Abstract
Purpose
To examine whether, in an adult intensive care unit (ICU), procalcitonin or C-reactive protein (CRP) levels discriminated between 2009 H1N1 influenza infection and community-acquired pneumonia of bacterial origin.
Methods
A retrospective observational study performed at an Australian hospital over a 4-month winter period during the 2009 H1N1 influenza pandemic. Levels on admission of procalcitonin and CRP were compared between patients admitted to the ICU with community-acquired pneumonia of bacterial and 2009 H1N1 origin.
Results
Compared to those with bacterial or mixed infection (n = 9), patients with 2009 H1N1 infection (n = 16) were significantly more likely to have bilateral chest X-ray infiltrates, lower APACHE scores, more prolonged lengths of stay in ICU and lower white cell count, procalcitonin and CRP levels. Using a cutoff of >0.8 ng/ml, the sensitivity and specificity of procalcitonin for detection of patients with bacterial/mixed infection were 100 and 62%, respectively. A CRP cutoff of >200 mg/l best identified patients with bacterial/mixed infection (sensitivity 100%, specificity 87.5%). In combination, procalcitonin levels >0.8 ng/ml and CRP >200 mg/l had optimal sensitivity (100%), specificity (94%), negative predictive value (100%) and positive predictive value (90%). Receiver-operating characteristic curve analysis suggested the diagnostic accuracy of procalcitonin may be inferior to CRP in this setting.
Conclusions
Procalcitonin measurement potentially assists in the discrimination between severe lower respiratory tract infections of bacterial and 2009 H1N1 origin, although less effectively than CRP. Low values, particularly when combined with low CRP levels, suggested bacterial infection, alone or in combination with influenza, was unlikely.
Keywords: Procalcitonin, C-reactive protein, 2009 H1N1, Influenza
Introduction
Within months of the detection of 2009 H1N1 influenza virus in North America, Australia experienced the rapid spread of the virus within the community. The burden of disease on hospitals and in particular intensive care units (ICU) was considerable [1]. Epidemiological and animal model data suggest that 2009 H1N1 may be more virulent than seasonal strains of influenza [2].
Procalcitonin is a novel biomarker with potential use for diagnostic and prognostic purposes. Under normal physiological conditions, its release from the thyroid gland assists in maintenance of calcium homeostasis. By contrast, release of lipopolysaccharide and cytokines (e.g. TNF alpha, interleukin-1, interleukin-6) during bacterial infection upregulate its expression from a variety of sites, including the liver, kidney and monocytes [3]. Compared to clinical features, radiological findings or traditional markers such as white cell count (WCC) or C-reactive protein (CRP), procalcitonin measurement may be a better means of distinguishing between viral and bacterial infections [4], and hence the need for antibiotic therapy [5]. However, in the respiratory setting, studies to date have focused on children [6] and less severe disease [7], and included few patients with influenza [8].
This study aimed to examine whether, on admission to an adult ICU, procalcitonin levels and/or CRP discriminated between 2009 H1N1 influenza infection and community-acquired pneumonia of bacterial origin.
Methods
The study was conducted in a 23-bed ICU at a 600-bed tertiary hospital in Western Australia. The study design was a retrospective observational study. All investigations were carried out as part of the routine clinical management of the patients, and patients were not identified. Ethics approval was not required. All patients admitted to the ICU between 10 May and 9 September 2009 with community-acquired pneumonia were considered for the study. The comparison was then limited to those with a microbiologically confirmed aetiology of 2009 H1N1 (positive viral culture or polymerase chain reaction performed on a respiratory specimen) or bacterial infection (culture of a recognised pathogen from blood stream and/or lower respiratory tract specimen, or seroconversion to Mycoplasma/Chlamydophila/Legionella). Patients with mixed 2009 H1N1 and bacterial infection were grouped together with those with bacterial infection. Demographic and clinical data from the first 24 h admission to ICU were collected from an existing database. Procalcitonin and CRP measurements were routinely performed at least twice weekly. Procalcitonin measurements were performed using a VIDAS BRAHMS enzyme-linked fluorescent assay (bioMerieux, France). The interpretative cutoff for bacterial sepsis is considered to be greater than 0.5–2 ng/ml in most studies [9]. CRP measurement was performed using an Architect ci16200 latex-enhanced immunoturbidimetric assay (Abbott Diagnostics, Abbott Park, IL). During the study period the empiric management of all patients admitted to the ICU with suspected pneumonia (bacterial or viral) included anti-bacterial therapy.
The Fisher’s exact test was used to compare proportions for categorical variables. For continuous variables, Student's t test and Mann-Whitney U test were used for comparing parametric and non-parametric data, respectively. Receiver-operator characteristic (ROC) curve analysis was performed using GraphPad Prism version 5.02.
Results
During the study period, 25 patients were considered for analysis. One patient with 2009 H1N1 infection had mixed infection (blood culture positive for Streptococcus pneumoniae). Separation of the patients according to aetiology created two groups for comparison: 16 patients with 2009 H1N1 infection (2009 H1N1 group) and 9 with bacterial/mixed infection (bacterial/mixed group) (Table 1). Three patients within the 2009 H1N1 group remain in the ICU. Comparison of the 2009 H1N1 and bacterial/mixed groups revealed the 2009 H1N1 group was significantly more likely to have bilateral chest X-ray infiltrates, lower APACHE scores, lower median white cell counts and more prolonged lengths of stay in ICU.
Table 1.
Demographic, clinical and laboratory features of the two groups
| Clinical and laboratory features | 2009 H1N1 group (n = 16) | Bacterial/mixed group (n = 9) | P-value |
|---|---|---|---|
| Pathogen | 2009 H1N1 influenza virus | Streptococcus pneumonia (3), Staphylococcus aureus (2), Legionella pneumophilia (1), Legionella longbeachae (1), Streptococcus pyogenes (1), Haemophilus influenzae (1) | |
| Male | 8 (50%) | 5 (56%) | 1.0 |
| Age (years) (mean ± SD) | 42 ± 17 | 45 ± 18 | 0.61 |
| Pregnant | 4 (25%) | 0 (0%) | 0.26 |
| Significant co-morbidities | 3 (17%) | 1 (11%) | 1.0 |
| APACHE score (mean ± SD) | 14.9 ± 4 | 20.8 ± 7 | 0.016a |
| Peak temperature (mean ± SD) | 38.0 ± 1 | 38.1 ± 1 | 0.83 |
| Bilateral (vs unilateral) infiltrate on chest X-ray | 16 (100%) | 4 (44%) | 0.002a |
| PaO2/FiO2 ratio (mean ± SD) | 102 ± 50 | 178 ± 162 | 0.169 |
| ECMO | 4 (25%) | 0 (0%) | 0.26 |
| Nitric oxide | 5 (31%) | 1 (11%) | 0.36 |
| Renal replacement therapy | 3 (19%) | 3 (33%) | 0.63 |
| Initial WCC (x 109/l) (median, IQR) | 6.8 (4.3–8.6) | 15.9 (5.7–40.0) | 0.02a |
| Initial C-reactive protein (mg/l) (median, IQR) | 103 (71–140) | 400 (270–425) | 0.0001a |
| Initial procalcitonin (ng/ml) (median, IQR) | 0.62 (0.21–5.9) | 13.0 (8.2–81.5) | 0.002a |
| Length of stay (ICU) (mean ± SD) | 18 ± 10 | 12 ± 16 | 0.006a |
| Death in ICU | 2 (12%) | 0 (0%) | 0.54 |
SD standard deviation, IQR inter-quartile range, APACHE acute physiologic and chronic health evaluation, ECMO extracorporeal membrane oxygenation
aSignificant difference
Following admission to ICU, the median day on which levels were first measured were day 2 [interquartile range (IQR) 1–3] for procalcitonin and day 1 (IQR 1–1) for CRP. Figure 1a, b demonstrates the initial procalcitonin and CRP values in the two groups on admission to ICU. In the 2009 H1N1 group, the median values for both procalcitonin (0.62 vs. 13.0 ng/ml, P = 0.002) and CRP (118 vs. 363 mg/l, P = 0.0001) were significantly lower than in the bacterial/mixed group. A cutoff of >0.8 ng/ml for procalcitonin best identified patients with bacterial/mixed infection [sensitivity 100%, specificity 62.5%, negative predictive value (NPV) 100%, positive predictive value (PPV) 60%]. For CRP a cutoff of >200 mg/l best identified patients with bacterial/mixed infection (sensitivity 100%, specificity 87.5%, NPV 100%, PPV 82%). Figure 2 presents the corresponding ROC curves for procalcitonin and CRP. The area under the ROC curve was 0.88 for procalcitonin [95% confidence interval (CI) 0.73–1.01] compared to 0.97 for CRP (CI 0.92–1.03). When used in combination, procalcitonin levels >0.8 ng/ml and CRP >200 mg/l had optimal sensitivity (100%), specificity (94%), NPV (100%) and PPV (90%) for the detection of patients with bacterial/mixed infection.
Fig. 1.
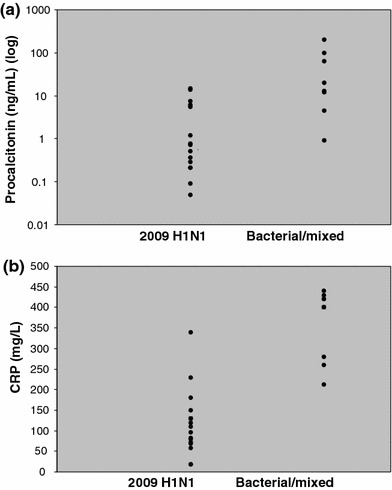
a Procalcitonin values on admission to ICU; b CRP values on admission to ICU
Fig. 2.
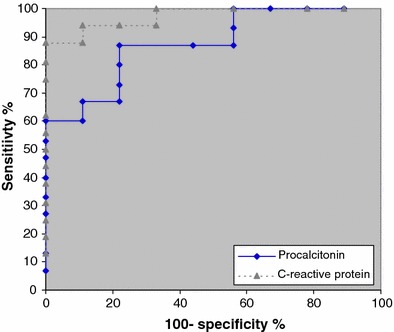
ROC curves of procalcitonin and CRP for the detection of patients with bacterial/mixed infection on admission to ICU
The two patients within the 2009 H1N1 group who died had higher mean procalcitonin values on admission to ICU compared to those who survived (14.5 vs. 1.7 ng/ml, P < 0.0001).
Discussion
Experience using biomarkers as diagnostic and prognostic adjuncts during influenza pandemics is lacking. Studies describing the ability of procalcitonin or CRP to discriminate between viral and bacterial infections have included few patients with influenza or severe disease, limiting their applicability to the scenarios currently faced by intensivists. Animal models using the avian H5N1 or recombinant 1918 H1N1 virus have shown dysregulated cytokine production to be a feature during severe infection with these more virulent strains of influenza [10]. Although the interplay between cytokine production and procalcitonin is complex [11], our findings suggest that increased expression of this biomarker appears not to occur despite significant viral associated pneumonitis, and on occasions carditis and myositis. Interestingly, in a small case series from Singapore [12], patients ventilated because of severe SARS coronavirus infections demonstrated procalcitonin levels of <1.0 ng/ml, comparable to the results of our study.
The diagnostic utility of procalcitonin compared to CRP is disputed. A recent meta-analysis concluded procalcitonin was more accurate than CRP for the distinction between viral and bacterial infections [13]; however, performance may vary depending on microbial factors [14], severity of disease [8], clinical syndrome [13] and the cutoffs used [13]. In our study setting, when appropriate cutoffs were applied, both markers had potentially useful negative predictive values; however, the lower specificity of procalcitonin favoured CRP for identification of patients with bacterial/mixed infection. Contrary to previous attempts to use combinations of biomarkers to improve differentiation between viral and bacterial pneumonia [15], we found the combination of procalcitonin and CRP improved the distinction between the two groups.
Our data suggested that 2009 H1N1-infected patients with higher procalcitonin levels had a worse prognosis. In the ICU setting, procalcitonin is known to have prognostic value [11] and, when combined with clinical judgement, may allow earlier cessation of antibiotic therapy [16]. Given the tendency to administer both anti-viral and anti-bacterial therapy to patients infected with 2009 H1N1, additional studies are required to determine whether low procalcitonin levels could safely be used to reduce antibiotic use in this setting.
Small sample sizes and the retrospective nature of our study necessitate cautious interpretation of our results. We also acknowledge that, by limiting our study to those in whom a microbiologically confirmed viral or bacterial diagnosis was made, we may have created a bias towards two more easily discernible groups. Finally, an assessment of the relative value of biomarkers may have been enhanced by knowledge of their kinetics: a property known to favour procalcitonin over CRP [17].
In conclusion, our study suggests that in adults, measurement of procalcitonin levels assists in the discrimination between severe lower respiratory tract infection of bacterial and 2009 H1N1 origin, although less effectively than CRP. Low values, particularly when combined with low CRP values, suggest that bacterial infection, either alone or in combination with influenza, is unlikely. When combined with clinical judgement, this potentially identifies a group of patients in whom anti-bacterial therapy may be withheld.
Acknowledgments
We wish to thank Brigit Roberts (research coordinator) for her assistance with data collection.
References
- 1.ANZIC Influenza Investigators. Webb SA, Pettilä V, Seppelt I, Bellomo R, Bailey M, Cooper DJ, Cretikos M, Davies AR, Finfer S, Harrigan PW, Hart GK, Howe B, Iredell JR, McArthur C, Mitchell I, Morrison S, Nichol AD, Paterson DL, Peake S, Richards B, Stephens D, Turner A, Yung M. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361:1991–1993. doi: 10.1056/NEJMe0909666. [DOI] [PubMed] [Google Scholar]
- 2.Munster VJ, de Wit E, van den Brand JM, Herfst S, Schrauwen EJ, Bestebroer TM, van de Vijver D, Boucher CA, Koopmans M, Rimmelzwaan GF, Kuiken T, Osterhaus AD, Fouchier RA. Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science. 2009;325:481–483. doi: 10.1126/science.1177127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niederman M. Biological markers to determine eligibility in trials for community-acquired pneumonia: a focus on procalcitonin. Clin Infect Dis. 2008;47:S127–S132. doi: 10.1086/591393. [DOI] [PubMed] [Google Scholar]
- 4.Erratum Clin Infect Dis. 2005;40:1386–1388. [Google Scholar]
- 5.Schuetz P, Christ-Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, Neidert S, Fricker T, Blum C, Schild U, Regez K, Schoenenberger R, Henzen C, Bregenzer T, Hoess C, Krause M, Bucher HC, Zimmerli W, Mueller B, ProHOSP Study Group Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections. JAMA. 2009;302:1059–1066. doi: 10.1001/jama.2009.1297. [DOI] [PubMed] [Google Scholar]
- 6.Gendrel D, Raymond J, Coste J, Moulin F, Lorrot M, Guérin S, Ravilly S, Lefèvre H, Royer C, Lacombe C, Palmer P, Bohuon C. Comparison of procalcitonin with C-reactive protein, interleukin 6 and interferon-alpha for differentiation of bacterial versus viral infections. Pediatr Infect Dis J. 1999;18:875–881. doi: 10.1097/00006454-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Masiá M, Gutiérrez F, Padilla S, Soldán B, Mirete C, Shum C, Hernández I, Royo G, Martin-Hidalgo A. Clinical characterisation of pneumonia caused by atypical pathogens combining classic and novel predictors. Clin Microbiol Infect. 2007;13:153–161. doi: 10.1111/j.1469-0691.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 8.Masiá M, Gutiérrez F, Shum C, Padilla S, Navarro JC, Flores E, Hernández I. Usefulness of procalcitonin levels in community-acquired pneumonia according to the patients outcome research team pneumonia severity index. Chest. 2005;128:2223–2229. doi: 10.1378/chest.128.4.2223. [DOI] [PubMed] [Google Scholar]
- 9.Tang BM, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis. 2007;7:210–217. doi: 10.1016/S1473-3099(07)70052-X. [DOI] [PubMed] [Google Scholar]
- 10.Baskin CR, Bielefeldt-Ohmann H, Tumpey TM, Sabourin PJ, Long JP, García-Sastre A, Tolnay AE, Albrecht R, Pyles JA, Olson PH, Aicher LD, Rosenzweig ER, Murali-Krishna K, Clark EA, Kotur MS, Fornek JL, Proll S, Palermo RE, Sabourin CL, Katze MG. Early and sustained innate immune response defines pathology and death in nonhuman primates infected by highly pathogenic influenza virus. Proc Natl Acad Sci. 2009;106:3455–3460. doi: 10.1073/pnas.0813234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christ-Cain M, Muller B. Biomarkers in respiratory tract infections: diagnostic guides to antibiotic prescription, prognostic markers and mediators. Eur Respir J. 2007;30:556–573. doi: 10.1183/09031936.00166106. [DOI] [PubMed] [Google Scholar]
- 12.Chua A, Lee K. Procalcitonin in severe acute respiratory syndrome (SARS) J Infect. 2004;48:303–306. doi: 10.1016/j.jinf.2004.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39:206–217. doi: 10.1086/421997. [DOI] [PubMed] [Google Scholar]
- 14.Moulin F, Raymond J, Lorrot M, Marc E, Coste J, Iniguez JL, Kalifa G, Bohuon C, Gendrel D. Procalcitonin in children admitted to hospital with community acquired pneumonia. Arch Dis Child. 2001;84:332–336. doi: 10.1136/adc.84.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korppi M. Non-specific host response markers in the differentiation between pneumococcal and viral pneumonia: what is the most accurate combination? Pediatr Int. 2004;46:545–550. doi: 10.1111/j.1442-200x.2004.01947.x. [DOI] [PubMed] [Google Scholar]
- 16.Nobre V, Harbarth S, Graf JD, Rohner P, Pugin J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients. Am J Respir Crit Care Med. 2008;177:498–505. doi: 10.1164/rccm.200708-1238OC. [DOI] [PubMed] [Google Scholar]
- 17.Boussekey N, Leroy O, Georges H, Devos P, d’Escrivan T, Guery B. Diagnostic and prognostic values of admission and procalcitonin levels in community-acquired pneumonia in an intensive care unit. Infection. 2005;33:257–263. doi: 10.1007/s15010-005-4096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


