Abstract
Objective
To study survival and to characterize long-term functional impairments and health-related quality of life (HRQOL) of patients with Lambert-Eaton myasthenic syndrome (LEMS).
Methods
In this observational study, survival of patients with LEMS, separately for nontumor (NT) and small cell lung cancer (SCLC), was compared to that of the Dutch general population and patients with SCLC. Disease course in patients with LEMS was recorded retrospectively. Several scales for functional impairments and health-related quality of life were assessed.
Results
We included 150 patients with LEMS. Survival was similar to that of the general population in 65 patients with NT-LEMS. Tumor survival was significantly longer in 81 patients with SCLC-LEMS compared to patients with non-LEMS SCLC (overall median survival 17 vs 7.0 months, p < 0.0001). At diagnosis, 39 (62%) of 63 patients with complete follow-up data were independent for activities of daily living, improving to 85% at the 1-year follow-up. The physical HRQOL composite score (55.9) was significantly lower than in the general population (76.3, p < 0.0001) and comparable to that of patients with myasthenia gravis (60.5). The mental HRQOL composite score was 71.8 in patients with LEMS, comparable to that of the general population (77.9, p = 0.19) and patients with myasthenia gravis (70.3).
Conclusions
This study shows that patients with NT-LEMS have normal survival. Patients with SCLC-LEMS have an improved tumor survival, even after correction for tumor stage. A majority of patients with LEMS report a stable disease course and remain or become independent for self-care after treatment.
Lambert-Eaton myasthenic syndrome (LEMS) is a rare autoimmune disorder characterized by fluctuating muscle weakness, loss of tendon reflexes, and autonomic dysfunction.1,2 Muscle weakness usually starts in the proximal leg muscles,1,3 which can severely limit mobility. Symptoms usually progress over the first months and can often be controlled by symptomatic and immunosuppressive treatment.4–6
After diagnosis, symptoms can vary between long-lasting remission on treatment, frequent fluctuations, and permanent disability. Distributions of symptoms and signs have been reported in several studies.1,3,7–9 Long-term follow-up of muscle strength scores, EMG, and voltage-gated calcium channel (VGCC) antibody results has been reported in 47 patients.10 Functional impairments of patients with LEMS over the disease course have been described in 12 patients only.11
Associated tumors are found in 50% to 60% of patients with LEMS, almost invariably small cell lung cancer (SCLC).1,3,7,12 Limited data suggest some improvement of symptoms in patients with LEMS with SCLC (SCLC-LEMS) after treatment of the tumor.13 Previous studies have shown a profound improved tumor survival in SCLC-LEMS,14–18 but no data exist on the quality of life of this period of improved survival. Hardly any data are available determining survival and quality of life of patients with LEMS without associated tumors.1
In this observational study, we aimed to characterize functional impairments over the disease course and the quality of life of patients with LEMS. We studied survival of all patients with LEMS with and without associated tumors.
Methods
Patient population
From July 1, 1998, to October 1, 2015, data from all consecutive Dutch patients with LEMS were collected prospectively, as described before.3,19 Leiden University Medical Center has a tertiary neuromuscular outpatient clinic and is the nationwide referral center for LEMS in the Netherlands. Patients were also identified through diagnosis registration databases and neuromuscular databases in university centers up to 2003. Afterward, we approached treating neurologists of all Dutch patients with positive results for VGCC antibodies (assay performed in Leiden and Rotterdam for all Dutch hospitals). This resulted in a small number of patients added retrospectively after a positive VGCC assay and verification of diagnosis (n = 7). One patient with LEMS who lacked most required data was excluded from this study.
The diagnosis of LEMS was based on characteristic clinical features, supported by either the presence of antibodies to VGCC or abnormal decrement and 60% increment on repetitive nerve stimulation.2,20 Increment testing was performed immediately after 10 to 30 seconds of voluntary contraction.
Survival
In the survival analysis, we separated the patients with LEMS with and without associated SCLC, excluding non-SCLC from the analysis (n = 3), as well as 1 patient with SCLC without a known date of tumor diagnosis. Patients with LEMS without associated tumor were compared to the general Dutch population as published by the Central Statistics office of the Netherlands, matching patients with LEMS for age and year at LEMS diagnosis and sex21 (Statline.cbs.nl22). Survival from diagnosis of tumors in patients with LEMS with associated SCLC was compared to survival in all patients with SCLC in the Netherlands from 1998 to 2012, as registered in the Netherlands Cancer Registry Netherlands Cancer Registry operated by Netherlands Comprehensive Cancer Organisation.23 As a secondary outcome measure, both patients with SCLC-LEMS and controls with SCLC were compared post hoc according to tumor stage (limited or extensive disease). Among patients with SCLC-LEMS, patients with and those without bulbar involvement or loss of weight within 3 months from onset were compared to show whether these variables predicted survival. Survival of these patients was also calculated according to patients' Dutch-English LEMS Tumour Association Prediction (DELTA-P) scores.24 In patients with follow-up data, medical events leading up to death were studied to determine their potential relation with LEMS.
Functional impairments
Disease course in patients with LEMS was recorded retrospectively with a semistructured interview in all available patients alive in 2014 to 2015 in combination with medical records. We used the modified Rankin Scale (mRS) and Karnofsky Performance Scale (KPS) to grade functional impairment. For the mRS, a structured interview was performed.25,26 For a limited number of patients (10 of 63), mRS and KPS scores were collected solely from medical records. In all of these patients, extensive follow-up data were available to derive functional limitations.
Treatment modalities, subjective response, and devices to assist mobility were recorded for all patients. Exacerbations were recorded, as defined by a subjective decrease in strength reported by patients supported by medical records and exacerbations requiring emergency treatment with either IV immunoglobulin or plasmapheresis as a more robust but less frequent criterion. Maximum disease severity was also recorded as reported by patients and supported by medical records.
Health-related quality of life
Health-related quality of life (HRQOL) was assessed with the Short Form-36 (SF-36), a self-administered validated questionnaire that was mailed to all known living patients with LEMS in March 2012. Nonresponders were reminded twice. Control cohorts were a population-based cohort of 464 patients with myasthenia gravis (MG) in the Netherlands collected at the same time27 and published normative data in the Dutch general population.28
The SF-36 is organized into 8 domains, with a score ranging from 0 (worst HRQOL) to 100 (best HRQOL). The 8 domains are physical functioning, role physical (role limitations due to physical problems), bodily pain, general health evaluation, vitality, social functioning, role emotional (role limitations due to emotional problems), and mental health. These domains produce a Physical Composite Score (PCS) and Mental Composite Score (MCS).29
The impact of baseline demographic and disease-related factors on both PCS and MCS quality of life was first assessed by univariate analysis. The predictors studies were chosen on the basis of expected baseline contributors to quality of life and likely clinical predicting factors. They were age at onset (>50 or <50 years), sex, partner, state of employment, presence of an associated tumor, presence of other autoimmune disease,30 pattern of muscle weakness, medication status, and mRS score. A second multivariate analysis was performed to determine which of these factors independently predicted HRQOL.
Statistics
Descriptive measures were presented as mean ± SD if appropriate or as median with interquartile range. Baseline variables between patients with LEMS with and without associated lung cancer were compared by use of t tests for linear and Fisher exact tests for categorical variables. Survival analysis was calculated with Kaplan-Meier plots and log-rank tests for nominal variables and log-rank test for trend for ordinal DELTA-P scores. HRQOL scores for all domains and composite scores were compared between LEMS, MG, and normative data in the Dutch general population with a 1-way between-group analysis of variance followed by post hoc comparison with the Tukey multiple-comparison test to test all pairwise comparisons. All individual predicting variables for PCS and MCS were first analyzed with a t test or 1-way analysis of variance for categorical variables and linear regression for mRS scores. Variables were included in a multivariate model only in case of a value of p < 0.20 in the univariate analysis.31 For missing values (2.4% of data) in this model, a 10-fold multiple imputation was performed. After missing data imputation, a generalized linear model was performed to determine which of these variables independently predicted HRQOL. Bonferroni correction for multiple comparisons was used, correcting for the number of categories for each variable. Data analysis was performed with SPSS version 23.0 (SPSS Institute Inc, Chicago, IL) and GraphPad Prism 6 (GraphPad, La Jolla, CA).
Standard protocol approvals, registrations, and patient consents
The study was approved by the Medical Ethics Committee of the Leiden University Medical Center. All patients included for follow-up of functional impairments and quality of life provided written informed consent.
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Results
We included 150 patients with LEMS, of whom 85 (59%) had an associated lung cancer (flowchart for inclusion available in figure e-1 from Dryad, doi.org/10.5061/dryad.g0t911k). Median time from onset to diagnosis was 18 months in patients without and 4 months in patients with an associated SCLC. Median time from LEMS diagnosis to detection of associated lung cancer was 0 months. A delay beyond 2 years was found in 2 patients (35 and 41 months). The first patient repeatedly avoided screening, while the latter was screened in 1988 according to standards of care that are currently considered insufficient. Baseline characteristics are shown in table 1 for the total LEMS population and subgroups in whom functional impairments and HRQOL were assessed.
Table 1.
Baseline characteristics for all patients with LEMS
Survival
In the 65 patients with LEMS without an associated tumor, life expectancy was similar to the average life expectancy in the Netherlands adjusted for sex, age, and year of diagnosis (log-rank test p = 0.63, hazard ratio [log-rank test] 1.16, 95% confidence interval [CI] 0.59–2.27, figure 1; survival percentages are available in table e-1 from Dryad, doi.org/10.5061/dryad.g0t911k). In 81 patients with LEMS with an associated SCLC, tumor survival was significantly longer compared to patients with SCLC without LEMS (median survival 17 vs 7.0 months, respectively, p < 0.0001). According to tumor stage, patients with SCLC-LEMS had a longer tumor survival both in limited (median survival 19 vs 12.1 months, p = 0.0015) and extensive (median survival 13 vs 4.9 months, p < 0.0001, figure 2 and table e-2 available from Dryad, doi.org/10.5061/dryad.g0t911k) disease. Data were similar after additional correction for sex, age, and year of tumor diagnosis (data not shown). Early bulbar muscle involvement, loss of weight, and DELTA-P scores did not significantly affect survival in patients with SCLC-LEMS (p = 0.41, 0.58, and 0.063, respectively).
Figure 1. Survival of NT-LEMS compared to matched Dutch life expectancy.
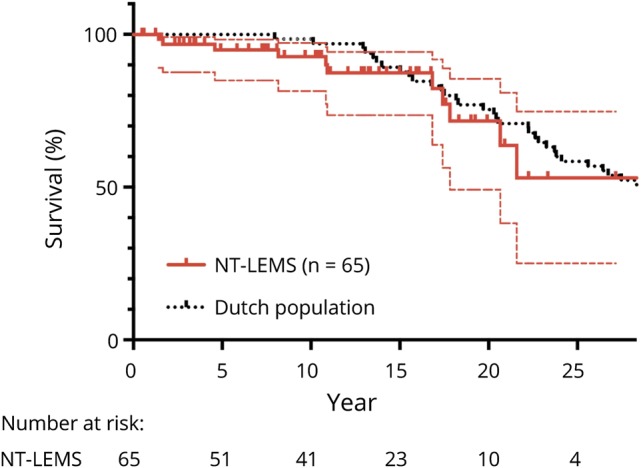
Kaplan-Meier curve showing survival of patients with Lambert-Eaton myasthenic syndrome without an associated tumor (NT-LEMS) compared to the average life expectancy in the Netherlands after adjustment for sex, age, and year of diagnosis. Dotted thin lines represent 95% confidence interval, and small vertical lines represent censored data for the patients with LEMS.
Figure 2. Survival of SCLC-LEMS compared to all Dutch patients with SCLC, LD or ED.
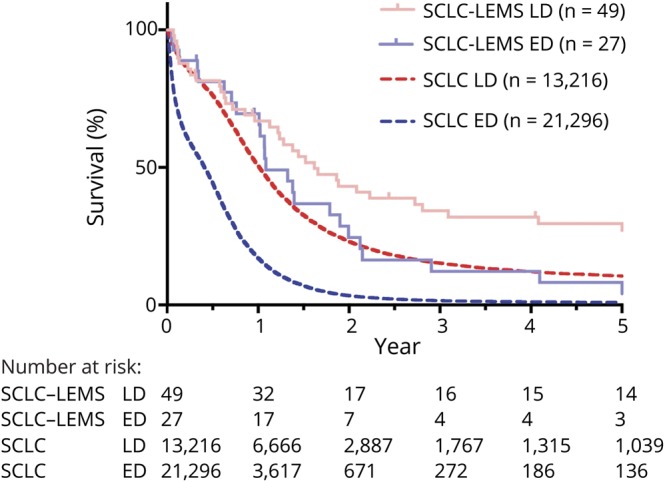
Kaplan-Meier curve showing tumor survival of patients with Lambert-Eaton myasthenic syndrome with an associated small cell lung cancer (SCLC-LEMS) (1998–2015) compared to the average life expectancy of patients with SCLC in the Netherlands (1998–2012) divided according to tumor stage. Small vertical lines represent censored data for the patients with LEMS. ED = extensive disease; LD = limited disease.
In contrast to the group-wise survival analysis, individually, LEMS likely contributed to death in 3 VGCC-positive patients. Two patients with SCLC-LEMS had respiratory insufficiency due to LEMS. The first had very limited response to aggressive treatment and died of abdominal sepsis while in the intensive care unit. The second died as a result of sudden respiratory deterioration just after a recent intensive care unit stay for respiratory muscle weakness. The third patient, with probably unrelated rectal carcinoma, experienced respiratory insufficiency shortly before his death. He had previously been admitted to the intensive care unit for respiratory muscle weakness but was not analyzed again for his dyspnea in a palliative setting. In all 3 patients, respiratory muscle weakness was likely a relevant contributing factor, although probably not the sole cause of death.
Functional impairments
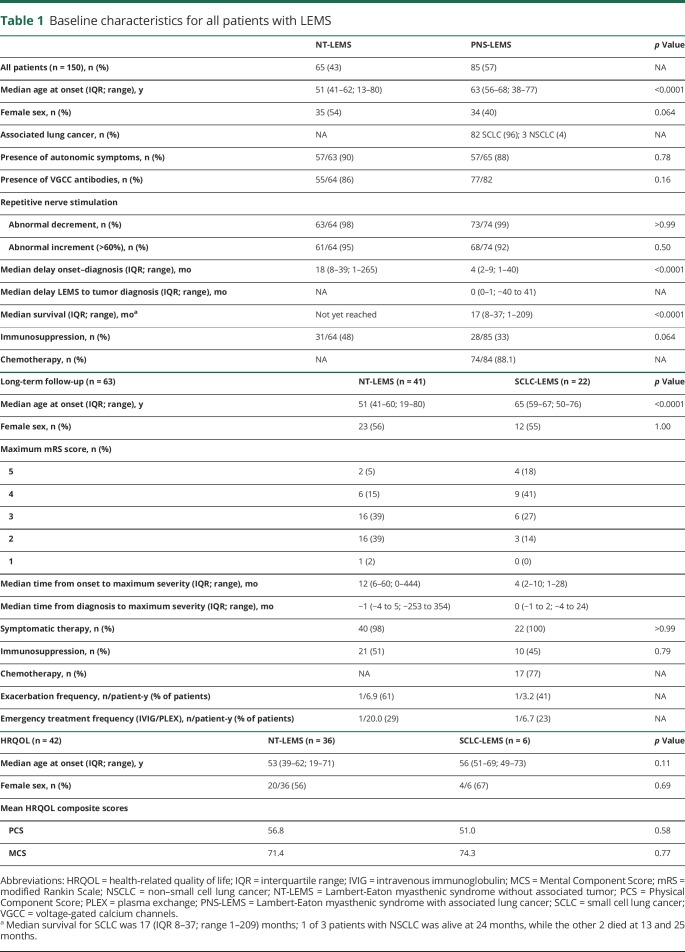
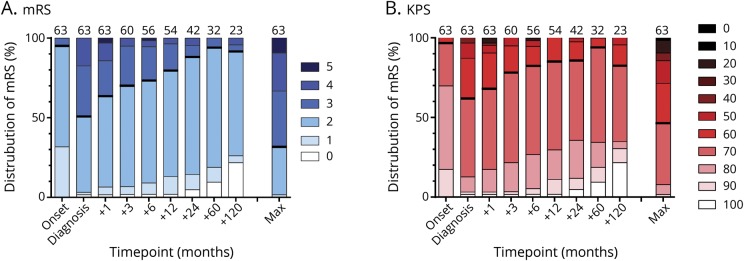
Detailed follow-up data for functional impairments were available for 63 patients (41 with LEMS without tumor and 22 with SCLC-LEMS). Median follow-up was 130 months for patients with LEMS without and 12 months for patients with an associated SCLC. At diagnosis, 39 of 63 patients (62%) were independent for self-care (KPS score ≥70), improving to 85% at the 1-year follow-up (figure 3 shows overall KPS and mRS distribution). Patients with lung cancer reported more functional impairments at any point in the disease course (figure 4, A and B). Maximal disease severity was reached at a median of 1 month before diagnosis, while 30% deteriorated beyond diagnosis. In the 32 patients with LEMS with at least 5 years of follow-up, 27 patients (84%) had reached their worst mRS score in or before the first year after diagnosis. Patient-reported maximal disease severity in this group was reached in the first 2 years in 75% of patients.
Figure 3. Distribution of functional impairments during the disease course.
Distribution of (A) modified Rankin Scale (mRS) and (B) Karnofsky Performance Scale (KPS) scores at onset of symptoms; diagnosis; 1, 3, 6, 12, 24, 60, and 120 months after diagnosis; and maximal (max) disease severity. At diagnosis, 62% of patients were independent for self-care (KPS score ≥70), increasing to 68% 1 month later and 85% 1 year later. At maximum disease severity, 46% of patients were independent for self-care. Number of patients available at top of bar for each time point.
Figure 4. Distribution of functional impairments during the disease course for subgroups.
Distribution of modified Rankin Scale (mRS) scores for patients (A) with and (B) without associated lung cancer and those treated (C) purely symptomatically and (D) with immunosuppressants as well. Data presented at onset of symptoms, diagnosis, 1 to 120 months after diagnosis, and at maximal (max) disease severity. Number of patients available at top of bar for each time point. DAP = 3,4-diaminopyridine.
During disease course, 73% of patients used any device to assist mobility. Fifty-two percent used a wheelchair, while only 6% were fully wheelchair dependent at any point in disease course. Most patients required a wheelchair for only a limited period of time.
Symptomatic treatment consisted of 3,4-diaminopyridine in 95% of patients and pyridostigmine in 68%. Of patients treated with 3,4-diaminopyridine and pyridostigmine, 88% noticed a subjective improvement in symptoms due to 3,4-diaminopyridine and 67% due to pyridostigmine. Immunosuppressive treatment was started in 49%, of which the most common therapies were either prednisone combined with azathioprine (29%) or prednisone alone (14%). The frequency of both immunosuppressive and emergency treatments showed no significant differences between patients with LEMS with and without SCLC (data not shown). Positive treatment effect, as shown by an improvement in mRS scores after diagnosis, was heterogeneous but generally reached in 6 to 12 months (figure 4, C and D). Patients treated with immunosuppressive drugs reported more functional impairments at diagnosis compared to those treated symptomatically but had a comparable mRS score distribution 2 and 5 years after diagnosis.
Exacerbations reported by patients occurred in 54% of patients. These self-reported exacerbations overall occurred once in every 5.7 patient-years. Exacerbations requiring emergency treatment with either or plasmapheresis were less frequent, occurring in 27% of patients, overall once in every 16 patient-years of follow-up. Nine patients with SCLC-LEMS had follow-up data available after tumor recurrence; of them, only 2 had a simultaneous worsening of LEMS. One of these patients, however, had concurrent pancreatitis, and the other had experienced 2 previous LEMS exacerbations without tumor recurrence. Five patients went into full remission and were able to stop all treatment at a median of 4 years after diagnosis; they remained in remission without any symptoms or treatment on long-term follow-up (median 12 years). One of these patients was treated for SCLC and one with immunosuppressants. Two other patients were treated only symptomatically, and the last patient with a short disease course received no treatment at all, suggesting that even spontaneous remission without immunomodulating therapy is possible.
Two patients in the SCLC-LEMS group had paraneoplastic cerebellar degeneration as a second paraneoplastic disease. This had relevant effects on physical limitations, but these patients ultimately had an mRS score time course comparable to that of other patients.
Health-related quality of life
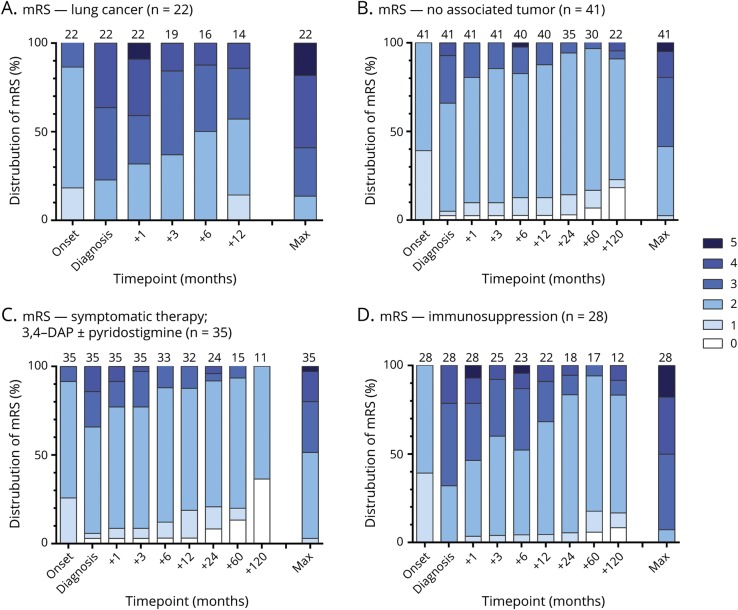
Forty-four of 67 (66%, 6 with SCLC) patients with LEMS alive and included at the time responded to our SF-36 questionnaire. Two questionnaires were excluded due to incomplete data. Patients with LEMS scored lower on the physical HRQOL than the general Dutch population (PCS 55.9 [95% CI 48.9–62.9] vs 76.3 [95% CI 75.0–77.5], respectively, p < 0.0001), reflected in lower scores in 3 of 4 related domains (physical functioning, role physical, general health, figure 5; scores for all domains are available in table e-3 available from Dryad, doi.org/10.5061/dryad.g0t911k). HRQOL scores were comparable for the MCS (71.8 [95% CI 65.4–78.3] vs 77.9 [95% CI 76.8–79.0], p = 0.19) but lower for the vitality and social functioning domains (table e-3 available from Dryad, doi.org/10.5061/dryad.g0t911k). Between LEMS and MG, the composite scores and most of the domain subscores were comparable except for lower scores for patients with LEMS in the physical functioning subdomain (45.8 vs 62.2 in MG, p = 0.0001), which is dominated by questions involving leg strength.
Figure 5. Health-related quality of life in LEMS compared to MG and the Dutch general population.
All scores range from 0 to 100. PCS = Physical Composite Score; MCS = Mental Composite Score; SF-36 = Short Form-36. *A significant difference between patients with Lambert-Eaton myasthenic syndrome (LEMS) and the Dutch general population. **A significant difference between LEMS and myasthenia gravis (MG).
Univariate analysis of potential predicting variables for physical and mental HRQOL composite scores showed employment status, pattern of muscle weakness, and mRS scores to be significantly associated with PCS (table 1). State of employment and whether patients had a partner were associated with MCS. A multivariate analysis aiming to detect independent predictors for quality of life (table 1) showed having a partner and employment status to be independently associated with higher PCS and MCS. In addition, a higher HRQOL was linked to a more limited pattern of muscle weakness for the PCS and male sex for the MCS. The mRS score did not affect either PCS or MCS in this multivariate model.
Discussion
This study shows that patients with LEMS without an associated tumor have a normal survival, confirms that patients with SCLC-LEMS have an improved tumor survival compared to patients with SCLC without LEMS, and shows that patients with LEMS can have a relatively well-controlled life with mainly physical limitations and normal mental quality of life.
In contrast to patients with MG, we show that survival in patients with LEMS without an associated tumor was similar to the average life expectancy in the Netherlands.32–34 The increased mortality in MG was at least partially related to an increase in respiratory disease as a cause of death,35 likely related to respiratory muscle weakness, which can occur in both MG and LEMS but might be less frequent in patients with LEMS given the lack of an increase in mortality.
Our study shows that tumor survival is increased in all patients with SCLC-LEMS with both limited and extensive disease. Median survival is doubled in patients with SCLC-LEMS with extensive disease compared to patients with SCLC without LEMS, and overall 5-year survival is increased from 4.4% to 21%. Survival in patients with SCLC without LEMS (limited disease) is comparable to that of patients with SCLC-LEMS with extensive disease. Several previous smaller studies have reported this improved survival,14,15,36 including a recent prospective cohort study of patients with SCLC with and without LEMS.17 Our study shows that this improved survival cannot merely be attributed to tumor stage (patients with SCLC-LEMS are more frequently found while still having limited disease). There will be an inevitable lead-time bias due to earlier diagnosis of SCLC because of neuromuscular symptoms, but this cannot fully explain the survival difference. This additional improvement in survival supports a biochemical or immunologic cause such as an antitumor immune response.
We show that the majority of patients with LEMS have a relatively stable disease course after diagnosis and treatment. Most patients either remain or become independent for self-care over time after appropriate treatment. Because disease severity directly affects treatment decisions, especially whether to add immunosuppressive treatment, we could not compare the effect of individual treatments. We did note that patients treated symptomatically improve sooner after diagnosis than those treated with immunosuppressive drugs (probably a confounder by indication), but both groups ultimately reach a relatively stable level of limitations ≈2 years after diagnosis. Maximum disease severity has already been reached before diagnosis in a majority of patients and within the first 2 years in ≈80%, the latter of which is very similar to that in MG.34,37 Patients with LEMS with associated lung cancer report more functional impairments over the entire disease course. Both LEMS symptoms, which can be more progressive in SCLC-LEMS,3,16,24 and lung cancer and related treatment are likely to contribute to disability in this group. Patients with SCLC-LEMS also seem to have a higher exacerbation rate, although this should be interpreted with caution because follow-up in this group is shorter and exacerbations seem more likely to occur in the first years after diagnosis. It should be noted, however, that most of these patients still become independent for activities of daily life after treatment and seem to have overall HRQOL comparable to that of patients with LEMS without an associated tumor (table 1). This supports the notion that low performance scores due to muscle weakness in SCLC-LEMS should not be a reason to refrain from tumor treatment, especially because tumor treatment can improve symptoms in paraneoplastic disease.13
In patients with LEMS with associated lung cancer, LEMS symptoms usually precede tumor diagnosis. However, after initial treatment and improvement of both diseases, frequently no exacerbation of LEMS occurs on tumor progression as a (repeated) warning. This could mean either that tumor progression does not elicit such a strong immune response as the initial tumor presentation or that an exacerbation of LEMS would require more time to develop, as is the case before the start of the disease.
The reduced HRQOL in patients with LEMS was comparable to that in patients with MG and related mostly to physical limitations. General demographic factors seemed to predict variation in HRQOL that was at least as strong as disease-specific variables in our population, especially for mental health. Several previous studies in MG have reported reduced HRQOL in patients with MG for most domains of the SF-36.27,38–40 Our study showed female sex, generalized disease, and lack of employment to be associated with reduced HRQOL, comparable to results in 2 large MG studies.27,41 In contrast to the pattern of muscle weakness, the mRS score as a marker for disease severity did not independently predict HRQOL. This could be related to the limited number of patients, limited overall variation in mRS scores, or confounding by also including the pattern of weakness in the model.
The largest previous study concerning disease course in LEMS (n = 47) focused on muscle strength scores and EMG and antibody results. In contrast, we report patient-oriented outcomes, including functional impairments and quality of life.10 This previous study10 also reported a variable prognosis, with sustained clinical remission in 43% of patients and about a quarter of patients remaining (at least partially) wheelchair dependent at follow-up. In this cohort, both treatment with immunosuppressants and sustained clinical remission occurred more frequently compared to our study. Although this might suggest an association between the two, we consider it more likely a difference in definition of clinical remission, because many patients in our study still report a decrease in their level of work and social activities even after substantial or apparent full clinical improvement without major objective weakness at the outpatient clinic. A smaller study of 12 patients with LEMS reported lifestyle limitations comparable to our cohort, with restrictions in activities of daily living in 75% of patients, poor reported health status, and low HRQOL scores as measured by EQ-5D utility scores.11 Previous follow-up of 16 patients with SCLC-LEMS reported sustained improvement of LEMS after tumor treatment.13 Our study confirms that patients with SCLC-LEMS can improve and regain independence for self-care, but these patients still experience limitations in daily life.
Limitations of our study include a relatively small sample size inherent to the rarity of this disease, the partly retrospective nature of the study, and the use of different subpopulations for disease course and HRQOL. The limited number of deaths in our study precludes a certain conclusion that survival is normal in patients with LEMS without associated tumor. Lacking sufficient EMG or laboratory parameters for comparison, we specifically focused on patient-oriented outcomes because they represent patients' limitations best. Functional impairments could have been influenced by comorbidity, but this effect is group-wise minimal in that only 2 of 22 patients with SCLC-LEMS had another paraneoplastic neurologic disease (cerebellar degeneration). In addition, in the few patients with relevant comorbidity, the level of physical functioning appeared to be determined mainly by LEMS.
This study provides detailed information on long-term prognosis and limitations in LEMS. This can guide expectations of doctors and patients and be of potential relevance for treatment choices. Although LEMS is usually a chronic disease with long-term physical limitations and reduced quality of life, appropriate treatment results in a relevant decrease in functional impairments for most patients.
Acknowledgment
The authors thank all referring physicians, especially A.J. van der Kooi, J. Poelen, L.H. van den Berg, M.H. de Baets, and N.C. Voermans.
Glossary
- CI
confidence interval
- DELTA-P
Dutch-English LEMS Tumour Association Prediction
- HRQOL
health-related quality of life
- KPS
Karnofsky Performance Scale
- LEMS
Lambert-Eaton myasthenic syndrome
- MCS
Mental Composite Score
- MG
myasthenia gravis
- mRS
modified Rankin Scale
- PCS
Physical Composite Score
- SCLC
small cell lung cancer
- SF-36
Short Form-36
- VGCC
voltage-gated calcium channel
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study funding
No targeted funding reported.
Disclosures
A. Lipka, M. Boldingh, E. van Zwet, M. Schreurs, J. Kuks, and C. Tallaksen report no disclosures relevant to the manuscript. M. Titulaer received research funds for serving on a scientific advisory board for MedImmune, fees for consultation from Guidepoint Global, an unrestricted research grant from Euroimmun AG, and a travel grant for lecturing in India from Sun Pharma, India. J. Verschuuren reports involvement in an NIH-sponsored thymectomy trial (ClinicalTrials.gov, number NCT00294658), reports a FP7 European MG grant (602420), and is a consultant for Alexion Pharmaceuticals and arGEN-X. Go to Neurology.org/N for full disclosures.
References
- 1.O'Neill JH, Murray NM, Newsom-Davis J. The Lambert-Eaton myasthenic syndrome: a review of 50 cases. Brain 1988;111:577–596. [DOI] [PubMed] [Google Scholar]
- 2.Titulaer MJ, Lang B, Verschuuren JJ. Lambert-Eaton myasthenic syndrome: from clinical characteristics to therapeutic strategies. Lancet Neurol 2011;10:1098–1107. [DOI] [PubMed] [Google Scholar]
- 3.Titulaer MJ, Wirtz PW, Kuks JB, et al. The Lambert-Eaton myasthenic syndrome 1988-2008: a clinical picture in 97 patients. J Neuroimmunol 2008;201-202:153–158. [DOI] [PubMed] [Google Scholar]
- 4.Verschuuren JJ, Wirtz PW, Titulaer MJ, Willems LN, van Gerven J. Available treatment options for the management of Lambert-Eaton myasthenic syndrome. Expert Opin Pharmacother 2006;7:1323–1336. [DOI] [PubMed] [Google Scholar]
- 5.Keogh M, Sedehizadeh S, Maddison P. Treatment for Lambert-Eaton myasthenic syndrome. Cochrane Database Syst Rev 2011:Cd003279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Sonderen A, Wirtz PW, Verschuuren JJ, Titulaer MJ. Paraneoplastic syndromes of the neuromuscular junction: therapeutic options in myasthenia gravis, Lambert-Eaton myasthenic syndrome, and neuromyotonia. Curr Treat Options Neurol 2013;15:224–239. [DOI] [PubMed] [Google Scholar]
- 7.Nakao YK, Motomura M, Fukudome T, et al. Seronegative Lambert-Eaton myasthenic syndrome: study of 110 Japanese patients. Neurology 2002;59:1773–1775. [DOI] [PubMed] [Google Scholar]
- 8.Wirtz PW, Smallegange TM, Wintzen AR, Verschuuren JJ. Differences in clinical features between the Lambert-Eaton myasthenic syndrome with and without cancer: an analysis of 227 published cases. Clin Neurol Neurosurg 2002;104:359–363. [DOI] [PubMed] [Google Scholar]
- 9.Waterman SA. Autonomic dysfunction in Lambert-Eaton myasthenic syndrome. Clin Auton Res 2001;11:145–154. [DOI] [PubMed] [Google Scholar]
- 10.Maddison P, Lang B, Mills K, Newsom-Davis J. Long term outcome in Lambert-Eaton myasthenic syndrome without lung cancer. J Neurol Neurosurg Psychiatry 2001;70:212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harms L, Sieb JP, Williams AE, et al. Long-term disease history, clinical symptoms, health status, and healthcare utilization in patients suffering from Lambert Eaton myasthenic syndrome: results of a patient interview survey in Germany. J Med Econ 2012;15:521–530. [DOI] [PubMed] [Google Scholar]
- 12.Lennon VA, Kryzer TJ, Griesmann GE, et al. Calcium-channel antibodies in the Lambert-Eaton syndrome and other paraneoplastic syndromes. New Engl J Med 1995;332:1467–1474. [DOI] [PubMed] [Google Scholar]
- 13.Chalk CH, Murray NM, Newsom-Davis J, O'Neill JH, Spiro SG. Response of the Lambert-Eaton myasthenic syndrome to treatment of associated small-cell lung carcinoma. Neurology 1990;40:1552–1556. [DOI] [PubMed] [Google Scholar]
- 14.Maddison P, Newsom-Davis J, Mills KR, Souhami RL. Favourable prognosis in Lambert-Eaton myasthenic syndrome and small-cell lung carcinoma. Lancet 1999;353:117–118. [DOI] [PubMed] [Google Scholar]
- 15.Maddison P, Lang B. Paraneoplastic neurological autoimmunity and survival in small-cell lung cancer. J Neuroimmunol 2008;201-202:159–162. [DOI] [PubMed] [Google Scholar]
- 16.Wirtz PW, Wintzen AR, Verschuuren JJ. Lambert-Eaton myasthenic syndrome has a more progressive course in patients with lung cancer. Muscle Nerve 2005;32:226–229. [DOI] [PubMed] [Google Scholar]
- 17.Maddison P, Gozzard P, Grainge MJ, Lang B. Long-term survival in paraneoplastic Lambert-Eaton myasthenic syndrome. Neurology 2017;88:1334–1339. [DOI] [PubMed] [Google Scholar]
- 18.Titulaer MJ, Verschuuren JJ. Lambert-Eaton myasthenic syndrome: tumor versus nontumor forms. Ann NY Acad Sci 2008;1132:129–134. [DOI] [PubMed] [Google Scholar]
- 19.Wirtz PW, van Dijk JG, van Doorn PA, et al. The epidemiology of the Lambert-Eaton myasthenic syndrome in the Netherlands. Neurology 2004;63:397–398. [DOI] [PubMed] [Google Scholar]
- 20.Oh SJ, Kurokawa K, Claussen GC, Ryan HF Jr. Electrophysiological diagnostic criteria of Lambert-Eaton myasthenic syndrome. Muscle Nerve 2005;32:515–520. [DOI] [PubMed] [Google Scholar]
- 21.Cox FM, Titulaer MJ, Sont JK, Wintzen AR, Verschuuren JJ, Badrising UA. A 12-year follow-up in sporadic inclusion body myositis: an end stage with major disabilities. Brain 2011;134:3167–3175. [DOI] [PubMed] [Google Scholar]
- 22.Central Statistics of the Netherlands. Available at statline.cbs.nl. Accessed September 24, 2015.
- 23.Netherlands Cancer Registry. Netherlands Cancer Registry Operated by Netherlands Comprehensive Cancer Organisation. Accessed October 26, 2015.
- 24.Titulaer MJ, Maddison P, Sont JK, et al. Clinical Dutch-English Lambert-Eaton Myasthenic syndrome (LEMS) tumor association prediction score accurately predicts small-cell lung cancer in the LEMS. J Clin Oncol 2011;29:902–908. [DOI] [PubMed] [Google Scholar]
- 25.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–607. [DOI] [PubMed] [Google Scholar]
- 26.Wilson JT, Hareendran A, Grant M, et al. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke 2002;33:2243–2246. [DOI] [PubMed] [Google Scholar]
- 27.Boldingh MI, Dekker L, Maniaol AH, et al. An up-date on health-related quality of life in myasthenia gravis: results from population based cohorts. Health Qual Life Outcomes 2015;13:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aaronson NK, Muller M, Cohen PD, et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol 1998;51:1055–1068. [DOI] [PubMed] [Google Scholar]
- 29.Ware JEJ, Kosinski MA. SF-36 Physical & Mental Health Summary Scales: A Manual for Users of Version 1. 2nd ed. Lincoln, RI: QualityMetric Incorporated; 2001. [Google Scholar]
- 30.de Meel RH, Lipka AF, van Zwet EW, Niks EH, Verschuuren JJ. Prognostic factors for exacerbations and emergency treatments in myasthenia gravis. J Neuroimmunol 2015;282:123–125. [DOI] [PubMed] [Google Scholar]
- 31.Mould R. Introductory Medical Statistics. 3rd ed. Bristol: Institute of Physics Pub., 1998. [Google Scholar]
- 32.Basta I, Pekmezovic T, Peric S, et al. Survival and mortality of adult-onset myasthenia gravis in the population of Belgrade, Serbia. Muscle Nerve 2018;58:708–712. [DOI] [PubMed] [Google Scholar]
- 33.Christensen PB, Jensen TS, Tsiropoulos I, et al. Mortality and survival in myasthenia gravis: a Danish population based study. J Neurol Neurosurg Psychiatry 1998;64:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Somnier FE, Keiding N, Paulson OB. Epidemiology of myasthenia gravis in Denmark: a longitudinal and comprehensive population survey. Arch Neurol 1991;48:733–739. [DOI] [PubMed] [Google Scholar]
- 35.Owe JF, Daltveit AK, Gilhus NE. Causes of death among patients with myasthenia gravis in Norway between 1951 and 2001. J Neurol Neurosurg Psychiatry 2006;77:203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wirtz PW, Lang B, Graus F, et al. P/Q-type calcium channel antibodies, Lambert-Eaton myasthenic syndrome and survival in small cell lung cancer. J Neuroimmunol 2005;164:161–165. [DOI] [PubMed] [Google Scholar]
- 37.Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve 2008;37:141–149. [DOI] [PubMed] [Google Scholar]
- 38.Padua L, Evoli A, Aprile I, et al. Quality of life in patients with myasthenia gravis. Muscle Nerve 2002;25:466–467. [DOI] [PubMed] [Google Scholar]
- 39.Paul RH, Nash JM, Cohen RA, Gilchrist JM, Goldstein JM. Quality of life and well-being of patients with myasthenia gravis. Muscle Nerve 2001;24:512–516. [DOI] [PubMed] [Google Scholar]
- 40.Winter Y, Schepelmann K, Spottke AE, et al. Health-related quality of life in ALS, myasthenia gravis and facioscapulohumeral muscular dystrophy. J Neurol 2010;257:1473–1481. [DOI] [PubMed] [Google Scholar]
- 41.Twork S, Wiesmeth S, Klewer J, Pohlau D, Kugler J. Quality of life and life circumstances in German myasthenia gravis patients. Health Qual Life Outcomes 2010;8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.