocated to the ECMO group did not receive ECMO because they improved with conventional treatment once at the ECMO center [15]. Similarly, we cannot exclude the possibility that some of the patients transported with ECMO might not have needed ECMO if treated from the beginning with other rescue therapies at the referral centers, where more therapeutic options were available. However, most of these patients were considered to be not safely transportable without ECMO.
Limitations of the study
Although the activity of the ECMOnet covered all of the Italian territory, a limited number of ECMO treatments were performed in centers outside the network and are not included in this report. The institution and funding of the ECMOnet was communicated officially by the Italian Ministry of Health to all Italian hospitals, but access to it was not compulsory. Thus, the study population does not include all ECMO treatments, nor all potentially eligible patients in Italy during the H1N1 pandemic.
The lack of a 6 or 12 month follow-up on respiratory function of surviving patients limits our knowledge on the effect of prolonged mechanical ventilation before ECMO assistance, and more generally, the long-term consequences of ventilatory treatment versus ECMO treatment in ARDS patients.
Conclusions
In summary, the institution of the Italian ECMO network allowed a high survival rate of patients with severe ARDS due to H1N1 infection treated by ECMO, providing effective and safe centralization and creating an important organization platform to face future possible epidemics with high demand for critical care services and specialized respiratory support.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Minister of Health Prof. Antonio Fazio and the Ministry of Health who funded the entire network organization. We thank Maria Grazia Pompa (Department of Malattie Infettive e Profilassi Internazionale, Ministry of Health, Italy) for providing incidence and mortality data of patients admitted to the Italian ICUs with influenza A(H1N1) during the study period. We thank the Coordination Center of GiViTi for providing data of patients with H1N1 included in GiViTi H1N1 registry. We thank the biostatisticians Teresa Greco and Rosalba Lembo (Department of Anesthesia and Intensive Care, Università Vita-Salute San Raffaele, Milan, Italy) for providing support on statistical analysis. We thank G. Tognoni (Consorzio Mario Negri Sud, Santa Maria Imbaro, Italy) for providing support on ethical and legal aspects. We thank the Italian Air Force whose indispensable support allowed long distance patient transfer. We thank all ambulance personnel whose indispensable support allowed interhospital transfer of all patients. We are indebted to all physicians, perfusionists, and nursing staff of the participating units for their priceless cooperation in caring for these complex patients.
Appendix
ECMOnet Italian ECMO network
G. Foti, M. Bombino (Dipartimento di Medicina Perioperatoria, Ospedale San Gerardo, Monza, Italy); A. M. Scandroglio, M. G. Calabrò, T. Bove, M. De Bonis (Terapia Intensiva Cardiovascolare, Dipartimento di Anestesia Cardiaca e Terapia Intensiva, Università Vita-Salute San Raffaele, Milano, Italy); A. Pasquini (Anestesia e Rianimazione di Emergenza, Dipartimento DAI e Medicina e Chirurgia Generale e d’Urgenza, Azienda Ospedaliero Universitaria Careggi, Firenze, Italy); F. Mojoli (Anestesia e Rianimazione I, Fondazione IRCCS Policlinico San Matteo, Università degli Studi, Pavia, Italy); M. Zanierato (Anestesia e Rianimazione I, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy); M. Belliato, L. Carnevale (Anestesia e Rianimazione II, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy); R. Urbino, L. Del Sorbo, V. Fanelli (Dipartimento di Anestesiologia e Medicina degli Stati Critici, Università di Torino, Ospedale S. Giovanni Battista-Molinette, Torino, Italy); G. Bello, R. Maviglia (Dipartimento di terapia Intensiva e Anestesiologia, Università Cattolica del Sacro Cuore, Rome, Italy); A. Lissoni, L. Caspani (Dipartimento di Anestesiologia, Fondazione Istituto Di Ricovero e Cura a Carattere Scientifico, Ospedale Maggiore Policlinico, Università degli Studi di Milano, Milan, Italy); P. Pietropaoli, M. Rocco, A. Morelli (Dipartimento di Scienze Anestesiologiche, Medicina Critica e Terapia del Dolore, Università la Sapienza, Rome, Italy); G. Frascaroli, F. Caramelli (Dipartimento Attività Integrate Cardio-Toraco-Vascolare, Azienda Ospedaliero-Universitaria S. Orsola-Malpighi di Bologna, Bologna, Italy); R. Tufano, M. Iannuzzi (Centro di Rianimazione, Dipartimento Assistenziale di Anestesia, Rianimazione, Terapia Intensiva, Terapia Iperbarica e Terapia Antalgica, Università degli Studi Federico II, Naples); F. Bruno, S. Grasso (Unità operativa di Anestesia e Rianimazione I, dipartimento di Emergenza e Trapianti d’Organo, Università di Bari, Bari, Italy); L. Lorini (Dipartimento di Anestesia e Rianimazione, Ospedali Riuniti di Bergamo, Bergamo, Italy); C. Ori, S. Rossi, P. Persona (Istituto di Anestesia e Rianimazione, Azienda Ospedaliera-Università di Padova, Padua, Italy).
Footnotes
This article is written for the ECMOnet Italian ECMO network. Members of the network are listed in the Appendix.
References
- 1.Fowler RA, Lapinsky SE, Hallett D, Detsky AS, Sibbald WJ, Slutsky AS, Stewart TE, Toronto SARS Critical Care Group Critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:367–373. doi: 10.1001/jama.290.3.367. [DOI] [PubMed] [Google Scholar]
- 2.Lew TW, Kwek TK, Tai D, Earnest A, Loo S, Singh K, Kwan KM, Chan Y, Yim CF, Bek SL, Kor AC, Yap WS, Chelliah YR, Lai YC, Goh SK. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:374–380. doi: 10.1001/jama.290.3.374. [DOI] [PubMed] [Google Scholar]
- 3.Li FC, Choi BC, Sly T, Pak AW. Finding the real case-fatality rate of H5N1 avian influenza. J Epidemiol Community Health. 2008;62:555–559. doi: 10.1136/jech.2007.064030. [DOI] [PubMed] [Google Scholar]
- 4.Dominguez-Cherit G, Lapinsky SE, Macias Ae, Pinto R, Espinosa-Perez L, de la Torre A, Poblano-Morales M, Baltazar-Torres JA, Bautista E, Martinez A, Martinez MA, Rivero E, Valdez R, Ruiz-Palacios G, Hernández M, Stewart TE, Fowler RA. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302:1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A, Zarychansky R, Pinto R, Cook DJ, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, Turgeon AF, Lapinsky S, Ahern SP, Smith O, Siddiqui F, Jouvet P, Khwaja K, McIntyre L, Menon K, Hutchison J, Hornstein D, Joffe A, Lauzier F, Singh J, Karachi T, Wiebe K, Olafson K, Ramsey C, Sharma S, Dodek P, Meade M, Hall R, Fowler RA, Canadian Critical Care Trials Group H1N1 Collaborative Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 6.The ANZIC Influenza Investigators Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361:1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 7.Miller RR, III, Markewitz BA, Rolfs RT, Brown SM, Dascomb KK, Grissom CK, Friedrichs MD, Mayer J, Hirshberg EL, Conklin J, Paine R, III, Dean NC. Clinical findings and demographic factors associated with ICU admission in Utah due to novel 2009 influenza A(H1N1) infection. Chest. 2010;137:752–758. doi: 10.1378/chest.09-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estenssoro E, Ríos FG, Apezteguía C, Reina R, Neira J, Ceraso DH, Orlandi C, Valentini R, Tiribelli N, Brizuela M, Balasini C, Mare S, Domeniconi G, Ilutovich S, Gómez A, Giuliani J, Barrios C, Valdez P, Registry of the Argentinian Society of Intensive Care SATI Pandemic 2009 influenza A in Argentina: a study of 337 patients on mechanical ventilation. Am J Respir Crit Care Med. 2010;182:41–48. doi: 10.1164/201001-0037OC. [DOI] [PubMed] [Google Scholar]
- 9.Rello J, Rodríguez A, Ibañez P, Socias L, Cebrian J, Marques A, Guerrero J, Ruiz-Santana S, Marquez E, Del Nogal-Saez F, Alvarez-Lerma F, Martínez S, Ferrer M, Avellanas M, Granada R, Maraví-Poma E, Albert P, Sierra R, Vidaur L, Ortiz P, Prieto del Portillo I, Galván B, León-Gil C, H1N1 SEMICYUC Working Group Intensive care adult patients with severe respiratory failure caused by influenza A(H1N1)v in Spain. Crit Care. 2009;13:R148. doi: 10.1186/cc8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, Jasuja S, Deutscher M, Chen S, Walker JD, Duchin JS, Lett S, Soliva S, Wells EV, Swerdlow D, Uyeki TM, Fiore AE, Olsen SJ, Fry AM, Bridges CB, Finelli L, 2009 Pandemic Influenza A(H1N1) Virus Hospitalizations Investigation Team Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 11.Lapinsky SE. Epidemic viral pneumonia. Curr Opin Infect Dis. 2010;23:139–144. doi: 10.1097/QCO.0b013e328336eaae. [DOI] [PubMed] [Google Scholar]
- 12.WHO (2011) National influenza pandemic plans. http://www.who.int/csr/disease/influenza/nationalpandemic/en/index.html
- 13.Sprung CL, Zimmerman JL, Christian MD, Joynt GM, Hick JL, Taylor B, Richards GA, Sandrock C, Cohen R, Adini B. Recommendations for intensive care unit and hospital preparations for an influenza epidemic or mass disaster: summary report of the European Society of Intensive Care Medicine’s Task Force for intensive care unit triage during an influenza epidemic or mass disaster. Intensive Care Med. 2010;36:428–443. doi: 10.1007/s00134-010-1759-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 15.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D, CESAR trial collaboration Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 16.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European consensus conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 17.Singh JM, MacDonald RD. Pro/con debate: do the benefits of regionalized critical care delivery outweigh the risks of interfacility patient transport? Crit Care. 2009;13:219. doi: 10.1186/cc7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Extracorporeal Life Support Organization (2010) www.elso.med.umich.edu/H1N1.htm. Accessed 28 May 2010
- 19.Freed DH, Henzler D, White CW, Fowler R, Zarychanski R, Hutchison J, Arora RC, Manji RA, Legare JF, Drews T, Veroukis S, Kesselman M, Guerguerian AM, Kumar A, Canadian Critical Care Trials Group Extracorporeal lung support for patients who had severe respiratory failure secondary to influenza A(H1N1) 2009 infection in Canada. Can J Anesth. 2010;57:240–247. doi: 10.1007/s12630-009-9253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norfolk SG, Hollingsworth CL, Wolfe CR, Govert JA, Que LG, Cheifetz IM, Hollingsworth JW. Rescue therapy in adult and pediatric patients with pH1N1 influenza infection: a tertiary center intensive care unit experience from April to October 2009. Crit Care Med. 2010;38:2103–2107. doi: 10.1097/CCM.0b013e3181f268f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roch A, Lepaul-Ercole R, Grisoli D, Bessereau J, Brissy O, Castanier M, Dizier S, Forel JM, Guervilly C, Gariboldi V, Collart F, Michelet P, Perrin G, Charrel R, Papazian L. Extracorporeal membrane oxygenation for severe influenza A(H1N1) acute respiratory distress syndrome: a prospective observational comparative study. Intensive Care Med. 2010;36:1899–1905. doi: 10.1007/s00134-010-2021-3. [DOI] [PubMed] [Google Scholar]
- 22.Davies A, Jones D, Gattas D. Extracorporeal membrane oxygenation for ARDS due to 2009 influenza A(H1N1) JAMA. 2010;303:942. doi: 10.1001/jama.2010.202. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell MD, Mikkelsen ME, Umscheid CA, Lee I, Fuchs BD, Halpern SD. A systematic review to inform institutional decisions about the use of extracorporeal membrane oxygenation during the H1N1 influenza pandemic. Crit Care Med. 2010;38:1398–1404. doi: 10.1097/CCM.0b013e3181de45db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park PK, Dalton HJ, Bartlett RH. Point: efficacy of extracorporeal membrane oxygenation in 2009 influenza A(H1N1): sufficient evidence? Chest. 2010;138:776–778. doi: 10.1378/chest.10-1791. [DOI] [PubMed] [Google Scholar]
- 25.Morris AH, Hirshberg E, Miller RR, 3rd, Statler KD, Hite RD. Counterpoint: efficacy of extracorporeal membrane oxygenation in 2009 influenza A(H1N1): sufficient evidence? Chest. 2010;138:778–781. doi: 10.1378/chest.10-1792. [DOI] [PubMed] [Google Scholar]
- 26.Hubmayr RD, Farmer JC. Should we “rescue” patients with 2009 influenza A(H1N1) and lung injury from conventional mechanical ventilation? Chest. 2010;137:745–747. doi: 10.1378/chest.09-2915. [DOI] [PubMed] [Google Scholar]
- 27.Dalton HJ, MacLaren G. Extracorporeal membrane oxygenation in pandemic flu: insufficient evidence or worth the effort? Crit Care Med. 2010;38:1484–1485. doi: 10.1097/CCM.0b013e3181e08fff. [DOI] [PubMed] [Google Scholar]
- 28.Brogan TV, Thiagarajan RR, Rycus PT, Bartlett RH, Bratton SL. Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-center database. Intensive Care Med. 2009;35:2105–2114. doi: 10.1007/s00134-009-1661-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


