Abstract
Introduction
Breast cancer is the most common malignancy in women in the United States and triple-negative breast cancer (TNBC) accounts for 15–20%. The standard of care for metastatic TNBC has been limited to cytotoxic chemotherapy with modest efficacy. TNBC is associated with high levels of tumor-infiltrating lymphocytes and PD-L1 expression, supporting the investigation of immune checkpoint inhibitors in this breast cancer subtype.
Areas Covered
This review summarizes the clinical data supporting the use of atezolizumab and nab-paclitaxel in the treatment of metastatic PD-L1-positive TNBC. It examines the pharmacology and toxicity profile of the combination in patients with metastatic TNBC.
Expert Opinion
The addition of atezolizumab to nab-paclitaxel prolonged progression-free survival in both the intention-to-treat and PD-L1-positive subgroups in the first line setting in patients with metastatic TNBC. The IMpassion 130 trial led to FDA-approval of this combination in patients with PD-L1-positive, metastatic TNBC and represents the first approval of immunotherapy for TNBC. This work supports ongoing investigations of other immunotherapy combinations in TNBC, predictive biomarker development and immunotherapy in patients with early stage TNBC. Immunotherapy combinations in TNBC have the potential to lead to improved survival in this group of patients with high risk disease.
Keywords: atezolizumab, immunotherapy, nab-paclitaxel, PD-L1, triple-negative breast cancer
1. Introduction
Breast cancer is the most common malignancy diagnosed in women in the United States (US) with an estimated 268,000 new cases and 42,260 cancer-related deaths predicted in 2019 [1]. Triple-negative breast cancer (TNBC) accounts for approximately 15% of all breast cancer cases [3].TNBC is associated with a higher nuclear grade, an increased risk of metastatic recurrence and inferior overall survival compared to other breast cancer subtypes treated with modern therapies [4,5]. TNBC is more common in younger patients, African Americans and those with deleterious BRCA mutations [2]. One likely contributing factor to the observed poorer outcomes in patients with TNBC, is the lack of targeted therapies such as anti-endocrine and HER2-targeted therapies that have significantly improved outcomes for other breast cancer subtypes [5,6]. To complicate things further, TNBC is a heterogenous disease comprised of molecular subtypes including two basal-like (BL) subtypes, a mesenchymal subtype and a luminal androgen receptor subtype and each subtype varies in response to therapy and prognosis[7]. Prior to the approval of atezolizumab, the standard of care for systemic treatment of metastatic TNBC was limited to cytotoxic chemotherapy, with the exception of PARP inhibitors in patients with deleterious BRCA mutations [8,9].
The majority of patients with TNBC are initially diagnosed with Stage I-III disease and only approximately 14% present with de novo metastatic disease [10]. Neoadjuvant chemotherapy has become the standard of care for patients with ≥ T2 tumors or node positive disease and patients experiencing a pathologic complete response (pCR) have a much lower risk of metastatic recurrence [9,11]. Patients who do not achieve a pCR with neoadjuvant chemotherapy have a 40–50% risk of metastatic recurrence which can be decreased somewhat with the addition of adjuvant capecitabine [12,13]. Patients with metastatic TNBC have a median survival of 13–15 months and a median duration of response to first-line palliative chemotherapy of approximately 12 weeks [10,14]. The duration of response to second-line therapy is approximately 9 weeks and only 4 weeks with third-line therapy [10]. Metastatic TNBC remains an area of unmet need for effective targeted therapies and large scale efforts have been underway over the last decade, including the investigation of immunotherapy for TNBC [15].
Harnessing the immune system to control metastatic cancer is promising treatment strategy in TNBC. Tumor-associated antigens can be recognized by the immune system and evoke an immune response which may result in tumor cell death due to a T-cell tumor-specific response [16]. However, tumor cell variants can also evade the immune system and escape immune destruction via upregulation of immune checkpoint molecules, activation of immune-suppressive metabolic pathways, and alteration of specific surface antigens [16].
Tumors with a higher mutational burden express more tumor neoantigens to immune cells, resulting in increased tumor-infiltrating lymphocytes (TILs) and a higher likelihood of anti-tumor immune response in many tumor types [16–21]. A higher frequency of TILs in primary TNBC tumors correlates with an increased pCR rate in response to neoadjuvant chemotherapy [16,21]. Factors impacting this association may include chemotherapy-induced alterations in the tumor microenvironment resulting in a more effective immune response, depletion of suppressor and regulatory T cells, and the generation of somatic mutations that increase tumor neoantigens [16].
The development of immune checkpoint inhibitors that block immune checkpoint proteins including programmed cell death protein 1 (PD-1), programmed-death ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) has dramatically improved long-term outcomes for patients with multiple malignancies, including melanoma, non-small cell lung cancer, and renal cell carcinoma [18,20]. Monoclonal antibody blockade of immune checkpoints results in the activation of T-cells targeting tumor cells [17]. TNBC is associated with high levels of TILs and PD-L1 expression, supporting the investigation of immune checkpoint inhibitors in this breast cancer subtype [19]. This article reviews the current treatment options for metastatic TNBC and the clinical data leading to the FDA approval of atezolizumab plus nab-paclitaxel in PD-L1-positive metastatic TNBC.
2. Treatment of Metastatic TNBC
Sequential lines of chemotherapy remain the mainstay of the treatment of metastatic TNBC. Sequential single agents are preferred, however, combination chemotherapy can be considered in patients with impending visceral crisis or rapid disease progression requiring prompt cytoreduction for symptom management. While there is no survival advantage associated with combination chemotherapy, response rates are higher [9].
Anthracyclines and taxanes are the most active chemotherapeutics in TNBC and typically incorporated early on in treatment. As most patients are diagnosed with Stage I-III disease, the receipt of anthracyclines and taxanes as part of adjuvant or neoadjuvant chemotherapy is common. In the front-line metastatic setting, anthracyclines such as doxorubicin have a 35–50% response rate, however, long term administration is limited by the cumulative risk of cardiomyopathy and reduced ejection fraction [22]. Taxanes, including docetaxel and paclitaxel, are active in metastatic breast cancer with a 25–35% response rate and improved time to progression and overall survival in all breast cancer subtypes previously treated with an anthracycline [23,24]. The TNT trial compared the efficacy of upfront carboplatin to docetaxel in patients with metastatic TNBC and demonstrated superior efficacy of carboplatin only in patients with deleterious BRCA mutations. In the intent-to-treat population, there was no difference in objective response rate (ORR) (31.4% with carboplatin vs. 34.0% with docetaxel; 95% CI, 12.1–6.9; p=0.66), however, in patients with germline BRCA1/2 mutations, the overall response rate with carboplatin was 68% compared to 33.3% with docetaxel (95% CI, 6.3–63.1;p=0.03). There was no difference in overall survival (OS) [25]. Testing for BRCA mutations is an important consideration in patients with metastatic TNBC given the superior efficacy of carboplatin compared to docetaxel in patients with deleterious BRCA mutations.
Other chemotherapy agents with activity in metastatic TNBC include eribulin, capecitabine, vinorelbine and gemcitabine [22]. Eribulin is a non-taxane microtubule inhibitor currently approved in the US for the treatment of patients with metastatic HER2-negative breast cancer previously treated with two prior lines of chemotherapy, typically an anthracycline and taxane [9]. The EMBRACE trial demonstrated an improved median OS in patients treated with eribulin, 13.1 months, compared to treatment of the physician’s choice (TPC), 10.6 months, in the intention to treat population (hazard ratio (HR) 0.81; 95% CI, 0.66–0.99; p=0.041). [26].
Capecitabine is a 5-fluorouracil prodrug and pyrimidine antimetabolite that inhibits thymidylate synthetase and is often favored due to oral administration and lack of alopecia as a side effect [22]. Capecitabine has a similar overall response rate, median PFS, and OS compared to eribulin, however, in a subset analysis of patients with TNBC, OS was improved with eribulin compared to capecitabine [28].
Vinorelbine is a semisynthetic vinca alkaloid which has also has activity in metastatic breast cancer [22]. There are no large series evaluating the effect of vinorelbine in TNBC specifically, however, in a study including patients with HER2-negative metastatic breast cancer who received at least one prior line of chemotherapy, overall response to vinorelbine monotherapy was about 27% with a medianPFS of 6 months and median OS of 22 months [29].
Gemcitabine is a pyrimide antimetabolite that inhibits DNA synthesis with limited toxicity and is commonly used in combination with carboplatin [30]. In patients with metastatic TNBC who had received no more than two prior lines of chemotherapy patients who received gemcitabine plus carboplatin had a median PFS of 4.1 months, ORR of 30.2%, and median OS of 11.1 months [31].
PARP inhibitors are effective in patients with germline BRCA1/2 mutations. The OlympiAD trial showed a superior ORR (60% vs 29%), and median PFS (7.0 months vs. 4.2 months; HR 0.58; 95% CI, 0.43–0.80; p<0.001) with olaparib compared to TPC (capecitabine, eribulin, or vinorelbine) in patients with BRCA1/2 mutations and HER2-negative breast cancer who had receive no more than two prior lines of chemotherapy for metastatic disease [32]. The EMBRACA trial showed benefit with talazoparib over TPC (capecitabine, eribulin, gemcitabine, or vinorelbine) in patients with advanced breast cancer and a germline BRCA1/2 mutation who received up to three prior lines of chemotherapy. In the intention-to-treat population, ORR was doubled with talazoparib compared to TPC (62.6% vs. 27.2%; odds ratio 5.0; 95% CI, 2.9–8.8; p<0.001). The median PFS was 8.6 months in the talazoparib group compared to 5.6 months with TPC (HR 0.54; 95% CI, 0.55–1.06; p=0.11) [33]. BRCA1/2 testing should be strongly considered in patients with metastatic TNBC [9]. If a BRCA1/2 mutation is identified, treatment with carboplatin and PARP inhibitors should be considered [25]
3. Pharmacology and Administration of Atezolizumab
Atezolizumab (MPDL3280A) is a humanized IgG4 antibody which consist of two heavy chains (448 amino acids) and two light chains (214 amino acids) and is produced in Chinese hamster ovary cells [34]. Atezolizumab is engineered to eliminate Fc-effector function via a single amino acid substitution (asparagine to alanine) at position 298 on the heavy chain, which results in a non-glycosylated antibody that has minimal binding to Fc receptors and prevents Fc-effector function at expected concentration in humans [35].
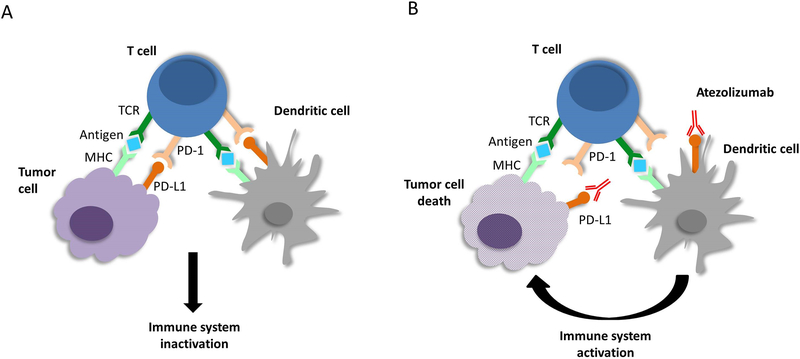
Atezolizumab is a high affinity monoclonal antibody that specifically binds to PD-L1 and prevents its interaction with PD-1 and B7.1 [36]. Interaction of PD-L1 with PD-1 and B7.1 receptors inhibits T cell activation [36]. As a result, when atezolizumab binds to PD-L1 and blocks interaction with PD-1 and B7.1, tumor specific T cell response is enhanced (Figure 1) [36]. The affinity of atezolizumab for PD-L1 is 400 pM [37].
Figure 1.
Mechanism of action of atezolizumab. (A) Tumor cells present antigens to T-cells and interaction of PD-L1 on tumor cells and PD-1 on T-cells results in inactivation of the immune system. (B) Atezolizumab blocks PD-L1 on tumor cells and prevents interaction with PD-1 on T-cells resulting in activation of anti-tumor immune response.
The recommended dose of atezolizumab in treatment of metastatic TNBC is 840 mg administered on days 1 and 15 for each 28-day cycle [38,39]. The initial infusion should be administered over 60 minutes with subsequent infusions over 30 minutes if the first infusion is tolerated [40]. The clearance is 0.20 L/day, volume of distribution at steady state is 6.7 L, and the terminal half-life is approximately 27 days [38]. After the completion of multiple doses, steady state is usually reached within 6 to 9 weeks. There is no recommended dose adjustment for mild to moderate renal impairment (eGFR 30–89 mL/min/1.73m2) and no dose adjustment recommended for mild hepatic impairment [38]. Atezolizumab has not been studied in moderate to severe hepatic impairment [38]. Atezolizumab should be administered prior to nab-paclitaxel and should not be administered as an IV push or bolus [38]
4. Pharmacology and Administration of Nab-Paclitaxel
Nab-paclitaxel is albumin-bound paclitaxel and is highly lipophilic and insoluble in water [41]. The paclitaxel is contained within nanoparticles that consist mostly of paclitaxel bound to human albumin with a mean particle size of approximately 130 nanometers [42]. Each vial of nab-paclitaxel contains paclitaxel and human albumin in a ratio of 1:9 [43]. Nab-paclitaxel is a solvent free formulation of paclitaxel unlike paclitaxel and docetaxel. Paclitaxel is combined with Cremaphor EL to solubilize paclitaxel for intravenous administration [41]. Both solvents used in paclitaxel and docetaxel are pharmacologically active and have been associated with hypersensitivity reactions [41]. As a result, premedication with corticosteroids and antihistamines are required to minimize the risk of reaction [41]. Because nab-paclitaxel is solvent free, hypersensitivity reactions are less common than with paclitaxel and it can be administered without premedication with corticosteroids [44].
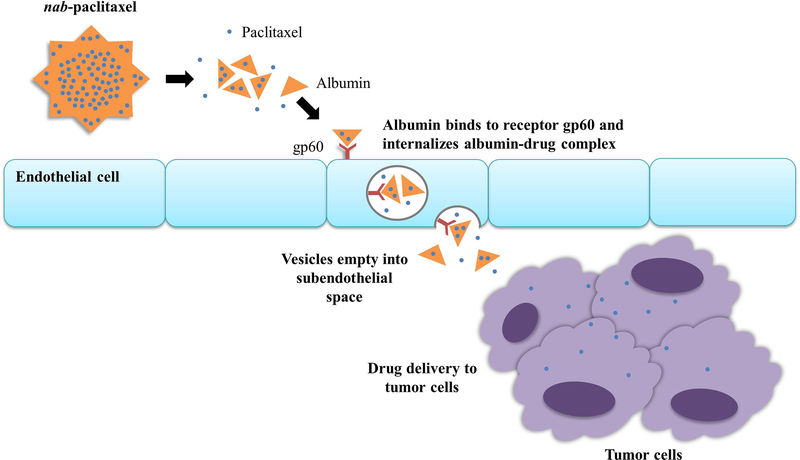
With nab-paclitaxel, albumin is used to deliver paclitaxel to tumor cells [41]. Albumin binds an endothelial glycoprotein receptor (gp60) which activates invagination into the plasma membrane of the albumin-paclitaxel complex [41]. This is followed by cellular transport across the endothelial cell to the subendothelial space and where paclitaxel can be delivered to tumor cells (Figure 2) [41]. Paclitaxel is a microtubule inhibitor that promotes assembly of microtubules from tubulin dimers and stabilizes microtubules by preventing depolymerization, a process necessary for normal cell division [42]. This stability inhibits the normal reorganization of the microtubule network that is necessary for vital interphase and mitotic cellular functions [42].
Figure 2.
Action of albumin-bound paclitaxel. Nanoparticle albumin-bound paclitaxel is carried in the bloodstream and is actively transported through the endothelial cell cytoplasm by binding to endothelial cell membrane glycoprotein, gp60. The albumin-paclitaxel complex is carried in vesicles to the extracellular space and tumor cells.
The recommended dose of nab-paclitaxel in the treatment of metastatic TNBC is 100 mg/m2 on days 1, 8 and 15 for each 28-day cycle [40,42]. Following the administration of nab-paclitaxel, plasma concentrations decline in a biphasic manner [42]. The initial rapid decline represents distribution to the peripheral compartment and the slower second phase represents drug elimination [42]. The mean terminal half-life ranges from 13 to 27 hours [42]. Albumin-bound paclitaxel is metabolized primarily to 6-alpha hydroxypaclitaxel by CYP2C8; and to 2 minor metabolites, 3’-p-hydroxypaclitaxel and 6-alpha, 3’-p-dihydroxypaclitaxel, by CYP3A4 [42]. Caution should be exercised when administering albumin-bound paclitaxel with medicines known to inhibit or induce either CYP2C8 or CYP3A4 [42]. Nab-paclitaxel is not clinically interchangeable with other paclitaxel formulations [42].
5. Atezolizumab in Combination with Nab-Paclitaxel in Metastatic TNBC
Atezolizumab was first evaluated in an open-label, multicenter, phase 1 first-in-human trial investigating the safety and preliminary efficacy of single-agent atezolizumab in patients with advanced solid and hematologic malignant neoplasms with multiple expansion cohorts including TNBC [46]. Preliminary signs of efficacy were meager with an ORR of 13% in all patients compared to 24% for patients treated in the first-line setting [47].
Cytotoxic tumor cell destruction by chemotherapy such as taxanes promotes tumor antigen release which can enhance antitumor response to immune checkpoint inhibition by activating toll-like receptor activity and promoting dendritic-cell activity [45]. Because glucocorticoid premedication could potentially decrease the efficacy of immunotherapy and nab-paclitaxel does not require premedication with steroids, it was felt to be most appropriate to use in combination with immunotherapy in TNBC [45].
The combination of atezolizumab plus nab-paclitaxel was then evaluated in a phase Ib study enrolling 33 patients with metastatic or locally recurrent TNBC previously treated with up to 2 prior lines of chemotherapy in the metastatic setting. The results were more promising with an ORR of 39.4%, including 1 complete response and 12 partial responses. The median PFS was 5.5 months and median OS was 14.7 months. In the first line setting, the ORR increased to 53.8% with a median PFS of 8.6 months [39,48].
The IMpassion130 trial was a phase III, international, randomized, double-blind, placebo-controlled study designed to evaluate the efficacy of atezolizumab in combination with nab-paclitaxel compared to nab-paclitaxel alone in patients with locally advanced or metastatic TNBC with no prior chemotherapy in the metastatic setting [40]. Patients previously treated with taxanes in the neoadjuvant or adjuvant setting were eligible provided the treatment was completed at least 12 months prior to randomization. Patients with asymptomatic treated brain metastasis were permitted and patients with prior autoimmune disease or glucocorticoid or immunosuppressive medications were excluded. The study enrolled 902 patients randomized 1:1 to receive atezolizumab 840 mg or placebo on days 1 and 15 in combination with nab-paclitaxel 100 mg/m2 on days 1, 8, and 15 of every 28 day cycle. Patients were stratified by the presence or absence of liver metastasis, use or nonuse of prior taxanes, and PD-L1 expression on tumor-infiltrating immune cells as a percentage of tumor area (<1% PD-L1-negative and ≥ 1% PD-L1-positive). The trial had two primary efficacy end points, investigator-assessed PFS and OS, that were evaluated in the intent-to-treat population and in the subgroup of patients with PD-L1-positive tumors. PD-L1-positive tumors were detected in 40.9% of patients and approximately 50% of patients were previously treated with neoadjuvant or adjuvant chemotherapy.
With a median follow-up of 12.9 months, PFS was significantly prolonged in the the intent-to-treat population with atezolizumab plus nab-paclitaxel compared to nab-paclitaxel plus placebo (7.2 months vs. 5.5 months, stratified HR for progression or death 0.80; 95% CI, 0.69–0.92; p=0.002) and the PD-L1-positive subgroup (5.0 months vs. 7.5 months, stratified HR for progression or death 0.62; 95% CI, 0.49–0.78; p<0.001) Median OS in the intent-to-treat population was not statistically different, however there was a trend towards improved survival from 17.6 months with nab-paclitaxel plus placebo to 21.3 months with atezolizumab plus nab-paclitaxel (HR for death 0.84; 95% CI, 0.69–1.02; p=0.08). Formal testing of OS in the PD-L1-positive subgroup was not performed due to the hierarchical statistical analysis procedure used in this trial, however, OS was numerically higher in the atezolizumab plus nab-paclitaxel group compared to the placebo plus nab-paclitaxel group (25.0 months vs. 15.5 months, stratified HR for death 0.62; 95% CI, 0.45–0.86) (Table 1).
Table 1.
Efficacy endpoints of IMpassion130
| Intention-to-treat population | PD-L1-positive subgroup | |||||
|---|---|---|---|---|---|---|
| atezolizumab + nab-paclitaxel | placebo + nab-paclitaxel | p value (95% CI) | atezolizumab + nab-paclitaxel | placebo + nab-paclitaxel | p value (95% CI) | |
| PFS | 7.2 mo | 5.5 mo | 0.0025 (0.69–0.92) | 7.5 mo | 5.0 mo | <0.001 (0.49–0.78) |
| OS | 21.3 mo | 17.6 mo | 0.08 (0.69–1.02) | 25.0 mo | 15.5 mo | (0.45–0.86) |
| ORR | 56.0% | 45.9% | 0.002 (3.4–16.8) | 58.9% | 42.6% | 0.002 (5.7–26.9) |
PFS: median progression-free survival, OS: median overall survival, ORR: objective response rate
The most common adverse events (AEs) seen in patient with TNBC who received atezolizumab in combination with nab-paclitaxel were alopecia (56%), peripheral neuropathy (47%), fatigue (47%), nausea (46%) and diarrhea (33%). AEs observed more frequently in patients treated with atezolizumab plus nab-paclitaxel compared to placebo plus nab-paclitaxel included nausea, cough, neutropenia, pyrexia, and hypothyroidism [40]. The incidence of Grade 3/4 AEs was modestly higher in patients who received atezolizumab plus nab-paclitaxel compared to placebo plus nab-paclitaxel (48.7% vs. 42.2%).
Adverse events of special interest which could be potentially immune-related were common in this trial and occurred in 57.3% of patients who received atezolizumab plus nab-paclitaxel compared to 41.8% who received placebo plus nab-paclitaxel. In the atezolizumab plus nab-paclitaxel arm, the most common any grade immune-related AEs included: rash (34.1%), (hepatitis (15.3%), hypothyroidism (17.3%), hyperthyroidism (4.4%) and pneumonitis (3.1%). The incidence of grade 3/4 AEs of special interest were much less common (7.5% with atezolizumab vs. 4.3% with placebo).
6. Conclusions
The IMpassion130 trial led to the accelerated US FDA approval of atezolizumab in combination with nab-paclitaxel for adult patients with unresectable locally advanced or metastatic TNBC whose tumors express PD-L1 by an FDA-approved assay [40]. The Ventana PD-L1 (SP142) Assay was approved as a companion diagnostic device for selecting TNBC patients for atezolizumab.
Atezolizumab is the first immunotherapy agent to be approved for the treatment of breast cancer. The addition of atezolizumab to nab-paclitaxel prolonged PFS in the intent-to-treat and PD-L1 positive subset of patients with metastatic TNBC treated in the first-line setting without a statistically significant prolongation of OS at this time. The combination of atezolizumab plus nab-paclitaxel is currently approved by the FDA and European Comission (EC) for the treatment of patients with PD-L1-positive, unresectable or metastatic TNBC. In the US, the FDA approval does not provide a limit on prior lines of therapy, whereas the EC approved this in the first-line setting. The addition of atezolizumab to nab-paclitaxel does increase the incidence of immune-related AEs, including rash and hypothyroidism. The incidence of serious immune-related AEs is infrequent and consistent with other trials of atezolizumab.
7. Expert Opinion
The approval of atezolizumab in combination with nab-paclitaxel for patients with metastatic, PD-L1-positive TNBC represents an important advance in the treatment of breast cancer. While the observed prolongation of median PFS in the IMpassion130 trial may be considered modest, there is a subset of patients who receive long-term benefit with maintenance atezolizumab after discontinuation of nab-paclitaxel. For these exceptional responders, we consider this therapy to be a breakthrough by providing prolonged control of disease without traditional chemotherapy-related toxicity. Efforts to develop predictive biomarkers going beyond PD-L1 expression to prospectively identify patients with metastatic TNBC who will benefit from the addition of atezolizumab to nab-paclitaxel would be of great benefit in increasing the magnitude of benefit.
The use of PD-L1 expression to determine patients deriving clinical benefit from immunotherapy remains suboptimal in TNBC. In the IMpassion130 trial immunohistochemistry (IHC) using the SP142 PD-L1 antibody was performed and scored for expression on tumor infiltrating lymphocytes (TILs) [50]. This is in contrast to other PD-L1 assays approved in non-small cell lung cancer and melanoma where antibodies SP263, 28–8 and 22C3 are used and expression is scored on tumor cells rather than immune cells [50]. In addition, these PD-L1 IHC assays use a different scoring systems and are limited by interobserver variability [50].
There remains a need to develop other predictive biomarkers beyond PD-L1 that may be able to better assist in identifying patients likely to respond to immunotherapy. TILs are associated with a higher pathologic complete response and overall response rate and may be able to predict benefit from immunotherapy when used in conjunction with PD-L1 expression [7]. Multiple gene signatures evaluating immune-related genes, tumor mutational burden, and microsattelite instability may also help to better classify tumor immunogenicity [7].
Although nab-paclitaxel was used in the IMpassion130 trial, there are ongoing trials evaluating the effects of other chemotherapy agents and targeted agents in combination with immunotherapy [7]. In addition to concurrent chemotherapy and immunotherapy, the TONIC trial is investigating the role of induction chemotherapy or radiation followed by anti-PD-1 monotherapy and what the best sequence of treatment may be [51].
The frequency of immune-related AES with atezolizumab in combination with nab-paclitaxel is similar to atezolizumab in combination with other chemotherapy agents or as a single agent. Most immune-related AEs are low grade and medically managed without the need to permanently discontinue atezolizumab, however, more severe immune-related AEs can occur and this must be discussed with patients prior to administering atezolizumab. Patients with prior autoimmune diseases were not eligible for enrollment in IMpassion130 and safety of immunotherapy in these patients is the topic of ongoing investigation.
In the IMpassion130 trial, patients were prospectively evaluated for PD-L1 expression on tumor-infiltrating immune cells which was expressed as a percentage of tumor area (<1% PD-L1 negative and ≥ 1% PD-L1 positive) and 40.9% of patients were PD-L1-positive. Unfortunately, approximately 60% of patients with metastatic TNBC will not be candidates for atezolizumab in the current landscape and there remains an urgent clinical need for new strategies to harness the immune system to treat PD-L1-negative tumors.
The investigation of immunotherapy strategies in TNBC continues to rapidly expand. The encouraging results from IMpassion130 have led to other trials investigating PD-1 or PD-L1 inhibitors in patients with early stage TNBC and in combination with other chemotherapy and targeted agents. Other ongoing efforts include trials investigating personalized cancer vaccines targeting specific tumor neoantigens, agonists of T-cell stimulatory molecules and bispecific antibodies binding tumor antiegns and immune cell surface proteins [52]. We remain hopeful that these ongoing studies will lead to an improvement in survival for patients with TNBC.
Article Highlights.
Patients with triple-negative breast cancer (TNBC) have a more aggressive disease course, higher risk of metastatic recurrence and limited systemic treatment options compared to other breast cancer subtypes.
TNBC has a higher tumor mutational burden, expression of tumor-infiltrating lymphocytes (TILs) and PD-L1 expression compared to other breast cancer subtypes, supporting the potential activity of immunotherapy.
The addition of atezolizumab to nab-paclitaxel prolonged progression-free survival (PFS) in the intent-to-treat population and in the PD-L1-positive subgroup of patients in the first-line treatment of metastatic TNBC with low rates of severe autoimmune toxicity.
Atezolizumab plus nab-paclitaxel should be considered in appropriate patients without clinically significant autoimmune disease with metastatic PD-L1-positive TNBC.
Acknowledgments
Funding
This investigation was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32CA236734-01. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewers Disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Howlader N NA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2016, National Cancer Institute. . (Ed.^(Eds) (Bethesda, MD, 2019) [Google Scholar]
- 2.Elias AD. Triple-negative breast cancer: a short review. Am J Clin Oncol, 33(6), 637–645 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Hammond ME, Hayes DF, Dowsett M et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol, 28(16), 2784–2795 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dent R, Trudeau M, Pritchard KI et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res, 13(15 Pt 1), 4429–4434 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Li X, Yang J, Peng L et al. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat, 161(2), 279–287 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Pal SK, Childs BH, Pegram M. Triple negative breast cancer: unmet medical needs. Breast Cancer Res Treat, 125(3), 627–636 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marra A, Viale G, Curigliano G. Recent advances in triple negative breast cancer: the immunotherapy era. BMC Med, 17(1), 90 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardoso F, Senkus E, Costa A et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4)dagger. Ann Oncol, 29(8), 1634–1657 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Network NCC. Breast Cancer (Version 3.2019). (Ed.^(Eds) (2019)
- 10.Kassam F, Enright K, Dent R et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer, 9(1), 29–33 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Cortazar P, Zhang L, Untch M et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet, 384(9938), 164–172 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Kuroi K, Toi M, Ohno S et al. Prognostic significance of subtype and pathologic response in operable breast cancer; a pooled analysis of prospective neoadjuvant studies of JBCRG. Breast Cancer, 22(5), 486–495 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Masuda N, Lee SJ, Ohtani S et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med, 376(22), 2147–2159 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Gobbini E, Ezzalfani M, Dieras V et al. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Cancer, 96, 17–24 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Emens LA. Breast Cancer Immunotherapy: Facts and Hopes. Clin Cancer Res, 24(3), 511–520 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Teijido P, Cabal ML, Fernandez IP, Perez YF. Tumor-Infiltrating Lymphocytes in Triple Negative Breast Cancer: The Future of Immune Targeting. Clin Med Insights Oncol, 10(Suppl 1), 31–39 (2016).* A review of the association of TNBC with the immune environment and immunotherapy.
- 17.Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med, 50(12), 165 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol, 11(1), 24–37 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Li M, Jiang Z, Wang X. A Comprehensive Immunologic Portrait of Triple-Negative Breast Cancer. Transl Oncol, 11(2), 311–329 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer, 12(4), 252–264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo L, Maltese A, Betancourt L et al. Locally advanced breast cancer: Tumor-infiltrating lymphocytes as a predictive factor of response to neoadjuvant chemotherapy. Eur J Surg Oncol, 45(6), 963–968 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Zeichner SB, Terawaki H, Gogineni K. A Review of Systemic Treatment in Metastatic Triple-Negative Breast Cancer. Breast Cancer (Auckl), 10, 25–36 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones SE, Erban J, Overmoyer B et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol, 23(24), 5542–5551 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Rugo HS, Barry WT, Moreno-Aspitia A et al. Randomized Phase III Trial of Paclitaxel Once Per Week Compared With Nanoparticle Albumin-Bound Nab-Paclitaxel Once Per Week or Ixabepilone With Bevacizumab As First-Line Chemotherapy for Locally Recurrent or Metastatic Breast Cancer: CALGB 40502/NCCTG N063H (Alliance). J Clin Oncol, 33(21), 2361–2369 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tutt A, Tovey H, Cheang MCU et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med, 24(5), 628–637 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortes J, O’Shaughnessy J, Loesch D et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet, 377(9769), 914–923 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Kaufman PA, Awada A, Twelves C et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol, 33(6), 594–601 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Twelves C, Awada A, Cortes J et al. Subgroup Analyses from a Phase 3, Open-Label, Randomized Study of Eribulin Mesylate Versus Capecitabine in Pretreated Patients with Advanced or Metastatic Breast Cancer. Breast Cancer (Auckl), 10, 77–84 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papaldo P, Fabi A, Ferretti G et al. A phase II study on metastatic breast cancer patients treated with weekly vinorelbine with or without trastuzumab according to HER2 expression: changing the natural history of HER2-positive disease. Ann Oncol, 17(4), 630–636 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Silvestris N, Cinieri S, La Torre I et al. Role of gemcitabine in metastatic breast cancer patients: a short review. Breast, 17(3), 220–226 (2008). [DOI] [PubMed] [Google Scholar]
- 31.O’Shaughnessy J, Schwartzberg L, Danso MA et al. Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J Clin Oncol, 32(34), 3840–3847 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Robson M, Im SA, Senkus E et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med, 377(6), 523–533 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Litton JK, Rugo HS, Ettl J et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med, 379(8), 753–763 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah NJ, Kelly WJ, Liu SV, Choquette K, Spira A. Product review on the Anti-PD-L1 antibody atezolizumab. Hum Vaccin Immunother, 14(2), 269–276 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santini FC, Rudin CM. Atezolizumab for the treatment of non-small cell lung cancer. Expert Rev Clin Pharmacol, 10(9), 935–945 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heimes AS, Schmidt M. Atezolizumab for the treatment of triple-negative breast cancer. Expert Opin Investig Drugs, 28(1), 1–5 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Lee HT, Lee SH, Heo YS. Molecular Interactions of Antibody Drugs Targeting PD-1, PD-L1, and CTLA-4 in Immuno-Oncology. Molecules, 24(6) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tecentriq (atezolizumab) [package insert]. San Francisco, CA: Genentech, Inc; 2019.). [Google Scholar]
- 39.Adams S, Diamond JR, Hamilton E et al. Atezolizumab Plus nab-Paclitaxel in the Treatment of Metastatic Triple-Negative Breast Cancer With 2-Year Survival Follow-up: A Phase 1b Clinical Trial. JAMA Oncol, 5(3), 334–342 (2019).* This study evaluated the safety profile of atezolizumab plus nab-paclitaxel.
- 40.Schmid P, Adams S, Rugo HS et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med, 379(22), 2108–2121 (2018).** This study led to the FDA approval of atezolizumab plus nab-paclitaxel in the first-line setting in PD-L1 positive metastatic TNBC
- 41.Vishnu P, Roy V. nab-paclitaxel: a novel formulation of taxane for treatment of breast cancer. Womens Health (Lond), 6(4), 495–506 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Abraxane (paclitaxel protein-bound particles for injectable suspension) (albumin-bound) [package insert]. Summit, NJ: Abraxis BioScience, LLC; 2013. .). [Google Scholar]
- 43.Yardley DA. nab-Paclitaxel mechanisms of action and delivery. J Control Release, 170(3), 365–372 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Picard M Management of Hypersensitivity Reactions to Taxanes. Immunol Allergy Clin North Am, 37(4), 679–693 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Soliman HH. nab-Paclitaxel as a potential partner with checkpoint inhibitors in solid tumors. Onco Targets Ther, 10, 101–112 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herbst RS, Soria JC, Kowanetz M et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature, 515(7528), 563–567 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Emens LA, Cruz C, Eder JP et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol, 5(1), 74–82 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu SV, Camidge DR, Gettinger SN et al. Long-term survival follow-up of atezolizumab in combination with platinum-based doublet chemotherapy in patients with advanced non-small-cell lung cancer. Eur J Cancer, 101, 114–122 (2018). [DOI] [PubMed] [Google Scholar]
- 49.CHMP recommends EU approval of Roche’s Tecentriq in combination with Abraxane as an initial treatment for people with PD-L1-positive, metastatic triple-negative breast cancer [news release]. Roche.). [Google Scholar]
- 50.Yeong J, Tan T, Chow ZL et al. Multiplex immunohistochemistry/immunofluorescence (mIHC/IF) for PD-L1 testing in triple-negative breast cancer: a translational assay compared with conventional IHC. J Clin Pathol, (2020). [DOI] [PubMed] [Google Scholar]
- 51.Voorwerk L, Slagter M, Horlings HM et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med, 25(6), 920–928 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Yu LY, Tang J, Zhang CM et al. New Immunotherapy Strategies in Breast Cancer. Int J Environ Res Public Health, 14(1) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]