Abstract
Lipid rafts are special microdomains in the plasma membrane. They are enriched in sphingolipids and cholesterol, playing critical roles in many biological processes. The purpose of this study is to analyze the requirement of cholesterol, a crucial component of lipid rafts for cell infection by pseudorabies virus (PrV). Cholesterol of plasma membrane or viral envelope was depleted with methyl-beta-cyclodextrin (MβCD), and the infectivity of three strains of PrV was determined with plaque assays. The effect of adding cholesterol to MβCD-treated cells and viruses on cell infection was analyzed. Furthermore, effect of post-adsorption cholesterol depletion on PrV infection was investigated. We show that cholesterol depletion of either the plasma membrane or the viral membrane by MβCD significantly impaired the infectivity of PrV strains Kaplan, Becker, and Bartha K-61. The virus was shown to have lower cholesterol content and to respond to lower MβCD concentrations. Exogenous cholesterol added to either MβCD-treated cells or virions partially restored the virus infectivity. Optimal PrV infection requires cholesterol in viral and plasma membranes.
Keywords: Cholesterol, Lipid Raft, Cholesterol Content, Canine Distemper Virus, Cholesterol Depletion
Introduction
Pseudorabies virus (PrV) is an enveloped virus and a member of alphaherpesviruses. It causes Aujeszky’s disease (AD) in its natural host (pig). In susceptible species, PrV infection is often fatal, and animals die from central nervous system disorders [10]. The PrV genome consists of linear, double-stranded, non-circularly permuted DNA molecules, and is closely similar to that of the prototypical herpes simplex virus (HSV). The PrV envelope contains at least 10 different viral glycoproteins. In PrV-infected cells, newly synthesized viral envelope glycoproteins travel from the endoplasmic reticulum via the Golgi network to the plasma membrane, rendering the cells recognizable for virus-specific antibodies [5].
Lipid rafts are special microdomains with distinct lipid and protein composition in the plasma membrane (PM). They are enriched in sphingolipids and cholesterol, and play a critical role in many biological processes [2, 21]. It has been documented that the lipid composition of envelopes from a variety of viruses is distinct from that of the host PM from which they are derived, leading to the hypothesis that viruses may bud from specific PM microdomains [18, 20].
Methyl-β-cyclodextrin (MβCD) is able to deplete the plasma membrane cholesterol and has been used to show the importance of cholesterol/lipid rafts for the entry of different viruses [3, 8, 16, 17, 19, 22]. Monospecific antibody-induced patching of some PrV glycoproteins indicated that the antibody-induced patches were enriched in monosialoganglioside GM1 (GM1), a marker of lipid raft microdomains, but were excluded for transferrin receptor, a non-raft marker, suggesting that these viral proteins associate with lipid rafts to a different extent. However, addition of MβCD only slightly reduced copatching efficiency between the different viral proteins, indicating that other factors, perhaps tegument–glycoprotein interactions, may be important for the observed copatching events [5]. Recently, it has been reported that cholesterol in the plasma membrane is required for efficient PrV entry [4]. Here, we confirm the importance of cholesterol in plasma membrane and extend these findings by (i) quantifying the decrease of the cholesterol content and (ii) by showing that three different strains are affected in the same way. Furthermore, we show for the first time that cholesterol depletion from the viral membrane is also detrimental for PrV infection.
Materials and Methods
Cells and Viruses
African green monkey kidney cells (Vero) and baby hamster kidney cells (BHK21) were maintained in Dulbecco Modified Eagle’s medium (DMEM) and Minimum Essential Medium (MEM), respectively, supplemented with 5% fetal calf serum (FCS, Excell Bio, Shanghai). PrV strain Bartha K-61 was available. The Kaplan and Becker strains of PrV were achieved by standard cell transfection procedure using Lipofectamine 2000 transfection reagent (Invitrogen, USA). The full-length genomes of PrV strains Kaplan and Becker were provided by Prof. Lynn Enquist and Ms. Marlies G. Eldridge, Princeton University, Princeton, USA. The PrV strains and vesicular stomatitis virus (VSV, strain Indiana) were propagated in Vero and BHK21 cells, respectively. All viruses used for the experiments were grown in serum-free medium.
Depletion and Replenishment of Cholesterol from Cells
In order to deplete cellular plasma membrane cholesterol, 2 × 105 cells diluted in 1 ml medium containing 5% FCS were seeded per well of 24-well plates and incubated in a CO2-incubator overnight. The next day, the cells were washed two times with phosphate-buffered saline (PBS) and incubated with serum-free DMEM in the absence (control cells) or presence (treated cells) of the indicated amounts of MβCD (Sigma, China) at 37°C. After 30 min, MβCD was removed by washing the cells three times with PBS. For cholesterol replenishment, the cells were treated with 12 mM MβCD, and the cholesterol-depleted cells were incubated with the indicated amounts of cholesterol (Sigma, China) to final concentrations ranging from 50 to 500 μM for 30 min at 37°C.
Depletion and Replenishment of Cholesterol from Virus
In order to extract viral cholesterol, aliquots of virus suspensions with a titer of 2 × 106 pfu/ml were treated with the indicated amounts of MβCD at 37°C for 30 min. For cholesterol replenishment, viral cholesterol was depleted with 1.2 mM MβCD, and then virions were incubated with cholesterol as described above.
Infection of Cells
Virus plaque assays were performed as previously described [11, 12, 23]. Briefly, to analyze the effect of plasma membrane cholesterol depletion on virus infection, MβCD-treated or mock-treated cells on 6-well plates were infected with 250 μl virus dilutions in medium at a multiplicity of infection (MOI) of 1 at 37°C for 1 h. Then, the cells were covered with 2.5 ml methylcellulose (Sigma, China) (1% (w/v) in medium). After incubation for 36–48 h, the overlaid medium was removed, and the cells were washed with PBS. After fixation with 3% paraformaldehyde in PBS (1 ml/well) for 30 min at room temperature, the cells were stained with 1% crystal violet (w/v) diluted in 5% ethanol in PBS for 20 min. After thorough washing, the virus titer in plaque-forming units (pfu) was calculated based on the recorded plaque number. In order to analyze the infection of cells after cholesterol replenishment, the cholesterol-replenished and non-replenished cells were washed three times with PBS, and then 250 μl of the virus suspensions at an MOI of 1 was added to the cells at 37°C for 1 h. Subsequently, cells were washed three times, overlaid with methylcellulose, and kept at 37°C for 48 h prior to plaque assays as above.
In order to analyze infection efficiency after cholesterol extraction of viruses, the cells were washed three times with PBS and then were infected with 250 μl MβCD-treated or mock-treated viruses, respectively, at 37°C for 1 h. The inoculums were diluted 103-fold to avoid adverse effects of MβCD on cells. In order to analyze infection efficiency after viral cholesterol replenishment, 250 μl cholesterol-replenished or non-replenished virus suspensions were used to infect cells at 37°C for 1 h. Then the cells were subjected to plaque assays as above.
Determination of Cholesterol
In order to determine the cholesterol content of plasma membranes, cells grown on 6-well plates were treated with MβCD at various concentrations as above. In parallel, confluent Vero cell monolayers were replenished with exogenous cholesterol, after they had been treated with 12 mM MβCD. Then, the cells were washed three times with PBS, trypsinized, and centrifuged at 1400 rpm at 4°C for 10 min. The cells were resuspended in PBS and subjected to cell counting. Equal cell numbers of the samples (3.3 × 104 cells/sample) were pelleted at 3000 rpm at 4°C for 5 min. Subsequently, the plasma cholesterol concentrations were determined with Amplex® Red Cholesterol Assay Kit (Molecular Probes, USA), according to the manufacturer’s instructions.
In order to determine viral cholesterol content, 1 ml PrV particles (2 × 107 pfu/ml) were sedimented by ultracentrifugation at 165,000×g at 4°C for 1 h. The pellets were suspended in 100 μl PBS. Seventy-five microliters of virus suspension was mixed with equal volume lysis buffer, incubated with 150 μl work solution, and subjected to the cholesterol assay as described above.
Effect of Post-Adsorption Cholesterol Depletion on the PrV Production
Cell monolayers in 12-well plates were washed with PBS and then infected with viruses at an MOI of 1 at 37°C. Then, 12 mM MβCD was added to the infected cells at 4, 8, 12, 16, 20, 24, 28, or 32 h post-infection. Mock-treated cells were used as control. Then, the cell supernatant was collected and subjected to plaque assays for determining the virus infection efficiency as above.
Results
Reduction of Cholesterol Content by MβCD
In order to determine the effect of cholesterol on virus infection, we depleted cholesterol from either the viral or the cellular membranes by MβCD. The efficiency of MβCD to deplete cholesterol from the plasma membrane is shown in Fig. 1a. MβCD treatment of cells resulted in a dose-dependent reduction of the cholesterol content in the plasma membrane. The amount of cholesterol was reduced by about 60% at a concentration of 12 mM MβCD. As shown in Fig. 1b, adding cholesterol to depleted cells resulted in an increase of the cholesterol content. At a concentration of 500 μM, the cholesterol values of the plasma membranes were similar to the values determined prior to MβCD treatment.
Fig. 1.
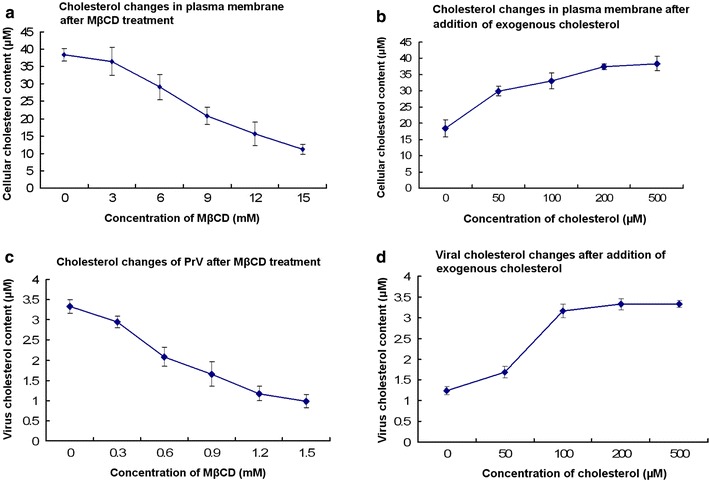
Determination of cholesterol. a After Vero cells were treated with MβCD of various concentrations, the cellular cholesterol content was determined with a colorimetric reaction based kit. b The recovery of cellular cholesterol was measured using the same kit. c After PrV was treated with MβCD of various concentrations, the viral cholesterol was determined with the cholesterol determination kit. d The MβCD-treated viruses were replenished with exogenous cholesterol and the viral cholesterol was measured. It is noted that all the viruses gave the similar results, and the representative results were from PrV Kaplan strain
MβCD was also effective in depleting cholesterol from the viral membrane. Incubation of PrV with the drug resulted in a dose-dependent decrease of the viral cholesterol content. At 1.2 mM, the amount of cholesterol in the virions was reduced by 65% (Fig. 1c). When cholesterol-depleted viruses were incubated with exogenous cholesterol, the viral cholesterol recovered to values measured prior to depletion by MβCD (Fig. 1d).
Importance of Cholesterol for PrV Infection
In order to determine the effect of plasma membrane cholesterol on virus infection, MβCD-treated cells were infected with three different strains of PrV. As shown in Fig. 2a, PrV infectivity was reduced in a dose-dependent manner. At a concentration of 12 mM MβCD, the infection rate was reduced by about 80%. There was no major difference between the three strains analyzed, Becker, Kaplan, and Bartha K-61. In contrast, infectivity of VSV was not decreased by cholesterol depletion. In order to confirm the importance of cellular cholesterol for PrV infection, we analyzed the effect of cholesterol replenishment on the susceptibility of the cells to infection. As shown in Fig. 2b, addition of cholesterol to depleted cells resulted in a dose-dependent increase of the infectivity values. At 500 μM cholesterol, the increase of the infectivity was about three- to four folds.
Fig. 2.
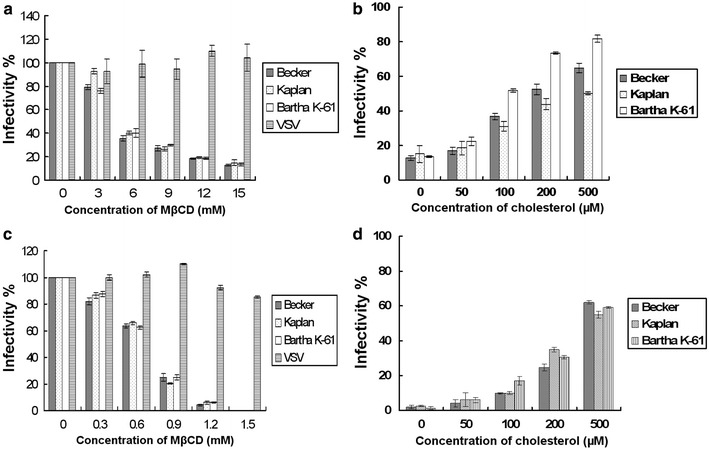
Importance of cholesterol on virus infection. Cells were treated with MβCD concentration range from 0 to 15 mM, and then cells were infected with three strains of PrV or VSV. The virus infection efficiency is shown in a. Vero cells treated with 12 mM MβCD were replenished with 50–500 μM exogenous cholesterol and then were subjected to PrV infection. The recovery of the viral infectivity is shown in b. The 100% infectivity values of PrV and VSV represent average plaque numbers of 300 and 150, respectively. Results are mean values of three independent experiments with standard deviation. PrV and VSV were treated with MβCD range from 0 to 1.5 mM, and the MβCD-treated viruses were used to infect cells. The infectivity of the viruses is provided in c. The PrV particles were treated with MβCD at the concentration of 1.2 mM and replenished with the exogenous cholesterol at the concentration ranging from 50 to 500 μM. d After replenishment, the infectivity of the viruses is determined. The 100% infectivity values of PrV and VSV represent average plaque numbers of 300 and 150, respectively
The effect of viral cholesterol on PrV infection is shown in Fig. 2c, and all three strains of PrV showed the same sensitivity to MβCD treatment. At a concentration of 1.2 mM, the viral infectivity was reduced by about 95%. The infectivity of control VSV was not affected by MβCD treatment significantly. In order to find out whether the effect of cholesterol depletion was reversible, virus was treated with 1.2 mM MβCD followed by the addition of exogenous cholesterol. As shown in Fig. 2d, replenishment of cholesterol resulted in an increase of the infectivity of MβCD-treated PrV. At a concentration of 500 μM exogenous cholesterol, viral infectivity recovered up to 60% value of that determined prior to cholesterol depletion.
Effect of Post-Adsorption Cholesterol Depletion on PrV Infection
The cholesterol in plasma membrane was depleted at various time points post-infection, and then the amount of infectious viruses released into culture supernatant was determined. The results showed that addition of MβCD to virus-infected cells did not decrease the virus titer determined in the medium (Fig. 3).
Fig. 3.
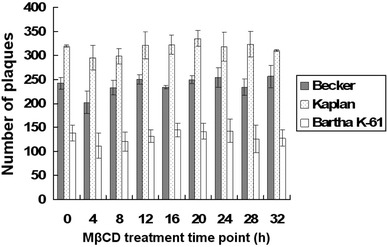
Effect of cholesterol depletion on the virus release. Vero cells were infected with viruses at an MOI of 1 at 37 C. Then, 12 mM MβCD was used to treat the infected cells at 4, 8, 12, 16, 20, 24, 28, or 32 h post-infection. Mock-treated cells were used as control. The number of plaques refers to the mean number of plaques of three wells
Discussion
For enveloped viruses, the fusion between the viral envelope and cellular membrane is a key step in the initiation of an infection. Cholesterol appears to play an important role in this process. The dependence of some viruses on cholesterol is restricted to either of the two membranes. Infectivities of influenza virus and canine distemper virus (CDV) are inhibited when cholesterol is depleted from the viral membrane [8, 24]. Infection by ecotropic murine leukemia virus is affected by depletion of cholesterol from the target cell [14]. In the case of human immunodeficiency virus (HIV), the infection is sensitive to variations in both the viral and PM cholesterol [13, 15]. The role of lipid rafts in cell infection by several herpesviruses including HSV, varicella-zoster virus (VZV), human herpesvirus 6 (HHV6), and Epstein-Barr virus (EBV) has been investigated [1, 6, 7, 9, 25]. These findings show that these viruses resemble HIV in that both viral and PM cholesterol are required for virus infection. Recently, it is shown that MβCD-mediated cholesterol depletion of PM specifically impairs entry of PrV [4]. We have confirmed this finding and extended it (i) by relatively quantifying the cholesterol changes induced by MβCD-treatment and (ii) by showing that cholesterol dependence is not a strain-dependent feature of PrV entry. PrV Bartha strain is not only deleted in gE, but also in gI and in Us9 and mutated in gC, and differs in the genetic background from the other two PrV strains. In our experiments, these strains showed similar sensitivities to MβCD treatment and cholesterol replenishment, indicating cholesterol depletion from either cells or viruses affects the infectivity of PrV. From these data and those reported by other groups, it appears that requirement for cholesterol is a general feature for herpesvirus entry. Cholesterol-containing membrane microdomains may serve as specialized platforms for the initiation of infection. They may be especially important for herpesviruses where a group of surface proteins interact to achieve attachment to and penetration into cells.
For the first time, we have relatively quantified the extent of cholesterol reduction that is associated with an impairment of the infection process. In this study, we found that the virions were more sensitive to MβCD treatment than cells as far as the initiation of infection is concerned. In the cholesterol assays, we found that the cholesterol content of both plasma and viral membranes was gradually decreased with the increase of MβCD concentration. The addition of exogenous cholesterol enhanced the cholesterol content of both membranes and restored virus infectivity in a dose-dependent fashion. The changes of membrane cholesterol are correlated with the PrV infectivity, supporting the conclusion that cholesterol dependence of PrV infection. Our recent report indicates that cell infection by transmissible gastroenteritis coronavirus (TGEV), interacting with host cells via its S glycoprotein, is also dependent on the cholesterol in both plasma and viral membranes [19]. Compared with TGEV, there are more viral glycoproteins on the surface of PrV, and efficient virus infection may be mediated by one or more glycoprotein complex. Cholesterol may be an indispensable molecule involved in such process, nevertheless, we cannot exclude the possibility that the reduction in virus infectivity is a direct effect of cholesterol on PrV fusion activity.
The finding that in the case of virions lower concentrations of MβCD result in a reduction of infectivity may be explained by the intracellular maturation of PrV. Like herpesviruses, in general, PrV matures by a budding process at membranes of the trans-Golgi network (TGN). Intracellular membranes are known to contain less cholesterol than the plasma membrane. As the envelope of PrV is derived from an intracellular membrane, it is expected to have lower cholesterol content than a viral envelope derived from the plasma membrane. Therefore, lower amounts of MβCD are required to deplete cholesterol from the PrV envelope compared to cholesterol depletion from the plasma membrane. This explanation is also consistent with our finding that MβCD treatment of infected cells, i.e. after the step of virus adsorption, did not affect virus infectivity.
Acknowledgments
X.R. was a recipient of a fellowship from Deutscher Akademischer Austauschdienst (DAAD). We acknowledge Dr. Lynn Enquist and Ms. Marlies G. Eldridge, Princeton University, Princeton, USA, for providing genomes of PrV strains Kaplan and Becker. The funds from National Natural Science Foundation of China (30700590; 30972195), Northeast Agricultural University (CXZ008-1) to X.R., National Natural Science Foundation of China (30700591) to J.Y., and Heilongjiang Provincial Science and Technology Department (ZJN0702-01) to G.L are acknowledged.
References
- 1.Bender FC, Whitbeck JC, Ponce de Leon M, Lou H, Eisenberg RJ, Cohen GH. Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J Virol. 2003;77:9542–9552. doi: 10.1128/JVI.77.17.9542-9552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown DA, London E. Structure and function of sphingolipid and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 3.Danthi P, Chow M. Cholesterol removal by methyl-beta-cyclodextrin inhibits poliovirus entry. J Virol. 2002;78:33–41. doi: 10.1128/JVI.78.1.33-41.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desplanques AS, Nauwynck HJ, Vercauteren D, Geens T, Favoreel HW. Plasma membrane cholesterol is required for efficient pseudorabies virus entry. Virology. 2008;376:339–345. doi: 10.1016/j.virol.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Favoreel HW, Mettenleiter TC, Nauwynck HJ. Copatching and lipid raft association of different viral glycoproteins expressed on the surfaces of pseudorabies virus-infected cells. J Virol. 2004;78:5279–5287. doi: 10.1128/JVI.78.10.5279-5287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hambleton S, Steinberg SP, Gershon MD, Gershon AA. Cholesterol dependence of varicella-zoster virion entry into target cells. J Virol. 2007;81:7548–7558. doi: 10.1128/JVI.00486-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang H, Li Y, Sadaoka T, Tang H, Yamamoto T, Yamanishi K, Mori Y. Human herpesvirus 6 envelope cholesterol is required for virus entry. J Gen Virol. 2006;87:277–285. doi: 10.1099/vir.0.81551-0. [DOI] [PubMed] [Google Scholar]
- 8.Imhoff H, von Messling V, Herrler G, Haas L. Canine distemper virus infection requires cholesterol in the viral envelope. J Virol. 2007;81:4158–4165. doi: 10.1128/JVI.02647-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katzman RB, Longnecker R. Cholesterol-dependent infection of Burkitt’s lymphoma cell lines by Epstein-Barr virus. J Gen Virol. 2003;84:2987–2992. doi: 10.1099/vir.0.19252-0. [DOI] [PubMed] [Google Scholar]
- 10.Klupp BG, Hengartner CJ, Mettenleiter TC, Enquist LW. Complete, annotated sequence of the pseudorabies virus genome. J Virol. 2004;78:424–440. doi: 10.1128/JVI.78.1.424-440.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Yin J, Sui X, Li G, Ren X. Comparative analysis of the effect of glycyrrhizin diammonium and lithium chloride on infectious bronchitis virus infection in vitro. Avian Pathol. 2009;38:215–221. doi: 10.1080/03079450902912184. [DOI] [PubMed] [Google Scholar]
- 12.Li G, Zeng Y, Yin J, Lillehoj HS, Ren X. Cloning, expression and biological analysis of recombinant chicken IFN-γ expressed in Escherichia coli. Hybridoma. 2010;29:1–6. doi: 10.1089/hyb.2009.0053. [DOI] [PubMed] [Google Scholar]
- 13.Liao Z, Graham DR, Hildreth JE. Lipid rafts and HIV pathogenesis: virion-associated cholesterol is required for fusion and infection of susceptible cells. AIDS Res Hum Retrovir. 2003;19:675–687. doi: 10.1089/088922203322280900. [DOI] [PubMed] [Google Scholar]
- 14.Lu X, Xiong Y, Silver J. Asymmetric requirement for cholesterol in receptor-bearing but not envelope-bearing membranes for fusion mediated by ecotropic murine leukemia virus. J Virol. 2002;76:6701–6709. doi: 10.1128/JVI.76.13.6701-6709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manes S, Del Real G, Lacalle RA, Lucas P, Gomez-Mouton C, Sanchez-Palomino S, Delgado R, Alcami J, Mira E, Martinez A. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 2000;1:190–196. doi: 10.1093/embo-reports/kvd025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marjomaki V, Pietiainen V, Matilainen H, Upla P, Ivaska J, Nissinen L, Reunanen H, Huttunen P, Hyypia T, Heino J. Internalization of echovirus 1 in caveolae. J Virol. 2002;76:1856–1865. doi: 10.1128/JVI.76.4.1856-1865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norkin LC. Simian virus 40 infection via MHC class I molecules and caveolae. Immunol Rev. 1999;168:13–22. doi: 10.1111/j.1600-065X.1999.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 18.Pessin JE, Glaser M. Budding of Rous sarcoma virus and vesicular stomatitis virus from localized lipid regions in the plasma membrane of chicken embryo fibroblasts. J Biol Chem. 1980;255:9044–9050. [PubMed] [Google Scholar]
- 19.Ren X, Glende J, Yin J, Schwegmann-Wessels C, Herrler G. Importance of cholesterol for infection of cells by transmissible gastroenteritis virus. Virus Res. 2008;137:220–224. doi: 10.1016/j.virusres.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheiffele P, Rietveld A, Wilk T, Simons K. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J Biol Chem. 1999;274:2038–2044. doi: 10.1074/jbc.274.4.2038. [DOI] [PubMed] [Google Scholar]
- 21.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 22.Stuart AD, Eustace HE, McKee TA, Brown TD. A novel cell entry pathway for a DAF-using human enterovirus is dependent on lipid rafts. J Virol. 2002;76:9307–9322. doi: 10.1128/JVI.76.18.9307-9322.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sui X, Yin J, Ren X. Antiviral effect of glycyrrhizinate diammonium and lithium chloride on cell infection by pseudorabies herpesvirus. Antiviral Res. 2010;85:346–353. doi: 10.1016/j.antiviral.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun XJ, Whittaker GR. Role for influenza virus envelope cholesterol in virus entry and infection. J Virol. 2003;77:12543–12551. doi: 10.1128/JVI.77.23.12543-12551.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang H, Kawabata A, Takemoto M, Yamanishi K, Mori Y. Human herpesvirus-6 infection induces the reorganization of membrane microdomains in target cells, which are required for virus entry. Virology. 2008;378:265–271. doi: 10.1016/j.virol.2008.05.028. [DOI] [PubMed] [Google Scholar]


