Abstract
Background: Familial adenomatous polyposis (FAP) is a condition typically caused by pathogenic germline mutations in the APC gene. In addition to colon polyps, individuals with FAP have a substantially increased risk of developing papillary thyroid cancer (PTC). Little is known about the events underlying this association, and the prevalence of somatic “second-hit” mutations in APC is controversial.
Methods: Whole-genome sequencing was performed on paired thyroid tumor and normal DNA from 12 FAP patients who developed PTC. Somatic mutation profiles were compared with clinical characteristics and previously sequenced sporadic PTC cases. Germline variant profiling was performed to assess the prevalence of variants in genes previously shown to have a role in PTC predisposition.
Results: All 12 patients harbored germline mutations in APC, consistent with FAP. Seven patients also had somatic mutations in APC, and seven patients harbored somatic mutations in KMT2D, which encodes a lysine methyl transferase. Mutation of these genes is extremely rare in sporadic PTCs. Notably, only two of the tumors harbored the somatic BRAF p.V600E mutation, which is the most common driver mutation found in sporadic PTCs. Six tumors displayed a cribriform–morular variant of PTC (PTC-CMV) histology, and all six had somatic mutations in APC. Additionally, nine FAP-PTC patients had rare germline variants in genes that were previously associated with thyroid carcinoma.
Conclusions: Our data indicate that FAP-associated PTCs typically have distinct mutations compared with sporadic PTCs. Roughly half of the thyroid cancers that arise in FAP patients have somatic “second-hits” in APC, which is associated with PTC-CMV histology. Somatic BRAF p.V600E variants also occur in some FAP patients, a novel finding. We speculate that in carriers of heterozygous pathogenic mutations of tumor suppressor genes such as APC, a cooperating second-hit somatic variant may occur in a different gene such as KTM2D or BRAF, leading to differences in phenotypes. The role of germline variance in genes other than APC (9 of the 12 patients in this series) needs further research.
Keywords: familial adenomatous polyposis, APC, papillary thyroid cancer, cribriform–morular variant, whole-genome sequencing
Introduction
In addition to colon polyps and colorectal cancer (CRC), patients with familial adenomatous polyposis (FAP) have an increased risk to develop extracolonic malignancies and benign conditions/tumors. Of particular interest is the frequent occurrence of papillary thyroid cancer (PTC), which is some 100 times more prevalent in FAP patients than in the general population (1). FAP is typically caused by germline mutations in the APC gene that result in a truncated APC protein and inevitably develops into CRC when somatic “second-hit” mutations in APC occur in colon cells (2). Previous studies have confirmed the existence of such “second hits” in the APC gene in a subset of thyroid cancers that occur in FAP patients, (3) but this remains controversial, as other reports have concluded that the APC gene is rarely somatically mutated in FAP-associated PTC (4–6).
This contradiction has not been resolved. FAP-associated PTCs often display a cribriform–morular variant (CMV) histology, which is otherwise extremely rare (∼0.2% of all thyroid cancers) (7). Altogether, about half of all CMV-PTCs occur in FAP patients (7,8). In general, PTC is about three times more common in females than in males, and this ratio is even higher in PTC-CMV patients and FAP-associated PTCs (7). Molecular characteristics of PTC-CMV include mutations in the CTNNB1 and/or PIK3CA genes and RET/PTC rearrangements. No oncogenic BRAF mutations have so far been reported in CMV-PTCs or FAP-associated PTCs; however, a comprehensive assessment of the somatic alterations that occur in FAP-associated PTCs has not been conducted.
To better understand the germline and somatic variants found in this unique tumor type and to try to learn more about the genetic mechanisms of thyroid cancer development in individuals with FAP-associated germline mutations in APC, we performed whole-genome sequencing of paired tumor and normal DNA from 12 FAP-associated PTC patients.
Materials and Methods
Patients
All patients with both FAP and PTC were selected from the PTC patient repositories at the Cleveland Clinic and the Helsinki University Hospital. Histological review of tumor sections was performed to confirm the presence of cancer in the resected thyroid. This study was limited to patients with germline APC mutations detected by clinically approved testing methods. All patients provided written informed consent, and studies were performed in accordance with the Declaration of Helsinki and approved by institutional review boards at both institutions.
DNA extraction and sequencing
DNA was extracted from paraffin-embedded thyroid tumors and adjacent normal tissue (patients 2, 4, 5, 7, 8, 9, 10, and 12) using QIAamp DNA FFPE Tissue Kits (Qiagen, Hilden, Germany) or from blood (patients 1, 3, 6, and 11) using a previously described nonenzymatic DNA extraction method (9). DNA samples were quantified using a Qubit 3.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA), and fragment size was assessed using a 2100 Bioanalyzer system (Agilent Technologies, Santa Clara, CA). All samples had average fragment sizes >1500 bp. Library preparation and paired-end genome sequencing were performed by Novogene (Beijing, China), using TruSeq Nano DNA HT Sample Preparation Kits (Illumina, San Diego, CA) and HiSeq 4000 instruments (Illumina, San Diego, CA). All samples were sequenced to >80 gigabytes of data.
Informatics
The quality of sequences was confirmed using FASTQC software. All samples had >99% of reads mapped, and all samples had >90% of bases with phred-scaled quality scores >30. Mapping was performed with Burrows–Wheeler Aligner to the Genome Reference Consortium Human Build 37. Samples were sorted with SamTools, duplicates were marked with Picard, and variants were called with GATK and then annotated with ANNOVAR. For somatic and germline variant analysis, paired .vcf files from each tumor and matching adjacent normal thyroid or blood sample were loaded into BasePlayer software (10). Variants present in the tumor sample and absent but covered in the paired germline DNA were considered somatic. Publicly available data from the American Association for Cancer Research Genomics Evidence Neoplasia Information Exchange (AACR-GENIE) and The Cancer Genome Research Atlas (TCGA) were accessed using cbioportal.org on August 19, 2019. Somatic variant signature analysis was performed using the R package DeconstructSigs (11), and the 30 signatures were described in the catalog of somatic mutations in cancer (COSMIC) mutational signatures (v2-March 2015). Somatic loss of heterozygosity (LOH) was determined using BasePlayer software (10) by comparing the allelic ratios of sequenced germline variants with their corresponding ratios in the matched tumor sample as described (12,13). A tumor to normal ratio of ≤0.6 or ≥1.67 was considered LOH, and a tumor to normal ratio of 0.6–0.8 or 1.25–1.67 was considered putative LOH.
Results
The 12 FAP-PTC patients in our cohort consisted of 4 males and 8 females (Table 1). Five patients displayed classic PTC histology (three males and two females), six patients had PTC-CMV (one male and five females), and one female had follicular variant of PTC. The number of tumors varied from one to two per individual and tumor sizes varied between 0.1 and 9 cm. All patients in our sample set were diagnosed with FAP before thyroid cancer, with an average age at FAP diagnosis of 25 years (range 12–44 years), and an average age at PTC diagnosis of 38 years (range 20–62 years). Patients were diagnosed with thyroid carcinoma on average 15 years after being diagnosed with FAP (range 1–46 years after FAP diagnosis). This cohort displayed characteristics typically associated with FAP, including CRC, ampullary cancer, and desmoid tumors.
Table 1.
Clinical Characteristics of the 12 Papillary Thyroid Cancer Patients with Familial Adenomatous Polyposis
| Patient no. | Sex | Histology | Age (FAP) | Age (thyroid cancer) | No. of thyroid tumors | Tumor size(s), cm | Ascertainment | Other FAP-related disease presentation |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | PTC-CMV | 12 | 30 | 1 | 9 | *** | Desmoid tumors |
| 2 | Female | PTC-CMV | 21 | 35 | 2 | 0.2 0.1 |
* | Desmoid tumors |
| 3 | Female | PTC-CMV | 43 | 45 | 1 | 2.4 | ** | — |
| 4 | Female | PTC-CMV | 33 | 35 | 2 | 0.9 0.3 |
* | Colon cancer |
| 5 | Female | PTC-CMV | 18 | 43 | 2 | 0.9 0.2 |
* and ** | — |
| 6 | Female | PTC-CMV | 21 | 21 | 2 | 2 3 |
** | Desmoid tumors |
| 7 | Female | PTC | 18 | 42 | 1 | 0.9 | * | Unspecified extrathyroid cancer |
| 8 | Male | PTC | 20 | 44 | 2 | 0.8 1 |
* | Desmoid tumors, adrenal adenoma |
| 9 | Female | PTC | 16 | 62 | 1 | 0.4 | * and ** | Ampullary cancer |
| 10 | Male | PTC | 23 | 28 | 1 | 1.6 | * | Desmoid tumors |
| 11 | Female | PTC-FV | 24 | 24 | 1 | 0.9 | *** | Desmoid tumors |
| 12 | Male | PTC | 44 | 45 | 2 | 0.6 0.4 |
* | Desmoid tumors |
Annual ultrasound screening of FAP patients.
Subjective symptoms.
Computed tomography scan for unrelated reasons.
CMV, cribriform–morular variant; FAP, familial adenomatous polyposis; PTC, papillary thyroid cancer.
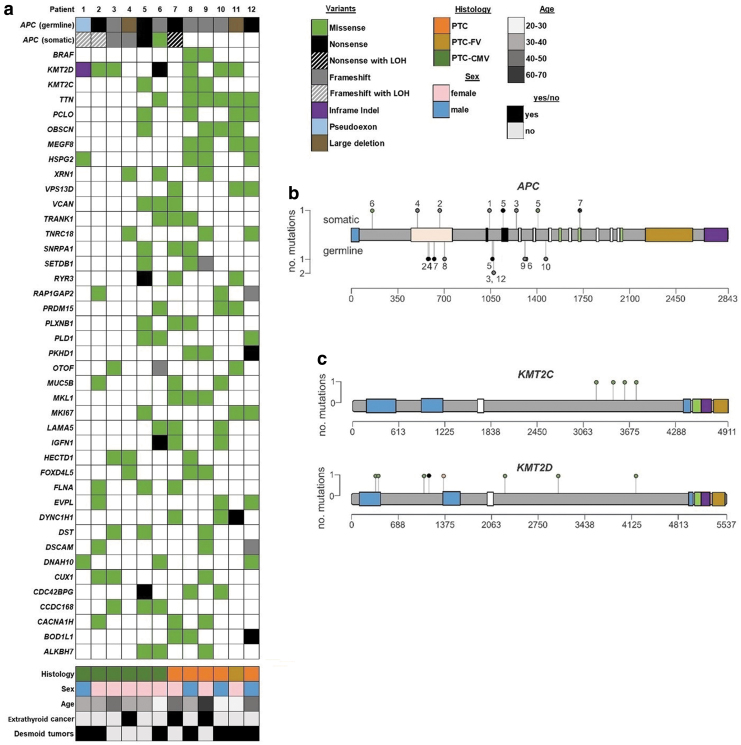
Whole-genome sequencing was performed on paired tumor and normal DNA from all 12 cases. An oncoprint of the most commonly mutated genes is presented in Figure 1a. Seven patients had somatic “second-hit” mutations in APC, including one patient with clear somatic LOH of APC and two patients with putative LOH. The lysine methyl transferase gene KMT2D was also mutated in 7 of the 12 PTC tumors, and another KMT2 family member, KMT2C, was mutated in three patients (four mutations).
FIG. 1.
Mutations and demographics of 12 FAP patients with PTC. (a) Oncoprint shows pathogenic germline APC variants and somatic nonsilent mutations in the indicated genes. Demographic and other clinical information is presented below the variants. (b) Lollipop plot shows all nonsilent somatic (top side) and germline (bottom side) variants in the APC coding sequence. Numbers adjacent to the mutation circles correspond to patient numbers. APC coding sequence is presented for NM_000038.6, and domains are colored as follows: blue, dimerization domain; tan, armadillo repeats; black, 15 amino acid repeats; white, 20 amino acid repeats; green, SAMP repeats; gold, basic domain; purple, EB1 binding. (c) Lollipop plot shows somatic mutations in KMT2C (NM_170606.3) and KMT2D (NM_003482.3). Domains are colored as follows: blue, plant homeodomain fingers; white, high mobility group box; green, FY-rich N-terminal domain; purple, FY-rich C-terminal domain; gold, SET methyl transferase domain. All variants are colored according to the legend. CMV, cribriform–morular variant; FAP, familial adenomatous polyposis; LOH, loss of heterozygosity; PTC, papillary thyroid cancer. Color images are available online.
The recurrently mutated genes in these samples were strikingly different from genes that are typically mutated in sporadic PTC, as evidenced by comparison with the AACR-GENIE and TCGA of PTC data sets (Supplementary Table S1). Specifically, mutations in KMT2C, KMT2D, and APC were only detected in <3% of sporadic cases. Conversely, BRAF is mutated in about 60% of all PTCs, and the vast majority of these mutations consist of the known cancer driver mutation BRAF p.V600E. However, in our sample set, only two cases harbored BRAF mutations (both p.V600E). Notably, neither of these samples had somatic APC mutations. RET/PTC translocations are also common in typical PTCs (14); however, in our cohort, there was no evidence of breakpoints between exons 11 and 12 in RET. Therefore, it is likely that none of the APC-associated PTCs we sequenced harbored RET/PTC or other RET translocations/inversions. We did not detect any somatic mutations in the RAS genes (NRAS, HRAS, and KRAS), which are recurrently mutated in sporadic PTC.
All clinically detected germline nonsense, frameshift, and insertion/deletion variants in the APC gene were validated in our genome sequencing. Three samples had complex APC germline mutations: patient 11 had a heterozygous ∼23 Mb deletion of chromosome 5q that contained APC, patient 4 had a deletion of exons 15–16 (NM_000038, coding exons 14–15), and patient 1 had a C to T transition within intron 11 that results in incorporation of an out-of-frame pseudoexon and underlies FAP, as we have previously described (Table 2) (15). The remaining nine patients all had frameshift and nonsense germline mutations in the APC coding sequence, located between codons 471 and 1465 (Fig. 1b and Table 2). The “second-hit” somatic variants in APC were more spread out, and there was not an obvious correlation between the location of the germline mutation in APC and the somatic second hit, in contrast to reports that the location of the germline FAP-associated APC mutation can influence the position of the somatic APC variant in CRC secondary to FAP (Fig. 1b and Table 2) (16).
Table 2.
Notable Germline and Somatic Variants Identified in 12 Familial Adenomatous Polyposis–Papillary Thyroid Cancers
| Patient no. | APC (germline) | APC (somatic) | BRAF (somatic) | KMT2C (somatic) | KMT2D (somatic) |
|---|---|---|---|---|---|
| 1 | c.1408 + 731C>T (p.Gly471Serfs*55) | c.3119_3149del (p.Arg1040Lysfs*6) and putative LOH | — | — | c.4059_4061del (p.Glu1354del) |
| 2 | c.1732G>T (p.Glu578*) | c.1995delA (p.Leu665Phefs*5) and LOH | — | — | c.1039C>T (p.His347Tyr) |
| 3 | c.3202_3205delTCAA (p.Ser1068Glyfs*57) | c.3729_3730delTC (p.Gln1244Lysfs*11) | — | — | c.12593G>A (p.Arg4198Gln) |
| 4 | c.1744_8532del (p.Glu582fs*14) | c.1490delT (p.Leu497Glnfs*9) | — | — | — |
| 5 | c.3183_3187delACAAA (p.Gln1062fs*) | c.3429T>A (p.Tyr1143*) and c.4195C>T (p.Arg1399Cys) | — | c.10681C>T (p.Pro3561Ser) and c.10235G>A (p.Arg3412Gln) | — |
| 6 | c.3927_3931delAAAGA (p.Glu1309Aspfs*4) | c.436G>A (p.Ala146Thr) | — | — | c.3415C>T (p.Gln1139*) |
| 7 | c.1873C>T (p.Gln625*) | c.5176G>T (p.Glu1726*) and putative LOH | — | — | — |
| 8 | c.2108_2109insG (p.Val704Serfs*2) | — | c.1799T>A (p.Val600Glu) | c.11137G>T (p.Ala3713Ser) | c.6797G>A (p.Gly2266Asp) |
| 9 | c.3920_3924delTAAAA (p.Ile1307Argfs*6) | — | c.1799T>A (p.Val600Glu) | c.9571C>T (p.Leu3191Phe) | — |
| 10 | c.4393_4394delAG (p.Ser1465Trpfs*3) | — | — | — | c.9136G>A (p.Glu3046Lys) |
| 11 | c.1-?_8532+?del (full gene deletion) | — | — | — | c.1168G>A (p.Val390Ile) and c.3190G>A (p.Val1064Ile) |
| 12 | c.3202_3205delTCAA (p.Ser1068Glyfs*57) | — | — | — | — |
Mutations are reported for the following transcripts: APC, NM_000038.6; BRAF, NM_004333.5; KMT2C, NM_170606.3; KMT2D, NM_003482.3.
LOH, loss of heterozygosity; no., number.
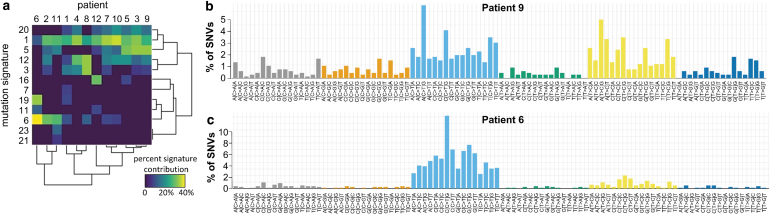
We examined the specific base pair substitutions in the mutations in these samples and compared them with described mutational signatures associated with different cancers and cancer subtypes (17). Most samples showed strong correlations with expression signatures 1 (pan-cancer deamination of 5-methylcytosine), 3 (failure of DNA double-strand break-repair by homologous recombination), 5 (pan-cancer with unknown etiology), 12 (unknown etiology signature found in liver cancer), and 20 (defective DNA mismatch repair) (Fig. 2a, b). The tumor of patient 6 exhibited a strong signal for mutation signature 6, which is seen in microsatellite unstable tumors that have DNA mismatch repair defects, and mutation signature 19, which is an unknown etiology signature found in pilocytic astrocytomas (Fig. 2c) (17–20). Notably, patient 6 did not have somatic mutations in MLH1, PMS2, MSH2, or MSH6 and also did not have likely pathogenic germline variants in these genes.
FIG. 2.
Signatures of somatic mutations in thyroid cancer patients with FAP. (a) Heat map shows the relative contributions of each of the 30 COSMIC v2 mutational signatures for the 12 thyroid cancers in patients with FAP. Signatures that did not contribute to any patients are not shown. (b) The profile displaying the fraction of somatic mutations found in each of the 96 possible trinucleotide combinations of single nucleotide variants for a typical sample in this cohort (patient 9), whose tumor showed mutation consistent with signatures 1, 5, 12, and 20. (c) The mutation profile for patient 7, whose tumor was mostly composed of signatures 6 and 19, is consistent with signatures 1, 5, 12, and 20. COSMIC, catalog of somatic mutations in cancer; SNVs, single nucleotide variant. Color images are available online.
Finally, we examined the germline variants in these samples for alleles that might play a role in thyroid cancer, specifically focusing on nonsynonymous variants in genes previously implicated in familial thyroid cancer (21). Because rare variants are more likely to be high-risk alleles for diseases than common variants, based on family studies (22), we only examined nonsynonymous variants with a population minor allele frequency less than 0.01 in the gnomAD database. We found that nine of the FAP-PTC patients had germline variants in 17 different genes, which were previously found to harbor variants associated with familial thyroid carcinoma (21) (Supplementary Table S2). Interestingly, there were several variants in the RNF213 gene in three different FAP-PTC patients. RNF213 has been found to be mutated in liver cancer (23), and all three of the patients (4,5,8) with RNF213 variants had a strong signal for mutational signature 12 (Fig. 2a), which is linked to liver cancer. The p.R752L FGD6 variant was found in two patients, one from the United States (patient 9) and one from Finland (patient 1). FGD6 is located on chromosome 12q22, and this band has been observed to be amplified in thyroid adenomas and might also play a role in thyroid carcinomas as well (24).
Discussion
It is surprising, although not entirely unexpected, that we detected somatic second hits in APC in over half of the FAP-PTC tumors we analyzed. Cetta et al. reported that somatic mutations in APC do not occur in FAP-associated PTC (4,25), but in contrast, somatic second-hit mutations in APC were previously reported by other groups (3,5,6). One explanation of this apparent contradiction is the recently improved sequencing methodology, and the failure of some researchers to examine the entire APC coding sequence.
Mechanistically, pathogenic nonsense and frameshift APC mutations lead to truncated APC protein products that are unable to interact with the cytoplasmic complex that mediates β-catenin degradation. Thus, the β-catenin/Lef/Tcf complex remains unchecked in the nucleus where it activates WNT signaling pathways responsible for enhanced cellular migration, proliferation, and loss of differentiation (26). Our finding is consistent with the idea that somatic second hits and/or LOH in APC further add cancerous properties to the cell and likely contribute to malignant transformation. Our identification of the somatic BRAF p.V600E mutation in two patients is a novel finding, as to our knowledge, no single case has been described in the literature where oncogenic BRAF mutations occur in either FAP-PTC or PTC-CMV (7,27–32). This implies that some PTCs arising in the context of germline pathogenic APC variants can share the same driver mutations as sporadic PTCs.
The mutual exclusivity of the somatic APC and BRAF mutations is consistent with different molecular subtypes of PTC occurring in different FAP patients. The tumors with BRAF p.V600E mutations displayed typical PTC histology and occurred in one male and one female. However, the patients with nonsilent somatic APC variants were almost entirely female (6 to 1, female to male), and in six of seven cases showed a PTC-CMV histology. Interestingly, the patient with a somatic APC mutation who did not have a PTC-CMV histology (patient 7) harbored the most 3′ APC mutation we detected. The mutated APC protein in patient 7 likely retains some beta-catenin binding ability, and we speculate this could explain that patient 7 did not have a CMV histology.
What does it mean that 58% and 33% of the PTC tumors in these FAP patients have somatic variants in the KMT2D and KMT2C genes, respectively? KMT2D and KMT2C are methyl transferase genes that encode important pieces of the COMPASS complex (33). Pathogenic somatic mutations in both of these genes have been detected in many different cancers, such as oropharyngeal squamous cell carcinoma, T cell lymphoma, bladder cancer, head and neck cancer, and breast and endometrial cancers but are extremely rare in sporadic PTC (34–37). Notably, KMT2D somatic variants have been shown to contribute to increased mutational burden and genome instability (38).
Prompted by the occurrence of KMT2D somatic variants, we looked for a biological link between APC and KMT2D, and to our surprise, it seems that KMT2D might also be involved in WNT signaling. KMT2D together with the ALK gene are connected with CTNNB1 (β-catenin) (39), thus, it is no surprise that in some tumors somatic mutations occur in KMT2D instead of APC.
We speculate that the detected somatic variants in these genes (particularly KMT2D) might be important in the context of activated WNT signaling caused by FAP-associated germline APC mutations, and evidence of epigenetic dysregulation in these cases warrants further investigation. Our data implicate that the mutations in KMT2D may be cancer drivers in the patients in which they were observed.
The overall female to male ratio in our cohort is less skewed toward females than other reports of FAP-PTC patient demographics (7,40). For example, Lam et al. (7) reported that the female to male ratio in PTC-CMV is 31 to 1, whereas in our cohort there was only a 5 to 1 ratio of females to males among patients with PTC-CMV. However, we do acknowledge that the modest size of our cohort does not lend itself to definitive conclusions regarding sex ratios. Seven of the 12 patients had desmoid tumors, which is not surprising given that this a common feature of FAP patients (29,41–45).
Our study is the first that suggests mutations in genes other than APC can cooperate with the germline APC variant in FAP patients to drive thyroid cancer. Also, our findings provide new information on the genetic steps that participate in the carcinogenesis process. Our data are consistent with a model where the pathogenic germline APC variants act as “gatekeepers” in the thyroid. Some patients, almost always females, acquire somatic second hits in APC that drive a thyroid cancer with CMV histology. In other cases, oncogenic activating mutations somatically occur in BRAF, similar to sporadic thyroid cancers. In patients who lack clear driver mutations in APC or BRAF, an intriguing possibility is that somatic variants in other genes (e.g., KTM2D, KMT2C, and others) may act as cancer drivers in the thyroid. This concept postulates that a somatic heterozygous variant in a gene such as KTM2D can act as a trigger of the malignant transformation of a cell heterozygous for a pathogenic variant in another gene (i.e., APC) and is in line with the concept that multiple events contributing different cancerous properties to a cell need to occur in order for a malignancy to develop and proliferate (46).
It is striking that 9 of the 12 patients we sequenced harbored rare nonsynonymous mutations in 17 selected genes known to be associated with familial thyroid cancer. This is consistent with the concept that additional germline variants other than of APC can contribute to PTC formation in FAP patients. Further studies are necessary to unequivocally prove a causative role for the implicated germline variants in FAP-associated PTC and explore their interactions with the altered WNT signaling caused by pathogenic APC variants.
Supplementary Material
Acknowledgments
The authors would like to acknowledge: the patients who consented to provide material for this study; Jan Lockman and Barbara Fersch for administrative help; the OSUCCC Genomics Shared Resource and the Plant Microbe Genomics Facility for sequencing assistance; the OSUCCC Human Cancer Genetics Sample Bank for sample processing and storage; and the Ohio Supercomputer Center for computational resources.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the Leukemia Clinical Research Foundation, the Pelotonia fellowship program, the Jane and Aatos Erkko Foundation, the Finnish Cancer Organizations, the Sigrid Juselius Foundation, the Inkeri and Mauri Vänskä Foundation, the HiLIFE Fellows 2017–2020, the National Cancer Institute Grants P01CA124570 and P50CA168505, and The Academy of Finland Grant 294643.
Supplementary Material
References
- 1. Herraiz M, Barbesino G, Faquin W, Chan-Smutko G, Patel D, Shannon KM, Daniels GH, Chung DC. 2007. Prevalence of thyroid cancer in familial adenomatous polyposis syndrome and the role of screening ultrasound examinations. Clin Gastroenterol Hepatol 5:367–373 [DOI] [PubMed] [Google Scholar]
- 2. Nallamilli BR, Hegde M. 2017. Detecting APC gene mutations in familial adenomatous polyposis (FAP). Curr Protoc Hum Genet 92: 10.1002/cphg.29 [DOI] [PubMed] [Google Scholar]
- 3. Soravia C, Sugg SL, Berk T, Mitri A, Cheng H, Gallinger S, Cohen Z, Asa SL, Bapat BV. 1999. Familial adenomatous polyposis-associated thyroid cancer: a clinical, pathological, and molecular genetics study. Am J Pathol 154:127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cetta F, Dhamo A, Malagnino G, Barellini L. 2007. Germ-line and somatic mutations of the APC gene and/or β-catenin gene in the occurrence of FAP associated thyroid carcinoma. World J Surg 31:1366–1369 [DOI] [PubMed] [Google Scholar]
- 5. Iwama T, Konishi M, Iijima T, Yoshinaga K, Tominaga T, Koike M, Miyaki M. 1999. Somatic mutation of the APC gene in thyroid carcinoma associated with familial adenomatous polyposis. Jpn J Cancer Res 90:372–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uchino S, Noguchi S, Yamashita H, Yamashita H, Watanabe S, Ogawa T, Tsuno A, Murukami A, Miyauchi A. 2006. Mutational analysis of the APC gene in cribriform-morular variant of papillary thyroid carcinoma. World J Surg 30:775–779 [DOI] [PubMed] [Google Scholar]
- 7. Lam AK, Saremi N. 2017. Cribriform-morular variant of papillary thyroid carcinoma: a distinctive type of thyroid cancer. Endocr Relat Cancer 24:R109- R121 [DOI] [PubMed] [Google Scholar]
- 8. Uchino S, Ishikawa H, Miyauchi A, Hirokawa M, Noguchi S, Ushiama M, Yoshida T, Michikura M, Sugano K, Sakai T. 2016. Age- and gender-specific risk of thyroid cancer in patients with familial adenomatous polyposis. J Clin Endocrinol Metab 101:4611–4617 [DOI] [PubMed] [Google Scholar]
- 9. Lahiri DK, Nurnberger JI Jr. 1991. A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. J Nucl Acids Res 19:5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katainen R, Donner I, Cajuso T, Kaasinen E, Palin K, Makinen V, Aaltonen LA, Pitkanen E. 2018. Discovery of potential causative mutations in human coding and noncoding genome with the interactive software BasePlayer. Nat Protoc 13:2580–2600 [DOI] [PubMed] [Google Scholar]
- 11. Rosenthal R, McGranahan N, Herrero J, Taylor BS, Swanton C. 2016. DeconstructSigs: delineating mutational processes in single tumors distinguishes DNA repair deficiencies and patterns of carcinoma evolution. Genome Biol 17:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Porkka N, Valo S, Nieminen T, Olkinuora A, Maki-Nevala S, Eldfors S, Peltomaki P. 2017. Sequencing of Lynch syndrome tumors reveals the importance of epigenetic alterations. Oncotarget 14:108020–108030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olllikainen M, Abdel-Rahman WM, Moisio AL, Lindroos A, Kariola R, Jarvela I, Poyhonen M, Butzow R, Peltomaki P. 2005. Molecular analysis of familial endometrial carcinoma: a manifestation of hereditary nonpolyposis colorectal cancer of a separate syndrome? J Clin Oncol 20:4609–4616 [DOI] [PubMed] [Google Scholar]
- 14. Cetta F, Chiappetta G, Melillo RM, Petracci M, Montalto G, Santoro M, Fusco A. 1998. The ret/ptc1 oncogene is activated in familial adenomatous polyposis- associated thyroid papillary carcinomas. J Clin Endocrinol Metab 83:1003–1006 [DOI] [PubMed] [Google Scholar]
- 15. Nieminen TT, Pavicic W, Porkka N, Kankainen M, Jarvinen HJ, Lepisto A, Peltomaki P. 2016. Pseudoexons provide a mechanism for allele-specific expression of APC in familial adenomatous polyposis. Oncotarget 7:70685–70698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Albuquerque C, Breukel C, van der Luijt R, Fidalgo P, Lage P, Slors FJ, Leitao CN, Fodde R, Smits R. 2002. The ‘just-right’ signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade. Hum Mol Genet 11:1549–1560 [DOI] [PubMed] [Google Scholar]
- 17. Alexandrov LB, Nik-Zainal S, Wedge DC, Campbell PJ, Stratton MR. 2013. Deciphering signature of mutational processes operative in human cancer. Cell Rep 3:246–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nik-Zainal S, Alexandrov LB, Wedge DC, Van Loo P, Greenman CD, Raine K, Jones D, Hinton J, Marshall J, Stebbings LA, Menzies A, Martin S, Leung K, Chen L, Leroy C, Ramakrishna M, Rance R, Lau KW, Mudie LJ, Varela I, McBride DJ, Bignell GR, Cooke SL, Shlien A, Gamble J, Whitmore I, MaddisonM, Tarpey PS, Davies HR, Papaemmanuil E, Stephens PJ, McLaren S, Butler AP, Teague JW, Jönsson G, Garber JE, Silver D, Miron P, Fatima A, Boyault S, Langerød A, Tutt A, Martens JW, Aparicio SA, Borg Å, Salomon AV, Thomas G, Børresen-Dale AL, Richardson AL, Neuberger MS, Futreal PA, Campbell PJ, Stratton MR; Breast Cancer Working Group of the International Cancer Genome Consortium. 2012. Mutational processes molding the genomes of 21 breast cancers. Cell 25:979–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Helleday T, Eshtad S, Nik-Zainal S. 2014. Mechanisms underlying mutational signatures in human cancers. Nat Rev Genet 15:585–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alexandrov LB, Stratton MR. 2014. Mutational signatures: the patterns of somatic mutations hidden in cancer genomes. Curr Opin Genet Dev 24:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Liyanarachchi S, Miller KE, Nieminen TT, Comiskey DF, Li W, Brock P, Symer DE, Akagi K, He H, Koboldt DC, de la Chapelle A. 2019. Whole genome sequencing reveals rare germline variants leading to familial non-medullary thyroid cancer. Thyroid 29:946–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Foulkes WD. 2008. Inherited susceptibility to common cancers. N Eng J Med 359:2143–2153 [DOI] [PubMed] [Google Scholar]
- 23. Li X, Xu W, Kang W, Wong SH, Wang M, Zhou Y, Fang X, Zhang X, Yang H, Wong CH, To KF, Chan SL, Chan MTV, Sung JJY, Wu WKK, Yu J. 2018. Genomic analysis of liver cancer unveils novel driver genes and distinct prognostic features. Theranostics 8:1740–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Y, Cope L, Sun W, Wang Y, Prasad N, Sangenario L, Talbot K, Somervell H, Westra W, Bishop J, Califano J, Zeiger M, Umbricht C. 2013. DNA copy number variations characterize benign and malignant thyroid tumors. J Clin Endocrinol Metab 98:E558–E566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cetta F, Pelizzo MR, Curia MC, Barbarisi A. 1999. Genetics and clinicopathological findings in thyroid carcinomas associated with familial adenomatous polyposis. Am J Pathol 155:7–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gao C, Wang Y, Broaddus R, Sun L, Xue F, Zhang W. 2018. Exon 3 mutations of CTNNB1 drive tumorigenesis: a review. Oncotarget 9:5492–5508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rossi ED, Revelli L, Martini M, Taddei A, Pintus C, Panunzi C, Fadda G. 2012. Cribriform-morular variant of papillary thyroid carcinoma in an 8-year-old girl: a case report with immunohistochemical and molecular testing. Int J Surg Pathol 20:629–632 [DOI] [PubMed] [Google Scholar]
- 28. Nakazawa T, Celestino R, Machado JC, Cameselle-Teijeiro JM, Vinagre J, Eloy C, Benserai F, Lameche S, Soares P, Sobrinho-Simoes M. 2013. Cribriform-morular variant of papillary thyroid carcinoma displaying poorly differentiated features. Int J Surg Pathol 21:379–389 [DOI] [PubMed] [Google Scholar]
- 29. Giannelli SM, McPhaul L, Nakamoto J, Gianoukakis AG. 2014. Familial adenomatous polyposis-associated, cribriform morular variant of papillary thyroid carcinoma harboring a K-RAS mutation: case presentation and review of molecular mechanisms. Thyroid 24:1184–1189 [DOI] [PubMed] [Google Scholar]
- 30. Kwon MJ, Rho YS, Jeong JC, Shin HS, Lee JS, Cho SJ, Nam ES. 2015. Cribriform-morular variant of papillary thyroid carcinoma: a study of 3 cases featuring the PIK3CA mutation. Hum Pathol 46:1180–1188 [DOI] [PubMed] [Google Scholar]
- 31. Brehar AC, Terzea DC, Ioachim DL, Procopiuc C, Brehar FM, Bulgar AC, Ghemigian MV, Dumitrache C. 2016. Cribriform-morular variant of papillary thyroid carcinoma at pediatric age—case report and review of the literature. Rom J Morphol Embryol 57:531–537 [PubMed] [Google Scholar]
- 32. Oh EJ, Lee S, Bae JS, Kim Y, Jeon S, Jung CK. 2017. TERT promoter mutation in an aggressive cribriform morular variant of papillary thyroid carcinoma. Endocr Pathol 28:49–53 [DOI] [PubMed] [Google Scholar]
- 33. Lang A, Yilmaz M, Hader C, Murday S, Kunz X, Wagner N, Wiek C, Petzsch P, Köhrer K, Koch J, Hoffmann MJ, Greife A, Schulz WA. 2019. Contingencies of UTX/KDM6A action in urothelial carcinoma. Cancers (Basel) 11:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haft S, Ren S, Xu G, Mark A, Fisch K, Guo TW, Khan Z, Pang J, Ando M, Liu C, Sakai A, Fukusumi T, Califano JA. 2019. Mutation of chromatin regulators and focal hotspot alterations characterize human papillomavirus-positive oropharyngeal squamous cell carcinoma. Cancer 125:2423–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fernandez-Pol S, Ma L, Joshi RP, Arber DA. 2019. A survey of somatic mutations in 41 genes in a cohort of T-cell lymphomas identifies frequent mutations in genes involved in epigenetic modification. Appl Immunohistochem Mol Morphol 27:416–422 [DOI] [PubMed] [Google Scholar]
- 36. Herz HM. 2016. Enhancer deregulation in cancer and other diseases. Bioessays 38:1003–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. 2013. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kantidakis T, Saponaro M, Mitter R, Horswell S, Kranz A, Boeing S, Aygün O, Kelly GP, Matthews N, Stewart A, Stewart AF, Svejstrup JQ. 2016. Mutation of cancer driver MLL2 results in transcription stress and genome instability. Genes Dev 15:408–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pinckney L, Collins J, Schammel CMG, Gevaert M, Schammel DP, Edenfield WJ, Elder J, Puls LE. 2018. Mutational analysis of selected high-grade malignancies in a premenopausal gynecologic cancer population: a potential for targeted therapies? Appl Cancer Res 38:1–12 [Google Scholar]
- 40. Cetta F. 2015. FAP associated papillary thyroid carcinoma: a peculiar subtype of familial nonmedullary thyroid cancer. Patholog Res Int 2015:309348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chong J, Koshiishi N, Kurihara K, Kubuno S, Kawai T, Fukayama M. 2000. Aspiration and imprint cytopathology of thyroid carcinoma associated with familial adenomatous polyposis. Diagn Cytopathol 23:101–105 [DOI] [PubMed] [Google Scholar]
- 42. Dalal KM, Moraitis D, Iwamoto C, Shaha AR, Patel SG, Ghossein RA. 2006. Clinical curiosity: cribriform-morular variant of papillary thyroid carcinoma. Head Neck 28:471–476 [DOI] [PubMed] [Google Scholar]
- 43. Crippa S, Saletti P, Barizzi J, Mazzucchelli L. 2012. The clinical management in familial adenomatous polyposis deserves continuous monitoring for thyroid carcinoma. BMJ Case Rep 2012:bcr2012007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abdullah Suhaimi SN, Nazri N, Nani Harlina ML, Md Isa N, Muhammad R. 2015. Familial adenomatous polyposis-associated papillary thyroid cancer. Malays J Med Sci 22:69–72 [PMC free article] [PubMed] [Google Scholar]
- 45. Alikhan M, Koshy A, Hyjek E, Stenson K, Cohen RN, Yeo KT. 2015. Discrepant serum and urine β-hCG results due to production of β-hCG by a cribriform- morular variant of thyroid papillary carcinoma. Clin Chim Acta 438:181–185 [DOI] [PubMed] [Google Scholar]
- 46. Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 4:646–674 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.