Abstract
Background
ONC201 is a dopamine receptor D2 (DRD2) antagonist that penetrates the blood–brain barrier. ONC201 efficacy has been shown in glioblastoma animal models and is inversely correlated with dopamine receptor DRD5 expression. ONC201 is well tolerated in adult recurrent glioblastoma patients with dosing every 3 weeks and has achieved an objective radiographic response in a patient harboring the H3 K27M mutation.
Methods
In a window-of-opportunity arm, 6 adult subjects initiated ONC201 prior to re-resection of recurrent glioblastoma with intratumoral concentrations as the primary endpoint. An additional 20 adults with recurrent glioblastoma received single agent weekly oral ONC201 at 625 mg, with progression-free survival at 6 months (PFS6) by Response Assessment in Neuro-Oncology (RANO) criteria as the primary endpoint.
Results
The window-of-opportunity arm achieved its primary endpoint with intratumoral ONC201 concentrations at ~24 hours following the second weekly dose ranging from 600 nM to 9.3 µM. Intratumoral pharmacodynamics assessed by activating transcriptional factor 4, death receptor 5, and apoptosis induction relative to archival samples were observed with the strongest intensity and uniformity among patients with low DRD5 tumor expression. The primary endpoint of PFS6 by RANO was not achieved at 5% in this molecularly unselected cohort; however, 1 of 3 patients enrolled with the H3 K27M mutation had a complete regression of enhancing multifocal lesions that remained durable for >1.5 years. No treatment modifications or discontinuations due to toxicity were observed, including in those who underwent re-resection.
Conclusions
Weekly ONC201 is well tolerated, and meaningful intratumoral concentrations were achieved. ONC201 may be biologically active in a subset of adult patients with recurrent glioblastoma.
Keywords: DRD2, DRD5, glioblastoma, H3 K27M, ONC201
Key Points.
Biologically active intratumoral drug concentrations are achieved with weekly ONC201 dosing.
Although the PFS6 endpoint in unselected patients was not achieved, ONC201 may be biologically active in a subset of adult patients with recurrent glioblastoma.
Importance of the Study.
Dopamine receptor D2 is overexpressed in high-grade gliomas and controls mitogenic signaling pathways. ONC201 is a small-molecule DRD2 antagonist that penetrates the blood–brain barrier and improves survival in glioblastoma animal models. We determined intratumoral concentrations, intratumoral pharmacodynamics, safety, and clinical activity of oral ONC201 administered once every week to adult recurrent glioblastoma patients. ONC201 achieved intratumoral concentrations that exceeded preclinical efficacy thresholds and induced biomarkers of pharmacodynamic signaling/apoptosis indicating target engagement. We report the second case of an adult recurrent H3 K27M–mutant glioblastoma patient undergoing radiographic regression of multifocal, multirecurrent disease with ONC201 administration. Our results indicate weekly ONC201 is well tolerated and may be biologically active in a subset of glioblastoma patients who may be identified by biomarkers that include DRD2, DRD5, and/or H3 K27M. These findings support ongoing clinical trials of ONC201 in diffuse midline gliomas with or without the H3 K27M mutation.
There is mounting evidence that gliomas and other cancers can exploit neurotransmitters such as dopamine to promote tumorigenesis, propagation, and dissemination.1,2 Paracrine sourcing of dopamine from the microenvironment or autocrine production by tumor cells can produce an immunosuppressive tumor microenvironment and activate prosurvival signaling pathways in tumor cells.3,4 Among the 5 dopamine receptor family members, dopamine receptor D2 (DRD2) has been the most extensively studied in cancer.5–8 Selective overexpression of DRD2 has been reported in glioblastoma, along with suppression of mitogen-activated protein kinase signaling and tumor growth in response to gene silencing.5,9
ONC201 is the first small-molecule DRD2 antagonist for oncology that has demonstrated p53-independent antitumor activity in preclinical models of glioblastoma and several other cancers.9–22 The compound crosses the intact blood–brain barrier in rodents and has exhibited antiglioma activity in an orthotopic model of glioblastoma.21 Downstream signaling effects of ONC201 in tumor cells include activation of the integrated stress response,23–25 mitochondrial stress,10 Akt/extracellular signal-regulated kinase inactivation, and induction of TRAIL (tumor necrosis factor–related apoptosis-inducing ligand)/death receptor 5 (DR5)11,21 that lead to cell cycle arrest and apoptosis. Prolonged pharmacodynamics have been reported in vitro and in vivo with single or infrequent doses.21,26 DRD5 is a member of the dopamine receptor family that causes downstream effects that oppose those of DRD2, and its expression influences anticancer efficacy of DRD2 antagonism. A DRD2+DRD5− expression signature is associated with improved ONC201 efficacy broadly in vitro across human cancers and in adult recurrent glioblastoma patients.9
Phase I clinical trials in advanced solid tumors have determined the recommended phase II dose of ONC201 to be 625 mg administered every 1 or 3 weeks.27 This dose was selected based on pharmacokinetic and pharmacodynamic studies without any dose-limiting toxicities. Once every 3 weeks, dosing was selected for clinical introduction; however, subsequent evaluation of weekly dosing has shown equivalent safety and enhanced immunostimulatory activity.28
We previously reported the first clinical study of ONC201 in adult recurrent glioblastoma patients.29 Similar to other clinical studies, 625 mg of ONC201 was well tolerated with every 3 week oral dosing. Low DRD5 expression was associated with relatively prolonged progression-free survival (PFS) and overall survival (OS).9 An objective response was observed in a 22-year-old female patient with recurrent thalamic glioblastoma harboring the H3 K27M mutation. This response has remained durable for 2.5 years with radiographic resolution of the primary thalamic lesion and continued treatment without drug-related toxicity.9 Subsequent preclinical studies have suggested that H3 K27M–mutant gliomas exhibit an altered dopamine receptor expression profile and increased sensitivity to ONC201.30 Additionally, we recently reported the clinical experience with ONC201 in a 10-year-old female patient with H3.3 K27M–mutant diffuse intrinsic pontine glioma.31 ONC201 was well tolerated when administered at the adult recommended phase II dose scaled by body weight. Radiographic tumor shrinkage was observed that remained durable for >18 months and was associated with sustained improvement in grade IV facial palsy and hearing loss.
The aim of this study was to determine the intratumoral drug concentrations, intratumoral pharmacodynamics, safety, and clinical activity of ONC201 administered as a 625 mg oral dose once every week to adult patients with recurrent glioblastoma.
Materials and Methods
Patients
Patients ≥18 years of age were enrolled on 2 arms as part of a phase II clinical trial (NCT02525692). This study was approved by local institutional review boards and complied with International Ethical Guidelines for Biomedical Research Involving Human Subjects, good clinical practice guidelines, and the Declaration of Helsinki. Written informed consent was obtained for each patient. Twenty-six patients were enrolled into 2 arms: 20 patients in a nonsurgical arm and 6 patients in a surgical arm. For both arms, patients had histologically confirmed World Health Organization glioblastoma with radiographic evidence of progressive disease by Response Assessment in Neuro-Oncology (RANO) criteria following initial therapy. Patients were at least 12 weeks from prior radiotherapy, corticosteroid dose was stable or decreasing for at least 2 weeks prior to study entry, Karnofsky performance status (KPS) was ≥60%, and prior bevacizumab was not allowed. Isocitrate dehydrogenase 1 and 2 mutant glioblastomas were not eligible. Archival tumor tissue was required. For the surgical arm, patients needed to be eligible for salvage surgical resection.
Procedures
ONC201 was administered orally once every week on an outpatient basis. Treatment was continued until radiographic or clinical progression, unacceptable toxicity, or patient decision. For patients in the surgical arm, ONC201 was initiated 8 days prior to scheduled surgical resection in order to obtain a resected specimen ~24 hours after the second dose of ONC201.
Endpoints and Assessments
For the surgical arm, the primary endpoint was determination of intratumoral concentrations of ONC201. For the nonsurgical arm, the primary endpoint was PFS at 6 months (PFS6) by RANO criteria. Secondary endpoints were objective response rate, PFS, OS, and safety. Exploratory endpoints were correlations between clinical outcome and tumor tissue predictive and pharmacodynamic biomarkers.
MRI for disease assessments was performed every 8 weeks according to RANO criteria. OS was calculated from time of study registration until death. PFS was measured from time of study registration until documented progression. PFS and OS are reported using the intention to treat concept. Safety data are reported using summary tables and data listings. PFS and OS were assessed using the Kaplan–Meier method. Toxicity was graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) v4.0. All patients who received therapy on study were considered evaluable for toxicity.
Tissue Analyses
Immunohistochemistry was performed with formalin-fixed paraffin-embedded tissue for DRD2 (Santacruz, sc-5303), DRD5 (Atlas, HPA048930), activating transcription factor 4 (ATF4) (Abcam, ab184909), DR5 (Novus, NB100-56618), and terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling (TUNEL) (Novus, #NBP2-31164) as previously described.26,27 For intratumoral drug concentrations, the previously described liquid chromatography–tandem mass spectrometry (LC-MS/MS) method for detection of ONC201 in K2EDTA human plasma was adapted.27 Resected tumor tissue was flash frozen until analysis. Tissue homogenates were prepared from weighed, thawed samples with a tissue homogenizer and 5-fold dilution into EDTA human plasma. Extraction was performed with acetonitrile, and reconstitution was in methanol:water.
Rat Biodistribution Study
Female Sprague-Dawley rats (n = 2 per time point) received 125 mg/kg oral ONC201. Plasma (ng/mL) and tissue (ng/g) concentrations were determined using LC-MS/MS as described above at the following time points (h): 0, 0.083, 0.5, 1, 2, 4, 8, 12, 24, 48, 72. The following tissues were evaluated: liver, kidney, large intestine, muscle, cerebrospinal fluid, bone marrow, skin, neck lymph, abdominal cavity lymph, spleen, brain, spinal cord, and adipose tissue. The studies were performed at Wuxi AppTec. The animal use and all procedures performed in the study were approved by the Institutional Animal Care and Use Committee of Wuxi AppTec. All federal, local, and institutional regulations were strictly followed while performing the study.
Statistical Analyses
For the surgical arm, a sample size of 6 was selected for an 80% power to detect a difference of −1.4 between the null hypothesis mean of 0.0 and the alternative hypothesis mean of 1.4 with an estimated standard deviation of 1.0 and with a significance level (alpha) of 0.05 using a two-sided one-sample t-test.
For PFS6, the study was designed to detect a difference of 20% using a one-sided binomial test with a null hypothesis of <10% against and an alternative hypothesis of ≥30%. The null hypothesis was based on a large meta-analysis of clinical trials that evaluated a wide array of salvage therapeutics for recurrent glioblastoma.32–34 This design yields 85% power to detect the 20% difference at an alpha level of 0.05. A Simon 2-stage phase II design was used with an interim analysis after 16 evaluable patients. Assuming exponential distribution, the null and alternative for the PFS6 are translated into 0.44 versus 0.67 rates for PFS2. Under these rates the chances of observing 7 or fewer progression-free patients out of the first 16 are 0.6 under the null and 0.046 under the alternative hypothesis. A total of 20 patients were accrued for this analysis because 4 patients did not receive at least 6 doses of ONC201 and were deemed replaceable per protocol; however, all patients were included in the presented analyses. The futility criteria were met in the first stage and further accrual to this arm was closed.
Results
Patient Characteristics and Treatment
A total of 26 patients were enrolled between March 2017 and April 2018—6 patients in a surgical arm to assess intratumoral drug concentrations and pharmacodynamics and 20 patients in a nonsurgical arm to evaluate PFS6. Patient characteristics are listed in Table 1 for each arm. All patients received 625 mg ONC201 as oral capsules once every week.
Table 1.
Baseline demographic, clinical, and molecular characteristics of patients treated with weekly ONC201 in nonsurgical and surgical arms
| Nonsurgical Arm (N = 20) | Surgical Arm (N = 6) | |
|---|---|---|
| Age, y, median (range) | 55 (20–74) | 65.5 (47–69) |
| Male:female | 10:10 | 1:5 |
| KPS, median (range) | 80 (20–80) | 85 (80–100) |
| Number of baseline lesions, median (range) | 1 (1–3) | 1 (1–2) |
| Number of recurrences | ||
| 1 | 16 (80%) | 5 (83%) |
| 2 | 3 (15%) | 1 (17%) |
| 3 | 1 (5%) | 0 (0%) |
| Prior low-grade diagnosis | 1 (5%) | 0 (0%) |
| Prior radiotherapy | 20 (100%) | 5 (83%) |
| Prior temozolomide | 20 (100%) | 6 (100%) |
| Extent of resection at the latest surgery | ||
| Subtotal (partial) | 3 (15%) | 0 (0%) |
| Gross total | 13 (65%) | 6 (100%) |
| Unknown | 4 (20%) | 1 (17%) |
| Salvage surgery at time of recurrence | 6 (30%) | 1 (17%) |
| MGMT | ||
| Methylated | 5 (25%) | 2 (33%) |
| Unmethylated | 11 (55%) | 4 (67%) |
| Unknown | 4 (20%) | 0 (0%) |
| H3 K27M | 3 (15%) | 0 (0%) |
| Corticosteroids at study entry | 18 (90%) | 5 (83%) |
For the 6 patients in the surgical arm, the median age was 65.5 years and 5 (83%) were female. Five of these patients had glioblastoma at first recurrence. All had previously received at least radiation and temozolomide. Four (67%) patients had unmethylated O6-methylguanine-DNA methyltransferase (MGMT) status. Five (83%) of these patients were receiving corticosteroids at study entry.
For the 20 patients in the nonsurgical arm, the median age was 55 years (range, 20–74) with an equal number of male to female patients. All patients had primary glioblastoma, except 1 (5%), who initially had a diagnosis of grade II astrocytoma that transformed into glioblastoma. All patients previously received radiation and temozolomide. Sixteen (80%) were at first recurrence, 3 (15%) at second recurrence, and 1 (5%) at third recurrence. Eleven (55%) patients had unmethylated MGMT status. Eighteen (90%) of these patients were receiving corticosteroids at study entry.
Safety
ONC201 was well tolerated, with no treatment modifications or interruptions due to drug-related toxicity in either arm. There were no reported grade 3 or 4 drug-associated adverse events (Table 2). For the nonsurgical arm, the most common adverse events attributed as possibly/probably related to study drug by investigators were grades 1 and 2 hypophosphatemia (35%), nausea (25%), vomiting (25%), and diarrhea (25%). For the surgical arm, the most common adverse event was headache in 4 (67%) patients (one grade 1, two grade 2, one grade 3) that was not attributed to study drug (Supplementary Table 1).
Table 2.
Adverse events in patients treated on the nonsurgical arm (N = 20)*
| All Adverse Events | Possibly/Probably Related | |||
|---|---|---|---|---|
| Adverse Events, N (%) | All Grades | Grades 3–4 | All Grades | Grades 3–4 |
| Nervous system disorders | ||||
| Headache | 10 (50%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Nervous system disorders, other | 6 (30%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Dizziness | 4 (20%) | 0 (0%) | 1 (5%) | 0 (0%) |
| Memory impairment | 4 (20%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Seizure | 4 (20%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Paresthesia | 3 (15%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Metabolism and nutrition disorders | ||||
| Hypophosphatemia | 9 (45%) | 0 (0%) | 7 (35%) | 0 (0%) |
| Hyperglycemia | 8 (40%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Anorexia | 4 (20%) | 0 (0%) | 3 (15%) | 0 (0%) |
| General disorders and administration site conditions | ||||
| Gait disturbance | 8 (40%) | 1 (5%) | 4 (20%) | 0 (0%) |
| Fatigue | 7 (35%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Injury, poisoning, and procedural complications | ||||
| Fall | 8 (40%) | 1 (5%) | 0 (0%) | 0 (0%) |
| Gastrointestinal disorders | ||||
| Nausea | 7 (35%) | 0 (0%) | 5 (25%) | 0 (0%) |
| Vomiting | 7 (35%) | 0 (0%) | 5 (25%) | 0 (0%) |
| Gastrointestinal disorders, other | 6 (30%) | 1 (5%) | 0 (0%) | 0 (0%) |
| Diarrhea | 5 (25%) | 0 (0%) | 5 (25%) | 0 (0%) |
| Dysphagia | 3 (15%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Investigations | ||||
| Platelet count decreased | 6 (30%) | 0 (0%) | 1 (5%) | 0 (0%) |
| Psychiatric disorders | ||||
| Confusion | 5 (25%) | 0 (0%) | 1 (5%) | 0 (0%) |
| Insomnia | 4 (20%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Musculoskeletal and connective tissue disorders | ||||
| Muscle weakness right-sided | 4 (20%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Muscle weakness left-sided | 3 (15%) | 2 (10%) | 0 (0%) | 0 (0%) |
| Muscle weakness lower limb | 3 (15%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Infections and infestations | ||||
| Urinary tract infection | 3 (15%) | 1 (5%) | 0 (0%) | 0 (0%) |
| Vascular disorders | ||||
| Hypertension | 3 (15%) | 2 (10%) | 0 (0%) | 0 (0%) |
*Adverse events by CTCAE reported in >10% of patients.
Molecular Assessments
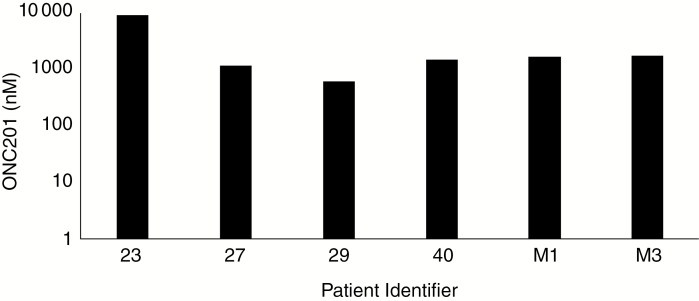
Analyzing tumor tissue resected ~24 hours after the second weekly dose of ONC201 revealed concentrations of 600 nM to 9.3 µM that exceeded the 600 nM half-maximal inhibitory concentration observed in glioblastoma neurosphere cultures (Fig. 1). There was a modest correlation between the intratumoral concentration of the drug and systemic exposure, as approximated from plasma concentrations at the previously reported Tmax of 2 hours (Supplementary Fig. 1). Blood–brain barrier penetrance is consistent with findings from a non-tumor-bearing rat biodistribution study that revealed ~5-fold higher concentrations in the brain and other organs relative to the plasma (Supplementary Fig. 2). However, significantly higher concentrations were sustained beyond 12 hours following administration in humans relative to rats.
Fig. 1.
ONC201 concentrations in tumor tissue homogenates from resected glioblastoma specimens ~24 hours after the second dose of 625 mg oral ONC201.
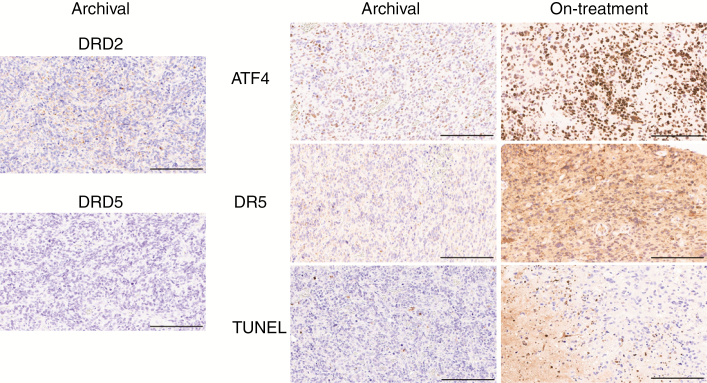
Immunohistochemistry analysis was conducted on archival tumor specimens and posttreatment samples for pharmacodynamic assessments associated with response to ONC201 in preclinical models: ATF4, DR5, and apoptosis. The patient exhibiting induction of all pharmacodynamic biomarkers had the lowest intratumoral concentration of ONC201 (Patient 29; Fig. 2), supporting the conclusion that therapeutic thresholds were exceeded. Heterogeneous induction across markers was observed in the cohort; however, consistent induction across markers was observed in 2 of 6 patients (Table 3).
Fig. 2.
Biomarker immunohistochemistry analysis for adult recurrent glioblastoma patient treated with ONC201. Left panel shows DRD2 and DRD5 expression in archival tumor sample. Right panel shows pharmacodynamic biomarkers of ONC201 in archival tumor sample (archival) and in tumor sample resected ~24 hours after the second weekly dose of 625 mg ONC201 (on-treatment). Scale bars: 200 µm.
Table 3.
Biomarker analysis of ONC201-treated patients undergoing resection of recurrent glioblastoma
| Patient Identifier | ATF4 Induction | DR5 Induction | TUNEL Induction | DRD5 |
|---|---|---|---|---|
| 23 | ✓ | ✓ | * | – |
| 27 | ✓ | ✘ | ✘ | + |
| 29 | ✓ | ✓ | ✓ | – |
| 40 | ✘ | ✓ | ✘ | – |
| M1 | ✓ | ✓ | ✘ | – |
| M3 | ✘ | ✘ | * | + |
*Insufficient tissue.
Recent studies have identified a DRD2+DRD5− biomarker signature as a predictor of sensitivity to ONC201.9 Immunohistochemistry analysis for these 2 biomarkers revealed DRD2 staining in all patients’ specimens with heterogeneity in DRD5 expression. Interestingly, the 2 patients with consistent biomarker panel induction had low DRD5 expression in their tumors.
Clinical Outcomes
Only patients in the nonsurgical arm were assessed for clinical efficacy endpoints. The median PFS was 1.8 months and PFS6 was 5%, which did not achieve the primary endpoint, and accrual was not continued due to futility (Supplementary Fig. 3). Median OS was 7.5 months.
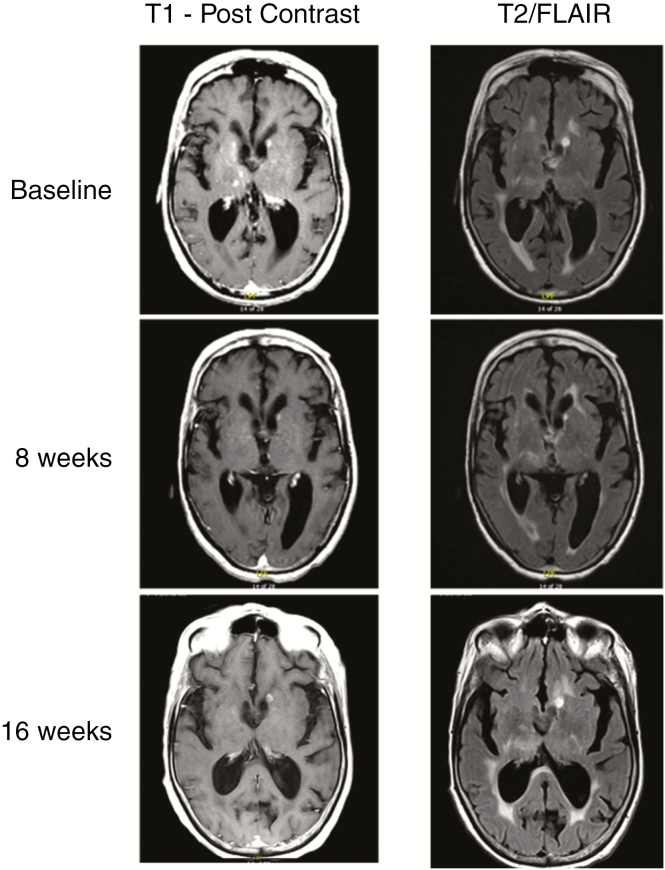
One patient with subcentimeter, multifocal, recurrent glioblastoma harboring the H3 K27M mutation experienced a complete regression of her enhancing lesions (Fig. 3). One T1-hyperintense lesion adjacent to the left lateral ventricle was of unclear etiology. Prior to initiating ONC201, her disease recurred following second-line CCNU that was preceded by surgery, radiation, and temozolomide. This tumor regression was not evaluable by RANO due to subcentimeter lesion sizes; however, these regressions remained durable for over 1.5 years. Tumor regressions were not observed in other patients in the arm, including 2 other patients with recurrent glioblastoma harboring the H3 K27M mutation.
Fig. 3.
Multifocal tumor regression in patient with recurrent glioblastoma harboring the H3 K27M mutation.
Discussion
Most cancer therapies do not cross the blood–brain barrier, often precluding their therapeutic potential for brain tumors and sometimes necessitating intrathecal injection, convection-enhanced delivery, or other alternative delivery methods. We report the results from the first surgical study of ONC201 in recurrent glioblastoma patients. These findings corroborate preclinical observations performed in rodents that ONC201 penetrates the blood–brain barrier and achieves biologically active concentrations that exceed the 600 nM half-maximal inhibitory concentration observed in glioblastoma neurosphere cultures.21 The ability of ONC201 to induce biomarkers such as ATF4 and DR5 suggests target engagement, as each has been independently shown to mediate the antitumor activity of the compound.21,23,24
The observation that all patients achieved intratumoral ONC201 concentrations without universally robust pharmacodynamic signaling or apoptosis suggests the need for appropriate molecular selection and consideration of combination therapy. A number of synergistic combinations with ONC201 have been identified in preclinical studies including radiation, chemotherapy, and targeted agents that merit further evaluation in gliomas.18,23,35,36 These results also support the recently reported findings that tumors with DRD5 low expression may be more sensitive to ONC201.9
ONC201 administered weekly was well tolerated, similar to that observed with every 3 week dosing in adult recurrent glioblastoma patients and reported in other tumor types.27,29 Patients did not require treatment interruption or modification for the duration of the study. Coupled with the observed activity in select patients, these findings continue to suggest that ONC201 is well tolerated and may be biologically active in a subset of glioblastoma patients who may be identified by biomarkers that include DRD2, DRD5, and/or H3 K27M.
This is the second case report of an adult recurrent glioblastoma patient undergoing radiographic regression of multifocal, multirecurrent disease. We previously reported the first H3 K27M–mutant glioma patient to receive ONC201, who continues to experience a durable radiographic response, including a complete regression of her primary thalamic lesion.29 Preliminary studies have suggested that the H3 K27M mutation may harbor dysregulated dopamine receptor expression and sensitize gliomas to ONC201.30 Clinical trials are under way to further investigate the activity of this compound in diffuse midline gliomas with or without the H3 K27M mutation (NCT03295396, NCT02525692, NCT03416530).
Supplementary Material
Funding
This work was supported by grants from the National Cancer Institute (4R44CA192427-02) to W.O., the Musella Foundation to J.E.A., and Oncoceutics.
Conflict of interest statement
V.V.P., R.S.T., K.M., J.E.A., M.S., and W.O. are employees and shareholders of Oncoceutics. M.M. is a board member and has ownership interest in Oncoceutics.
I.A-R. has served on advisory boards for Agios, Karus Therapeutics, Boehringer Ingelheim and FORMA Therapeutics. She has research support from Astex Pharmaceuticals.
Y.O. has served on advisory boards for Novocure and Abbvie and has research support from BMS and Novocure.
T.T.B. has consulted for Merck & Co., Inc., NXDC, Amgen, Roche, Oxigene, Foundation Medicine, Proximagen/Upsher, Champions Biotechnology and Genomicare. He has had grant support from Pfizer, AstraZeneca and Millennium.
P.Y.W. has research support from Agios, Astra Zeneca, Beigene, Eli Lily, Genentech/Roche, Kazia, MediciNova, Merck, Novartis, Oncoceutics, Sanofi-Aventis, Vascular Biogenics, VBI Vaccines. He has served on advisory boards for Agios, Astra Zeneca, Bayer, Blue Earth Diagnostics, Immunomic Therapeutics, Karyopharm, Kiyatec, Puma, Taiho, Vascular Biogenics, Deciphera, VBI Vaccines and Tocagen. He has been a speaker for Merck and Prime Oncology.
Authorship statement
I.A-R., Y.O., M.M., T.T.B., and P.Y.W. performed the clinical trial. I.A-R., J.E.A, W.O., M.S., T.T.B., and P.Y.W. were responsible for the clinical trial design. K.M. was responsible for data collection and monitoring. V.V.P., R.S.T., M.S., and J.E.A. performed clinical correlatives, rat biodistribution studies, and data analysis. I.A-R., V.V.P., and J.E.A. wrote the manuscript. All authors reviewed the final manuscript.
References
- 1. Caragher SP, Hall RR, Ahsan R, Ahmed AU. Monoamines in glioblastoma: complex biology with therapeutic potential. Neuro Oncol. 2018;20(8):1014–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gillespie S, Monje M. An active role for neurons in glioma progression: making sense of Scherer’s structures. Neuro Oncol. 2018;20(10):1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caragher SP, Shireman JM, Huang M, et al. . Activation of dopamine receptor 2 prompts transcriptomic and metabolic plasticity in glioblastoma. J Neurosci. 2019;39(11):1982–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang X, Wang ZB, Luo C, et al. . The prospective value of dopamine receptors on bio-behavior of tumor. J Cancer. 2019;10:1622–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li J, Zhu S, Kozono D, et al. . Genome-wide shRNA screen revealed integrated mitogenic signaling between dopamine receptor D2 (DRD2) and epidermal growth factor receptor (EGFR) in glioblastoma. Oncotarget. 2014;5(4):882–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mu J, Huang W, Tan Z, et al. . Dopamine receptor D2 is correlated with gastric cancer prognosis. Oncol Lett. 2017;13(3):1223–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jandaghi P, Najafabadi HS, Bauer AS, et al. . Expression of DRD2 is increased in human pancreatic ductal adenocarcinoma and inhibitors slow tumor growth in mice. Gastroenterology. 2016;151:1218–1231. [DOI] [PubMed] [Google Scholar]
- 8. Meredith EJ, Holder MJ, Rosén A, et al. . Dopamine targets cycling B cells independent of receptors/transporter for oxidative attack: implications for non-Hodgkin’s lymphoma. Proc Nat Acad Sci U S A. 2006;103:13485–13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prabhu VV, Madhukar NS, Gilvary C, et al. . Dopamine receptor D5 is a modulator of tumor response to dopamine receptor D2 antagonism. Clin Cancer Res. 2019;25(7):2305–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greer YE, Porat-Shliom N, Nagashima K, et al. . ONC201 kills breast cancer cells in vitro by targeting mitochondria. Oncotarget. 2018;9(26):18454–18479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hayes-Jordan AA, Ma X, Menegaz BA, et al. . Efficacy of ONC201 in desmoplastic small round cell tumor. Neoplasia. 2018;20(5):524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lev A, Lulla AR, Ross BC, et al. . ONC201 Targets AR and AR-V7 signaling, reduces PSA, and synergizes with everolimus in prostate cancer. Mol Cancer Res. 2018;16(5):754–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lev A, Lulla AR, Wagner J, et al. . Anti-pancreatic cancer activity of ONC212 involves the unfolded protein response (UPR) and is reduced by IGF1-R and GRP78/BIP. Oncotarget. 2017;8(47):81776–81793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ni X, Zhang X, Hu CH, et al. . ONC201 selectively induces apoptosis in cutaneous T-cell lymphoma cells via activating pro-apoptotic integrated stress response and inactivating JAK/STAT and NF-κB pathways. Oncotarget. 2017;8(37):61761–61776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prabhu VV, Allen JE, Dicker DT, El-Deiry WS. Small-molecule ONC201/TIC10 targets chemotherapy-resistant colorectal cancer stem-like cells in an Akt/Foxo3a/TRAIL-dependent manner. Cancer Res. 2015;75(7):1423–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prabhu VV, Talekar MK, Lulla AR, et al. . Single agent and synergistic combinatorial efficacy of first-in-class small molecule imipridone ONC201 in hematological malignancies. Cell Cycle. 2018;17(4): 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ralff MD, Kline CLB, Küçükkase OC, et al. . ONC201 demonstrates antitumor effects in both triple-negative and non-triple-negative breast cancers through TRAIL-dependent and TRAIL-independent mechanisms. Mol Cancer Ther. 2017;16(7):1290–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Talekar MK, Allen JE, Dicker DT, El-Deiry WS. ONC201 induces cell death in pediatric non-Hodgkin’s lymphoma cells. Cell Cycle. 2015;14(15):2422–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tu YS, He J, Liu H, et al. . The imipridone ONC201 induces apoptosis and overcomes chemotherapy resistance by up-regulation of bim in multiple myeloma. Neoplasia. 2017;19(10):772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Q, Wang H, Ran L, Zhang Z, Jiang R. The preclinical evaluation of TIC10/ONC201 as an anti-pancreatic cancer agent. Biochem Biophys Res Commun. 2016;476(4):260–266. [DOI] [PubMed] [Google Scholar]
- 21. Allen JE, Krigsfeld G, Mayes PA, et al. . Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci Transl Med. 2013;5(171):171ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ishida CT, Zhang Y, Bianchetti E, et al. . Metabolic reprogramming by dual AKT/ERK inhibition through imipridones elicits unique vulnerabilities in glioblastoma. Clin Cancer Res. 2018;24(21):5392–5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ishizawa J, Kojima K, Chachad D, et al. . ATF4 induction through an atypical integrated stress response to ONC201 triggers p53-independent apoptosis in hematological malignancies. Sci Signal. 2016;9(415):ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kline CL, Van den Heuvel AP, Allen JE, Prabhu VV, Dicker DT, El-Deiry WS. ONC201 kills solid tumor cells by triggering an integrated stress response dependent on ATF4 activation by specific eIF2α kinases. Sci Signal. 2016;9(415):ra18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yuan X, Kho D, Xu J, Gajan A, Wu K, Wu GS. ONC201 activates ER stress to inhibit the growth of triple-negative breast cancer cells. Oncotarget. 2017;8(13):21626–21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Romaguera JE, Lee HJ, Tarapore R, et al. . Integrated stress response and immune cell infiltration in an ibrutinib-refractory mantle cell lymphoma patient following ONC201 treatment. Br J Haematol. 2019;185(1):133–136. [DOI] [PubMed] [Google Scholar]
- 27. Stein MN, Bertino JR, Kaufman HL, et al. . First-in-human clinical trial of oral ONC201 in patients with refractory solid tumors. Clin Cancer Res. 2017;23(15):4163–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stein MN, Malhotra J, Tarapore RS, et al. . Safety and enhanced immunostimulatory activity of the DRD2 antagonist ONC201 in advanced solid tumor patients with weekly oral administration. J Immunother Cancer. 2019;7(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arrillaga-Romany I, Chi AS, Allen JE, Oster W, Wen PY, Batchelor TT. A phase 2 study of the first imipridone ONC201, a selective DRD2 antagonist for oncology, administered every three weeks in recurrent glioblastoma. Oncotarget. 2017;8(45):79298–79304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chi AS, Stafford JM, Sen N, et al. . H3 K27M mutant gliomas are selectively killed by ONC201, a small molecule inhibitor of dopamine receptor D2. Neuro Oncol. 2017;19:vi81. [Google Scholar]
- 31. Hall MD, Odia Y, Allen JE, et al. . First clinical experience with DRD2/3 antagonist ONC201 in H3 K27M-mutant pediatric diffuse intrinsic pontine glioma: a case report. J Neurosurg Pediatr. 2019;23:1–7. [DOI] [PubMed] [Google Scholar]
- 32. Ballman KV, Buckner JC, Brown PD, et al. . The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol. 2007;9(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lamborn KR, Yung WK, Chang SM, et al. ; North American Brain Tumor Consortium Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10(2):162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu W, Lamborn KR, Buckner JC, et al. . Joint NCCTG and NABTC prognostic factors analysis for high-grade recurrent glioma. Neuro Oncol. 2010;12(2):164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karpel-Massler G, Bâ M, Shu C, et al. . TIC10/ONC201 synergizes with Bcl-2/Bcl-xL inhibition in glioblastoma by suppression of Mcl-1 and its binding partners in vitro and in vivo. Oncotarget. 2015;6(34):36456–36471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Allen JE, Prabhu VV, Talekar M, et al. . Genetic and pharmacological screens converge in identifying FLIP, BCL2, and IAP proteins as key regulators of sensitivity to the TRAIL-inducing anticancer agent ONC201/TIC10. Cancer Res. 2015;75(8):1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.