Abstract
Relapse of chronic lymphocytic leukaemia and non-Hodgkin’s lymphoma after standard of care treatment is common and new therapies are needed. The targeted alpha therapy with 212Pb-NNV003 presented in this study combines cytotoxic α-particles from 212Pb, with the anti-CD37 antibody NNV003, targeting B-cell malignancies. The goal of this study was to explore 212Pb-NNV003 for treatment of CD37 positive chronic lymphocytic leukaemia and non-Hodgkin’s lymphoma in preclinical mouse models.An anti-proliferative effect of 212Pb-NNV003 was observed in both chronic lymphocytic leukaemia (MEC-2) and Burkitt’s lymphoma (Daudi) cells in vitro. In biodistribution experiments, accumulation of 212Pb-NNV003 was 23%ID/g and 16%ID/g in Daudi and MEC-2 tumours 24 h post injection. In two intravenous animal models 90% of the mice treated with a single injection of 212Pb-NNV003 were alive 28 weeks post cell injection. Median survival times of control groups were 5–9 weeks. There was no significant difference between different specific activities of 212Pb-NNV003 with regards to therapeutic effect or toxicity. For therapeutically effective activities, a transient haematological toxicity was observed. This study shows that 212Pb-NNV003 is effective and safe in preclinical models of CD37 positive chronic lymphocytic leukaemia and non-Hodgkin’s lymphoma, warranting future clinical testing.
Introduction
In the USA, chronic lymphocytic leukaemia (CLL) and non-Hodgkin’s lymphoma (NHL) account for 1.2% and 4.3% of all new cancer incidence, with a combined estimated number of new cases of approximately 95,000 in 2019 [1, 2]. The standard of care for CLL is chemotherapy in combination with anti-CD20 antibodies. However, small molecular inhibitors are emerging as new therapies. While these regimens are initially effective in inducing responses, most patients eventually relapse and become refractory to further treatments [3–5]. NHL comprises a more heterogeneous group of diseases where treatment varies between subtypes. The backbone for most patients is chemotherapy combined with anti-CD20 antibodies. Indolent types of NHL are commonly diagnosed at advanced stage and thus incurable, but median survival is expected to be 15–20 years [6]. More aggressive subtypes of NHL are curable with intensive therapies, but patients who experience relapse often have a dismal outcome [7]. Both for CLL and NHL, new therapies with different mechanisms of actions and targets are needed.
In this study we explore a novel strategy, a targeted alpha therapy (TAT). We have conjugated the IgG1 chimeric antibody NNV003 with the chelator TCMC and labelled it with the alpha-particle generating radionuclide 212Pb (212Pb-NNV003). NNV003 binds with high affinity to CD37 and has been shown to internalise in some cell lines and induce antibody-dependent cellular phagocytosis and antibody-dependent cellular cytotoxicity [8]. CD37 is a glycosylated transmembrane protein, which has emerged as a therapeutic target in the recent years. It is highly and selectively expressed by B-lymphocytes and B-cell malignancies [9]. There are currently three CD37 targeting therapies in clinical development [10–13]. One of these compounds, 177Lu-lilotomab satetraxetan (Betalutin®), applies the β-emitter lutetium-177 as the cytotoxic payload, and is currently in clinical phase 2b for patients with relapsed follicular lymphoma (NCT01796171) [13]. Unlike NHL, where enlarged lymph nodes and tumours dominate the clinical picture, CLL more often presents as a disseminated leukemic disease. In theory, it would be more advantageous to use an α-emitter as the cytotoxic payload in CLL. Due to the α-particles’ short range of 50–100 μm, the radiation will be more localised to target cells than β-particles that have a range of 0.05–12 mm. The α-particles’ high LET of 100 keV/μm creates irreparable DNA double strand breaks. Consequently, only 2–3 α-particles are needed to kill a single cell, compared to 100–1000 low LET β-particles [14]. 212Pb has two alternative decay pathways through α-emitting daughters, 212Bi or 212Po, and can therefore be used as an in vivo generator of α-particles [15].
The anti-tumour efficacy of 212Pb has been demonstrated in preclinical studies; in several animal models of peritoneal cancer [16–22], prostate cancer, melanoma, pancreatic cancer and breast cancer [23–26]. It has also been applied in a pre-targeting setting [27, 28]. Recently, a phase 1 trial with 212Pb-TCMC-trastuzumab documented safety and feasibility in patients with human HER2 expressing malignancies [29]. Furthermore, a phase 1 study of 212Pb-DOTAMTATE for treatment of neuroendocrine tumours has been initiated (NCT03466216). In our study, we have investigated the in vitro cytotoxic effect of 212Pb-NNV003 in a CLL and a Burkitt’s lymphoma cell line. The in vivo tumour targeting of the TAT was studied in subcutaneous xenograft models. Two different disseminated models of CLL and NHL were used to evaluate the in vivo anti-tumour efficacy and toxicity of 212Pb-NNV003.
Materials and methods
Ethics statement–animal research
All studies were conducted under the approval of the institutional IACUC committee, Orano Med Institutional Animal Care and Use Committee, ethical approval number IAC-PR-006. Mice were kept under pathogen-free condition in a 12-hour light/dark cycle, with ad libitum access to food and water. Temperature, humidity and air-flow was continuously monitored. The cages contained enrichments and the bedding was changed once a week. Animal health was monitored by trained staff. The mice were euthanised by cervical dislocation when humane end point was reached. ARRIVE guidelines were followed (S2 File). See Supplementary S1 File and S1 Table for more information.
Labelling antibodies with 212Pb
NNV003 and cetuximab (binding to EGFR, used as unspecific control, Merck KGaA, Germany) were conjugated with a customised bifunctional version of TCMC (1,4,7,10-Tetrakis(carbamoylmethyl)-1,4,7,10-tetraazacyclododecane, Macrocyclics, USA), using an enzymatic procedure based on a process described by Jeger [30] and Dennler [31] resulting in up to two TCMC molecules conjugated to a specific amino acid in the Fc part of the antibody. An over 99.9% radiochemically pure 212Pb was eluted with 0.4 M ammonium acetate from a 224Ra generator (Orano Med LLC, USA). TCMC-NNV003 and TCMC-cetuximab in 150 mM ammonium acetate were added to purified 212Pb at ratios of 3.7, 37 or 370 MBq/mg and incubated at 37°C for 10 min with shaking at 300 rpm. ITLC was used to confirm a chelation > 95%. Samples were diluted in 0.9% NaCl prior to injection. Specific activities (SA) used: 37 MBq/mg (biodistribution and acute toxicity studies), 370 MBq/mg (cytotoxicity assay) and 3.7–370 MBq/mg (therapy studies). The immunoreactivity (IRF) of 212Pb-NNV003 was measured as previously described [8].
Cell lines
The human CLL cell line MEC-2 (Creative Bioarray, USA) and the Burkitt’s lymphoma cell line Daudi (ATCC, USA) were cultured in IMDM and RPMI medium. Media were supplemented with 10% heat inactivated fetal bovine serum and 1% Penicillin-Streptomycin (ATCC, USA).
In vitro studies
MEC-2 and Daudi cells (1x106) were fixed in 1% formaldehyde for 15 min at 4°C, stained with 5 μL Alexa Fluor® 647 mouse anti-human CD37 (Clone M-B371, BD Bioscience, USA) for 30 min on ice in the dark and analysed in a Guava easyCyte 8HT (Millipore, USA).
MEC-2 and Daudi cells were plated in 96 well-plates with 5000 cells/well. 212Pb-NNV003 or 212Pb-cetuximab was added to the cells at final concentrations of 57.8 Bq/ml to 14.8 kBq/ml (n = 8 wells per concentration). The cells were incubated for 5 h before washing. After resuspending in fresh medium, the cells were kept in culture for 6 more days. The CyQUANT™ NF Cell Proliferation Assay Kit (Thermo Fisher Scientific, USA) was used to measure cell proliferation.
Biodistribution
10x106 Daudi or 2.5x106 MEC-2 cells were injected subcutaneously (s.c) in the flank of 15 female CB17 SCID mice (CB17/Icr-Prkdcscid/IcrIcoCrl, Charles River Laboratories, USA) or 24 female R2G2 mice (B6;129-Rag2tm1FwaII2rgtm1Rsky/DwlHsd, Envigo, USA). When tumours reached a volume of 200–300 mm3, 200 μg murine IgG2a (M7769-5MG, Sigma Aldrich, USA) was injected intraperitoneally (i.p.). Next day, 370 kBq 212Pb-NNV003 was injected intravenously (i.v.). Mice were euthanised at predetermined time-points: 1 h (n = 5 CB17 SCID, n = 10 R2G2), 6 h (n = 5 CB17 SCID, n = 4 R2G2) and 24 h (n = 5 CB17 SCID, n = 10 R2G2). Organs and tumours were harvested, weighted and the activity was measured by a calibrated gamma counter (Wizard2, Perkin Elmer, USA). The background was subtracted from the measurements and values were decay corrected. Percent injected dose/g (%ID/g) was calculated for each tissue.
Radiation dosimetry
The biodistribution data from the two s.c. models was used to calculate the absorbed radiation doses from 212Pb-NNV003, performed by Rapid (Maryland, USA). Time-integrated activity coefficients were obtained by the trapezoidal method as the data could not be exponentially fitted. Physical decay was used to extrapolate after the last time point.
Toxicity studies of 212Pb-NNV003
Female CB17 SCID mice (30 total, n = 5 per group) were injected i.v. with 212Pb-NNV003 or PBS. Female R2G2 mice (29 total) were injected i.v. with 212Pb-NNV003 (n = 5 per group), 0.9% NaCl or 212Pb-cetuximab (n = 3 per group), to ensure similar tolerability of the two TATs. 200 μg murine IgG2a was injected i.p. one day before TAT injection. The mice were weighed three times a week and observed daily for clinical signs of radiotoxicity. Mice were euthanised when termination criteria were met (see Termination criteria section). Histopathological examinations were performed by Comparative Bioscience Inc (USA) on organs collected from R2G2 mice.
In both therapy models described below the concentration of platelets, red blood cells and white blood cells were monitored (see Haematological toxicity section).
Therapy studies
To mimic disseminated CLL disease, 68 female R2G2 mice were i.v. injected with 2.5x106 MEC-2 cells two days prior to treatment with 212Pb-NNV003 (370 MBq/mg), 212Pb-cetuximab (370 MBq/mg), NNV003-TCMC or 0.9% NaCl (n = 10). This model was also used to test different SAs of 212Pb-NNV003. 70 R2G2 mice were i.v. injected with MEC-2 cells, and received 370 kBq 212Pb-NNV003 (3.7, 37 or 370 MBq/mg), 370 kBq 212Pb-cetuximab (3.7 MBq/mg), NNV003-TCMC or 0.9% NaCl (n = 10 per group).
67 female CB17 SCID mice were i.v. injected with 10x106 Daudi cells two days before treatment with 212Pb-NNV003 (370 MBq/mg), 212Pb-cetuximab, NNV003-TCMC or 0.9% NaCl (n = 12 for 280 kBq 212Pb-NNV003 and n = 11 for the other groups). In all studies, animals received 200 μg murine IgG2a i.p. the day before treatment. The mice were checked daily for clinical symptoms and body weights were monitored. They were euthanised when termination criteria were met (see Termination criteria section). Statistical analysis performed as described in Statistics section.
Haematological toxicity
In both therapy models in the study, the concentration of platelets, red blood cells and white blood cells were monitored. 100 μL blood was collected prior to treatment and every two weeks thereafter from the retro-orbital sinus. The cell concentrations were determined using Vetscan HM5 hematology analyzer (Abaxis, USA). Animals received 300 μL 0.9% NaCl i.p. after blood collection. Statistical analysis was performed as described in the Statistics section.
Termination criteria
Animals were euthanised by cervical dislocation when a combination of following humane end-points occurred: weight loss > 15% over two consecutive days, lack of grooming over 5 days, weakness over 3 days, reduced motility, paralysis, palpable abdominal tumour > 1000 mm3, hunched back, severe anaemia and diarrhoea.
Statistics
All statistical analysis were done in GraphPad Prism 7.00 (GraphPad Software, USA). Log rank tests were performed for pairwise comparisons of treatment groups in the therapy studies. The Holm-Sidak method for multiple comparisons correction was used, with a significance level of α = 0.05. The platelet counts of the 212Pb-NNV003 treated mice were compared with the NaCl treated mice by one-way ANOVA followed by the Dunnett’s multiple comparison test with significance level of α = 0.05.
Results
Immunoreactivity of 212Pb-NNV003
The IRF of 212Pb-NNV003 was measured after initiation of the studies and was found to be around 57%. This suboptimal binding was due to radiation induced oxidation of the antibody after labelling and not the conjugation method. The addition of ascorbic acid during labelling restored the binding of 212Pb-NNV003 to the cells to approximately 80%, which is normally obtained with NNV003 labelled with lutetium-177 [8].
CD37 expression and cytotoxicity of 212Pb-NNV003
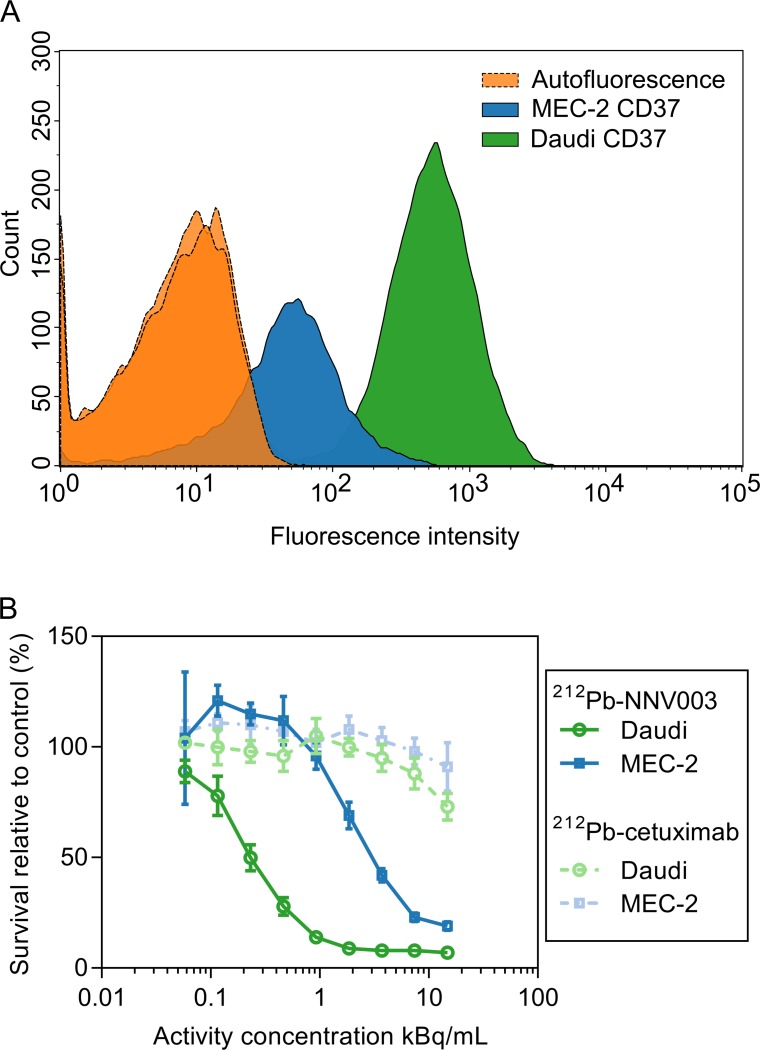
CD37 expression was approximately 20 times higher in Daudi cells than MEC-2 cells (Fig 1A). 212Pb-NNV003 had a dose dependent anti-proliferative effect on both cell lines, while 212Pb-cetuximab only had modest effect at the highest concentrations (Fig 1B). Daudi cells appeared more sensitive than MEC-2 cells. The experiment was repeated, and the trend was confirmed (S2 Fig).
Fig 1. CD37 expression and cytotoxic effect of 212Pb-NNV003.
(A) Flow cytometry histograms of cells only and cells incubated with 5 μl Alexa Fluor® 647 anti-human CD37. (B) Proliferation of Daudi and MEC-2 cells treated with 212Pb-NNV003 or 212Pb-cetuximab. Data represented as average of n = 8 replicates and error bars = SD.
Biodistribution and dosimetry of 212Pb-NNV003
In each animal study the mice were predosed with murine IgG2a before TAT injection to decrease the binding of 212Pb-NNV003 to murine Fc receptors and thus prevent clearance of antibody to spleen and liver in immune deficient mice with low amounts of endogenous antibodies [32]. Murine IgG2a binds with a similar affinity as human IgG1 to murine Fc receptors [33, 34]. A biodistribution performed in CB17 SCID mice revealed significant decrease in 212Pb-NNV003 uptake in spleen, kidneys and liver (S3A Fig). In immune competent Balb/c mice, however, the biodistribution was not altered by the predosing with IgG2a (S3B Fig).
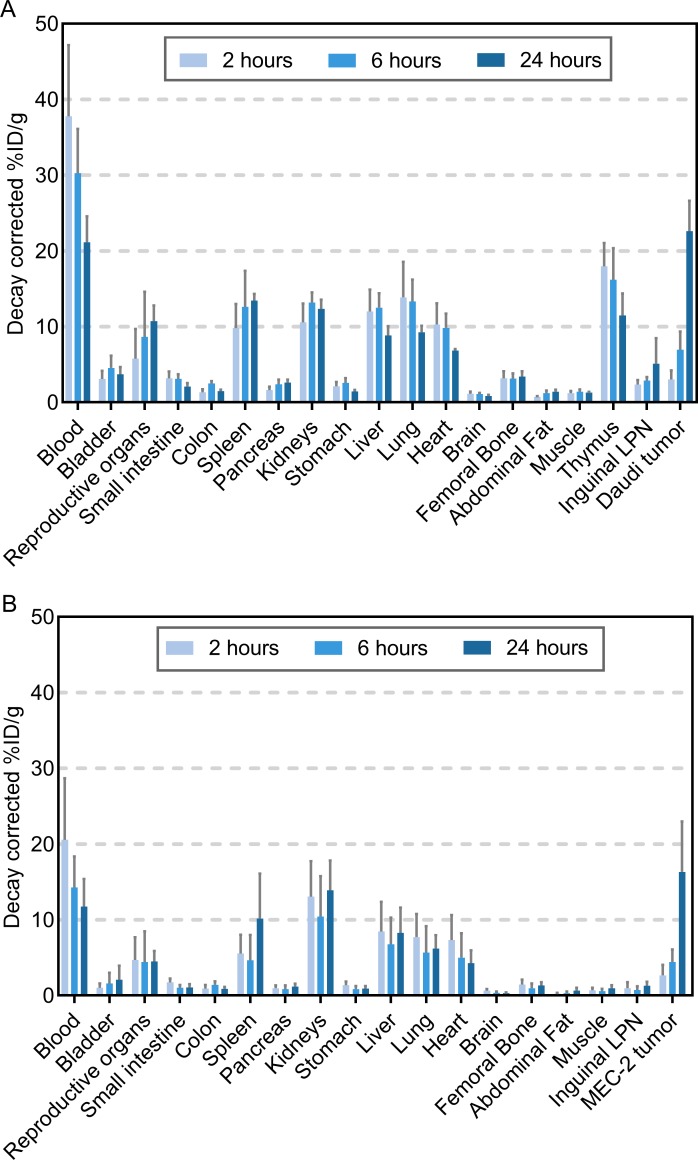
212Pb-NNV003 was rapidly taken up in blood rich organs and thymus. Accumulation in tumour was slower but reached approximately 23%ID/g in Daudi tumours and 16%ID/g in MEC-2 tumours after 24 h (Fig 2A and 2B). The lack of redistribution of the radionuclide after initial uptake in organs indicates in vivo stability of 212Pb-NNV003.
Fig 2. Biodistribution of 212Pb-NNV003.
%ID/g of 212Pb-NNV003 in tissues of (A) CB17 SCID mice with Daudi s.c. xenografts (n = 5 per time point) and (B) R2G2 mice with MEC-2 s.c. xenografts (n = 4 at 6 h, n = 10 at 2 and 24 h). Data presented as averages with error bars = SD, LPN = Lymph Node.
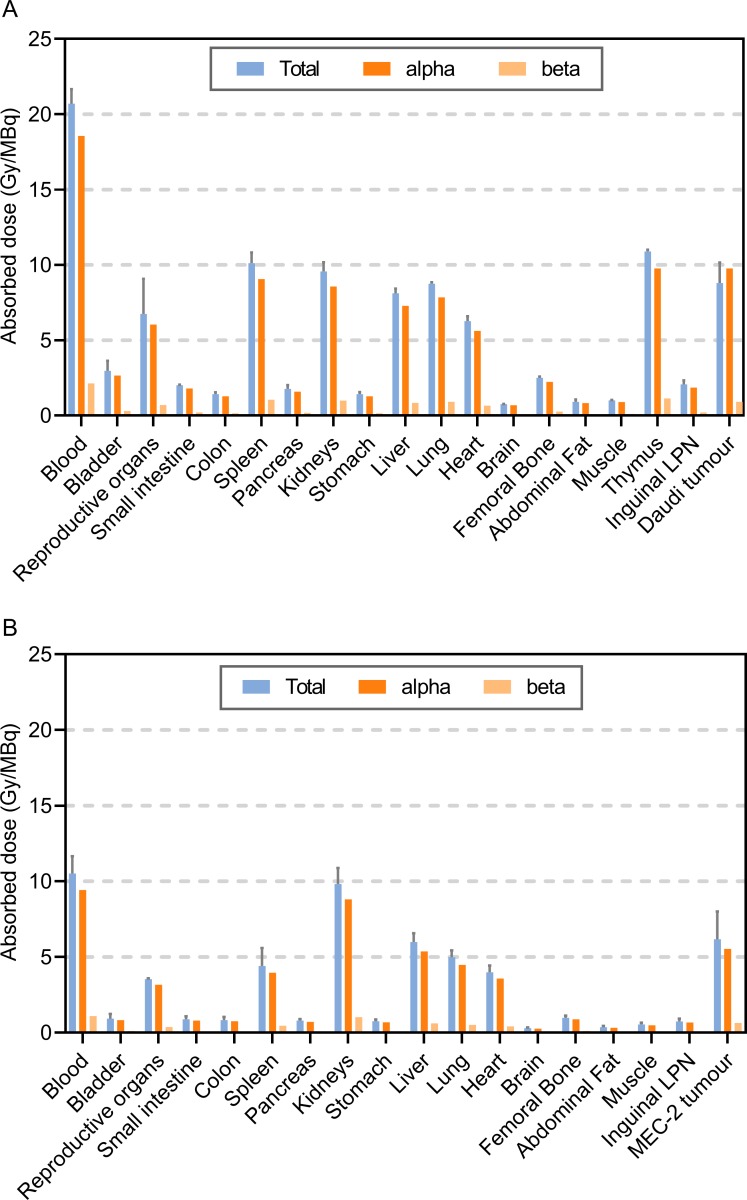
The tissue absorbed doses from the TAT is presented in Fig 3. Alpha radiation contributes most to the total absorbed dose, which was highest in blood rich organs and in the tumours.
Fig 3. Dosimetry of 212Pb-NNV003.
Absorbed radiation dose (Gy/MBq) to tissues of (A) CB17 SCID mice with Daudi s.c. xenografts (n = 5 per time point) and (B) R2G2 mice with MEC-2 s.c. xenografts (n = 4 at 6 h, n = 10 at 2 and 24 h). Error bars = SD of total absorbed radiation dose.
Acute toxicity of 212Pb-NNV003
The CB17 SCID mice that reached termination criteria were euthanised because of acute radiation toxicity. One mouse treated with 740 kBq was found dead in the cage, assumed dead of radiation toxicity. No R2G2 mice reached termination criteria. Remaining CB17 SCID and the R2G2 mice were euthanised at the end of the studies, 29 (CB17 SCID) or 33 (R2G2) days after TAT injection.
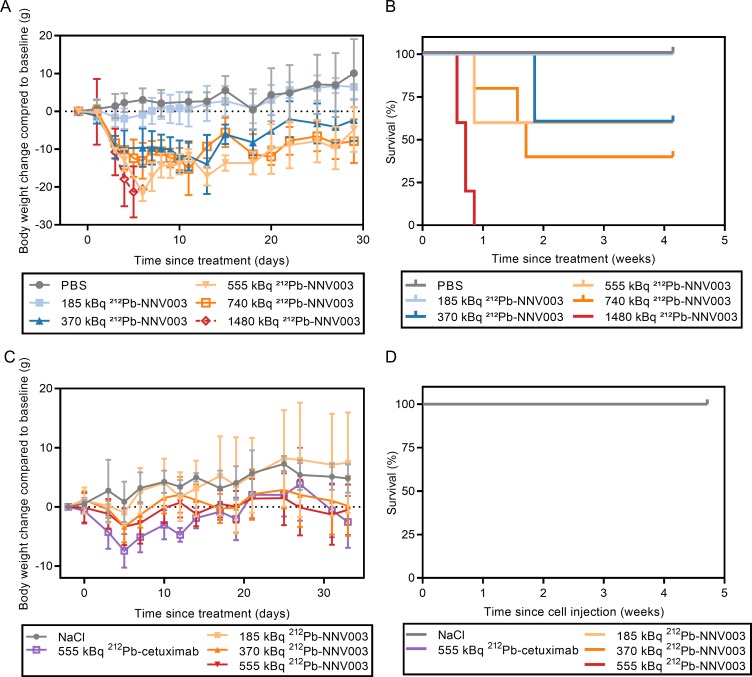
1480 kBq 212Pb-NNV003 was too toxic for the CB17 SCID mice, and within a week after injection, all mice had been euthanised due to weight loss (Fig 4A and 4B). Doses of 370–740 kBq 212Pb-NNV003 also caused radiotoxicity with weight loss and 40–60% of the mice had to be euthanised within three weeks post injection. However, the lowest dose, 185 kBq, was well tolerated. CB17 SCID mice are known to have low tolerance to ionising radiation, due to a deficiency in the DNA double strand break repair mechanism [35]. Therefore, R2G2 mice were used for the MEC-2 model since they do not possess the SCID mutation and are therefore less sensitive to radiation. Indeed, doses of 185–555 kBq of 212Pb-NNV003 and 555 kBq of 212Pb-cetuximab could be administered in R2G2 mice without mortality, with only a mild and reversible initial weight loss (Fig 4C and 4D). Histopathological examination of the treated R2G2 mice showed no signs of radiation induced damage. From these results, the highest non-severely toxic doses (HNSTD) were established: 185 kBq in CB17 SCID mice and 555 kBq in R2G2 mice, and the following 212Pb-NNV003 doses were chosen for therapy studies: 90, 185 and 280 kBq (CB17 SCID) and 185, 370, 555 and 740 kBq (R2G2). The two additional doses of 280 kBq (CB17 SCID) and 740 kBq (R2G2) that were not tested in the acute toxicity studies were included to test the range of the therapeutic window.
Fig 4. Acute toxicity of 212Pb-NNV003.
CB17 SCIDs were injected i.v. with increasing dose of 212Pb-NNV003 or PBS. (A) Body weights (average of n = 5, error bars = SD) and (B) survival of the mice. R2G2 mice were injected with increasing dose of 212Pb-NNV003 (n = 5), 212Pb-cetuximab or NaCl (n = 3). (C) Body weight (average with error bars = SD) and (D) survival of the mice (overlapping curves).
Anti-tumour effect of 212Pb-NNV003
In the disseminated model of NHL, the injected Daudi cells infiltrated the bone marrow of the mice causing hind leg paralysis. The CLL model had a more aggressive profile, where the MEC-2 cells mostly infiltrated abdominal tissues, forming tumours in and around ovaries, kidneys, liver and spleen, and therefore represented a more difficult model to treat than the NHL model. Because of the aggressive profile, R2G2 mice were used for the MEC-2 model, permitting treatment with a higher dose than is possible in CB17 SCID mice. The SCID mutation impairs the DNA double strand break repair pathway, making the mouse strain inherently sensitive to radiation [35]. The mice that reached termination criteria were euthanised because of tumour infiltration or acute radiation toxicity (2 mice treated with 370 and 740 kBq 212Pb-NNV003). Three R2G2 mice, treated with either 185 kBq 212Pb-NNV003, 370 kBq 212Pb-cetuximab or 10 μg NNV003, and one CB17 SCID mouse treated with 185 kBq 212Pb-NNV003 were found dead in the cage and their cause of death was presumably related to tumour infiltration. The remaining mice were euthanised at the end of the study, 201 (Fig 5A), 197 (Fig 5B) or 150 (Fig 5C) days after cell injection.
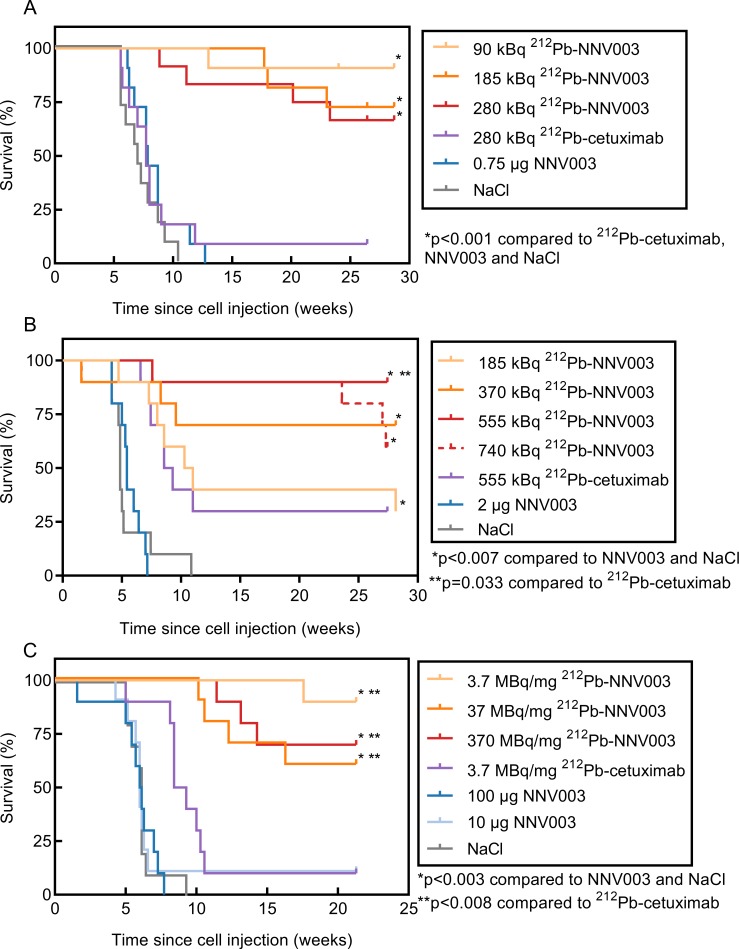
Fig 5. Anti-tumour effect of 212Pb-NNV003.
Survival of (A) CB17 SCID mice (n = 11 or 12) i.v. injected with Daudi cells and of (B) R2G2 mice (n = 10) i.v. injected with MEC-2 cells two days prior to treatment with 212Pb-NNV003, 212Pb-cetuximab, NNV003 or NaCl. (C) Survival of R2G2 mice (n = 10) i.v. injected with MEC-2 cells two days prior to treatment with 370 kBq 212Pb-NNV003 with increasing SAs, 212Pb-cetuximab, NNV003 or NaCl. Mice were censored at the end of the study.
In both models, a single injection of 212Pb-NNV003 significantly prolonged median survival compared to controls (Fig 5A and 5B). At study termination 28 weeks post cell injection, 67–91% of the Daudi injected CB17 SCID mice and 30–90% of the MEC-2 injected R2G2 mice treated with 212Pb-NNV003 were still alive. In the MEC-2 model, 555 kBq unspecific 212Pb-cetuximab showed an anti-tumour effect comparable to the effect of 185 kBq 212Pb-NNV003 (Fig 5B). There was a dose-dependent response to doses of 370 to 740 kBq 212Pb-NNV003, which were more effective than 212Pb-cetuximab, but only the 555 kBq dose of 212Pb-NNV003 was statistically superior.
A single i.v. injection of 370 kBq 212Pb-NNV003, with SA between 3.7 and 370 MBq/mg, improved survival of R2G2 mice i.v. injected with MEC-2 cells, compared to controls (Fig 5C). 60–90% of the R2G2 mice treated with 212Pb-NNV003 were still alive at the end of the study, 21 weeks post cell injection. No significant difference between 212Pb-NNV003 SAs was observed.
Haematological toxicity of 212Pb-NNV003
In the R2G2 therapy study, two animals treated with 370 and 740 kBq 212Pb-NNV003 died 9 days post injection of suspected acute radiotoxicity. 70% of the mice in the 370 kBq group survived for more than 28 weeks with no signs of toxicity, therefore we suspect that cause of death was poisoning during grooming. Further, two mice treated with 740 kBq were euthanised due to weight loss 165 and 191 days post cell inoculation. Necropsy observations showed no macroscopic tumours; however, small spleens and pale organs might indicate radiation damage. These results indicate that 740 kBq was a too high dose and confirm the HNSTD of 555 kBq in R2G2 mice. No toxicity was observed in the CB17 SCID mice treated with 280 kBq, thus this dose was determined as HNSTD for CB17 SCID mice.
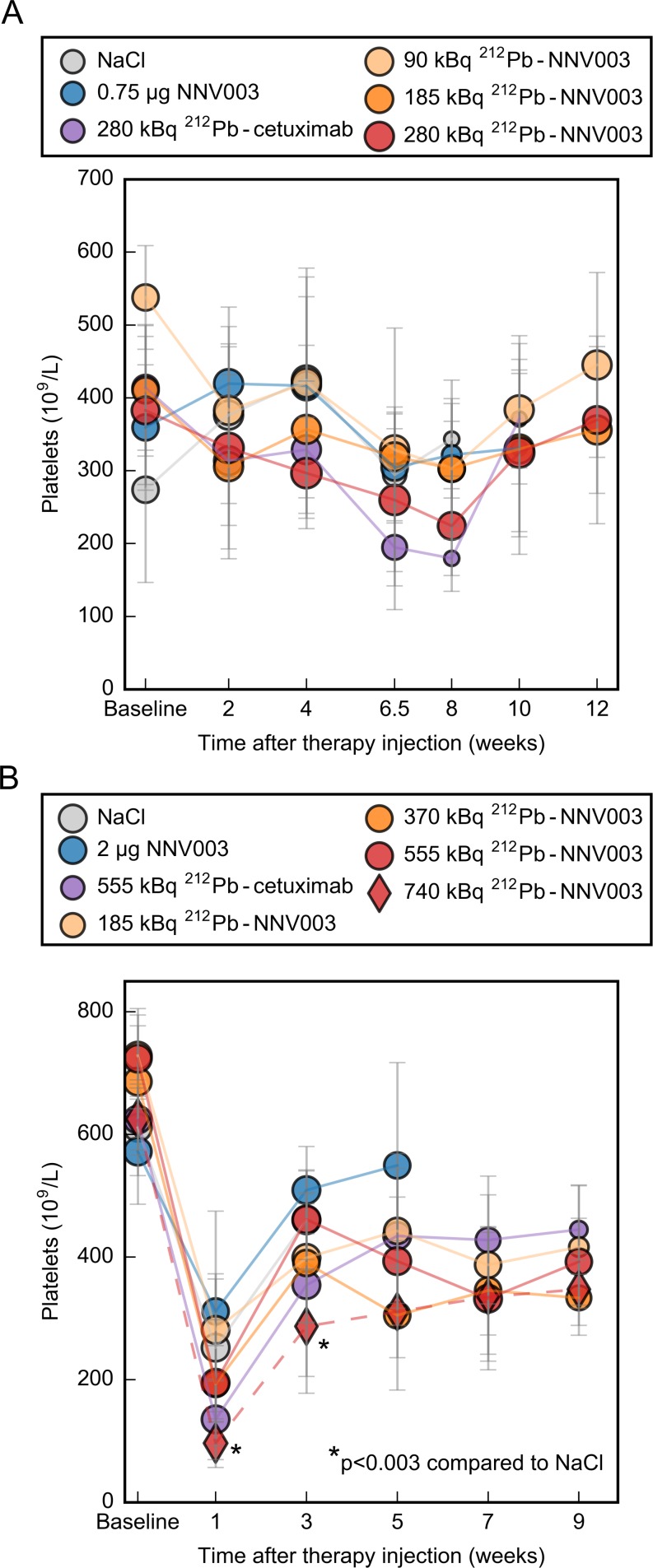
At doses ranging from 185 to 555 kBq (R2G2) and 90 to 280 kBq (CB17 SCID), the haematological toxicity was modest. In R2G2 mice, but not in CB17 SCIDs, the platelet counts decreased one week after TAT injection, but only the 555 kBq treatment was significantly different from the NaCl treatment at week 1 and 3 (Fig 6A and 6B). The platelet counts also decreased in the untreated control group. We suggest this initial decrease in platelet counts to be due to the stress of being handled (three injections during three consecutive days) before blood sampling. Due to a shorter lifespan of controls, no reliable comparisons could be made after 3–4 weeks. The white blood cell- and red blood cell levels are presented in S5 Fig. Compared to controls and to baseline, no decrease in white blood cell- or red blood cell counts was observed in any of the studies.
Fig 6. Haematological toxicity of 212Pb-NNV003.
Platelet counts from (A) CB17 SCID mice i.v. injected with Daudi cells two days before treatment and from (B) R2G2 mice i.v. injected with MEC-2 cells two days before treatment. Average with error bars = SD. Marker size represents the number of mice at each measurement, ranging from (A) 3–11 and (B) 4–10.
Discussion
For the treatment of CLL and NHL patients, new therapies with different mechanisms of actions and targets are needed to further improve outcome. In the current study, a novel anti-CD37 TAT 212Pb-NNV003 induced cytotoxicity in cell lines and was rapidly taken up in CD37 positive tumours. Furthermore, the TAT efficiently prolonged survival in CLL and NHL mouse models with up to 90% survival at the end of the study and low levels of haematological toxicity.
In mice injected with Daudi cells, 91% of the animals were still alive 28 weeks after receiving 90 kBq of 212Pb-NNV003. In the MEC-2 model, doses of 370 kBq or more were needed to achieve similar effects. This corresponds well with the in vitro data showing that Daudi cells were more sensitive to 212Pb-NNV003 compared to MEC-2 cells. Further, Daudi cells had higher and more homogeneous expression of CD37 than MEC-2 cells. MEC-2 cells present a more aggressive and invasive growth in vivo. Mice i.v. injected with MEC-2 cells were often euthanised due to weight loss after massive infiltration in several critical organs, while mice injected with Daudi cells were euthanised due to hind leg paralysis caused by localised infiltration of the bone marrow. Moreover, CB17 SCID mice have functional natural killer cells, whereas R2G2 do not, and the chimeric antibody NNV003 could induce some immunotherapeutic effect in this strain [8]. Accordingly, in a separate therapy study with Daudi-bearing CB17 SCID (S4 Fig) we observed that 5 μg NNV003 had a similar anti-tumour effect as 185 kBq 212Pb-NNV003, while 100 μg NNV003 had no effect in the MEC-2 model. Therefore, the specific activity was increased to avoid any contribution of the antibody to the therapeutic effect, and the 0.75 μg NNV003 for 280 kBq used in the present study had no effect (Fig 5A)
In theory, α-particles are more suitable than β-particles for treatment of disseminated leukemic disease due to their short range and high level of cytotoxicity. Indeed, the survival rate after 90 kBq 212Pb-NNV003 treatment, at less than half the HNSTD, was 91% in the Daudi model, whereas treatment with β-emitting 177Lu-lilotomab satetraxetan at half the HNSTD, in the same animal model, led to 10% survival 28 weeks after therapy injection [9]. It is worth noting that lilotomab is the murine version of NNV003 and although they share the same epitope, it is not a direct comparison because of the difference in immunotherapeutic capacity [8]. Human IgG1 antibodies have been shown to bind stronger to mouse Fc receptors and induce more immunotherapeutic effect than mouse IgG1 [34]. However, the large difference between the treatments cannot be explained by this as the NNV003 dose was too low to have significant anti-tumour effect. This comparison supports the theory that α-particles are more advantageous against disseminated leukemic diseases.
The effect of different SAs was investigated to select a SA for a clinical trial. A clinically relevant SA is expected to be 3.7–7.4 MBq/mg, assuming that binding of 3–4 212Pb-NNV003 molecules is sufficient to eradicate a targeted cell [14]. In a phase 1 study of 212Pb-TCMC-trastuzumab treatment, a single i.p. infusion of up to 27.4 MBq/m2 was well tolerated [29], corresponding to approximately 50 MBq per patient. In our calculations we have assumed 5x106 B-lymphocytes/ml in a patient [36], 5 l blood and a CD37 expression of 1x105 antigens per cell [8]. An injection of 50 MBq 212Pb-NNV003 with the SA of 3.7 MBq/mg (corresponding to 13.5 mg 212Pb-NNV003), would lead to over 2x106 NNV003 molecules per cell and we can thus assume 100% occupancy of the antigen. The SA of 3.7 MBq/mg means that there is 51x10-6 212Pb nuclei per NNV003 antibody, which leads to 5.1 212Pb nuclei per cell. Consistently, we demonstrated that the anti-tumour effect of 212Pb-NNV003 was independent of SAs in the range of 3.7 to 370 MBq/mg. The increase in unlabelled NNV003 in the 370 kBq treatment did not have a negative impact on the therapeutic effect of 212Pb-NNV003.
The biodistribution of 212Pb-NNV003 did not reveal any unexpected accumulation in normal organs and was similar to biodistributions of other 212Pb labelled antibodies i.v. injected in mice. The uptake of 212Pb-NNV003 in liver and kidneys is consistent with results from other studies [24, 37], while the accumulation in spleen of 212Pb-NNV003 is lower than has been measured for another 212Pb labelled antibody [24]. The accumulation of 212Pb-NNV003 reached maximum after 24 h, resulting in an absorbed dose of 9.1 and 6.2 in the Daudi and MEC-2 tumours, respectively. A more rapid tumour targeting is expected in the i.v. models since the tumour cells are more accessible, and therefore a higher absorbed dose.
In the MEC-2 model, a modest anti-tumour effect was observed with the 212Pb-labeled cetuximab treatment. Cetuximab does not bind to MEC-2 cells (S1 Fig). Thus, we speculate that the observed effect may be related to the co-localisation of tumour cells in blood-rich organs. MEC-2 cells infiltrated mostly organs with a high flow of radioactive blood in the hours after injection. Especially cells localised in the kidneys would be expected to receive a significant dose due to renal excretion of the TAT.
Female mice were used in these studies for practical reasons. Although they are less prone to kidney injuries than male mice, they are more sensitive to haematological toxicity of ionising radiation, and therefore represent the worst case scenario [38, 39]. As with other TATs for treatment of diseases with bone marrow involvement, haematological toxicity was expected to be dose limiting for 212Pb-NNV003 [40–43]. A modest thrombocytopenia was observed in the MEC-2 model, but not in the Daudi model. No reduction in white blood cells or red blood cells was observed (S5 Fig). However, the white blood cell count in these immune deficient mice is generally lower at baseline than in other immunocompetent strains and are thus not well suited for monitoring haematological toxicity [44]. Furthermore, the total dose to femoral bone was low in both models, which might explain the modest haematological toxicity observed. However, NNV003 does not bind to murine CD37 and hence only non-specific binding of 212Pb-NNV003 will contribute to the absorbed radiation dose in the mice.
Around 35% of the gamma rays from 212Pb decay to 212Bi are internally converted, which can cause a 212Bi-chelate complex to become unstable, and when using DOTA, 30% of the 212Bi is released [45]. When using TCMC, 16% of 212Bi is released (publication in preparation). If the released 212Bi is circulating in the blood, it could have enough time to accumulate in the kidneys prior to decay, which could potentially be a source of non-targeted toxicity. Some indication of late radiation toxicity was observed in the 740 kBq group in the MEC-2 study, which might be due to this effect. However, the absorbed radiation doses to the kidneys at the HNSTDs (HNSTD based on haematological toxicity) were 2.5 Gy and 5.1 Gy (corresponding to 9.1 Gy/MBq, Fig 3), which is half of the dose that was found acceptable in a study with the alpha-emitter 211At-MX35-F(ab’)2 [46].
The IRF of 212Pb-NNV003 to CD37 was not measured before the studies were initiated and was probably around 50–60% based on post study measurements. The addition of ascorbic acid during labelling lead to an optimal IRF of around 80%, indicating that the problem was due to radiation induced oxidation and not due to the conjugation method. The IRF of 212Pb-NNV003 did not seem to affect the efficacy of the TAT since the therapy studies showed long term efficacy even with a suboptimal binding, and a significantly higher anti-tumour effect than the non-binding control 212Pb-cetuximab. We hypothesise that the increase in specific binding would lead to a better effect at lower doses of 212Pb-NNV003 and studies have been initiated to confirm this.
We have shown that 212Pb-NNV003 is effective and has a favourable safety profile in preclinical models of CD37 positive CLL and NHL. Future clinical testing is warranted.
Supporting information
Detailed description of animal models and supplementary method description.
(PDF)
(PDF)
(XLSX)
Strain, age and average weight of experimental animals at the start of the studies.
(PDF)
Flow cytometry histograms of cells (autofluorescence), cells blocked with unlabelled cetuximab and incubated with fluorescently labelled cetuximab (unspecific binding) and cells incubated with only fluorescently labelled cetuximab (total binding).
(TIF)
Proliferation of Daudi and MEC-2 cells treated with 212Pb-NNV003 or 212Pb-cetuximab. Data represented as average of n = 8 replicates (n = 1–8 for 212Pb-cetuximab) and error bars = SD.
(TIF)
%ID/g of 212Pb-NNV003 in tissues of (A) CB17 SCID or (B) Balb/c mice with or without IgG2a predosing. n = 3 (no predosing Balb/c at 4 hours) or n = 5 (all other groups). Data presented as averages with error bars = SD, R = right, L = left, LPN = Lymph Node.
(TIF)
Survival of CB17 SCID mice (n = 10 or 20) i.v. injected with Daudi cells two days prior to treatment with 212Pb-NNV003 (37 MBq/mg), 212Pb-cetuximab, NNV003 or NaCl. Mice were censored at the end of the study.
(TIF)
White blood cell counts (A and C) and red blood cell counts (B and D), measured in CB17 SCID mice i.v. injected with Daudi cells (A and B) and R2G2 mice i.v. injected with MEC-2 cells (C and D). There were 10–11 mice in each group at baseline. Marker size represents the number of mice at each measurement. Data is presented as average with error bars = SD.
(TIF)
Acknowledgments
The authors would like to thank Rapid (Maryland, USA) for the work on the dosimetry calculations.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was partially funded by the Norwegian Research Council, grant number 25690, and by Nordic Nanovector ASA and Orano Med. The funders provided support in the form of salaries for authors AFM, AS, JT, HH, TARS and JD, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section. No additional external funding received for this study.
References
- 1.Surveillance, Epidemiology, and End Results (SEER) Program Cancer Stat Facts—Chronic Lymphocytic Leukemia seer.cancer.gov2019 Available from: https://seer.cancer.gov/statfacts/html/clyl.html
- 2.Surveillance, Epidemiology, and End Results (SEER) Program Cancer Stat Facts—Non-Hodkin Lymphoma seer.cancer.gov2019 Available from: https://seer.cancer.gov/statfacts/html/nhl.html
- 3.Furman RR, Cheng S, Lu P, Setty M, Perez AR, Guo A, et al. Ibrutinib Resistance in Chronic Lymphocytic Leukemia. New England Journal of Medicine. 2014;370(24):2352–4. 10.1056/NEJMc1402716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rezvani AR, Maloney DG. Rituximab resistance. Best practice & research Clinical haematology. 2011;24(2):203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herling CD, Abedpour N, Weiss J, Schmitt A, Jachimowicz RD, Merkel O, et al. Clonal dynamics towards the development of venetoclax resistance in chronic lymphocytic leukemia. Nat Commun. 2018;9(1):727 10.1038/s41467-018-03170-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan D, Horning SJ, Hoppe RT, Levy R, Rosenberg SA, Sigal BM, et al. Improvements in observed and relative survival in follicular grade 1–2 lymphoma during 4 decades: the Stanford University experience. Blood. 2013;122(6):981–7. 10.1182/blood-2013-03-491514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armitage JO, Gascoyne RD, Lunning MA, Cavalli F. Non-Hodgkin lymphoma. The Lancet. 2017;390(10091):298–310. [DOI] [PubMed] [Google Scholar]
- 8.Maaland AF, Heyerdahl H, O’Shea A, Eiriksdottir B, Pascal V, Andersen JT, et al. Targeting B-cell malignancies with the beta-emitting anti-CD37 radioimmunoconjugate 177Lu-NNV003. European Journal of Nuclear Medicine and Molecular Imaging. 2019;46(11):2311–21. 10.1007/s00259-019-04417-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahle J, Repetto-Llamazares AH, Mollatt CS, Melhus KB, Bruland OS, Kolstad A, et al. Evaluating antigen targeting and anti-tumor activity of a new anti-CD37 radioimmunoconjugate against non-Hodgkin's lymphoma. Anticancer Res. 2013;33(1):85–95. [PubMed] [Google Scholar]
- 10.Beckwith KA, Frissora FW, Stefanovski MR, Towns WH, Cheney C, Mo X, et al. The CD37-targeted antibody–drug conjugate IMGN529 is highly active against human CLL and in a novel CD37 transgenic murine leukemia model. Leukemia. 2014;28:1501 10.1038/leu.2014.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deckert J, Park PU, Chicklas S, Yi Y, Li M, Lai KC, et al. A novel anti-CD37 antibody-drug conjugate with multiple anti-tumor mechanisms for the treatment of B-cell malignancies. Blood. 2013;122(20):3500–10. 10.1182/blood-2013-05-505685 [DOI] [PubMed] [Google Scholar]
- 12.Heider K-H, Kiefer K, Zenz T, Volden M, Stilgenbauer S, Ostermann E, et al. A novel Fc-engineered monoclonal antibody to CD37 with enhanced ADCC and high proapoptotic activity for treatment of B-cell malignancies. Blood. 2011;118(15):4159–68. 10.1182/blood-2011-04-351932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolstad A, Madsbu U, Beasley M, Bayne M, Illidge TM, O'Rourke N, et al. LYMRIT 37–01: A Phase I/II Study of 177lu-Lilotomab Satetraxetan (Betalutin®) Antibody-Radionuclide-Conjugate (ARC) for the Treatment of Relapsed Non-Hodgkin's Lymphoma (NHL)—Analysis with 6-Month Follow-up. Blood. 2018;132(Suppl 1):2879-. [Google Scholar]
- 14.Gudkov SV, Shilyagina NY, Vodeneev VA, Zvyagin AV. Targeted Radionuclide Therapy of Human Tumors. International Journal of Molecular Sciences. 2016;17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edem PE, Fonslet J, Kjaer A, Herth M, Severin G. In Vivo Radionuclide Generators for Diagnostics and Therapy. Bioinorganic Chemistry and Applications. 2016:6148357 10.1155/2016/6148357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rotmensch J, Atcher RW, Schlenker R, Hines J, Grdina D, Block BS, et al. The effect of the α-emitting radionuclide lead-212 on human ovarian carcinoma: A potential new form of therapy. Gynecologic Oncology. 1989;32(2):236–9. 10.1016/s0090-8258(89)80040-x [DOI] [PubMed] [Google Scholar]
- 17.Horak E, Hartmann F, Garmestani K, Wu CC, Brechbiel M, Gansow OA, et al. Radioimmunotherapy targeting of HER2/neu oncoprotein on ovarian tumor using lead-212-DOTA-AE1. Journal of Nuclear Medicine. 1997;38(12):1944–50. [PubMed] [Google Scholar]
- 18.Milenic DE, Garmestani K, Brady ED, Albert PS, Ma DS, Abdulla A, et al. alpha-particle radioimmunotherapy of disseminated peritoneal disease using a Pb-212-labeled radioimmunoconjugate targeting HER2. Cancer Biotherapy and Radiopharmaceuticals. 2005;20(5):557–68. 10.1089/cbr.2005.20.557 [DOI] [PubMed] [Google Scholar]
- 19.Boudousq V, Bobyk L, Busson M, Garambois V, Jarlier M, Charalambatou P, et al. Comparison between Internalizing Anti-HER2 mAbs and Non-Internalizing Anti-CEA mAbs in Alpha-Radioimmunotherapy of Small Volume Peritoneal Carcinomatosis Using Pb-212. Plos One. 2013;8(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milenic DE, Baidoo KE, Kim YS, Brechbiel MW. Evaluation of cetuximab as a candidate for targeted alpha-particle radiation therapy of HER1-positive disseminated intraperitoneal disease. mAbs. 2015;7(1):255–64. 10.4161/19420862.2014.985160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasten BB, Arend RC, Katre AA, Kim H, Fan J, Ferrone S, et al. B7-H3-targeted 212Pb radioimmunotherapy of ovarian cancer in preclinical models. Nucl Med Biol. 2017;47:23–30. 10.1016/j.nucmedbio.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milenic DE, Baidoo KE, Kim YS, Barkley R, Brechbiel MW. Targeted alpha-Particle Radiation Therapy of HER1-Positive Disseminated Intraperitoneal Disease: An Investigation of the Human Anti-EGFR Monoclonal Antibody, Panitumumab. Translational Oncology. 2017;10(4):535–45. 10.1016/j.tranon.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasten BB, Gangrade A, Kim H, Fan JD, Ferrone S, Ferrone CR, et al. Pb-212-labeled B7-H3-targeting antibody for pancreatic cancer therapy in mouse models. Nuclear Medicine and Biology. 2018;58:67–73. 10.1016/j.nucmedbio.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasten BB, Oliver PG, Kim H, Fan JD, Ferrone S, Zinn KR, et al. Pb-212-Labeled Antibody 225.28 Targeted to Chondroitin Sulfate Proteoglycan 4 for Triple-Negative Breast Cancer Therapy in Mouse Models. International Journal of Molecular Sciences. 2018;19(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miao YB, Hylarides M, Fisher DR, Shelton T, Moore H, Wester DW, et al. Melanoma therapy via peptide-targeted alpha-radiation. Clinical Cancer Research. 2005;11(15):5616–21. 10.1158/1078-0432.CCR-05-0619 [DOI] [PubMed] [Google Scholar]
- 26.Tan ZQ, Chen PP, Schneider N, Glover S, Cui LL, Torgue J, et al. Significant systemic therapeutic effects of high-LET immunoradiation by (212)pb-trastuzumab against prostatic tumors of androgen-independent human prostate cancer in mice. International Journal of Oncology. 2012;40(6):1881–8. 10.3892/ijo.2012.1357 [DOI] [PubMed] [Google Scholar]
- 27.Su FM, Beaumier P, Axworthy D, Atcher R, Fritzberg A. Pretargeted radioimmunotherapy in tumored mice using an in vivo Pb-212/Bi-212 generator. Nuclear Medicine and Biology. 2005;32(7):741–7. 10.1016/j.nucmedbio.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 28.Shah MA, Zhang XL, Rossin R, Robillard MS, Fisher DR, Bueltmann T, et al. Metal-Free Cycloaddition Chemistry Driven Pretargeted Radioimmunotherapy Using alpha-Particle Radiation. Bioconjugate Chemistry. 2017;28(12):3007–15. 10.1021/acs.bioconjchem.7b00612 [DOI] [PubMed] [Google Scholar]
- 29.Meredith RF, Torgue JJ, Rozgaja TA, Banaga EP, Bunch PW, Alvarez RD, et al. Safety and Outcome Measures of First-in-Human Intraperitoneal alpha Radioimmunotherapy With 212Pb-TCMC-Trastuzumab. Am J Clin Oncol. 2018;41(7):716–21. 10.1097/COC.0000000000000353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeger S, Zimmermann K, Blanc A, Grunberg J, Honer M, Hunziker P, et al. Site-specific and stoichiometric modification of antibodies by bacterial transglutaminase. Angew Chem Int Ed Engl. 2010;49(51):9995–7. 10.1002/anie.201004243 [DOI] [PubMed] [Google Scholar]
- 31.Dennler P, Chiotellis A, Fischer E, Bregeon D, Belmant C, Gauthier L, et al. Transglutaminase-based chemo-enzymatic conjugation approach yields homogeneous antibody-drug conjugates. Bioconjug Chem. 2014;25(3):569–78. 10.1021/bc400574z [DOI] [PubMed] [Google Scholar]
- 32.Reddy N, Lin Ong G, Behr TM, Sharkey RM, Goldenberg DM, Mattes MJ. Rapid blood clearance of mouse IgG2a and human IgG1 in many nude and nu/+ mouse strains is due to low IgG2a serum concentrations. Cancer Immunology, Immunotherapy. 1998;46(1):25–33. 10.1007/s002620050456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dekkers G, Bentlage AEH, Stegmann TC, Howie HL, Lissenberg-Thunnissen S, Zimring J, et al. Affinity of human IgG subclasses to mouse Fc gamma receptors. mAbs. 2017;9(5):767–73. 10.1080/19420862.2017.1323159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Overdijk MB, Verploegen S, Ortiz Buijsse A, Vink T, Leusen JH, Bleeker WK, et al. Crosstalk between human IgG isotypes and murine effector cells. J Immunol. 2012;189(7):3430–8. 10.4049/jimmunol.1200356 [DOI] [PubMed] [Google Scholar]
- 35.Fulop GM, Phillips RA. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990;347(6292):479–82. 10.1038/347479a0 [DOI] [PubMed] [Google Scholar]
- 36.Eichhorst B, Robak T, Montserrat E, Ghia P, Hillmen P, Hallek M, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v78–84. [DOI] [PubMed] [Google Scholar]
- 37.Schneider NR, Lobaugh M, Tan Z, Sandwall P, Chen P, Glover SE, et al. Biodistribution of Pb-212 conjugated trastuzumab in mice. Journal of Radioanalytical and Nuclear Chemistry. 2013;296(1):75–81. [Google Scholar]
- 38.Billings. Effect of Gender on the Radiation Sensitivity of Murine Blood Cells. Gravit Space Res. 2015. [PMC free article] [PubMed] [Google Scholar]
- 39.Kang KP, Lee JE, Lee AS, Jung YJ, Kim D, Lee S, et al. Effect of gender differences on the regulation of renal ischemia-reperfusion-induced inflammation in mice. Molecular medicine reports. 2014;9(6):2061–8. 10.3892/mmr.2014.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jurcic JG, Larson SM, Sgouros G, McDevitt MR, Finn RD, Divgi CR, et al. Targeted α particle immunotherapy for myeloid leukemia. Blood. 2002;100(4):1233–9. [PubMed] [Google Scholar]
- 41.Rosenblat TL, McDevitt MR, Mulford DA, Pandit-Taskar N, Divgi CR, Panageas KS, et al. Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia. Clin Cancer Res. 2010;16(21):5303–11. 10.1158/1078-0432.CCR-10-0382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jurcic JG, Levy MY, Park JH, Ravandi F, Perl AE, Pagel JM, et al. Phase I Trial of Targeted Alpha-Particle Therapy with Actinium-225 (Ac-225)-Lintuzumab and Low-Dose Cytarabine (LDAC) in Patients Age 60 or Older with Untreated Acute Myeloid Leukemia (AML). Blood. 2016;128(22). [Google Scholar]
- 43.Atallah EL, Orozco JJ, Craig M, Levy MY, Finn LE, Khan SS, et al. A Phase 2 Study of Actinium-225 (Ac-225)-Lintuzumab in Older Patients with Untreated Acute Myeloid Leukemia (AML)—Interim Analysis of 1.5 mu ci/Kg/Dose. Blood. 2018;132 10.1182/blood-2018-01-769018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nemzek JA, Bolgos GL, Williams BA, Remick DG. Differences in normal values for murine white blood cell counts and other hematological parameters based on sampling site. Inflamm Res. 2001;50(10):523–7. 10.1007/PL00000229 [DOI] [PubMed] [Google Scholar]
- 45.Mirzadeh S, Kumar K, Gansow OA. The Chemical Fate of 212Bi-DOTA Formed by β- Decay of 212Pb(DOTA)2-. Radiochimica Acta. 1993;60(1):1–10. [Google Scholar]
- 46.Back T, Haraldsson B, Hultborn R, Jensen H, Johansson ME, Lindegren S, et al. Glomerular filtration rate after alpha-radioimmunotherapy with 211At-MX35-F(ab')2: a long-term study of renal function in nude mice. Cancer Biother Radiopharm. 2009;24(6):649–58. 10.1089/cbr.2009.0628 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed description of animal models and supplementary method description.
(PDF)
(PDF)
(XLSX)
Strain, age and average weight of experimental animals at the start of the studies.
(PDF)
Flow cytometry histograms of cells (autofluorescence), cells blocked with unlabelled cetuximab and incubated with fluorescently labelled cetuximab (unspecific binding) and cells incubated with only fluorescently labelled cetuximab (total binding).
(TIF)
Proliferation of Daudi and MEC-2 cells treated with 212Pb-NNV003 or 212Pb-cetuximab. Data represented as average of n = 8 replicates (n = 1–8 for 212Pb-cetuximab) and error bars = SD.
(TIF)
%ID/g of 212Pb-NNV003 in tissues of (A) CB17 SCID or (B) Balb/c mice with or without IgG2a predosing. n = 3 (no predosing Balb/c at 4 hours) or n = 5 (all other groups). Data presented as averages with error bars = SD, R = right, L = left, LPN = Lymph Node.
(TIF)
Survival of CB17 SCID mice (n = 10 or 20) i.v. injected with Daudi cells two days prior to treatment with 212Pb-NNV003 (37 MBq/mg), 212Pb-cetuximab, NNV003 or NaCl. Mice were censored at the end of the study.
(TIF)
White blood cell counts (A and C) and red blood cell counts (B and D), measured in CB17 SCID mice i.v. injected with Daudi cells (A and B) and R2G2 mice i.v. injected with MEC-2 cells (C and D). There were 10–11 mice in each group at baseline. Marker size represents the number of mice at each measurement. Data is presented as average with error bars = SD.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.