Abstract
We formulate an epidemic model for the spread of an infectious disease along with population dispersal over an arbitrary number of distinct regions. Structuring the population by the time elapsed since the start of travel, we describe the infectious disease dynamics during transportation as well as in the regions. As a result, we obtain a system of delay differential equations. We define the basic reproduction number as the spectral radius of a next generation matrix. For multi-regional systems with strongly connected transportation networks, we prove that if then the disease will be eradicated from each region, while if there is a globally asymptotically stable equilibrium, which is endemic in every region. If the transportation network is not strongly connected, then the model analysis shows that numerous endemic patterns can exist by admitting a globally asymptotically stable equilibrium, which may be disease free in some regions while endemic in other regions. We provide a procedure to detect the disease free and the endemic regions according to the network topology and local reproduction numbers. The main ingredients of the mathematical proofs are the inductive applications of the theory of asymptotically autonomous semiflows and cooperative dynamical systems. We visualise stability boundaries of equilibria in a parameter plane to illustrate the influence of the transportation network on the disease dynamics. For a system consisting of two regions, we find that due to spatial heterogeneity characterised by different local reproduction numbers, may depend non-monotonically on the dispersal rates, thus travel restrictions are not always beneficial.
Keywords: Epidemic models, Transportation networks, Global dynamics, Delay differential systems
Introduction
The increasing volume of international trade and tourism highly facilitates the rapid spread of infectious diseases around the world. The outbreaks of severe acute respiratory syndrome (SARS) in 2002–2003 and influenza A virus subtype H1N1 in 2009 highlighted the important role of human transportation on the global spread of infectious diseases, see the reviews WHO (2003) for the spread of SARS, and Khan et al. (2009) for the spread of influenza along international air traffic routes.
There are several well-known studies which constructed and analysed various metapopulation models, based on differential equations, to describe the spatial spread of infectious diseases in connected regions, see Arino (2009), Wang (2007), Arino and Driessche (2003), Gao and Ruan (2012), Wang and Zhao (2004, 2005), Arino et al. (2005, 2006, 2007), Li and Zou (2010) and references therein. In this framework, the spatial structure is represented by a finite number of distinct patches, and the population dynamics in the patches is coupled to the dynamics of other patches, to account for the mobility between regions.
The network science approach, focusing on the structure of the network formed by the connections among regions, provided further important insights to understand the role of mobility patterns and heterogeneity in the transmission dynamics and the global invasion of infectious diseases, see Balcan and Vespignani (2011), Colizza and Vespignani (2007, 2008), Meloni et al. (2011), Poletto et al. (2012), Colizza et al. (2006).
The above mentioned works studied mostly the impact of spatial dispersal of infected individuals from one region to another, and did not consider transportation as a platform of disease transmission. However, contact tracing of passengers provided evidence that during long distance travel, such as intercontinental commercial flights, a single infected passenger may infect several other persons during the flight, see the comprehensive summary for several infectious diseases by European Centre for Disease Prevention and Control (2009a), and references (Bell 2004; European Centre for Disease Prevention and Control 2009b; Olsen et al. 2003).
To properly describe the number of generated infections via transportation in the destination region, transport related infections were incorporated into the compartmental model approach in Cui et al. (2006), Takeuchi et al. (2007), Liu and Takeuchi (2006), where it was illustrated that the disease can persist in regions connected by human transportation even if the infection died out in all regions in the absence of travel.
These models assumed that individuals, who left a certain region, arrived immediately to their destination region. In reality, animal transportation can take rather long time; and in the case of human travel, for rapidly progressing diseases such as SARS and influenza, even a fraction of a day can be significant. During travel, passengers are in a closed environment with a high-density layout of seating, exposed to low humidity and hypobaric hypoxia (Mangili and Gendreau 2005; Silverman and Gendreau 2009). Thus, it is more precise to consider the number of infected passengers as a dynamical variable in an environment that is different from residential areas, and then the time needed to complete the travel naturally becomes an important parameter of the model. The pioneering works (Liu et al. 2008; Nakata 2011) formulated submodels for disease transmission dynamics during travel, combining with compartmental models in the regions, but, due to the apparent mathematical difficulties, their analysis is restricted to only two identical regions.
In this manuscript we analyse an epidemic model that includes both infectious disease dynamics during transportation, and an arbitrary number of heterogeneous regions forming a transportation network. These two features together have not been studied before. Our aim is to obtain a qualitative picture of the disease transmission dynamics in a heterogeneous multi-regional environment characterised by respective risk of infection in regions as well as during travel, and to understand the role of the transportation network in the disease transmission dynamics.
The description of the structure of the transportation network is based on directed graphs: the regions are the nodes of the graph, and nodes and are connected by a directed link if there is transportation of individuals from region to region . We explicitly incorporate the time needed to complete a one-way travel from one region to another, and consider the disease dynamics along such directed links. A network is called strongly connected, if for any ordered pair of nodes, there is a directed path (a sequence of directed links) from node to node . Otherwise, we say that the network is not strongly connected. For example, having two sets of nodes and , where there are no directed links from any node in to any node in , but there are directed links for any other ordered pair of nodes, gives a connected, but not strongly connected network.
Many transportation networks are naturally strongly connected (one can go from any region to any other region, possibly via other regions), but there are significant biological reasons to consider not strongly connected networks as well. When an outbreak of an infectious disease is reported, the structure of the transportation network may change from strongly connected to not strongly connected, since individuals likely do not travel to the endemic region, and public health authorities may advise against travelling to high risk regions. Some transportation connections may be shut down in order to implement a disease control policy. The simplest example is the case of two connected regions, when the transportation becomes unidirectional. We explore this case in details in Sect. 6. The displacement network for the transportation of livestock is a typical example of not strongly connected networks, as animals are moved from farms to slaughterhouses (possibly via several intermediate steps, such as assembling centres), but there is no movement of livestock from the slaughterhouses back to the farms. During the transportation, animals are kept in crowded cages, therefore there is an elevated risk of disease transmission. Such livestock transportation network can be very complex (Bajardi et al. 2011). Migration routes in ecology typically follow a directional trend, such as from South to North because of climate change, downstream in rivers, etc. In those cases the network of the habitats of the species is not strongly connected. Human networks are usually strongly connected. The rural-to-urban migration, however, can be seen as an example for unidirectional transportation if we neglect the short-term mobility such as tourism and business travels. The vector of Chagas disease appeared this way in major cities in South America (Alirol et al. 2011). The presence of tuberculosis in Canada (Zhou et al. 2008) is driven by the constant flow of immigration from developing countries. TB is one of the diseases which is transmissible during travel (European Centre for Disease Prevention and Control 2009a). Even if the reproduction number of TB in Canada is 1, TB can persist in Canada due to the endemicity in the regions which are the source of immigration. Immigration from Canada to those regions is negligible, hence by ignoring short-term travels, this can be viewed as an example of a not strongly connected network.
Motivated by these examples, we perform a systematic study to analyse the global dynamics for not strongly connected networks. In the literature, most qualitative studies focus only on the case of strongly connected transportation network, see e.g. Li and Zou (2010), Arino and Driessche (2003), Arino (2009), Wang and Zhao (2004). It seems that there is no established approach to analyse the dynamics with not strongly connected networks. Here we develop new analytical tools so that the long term behaviour of systems with not strongly connected transportation networks can also be understood.
The paper is organised as follows. The formulation and the detailed mathematical analysis of the model is given in Sects. 2–5 (some detailed calculations are collected in the Appendix), which may be skipped by a mathematically less inclined reader. We provide a rigorously proven and complete characterisation of the asymptotic behaviour of the system for an arbitrary number of regions for any network structure. The main techniques we use are stability theory of delay differential equations, cooperative sublinear systems, and an iterative application of the theory of asymptotically autonomous semiflows. In particular, we show that there always exists a globally asymptotically stable equilibrium. In the case of strongly connected transportation network, the basic reproduction number of the full system (defined as the spectral radius of a next generation matrix), as usual, serves as a threshold: either the disease dies out in every region, or the disease persists in every region. However, if the network is not strongly connected, multiple endemic patterns may emerge: some regions may become endemic, while the disease may be eradicated in some other regions. We provide a systematic method to determine, based on the network structure and local reproduction numbers, which regions become endemic and which regions become disease free. The results are illustrated for the case of two patches in Sects. 6–7. In Sect. 8 we numerically investigate the scenario when the dispersal rates of susceptible and infective individuals are different. Finally, we give a biological interpretation to each analytical result in Sect. 9.
Model formulation
We consider an arbitrary number of distinct regions. For with we define a set containing all indices of the regions. For , we denote by and the numbers of susceptible and infected individuals at time in region , respectively. Let be the recruitment rate, the natural death rate and the recovery rate of the infected individuals in region . We use standard incidence , where is the effective contact rate, which is the total contact rate multiplied by the probability of transmission of infection, in region . Then we obtain the basic SIS epidemic model
| 2.1a |
| 2.1b |
for , where , and are positive and is nonnegative for . Following Liu et al. (2008), we incorporate transportation where it is assumed that individuals neither die nor give birth during the transportation. If there is a transport connection from region to region , where and , then we denote by and the density of susceptible and infective individuals at time with respect to , where represents the time that they spent in the transportation from region to region at time (thus they left region at time ), where is the time required to complete a one-way travel from region to region . Let . Thus, is the number of individuals who left region in the time interval , where . In particular, for and , this gives the total number of individuals who are being in travel from region to at time . We assume that susceptible and infected individuals continuously leave region to region at a per capita rate and , respectively. Respective numbers of susceptible and infected individuals who leave region to per unit of time at each time are given as
| 2.2 |
Then the disease dynamics in the transportation from region to region is governed by
| 2.3a |
| 2.3b |
where we use the index to denote parameters during the transportation, thus and are respectively the effective contact rate and recovery rate in the transportation. Note that it is assumed that individuals neither die nor give birth during the transportation. Then
| 2.4 |
Here, the identity is due to the assumption that there is neither death nor giving birth on the transportation. From (2.3b) we obtain a logistic equation as
| 2.5 |
Using (2.2) as an initial condition, one can explicitly solve (2.5) along the characteristic lines. For simplicity, let us assume that for any . Then we have
| 2.6 |
Note that for and . One can also compute explicitly as
| 2.7 |
Note that and are respectively the population densities of susceptible and infective individuals entering region from region at time .
For it is convenient to define
We arrive at the following model:
| 2.8a |
| 2.8b |
for . One can see that the transport-related infection model formulated in Liu et al. (2008) is a special case of system (2.8). If there is no transportation from region to region then we set .
The basic reproduction number
For system (2.8), we construct a next generation matrix to define the basic reproduction number (Diekmann et al. 1990). In absence of infected individuals coming from other regions via the transportation into a region , the basic reproduction number in region is given as
| 3.1 |
Assuming that there is a transportation connection from region to region , we consider the expected number of infected individuals appearing in region due to the transport infection caused by a typical infective individual who was introduced into region . Since the probability of leaving the infective population of region by means of travel is , and the expected number of infected individuals who arrive at region if the travel was started with a single infective is [this follows from the linear part of (2.5)], taking the product of these two quantities, we get
Thus we define a next generation matrix for (4.1) as
| 3.2 |
Since is a nonnegative matrix, one of the eigenvalues gives the spectral radius of , see Theorem 1.1 in Chapter 2 in Berman and Plemmons (1994). We define the basic reproduction number as the spectral radius of and denote it by .
The following inequality gives a biologically meaningful estimation for the basic reproduction number.
Proposition 3.1
One has
Proof
Since
we can apply Corollary 1.5 in Chapter 2 in Berman and Plemmons (1994) to get the conclusion.
From Proposition 3.1 one can see that if the basic reproduction number is less than or equal to one, then each regional reproduction number is also less than or equal to one. On the other hand, if there exists a regional reproduction number which is 1, then the basic reproduction number is also greater than one.
For a square matrix we denote by the stability modulus of , which is defined as
where is the identity matrix. Let
We relate the basic reproduction number with the stability modulus of .
Proposition 3.2
It holds that
Proof
We define two matrices as
Now one has and . Then as in the proof of Theorem 2 in Driessche and Watmough (2002) we obtain the conclusion.
Finally, if is an irreducible matrix, then by the Perron-Frobenius theorem, the basic reproduction number is given by a simple eigenvalue of .
Population dynamics
To facilitate the mathematical analysis of the global dynamics of (2.8), here we assume that i.e., susceptible and infected individuals continuously leave region to region at the same rate (the general case is discussed in Sect. 8). Then we can consider a system which is described in terms of the total and infectious population instead of (2.8). The total population dynamics can be written as a system of linear delay differential equations, which is decoupled from the dynamics of the infectious population. To denote the total population at region at time , let . As an equivalent system to (2.8), with the assumption one has
| 4.1a |
| 4.1b |
where
| 4.2 |
for . We obtain a closed system of delay differential equations (4.1) with (4.2) being an alternative expression of (2.6), which results from the disease transmission in the transportation. In the sequel we analyse dynamical properties of (4.1) with (4.2).
Asymptotic stability of the total population
To analyse the dynamics of the total population, we introduce the vector valued function defined as
We denote by the Banach space of continuous functions mapping the interval into equipped with the sup-norm, where . The nonnegative cone of is defined as . Let
which is the set that contains only the strictly positive functions. Due to the biological interpretation, for (4.1a) we consider initial conditions for , where . Then one can see that every component of the solution of (4.1a) is strictly positive for .
Remark 4.1
For any nonnegative initial function, system (4.1a) generates a strictly positive solution. However, we require the initial function of (4.1a) to be in , in order to define (4.2) for small .
To prove asymptotic stability of (4.1a), we use some properties of -matrices and diagonally dominant matrices. Let be an real square matrix. For with non-positive off-diagonal entries, is said to be a nonsingular -matrix if all principal minors of are positive. See also Theorem 5.1 in Chapter 5 in Fiedler (1986) for equivalence conditions which characterise nonsingular -matrices (matrices of class ). Following Chapter 5 in Fiedler (1986), we say that is a diagonally dominant matrix if there exist positive numbers such that
We also refer to Theorem 5.14 in Chapter 5 in Fiedler (1986) to associate diagonally dominant matrices with -matrices.
Theorem 4.2
There exists a unique positive equilibrium of (4.1a), where each component is strictly positive. The positive equilibrium is globally asymptotically stable.
Proof
Let us assume that there exists an equilibrium. Denote it by a column vector given as . We define a column vector and a square matrix as
and
respectively. Then the equilibrium satisfies the linear equation
| 4.3 |
Since is a diagonally dominant matrix, is a diagonally dominant matrix as well by applying Theorem 5.15 in Fiedler (1986). Moreover, one can prove that is an -matrix by Theorem 5.14 in Fiedler (1986). Thus is a non-singular matrix and , see Theorem 5.1 in Fiedler (1986). Hence, one can solve (4.3) as , where the inequality holds componentwise. To prove that each component of the equilibrium is strictly positive, we suppose that there exists such that . Then it follows that
which is a contradiction. Thus each component of the equilibrium is strictly positive. To show the asymptotic stability, we define for . Then
| 4.4 |
for . Now it is straightforward to apply Theorem 2.1 in Győri (1992) or Theorem 1 in Hofbauer and So (2000), using the property of the square matrix as an -matrix, to conclude that the zero solution of (4.4) is asymptotically stable.
Disease transmission dynamics
We introduce a vector valued function defined as
Consider a product space of continuous functions given as , equipped with the sup-norm, where . We use a convention such that if . Let us define the set
From the biological motivation, the initial function for (4.1) is taken from , i.e.
where . Finally, we assume that
which has the obvious biological interpretation that in each region the initial infected population is a part of the total population. Then we prove well-posedness of the system (4.1) in Appendix A.1.
Lemma 5.1
For each initial function, system (4.1) generates a unique nonnegative bounded solution defined for all . In particular, it holds that for .
Let us define
For this matrix we can associate a directed graph [see Fiedler (1986)] with vertices, where there is a directed edge from vertex to vertex if and only if . Then the graph of reflects the structure of the transport connection among regions. For example, is an irreducible matrix if and only if for any pair of two regions there is a path from one to the other region, i.e., the associated directed graph is strongly connected. We refer to the scenario in which is an irreducible matrix as strongly connected transportation network. We refer to the other scenario in which is a reducible matrix as not strongly connected transportation network.
Strongly connected transportation network
To consider positive solutions, from the phase space we exclude the disease free subspace , where
Then system (4.1) generates a positive solution for a sufficiently large , see e.g. Theorem 1.2 in Chapter 5 in Smith (1995) for the proof. Thus there exists such that for all and .
Remark 5.2
If , then for holds. It has an obvious biological interpretation that if there is no infected individual in any of the regions, then the disease does not spread.
One can consider (4.1b) as a system of non-autonomous delay differential equations with a non-autonomous term , which is governed by system (4.1a). We derive a limiting system of (4.1b) using Theorem 4.2. We define a positive function as
| 5.1 |
for . By Theorem 4.2, one can obtain
for any . As an asymptotically autonomous system of (4.1b), we get the following system of delay differential equations
| 5.2 |
for .
In Appendix A.1 we apply a threshold type result for cooperative systems of functional differential equations in Zhao and Jing (1996) to prove the following theorem.
Theorem 5.3
For (5.2), if , then the trivial equilibrium is globally asymptotically stable in , whereas if , then there exists a positive equilibrium, where each component is strictly positive. The positive equilibrium is globally asymptotically stable in .
We return to the analysis of (4.1) by exploiting the result in Theorem 5.3. We denote by the positive equilibrium of (4.1a), which is given in Theorem 4.2. Then one can see that (4.1) has the disease free equilibrium given as
| 5.3 |
We denote by the positive equilibrium of (5.2). Then we immediately see that (4.1) has an endemic equilibrium given as
| 5.4 |
if . We prove global asymptotic stability of equilibria of (4.1) in Appendix A.1, where we apply the theory of asymptotically autonomous systems, see Thieme (1992).
Theorem 5.4
For (4.1), if , then the disease free equilibrium is globally asymptotically stable in , whereas if , then the endemic equilibrium is globally asymptotically stable in .
Not strongly connected transportation network
For not strongly connected transportation networks, is a reducible matrix. After operating a suitable permutation matrix, one can see that there exists such that has a triangular block form given as
| 5.5 |
where each diagonal entry is a square matrix that is either an irreducible matrix or a null matrix, see Chapter 2.3 in Berman and Plemmons (1994). We assume that is a square matrix, where is a positive integer. We define a set , containing indices of diagonal entries in (5.5). For every we then define , where with . Now one has that , which implies that the whole system can be divided into sets of regions. For every , if , then the transportation network among the regions is strongly connected, whereas if , the set consists of a single region .
Finally, for all we refer to the set of regions for as the th block, see Fig. 1 for an example.
Fig. 1.
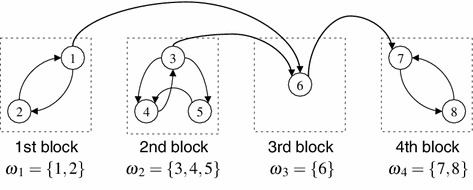
Diagram for transmission of the disease when the transportation network is not strongly connected. In this example, there are eight regions categorised by four blocks. The arrows indicate the transport connections
Remark 5.5
For a given reducible matrix , in general, the triangular matrix form (5.5) is not uniquely determined, thus some blocks are not necessary to be labelled uniquely. In the system described as in Fig. 1, one can reorder the st and the nd blocks.
As in Sect. 5.1, to consider positive solutions, we exclude the disease free subspace from the phase space. Let
for each . Note that now . For each we give the disease free subspace as
Let us define . We choose the initial function . Then system (4.1) generates a positive solution for a sufficiently large , in particular, in absence of the transportation connecting blocks.
We now define a reproduction number for each block. Now the next generation matrix has a triangular form:
where each diagonal entry is a square matrix that is either an irreducible matrix or a matrix, see again Chapter 2.3 in Berman and Plemmons (1994). For every we denote the spectral radius of by , which is the basic reproduction number for the th block in absence of infected individuals coming from other blocks into the th block via the transportation.
Remark 5.6
For such that , one has .
We analyse the disease transmission dynamics step by step starting from the st block. It is convenient to introduce the following terminology.
Definition 5.7
For all , we say that the th block is disease free if
for any solutions in . We say that the th block is endemic if there exists a vector with strictly positive components, and
for any solutions in .
It is straightforward to get the following threshold type result from Theorem 5.4.
Proposition 5.8
The st block is disease free if , whereas it is endemic if .
We employ mathematical induction to analyse the disease dynamics in the whole system. Let us choose arbitrarily. Suppose that all blocks from the st block to the th block are already classified as endemic or disease free.
Definition 5.9
We say that the th block is accessible from an endemic block if there exists such that the th block is endemic and .
The disease dynamics in the th block is determined as follows, see Appendix A.1.2 for the proof.
Proposition 5.10
For every the following statements hold.
-
(i)
Let us assume that the th block is accessible from an endemic block. Then the th block is endemic.
-
(ii)
Let us assume that the th block is not accessible from an endemic block. Then the th block is disease free if , whereas it is endemic if .
We note that the first statement of Proposition 5.10 implies that one endemic block becomes a trigger to spread the disease to all directly and indirectly accessible blocks via the transportation. The same structure of the equilibrium is found in a multi-patch epidemic model without infection during the transportation in Theorem 4 in Arino and Driessche (2003).
With Proposition 5.10 we can classify each block as endemic or disease free, which forms an endemic pattern in the whole system. The classification can be done in the following steps.
Form of the endemic pattern
-
(i)Determine . If ,
- then the st block is endemic,
- else the st block is disease free.
-
(ii)For
- if the th block is accessible from an endemic block,
-
(i)then the th block is endemic,
-
(ii)else determine . If
-
(A)then the th block is endemic,
-
(B)else the th block is disease free.
-
(A)
-
(i)
Consider the network described as in Fig. 1 for an example. Note that now has the form
where diagonal entries
are irreducible blocks and off-diagonal entries are given as
According to the procedure for the classification, we can determine the disease free and endemic blocks as in Table 1. This example illustrates that it is possible that the system admits numerous endemic patterns by having partially endemic equilibria, where some blocks are disease free and other blocks are endemic. This is in contrast with a strongly connected network, where all regions are endemic or all of them are disease free.
Table 1.
Classification of the disease free and endemic blocks for the network described in Fig. 1
| Disease free blocks | Endemic blocks | Globally stable equilibrium | ||||
|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | No such block | ||
| 1 | 1 | 1 | 1 | |||
| 1 | 1 | 1 | Any | |||
| 1 | 1 | Any | Any | |||
| 1 | 1 | Any | Any | |||
| 1 | 1 | Any | Any | No such block |
In Appendix A.1.2 we prove the following result.
Theorem 5.11
System (4.1) always has an equilibrium that is globally asymptotically stable. Depending on the structure of the transportation network and reproduction numbers , one can identify the endemic pattern of the equilibrium that is globally asymptotically stable.
We close this section by describing the complete dynamics for the simplest case, as an application of Theorem 5.11. If then, applying Theorem 5.4, the disease dynamics can be determined independently for each block. Thus we consider the case that , i.e., the nd block is accessible from the st block.
Corollary 5.12
Let and . Then the following statements hold.
-
(i)
If then the disease free equilibrium given as is globally asymptotically stable.
-
(ii)
If then the equilibrium , which is endemic only for region , is globally asymptotically stable.
-
(iii)
If then the equilibrium , which is endemic for both regions, is globally asymptotically stable.
Stability boundaries in a two-parameter plane
We visualise stability boundaries in a two-parameter plane for a system of two regions, i.e., . For the two-region system we consider two types of transportation connection as in Sect. 5, namely bidirectional transportation and unidirectional transportation. Unidirectional transportation may arise in several real scenarios. When an outbreak of an infectious disease in a two-region system is reported, the structure of the transportation network may vary, from bidirectional to unidirectional transportation, since individuals do not likely to travel to the endemic region (Meloni et al. 2011) or one way of transportation may be shut down to implement a disease control program. Rural-to-urban migration can be another example for unidirectional transportation. From the visualization of stability boundaries in a two-parameter plane one can see how the network structure of the transportation affects the disease transmission dynamics.
Bidirectional transportation
First consider a situation in which the two regions are connected to each other via bidirectional transportation. We assume that
| 6.1 |
Then one obtains as an irreducible matrix. From Theorem 5.4, we can conclude that the condition
| 6.2 |
plays as a threshold condition for the global stability of equilibria. The next generation matrix (3.2) is given as
where
| 6.3 |
for . Here the notation means the basic reproduction number in region in absence of infected individuals from another region , as in Sect. 5.2. Note that the biological meaning is consistent with defined in Sect. 5.2. For the interpretation of , see Sect. 4. We give an explicit expression for the basic reproduction number.
Proposition 6.1
It holds that
| 6.4 |
Proof
The eigenvalues of are roots of the equation
The roots of this quadratic equation can be computed as
Since the larger root gives , we get (6.4).
From (6.4), if , then one can easily deduce that holds for any . Thus the endemic equilibrium is globally asymptotically stable everywhere in the -parameter plane. In this case the transport-related infection has enough potential to spread the disease in both regions although regional reproduction numbers might be arbitrarily small. We fix and such that
| 6.5 |
holds, and define a positive function as
| 6.6 |
Proposition 6.2
Let us assume that (6.5) holds. Then if and only if
| 6.7 |
Proof
First, let us assume that . Since it holds that
one can see that . From (6.4), if and only if
| 6.8 |
Squaring both sides we get
Since , one can obtain (6.7). Next we assume that (6.7) holds. One can compute that
Then it is easy to obtain (6.8), which implies .
One can see that the condition (6.2) can be expressed as , which we call the stability boundary in the -parameter plane. For the visualization of the stability boundary, we plot this curve in Fig. 2. One can see that the stability boundary separates the parameter plane into two distinct regions. We can determine that the region above the stability boundary is the global stability region of the endemic equilibrium, whereas the region below the stability boundary is the global stability region of the disease free equilibrium. It is easy to prove that the stability region of the disease free equilibrium is smaller than the region , as shown in Fig. 2. Thus, as in Liu and Takeuchi (2006), Liu et al. (2008), it is possible that both regional reproduction numbers are 1, but the disease is endemic in both regions.
Fig. 2.
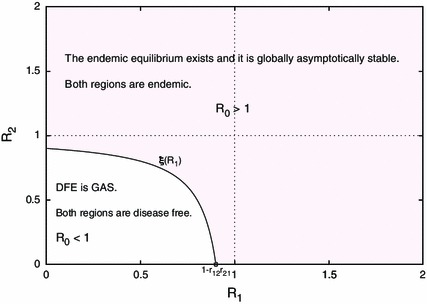
Stability regions of the disease free and the endemic equilibrium in -parameter plane for when two regions are connected via bidirectional transportation. The curve is the stability boundary defined in (6.6). DFE denotes the disease free equilibrium and GAS denotes globally asymptotically stable
Unidirectional transportation
Next we consider a system with one-way transportation from region to region , when the transportation network is not strongly connected. We assume that . For this scenario we have a complete picture of the disease dynamics from Corollary 5.12 in Sect. 5.2. To visualise the results of Corollary 5.12, it is natural to choose regional reproduction numbers as two free parameters, then we can express respective stability regions of equilibria in the -parameter plane in Fig. 3. One can see that if the reproduction number for region exceeds one, then both regions become endemic, even if the reproduction number for region is 1. This clearly shows the impact of the unidirectional transportation on the disease transmission dynamics.
Fig. 3.
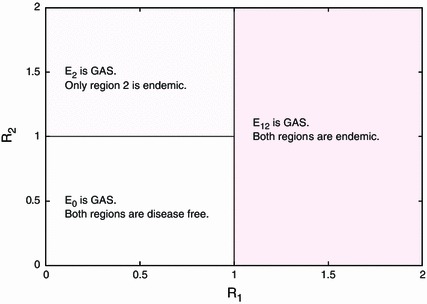
Stability boundaries of the disease free and two endemic equilibria in the -parameter plane for unidirectional transportation. We denote by , and the disease free equilibrium, the endemic equilibrium for only region and the endemic equilibrium for both regions, respectively. GAS denotes globally asymptotically stable
Travel restrictions for a two-regional system
Since, for multi-patches epidemic models, the basic reproduction number is given as a spectral radius of the “large” next generation matrix, it is not straightforward to derive biological interpretations. Limiting the number of regions to two, it is possible to derive more analytical results for the basic reproduction number, which may give some insight into the impact of population dispersal on the disease transmission dynamics (Arino and Driessche 2003; Gao and Ruan 2012; Hsieh et al. 2007; Li and Zou 2010). From (6.4) with (6.3), one can observe that the basic reproduction number monotonically increases with respect to the contact rates in the regions, the contact rates in the transportation and the duration of the transportation; but decreases with respect to the mortality rate and recovery rate. This dependency has obvious biological meaning. In the following we elaborate on the influence of two dispersal rates, and . We define a constant
| 7.1 |
which is the basic reproduction number in the limit case when and tend to infinity:
To show the parameter dependency, we write for . Without loss of generality, one can assume that , which implies that, in absence of the transportation, the basic reproduction number in region is larger than that in region . Finally, we denote
In Appendix A.2 we prove monotonicity of the basic reproduction number with respect to one dispersal rate:
Theorem 7.1
For the following statements hold.
-
(i)Assume that holds.
- If , then there exists such that
and . - If either or , then and .
- If , then and there exists such that
-
(ii)Assume that holds.
- If , then and .
- If , then .
- If , then and .
Theorem 7.1 suggests that it is important to know the order of the three quantities, , and , which measure the risk of infection in region , region and in the transportation. As an example we fix the parameters, except two dispersal rates, as in Table 2, where holds. Using the formula (6.4), we plot the basic reproduction number as a function of in Fig. 4.
Table 2.
Parameter values for numerical examples
| In region , | In region , | |
|---|---|---|
| Effective contact rate | ||
| Mortality rate | ||
| Recovery rate | ||
| Reproduction number |
| From region to region | From region to region | |
|---|---|---|
| , | , | |
| Effective contact rate in the transportation | ||
| Recovery rate in the transportation | ||
| Duration of the transportation | ||
| Reproduction number in the transportation | ||
Effective contact rate in the region is based on Nichol et al. (2010) for human influenza. In this parameter setting holds
Fig. 4.
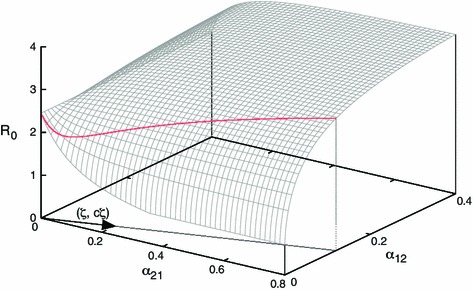
Shape of the basic reproduction number as a function of two dispersal rates
Theorem 7.1 implies that travel restrictions via reducing dispersal rates are not always helpful to decrease the basic reproduction number, thus one should carefully see how dispersal rates affect the basic reproduction number. To understand this we assume that , which implies that, in absence of the transportation, individuals in region are exposed to relatively high risk of infection. One can notice that the expected sojourn time of an infected individual in region , given as , decreases as increasing . Thus infected individuals in region likely start a journey to the safer place, region , as is increasing. If , then the environment inside the transportation is also relatively safe from the infection. Hence the dispersal rate from region to has a positive effect for reducing the basic reproduction number as shown in (i)-(b) and (i)-(c) in Theorem 7.1. Let us focus on the scenario described as
| 7.2 |
If is small then infected individuals in region likely to stay at this safer place. Thus the dispersal rate from region to reduces the basic reproduction number [see (i)-(a) for small in Theorem 7.1]. On the other hand, if is large, then the expected sojourn time of an infected individual in region becomes short. Thus infected individuals in region are likely to start a journey to the more risky region , rather than staying at region . Hence increasing , the infectious disease is mainly transmitted in the transportation, where the risk of infection is highest among the three different environments [see (7.2)], thus the basic reproduction number increases as well. The dependency with respect to can be discussed similarly.
Monotonicity of the basic reproduction number with respect to the mobility rate is also investigated in Gao and Ruan (2012), Hsieh et al. (2007). The authors analytically give sufficient conditions for the monotonicity of the basic reproduction number and present some numerical examples that shows the basic reproduction number nonmonotonically changes with respect to the mobility rate. Here, in Theorem 7.1, we completely characterise the monotone dependency of the basic reproduction number, which takes into account infection during transportation, with respect to one mobility rate. The result in Theorem 7.1 implies that the travel restriction can have both negative and positive impact for disease eradication. The authors in Hsieh et al. (2007) also find the dilution effect that the basic reproduction number decreases as the mobility rate from a high prevalence patch to a low prevalence patch increases, without assuming the infection during the transportation.
We can numerically observe how the basic reproduction number changes as the dispersal rates vary together. In the -parameter plane we consider a parametrised straight line by , along which we vary two parameters. The straight line can be represented as
where is a fixed constant characterising the slope, see Fig. 4 for a graphical explanation. Using the parameter values given in Table 2 we plot the basic reproduction number as a function of in Fig. 5. For one can temporarily decrease the basic reproduction number below unity by reducing dispersal rates, but further reduction increases the basic reproduction number. For small , as increases, increases much slower than . Thus the basic reproduction number decreases with respect to the dispersal rates, as explained above, by letting infected individuals in region board the transportation to region , which is the safer place. By further increasing , infected individuals in region return to region while spreading the disease in the transportation. Thus, by the same mechanism described above, the basic reproduction number increases as increases. From Theorem 7.1 it is easy to see that if then the basic reproduction number monotonically either decreases or increases with respect to . We can conclude that the regional heterogeneity due to the different infectious risks in regions is responsible for the non-monotonicity of the basic reproduction number with respect to population dispersal rates.
Fig. 5.
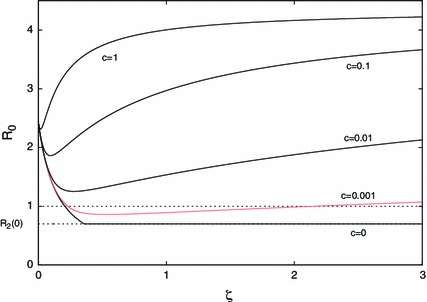
Non-monotonicity of the basic reproduction number for five different
Simulations for the impact of the reduced travel of infectives
The assumption allowed us to perform a complete rigorous global analysis of our system, however it is restrictive, whenever infected individuals are less capable of travelling. In this section we numerically investigate the general situation, where the dispersal rates of susceptible and infective individuals are different. It is reasonable to suppose that , thus here we assume , where the parameter represents the relative travel rate of infected individuals. The special case means that the disease is so mild that infected individuals travel at the same rate as susceptibles (this case was analysed in the previous sections), while means that infected individuals do not travel at all. Generally, we can see from the simulations on a wide range of parameters that our system shows global convergence of solutions. However, the parameter has an important role not only in determining the values of the steady states, but also in selecting which of the equilibria is globally attractive. We highlighted two interesting situations in Figs. 6 and 7.
Fig. 6.
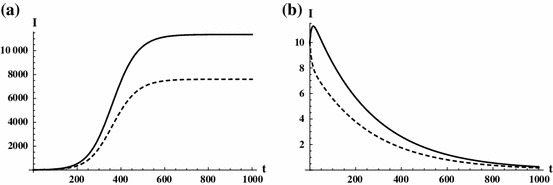
The figure depicts a situation when the disease is sustained in both patches with (a), but dies out for (b). Parameters are taken as in Table 2 with the modification , and setting , where and are set so that the total population of patch one and two are and . Solid curve is , dashed curve is . In this case both local reproduction numbers are 1, and the disease is endemic only if sufficiently many infected individuals travel
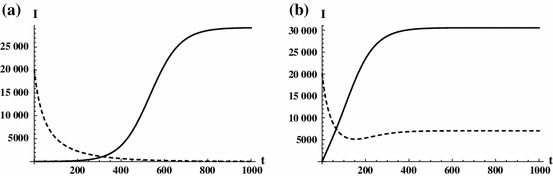
Fig. 7.
The figure depicts a situation when for (a) the disease is endemic in patch one but dies out in patch 2. For (b), the disease is endemic in both patches. Parameters are the same as in Fig. 6, only the transmission rates are modified to and . For a better visualization, we started with large density of infection in patch two
Figure 6 shows a scenario where the local reproduction numbers are 1, but the disease is sustained in both patches due to travel related infections for . Reducing means that infected individuals travel less, hence the number of travel related infections decreases, thus one suspects that for small enough, the disease will be eradicated, and indeed this is the case [see (b) for ].
In Fig. 7, the reproduction number of patch one is 1, while it is smaller than one on patch two. This implies that in the absence of travel of infected individuals (), the disease is endemic only in patch one and dies out in patch two. Allowing the travel of infected individuals even with a small rate makes the disease endemic in both patches.
The dependence of the endemic equilibria on in the above cases is illustrated in Fig. 8. In (a), one can observe a critical , that is a threshold between disease eradication and persistence. Notice that the endemic equilibria are very sensitive to when . In (b), the disease persists on both patches for all . The role of is particularly interesting in the case of strong heterogeneity, when the two patches poses very different risk. Then, by means of transportation, infected individuals move to a completely different environment. A particular case is shown in Fig. 9a, where patch one has high prevalence, while patch two is disease free in the absence of infected travellers. One can observe a nonmonotone behaviour with respect to : for a small travel rate of infected individuals, by transporting them to a safer patch the density of infection is reduced in patch one. However, increasing the number of infected travellers, this effect diminishes, as patch two becomes more risky, and at the same time the increased volume of infected travellers generates more transport related infections, thus the level of endemicity becomes increasing in patch one as well as is further increased. Figure 9b represents the extreme situation, when there is no transmission at all in patch two (i.e. ). Then, the density of infection is a monotone decreasing function of on patch one, while it is increasing in in patch two.
Fig. 8.
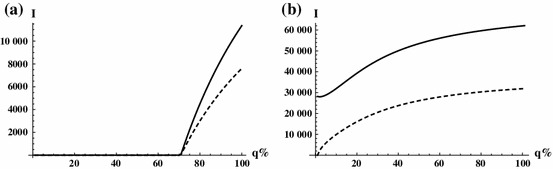
Endemic equilibria (solid) and (dashed) as function of for Figs. 6. and 7
Fig. 9.
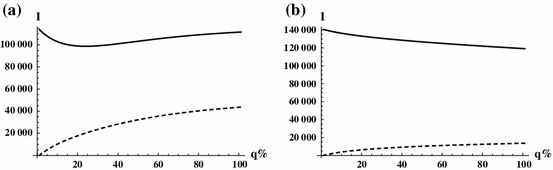
Monotone increasing, monotone decreasing, and non-monotone dependence of the endemic equilibrium on . Parameters are the same as before, except that in a , and in b
Discussion
We formulated an epidemic model for the spread of an infectious disease along with population dispersal by a system of delay differential equations. The disease transmission dynamics during transportation is described by a system of partial differential equations, structuring the population by the time elapsed since the start of the travel as in Liu et al. (2008). We improve the submodel for disease transmission dynamics during transportation, by adding the possibility of recovery during travel, and by allowing different mobility rates for susceptible and infective populations. Here, the mobility rate is assumed to depend on the region where the individual currently resides, and the individuals are homogeneously mixed into the local population upon arrival, thus our model fits into the framework of the usual patch models in the literature (Arino 2009; Brauer and Driessche 2001; Cui et al. 2006; Gao and Ruan 2012; Hsieh et al. 2007; Li and Zou 2010; Wang and Zhao 2004, 2005), that accounts for long-term mobility such as immigration of infectives. As examples describing short-term mobility such as tourism and business travels, we refer to Arino and Driessche (2003) and Knipl et al. (2013), where the mobility rates depend on the individual’s original and current locations as well.
To describe the spatial structure and the connectivity of distinct regions, we adopt the concept of directed graph (Fiedler 1986), which is widely used in the context of metapopulation type epidemic models, see Arino (2009), Arino and Driessche (2003). See also Colizza and Vespignani (2007, 2008), Meloni et al. (2011), Poletto et al. (2012), where the authors use graphs with various degree distributions to capture human mobility patterns during an epidemic. In the present manuscript, the travel matrix describes the connectivity of regions, and from one can construct a directed graph, representing the transportation network. Any transportation networks characterised by directed graphs can be classified as either strongly connected or not strongly connected, in other words, the travel matrix is either an irreducible or a reducible matrix. Here we consider both types of transportation network and then show that the disease dynamics can be characterised by the structure of the transportation network.
For the multi-regional models without incorporating transport-related infection, it is known that if the transportation network is strongly connected, then the basic reproduction number determines whether the disease free equilibrium is globally stable or the disease is uniformly persistent everywhere, see Wang and Zhao (2004, 2005), Li and Zou (2010). In Theorem 5.4 we also show that our model admits a threshold-type dynamics: if the basic reproduction number is less than or equal to one then the disease free equilibrium is globally asymptotically stable, while if the basic reproduction number is 1 then the endemic equilibrium is globally asymptotically stable. Subsequently we analyse the system when the transportation network is not strongly connected. In the literature, qualitative analysis for multi-regional systems without strongly connected transportation network seems to be limited (except Li and Zou 2010; Arino et al. 2006, where only and patches are considered). In Sect. 5.2, keeping the generality for the number of regions, we consider the disease dynamics in the reducible case. First we show that the whole multi-regional system can be seen as a set of blocks, where regions within each block are strongly connected via the transportation. Then we provide a systematic way to determine the endemic situation in each block, see Proposition 5.10 and the preceding procedure. Proposition 5.10 gives a simple rule: if the block is accessible from other endemic blocks then that block is also endemic, otherwise the basic reproduction number for the particular block determines the endemic situation as stated in Theorem 5.4. Thus, in general, the system admits partially endemic equilibria, where endemic blocks and disease free blocks coexist. We further prove that our model always has an equilibrium that is globally asymptotically stable.
To understand the disease dynamics in a heterogeneous environment, our modelling and analysis suggest that the first priority is to confirm the strong connectivity of the transportation network. If it is strongly connected then the basic reproduction number becomes an important quantity to determine the disease transmission dynamics. If the transportation network is not strongly connected, then we may relabel the regions so that the travel matrix has a triangular form (5.5). From this reordering process, the whole multi-regional system will be divided into blocks. By following the procedure in Sect. 5.2 one can determine every possible disease dynamics. Since there may be a source block that spreads the disease to other blocks, one may try to decrease the basic reproduction number of the source block to eradicate the disease.
Our model explicitly incorporates transport-related infection, taking into account the time to complete travel between regions, differently from the models in Arino and Driessche (2003), Arino et al. (2007), Bajardi et al. (2011), Colizza et al. (2006), Colizza and Vespignani (2007, 2008), Meloni et al. (2011), Poletto et al. (2012), Cui et al. (2006), Liu and Takeuchi (2006), Wang and Zhao (2004). The potential impact of the transport-related infection can be also seen from the expression of the basic reproduction number, obtained in (6.4) in Sect. 6, for a two-regional system. If there is no transport-related infection, one always has . However, infection during the travel may allow to exceed one, from which the basic reproduction number also exceeds one. Thus the transport-related infection itself may have enough potential to spread the disease in the host population.
We also consider the effect of travel restrictions for the two-regional system via analysing how population dispersal rates change the basic reproduction number in Sect. 7. Using mathematical models it is reported that travel restriction can delay the outbreak of influenza (Hollingsworth et al. 2006; Bajardi et al. 2011; Epstein et al. 2007). Our results suggest that travel restrictions may not be efficient to control the basic reproduction number. Similar results are obtained in different models (Hsieh et al. 2007; Gao and Ruan 2012). It is also shown that the basic reproduction number does not necessarily decrease as population mobility decreases, see Fig. 5. Controlling the local infectious process in each region by e.g. reducing contact rates via isolation policy seems to be more efficient to decrease the basic reproduction number than reducing the population mobility. In most real situations, infected individuals are less likely to travel than susceptibles. The impact of this difference on the dynamics is discussed in details in Sect. 8.
In this manuscript we assume a continuous process of population dispersal. In reality, airline flights or trains, connecting distinct areas, are periodically scheduled, thus mobility can be given by periodic (and possibly discontinuous) functions. This assumption leads to a model by delay differential equations with periodic coefficients, where it is much harder to draw biological conclusions, due to the difficulties in the qualitative analysis. Stochastic components may also play a role in the infectious process, as during the transportation, only a limited number of individuals are confined into one carrier, even when the total volume of transportation is very large (this holds for human travel as well as animal transportation). Coupling the stochastic process during transportation with time delay to a deterministic system on the patches is also challenging. We leave these considerations as future work.
Acknowledgments
The authors are grateful for Professor Eduardo Liz for his kind hospitality at the Universidade de Vigo, October, 2010, where YN and GR started this project. The authors are grateful for Professor Teresa Faria for her kind hospitality at the University of Lisbon, July 2012, where the authors had stimulating discussions about multipatch models. YN and GR were supported by European Research Council StG Nr. 259559. YN was also supported by Spanish Ministry of Science and Innovation (MICINN), MTM2010-18318 and by the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TÁMOP-4.2.4. A/2-11-1-2012-0001 ‘National Excellence Program’. Since April 2014 YN was also supported by JSPS Fellowship, No. 268448. GR was supported by European Union and the European Social Fund through project FuturICT.hu (Grant No. TÁMOP-4.2.2.C-11/1/KONV-2012-0013), and Hungarian Scientific Research Fund OTKA K75517. The authors are particularly grateful for the three referees, whose comments significantly improved the manuscript.
Appendix A
We introduce a partial order for real vectors as well as real matrices, which will be used throughout the appendix. For two real matrices and we write
Moreover, we write
A.1 Disease transmission dynamics
Proof of Lemma 5.1
We define as
where has to be understood as a nonautonomous term determined by (4.1a). Since is locally Lipschitzian with respect to the second argument, there exists such that (4.1b) has a unique local solution on , see Theorem 2.3, Chapter 2 in Hale and Verduyn Lunel (1993). It is easy to see that if and then for . Thus from Theorem 2.1 of Chapter 5 in Smith (1995), the solution of (4.1b) is nonnegative. We show the boundedness of the solution. Suppose that there exists and such that
Since from (2.4), for any , one has , we get
which is a contradiction. Thus follows for . Boundedness of follows from Theorem 4.2, thus is also bounded. Finally, one can take by continuation of the solution, see Chapter 2 in Hale and Verduyn Lunel (1993).
A.1.1 Strongly connected transportation network
The limit system (5.2) can be written as
where the map is defined by
for .
Proof of Theorem 5.3
We apply Theorem 3.2 in Zhao and Jing (1996). We verify that is a cooperative and sublinear map. It is straightforward to see that other assumptions in Theorem 3.2 in Zhao and Jing (1996) hold, thus we omit it. The Frechét derivative of evaluated at is given as
| 10.1 |
for and . Then one can see that is continuously Frechét differentiable. For any with and one has
Hence, is a cooperative map in . Now we define a map by
where denotes the natural inclusion from to . We show that is sublinear and that is strictly sublinear, i.e., for any it holds that
| 10.2 |
for any and . Choose arbitrarily. For any one can compute that
for any and . From the definition of in (5.1), for any it holds that
thus (10.2) follows. We show that is bounded. Since there exists such that
one can derive a comparison system:
for . It is easy to see that is bounded, thus so is . Finally, one can see that holds. Thus by Theorem 3.2 in Zhao and Jing (1996) and Proposition 3.2 the threshold dynamics can be expressed in terms of .
To discuss the asymptotic stability of equilibria of (4.1), we apply the principle of linearised stability, see Theorem 6.8 in Chapter VII in Diekmann et al. (1995). Denote for and by the identity matrix. We prove the following result.
Lemma 10.1
Let be an equilibrium of (4.1). If all roots of the following equation
| 10.3 |
have negative real parts then the equilibrium is asymptotically stable.
Proof
We define two maps and for the right hand side of (4.1), i.e., (4.1) can be written as
| 10.4a |
| 10.4b |
For an equilibrium of (4.1), one can derive the characteristic equation as
| 10.5 |
From Theorem 4.2 we know that every root of the equation:
has negative real part. Thus we consider the location of the roots of the equation
| 10.6 |
which is equivalent to (10.3). Thus we obtain the conclusion.
Proof of Theorem 5.4
First we prove asymptotic stability of equilibria.
Proposition 10.2
For (4.1) if then the disease free equilibrium is asymptotically stable, whereas if then the endemic equilibrium is asymptotically stable.
Proof
All roots of (10.3) are located in the right half complex plane if and only if the trivial equilibrium of the equation
| 10.7 |
is asymptotically stable. The linearised system (10.7) can be written as
| 10.8 |
for . We apply Theorem 1 in Hofbauer and So (2000) to (10.8). System (10.8) with becomes
for . Let us assume that holds. Then by Proposition 3.2, one has . Since off-diagonal entries of are nonpositive, is a non-singular -matrix, see Lemma 2.1 in Faria (2011) for the proof. From Proposition 3.1 it holds that
Hence by Theorem 1 in Hofbauer and So (2000) the trivial equilibrium of (10.7) is asymptotically stable. Next we assume that . We consider system (10.8) with . We define a matrix
One can see that the equilibrium condition is given as
| 10.9 |
where
Since holds for , one can see that . Then one has
| 10.10 |
Since off-diagonal entries of are nonpositive, (10.10) implies that is an -matrix (a matrix of class ), see Theorem 5.1 in Fiedler (1986). Thus is a non-singular matrix and all principal minors of are positive, i.e., and is weakly diagonally dominant in the sense of Hofbauer and So (2000). Finally, from (10.9) one can see that
which is equivalent to
for . Thus every diagonal entry of is negative. Therefore, by Theorem 1 in Hofbauer and So (2000) we conclude that (10.8) is asymptotically stable. Finally we prove the stability for the case by a comparison argument. For any there exists such that for . We write instead of . It holds that
for and . We consider an auxiliary system given by
| 10.11 |
for with . We define a matrix as
Since follows, one can notice that implies by Proposition 3.2. Now it is straightforward to apply Theorem 3.2 in Zhao and Jing (1996), see also the proof of Theorem 5.3, to conclude that the trivial equilibrium of (10.11) is asymptotically stable for . The comparison argument shows the stability of the disease free equilibrium of (4.1).
Proposition 10.3
For (4.1) if then the disease free equilibrium is globally attractive in , whereas if then the endemic equilibrium is globally attractive in .
Proof
Since we have the boundedness of solutions from Lemma 5.1, one can show that forward orbits of (4.1b) are precompact thus the -limit sets are not empty, see e.g. Chapter 5 in Smith (2011). We apply Theorem 4.1 in Thieme (1992). First we consider the case . From Theorem 5.3 and Remark 5.2 the basin of attraction of the trivial equilibrium of (5.2) is . Hence the -limit set of every forward orbit of (4.1b) intersects the basin of attraction. By Theorem 4.1 in Thieme (1992) we can conclude that every solution of (4.1b) converges to the disease free equilibrium. Next we consider the case . We exclude the possibility that the -limit set of a forward orbit of (4.1b) contains the trivial element . Suppose that there is a solution of (4.1b) such that
| 10.12 |
Since, from Lemma 4.2, it holds that for , for any and for all there exists a sufficiently large such that
For from (4.1b) we find an estimate
We consider the following auxiliary system
For we define a matrix as
Since we have from Proposition 3.2, for sufficiently small one has . We fix so that . Since is an irreducible matrix with non-negative off-diagonals, is a simple eigenvalue with a positive eigenvector, see Theorem A.5 in Smith and Waltman (1995). Let
be the positive eigenvector corresponding to for i.e. one has that
We define a functional as
Then
where denotes the scalar product. Since one has that
we get . Hence is increasing with respect to . From a comparison argument it is easy to see that a positive solution of (4.1b) can not converge to the trivial equilibrium, which contradicts the assumption (10.12). Thus the -limit set of any forward orbit of (4.1b) does not contain the trivial element. By Theorem 4.1 in Thieme (1992), each solution of (4.1) converges to the endemic equilibrium.
From Propositions 10.2 and 10.3 we obtain Theorem 5.4.
A.1.2 Not strongly connected transportation network
Let us choose arbitrarily. The population dynamics in the th block is described as
| 10.13a |
| 10.13b |
for .
Proof of Proposition 5.10
(i) From the induction hypothesis there exists such that
| 10.14 |
Note that there exists such that from the assumption that th block is accessible from an endemic block. We obtain the following limit system
| 10.15 |
for . We prove global attractivity of (10.15).
Lemma 10.4
There exists a positive equilibrium of (10.15) which is globally asymptotically stable.
Proof
First we show that if a nonnegative equilibrium exists then it is positive and unique. The existence will be proved in the end. We define a map by
We denote by the equilibrium. The equilibrium satisfies
| 10.16 |
From the irreducibility there is a path starting from region , where one has , passing through all regions . Along this path we relabel the regions as . Suppose that . Since we have , (10.16) implies that
which is a contradiction. Thus . We show that if for . Suppose that . Then (10.16) implies that
which is a contradiction. Thus we get . The mathematical induction shows that each component of the equilibrium is strictly positive. We show the uniqueness of the equilibrium. We assume that there exist two equilibria, which we denote by and with . One can, without loss of generality, assume that there exists such that holds. Then there exists such that
We define
Then we have . It is easy to see that
As in the proof of Theorem 5.3, one can see that is strictly sublinear, i.e., Thus
Hence it follows
which is a contradiction. Thus the positive equilibrium is unique. To show the existence of the equilibrium we define a map by
for . We apply Corollary 2.2 in Chapter 5 in Smith (1995) to show existence and global attractivity of the equilibrium. Using the monotonicity of one can see that satisfies the quasimonotone condition, see Chapter 5 in Smith (1995). For any initial function there exists sufficiently large such that and that , where is the natural inclusion from to . One also has that . As in the proof of Theorem 5.3 one can prove that the solution is bounded. Thus the forward orbits of are precompact and thus the -limit set is not empty, see e.g. Chapter 5 in Smith (2011). Since the equilibrium is unique, it holds that
As the semiflow is monotone, from Theorem 1.1 in Chapter 5 in Smith (1995), one has , i.e. the equilibrium is globally attractive.
Let us fix such that . From (10.14) there exists sufficiently large such that
for . Consider an auxiliary equation given as
It is easy to see that there exists a unique positive equilibrium that is globally asymptotically stable. We denote by the positive equilibrium. Then one can see that , which implies that the -limit set of any forward orbit of (10.13) does not contain the trivial equilibrium. By Theorem 4.1 in Thieme (1992) we conclude that solutions of (10.13) converge to the endemic equilibrium.
(ii) First we notice that
| 10.17 |
holds. Then we get the following limit system
| 10.18 |
By Theorem 5.3 we can determine the dynamics of (10.18) in terms of as that if then the trivial equilibrium is globally asymptotically stable in , whereas if then a positive equilibrium exists and it is globally asymptotically stable in . Applying Theorem 4.1 in Thieme (1992) as in the proof of Proposition 10.3, one can obtain the conclusion.
Proof of Theorem 5.11
Since we have the global attractivity of the equilibrium from Proposition 5.10, here we only prove stability. For every one has that
for . For every we define a map as
We denote by a given globally attractive equilibrium. It holds that
where is the identity matrix. We get that
We choose arbitrary. Roots of
are in the right half complex plane if and only if the trivial equilibrium of the following equation is asymptotically stable:
| 10.19 |
which can be written as
for . Let us assume that . Note that implies . As in the proof of Proposition 10.2 one can see that the trivial equilibrium of (10.19) is asymptotically stable if and that (10.19) is asymptotically stable if . For the case that there exists such that with , one can construct a comparison system, as in the proof of Proposition 10.2, from which stability is deduced. The proof is tedious but straightforward, thus we omit it here.
A.2 Travel restrictions for a two-regional system
We define a parameterised function as
for and . The basic reproduction number is the larger root of . Thus we have
| 10.20 |
for . Notice that
| 10.21 |
for and .
Proposition 10.5
For it holds that
| 10.22 |
for .
Proof
We only prove (10.22) for . From the symmetry one can get similarly (10.22) for . In the following we omit arguments from , and for for a simple presentation. By differentiating (10.20) with respect to we get
which is equivalent to
Note that for . Since from (10.21) it holds that
we obtain that
| 10.23 |
From (10.20) one has
Using (10.21) we get
then we compute that
Therefore, from (10.23) we obtain
thus we arrive to the conclusion.
Next we define two functions of as
where is defined in (7.1), for and . Then we prove
Lemma 10.6
For and it holds that
for .
Proof
Using (10.21) an equivalent form of is given as
We compute for as
Similarly, one can get
and the conclusion is reached.
Now we show a classification for the sign of . For we define a constant via the relation
Lemma 10.7
For the following statements hold.
-
(i)Let us assume that .
- If then there exists such that
and . - If either or then and .
- If then and there exists such that
and that10.24
-
(ii)Let us assume that . For and one has that
- If then .
- If then .
- If then .
Proof
One can compute that
| 10.25 |
and that
| 10.26 |
for and . One can see that is either a monotone or a constant function [depending on the sign of . Thus the combination of two boundary values given in (10.25) and (10.26) determines the sign of for as listed. We prove (10.24). One can see that is equivalent to
We compute that
which implies (10.24), since is a decreasing function.
Proof of Theorem 7.1
First we consider the sign of . We compute that
| 10.27 |
It is easy to see that . Since, for given parameters and , is a quadratic function of the third argument with a positive coefficient of , it holds that
by Lemma 10.6. Since from Lemma 10.7 one obtains the sign of , by Lemma 10.5 we get the sign of as in the conclusion. Next we consider the sign of . If then one can obtain the sign as in the same argument above. Let us assume that . For we have
from Lemmas 10.7 and 10.6. This implies that , thus by Proposition 10.5 we have for . For we have
Computing
we obtain that
Similarly to the argument for , we can determine the sign of .
Contributor Information
Yukihiko Nakata, Email: nakata@math.u-szeged.hu, Email: nakata@ms.u-tokyo.ac.jp.
Gergely Röst, Email: rost@math.u-szeged.hu.
References
- Alirol E, Getaz L, Stoll B, Chappuis F, Loutan L. Urbanisation and infectious diseases in a globalised world. Lancet Infect Dis. 2011;11(2):131–141. doi: 10.1016/S1473-3099(10)70223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arino J (2009) Diseases in metapopulations. Modeling and dynamics of infectious diseases. In: Series in contemporary applied mathematics, vol 11. World Scientific Publishing, Singapore, pp 65–123
- Arino J, Brauer F, van den Driessche P, Watmough J, Wu J. Simple models for containment of a pandemic. J R Soci Interface. 2006;3(8):453–457. doi: 10.1098/rsif.2006.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arino J, Davis J, Hartley D, Jordan R, Miller J, van den Driessche P. A multi-species epidemic model with spatial migration. Math Med Biol. 2005;22(2):129–142. doi: 10.1093/imammb/dqi003. [DOI] [PubMed] [Google Scholar]
- Arino J, van den Driessche P. A multi-city epidemic model. Math Popul Stud. 2003;10(3):175–193. doi: 10.1080/08898480306720. [DOI] [Google Scholar]
- Arino J, Jordan R, van den Driessche P. Quarantine in a multi-species epidemic model with spatial dynamics. Math Biosci. 2007;206(1):46–60. doi: 10.1016/j.mbs.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Bajardi P, Barrat A, Natale F, Savini L, Colizza V. Dynamical patterns of cattle trade movements. PLoS One. 2011;6(5):e19869. doi: 10.1371/journal.pone.0019869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajardi P, Poletto C, Ramasco JJ, Tizzoni M, Colizza V, Vespignani A. Human mobility networks, travel restrictions, and the global spread of 2009 H1N1 pandemic. PLoS One. 2011;6(1):e16591. doi: 10.1371/journal.pone.0016591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcan D, Vespignani A. Phase transitions in contagion processes mediated by recurrent mobility patterns. Nat Phys. 2011;7:581–586. doi: 10.1038/nphys1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell DM (2004) World Health Organization Working Group on Prevention of International and Community Transmission of SARS.: Public health interventions and SARS spread, 2003. Emerg Infect Dis. http://wwwnc.cdc.gov/eid/article/10/11/04-0729.htm [DOI] [PMC free article] [PubMed]
- Berman A, Plemmons RJ (1994) Nonnegative matrices in the mathematical sciences. Classics in Applied Mathematics. SIAM, Philadelphia
- Brauer F, van den Driessche P. Models for transmission of disease with immigration of infectives. Math Biosci. 2001;171:143–154. doi: 10.1016/S0025-5564(01)00057-8. [DOI] [PubMed] [Google Scholar]
- Colizza V, Barrat A, Barthélemy M, Vespignani A. The role of the airline transportation network in the prediction and predictability of global epidemics. Proc Natl Acad Sci USA. 2006;103(7):2015–2020. doi: 10.1073/pnas.0510525103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colizza V, Vespignani A. Invasion threshold in heterogeneous metapopulation networks. Phys Rev Lett. 2007;99(14):148701. doi: 10.1103/PhysRevLett.99.148701. [DOI] [PubMed] [Google Scholar]
- Colizza V, Vespignani A. Epidemic modeling in metapopulation systems with heterogeneous coupling pattern: theory and simulations. J Theor Biol. 2008;251(3):450–467. doi: 10.1016/j.jtbi.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Cui J, Takeuchi Y, Saito Y. Spreading disease with transport-related infection. J Theor Biol. 2006;239(3):376–390. doi: 10.1016/j.jtbi.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Diekmann O, van Gils SA, Walther HO (1995) Delay equations: functional-, complex-, and nonlinear analysis. In: Applied mathematical sciences vol 110. Springer, New York
- Diekmann O, Heesterbeek JAP, Metz JAJ. On the definition and the computation of the basic reproduction ratio in models for infectious diseases in heterogeneous populations. J Math Biol. 1990;28(4):365–382. doi: 10.1007/BF00178324. [DOI] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control (2009a) Risk assessment guidelines for diseases transmitted on aircraft. ECDC Technical Report. http://ecdc.europa.eu/en/publications/Publications/0906_TER_Risk_Assessment_Guidelines_for_Infectious_Diseases_Transmitted_on_Aircraft.pdf
- European Centre for Disease Prevention and Control (2009b) Risk assessment guidelines for diseases transmitted on aircraft. Part 2: operational guidelines for assisting in the evaluation of risk for transmission by disease. ECDC Technical Report. http://ecdc.europa.eu/en/publications/Publications/1012_GUI_RAGIDA_2.pdf
- Epstein JM, Goedecke DM, Yu F, Morris RJ, Wagener DK, Bobashev GV. Controlling pandemic flu: the value of international air travel restrictions. PLoS One. 2007;2(5):e401. doi: 10.1371/journal.pone.0000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria T. Global asymptotic behaviour for a Nicholson model with patch structure and multiple delays. Nonlinear Anal Theory Methods Appl. 2011;74(18):7033–7046. doi: 10.1016/j.na.2011.07.024. [DOI] [Google Scholar]
- Fiedler M. Special matrices and their applications in numerical mathematics. The Hague: Martinus Nijhoff Publishers; 1986. [Google Scholar]
- Gao D, Ruan S. A multipatch malaria model with logistic growth populations. SIAM J Appl Math. 2012;72(3):819–841. doi: 10.1137/110850761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Győri I (1992) Stability in a class of integrodifferential systems. Recent trends in differential equations. World Scientific Publishing, Singapore, pp 269–284
- Hale JK, Verduyn Lunel SM (1993) Introduction to functional-differential equations. In: Applied mathematical sciences, vol 99. Springer, New York
- Hofbauer J, So JWH (2000) Diagonal dominance and harmless off-diagonal delays. Proc Am Math Soc 128(9):2675–2682
- Hollingsworth TD, Ferguson NM, Anderson RM. Will travel restrictions control the international spread of pandemic influenza? Nat Med. 2006;12(5):497–499. doi: 10.1038/nm0506-497. [DOI] [PubMed] [Google Scholar]
- Hsieh YH, van den Driessche P, Wang L. Impact of travel between patches for spatial spread of disease. Bull Math Biol. 2007;69(4):1355–1375. doi: 10.1007/s11538-006-9169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K, Arino J, Hu W, Raposo P, Sears J, Calderon F, Heidebrecht C, Macdonald M, Liauw J, Chan A, Gardam M. Spread of a novel influenza A (H1N1) virus via global airline transportation. N Engl J Med. 2009;361(2):212–214. doi: 10.1056/NEJMc0904559. [DOI] [PubMed] [Google Scholar]
- Knipl DH, Röst G, Wu J. Epidemic spread and variation of peak times in connected regions due to travel-related infections–dynamics of an antigravity-type delay differential model. SIAM J Appl Dyn Syst. 2013;12(4):1722–1762. doi: 10.1137/130914127. [DOI] [Google Scholar]
- Li J, Zou X. Dynamics of an epidemic model with non-local infections for diseases with latency over a patchy environment. J Math Biol. 2010;60(5):645–686. doi: 10.1007/s00285-009-0280-9. [DOI] [PubMed] [Google Scholar]
- Liu J, Wu J, Zhou Y. Modeling disease spread via transport-related infection by a delay differential equation. Rocky Mt J Math. 2008;38(5):1525–1540. doi: 10.1216/RMJ-2008-38-5-1525. [DOI] [Google Scholar]
- Liu X, Takeuchi Y. Spread of disease with transport-related infection and entry screening. J Theor Biol. 2006;242(2):517–528. doi: 10.1016/j.jtbi.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Mangili A, Gendreau MA. Transmission of infectious diseases during commercial air travel. Lancet. 2005;365(9463):989–996. doi: 10.1016/S0140-6736(05)71089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni S, Perra N, Arenas A, Gómez S, Moreno Y, Vespignani A. Modeling human mobility responses to the large-scale spreading of infectious diseases. Sci Rep. 2011;1:62. doi: 10.1038/srep00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata Y. On the global stability of a delayed epidemic model with transport-related infection. Nonlinear Anal Real World Appl. 2011;12(6):3028–3034. doi: 10.1016/j.nonrwa.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol KL, Tummers K, Hoyer-Leitzel A, Marsh J, Moynihan M, McKelvey S. Modeling seasonal influenza outbreak in a closed college campus: impact of pre-season vaccination, in-season vaccination and holidays/breaks. PLoS One. 2010;5(3):e9548. doi: 10.1371/journal.pone.0009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SJ, Chang HL, Cheung TYY, Tang AFY, Fisk TL, Ooi SPL, Kuo HW, Jiang DDS, Chen KT, Lando J, Hsu KH, Chen TJ, Dowell SF. Transmission of the severe acute respiratory syndrome on aircraft. N Engl J Med. 2003;349(25):2416–2422. doi: 10.1056/NEJMoa031349. [DOI] [PubMed] [Google Scholar]
- Poletto C, Tizzoni M, Colizza V. Heterogeneous length of stay of hosts’ movements and spatial epidemic spread. Sci Rep. 2012;2:476. doi: 10.1038/srep00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D, Gendreau M. Medical issues associated with commercial flights. Lancet. 2009;373(9680):2067–2077. doi: 10.1016/S0140-6736(09)60209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H, Waltman P (1995) The theory of the chemostat: dynamics of microbial competition. In: Cambridge studies in mathematical biology, vol 13. Cambridge University Press, Cambridge
- Smith HL (1995) Monotone dynamical systems: an introduction to the theory of competitive and cooperative systems. In: Mathematical surveys and monographs, vol 41. American Mathematical Society
- Smith HL (2011) An introduction to delay differential equations with applications to the life sciences. In: Texts in applied mathematics, vol 57. Springer, New York
- Takeuchi Y, Liu X, Cui J. Global dynamics of SIS models with transport-related infection. J Math Anal Appl. 2007;329(2):1460–1471. doi: 10.1016/j.jmaa.2006.07.057. [DOI] [Google Scholar]
- Thieme HR. Convergence results and a Poincaré–Bendixson trichotomy for asymptotically autonomous differential equations. J Math Biol. 1992;30(7):755–763. doi: 10.1007/BF00173267. [DOI] [Google Scholar]
- van den Driessche P, Watmough J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci. 2002;180(1–2):29–48. doi: 10.1016/S0025-5564(02)00108-6. [DOI] [PubMed] [Google Scholar]
- Wang W (2007) Epidemic models with population dispersal. In: Mathematics for life science and medicine, biological and medical physics, biomedical engineering. Springer, New York
- Wang W, Zhao X. An epidemic model in a patchy environment. Math Biosci. 2004;190(1):97–112. doi: 10.1016/j.mbs.2002.11.001. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhao X. An age-structured epidemic model in a patchy environment. SIAM J Appl Math. 2005;65(5):1597–1614. doi: 10.1137/S0036139903431245. [DOI] [Google Scholar]
- WHO (2003) Severe acute respiratory syndrome (SARS): status of the outbreak and lessons for the immediate future. Geneva. http://www.who.int/csr/media/sars_wha.pdf
- Zhou Y, Khan K, Feng Z, Wu J. Projection of tuberculosis incidence with increasing immigration trends. J Theor Biol. 2008;254(2):215–228. doi: 10.1016/j.jtbi.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Zhao XQ, Jing ZJ. Global asymptotic behavior in some cooperative systems of functional differential equations. Can Appl Math Q. 1996;4(4):421–444. [Google Scholar]


