Abstract
Within the last 5 years, protein microarrays have been developed and applied to multiple approaches: identification of protein–protein interactions or protein–small molecule interactions, cancer profiling, detection of microorganisms and toxins, and identification of antibodies due to allergens, autoantigens, and pathogens. Protein microarrays are small size (typically in the microscopy slide format) planar analytical devices with probes arranged in high density to provide the ability to screen several hundred to thousand known substrates (e.g., proteins, peptides, antibodies) simultaneously. Due to their small size, only minute amounts of spotted probes and analytes (e.g., serum) are needed; this is a particularly important feature, for these are limited or expensive. In this review, different types of protein microarrays are reviewed: protein microarrays (PMAs), with spotted proteins or peptides; antibody microarrays (AMAs), with spotted antibodies or antibody fragments (e.g., scFv); reverse phase protein microarrays (RPMAs), a special form of PMA where crude protein mixtures (e.g., cell lysates, fractions) are spotted; and nonprotein microarrays (NPMAs) where macromolecules other than proteins and nucleic acids (e.g., carbohydrates, monosaccharides, lipopolysaccharides) are spotted. In this study, exemplary experiments for all types of protein arrays are discussed wherever applicable with regard to investigations of microorganisms.
Keywords: Severe Acute Respiratory Syndrome, Staphylococcal Enterotoxin, Protein Microarrays, Rolling Circle Amplification, Yersinia Pestis
Introduction
Protein microarrays have been developed within the last 5 years using the knowledge and technical innovations developed for DNA microarrays. DNA microarray technology has evolved, as a consequence to handle sets of thousands of probes from the huge sequencing programs, beginning with microbial genomes (Sanger et al. 1982; Fleischmann et al. 1995) and reaching the top with the mouse (Nadeau et al. 2001) and the human (Venter et al. 2001) genomes. The technical aspect of miniaturizing traditional methods, such as Western-Blotting and protein dotting onto classical surfaces such as nitrocellulose and nylon membranes could be adapted to protein microarray technology.
First protein arrays were constructed as high-density protein arrays to analyze protein expression cDNA libraries (Bussow et al. 1998). These arrays bear the feature of high-density (112 spots/cm2) but have a format of about 500 cm2, and therefore were called protein macroarrays. In this way, 27,648 E. coli clones were spotted in duplicate onto one 222×222 mm Nylon filter membrane (Hybond-N+, Amersham). Subsequently, protein-expression and the detection of recombinant expression products were performed directly on these filters, without further purification.
In a further development of this high-density spotting, crude cell lysates or purified proteins were spotted onto PVDF filters cut to microscopic slide format. This way, protein microarrays were generated with a theoretical density of 300 spots/cm2. In this study, a detection limit could be reached for spotted GAPDH protein detected by a monoclonal anti-GAPDH antibody of 250 amol/spot, respectively, 10 pg/spot (Lueking et al. 1999).
A Short time later, two publications demonstrating the potential of protein microarrays to screen whole proteomes were presented. The first proteome wide-protein array was used to analyze protein–protein interactions of Saccharomyces cerevisiae proteins. This array consisted of 6,000 yeast transformants, each with one cloned ORF fused to an activation domain and screened with 192 different yeast proteins (Uetz et al. 2000). This approach is from the technical aspect of in situ expression similar to the work of Bussow et al. (1998). The second proteome wide protein microarray consisted of 5,800 unique yeast proteins on a modified microscopic slide, which bear all adjectives of a protein-microarray. With this array, they demonstrated the usability of protein microarray technology to screen for protein–protein interactions by identification of calmodulin- and phospholipid-binding proteins (Zhu et al. 2001).
While these exemplary studies belong to the group of protein microarrays (PMAs) discussed below, they represent pioneer works in the field of all protein microarrays.
The types of protein microarrays
Protein microarrays (PMAs)
PMAs are named after the spotted compound basically purified recombinant proteins or peptides. These components can be used for a broad range of applications and some of them are discussed in this section.
A common application of PMAs is the detection of antibody reactions (e.g., serum screening), which was used in several applications for human (Lueking et al. 2003), bacterial (Li et al. 2005; Steller et al. 2005) or plant proteins (Kersten et al. 2003). The systematical search for antibody specificities and cross-reactivities, as done for eleven polyclonal and monoclonal antibodies on PMAs containing 5,000 yeast proteins is an alternative strategy (Michaud et al. 2003).
Furthermore, PMAs can be used for the high-throughput identification of kinase targets, to identify, e.g., potential substrates for, e.g., Arabidopsis thaliana mitogen-activated protein kinases (MPK). For this purpose, a novel method, allowing high throughput study of protein phosphorylation was used on a microarray including 1,690 nonredundant Arabidopsis proteins. Using a threshold-based quantification method to evaluate the microarray results, 48 potential substrates of MPK3, and 39 of MPK6 could be identified; of which, 26 are common for both kinases (Feilner et al. 2005).
Polypeptides, protein domains or in general not complete proteins can be spotted as well to generate PMAs. Peptide microarrays can be used for the detection of molecular interactions in cellular signal transduction (Stoevesandt et al. 2005). Therefore, phosphorylated CD3ζ ITAM and the nonphosphorylated counterpart ITAM peptides were spotted onto silanizated glass surfaces and incubated with lysates of cells expressing a fusion protein of the interaction partner ZAP-70.
Peptide microarrays were also used for simultaneous detection of pathogen infection. For this purpose, peptides, specific for hepatitis B and C viruses, human immunodeficiency virus, Epstein Barr virus, and syphilis were made functional by a glyoxyl group and anchored on the glass surface by site-specific α-oxosemicarbazene ligation. This combined assay displays high sensitivity and specificity (Duburcq et al. 2004).
Peptide microarrays are perfectly suited for epitope mapping of antibodies and antibody fragments: A set of peptides with N- and C-terminal truncations of the peptide of interest were spotted to aldehyde slides (Poetz et al. 2005). The authors have introduced quantifier spots to perform a relative affinity ranking of recombinant Fab fragments. For this system, two spots are required: One spot contains the antigen, while the other a capture molecule, which recognizes the constant part of the Fab (Poetz et al. 2005). Affinity values are calculated from both signal intensities using the ambient analyte assay conditions first defined by Ekins 1989.
Reverse phase microarrays (RPMAs)
Reverse phase microarrays (RPMAs) are a special form of PMA, because here, protein mixtures derived from cell or tissue lysates are spotted. This approach offered the possibility to analyze modified proteins, which can not be obtained from E. coli or yeast. The first RPMA was used to quantify the abundance of selected proteins during the progression of normal epithelium into prostate carcinoma (Paweletz et al. 2001). Cancer profiling is a predominant application of RPMAs identifying, for instance, potential serum markers for colon cancer (Nam et al. 2003). In this study, fractionated, solubilized proteins from the LoVo colon cancer cell line were arrayed and incubated with autoantibodies of the same patient. Subsequently, protein fractions were selected and the proteins within the fractions identified by MS analysis.
Furthermore, RPMAs can be used to simultaneously monitor dynamics of the site-specific phosphorylation of signaling molecules during stimulation of Jurkat T-Cells (Chan et al. 2004). The authors determined a dynamic range of four orders of magnitude with a detection limit of one protein in 105 to 106 lysate proteins.
Reproducible protein quantification can be obtained by multiple replica and dilution series on the array (Mircean et al. 2005). Until now, RPMAs for analyzing microorganisms have not been published, and RPMAs are discussed in more detail elsewhere (Hultschig et al. 2006).
Antibody microarrays (AMAs)
Antibody microarrays (AMAs) consists of antibodies or antibody fragments. They are used for, e.g., the identification of proteins and bacteria. For example, biotinylated capture antibodies are immobilized on NeutrAvidin-coated slides and after incubation with solutions containing toxins or bacteria, these are detected by subsequent incubation with fluorescently labeled secondary antibodies. The detection limits in the case of the tested cholera toxin, staphylococcal enterotoxin B, Ricin, and Bacillus globigii were as low as 8, 4, 10 ng/ml and 6.2×104 cfu/ml, respectively (Delehanty and Ligler 2002).
Furthermore, AMAs are useful tools for analyzing protein content, and its changes due to development, disease, signaling, and other factors. Such an analysis was performed with an AMA consisting of 224 different antibodies on a nitrocellulose-coated glass slide, probed with protein extracts of mouse F9 embryonic carcinoma cells stimulated to differentiate and 43 proteins were identified to be upregulated and a sensitivity of 3 ng/ml protein detection was reached (Kopf et al. 2005).
In a different study, 378 antibodies containing commercial microarray (BD Bioscience) was applied to profile the effect of OxLDL (oxidized low density lipoprotein) on human aortic smooth muscle cells. Out of the 298 detected proteins in the cell lysates, 54 were differentially expressed (Sukhanov and Delafontaine 2005).
Besides the antibodies, antibody fragments such as scFv’s can be used to generate AMAs. However, although the approach is particularly interesting, the bottleneck in analyzing proteins and tissues is the availability of suitable antibodies. To overcome this bottleneck, in vitro selection methods such as phage display were implemented (Konthur et al. 2005). To advance the effectiveness of the in vitro selection, scFv’s can be spotted directly onto microarrays (Wingren et al. 2005) or analyzed by doublespotting the scFv directly onto the prior insolublezed target proteins (Angenendt et al. 2004).
Non-protein microarrays (NPMAs)
Besides proteins (or nucleic acids), other components can be spotted to surfaces that bind proteins or microorganisms. Although these are not protein microarrays, they are nevertheless presented here because of their similar application possibilities.
The first example is a NPMA containing carbohydrates, which was subsequently probed with different E. coli strains. E. coli cells were bound by adhesion to mannose spotted on the microarray. An advantage of this approach is the nondestructive nature of this technique because the microorganisms could be recovered and used for further investigations, e.g., antibacterial susceptibility or substrate acquisition tests. In this study, the bacteria were stained using a nucleic acid dye and routinely 109E. coli cells were used for detection, but the limit observed was 105–106 cells (Disney and Seeberger 2004).
Another example for a NPMA is a monosaccharide array for the detection of bacterial toxins. Arrays of N-acetyl galactosamine (GalNAc) or N-acetylneuraminic acid (Neu5Ac) derivates were immobilized on the surface and used as receptors. The bacterial toxins (cholera and tetanus) were bound specifically to the monosaccharides, whereas labeled cells of E. coli, Salmonella typhimurium, Listeria monocytogenes, or staphylococcal enterotoxin B were not bound. For both toxins, the limit of detection is around 100 ng/ml (Ngundi et al. 2005).
In another experiment, lipopolysaccharide (LPS) microarray were used for the detection of antibodies (Thirumalapura et al. 2005). Therefore, LPS was isolated from different bacteria and immobilized on nitrocellulose-coated glass slides. The specificity of these LPS arrays was tested using four monoclonal antibodies against these bacteria and the detection limit for antibodies was 10 ng/ml.
Limitations and solutions of protein microarray technology
Besides the obvious advantages of protein microarrays, such as high-throughput, high-density, improved reproducibility, and low sample consumption, there are also some limitations and bottlenecks. In this part of the review, I discuss more general aspects applicable to all protein microarrays.
Availability of purified proteins
The on demand availability of purified proteins is in the case, were hundred to thousands proteins should be spotted a major limitation.
A very fascinating approach to circumvent this limitation was done by Ramachandran et al.(2004). They have generated protein microarrays by printing cDNAs onto glass slides besides capture antibodies (anti-GST). After adding a cell-free expression system, the proteins were generated directly on the microarray and bound with the fused GST-part to the capture antibodies. The proteins could now be analyzed as in a conventional protein-microarray. They demonstrated the feasibility with specific antibody binding to distinct fusion proteins and to map pairwise interactions among 29 human DNA replication proteins (Ramachandran et al. 2004).
To circumvent purification it might be useful for some applications to spot unpurified proteins if possible. In a recent approach, a cellulose surface was used and proteins were bound indirectly via a cellulose binding protein (Ofir et al. 2005).
A further possibility to circumvent the limitation of availability of purified and/or modified proteins is the RPMA approach presented above.
Availability of suitable surfaces
Proteins are, due to their composition from many different amino acids, a class of very heterogeneous macromolecules with variable properties. This makes it extremely complicated to find a common surface suitable for different proteins with a broad range in molecular weight and physico–chemical properties such as charge and hydrophobicity. Therefore, multiple surfaces have been developed during the last 5 years; some of them are listed in Table 1 with their applications. Thereby, it is not important if the binding is due to a covalent binding or a physical adsorption. Binding could be from chemical reactions or physical adsorption directly between the surface structures and the spotted molecule (protein/antibody), or indirectly by a linker molecule. This linker molecule can be part of the protein (fusion protein) or a separate molecule.
Table 1.
Surface and applications
| Surface | Type | Application | Reference |
|---|---|---|---|
| Aldehyde | PMA | Epitope mapping using peptide microarrays. | (Poetz et al. 2005) |
| Amine | AMA | Comparison study of some monoclonal or polyclonal antibodies on different surfaces. | (Angenendt et al. 2002) |
| Aminosilane | NPMA | Detection of tetanus and cholera toxins using monosaccharide arrays. | (Ngundi et al. 2005) |
| Avidine | PMA | Immobilization of biotinylated peptides for identification of kinase substrates using fluorescent dyes. | (Lesaicherre et al. 2002) |
| BSA-NHS | PMA | Protein–protein, kinase substrate and small molecule interaction experiments. | (MacBeath and Schreiber 2000) |
| Cellulose coated | PMA/AMA | Proteins/peptides bind via a cellulose binding protein to a cellulose-coated surface. Also good results with unpurified proteins or antibodies. | (Ofir et al. 2005) |
| Copolymer | AMA | Assay for rheumatoid factor. The detection limit was 54 amol/spot. | (Cretich et al. 2004) |
| DNA-coating | PMA | Binding of proteins via a GAL4 DNA binding domain on a DNA-coated surface. | (Choi et al. 2005) |
| Epoxy | PMA/NPMA | Serological tuberculosis assay. | (Tong et al. 2005) |
| NAPPA | PMA/AMA | Cell-free protein expression directly on microarray, subsequent to capture antibodies. With this interaction studies of recombinant human proteins. | (Ramachandran et al. 2004) |
| NC FAST | AMA | Protein levels from different cell types were analyzed on AMA containing 224 different antibodies. Detection of 3 ng/ml Caspase 9. | (Kopf et al. 2005) |
| NC FAST | PMA | First bacterial protein microarray used for N. meningitidis serum screening. | (Steller et al. 2005) |
| NC FAST | AMA | Analysis of antibody specificities on yeast proteome arrays. | (Michaud et al. 2003) |
| NC FAST | RPMA | Signal transduction analysis of cell culture lysates. Spot size 400 μm. | (Chan et al. 2004) |
| NC | NPMA | Lipopolysaccharide spotted on NC to detect bacterial species-specific antibodies. | (Thirumalapura et al. 2005) |
| Ni-coated | PMA | 5,800 yeast proteins spotted for interaction screen with other proteins and phospholipids. | (Zhu et al. 2001) |
| Octyltri-chlorosilane | PMA | Protein binding via leucine zipper with UV crosslinking. | (Zhang et al. 2005) |
| Polyacrylamide | PMA | First plant protein microarray used for antibody reactions. | (Kersten et al. 2003) |
| Poly-l-Lysine | AMA/PMA | Investigation of 115 antibody/antigen pairs. | (Haab et al. 2001) |
| PS modified | AMA | Comparison study of some monoclonal or polyclonal antibodies on different surfaces. | (Angenendt et al. 2002) |
| Silicon | AMA | Anti rabbit IgG was spotted and detected with rabbit IgG-FITC. They reach by small spots sizes (55 μm) a theoretical density of 4,400 spots/cm2. | (Ressine et al. 2003) |
| Sol–Gel | AMA | Detection of a pathogenic E. coli strain using specific antibodies. | (Lee et al. 2005) |
| Streptavidin | AMA | Orientated binding of antibodies and Fab fragments on streptavidin-coated AMA. Theoretical density of 10,000 spots/cm2. | (Peluso et al. 2003) |
Here, some surfaces are listed with their classification and applications. NC nitrocellulose (Super Nitro, Telechem International, USA); NCFAST nitrocellulose coated FAST slides (Schleicher&Schuell, Germany); PS polystyrol; PMA protein microarray; AMA antibody microarray; RPMA reverse phase protein microarray; NPMA non-protein microarray; NAPPA nucleic acid programmable protein array
To find a surface suitable for all spotted components in a project is a major challenge, and there is more than one recommendation to use a distinct surface, and only few publications are dealing with this aspect, globally. Most investigations are driven by a discrete scientific problem, and for this problem-optimized conditions for this special approach have been found to obtain results. From this reason, available data are often not conferrable because they are different in the spotted material (e.g., different proteins), in analytes (diverse sera, proteins), and are spotted on different surfaces, and processed under different conditions. However, the few systematic studies for finding optimal processing conditions and suitable surfaces (Angenendt et al. 2002, 2003a,b; Gutmann et al. 2005) are often without a study-driven relevance.
Orientation of spotted components
A further aspect is the orientation of spotted compounds. For some applications where sufficient amounts of reactive parts (e.g., antigenic epitopes or proteins on the surface of a spotted microorganism) are present, this aspect is of less relevance. For others, like quantitative analysis, enzymatic reactions, interaction studies, phosphorylations, or protein drug screens, it becomes of essential relevance because reactive sites of proteins are usually limited.
The effect of randomly vs specifically oriented capture agents based on both full-sized antibodies and Fab fragments on two types of streptavidin-coated slides was studied (Peluso et al. 2003). The orientation of capture agents increases the analyte-binding capacity up to 10-fold. By using surface plasmon resonance analysis, a dense monolayer of 90% active Fab fragments has been detected (Peluso et al. 2003).
This aspect has also been studied for enzymatic activity because activity can be completely lost or lowered (Cha et al. 2005). A different approach is the linking of proteins to DNA-coated arrays mediated from PCR-amplified DNA to a GAL4 DNA binding domain (Choi et al. 2005).
A new method for stable binding of proteins to a surface has recently been developed by linking proteins via a Leucine zipper (Zhang et al. 2005). Therefore an elastin mimetic domain is covalently bound to an Octyltrichlorosilane surface by a photoreactive amino acid and UV irradiation, whereas the target protein is fused to the complementary leucine zipper helix. The strong noncovalent binding of the helices leads to effective immobilizing of the target protein.
Sensitivity and detection limit
Sensitivity and specificity are also very important features which are linked to other parameters like signal to noise ratio, background, and binding of (serum)–proteins to the background. These parameters are not only influenced by the surface; processing and detection also affect them. The detection limit is a good way to measure parameter, but is in most cases only a value for a certain couple of action partners (e.g., protein-antibody), and often detected only for an individual experimental setup.
Conventional detection methods for microarrays often use fluorescent dyes, which were detected by irradiation from a laser scanner. Detection methods are discussed intensively elsewhere (Espina et al. 2004; Feilner et al. 2004), while in this study, only new approaches should be pointed out to reach much higher sensitivities. With a special method called MIST (multiple spotting technique) (Angenendt et al. 2003a,b), multiple spotting steps are performed successively to one position. In such an experiment, protein is spotted in the first spotting round, and after washing and blocking steps, the corresponding antibody was spotted in the second spotting round to the same position. By this technique, it is possible to perform an enzymatic reaction and reach with this approach an extremely high sensitivity of 60 ymol (10−24 mol) (Angenendt et al. 2005) because chemical reactions take place in small “reaction compartments” formed by droplets. Normally enzymatic reactions, producing soluble products, were not possible on conventional protein microarrays, as products could mix on the planar surface.
A further technical approach for better sensitivity is the rolling circle amplification (RCA) (Lizardi et al. 1998). The system requires the direct conjugation of the proteins to be analyzed with a label (e.g., biotin or digoxigenin). This label was detected by an antibody, which is bound to a primer. After hybridization of a circular DNA molecule, the primer is extended by a polymerase. For detection, fluorescently labeled oligonucleotides complementary to the elongated DNA were used. AMAs consisting of 84 distinct antibodies specific to serum proteins were used with detection via RCA, for protein expression profiling of lung cancer patients (Gao et al. 2005).
Stability of spotted compounds
Very important for use, e.g., in diagnosis is the stability of diagnostic protein microarray test systems. Two kinds of stability have to be distinguished: one is the stability of the surface itself, and the other is the stability of spotted proteins on these. In a stability analysis, different antibodies were spotted on four different surfaces and stored under two different conditions (4°C, dried or in blocking solution) over 8 weeks, without crucial differences (Angenendt et al. 2002). A shelf life was detected of more than 1 year at 2–8°C for a RheumaChip, with 14 different autoantigens suitable for the simultaneous determination of the most significant autoantibodies associated with rheumatic diseases (Schulte-Pelkum et al. 2005).
Multiplexing
With protein microarrays, multiple (up to several thousands) spotted compounds can be analyzed, but in most applications only with one single analyte or a mixture of few at one time. With multiplexing approaches, multiple analytes should also be investigated at the same time. Multiplexing is an aspect not important for all approaches, but for approaches where many probes should be screened (e.g., medical routine diagnosis), it becomes of tremendous importance. Recently, the aspect of multiplexing has been discussed in detail (Kersten et al. 2005), pointing out two major strategies: One is the multiplexing by different fluorophores, but this approach enables multiplexing with the limitation of detectable labels on the microarray. This approach could be performed better with nonarray systems, like the LiquiChip system of Qiagen. The second approach is to perform multiplexing by compartmentation, with physical compartments as multiwell slides (from different manufactures) or nonphysical compartmentation by MIST (Angenendt et al. 2003a,b, 2004, 2005).
Microarrays and microorganisms
Besides the general applications of protein microarrays mentioned above, there are only a few publications dealing with microorganisms. In this section, some exemplary publications in the field of microbiology are presented, dealing with protein microarrays and possible detection methods are summarized in Fig. 1.
Fig. 1.
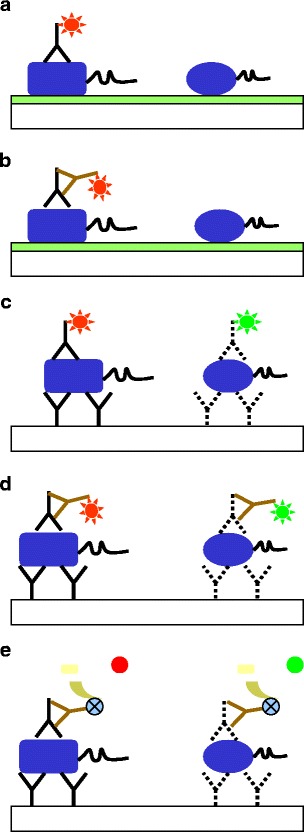
Possible identification of microorganisms. Microorganisms (dark blue symbols) will be fixed directly to coated microarrays (possible species-specific monosaccharides; light green in a and b). Alternatively, microorganisms can be bound via species-specific antibodies (black Ys in c–e). Direct detection with species-specific labeled antibodies (black Ys in a and c), sandwich assays with species-specific antibodies (black Ys), and secondary labeled antibodies (brown Ys with red or green stars in b and d) could be performed. With the MIST technology (Angenendt et al. 2003a,b), detection could be performed by using an antibody bound enzyme (blue circle bound to Ys) which activity converts a substrate (yellow) to a detectable product (green or red circle in e)
The first PMA used for serodiagnosis of infectious disease contained a dilution row of human IgG (2–50 pg) and IgM (0.4–8 pg), and antigens (50 pg) from Toxoplasma gondii, rubella virus, cytomegalovirus, and herpes simplex virus type I and II spotted on amino–silane-activated glass slides (Mezzasoma et al. 2002). The analysis showed a linear detection of internal calibration curves (IgG and IgM) and antigens, respectively, and results similar to ELISA analysis.
In my group, we have performed serum-screening experiments with serum probes from Meningitis patients on PMAs with 67 different recombinant Neisseria proteins. From these proteins coding from phase variable genes, 47 showed an immune response to at least one out of 20 patient sera. Nine proteins elicited an immune response in more than three patients, and the phase-variable opacity protein OpaV, showing responses in 11 patient sera. This is also an interesting aspect as this protein is annotated as a hypothetical protein with an authentic frameshift from the Neisseria genome. So protein microarray technology could be used to verify predictions from genome data (Steller et al. 2005).
Another bacterial PMA containing about 150 Yersinia pestis proteins was used to profile antibody responses in immunized rabbits. In this study, antibodies to 50 proteins could be detected. Out of these 11 proteins are F1 and V antigens to which the predominant antibody responses occurred. These identified targets could be used for further evaluation as candidates for vaccines and as diagnostic markers (Li et al. 2005).
PMAs for serum screening of bacterial proteins have been established as shown above, and the use of viral proteins instead of bacterial proteins is technically not a difference, and could be used for identification and classification of viruses.
Recently, PMAs were used for investigation of a coronavirus, the severe acute respiratory syndrome coronavirus (SARS-CoV). An important step in the development of vaccines and of diagnostic markers is to profile the antibodies to individual proteins. In this study, 13 recombinant proteins associated with structural and putative uncharacterized proteins of the SARS-CoV were prepared and used for the generation of SARS-CoV PMA. These were subsequently screened with SARS patient sera for their IgG antibodies, and antibody reactions to some of these proteins could be observed and found in one immunogenic protein as a good vaccine candidate. Moreover, they identified potential diagnostic markers and could support a hypothesis for some of the proteins to be synthesized during virus cycle deduced from the presence of antibodies against these (Qiu et al. 2005).
A PMA/NPMA array, consisting of 54 different Mycobacterium tuberculosis antigens, was used for a serological tuberculosis (TB) assay (Tong et al. 2005). This array consists of recombinant antigens, purified polysaccharides and lipopolysaccharides, oligosaccharides bound to bovine serum albumin, and antigens from fractionation of M. tuberculosis cells and culture fluids, spotted on epoxy-coated slides. This array could be stored at 4°C for 7 weeks. One antigen, Ara6-BSA, discriminated well between TB and non-TB, whereas most antigens gave poor discrimination, due to E. coli protein contamination or lack of glycosylation in E. coli.
Conclusions
In this review, protein microarray technology is presented from the beginning to recent applications. Protein microarrays were divided in the major groups of PMA, RPMA and AMA, and the NPMA approaches. Limitations of protein microarray technology and possible solutions are discussed, and the use of protein microarrays for studying microorganisms is pointed out.
Until now, there are only limited advances in the identification of microorganisms using protein microarrays. A major limitation is, until now, the high detection limit that could be reached, but there has been some new technological advancement. Fortunately, there are some encouraging approaches in indirect identification of pathogenic interactions by identifying serum antibodies. But this is only the matter of time that protein microarrays and, here especially, antibody-microarrays will be used for identification. This will be done first for in vitro models, but the most important are the identification of pathogens in medical samples (e.g., stool, blood, other body fluids) and from environmental sources (e.g., soil, air filtrates). These two fields will benefit from the addressed problems from the personalized medicine as well as from aspects coming from bioterrorism. So, one could expect in the near future that protein microarrays will be established for many applications in microbiological investigation.
Acknowledgements
The author thanks Zoltán Konthur for the helpful discussion and critical reading of the manuscript. The author wants to thank the Deutsche Forschungsgemeinschaft (DFG SFB TR 19).
References
- Angenendt P, Glokler J, Murphy D, Lehrach H, Cahill DJ. Toward optimized antibody microarrays: a comparison of current microarray support materials. Anal Biochem. 2002;309:253–260. doi: 10.1016/s0003-2697(02)00257-9. [DOI] [PubMed] [Google Scholar]
- Angenendt P, Glokler J, Konthur Z, Lehrach H, Cahill DJ. 3D protein microarrays: performing multiplex immunoassays on a single chip. Anal Chem. 2003;75:4368–4372. doi: 10.1021/ac034260l. [DOI] [PubMed] [Google Scholar]
- Angenendt P, Glokler J, Sobek J, Lehrach H, Cahill DJ. Next generation of protein microarray support materials: evaluation for protein and antibody microarray applications. J Chromatogr A. 2003;1009:97–104. doi: 10.1016/s0021-9673(03)00769-6. [DOI] [PubMed] [Google Scholar]
- Angenendt P, Wilde J, Kijanka G, Baars S, Cahill DJ, Kreutzberger J, Lehrach H, Konthur Z, Glokler J. Seeing better through a MIST: evaluation of monoclonal recombinant antibody fragments on microarrays. Anal Chem. 2004;76:2916–2921. doi: 10.1021/ac035357a. [DOI] [PubMed] [Google Scholar]
- Angenendt P, Lehrach H, Kreutzberger J, Glokler J. Subnanoliter enzymatic assays on microarrays. Proteomics. 2005;5:420–425. doi: 10.1002/pmic.200400955. [DOI] [PubMed] [Google Scholar]
- Bussow K, Cahill D, Nietfeld W, Bancroft D, Scherzinger E, Lehrach H, Walter G. A method for global protein expression and antibody screening on high-density filters of an arrayed cDNA library. Nucleic Acids Res. 1998;26:5007–5008. doi: 10.1093/nar/26.21.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha T, Guo A, Zhu XY. Enzymatic activity on a chip: the critical role of protein orientation. Proteomics. 2005;5:416–419. doi: 10.1002/pmic.200400948. [DOI] [PubMed] [Google Scholar]
- Chan SM, Ermann J, Su L, Fathman CG, Utz PJ. Protein microarrays for multiplex analysis of signal transduction pathways. Nat Med. 2004;10:1390–1396. doi: 10.1038/nm1139. [DOI] [PubMed] [Google Scholar]
- Choi YS, Pack SP, Yoo YJ. Development of a protein microarray using sequence-specific DNA binding domain on DNA chip surface. Biochem Biophys Res Commun. 2005;329:1315–1319. doi: 10.1016/j.bbrc.2005.01.167. [DOI] [PubMed] [Google Scholar]
- Cretich M, Pirri G, Damin F, Solinas I, Chiari M. A new polymeric coating for protein microarrays. Anal Biochem. 2004;332:67–74. doi: 10.1016/j.ab.2004.05.041. [DOI] [PubMed] [Google Scholar]
- Delehanty JB, Ligler FS. A microarray immunoassay for simultaneous detection of proteins and bacteria. Anal Chem. 2002;74:5681–5687. doi: 10.1021/ac025631l. [DOI] [PubMed] [Google Scholar]
- Disney MD, Seeberger PH. The use of carbohydrate microarrays to study carbohydrate-cell interactions and to detect pathogens. Chem Biol. 2004;11:1701–1707. doi: 10.1016/j.chembiol.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Duburcq X, Olivier C, Malingue F, Desmet R, Bouzidi A, Zhou F, Auriault C, Gras-Masse H, Melnyk O. Peptide-protein microarrays for the simultaneous detection of pathogen infections. Bioconjug Chem. 2004;15:307–316. doi: 10.1021/bc034226d. [DOI] [PubMed] [Google Scholar]
- Ekins RP. Multi-analyte immunoassay. J Pharm Biomed Anal. 1989;7:155–168. doi: 10.1016/0731-7085(89)80079-2. [DOI] [PubMed] [Google Scholar]
- Espina V, Woodhouse EC, Wulfkuhle J, Asmussen HD, Petricoin EF, 3rd, Liotta LA. Protein microarray detection strategies: focus on direct detection technologies. J Immunol Methods. 2004;290:121–133. doi: 10.1016/j.jim.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Feilner T, Hultschig C, Lee J, Meyer S, Immink RG, Koenig A, Possling A, Seitz H, Beveridge A, Scheel D, Cahill DJ, Lehrach H, Kreutzberger J, Kersten B. High-throughput identification of potential Arabidopsis MAP kinases substrates. Mol Cell Proteomics. 2005;4:1558–1568. doi: 10.1074/mcp.M500007-MCP200. [DOI] [PubMed] [Google Scholar]
- Feilner T, Kreutzberger J, Niemenn B, Kramer A, Possling A, Seitz H, Kersten B. Proteomic studies using microarrays. Current Proteomics. 2004;1:283–295. [Google Scholar]
- Fleischmann RD, Adams MD, White O, Clayton RA, Kirkness EF, Kerlavage AR, Bult CJ, Tomb JF, Dougherty BA, Merrick JM, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- Gao WM, Kuick R, Orchekowski RP, Misek DE, Qiu J, Greenberg AK, Rom WN, Brenner DE, Omenn GS, Haab BB, Hanash SM. Distinctive serum protein profiles involving abundant proteins in lung cancer patients based upon antibody microarray analysis. BMC Cancer. 2005;5:110. doi: 10.1186/1471-2407-5-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann O, Kuehlewein R, Reinbold S, Niekrawietz R, Steinert CP, de Heij B, Zengerle R, Daub M. Fast and reliable protein microarray production by a new drop-in-drop technique. Lab Chip. 2005;5:675–681. doi: 10.1039/b418765b. [DOI] [PubMed] [Google Scholar]
- Haab BB, Dunham MJ, Brown PO (2001) Protein microarrays for highly parallel detection and quantitation of specific proteins and antibodies in complex solutions. GenomeBiology 2:RESEARCH0004 [DOI] [PMC free article] [PubMed]
- Hultschig C, Kreutzberger J, Seitz H, Konthur Z, Bussow K, Lehrach H (2006) Recent advances of protein microarrays. Curr Opin Chem Biol (Epub 2005 Dec 20 ahead of print) [DOI] [PMC free article] [PubMed]
- Kersten B, Feilner T, Kramer A, Wehrmeyer S, Possling A, Witt I, Zanor MI, Stracke R, Lueking A, Kreutzberger J, Lehrach H, Cahilll DJ. Generation of Arabidopsis protein chips for antibody and serum screening. Plant Mol Biol. 2003;52:999–1010. doi: 10.1023/a:1025424814739. [DOI] [PubMed] [Google Scholar]
- Kersten B, Wanker EE, Hoheisel JD, Angenendt P. Multiplex approaches in protein microarray technology. Expert Rev Proteomics. 2005;2:499–510. doi: 10.1586/14789450.2.4.499. [DOI] [PubMed] [Google Scholar]
- Konthur Z, Hust M, Dubel S. Perspectives for systematic in vitro antibody generation. Gene. 2005;25(364):19–29. doi: 10.1016/j.gene.2005.05.042. [DOI] [PubMed] [Google Scholar]
- Kopf E, Shnitzer D, Zharhary D. Panorama Ab microarray cell signaling kit: a unique tool for protein expression analysis. Proteomics. 2005;5:2412–2416. doi: 10.1002/pmic.200401305. [DOI] [PubMed] [Google Scholar]
- Lee W, Park KS, Kim YW, Lee WH, Choi JW. Protein array consisting of sol–gel bioactive platform for detection of E. coli O157:H7. Biosens Bioelectron. 2005;20:2292–2299. doi: 10.1016/j.bios.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Lesaicherre ML, Uttamchandani M, Chen GY, Yao SQ. Developing site-specific immobilization strategies of peptides in a microarray. Bioorg Med Chem Lett. 2002;12:2079–2083. doi: 10.1016/s0960-894x(02)00379-7. [DOI] [PubMed] [Google Scholar]
- Li B, Jiang L, Song Q, Yang J, Chen Z, Guo Z, Zhou D, Du Z, Song Y, Wang J, Wang H, Yu S, Wang J, Yang R. Protein microarray for profiling antibody responses to Yersinia pestis live vaccine. Infect Immun. 2005;73:3734–3739. doi: 10.1128/IAI.73.6.3734-3739.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizardi PM, Huang X, Zhu Z, Bray-Ward P, Thomas DC, Ward DC. Mutation detection and single-molecule counting using isothermal rolling-circle amplification. Nat Genet. 1998;19:225–232. doi: 10.1038/898. [DOI] [PubMed] [Google Scholar]
- Lueking A, Horn M, Eickhoff H, Bussow K, Lehrach H, Walter G. Protein microarrays for gene expression and antibody screening. Anal Biochem. 1999;270:103–111. doi: 10.1006/abio.1999.4063. [DOI] [PubMed] [Google Scholar]
- Lueking A, Possling A, Huber O, Beveridge A, Horn M, Eickhoff H, Schuchardt J, Lehrach H, Cahill DJ. A nonredundant human protein chip for antibody screening and serum profiling. Mol Cell Proteomics. 2003;2:1342–1349. doi: 10.1074/mcp.T300001-MCP200. [DOI] [PubMed] [Google Scholar]
- MacBeath G, Schreiber SL. Printing proteins as microarrays for high-throughput function determination. Science. 2000;289:1760–1763. doi: 10.1126/science.289.5485.1760. [DOI] [PubMed] [Google Scholar]
- Mezzasoma L, Bacarese-Hamilton T, Di Cristina M, Rossi R, Bistoni F, Crisanti A. Antigen microarrays for serodiagnosis of infectious diseases. Clin Chem. 2002;48:121–130. [PubMed] [Google Scholar]
- Michaud GA, Salcius M, Zhou F, Bangham R, Bonin J, Guo H, Snyder M, Predki PF, Schweitzer BI. Analyzing antibody specificity with whole proteome microarrays. Nat Biotechnol. 2003;21:1509–1512. doi: 10.1038/nbt910. [DOI] [PubMed] [Google Scholar]
- Mircean C, Shmulevich I, Cogdell D, Choi W, Jia Y, Tabus I, Hamilton SR, Zhang W. Robust estimation of protein expression ratios with lysate microarray technology. Bioinformatics. 2005;21:1935–1942. doi: 10.1093/bioinformatics/bti258. [DOI] [PubMed] [Google Scholar]
- Nadeau JH, Balling R, Barsh G, Beier D, Brown SD, Bucan M, Camper S, Carlson G, Copeland N, Eppig J, Fletcher C, Frankel WN, Ganten D, Goldowitz D, Goodnow C, Guenet JL, Hicks G, Hrabe de Angelis M, Jackson I, Jacob HJ, Jenkins N, Johnson D, Justice M, Kay S, Kingsley D, Lehrach H, Magnuson T, Meisler M, Poustka A, Rinchik EM, Rossant J, Russell LB, Schimenti J, Shiroishi T, Skarnes WC, Soriano P, Stanford W, Takahashi JS, Wurst W, Zimmer A. Sequence interpretation. Functional annotation of mouse genome sequences. Science. 2001;291:1251–1255. doi: 10.1126/science.1058244. [DOI] [PubMed] [Google Scholar]
- Nam MJ, Madoz-Gurpide J, Wang H, Lescure P, Schmalbach CE, Zhao R, Misek DE, Kuick R, Brenner DE, Hanash SM. Molecular profiling of the immune response in colon cancer using protein microarrays: occurrence of autoantibodies to ubiquitin C-terminal hydrolase L3. Proteomics. 2003;3:2108–2115. doi: 10.1002/pmic.200300594. [DOI] [PubMed] [Google Scholar]
- Ngundi MM, Taitt CR, McMurry SA, Kahne D, Ligler FS. Detection of bacterial toxins with monosaccharide arrays. Biosens Bioelectron. 2005;7:7. doi: 10.1016/j.bios.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofir K, Berdichevsky Y, Benhar I, Azriel-Rosenfeld R, Lamed R, Barak Y, Bayer EA, Morag E. Versatile protein microarray based on carbohydrate-binding modules. Proteomics. 2005;5:1806–1814. doi: 10.1002/pmic.200401078. [DOI] [PubMed] [Google Scholar]
- Paweletz CP, Charboneau L, Bichsel VE, Simone NL, Chen T, Gillespie JW, Emmert-Buck MR, Roth MJ, Petricoin IE, Liotta LA. Reverse phase protein microarrays which capture disease progression show activation of pro-survival pathways at the cancer invasion front. Oncogene. 2001;20:1981–1989. doi: 10.1038/sj.onc.1204265. [DOI] [PubMed] [Google Scholar]
- Peluso P, Wilson DS, Do D, Tran H, Venkatasubbaiah M, Quincy D, Heidecker B, Poindexter K, Tolani N, Phelan M, Witte K, Jung LS, Wagner P, Nock S. Optimizing antibody immobilization strategies for the construction of protein microarrays. Anal Biochem. 2003;312:113–124. doi: 10.1016/s0003-2697(02)00442-6. [DOI] [PubMed] [Google Scholar]
- Poetz O, Ostendorp R, Brocks B, Schwenk JM, Stoll D, Joos TO, Templin MF. Protein microarrays for antibody profiling: specificity and affinity determination on a chip. Proteomics. 2005;5:2402–2411. doi: 10.1002/pmic.200401299. [DOI] [PubMed] [Google Scholar]
- Qiu M, Shi Y, Guo Z, Chen Z, He R, Chen R, Zhou D, Dai E, Wang X, Si B, Song Y, Li J, Yang L, Wang J, Wang H, Pang X, Zhai J, Du Z, Liu Y, Zhang Y, Li L, Wang J, Sun B, Yang R. Antibody responses to individual proteins of SARS coronavirus and their neutralization activities. Microbes Infect. 2005;7:882–889. doi: 10.1016/j.micinf.2005.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran N, Hainsworth E, Bhullar B, Eisenstein S, Rosen B, Lau AY, Walter JC, LaBaer J. Self-assembling protein microarrays. Science. 2004;305:86–90. doi: 10.1126/science.1097639. [DOI] [PubMed] [Google Scholar]
- Ressine A, Ekstrom S, Marko-Varga G, Laurell T. Macro-nanoporous silicon as a support for high-performance protein microarrays. Anal Chem. 2003;75:6968–6974. doi: 10.1021/ac034425q. [DOI] [PubMed] [Google Scholar]
- Sanger F, Coulson AR, Hong GF, Hill DF, Petersen GB. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982;162:729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Schulte-Pelkum J, Hentschel C, Kreutzberger J, Hiepe F, Schoessler W. A chip for the detection of antibodies in autoimmune diseases. Clin Lab Int. 2005;29:14–15. [Google Scholar]
- Steller S, Angenendt P, Cahill DJ, Heuberger S, Lehrach H, Kreutzberger J. Bacterial protein microarrays for identification of new potential diagnostic markers for Neisseria meningitidis infections. Proteomics. 2005;5:2048–2055. doi: 10.1002/pmic.200401097. [DOI] [PubMed] [Google Scholar]
- Stoevesandt O, Elbs M, Kohler K, Lellouch AC, Fischer R, Andre T, Brock R. Peptide microarrays for the detection of molecular interactions in cellular signal transduction. Proteomics. 2005;5:2010–2017. doi: 10.1002/pmic.200401095. [DOI] [PubMed] [Google Scholar]
- Sukhanov S, Delafontaine P. Protein chip-based microarray profiling of oxidized low density lipoprotein-treated cells. Proteomics. 2005;5:1274–1280. doi: 10.1002/pmic.200400985. [DOI] [PubMed] [Google Scholar]
- Thirumalapura NR, Morton RJ, Ramachandran A, Malayer JR. Lipopolysaccharide microarrays for the detection of antibodies. J Immunol Methods. 2005;298:73–81. doi: 10.1016/j.jim.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Tong M, Jacobi CE, van de Rijke FM, Kuijper S, van de Werken S, Lowary TL, Hokke CH, Appelmelk BJ, Nagelkerke NJ, Tanke HJ, van Gijlswijk RP, Veuskens J, Kolk AH, Raap AK. A multiplexed and miniaturized serological tuberculosis assay identifies antigens that discriminate maximally between TB and non-TB sera. J Immunol Methods. 2005;301:154–163. doi: 10.1016/j.jim.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Uetz P, Giot L, Cagney G, Mansfield TA, Judson RS, Knight JR, Lockshon D, Narayan V, Srinivasan M, Pochart P, Qureshi-Emili A, Li Y, Godwin B, Conover D, Kalbfleisch T, Vijayadamodar G, Yang M, Johnston M, Fields S, Rothberg JM. A comprehensive analysis of protein–protein interactions in Saccharomyces cerevisiae. Nature. 2000;403:623–627. doi: 10.1038/35001009. [DOI] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Kodira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Gabor Miklos GL, Nelson C, Broder S, Clark AG, Nadeau J, McKusick VA, Zinder N, Levine AJ, Roberts RJ, Simon M, Slayman C, Hunkapiller M, Bolanos R, Delcher A, Dew I, Fasulo D, Flanigan M, Florea L, Halpern A, Hannenhalli S, Kravitz S, Levy S, Mobarry C, Reinert K, Remington K, Abu-Threideh J, Beasley E, Biddick K, Bonazzi V, Brandon R, Cargill M, Chandramouliswaran I, Charlab R, Chaturvedi K, Deng Z, Di Francesco V, Dunn P, Eilbeck K, Evangelista C, Gabrielian AE, Gan W, Ge W, Gong F, Gu Z, Guan P, Heiman TJ, Higgins ME, Ji RR, Ke Z, Ketchum KA, Lai Z, Lei Y, Li Z, Li J, Liang Y, Lin X, Lu F, Merkulov GV, Milshina N, Moore HM, Naik AK, Narayan VA, Neelam B, Nusskern D, Rusch DB, Salzberg S, Shao W, Shue B, Sun J, Wang Z, Wang A, Wang X, Wang J, Wei M, Wides R, Xiao C, Yan C, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Wingren C, Steinhauer C, Ingvarsson J, Persson E, Larsson K, Borrebaeck CA. Microarrays based on affinity-tagged single-chain Fv antibodies: sensitive detection of analyte in complex proteomes. Proteomics. 2005;5:1281–1291. doi: 10.1002/pmic.200401009. [DOI] [PubMed] [Google Scholar]
- Zhang K, Diehl MR, Tirrell DA. Artificial polypeptide scaffold for protein immobilization. J Am Chem Soc. 2005;127:10136–10137. doi: 10.1021/ja051457h. [DOI] [PubMed] [Google Scholar]
- Zhu H, Bilgin M, Bangham R, Hall D, Casamayor A, Bertone P, Lan N, Jansen R, Bidlingmaier S, Houfek T, Mitchell T, Miller P, Dean RA, Gerstein M, Snyder M. Global analysis of protein activities using proteome chips. Science. 2001;293:2101–2105. doi: 10.1126/science.1062191. [DOI] [PubMed] [Google Scholar]


