Abstract
Purpose
During the 2009 H1N1 influenza A virus pandemic, a minority of patients developed rapidly progressive pneumonia leading to acute lung injury (ALI)—acute respiratory distress syndrome (ARDS). A recent meta-analysis provides support for prolonged corticosteroid treatment in ALI-ARDS. We prospectively evaluated the response to oseltamivir and prolonged corticosteroid treatment in patients with ALI-ARDS and suspected H1N1 influenza.
Methods
From June 24 through 12 July 2009, 13 patients with suspected H1N1 pneumonia and ALI-ARDS were admitted to the intensive care unit (ICU) of a tertiary care hospital. H1N1 influenza was confirmed with real-time reverse transcriptase-polymerase chain reaction assay in eight patients. Oseltamivir and corticosteroid treatment were initiated concomitantly at ICU admission; those with severe ARDS received methylprednisolone (1 mg/kg/day), and others received hydrocortisone (300 mg/day) for a duration of 21 ± 6 days.
Results
Patients with and without confirmed H1N1 influenza had similar disease severity at presentation and a comparable response to treatment. By day 7 of treatment, patients experienced a significant improvement in lung injury and multiple organ dysfunction scores (P < 0.001). Twelve patients (92%) improved lung function, were extubated, and discharged alive from the ICU. Hospital length of stay and mortality were 18.7 ± 9.6 days and 15%, respectively. Survivors were discharged home without oxygen supplementation.
Conclusions
In ARDS patients, with and without confirmed H1N1 influenza, prolonged low-to-moderate dose corticosteroid treatment was well tolerated and associated with significant improvement in lung injury and multiple organ dysfunction scores and a low hospital mortality. These findings provide the rationale for developing a randomized trial.
Keywords: H1N1 influenza A virus, Acute respiratory distress syndrome, Corticosteroid treatment, Mechanical ventilation, Mortality
Introduction
During the 2009 H1N1 influenza A virus pandemic, a minority of patients have developed severe pneumonia leading to acute respiratory distress syndrome (ARDS) and multiple organ dysfunction (MODS), associated with prolonged intensive care unit (ICU) stay and high (17–54%) mortality [1–7]. In influenza, including H1N1, experimental and clinical studies have identified dysregulated systemic inflammation as an important pathogenetic mechanism correlating with disease severity and progression [1, 8–11]. There has been significant advancement in our understanding of the cellular mechanisms of corticosteroid action [12] and the interaction between corticosteroids and transcription factors for inflammatory cytokines in critical illness and ARDS [13, 14]. Within this new pathogenetic construct [15], an extensive rationale was recently provided for low-to-moderate dose prolonged corticosteroid treatment as an adjunct to antiviral therapy in severe cases of H5N1 influenza [8].
In the event of a growing pandemic, even a small fraction of patients developing severe pneumonia with respiratory failure can overwhelm any country’s critical care and hospital infrastructure. A recent report by the US President’s Council of Advisors on Science and Technology presented as a “plausible scenario” 300,000 patients requiring ICU admission in the United States alone [16]. Following the reports that most patients initially died despite the best supportive treatment (including antiviral) alone, corticosteroids were widely used during the 2002–2003 severe acute respiratory syndrome (SARS) epidemic [17]. While some studies indicated a possible reduction in hospital mortality and length of stay [17–19], the implementation of extremely heterogeneous treatment regimens that varied widely for timing of initiation (early vs. late), dosage (hydrocortisone equivalent 300–6,000 mg/day), and duration (2–36 days) of treatment made any assessment of efficacy unfeasible [20]. Since then, however, three randomized trials [21–23] investigating prolonged low-to-moderate dose corticosteroid treatment in early acute lung injury (ALI) and ARDS and the finding of recent meta-analyses [24, 25] have provided a stronger foundation to guide anti-inflammatory treatment in ALI-ARDS. Not surprisingly, the two largest studies of severe H1N1 influenza A-associated respiratory failure report the use of corticosteroids in 51–69% of patients [6, 7].
In the southern hemisphere, May through September is the peak season for influenza. As of 6 July 2009, 2,485 cases of H1N1 influenza A virus were confirmed in Argentina with 60 (2.5%) deaths [26]. In response to the influenza outbreak, and taking into account recent randomized trials [21, 23], we developed and prospectively evaluated a comprehensive protocol to manage patients with suspected H1N1 influenza A virus pneumonia and acute hypoxemic respiratory failure admitted to the medical ICU. This case series describes the clinical presentation of 13 consecutive patients and their response to combination high-dose oseltamivir and prolonged low-to-moderate dose corticosteroid treatment. Patients with and without confirmed H1N1 influenza had similar disease severity at presentation and similar response to treatment. Twelve patients (92%) improved lung function, were extubated, and discharged alive from the ICU. Hospital and 60-day mortality was 15%.
Methods
Hospital Municipal de Agudos Dr. Leónidas Lucero is a tertiary acute care hospital in Bahía Blanca, Buenos Aires, Argentina. All 13 patients admitted to the medical ICU with influenza-like illness, abnormal chest radiograph, and severe hypoxemia requiring invasive mechanical ventilation were managed in accordance with a preestablished protocol (see below). This case series was determined to be exempt from institutional review board approval. H1N1 influenza A virus infection was diagnosed by testing nasopharyngeal swab specimens with real-time reverse transcription-polymerase chain reaction (rRT-PCR) in accordance with published guidelines from the US Centers for Disease Control and Prevention (CDC) [27].
The severity of illness and severity of MODS were quantified with measurements (day 1 and 7) of the Acute Physiology and Chronic Health Evaluation II [28] and Sequential Organ Failure Assessment (SOFA) scores [29]. With the latter, acute organ dysfunction was defined as an acute deterioration in a previously normal organ with a SOFA score greater than 2 [29]. C-reactive protein levels were used as a marker of systemic inflammation [21, 23]. All but one patient met diagnostic criteria for ARDS according to the American-European Consensus definition [30]. The lung injury score (LIS) quantifies the physiologic respiratory impairment in ARDS through the use of a 4-point score based on the levels of positive end-expiratory pressure (PEEP), ratios of PaO2 to fraction of inspired oxygen (FiO2) (PaO2:FiO2), the static lung compliance (CST), and the degree of infiltration present on chest radiograph [31]. ARDS was defined as severe if the PaO2:FiO2 was ≤120 on PEEP ≥ 12 cmH2O. Patients were defined as improvers if, by day 7 of ARDS, they achieved a lung injury score equal to or lower than 2 or a reduction from day 1 equal to or greater than 1 point.
At ICU admission, before results of microbiological analysis (nasopharyngeal swab, respiratory secretions, and blood) were available, patients were started on intravenous empiric antimicrobial therapy in agreement with recent guidelines [32] and antiviral treatment via nasogastric tube with high-dose oseltamivir (rationale provided in [2]), 150 mg twice/day for 5 days followed by 75 mg twice/day for 3–5 days (dictated by clinical evolution). Corticosteroid treatment was initiated at ICU entry concomitantly with antiviral treatment. Similar to the methodology of recent randomized trials [21, 33], all patients were initially started on hydrocortisone, 100 mg intravenously every 8 h. This dosage was continued until ICU discharge, followed by a lower dose until hospital discharge (100 mg every 12 h for 7 days and 100 mg per day for 7 days). The longer duration of hydrocortisone treatment, in comparison to the prior trials [21, 33, 34], was dictated by recent reports indicating that shorter duration [35] or rapid tapering [24] of treatment is associated with rebound systemic inflammation and clinical deterioration. Similar to the methodology of another recent randomized trial [23], if the patient deteriorated and developed severe ARDS (PaO2:FiO2 ≤ 120 on PEEP ≥ 12 cmH2O), hydrocortisone was changed to methylprednisolone infusion at an initial dose of 1 mg/kg/day for 14 days followed by 0.5 mg/kg/day for 7 days and then tapered over 6 days [23]. Corticosteroid treatment administration was changed from intravenous to enteral once oral intake was restored following extubation. The anti-inflammatory potency of hydrocortisone 300 mg is equivalent to methylprednisolone 60 mg. In the six patients with severe ARDS, the body weight (kg) and initial daily methylprednisolone dosage (mg/day) patients ranged from 76 to 100.
Most aspects of care were managed following a preestablished protocol that, in addition to antimicrobial, antiviral, and corticosteroid treatment, followed recent guidelines for fluid resuscitation, sedation and analgesia, gastrointestinal and thromboembolic prophylaxis, and enteral nutrition [36]. Ventilator management incorporated tidal volume and PEEP conforming with the ARDSnet recommendations [37], and standardized procedures for recruitment maneuvers, prone position ventilation for refractory hypoxemia, and weaning from mechanical ventilation. Prone position ventilation was instituted when PaO2:FiO2 was 150 or less on a PEEP equal or greater than 10 cmH2O and the patient failed to improve to at least four recruitment maneuvers over 12 h. Patients were continuously kept in prone position for at least 6 h per day. During mechanical ventilation, intubated patients had weekly nonbronchoscopic bronchoalveolar lavage; the diagnostic threshold for bacterial pneumonia was ≥104 CFU/ml [23].
Statistical analysis
All statistical calculations were preformed using the SAS System for Windows (Version 9.2, SAS Institute, Cary, NC). We compared clinical characteristics on admission and over time between patients positive and negative for H1N1 and patients with and without severe ARDS. Standard descriptive statistics were obtained by Student’s t-test, χ 2 Fisher’s exact test, and Wilcoxon rank sum test. All reported probabilities are two-sided without adjustment.
Results
From June 24 through 12 July 2009, 13 patients with severe hypoxemia and suspected H1N1 influenza A virus pneumonia were admitted to the medical ICU of Hospital Municipal de Agudos Dr. Leónidas Lucero. Except for one patient transferred from another medical center, the reported hospitalization was the first hospitalization related to the disease. All patients presented with fever, myalgia, cough, progressive dyspnea, and clinical criteria of severe sepsis. Eight patients had laboratory-confirmed H1N1 infection, one patient had positive rRT-PCR for influenza A but negative H1N1, and four patients were negative for influenza A. We cannot exclude false-negative rRT-PCR results, recently reported in ten percent of ICU patients with H1N1 pneumonia [5]. Chest radiographs in all but one patient (who had one quadrant involvement) showed bilateral infiltrates consistent with severe multilobar pneumonia or ARDS. Eight (62%) patients had vasopressor-dependent shock.
Table 1 provides baseline characteristics for the entire group (n = 13), for patients with positive (H1N1+; n = 8) and negative H1N1 (H1N1−; n = 5) influenza A virus rRT-PCR, and for patients with (n = 6) and without severe ARDS (n = 7). Patients with positive and negative RT-PCR had similar disease severity at presentation. Among H1N1+ patients, two had chronic obstructive pulmonary disease (COPD), one (15 years old) had Prader Willi syndrome and diabetes, and two additional patients were moderately obese (BMI >35). Among H1N1− patients, one had asthma and one had epilepsy. Five patients were cigarette smokers (three severe ARDS, three H1N1+), and two had a history of alcohol abuse (two severe ARDS, two H1N1−).
Table 1.
Findings at intensive care unit (ICU) admission
| Variables | Combined | H1N1 rRT-PCR | Severe ARDSa | ||||
|---|---|---|---|---|---|---|---|
| (N = 13) | Positive (N = 8) | Negative (N = 5) | P value | Yes (N = 6) | No (N = 7) | P value | |
| Age | 39 ± 11 | 39.5 ± 12.8 | 38.2 ± 9 | 0.83 | 36.2 ± 11.7 | 41.2 ± 10.9 | 0.42 |
| Male sex, no. (%) | 9 (69.2%) | 4 (50%) | 5 (100%) | 0.10 | 4 (67%) | 5 (71%) | 1.00 |
| Symptoms onset to ICU admission, days | 3 (1) | 3 (3) | 2 (0) | 0.05 | 2.5 (1) | 3 (5) | 0.40 |
| APACHE II score | 13.7 ± 5.3 | 12.1 ± 4.3 | 16.2 ± 6.4 | 0.25 | 15.7 ± 5.7 | 12.0 ± 4.8 | 0.24 |
| SOFA score | 6.5 ± 2.8 | 5.9 ± 1.6 | 7.4 ± 4.1 | 0.46 | 8.2 ± 3.0 | 5.0 ± 1.5 | 0.05 |
| C-reactive protein (mg/dl)b | 97.1 ± 70.4 | 83.5 ± 78.7 | 116.1 ± 59.6 | 0.39 | 108.8 ± 72.6 | 85.4 ± 69.4 | 0.43 |
| Leukocyte count per mm3 | 10,577 ± 8,065 | 10,788 ± 7,890 | 1,024 ± 9,272 | 0.92 | 8,883 ± 8,593 | 12,029 ± 7,953 | 0.51 |
| Lymphocyte count per mm3 | 949 ± 640 | 1,065 ± 644 | 787 ± 670 | 0.49 | 970 ± 815 | 928 ± 485 | 0.92 |
| Serum creatine kinase, IU/lb | 957 ± 2122 | 235 ± 206 | 2,203 ± 3,410 | 0.33 | 1,608 ± 2,801 | 162 ± 139 | 0.26 |
| Serum lactate dehydrogenase, IU/lb | 417 ± 236 | 337 ± 200 | 537 ± 261 | 0.25 | 232 ± 55 | 541 ± 229 | 0.02 |
| Lung injury scorea | 3.01 ± 0.7 | 2.83 ± 0.77 | 3.45 ± 0.33 | 0.07 | 3.6 ± 0.4 | 2.6 ± 0.6 | 0.32 |
| PEEP (cmH2O2) | 14 ± 5 | 13 ± 5 | 16 ± 5 | 0.25 | 18.3 ± 3.7 | 10.0 ± 1.6 | 0.002 |
| PaO2:FiO2 | 118 ± 48 | 126 ± 49 | 105 ± 47 | 0.46 | 88 ± 23 | 144 ± 49 | 0.02 |
| Vasopressor-dependent septic shock | 8 (62%) | 4 (50%) | 4 (80%) | 0.56 | 6 (100%) | 2 (29%) | 0.02 |
| Renal dysfunction | 2 (15.4) | 1 (12.5%) | 1 (20%) | 1.0 | 2 (33%) | 0 | 0.19 |
Data are reported as mean and standard deviation or median and (range). PaO2:FiO2 reflect the first value obtained at ICU admission
aOf the eight patients with positive H1N1 rRT-PCR, three had severe ARDS
bMissing values: 1 C-reactive protein levels (1 H1N1 positive), 2 serum creatine kinase (1 H1N1 positive), 2 serum lactate dehydrogenase (1 H1N1 negative)
At ICU admission, five patients had more than 10,000 leukocytes per cubic millimeter (4 H1N1+), six other patients (3 H1N1+) had lymphopenia (all less than 600 leukocytes per cubic millimeter), seven (3 H1N1+) had increased creatine kinase levels (one above 1,000 IU per liter), and six (3 H1N1+) had elevated lactate dehydrogenase levels (all below 1,000 IU per liter). Blood cultures obtained in eight patients were without growth. All patients had PaO2:FiO2 < 200 while receiving PEEP ≥ 8 cmH2O2; all but one patient (one quadrant involvement on chest radiograph) had a lung injury score ≥2. As shown in Table 1, patients with severe ARDS (n = 6; 3 H1N1 positive) had more septic shock, higher SOFA score, worse gas exchange, and higher PEEP requirements; all but one required prone ventilation. Four patients were switched to methylprednisolone within 24 h of ICU admission, two (one nonsurvivor) on day 4 of hydrocortisone treatment. Seven patients with either severe pneumonia (n = 1) or less severe ARDS (n = 6) received prolonged hydrocortisone treatment, and none required prone ventilation; all ARDS patients had an improved lung injury score by day 7.
Table 2 shows the findings by day 7 of treatment based on H1N1 status and severity of lung injury at ICU entry. By day 7, treatment was associated with a significant reduction in C-reactive protein levels (P = 0.04), APACHE II (P = 0.002), lung injury (P < 0.001), and SOFA (P < 0.001) scores. By day 7, ≥50% reduction in the SOFA score was observed in 8 of 11 survivors and none of hospital nonsurvivors, while the lung injury score improved in all but two patients (one nonsurvivor). Figure 1 shows changes in PaO2:FiO2, lung injury, and SOFA scores. Importantly, the response in patients with positive and negative H1N1 influenza was comparable, and the degree of improvement with methylprednisolone (severe ARDS) and hydrocortisone treatment was similar. Patients with severe ARDS had a higher rate of tracheotomy (4 vs. 0; P = 0.02) and ventilator-associated pneumonia (4 vs. 0; P = 0.02) and a trend toward higher lung injury score and longer duration of mechanical ventilation (Table 2). Five patients were extubated by day 7 and an additional six by day 14. Four patients (all survivors, three H1N1+) developed ventilator-associated pneumonia caused by Klebsiella pneumoniae (2), Acinetobacter baumani (1), and Proteus mirabilis (1). Five nondiabetic patients required low doses of insulin for glycemic control. Gastrointestinal bleeding requiring transfusion and neuromuscular weakness were not observed.
Table 2.
Findings by day 7 and outcome variables
| Variables | Combined | H1N1 rRT-PCR | Severe ARDS | ||||
|---|---|---|---|---|---|---|---|
| (N = 13) | Positive (N = 8) | Negative (N = 5) | P value | Yes (N = 6) | No (N = 7) | P value | |
| C-reactive protein day 7 (mg/dl)a | 41 ± 34 | 49 ± 38 | 28 ± 26 | 0.55 | 41 ± 43 | 28 ± 15 | 0.26 |
| Lung injury score (LIS) on day 7 | 2.1 ± 0.6 | 2 ± 0.5 | 2.2 ± 0.8 | 0.74 | 2.4 ± 0.7 | 1.7 ± 0.3 | 0.06 |
| Improvement in LIS by day 7b | 11 (84.6%) | 8 (100%) | 3 (60%) | 0.12 | 4 (67%) | 7 (100%) | 0.19 |
| APACHE II score on day 7 | 9.2 ± 5.3 | 8.7 ± 5.9 | 10 ± 4.7 | 0.68 | 11.5 ± 5.6 | 7.3 ± 4.5 | 0.17 |
| SOFA score on day 7 | 3.2 ± 2.5 | 3.3 ± 2.0 | 3.0 ± 3.5 | 0.89 | 4.2 ± 2.8 | 2.3 ± 2.1 | 0.21 |
| Serum creatine kinase, U/la | 393 ± 508 | 370 ± 490 | 456 ± 663 | 0.85 | 510 ± 647 | 253 ± 278 | 0.41 |
| Serum lactate dehydrogenase, U/la | 270 ± 72 | 267 ± 81 | 279 ± 55 | 0.79 | 310 ± 72 | 237 ± 58 | 0.10 |
| Duration mechanical ventilation (days) | 11.3 ± 6.5 | 12.4 ± 6.2 | 9.6 ± 4.6 | 0.38 | 14.7 ± 6.3 | 8.4 ± 3.0. | 0.06 |
| Duration of corticosteroid treatment (days) | 21.2 ± 6.1 | 22.5 ± 5.9 | 19 ± 6.4 | 0.35 | 24.5 ± 5.4 | 18.3 ± 5.4 | 0.06 |
| ICU length of stay (days) | 13.6 ± 6.5 | 15.4 ± 7 | 10.8 ± 5 | 0.19 | 16.7 ± 7.3 | 11.0 ± 4.7 | 0.14 |
| Hospital length of stay (days)a | 18.7 ± 9.6 | 21 ± 11.3 | 15 ± 4.7 | 0.21 | 23 ± 12.0 | 15 ± 5.2 | 0.18 |
| Hospital mortalitya | 2 (15.4%) | 1 (12.5%) | 1 (20%) | 1.0 | 1 (17%) | 1 (14%) | 0.71 |
Data are reported as mean and standard deviation or median and (range)
aMissing values: 2 C-reactive protein levels (1 H1N1 positive), 2 serum creatine kinase (2 H1N1 negative), and 2 serum lactate dehydrogenase (1 H1N1 positive)
bIn patients with ARDS (n = 12) improvement in LIS by day 7 was defined as a lung injury score ≤2 or a reduction from day 1 ≥1 point
Fig. 1.
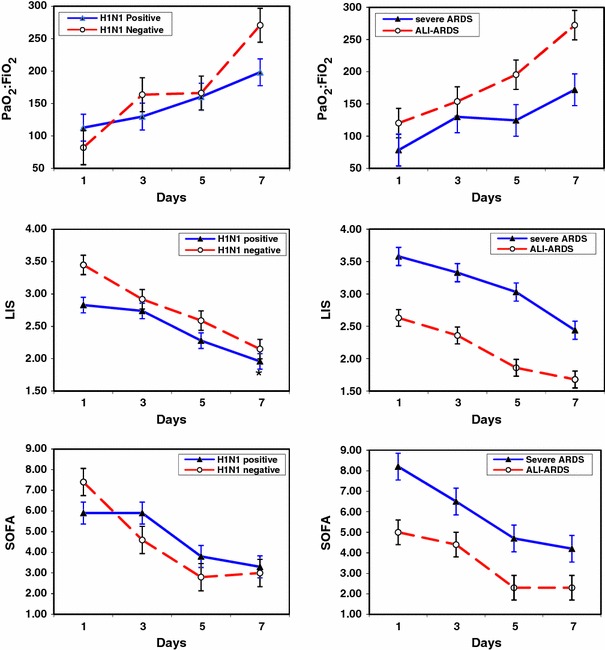
Changes in PaO2:FiO2 (top), lung injury (middle), and SOFA (bottom) scores during the first 7 days of treatment in patients with positive and negative H1N1 rRT-PCR (left) and in patients with and without severe ARDS (right). SOFA score Sequential Organ Failure Assessment score [29]. Patients with severe ARDS were treated with prolonged methylprednisolone treatment at an initial dose of 1 mg/kg/day; patients with ALI-ARDS were treated with hydrocortisone at an initial dose of 300 mg/day. PaO2:FiO2 reflect the worst daily value. From day 1 to day 7, the PaO2:FiO2 increased from 113 ± 21 to 198 ± 21 (P = 0.006) in H1N1 positive, from 82 ± 26 to 271 ± 26 (<0.0001) in H1N1 negative, from 78 ± 25 to 172 ± 25 (P = 0.01) in severe ARDS, and from 120 ± 23 to 272 ± 23 (P < 0.001) in ALI-ARDS. From day 1 to day 7, the LIS decreased from 2.83 ± 0.8 to 2.01 ± 0.5 (P = 0.003) in H1N1 positive, from 3.45 ± 0.3 to 2.15 ± 0.8 (P = 0.02) in H1N1 negative, from 3.58 ± 0.4 to 2.44 ± 0.7 (P = 0.009) in severe ARDS, and from 2.63 ± 0.6 to 1.73 ± 0.3 (P = 0.007) in ALI-ARDS. From day 1 to day 7, the SOFA score decreased from 5.9 ± 1.6 to 3.3 ± 2.0 (P = 0.01) in H1N1 positive, from 7.4 ± 4.1 to 3.0 ± 3.5 (P = 01) in H1N1 negative, from 8.2 ± 3.0 to 4.2 ± 2.9 (P = 0.04), and from 5.0 ± 1.5 to 2.3 ± 2.1 (P = 0.02) in ALI-ARDS
Duration of corticosteroid treatment was 21.2 ± 6.1 days (range 11–28). All but one patient improved lung function, were extubated, and discharged alive from the ICU. The ICU nonsurvivor was a 46-year-old male smoker with a history of alcohol abuse, admitted with septic shock and severe ARDS, negative H1N1 RT-PCR, dysfunction of five vital organs (APACHE II and SOFA scores 20 and 16, respectively), failed to improve lung injury score by day 7 of ARDS, and died on ICU day 15 with progression of MODS. One hospital nonsurvivor, a 60-year-old moderately obese (BMI >35) female with COPD, positive H1N1 RT-PCR, without shock at study entry, was extubated after 7 days of mechanical ventilation and died after ICU discharge as a result of cardiac arrest (electromechanical dissociation) preceded by sudden onset of severe dyspnea. Pulmonary embolism was suspected, but autopsy was not obtained. Hospital mortality in patients with and without shock was similar (12.5 vs. 20%). All hospital survivors were discharged home without oxygen supplementation and were alive at day 60. At the physician discretion, outpatient tapering was continued in all those with severe ARDS (5.3 ± 2.8 days) and in four without severe ARDS (3.4 ± 3.2 days).
Discussion
This is the first case series of patients with suspected H1N1 influenza A virus pneumonia and ALI-ARDS prospectively evaluating the response to combination oseltamivir and prolonged low-to-moderate dose corticosteroid treatment. Similar to prior reports [1–7], our patients were young (39 ± 11 years old) and otherwise healthy, with the most common comorbidity being chronic lung disease. Common laboratory findings included a total leukocyte count within normal limits, lymphopenia, and increased lactate dehydrogenase and creatine kinase levels. Eight patients tested positive for H1N1 influenza A virus, and their clinical presentation was similar to those with a negative test. Acute hypoxemic respiratory failure developed rapidly (median 3 days) and was frequently associated with vasopressor-dependent shock (62%). All but one patient met criteria for ARDS (single lung involvement on chest radiograph) [30], and six patients had severe ARDS, a condition associated with ICU mortality in excess of 50% [38].
By day 7 of treatment, patients experienced a significant reduction in C-reactive protein levels, PaO2:FiO2, lung injury, and multiple organ dysfunction scores (Fig. 1). The response in patients with positive and negative H1N1 influenza was similar and comparable to the findings of recent early ALI-ARDS randomized trials [21, 23]. Treatment was well tolerated. Twelve patients (92%) had improved lung function, were extubated, and discharged alive from the ICU. Our 60-day mortality (15%) is similar to the one recently reported by the Canadian Critical Care Trials Group H1N1 Collaborative (17.3%) [7] and lower than the 30–58% reported by others [1–3, 5, 6]. Our patient population differed for the lack of pregnant [3, 5] or morbidly obese patients [2, 5], the inclusion of only one pediatric patient [1, 3, 7], and a shorter duration of symptoms at presentation [1, 2, 5]. While two of the largest studies report corticosteroid use in 51 [7] and 69% [6] of patients, subgroup analyses on response to corticosteroid treatment were not provided.
Severe influenza [8, 9] shares a common pathogenetic mechanism—dysregulated systemic inflammation—with other life-threatening diseases leading to ALI-ARDS and MODS [14]. In ARDS patients, deficient corticosteroid-mediated downregulation of inflammatory cytokines transcription is associated with disease progression and mortality [15, 39]. In a randomized trial, prolonged low-to-moderate dose corticosteroid treatment (in comparison to placebo) was associated with a significant downregulation of inflammatory cytokine transcription, reduction in pulmonary and systemic inflammation, and improvement in pulmonary and multiple organ dysfunction [14, 15].
Although neuraminidase inhibitors are effective in treating H1N1 influenza A virus when given within 48 h of infection, all of our patients presented 2 days after onset of symptoms, and timing to presentation did not affect disease severity. These antiviral agents do not, however, directly reduce the inflammatory response. While lacking a randomized trial in patients with severe influenza, valuable data are available from recent meta-analyses of randomized trials in patients with severe sepsis (n = 1,228) [34] and ALI-ARDS (n = 648) [25]. All ALI-ARDS [40] and many severe sepsis studies [34] reported that prolonged corticosteroid treatment was associated with a significant reduction in markers of systemic inflammation. Consistently, both meta-analyses found that prolonged low-to-moderate dose corticosteroid treatment was associated with a significant physiological improvement (accelerated resolution of shock [34] and respiratory failure [25]), a reduction in duration of ICU stay (4.5 days) [25, 34], and no increased risk for nosocomial infections, neuromuscular weakness, or gastrointestinal bleeding [25, 34]. Importantly, in the SARS experience, most serious side effects—psychosis, fatal aspergillosis, and avascular necrosis—were reported in those receiving high-dose methylprednisolone (3–10 mg/kg/day) [20].
For ALI-ARDS, all controlled studies (n = 334) investigating treatment initiated within 3 days of disease onset (similar to our protocol) showed a significant improvement in survival, with an overall 24% absolute reduction in mortality (risk ratio 0.69; 95% confidence interval 0.56–0.84) [25, 40]. The meta-analysis of randomized trials in severe sepsis reported that the risk of death was decreased with low-to-moderate doses (less than 1,000 mg hydrocortisone equivalent) and with longer duration of treatment [34]. While both meta-analyses [34] [40] found a survival advantage, the lack of a large confirmatory trial and the moderate degree of heterogeneity across the studies led a recent consensus statement to grade the evidence for a survival benefit in severe sepsis and ALI-ARDS as weak (grade 2b) [13].
The size of this small case controlled series is the major limitation of this study. While a randomized trial in H1N1 influenza A virus-associated ARDS is needed to evaluate the effect of this treatment on mortality, it is unlikely to be realized in the foreseeable future. Importantly, ARDS patients with and without documented H1N1 influenza had a similar response to treatment, comparable to that of recent ALI-ARDS trials in patients without influenza [25, 40]. Also, the degree of improvement with methylprednisolone (more severe disease and higher dose) and hydrocortisone (less severe disease and lower dose) treatment was similar. At present, information on the natural course of severe H1N1 infection is still limited [1–7], but it is likely that this single ICU experience will be replicated worldwide.
Based on our observations, we conclude that prolonged low-to-moderate dose corticosteroid treatment is safe and associated with significant improvement in lung injury and multiple organ dysfunction scores [40]. This report is too small to reach conclusions on mortality, but it provides the rationale and preliminary data for the design of randomized trial. Because ARDS is the leading cause of death for patients admitted to the hospital with H1N1 influenza A virus pneumonia [1–5], it is probable that physicians—similar to the SARS [17–19] and the recent international H1N1 response [1–7]—will consider adjunctive corticosteroid therapy. To simplify management and taking into consideration the methodology of the early ARDS trial [23], we recommend using methylprednisolone. Following an initial 60 mg intravenous bolus, methylprednisolone is administered as continuous infusion at (1) 60 mg/day (day 1–14), (2) 30 mg/day (day 15–21), (3) 15 mg/day (day 22–25), and (4) 10 mg/day (day 26–28). If the patient has, or later develops, severe ARDS, the methylprednisolone dosage is increased to 1 mg/kg/day [23]. If between days 1–14 the patient is extubated, the patient is advanced to day 15 of drug therapy and tapered according to schedule. Following extubation, the infusion is changed to intravenous boluses given every 12 h until the patient tolerates enteral intake. Treatment response should be monitored with daily measurement of lung injury and SOFA scores [21, 23]. Finally, secondary prevention (including infection surveillance and avoidance of paralysis) is important to minimize complications associated with, or masked by, prolonged glucocorticoid treatment [15, 23].
Acknowledgments
The study was not funded. We are grateful to Drs. Mark Holodniy, David Armbruster, and Scott Sinclair for critical review of the manuscript.
Conflicts of interest statement
We declare that we have no conflicts of interest.
References
- 1.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quinones-Falconi F, Bautista E, Ramirez-Venegas A, Rojas-Serrano J, Ormsby CE, Corrales A, Higuera A, Mondragon E, Cordova-Villalobos JA. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quinones-Falconi F, Bautista E, Ramirez-Venegas A, Rojas-Serrano J, Ormsby CE, Corrales A, Higuera A, Mondragon E, Cordova-Villalobos JA. Intensive-care patients with severe novel influenza A (H1N1) virus infection—Michigan, June 2009. MMWR Morb Mortal Wkly Rep. 2009;58:749–752. [PubMed] [Google Scholar]
- 3.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 4.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Hospitalized patients with novel influenza A (H1N1) virus infection—California, April–May, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:536–541. [PubMed] [Google Scholar]
- 5.Rello J, Rodriguez A, Ibanez P, Socias L, Cebrian J, group THNSw (2009) Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit Care 13:R148. http://ccforum.com/content/13/5/R148 [DOI] [PMC free article] [PubMed]
- 6.Domínguez-Cherit G, Lapinsky S, Macias A, Pinto R, Espinosa-Perez L, de la Torre A, Poblano-Morales M, Baltazar-Torres J, Bautista E, Martinez A, Martinez M, Rivero E, Valdez R, Ruiz-Palacios G, Hernández M, Stewart T, Fowler R. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302:1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Zarychanski R, Pinto R, Cook D, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, Turgeon A, Lapinsky S, Ahern S, Smith O, Siddiqui F, Jouvet P, Khwaja K, McIntyre L, Menon K, Hutchison J, Hornstein D, Joffe A, Lauzier F, Singh J, Karachi T, Wiebe K, Olafson K, Ramsey C, Sharma S, Dodek P, Meade M, Hall R, Fowler R, Collaborative ftCCCTGHN Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 8.Carter MJ. A rationale for using steroids in the treatment of severe cases of H5N1 avian influenza. J Med Microbiol. 2007;56:875–883. doi: 10.1099/jmm.0.47124-0. [DOI] [PubMed] [Google Scholar]
- 9.Van Reeth K, Van Gucht S, Pensaert M. Correlations between lung proinflammatory cytokine levels, virus replication, and disease after swine influenza virus challenge of vaccination-immune pigs. Viral Immunol. 2002;15:583–594. doi: 10.1089/088282402320914520. [DOI] [PubMed] [Google Scholar]
- 10.de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, Ha do Q, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar J. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, Hatta M, Muramoto Y, Tamura D, Sakai-Tagawa Y, Noda T, Sakabe S, Imai M, Hatta Y, Watanabe S, Li C, Yamada S, Fujii K, Murakami S, Imai H, Kakugawa S, Ito M, Takano R, Iwatsuki-Horimoto K, Shimojima M, Horimoto T, Goto H, Takahashi K, Makino A, Ishigaki H, Nakayama M, Okamatsu M, Warshauer D, Shult PA, Saito R, Suzuki H, Furuta Y, Yamashita M, Mitamura K, Nakano K, Nakamura M, Brockman-Schneider R, Mitamura H, Yamazaki M, Sugaya N, Suresh M, Ozawa M, Neumann G, Gern J, Kida H, Ogasawara K, Kawaoka Y. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009;460:1021–1025. doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 13.Marik PE, Pastores S, Annane D, Meduri G, Sprung C, Arlt W, Keh D, Briegel J, Beishuizen A, Dimopoulou I, Tsagarakis S, Singer M, Chrousos GP, Zaloga GP, Bokhari F, Vogeser M. Clinical practice guidelines for the diagnosis and management of corticosteroid insufficiency in critical illness: recommendations of an international task force. Crit Care Med. 2008;36:1937–1949. doi: 10.1097/CCM.0b013e31817603ba. [DOI] [PubMed] [Google Scholar]
- 14.Meduri GU, Tolley EA, Chrousos GP, Stentz F. Prolonged methylprednisolone treatment suppresses systemic inflammation in patients with unresolving acute respiratory distress syndrome. Evidence for inadequate endogenous glucocorticoid secretion and inflammation-induced immune cell resistance to glucocorticoids. Am J Respir Crit Care Med. 2002;165:983–991. doi: 10.1164/ajrccm.165.7.2106014. [DOI] [PubMed] [Google Scholar]
- 15.Meduri G, Annane D, Chrousos G, Marik P, Sinclair S (2009) Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy. Chest 136. doi:10.1378/chest.1308-2408 [DOI] [PubMed]
- 16.(August 7, 2009) Report to the President on US preparation for 2009-H1N1 Influenza. Office of the Press Secretary
- 17.Yam LY, Lau AC, Lai FY, Shung E, Chan J, Wong V. Corticosteroid treatment of severe acute respiratory syndrome in Hong Kong. J Infect. 2007;54:28–39. doi: 10.1016/j.jinf.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen RC, Tang XP, Tan SY, Liang BL, Wan ZY, Fang JQ, Zhong N. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129:1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng VC, Tang BS, Wu AK, Chu CM, Yuen KY. Medical treatment of viral pneumonia including SARS in immunocompetent adult. J Infect. 2004;49:262–273. doi: 10.1016/j.jinf.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, Della Porta R, Giorgio C, Blasi F, Umberger R, Meduri GU. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005;171:242–248. doi: 10.1164/rccm.200406-808OC. [DOI] [PubMed] [Google Scholar]
- 22.Annane D, Sebille V, Bellissant E. Effect of low doses of corticosteroids in septic shock patients with or without early acute respiratory distress syndrome. Crit Care Med. 2006;34:22–30. doi: 10.1097/01.CCM.0000194723.78632.62. [DOI] [PubMed] [Google Scholar]
- 23.Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, Gibson MRU. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131:954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 24.Meduri GU, Marik PE, Chrousos GP, Pastores SM, Arlt W, Beishuizen A, Bokhari F, Zaloga G, Annane D. Steroid treatment in ARDS: a critical appraisal of the ARDS network trial and the recent literature. Intensive Care Med. 2008;34:61–69. doi: 10.1007/s00134-007-0933-3. [DOI] [PubMed] [Google Scholar]
- 25.Tang B, Craig J, Eslick G, Seppelt I, McLean A. Use of corticosteroids in acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med. 2009;37:1594–1603. doi: 10.1097/CCM.0b013e31819fb507. [DOI] [PubMed] [Google Scholar]
- 26.Tang B, Craig J, Eslick G, Seppelt I, McLean A (2009) World Health Organization. Pandemic (H1N1) 2009—update 58, 6 July 2009
- 27.Tang B, Craig J, Eslick G, Seppelt I, McLean A (2009) United States Centers for Disease Control and Prevention. Interim guidance on specimen collection, processing, and testing for patients with suspected swine-origin influenza A (H1N1) virus infection. http://www.cdc.gov/h1n1flu/specimencollection.htm
- 28.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 30.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 31.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 32.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr, Musher DM, Niederman MS, Torres A, Whitney CG. Infectious diseases society of America/American thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troche G, Chaumet-Riffaut P, Bellissant E. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 34.Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, De Gaudio R, Keh D, Kupfer Y, Oppert M, Meduri GU. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA. 2009;301:2362–2375. doi: 10.1001/jama.2009.815. [DOI] [PubMed] [Google Scholar]
- 35.Nawab Q, Golden E, Confalonieri M, Umberger R, Meduri G. Glucocorticoid treatment in severe community-acquired pneumonia. Am J Respir Crit Care Med. 2007;175:A594. [Google Scholar]
- 36.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 38.Ferguson ND, Kacmarek RM, Chiche JD, Singh JM, Hallett DC, Mehta S, Stewart TE. Screening of ARDS patients using standardized ventilator settings: influence on enrollment in a clinical trial. Intensive Care Med. 2004;30:1111–1116. doi: 10.1007/s00134-004-2163-2. [DOI] [PubMed] [Google Scholar]
- 39.Meduri GU, Muthiah MP, Carratu P, Eltorky M, Chrousos GP. Nuclear factor-kappaB—and glucocorticoid receptor alpha—mediated mechanisms in the regulation of systemic and pulmonary inflammation during sepsis and acute respiratory distress syndrome. Evidence for inflammation-induced target tissue resistance to glucocorticoids. Neuroimmunomodulation. 2005;12:321–338. doi: 10.1159/000091126. [DOI] [PubMed] [Google Scholar]
- 40.Meduri G, Marik P, Annane D. Prolonged glucocorticoid treatment in ARDS: evidence supporting effectiveness and safety. Crit Care Med. 2009;37:1800–1803. doi: 10.1097/CCM.0b013e31819d2b43. [DOI] [PubMed] [Google Scholar]


